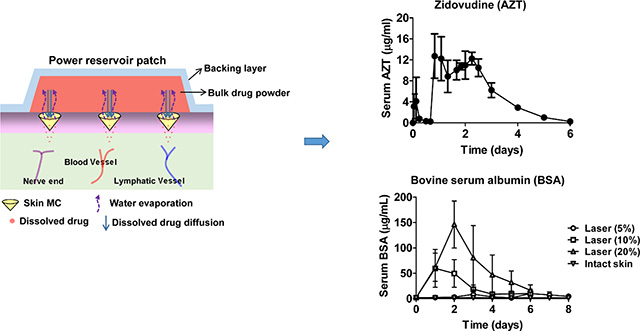
Abstract
Traditional patches are most successful in transdermal delivery of low-dose hydrophobic drugs. Week-long transdermal delivery of high-dose hydrophilic drugs remains a big challenge. This study explored ablative fractional laser (AFL) to assist 3-day to week-long sustained transdermal delivery of powder hydrophilic drugs in murine models. Bulk drug powder was coated into reservoir patches followed by topical application onto AFL-treated skin. Water evaporated from AFL-generated skin microchannels (MCs) gradually dissolve topical drug powder to elicit multi-day sustained drug delivery. Using sulforhodamine b, zidovudine, and bovine serum albumin as model hydrophilic drugs, we found tapped coating could coat 10–20 mg drug per 0.5 cm2 reservoir patch to elicit 3-day sustained delivery, while compression coating could coat ~35–70 mg drug per 0.5 cm2 reservoir patch to elicit week-long sustained delivery. Besides sustained drug delivery, AFL-assisted powder reservoir patch delivery also showed a good safety. AFL-generated skin MCs resealed in 1–2 days and completely recovered in 3 days after the week-long sustained delivery. AFL-assisted powder reservoir patch delivery involves no complex powder formulation and only requires incorporation of highly water-soluble mannitol or a similar excipient to elicit the high-efficient delivery. Enlarging reservoir patch size to 10 cm2 can conveniently expands the delivery capacity to gram scale. To our knowledge, this is the first time that high-dose week-long sustained transdermal delivery of hydrophilic drugs was achieved via a simple laser-based powder delivery platform.
Keywords: Transdermal, Powder, High-dose, Laser, Microchannel, Week-long
Graphical Abstract
Ablative fractional laser (AFL) enables high-dose week-long sustained delivery of powder hydrophilic drugs coated in reservoir patches.
Introduction
Transdermal delivery is highly attractive for alternative drug delivery and yet faces significant challenges due to the barrier function of the stratum corneum (SC) layer [1–3]. Only a small number of hydrophobic drugs with molecular weight ≤500 Da are able to penetrate the SC layer. The first transdermal patch (Scopolamine) was approved in 1979 and since then, only two dozen transdermal products have been approved for human use [1, 4]. Different strategies, like chemical enhancers, iontophoresis, and ultrasound, were explored to disrupt SC layer to facilitate transdermal drug delivery and yet received limited success due to their low efficiency in SC disruption or their safety concerns [1].
Laser is a monochromatic and coherent light with broad applications in surgery and medicine. Laser has also been explored to ablate SC layer to facilitate transdermal drug delivery by taking advantage of its photothermal and photomechanical effects [5, 6]. Different lasers, like excimer laser, Q-switched neodymium-doped yttrium aluminum garnet (Nd:YAG) laser, ruby laser, erbium:yttrium scandium gallium garnet (YSGG) laser, and CO2 laser, have been utilized to ablate full-surface SC layer to facilitate transdermal drug delivery [5–9]. The degree of SC ablation was highly correlated with laser energy [5–9]. Low laser energy led to inefficient SC ablation, while high laser energy caused overt skin reactions [10].
This dilemma was effectively addressed with the advent of ablative fractional laser (AFL). AFL was fabricated based on ‘fractional photothermolysis’ conceptualized for improved skin resurfacing in cosmetic field [11]. AFL emits high-energy micro-laser beams to ablate tiny skin tissues and generate skin microchannels (MCs). AFL-generated skin MCs are often tens to hundreds of micrometers in diameter and can span from skin surface to epidermal or dermal tissue depending on laser settings [12, 13]. Due to the sparing of the majority of the skin from laser ablation, AFL-treated skin can be quickly and completely recovered in days. Due to significantly improved safety, AFL has been actively explored to facilitate transdermal delivery of hydrophilic drugs. AFL was found to significantly enhance transdermal delivery of imiquimod, polypeptide, fluorescein isothiocyanate-labeled dextran, small interfering RNA, and plasmid DNA in mouse skin [14, 15]. AFL was also found to significantly enhance transdermal uptake of porphyrin precursors (methyl 5-aminolevulinate and 5’-aminolevulinic acid) and increase deep penetration of chemotherapy drugs (5-fluorouracil and cisplatin) in pig skin [16–19]. AFL was also utilized to enhance transdermal delivery of monoclonal antibodies, nanoparticles and microparticles [20–22]. Even living human cells could be efficiently delivered into AFL-treated epidermal and dermal tissues [23]. Besides preclinical evaluations, AFL was also explored to enhance topical delivery of corticosteroids and anesthetics in human subjects [24–27].
Most of the above studies focused on exploring AFL to assist short-term (≤24 hours) delivery of hydrophilic drugs across the skin. The potential of AFL to facilitate 3-day to week-long sustained delivery of hydrophilic drugs at high doses remains elusive. Recently, we explored AFL to facilitate 12-hour sustained transdermal delivery of hydrophilic drugs in a powder form [28]. Such a delivery took advantage of water evaporation from AFL-generated skin MCs to gradually dissolve topical drug powder coated in volumetric channels of thick patches to elicit ~12-hour sustained delivery [28]. Increasing powder coating amount may prolong the duration of drug delivery to multiple days. The current study coated bulk drug powder in cylindrical reservoir patches followed by topical application onto AFL-treated skin to elicit 3-day to week-long sustained drug delivery in murine models. We found tapped coating could coat 10–20 mg drug per 0.5 cm2 reservoir patch to elicit 3-day sustained delivery, while compression coating could coat ~35–70 mg drug per 0.5 cm2 reservoir patch to elicit week-long sustained delivery. We further found AFL-generated skin MCs could reseal in 1–2 days and completely recover in 3 days after the week-long sustained drug delivery, indicating the safety of AFL-assisted powder reservoir patch delivery.
Materials and Methods
Reagents
Sulforhodamine B (SRB, 230161), zidovudine (AZT, PHR1292), bovine serum albumin (BSA, A2733), and mannitol (M4125) were purchased from Sigma (St. Louis, MO). Texas Red (TR)-conjugated BSA (TR-BSA, A23017) was purchased from Thermo Fisher Scientific (Waltham, MA).
Laser device
Lumenis Ultrapulse ablative fractional CO2 laser at different energies (2.5, 5, and 10 mJ) and percent coverages (5, 10, and 20%) was used in this study to generate skin MCs to support transdermal powder drug delivery. AFL at 5, 10, and 20% coverage was able to generate 11×11, 15×15, and 21×21 arrays of skin MCs in 8×8 mm2 area, respectively.
Animals
BALB/c mice (female, 6–8 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). Mice were anesthetized by intraperitoneal injection of Ketamine (80 mg/kg) and Xylazine (10 mg/kg) for hair removal, AFL treatment, and patch application. All animal-related procedures were approved by the Institutional Animal Care and Use Committee of the University of Rhode Island.
Reservoir patch coating
Sterile cylindrical containers with 8 mm in diameter and 5 mm in depth were used as reservoir patches in this study. Drugs were mixed with highly water-soluble mannitol in water and then lyophilized for reservoir patch coating. For tapped coating, drug/mannitol powder was loaded into reservoir patches with gentle tapping. For compression coating, blank reservoir patches were laid on flat surface of cured polydimethylsiloxane (PDMS) (Sylgard 184) in ultracentrifuge tubes (357007, Beckman Coulter Life Sciences) and an excessive amount of drug/mannitol powder was poured to cover the blank reservoir patches followed by centrifugation at 20,000 rpm for 90 minutes in Beckman Ultracentrifuge (Avanti J-E series). Powder above the surface of the reservoir patches was removed by a scalpel. Powder reservoir patches were kept in a desiccator before use.
In vitro Franz Cell delivery
Franz Cells with an orifice diameter of 5 mm and recipient chamber volume of 1.5 ml were custom-made by PermeGear (Hellertown, PA). AFL-treated skin or non-treated intact skin was dissected and mounted on recipient chambers. Powder reservoir patches were topically applied and further assembled with blank donor chambers to form Franz Cell systems. Phosphate-buffered saline (PBS, 1.5 ml) was added to recipient chambers and bubbles under the skin were carefully removed. Franz Cell systems were put on a temperature-controlled magnetic stirrer to maintain PBS temperature at ~34°C, similar to skin surface temperature of live mice [29]. A small volume of PBS (100 μl) was collected from the recipient chamber daily for 7 days and 100 μl fresh PBS was added back to maintain PBS volume the same during the entire study period.
In vivo delivery
Hair on the lateral back skin of BALB/c mice was removed one day before experiment by clipping and application of hair-removal lotion. Hair-removed lateral back skin of 8×8 mm2 area was exposed to AFL at indicated settings to generate skin MCs. Powder reservoir patches were topically applied and further sealed with Tegaderm film to form an air-tight system. A narrow bandage was used to keep the entire system in position.
SRB extraction and quantification
Patch-coated SRB before delivery and patch-remaining SRB after delivery was extracted into PBS. SRB remaining on skin surface was harvested by washing skin surface with 50–100 μl PBS for 3 times. SRB amounts in PBS extracts, PBS washes, and PBS samples in Franz Cell studies were quantified by measurement of fluorescence intensity at 565/586nm. Serum SRB levels were measured similarly as in our previous report [28]. In brief, blood (~25 μl) was collected into heparin-containing tubes and immediately centrifuged. Supernatants (serum) were collected and serum SRB levels were quantified by measurement of fluorescence intensity at 565/585nm after more than a 20-fold dilution in PBS.
AZT extraction and quantification
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to quantify AZT levels as in our previous report [28]. In brief, patch extracts, skin surface washes, and AZT standards (5, 20, 50, 100, 200, 400 ng/ml) were mixed with 100 ng/ml AZT-internal standard (AZT-IS, 3’-azido-3’-deoxythymidine, MG103, Moravek Biochemicals, Brea, CA). A small amount of blood (~25 μl) was collected before delivery and at different times after delivery to measure serum AZT levels. Serum samples were diluted 60 times with PBS, mixed with 100 ng/ml AZT-IS, filtered through 10 kDa cutoff Amicon filter. The different samples were loaded into an AB Sciex 4500 QTRAP LC-MS/MS equipped with Shimadzu LC-20AD pumps and a QTRAP 4500 System. A Synergi Hydro-RP 80A, 2.0×150 mm, 4μm particle size analytical column (Phenomonex, Torrance, CA) was used for sample separation. Acquisition was performed in multiple reaction monitoring (MRM) mode using m/z 268/127 for AZT detection and 271/130 for AZT-IS detection. A standard curve of peak area ratios of AZT to AZT-IS against AZT concentrations was generated to quantify AZT levels in unknown samples.
BSA extraction and quantification
A small amount of blood (~25 μl) was collected daily for 6–8 days in AFL-assisted powder BSA delivery. Serum samples were diluted 1,000 times with PBS for measurement of serum BSA levels with a commercial ELISA kit (F030, Cygnus Technologies). For accurate quantification of BSA amount in recipient chambers in Franz Cell studies, 0.5 mg TR-BSA was mixed with 1.42 g BSA and TR-BSA/BSA mixture was then mixed with mannitol at 1:2.5 weight ratio followed by lyophilization and compression patch coating. Fluorescence intensity of TR-BSA in unknown samples was read at 596/615nm together with a 2-fold serial dilution of above prepared TR-BSA-admixed BSA/mannitol samples. A standard curve was generated by plotting fluorescence intensities of TR-BSA against BSA concentrations in the 2-fold serial diluted samples, by which BSA amounts in unknown samples were calculated.
Delivery efficiency
Delivery efficiency of AFL-assisted powder reservoir patch delivery was quantified using the following formula: (patch coating amount - patch remaining amount - skin surface amount)/patch coating amount × 100%.
Transepidermal water loss (TEWL)
TEWL of mouse skin was measured by Tewameter TM300 (Courage-Khazaka, Köln, Germany) equipped with an open chamber probe with the probe head 1 cm in diameter.
Oral gavage
Oral gavage was performed as in our previous report [28]. In brief, a sterile plastic mouse-specific feeding tube (Cadence Science, Inc.) was inserted and advanced to the stomach when mice were restrained. AZT solutions were then administered followed by gentle retraction of the plastic feeding tubes.
Histological analysis
Skin was dissected at indicated times and fixed in formalin, embedded in paraffin, and sectioned at 5 μm thickness followed by hematoxylin and eosin (H & E) staining. Images of H & E-stained sections were taken under Nikon Eclipse E600 microscope.
Statistical analysis
All values were expressed as Mean ± SD (standard deviations). Student’s t-test was used to compare differences between groups. One-way ANOVA with Bonferroni’s multiple comparison test was used to compare differences for more than 2 groups. P value was calculated by PRISM software (GraphPad, San Diego, CA) and considered significant if it was less than 0.05.
Results
AFL-assisted powder reservoir patch delivery of SRB
Reservoir patches significantly increased powder coating amount. Due to the slow water evaporation from AFL-generated skin MCs, it remained to be explored whether the bulk amount of drug powder coated in reservoir patches could be fully dissolved to elicit efficient delivery. Using hydrophilic SRB as a fluorescence model drug, we conducted a pilot study and found pure SRB power coated in reservoir patches could not be completely dissolved by evaporated water from AFL-generated skin MCs both in vitro and in vivo (data not shown). Based on our previous experience [28], highly water-soluble mannitol was mixed with SRB at 1:1 weight ratio with a promise to increase powder SRB delivery. We found ~40 mg SRB/mannitol powder containing ~20 mg SRB could be coated per reservoir patch with 0.5 cm2 surface area. AFL-assisted powder reservoir patch delivery of SRB in the presence of mannitol was first explored in Franz Cell systems. Skin of BALB/c mice was treated with AFL at low (2.5 mJ/5%), medium (5 mJ/10%), or high laser setting (10 mJ/20%). AFL-treated skin or non-treated intact skin was dissected and mounted on recipient chambers of Franz Cell systems followed by topical application of powder SRB/mannitol (1:1)-coated reservoir patches (hereafter powder SRB-coated reservoir patches), as shown in Fig.1A. SRB levels in recipient chambers were measured daily for 7 days.
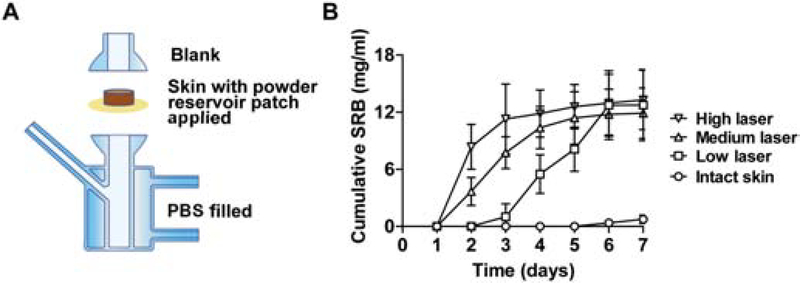
Fig. 1. AFL-assisted week-long sustained SRB delivery in vitro.
A. Illustration of Franz Cell systems to explore AFL-assisted powder reservoir patch delivery. AFL-treated or intact skin was mounted on recipient chambers followed by topical application of powder drug-coated reservoir patches. Donor chambers were then put on top to complete Franz Cell assembly. Recipient chambers were filled with 1.5 mL PBS and donor chambers were left blank. B. Lateral back skin of BALB/c mice was exposed to AFL at low, medium, or high laser setting, or left non-treated. Skin was dissected and mounted on recipient chambers of Franz Cell systems followed by topical application of powder SRB-coated reservoir patches. Cumulative SRB delivery in recipient chambers was explored daily for 7 days. n=6
No significant SRB delivery was observed on day 1 (Fig.1B). SRB levels quickly increased from day 1 to 3 in high laser group and then slowly increased afterwards (Fig.1B). SRB levels quickly increased from day 1 to 4 in medium laser group and then slowly increased afterwards (Fig.1B). SRB levels remained at low levels in the first 2 days in low laser group, slowly increased from day 2 to 3, quickly increased from day 3 to 6, and plateaued on day 7 (Fig.1B). The above data indicated a positive correlation between laser intensities and SRB delivery rates. SRB levels reached 13.3, 11.9, and 12.7 mg/mL on day 7 in high, medium, and low laser groups, respectively (Fig.1B). Considering ~20 mg SRB was coated per reservoir patch and 1.5 mL PBS was added to recipient chambers, the overall SRB delivery efficiency was estimated to be at least 90% for all laser conditions. In comparison, SRB levels remained below 0.8 mg/mL in intact skin group (Fig.1B), indicating no significant SRB delivery across intact skin. At the end of delivery, no SRB powder could be found in laser groups, while the majority of SRB powder remained in intact skin group. The lag time of one day in medium and high laser groups and 2 days in low laser group is unique to AFL-assisted powder reservoir patch delivery considering the bulk amount of drug powder needs to be first dissolved to elicit efficient delivery across AFL-treated skin. The Franz Cell study indicated AFL-assisted powder reservoir patch delivery could elicit week-long sustained SRB delivery in vitro.
Next, we explored AFL-assisted powder reservoir patch delivery of SRB in vivo. Lateral back skin of BALB/c mice was treated with low, medium, or high laser, or left non-treated followed by topical application of powder SRB-coated reservoir patches. Patches were removed daily for 3 days to measure the delivery efficiency. As shown in Fig.2A, ~67%, 51%, and 25% SRB was delivery at 24 hours in high, medium, and low laser groups, respectively. Delivery efficiency increased to ~92%, 75%, and 54% at 48 hours in high, medium, and low laser groups, respectively (Fig.2A). Delivery efficiency reached more than 95% in high and medium laser groups and more than 85% in low laser group at 72 hours (Fig.2A). In comparison, less than 1% SRB was delivered in intact skin group and the majority of SRB remained as a powder form at each time point (Fig.2A). The above study indicated AFL could assist 3-day sustained powder SRB delivery in live mice.
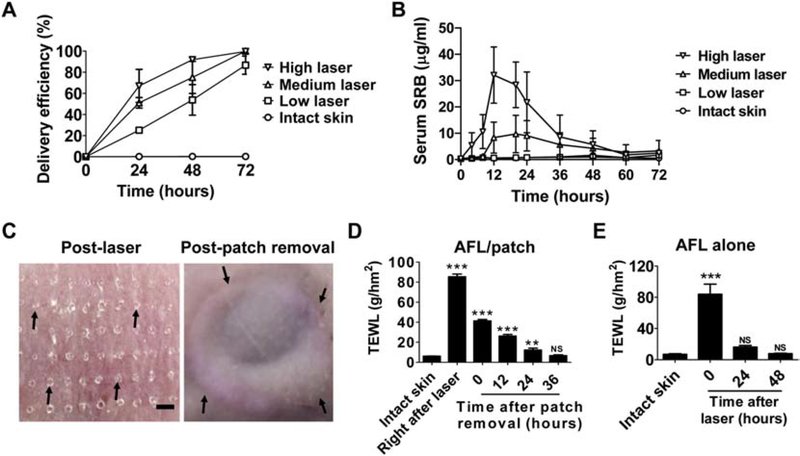
Fig. 2. AFL-assisted 3-day sustained SRB delivery in vivo.
A. Powder SRB-coated reservoir patches were topically applied onto AFL-treated or intact skin of BALB/c mice. Patches were removed daily for 3 days to evaluate the delivery efficiency. n=4–5. B. Powder SRB-coated reservoir patches were topically applied onto AFL-treated skin or intact skin of BALB/c mice followed by patch removal 3 days later. A small amount of blood was collected at different time points (3, 6, 9, 12, 16, 20, 26, 32, 36, 42, 48, 54, 60 and 72 hours) to quantify serum SRB levels. C. Lateral back skin of BALB/c mice was exposed to AFL (2.5 mJ/5%) followed by topical application of powder SRB-coated reservoir patches. Patches were removed 3 days later. Skin pictures were taken right after AFL treatment (left) and patch removal (right). Arrows in the left panel pointed to AFL-generated skin MCs. Arrows in the right panel pointed to the boundaries of reservoir patch application. Scale: 667 μm. D. Lateral back skin of BALB/c mice was exposed to AFL (2.5 mJ/5%) followed by topical application of powder SRB-coated reservoir patches as in C. Patches were removed 3 days later. TEWL was measured before and right after AFL treatment, right after and at different time points after patch removal. E. Lateral back skin of BALB/c mice was exposed to AFL (2.5 mJ/5%). TEWL was measured before AFL treatment or at different time points after AFL treatment. n=4–5. One-way ANOVA with Bonferroni’s multiple comparison test was used to compare differences between groups in D and E. **, p<0.01; ***, p<0.001. NS: not significant.
Systemic SRB delivery was further explored in AFL-assisted 3-day sustained powder delivery. As shown in Fig.2B, serum SRB quickly increased and peaked at ~32 μg/mL at 12 hours in high laser group and then gradually reduced to below 3 μg/mL at 72 hours (Fig.2B). Serum SRB remained at relatively low levels in the first 8 hours in medium laser group, quickly increased to peak levels (8–10 μg/mL) between 12–24 hours, and gradually reduced to below 4 μg/mL at 72 hours (Fig.2B). Serum SRB levels remained below 2 μg/mL in low laser and intact skin groups in entire delivery period (Fig.2B). Area-under-the Curve (AUC) was calculated to be 810, 361, and 61 μg·hr/ml in high, medium, and low laser groups, respectively. Lack of significant increase of serum SRB in low laser group is expected to be a net result of slow SRB delivery (Fig.1B) and quick SRB clearance. According to one report [30], half SRB could be cleared from peripheral blood in minutes. The above results indicated the positive correlation between serum SRB levels and laser intensities in AFL-assisted powder reservoir patch delivery. The above results also indicated more rapid SRB delivery in live mice than in Franz Cell systems.
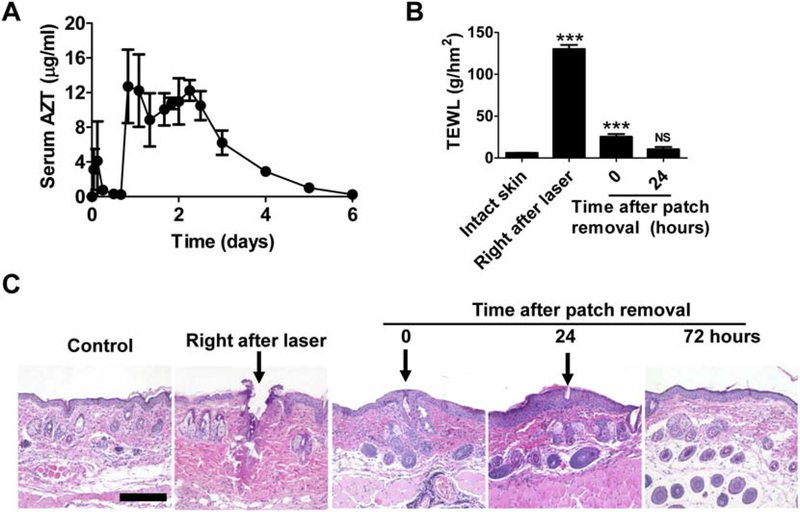
We also explored local safety and MC resealing kinetics in AFL-assisted powder SRB delivery at low laser condition. Skin MCs were clearly visible right after AFL treatment (left, Fig.2C) and invisible at the end of 3-day delivery (right, Fig.2C). No significant skin reactions were found except clear signs of the boundaries of reservoir patch application (right, Fig.2C). Kinetics of skin MC resealing were studied by measurement of TEWL of AFL-treated skin at different time points. As shown in Fig.2D, AFL treatment (2.5 mJ/5%) instantly increased TEWL of the skin from baseline (~6 g/hm2) to ~85 g/hm2. TEWL of AFL-treated skin reduced to ~40 g/hm2 measured shortly after patch removal 3 days later, hinting partial resealing of skin MCs. TEWL of AFL-treated skin returned to baseline levels 36 hours after patch removal, hinting complete resealing of skin MCs (Fig.2D). Quick resealing of skin MCs was expected to reduce infection risks of environmental pathogens. To explore impacts of reservoir patch application on skin MC resealing, we measured TEWL of AFL-treated skin at different time points in the absence of reservoir patch application. We found TEWL of AFL-treated skin returned to baseline levels in 1–2 days (Fig.2E). The above results indicated reservoir patch application could delay skin MC resealing. We further found blank patch application could also delay skin MC resealing but was less effective than powder patch application (data not shown).
Dynamics of powder dissolution
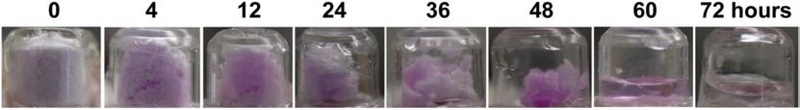
Dynamics of powder dissolution were then explored after topical application of powder SRB-coated reservoir patches onto AFL (2.5 mJ/5%)-treated skin of BALB/c mice. SRB is a black powder and turns into a strong pink color after wetting or dissolution. Here, a trace amount of SRB was mixed with bulk amount of mannitol for sensitive detection of powder dissolution and delivery. As shown in Fig.3, SRB/mannitol powder mainly showed white mannitol color at the beginning of the delivery due to the dominant mannitol content. SRB/mannitol powder turned into light pink at 4 hours, indicating absorption of evaporated water (Fig.3). Entire powder turned into pink-colored wet mass at 12 hours due to continuous absorption of evaporated water and increased water content (Fig.3). Wet mass gradually shrank and turned into a pink-colored liquid at 60 hours and a clear liquid with little or no pink color at 72 hours (Fig.3). This study clearly indicated the gradual physical change of powder during the 3-day in vivo delivery from the initial dry powder, to powder with increased water content, to a wet mass, and lastly to a liquid form. This study also indicated significant drug delivery occurred when drug powder was converted to a wet mass before transition to a liquid form.
Fig. 3. Dynamics of powder dissolution and delivery in AFL-assisted powder reservoir patch delivery in vivo.
Powder SRB/mannitol (1:50,000)-coated reservoir patches were topically applied onto AFL (2.5 mJ/5%)-treated skin of BALB/c mice. Patch images were taken at indicated times and representative patch images were shown.
AFL-assisted 3-day sustained AZT delivery
Using model drug SRB, we found AFL could assist 3-day sustained SRB delivery. Next, we explored whether the same delivery platform could be used for 3-day sustained AZT delivery. AZT is a nucleoside reverse transcriptase inhibitor and was the first antiretroviral drug approved to treat human immunodeficiency virus (HIV) [31]. Although more than 2 dozen antiretroviral drugs have been approved to treat HIV ever since, poor medication adherence was identified as a major barrier in antiretroviral therapy [32]. Sustained delivery technologies are highly demanded to reduce dosing frequency and improve the efficacy of antiretroviral therapy. To explore whether AFL could assist 3-day sustained AZT delivery, AZT was mixed with mannitol at 1:1 and also 1:2.5 and 1:5 weight ratios to prepare lyophilized powder for reservoir patch coating considering AZT had less water solubility than SRB (20 vs. 100 mg/ml). We found 20.3, 11.4, and 6.6 mg AZT could be coated per reservoir patch at 1:1, 1:2.5, and 1:5 mixing ratios, respectively (Table 1). Patches were topically applied onto AFL (2.5 mJ/5%)-treated skin of BALB/c mice and removed 3 days later. We found 51% AZT at 1:1 mixing ratio and more than 90% AZT at 1:2.5 and 1:5 mixing ratios were delivered in 3 days (Table 1). These results indicated AZT needed to be mixed with at least 2.5 times more mannitol to elicit efficient delivery across AFL (2.5 mJ/5%)-treated skin.
Table 1.
Pharmacokinetics of AFL-assisted powder AZT delivery under tapped coating
| AZT (mg) | Delivery efficiency (%) | Cmax (μg/ml) | Tmax (hours) | AUC (μg·hr/ml) | Relative bioavailability* | |
|---|---|---|---|---|---|---|
| AFL (1:1) | 20.3±0.7 | 51±12 | 10.0±2.2 | 20 | 265 | 49% |
| AFL (1:2.5) | 11.4±0.4 | 93±3 | 9.8±2.6 | 26 | 249 | 80% |
| AFL (1:5) | 6.6±0.2 | 90±10 | 6.8±3.9 | 26 | 131 | 72% |
| Oral | 0.32 | 100 | 7.0±0.8 | 0.5 | 8.7 | 100% |
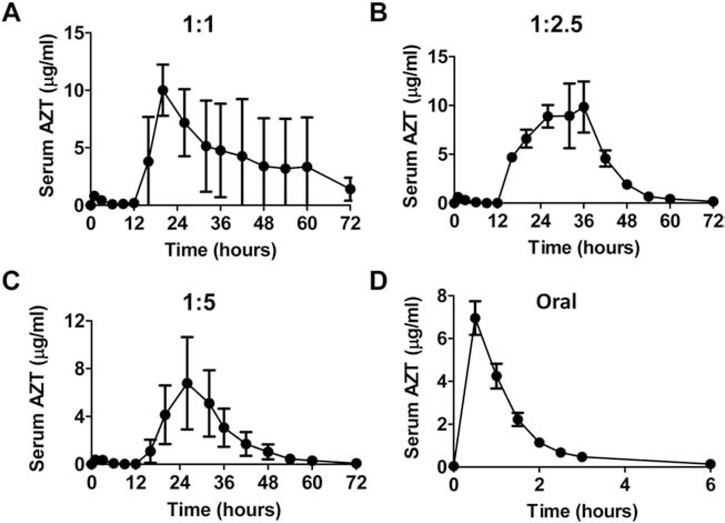
Serum AZT levels were also measured in above studies. As shown in Fig.4A–C, dual-phase delivery was observed with the first-phase delivery started right after patch application and the second-phase delivery started 12 hours after patch application. At 1:1 mixing ratio with 51% delivery efficiency, big variations of serum AZT levels were observed in the second-phase delivery (Fig.4A). At 1:2.5 and 1:5 mixing ratios with more than 90% delivery efficiency, less variability of serum AZT levels was observed in the second-phase delivery (Fig.4B & C). By comparing AUC of the first-phase and second-phase deliveries at 1:2.5 and 1:5 mixing ratios, we found less than 2% AZT was delivered in the first phase and more than 98% AZT was delivered in the second phase. Serum AZT levels peaked between 20–26 hours and then gradually reduced to baseline levels at 72 hours (Fig.4B & C). Powder AZT-coated reservoir patches were also topically applied on intact skin surface and the majority of AZT powder remained at the end of the 3-day delivery, indicating no significant SRB delivery in the absence of AFL treatment (data not shown). We also conducted oral AZT delivery to compare with the above AFL-assisted powder reservoir patch delivery. As shown in Fig.4D, oral delivery only lasted for 6 hours and serum AZT levels peaked at 0.5 hour. AUC was then compared among the different deliveries. As shown in Table 1, AUC of AFL-assisted powder AZT delivery at 1:1, 1:2.5, and 1:5 mixing ratios was ~265, 249 and 131 μg·hr/ml, respectively, and AUC of oral AZT delivery was ~8.7 μg·hr/ml. Relative bioavailability of AFL-assisted powder AZT delivery at 1:1, 1:2.5, and 1:5 mixing ratios to oral delivery was 49, 80, and 72%, respectively (Table 1). These data indicated a maximum of 11.4 mg AZT could be coated per reservoir patch to elicit 3-day sustained delivery across AFL-treated skin.
Fig. 4. AFL-assisted 3-day sustained AZT delivery in vivo.
A-C. Lateral back skin of BALB/c mice was exposed to AFL (2.5 mJ5/%) followed by topical application of powder AZT-coated reservoir patches at 1:1 (A), 1:2.5 (B), or 1:5 mixing ratio (C). A small amount of blood (~25 μl) was collected before delivery and 1, 3, 6, 9, 12, 16, 20, 26, 32, 36, 42, 48, 54, 60, and 72 hours after delivery to measure serum AZT levels. D. BALB/c mice were subjected to oral delivery of 0.32 mg AZT. A small amount of blood (~25 μl) was collected before delivery and 0.5, 1, 1.5, 2, 2.5, 3 and 6 hours after delivery to measure serum AZT levels. n=6
AFL-assisted week-long sustained AZT delivery
After achieving 3-day sustained AZT delivery, we explored whether AFL could support week-long sustained AZT delivery. To this end, centrifugal forces were applied to increase powder coating amount with a promise to extend drug release beyond 3 days. AZT was mixed with mannitol at 1:2.5 weight ratio for powder preparation and compression coating. We found ~67.5 mg AZT could be coated per reservoir patch under compression coating, corresponding to a 6-fold increase of coating amount as compared to tapped coating. Lateral back skin of mice was exposed to AFL (10 mJ/5%) followed by topical application of compression-coated AZT patches. Here, 10 mJ laser energy was used to generate bigger skin MCs that were more promising to elicit efficient delivery under compression coating than the previously explored 2.5 mJ energy. As shown in Fig.5A, dual-phase 6-day sustained AZT delivery was observed. The first-phase delivery started right after patch application and lasted for 16 hours (Fig.5A). The second-phase delivery started 16 hours after patch application and lasted for ~5 days (Fig.5A). Around 2.5% AZT was delivered in the first phase and more than 97% AZT was delivered in the second phase (Fig.5A). As compared to tapped coating, compression coating slightly extended the first-phase delivery and more significantly extended the second-phase delivery. Serum AZT levels peaked 20 hours after delivery and were maintained at peak levels till 60 hours after delivery (Fig.5A). Serum AZT levels gradually reduced to baseline levels afterwards (Fig.5A). At the end of delivery, only a small volume of liquid was found in reservoir patches and the overall delivery efficiency reached more than 94%. This study support compression coating to increase powder coating amount and extend drug release to 6 days. In comparison, no significant delivery was observed when compression-coated powder AZT patches were topically applied on intact skin (data not shown).
Fig. 5. AFL-assisted 6-day sustained AZT delivery in vivo.
A. Lateral back skin of mice was exposed to AFL (10 mJ/5%) followed by topical application of compression-coated powder AZT patches. Patches were removed 6 days later. A small amount of blood (~25 μl) was collected before delivery and 1, 3, 6, 12, 16, 20, 26, 32, 40, 44, 48, 54, 60, 72, 96, 120, and 144 hours after delivery to measure serum AZT levels. B. BALB/c mice were subjected to AFL-assisted powder AZT delivery as in A. Patches were removed 7 days later. TEWL was measured before and right after AFL treatment, and right after and 24 hours after patch removal. C. BALB/c mice were subjected to AFL-assisted powder AZT delivery as in B. Skin was dissected at indicated time points followed by standard histological analysis. Representative images of H & E-stained skin sections were shown. Arrows pointed to AFL-generated skin MCs. Scale: 200 μm. n=4–5. One-way ANOVA with Bonferroni’s multiple comparison test was used to compare differences between groups in B. ***, p<0.001. NS: not significant.
We further explored whether AFL-generated skin MCs could remain open for 7 days to support week-long sustained delivery. Lateral back skin of mice was exposed to AFL (10 mJ/5%) followed by topical application of powder AZT-coated reservoir patches as in above studies. Patches were removed 7 days later and TEWL was monitored before and after AFL treatment, and right after and 24 hours after patch removal. As shown in Fig.5B, TEWL of the skin increased to ~130 g/hm2 right after AFL treatment and reduced to ~25 g/hm2 after patch removal 7 days later. TEWL of the skin returned to near baseline level 24 hours after patch removal (Fig.5B). Histological analysis was further conducted to explore skin MC resealing and microscopic skin recovery after the week-long sustained AZT delivery. Right after AFL (10 mJ/5%) treatment, skin MCs with ~100 μm in diameter were visible (Fig.5C). After week-long sustained AZT delivery, skin MCs partially resealed and re-epithelialization occurred around skin MCs (Fig.5C). Re-epithelialization almost completed 24 hours after patch removal and skin completely recovered 72 hours after patch removal (Fig.5C). These data indicated AFL-treated skin could be completely recovered in 3 days after AFL-assisted week-long sustained powder AZT delivery.
AFL-assisted week-long sustained BSA delivery
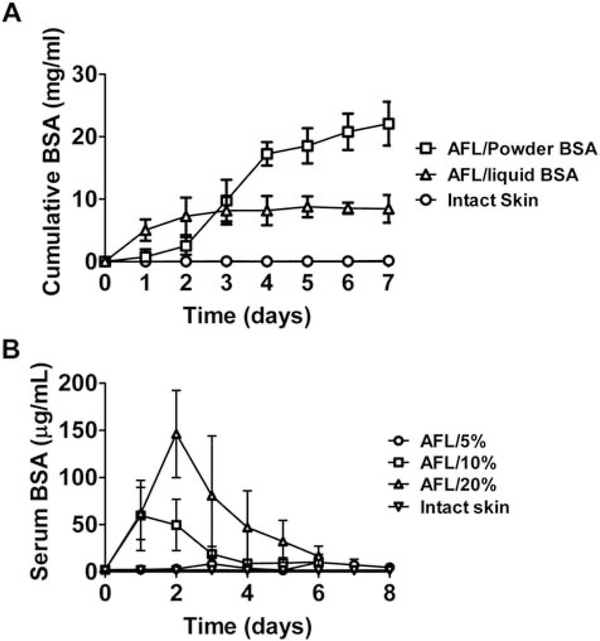
Besides small chemicals, we also explored whether macromolecules could be sustainably delivered via AFL-assisted powder reservoir patch delivery. BSA (~66 kDa) was used as a model drug to explore this first in Franz Cell systems. BSA was mixed with mannitol at 1:2.5 weight ratio and then lyophilized for compression coating. A trace amount of TR-BSA was added to BSA/mannitol mixture for accurate quantification of BSA amount without interference of proteins that might release from dissected skin during the 7-day incubation period. AFL (10 mJ/10%)-treated or intact skin was mounted on recipient chambers followed by topical application of powder BSA-coated reservoir patches that contained ~38 mg BSA per patch. Considering slow powder dissolution contributes to AFL-assisted multi-day sustained powder drug delivery, topical application of liquid drugs is expected to elicit more rapid delivery. To this end, the same amount of BSA/mannitol powder as coated per reservoir patch was dissolved in 1 mL PBS and then added to donor chambers to explore liquid BSA delivery across AFL (10 mJ/10%)-treated skin in Franz Cell systems. Cumulative BSA delivery in recipient chambers was monitored daily for 7 days. As shown in Fig.6A, BSA levels slowly increased in the first 2 days of AFL-assisted powder BSA delivery, quickly increased from day 2 to 4, and then slowly increased from day 4 to 7. In AFL-assisted liquid BSA delivery, BSA levels quickly increased in the first 2 days and then slowly increased from day 2 to 7 (Fig.6A). BSA levels reached 22.1 mg/mL at the end of AFL-assisted powder BSA delivery and 8.5 mg/mL at the end of AFL-assisted liquid BSA delivery (Fig.6A). Due to 1.5 mL PBS added to recipient chambers, the delivery efficiency was estimated to be more than 85% in AFL-assisted powder BSA delivery and ~34% in AFL-assisted liquid BSA delivery. Higher efficiency of AFL-assisted powder BSA delivery might be due to the steep concentration gradient established between reservoir patches and the skin when the majority of BSA powder was converted to a wet mass. In comparison, the same amount of BSA was dissolved in 1 mL PBS and added to donor chambers in AFL-assisted liquid BSA delivery, which was expected to generate less steep concentration gradient between donor chambers and the skin considering reservoir patches had a volume of only 0.25 mL and powder remained as a wet mass without complete dissolution during an extended period in AFL-assisted powder BSA delivery. As expected, no significant BSA delivery was observed in intact skin group (Fig.6A).
Fig. 6. AFL-assisted week-long sustained BSA delivery.
A. AFL (10 mJ/10%)-treated or intact skin of BALB/c mice was mounted on recipient chambers of Franz Cell systems followed by topical application of compression-coated powder BSA patches. For liquid BSA delivery, the same amount of BSA/mannitol powder as coated per reservoir patch was dissolved in 1 mL PBS and then added to donor chambers of Franz Cell systems. Cumulative SRB delivery in recipient chambers was monitored daily for 7 days. B. Lateral back skin of BALB/c mice was exposed to AFL at 10 mJ energy and different coverages (5, 10, and 20%) or left non-treated followed by topical application of compression-coated powder BSA patches. Serum BSA levels were quantified daily for 6 days in 10 and 20% laser groups and 8 days in 5% laser and intact skin groups. n=4–6.
Next, we explored AFL-assisted powder BSA delivery in vivo. AFL at 10 mJ energy and different percent coverages (5, 10, and 20%) was used to generate skin MCs to explore whether powder BSA delivery was affected by laser percent coverage. Compression-coated powder BSA patches were topically applied onto AFL-treated skin or intact skin surface. Serum BSA levels were quantified daily for 6–8 days. Serum BSA levels quickly increased and peaked at 146 μg/mL on day 2 in 20% laser group, and then slowly reduced to 16.6 μg/mL on day 6 (Fig.6B). Serum BSA levels quickly increased and peaked between 50–60 μg/mL in 2 days in 10% laser group, and then gradually reduced to 10.4 μg/mL on day 6 (Fig.6B). Serum BSA levels in 5% laser group were relatively low (≤10 μg/mL) in the entire delivery period (Fig.6B). Here, we also compared the relative rate of AFL-assisted powder BSA delivery in vivo and in vitro at the same laser condition (10 mJ/10%). We found the most rapid BSA delivery occurred in 2 days in vivo and between day 2–4 in vitro. This indicated more rapid AFL-assisted in vivo versus in vitro BSA delivery, as observed in SRB studies (Fig.1 & 2). Powder-liquid transition occurred between day 2–3 in 10 and 20% laser groups and between day 5–6 in 5% laser group. Delivery efficiency reached more than 85% in all laser groups at the end of the delivery. In comparison, no significant BSA delivery was observed in intact skin group and the majority of BSA remained as a powder form at the end of the delivery. When comparing serum BSA levels at the late stage of delivery, we found serum BSA levels in all laser groups were significantly higher than that in intact skin group on day 6 and serum BSA levels in 5% laser group were significantly higher than that in intact skin group on day 7 and 8 (Table 2). These data indicated AFL-assisted powder reservoir patch delivery could support at least week-long sustained BSA delivery in murine models. Interestingly, despite nearly complete BSA delivery in all laser groups, AUC showed a positive correlation to laser percent coverage: 41 μg∙day/mL in 5% laser group, 153 μg∙day/mL in 10% laser group, and 378 μg∙day/mL in 20% laser group.
Table 2.
Comparison of serum BSA levels at late stage of week-long delivery
| AFL/20% | AFL/10% | AFL/5% | Intact skin | p value (vs. Intact skin) |
|||
|---|---|---|---|---|---|---|---|
| AFL/20% | AFL/10% | AFL/5% | |||||
| Day 6 | 16.6±10.7 | 10.4±5.7 | 10.0±8.6 | 1.7±0.6 | 0.008 (**) | 0.006 (**) | 0.040 (*) |
| Day 7 | - | - | 7.4±6.0 | 1.7±0.7 | - | - | 0.042 (*) |
| Day 8 | - | - | 4.8±2.6 | 1.8±0.5 | - | - | 0.022 (*) |
Note:
p<0.05
p<0.01.
Discussion
This study explored AFL to assist 3-day to week-long sustained transdermal delivery of hydrophilic drugs in murine models. Such a delivery was based on AFL treatment to generate skin MCs followed by topical application of powder drug-coated reservoir patches to deliver drugs into the skin via skin MCs. Water evaporation from AFL-generated skin MCs played a crucial role in converting the bulk drug powder into a wet mass to elicit the multi-day sustained delivery. AFL-assisted powder reservoir patch delivery could efficiently deliver both small chemical drugs and macromolecules. This study found tapped coating could coat ~11 mg AZT and 20 mg SRB per reservoir patch to elicit 3-day sustained delivery (Fig.2 & 4), while compression coating could coat ~38 mg BSA and 68 mg AZT per reservoir patch to elicit 6–8-day sustained delivery (Fig.5 & 6). Considering reservoir patches used in our studies have a surface area of 0.5 cm2, compression coating achieved a coating capacity of 76 mg/m2 for BSA and 136 mg/m2 for AZT. High-dose transdermal delivery of hydrophilic drugs were also explored in two previous studies [33, 34]. In one study, ibuprofen sodium formulated with poly(methylvinyl ether/maleic acid) (PMVE/MA) polymer was loaded into the microneedle (MN) baseplate with an estimated coating capacity of ~74 mg/cm2 [33]. In the other study, lyophilized wafers were combined with hydrogel-forming MNs to deliver high-dose small chemical and macromolecular drugs across the skin with an estimated coating capacity of 53 mg/cm2 for ibuprofen sodium and 1.1 mg/cm2 for ovalbumin [34]. We achieved ~2 times higher coating and delivery capacity for small chemical drugs and ~70 times higher coating and delivery capacity for macromolecules in this study. Delivery capacity of AFL-assisted powder reservoir patch delivery can be further expanded by enlarging patch size. A reservoir patch size of 10 cm2, similar to traditional buprenorphine patch BuTrans® and estradiol patch Climara®, is expected to have a coating capacity of 220–400 mg with tapped coating and 760–1,360 mg with compression coating. The reservoir patches used in our studies have a depth of 5 mm, which are expected to have a good wearability in humans considering typical watches have a thickness of 7 or 9 mm. Increasing reservoir patch depth to 7 or 9 mm is expected to increase the delivery capacity to over 1 g under compression coating. The high-dose AFL-assisted powder reservoir patch delivery is expected to significantly increase the number of hydrophilic drugs that can be delivered via transdermal route.
AFL-assisted high-dose powder drug delivery is attributed to the following factors. Powder drugs can be directly coated and only one excipient (mannitol) is required to mix with hydrophilic drugs to elicit high-efficient delivery. Compression coating also significantly increases powder coating amount and extends drug release. In AFL-assisted powder AZT delivery, we found compression coating slightly extended the first-phase delivery and more significantly extended the second-phase delivery (Fig.5A). The reason of compression coating to extend drug release remains to be explored but may be related to longer time for evaporated water to convert compression-coated drug powder to a wet mass and longer time for more drugs under compression coating to diffuse into the skin via skin MCs. This study found highly water-soluble mannitol could facilitate AFL-assisted powder reservoir patch delivery. Mannitol most likely works to facilitate water absorption and transition of bulk drug powder to a wet mass. As indicated by the imaging study (Fig.3), significant drug delivery occurred when the bulk drug powder was converted to a wet mass. Mannitol is able to absorb large amounts of water and deliquesce after the flow moisture point was reached [35]. Thus, condensed water during the process of deliquescence is likely to facilitate dissolution of less water-soluble drugs to facilitate their transdermal delivery via skin MCs.
Dual-phase SRB and AZT delivery was observed following AFL-assisted powder reservoir patch delivery in vivo. The first-phase delivery started right after patch application and the second-phase delivery started 8–16 hours after patch application. The first-phase delivery was likely contributed by powder in close proximity to skin MCs, while the second-phase delivery was likely contributed by the remaining powder after conversion to a wet mass. AFL-assisted powder reservoir patch deliery of BSA also showed dual-phase delivery as indicated by the Franz Cell studies: slow BSA delivery in the first 2 days and fast BSA delivery between day 2 to 4 (Fig.6A). The lack of dual-phase delivery of BSA in vivo was most likely due to no blood collection in the first 24 hours to analyze serum BSA levels. AUC of AFL-assisted powder SRB and BSA delivery was positively correlated with laser intensity (Fig.2B & Fig.6B), which seemed contradictive to the high delivery efficiency in all laser conditions. Such a contradiction can be explained by the net result of drug delivery and systemic clearance (for SRB) or local uptake (for BSA). Fast delivery in strong laser conditions overcame drug clearance or local uptake effect, resulting in an increase of serum drug levels. In contrast, slow delivery in weak laser condition failed to overcome drug clearance or local uptake effect, leading to minimal increase of serum drug levels. Our studies found Franz Cell systems could be used to predict the relative rate of AFL-assisted powder drug delivery in vivo under different laser conditions. Yet, the overall rate of drug delivery was found to be slower in Franz Cell systems than in vivo studies. This might be caused by slower rate of water evaporation in vitro than in vivo. Due to the lack of blood flow in dissected skin, dermal interstitial water couldn’t be replenished as in vivo studies. Although water in recipient chambers might replenish the lost water in dissected skin, such a process was expected to be less efficient than in vivo systems.
AFL-assisted week-long sustained powder drug delivery was found to have a good safety. Skin MCs resealed in 2 days and completely recovered in 3 days at the end of the week-long AZT delivery (Fig.5B–C). One major safety concern of AFL-assisted powder drug delivery is the potential pathogen infection via open skin MCs. Disinfection of skin surface before delivery, patch occlusion during delivery, and quick resealing of skin MCs at the end of delivery are expected to significantly reduce local infection risks. AFL-assisted powder drug delivery takes advantage of water evaporation from skin MCs to dissolve topical drug powder. Although reservoir patches used in our study had a volume of 0.25 mL, water evaporated into reservoir patches was expected to be much less than 0.25 mL due to the occupation of the majority of patch volume by drug powder. At the end of most of the in vivo deliveries, only a small volume of liquid could be recovered (<50 μl), indicating minimal water loss and little impact on animal health in AFL-assisted powder reservoir patch delivery. For potential clinical translation, a proportionally increased water loss (<1 mL) with 10 cm2 patch size is not expected to pose a significant risk to human health.
MNs have been also explored to generate skin MCs to support week-long sustained drug delivery. Due to the quick resealing of MN-generated skin MCs in 2 hours without occusion and in 2 days with occlusion [36], HMG-CoA reductase inhibitor Fluvastatin and non-specific COX inhibitor Diclofenac were used to delay skin MC resealing to support week-long sustained drug delivery [37, 38]. We found AFL (10 mJ/5%)-generated skin MCs could remain open for 7 days with reservoir patch occlusion to support week-long sustained drug delivery (Fig.5). The ability of AFL to generate skin MCs to support week-long sustained drug delivery without application of micropore lifetime extenders is expected to be due to the relatively big MCs generated by AFL. AFL ablates tiny skin tissues to generate skin MCs, while MN insertion forces skin tissues to separate to generate skin MCs and MN retraction causes partial MC closure [39]. As of such, relatively small MCs were formed even with the use of relatively big MNs [39]. From this regard, laser may be a more promising technology to generate skin MCs to support week-long sustained drug delivery.
This study found AFL could facilitate week-long sustained AZT and BSA delivery. Week-long sustained delivery is promising to reduce dosing frequency and improve medication adherence in antiretriviral therapy or treatment of growth hormone deficiencies if a small handheld AFL device can be fabricated to support powder reservoir patch delivery. Furthermore, an integrated laser ablation and patch application system that combines the two-step delivery into one and a flexible reservoir patch that responds to delivery volume changes would greatly facilitate clinical translation of AFL-assisted week-long powder drug delivery. Drugs are mostly maintained in a wet and warm environment (~34°C) on skin surface in AFL-assisted week-long sustained powder drug delivery. Most of the chemical drugs are expected to have a good stability in AFL-assisted week-long powder delivery. For protein-based drugs that are sensitive to increased temperatures, trehalose known to stabilize proteins in various harsh environments may be used to maintain protein activity during the week-long sustained drug delivery [40]. Also guinea pigs or miniature pigs with similar skin to human skin are required to validate AFL-assisted week-long sustained powder drug delivery. Laser parameters and drug/mannitol mixing ratios can be modified to obtain similar delivery kinetics in guinea pigs or miniature pigs.
Conclusion
This study explored AFL to assist 3-day to week-long sustained delivery of high-dose hydrophilic drugs in murine models. Our data indicate bulk drug powder can be coated in reservoir patches followed by topical application onto AFL-treated skin to elicit multi-day sustained delivery with a good efficiency and safety. About 10–20 mg drug can be coated per 0.5 cm2 reservoir patch to elicit 3-day sustained delivery with tapped coating. About 35–70 mg drug could be coated per 0.5 cm2 reservoir patch to elicit week-long sustained delivery with compression coating. Enlarging reservoir patch size to 10 cm2 is expected to increase the maximal coating and delivery capacity to gram scale to meet the delivery needs of many hydrophilic drugs in clinics. Week-long sustained transdermal drug delivery is promising to reduce dosing frequency and improve medication adherence in treatment of long-term chronic diseases, such as HIV, hypertension, and diabetes. AFL-assisted powder reservoir patch delivery involves no complex powder formulation and only highly water-soluble mannitol (or a similar excipient) is required to facilitate the high-efficient delivery. To our knowledge, this is the first time that week-long sustained transdermal delivery of high-dose hydrophilic chemical and macromolecular drugs was achieved with a simple laser-based delivery platform.
Highlights.
Laser facilitates week-long transdermal powder drug delivery
Laser facilitates high-dose transdermal powder drug delivery
Laser facilitates both small chemical and macromolecular drug delivery
Laser-treated skin can be recovered in 3 days
Acknowledgements
This work is partly supported by the National Institutes of Health grants DA033371, AI107678, and AI139473 (to X.Y.C.). The LC-MS/MS machine at a Rhode Island NSF EPSCoR research facility is supported in part by the National Science Foundation EPSCoR Cooperative Agreement #EPS-1004057. Microplate reader and Nikon Eclipse E600 microscope used in this work is supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health grant P20GM103430. Histological analysis was supported by the Molecular Pathology Core of the COBRE Center for Cancer Research Development, funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P30GM110759.
Footnotes
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Prausnitz MR, Langer R, Transdermal drug delivery, Nature biotechnology, 26 (2008) 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Naik A, Kalia YN, Guy RH, Transdermal drug delivery: overcoming the skin’s barrier function, Pharmaceutical science & technology today, 3 (2000) 318–326. [DOI] [PubMed] [Google Scholar]
- [3].Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL, Challenges and opportunities in dermal/transdermal delivery, Therapeutic delivery, 1 (2010) 109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pastore MN, Kalia YN, Horstmann M, Roberts MS, Transdermal patches: history, development and pharmacology, British journal of pharmacology, 172 (2015) 2179–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jacques SL, McAuliffe DJ, Blank IH, Parrish JA, Controlled removal of human stratum corneum by pulsed laser, The Journal of investigative dermatology, 88 (1987) 88–93. [DOI] [PubMed] [Google Scholar]
- [6].Lee S, McAuliffe DJ, Flotte TJ, Kollias N, Doukas AG, Photomechanical transdermal delivery: the effect of laser confinement, Lasers in surgery and medicine, 28 (2001) 344–347. [DOI] [PubMed] [Google Scholar]
- [7].Nelson JS, McCullough JL, Glenn TC, Wright WH, Liaw LH, Jacques SL, Mid-infrared laser ablation of stratum corneum enhances in vitro percutaneous transport of drugs, The Journal of investigative dermatology, 97 (1991) 874–879. [DOI] [PubMed] [Google Scholar]
- [8].Gomez C, Costela A, Garcia-Moreno I, Llanes F, Teijon JM, Blanco MD, Skin laser treatments enhancing transdermal delivery of ALA, Journal of pharmaceutical sciences, 100 (2011) 223–231. [DOI] [PubMed] [Google Scholar]
- [9].Lee WR, Shen SC, Wang KH, Hu CH, Fang JY, The effect of laser treatment on skin to enhance and control transdermal delivery of 5-fluorouracil, Journal of pharmaceutical sciences, 91 (2002) 1613–1626. [DOI] [PubMed] [Google Scholar]
- [10].Preissig J, Hamilton K, Markus R, Current Laser Resurfacing Technologies: A Review that Delves Beneath the Surface, Seminars in plastic surgery, 26 (2012) 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR, Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury, Lasers in surgery and medicine, 34 (2004) 426–438. [DOI] [PubMed] [Google Scholar]
- [12].Chen X, Shah D, Kositratna G, Manstein D, Anderson RR, Wu MX, Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology, Journal of controlled release : official journal of the Controlled Release Society, 159 (2012) 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scheiblhofer S, Thalhamer J, Weiss R, Laser microporation of the skin: prospects for painless application of protective and therapeutic vaccines, Expert opinion on drug delivery, 10 (2013) 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee WR, Shen SC, Al-Suwayeh SA, Yang HH, Yuan CY, Fang JY, Laser-assisted topical drug delivery by using a low-fluence fractional laser: imiquimod and macromolecules, Journal of controlled release : official journal of the Controlled Release Society, 153 (2011) 240–248. [DOI] [PubMed] [Google Scholar]
- [15].Lee WR, Shen SC, Chen WY, Aljuffali IA, Suen SY, Fang JY, Noninvasive delivery of siRNA and plasmid DNA into skin by fractional ablation: erbium:YAG laser versus CO(2) laser, European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 86 (2014) 315–323. [DOI] [PubMed] [Google Scholar]
- [16].Wenande E, Olesen UH, Nielsen MM, Janfelt C, Hansen SH, Anderson RR, Haedersdal M, Fractional laser-assisted topical delivery leads to enhanced, accelerated and deeper cutaneous 5-fluorouracil uptake, Expert opinion on drug delivery, 14 (2017) 307–317. [DOI] [PubMed] [Google Scholar]
- [17].Wenande E, Olesen UH, Boesen MR, Persson DP, Lerche CM, Sturup S, Gammelgaard B, Husted S, Anderson RR, Haedersdal M, Laser-assisted delivery enhances topical uptake of the anticancer agent cisplatin, Drug delivery, 25 (2018) 1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Haedersdal M, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, Anderson RR, Fractional CO(2) laser-assisted drug delivery, Lasers in surgery and medicine, 42 (2010) 113–122. [DOI] [PubMed] [Google Scholar]
- [19].Haedersdal M, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, Anderson RR, Pretreatment with ablative fractional laser changes kinetics and biodistribution of topical 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL), Lasers in surgery and medicine, 46 (2014) 462–469. [DOI] [PubMed] [Google Scholar]
- [20].Lapteva M, Del Rio-Sancho S, Wu E, Carbonell WS, Bohler C, Kalia YN, Fractional laser ablation for the targeted cutaneous delivery of an anti-CD29 monoclonal antibody - OS2966, Scientific reports, 9 (2019) 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Engelke L, Winter G, Engert J, Application of water-soluble polyvinyl alcohol-based film patches on laser microporated skin facilitates intradermal macromolecule and nanoparticle delivery, European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 128 (2018) 119–130. [DOI] [PubMed] [Google Scholar]
- [22].Genina EA, Bashkatov AN, Dolotov LE, Maslyakova GN, Kochubey VI, Yaroslavsky IV, Altshuler GB, Tuchin VV, Transcutaneous delivery of micro- and nanoparticles with laser microporation, Journal of biomedical optics, 18 (2013) 111406. [DOI] [PubMed] [Google Scholar]
- [23].Yu J, Dubey S, Kalia YN, Needle-free cutaneous delivery of living human cells by Er:YAG fractional laser ablation, Expert opinion on drug delivery, 15 (2018) 559–566. [DOI] [PubMed] [Google Scholar]
- [24].Park JH, Chun JY, Lee JH, Laser-assisted topical corticosteroid delivery for the treatment of keloids, Lasers in medical science, 32 (2017) 601–608. [DOI] [PubMed] [Google Scholar]
- [25].Yu J, Bachhav YG, Summer S, Heinrich A, Bragagna T, Bohler C, Kalia YN, Using controlled laser-microporation to increase transdermal delivery of prednisone, Journal of controlled release : official journal of the Controlled Release Society, 148 (2010) e71–73. [DOI] [PubMed] [Google Scholar]
- [26].Tian T, Luo Y, Jiang T, Dong Y, Yu A, Chen H, Gao X, Li Y, Clinical effect of ablative fractional laser-assisted topical anesthesia on human skin: A randomized pilot study, Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology, 18 (2016) 409–412. [DOI] [PubMed] [Google Scholar]
- [27].Meesters AA, Bakker MM, de Rie MA, Wolkerstorfer A, Fractional CO2 laser assisted delivery of topical anesthetics: A randomized controlled pilot study, Lasers in surgery and medicine, 48 (2016) 208–211. [DOI] [PubMed] [Google Scholar]
- [28].Cao Y, Kakar P, Hossen MN, Wu MX, Chen X, Sustained epidermal powder drug delivery via skin microchannels, Journal of controlled release : official journal of the Controlled Release Society, 249 (2017) 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gordon CJ, The mouse thermoregulatory system: Its impact on translating biomedical data to humans, Physiol Behav, 179 (2017) 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Verant P, Ricard C, Serduc R, Vial JC, van der Sanden B, In vivo staining of neocortical astrocytes via the cerebral microcirculation using sulforhodamine B, Journal of biomedical optics, 13 (2008) 064028. [DOI] [PubMed] [Google Scholar]
- [31].Broder S, The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic, Antiviral research, 85 (2010) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kirtane AR, Abouzid O, Minahan D, Bensel T, Hill AL, Selinger C, Bershteyn A, Craig M, Mo SS, Mazdiyasni H, Cleveland C, Rogner J, Lee YL, Booth L, Javid F, Wu SJ, Grant T, Bellinger AM, Nikolic B, Hayward A, Wood L, Eckhoff PA, Nowak MA, Langer R, Traverso G, Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy, Nature communications, 9 (2018) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McCrudden MT, Alkilani AZ, McCrudden CM, McAlister E, McCarthy HO, Woolfson AD, Donnelly RF, Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs, Journal of controlled release : official journal of the Controlled Release Society, 180 (2014) 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Donnelly RF, McCrudden MT, Zaid Alkilani A, Larraneta E, McAlister E, Courtenay AJ, Kearney MC, Singh TR, McCarthy HO, Kett VL, Caffarel-Salvador E, Al-Zahrani S, Woolfson AD, Hydrogel-forming microneedles prepared from “super swelling” polymers combined with lyophilised wafers for transdermal drug delivery, PloS one, 9 (2014) e111547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Szakonyi G, Zelko R, The effect of water on the solid state characteristics of pharmaceutical excipients: Molecular mechanisms, measurement techniques, and quality aspects of final dosage form, Int J Pharm Investig, 2 (2012) 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gupta J, Gill HS, Andrews SN, Prausnitz MR, Kinetics of skin resealing after insertion of microneedles in human subjects, Journal of controlled release : official journal of the Controlled Release Society, 154 (2011) 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ghosh P, Brogden NK, Stinchcomb AL, Fluvastatin as a micropore lifetime enhancer for sustained delivery across microneedle-treated skin, Journal of pharmaceutical sciences, 103 (2014) 652–660. [DOI] [PubMed] [Google Scholar]
- [38].Brogden NK, Milewski M, Ghosh P, Hardi L, Crofford LJ, Stinchcomb AL, Diclofenac delays micropore closure following microneedle treatment in human subjects, Journal of controlled release : official journal of the Controlled Release Society, 163 (2012) 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Coulman SA, Birchall JC, Alex A, Pearton M, Hofer B, O’Mahony C, Drexler W, Povazay B, In vivo, in situ imaging of microneedle insertion into the skin of human volunteers using optical coherence tomography, Pharmaceutical research, 28 (2011) 66–81. [DOI] [PubMed] [Google Scholar]
- [40].Kaushik JK, Bhat R, Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose, The Journal of biological chemistry, 278 (2003) 26458–26465. [DOI] [PubMed] [Google Scholar]