Abstract
Background
Over 320 million individuals are living with Major Depressive Disorder (MDD), a leading cause of disability worldwide. Thus, there is a crucial need to identify processes that contribute to the maintenance of depressive episodes. Difficulty removing negative information from working memory (WM) is posited to exacerbate affective, cognitive, and biological dysregulation in Major Depressive Disorder (MDD), but this has not yet been tested empirically.
Methods
In this study we examined whether training depressed individuals to remove negative information from WM (RNI training) would reduce symptoms of depression and levels of rumination, and would be associated with attenuated biological responsivity to stress. Individuals diagnosed with MDD were randomly assigned to complete Real-RNI training or Sham-RNI training for six days.
Results
Across conditions, participants exhibited significant improvements from pre- to post-training in removing negative information from WM, symptoms of depression, and rumination. Furthermore, participants in the Real-RNI condition showed a more attenuated pattern of cortisol and respiratory sinus arrhythmia (RSA) responses to stress than did participants in the Sham-RNI training condition.
Limitations
We did not assess the long-term effects of training. It will be important for future research to examine whether the documented training-related effects persist across time.
Conclusions
This study is the first to examine the effects of RNI training on clinical symptoms and biological responses to stress in MDD, and it provides experimental evidence that training individuals with depression to remove negative information from WM can help to modulate the heightened biological responses to stress seen in depression.
Keywords: Cognitive bias modification, depression, rumination, biological stress response
Major Depressive Disorder (MDD) is characterized by difficulty removing negative information from working memory (WM; LeMoult & Gotlib, 2018; World Health Organization, 2012). Although researchers have posited that difficulty removing negative information from WM contributes to depressive symptoms, rumination, and exaggerated biological responses to stress (Joormann, 2010; LeMoult & Gotlib, 2018; Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008), there has been no research to date that experimentally examines the associations among these variables in the context of depression. Consequently, we do not fully understand the ways in which difficulty removing negative information from WM contributes to MDD.
To date, only one study has experimentally examined the effects of difficulty removing information from WM on symptoms of depression and levels of rumination (Onraedt & Koster, 2014). In that study, participants with MDD and high levels of trait rumination completed six sessions of training on a dual n-back task. Although performance on the dual n-back task significantly improved from pre- to post-training, this gain did not transfer to measures of depression, rumination, or alternative measures of WM. It is possible that Onraedt and Koster (2014) did not find transfer effects because participants were trained to remove non-valenced information from WM, which does not directly target the core difficulty underlying levels of rumination and depressive symptoms observed in MDD: difficulty removing negatively valenced information from WM (for reviews see Joormann & Stanton, 2016; LeMoult & Gotlib, 2018). While there is some evidence that affective WM training can result in improved control over affective information in non-clinical samples (Schweizer et al., 2011, 2013), the associations among removing negative information from WM, rumination, and depression have not been examined experimentally, and not within a sample of participants with MDD.
Although researchers have also not yet examined the association between difficulties in WM disengagement and dysregulated biological responses to stress, studies assessing constructs related to WM disengagement provide a degree of support for this association. For example, researchers have documented that ruminative responses to stress are associated with impaired recovery of both the neuroendocrine and autonomic nervous systems (Key, Campbell, Bacon, & Gerin, 2008), particularly for individuals with MDD (LeMoult & Joormann, 2014). For example, both MDD and ruminative responses to stress have been linked with excessive respiratory sinus arrhythmia (RSA) withdrawal in response to stress (Beauchaine, 2015; LeMoult, Yoon, & Joormann, 2015). Given the association between difficulty disengaging from negative material in WM and rumination (LeMoult & Gotlib, 2018), there is reason to expect that difficulty disengaging from negative information in WM might underlie the dysregulated biological responses to stress documented in MDD. However, this possibility has not yet been experimentally examined.
The cognitive bias modification (CBM) literature highlights the importance of manipulating cognitive processes to test causal relations and to identify mechanisms that might underlie the onset of MDD (Hallion & Ruscio, 2011). Investigators have contended that the strongest advances in our understanding of how CBM paradigms achieve their effects will come from the assessment of training-related changes across multiple domains. Underscoring this point, previous studies using CBM paradigms have demonstrated that training individuals to alter the way they process information can result in changes in cognition, affect, and biology. These studies help to establish causal associations between cognitive biases and both affective and biological functioning (Hertel & Mathews, 2011). To date, much of the CBM literature has targeted attentional and interpretation biases in the context of anxiety disorders (Hallion & Ruscio, 2011; Macleod & Mathews, 2012). Thus, studies are needed that investigate the cognitive, affective, and biological effects of training participants with MDD to remove negative information from WM.
The present study was designed to extend the CBM literature by testing the effects of training depressed individuals to remove negative information (RNI training) from WM on cognitive, affective, and biological functioning. We assigned individuals diagnosed with MDD to either the Real-RNI or Sham RNI condition for six days. We measured individual differences in the ability to remove negative information from WM, symptoms of depression, and levels of rumination at both pre- and post-training sessions. In addition, at post-training, participants completed a standardized laboratory-based stressor, during which we measured biological responsivity. We predicted that, compared to participants in the Sham-RNI condition, participants in the Real-RNI condition would exhibit greater improvements from pre- to post-training in removing negative information from WM (Hypothesis 1). Further, we expected that, compared to participants assigned to the Sham-RNI condition, participants assigned to the Real-RNI condition would report greater pre- to post-training decreases in symptoms of depression (Hypothesis 2) and levels of rumination (Hypothesis 3), and would exhibit attenuated biological responses across the laboratory stressor as measured by cortisol (Hypothesis 4) and RSA (Hypothesis 5).
Method
Participants
Adults between 18 and 60 years of age were eligible to participate in this study if they were fluent in English and met criteria for current MDD or had no past or current psychopathology (CTLs). We recruited CTL participants in order to confirm that our sample of depressed participants had, at pre-training, significantly higher self-reported symptoms of depression and rumination, and significantly greater difficulty removing negative information from WM. Diagnostic status was determined using the Structured Clinical Interview for DSM-IV (SCID-IV; First, spitzer, Gibbon, & Williams, 1996). Participants were excluded if they had major medical conditions, head trauma, bipolar disorder, symptoms of psychosis, an alcohol or substance use disorder in the past 6 months, or conditions know to affect the neuroendocrine or autonomic nervous systems.
Of the 90 individuals (54 with MDD and 36 CTLs) who were eligible following the SCID-IV, seven participants with MDD did not complete the post-training session and five participants with MDD had substantial difficulties following directions (e.g., did not properly complete the baseline assessment or did not complete any at-home trainings). Thus, the final sample consisted of 78 participants (42 with MDD and 36 CTLs) who were between 19 and 55 years of age. The final sample of participants did not differ at pre-training from the 12 individuals without complete data on any clinical or demographic characteristics, all ps>.05.
Working Memory Bias Task
Difficulty eliminating positive and negative information from WM was assessed using the affective version of the Sternberg task (Joormann & Gotlib, 2008). The task consists of three blocks of 40 trials. Each trial consisted of a learning display, a cue display, and a probe-recognition display. During the learning display, participants viewed two sets of three words. Following the offset of the word sets, participants viewed a cue that indicated which set of words would be relevant for the probe-recognition display; this prompted participants to remove the other (irrelevant) set of words from WM. Of the 120 trials, 40% required that participants remove the positive word set and 40% required that participants remove the negative word set. The final 20% of trials were control trials, which included positive and negative words in both sets; these trials were included to ensure that participants did not learn to make decisions about the probe based on word valence. Finally, in the probe-recognition display, a single word was presented and participants were given 3,000ms to indicate as quickly and as accurately as possible whether the probe word was from the relevant word set. The probe word could be from the relevant set, the irrelevant set, or a novel word that was not included in either set. Consistent with previous research (Joormann & Gotlib, 2008), individual differences in the ability to remove irrelevant information from WM were modeled as decision latencies to words from the irrelevant set minus the decision latencies to new words of the same valence. These differences in response times are termed intrusion effects and were calculated separately for positive and negative words. Difficulty removing information from WM results in larger intrusion effects. Psychometric properties for the Sternberg were good: split-half reliability coefficients on critical trial RTs ranged from .78 to .96.
Working Memory Training
Using stratified random assignment based on their scores on the Beck Depression Inventory-II, participants with MDD were assigned to either the Real-RNI (n=22) or the Sham-RNI (n=20) condition. In both conditions, participants completed an at-home training session each day for six days. Training tasks were administered using E-Prime software version 2 on laptop computers provided to participants. Each training session lasted 15–20 minutes and performance files were stored on the computer until participants returned the laptop at their post-training session. Consistent with previous research, analyses were restricted to trials in which participants made correct responses and in which reaction times (RTs) were less than 3,000ms (Joormann & Gotlib, 2008). This resulted in the loss of 9.21% of trials at pre-training and 7.86% of trials at post-training, which is consistent with previous studies (Yoon, LeMoult, & Joormann, 2014). Participants in the two conditions did not differ from each other in the percentage of trials lost at pre-training or post-training, ps>.201.
Real RNI
Participants in the Real-RNI condition were presented with an adapted version of the original affective Sternberg Task that was completed during the pre-training session. The original and training tasks differed in length and in percentage of each type of trial. Of the 60 trials completed during each training session, 80% of trials required that participants remove the negative word set, 10% required that participants remove the positive word set (to ensure that participants had reason to continue learning both positive and negative word sets), and 10% were control trials.
Sham RNI
Participants in the Sham-RNI condition were presented with a modified lexical decision task. In each trial, participants were presented with a string of letters and were required to indicate whether the string formed a real word. Of the 360 trials, 50% of trials presented nonsense words, and 50% presented the same words that were used in the Real-RNI task. Thus, participants in the Real-RNI and Sham-RNI conditions were exposed to the same wordlists each day (additional details in the online supplement).
Psychosocial stressor
To examine participants’ biological responses to stress, participants completed the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993) during the post-training lab session. The TSST is a standardized stressor that has been found to be effective in eliciting a biological stress response (Dickerson & Kemeny, 2004). It consists of four phases: baseline (20 minutes), anticipation (10 minutes), stressor (10 minutes), and recovery (30 minutes). Neuroendocrine (salivary cortisol) and autonomic (RSA) responses were measured throughout the task. See online supplement for further details.
Cortisol
Saliva samples were collected immediately before the baseline period (S1), immediately after the baseline period (S2), immediately after the stressor (S3), 15 minutes after stressor offset (S4), and 30 minutes after stressor offset (S5). This collection schedule is based on meta-analytic findings of the timing of cortisol reactivity and recovery (Dickerson & Kemeny, 2004). Cortisol values were winsorized to 2 standard deviations above the mean as is consistent with previous research (Doom, Cicchetti, Rogosch, & Dackis, 2013; Gotlib et al., 2015).
RSA
Autonomic data were recorded continuously using the Biopac system. ECG signals were transmitted using three electrodes positioned in a modified lead II configuration. Respiration was measured using a respiratory belt placed around the chest and the abdomen. RSA was scored in 1-minute epochs using AcqKnowledge 4.0 software, and was calculated as the natural log of the high frequency power (.15-.40 Hz). Consistent with Kircanski, Waugh, Camacho, and Gotlib (2016), we created the following segments: baseline (10 minutes), anticipation (5 minutes), speech task (5 minutes), arithmetic task (5 minutes), initial recovery (15 minutes), and final recovery (15 minutes).
Self-Report Measures
Symptoms of Depression
The severity of participants’ symptoms of depression was assessed with the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996), a 21-item self-report questionnaire assessing depressive symptoms experienced in the previous two weeks. In addition, at the pre- and post-training sessions, participants completed a modified BDI-II scale (M-BDI-II) that assessed depressive symptoms in the previous three days. In the present study, there was excellent internal consistency in both the original and modified versions of the BDI-II (αs ≥ .92).
Rumination
Rumination was assessed with the Ruminative Responses Scale (Nolen-Hoeksema & Morrow, 1991), a 22-item self-report questionnaire designed to measure individual differences in the tendency to ruminate when feeling depressed. In addition, at the pre- and post-training session, participants completed a modified RRS (M-RRs) that assessed rumination in the previous three days. In the current study, there was excellent internal consistency in both the original and modified versions of the RRS (αs ≥ .93).
Procedure
The study was approved by the Stanford University Institutional Review Board. Following previous CBM studies (Hallion & Ruscio, 2011; Koster & Hoorelbeke, 2015), data were collected in a multi-session procedure. Participants first came into the lab to complete the SCID and self-report measures of symptoms of depression (BDI-II) and rumination (RRS). Eligible participants were invited to return to the lab within two weeks for the pre-training session, during which they completed the Sternberg Task and reported on their symptoms of depression (M-BDI-II) and rumination (M-RRS) within the past three days. Participants were then assigned to complete either Real-RNI or Sham-RNI training at home for the next six consecutive days. On the seventh day, participants returned to the lab for the post-training session, during which participants completed the same cognitive and self-report measures completed during the pre-training session, and the TSST.
Statistical Analyses
To examine whether depressed individuals who were assigned to the Real-RNI condition exhibited greater improvements from pre- to post-training than did depressed individuals who were assigned to the Sham-RNI condition in removing negative information from WM (Hypothesis 1), we will conduct a three-way Condition (Real RNI, Sham RNI) by Valence (positive, negative) by Time (pre-training, post-training) repeated-measures analysis of variance (ANOVA) on intrusion effects. If the expected three-way interaction is significant, we will conduct follow-up two-way ANOVAs within each condition, for each valence, and at each time point, followed by paired or independent sample t-tests as indicated. To examine whether depressed individuals assigned to the Real-RNI condition exhibit greater improvements from pre- to post-training than depressed individuals assigned to the Sham-RNI condition in symptoms of depression (Hypothesis 2) and levels of rumination (Hypothesis 3), we will conduct a two-way Condition by Time repeated-measures ANOVAs on BDI-II and on RRS scores. If the expected two-way interaction is significant, we will conduct independent-samples t-tests at pre- and post-training, and paired-samples t-tests for participants in both the Real-RNI and Sham-RNI condition.
To examine whether depressed individuals assigned to the Real-RNI condition exhibited attenuated cortisol (Hypothesis 4) and RSA (Hypothesis 5) responses to stress than did depressed individuals assigned to the Sham-RNI condition, we will use hierarchical linear modeling (HLM; Raudenbush & Bryk, 2002). This approach allows us to model the repeated measurements of both cortisol and RSA within persons as a function of time, and permits the examination of unevenly spaced measurement occasions (Hruschka, Kohrt, & Worthman, 2005). Thus, the exact time of cortisol and RSA collection will be allowed to vary by individual, which provides a precise estimation of collection timepoints for each participant. Linear, quadratic, and piecewise models will be evaluated for both cortisol and RSA. We will select the model that best fits the data based on the smallest value of Akaikie’s Information Criteria (AIC) and visual inspection of the data. Condition will be dummy-coded with the Sham-RNI condition as the referent group, and will be included at Level 2. Models will be fit using full information maximum likelihood for the calculation of deviance and AIC, and fit using restricted maximum likelihood for the estimation of model parameters. See supplement for all HLM equations.
Results
Differences Between MDD and CTL Groups
Clinical and demographic characteristics of participants are presented in Table 1. Participants with MDD did not differ significantly from CTLs with respect to age, gender, racial background, marital status, years of education, or income, all ps>.05. As expected, however, at pre-training, participants with MDD had significantly higher levels of depressive symptoms, t(49)=−16.09, p<.001, g=3.43, and rumination, t(71 )=−15.46, p<.001, g=3.42, than did CTLs. MDD participants also had significantly greater negative intrusions effects on the Sternberg task than did CTL participants, t(76)=−2.05, p=.044, g=0.46, indicating that they had more difficulty removing negative information from WM.
Table 1.
Clinical and Demographic Characteristics
| Participants with MDD | Controls | |||
|---|---|---|---|---|
| Variable | Real RNI | Sham RNI | Total | |
| Age, M (SD) | 28.00 (5.58) | 29.50 (9.40) | 28.71 (7.58) | 29.71 (10.25) |
| Gender, % female | 68.2 | 70.0 | 69.0 | 66.7 |
| Racial background, % white | 69.6 | 600 | 64.3 | 60.6 |
| Marital status, % single | 72.7 | 80.0 | 76.2 | 62.9 |
| Years of education, M (SD) | 15.32 (3.58) | 16.70 (2.62) | 15.98 (3.20) | 14.79 (3.58) |
| Number trainings completed, M (SD) | 5.82 (0.50) | 5.95 (0.39) | 5.88 (0.45) | --- |
| BDI-II, M (SD) | 30.14 (11.30) | 27.45 (9.34) | 28.86 (10.38) | 1.53 (2.36) |
| RRS, M (SD) | 62.55 (12.16) | 58.45 (8.86) | 60.60 (10.79) | 30.44 (9.25) |
| Pre-Training | ||||
| M-BDI-II, M (SD) | 31.90 (10.31) | 26.47 (9.14) | 29.39 (10.05) | 2.69 (3.20) |
| M-RRS, M (SD) | 56.46 (9.82) | 52.32 (7.36) | 54.54 (8.91) | 27.80 (6.09) |
| Negative intrusion effects, M (SD) | 35 2.01 (220.87) | 446.84 (218.32) | 397.17 (222.20) | 298.71 (199.04) |
| Positive intrusion effects, M (SD) | 397.44 (201.67) | 411.78 (242.13) | 404.27 (219.20) | 382.84 (196.98) |
| Post-Training | ||||
| M-BDI-II, M (SD) | 28.14 (10.55) | 22.50 (11.35) | 25.45 (11.18) | --- |
| M-RRS, M (SD) | 52.50 (11.02) | 49.05 (9.76) | 50.86 (10.46) | --- |
| Negative intrusion effects, M (SD) | 297.24 (177.82) | 301.87 (224.58) | 299.44 (198.93) | --- |
| Positive intrusion effects, M (SD) | 308.62 (208.71) | 313.92 (323.18) | 311.14 (265.93) | --- |
Note: BDI-II = Beck Depression Inventory-II, RRS = Ruminative Responses Scale, M-BDI-II = Modified Beck Depression Inventory-II (previous three days), M-RRS = Modified Ruminative Response Scale (previous three days).
Differences Between Depressed Participants in the Real-RNI and Sham-RNI Conditions
Participants with MDD who received Real-RNI training did not differ significantly from those who received Sham-RNI training with respect to age, gender, racial background, marital status, years of education, income, number of at-home training sessions completed, or baseline symptoms of depression, rumination, negative intrusion effects, or positive intrusion effects, ps>.05.
Effects of RNI Training
Pre- and post-training negative intrusions, symptoms of depression, and levels of rumination are presented in Table 1.
Working memory biases (Hypothesis 1)
The three-way repeated-measures ANOVA yielded a significant main effect of time, F(1,40)=10.84, p=.002, η2=.21; participants with MDD significantly improved from pre- to post-training in removing positively and negatively valenced irrelevant information from WM. No other effects were significant, ps>.302.1
Depression (Hypothesis 2)
The repeated-measures ANOVA conducted on symptoms of depression yielded a main effect of time, F(1,39)=6.97, p=.012, η2=.15, reflecting a significant decline from pre- to post-training in depressive symptoms. No other effects were significant, ps>.078.
Rumination (Hypothesis 3)
The repeated-measures ANOVA on rumination yielded a significant main effect of time, F(1,39)=10.62, p=.002, η2=.21, reflecting a significant pre- to post-training decline in levels of rumination. No other effects were significant, ps>.160.
Cortisol responses to stress (Hypothesis 4)
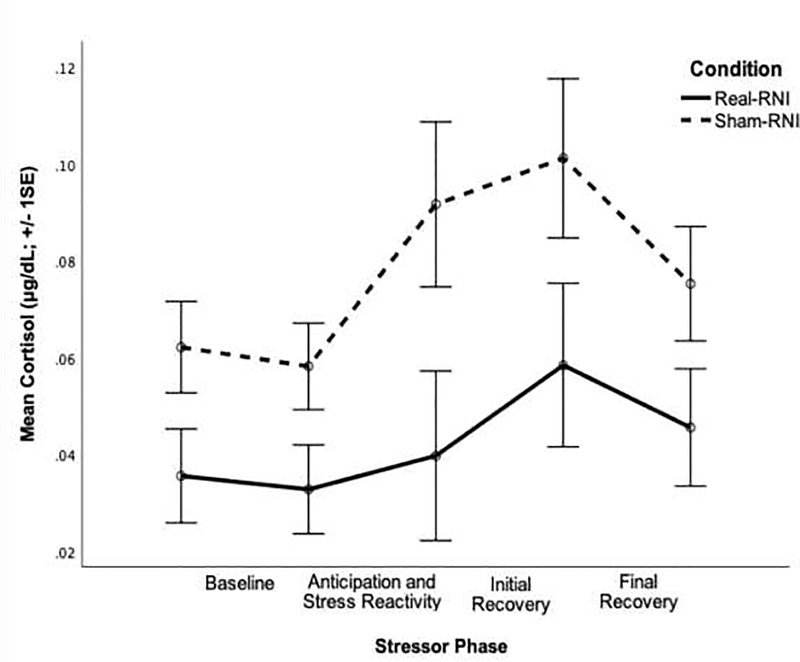
Cortisol response to stress is presented by condition in Figure 1 and all results can be found in Table S1 of the online supplement. Based on visual inspection of the data, deviance statistics, and the AIC value, the quadratic growth model best fit the pattern of cortisol production.
Figure 1.
Pattern of cortisol response to stress by condition.
To examine the basic pattern of cortisol response to the TSST, we first ran a baseline model without any variables at Level 2. Participants’ average cortisol level was significantly different than zero at baseline, B=−0.24, t(38)=−2.32, p=.026, and significantly increased in response to the stressor, B=0.01, t(38)=2.27, p=.029. Although the rate of change in cortisol level over time (i.e. the quadratic slope) was not significant, B=−0.00, t(109)=−1.65, p=.103, all estimates of variance components were significant (all ps<.001), indicating individual differences in the variation in cortisol levels at baseline and over time (linear and quadratic slopes).
Next, we tested variables shown to influence cortisol responses to stress as potential covariates: age, use of oral contraceptives, current use of psychotropic medication, past use of psychotropic medication, and engagement in exercise on the day of the session (Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004; Kudielka, Hellhammer, & Wüst, 2009). Use of oral contraceptives and psychotropic medication predicted baseline cortisol, and use of oral contraceptives predicted the rate of change in cortisol over time (i.e. the quadratic slope), ps<.049. Thus, we controlled for these variables in the corresponding Level 2 equation.
As expected, condition significantly predicted the quadratic slope, B=0.0002, t(107)=2.00, p=.048. Compared to participants in the Sham-RNI condition, individuals in the Real-RNI condition exhibited an accelerated rate of change in cortisol secretion, consistent with improved recovery from the stressor.
RSA response to stress (Hypothesis 5)
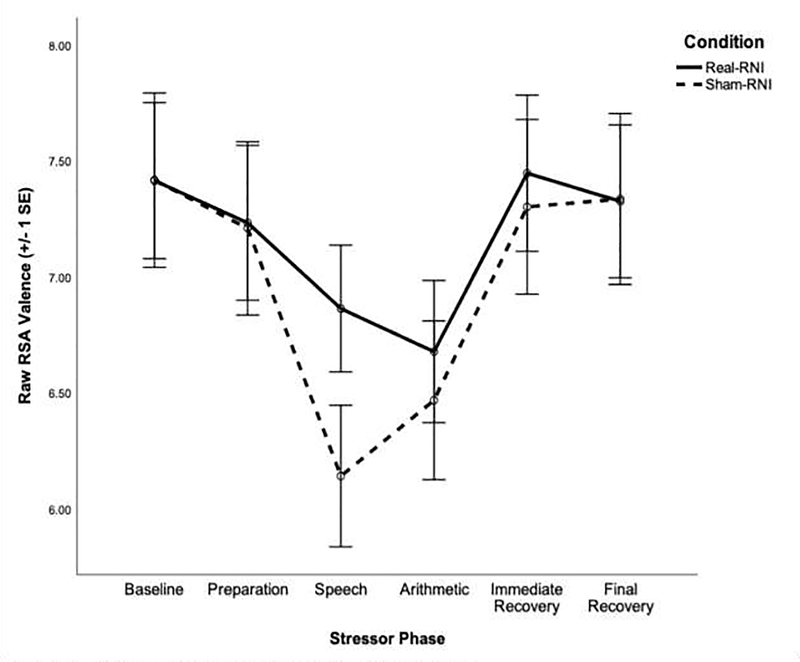
RSA response to stress is presented by condition in Figure 2 and all results can be found in Table S2 of the online supplement. Based on visual inspection of the data, deviance statistics, and the AIC value, RSA data were best fit with a piecewise linear growth model, which estimated the slope of RSA across baseline, anticipation, initial stress reactivity, subsequent stress reactivity, initial recovery, and final recovery phases of the stressor, as is consistent with previous work (Waugh, Panage, Mendes, & Gotlib, 2010).
Figure 2.
Pattern of RSA response to stress by condition.
A baseline model without any Level 2 predictors indicated that participants’ level of RSA at baseline was significantly different from zero, B=7.43, t(38)=32.88, p<.001, remained constant during the anticipation period, B=−0.02, t(38)=−1.88, p=.067, and then significantly declined during the initial stress reactivity period, B=−0.14, t(38)=−5.28, p<.001. Levels of RSA remained constant across the subsequent stress reactivity phase, B=0.003, t(38)=0.33, p=.742, significantly increased across the initial recovery period, B=0.05, t(38)=8.37, p<.001, and remained constant across the final recovery period, B=−0.003, t(38)= −0.67, p=.508.
Next, we tested variables shown to influence RSA response to stress as potential covariates: age, current use of psychotropic medication, past use of psychotropic medication, and engagement in exercise on the day of the session (Grossman, Wilhelm, & Spoerle, 2004; O’Regan, Kenny, Cronin, Finucane, & Kearney, 2015; Voss, Schroeder, Heitmann, Peters, & Perz, 2015). Both past and current use of psychotropic medication predicted the slope of RSA during the final recovery period, ps<.018. Consequently, these variables were included as covariates in the corresponding Level 2 equation.
As expected, participants in the Real-RNI condition exhibited a more attenuated pattern of RSA response to stress than did participants in the Sham-RNI condition. Specifically, compared to participants in the Sham-RNI condition, participants in the Real-RNI condition had less RSA withdrawal in response to the speech (initial stress reactivity), B=0.15, t(37)=3.14, p=.003, and less RSA augmentation in response to the arithmetic task (subsequent stress reactivity), B=−0.05, t(37)=−2.63, p=.012.
Associations between Change in Negative Intrusion Effects and Key Outcomes
We conducted exploratory analyses to examine whether change in negative intrusions was associated with change in symptoms of depression, change in rumination, and degree of biological responses to stress, and to test whether these associations differed by condition. Analyses indicated that neither change in negative intrusions nor the interaction between condition and change in negative intrusions was associated with change in symptoms of depression or with change in levels of rumination, ps > .150 (detailed results presented in Table S3 of the online supplement). Similarly, neither change in negative intrusions nor the interaction between condition and change in negative intrusions predicted cortisol responses to stress, ps > .435 (detailed results presented in the text of the online supplement). However, a significant interaction between condition and change in negative intrusions predicted RSA trajectory across both the initial stress reactivity, B=0.001, t(35)=3.24, p=.003, and subsequent stress reactivity phase of the TSST, B=−0.0001, t(35)=−2.13, p=.040. Further, the interaction between condition and change in negative intrusion effects was significant at a trend level for baseline levels of RSA, B=−0.004, t(35)=−2.00, p=.053. Within the Real-RNI condition, greater change in negative intrusions was associated with a briefer RSA withdrawal during the anticipation period, B=−0.0001, t(19)=−2.14, p=.045, and with faster RSA recovery during the initial stress reactivity period, B=0.0004, t(19)=2.73, p=.013. Within the Sham-RNI condition, greater change in negative intrusions predicted higher levels of RSA at baseline, B=0.003, t(16)=2.70, p=.016, with greater pre- to post-training improvement in removing negative information from WM predicting higher levels of RSA at baseline. Detailed results are presented in the text of the online supplement.
Discussion
This study is the first to experimentally examine the effects of training depressed individuals to remove negative information from WM on cognition, affect, and biological responses to stress. Previous WM training paradigms have focused predominantly on nonvalenced stimuli (Course-Choi, Saville, & Derakshan, 2017; Onraedt & Koster, 2014; Owens, Koster, & Derakshan, 2013); this study is the first to examine the effects of affective WM training with a clinically depressed sample. Using multiple indicators of biological functioning, we found that depressed individuals assigned to the Real-RNI training condition exhibited less biological reactivity in response to the stressor than did depressed individuals assigned to the Sham-RNI training condition. In addition, contrary to our hypotheses, all participants significantly improved in their ability to remove negative information from WM and reported significant decreases in both symptoms of depression and levels of rumination. In other words, there was no significant effect of Real-RNI compared to Sham-RNI training on pre- to post-training change in negative intrusions, symptoms of depression, or levels of rumination.
Unexpectedly, participants in both conditions significantly improved from pre- to post-training in removing emotional information from WM. There are several possible explanations for this finding. First, the lexical decision task completed by participants in the Sham-RNI condition may have engaged the neural systems involved in WM, such as subregions of the prefrontal cortex (Courtney, Ungerleider, Keil, & Haxby, 1997; Curtis & D’Esposito, 2003). Indeed, there is evidence to suggest that both lexical decision tasks and verbal WM tasks are associated with increased activation of regions such as the anterior cingulate gyrus and left inferior frontal gyrus (Chen & Desmond, 2005; Phan et al., 2005; Zhang, Leung, & Johnson, 2003). Thus, it is possible that the real and sham tasks may have been activating structures in the same neural system (McNamara & Altarriba, 1988). However, it is important to note that the lexical decision task does not involve the same degree of active engagement that is required by the modified Sternberg task (Roediger, 1990). Reviews of the CBM literature argue that, while training and control conditions may involve exposure to identical stimuli, the critical difference between conditions is whether the stimuli are actively processed in a manner that is transfer-appropriate to the variables of interest (Hertel & Mathews, 2011). This argument is supported by a number of empirical studies demonstrating that active training (beyond stimulus exposure) is required for successful modification of cognitive processes (Hoppitt, Mathews, Yiend, & Mackintosh, 2010; Mathews & Mackintosh, 2000). It is also possible that participants in both conditions improved significantly from pre- to post-training in negative intrusions, symptoms of depression, and levels of rumination because of a regression to the mean or because of natural temporal fluctuations in these phenomena (Barnett, van der Pols, & Dobson, 2005). The equivalent changes across conditions could also be attributed to our use of an active control group, which may have served as a form of behavioral activation, as participants in both conditions completed cognitive tasks daily. To test for this possibility, future work should consider the use of a wait-list control condition, which could allow us to gain a better understanding of the reasons why individuals in the control condition improved on both affective and cognitive measures. Finally, all participants completed the affective Sternberg task at baseline: this single training session may have been sufficient to improve participants’ ability to control the contents of WM. Consistent with this possibility, researchers have found training-related effects to emerge after a single session of cognitive bias modification training in samples of depressed individuals (Cohen, Mor, & Henik, 2015; Tran, Hertel, & Joormann, 2011; Yiend et al., 2014).
Importantly, we found that biological responses to stress at post-training differed by training condition. Compared to participants in the Sham-RNI condition, participants in the Real-RNI condition exhibited attenuated cortisol and RSA responses to stress. Moreover, for individuals who completed Real-RNI training, greater pre- to post-training improvement in removing negative information from WM was associated with more flexible RSA withdrawal and augmentation across the preparation and initial stress reactivity periods. Among participants who completed Sham-RNI training, greater pre- to post-training improvement in removing negative information from WM was associated with higher levels of RSA at baseline. Thus, the current study provides evidence that difficulty removing negative information from WM is associated with exaggerated biological responses to stress. This finding is particularly important given the effects of chronic biological dysregulation on neural, cardiovascular, autonomic, and immune systems (Golbidi, Frisbee, & Laher, 2015; McEwen, 2008), and on risk for the recurrence of depressive episodes (Appelhof et al., 2006; Morris, Rao, & Garber, 2012; Yaroslavsky, Rottenberg, & Kovacs, 2014). It is interesting to speculate why we might have found significant differences between training conditions in biological responses to stress, but not in negative intrusions, depression, or rumination. One possibility is that the benefits of CBM protocols are not fully realized until participants encounter stress. Indeed, this proposition is consistent with results from the meta- analysis conducted by Hallion and Ruscio (2011), in which they found more robust effects of CBM procedures following a stressor. However, in light of the finding that participants in the Real-RNI and Sham-RNI training conditions did not differ in pre- to post-training changes in working memory biases, symptoms of depression, or levels of rumination, it is important to acknowledge that the observed group differences in biological responses to stress could reflect false positive results. Thus, it is critical to replicate the findings reported here.
Two limitations of the present study warrant discussion. First, all participants completed the affective Sternberg task at pre-training. Researchers have suggested that exposing a control group (i.e. Sham-RNI condition) to tasks similar to those used in active training has the potential to improve their WM performance (Onraedt & Koster, 2014). Thus, future work should use different tasks for assessment and training. Second, a long-term follow-up was not included in the present study. Although we did document training-related effects, it will be important for future work to assess long-term changes in memory performance following RNI training.
The present study extends the CBM literature by manipulating a cognitive process that is central to depression and by examining the effects of this manipulation on cognitive, affective, and biological functioning. Contrary to our hypotheses, we found that participants in both the Real-RNI and Sham-RNI training condition improved from pre- to post-training in negative intrusions, symptoms of depression, and levels of rumination. However, our results do provide preliminary experimental evidence that cognitive control deficits contribute to dysregulated biological responses to stress, a finding that is critical given the prospective association between dysregulated biological responses to stress and trajectories of illness in MDD (Morris & Rao, 2014; Morris et al., 2012). Indeed, the present study is the first to experimentally test the associations among difficulties removing negative information from WM, symptoms of depression, levels of rumination, and biological responses to stress in depressed individuals. It is critical that investigators continue to conduct research in this area. In particular, future research should investigate whether the effects of training observed in the current study persist across time. Further, it will be important for future studies to examine the neural mechanisms underlying RNI training to elucidate precisely the ways in which CBM paradigms achieve their beneficial effects.
Supplementary Material
Highlights.
Depressed participants completed working memory (WM) training or sham training
Compared to sham training, Real WM training resulted in attenuated biological responses to stress
Participants in both conditions showed improvements in WM biases, depression, and rumination
Acknowledgements
We thank Christina Schreiner, Emily Livermore, and Brooke Gilbert for their assistance in scheduling and running the participants.
Funding: This research was supported by National Institute of Mental Health (NIMH) Grants R21-MH101545 (to IHG), F32-MH102013 (to JL), and by the Brain & Behavior Research Foundation (formerly NARSAD) Young Investigator Award 22337 (to JL). These funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Declarations of interest: None.
Including age as a covariate did not change the findings reported here. The main effect of age was not significant, and age did not interact significantly with time, valence, or condition to predict intrusion scores, ps > .211.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appelhof BC, Huyser J, Verweij M, Brouwer JP, Van Dyck R, Fliers E, … Schene AH (2006). Glucocorticoids and relapse of major depression (dexamethasone/ corticotropin-releasing hormone test in relation to relapse of major depression). Biological Psychiatry, 59(8), 696–701. [DOI] [PubMed] [Google Scholar]
- Barnett AG, van der Pols JC, & Dobson AJ (2005). Regression to the mean: What it is and how to deal with it. International Journal of Epidemiology, 34(1), 215–220. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Chen SHA, & Desmond JE (2005). Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage, 24(2), 332–338. [DOI] [PubMed] [Google Scholar]
- Cohen N, Mor N, & Henik A (2015). Linking executive control and emotional response: A training procedure to reduce rumination. Clinical Psychological Science, 31(1), 15–25. [Google Scholar]
- Course-Choi J, Saville H, & Derakshan N (2017). The effects of adaptive working memory training and mindfulness meditation training on processing efficiency and worry in high worriers. Behaviour Research and Therapy, 89, 1–13. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, & Haxby JV (1997). Transient and sustained activity in a distributed neural system for human working memory. Nature, 386(6625), 608. [DOI] [PubMed] [Google Scholar]
- Curtis CE, & D’Esposito M (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences, 7(9), 415–423. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. [DOI] [PubMed] [Google Scholar]
- Doom JR, Cicchetti D, Rogosch FA, & Dackis MN (2013). Child maltreatment and gender interactions as predictors of differential neuroendocrine profiles. Psychoneuroendocrinology, 38(8), 1442–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1996). Structured clinical interview for DSM-IV axis I disorders-clinician version (SCID-CV). Washington: American Psychiatric Press. [Google Scholar]
- Golbidi S, Frisbee JC, & Laher I (2015). Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. American Journal of Physiology-Heart and Circulatory Physiology, 308(12), H1476–H1498. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Lemoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, … Wolkowitz OM (2015). Telomere length and cortisol reactivity in children of depressed mothers. Molecular Psychiatry, 20(5), 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Wilhelm FH, & Spoerle M (2004). Respiratory sinus arrhythmia, cardiac vagal control, and daily activity. American Journal of Physiology-Heart and Circulatory Physiology, 287(2), H728–H734. [DOI] [PubMed] [Google Scholar]
- Hallion LS, & Ruscio AM (2011). A Meta-Analysis of the Effect of Cognitive Bias Modification on Anxiety and Depression. Psychological Bulletin, 137(6), 940–958. [DOI] [PubMed] [Google Scholar]
- Hertel PT, & Mathews A (2011). Cognitive bias modification: Past perspectives, current findings, and future applications. Perspectives on Psychological Science, 6(6), 521–536. [DOI] [PubMed] [Google Scholar]
- Hoppitt L, Mathews A, Yiend J, & Mackintosh B (2010). Cognitive Bias Modification: The Critical Role of Active Training in Modifying Emotional Responses. Behavior Therapy, 41(1), 73–81. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, & Worthman CM (2005). Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology, 30(7), 698–714. [DOI] [PubMed] [Google Scholar]
- Joormann J (2010). Cognitive inhibition and emotion regulation in depression. Current Directions in Psychological Science, 19(3), 161–166. [Google Scholar]
- Joormann J, & Gotlib IH (2008). Updating the Contents of Working Memory in Depression: Interference From Irrelevant Negative Material. Journal of Abnormal Psychology, 117(1), 182–192. [DOI] [PubMed] [Google Scholar]
- Joormann J, & Stanton CH (2016). Examining emotion regulation in depression: A review and future directions. Behaviour Research and Therapy, 86, 35–49. [DOI] [PubMed] [Google Scholar]
- Joormann J, Yoon KL, & Zetsche U (2007). Cognitive inhibition in depression. Applied and Preventive Psychology, 12(3), 128–139. [Google Scholar]
- Key BL, Campbell TS, Bacon SL, & Gerin W (2008). The influence of trait and state rumination on cardiovascular recovery from a negative emotional stressor. Journal of Behavioral Medicine, 31(3), 237–248. [DOI] [PubMed] [Google Scholar]
- Kircanski K, Waugh CE, Camacho MC, & Gotlib IH (2016). Aberrant parasympathetic stress responsivity in pure and co-occurring major depressive disorder and generalized anxiety disorder. Journal of Psychopathology and Behavioral Assessment, 38(1), 5–19. [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Koster EHW, & Hoorelbeke K (2015). Cognitive bias modification for depression. Current Opinion in Psychology, 4, 119–123. [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, & Kirschbaum C (2004). HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology, 29(1), 83–98. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, & Wüst S (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology, 34(1), 2–18. [DOI] [PubMed] [Google Scholar]
- LeMoult J, & Gotlib IH (2019). Depression: A cognitive perspective. Clinical Psychology Review, 69, 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J, & Joormann J (2014). Depressive rumination alters cortisol decline in Major Depressive Disorder. Biological Psychology, 100(1), 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J, Yoon KL, & Joormann J (2015). Rumination and Cognitive Distraction in Major Depressive Disorder: an Examination of Respiratory Sinus Arrhythmia. Journal of Psychopathology and Behavioral Assessment, 38(1), 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod C, & Mathews A (2012). Cognitive Bias Modification Approaches to Anxiety. Annual review of clinical psychology, 8, 189–217. [DOI] [PubMed] [Google Scholar]
- Mathews A, & Mackintosh B (2000). Induced emotional interpretation bias and anxiety. Journal of Abnormal Psychology, 109(4), 602–615. [PubMed] [Google Scholar]
- McEwen BS (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology, 583(2–3), 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara TP, & Altarriba J (1988). Depth of spreading activation revisited: Semantic mediated priming occurs in lexical decisions. Journal of Memory and Language, 27(5), 545–559. [Google Scholar]
- Morris MC, & Rao U (2014). Cortisol response to psychosocial stress during a depressive episode and remission. Stress, 17(1), 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Rao U, & Garber J (2012). Cortisol responses to psychosocial stress predict depression trajectories: Social-evaluative threat and prior depressive episodes as moderators. Journal of Affective Disorders, 143(1–3), 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Morrow J (1991). A Prospective Study of Depression and Posttraumatic Stress Symptoms After a Natural Disaster: The 1989 Loma Prieta Earthquake. Journal of Personality and Social Psychology, 61(1), 115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, & Lyubomirsky S (2008). Rethinking Rumination. Perspectives on Psychological Science, 3(5), 400–424. [DOI] [PubMed] [Google Scholar]
- O’Regan C, Kenny RA, Cronin H, Finucane C, & Kearney PM (2015). Antidepressants strongly influence the relationship between depression and heart rate variability: Findings from The Irish Longitudinal Study on Ageing (TILDA). Psychological Medicine, 45(3), 623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onraedt T, & Koster EHW (2014). Training working memory to reduce rumination. PLoS ONE, 9(3), e90632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M, Koster EHW, & Derakshan N (2013). Improving attention control in dysphoria through cognitive training: Transfer effects on working memory capacity and filtering efficiency. Psychophysiology, 50(3), 297–307. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, & Tancer ME (2005). Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry, 57(3), 210–219. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, & Bryk AS (2002). Hierarchical Linear Models: Applications and Data Analysis Methods. Advanced quantitative techniques in the social sciences 1 (Vol. 2nd). [Google Scholar]
- Roediger HL (1990). Implicit memory: Retention without remembering. American Psychologist, 45(9), 1043. [DOI] [PubMed] [Google Scholar]
- Schweizer S, Grahn J, Hampshire A, Mobbs D, & Dalgleish T (2013). Training the emotional brain: Improving affective control through emotional working memory training. Annals of Internal Medicine, 33(12), 5301–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Hampshire A, & Dalgleish T (2011). Extending brain-training to the affective domain: Increasing cognitive and affective executive control through emotional working memory training. PLoS ONE, 6(9), e24372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TB, Hertel PT, & Joormann J (2011). Cognitive Bias Modification: Induced Interpretive Biases Affect Memory. Emotion, 11(1), 145. [DOI] [PubMed] [Google Scholar]
- Voss A, Schroeder R, Heitmann A, Peters A, & Perz S (2015). Short-term heart rate variability - Influence of gender and age in healthy subjects. PLoS ONE, 10(3), e0118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Panage S, Mendes WB, & Gotlib IH (2010). Cardiovascular and affective recovery from anticipatory threat. Biological Psychology, 84(2), 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2012). Depression: Fact sheet no. 369. Retrieved from http://www.who.int/mediacentre/factsheets/fs369/en/index.html
- Yaroslavsky I, Rottenberg J, & Kovacs M (2014). Atypical patterns of respiratory sinus arrhythmia index an endophenotype for depression. Development and Psychopathology, 26(4), 1337–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiend J, Lee JS, Tekes S, Atkins L, Mathews A, Vrinten M, … Shergill S (2014). Modifying interpretation in a clinically depressed sample using “cognitive bias modification-errors”: A double blind randomised controlled trial. Cognitive Therapy and Research, 38(2), 146–159. [Google Scholar]
- Zhang JX, Leung HC, & Johnson MK (2003). Frontal activations associated with accessing and evaluating information in working memory: An fMRI study. NeuroImage, 20(3), 1531–1539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.