Abstract
Use of the herbicide atrazine is banned in the European Union, yet it is still widely used in the USA and Australia. Atrazine is known to alter testosterone and oestrogen production and thus reproductive characteristics in numerous species. In this proof of concept study, we examined the effect of atrazine exposure, at a supra-environmental dose (5 mg/kg bw/day), beginning on E9.5 in utero, prior to sexual differentiation of the reproductive tissues, until 26 weeks of age, on the development of the mouse penis. Notably, this is the first study to specifically investigate whether atrazine can affect penis characteristics. We show that atrazine exposure, beginning in utero, causes a shortening (demasculinisation) of penis structures and increases the incidence of hypospadias in mice. These data indicate the need for further studies of atrazine on human reproductive development and fertility, especially considering its continued and widespread use.
Keywords: Endocrine disruptor, atrazine, hypospadias, penis, mouse
Introduction
Hypospadias is the abnormal placement of the urethral opening, resulting from disrupted urethral closure during development. It affects 1 in 125 live male births in developed countries and its incidence doubled in the United States of America between 1970 and 19931, with a similar prevalence and increase in incidence reported in Australia between 1980 and 2000.2 This increase is too rapid to be accounted for by genetic mutations and is not due to increased reporting.1 Males clinically diagnosed with hypospadias often exhibit reduced fertility and chordee (curvature of the penis), which can have profound medical and psychological consequences for the individual and family, as well as placing a significant burden on the health care budgets.3
Correct urethral closure is dependent on androgens and blocked by exogenous oestrogens.4 Environmental toxicants, namely endocrine disrupting chemicals (EDCs), can interfere with both androgen and oestrogen signalling pathways.5 Endocrine disrupting chemicals (EDCs) are increasing exponentially in their abundance and are highly pervasive in our environment, due to their beneficial properties, such as being effective plasticisers, preservatives or pesticides, and their use in numerous industrial and manufacturing processes.5 Hence, it is postulated that exposure to EDCs may explain the increasing incidence of hypospadias. This statement is supported by a growing body of evidence from human and animal studies, most notably the increased incidence of hypospadias and other male reproductive tract abnormalities (i.e. cryptorchidism) in the sons of the greater than 10 million women prescribed the synthetic oestrogen diethylstilbestrol, to reduce the risk of miscarriage, as well as females and their offspring exposed to phytoestrogens, polychlorinated biphenyls (PCBs), perflurooctanoic acid (PFOA), and especially, organochloride pesticides used in agriculture (e.g. dichlorodiphenyltrichloroethane (DDT), hexachlorobenezene and methoxychlor) (see reviews5,6,7,8).
Atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine, ATR) is one of the most commonly used herbicides in agriculture in the USA9 and Australia.10 ATR is known to have endocrine disrupting effects in amphibians, fish, reptiles and rodents.5 ATR can increase the expression of the gene cytochrome P450 family 19 subfamily A member 1 (Cyp19a1) in vivo in a wide spectrum of animals ranging from fish to rodents11, leading to an increase in the enzyme aromatase, that is responsible for converting testosterone to oestradiol.12 In addition, ATR has anti-androgenic properties, as it causes a decrease in the conversion of testosterone to the more potent dihydrotestosterone (DHT), via suppression of the enzyme 5α-reductase.13 Thus, ATR can have both oestrogenic and anti-androgenic actions, which can markedly affect male reproductive development. Here we examined the effects of ATR exposure, specifically on penis development which has not yet been investigated.
Materials and Methods
Animal treatments
Pregnant C57BL/6J mice were treated with atrazine (ATR, 5 mg/kg bw/day; Sigma-Aldrich, NSW, Australia, n = 3) in distilled water or vehicle control (0.5% DMSO, Control, n = 3) in distilled water from embryonic day 9.5 through to weaning. Male mice were housed individually and maintained on ATR water (n = 7) or control (n = 8) water treatment from weaning (three weeks of age) until sexual maturity (26 weeks post-partum). All mice were maintained under a 12 hr light: 12 hr dark lighting regimen and fed ad libitum a soy-free diet (Specialty Feeds, Perth, WA, Australia) throughout their gestation, lactation and post-weaning to avoid the effects of phytoestrogens. Water intake and food consumption was monitored for the duration of the study. Body weights were recorded weekly.
Analyses of body and testis weight, and examination of external genitalia
At 26 weeks of age mice were anesthetised with isoflurane (Forane; Abbott, NSW, Australia) and euthanised via cervical dislocation. At post mortem body weight was recorded, testes dissected and weighed, while the penis was dissected and imaged for gross morphology (n = 7 ATR and n = 8 control males). Comprehensive assessment of the morphological distinctions between the mouse and human penis, including similarities in their embryological origin, allows for the accurate comparison of analogous structures and hypospadias phenotypes.14,15 Mouse penis development differs from that of humans in that they have an os penis bone and male urogenital mating protuberance (MUMP). MUMP development is highly androgen dependent and forms a cartilaginous distal projection from the os penis bone that ensures the correct placement of the penis in the female urogenital tract for effective fertilisation.15 Thus, measurement of the MUMP provides a reliable biological proxy for how endocrine factors can affect the penis. The MUMP was imaged using a Nikon Digital Sight DS-U3 (Nikon Australia, VIC, Australia) and all images were analysed and MUMP height measured with NIS Elements Analysis D 4.300.00 64-bit software.
The penis was then fixed in 4% paraformaldehyde and processed for histology. The tissue was serially sectioned at 7 μm and stained with Hematoxylin and Eosin Y (H & E; Sigma-Aldrich, NSW, Australia) following standard protocols.16 To determine the presence of hypospadias, we examined how proximal the urethral opening persisted in the penis using the os penis bone as a distal landmark. This is a well-established standard methodology for determining hypospadias in the mouse penis.4,17
Statistical analyses
All data were tested for normality using the Shapiro-Wilk test. Data subjected to repeated measures analysis were tested for sphericity using the Mauchly’s sphericity test. Body weight, cumulative weight gain, as well as food and water intake were analysed using a repeated measures ANOVA, with treatment as a fixed factor, using SAS version 9.2 (SAS Institute). Birth weight, relative testis weight at post mortem using a one-way ANOVA. Non-normally distributed data (survival to weaning and MUMP height) were analysed by a Mann Whitney test using R studio version 1.0.143. Sex ratio was compared with an expected 50:50 ratio as well as between groups by a corrected χ2 procedure and was double-checked by binominal analysis. Results were considered significant when P ≤ 0.05 and all data are expressed as mean ± SEM, unless otherwise stated.
Results
Litter sex ratio, birth weight and survivability
ATR treatment had no effect on the sex ratio of pups when compared with controls (proportion male; control 0.44 (n = 8 male, n = 10 female), ATR 0.41 (n = 7 male, n = 10 female; P > 0.1). Also, ATR treatment had no effect compared with controls on average pup birth weight (control 4.1 ± 0.2 g, n = 18; ATR 3.9 ± 0.4 g, n =17; P > 0.1) or survival to weaning (control n = 18/18 (100%); ATR n = 17/17 (100%; P > 0.1).
Food and water intake, growth rate, body and relative testes weights at post mortem
Food and water intake did not differ between control and ATR exposed males (P > 0.1). Average water intake for control and ATR exposed males were 25.9 ± 0.6 ml/week and 25.7 ± 0.8 ml/week respectively, whilst average food intake was 27.6 ± 0.9 g/week and 27.9 ± 0.9 g/week, respectively. Equally, cumulative weight gain (growth rate from weaning to post mortem; data not shown), body weight (control 37.2 ± 0.8 g, ATR 36.7 ± 1.1 g) and relative testes weight (control 0.0058 ± 0.0003 g/g bw, ATR 0.0063 ± 0.0004 g/g bw) at post mortem were not different between control and ATR exposed males (P > 0.1).
ATR alters MUMP development
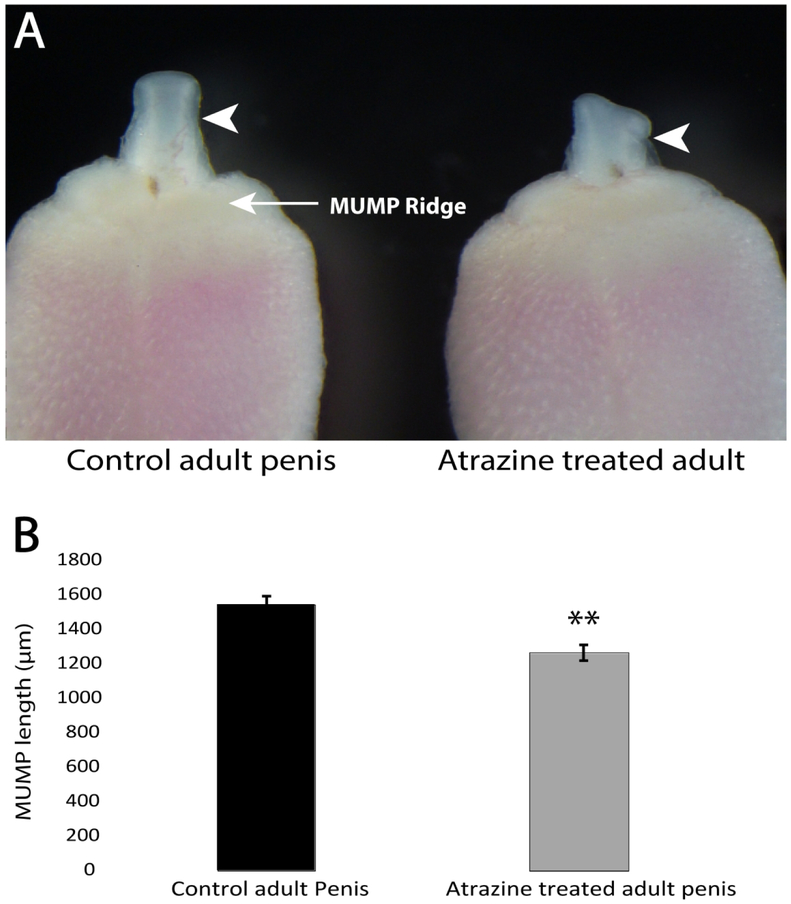
Males exposed to ATR displayed abnormal MUMP morphology (Figure 1A, white arrowhead indicating the MUMP), being consistently asymmetric in shape. This phenotype was observed in all seven ATR exposed males examined. In addition, the MUMP was also significantly shorter in ATR exposed males when compared to controls (P = 0.0014, Figure 1B). Again, this was consistent across all ATR exposed males.
Fig. 1.
Effects of atrazine (ATR) on penis development in a mouse. A) Gross morphology of the MUMP (indicated by white arrow heads) and MUMP ridge of the glans penis in the control (left) and the atrazine treated male (right) at 26 weeks of age. B) The mean (± SEM) length of the MUMP measure from the distal tip of the MUMP till the proximal base of the MUMP located in the MUMP ridge of the control (n = 8) and atrazine treated (n = 7) adult mouse penis (** indicates P = 0.0014).
ATR induced hypospadias
Analyses of the urethral opening showed it was distal to the tip of the os penis bone, in its normal (wild type) location for control and the majority of ATR exposed males (Figure 2 A-B). However, hypospadias (the proximal placement of the urethral opening) was observed in one of the seven ATR males examined (14.3% incidence), as indicated by the persistent urethral opening (ventral cleft) (Figure 2D, black arrowhead) in proximal sections of the penis where the os penis is visible (Figure 2D; os - dark pink condensed cells). This phenotype is very rare in wild type mice and was not observed in any vehicle control treated males in this cohort (n = 8, Figure 2C).
Fig. 2.
Comparison of distal transverse section histology of the adult control mouse penis (A), and the adult atrazine treated mouse penis (B), the ventral cleft (urethra opening) indicated by the black arrowhead. Comparison of proximal transverse section histology of the adult control mouse penis (C) where the ventral cleft is no longer present, and the adult atrazine treated mouse penis with a persisting ventral cleft (D). The ventral cleft indicated by the black arrowhead, U = urethra, Os = os penis bone. Scale bars = 500 μm.
Discussion
Although ATR was banned in the European Union in 2003,20 due to its highly persistent levels in ground water and risks to human health, it continues to be used at very high rates especially across the USA and in Australia.5 ATR is extremely stable in the environment with a half-life of more than 100 days in water21 and 240 days in soil.22 The dose given in this study, although supra-environmental, was based on previous papers that showed impacts on male reproductive development in rodents. These included decreased testis weight, altered testis morphology and steroidogenic enzyme expression, as well as reduced testosterone concentrations, the number of epididymal spermatozoa and delayed meiosis of spermatozoa.11,23, 24 However, no previous study had examined the potential impacts of ATR on penis development.
Development of the penis, including the processes of urethral closure and differentiation of the MUMP, are all highly androgen dependent processes.17 Furthermore, exposure of the developing penis to exogenous oestrogen is known to prevent urethral closure and demasculinise the developing genitalia leading to a smaller overall penis size and reduction of structures including the MUMP.4,18,19 Therefore, the stunted growth and altered morphology of the MUMP, evident in all the ATR exposed mice, is consistent with an endocrine disruption caused by the exposure. This is further validated by the incidence of hypospadias observed in one ATR exposed mouse (14.3% incidence in the ATR cohort). The ATR hypospadias phenotype was similar to that of mice exposed to the potent oestrogen diethylstilbestrol.4,17 Collectively, the stunted growth and disrupted morphology of the MUMP, as well as hypospadias phenotype of ATR exposed mice are consistent with ATR eliciting an anti-androgenic and pro-oestrogenic response during penis development. Given the association between EDCs and the increasing incidence of hypospadias in humans, these data implicate ATR to be one of many potential causative agents in the etiology of this common disease.
Further rodent studies are required using lower, environmentally relevant doses and greater sample number, to determine if they also cause hypospadias. This is supported by the finding that men with higher urinary atrazine concentrations had poor semen quality.25 Given the rapidly increasing incidence of hypospadias and our demonstrated effect of ATR exposure on urethral closure and penis development the association between human exposures to ATR and the incidence of hypospadias warrants further investigations.
Acknowledgements
The authors thank Tania Long and Darren Cipolla for their technical contributions and help with animal husbandry.
Funding Sources
This work was supported by University of Melbourne internal funds [R06000010 to M.P.G.], as well as NIH R01DK096263 and NHMRC APP1098480 to A.J.P.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to declare.
Statement of Ethics
Animal experiments conform to internationally accepted standards and were approved by the University of Melbourne Animal and Ethics committee (AEC 1513481.5) in accordance with the Australian National Health and Medical Research Council guidelines.
References
- 1.Paulozzi LJ. International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect. 1999; 107, 297–302. DOI: 10.1289/ehp.99107297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nassar N, Bower C, Barker A. Increasing prevalence of hypospadias in Western Australia, 1980-2000. Arch Dis Child. 2007; 92, 580–584. DOI: 10.1136/adc.2006.112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouty A, Ayers KL, Pask AJ, Helourry Y, Sinclair AH. The genetic and environmental factors underlying hypospadias. Sex Dev. 2015; 9, 239–259. DOI: 10.1159/000441988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govers LC, Phillips TR, Mattiske DM et al. A critical role for estrogen signaling in penis development. FASEB J. 2019; accepted 3rd June 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: The Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015; 36, E1–E150. DOI: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skakkebaek NE, Raipert-De Meyts E, Buck GM et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev. 2016; 96, 55–97. DOI: 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonde JP, Flachs EM, Rimborg S, et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders:a systemic review and meta-analysis. Hum Reprod Update. 2017; 23, 104–125. DOI: 10.1093/humupd/dmw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United Nations Environmental Programme & World Health Organization. State of the science of endocrine disrupting chemicals- 2012 (eds. Bergman A, Heindel JJ, Jobling S, Kidd SA, Zoeller RT). 2013; pp. 1–289. United Nations Environmental Programme & World Health Organization, Geneva, Switzerland. [Google Scholar]
- 9.Farruggia FT, Rossmeisl CM, Hetrick JA, Biscoe M, Branch MER III. Refined Ecological Risk Assessment for Atrazine; 2016, US Environmental Protection Agency, Office of Pesticide Programs, Washington D.C., U.S.A. [Google Scholar]
- 10.Mnif W, Hassine AI, Bouaziz A, Bartegi A, Thomas O, Roig B. Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health. 2011; 8, 2265–2303. DOI: 10.3390/ijerph8062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin YX, Wang LG, Fu ZW. Oral exposure to atrazine modulates hormone synthesis and the transcription of steroidogenic genes in male peripubertal mice. Gen Comp Endocrinol. 2013; 184, 120–127. DOI: 10.1016/j.ygcen.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Holloway AC, Anger DA, Crankshaw DJ, Wu M, Foster WG. Atrazine-induced changes in aromatase activity in estrogen sensitive target tissues. J Appl Toxicol. 2008; 28, 260–270. DOI: 10.1002/jat.1275. [DOI] [PubMed] [Google Scholar]
- 13.Kniewald J, Osredecki V, Gojmerac T, Zechner V, Kniewald Z. Effect of s-triazine compounds on testosterone metabolism in the rat prostate. J Appl Toxicol. 1995; 15, 215–218. DOI: 10.1002/jat.2550150312. [DOI] [PubMed] [Google Scholar]
- 14.Blaschko SD, Mahawong P, Ferretti M, et al. Analysis of the effect of estrogen/androgen perturbation on penile development in transgenic and diethystilbesterol-treated mice. Anat Rec. 2013; 296, 1127–1141. DOI: 10.1002/ar.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips TR, Wright DK, Gradie PE, Johnston LA., Pask AJ. A comprehensive atlas of the adult mouse penis. Sex Dev. 2015; 9, 162–172. DOI: 10.1159/000431010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008; 5, pdb.prot4986. DOI: 10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 17.Mahawong P, Sinclair A, Li Y, Schlomer B, et al. Prenatal diethylstilbestrol induces malformation of the external genitalia of male and female mice and persistent second-generation developmental abnormalities of the external genitalia in two mouse strains. Differentiation. 2014; 88, 51–69. DOI: 10.1016/j.diff.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Yu HS, Pask AJ, et al. Hormone-responsive genes in the SHH and WNT/beta-catenin signaling pathways influence urethral closure and phallus growth. Biol Reprod. 2018; 99, 806–816. DOI: 10.1093/biolre/ioy117. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair AW, Cao M, Baskin L, Cunha GR. Diethylstilbesterol-induced mouse hypospadias:”window of susceptibility”. Differentiation. 2016; 91, 1–18. DOI: 10.1016/j.diff.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EU Commission. Commission decision of 10 March 2004 concerning the non-inclusion of atrazine in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing this active substance. J Eur Union. 2004; 78, 53–55. [Google Scholar]
- 21.Australian Pesticides and Veterinary Medicines Authority. Technical report: Environmental assessment. 2004; Canberra, Australia: Available from: https://apvma.gov.au/node/14356 [cited 2018 Dec 23]. [Google Scholar]
- 22.US Environmental Protection Agency (EPA) Interim Reregistration Eligibility Decision for Atrazine 2005. 2003; US EPA, Washington D.C., U.S.A. [Google Scholar]
- 23.Victor-Costa AB, Bandeira SM, Oliveira AG, Mahecha GA, Oliveira CA. Changes in testicular morphology and steroidogenesis in adult rats exposed to atrazine. Reprod Toxicol. 2010; 29, 3, 323–331. DOI: 10.1016/j.reprotox.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Gely-Pernot A, Hao C, Becker E, et al. The epigenetic processes of meiosis in male mice are broadly affected by the widely used herbicide atrazine. BMC Genomics. 2015; 16, 885 DOI: 10.1186/s12864-015-2095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swan SH, Kruse RL, Liu F, et al. Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect. 2003; 111, 12, 1478–1484. DOI: 10.1289/ehp.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]