Abstract
Overdoses caused by the opioid agonist fentanyl have increased exponentially in recent years. Identifying mechanisms to counter progression to fatal respiratory apnea during opioid overdose is desirable, but difficult to study in vivo. The pontine Kölliker-Fuse/Parabrachial complex (KF/PB) provides respiratory drive and contains opioid-sensitive neurons. The contribution of the KF/PB complex to fentanyl-induced apnea was investigated using the in situ arterially perfused preparation of rat. Systemic application of fentanyl resulted in concentration-dependent respiratory disturbances. At low concentrations, respiratory rate slowed and subsequently transitioned to an apneustic-like, 2-phase pattern. Higher concentrations caused prolonged apnea, interrupted by occasional apneustic-like bursts. Application of CTAP, a selective mu opioid receptor antagonist, directly into the KF/PB complex reversed and prevented fentanyl-induced apnea by increasing the frequency of apneustic-like bursting. These results demonstrate that countering opioid effects in the KF/PB complex is sufficient to restore phasic respiratory output at a rate similar to pre-fentanyl conditions, which could be beneficial in opioid overdose.
Keywords: Pons, Kölliker-Fuse, Mu opioid, Respiratory depression
1. Introduction
Opioid overdose deaths have rapidly increased largely due to an exponential rise in fentanyl overdoses, leading to the current opioid epidemic in the United States (Rudd et al., 2016; Seth et al., 2018; Volkow and Collins, 2017). The main causative agent of opioid overdose is respiratory depression which progresses to complete apnea (Pattinson, 2008; Stoeckel et al., 1982). Opioid-induced respiratory depression is due to activation of mu opioid receptors (Dahan et al., 2001), which are located throughout the pontomedullary respiratory circuit (Lonergan et al., 2003; Mansour et al., 1994). Opioid-induced respiratory depression is characterized by a decrease in respiratory rate, tidal volume, and chemosensory drive, accompanied by an irregular breathing pattern (Bouillon et al., 2003; Pattinson, 2008; Stoeckel et al., 1982) and upper airway dysfunction (Christ et al., 2006; Savilampi et al., 2014, 2013).
Respiratory depressant effects of opioids have a striking resemblance to the physiological actions of the pontine Kölliker-Fuse/parabrachial complex (KF/PB). The KF/PB plays a vital role in respiratory rate control (Chamberlin and Saper, 1994; Levitt et al., 2015; Zuperku et al., 2016), chemosensory reflex control (Damasceno et al., 2014), eupneic respiratory pattern formation (Dhingra et al., 2019; Dutschmann and Herbert, 2006; Fung and St John, 1995; Lumsden, 1923; Smith et al., 2009), and hypoglossal motor output (Bautista and Dutschmann, 2014; Yokota et al., 2011), all of which fail during opioid-induced respiratory depression (Ehsan et al., 2016; Overdyk. et al., 2014; Hajiha et al., 2009; Pattinson, 2008). Furthermore, the KF has been implicated in generating apnea in the context of Rett syndrome and trigeminal stimulation (Abdala et al., 2016; Dutschmann and Herbert, 1996). Thus, we predict the opioid-induced depression of KF/PB neurons contributes to respiratory failure observed during opioid overdose.
Prior studies performed in vivo have shown that the KF/PB complex contributes to opioid effects on rate and variability (Hurlé et al., 1985; Levitt et al., 2015; Miller et al., 2017; Prkic et al., 2012; Varga et al., 2019). However, in vivo experiments are infrequently studied in the context of complete apnea. The goals of our studies were to 1) determine the neurophysiological progression to apnea caused by fentanyl and 2) determine the role of KF/PB opioid receptors in fentanyl-induced apnea. We used the in situ arterially perfused working heart-brainstem preparation of rat, with emphasis on phrenic and vagal nerve patterning (Paton, 1996; St.-John and Paton, 2004; Wilson et al., 2001). This preparation maintains in vivo-like, 3-phase respiratory motor output in the absence of anesthesia, and allowed us to investigate KF/PB-mediated overdose reversal in the context of complete central apnea without confounding peripheral and chemosensory factors that arise from cessation of ventilation and consequent decrease in systemic oxygenation.
In the arterially perfused working heart-brainstem preparation, application of mu opioid agonist DAMGO into the KF causes apneusis characterized by prolonged inspiratory time, loss of the post-inspiratory phase of respiration, and increased variability in phase timing (Levitt et al., 2015). The KF contains a significant population of neurons that are directly hyperpolarized by opioids (Levitt et al., 2015). We hypothesize that these opioid-sensitive KF neurons mediate effects of systemic opioid exposure, including progression to apnea. We tested this hypothesis using microinjection of the selective mu opioid receptor antagonist, d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), into the KF/PB complex. We found that microinjection of CTAP into the KF/PB complex prevented and reversed fentanyl-induced apnea by increasing the frequency of low-amplitude, long-duration, 2-phase, apneustic-like phrenic bursts. Thus, the KF/PB complex contributes to fentanyl-induced apnea, and the reversal of opioid effects in the KF/PB complex increases the frequency of a 2-phase respiratory pattern, but does not restore eupneic output.
2. Methods
2.1. Animals
Rats (Sprague–Dawley; male and female; P26-32; Charles River Laboratories) were used for all experiments. Rats were group housed on a 12-hour light-dark cycle with ad libitum access to food and water. Experiments were conducted in accordance with the National Institutes of Health guidelines and with approval from the Institutional Animal Care and Use Committee of the University of Florida. Mu opioid receptor (MOR)-knockout Sprague-Dawley rats with ZFN target site (GCTGTCTGCCACCCAGTCAAAGCCCTGGATTTC within exon 2) were generated by Horizon (St. Louis, MO), as described by Arttamangkul et al., 2018. The animals were bred and raised in house for two more generations before use in experiments. All care and use of MOR-knockout rats was performed at Oregon Health and Science University (Portland, OR) with approval from the OHSU Institutional Animal Care and Use Committee.
2.2. Drugs
d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) and l-Glutamic acid were from Tocris (Minneapolis, MN, USA). Fentanyl citrate was from Sigma-Aldrich (St Louis, MO, USA). Naloxone was from Abcam (Cambridge, MA, USA).
2.3. In situ arterially perfused working heart-brainstem preparation
Phrenic and vagus nerve recordings were obtained using in situ arterially perfused working heart-brainstem preparation, as described previously (Levitt et al., 2015; Paton, 1996). Rats were pretreated with heparin (1000 U, subcutaneous) 20 min prior to the start of surgical dissection. Rats were deeply anaesthetized with isoflurane until visible respirations had nearly ceased. Rats were then bisected inferior to the diaphragm, exsanguinated and placed in ice-cold Ringers solution containing, in mM: 125 NaCl, 3 KCl, 2.5 CaCl2, 1.25 MgCl2, 1.25 KH2PO4, 10 D-glucose and 24 NaHCO3. Rats were decerebrated at the precollicular level. The lungs were removed and the left phrenic nerve (PN) was isolated from the diaphragm and cut distally. The left central vagus nerve (cVN) was isolated from the neck and cut distally. The cerebellum was removed to allow accessibility to the dorsal surface of the brainstem.
The preparation was placed in the recording chamber in a right lateral recumbent position, a double lumen catheter was placed in the descending aorta and perfused, via peristaltic pump (Watson-Marlow Pump Pro MPL 580; flow rate 21-24 ml min−1), with warmed (32°C), carbogenated (95% O2/5% CO2), modified Ringers solution containing polyethylene glycol MW 20,000 (1.25 %) for oncotic pressure. The second lumen of the catheter output to a pressure transducer that allowed for monitoring and maintenance of perfusion pressure (50-70 mmMg). Vasopressin (200-400 pM, Sigma-Aldrich, St Louis, MO) was added to the perfusate to induce vasoconstriction and stabilize perfusion pressure. The preparation was observed during recovery until respiratory-related movements became evident. Vecuronium bromide (4 μg ml−1, Patterson Veterinary, Greeley, CO) was then added to the perfusate to paralyze skeletal muscle. The preparation was then placed in a prone position and mounted on ear bars of a stereotaxic frame (Kopf, Tujunga, CA).
The phrenic and vagus nerves were desheathed and whole nerve activity was recorded using glass suction electrodes (15 – 25 μm tip diameter). Recordings were AC amplified at 10 kHz (A-M Systems Model 1700, A-M Systems, Carlsborg, WA, USA), band-pass filtered (300 Hz–5 kHz), and digitized at 3–10 kHz (A–D converter and Spike2 software, Cambridge Electronic Design, Cambridge, UK). Nerve activity was positively rectified and integrated (time constant = 50 ms).
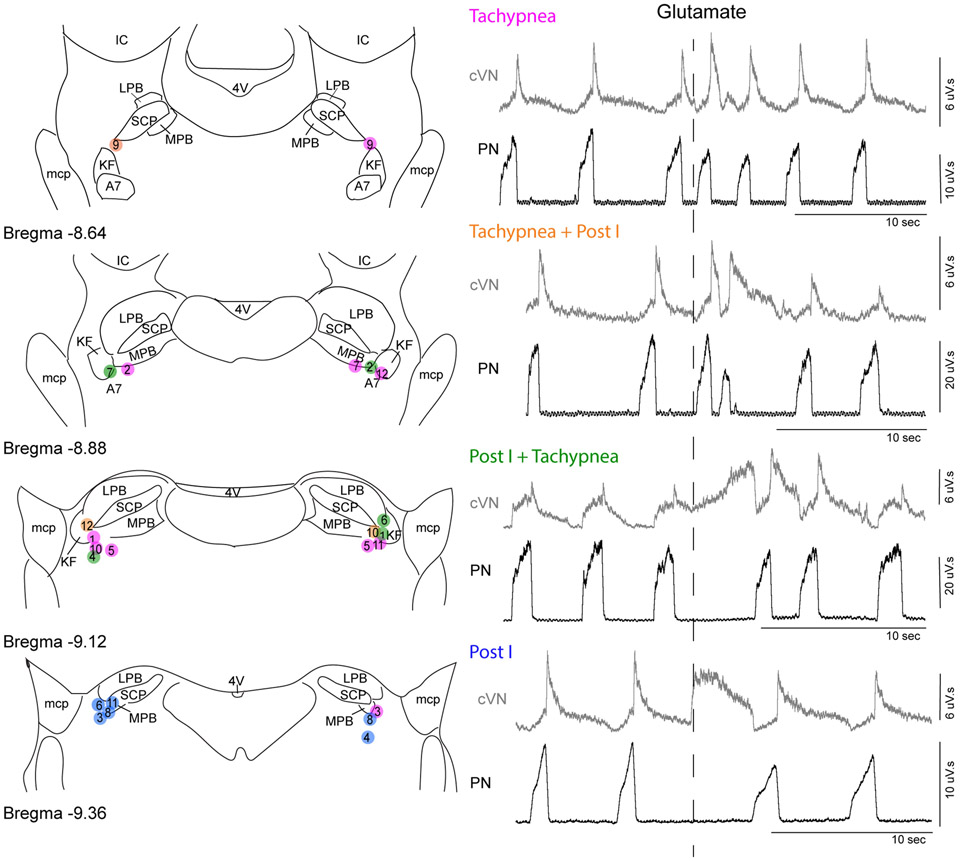
A custom-pulled triple barrel pipet was used for microinjections. The pipet contained glutamate (10 mM), CTAP (100 μM), and red fluorescent microspheres (Fluospheres 580/605, Invitrogen, 2 %) diluted in Ringers solution. Microinjection pipettes were placed just caudal to the inferior colliculus, 2.0-2.5 mm lateral from midline. The KF/PB was functionally identified with sequential (dorsal to ventral) glutamate microinjections (60 nl) until post-inspiratory apnea (pause in phrenic nerve firing with corresponding increase in cVN activity) or increase in phrenic rate, or mixed response was observed (Figure 1). These responses were similar to interleaved effects of glutamate in the KF/PB complex observed previously (Chamberlin and Saper, 1994; Dutschmann and Herbert, 2006; Gestreau et al., 2005). Respiratory effects occurred about 1 second following glutamate injection, lasted for 2 - 4 respiratory cycles, and were typically elicited 2.5 – 3.0 mm ventral from the exposed brainstem surface. Post-experiment histology revealed that injection sites were located ventrolateral to the tip of the superior cerebellar peduncle overlapping the KF, external medial PB and external lateral PB. Post-inspiratory apneas were generally, but not exclusively, elicited from more caudal injections (Figure 1). Stereotaxic coordinates of the positive injection site were recorded for further injections into the same location, and the contralateral KF/PB was identified as above.
Figure 1. Glutamate injections into KF/PB cause mixed tachypnea and post-inspiratory apnea responses.
Left, Semi-schematic drawings of coronal slices through rat dorsolateral pons containing KF/PB. Location of glutamate injections were identified post-hoc in coronal slices (n = 12 rats). Circles represent center of glutamate injection sites marked by fluorescent beads. Right, example recordings of integrated phrenic nerve activity (PN) and central vagus nerve activity (cVN) surrounding glutamate microinjection (60 nl, 10 mM) into KF/PB. Color of circles on the map correspond to the response observed: tachypnea only (red), tachypnea followed by post-I apnea (pink), post-I apnea followed by tachypnea (purple), post-I apnea only (blue). Bilateral pairs of injections are identified numerically and correspond to the same numbers used in Figure 7. KF, Kölliker-Fuse; SCP, superior cerebellar peduncle; LPB, lateral parabrachial area; MPB, medial parabrachial area; mcp, middle cerebellar peduncle; IC, inferior colliculus; 4V, fourth ventricle; A7, noradrenergic neurons.
Once the KF/PB was identified bilaterally, baseline nerve activity was recorded for > 5 minutes before experimental manipulations were made. CTAP (60-90 nl) was microinjected into the KF/PB unilaterally. Within 3-5 min, CTAP was injected into the contralateral KF/PB. CTAP microinjections were performed before or after addition of fentanyl (300 nM) to the perfusion solution as described in the Results. Naloxone (1 μM) was applied to the reperfusion solution to reverse opioid effects. At the end of the experiment, fluorescent beads (60-90 nl) were injected bilaterally at the same stereotaxic coordinates to mark the center of the injection sites. Injection volume was determined by visualizing the drop in the meniscus level in the pipette using a binocular microscope with a calibrated graticule eyepiece positioned perpendicular to the injection pipette. Following the experiment, the head was removed, fixed in paraformaldehyde (4%) for at least 24 h at 4°C and stored in PBS (4°C) until further processing. The brainstem was removed and coronal slices of brainstem (100 μm) containing the injection site were cut in PBS using a vibratome (Leica VT1200S). Slices were imaged using a Dinolite fluorescent microscope (Model AM4115T-GRFBY, Dunwell Tech Inc, Torrance, CA) or Nikon AZ100 fluorescent microscope. Brightfield and fluorescent images were superimposed in Fiji (Schindelin et al., 2012) for injection site identification. The map of injection sites is shown in Figures 1 and 7. Injections were targeted ventrolateral from the tip of the superior cerebellar peduncle (scp), and overlapped the KF and nearby structures including external medial PB, external lateral PB and A7 (Figures 1 and 7). Experiments with both injections that were in or near the KF/PB were included in analysis. Experiments with microinjections that missed the KF on either side by more than ~250 μm were excluded (n = 3).
2.4. Microinjection Spread
Diffusion of CTAP to nearby pontine centers was estimated using an equation from Sykova and Nicholson, 2008 (Sykovα and Nicholson, 2008) that describes drug diffusion from a pressure injection throughout an isotropic medium to estimate drug concentrations at various distances throughout time, from the injection site.
The hydrodynamic radius, R, of CTAP was estimated using a variation of the Flory-Fox expression, assuming CTAP has a spherical volume.
where MW is molecular weight, N is Avogadro’s number, and ρ is the density of water at 32 degrees celcius.
Once the hydrodynamic radius was estimated, free diffusion constant of CTAP was estimated using the Stokes Einstein relation.
Where k is Boltzmann constant, T is temperature in Kelvin, η is the dynamic viscosity of water, and R is the hydrodynamic radius.
The free diffusion value for CTAP was then used in the pressure injection expression developed by Sykova and Nicholson, without accounting for uptake or clearance of CTAP.
U is injected volume (maximum = 90 nl), Cf concentration of injection (100 μM), D is free diffusion constant (3.84 x 10−7 cm2/s), t is time in seconds (600 s), r is the hydrodynamic radius (7.59x10−10 meters), α is the volume fraction, and λ is the tortuosity. The volume fraction (0.23) and tortuosity (1.63) values were from Sykova and Nicholson (table 4a, rat P20-23, 37°C). Concentrations at various radii were determined at 10 minutes (600 s) post injection, the last time point for all CTAP bilateral data analysis. The calculated concentration at a radius of 600 μm was 34 nM (too low to effectively compete with fentanyl (300 nM)), and at a radius of 1 mm was 0.34 fM. Thus, the effective concentration of CTAP needed to antagonize opioid receptors was located in and around the KF/PB complex and other respiratory regions nearby, such as A7.
2.5. Data analysis
Respiratory parameters were determined from integrated phrenic and central vagus nerve signals using active cursors and x, y plot function in Spike2. Inspiratory time (Ti) was calculated as the duration of individual phrenic nerve bursts. Expiratory time (Te) was calculated as the time interval between the end of a phrenic nerve burst and the beginning of the next phrenic burst. The total cycle time (Ttot) was determined from the beginning of a phrenic nerve burst to the beginning of the next phrenic nerve burst (Ti + Te). Respiratory rate was determined as number of cycles per minute. Post-inspiratory time and late expiration (E2) was obtained using the integrated vagus nerve activity. The vagus nerve is active during post-inspiration and inactive during E2. Post-inspiration was measured as the time interval between the end of a phrenic nerve burst and the corresponding end of the vagus nerve burst. Phrenic nerve amplitude was calculated using the max value of each phrenic burst. Averages of each of these parameters were obtained for each experimental treatment in 5 minute bins. Statistical comparisons of grouped data were made using repeated-measures one-way ANOVA with Tukey’s post-hoc test or one-way ANOVA with Dunnett's post-hoc test in GraphPad Prism (version 7).
3. Results
3.1. Fentanyl caused 2-phase breathing followed by apnea in the arterially perfused preparation
To study role of the KF/PB on the multiple aspects of opioid-induced respiratory depression, including apnea, we used the in situ arterially perfused working heart-brainstem preparation of rat (Paton, 1996; St.-John and Paton, 2004; Wilson et al., 2001). This preparation maintains an intact brainstem respiratory network with output to motor nerves, a three-phase respiratory cycle without anesthesia, and with pH and oxygenation maintained by the perfusion solution. The respiratory cycle was monitored by simultaneously recording the phrenic nerve (PN), which is active during inspiration, and the central vagus nerve (cVN), which was used to monitor post-inspiration.
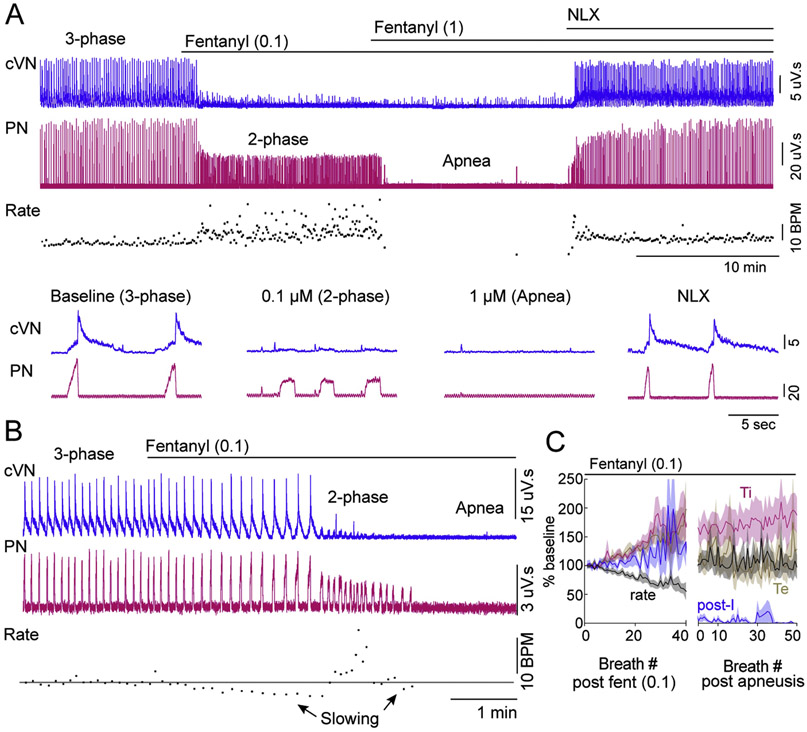
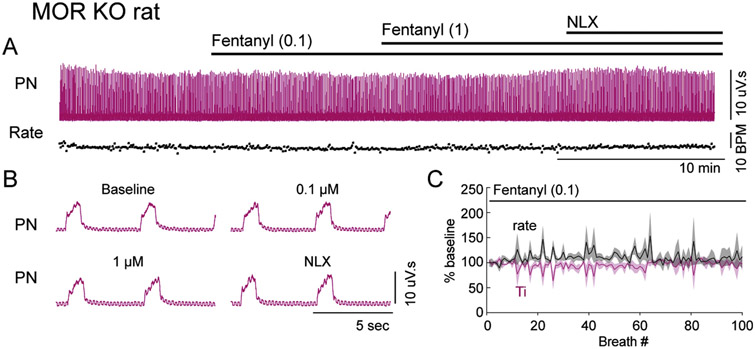
Systemic administration of fentanyl (100 nM) to the perfusion solution resulted in time-dependent changes in the respiratory motor output pattern (Figure 2; n = 12 rats). First, respiratory rate slowed, with a corresponding increase in duration of inspiration (Ti), total expiration (Te) and post-inspiration (post-I) (Figure 2B and C). Second, there was an abrupt transition to 2-phase patterned respiration, characterized by low-amplitude, long-Ti phrenic nerve bursts and a lack of post-inspiration activity in the vagus nerve (Figure 2A-C). The number of breaths that occurred prior to the transition to 2-phase respiration was variable between preparations (range = 8 – 42 breaths; mean = 26 ± 3.5 breaths). Additionally, in the 1-2 breaths preceding 2-phase respiration, post-inspiratory activity decreased as late expiratory activity increased. Finally, in 4 out of 12 rats, apnea occurred during which both phrenic and vagus nerve activity was absent (Figure 2B). In these preparations, Te increased by 313 ± 68 % for several breaths (range = 3 – 8 breaths) prior to apnea. Perfusion of a saturating concentration of fentanyl (1 μM) resulted in a rapid transition to apnea in all rats tested (n = 5) (Figure 2A). The effects of fentanyl were reversed by perfusion of the opioid antagonist naloxone (Figure 2A), indicating that they were due to activation of opioid receptors. To ensure that the effects of fentanyl were due to activation of mu opioid receptors specifically, rats lacking mu opioid receptors were used (Arttamangkul et al., 2018). In mu opioid receptor knockout rats, fentanyl (100 nM and 1 μM) had no effect on fictive respiratory rate, Ti or amplitude (Figure 3; n = 3).
Figure 2. Dose dependent fentanyl-induced changes in respiratory motor output.
Recordings from central vagus nerve (cVN) and phrenic nerve (PN) were made using an in situ preparation of wild-type rat. A,B Continuous recording of integrated cVN (blue) and PN (pink) activity in rat. Instantaneous respiratory rate (fictive breaths per minute (BPM)) is shown below the nerve recordings. Expanded time scale of nerve recordings is shown below rate for example A. A, At baseline a eupnea-like, 3-phase respiratory pattern was observed with augmenting PN discharge and post-I cVN activity. Systemic perfusion of fentanyl (0.1 μM) produced 2-phase breathing (long Ti, low-amplitude phrenic bursts with no post-I cVN activity) in 8/12 rats. Sequential perfusion of fentanyl (1 μM) produced apnea (no PN or cVN activity). Addition of naloxone (NLX; 1 μM) to the perfusion restored respiratory motor output. B, At baseline a eupnea-like, 3-phase respiratory pattern was observed, as in A. Systemic perfusion of fentanyl (0.1 μM) caused gradual slowing of 3-phase pattern, then produced transient 2-phase breathing (long Ti, low-amplitude phrenic bursts with no post-I cVN activity), followed by slowing and eventual progression to apnea (no PN or cVN activity) in 4/12 rats. C, Summary of normalized instantaneous rate (black), inspiratory duration (Ti, pink), expiratory duration (Te, brown) and post-inspiratory duration (post-I, blue) during perfusion of fentanyl (0.1 μM) (n = 12 rats). X-axis is number of breaths from addition of fentanyl to the perfusion (left section) or from first apneustic-like breath (right section). Respiratory rate first slowed (left), and then abruptly transitioned to 2-phase pattern (right). Time to transition to 2-phase pattern was variable (8 - 42 breaths).
Figure 3. Mu opioid receptors are necessary for fentanyl-induced changes in respiratory motor output.
Recordings from phrenic nerve (PN) were made using an in situ preparation of mu opioid receptor knockout (MOR KO) rat. A, Continuous recording of integrated PN (pink) activity in rat. Instantaneous respiratory rate (fictive breaths per minute (BPM)) is shown below the nerve recording. B, Expanded time scale of integrated PN activity during the example experiment shown in A. Perfusion of fentanyl (0.1 μM and 1 μM) had no effect on phrenic motor output or pattern. C, Summary of normalized instantaneous rate (black) and inspiratory duration (Ti, pink) during perfusion of fentanyl (0.1 μM) (n = 3 rats). X-axis is number of breaths from addition of fentanyl to the perfusion.
To achieve a fast and reliable transition to apnea, a sub-saturating concentration of fentanyl (300 nM) was administered systemically to the perfusion solution (n = 5). This caused a rapid transition within a single respiratory cycle, from a 3-phase eupnea-like respiratory pattern to a 2-phase, apneustic-like respiratory pattern, frequently leading to lengthy apnea (average duration = 8.5 ± 5.4 min, Figure 4A and G). There was some gradual recovery in fictive respiratory rate over 30 minutes of fentanyl perfusion (Figure 4). Thus, respiratory parameters were quantified 10 minutes (early) and 25-30 minutes (late) into the fentanyl perfusion. The fictive respiratory rate was significantly reduced from baseline both at the early and late time points during fentanyl perfusion (Figure 4C). The phrenic nerve bursts that occurred during fentanyl perfusion were apneustic-like, low-amplitude (Figure 4D), long Ti (Figure 4E) events. In contrast to slight recovery of rate, the reduction of phrenic nerve amplitude and lengthening of Ti persisted for up to 60 minutes with no recovery (Figure 4A, D and E). In addition, activity of the central vagus nerve during post-inspiration was eliminated and did not recover during perfusion of fentanyl (Figure 4F). Synchronous, very low-amplitude vagal output was observed with these apneustic-like phrenic bursts, similar to 2-phase pattern reported in previous studies (Dutschmann and Herbert, 2006; Smith et al., 2009). All of the fentanyl-induced changes were reversed when the opioid antagonist naloxone (1 μM) was added to the perfusion solution (Figure 4).
Figure 4. Fentanyl perfusion causes prolonged apnea interrupted by low-amplitude, 2-phase bursts.
Recordings from central vagus nerve (cVN) and phrenic nerve (PN) were made using an in situ preparation of wild-type rat. A, Continuous recording of integrated cVN (top) and PN (bottom) activity. Instantaneous respiratory rate (fictive breaths per minute (BPM)) is shown below. B, Expanded time scale of integrated cVN and PN activity during the experiment shown in A (location of sweep indicated by numbers in parentheses). (1) Baseline, a 3-phase respiratory pattern was observed with augmenting PN discharge and post-inspiratory cVN activity. (2) Systemic perfusion of opioid agonist fentanyl (300 nM) rapidly produced apnea (lack of PN and cVN activity). (3) Fictive respiratory rate increased gradually over time with the occurrence of 2-phase, low-amplitude, long-Ti, square wave PN bursts. (4) Systemic perfusion of naloxone (NLX, 1 μM) restored baseline respiratory motor output and pattern. C–G, Summary of fictive respiratory rate (min−1), phrenic amplitude (% baseline), inspiratory duration (Ti), post-inspiratory duration, and total expiratory duration (Te) calculated using averages of 5 minute bins during each condition per rat, as described in section 3.1. Exception was that the last one to five breaths were used for PN amplitude, Ti and post-inspiration in condition (2) if preparation went to apnea within one to five apneustic-like breaths. Symbols are group means ± SEM, n = 5 rats. *p < 0.05, **p < 0.01, ***p < 0.001 compared to baseline by repeated measures one-way ANOVA and Tukey’s post-hoc test.
3.2. Pretreatment with mu opioid antagonist CTAP into KF/PB prevents fentanyl-induced apnea
The contribution of mu opioid receptors in the KF/PB to fentanyl-induced respiratory changes was tested by local application of the selective mu opioid receptor antagonist CTAP into the KF/PB. In the first set of experiments (n = 7), CTAP (100 μM, 60-90 nl) was microinjected into KF/PB bilaterally prior to systemic fentanyl (300 nM) administration (Figure 5). Respiratory parameters, including rate, phrenic nerve burst amplitude, inspiratory duration, total expiratory duration, and post-inspiratory duration following CTAP microinjection into the KF were not significantly different from baseline (Figure 5C-G). Fentanyl (300 nM) was added to the perfusion solution 5-10 minutes after CTAP microinjection. The pre-application of CTAP into the KF prevented fentanyl-induced apnea. Instead, apneustic-like (2-phase, low-amplitude, long Ti) phrenic nerve bursts occurred at a higher frequency than baseline (Figure 5A,C). Furthermore, the transition between a 3-phase eupnea-like pattern and a 2-phase apneustic-like pattern was slowed as compared to fentanyl alone. In 4 of 7 preparations, 3-phase and 2-phase patterns were intermingled before ultimately progressing to a rapid 2-phase pattern. Respiratory parameters were quantified 25-30 minutes into the fentanyl perfusion. Unlike control experiments without CTAP pre-application, respiratory rate was faster than baseline (Figure 5C), and Te was unchanged (Figure 5G) following fentanyl perfusion, consistent with prevention of apnea. Similar to control experiments without CTAP application, there was a significant decrease in phrenic burst amplitude (Figure 5D), increase in Ti (Figure 5E) and loss of post-inspiratory activity (Figure 5F) following fentanyl perfusion. Finally, naloxone (1 μM) was administered into the perfusion solution to reestablish initial baseline. Thus, CTAP pre-application into the KF/PB prevented fentanyl-mediated apnea but failed to prevent the transition to low-amplitude, long-Ti, 2-phase breathing.
Figure 5. Bilateral application of CTAP, a selective mu opioid receptor antagonist, into the KF/PB prevents fentanyl-induced apnea.
Recordings from central vagus nerve (cVN) and phrenic nerve (PN) were made using an in situ preparation of wild-type rat. A, Continuous recording of integrated cVN (top) and PN (bottom) activity. Instantaneous respiratory rate (fictive breaths per minute (BPM)) is shown below. B, Expanded time scale of integrated cVN and PN activity during the experiment shown in A (location of sweep indicated by numbers in parentheses). (1) Baseline, a 3-phase respiratory pattern was observed with augmenting PN discharge and post-inspiratory cVN activity. (2) CTAP (60 nl, 100 μM) into KF bilaterally (unilateral injection at each arrow in A) did not change respiratory pattern. (3) Systemic perfusion of fentanyl (300 nM) induced 2-phase, low-amplitude, long-Ti, PN bursting faster than baseline rate. (4) Systemic perfusion of naloxone (NLX; 1 μM) restored baseline rate and pattern. C–G, Summary of fictive respiratory rate (min−1), phrenic amplitude (% baseline), inspiratory duration (Ti), post-inspiratory duration, and total expiratory duration (Te) were calculated using averages of 5 minute bins during each condition per rat, as described in section 3.2. Symbols are group means ± SEM, n = 7 rats. *p < 0.05, **p < 0.01, ***p < 0.001 compared to baseline, #p < 0.05 compared to CTAP KF by repeated measures one-way ANOVA and Tukey's post-hoc test.
3.3. Mu opioid antagonist CTAP in KF/PB reverses fentanyl-induced apnea
To determine if fentanyl-mediated apnea and severe rate depression could be reversed, the selective mu opioid receptor antagonist CTAP (100 μM, 60-90 nl) was microinjected into the KF/PB, bilaterally, approximately 10 minutes following the addition of fentanyl (300 nM) to the perfusion (n = 5). This caused a gradual increase in fictive respiratory rate, but not amplitude, that reached a plateau ~10 minutes after CTAP injection (Figure 6A). Respiratory parameters were measured 10-15 minutes following bilateral KF/PB CTAP microinjection, which was 22-30 minutes after systemic fentanyl administration. Following CTAP application into the KF/PB, there was an increase in respiratory rate (Figure 6C) and decrease in Te (Figure 6G) back to baseline levels, but no significant change in phrenic burst amplitude (Figure 6D), Ti (Figure 6E) or post-inspiration (Figure 6F). Finally, naloxone (1 μM) was administered into the perfusion solution to reestablish the initial baseline. In conclusion, microinjection of CTAP into the KF/PB can reverse fentanyl-mediated apnea and severe rate depression, but low-amplitude, long Ti, apneustic-like bursting persists. Thus, opioid-sensitive KF/PB neurons are sufficient to drive rate increases, however they not sufficient to recover eupneic 3-phase respiratory patterning.
Figure 6. Bilateral application of CTAP, a selective mu opioid receptor antagonist, into the KF reverses fentanyl-induced apnea.
Recordings from central vagus nerve (cVN) and phrenic nerve (PN) were made using an in situ preparation of wild-type rat. A, Continuous recording of integrated cVN (top) and PN (bottom) activity. Instantaneous respiratory rate (fictive breaths per minute (BPM)) is shown below. B, Expanded time scale of integrated cVN and PN activity during the experiment shown in A (location of sweep indicated by numbers in parentheses). (1) Baseline, a 3-phase respiratory pattern was observed with augmenting PN discharge and post-inspiratory cVN activity. (2) Systemic perfusion of fentanyl (300 nM) rapidly produced apnea. (3) CTAP (60 nl, 100 μM) into KF bilaterally (unilateral injection at each arrow in A) gradually restored fictive respiratory rate to baseline by increasing frequency of 2-phase phrenic bursts. (4) Systemic perfusion of naloxone (NLX; 1 μM) restored baseline rate and pattern. C-G, Summary of fictive respiratory rate (min−1), phrenic amplitude (% baseline), inspiratory duration (Ti), post-inspiratory duration, and total expiratory duration (Te) were calculated using averages of 5 minute bins during each condition per rat, as described in section 3.3. Symbols are group means ± SEM, n = 5 rats. *p < 0.05, **p < 0.01, ***p < 0.001 by repeated measures one-way ANOVA and Tukey's post-hoc test.
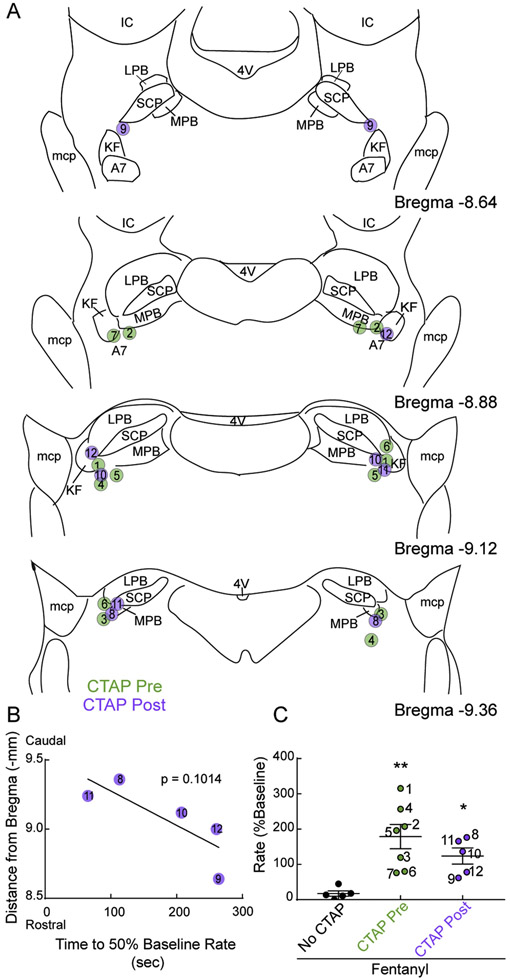
3.4. Comparison of CTAP effect and injection locations
A summary of injection site locations per experiment is shown in Figure 7. Post-experiment histology revealed that injection sites were located ventrolateral to the tip of the superior cerebellar peduncle overlapping the KF, external medial PB, external lateral PB, and A7 (Figures 1 and 7A). Injections of CTAP that were more caudal tended to more quickly reverse respiratory rate, but the correlation was not statistically significant (p = 0.1014; Figure 7B). We also compared respiratory rate following 25 – 30 minutes of fentanyl perfusion in all three conditions (control, CTAP pre-application, CTAP post-application). Respiratory rate was substantially faster than control in experiments with CTAP pre- or post-application (Figure 7C).
Figure 7. Summary of CTAP injection locations per experiment.
A, Semi-schematic drawings of coronal slices through rat dorsolateral pons containing KF/PB. Location of CTAP injections before (“CTAP Pre”, green) and after (“CTAP post”, purple) systemic fentanyl perfusion were identified post-hoc in coronal slices (n = 12 rats). Circles represent center of CTAP injection sites marked by fluorescent beads (90 nl). Bilateral pairs of injections are identified numerically and correspond with data indicated in B and C. KF, Kölliker-Fuse; SCP, superior cerebellar peduncle; LPB, lateral parabrachial area; MPB, medial parabrachial area; mcp, middle cerebellar peduncle; IC, inferior colliculus; 4V, fourth ventricle; A7, noradrenergic neurons. B, Correlation between time from last CTAP injection until rate recovery to 50% of baseline (x-axis) vs. distance from bregma (y-axis) in experiments with CTAP injected into KF post fentanyl perfusion (“CTAP post”). For experiments with injections in two rostro-caudal sections, the median of the distances was used. Each data point is an individual animal. The slope of the linear regression was not statistically different from zero (p = 0.1014). C, Summary of normalized fictive respiratory rate in all experimental groups with fentanyl (300 nM) added to the perfusion, and without (black symbols) or with bilateral CTAP into the KF either before (CTAP pre, green symbols) or after (CTAP post, purple symbols) fentanyl perfusion. Rate values are from the last 5 minutes of fentanyl perfusion before reversal with systemic naloxone (22 – 30 minutes into fentanyl perfusion for all groups). Symbols are individual rats, line and error are mean ± SEM. Numbers to the right of each symbol corresponds with numbered symbols in A and B. *p < 0.05, **p < 0.01 by one-way ANOVA and Dunnett's post-hoc test.
4. Discussion
Opioid overdose leads to prolonged apnea, which can be fatal. The major findings of this study were that application of a selective mu opioid receptor antagonist into the KF/PB both prevented and reversed fentanyl-induced apnea, leading to a faster respiratory rate. This aligns with other studies implicating the KF and nearby parabrachial areas as modulators of respiratory rate (Chamberlin and Saper, 1994; Dutschmann et al., 2007; Lara et al., 1994; Miller et al., 2017; Prkic et al., 2012; Zuperku et al., 2016), especially opioid-mediated rate depression (Levitt et al., 2015; Miller et al., 2017; Prkic et al., 2012; Varga et al., 2019). Notably, rhythmic motor output was restored even though fentanyl was still suppressing activity in the medulla and spinal cord, including inspiratory, excitatory neurons in the pre-Bötzinger complex (Montandon et al., 2011). In contrast, amplitude and post-inspiration were not restored by opioid antagonist application into KF/PB, indicating that these functions are mediated in part, or in full, by areas outside of the KF/PB that are still inhibited by fentanyl. Similarly, reversal of remifentanil-induced depression of respiratory rate, but not amplitude, was observed with injections of the non-selective opioid antagonist naloxone into the parabrachial area of ventilated, and anesthetized or decerebrate, canines and rabbits (Miller et al., 2017; Prkic et al., 2012).
The 2-phase, apneustic-like respiratory pattern (low-amplitude, long-duration, post-inspiration-lacking) was an intermediate state between normal 3-phase breathing (eupnea-like) and apnea that is observed in situ with multiple experimental conditions, including 1) perfusion of low-dose fentanyl (Figure 2), 2) perfusion of opioid agonist DAMGO (Koganezawa et al., 2011), 3) opioid antagonist in the KF during perfusion of fentanyl (Figures 5-6), and 4) opioid agonist in the KF (Levitt et al., 2015). Our interpretation of these seemingly conflicting data is that 3-phase eupnea-like respiratory output requires a fully intact pontomedullary circuitry. In summary, the KF/PB is sufficient to restore rhythmic respiratory motor output during fentanyl-induced apnea, but recovery to a 3-phase, eupnea-like respiratory pattern requires activity of a larger opioid-sensitive pontomedullary network.
4.1. Potential mechanisms for recovery of rate
A subregion of the parabrachial complex has been termed the tachypneic region (Miller et al., 2017; Zuperku et al., 2016). Stimulation of this region leads to increases in rate by shortening expiratory duration, and opioid antagonist in this region reversed remifentanil respiratory rate depression. We also observed increases in rate with opioid antagonist microinjection in the KF/PB that were due to shortening expiratory duration. The latency to rate increase tended to be shorter in caudal regions than rostral regions of the KF/PB complex (Figure 7B). Our injections were more lateral than the tachypneic region identified in medial PB (Miller et al., 2017), suggesting that the “hotspot” for opioid-induced rate depression in the pons may be larger than previously described, and includes caudal KF, medial PB and external lateral PB.
In our CTAP pre-treatment experiments, rate was increased further than baseline. Opioid agonist application to certain areas of the ventral respiratory column have been previously shown to increase respiratory rate (Langer et al., 2017; Lonergan et al., 2003; Mustapic et al., 2010). Removal of opioid receptor-mediated rate depressing mechanisms in the KF/PB may have unveiled a paradoxical opioid-mediated stimulation of rate in ventral respiratory column.
In addition, the KF/PB provides excitatory input to inhibitory neurons in medullary respiratory rhythm generating areas, including Bötzinger and pre-Bötzinger complexes (Barnett et al., 2017; Geerling et al., 2017; Jones and Dutschmann, 2016; Poon and Song, 2014; Song et al., 2012; Yokota et al., 2011). It is becoming evident that increases in phasic inhibition can increase respiratory rate (Baertsch et al., 2018; Cregg et al., 2017). Phasic excitation of inhibitory neurons by opioid-sensitive KF/PB neurons may explain the increase in rate with opioid antagonist in the KF/PB.
A schematic was created to depict a possible mechanism for fentanyl-induced apnea (Figure 8, top), and restoration of 2-phase breathing by opioid antagonist in the KF/PB complex (Figure 8, bottom), using existing models for reference (Molkov et al., 2017; Smith et al., 2009; Zuperku et al., 2019). During fictive overdose, opioids inhibit a population of inspiratory neurons in the medulla (Gray et al., 1999; Montandon et al., 2011; Takeda et al., 2001) as well as inspiratory and post-inspiratory populations in the KF/PB (unpublished observations) (Figure 8, top). GABAergic transmission in the KF gates expiration with decreased GABAergic activity resulting in increased expiratory activity and production of central apneas (Abdala et al., 2016, 2010). Opioid inhibition of inspiratory neurons in the KF could reduce GABAergic interneuron activity through pontine local connections and promote tonic expiratory activity. Tonic pontine expiratory activity could excite medullary (Ezure and Tanaka, 2006) expiration interneurons, leading to further suppression of medullary inspiratory rhythm, and thus, promoting apnea.
Figure 8. Hypothetical schematic of pontine mechanism of apnea reversal.
Neuron populations in the KF/PB (left) and medulla (right) are designated inspiratory (I), expiratory (E) or post-I. Excitatory neurons are blue with arrowhead projections. Inhibitory neurons are red with ball-head projections. Mu opioid receptors are indicated by stars; yellow filled stars are occupied by agonist, white stars are blocked by antagonist. Dashed lines indicate inhibition of the neuron and/or neuron terminals. Proposed populations of neurons directly inhibited by opioids are KF/PB inspiratory, KF/PB post-inspiratory, and a sub-population of medullary inspiratory neurons. Top, fentanyl perfusion: During Active overdose, opioid inhibition of KF/PB inspiratory neurons reduces excitation of inhibitory interneurons and leads to KF/PB tonic expiratory drive, causing downstream tonic inhibition of medullary I rhythm and apnea. Bottom, CTAP in KF + fentanyl perfusion: KF/PB inspiratory neurons are not inhibited, permitting appropriate phasic inhibition of KF/PB expiratory neurons, thus removing tonic inhibition of medullary inspiratory neurons. PN amplitude and duration are not restored possibly due to presynaptic receptor inhibition of terminals onto medullary inspiratory neurons. Post-I pattern is not restored possibly due to presynaptic receptor inhibition of post-I neuron terminals in the medulla. This schematic is an adaptation from existing models (Molkov et al., 2017; Smith et al., 2009; Zuperku et al., 2019).
When opioid antagonist is injected into the KF/PB in the presence of systemic fentanyl (Figure 8, bottom), apnea could be rescued by restoring GABAergic activity in the KF, and thus, phasic expiratory activity. Medullary inspiratory neurons would no longer be tonically inhibited, and phasic release from inhibition at the end of expiration would promote activation. Inspiratory output can be restored through a population of inspiratory neurons not directly inhibited by opioids. A 3-phase pattern is not restored by KF/PB CTAP microinjection possibly due to presynaptic inhibition of post-inspiratory inputs onto medullary neurons (Lalley, 2005 and 2003), or inhibition of medullary post-inspiratory neurons directly. Notably, opioid inhibition of the excitatory, inspiratory, putative I-driver neurons in the medulla (Montandon et al., 2011) was not sufficient to maintain apnea. Rather, both the medulla and KF/PB need to be inhibited for complete apnea to occur.
4.2. Eupnea requires a fully intact pontomedullary circuitry
The KF has long been implicated in upper airway function and normal 3-phase eupneic breathing (Bautista and Dutschmann, 2014; Dhingra et al., 2019; Dutschmann and Herbert, 2006a; Fung and St John, 1995; Lara et al., 2002; Lumsden, 1923; Smith et al., 2009). Opioid agonist DAMGO into the KF causes loss of post-inspiration (Levitt et al., 2015). However, in the current experiments, post-inspiration did not return with opioid antagonist microinjection into the KF/PB during systemic fentanyl. Thus, the KF is necessary for generation of post-inspiration, but not sufficient. Therefore, opioid sensitivity in other respiratory centers must interfere with formation of a 3-phase respiratory cycle, consisting of inspiration, post-inspiration, and late expiration. Alternatively, vagal premotor or motor neurons could be directly inhibited by opioids (Erbs et al., 2015).
Respiratory regions in the medulla, including the Bötzinger complex and post-inspiratory complex (PiCo) also contribute to post-inspiration (Anderson et al., 2016; Burke et al., 2010). The PiCo is highly opioid-sensitive (Anderson et al., 2016). Based on immunohistochemistry, mu opioid receptors are more abundant on presynaptic terminals than somas in the Bötzinger complex (Lonergan et al., 2003). The KF sends dense glutamatergic input to the Bötzinger complex (Geerling et al., 2017; Song et al., 2012; Yokota et al., 2015, 2007), and post-inspiratory activity of Bötzinger complex neurons is lost when the KF is inhibited (Song et al., 2015). In our experiments, presynaptic terminals from opioid-sensitive KF/PB neurons in projection areas, such as Bötzinger complex and/or PiCo, would remain inhibited by circulating fentanyl (even though the receptors on KF/PB neuron cell bodies are blocked by CTAP) (Figure 8). Thus, CTAP microinjections into KF/PB may only revive one component of an opioid-sensitive post-inspiratory rhythm generating circuit, leading to failure to recover post-inspiratory motor output.
Regulation of the medullary respiratory circuitry by presynaptic opioid receptors has been reported previously (Ballanyi et al., 2010, 1997; Haji et al., 2003; Lorier et al., 2010; Takeda et al., 2001). Small doses of fentanyl slow discharge frequency and prolong duration of firing of bulbospinal and propriobulbar inspiratory neurons presumably through presynaptic mechanisms (Lalley, 2005, 2003). Thus, in our experiments, long-duration phrenic bursts may occur due to high sensitivity of opioid receptors on presynaptic terminals onto medullary neurons (Figure 8).
4.3. Considerations of the in situ arterially perfused preparation
The in situ arterially perfused preparation has an intact respiratory circuitry that produces an “in vivo-like” 3-phase respiratory motor output pattern in the absence of anesthesia, but lacks pulmonary stretch receptor feedback, and has diminished chemosensory reflex due to constant tissue oxygenation and pH maintenance by the perfusion solution. We used the latter to our advantage since we were able to maintain healthy preparations in the absence of respiratory motor output. The lack of pulmonary stretch receptor feedback requires consideration. Slowly adapting pulmonary stretch receptors (PSRs) modulate respiratory phase timing through vagal afferents that project to the NTS and from there to corresponding pontine and medullary respiratory centers (Bruce, 1997; Kubin et al., 2006; Schelegle and Green, 2001). The KF functions as a failsafe, or auxiliary inspiratory off-switch (IOS) (Bruce, 1997; Cohen and Shaw, 2004; Greer et al., 1990; Molkov et al., 2013; Morschel and Dutschmann, 2009; Song and Poon, 2004; Stella, 1938). Apneusis, caused by loss of all IOS, is pronounced following pontine lesions in vagotomized animals (St.John, 1998; Stella, 1938) or following inactivation of the KF with opioid or GABA-A agonist in the in situ preparation (Dutschmann and Herbert, 2006; Levitt et al., 2015). However, changes in rate (due to increases in expiratory duration) in these experiments are inconsistent, likely because the populations of KF neurons inhibited by opioid and GABA-A agonist are different. Opioids inhibit 60 % of KF neurons (Levitt et al., 2015). In vagi-intact animals, opioid or GABA-A agonist injection into KF consistently leads to decreases in rate and smaller increases in inspiratory duration with low variability presumably due to intact PSRs (Damasceno et al., 2014; Levitt et al., 2015; St.John, 1998). Therefore, effects of manipulations in the KF on rate may differ depending on the presence of peripheral feedback.
The increase in rate we observed when CTAP was microinjected into the KF is consistent with the increase in rate following opioid antagonist injected into KF/PB of artificially ventilated dogs (Prkic et al., 2012). However, each of these preparations investigate pontine-mediated opioid overdose recovery with lack of peripheral feedback. In awake mice with fully functioning feedback mechanisms, deletion of mu opioid receptors from KF neurons significantly attenuated morphine-induced respiratory rate depression and reduced the occurrence of apneas (Varga et al., 2019). Thus, the KF also contributes to respiratory rate depression caused by systemic opioids in a fully intact animal with functioning PSRs.
4.4. Opioids consistently depress breathing in the pons
Numerous studies have been performed throughout the respiratory column to find the “critical site” of opioid-induced respiratory depression (Hurlé et al., 1985; Krause et al., 2009; Langer et al., 2017; Levitt et al., 2015; Lonergan et al., 2003; Miller et al., 2017; Montandon et al., 2011; Mustapic et al., 2010; Prkic et al., 2012; Stucke et al., 2015; Varga et al., 2019). These studies have been conducted with a variety of animal models (rat, mouse, cat, dog, rabbit, goat), opioid agonist (fentanyl, remifentanil, DAMGO), opioid doses, respiratory network locations (KF/PB, preBötC, VRC), methods of pharmacological manipulation (microinjection, microdialysis), and state of CNS (awake, anesthetized, decerebrate, sleep). These closely related, but critically different, studies have produced confusion and disagreement about the roles of specific respiratory centers in opioid overdose, due to the differences in obtained results. Unlike inconsistency among studies in the ventral respiratory column, especially the pre-Bötzinger complex (Krause et al., 2009; Langer et al., 2017; Lonergan et al., 2003; Montandon et al., 2011; Mustapic et al., 2010; Stucke et al., 2015; Varga et al., 2019), studies in the pons have consistently shown that opioid actions in the KF/PB suppress breathing in all species tested (rat, mouse, cat, dog, rabbit) (Hurlé et al., 1985; Levitt et al., 2015; Miller et al., 2017; Prkic et al., 2012; Varga et al., 2019).
4.5. State dependence of opioid action: Implications for targeting the KF/PB
At analgesic doses, opioids have marked effects on CNS sleep states (Bonafide et al., 2008; Dimsdale et al., 2007; Shaw et al., 2005), and contribute to worsening or generation of sleep disordered breathing (Correa et al., 2015; Van Ryswyk and Antic, 2016). At higher doses, opioids have been shown to decrease cortical arousal state (Montandon et al., 2016; Montandon and Horner, 2019) and depress cortical centers (Pattinson et al., 2009), and thus, during overdose elicit sedation. Taken together, systemic opioids disrupt sleep states at low doses and decrease cortical arousal at high doses. Studies using opioid microinjection into respiratory centers in awake animals fail to model the state of the CNS in the context of systemic opioids and subsequent overdose, with compensation from centers that would, during overdose, be inhibited by opioids. Studies done under anesthesia or decerebration may more closely resemble the reduced CNS state in opioid overdose. The decerebration in our preparation may provide a useful method to investigate breathing generation during overdose in a state lacking “cortical arousal”, specifically in the context of opioid overdose. Although decerebrate, the preparation is likely exhibiting a state which is physiologically relevant with careful interpretation (Kubin, 2001).
Activity of the KF may have additional importance for opioid-induced respiratory depression due to its association with respiratory modulation during sleep and/or sedation (Bonis et al., 2010). The KF has been shown to impact breathing in anesthetized, ventilated goats during nighttime, as well as anesthetized rats (Bonis et al., 2010; Levitt et al., 2015). Furthermore, hypoplasia of the KF in infants has been implicated in the neuropathology of Sudden Infant Death Syndrome, characterized by failure to stimulate breathing during sleep, indicating a decreased or absent chemosensory response (Lavezzi et al., 2019, 2004). Taken together, KF modulation can impact respiration during sleep and sedation. Thus, opioid inhibition of KF neurons during opioid-induced sedation (overdose) may signifcantly contribute to decreases in respiratory output.
4.6. Other factors contributing to fatal opioid overdose
Increased chest wall muscle tone has been suggested to cause impaired ventilation and reduced tidal volume during administration of fentanyl and related compounds (Burns et al., 2016; Comstock et al., 1981; Çoruh et al., 2013). This muscle rigidity is due to opioid receptor activation in the dopaminergic and adrenergic systems (Campbell et al., 1995; Genç et al., 1983; Jerussi et al., 1987). In our experiments, fentanyl decreases amplitude (fictive tidal volume) of phrenic motor output in the absence of chest wall rigidity due to the paralyzed nature of the preparation. This suggests fentanyl-induced decreases in tidal volume also have a significant central origin.
Pulmonary hypertension has also been hypothesized to contribute to opioid-induced hypoxia (Hakim et al., 1992; Meyer et al., 2015), occurring through both opioid receptor and histamine receptor sensitive pathways. The preparation used for our experiments lacks lungs and is provided with sufficient oxygenation through the perfusion solution. Therefore, pulmonary hypertension was not investigated in our experiments, but may be of importance in vivo.
5. Conclusions
Systemic fentanyl perfusion in the in situ arterially perfused preparation caused a concentration-dependent respiratory pattern breakdown characterized by initial slowing of fictive respiratory rate, followed by a transition to a 2-phase pattern, and eventually complete respiratory apnea. Application of the selective mu opioid receptor antagonist CTAP into the KF/PB prevented and reversed fentanyl-induced apnea by increasing the frequency of apneustic-like bursting without changing respiratory patterning. This suggests opioid-sensitive neurons in the KF/PB are sufficient to restore phasic respiratory motor output, but functional recovery to a eupneic 3-phase pattern requires activity of a larger opioid-sensitive pontomedullary network, potentially including peripheral stretch receptor feedback. Countering opioid effects in the KF/PB warrants further testing as a means to prevent opioid overdose in fully intact animals.
Highlights.
Fentanyl causes dose-dependent 2-phase breathing followed by apnea in rat in situ preparations.
Inhibition of mu opioid receptors in the KF/PB reverses fentanyl-induced apnea.
Mu opioid receptors in the KF/PB contribute to respiratory rate decreases by fentanyl.
The KF can provide drive to restore phasic respiratory motor output in the context of fentanyl overdose.
Acknowledgements
We thank Dr. Adrienn Varga for helpful comments on this manuscript. This work was supported by the National Institutes of Health Grant DA038069.
Footnotes
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdala AP, Toward MA, Dutschmann M, Bissonnette JM, Paton JFR, 2016. Deficiency of GABAergic synaptic inhibition in the Kölliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett syndrome. J. Physiol 594, 223–237. 10.1113/JP270966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala APL, Dutschmann M, Bissonnette JM, Paton JFR, 2010. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U. S. A 107, 18208–18213. 10.1073/pnas.1012104107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TM, Garcia AJ, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG, Ramirez JM, 2016. A novel excitatory network for the control of breathing. Nature 536, 76–80. 10.1038/nature18944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Heinz DA, Bunzow JR, Song X, Williams JT, 2018. Cellular tolerance at the μ-opioid receptor is phosphorylation dependent. Elife 7 10.7554/eLife.34989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baertsch NA, Baertsch HC, Ramirez JM, 2018. The interdependence of excitation and inhibition for the control of dynamic breathing rhythms. Nat. Commun 9 10.1038/s41467-018-03223-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Lalley PM, Hoch B, Richter DW, 1997. cAMP-dependent reversal of opioid- and prostaglandin-mediated depression of the isolated respiratory network in newborn rats. J. Physiol 504, 127–134. 10.1111/j.1469-7793.1997.127bf.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Panaitescu B, Ruangkittisakul A, 2010. Indirect opioid actions on inspiratory pre-bötzinger complex neurons in newborn rat brainstem slices, in: Advances in Experimental Medicine and Biology, pp. 669 75–79. 10.1007/978-1-4419-5692-7_16 [DOI] [PubMed] [Google Scholar]
- Barnett WH, Jenkin SEM, Milsom WK, Paton JFR, Abdala AP, Molkov YI, Zoccal DB, 2017. The Kölliker-Fuse nucleus orchestrates the timing of expiratory abdominal nerve bursting. J. Neurophysiol 119, 401–412. 10.1152/jn.00499.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista TG, Dutschmann M, 2014. Inhibition of the pontine Kölliker-Fuse nucleus abolishes eupneic inspiratory hypoglossal motor discharge in rat. Neuroscience 267, 22–29. 10.1016/j.neuroscience.2014.02.027 [DOI] [PubMed] [Google Scholar]
- Bonafide CP, Aucutt-Walter N, Divittore N, King T, Bixler EO, Cronin AJ, 2008. Remifentanil inhibits rapid eye movement sleep but not the nocturnal melatonin surge in humans. Anesthesiology 108, 627–633. 10.1097/ALN.0b013e3181684bc3 [DOI] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Krause KL, Kiner A Smith T, Marshall BD, Qian B, Pan LG, Forster HV, 2010. A role for the Kölliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J. Appl. Physiol 109, 159–170. 10.1152/japplphysiol.00933.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon T, Bruhn J, Roepcke H, Hoeft A, 2003. Opioid-induced respiratory depression is associated with increased tidal volume variability. Eur. J. Anaesthesiol 20, 127–133. 10.1017/S0265021503000243 [DOI] [PubMed] [Google Scholar]
- Bruce EN, 1997. Chemoreflex and vagal afferent mechanisms enhance breath to breath variability of breathing. Respir. Physiol 110, 237–244. 10.1016/S0034-5687(97)00088-1 [DOI] [PubMed] [Google Scholar]
- Burke PGR, Abbott SBG, McMullan S, Goodchild AK, Pilowsky PM, 2010. Somatostatin selectively ablates post-inspiratory activity after injection into the Bötzinger complex. Neuroscience 167, 528–539. 10.1016/j.neuroscience.2010.01.065 [DOI] [PubMed] [Google Scholar]
- Burns G, Derienz RT, Baker DD, Casavant M, Spiller HA, 2016. Could chest wall rigidity be a factor in rapid death from illicit fentanyl abuse? Clin. Toxicol 54, 420–423. 10.3109/15563650.2016.1157722 [DOI] [PubMed] [Google Scholar]
- Campbell C, Weinger MB, Quinn M, 1995. Alterations in diaphragm EMG activity during opiate-induced respiratory depression. Respir. Physiol 100, 107–117. 10.1016/0034-5687(94)00119-K [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB, 1994. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci 14, 6500–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ A, Arranto CA, Schindler C, Klima T, Hunziker PR, Siegemund M, Marsch SC, Eriksson U, Mueller C, 2006. Incidence, risk factors, and outcome of aspiration pneumonitis in ICU overdose patients. Intensive Care Med. 32, 1423–1427. 10.1007/s00134-006-0277-4 [DOI] [PubMed] [Google Scholar]
- Cohen MI, Shaw CF, 2004. Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir. Physiol. Neurobiol 143, 127–140. 10.1016/j.resp.2004.07.017 [DOI] [PubMed] [Google Scholar]
- Comstock MK, Carter JG, Moyers JR, Stevens WC, 1981. Rigidity and hypercarbia associated with high dose fentanyl induction of anesthesia. Anesth. Analg 60, 362–363. 10.1213/00000539-198105000-00018 [DOI] [PubMed] [Google Scholar]
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J, 2015. Chronic opioid use and central sleep apnea: A review of the prevalence, mechanisms, and perioperative considerations. Anesth. Analg 120, 1273–1285. 10.1213/ANE.0000000000000672 [DOI] [PubMed] [Google Scholar]
- Çoruh B, Tonelli MR, Park DR, 2013. Fentanyl-induced chest wall rigidity. Chest 143, 1145–1146. 10.1378/chest.12-2131 [DOI] [PubMed] [Google Scholar]
- Cregg JM, Chu KA, Dick TE, Landmesser LT, Silver J, 2017. Phasic inhibition as a mechanism for generation of rapid respiratory rhythms. Proc. Natl. Acad. Sci 114, 12815–12820. 10.1073/pnas.1711536114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Sarton E, Teppema L, Olievier C, Nieuwenhuijs D, Matthes HW, Kieffer BL, 2001. Anesthetic potency and influence of morphine and sevoflurane on respiration in mu-opioid receptor knockout mice. Anesthesiology 94, 824–832. [DOI] [PubMed] [Google Scholar]
- Damasceno RS, Takakura AC, Moreira TS, 2014. Regulation of the chemosensory control of breathing by Kölliker-Fuse neurons. Am. J. Physiol. Integr. Comp. Physiol 307 10.1152/ajpregu.00024.2014 [DOI] [PubMed] [Google Scholar]
- Dhingra RR, Furuya WI, Bautista TG, Dick TE, Galán RF, Dutschmann M, 2019. Increasing Local Excitability of Brainstem Respiratory Nuclei Reveals a Distributed Network Underlying Respiratory Motor Pattern Formation. Front. Physiol 10 10.3389/fphys.2019.00887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale JE, Norman D, DeJardin D, Wallace MS, 2007. The effect of opioids on sleep architecture. J. Clin. Sleep Med 3, 33–36. [PubMed] [Google Scholar]
- Dutschmann M, Herbert H, 2006a. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur. J. Neurosci 24, 1071–1084. 10.1111/j.1460-9568.2006.04981.x [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H, 2006b. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur. J. Neurosci 24, 1071–1084. 10.1111/j.1460-9568.2006.04981.x [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Kron M, Mörschel M, Gestreau C, 2007. Activation of Orexin B receptors in the pontine Kölliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir. Physiol. Neurobiol 159, 232–235. 10.1016/j.resp.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Ehsan Z, Mahmoud M, Shott SR, Amin RS, Ishman SL, 2016. The effects of Anesthesia and opioids on the upper airway: A systematic review, in: Laryngoscope, pp. 270–284. 10.1002/lary.25399 [DOI] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, Koch M, Kessler P, Hentsch D, Birling MC, Koutsourakis M, Vasseur L, Veinante P, Kieffer BL, Massotte D, 2015. A mu–delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct. Funct 220, 677–702. 10.1007/s00429-014-0717-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, 2006. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience 141, 1011–1023. 10.1016/j.neuroscience.2006.04.020 [DOI] [PubMed] [Google Scholar]
- Fung ML, St John WM, 1995. The functional expression of a pontine pneumotaxic centre in neonatal rats. J. Physiol 489, 579–591. 10.1113/jphysiol.1995.sp021074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Yokota S, Rukhadze I, Roe D, Chamberlin NL, 2017. Kölliker-Fuse GABAergic and glutamatergic neurons project to distinct targets. J. Comp. Neurol 525, 1844–1860. 10.1002/cne.24164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genç E, Havemann U, Tzoneva-Tyutyulkova N, Kuschinsky KK, 1983. Motility, rigidity and turnover of dopamine in the striatum after administration of morphine to rats: A re-evaluation of their mechanisms. Neuropharmacology 22, 471–476. 10.1016/0028-3908(83)90165-X [DOI] [PubMed] [Google Scholar]
- Gestreau C, Dutschmann M, Obled S, Bianchi AL, 2005. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir. Physiol. Neurobiol 147, 159–176. 10.1016/j.resp.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL, 1999. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science (80-. ). 286, 1566–1568. 10.1126/science.286.5444.1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL, Liu GS, 1990. Neural mechanisms generating respiratory pattern in mammalian brain stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J. Neurophysiol 64, 1149–1169. [DOI] [PubMed] [Google Scholar]
- Haji A, Yamazaki H, Ohi Y, Takeda R, 2003. Distribution of mμ receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neurosci. Lett 351, 37–40. 10.1016/S0304-3940(03)00951-0 [DOI] [PubMed] [Google Scholar]
- Hajiha M, Dubord MA, Liu H, Horner RL, 2009. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J. Physiol 587, 2677–2692. 10.1113/jphysiol.2009.171678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim TS, Grunstein MM, Michel RP, 1992. Opiate action in the pulmonary circulation. Pulm. Pharmacol 5, 159–165. 10.1016/0952-0600(92)90036-G [DOI] [PubMed] [Google Scholar]
- Hurlé MA, Mediavilla A, Flórez J, 1985. Differential respiratory patterns induced by opioids applied to the ventral medullary and dorsal pontine surfaces of cats. Neuropharmacology 24, 597–606. 10.1016/0028-3908(85)90100-5 [DOI] [PubMed] [Google Scholar]
- Jerussi TP, Capacchione JF, Benvenga MJ, 1987. Reversal of opioid-induced muscular rigidity in rats: Evidence for alpha-2 adrenergic involvement. Pharmacol. Biochem. Behav 28, 283–289. 10.1016/0091-3057(87)90226-7 [DOI] [PubMed] [Google Scholar]
- Jones SE, Dutschmann M, 2016. Testing the hypothesis of neurodegeneracy in respiratory network function with a priori transected arterially perfused brain stem preparation of rat. J. Neurophysiol 115, 2593–2607. 10.1152/jn.01073.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganezawa T, Okada Y, Terui N, Paton JFR, Oku Y, 2011. A μ-opioid receptor agonist DAMGO induces rapid breathing in the arterially perfused in situ preparation of rat. Respir. Physiol. Neurobiol 177, 207–211. 10.1016/j.resp.2011.04.002 [DOI] [PubMed] [Google Scholar]
- Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, Qian B, Pan LG, 2009. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Botzinger complex and the surrounding region. J. Appl. Physiol 106, 605–619. 10.1152/japplphysiol.90966.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, 2001. Carbachol models of REM sleep: Recent developments and new directions. Arch. Ital. Biol 139, 147–168. 10.4449/aib.v139i1.210 [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR, 2006. Central pathways of pulmonary and lower airway vagal afferents. J. Appl. Physiol 101, 618–627. 10.1152/japplphysiol.00252.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, 2005. Opiate slowing of feline respiratory rhythm and effects on putative medullary phase-regulating neurons. Am. J. Physiol. Integr. Comp. Physiol 290, R1387–1396. 10.1152/ajpregu.00530.2005 [DOI] [PubMed] [Google Scholar]
- Lalley PM, 2003. μ-Opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am. J. Physiol. - Regul. Integr. Comp. Physiol 285, R1287–R1304. 10.1152/ajpregu.00199.2003 [DOI] [PubMed] [Google Scholar]
- Langer TM, Neumueller SE, Crumley E, Burgraff NJ, Talwar S, Hodges MR, Pan L, Forster HV, 2017. Effects on breathing of agonists to μ-opioid or GABAAreceptors dialyzed into the ventral respiratory column of awake and sleeping goats. Respir. Physiol. Neurobiol 239, 10–25. 10.1016/j.resp.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara JP, Dawid-Milner MS, López MV, Montes C, Spyer KM, González-Barón S, 2002. Laryngeal effects of stimulation of rostral and ventral pons in the anaesthetized rat. Brain Res. 934, 97–106. 10.1016/S0006-8993(02)02364-8 [DOI] [PubMed] [Google Scholar]
- Lara JP, Parkes MJ, Silva-Carvhalo L, Izzo P, Dawid- Milner MS, Spyer KM, 1994. Cardiovascular and respiratory effects of stimulation of cell bodies of the parabrachial nuclei in the anaesthetized rat. J. Physiol 477, 321–329. 10.1113/jphysiol.1994.sp020193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi AM, Ferrero S, Paradiso B, Chamitava L, Piscioli F, Pusiol T, 2019. Neuropathology of Early Sudden Infant Death Syndrome--Hypoplasia of the Pontine Kolliker-Fuse Nucleus: A Possible Marker of Unexpected Collapse during Skin-to-Skin Care. Am. J. Perinatol 36, 460–471. 10.1055/s-0038-1669398 [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Ottaviani G, Rossi L, Matturri L, 2004. Hypoplasia of the parabrachial/Kölliker-Fuse complex in perinatal death. Biol. Neonate 86, 92–97. 10.1159/000078310 [DOI] [PubMed] [Google Scholar]
- Levitt ES, Abdala AP, Paton JFR, Bissonnette JM, Williams JT, 2015. μ opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive. J. Physiol 593, 4453–4469. 10.1113/JP270822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM, 2003. Mu opioid receptors in rat ventral medulla: Effects of endomorphin-1 on phrenic nerve activity. Respir. Physiol. Neurobiol 138, 165–178. 10.1016/S1569-9048(03)00173-3 [DOI] [PubMed] [Google Scholar]
- Lorier AR, Funk GD, Greer JJ, 2010. Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PLoS One 5, e8766 10.1371/journal.pone.0008766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden T, 1923. Observations on the respiratory centres in the cat. J. Physiol 57, 153–160. 10.1113/jphysiol.1923.sp002052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ, 1994. μ-Opioid receptor mRNA expression in the rat CNS: comparison to μ-receptor binding. Brain Res. 643, 245–265. 10.1016/0006-8993(94)90031-0 [DOI] [PubMed] [Google Scholar]
- Meyer RCR, Hetem SS, Mitchell D, Fuller A, 2015. Hypoxia following etorphine administration in goats (Capra hircus) results more from pulmonary hypertension than from hypoventilation. BMC Vet. Res 11 10.1186/s12917-015-0337-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Zuperku EJ, Stuth EAE, Banerjee A, Hopp FA, Stucke AG, 2017. A Subregion of the Parabrachial Nucleus Partially Mediates Respiratory Rate Depression from Intravenous Remifentanil in Young and Adult Rabbits. Anesthesiology 127, 502–214. 10.1097/ALN.0000000000001719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkov YI, Bacak BJ, Dick TE, Rybak IA, 2013. Control of breathing by interacting pontine and pulmonary feedback loops. Front. Neural Circuits 7 10.3389/fncir.2013.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkov YI, Rubin JE, Rybak IA, Smith JC, 2017. Computational models of the neural control of breathing. Wiley Interdiscip. Rev. Syst. Biol. Med 9 10.1002/wsbm.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Cushing SL, Campbell F, Propst EJ, Horner RL, Narang I, 2016. Distinct Cortical Signatures Associated with Sedation and Respiratory Rate Depression by Morphine in a Pediatric Population. Anesthesiology 125, 889–903. 10.1097/ALN.0000000000001303 [DOI] [PubMed] [Google Scholar]
- Montandon G, Horner RL, 2019. Electrocortical changes associating sedation and respiratory depression by the opioid analgesic fentanyl. Sci. Rep 9, 14122 10.1038/s41598-019-50613-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL, 2011. PreBotzinger Complex Neurokinin-1 Receptor-Expressing Neurons Mediate Opioid-Induced Respiratory Depression. J. Neurosci 31, 1292–1301. 10.1523/JNEUROSCI.4611-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschel M, Dutschmann M, 2009. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos. Trans. R. Soc. B Biol. Sci 364, 2517–2526. 10.1098/rstb.2009.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp F. a, Stuth E. a E., Zuperku EJ, 2010. Clinically relevant infusion rates of mu-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Bötzinger complex region. J. Neurophysiol 103, 409–418. 10.1152/jn.00188.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M, 2014. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 4, 317–325. [DOI] [PubMed] [Google Scholar]
- Paton JFR, 1996. A working heart-brainstem preparation of the mouse. J. Neurosci. Methods 65, 63–68. 10.1016/0165-0270(95)00147-6 [DOI] [PubMed] [Google Scholar]
- Pattinson KTS, 2008. Opioids and the control of respiration. Br. J. Anaesth 100, 747–758. 10.1093/bja/aen094 [DOI] [PubMed] [Google Scholar]
- Pattinson KTS, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, Wise RG, 2009. Opioids depress cortical centers responsible for the volitional control of respiration. J. Neurosci 29, 8177–8186. 10.1523/JNEUROSCI.1375-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CS, Song G, 2014. Bidirectional plasticity of pontine pneumotaxic postinspiratory drive: Implication for a pontomedullary respiratory central pattern generator. Prog. Brain Res 209, 235–254. 10.1016/B978-0-444-63274-6.00012-6 [DOI] [PubMed] [Google Scholar]
- Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EAE, Hopp FA, Dean C, Zuperku EJ, 2012. Pontine μ-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. J. Neurophysiol 108, 2430–2441. 10.1152/jn.00185.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L, 2016. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010-2015. MMWR. Morb. Mortal. Wkly. Rep 65, 1445–1452. 10.15585/mmwr.mm655051e1 [DOI] [PubMed] [Google Scholar]
- Savilampi J, Ahlstrand R, Magnuson A, Geijer H, Wattwil M, 2014. Aspiration induced by remifentanil: A double-blind, randomized, crossover study in healthy volunteers. Anesthesiology 121, 52–58. 10.1097/ALN.0000000000000202 [DOI] [PubMed] [Google Scholar]
- Savilampi J, Ahlstrand R, Magnuson A, Wattwil M, 2013. Effects of remifentanil on the esophagogastric junction and swallowing. Acta Anaesthesiol. Scand 57, 1002–1009. 10.1111/aas.12134 [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Green JF, 2001. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir. Physiol 125, 17–31. 10.1016/S0034-5687(00)00202-4 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, Bacon S, 2018. Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants — United States, 2015–2016. MMWR. Morb. Mortal. Wkly. Rep 67, 349–358. 10.15585/mmwr.mm6712a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw IR, Lavigne G, Mayer P, Choinière M, 2005. Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: A preliminary study. Sleep 28, 677–682. 10.1093/sleep/28.6.677 [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala APL, Rybak IA, Paton JFR, 2009. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos. Trans. R. Soc. B Biol. Sci 364, 2577–2587. 10.1098/rstb.2009.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Poon CS, 2004. Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir. Physiol. Neurobiol 143, 281–292. 10.1016/j.resp.2004.05.009 [DOI] [PubMed] [Google Scholar]
- Song G, Tin C, Poon CS, 2015. Multiscale fingerprinting of neuronal functional connectivity. Brain Struct. Funct 220, 2967–2982. 10.1007/s00429-014-0838-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Wang H, Xu H, Poon CS, 2012. Kölliker-Fuse neurons send collateral projections to multiple hypoxia-activated and nonactivated structures in rat brainstem and spinal cord. Brain Struct. Funct 217, 835–858. 10.1007/s00429-012-0384-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St.John WM, 1998. Neurogenesis of patterns of automatic ventilatory activity. Prog. Neurobiol 56, 97–117. 10.1016/S0301-0082(98)00031-8 [DOI] [PubMed] [Google Scholar]
- St.John WM, Paton JFR, 2004. Role of pontile mechanisms in the neurogenesis of eupnea. Respir. Physiol. Neurobiol 143, 321–332. 10.1016/j.resp.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Stella G, 1938. On the mechanism of production, and the physiological significance of ‘apneusis.’ J. Physiol 93, 10–23. 10.1113/jphysiol.1938.sp003621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel H, Schüttler J, Magnussen H, Hengstmann JH, 1982. Plasma fentanyl concentrations and the occurrence of respiratory depression in volunteers. Br. J. Anaesth 54, 1087–1095. 10.1093/bja/54.10.1087 [DOI] [PubMed] [Google Scholar]
- Syková E, Nicholson C, 2008. Diffusion in Brain Extracellular Space. Physiol. Rev 88, 1277–1340. 10.1152/physrev.00027.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Eriksson LI, Yamamoto Y, Joensen H, Onimaru H, Sten SG, 2001. Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology 95, 740–749. 10.1097/00000542-200109000-00029 [DOI] [PubMed] [Google Scholar]
- Van Ryswyk E, Antic NA, 2016. Opioids and Sleep-Disordered Breathing. Chest 150, 934–944. 10.1016/j.chest.2016.05.022 [DOI] [PubMed] [Google Scholar]
- Varga AG, Reid BT, Kieffer BL, Levitt ES, 2019. Differential impact of two critical respiratory centers in opioid- induced respiratory depression in awake mice. J. Physiol 10.1113/jp278612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Collins FS, 2017. The Role of Science in Addressing the Opioid Crisis. N. Engl. J. Med 377, 391–394. 10.1056/nejmsr1706626 [DOI] [PubMed] [Google Scholar]
- Wilson RJA, Remmers JE, Paton JFR, 2017. Brain stem PO(2) and pH of the working heart-brain stem preparation during vascular perfusion with aqueous medium. Am. J. Physiol. Integr. Comp. Physiol 281, R528–538. 10.1152/ajpregu.2001.281.2.r528 [DOI] [PubMed] [Google Scholar]
- Yokota S, Kaur S, Vanderhorst VG, Saper CB, Chamberlin NL, 2015. Respiratory-related outputs of glutamatergic, hypercapnia-responsive parabrachial neurons in mice. J. Comp. Neurol 523, 907–920. 10.1002/cne.23720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Niu JG, Tsumori T, Oka T, Yasui Y, 2011. Glutamatergic Kölliker-Fuse nucleus neurons innervate hypoglossal motoneurons whose axons form the medial (protruder) branch of the hypoglossal nerve in the rat. Brain Res. 1404, 10–20. 10.1016/j.brainres.2011.06.025 [DOI] [PubMed] [Google Scholar]
- Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y, 2007. Glutamatergic neurons in the Kölliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: A combined retrograde tracing and in situ hybridization study in the rat. Neurosci. Res 59, 341–346. 10.1016/j.neures.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Zuperku EJ, Stucke AG, Hopp FA, Stuth EAE, 2016. Characteristics of breathing rate control mediated by a subregion within the pontine parabrachial complex. J. Neurophysiol 117, 1030–1042. 10.1152/jn.00591.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuperku EJ, Stucke AG, Krolikowski JG, Tomlinson J, Hopp FA, Stuth EA, 2019. Inputs to medullary respiratory neurons from a pontine subregion that controls breathing frequency. Respir. Physiol. Neurobiol 265, 127–140. 10.1016/j.resp.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]