Abstract
Introduction
Progestin therapy is the only fertility-sparing treatment option for patients with atypical endometrial hyperplasia (AEH) and endometrial cancer (EC). However, the results of three meta-analyses revealed a high remission rate, as well as an association with a high rate of relapse. We previously conducted a phase II of medroxyprogesterone acetate (MPA) plus metformin as a fertility-sparing treatment for AEH and EC patients, and reported that metformin inhibited disease relapse after remission.
Methods and analysis
A randomised, open, blinded-endpoint design phase IIb dose response trial was planned to commence in July 2019. The trial aims to identify the appropriate dose of metformin to be combined with MPA therapy for fertility-sparing treatment of patients with AEH and EC. The primary endpoint of the trial is the 3-year relapse-free survival (RFS) rate. The secondary endpoints are RFS rate, the overall rate of response to MPA therapy, the conception rate after treatment, the outcome of pregnancy, toxicity evaluation and changes in insulin resistance and body mass index. A total of 120 patients will be enrolled from 15 Japanese institutions within a 2.5-year period and followed up for at least 3 years.
Ethics and dissemination
The protocol was approved by the institutional review board at Chiba University Hospital and boards at 14 other institutions. The trial will be conducted according to the principles of the World Medical Association’s Declaration of Helsinki and in accordance with Good Clinical Practice (GCP) standards. The trial findings will be published in a peer-reviewed journal.
Trial registration number
Japan Registry of Clinical Trials (jRCT2031190065).
Keywords: gynaecological oncology, adult oncology, gynaecological oncology
Strengths and limitations of this study.
This is the first randomised controlled trial that aims to identify the appropriate metformin dose to be added to medroxyprogesterone acetate (MPA) therapy for fertility-sparing treatment of patients with atypical endometrial hyperplasia (AEH) and endometrial cancer (EC).
The trial has been designed to meet high-quality randomised clinical trial criteria by performing central randomisation and ensuring multicentre participation.
Since the association between the trial primary endpoint, 3-year relapse-free survival rate and MPA plus metformin treatment will be examined, this study can evaluate the efficacy of the addition of metformin to medroxyprogesterone in fertility-sparing treatment of AEH and EC patients.
To reduce the bias associated with the open-label design, and to maintain objectivity, we used blinded investigators to evaluate the endpoint, and judgment was based on standardised criteria for central pathological diagnosis.
A limitation of this trial is that it is not confirmatory in the evaluation of the primary endpoint.
Introduction
Progestin therapy is the only fertility-sparing treatment option for patients with atypical endometrial hyperplasia (AEH) and endometrial cancer (EC). The guidelines of the National Comprehensive Cancer Network and the European Society of Gynecological Oncology Task Force regarding fertility-sparing treatment recommend progestin therapy for patients with AEH and EC who wish to conceive.1 2 However, the results of three meta-analyses revealed a high remission rate, as well as an association with a high rate of relapse.3–5
EC is often associated with obesity and/or diabetes mellitus, indicating that insulin resistance is a risk factor for EC.6–8 We previously reported the incidence of obesity, insulin resistance and abnormal glucose metabolism among young EC patients as 84%, 83% and 78%, respectively.9 Hyperinsulinemia due to insulin resistance plays an important role in carcinogenesis.10 Epidemiological and basic research studies support this notion; thus, insulin resistance may be a promising target for the treatment and prevention of EC.10 11
Metformin is a biguanide that is widely used for the treatment of type 2 diabetes mellitus. Several recent epidemiological studies have revealed that metformin reduces the incidence of cancer and cancer-related mortality in diabetes patients.12 13
Moreover, metformin inhibited the growth of breast, ovarian, endometrial and prostate cancer cells, probably through the suppression of mitogen-activated protein kinase (MAPK), cyclin D1 and mammalian target of rapamycin activity.14 15 Furthermore, metformin enhanced ovulation in patients with polycystic ovary syndrome and improved pregnancy rates.16 17
Based on this information, we previously conducted a phase II study of medroxyprogesterone acetate (MPA) plus metformin as a fertility-sparing treatment for AEH and EC patients and reported that metformin had inhibited disease relapse after remission. Additionally, metformin prevented weight gain caused by MPA and had improved insulin sensitivity among the registered patients.18
Therefore, we conducted a dose–response randomised phase II study of MPA plus metformin for fertility-sparing treatment of AEH and EC. The trial protocol was approved by the Pharmaceuticals and Medical Devices Agency (PMDA), Japan, in June 2019 and the trial was initiated in July 2019. Approval was obtained from the institutional review board (IRB) prior to patient recruitment at each institution.
Methods and analysis
Objectives
The purpose of this trial is to verify the appropriate dose of metformin in a new fertility-sparing treatment involving a combination of metformin and MPA among patients with AEH and EC. Furthermore, this trial aims to investigate the long-term efficacy and safety of this combination therapy.
Design of the trial
This trial is a prospective, randomised, open, blinded-endpoint design (PROBE), dose–response trial. Original Japanese protocol and informed consent form are provided in the online supplementary appendixes 1 and 2, and this protocol meets the criteria of the Standard Protocol Items: Recommendations for Interventional Trials 2013 statement. The trial is being conducted in 15 institutions across Japan. A list of recruiting sites is provided in online supplementary appendix 3. A local principal investigator (PI), supported by at least two other staff members (such as a research nurse or clinical research coordinator), conducts the study at each participating site.
bmjopen-2019-035416supp001.pdf (3.4MB, pdf)
bmjopen-2019-035416supp002.pdf (3.7MB, pdf)
bmjopen-2019-035416supp003.pdf (48.5KB, pdf)
Endpoints
The primary endpoint of the trial is the 3-year relapse-free survival (RFS) rate, which indicates the achievement of remission without recurrence 3 years from the date of trial entry for all subjects.
The secondary endpoints are RFS rate, the overall response rate to MPA therapy, conception rate following the treatment, the outcome of pregnancy, results of toxicity assessment and changes in insulin resistance and body mass index (BMI). The RFS period was defined as the period from the date of trial entry to the time of recurrence after remission or to the time of exclusion from the trial due to non-remission. Cases of non-remission are defined as those that experience disease progression before 32 weeks and those that do not achieve remission by 32 weeks. The conception rate was defined as the rate of successful pregnancies among patients who were in remission and tried to have a child.
Eligibility criteria
All patients underwent a 75 g oral glucose tolerance test before registration for the evaluation of glucose intolerance and insulin resistance.
Inclusion criteria
Histologically confirmed AEH or well-differentiated endometrioid adenocarcinoma, presumed to be International Federation of Gynecology and Obstetrics stage IA, and in which the EC was limited to the endometrium. Endometrial tissue sampling for diagnosis was performed by dilatation and curettage.
No prior treatment with a high dose of progestin, the levonorgestrel-releasing intrauterine system or chemotherapy for an endometrial lesion (the uses of low or medium doses of a progestin for menstrual cycle regulation were permitted).
Over 20 years of age and under 42 years of age.
Eastern Cooperative Oncology Group Performance Status 0.
-
Fulfilment of each of the following parameters:
Leucocyte count ≥ 3.0×109/L.
Platelet count ≥ 100×109/L.
Aspartate aminotransferase (glutamic-oxaloacetic transaminase) level less than twice the maximum institutional standard.
Alanine aminotransferase (glutamic-pyruvic transaminase) level less than twice the maximum institutional standard.
Serum creatinine concentration ≤ 1.0 mg/dL.
Creatinine clearance ≤ 60 L/min.
Total bilirubin level ≤ 1.5 mg/dL.
D-dimer level < 1.5 µg/mL.
No prior medical history of chemical sensitivity to any of the chemicals being used in the trial (MPA and metformin).
No clinical problem on electrocardiography.
The patient provided written informed consent.
Exclusion criteria
-
Contraindication to the drugs used in this trial (MPA and metformin):
-
Susceptible to lactic acidosis.
A history of lactic acidosis.
Moderate to severe kidney damage or dialysis.
Severe liver damage.
Shock, cardiac arrest, cardiac infarction, thrombosis of the lung or severe cardiovascular and/or lung damage.
Ingestion of excessive amounts of alcohol.
Gastrointestinal damage such as dehydration or diarrhoea and/or vomiting that could cause dehydration.
Serious ketosis, diabetic coma or pre-coma, or type 1 diabetes.
Serious infections and/or injuries, a medical history, including recent surgery, or the expectation that the patient will soon undergo surgery.
Malnourishment or starvation, hyposthenia or pituitary and/or adrenal gland disorders.
Pregnancy or the expectation that the woman could be pregnant.
Prior medical history of chemical sensitivity to biguanides or the compounds present in the drugs being tested.
-
High risk of thrombosis.
Surgery within the past week.
Cerebral and/or cardiac infarction, thrombophlebitis, or a prior history thereof.
Valvular heart disease, atrial fibrillation, endocarditis or other serious cardiac conditions.
The current administration of hormone treatments.
Undiagnosed breast lesions or vaginal and/or urinary bleeding.
Serious liver damage.
Hypocalcemia.
-
Psychosis or mental symptoms that could make it difficult for patients to participate in the trial.
Uncontrolled diabetes mellitus.
Diabetic patients who already take biguanides, including metformin (patients who manage their diabetes via diet and exercise therapy, or those who take medicines apart from metformin are eligible).
Concomitant malignancies.
Systemic administration of steroids.
Determined to be ineligible by the physician in charge for any other reason.
Randomisation
After confirming the fulfilment of the eligibility criteria, patients are randomly assigned to arm A (MPA alone group), arm B (MPA + metformin 750 mg/day group) and arm C (MPA + metformin 1500 mg/day group) in a 1:1:1 allocation via a dynamic and centralised randomisation procedure implemented with the DATATRAK Electronic Data Capture system (DATATRAK ONE V.14.1.0; https://secure.datatrak.net). Minimisation imbalance method with a probability of 0.9 is used for randomisation.19 The stratification factors to be balanced across treatment arms are BMI, histology and marital status.
Treatment methods
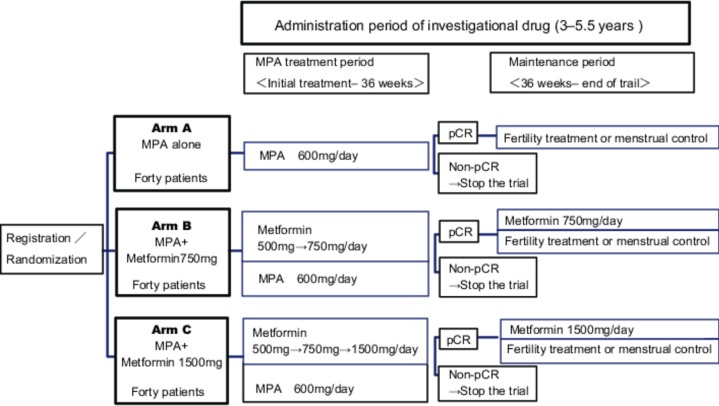
The patients are randomised into three treatment arms (figure 1).
Figure 1.
Trial design. MPA, medroxyprogesterone acetate; pCR, pathological complete response.
Arm A (control) MPA alone group: patients receive a daily oral dose of 600 mg of MPA for 32 weeks.
Arm B (experimental) MPA+ metformin 750 mg/day group: patients receive a daily oral dose of 600 mg of MPA for 32 weeks.
Patients simultaneously receive a daily dose of metformin (initial dose, 500 mg/day; increased monthly up to 750 mg/day if no adverse effects have developed) in combination with MPA from the initial point of treatment.
After MPA administration, metformin therapy is continued until conception or disease recurrence.
Arm C (experimental): MPA + metformin 1500 mg/day group: patients receive a daily oral dose of 600 mg of MPA for 32 weeks.
Patients simultaneously receive a daily dose of metformin (initial dose, 500 mg/day; increased monthly up to 1500 mg/day if no adverse effects have developed) in combination with MPA from the initial point of treatment.
After MPA administration, metformin therapy is continued until conception or disease recurrence.
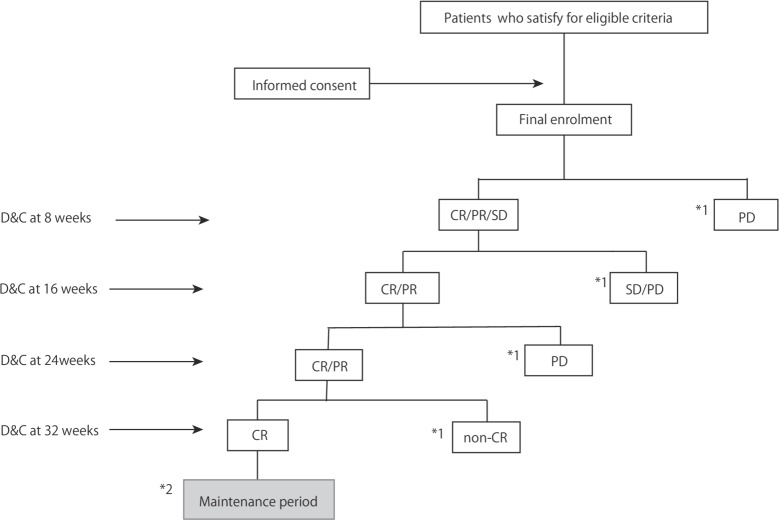
Endometrial curettage under anaesthesia will be performed to evaluate pathological response every 8 weeks.(figure 2).
Figure 2.
Judgement of remission flow chart during treatment period. *1, the protocol treatment is halted; *2, fertility treatment or menstrual control. CR, complete response; D&C, dilation and curettage; PD, progressive disease; PR, partial response; SD, stable disease.
The protocol treatment was halted if MPA therapy did not result in remission, according to the following criteria: the absence of any hormonal effect by 16 weeks; disease progression at 8, 16 and 24 weeks or no remission at 32 weeks. A combination of 100 mg of aspirin was permissible if patients received MPA.
Maintenance period
After completing the MPA treatment, only patients who achieved remission will enter the maintenance period. Follow-up will be performed by conducting endometrial sampling with a pipelle or with any other appropriate equipment. Patients are examined every 3 months until 3 years after the initial treatment (at the time of evaluation for the primary endpoint). After that, patients are examined every 6 months. If a patient desires to conceive in the immediate future, they are allowed to become pregnant immediately after achieving remission. Fertility treatment is recommended. A low dose of estrogen–progestin or medium doses of progestin is recommended for patients who do not desire to conceive, to control the menstrual cycle until they wish to conceive.
Criteria for discontinuation of trial treatment
The defined criteria for the discontinuation of trial medication are as follows:
The protocol treatment was halted if MPA therapy did not result in remission, according to the following criteria: the absence of any hormonal effect by 16 weeks; disease progression at 8, 16 and 24 weeks or no remission at 32 weeks.
If the patients relapse after remission.
Severe adverse effects (progressive or persistent), which in the opinion of the PI may be related to the study medication.
Other situations, judged by the PI, in which MPA or metformin cannot be continued.
Data management, monitoring, safety and auditing
Monitors will ensure that the investigational team is complying with the study protocol and Good Clinical Practice (GCP) standards, that the data and AEs are accurately and appropriately recorded in the electric case report forms (eCRFs), that severe AEs (SAEs) are reported to the trial coordinator and the investigational drug provider and that those meeting the SAE reporting criteria are reported to the IRB. AEs will be classified in accordance with the Medical Dictionary for Regulatory Activities, Japanese translation MedDRA/J V.22.0 (MedDRA Japanese Maintenance Organization, Tokyo, Japan). All participants with AEs are to be followed up during the course of the AE until their resolution, or for 4 weeks after the end of the trial. All SAEs will be reported to all investigators, discussed through a web-based AE reporting system, and will be reported to the PMDA, if necessary.
Data monitoring committee
The data monitoring committee comprises three clinical trial specialists, including a biostatistician, who were not associated with this study. The committee will meet at least twice a year and all the data obtained from the current trial will be checked by the committee.
Statistical analysis
Primary endpoints
An analysis of the effectiveness of primary endpoints will be conducted on the full analysis set. The following closed testing procedure will be used to assess the three groups: MPA-alone group as the control group, the group with MPA plus metformin (750 mg) and the group with MPA plus metformin (1500 mg).
The MPA-alone, MPA plus metformin (750 mg) and MPA plus metformin (1500 mg) groups will be pooled and analysed via a log-rank test. If the difference is significant, move to the next step; if not, terminate the procedure.
For the MPA-alone and the MPA plus metformin (1500 mg) groups, a log-rank test will be performed. If the difference is significant, move to the next step; if not, terminate the procedure.
For the MPA-alone and MPA plus metformin (750 mg) groups, a log-rank test will be performed. If the difference is significant, move to the next step; if not, terminate the procedure.
For the MPA plus metformin (750 mg) and MPA plus metformin (1500 mg) groups, a log-rank test will be performed.
Secondary endpoints
The same analysis performed on primary endpoints will be applied to the per protocol set. In addition, an analysis based on the Cox proportional hazards model will be carried out by adjusting for necessary allocation factors and layers.
Other items to be evaluated are the complete response rate for the MPA treatment period; the rate of trial continuation for the MPA-alone and MPA plus metformin groups; the recurrence-free survival time and recurrence-free rate in each year for the maintenance period; BMI values and homeostatic model assessment of insulin resistance results of all response cases that expressed the desire to become pregnant; the proportion of patients that became pregnant at least once during the trial period; outcomes of pregnant patients (miscarriage, stillbirth, live birth and weeks of gestation), and of all response cases that expressed the desire to become pregnant; and the proportion of patients that gave birth to a child.
Safety endpoints
In the analysis of secondary safety endpoints, the number and rate of adverse events in an adverse event rate safety analysis set will be evaluated. Particularly, we will evaluate items in which a grade 3 or greater adverse event will occur (grade 2 or greater in the case of neurotoxicity).
Target number of cases and the grounds for setting it
Based on previous studies, the non-recurrence rate for the MPA-alone group was assumed to be 60% and the non-recurrence rate for the MPA plus metformin group 85%. The same setting was applied for both groups for the following items: the response rate during the MPA treatment period was 80%, the probability of withdrawal during the maintenance period was 5%, the probability of pregnancy was 10%, patients for whom the trial was terminated during pregnancy were treated as non-recurrence-in-trial cases, the two-tailed significance level was 5% and the power was 80%.
Based on these configurations, the number of cases was calculated through simulation. As a result, the necessary number of cases per group is 40, and 120 in total. The number of patient allocation between AEH and EC has not been decided.
Interim analysis and monitoring
No interim analysis is planned.
Patient and public involvement
Patients and the public were not involved in the design of this trial.
Ethics and dissemination
We intend to publish results of the FELICIA trial in a major journal.
Discussion
This trial was planned to determine the appropriate dose of metformin in a new fertility-sparing treatment involving a combination of metformin and MPA among patients with AEH and EC. To confirm the appropriate dose of metformin, the RFS rate was set as the primary endpoint.
In Japan, pharmaceutical approval for the dose of metformin was for a maximum of 2250 mg/day and only for patients with diabetes mellitus. The recommended maintenance dose of metformin was 750–1500 mg/day. In a previous phase II trial, metformin (initial dose, 750 mg/day; increased weekly in increments of 750 mg/day up to 2250 mg/day if no adverse effects occurred) was administered concurrently with MPA since initiation of the treatment.18 Although the dose was reduced to under 1500 mg/day in some patients, no difference was found in treatment efficacy between the 1500 and 2250 mg groups (unpublished data). To consider long-term feasibility, we set the maximum dose of this trial as 1500 mg per day. This protocol was agreed on with regulatory strategy consultation of the PMDA.
Most studies of fertility-sparing treatments, including our previous phase II trial, have only evaluated relapse rates in remission patients.3 4 An evaluation of the efficacy of metformin depends on both the response rate and recurrence rate; however, even if remission rates are low, if response rates are also low, it may not necessarily mean that metformin is effective.
Therefore, we defined the 3-year RFS rate as the primary endpoint of this trial. This means the achievement of remission without recurrence 3 years from the date of trial enrolment.
This trial has some strengths. First, we are able to evaluate the long-term efficacy of metformin because the primary endpoint of this trial is the 3-year RFS rate. Following the first phase II trial of metformin combined with progestin for fertility-sparing treatment of AEH and EC started in 2009 and reported on in 2016, there are five trials currently examining the effect of metformin in fertility-sparing treatment for AEH and EC (NCT02035787, NCT03538704, NCT01968317, NCT02990728 and NCT01686126). In these studies, the primary endpoint is set as the pathological response rate. However, only one phase II trial has reported on relapse rate as a secondary endpoint. Second, further evidence of the long-term efficacy of metformin will be based on continued metformin therapy following the conclusion of MPA administration, until conception or disease recurrence. Based on our previous phase II trial results, we hypothesised that metformin might prevent recurrence after remission. Metformin had indirect effects that were caused by the lowering of glucose and insulin levels, as described above. However, in ongoing trials, metformin is administered combined with progestin and is discontinued following remission. In these trials, only an anticancer effect of metformin was expected as an outcome. Finally, this trial will evaluate metabolic status. Most of the candidate patients for this trial were obese, consistent with insulin resistance and abnormal glucose metabolism. Based on our previous phase II trial, we will evaluate improvements in the metabolic profiles of these patients that are anticipated with the addition of metformin.
A limitation of this trial is that design is not that of a confirmatory trial. In addition, this trial will not use a placebo control group. However, evaluation of the remission and relapse rates, which are associated with the primary endpoint, will be performed by a pathological review board. The evaluation will occur under blind and independent conditions. Therefore, we believe that it will be possible to maintain objectivity and to reduce potential bias. Finally, the PMDA approved our protocol for conducting this study based on the PROBE design. Another limitation is the small amount of participants. Subgroup analyses in AEH versus EC groups, and analyses based on the BMI or status of insulin resistance are particularly interesting. However, since there are only 40 patients in each group, we speculate that the subgroup analysis may have insufficient power for detecting a significant difference between the groups.
This trial is expected to clarify the clinical advantages of the combination of metformin with progestin. If this trial reveals the appropriate dose of metformin and shows long-term efficacy, the clinical use of metformin for fertility-sparing treatment of EC will be advanced.
bmjopen-2019-035416supp004.pdf (28.5KB, pdf)
Supplementary Material
Acknowledgments
We would like to thank all the staff and patients involved with the study at the 15 research sites, and the Trial Steering Committee and Central Pathology Committee and Data Monitoring Committee for their ongoing support throughout the trial. We would like to acknowledge the role of the Clinical Research Centre, Chiba University Hospital, in supporting the ongoing delivery of the trial at the sites.
Footnotes
Contributors: All authors made a significant contribution to the conception and design of the trial protocol. AM made major contributions to the design of this trial, development of the original trial protocol and drafting of the initial manuscript. YK developed the statistical analysis plan. All authors read and approved the final protocol.
Funding: This research is supported by the Japan Agency for Medical Research and Development under grant number JP19lk0201099.
Competing interests: HH has received personal fees from Torii Yakuhin, outside the submitted work.
Patient consent for publication: Obtained.
Ethics approval: The institutional review board (IRB) of each institution has approved the trial. The trial will be conducted in accordance with the Ordinance on the Standards for the Implementation of Clinical Trials (GCP Ordinance) and the principles of the World Medical Association's Declaration of Helsinki. All participants will receive adequate information about the nature, purpose, possible risk and benefits of the trial, and about alternative therapeutic choices, using an informed consent form approved by the IRB at Chiba University Hospital and boards at 14 other institutions (for the names of ethics committee, see online supplementary appendix 4). The informed consent form, signed by the participant, is required for enrolment in the trial.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. National Comprehensive Cancer Network Uterine neoplasms: NCCN clinical practice guidelines in oncology (NCCN Guidelines®). Available: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf [Accessed 11 Feb 2019].
- 2. Rodolakis A, Biliatis I, Morice P, et al. European Society of gynecological oncology Task force for fertility preservation: clinical recommendations for fertility-sparing management in young endometrial cancer patients. Int J Gynecol Cancer 2015;25:1258–65. 10.1097/IGC.0000000000000493 [DOI] [PubMed] [Google Scholar]
- 3. Gunderson CC, Fader AN, Carson KA, et al. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 2012;125:477–82. 10.1016/j.ygyno.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 4. Gallos ID, Yap J, Rajkhowa M, et al. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol 2012;207:266.e1–12. 10.1016/j.ajog.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 5. Wei J, Zhang W, Feng L, et al. Comparison of fertility-sparing treatments in patients with early endometrial cancer and atypical complex hyperplasia: a meta-analysis and systematic review. Medicine 2017;96:e8034 10.1097/MD.0000000000008034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579–91. 10.1038/nrc1408 [DOI] [PubMed] [Google Scholar]
- 7. Renehan AG, Tyson M, Egger M, et al. Body-Mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. 10.1016/S0140-6736(08)60269-X [DOI] [PubMed] [Google Scholar]
- 8. Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol 2002;3:565–74. 10.1016/S1470-2045(02)00849-5 [DOI] [PubMed] [Google Scholar]
- 9. Mitsuhashi A, Uehara T, Hanawa S, et al. Prospective evaluation of abnormal glucose metabolism and insulin resistance in patients with atypical endometrial hyperplasia and endometrial cancer. Support Care Cancer 2017;25:1495–501. 10.1007/s00520-016-3554-y [DOI] [PubMed] [Google Scholar]
- 10. Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 2012;12:159–69. 10.1038/nrc3215 [DOI] [PubMed] [Google Scholar]
- 11. Hernandez AV, Pasupuleti V, Benites-Zapata VA, et al. Insulin resistance and endometrial cancer risk: a systematic review and meta-analysis. Eur J Cancer 2015;51:2747–58. 10.1016/j.ejca.2015.08.031 [DOI] [PubMed] [Google Scholar]
- 12. Evans JMM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304–5. 10.1136/bmj.38415.708634.F7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, et al. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017;60:1639–47. 10.1007/s00125-017-4372-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quinn BJ, Kitagawa H, Memmott RM, et al. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab 2013;24:469–80. 10.1016/j.tem.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 15. Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 2014;10:143–56. 10.1038/nrendo.2013.256 [DOI] [PubMed] [Google Scholar]
- 16. Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med 2008;358:47–54. 10.1056/NEJMct0707092 [DOI] [PubMed] [Google Scholar]
- 17. Practice Committee of the American Society for Reproductive Medicine Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): a guideline. Fertil Steril 2017;108:426–41. 10.1016/j.fertnstert.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 18. Mitsuhashi A, Sato Y, Kiyokawa T, et al. Phase II study of medroxyprogesterone acetate plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Ann Oncol 2016;27:262–6. 10.1093/annonc/mdv539 [DOI] [PubMed] [Google Scholar]
- 19. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15. 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-035416supp001.pdf (3.4MB, pdf)
bmjopen-2019-035416supp002.pdf (3.7MB, pdf)
bmjopen-2019-035416supp003.pdf (48.5KB, pdf)
bmjopen-2019-035416supp004.pdf (28.5KB, pdf)