Abstract
Purpose:
Pharmacy dispensing databases are often used to identify patients' medications at a particular time point, for example to measure prescribing quality or the impact of medication use on clinical outcomes. We performed a systematic review of studies that examined methods to assess medications in use at a specific point in time.
Methods:
Comprehensive literature search to identify studies that compared active medications identified using pharmacy databases to medications identified using non-automated data sources. Two investigators independently reviewed abstracts and full-text material.
Results:
Of 496 studies screened, 29 studies evaluating 50 comparisons met inclusion criteria. Twenty-nine comparisons evaluated fixed look-back period approaches, defining active medications as those filled in a specified period prior to the index date (range 84-730 days). Fourteen comparisons evaluated medication-on-hand approaches, defining active medications as those for which the most recent fill provided sufficient supply to last through the study index date. Sensitivity ranged from 48% to 93% for fixed look-back period approaches and 35% to 97% for medication-on-hand approaches. Interpretation of comparative performance of methods was limited by use of different reference sources, target medication classes, and databases across studies. In four studies with head-to-head comparisons of these methods, sensitivity of the medication-on-hand approach was a median of 7% lower than the corresponding fixed look-back approach.
Conclusions:
The reported accuracy of methods for identifying active medications using pharmacy databases differs greatly across studies. More direct comparisons of common approaches are needed to establish the accuracy of methods within and across populations, medication classes, and databases.
Keywords: databases, drug prescribing, pharmaceutic, Pharmacoepidemiology, quality of care, research methodology, systematic review
1 ∣. INTRODUCTION
Automated pharmacy dispensing and claims databases are commonly used to measure medication exposure. Despite well-described limitations, pharmacy dispensing data are convenient, objective, and allow the study of large populations for which other methods of assessing medication exposure may be infeasible.1 Increasingly researchers and health systems have focused on examining prescribing quality and clinical outcomes, which includes topics of polypharmacy, duplication of therapy, medication interactions, adherence to prescribing guidelines, and changes in medication use after clinical events. These types of research questions frequently require that investigators establish a list of medications being taken by a patient at a fixed point in time, also referred to as an active medication list or cross-sectional medication list.2,3
In contrast to studies focused on adherence, where standard measures have been established and compared,4-7 there are no consensus measures for establishing active medication lists, and in many studies, approaches used to identify active medications are incompletely described.8 Adherence studies generally examine longitudinal medication use, and cohort entry is often defined as the first fill of the study medication being examined, ie, new user cohort study designs. However, these approaches are not suited to evaluating cross-sectional medication use, as cohort entry may be defined by a clinical event, such as occurrence of a potentially medication related adverse event, hospitalization, test result, or new diagnosis. Furthermore, case-control studies examining the association between a clinical event (eg, falls, acute coronary syndrome, infection) and prior drug exposure require careful attention to defining medication exposure at the time of the clinical outcome of interest.
In the absence of consensus measures, researchers have employed a variety of definitions of active medication use. Two broad approaches are commonly used. The fixed look-back period approach examines all medication filled within a set period prior to the study index date. The medication-on-hand approach (also termed legend duration approach) classifies medications for which the most recent fill provided sufficient supply to last through the study index date as active. However, there is a paucity of literature methodically examining and comparing these common approaches for establishing active medication lists. The lack of a comprehensive assessment of validated measures is a major impediment to identifying best practices and for defining what future work is needed to improve the accuracy of measurement of active medication use in both pharmacoepidemiology research and quality improvement initiatives. In the study, we systematically review the literature to identify studies which evaluated the accuracy of pharmacy database methods for establishing active medication lists.
2 ∣. METHODS
2.1 ∣. Search strategy
We conducted a search of English language literature using PubMed, Embase, and Google Scholar to identify studies that evaluated active medication use using pharmacy databases which were published between January 1 1990 and October 1 2017. The search strategy was developed in PubMed using Medical Subject Headings (MeSH) search terms and translated into searches tailored to Embase and GoogleScholar with the assistance of a research librarian (EW). Complete search strings for each database listed in Appendix 1. We additionally searched the reference list for each article which met study inclusion criteria to identify additional articles of possible interest.
2.2 ∣. Identification and selection of articles
Two study investigators (TA and EX) independently reviewed study abstracts to identify potentially relevant articles for retrieval using Covidence. The study protocol was registered on PROSPERO (CRD42018085584) in February 2018.
For title and abstract screening, we examined all original research publications which used pharmacy databases to establish a patient-level list of medications being used at a fixed point in time, regardless of the characteristics of the study population, sample size, setting, or type of pharmacy database. We included studies which established a comprehensive list of medications used by a patient and those which established a focused list medication classes (ie, cardiac medications). Studies which examined medication use over a period of time (period prevalence) rather than at a fixed point were not included (ie, studies of medication use during pregnancy).
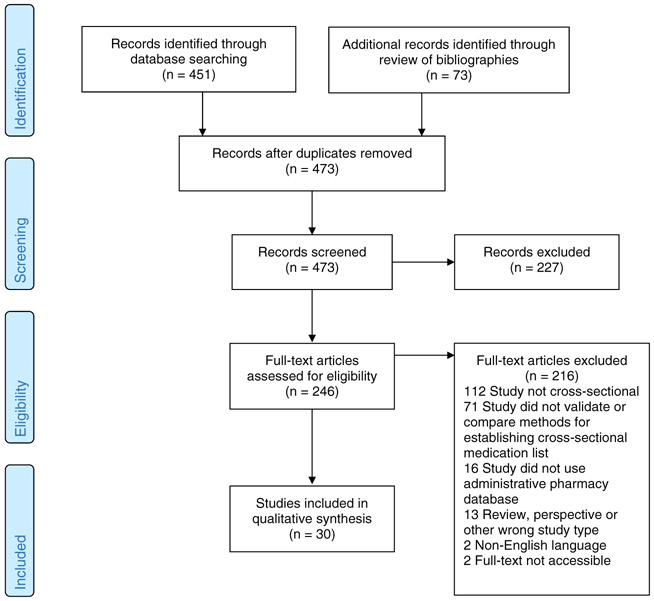
Two study investigators (TA and EX) independently reviewed the full-text articles, applying the above exclusion criteria. For studies meeting the initial criteria, we then determined whether the pharmacy database approaches used to identify active medications were compared with an alternative approach (ie, patient self-report or medical records) and included only studies with validation components. Disagreements between reviewers were resolved by consensus with the input of a third investigator (MAS). See PRISMA flow diagram for final study population (Figure 1).
FIGURE 1.
Systematic review PRISMA flow diagram [Colour figure can be viewed at wileyonlinelibrary.com]
2.3 ∣. Data analysis
First, we extracted study characteristics including primary aim, country, pharmacy database examined, clinical setting, study population, sample size, year, and alternative approach against which pharmacy databases were compared.
Second, we identified the criteria for classifying a medication as active, based upon a published framework for determining the number of drugs used by a patient.8 Extracted elements included the scope of medications examined, consideration of prior fill history (medication fills which occurred prior to the most recent fill in determining if a medication was active), allowable index gap (the length of time before the index date in which a medication must be filled to be counted as active), consideration of postindex fills (fills which occurred after the index date), and any other factors used to determine active medications.
Third, we extracted the results of the pharmacy database comparison. For each study, we extracted the number of medications identified as active by the pharmacy database approach and the comparator approach. This data was used to construct 2 × 2 tables from which we calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of pharmacy records compared with alternative methods. Though some studies referred to the pharmacy database as the gold standard, we converted all reported test characteristics such that the pharmacy database is the test metric compared with the alternative “gold-standard” approach. While establishing a true gold standard for drug exposure is challenging, calculating sensitivity and specificity provides important detail on the direction of misclassification between methods, and thus were our primary analysis. We secondarily calculated Cohen kappa statistic9 and the prevalence-adjusted and bias-adjusted kappa (PABAK)10 to assess concordance across studies. For studies which did not report the raw data necessary to construct a complete 2 × 2 table, we extracted the published test characteristics. For studies which reported test characteristics separately for individual drug classes, raw data for each class were summed when available; if not available, the median and range of test characteristics across drug classes were reported.
Lastly, we conducted a qualitative evaluation of the strengths and limitations of each pharmacy database method for establishing active medication lists.
3 ∣. RESULTS
We identified 30 studies from eight countries which validated methods for establishing active medication lists using pharmacy databases against medication use data obtained from other sources (Table 1).11-40 Studies had variable patient inclusion criteria, often requiring evidence of medication claims in the database prior to the study period. The majority of studies (90%) involved patients recruited for a cohort study or interventional trial. Participation rates ranged from 38% to 86% but were often unreported. Eleven studies evaluated national government databases, five evaluated regional government databases, seven evaluated health system databases, and seven evaluated outpatient pharmacy databases. Fourteen studies compared pharmacy database approaches with patient interview, seven to patient survey, four to chart review, one to pharmacist review of prescribing records, one to serum drug levels, and two to a combination of sources.
TABLE 1.
Descriptive characteristics of included studies
| Author, Publication Year | Country | Database Type | Database Name | Patient Population | Study Context | N | Comparison |
|---|---|---|---|---|---|---|---|
| Johnson, 199111 | USA | Integrated health system pharmacy database | Kaiser Permanente | Adults age ≥65 enrolled in the health plan and reporting being bedbound or having ≥2 deficits in activities of daily living | All study respondents (response rate not listed) | 83 | Patient interview |
| Heerdink, 199512 | Netherlands | Single outpatient pharmacy | Not specified | Adults age ≥65 in one city district and who filled ≥1 prescription in the database in prior 6 months | Participants selected by general practitioners, selection approach not specified | 100 | Patient interview |
| Lau 199513 | Netherlands | Single outpatient pharmacy | Not specified | Adults age ≥70 who were either elder home residents or community dwelling in a single city district and who filled ≥1 prescription in the database in prior 6 months | Random sample recruited from participating general practices | 115 | Patient interview |
| Sjahid, 199814 | Netherlands | Single outpatient pharmacy | Not specified | Adults age >55 in one city district | Participants in a prospective cohort study | 1682 | Patient interview |
| Klungel, 199915 | Netherlands | Regional network of community pharmacy databases | PHARMO database | Adults age 20-59 with hypertension from a single city district with available records in the PHARMO database | Survey respondents (response rate not listed) | 372 | Mailed survey |
| Lau, 200016 | Netherlands | Multiple individual outpatient pharmacy databases | Not specified | Adults age ≥40 hospitalized for ≥2 days in the general medicine ward of 2 hospitals with at ≥1 pharmacy fill in the prior year | Participants in a prospective cohort study | 304 | Chart review and patient survey |
| King, 200117 | Australia | National government pharmacy claims database | Australian pharmaceutical benefits scheme | Multicenter study of nursing home residents with evidence of ≥1 prescription in pharmacy claims database in prior year | All nursing home residents with eligible records | 942 | Chart review of nursing home records |
| Monster, 200218 | Netherlands | Multiple individual outpatient pharmacy databases | Not specified | Adults age 28-75 sampled from a single city | Survey participants (response rate 48%) | 8592 | In-clinic survey |
| Kwon, 200319 | USA | Managed care organization pharmacy database | Tufts health plan | Adults age ≥18 who screened positive for depression and were members of a single health plan | Randomized controlled trial participants | 200 | Mailed survey |
| Lund, 200320 | USA | Regional government health system | Iowa Department of Health Services pharmacy claims | Community-dwelling adults with Medicaid, ≥1 chronic disease, and ≥4 medications | Random sample of participants in a pharmaceutical case management program | 100 | Pharmacist review of the pharmacy databasea |
| Boudreau, 200521 | USA | Integrated health system pharmacy database | Group health cooperative | Community-dwelling adults age ≥67 with ≥1 chronic diseases and 2 years of health plan enrollment | Survey respondents (response rate 86%) | 3610 | Mailed survey |
| Caskie, 200622 | USA | Integrated health system pharmacy database | Group health cooperative | Adults with available pharmacy data | Prospective study cohort participants | 430 | Patient interview |
| Curtis, 200623 | USA | Managed care organization pharmacy database | Unspecified | Adults age ≥18 with chronic glucocorticoid use and enrolled in the health plan for ≥6 months | Survey respondents (response rate 38%) | 2363 | Mailed survey |
| Brown, 200724 | USA | Integrated health system pharmacy database | Kaiser Permanente | Adults age ≥45 and members of a regional health plan | Prospective study cohort participants | 7918 | Patient interview |
| Glintborg, 200725 | Denmark | National government pharmacy database | Danish Register of Medicinal Product Statistics | Single-center study of patients admitted to a general medicine ward | Prospective study cohort participants | 500 | Blood testing of drug levels |
| Haukka, 200726 | Finland | National government pharmacy database | Finnish National Prescription Register | Participants from a population-based genetic study of schizophrenia | Retrospective cohort study participants | 905 | Patient interview |
| Shalansky, 200727 | Canada | Regional government pharmacy database | PharmaNet (British Columbia) | Single-center study of patients with a clinic visit for heart failure OR prescribed one of three medications used for heart failure treatment | Interview participants (response rate 53%) | 194 | Patient interview |
| Nielsen, 200828 | Denmark | National government pharmacy database | Danish Register of Medicinal Product Statistics | Adults age ≥16 | Survey respondents (response rate 74%) | 16 688 | Patient interview |
| Pit, 200829 | Australia | National government pharmacy claims database | Australian Pharmaceutical Benefits Scheme | Adults age ≥65 attending 20 general practice clinics | Randomized controlled trial participants | 566 | Patient interview |
| Warholak, 200930 | USA | Aggregated pharmacy claims database from multiple pharmacy benefit managers | RxAccord | Hospitalized adults age ≥18 enrolled in participating insurance plan | Random sample | 78 | Chart review of admission medication list |
| Noize, 200931 | France | National government pharmacy database | CNAM-TS | Community-dwelling adults ≥age 67 from 3 French cities | Prospective cohort participants with available pharmacy data (44% of cohort) | 4112 | Patient interview with examination of drug packaging |
| Haapea, 201032 | Finland | National government pharmacy database | Finnish National Prescription Register | Patients born in 2 Finnish provinces in 1966 and alive in 1997 | Survey respondents (response rate 66%) | 7625 | Mailed survey |
| Rikala, 201033 | Finland | National government pharmacy database | Finnish National Prescription Register | Adults age ≥75 in a single city | Randomized controlled trial participants and agreeing to follow-up study | 570 | Patient interview |
| Allin, 201334 | Canada | Regional government pharmacy database | Ontario Drug Benefit Program | Community-dwelling adults age ≥65 | Survey respondents (response rate not listed) | 32 848 | Patient interview |
| Richardson, 201335 | Ireland | National government pharmacy database | Health Service Executive-Primary Care Reimbursement Services | Community-dwelling adults age ≥50 and providing consent for pharmacy linkage | Participants in an observational cohort study (response rate 62%) | 2621 | Patient interview |
| Fujita, 201536 | Japan | Regional government pharmacy database | National Health Insurance of Chiba City | Adults age 40-74 | All adults receiving a regular check up | 54 712 | Chart review of questionnaire used in routine clinic practice |
| Colantonio, 201637 | USA | National government pharmacy database | Medicare part D | Adults age ≥45 receiving Medicare pharmacy benefits | Participants in a national population-based observational cohort study | 899 | Telephone survey and patient interview with examination of drug packaging |
| Drieling, 201638 | USA | Integrated health system pharmacy database | Group Health Cooperative | Women age ≥60, participating in a longitudinal cohort study, and enrolled in the same health plan for ≥5 years | Survey respondents (response rate 48%) | 223 | Mailed survey |
| Lacasse, 201639 | Canada | Regional government pharmacy database | Regie de I'assurance Maladie du Quebec (RAMQ) | Adults ≥18 seen in participating chronic pain clinics with records in the pharmacy database in the prior year | Study respondents (response rate 44%) | 272 | Chart review of questionnaire used in routine clinic practice |
| Taipale, 201640 | Finland | National government pharmacy database | Finnish National Prescription Register | Community-dwelling adults age ≥75 from a single city | Randomized controlled trial participants | 569 | Patient interview |
Lund (2013) compared active medication lists generated by the pharmacy database algorithms to active medication lists generated by pharmacist review of the same pharmacy review database; no alternative source of medication use data was examined.
3.1 ∣. Pharmacy database methods used to define an active medication
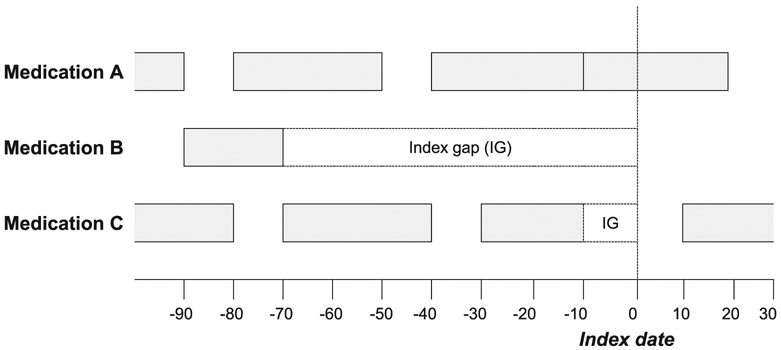
Eighteen studies evaluated a single approach for establishing an active medication list, and 12 compared multiple approaches yielding 51 total comparisons (Table 2). The scope of medications examined ranged from a single drug class to all prescribed medications. Twenty-nine comparisons in 20 studies used a fixed look-back period approach, classifying all medication filled by a patient in a specified time period (range 84-730 days) prior to the study index date as active. Only one study required more than one fill to classify a medication as active.36 No studies required fills to have a minimum number of days supplied. For illustration, Figure 2 depicts hypothetical classifications of medications using different timeframes for both fixed look-back period and medication-on-hand approaches.
TABLE 2.
Definition of active medications using pharmacy records
| Author, Year | Versiona | Scope | Index Date | Criteria for Active Medications Using Pharmacy Database |
|---|---|---|---|---|
| Fixed look-back period approaches | ||||
| Johnson, 199111 | - | 18 medication classes | Interview date | Any fills in the 90 days prior to index date. |
| Heerdink, 199512 | - | All medications | Interview date | Any fills in the 2 years prior to index date. |
| Lau, 199513 | A | 8 medication classes | Interview date | Any fills in the 90 days prior to index date. |
| B | 8 medication classes | Interview date | Any fills in the 30 days prior to index date. | |
| Sjahid, 199814 | A | 8 cardiac medication classes | Interview date | Any fills in the 6 months prior to index date. |
| Klungel, 199915 | A | Antihypertensive medications | Date of survey completion | Any fills in available records. Range 90 days to 16 months prior to index date. |
| King, 200117 | - | All medications | Date of nursing home visit | Any fills in the 12 weeks prior to index date. |
| Monster, 200218 | - | 7 medication classes | Date of survey completion | Any fills in the 1 year prior to index date. |
| Kwon, 200319 | - | Antidepressant medications | Date of survey completion | Any fills in the 90 days prior to index date. |
| Curtis, 200623 | - | Osteoporosis medications | Date of survey completion | Any fills in the 180 days prior to index date. |
| Glintborg, 200725 | - | 5 medications | Date of hospital admission | Any fills in the 6 month prior to index date. |
| Haukka, 200726 | - | 8 psychotropic medication classes | Interview date | Any fills in the 6 months prior to index date. |
| Nielsen, 200828 | A | 16 medication classes | Interview date | Any fills in the 90 days prior to index date. |
| Pit, 200829 | A | 6 medication classes and 3 individual medications | Interview date | Any fills in the 30 days prior to index date. |
| B | 6 medication classes and 3 individual medications | Interview date | Any fills in the 60 days prior to index date. | |
| C | 6 medication classes and 3 individual medications | Interview date | Any fills in the 90 days prior to index date. | |
| D | Benzodiazepines and NSAIDS | Interview date | Any fills in the 180 days prior to index date. | |
| E | Benzodiazepines and NSAIDS | Interview date | Any fills in the 365 days prior to index date. | |
| Noize, 200930 | - | 38 medication classes | Interview date | Any fills in the 60 days prior to index date. |
| Haapae, 201032 | - | 5 medication classes | Date of survey completion | Any fills in the 6 months prior to index date. |
| Rikala, 201033 | A | 3 classes of psychotropic medications | Interview date | Any fills in the 4 months prior to index date. |
| B | 3 classes of psychotropic medications | Interview date | Any fills in the 6 months prior to index date. | |
| C | 3 classes of psychotropic medications | Interview date | Any fills in the 1 year prior to index date. | |
| Allin, 201334 | A | Antihypertensives and oral diabetes medications | Interview date | Any fills in the 30 days prior to index date. |
| B | Antihypertensives and oral diabetes medications | Interview date | Any fills in the 100 days prior to index date. | |
| C | Antihypertensives and oral diabetes medications | Interview date | Any fills in the 130 days prior to index date. | |
| Richardson, 201335 | - | 19 medication classes | Interview date | ≥3 Fills in the 6 months prior to index date and ≥1 fill in the 1 month prior to index date |
| Colantonio, 201636 | A | Lipid-lowering medications | Phone interview date | Any fills in the 120 days prior to index date. |
| B | Lipid-lowering medications | In-person interview date | Any fills in the 120 days prior to index date. | |
| Lacasse, 201639 | - | 8 classes of analgesic medications | Date of questionnaire completion | Any fills in the 1 year prior to index date. |
| Medication-on-hand (legend duration) approaches | ||||
| Lau, 199513 | C | 8 medication classes | Interview date | Index date falls within the period from the [fill date] for a medication through the [fill date + 110% of the days' supply]. |
| Sjahid, 199814 | B | All cardiovascular medications | Interview date | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply]. |
| Klungel, 199915 | B | Antihypertensive medications | Date of survey completion | Index date falls within the period from the [fill date] for a medication through the [fill date + 110% of the days' supply]. |
| Lau, 200016 | - | All medications | Interview date | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply + 7-day grace period]. |
| Lund, 200320 | A | All medications | October 1, 2000 | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply]. |
| B | All medications | October 1, 2000 | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply] AND there is a fill both before and after the index date with a time period between fills of <90 days. | |
| C | All medications | October 1, 2000 | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply] AND there is a fill both before and after the index date with a time period between fills of <90 days. PLUS: A fill is required within 90 days before the index data for as-needed medications only. | |
| D | All medications | October 1, 2000 | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply] AND there is a fill both before and after the index date with a time period between fills of <90 days. PLUS: For as needed medications, there is a fill both ≤180 days before the index date AND ≤60 days after the index date. | |
| Boudreau, 200521 | - | 7 medication classes | Date of survey completion | At least 2 prescriptions for the medication were filled in the 12 months prior to index date AND the index date falls within the period from the [fill date] for a medication through the [fill date + days' supply + 60-day grace period]. |
| Caskie, 200622 | - | 16 medication classes | Interview date | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply + 7-day grace period]. For anti-infective medications only the grace period was 2 days rather than 7 days. |
| Shalansky, 200727 | All medications | Interview date | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply]. | |
| Nielsen, 200828 | B | 16 medication classes | Interview date | Index date falls within the period from the [fill date] for a medication through the [fill date + 110% of the days' supply]. |
| Warholak, 200930 | - | All medications | Date of hospital admission | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply]. |
| Drieling, 201638 | - | 4 medication classes | Date of survey completion | Index date falls within the period from the [fill date] for a medication through the [fill date + day's supply] OR gap of <60 days was present between the most recent preindex [fill date + days' supply] and the first postindex fill. |
| Other approaches | ||||
| Brown, 200724 | A | Lipid-lowering medications | Interview date | Any fills in the 15 days before or after the index date |
| B | Lipid-lowering medications | Interview date | Any fills in the 30 days before or after the index date | |
| C | Lipid-lowering medications | Interview date | Any fills in the 60 days before or after the index date | |
| D | Lipid-lowering medications | Interview date | Any fills in the 90 days before or after the index date | |
| Fujita, 201537 | A | Hypertension, diabetes, and lipid-lowering medications | Date of clinic visit | Any fills in the same month as the index date |
| B | Hypertension, diabetes, and lipid-lowering medications | Date of clinic visit | Any fills in 2 months prior or the same month as the index date | |
| Taipale, 201640 | - | 34 medication classes | Interview date | Variable and based on mathematical modeling of personal medication purchasing behaviors (PRE2DUP model) |
The following studies compared multiple approaches using different criteria to define active medications using pharmacy databases, noted by the version A-D: Lau (1995), Sjahid (1998), Klungel (1999), Nielsen (2008), Pit (2008), Rikala (2010), Allin (2013), Lund (2003), Brown (2007), and Fujita (2015). King (2001), Curtis (2006), Haukka (2007), and Noize (2009) also examined alternative approaches for defining active medications; however, the manuscripts only reported numeric results for the approaches listed in the tables.
FIGURE 2.
Comparison of common approaches for establishing active medications at a fixed time point.
In the above diagram, grey bars represent days supplied by medication fills in hypothetical refill patterns for three medications prescribed to a patient. The index gap (IG) is the period between the last day supplied by the most recent fill and the index date. Medication A has an index gap of 0 days, medication B has an index gap of 70 days, and medication C has an index gap of 10 days. Two common approaches to establishing an active medication list are demonstrated as follows:
1. Fixed look-back period approach: Defines active medications as those for which any fill occurred during a specified period prior to the index date. Using a fixed look-back period of 30 days would classify medications A and C as active, while using a fixed look-back period of 90 days would classify medications A, B, and C as active.
2. Medication-on-hand approach: Also termed legend duration approach. Defines active medications as those for which the most recent refill date provided sufficient supply to last through the study index date. Using this approach, only medication A would be classified as active. An allowable index gap may be added to the medication-on-hand approach, and would define active medications as those for which the most recent refill provided sufficient supply to last through the study index date including a grace period, which can be defined either as a fixed number of days or as a given percentage of the duration of the most recent fill. Using an allowable index gap of 14 days would classify medications A and C as active.
Fourteen comparisons in 11 studies used a medication-on-hand approach. Of these, seven comparisons had an allowable index gap of zero (no grace period), six included a grace period or fixed allowable index gap (range 3-60 days), and one included a flexible allowable index gap (requiring a gap of less than 60 days between the preindex fill and first postindex fill).38 Only one comparison required multiple fills prior to the index date to be counted as active.21 Two studies included additional criteria for medications typically used short term or as needed.20,22
Seven comparisons in three studies used an alternative method to define active medications. Six comparisons defined active medications as those filled within a fixed period which included both preindex fills and postindex fills.24,38 One study used a mathematical modeling approach to calculate personalized drug use periods for each patient based upon individual drug refill patterns.40
3.2 ∣. Performance of pharmacy database methods
The test characteristics of each pharmacy database method compared with other sources of active medication lists are listed in Table 3. Assessment of measure performance across studies was limited due to the use of different inclusion criteria, study populations, and comparator metrics. Comparisons examining fixed look-back period approaches reported variable sensitivity (48%-93% with majority between 70% and 85%) but high specificity (82%-100%, with majority greater than 90%). Among comparisons reporting results stratified by medication class, the sensitivity ranged from 29% to 100% with medications taken as needed and medications taken for short duration (eg, antibiotics and analgesics) having lower reported sensitivity than chronic medications. Similarly, the reported sensitivity of medication-on-hand approaches ranged from 35% to 96% (majority between 75% and 90%), while the reported specificity was generally higher (89%-100%).
TABLE 3.
Results of studies comparing pharmacy records to other sources of active medication lists
| Author, Year | Versiona | Pharmacy Database Criteria for Active Medications |
Comparison | Sens. | Spec. | PPV | NPV | Kappa | PABAK |
|---|---|---|---|---|---|---|---|---|---|
| Fixed look-back period approaches | |||||||||
| Johnson, 199111 | - | Any fills in the 90 days prior to index date. | Patient interview | 50-100 (Median 80) | 88-100 (Median 96) | 22-100 (Median 82) | - | - | - |
| Heerdink, 199512 | - | Any fills in the 2 years prior to index date. | Patient interview | 79 | - | - | - | - | - |
| Lau, 199513 | A | Any fills in the 90 days prior to index date | Patient interview | 69-100 (Median 82) | 88-100 (Median 99) | 19-100 (Median 90) | - | - | - |
| B | Any fills in the 30 days prior to index date | Patient interview | 38-73 (Median 50) | 93-100 (Median 100) | 33-100 (Median 100) | - | - | - | |
| Sjahid, 199814 | A | Any fills in the 6 months prior to index date. | Patient interview | 93 | - | 85 | - | 0.36-0.97 (Median 0.91) | |
| Klungel, 199915 | A | Any fills in available records. Range 90 days to 16 months prior to index date. | Mailed patient survey | 93 | 91 | 90 | 94 | 0.89 | 0.89 |
| King, 200117 | A | Any fills in the 12 weeks prior to index date. | Chart review of nursing home records | 78 | - | 60 | - | - | - |
| B | Any fills in the 12 weeks prior to index date. | Chart review of nursing home records | 48 | - | 56 | - | - | - | |
| Monster, 200218 | - | Any fill in the 1 year prior to index date. | Patient survey | 54 | 96 | 64 | 94 | 0.53 | 0.81 |
| Kwon, 200319 | - | Any fills in the 90 days prior to index date. | Mailed patient survey | 90 | 82 | 74 | 94 | 0.69 | 0.7 |
| Curtis, 200623 | - | Any fills in the 180 days prior to index date. | Mailed patient survey | 68 | 99 | 90 | 98 | 0.76 | 0.95 |
| Glintborg, 200725 | - | Any fills in the 2 years prior to index date. | Blood testing of drug levels | 93 | - | - | - | - | - |
| Haukka, 200726 | - | Any fills in the 6 months prior to index date. | Patient interview | 71 | 99 | 90 | 96 | 0.77 | 0.91 |
| Nielsen, 200828 | A | Any fills in the 90 days prior to index date. | Patient interview | 79 | 98 | 55 | 99 | 0.64 | 0.95 |
| Pit, 200829 | A | Any fills in the 30 days prior to index date. | Telephone interview | 33-81 (Median 60) | 96-100 (Median 99) | 81-100 (Median 93) | 69-98 (Median 91) | - | 0.62-0.97 (Median 0.83) |
| B | Any fills in the 60 days prior to index date. | Telephone interview | 54-93 (Median 78) | 91-100 (Median 97) | 71-100 (Median 90) | 86-98 (Median 96) | - | 0.74-0.98 (Median 0.89) | |
| C | Any fills in the 90 days prior to index date. | Telephone interview | 71-100 (Median 83) | 86-100 (Median 96) | 64-97 (Median 90) | 90-100 (Median 97) | - | 0.71-1.00 (Median 0.90) | |
| D | Any fills in the 180 days prior to index date. | Telephone interview | 83-89 (Median 86) | 78-95 (Median 87) | 54-66 (Median 60) | 96-98 (Median 97) | - | 0.61-0.88 (Median 0.75) | |
| E | Any fills in the 365 days prior to index date. | Telephone interview | 90-95 (Median 93) | 66-92 (Median 79) | 45-57 (Median 51) | 98-99 (Median 98) | - | 0.45-0.84 (Median 0.65) | |
| Noize, 200931 | - | Any drug reimbursement claims in the 60 days prior to index date. | Patient interview with examination of drug packaging | 1-91 (Median 73) | 81-100 (Median 99) | 14-100 (Median 79) | 76-100 (Median 97) | 0.02-0.93 (Median 0.69) | - |
| Haapae, 201032 | - | Any fills in the 6 months prior to index date. | Mailed patient survey | 74 | 99 | 71 | 99 | 0.72 | 0.99 |
| Rikala, 201033 | A | Any drug reimbursement claims in the 4 months prior to index date | Patient interview | 70 | 98 | 87 | 94 | 0.73 | 0.85 |
| B | Any drug reimbursement claims in the 6 months prior to index date | Patient interview | 78 | 97 | 86 | 95 | 0.77 | 0.87 | |
| C | Any drug reimbursement claims in the 12 months prior to index date | Patient interview | 84 | 96 | 82 | 96 | 0.79 | 0.87 | |
| Allin, 201334 | A | Any fills in the 30 days prior to index date. | Patient interview | - | - | 32-47 (Median 37) | 91-99 (Median 95) | 0.24-0.55 (Median 0.38) | - |
| B | Any fills in the 100 days prior to index date. | Patient interview | - | - | 70-86 (Median 79) | 78-99 (Median 89) | 0.46-0.87 (Median 0.67) | - | |
| C | Any fills in the 130 days prior to index date. | Patient interview | - | - | 73-88 (Median 83) | 77-99 (Median 89) | 0.47-0.89 (Median 0.68) | - | |
| Richardson, 201335 | - | ≥ 3 fills in the 6 months prior to index date and ≥ 1 fill in the one month prior to index date. | Patient interview | 78 | 96 | 76 | 96 | 0.73 | 0.86 |
| Colantonio, 201636 | A | Any fills in the 120 days prior to index date. | Phone interview | 78 | 90 | 86 | 86 | 0.72 | 0.73 |
| B | Any fills in the 120 days prior to index date. | Interview with examination of drug packaging | 81 | 90 | 86 | 87 | 0.68 | 0.69 | |
| Lacasse, 201639 | - | Any fills in the 365 days (1 year) prior to index date. | Patient questionnaire used in routine clinic practice | 53-100 (Median 78) | 63-99 (Median 93) | 27-81 (Median 74) | 84-100 (Median 95) | 0.11-0.83 (Median 0.66) | |
| Medication-on-hand approaches | |||||||||
| Lau, 199513 | C | Index date falls within the period from the [fill date] for a drug through the [fill date + 110% of the days' supply]. | Patient interview | 69-100 (Median 78) | 93-100 (Median 100) | 84-100 Median (100) | - | - | - |
| Sjahid, 199814 | B | Index date falls within the period from the [fill date] for a drug through the [fill date + days' supply]. | Patient interview | 85 | - | 94 | - | 0.27-0.97 (Median 0.89) | - |
| Klungel, 199915 | B | Index date falls within the period from the [fill date] for a drug through the [fill date + 110% of the days' supply]. | Mailed patient survey | 76 | 91 | 88 | 82 | 0.67 | 0.68 |
| Lau, 200016 | - | Index date falls within the period from the [fill date] for a drug through the [fill date + days' supply + 7-day grace period]. | Patient questionnaire and hospital medical record | 89 | - | 93 | - | - | - |
| Lund, 200320 | A | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply]. | Pharmacist review of the pharmacy database | 78 | 99 | - | - | - | - |
| B | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply] AND there is a fill both before and after the index date with a time period between fills of <90 days. | Pharmacist review of the pharmacy database | 92 | 96 | - | - | - | - | |
| C | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply] AND there is a fill both before and after the index date with a time period between fills of <90 days. PLUS: A fill is required within 90 days before the index data for as-needed medications only. | Pharmacist review of the pharmacy database | 94 | 92 | - | - | - | - | |
| D | Index date falls within the period from the [fill date] for a medication through the [fill date + days' supply] AND there is a fill both before and after the index date with a time period between fills of <90 days. PLUS: For as needed medications, there is a fill both ≤180 days before the index date AND ≤ 60 days after the index date. | Pharmacist review of the pharmacy database | 94 | 92 | - | - | - | - | |
| Boudreau, 200521 | - | At least 2 prescriptions for the medication were filled in the 12 months prior to index date AND the index date falls within the period from the [fill date] for a drug through the [fill date + days' supply + 60-day grace period]. | Patient mailed survey | - | - | - | - | 0.43-0.88 (Median 0.81) | 0.51-0.92 (Median 0.88) |
| Caskie, 200622 | - | Index date falls within the period from the [fill date] for a drug through the [fill date + days' supply + 7-day grace period]. | Patient interview | 38-82 | 89-100 | - | - | 0.00-0.92 | - |
| Shalansky, 200727 | - | Index date falls within the period from the [fill date] for a drug through the [fill date + days' supply]. | Patient interview | 84 | - | 97 | - | - | - |
| Nielsen, 200828 | B | Index date falls within the period from the [fill date] for a drug through the [fill date + 110% of the days' supply]. | Patient interview | 68 | 99 | 65 | 99 | 0.65 | 0.96 |
| Warholak, 200930 | - | Index date falls within the period from the [fill date] for a drug through the [fill date + days' supply]. | Chart review of admission medication list | 35 | - | 24 | - | - | - |
| Drieling, 201638 | - | Index date falls within the period from the [fill date] for a drug through the [fill date + days' supply] OR gap of ≤60 days was present between the most recent preindex [fill date + days' supply] and the first postindex fill. | Mailed patient survey | 96 | 99 | 98 | 99 | 0.96 | 0.97 |
| Other approaches | |||||||||
| Brown, 200724 | A | Any fills in the 15 days before or after the index date | Patient interview | 21 | 99 | 98 | 93 | - | - |
| B | Any fills in the 30 days before or after the index date | Patient interview | 35 | 99 | 96 | 95 | - | - | |
| C | Any fills in the 60 days before or after the index date | Patient interview | 54 | 99 | 94 | 97 | 0.67 | 0.92 | |
| D | Any fills in the 90 days before or after the index date | Patient interview | 59 | 99 | 91 | 96 | - | - | |
| Fujita, 201537 | A | Any fills in the same month as the index date | Patient questionnaire used in routine clinic practice | 56 | 98 | 90 | 90 | 0.63 | 0.79 |
| B | Any fills in 2 months prior or the same month as the index date | Patient questionnaire used in routine clinic practice | 69 | 98 | 89 | 93 | 0.73 | 0.84 | |
| Taiplae, 201640 | - | Variable and based on mathematical modeling of personal drug purchasing behaviors (PRE2DUP model) | Patient interview | 82 | 99 | 93 | 97 | 0.85 | 0.93 |
Abbreviations: PABAK, prevalence-adjusted and bias-adjusted kappa; Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value.
The following studies compared multiple approaches using different criteria to define active medications using pharmacy databases, noted by the version A-D: Lau (1995), Sjahid (1998), Klungel (1999), Nielsen (2008), Pit (2008), Rikala (2010), Allin (2013), Lund (2003), Brown (2007), and Fujita 2015.
3.3 ∣. Comparisons of pharmacy database methods within studies
Eleven studies compared multiple pharmacy database approaches within the same study population. Four studies compared different look-back period durations,13,29,33,34 consistently demonstrating that longer durations resulted in higher sensitivity but lower specificity and PPV. Two studies which also included postindex fills also demonstrated that increasing the overall duration examined resulted in higher sensitivity.24,37
Four studies compared a fixed look-back period approach to a medication-on-hand approach.13-15,28 In these four studies, the sensitivity of the medication-on-hand approach was a median of 7% lower (range, 18% higher to 17% lower) than the corresponding fixed look-back approach, while the PPV was a median of 9% higher (range, 2% lower to 10% higher). One study compared multiple medication-on-hand approaches to pharmacists' expert opinion of each subject's active medications based on individualized review of each subject's pattern of medication dispensing but did not examine a separate source of medication use data.20
4 ∣. DISCUSSION
Our systematic review indicates that methods for establishing active medication lists using pharmacy databases have not been comprehensively validated in the literature, and while many approaches have been proposed, they have rarely been compared with one another. Pharmacy database approaches have primarily been validated against patient self-report of medication use, through interviews or surveys. The majority of studies are drawn from cohorts and intervention trials with complex inclusion criteria, which may not be generalizable to real-world medication use reporting or refill patterns. Prior studies have evaluated approaches using fixed look-back periods ranging from 12 weeks to 2 years and medication-on-hand approaches. While reported sensitivity ranged from 48% to 93% for fixed look-back period approaches and 35% to 96% for medication-on-hand approaches, interpretation of these results are limited by the use of heterogeneous pharmacy databases, populations, comparison metrics, and clinical settings.
As researchers increasingly investigate prescribing quality and the temporal relationship between medication use and clinical outcomes, there remains an urgent need for development and rigorous testing of pharmacy database approaches to identify active medications, and a clear understanding of the accuracy of these approaches in commonly used data sources. Our findings have immediate implications for pharmacoepidemiology and drug safety researchers seeking to determine cross-sectional drug exposure for which new-user cohort designs may not be feasible. While new-user cohort study designs are convenient for examining exposure to a single drug, this approach is not feasible for examining multiple drug classes (eg, studies of polypharmacy) or populations defined by a clinical event such as an adverse event or clinical encounter. For example, multiple prior case-control studies examining the association between drug exposures and hip fracture have used pharmacy databases to define active mediation use.41-43 In the absence of best practice, these studies have employed a wide range of active medication definitions: requiring one fill in the prior 30 days,41 one fill in the prior 180 days,42 and a medication-on-hand approach with a grace period equaling half of the days' supply.43 This heterogeneity of approaches and the resultant differential misclassification may be one factor contributing to inconsistent observed associations. Thus, our review of the current literature raises several important themes, which may inform both future efforts to utilize pharmacy databases for studies of drug safety and future studies seeking to validate algorithms for establishing active medication lists (Table 4).
TABLE 4.
Considerations for designing and validating studies using administrative pharmacy records to identify active medication at a fixed time point
| Comparison group for validation studies |
|
| Timeframe |
|
| Scope of medications |
|
| Medication sources |
|
| Multiple measures |
|
First, it will be important to validate database algorithms by comparison with alternative sources of information on medication use. The consensus measures now routinely used for studies of adherence were the result of synthesis of studies comparing pharmacy database adherence algorithms to alternative sources of medication use information.4,5 Each comparison metric has important strengths and drawbacks. Patient interviews, the most common comparison in this review, are time intensive and susceptible to participation bias and recall errors. Patient surveys may suffer from low participation rates, unless included as part of routine clinical care.37,39 Biochemical measures are costly and may misclassify medication exposure for intermittently used medications or patients with transient nonadherence. Medication lists documented in electronic health records are likely to be of variable quality, as they may often reflect automated information populated from ordering systems which may not have been filled or may have been canceled. When available, medication reconciliation notes may provide a promising comparison, as they combine patient interviews conducted by clinicians, as part of regular clinical care, with a review of ordered prescriptions. However, it will be important for researchers to ensure the quality of these notes, which may also be impacted by the use of autopopulated medication lists from electronic health records and by experience of the person conducting the medication reconciliation, the information available to him or her, and by the degree of effort expended to obtain an accurate list.
Second, timeframe is likely the most important study design element in choosing algorithms for identifying active medications as this choice may directly impact the direction of potential misclassification bias. As depicted in Figure 2, the common approach of using a fixed look-back period may lead to both false positive and false negative classifications depending on the chosen look-back duration. Narrow look-back periods, such as 30 days, may miss active medications which were filled for extended supplies (eg, 90 days), medications used intermittently, medications taken with imperfect adherence, and medications which patients had stockpiled from prior fills. Conversely, broad look-back periods, such as 1 year, may classify medications no longer in use as active. The studies which compared different look-back periods consistently found that longer periods resulted in higher sensitivity but lower specificity and PPV.13,29,33,34 The second common approach identified in our review, the medication-on-hand approach, may improve upon fixed look-back period approaches by considering the days supplied in a fill, but may also misclassify active medications as nonactive, such as when patients fill medications late due to transient nonadherence or previous stockpiling of medications. The four studies to compare fixed look-back period approaches to medication-on-hand approaches found that medication-on-hand approaches were generally less sensitive but had a higher PPV than fixed look-back duration approaches.13-15,28 Investigators may consider adding grace periods to account for transient nonadherence6,44 as described in Figure 2. No studies directly compared the accuracy of medication-on-hand approaches with and without grace periods. Studies which considered fills following the index date were not directly compared with other approaches but risk misclassifying medications newly prescribed following the index date as active during the index date.
Third, future validation studies should pay careful attention to the scope of medications examined in pharmacy databases. As Goedken and colleagues have previously described, certain medication types are less likely to be included in pharmacy databases, such as over-the-counter medications, vitamins, and supplements.8 Pharmacy database approaches may need to be specified differently for medications used intermittently, those used with varying dosages (eg, insulin), and those used in nonstandardized dosage forms (eg, topical medications). Thus, a tailored rather than uniform approach to establishing active medications will likely be needed. The reviewed studies indicate that approaches with high sensitivity and specificity for chronic medications often have poorer accuracy for short duration and as-needed medications. Future validation studies might establish the accuracy of general approaches for broad medication categories, such as chronic prescribed medications versus as-needed medications, while individual research project goals should dictate which medications and methods are included in observational research studies.
Fourth, researchers should consider whether the pharmacy database of interest is likely to include all sources of medication of interest.45-47 In addition to nonprescription medications, prescriptions paid for with cash or using pharmaceutical samples may not be captured by certain pharmacy databases.48,49 Medications may not be captured by available claims data due to payment source, for example, Medicare Part D does not capture medications included as part of hospice care. Approaches for establishing active medication lists which have been validated in one database may not be as accurate in another, particularly when approaches validated in comprehensive national prescription registrars are extrapolated to more limited insurance or health system databases. This is particularly relevant in the United States, as we found few studies validating the use of Veterans Affairs, Medicare Part D, or private insurance databases, though all are commonly used in pharmacoepidemiology research. Consideration of site of care is important, as accuracy of approaches may differ in outpatient, nursing facility, and hospital settings. Medications used during hospital and skilled nursing facility stays may be supplied by internal pharmacies and thus not captured in other pharmacy databases.45
Fifth, future validation efforts should seek to compare multiple pharmacy database approaches within the same cohort, as our findings indicate that comparing approaches across different cohorts in different settings and against different comparison groups provides limited generalizable knowledge. Beyond validation studies, for many research goals, there may be value in conducting sensitivity analyses comparing more and less restrictive approaches for identifying active medications in the same cohort. For example, researchers might use an extended fixed look-back period of 6 months to define possibly active medications in conjunction with a more restrictive medication-on-hand approach to define medications very likely to be in active use.
In conclusion, use of pharmacy databases to examine active medication use at specific time points is increasing as studies focusing on prescribing quality and clinical outcomes become more common. However, methods for establishing active medication lists using pharmacy databases have not yet been extensively assessed or validated in the literature. Researchers planning studies of cross-sectional medication exposure should carefully weigh the tradeoffs of aforementioned study design choices and must clearly define the approaches used in final research products. Rigorous validation studies will be essential to establish best practices and improve the accuracy of measurement of active medication use.
Supplementary Material
KEY POINTS.
Use of pharmacy databases to examine patients’ medication use at specific time points is increasing, but few prior studies have systematically examined the validity of pharmacy database methods for establishing cross-sectional active medication lists.
Pharmacy database algorithm sensitivity ranged from 48% to 93% for fixed look-back period approaches and 35% to 97% for medication-on-hand approaches.
Interpretation of reported performance was limited by use of different nonpharmacy comparison metrics and lack of comparison of different pharmacy database approaches within study cohorts.
There remains an urgent need for rigorous validation studies comparing pharmacy database approaches within and across study cohorts to establish best practices and improve the accuracy of measurement of cross-sectional active medication use.
ACKNOWLEDGEMENTS
The authors would like to thank Bocheng Jing, Charlie M. Wray, Sarah Ngo, Kathy Fung, and John W. Boscardin from the San Francisco VA Medical Center and Michael A. Kelsh from Amgen Inc. for providing valuable feedback on the conceptualization of this manuscript.
FUNDING/SUPPORT
Dr. Steinman was supported by grants from the National Institute on Aging (K24AG049057 and P30AG044281). Dr. Anderson was supported by a National Research Service Award training grant (NRSA T32HP19025-14-00) to the University of California San Francisco. All other authors report receiving no external funding.
Funding information
NIH, Grant/Award Number: T32HP19025-14-00; National Institute on Aging, Grant/Award Numbers: K24AG049057 and P30AG044281
ROLE OF THE FUNDER/SPONSOR
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors state that no ethical approval was needed.
DISCLAIMER
The views expressed herein are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the University of California, San Francisco.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Christensen DB, Williams B, Goldberg HI, Martin DP, Engelberg R, LaGerfo JP. Assessing compliance to antihypertensive medications using computer-based pharmacy records. Med Care. 1997;35(11):1164–1170. [DOI] [PubMed] [Google Scholar]
- 2.Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med. 2015;175(12):1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lund BC, Schroeder MC, Middendorff G, Brooks JM. Effect of hospitalization on inappropriate prescribing in elderly Medicare beneficiaries. J Am Geriatr Soc. 2015;63(4):699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7-8):1280–1288. [DOI] [PubMed] [Google Scholar]
- 5.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574. [DOI] [PubMed] [Google Scholar]
- 6.Sattler EL, Lee JS, Perri M. Medication (re) fill adherence measures derived from pharmacy claims data in older Americans: a review of the literature. Drugs Aging. 2013;30(6):383–399. [DOI] [PubMed] [Google Scholar]
- 7.Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51(803):S11–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goedken AM, Lund BC, Cook EA, Schroeder MC, Brooks JM. Application of a framework for determining number of drugs. BMC Res Notes. 2016;9(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213–220. [DOI] [PubMed] [Google Scholar]
- 10.Byrt T, Bishop J, Carlin JB. Bias, prevalence, and kappa. J Clin Epidemiol. 1993;46(5):423–429. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RE, Vollmer WM. Comparing sources of drug data about the elderly. J Am Geriatr Soc. 1991;39(11):1079–1084. [DOI] [PubMed] [Google Scholar]
- 12.Heerdink ER, Leufkens HG, Koppedraaijer C, Bakker A. Information on drug use in the elderly: a comparison of pharmacy, general-practitioner and patient data. Pharm World Sci. 1995;17(1):20–24. [DOI] [PubMed] [Google Scholar]
- 13.Lau HS,de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–625. [DOI] [PubMed] [Google Scholar]
- 14.Sjahid SI, van der Linden PD, Stricker BH. Agreement between the pharmacy medication history and patient interview for cardiovascular drugs: the Rotterdam elderly study. Br J Clin Pharmacol. 1998;45(6):591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klungel OH, de Boer A, Paes AH, Herings RM, Seidell JC, Bakker A. Agreement between self-reported antihypertensive drug use and pharmacy records in a population-based study in the Netherlands. Pharm World Sci. 1999;21(5):217–220. [DOI] [PubMed] [Google Scholar]
- 16.Lau HS, Florax C, Porsius AJ, De Boer A. The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. Br J Clin Pharmacol. 2000;49(6):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King MA, Purdie DM, Roberts MS. Matching prescription claims with medication data for nursing home residents: implications for prescriber feedback, drug utilisation studies and selection of prescription claims database. J Clin Epidemiol. 2001;54(2):202–209. [DOI] [PubMed] [Google Scholar]
- 18.Monster TB, Janssen WM, de Jong PE. de Jong-van den berg LT. Pharmacy data in epidemiological studies: an easy to obtain and reliable tool. Pharmacoepidemiol Drug Saf. 2002;11(5):379–384. [DOI] [PubMed] [Google Scholar]
- 19.Kwon A, Bungay KM, Pei Y, et al. Antidepressant use: concordance between self-report and claims records. Med Care. 2003;41(3):368–374. [DOI] [PubMed] [Google Scholar]
- 20.Lund BC, Chrischilles EA, Carter BL, Ernst ME, Perry PJ. Development of a computer algorithm for defining an active drug list using an automated pharmacy database. J Clin Epidemiol. 2003;56(8):802–806. [DOI] [PubMed] [Google Scholar]
- 21.Boudreau DM, Doescher MP, Saver BG, Jackson JE, Fishman PA. Reliability of Group Health Cooperative automated pharmacy data by drug benefit status. Pharmacoepidemiol Drug Saf. 2005;14(12):877–884. [DOI] [PubMed] [Google Scholar]
- 22.Caskie GI, Willis SL, Warner Schaie K, Zanjani FA. Congruence of medication information from a brown bag data collection and pharmacy records: findings from the Seattle longitudinal study. Exp Aging Res. 2006;32(1):79–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis JR, Westfall AO, Allison J, Freeman A, Kovac SH, Saag KG. Agreement and validity of pharmacy data versus self-report for use of osteoporosis medications among chronic glucocorticoid users. Pharmacoepidemiol Drug Saf. 2006;15(10):710–718. [DOI] [PubMed] [Google Scholar]
- 24.Brown DW, Anda RF, Felitti VJ. Self-reported information and pharmacy claims were comparable for lipid-lowering medication exposure. J Clin Epidemiol. 2007;60(5):525–529. [DOI] [PubMed] [Google Scholar]
- 25.Glintborg B, Hillestrøm PR, Olsen LH, Dalhoff KP, Poulsen HE. Are patients reliable when self-reporting medication use? Validation of structured drug interviews and home visits by drug analysis and prescription data in acutely hospitalized patients. J Clin Pharmacol. 2007;47(11):1440–1449. [DOI] [PubMed] [Google Scholar]
- 26.Haukka J, Suvisaari J, Tuulio-Henriksson A, Lönnqvist J. High concordance between self-reported medication and official prescription database information. Eur J Clin Pharmacol. 2007;63(11):1069–1074. [DOI] [PubMed] [Google Scholar]
- 27.Shalansky S, Jang L, Ignaszewski A, Clark C, Jung L, Marra C. Accuracy of a prescription claims database for medication reconciliation for out-patients with heart failure. Can J Hosp Pharm. 2007;60(3):169–176. [Google Scholar]
- 28.Nielsen MW, Saødergaard B, Kjøller M, Hansen EH. Agreement between self-reported data on medicine use and prescription records vary according to method of analysis and therapeutic group. J Clin Epidemiol. 2008;61(9):919–924. [DOI] [PubMed] [Google Scholar]
- 29.Pit SW, Byles JE, Cockburn J. Accuracy of telephone self-report of drug use in older people and agreement with pharmaceutical claims data. Drugs Aging. 2008;25(1):71–80. [DOI] [PubMed] [Google Scholar]
- 30.Noize P, Bazin F, Dufouil C, et al. Comparison of health insurance claims and patient interviews in assessing drug use: data from the Three-City (3C) study. Pharmacoepidemiol Drug Saf. 2009;18(4): 310–319. [DOI] [PubMed] [Google Scholar]
- 31.Warholak TL, McCulloch M, Baumgart A, Smith M, Fink W, Fritz W. An exploratory comparison of medication lists at hospital admission with administrative database records. J Manag Care Pharm. 2009;15(9):751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haapea M, Miettunen J, Lindeman S, Joukamaa M, Koponen H. Agreement between self-reported and pharmacy data on medication use in the northern Finland 1966 birth cohort. Int J Methods Psychiatr Res. 2010;19(2):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rikala M, Hartikainen S, Sulkava R, Korhonen MJ. Validity of the Finnish prescription register for measuring psychotropic drug exposures among elderly finns: a population-based intervention study. Drugs Aging. 2010;27(4):337–349. [DOI] [PubMed] [Google Scholar]
- 34.Allin S, Bayoumi AM, Law MR, Laporte A. Comparability of self-reported medication use and pharmacy claims data. Health Rep. 2013;24(1):3–9. [PubMed] [Google Scholar]
- 35.Richardson K, Kenny RA, Peklar J, Bennett K. Agreement between patient interview data on prescription medication use and pharmacy records in those aged older than 50 years varied by therapeutic group and reporting of indicated health conditions. J Clin Epidemiol. 2013;66(11):1308–1316. [DOI] [PubMed] [Google Scholar]
- 36.Colantonio LD, Kent ST, Kilgore ML, et al. Agreement between Medicare pharmacy claims, self-report, and medication inventory for assessing lipid-lowering medication use. Pharmacoepidemiol Drug Saf. 2016;25(7):827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujita M, Sato Y, Nagashima K, Takahashi S, Hata A. Validity assessment of self-reported medication use by comparing to pharmacy insurance claims. BMJ Open. 2015;5(11):e009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drieling RL, LaCroix AZ, Beresford SA, Boudreau DM, Kooperberg C, Heckbert SR. Validity of self-reported medication use compared with pharmacy records in a cohort of older women: findings from the Women's Health Initiative. Am J Epidemiol. 2016;184(3):233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacasse A, Ware MA, Bourgault P, et al. Accuracy of self-reported prescribed analgesic medication use: linkage between the Quebec pain registry and the Quebec administrative prescription claims databases. Clin J Pain. 2016;32(2):95–102. [DOI] [PubMed] [Google Scholar]
- 40.Taipale H, Tanskanen A, Koponen M, Tolppanen AM, Tiihonen J, Hartikainen S. Agreement between PRE2DUP register data modeling method and comprehensive drug use interview among older persons. Clin Epidemiol. 2016;8:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray WA, Griffin MR, Downy W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262(23):3303–3307. [PubMed] [Google Scholar]
- 42.Bolton JM, Metge C, Lix L, Prior H, Sareen J, Leslie WD. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384–391. [DOI] [PubMed] [Google Scholar]
- 43.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Zolpidem use and hip fractures in older people. J Am Geriatr Soc. 2001;49(12):1685–1690. [DOI] [PubMed] [Google Scholar]
- 44.Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15(7):457–464. [PMC free article] [PubMed] [Google Scholar]
- 45.Dong YH, Choudhry NK, Krumme A, et al. Impact of hospitalization on medication adherence estimation in claims data. J Clin Pharm Ther. 2017;42(3):318–328. [DOI] [PubMed] [Google Scholar]
- 46.Lauffenburger JC, Balasubramanian A, Farley JF, et al. Completeness of prescription information in US commercial claims databases. Pharmacoepidemiol Drug Saf. 2013;22(8):899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polinski JM, Schneeweiss S, Levin R, Shrank WH. Completeness of retail pharmacy claims data: implications for Pharmacoepidemiologic studies and pharmacy practice in elderly adults. Clin Ther. 2009;31(9):2048–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pauly NJ, Talbert JC, Brown J. Low-cost generic program use by Medicare beneficiaries:implications for medication exposure misclassification in administrative claims data. J Manag Care Spec Pharm. 2016;22(6):741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Stürmer M, Brookhart MA. Evidence of sample use among new users of statins: implications for Pharmacoepidemiology. Med Care. 2014;52(9):773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.