Abstract
Objective
We evaluated potential circulating biomarkers of disease activity in giant cell arteritis (GCA), Takayasu’s arteritis (TAK), polyarteritis nodosa (PAN), and eosinophilic granulomatosis with polyangiitis (EGPA, Churg-Strauss).
Methods
A panel of 22 serum proteins was tested in patients enrolled in the Vasculitis Clinical Research Consortium Longitudinal Studies of GCA, TAK, PAN, or EGPA. Mixed models were used for most analyses. A J48 classification tree method was used to find the most relevant markers to differentiate between active and inactive GCA.
Results
418 samples from 152 patients (60 GCA, 29 TAK, 26 PAN, 37 EGPA), during both active vasculitis and remission, were tested. In GCA, BCA-1/CXCL13, ESR, IP-10/CXCL10, sIL-2Rα, and TIMP-1 showed significant (P<0.05) differences between disease states. In EGPA, G-CSF, GM-CSF, IL-6, IL-15, and sIL-2Rα showed significant increases during active disease, as did BCA-1/CXCL13 but only after adjustment for treatment. In PAN, ESR and MMP-3 showed significant differences between disease states. Differences in biomarker levels between diseases were significant for 11 markers and were more striking (all P<0.01) than differences related to disease activity. A combination of lower values of TIMP-1, IL-6, INFƔ, and MMP-3 correctly classified 87% of samples with inactive GCA.
Discussion
We identified novel biomarkers of disease activity in GCA and EGPA. Differences of biomarker levels between diseases, independent of disease activity, were more apparent than differences related to disease activity. Further studies are needed to determine whether these serum proteins have potential for clinical use in distinguishing active disease from remission or in predicting longer-term outcomes.
Keywords: Vasculitis, biomarkers, Takayasu’s arteritis (TAK), eosinophilic granulomatosis with polyangiitis (EGPA), polyarteritis nodosa (PAN)
INTRODUCTION
Different forms of vasculitis share the feature of inflammation of the blood vessels and damage to blood vessel walls but are otherwise heterogeneous. The clinical heterogeneity is observed between vasculitides, between patients with the same form of vasculitis, and in the same patient along the course of the disease (1), making the diagnosis and management of patients with vasculitis challenging (2). Although in some patients vasculitis has a monophasic course, many patients achieve remission with substantial immunosuppressive treatment but then relapse, with time to relapse difficult to predict (3).
Biomarkers in different forms of vasculitis are needed for diagnosis, including differentiation from infection or other conditions with similar symptoms, staging of organ systems involved, assessment of current disease activity, assessment of risk of relapse, predicting response to a particular treatment, and predicting long-term outcomes. Discovery of biomarkers for diagnosis and staging may best be performed in untreated patients with known diagnoses determined by other means. Longitudinal cohorts are best suited for the other unmet needs. For discovery of biomarkers of current disease activity that may prove useful clinically, it is essential to include patients on immune-suppressive medications, since this is the group which best matches the challenge in clinical practice.
Circulating proteins are particularly appealing as biomarkers in vasculitis due to their accessibility and potential clinical use, including to avoid the need for biopsy or provide information not attainable from a biopsy. There have been many studies of biomarkers in the vasculitides as it is recognized that improved tools are needed to identify active disease, predict relapse, and assist with treatment decisions (4). Unfortunately, the findings of these studies have been unsatisfactory and there remains a strong need for better biomarkers of disease activity in vasculitis, especially once treatment is started (4–7).
In the present study we aimed to identify circulating proteins that distinguish between active vasculitis and remission in giant cell arteritis (GCA), Takayasu’s arteritis (TAK), polyarteritis nodosa (PAN), and eosinophilic granulomatosis with polyangiitis (EGPA, Churg-Strauss). In order to compare among these diverse diseases, we used the same panel of markers that we previously tested in patients with highly active anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) (8). Twenty-two serum proteins (ACE, BCA-1/CXCL13, G-CSF, GM-CSF, IFNγ, IL-6, IL-8/CXCL8, IL-15, IL-18, IL-18BP, IP-10/CXCL10, MMP-3, NGAL, osteopontin, PAI-1, PDGF-AB, RANTES/CCL5, sICAM-1, slL-2Rα, sIL-6R, sTNFRII, and TIMP-1) linked to possible pathways relevant to vasculitis were measured, as were the clinical markers CRP and ESR. The 22 experimental markers were among 28 originally chosen to reflect a range of disease-related processes different from autoantibody specificity or the liver-derived markers of systemic inflammation, broadly categorized as cytokines, chemokines, soluble receptors, markers of microvascular damage and markers of tissue damage and repair. The proteins chosen for this study included those that were most strongly associated with active AAV in our previous study (8), but osteopontin, RANTES/CCL5, and sICAM-1 were also retained on the basis of previous studies in GCA (9–11). Several biomarkers elevated in highly active EGPA have previously been studied in this partially treated cohort (5, 6). The few biomarkers previously identified as associated with TAK or PAN (4), and many markers identified as being elevated in untreated GCA (12, 13), could not be included in this study.
MATERIAL AND METHODS
Patients
Patients with GCA, TAK, PAN, and EGPA were enrolled in the Vasculitis Clinical Research Consortium (VCRC) Longitudinal Study from 2006 to 2012. The VCRC is a multi-center research infrastructure dedicated to conducting clinical research in different forms of vasculitis. The 1990 ACR classification criteria for the respective disease were used to classify patients as having either GCA (14), TAK (15), or EGPA (16). A modified version of the 1990 ACR classification criteria for PAN was used to classify PAN, as these criteria may fail to differentiate PAN from MPA (17) and require disease to affect multiple organ systems.
Clinical data, including measures of disease activity, were collected on a quarterly or annual basis and at times of increased activity of vasculitis. Disease duration was defined as time between diagnosis and sample collection.
Patients were chosen for this study based on having a visit during active disease of at and at least 1 visit during remission. A minimum of 25 patients with each disease were chosen, using samples from patients with the highest recorded PGA. The resulting minimum PGA cut-offs were 4 for GCA, 3 for PAN, and 2 for TAK and EGPA. Samples from two remission visits were assayed if available and were chosen to include remission visits both before and after the active visit if available.
Circulating Markers
The twenty-two experimental serum proteins were measured using a microarray platform that effectively miniaturizes a capture ELISA, as described (8). CRP and ESR were measured at the clinical labs of the participating sites.
Statistical Methods
Distributions of marker concentrations were inspected to see whether natural-log (ln)-transformation produced distributions closer to normal. As a result, all markers were analyzed after ln-transformation, but some were also analyzed in parallel without transformation because distributions before and after transformation showed similar degrees of skewing. Significance was defined conventionally as P<0.05, either with or without adjustment for false discovery rate using the Benjamini-Hochberg method (19), because power to detect differences is low for cohorts of this size if such adjustment is used.
Mixed effects models were used to compare marker values between active disease and remission while accounting for within-patient repeated measures. Marker concentration was the dependent variable, with disease activity, use of prednisone, and use of other immunosuppressive drugs as dichotomous independent variables, and the patient as the random effect. Analyses were done with and without inclusion of the treatment variables. Data were missing for CRP at 18/418 visits and for ESR at 20/418 visits. There were no missing data for the experimental biomarkers, disease activity, or treatment.
For the primary analysis of assessing association of a marker with active disease, separate analyses were done in GCA, TAK, PAN, and EGPA. To determine whether markers differed between diseases, mixed models were used with the specific disease added as an independent categorical variable. Logistic regression was then used with disease activity as the outcome and difference-from-mean as the predictor (20). This approach carries the caveat that data from patients with only one remission visit had to be excluded. This approach also still includes repeated measures (3 per patient) but probably provides the best estimate of the AUC-ROC, which is commonly used in assessing and comparing predictive models in clinical research. Correlation coefficients (Pearson on ln-transformed data, Spearman on non-transformed data) were calculated to study the association between markers.
In a complementary, exploratory approach that can sometimes be more effective than linear models in using multivariable data to predict a binary outcome, the J48 classification tree method was used to find cut-off points of the most relevant markers to differentiate between active and inactive GCA, using WEKA Data Mining Software (21). The number of data-points was too small to consider this approach in the other diseases. We initially used all markers, allowing for the classifier to choose the ones leading to the most accurate classification. After generating the tree, we performed a 5-fold cross-validation.
RESULTS
Patients and samples
Four-hundred eighteen samples from 152 patients (60 GCA, 29 TAK, 26 PAN, 37 EGPA), each with samples from 1–2 active visits and 1–3 remission visits, were tested (Table 1). Fifty-five of the 60 GCA patients had the diagnosis confirmed by temporal artery biopsy or angiography. Most patients were on treatment at the time of sample collection: 93% GCA, 87% TAK, 93% PAN, 87% EGPA. In most patients, current treatment included prednisone: 88% GCA, 71% TAK, 83% PAN, 83% EGPA. Data on treatment, separated by disease and by current disease activity, are shown in Supplementary Table 1. In patients with active disease, severity ranged from PGA 1 to 9. Summaries of biomarker concentrations are shown in Table 2.
Table 1.
Characteristics of patients in this study
| GCA (n=60) | TAK (n=29) | PAN (n=26) | EGPA (n=37) | |
|---|---|---|---|---|
| Age | 71 (64,80) | 31 (26,42) | 50 (40,60) | 53 (36,65) |
| Female sex | 48 (80%) | 24 (83%) | 16 (62%) | 22 (59%) |
| Disease duration (months) | 7 (3,14) | 31 (18,51) | 14 (7,36) | 16 (7,32) |
| Severity (PGA) | 5 (4,7) | 3 (2,5) | 4.5 (4,5) | 4 (2,5) |
| Creatinine > 1.5 mg/dL | 2 | 1 | 2 | 1 |
Numbers for age, disease duration, and severity indicate median (25th percentile, 75th percentile). Numbers for female sex and creatinine > 1.5 mg/dL indicate numbers of patients, with percentages calculated for female sex. GCA = giant cell arteritis; TAK = Takayasu arteritis; PAN = polyarteritis nodosa; EGPA = eosinophilic granulomatosis with polyangiitis (Churg-Strauss). PGA = physician global assessment of disease activity (0–10 scale) during the active visit. All patients also had at least one visit during remission (PGA=0).
Table 2.
Biomarker concentrations in vasculitides during active disease and remission, and in healthy controls.
| GCA (n=60) | TAK (n=29) | PAN (n=26) | EGPA (n=37) | Controls (n=34) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Remission | Active | Remission | Active | Remission | Active | Remission | Active | ||
| ACE | 141 (106 – 205) | 129 (95 – 174) | 118 (76 – 178) | 94 (77 – 146) | 147 (91 – 207) | 148 (85 – 203) | 145 (75 – 219) | 131 (66 – 210) | 90 (67 – 117) |
| BCA-1 | 78 (35 – 154) | 95 (47 – 173) | 171 (98 – 322) | 237 (146 – 288) | 342 (170 – 1005) | 424 (185 – 816) | 74 (41 – 232) | 96 (55 – 188) | 38 (21 – 60) |
| CRP | 5 (3 – 12) | 6.5 (3 – 29) | 6.9 (3 – 15) | 5.8 (2 – 10) | 6.3 (3.0 – 11) | 10 (1.8 – 26) | 2.8 (1 – 4.9) | 3 (1 – 6.1) | |
| ESR | 20 (10 – 27) | 26 (12 – 44) | 19 (9 – 32) | 18 (13 – 41) | 13 (5 – 33) | 18 (7 – 39) | 8 (5 – 16) | 11 (5 – 21) | |
| G-CSF | 2.4 (1.2 – 5.5) | 3.3 (1.4 – 12.2) | 7.0 (1.5 – 117) | 7.0 (1.5 – 141) | 4.0 (1.2 – 4.0) | 1.5 (1.2 – 7.6) | 4.6 (1.5 – 6.4) | 6.4 (4.3 – 19) | 7.1 (4.6 – 12.2) |
| GM-CSF | <1 (<1 – <1) | <1 (<1 – <1) | <1 (<1 – <1) | <1 (<1 – <1) | <1 (<1 – <1) | <1 (<1 – <1) | <1 (<1 – 3.0) | <1 (<1 – 12) | 2.2 (<1 – 11) |
| IFNγ | <0.5 (<0.5 – <0.5) | <0.5 (<0.5 – <0.5) | <0.5 (<0.5 – <0.5) | <0.5 (<0.5 – <0.5) | <0.5 (<0.5 – <0.5) | <0.5 (<0.5 – <0.5) | <0.5 (<0.5 – <0.5) | <0.5 (<0.5 – <0.5) | <0.5 (<0.5 – <0.5) |
| IL-6 | 2.8 (<0.7 – 6.6) | 2.5 (<0.7 – 7.4) | 1.6 (<0.5 – 2.7) | 1.6 (<0.5 – 2.7) | 0.9 (<0.5 – 3.8) | 0.9 (<0.5 – 3.2) | 3.6 (<0.5 – 17) | 3.6 (0.6 – 26) | <0.5 (<0.5 – <0.5) |
| IL-8 | 9.8 (4.9 – 22) | 8.9 (2.8 – 30) | 7.0 (3.4 – 15) | 7.0 (2.3 – 9.4) | 12 (4.8 – 25) | 11 (3.8 – 16) | 3.8 (2.1 – 8.4) | 3.6 (1.8 – 8.0) | <1 (3.2 – 5.8) |
| IL-15 | 2.5 (1.5 – 4.2) | 2.4 (1.2 – 4.9) | 2.7 (1.5 – 4.4) | 2.6 (1.7 – 3.5) | 2.2 (1.0 – 5.0) | 2.4 (1.0 – 4.5) | 2.3 (1.5 – 6.3) | 4.4 (1.6 – 11) | 3.6 (1.6 – 5.2) |
| IL-18 | 241 (82 – 526) | 331 (143 – 506) | 295 (163 – 493) | 282 (181 – 412) | 461 (277 – 636) | 458 (317 – 639) | 175 (81 – 907) | 286 (89 – 782) | 52 (26 – 75) |
| IL-18BP | 82 (51 – 121) | 89 (56 – 153) | 41 (24 – 113) | 52 (25 – 107) | 225 (130 – 398) | 194 (128 – 390) | 112 (79 – 167) | 128 (85 – 181) | <6.1 (17 – 65) |
| IP-10 | 20 (11 – 35) | 16 (6.6 – 33) | 10 (6.1 – 22) | 9.7 (7.1 – 23) | 38 (19 – 58) | 26 (14 – 44) | 21 (12 – 42) | 20 (11 – 39) | 4.7 (3.2 – 7.3) |
| MMP-3 | 57 (37 – 93) | 64 (40 – 123) | 72 (23 – 178) | 62 (24 – 142) | 163 (125 – 285) | 130 (72 – 261) | 36 (23 – 78) | 46 (28 – 104) | 9.9 (5.2 – 20) |
| NGAL | 216 (143 – 373) | 217 (132 – 297) | 176 (128 – 236) | 179 (147 – 240) | 175 (95 – 296) | 182 (122 – 321) | 160 (86 – 210) | 190 (67 – 280) | 135 (99 – 166) |
| Osteopontin | 46 (18 – 76) | 56 (32 – 72) | 34 (12 – 79) | 41 (19 – 88) | 39 (18 – 63) | 44 (30 – 87) | 29 (9.3 – 60) | 24 (9.2 – 54) | 32 (29 – 48) |
| PAI-1 | 2.2 (<1 – 4.6) | 2.9 (<1 – 4.0) | 1.7 (<1 – 3.0) | 1.2 (<1 – 2.2) | 1.6 (<1 – 4.0) | 2.4 (<1 – 4.0) | 2.0 (<1 – 6.2) | 2.4 (<1 – 4.9) | 2.2 (<1 – 7.8) |
| PDGF-AB | 3.8 (2.6 – 4.9) | 3.5 (2.6 – 4.9) | 3.9 (2.4 – 4.9) | 3.6 (2.5 – 4.6) | 2.6 (1.5 – 4.8) | 2.4 (0.8 – 4.6) | 3.4 (2.4 – 5.0) | 4.3 (2.8 – 5.7) | 8.0 (3.6 – 13.0) |
| RANTES | 64 (46 – 97) | 73 (38 – 111) | 78 (54 – 106) | 73 (59 – 87) | 65 (42 – 83) | 61 (40 – 90) | 75 (51 – 107) | 80 (52 – 116) | 56 (22 – 110) |
| sICAM-1 | 670 (464 – 870) | 594 (425 – 842) | 464 (300 – 673) | 435 (220 – 628) | 488 (387 – 598) | 528 (371 – 841) | 544 (389 – 798) | 482 (397 – 846) | 247 (217 – 352) |
| sIL-2Rα | <2.5 (<2.5 – 12.6) | <2.5 (<2.5 – 59) | 27 (21 – 60) | 21 (<2.5 – 94) | 60 (6.5 – 166) | 46 (6.6 – 164) | 84 (3.2 – 434) | 133 (6.1 – 769) | <2.5 (<2.5 – <2.5) |
| sIL-6R | 31 (25 – 37) | 31 (26 – 38) | 21 (14 – 32) | 21 (15 – 37) | 25 (19 – 34) | 31 (24 – 37) | 29 (21 – 34) | 29 (20 – 39) | 16 (11 – 19) |
| sTNFRII | 500 (364 – 664) | 531 (361 – 704) | 458 (356 – 587) | 422 (340 – 551) | 687 (442 – 911) | 676 (424 – 1145) | 375 (252 – 662) | 461 (312 – 884) | 510 (322 – 916) |
| TIMP-1 | 467 (358 – 518) | 505 (404 – 631) | 373 (255 – 575) | 327 (219 – 501) | 377 (290 – 470) | 419 (340 – 520) | 380 (310 – 489) | 352 (254 – 536) | 115 (58 – 190) |
Values are medians and interquartile ranges. Results significanty (P<0.01) higher in a disease in comparison to at least one other disease in mixed models, adjusting for disease activity and treatment, are shown in bold. Results significantly lower than in all other diseases are not highlighted, since concentrations in these cases remained higher than in healthy controls. Units are mg/L (=mcg/mL) for CRP, mm/hr for ESR, ng/ml for ACE, MMP-3, NGAL, osteopontin, PAI-1, PDGF-AB, RANTES, ICAM-1, sIL-6R, and TIMP-1, and pg/ml for the remaining proteins, referring to the concentration in serum before dilution.
Biomarkers in giant cell arteritis and Takayasu’s arteritis
In GCA, BCA-1/CXCL13, ESR, IP-10/CXCL10, sIL-2Rα, and TIMP-1 showed significant (P<0.05) differences during active disease, with or without adjustment for treatment. Most of these markers were higher during active GCA, but IP-10/CXCL10 decreased. Only ESR remained significantly higher during active disease after adjustment for 24 markers being tested simultaneously (P≤0.001) (Table 3 and Supplementary Table 2). Changes with active disease were modest, with the greatest increases being 22% for G-CSF or 11 mm/hr for ESR (Table 3). Results were nearly identical when analysis was limited to the 55 patients in whom the diagnosis of GCA was confirmed by biopsy or angiography (Supplementary Table 3). Using conditional logistic regression, the OR of active disease with a 2.72-fold increase in sIL-2Rα was 1.53 (P=0.02). Change in slL-2Rα concentration compared to a patient’s mean during remission had an AUC-ROC of only 0.61 (P=0.007), and absolute sIL-2Rα concentration in an unadjusted logistic regression had an AUC-ROC of 0.57 (P=0.03)
Table 3.
Selected analyses of markers showing association with disease activity. Mixed effects models included marker concentration as the dependent variable, disease activity as a dichotomous independent variable, the patient as the random effect, with or without current use of prednisone and other immunosuppressive drugs (“Meds”) as independent dichotomous variables. Numbers indicate beta-coefficients associating an increase (if > 0) or decrease (if <0) in marker concentration with active disease, with 95% confidence intervals in parentheses, and P-values. All marker values except ESR were ln-transformed. Therefore, the beta-coefficient for ESR represents absolute change (mm/hr), whereas for other markers, the beta-coefficient multiplied by 2.72 represents fold-change. Only analyses with P<0.1 are included in this table. Analyses with P<0.05 are shown in bold. For results of all analyses, see Supplementary Table 1.
| Marker | GCA | GCA Meds | TAK Meds | PAN | PAN Meds | EGPA | EGPA Meds | LVV | LVV Meds |
|---|---|---|---|---|---|---|---|---|---|
| ACE | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | −0.27(−0.57 −0.04) P=0.09 | −0.32(−0.64– −0.01) P=0.05 | ||
| BCA-1 | 0.23 (0.01– 0.45) P=0.04 | 0.33 (0.10 – 0.56) P=0.005 | ----------------- | ----------------- | ----------------- | 0.27 (−0.01 -0.55) P=0.06 | 0.34 (0.05 – 0.62), P=0.02 | 0.21 (0.03 – 0.38) P=0.02 | 0.24 (0.07 – 0.42) P=0.006 |
| CRP | 0.35 (0.01 – 0.70) P=0.05 | ----------------- | −0.47 (−1.02 – 0.07) P=0.09 | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- |
| ESR | 10.6 (5.3 – 15.8) P=0.0001 | 10.3 (4.9 – 15.8) P=0.0003 | ----------------- | 8.12 (1.6 – 14.6) P=0.02 | 8.53 (1.79 – 15.3) P=0.01 | ----------------- | ----------------- | 7.86 (3.76 – 12) P=0.0002 | 7.69 (3.55 – 11.8) P=0.0003 |
| G-CSF | 0.45 (0.03 – 0.87) P=0.04 | 0.39 (−0.04 – 0.83) P=0.08 | ----------------- | ----------------- | ----------------- | 0.53 (0.15 – 0.92) P=0.007 | 0.62 (0.23 – 1.02) P=0.002 | ----------------- | ----------------- |
| GM-CSF | 0.31 (0.04 – 0.57) P=0.03 | 0.27 (−0.01 – 0.55) P=0.05 | ----------------- | ----------------- | ----------------- | 0.63 (0.21 – 1.06) P=0.004 | 0.75 (0.32 – 1.19) P=0.0009 | 0.21 (0.02 – 0.39) P=0.03* | 0.19 (0.01 – 0.38) P=0.04 |
| IL-6 | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | 0.45 (0.02 – 0.88) P=0.04 | 0.49 (0.04 – 0.94) P=0.03 | ----------------- | ----------------- |
| IL-15 | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | 0.44 (0.07 – 0.81) P=0.02 | 0.58 (0.2 – 0.95) P=0.003 | ----------------- | ----------------- |
| IL-18 | 0.21 (-0.01 - 0.43) P=0.06 | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | 0.14 (0.02 – 0.25) P=0.02 | 0.14 (−0.02 – 0.31) P=0.09 |
| IL-18BP | 0.14 (0.00 − 0.29) P=0.05 | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | 0.16 (−0.01 – 0.32) P=0.06 | 0.11 (−0.00 – 0.23) P=0.05 |
| IP-10 | −0.26 (−0.47 – −0.05) P=0.02 | −0.3(−0.52 – −0.08) P=0.008 | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | −0.18(−0.37 – 0.01) P=0.06 |
| MMP-3 | ----------------- | ----------------- | ----------------- | −0.58 (−1.12 – −0.04) P=0.04 | −0.68 (−1.24 – −0.13) P=0.02 | ----------------- | ----------------- | ----------------- | ----------------- |
| Osteopontin | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | 0.20 (−0.01 – 0.4) P=0.06 | ----------------- |
| PDGF-AB | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | 0.32 (−0.02 – 0.66) P=0.06 | 0.34 (−0.00 – 0.69) P=0.05 | ----------------- | ----------------- |
| sIL-2Rα | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | 0.60(0.12 –1.08) P=0.01 | 0.68 (0.18 – 1.18) P=0.008 | ----------------- | ----------------- |
| TIMP-1 | 0.12 (0.02 – 0.21) P=0.02 | 0.13 (0.03 – 0.23) P=0.01 | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- | ----------------- |
In TAK, no markers showed statistically significant (P<0.05) differences between active disease and remission. Because of similar pathology in GCA and TAK, these patients were pooled as “large-vessel vasculitis” (LVV) for a secondary analysis. BCA- 1/CXCL13, ESR, and GM-CSF were nominally increased (P<0.05) in active LVV regardless of treatment. IL-18 was only significantly higher in active LVV when treatment was not included in the model. Several markers significantly increased in active GCA alone no longer showed statistically significant increases in pooled LVV (P>0.05) (Table 3, Supplementary Table 2). Magnitude of change was invariably lower in pooled LVV, compared to GCA alone.
Biomarkers in polyarteritis nodosa
In PAN, ESR was higher (by only 8–9 mm/hr) and MMP-3 lower (by 58–85%) during active PAN, with or without treatment (Table 3, Supplementary Table 2).
Biomarkers in eosinophilic granulomatosis with polyangiitis
In EGPA, G-CSF, GM-CSF, IL-6, IL-15, and sIL-2Rα showed significant increases in active disease with or without adjustment for treatment, and BCA-1/CXCL13 was significantly increased only with adjustment for treatment (Table 3, Supplementary Table 2). Most of these markers were associated with disease activity in either the 18 ANCA-positive patients (BCA-1/CXCL13, sIL-2Rα) or the 19 ANCA-negative patients (G-CSF, GM-CSF), but not both (Supplementary Table 3). The largest magnitude of change was again modest: 2.7-fold for sIL-2Rα in ANCA-negative patients. Using conditional logistic regression, the OR of active disease with a 2.72-fold increase in GM-CSF was 1.76 (P=0.02). Change in GM-CSF concentration compared to a patient’s mean during remission had an AUC-ROC of only 0.59 (P=0.04), and absolute GM-CSF concentration in an unadjusted logistic regression had an AUC-ROC of 0.56 (P=0.11).
Correlations among tested biomarkers
The correlation of ESR or CRP with the experimental markers was weak, no higher than r = 0.25. The markers of systemic inflammation produced by the liver (ESR and CRP) were well-correlated with each other (r = 0.53). Most of the cytokines, chemokines, and soluble receptors (BCA-1/CXCL13, G-CSF, GM-CSF, IFNγ, IL-6, IL-8, IL-15, IL-18, IL-18BP, IP-10/CXCL10, sIL-2Rα, and sTNFRII) were weakly to moderately correlated (r between 0.25 and 0.50) in all 4 diseases (Supplementary Figure 1), and included all of the markers that were associated with EGPA. Another block lacked a clear inflammatory theme (ACE, osteopontin, PAI-1, PDGF-AB, RANTES/CCL5, sICAM-1, sIL-6R, and TIMP-1) and had weaker correlations in GCA than in the other diseases. MMP-3 and NGAL did not fall into larger blocks.
There was little if any correlation of biomarker concentrations with age. Among samples taken during remission, correlation coefficients of biomarker concentrations with age varied between r = −0.23 and 0.19. Similarly, there was no apparent correlation, in samples taken during active disease or remission, between biomarker concentrations and disease duration, r = −0.13 – 0.11.
Comparison of biomarkers between diseases
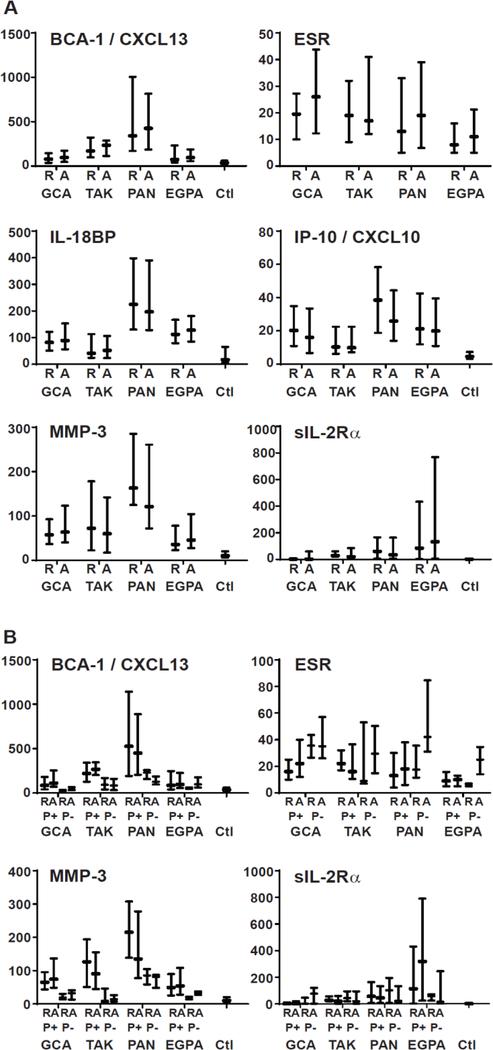
Differences in marker levels between diseases were apparent by inspection and were shown to be significant in mixed models for 11 markers: BCA-1/CXCL13, CRP, ESR, G-CSF, GM-CSF, IL-6, IL-8, IL-18BP, IP-10/CXCL10, MMP-3, and sIL-2Rα (Table 2). Plots of distributions of concentrations of the 6 markers that differed across diseases and differed with disease activity in at least one disease (BCA-1/CXCL13, ESR, IL-18BP, IP-10/CXCL10, MMP-3, sIL-2Rα) are shown in Figure 1A.
Figure 1.
Selected biomarker concentrations in different forms of vasculitis and in healthy controls (Ctl), separated by disease activity (A) and additionally by use (P+) or non-use (P−) of prednisone (B). Plots show medians and interquartile ranges. R = remission; A = active vasculitis. Units are mm/hr for ESR, ng/ml for MMP-3, and pg/ml for BCA-1, IL-18BP, IP-10, and sIL-2Rα.
Although association of markers with disease activity did not vary greatly with or without adjustment for treatment, association of marker concentration with prednisone treatment, after adjustment for disease activity, was convincing for 3 markers (BCA-1/CXCL13, ESR, MMP-3), in which P<0.01 across all diseases and in at least two individual diseases. Plots of data separated by disease, activity, and prednisone use are shown in Figure 1B.
Classification tree to differential disease states in giant cell arteritis
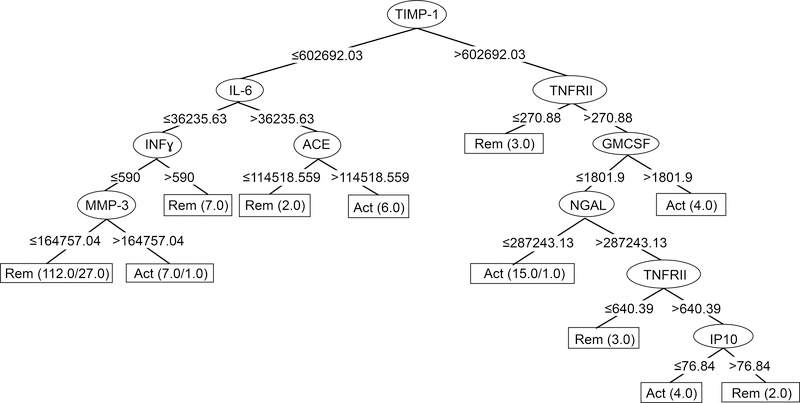
A J48 classification tree to differentiate between active and inactive GCA cases was generated starting with the full list of markers and resulted in the markers shown in Figure 2. TIMP-1 was the most important classifier, as it divided the tree in two major branches. A combination of lower values of TIMP-1, IL-6, INFy, and MMP-3 was found in 85 of the 104 samples during remission, and overall the tree correctly classified 102/104 (98%) of cases during remission. Different combinations of 8 markers correctly classified patients with active disease, but only in 36/61 (56%) cases. The 5-fold crossvalidation results showed only 66% of cases classified correctly: 87% of inactive cases but only 31 % of active cases. A J48 classification tree was also built with inclusion of ESR, but the accuracy was lower and complexity higher (data not shown).
Figure 2.
J48 Classification Tree for GCA biomarkers. Starting at the top, each decision node (oval) shows the marker to be used in a classification step. The marker level cut-point (in mm/hr for ESR, pg/ml for the others) is shown at each branch point, with values less than the cut-point moving a sample to the left and values greater than the cut-point going to the right. Classification of the sample by the algorithm as active disease (Act) or remission (Rem) is complete when it reaches one of the terminal nodes, shown as rectangles. Numbers in the terminal nodes show the total number of samples classified into the node followed by the number incorrectly classified, if any. For example, in the left-most terminal node, the tree has classified 112 samples sharing the properties of low TIMP-1, low IL-6, low IFNγ, and low MMP-3 as remission: 85 remission samples classified correctly, and 27 active samples classified incorrectly. Evaluation of the overall tree in classifying remission is determined by adding the numbers of samples correctly classified in Rem terminal nodes (102) and comparing to the number incorrectly classified in Act terminal nodes (2). Conversely, performance in classifying active disease involves comparing the numbers correctly classified in Act terminal nodes (34) to the numbers incorrectly classified in Rem terminal nodes (27).
DISCUSSION
This study was done to identify potential biomarkers of disease activity identified in GCA, TAK, PAN, and EGPA, and to compare biomarkers across diseases. Five markers were associated with GCA and 5 with EGPA, and only sIL2Ra was associated with both diseases. The magnitudes of marker change with disease activity were small, under 2-fold. Marker concentrations differed more between diseases, independent of apparent clinical activity or concurrent treatment, than they did with level of disease activity longitudinally within-patient.
The only marker associated with disease activity in different types of vasculitis was sIL-2Rα. The IL-2/IL-2Ra (CD25) pathway plays an essential role in regulating immune responses, both positive (activation and replication of effector T cells) and negative (development of Treg cells). sIL-2Rα can inhibit IL-2 signaling and enhance T cell proliferation and expansion (22) and it has been implicated in autoimmune conditions, including multiple sclerosis (23), and in macrophage activation syndrome arising from a range of causes (24).
The potential markers of active disease in GCA identified in this study include BCA-1/CXCL13, ESR, sIL-2Rα, and TIMP-1, which were increased in active disease, and IP-10/CXCL10, which was decreased. TIMP-1 was also the most important classifier when we used a different analytical approach with a J48 classification tree, which establishes a cut point for above-versus-below. The tree classified samples in remission much better than during active disease: 87% vs. 31% in the cross-validation step, which is an appropriately conservative way to interpret a classification scheme derived from a single dataset. ESR and CRP have both been shown to be associated with active GCA (25), and at least one of them is elevated in almost all patients with untreated, biopsy-proven GCA (26), in line with clinical practice. The fact that ESR and CRP are widely used clinically in determining disease activity in GCA could have biased this study to detect them as significant markers, if the investigator used them to determine whether a confusing clinical situation constituted a flare. To our knowledge, associations of elevated levels of sIL-2Rα and TIMP-1 and decreased levels of IP-10/CXCL10 with active GCA have not been previously reported.
MMPs have gelatinolytic activity and some of them have been found to be expressed (27) and up-regulated (28) in GCA lesions whereas their natural inhibitors TIMP-1 and TIMP-2 are down-regulated yielding an increase in proteolytic balance (29). Furthermore, dexamethasone has proven to downregulate several pro-inflammatory mediators, including TIMP-1 in vitro (30). In this context, finding an increase of TIMP-1 in active GCA is surprising. However, it has been recognized that TIMP-1 is a multifunctional protein which is not only an inhibitor of MMPs but also has a possible “cytokine-like” action, as well as growth factor-like and anti-apoptotic properties. TIMP-1 expression can be stimulated by a wide variety of agents including serum, growth factors, phorbol esters, cytokines, interleukins, including IL-6, and viruses (31). Therefore, we postulate that its increased circulating concentration in active GCA can be related to its cytokine-like action rather than its interaction with MMP-3, although we cannot predict a specific role, and circulating biomarkers in general may not reflect the local pathology. The lack of correlation between MMP-3 and TIMP-1 in this study (r=0.11 in GCA, and −0.15 – 0.13 in TAK, PAN, and EGPA) is consistent with this interpretation.
BCA-1/CXCL13, a chemokine for B cells, was associated with disease activity in GCA, and possibly in EGPA but only when the model was adjusted for treatment. This marker was of particular interest because it was strongly associated with highly active GPA or MPA in our previous study (8). However, prednisone use was associated with increased BCA-1/CXCL13 across multiple diseases in the current study. It appears likely that either active vasculitis or prednisone increases BCA-1/CXCL13, which may limit its usefulness as a clinical biomarker. We are not aware of previous data showing a rise in CXCL13/BCA-1 with prednisone, but this has been well-described for the other marker in which we saw such an effect, MMP-3 (8).
In TAK, plasma levels of cellular adhesion molecules and coagulation-related proteins were unrelated to activity status in a previous study (32). Small studies have reported higher serum levels of IL-6 (33, 34) and IL-8/CXCL8 (35) in patients with active TAK. We did not find such an association in our study but determining disease activity in TAK after treatment is started is notoriously difficult, and one or more forms of imaging will likely serve prominently as the gold standard in future biomarker studies.
In EGPA, G-CSF, GM-CSF, IL-6, IL-15, and sIL2-Ra seem the most promising biomarkers of disease activity within the tested panel, although likely differing in ANCApositive and ANCA-negative patients. Although all of these proteins were associated with highly active GPA and MPA in our previous study (8), the 3 markers most strongly associated with active GPA and MPA (BCA-1/CXCL13, MMP-3, TIMP-1) were not associated with active EGPA in the current study. In unpublished data from later stages of the same trial, a study in which many patients were on treatment and many flares were mild, similar to the current study, IL-6 and sIL-2Rα were among 10 markers associated with GPA and MPA, whereas G-CSF and GM-CSF were not (unpublished data).
The 3 blocks of markers with significantly correlated concentrations was similar to what we observed in our previous study of GPA and MPA in patients with severe disease and in remission (8). In both studies, CRP and ESR correlated well with each other but weakly and inconsistently with any other marker. The block of cytokines, chemokines, and soluble receptors was particularly similar to what was reported previously, except that sTNFRII did not correlate well with markers in that block in the previous study (8), but did in all 4 diseases in the current study. Finally, a block of other, generally noncytokine proteins was not apparent in the GPA/MPA study but was apparent in TAK, PAN, and EGPA in the current study, with no clear explanation.
Treatment with either prednisone or other immunosuppressive agents had a significant effect on concentrations of some measured biomarkers. Although estimates of association of a marker with active disease did not change much with or without adjustment for treatment, our ability to determine the effects of treatment and effects of active disease independent of treatment was limited by the fact that only about 10% of samples were obtained off treatment, in a cohort in which disease activity was also changing. Other studies in the VCRC EGPA cohort have shown substantial effects of treatment on levels of circulating biomarkers (eotaxin-3, ESR, CRP, and eosinophil count) (5, 6), but these markers are specifically related to eosinophils or are markers of systemic inflammation, two aspects of inflammation known to be particularly responsive to glucocorticoids.
A focus on patients during treatment is an appropriate assessment of the potential clinical utility of a biomarker’s association with disease activity, one that is not generally seen in the first study reporting a new biomarker. In vasculitis, study of untreated patients is best if the goal is to allow distinction from other potential diagnoses, or to gain insight into pathophysiology, or to provide non-invasive assessment of the involvement of particular organ systems (staging), or to provide prognostic information about likely response to treatment.. Our study thus could not address any of these questions and was not expected to provide a platform to discover disease-specific biomarkers, either between the vasculitides in this study or with AAV from our previous study. It is hard to predict whether biomarkers associated with future relapse risk will be found more readily in patients before or after treatment, and we did not attempt to address this question either. Although a comparison between diseases would ideally include untreated patients, we nevertheless were able to detect some differences in association with activity and during remission. IFNγ, IL-6, IL-8, osteopontin, PAI-1, PDGF, sICAM-1, and sIL-6R have been associated with active GCA (13), and we propose that our results simply reflect reduction in biomarker concentrations by treatment rather than being irreconcilable with earlier reports (13).
An additional limitation of this study was that dosing of medications, particularly prednisone, was not available, and we did not attempt to discern whether medications had been started or stopped in the month before the visit. Using the investigator’s assessment as the gold standard for disease activity is potentially problematic, but unavoidably so for this type of pilot study. Finally, the results of our J48 classification tree should be taken with caution given that the cross-validation was not very satisfactory as the accuracy dropped, and validation in a separate cohort is important before drawing conclusions about any prediction algorithm involving multiple predictor variables.
The unexpected finding that differences between diseases during clinical remission were more evident than differences related to disease activity or treatment deserves further study in a different direction. There was no clear biologic association of the markers in which this was observed, e.g., elevation of BCA-1/CXCL13, IL-18BP, and MMP-3 in PAN, or sIL-2Rα in EGPA. From a practical point of view, however, a focus on these proteins during clinical remission, including study of patients who remain in remission long-term (not represented in this study), may lead to discovery of biomarkers associated with a tendency to relapse or with long-term outcomes.
In conclusion, we identified several biomarkers of disease activity in both GCA and EGPA. The most promising markers of active disease in GCA were BCA-1/CXCL13, ESR, IP-10/CXCL10, sIL-2Rα, and TIMP-1, and in EGPA were G-CSF, GM-CSF, IL-6, IL-15, and sIL2-Ra. Differences of biomarker levels between diseases were more striking than differences related to disease activity or treatment, especially for 6 markers: BCA-1/CXCL13, ESR, IL-18BP, IP-10/CXCL10, MMP-3, and sIL-2Rα. Further studies in other cohorts are needed to confirm or refute these findings, to clarify the potential role of these cytokines for diagnosis and/or monitoring of clinical activity in GCA and EGPA, and to understand the roles of those molecules in the inflammatory cascade in the vascular wall.
Supplementary Material
Acknowledgments
Funding: This work was sponsored by the Vasculitis Clinical Research Consortium. The Vasculitis Clinical Research Consortium (VCRC) is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Science (NCATS). The VCRC is funded through collaboration between NCATS, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), U54 AR057319), and has received funding from the National Center for Research Resources (U54 RR019497). Additional funding for this project included RC1 AR 058303 and P60 AR047785.
Conflict of interest: Dr. Merkel reports receiving funds for the following activities: Consulting: AbbVie, AstraZeneca, Biogen, Boeringher-Ingelheim, Bristol-Myers Squibb, Celgene, ChemoCentryx, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Insmed, Jannsen, Kiniksa; Research Support: AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, Celgene, ChemoCentryx, Genentech/Roche, GlaxoSmithKline, Kypha, TerumoBCT; Royalties: UpToDate. Dr. Monach reports receiving funds for the following activities: Consulting: Celgene, ChemoCentryx.
REFERENCES
- 1.Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, et al. Eular/era-edta recommendations for the management of anca-associated vasculitis. Ann Rheum Dis 2016;75:1583–94. [DOI] [PubMed] [Google Scholar]
- 2.Kallenberg CG. Key advances in the clinical approach to anca-associated vasculitis. Nat Rev Rheumatol 2014;10:484–93. [DOI] [PubMed] [Google Scholar]
- 3.Cornec D, Cornec-Le Gall E, Specks U. Clinical trials in antineutrophil cytoplasmic antibody-associated vasculitis: What we have learnt so far, and what we still have to learn. Nephrol Dial Transplant 2017;32:i37–i47. [DOI] [PubMed] [Google Scholar]
- 4.Monach PA. Biomarkers in vasculitis. Curr Opin Rheumatol 2014;26:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dejaco C, Oppl B, Monach P, Cuthbertson D, Carette S, Hoffman G, et al. Serum biomarkers in patients with relapsing eosinophilic granulomatosis with polyangiitis (churg-strauss). PLoS One 2015;10:e0121737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grayson PC, Monach PA, Pagnoux C, Cuthbertson D, Carette S, Hoffman GS, et al. Value of commonly measured laboratory tests as biomarkers of disease activity and predictors of relapse in eosinophilic granulomatosis with polyangiitis. Rheumatology (Oxford) 2015;54:1351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomasson G, Lavalley M, Tanriverdi K, Finkielman JD, Davis JC Jr., Hoffman GS, et al. Relationship between markers of platelet activation and inflammation with disease activity in wegener’s granulomatosis. J Rheumatol 2011;38:1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monach PA, Warner RL, Tomasson G, Specks U, Stone JH, Ding L, et al. Serum proteins reflecting inflammation, injury and repair as biomarkers of disease activity in anca-associated vasculitis. Ann Rheum Dis 2013;72:1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruhl H, Vielhauer V, Weiss M, Mack M, Schlondorff D, Segerer S. Expression of darc, cxcr3 and ccr5 in giant cell arteritis. Rheumatology (Oxford) 2005;44:309–13. [DOI] [PubMed] [Google Scholar]
- 10.Remahl AI, Bratt J, Mollby H, Nordborg E, Waldenlind E. Comparison of soluble icam-1, vcam-1 and e-selectin levels in patients with episodic cluster headache and giant cell arteritis. Cephalalgia 2008;28:157–63. [DOI] [PubMed] [Google Scholar]
- 11.Prieto-Gonzalez S, Terrades-Garcia N, Corbera-Bellalta M, Planas-Rigol E, Miyabe C, Alba MA, et al. Serum osteopontin: A biomarker of disease activity and predictor of relapsing course in patients with giant cell arteritis. Potential clinical usefulness in tocilizumab-treated patients. RMD Open 2017;3:e000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Geest KS, Abdulahad WH, Rutgers A, Horst G, Bijzet J, Arends S, et al. Serum markers associated with disease activity in giant cell arteritis and polymyalgia rheumatica. Rheumatology (Oxford) 2015;54:1397–402. [DOI] [PubMed] [Google Scholar]
- 13.Burja B, Kuret T, Sodin-Semrl S, Lakota K, Rotar Z, Jese R, et al. A concise review of significantly modified serological biomarkers in giant cell arteritis, as detected by different methods. Autoimmun Rev 2018;17:188–94. [DOI] [PubMed] [Google Scholar]
- 14.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The american college of rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122–8. [DOI] [PubMed] [Google Scholar]
- 15.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The american college of rheumatology 1990 criteria for the classification of takayasu arteritis. Arthritis Rheum 1990;33:1129–34. [DOI] [PubMed] [Google Scholar]
- 16.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The american college of rheumatology 1990 criteria for the classification of churg-strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990;33:1094–100. [DOI] [PubMed] [Google Scholar]
- 17.Watts RA, Suppiah R, Merkel PA, Luqmani R. Systemic vasculitis--is it time to reclassify? Rheumatology (Oxford) 2011;50:643–5. [DOI] [PubMed] [Google Scholar]
- 18.Grayson PC, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, Koening CL, et al. New features of disease after diagnosis in 6 forms of systemic vasculitis. J Rheumatol 2013;40:1905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc B 1995;57:289–300. [Google Scholar]
- 20.Lieberthal JG, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, Koening CL, et al. Urinary biomarkers in relapsing antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol 2013;40:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank E HM, Witten IH The weka workbench. Online appendix for “data mining: Practical machine learning tools and techniques”. Fourth Edition ed.; 2016. [Google Scholar]
- 22.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on il-2. Nat Rev Immunol 2004;4:665–74. [DOI] [PubMed] [Google Scholar]
- 23.Maier LM, Anderson DE, Severson CA, Baecher-Allan C, Healy B, Liu DV, et al. Soluble il-2ra levels in multiple sclerosis subjects and the effect of soluble il-2ra on immune responses. J Immunol 2009;182:1541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin M, Park S, Hayden A, Giustini D, Trinkaus M, Pudek M, et al. Clinical utility of soluble interleukin-2 receptor in hemophagocytic syndromes: A systematic scoping review. Ann Hematol 2017;96:1241–51. [DOI] [PubMed] [Google Scholar]
- 25.Salvarani C, Cantini F, Boiardi L, Hunder GG. Laboratory investigations useful in giant cell arteritis and takayasu’s arteritis. Clin Exp Rheumatol 2003;21:S23–8. [PubMed] [Google Scholar]
- 26.Kermani TA, Schmidt J, Crowson CS, Ytterberg SR, Hunder GG, Matteson EL, et al. Utility of erythrocyte sedimentation rate and c-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum 2012;41:866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Pla A, Bosch-Gil JA, Rossello-Urgell J, Huguet-Redecilla P, Stone JH, Vilardell-Tarres M. Metalloproteinase-2 and −9 in giant cell arteritis: Involvement in vascular remodeling. Circulation 2005;112:264–9. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Pla A, Martinez-Murillo F, Savino PJ, Eagle RC Jr., Seo P, Soloski MJ. Mmp-12, a novel matrix metalloproteinase associated with giant cell arteritis. Rheumatology (Oxford) 2009;48:1460–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segarra M, Garcia-Martinez A, Sanchez M, Hernandez-Rodriguez J, Lozano E, Grau JM, et al. Gelatinase expression and proteolytic activity in giant-cell arteritis. Ann Rheum Dis 2007;66:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corbera-Bellalta M, Garcia-Martinez A, Lozano E, Planas-Rigol E, Tavera-Bahillo I, Alba MA, et al. Changes in biomarkers after therapeutic intervention in temporal arteries cultured in matrigel: A new model for preclinical studies in giant-cell arteritis. Ann Rheum Dis 2014;73:616–23. [DOI] [PubMed] [Google Scholar]
- 31.Guo SY, Shen X, Yang J, Yuan J, Yang RL, Mao K, et al. Timp-1 mediates the inhibitory effect of interleukin-6 on the proliferation of a hepatocarcinoma cell line in a stat3-dependent manner. Braz J Med Biol Res 2007;40:621–31. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman GS, Ahmed AE. Surrogate markers of disease activity in patients with takayasu arteritis. A preliminary report from the international network for the study of the systemic vasculitides (inssys). Int J Cardiol 1998;66 Suppl 1:S191–4; discussion S5. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Ma L, Yan F, Liu H, Ding Y, Hou J, et al. Mmp-9 and il-6 are potential biomarkers for disease activity in takayasu’s arteritis. Int J Cardiol 2012;156:236–8. [DOI] [PubMed] [Google Scholar]
- 34.Noris M, Daina E, Gamba S, Bonazzola S, Remuzzi G. Interleukin-6 and rantes in takayasu arteritis: A guide for therapeutic decisions? Circulation 1999;100:55–60. [DOI] [PubMed] [Google Scholar]
- 35.Park MC, Lee SW, Park YB, Lee SK. Serum cytokine profiles and their correlations with disease activity in takayasu’s arteritis. Rheumatology (Oxford) 2006;45:545–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.