Abstract
Immune responses are both pathogen and cell type-specific. The innate arm of immunity is characterized by rapid intracellular signaling cascades resulting in the production of hundreds of antimicrobial effectors that protect the host organism. Long noncoding RNAs have been shown to operate as potent modulators of both RNA and protein function throughout cell biology. Emerging data suggest that this is also true within innate immunity. LncRNAs have been shown to regulate both innate immune cell identity and the transcription of gene expression programs critical for innate immune responses. Here, we review the diverse roles of lncRNAs within innate defense with a specific emphasis on host-virus interactions.
INTRODUCTION:
A longstanding observation within evolution has been the discrepancy between species complexity and coding gene number1. For example, a nematode possesses more genes than a human while harboring fewer cells and cell types. However, with the advent of high-throughput sequencing efforts within the last fifteen years, it has been observed that noncoding, regulatory DNA has expanded in correlation with organismal complexity2,3. Indeed, the entire mammalian proteome is encoded in only 2% of the genome4. The remaining 98% of our DNA is not silent, however; nearly 70% of the genome is actively transcribed at some point within a cell in a time- and context-specific manner2,3. Moreover, approximately 90% of all RNA within a given cell is noncoding (ncRNA)5 This includes a variety of ncRNA subtypes: mainly ribosomal RNA (rRNA) and transfer RNA (tRNA) as well as microRNA (miRNA), small nuclear RNA (snRNA), and small nucleolar RNA (snoRNA). Long noncoding RNAs (lncRNAs) are a newly described ncRNA subclass for which little is known regarding both broad relevance and function throughout biology.
LncRNAs are defined as any ncRNA that is greater than 200 nucleotides in length. Much like mRNAs, lncRNAs are transcribed by Polymerase II (PolII), are capped at the 5’ end and can be polyadenylated and spliced. LncRNAs are distinguished by a definitive lack of open reading frames capable of producing a peptide larger than 100 amino acids. Although 100 aa has been the classical defining demarcation for putative proteins, recent work has described functional polypeptides of less than 100 aa (termed micropeptides) encoded in genes originally annotated as lncRNAs. As the study of lncRNA biology continues to expand it will be essential to empirically assess the coding potential of lncRNAs of interest in order to accurately understand underlying mechanisms.
LncRNAs are further subcategorized by both the directionality of their transcription as well as the vicinity of their loci to neighboring annotated genes. LncRNA genes that reside between annotated genes are termed long intergenic noncoding RNAs (lincRNAs). Conversely, lncRNA loci can also be encoded within coding genes often times within introns or with some portion of the lncRNA overlapping a coding exon. LncRNAs are further delineated as sense or antisense depending on the directionality of transcription relative to the nearest gene.
As interest in lncRNA function has grown, diverse mechanisms have been identified. However, the overarching commonality between many of these described functions denotes lncRNAs as versatile regulators of transcription. LncRNAs can control multiple steps of RNA biogenesis, starting from epigenetic control of transcription initiation all the way through modulation of mature transcript stability6–10. Innate immunity to microbial infection is characterized by the rapid induction of transcriptional programs leading to the timely production of cytokines and other effectors which are required for pathogen clearance11,12. LncRNAs have therefore become attractive candidates for the control of these responses. Indeed, a growing body of literature has defined essential roles for lncRNAs in all aspects of innate immunity including the selection and maintenance of professional innate immune cell identity and function as well as the induction and suppression of classical innate immune genes13–17. Here, we summarize our current understanding of lncRNA mechanisms as well as recent examples of innate-associated lncRNA functions with a specific emphasis on virus-host interactions.
GENERAL MECHANISMS OF LNCRNA FUNCTION:
LncRNA functional modalities can be subcategorized via various criteria including subcellular localizaion. Typically, lncRNAs are enriched in either the nucleus or the cytoplasm. Their relative intracellular residency can subsequently confer specific functionality. In general, nuclear lncRNAs either directly or indirectly modulate gene expression by changing chromatin accessibility, 3D DNA structures, etc. in a manner that can either promote or inhibit transcription from a given genetic locus. In contrast, cytoplasmic lncRNAs predominantly control protein function and/or modify mature coding transcript stability. We discuss these basic mechanisms in greater detail below.
Nuclear LncRNAs
The majority of characterized lncRNAs are nuclear and are thought to function as guides which bind and recruit proteins such as epigenetic modifiers or transcription factors, to relevant genomic loci in a manner that affects gene expression (Figure 1A). This can occur in either cis (regulation of the same allele from which the lncRNA is transcribed) or trans (allele-independent). Perhaps the most illustrative example of this mode of action is X-inactive specific transcript (Xist), a ~19 kb noncoding transcript required for gene dosage compensation and X-chromosome inactivation in mammalian placental females. Xist has been reported to bind a variety of different protein complexes required to epigenetically silence the inactive-X (Xi). These include polycomb repressive complex 2 (PRC2) and SMRT1/HDAC1-associated repressor protein (SHARP) among others18–20. Additional studies have also cited PRC2 as the primary effector for other lncRNA phenotypes including Hox transcript antisense RNA (HOTAIR) and the immune-associated lncRNA myeloid RNA regulator of Bim-induced death (Morrbid) which will be discussed later21,22.
Figure 1: General mechanisms of lncRNA function.
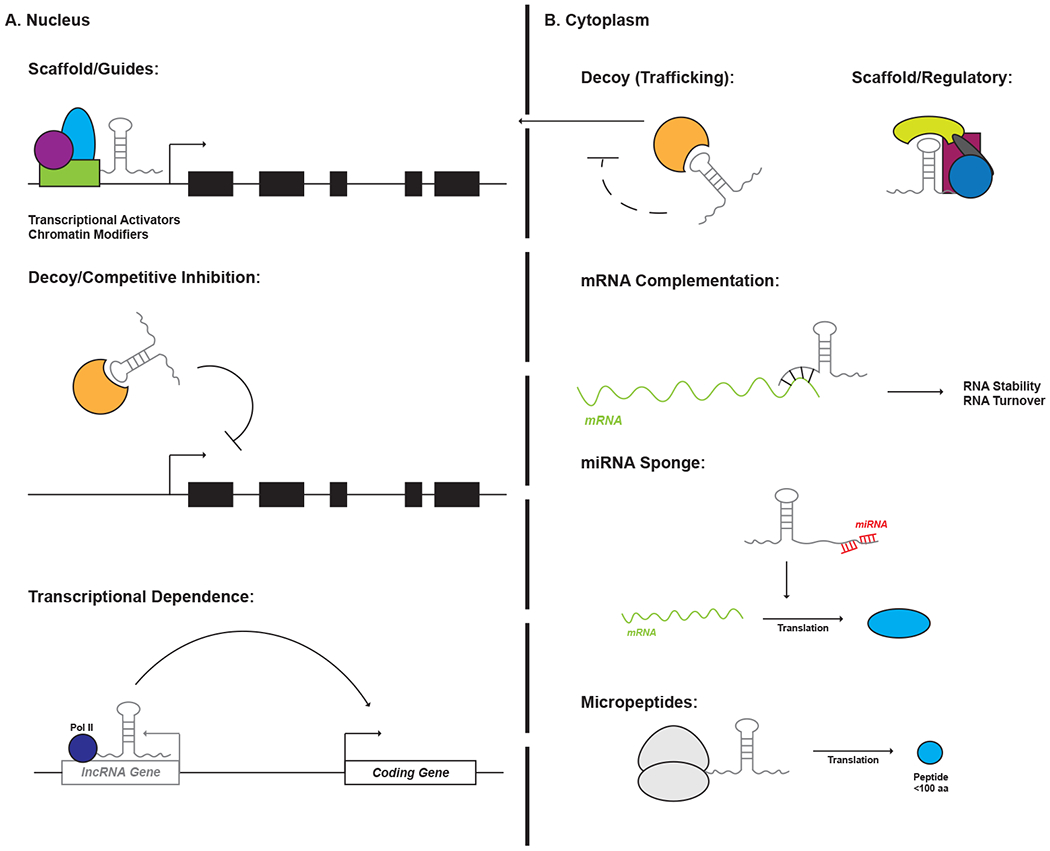
LncRNAs exhibit extensive versatility in regulating transcription and translation within a cell. These modes of action can be delineated in part by the intracellular compartment in which a lncRNA resides. The majority of nuclear lncRNAs function by regulating transcription initiation. This can be accomplished via interactions with proteins such as epigenetic modifiers and transcription factors to either recruit or inhibit these effectors from binding to target loci within the genome. In some cases, the lncRNA itself is dispensable and transcription through a lncRNA gene is what is required for transcription of a neighboring, coding locus. Cytoplasmic lncRNAs function to directly modulate protein function or affect transcript stability. Moreover, some cytoplasmic lncRNAs may encode short open reading frames that produce functional micropeptides of <100 amino acids.
Further evidence supporting a guide function for nuclear lncRNAs came from early work describing HOXA transcript at distal tip (HOTTIP). HOTTIP is transcribed from the 5’ end of the HOXA gene cluster and recruits the WDR5/MLL complex to HOXA loci23. This leads to deposition of the activating epigenetic mark H3K4me3, across the HOXA cluster, promoting transcription. This was also one of the first studies to describe 3D genomic architectural changes as part of the mechanism by which lncRNAs function, as chromosome looping is required for the correct localization of HOTTIP to HOXA loci.
Nuclear lncRNAs also function in alternative ways although fewer descriptions of these mechanisms exist at this point. For example, nuclear lncRNAs can also serve as decoy molecules by competitively sequestering a given regulatory protein and preventing localization to a gene locus. This is the case for growth arrest-specific 5 (Gas5) which is highly expressed in cells that have undergone growth arrest24. Gas5 functions as a competitive mimic of the GRE DNA motif recognized by the glucocorticoid receptor (GR). Gas5 consequently sequesters GR, prohibiting GR from binding to relevant metabolic genes and thus diminishing transcription from these loci.
Finally, it has been recently recognized that some lncRNAs are not actually required for observed phenotypes; rather, DNA features associated with the lncRNA gene are necessary for function25 (Figure 1, left). This was demonstrated for the Blustr locus which regulates transcription of the closest neighboring coding gene, Sfmbt226. The Blustr transcript itself is dispensable for this phenotype; however, introduction of truncating poly A-signals (PAS) throughout the body of the Blustr gene results in correlative decreases in Sfmbt2 expression. Thus, transcription through the Blustr gene is required for regulation of Sfmbt2 rather than the Blustr lncRNA itself. LncRNAs can also serve as proxy signals for cis-regulatory elements within the genome. This is the case for the group 1 innate lymphocyte (ILC1)-specific lncRNA RNA-demarcated Regulatory region of Id2 (Rroid), which will be discussed in later sections27.
Cytoplasmic LncRNAs
Cytoplasmic lncRNAs display distinct modes of activity relative to nuclear lncRNAs. These lncRNAs function in two general ways: direct modulation of protein function and control of mature transcript stability (Figure 1B). Like nuclear lncRNAs, cytoplasmic lncRNAs can directly bind to proteins and modify the function of the protein partner. This is exemplified by the lncRNA NFκB-Interacting LncRNA (NKILA), which regulates the master transcription factor NFκB28. IκBα protein is a major repressor of NFκB function. Upon stimulation, IκBα is phosphorylated and degraded, releasing NFκB. NFκB can then translocate into the nucleus where it induces gene expression programs. In human breast cancer cells, NKILA was found to bind IκBα in a manner that prevents phosphorylation. NFκB thus remains repressed, resulting in diminished expression of NFκB-dependent genes. Interestingly, NKILA is itself induced by NFκB implying a general negative feedback loop that may prevent prolonged inflammation. The role of NKILA has not been studied in the context of innate immunity; however, given its role as a regulator of NFκB, NKILA likely impacts immune function as well. In addition to NKILA, other cytoplasmic lncRNAs have also been shown to regulate protein function. Lnc-DC, lncRNA-ACOD1, and lnc-Lsm3b all have integral roles in innate immunity and thus will be discussed in detail later29–31.
In addition to binding and modulating proteins, cytoplasmic lncRNAs can also control the stability and turnover of other RNAs. For example, lncRNAs can behave as microRNA (miRNA) sponges by encoding seed sequences for a specific miRNA or miRNA family32–36. miRNAs bind to these lncRNA-encoded target sequences, resulting in miRNA sequestration. This ultimately protects the true, cognate mRNA target and leads to increased translation and protein production from the mRNA. For example, the lncRNA phosphatase and tensin homolog pseudogene 1 (PTENP1) sequesters miRNAs which normally target PTEN mRNA32. H19 sequesters miRNAs from the let-7 family33–35. Linc-regulator of reprogramming (Linc-RoR) competitively binds miR-145 which promotes the expression of a number of developmental genes including Oct4 and Nanog required for cellular differentiation36. Further studies will likely reveal that other lncRNAs function in a similar way in a context-specific manner.
An interesting consequence of lncRNA localization in the cytoplasm is proximity to ribosomes. A recent study found that ~40-70% of cytoplasmic lncRNAs can be found bound to ribosomes37–39. The consequences of these interactions are not fully elucidated40. This may simply be a snapshot of ribosome scanning to identify open reading frames. In support of this, many of these ribosome-bound lncRNAs were shown to be subject to rapid degradation39. However, recent examples have described lncRNAs capable of producing functional proteins of <100 amino acids (aa) termed, micropeptides (Figure 1B). The first of these was myoregulin (MLN), a 46 aa protein specifically expressed in skeletal muscle41. This micropeptide interacts with and inhibits the Ca2+ pump, SERCA, which regulates muscular function. MLN also functions in vivo, as MLN-deficient mice show altered Ca2+ regulation and fitness when challenged with exercise. Follow-up work by the same group has since identified other micropeptides which cooperatively regulate SERCA, suggesting a global regulatory role for micropeptides within muscle function42,43. Concordant with these findings, the micropeptide small regulatory polypeptide of amino acid response (SPAR) was found encoded within LINC0096144. SPAR localizes in lysosomes to negatively modulate mTORC1 and promote muscle regeneration in vivo. Finally, global assessment of translational potential in murine macrophages using the RiboTag/Cre system revealed that many annotated lncRNAs encode small ORFs driven by non-canonical translational start codons (i.e. non-AUG) 45. This work identified a subset of these ORFs which are induced following stimulation with the bacterial ligand lipopolysaccharide (LPS), including Aw112010. Mutant mice harboring a premature stop codon in the Aw112010 ORF (Aw112010Stop) succumb more readily to Salmonella infection and bear increased bacterial burden. The authors further demonstrate that Aw112010Stop macrophages produce diminished levels of interleukin(IL)-12 following LPS stimulation.
The spectrum of lncRNAs that produce functional micropeptides is unclear. Regardless, these studies call into question the current nomenclature (long non-coding RNAs), how we define translational potential and the parameters we use to identify novel proteins within biology.
LNCRNAS IN INNATE IMMUNITY
LncRNA regulation of innate immune cell differentiation and homeostasis
LncRNAs display remarkable cell and tissue-specific expression relative to mRNAs3. This suggests that lncRNAs may play important regulatory roles in the determination of cellular identity46. Indeed, lncRNAs have been implicated in the differentiation of a number of cellular lineages ranging from neurons to muscular tissue47–51. Although limited, a growing body of evidence indicates an analogous role for lncRNAs in the differentiation of professional innate immune cells beginning from early hematopoiesis through homeostatic maintenance of mature myeloid cell subsets (Table 1).
TABLE 1.
LncRNAs in Innate Immune Cell Function
| LncRNA | Species | Function | Reference |
|---|---|---|---|
| Lnc-DC | Hu, Ms | Binds to and inhibits dephosphorylation of STAT3, subsequently promoting expression of DC-associated genes including CD40 and CD80 | 31 |
| LncHSC1/2 | Hs | Drives myeloid cell differentiation | 52 |
| HOTAIRM1 | Hu | RA-induced lncRNA necessary for in vitro differentiation of monocyte lines into granulocytes; promotes CD11b and CD18 expression | 55 |
| Morrbid | Ms | Recruits the polycomb repressive complex to the Bcl2l11 promoter to support survival of short lived myeloid cell populations | 22 |
| Rroid | Ms | Regulates STAT5 deposition at the Id2 locus via retention of 3D chromatin architecture in a lncRNAindependent manner | 27 |
| Lnckdm2b | Ms | Supports ILC3 homeostatic proliferation via interaction with Satb1 and NURF to induce expression of Zfp292 and Bptf | 83 |
Lnc hematopoietic stem cells (HSC)-1 was one of ~150 IncRNAs specifically enriched in HSCs compared to B cells and granulocytes52. Depletion of LncHSC-1 in Sca1+ stem and progenitor cells resulted in an increase in the relative percentage of myeloid cells both in vitro and in vivo. This finding indicates that LncHSC-1 is a negative regulator of myelopoiesis although the exact mechanism has not yet been defined.
HOX antisense intergenic RNA myeloid 1 (HOTAIRM1) resides within the HOXA gene cluster. In the human acute promyelocytic leukemia cell, NB4, HOTAIRM1 is induced in a retinoic-acid (RA) and PU.1-dependent manner53,54. Depletion of HOTAIRM1 resulted in diminished RA-dependent granulocyte differentiation and reduced expression of the myeloid markers, CD11b and CD18. HOTAIRM1 has also been shown to function as a microRNA (miRNA) sponge, protecting the translation of a number of autophagy-associated proteins and promoting an increase in autophagy that is required for normal granulopoiesis55,56. HOTAIRM1 expression also decreases in primary human dendritic cells differentiated from peripheral blood monocytes suggesting physiological relevance during development in vivo.
Human monocyte-derived conventional dendritic cells (cDCs) are highly enriched for lnc-DC, a PU.1-dependent cytoplasmic lncRNA31. Depletion of lnc-DC leads to abrogated DC differentiation and function in vitro. Lnc-DC binds to and prevents the dephosphorylation of STAT3, thus allowing for increased expression of STAT3-dependent genes that are required for cDC function. Lnc-DC knockout mice also display reduced DC differentiation in vivo. Interestingly, murine lnc-DC has since been shown to produce a micropeptide. Whether this protein is functional is unknown.
In addition to controlling myeloid differentiation, lncRNAs have also been shown to regulate the homeostatic function of fully differentiated innate immune cells. We identified Morrbid as an essential modulator of cellular lifespan in highly inflammatory, short-lived myeloid cells--specifically eosinophils, neutrophils, and Ly6c+ monocytes22. Morrbid is localized to the nucleus where it regulates the allele-specific expression of the pro-apoptotic gene Bcl12l11 in cis. Mechanistically, Morrbid directly interacts with PRC2, promoting its residency at the Bcl2l11 locus and maintaining Bcl2l11 in a poised state. Through this mechanism, Morrbid regulates cellular turnover and the lifespan of inflammatory, innate immune cells. Furthermore, human MORRBID is dysregulated in patients with hypereosinophilia syndromes, further highlighting the importance of this lncRNA in regulating inflammatory innate immune cells.
We have also identified Rroid as a regulator of ILC1 cell function in mice. In this case, the Rroid RNA itself is not required but instead marks a regulatory element which is essential for DNA looping27. Maintenance of three-dimensional DNA structure allows for the deposition of STAT5 and the subsequent expression of Id2, a neighboring gene to the Rroid locus and an essential transcriptional regulator of ILC1 cellular identity and function. Whether transcription through the Rroid locus is required for Id2 expression is a topic of current investigation.
LncRNAs in innate signaling
Innate immune responses to microbes are characterized by the rapid induction of transcriptional programs downstream of pattern recognition receptors such as Toll-like receptors (TLRs). Given their role in modulating transcription and translation, lncRNAs are excellent candidates for the regulation of innate responses. Early work in this field focused on identifying lncRNAs which are induced following synthetic TLR stimulation using microbial ligands (Figure 2). This approach proved highly successful, beginning with the identification of lincRNA-Cox257,58. This lncRNA is upregulated following TLR2 activation of bone marrow-derived macrophages and can both promote and inhibit the expression of a number of essential anti-microbial genes. The repressive action of lincRNA-Cox2 is mediated by interaction with hnRNPA/B and hnRNPA2/B1. Additional work has since shown that lincRNA-Cox2 also binds to the SWI/SNF complex, leading to the activation of distinct innate-associated genes in a murine macrophage cell line (RAW264.7)59.
Figure 2: LncRNAs in innate immune signaling.
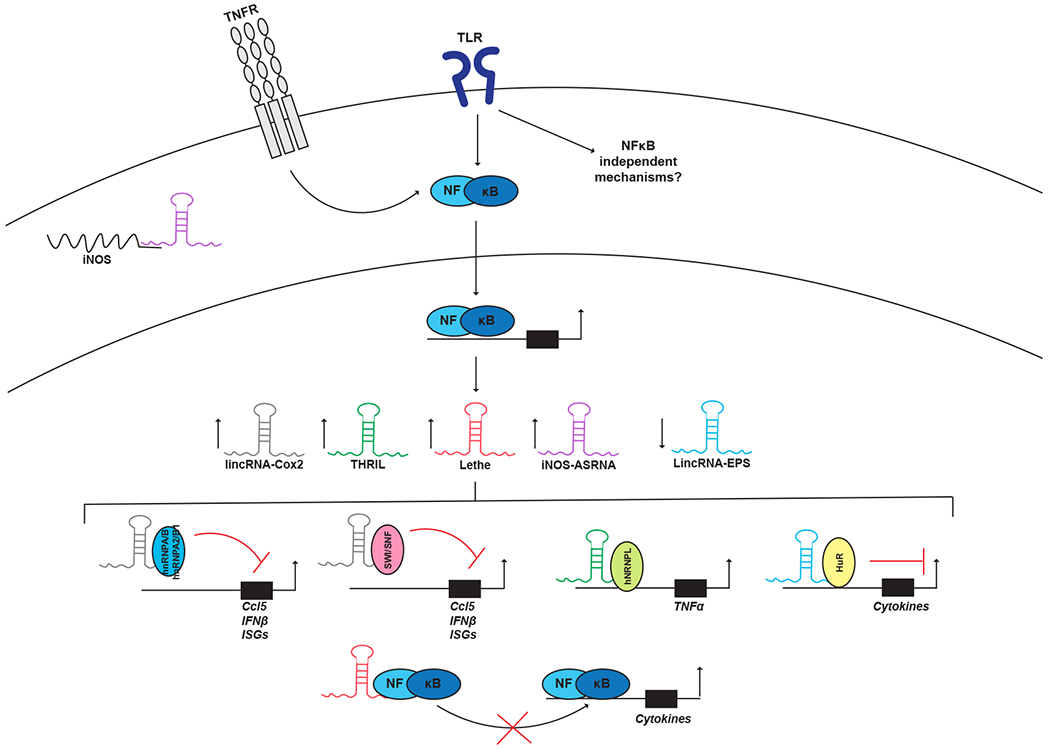
TLR stimulation leads to dynamic changes in the expression of a number of lncRNAs some of which function within the context of innate immunity. LincRNA-Cox2 acts to both activate and repress cytokines via interactions with SW/SNF or hnRNPA/B/hnRNPA2/B1 respectively. THRIL recruits hnRNPL to the Tnfa locus to promote transcription. LincRNA-EPS serves as a negative regulator of several inflammatory genes by recruiting hnRNPL. The iNOS-ASRNA is upregulated by IL-1β stimulation and localizes to the cytoplasm where it promotes the stability and subsequent translation of the iNOS coding transcript through direct base-pair complementation. Lethe is induced by TNFα signaling and functions as a decoy molecule, inhibiting NFκB function. Although the examples illustrated in the figure have focused on NFκB-associated lncRNAs there are like many other lncRNAs which regulate alternative signaling pathways associated with other transcription factors. Additional focus on lncRNA studies will likely reveal these important regulators.
Since the initial description of lincRNA-Cox2, additional studies have identified additional lncRNAs which are essential for the transcription of canonical innate genes. TNFα and hnRNPL related immunoregulatory lncRNA (THRIL) was identified shortly after lincRNA-Cox2 and was found to behave in a similar manner60. THRIL is also induced by TLR2 activation and binds an hnRNP protein (hnRNPL) to regulate the expression of TNFα in the human macrophage cell line, THP-1.
Lethe is induced by TNFα and functions as a competitive inhibitor downstream of the TNF receptor (TNFR) in mouse embryonic fibroblasts61. More specifically, Lethe binds the canonical transcription factor NFκB, leading to reduced NFκB occupancy at cognate, innate immune loci including Nfkbia. Interestingly, Lethe upregulation is dependent on NFκB speaking to a potential negative feedback mechanism required to prevent TNF-associated immunopathology.
The iNOS locus encodes an antisense lncRNA which is induced by IL-1β and functions in the cytoplasm of rat hepatocytes62. The iNOS AS transcript base-pair complements with the 3’ UTR of the iNOS mRNA, stabilizing the transcript and promoting its translation. Both iNOS mRNA and the iNOS AS transcript were shown to bind to AU-rich element binding protein HuR in a complex with hnRNPL in the cytoplasm of rat hepatocytes. The consequence of this interaction has not been elucidated. The iNOS AS transcript was later shown to be conserved in human and induced following stimulation with IL-1β and LPS in the HepG2 cells63.The exact mechanism of the human iNOS AS transcript has not been studied; however, preliminary data suggests it can bind to the 3’ UTR of iNOS mRNA similarly to what was initially described for the rat orthologue.
Finally, Linc-erythroid prosurvival (LincRNA-EPS) is downregulated following TLR stimulation64. Reduction in lincRNA-EPS expression results in increased expression of a panel of cytokines. Thus, lincRNA-EPS functions as a negative regulator of the innate immune response. In support of this finding, lincRNA-EPS-knockout mice challenged with LPS were more susceptible to septic shock due to increased expression of serum cytokines. Additional mechanistic work revealed that lincRNA-EPS interacts with hnRNPL to regulate nucleosome positioning at relevant immune response genes.
It is clear that lncRNAs are essential regulators of all aspects of innate immunity ranging from immune cell homeostasis to the modulation of intracellular effectors (Table 2). Greater focus on innate cell types and anti-pathogen responses that have not been explored will likely broaden our understanding of lncRNA function within immunity.
TABLE 2.
LncRNAs in Intracellular Immunity
| LncRNA | Species | Function | Reference |
|---|---|---|---|
| LincRNA-Cox2 | Ms | Both activates and suppresses cytokine expression downstream of TLR2 activation via interaction with SWI/SNF or hnRNPA/B/hnRNPA2/B1 respectively | 58,59 |
| THRIL | Hu | Promotes transcription of TNFα via hnRNPL | 60 |
| Lethe | Ms | Operates as a molecular decoy preventing NFκB localization to innate genes | 61 |
| LincRNA-EPS | Ms | Represses upregulation of a number of cytokines at homeostasis both in vitro and in vivo by recruiting hnRNPL | 64 |
| iNOS-AS Transcript | Rt | Basepair complements with the 3’UTR of iNOS mRNA, promoting its stability and iNOS translation | 62 |
| PACER | Hu | Interacts with the p50 subunit of NFκB to induce expression of COX2 | 84 |
| AS-IL-1α | Ms | Supports recruitment and deposition of RNA Polymerase II to the Il1a coding locus | 85 |
| AS-IL-1β | Ms | Modulates H3K4me3 deposition at the promoter of the Il1b locus in a manner that suppresses transcription | 86 |
| Lnc13 | Hu, Ms | Transcript produced from a risk allele identified in GWAS studies of celiac disease that associates with Hdac1 and hnRNPD | 87 |
| FIRRE | Hu | Interacts with hnRNPU and promotes the stability of innate coding mRNAs | 88 |
LNCRNAS IN VIRUS-HOST INTERACTIONS
LncRNAs in antiviral immunity
Initial studies on the role of long noncoding RNAs in innate immunity have primarily focused on bacterial stimulation. Viral infection is also sensed by pattern recognition receptors (PRRs) and leads to the induction of cytokines--particularly Type I IFN--as well as direct effectors and hundreds of interferon stimulated genes (ISGs). Recent studies have begun to explore the role of lncRNAs in antiviral defense (Figure 3, Table 3).
Figure 3: LncRNAs in host-virus interactions.
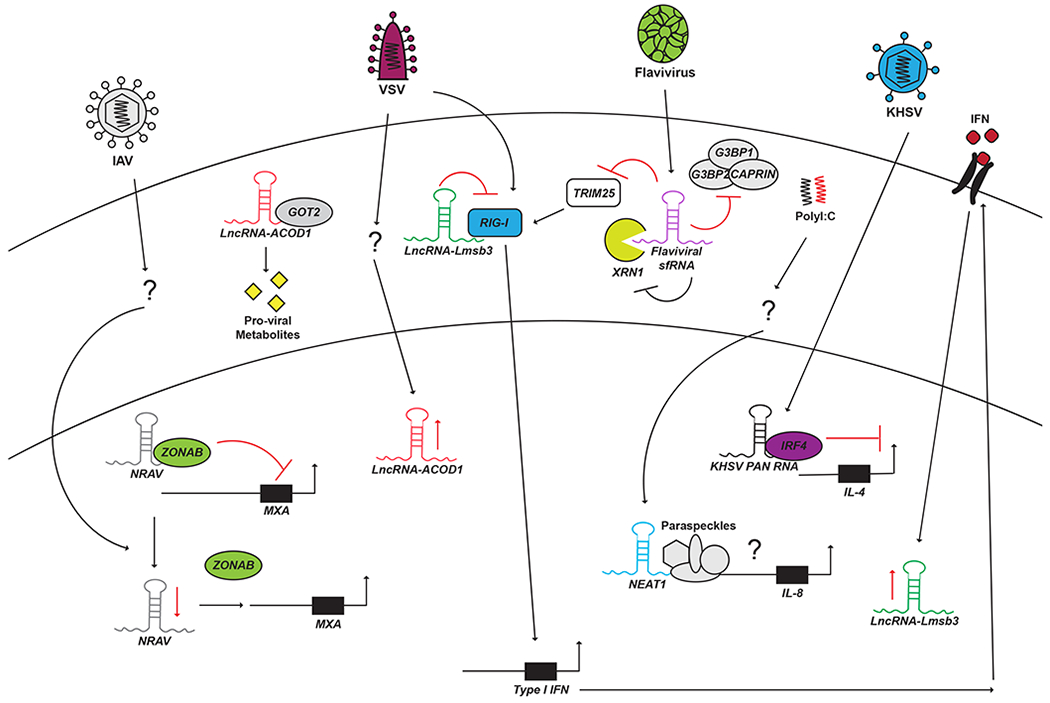
Innate immune responses to viruses are characterized by intracellular signaling cascades, cytokines, and protein effectors. Antiviral immunity thus requires specific regulatory mechanisms which include lncRNAs. NRAV is downregulated following Influenza A virus infection and functions to suppress the transcription of ISGs such as MXA. NEAT1 is induced by the viral RNA mimic PolyI:C and promotes IL-8 expression possibly through modulating paraspeckle formation. Lnc-Lsm3b is induced late in VSV infection and directly binds to and inhibits the canonical PRR RIG-I, thus attenuating downstream IFN and ISG induction. LncRNA-ACOD1 is also induced by VSV infection but functions in a pro-viral capacity by interacting with the metabolic enzyme GOT2 and promoting the production of metabolites required for VSV replication. Interestingly, viruses can also encode lncRNAs that can both promote the viral life cycle and also antagonize innate immune function. KSHV encodes PAN RNA which operates as decoy molecule for IRF4. Flaviviral sfRNA inhibits a number of host proteins. sfRNA is produced by XRN1-mediated nuclease activity. Once synthesized sfRNA then inhibits XRN1 function. sfRNA also blocks the function of TRIM25 as well the CAPRIN/G3BP1/G3BP2 complex both of which are required for canonical antiviral signaling.
TABLE 3.
LncRNAs in Innate Antiviral Immunity
| LncRNA | Species | Function | Reference |
|---|---|---|---|
| NRAV | Hs | Downregulated in human epithelial cells following infection with Influenza A; suppresses the expression of ISGs including MXA potentially through interaction with ZONAB | 65 |
| NEAT1 | Hs | Induced by PolyI:C stimulation; required for the upregulation of IL-8 by modulating the formation of paraspeckles | 66 |
| LncRNA-ACOD1 | Ms | Induced by VSV in an IFN-independent manner; binds to and promotes the function of the metabolic enzyme GOT2 to support VSV replication | 29 |
| Lnc-Lsm3b | Ms | Induced late in VSV infection in an IFN-dependent manner; interacts with and inhibits RIG-I activation thus suppressing the induction of innate transcriptional programs | 30 |
| NeST | Ms | Modulates susceptibility to Theiler’s virus persistence by recruiting WDR5/MLL to the Ifng locus to bolster transcription in T cells | 67 |
| lncRHOXF1 | Hu | Suppresses Type I IFN and ISG transcription in human placental cells | 89 |
| GAS5 | Hu | Upregulated by HCV infection; inhibits HCV replication by directly binding the HCV protein NS3 | 90 |
| LncITPRIP-1 | Hu | Induced by HCV infection in an IFNα-dependent manner; binds to and promotes MDA-5 function and the consequent induction of ISGs | 91 |
One of the first antiviral lncRNAs to be described was negative regulator of the antiviral response (NRAV) 65. NRAV is downregulated in human epithelial cells upon infection with a number of viruses including Influenza A, Sendai, and Herpes Simplex Virus-1. NRAV overexpression resulted in diminished production of a number of ISGs including MXA, an essential antiviral factor that targets Influenza A virus. NRAV was shown to directly bind to the multifunctional transcription factor, ZONAB. However, how this protein regulates ISG expression and how it interacts with NRAV is still unclear.
Nuclear enriched abundant transcript 1 (NEAT1) has also been ascribed an antiviral role66. NEAT1 is induced upon stimulation with the viral mimic PolyI:C and loss of NEAT1 results in a reduction in IL-8 mRNA expression. Previous studies have shown that NEAT1 is required for the formation of paraspeckles, granular bodies which impact transcription in the nucleus. The authors speculate that the regulation of IL-8 is similarly dependent on paraspeckle formation; however, additional work is required to support this hypothesis.
Recent work has identified lnc-Lsm3b as a direct regulator of the canonical viral PRR, RIG-I30. Lnc-Lsm3b is upregulated following vesicular stomatitis virus (VSV) infection in bone marrow-derived murine macrophages in a time- and Type I IFN-dependent manner. Lnc-Lsm3b subsequently binds to and sequesters RIG-I molecules late in infection, shutting down RIG-I activation and thus inhibiting the downstream antiviral effector response. Furthermore, Lnc-Lsm3b-knockout mice are protected from lethal VSV infection presumably due to deregulated enhanced production of IFN and other critical cytokines.
Outside of innate immunity, the T cell-specific lncRNA Nettoie Salmonella pas Theiler’s (NeST) was identified as a key determinant of sensitivity to Theiler’s virus persistence67. Theiler’s virus is a natural mouse pathogen; however, only certain inbred strains are susceptible to persistent viremia. Previous genetic studies mapped these differences to the NeST locus (originally termed Tmevpg1) 68 Interestingly, the NeST locus resides downstream of the Ifng gene, a critical antiviral cytokine. It was found that NeST RNA interacts with epigenetic modifier WDR5 to promote the transcription of Ifng in CD4+ and CD8+ T cells conferring the observed differences in Theiler’s virus infection between mouse strains. These data have been further corroborated in human T cells--specifically Th1 cells, which display diminished IFNg induction upon depletion of NEST69,70.
Finally, a lncRNA has been identified which promotes viral infection. LncRNA-ACOD1 is poorly expressed at baseline in bone marrow-derived-macrophages and murine tissue but is highly induced upon infection with VSV in an IFN-independent manner29. Loss of this lncRNA results in diminished VSV viremia in vivo. LncRNA-ACOD1 is localized in the cytoplasm where it binds to the metabolic enzyme GOT2. This interaction leads to the production of a number of metabolites such as α-ketoglutarate, which are required for VSV replication through an as yet unknown mechanism.
Virally-encoded lncRNAs
The majority of recent work done within lncRNA biology has focused on animal species. However, viruses themselves can encode long noncoding RNAs. Indeed, some of the earliest characterized lncRNAs are those transcribed from viral genomes. Viral lncRNAs have thus far been shown to have diverse roles both in regulating the viral life cycle as well as modulating host transcription to promote viral replication (Table 4). Two of the best studied viral lncRNAs are described below (Figure 3).
TABLE 4.
Virally Encoded LncRNAs
| LncRNAs | Virus | Phenotype | Reference |
|---|---|---|---|
| PAN RNA | KSHV | Interacts with IRF4 preventing correct localization to immune loci; binds to JMDJ3, UTX, MLL to mediate the switch from latent to lytic infection | 75,76 |
| sfRNA | Flavivirus | Generated by XRN1 degradation of flaviviral genomes; inhibits the function of XRN1, TRIM25 and the CAPRIN/G3BP1/G3BP2 complex | 77–79,81,82 |
| LAT | HSV-1, HSV-2 | The only transcript expressed during latent infection; required to maintain latency | 92,93 |
| Beta2.7 | HCMV | Prevents host cell apoptosis via interactions with mitochondrial complex 1 | 94 |
Kaposi sarcoma-associated herpesvirus (KSHV) expresses a 1.1 kb, polyadenylated nuclear RNA (PAN RNA) which lacks coding potential and is robustly induced in the lytic phase of infection71. PAN RNA displays self-contained post-transcriptional regulation. The 3’ end of the PAN transcript encodes a 79 nt element termed ENE, which prevents deadenylation-dependent RNA decay and thus allows for the rapid accumulation of PAN RNA during lytic infection72–74. PAN RNA can subsequently bind to a number of host proteins impacting both viral replication as well as the cellular response to virus infection. For example, PAN RNA was shown to bind directly to IRF4, a transcription factor essential for expression of a number of cytokines75. The interaction between IRF4 and PAN RNA was shown to attenuate IRF4 occupancy at the IL-4 promoter and prevent IL-4 production. Ectopic expression of PAN RNA similarly diminishes the expression of several other cytokines including IL-18 and IFNγ. PAN RNA also mediates the switch from latency to the lytic phase in infected cells via interactions with the epigenetic modifiers JMDJ3, UTX, and MLL276. These proteins remove repressive marks from integrated KSHV genomes subsequently reactivating the transcription of viral RNA. It is possible that these interactions also impact endogenous loci in a manner that may indirectly promote the transition to lytic infection, though this has not been systemically characterized.
LncRNAs are also produced by RNA viruses--specifically flaviviruses. The flaviviral family includes a number of globally important emerging pathogens, including Dengue (DENV), West Nile (WNV), and Zika virus (ZIKV). These viruses encode a subgenomic flaviviral transcript (sfRNA) that is 300-500 nt in length and lacks an open reading frame. This lncRNA promotes viral replication as well as pathogenicity across flaviviruses77. Interestingly, sfRNA is not an independently transcribed RNA but rather a product of XRN1-dependent decay of the viral genome77–79. Secondary structure in the sfRNA stalls further XRN1 function, leading to the accumulation of classically aberrant cellular RNAs (uncapped RNAs, etc.). sfRNAs from different flaviviruses have also been shown to evade and abrogate antiviral immune responses. As an example, sfRNA-deficient WNV is much more sensitive to recombinant IFNα in vitro and ectopic expression of sfRNA alone diminishes IFN-responsiveness in human cells80. These activities were dependent on IRF3 and IFNAR suggesting a direct relationship between sfRNA and the IFN response. An additional study found that DENV sfRNA binds directly to host factors G3BP1, G3BP2 and CAPRIN, proteins required for ISG production81. The interaction between DENV sfRNA and these proteins results in diminished translation of ISG transcripts including PKR and IFITM2 which are functional against flaviviruses. Finally, DENV sfRNA has also been shown to bind and inhibit the E3-ligase TRIM25, which is required for activation of the canonical antiviral PRR, RIG-I82. The association between sfRNA and TRIM25 subsequently inhibits RIG-I activation and downstream IFN induction.
CONCLUSION
LncRNAs have now been widely defined as potent regulators of both RNA and protein function throughout cell biology. Moreover, versatility in molecular function has made lncRNAs attractive candidates for regulators of all facets of immunity. The vast majority of characterized mechanisms have identified nuclear lncRNA-protein interactions which affect transcription initiation. However, it is likely that cytoplasmic lncRNAs also broadly contribute to these responses. Antiviral immunity may be a particularly relevant context to study cytoplasmic lncRNAs as many viruses replicate entirely within the cytosolic compartment. Defining if and how lncRNAs directly interface with these viruses to either promote or inhibit infection may prove revealing both in terms of host-virus interactions as well as overall lncRNA biology. In general, the study of lncRNA-mediated immune regulation will be essential in generating a complete narrative of what constitutes an immune response. This knowledge will add to our understanding of lncRNA biology as a whole and may offer novel targets for therapeutic development to treat infections and ameliorate immunological disease.
ACKNOWLEDGEMENTS
The work in this manuscript was supported by funds from CHOP, NIH R21AI128060, R21DK111755, and R01HL136572, the PEW Biomedical Scholars award, and the Burroughs Wellcome Fund investigator in the pathogenesis of infectious diseases award (J.H.M.); NIH T32AID055400-14 (M.B); and NIH R01AI122749, Burroughs Wellcome Fund investigator in the pathogenesis of infectious diseases award (S.C.)
REFERENCES
- 1.Taft RJ, Pheasant M & Mattick JS The relationship between non-protein-coding DNA and eukaryotic complexity. Bioessays 29, 288–299 (2007). [DOI] [PubMed] [Google Scholar]
- 2.ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derrien T et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Research 22, 1775–1789 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djebali S et al. Landscape of transcription in human cells. Nature 489, 101–108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palazzo AF & Lee ES Non-coding RNA: what is functional and what is junk? Front Genet 6, 2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn JJ & Chang HY Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet 17, 47–62 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Ransohoff JD, Wei Y & Khavari PA The functions and unique features of long intergenic non-coding RNA. Nature Publishing Group 1–15 (2017). doi: 10.1038/nrm.2017.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopp F & Mendell JT Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 172, 393–407 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulitsky I & Bartel DP lincRNAs: genomics, evolution, and mechanisms. Cell 154, 26–46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisler S & Coller J RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nature Publishing Group 14, 699–712 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan N & Chen ZJ Intrinsic antiviral immunity. Nat. Immunol 13, 214–222 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brubaker SW, Bonham KS, Zanoni I & Kagan JC Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol 33, 257–290 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atianand MK, Caffrey DR & Fitzgerald KA Immunobiology of Long Noncoding RNAs. Annu. Rev. Immunol 35, 177–198 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atianand MK & Fitzgerald KA Long non-coding RNAs and control of gene expression in the immune system. Trends Mol Med 20, 623–631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elling R, Chan J & Fitzgerald KA Emerging role of long noncoding RNAs as regulators of innate immune cell development and inflammatory gene expression. Eur. J. Immunol 46, 504–512 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y & Cao X Long noncoding RNAs in innate immunity. Cell. Mol. Immunol 13, 138–147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mowel WK, Kotzin JJ, McCright SJ, Neal VD & Henao-Mejia J Control of Immune Cell Homeostasis and Function by lncRNAs. Trends Immunol. (2017). doi: 10.1016/j.it.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Sun BK, Erwin JA, Song J-J & Lee JT Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322, 750–756 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McHugh CA et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521, 232–236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smola MJ et al. SHAPE reveals transcript-wide interactions, complex structural domains, and protein interactions across the Xist lncRNA in living cells. Proc. Natl. Acad. Sci. U.S.A 113, 10322–10327 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai M-C et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotzin JJ et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 537, 239–243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang KC et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kino T, Hurt DE, Ichijo T, Nader N & Chrousos GP Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal 3, ra8–ra8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engreitz JM, Ollikainen N & Guttman M Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nature Publishing Group. 17, 756–770 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Engreitz JM et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 539, 452–455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mowel WK et al. Group 1 Innate Lymphoid Cell Lineage Identity Is Determined by a cis-Regulatory Element Marked by a Long Non-coding RNA. Immunity 47, 435–449.e8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B et al. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell 27, 370–381 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Wang P, Xu J, Wang Y & Cao X An interferon-independent lncRNA promotes viral replication by modulating cellular metabolism. Science eaao0409 (2017). doi: 10.1126/science.aao0409 [DOI] [PubMed] [Google Scholar]
- 30.Jiang M et al. Self-Recognition of an Inducible Host lncRNA by RIG-I Feedback Restricts Innate Immune Response. Cell 173, 906–919.e13 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Wang P et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 344, 310–313 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Poliseno L et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465, 1033–1038 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallen AN et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 52, 101–112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng F et al. H19/let-7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance. Cell Death Dis 8, e2569–e2569 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Y et al. The H19/let-7 double-negative feedback loop contributes to glucose metabolism in muscle cells. Nucleic Acids Res. 42, 13799–13811 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y et al. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev. Cell 25, 69–80 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Ingolia NT, Lareau LF & Weissman JS Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell 147, 789–802 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Heesch S et al. Extensive localization of long noncoding RNAs to the cytosol and mono- and polyribosomal complexes. Genome Biol. 15, R6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlevaro-Fita J, Rahim A, Guigó R, Vardy LA & Johnson R Cytoplasmic long noncoding RNAs are frequently bound to and degraded at ribosomes in human cells. RNA 22, 867–882 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guttman M, Russell P, Ingolia NT, Weissman JS & Lander ES Ribosome profiling provides evidence that large noncoding RNAs do not encode proteins. Cell 154, 240–251 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson DM et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 160, 595–606 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson BR et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 351, 271–275 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson DM et al. Widespread control of calcium signaling by a family of SERCA-inhibiting micropeptides. Sci Signal 9, ra119–ra119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumoto A et al. mTORC1 and muscle regeneration are regulated by the LINC00961 -encoded SPAR polypeptide. Nature 541, 228–232 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Jackson R et al. The translation of non-canonical open reading frames controls mucosal immunity. Nature 564, 434–438 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flynn RA & Chang HY Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell 14, 752–761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kretz M et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 493, 231–235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin N et al. An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol. Cell 53, 1005–1019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson KM et al. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539, 433–436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun L et al. Long noncoding RNAs regulate adipogenesis. Proc. Natl. Acad. Sci. U.S.A 110, 3387–3392 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alvarez-Dominguez JR et al. De Novo Reconstruction of Adipose Tissue Transcriptomes Reveals Long Non-coding RNA Regulators of Brown Adipocyte Development. Cell Metab. 21, 764–776 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo M et al. Long non-coding RNAs control hematopoietic stem cell function. Cell Stem Cell 16, 426–438 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X et al. A myelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood 113, 2526–2534 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei S, Zhao M, Wang X, Li Y & Wang K PU.1 controls the expression of long noncoding RNA HOTAIRM1 during granulocytic differentiation. J Hematol Oncol 9, 44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xin J et al. Downregulation of long noncoding RNA HOTAIRM1 promotes monocyte/dendritic cell differentiation through competitively binding to endogenous miR-3960. Onco Targets Ther 10, 1307–1315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Z-H et al. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ. 24, 212–224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guttman M et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458, 223–227 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carpenter S et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science 341, 789–792 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu G et al. LincRNA-Cox2 Promotes Late Inflammatory Gene Transcription in Macrophages through Modulating SWI/SNF-Mediated Chromatin Remodeling. J. Immunol 196, 2799–2808 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z et al. The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proc. Natl. Acad. Sci. U.S.A 111, 1002–1007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rapicavoli NA et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife 2, e00762 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsui K et al. Natural antisense transcript stabilizes inducible nitric oxide synthase messenger RNA in rat hepatocytes. Hepatology 47, 686–697 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Yoshigai E et al. Natural antisense transcript-targeted regulation of inducible nitric oxide synthase mRNA levels. Nitric Oxide 30, 9–16 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Atianand MK et al. A Long Noncoding RNA lincRNA-EPs Acts as a Transcriptional Brake to Restrain Inflammation. Cell 165, 1672–1685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouyang J et al. NRAV, a long noncoding RNA, modulates antiviral responses through suppression of interferon-stimulated gene transcription. Cell Host Microbe 16, 616–626 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imamura K et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol. Cell 53, 393–406 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Gomez JA et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 152, 743–754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vigneau S, Rohrlich P-S, Brahic M & Bureau J-F Tmevpg1, a candidate gene for the control of Theiler’s virus persistence, could be implicated in the regulation of gamma interferon. J. Virol 77, 5632–5638 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collier SP, Collins PL, Williams CL, Boothby MR & Aune TM Cutting edge: influence of Tmevpg1, a long intergenic noncoding RNA, on the expression of Ifng by Th1 cells. J. Immunol 189, 2084–2088 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spurlock CF et al. Profiles of Long Noncoding RNAs in Human Naive and Memory T Cells. J. Immunol 199, 547–558 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun R, Lin SF, Gradoville L & Miller G Polyadenylylated nuclear RNA encoded by Kaposi sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. U.S.A 93, 11883–11888 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conrad NK & Steitz JA A Kaposi’s sarcoma virus RNA element that increases the nuclear abundance of intronless transcripts. EMBO J. 24, 1831–1841 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conrad NK, Mili S, Marshall EL, Shu M-D & Steitz JA Identification of a rapid mammalian deadenylation-dependent decay pathway and its inhibition by a viral RNA element. Mol. Cell 24, 943–953 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Mitton-Fry RM, DeGregorio SJ, Wang J, Steitz TA & Steitz JA Poly(A) tail recognition by a viral RNA element through assembly of a triple helix. Science 330, 1244–1247 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rossetto CC & Pari GS Kaposi’s sarcoma-associated herpesvirus noncoding polyadenylated nuclear RNA interacts with virus- and host cell-encoded proteins and suppresses expression of genes involved in immune modulation. J. Virol 85, 13290–13297 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rossetto CC & Pari G KSHV PAN RNA associates with demethylases UTX and JMJD3 to activate lytic replication through a physical interaction with the virus genome. PLoS Pathog. 8, e1002680 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pijlman GP et al. A highly structured, nuclease-resistant, noncoding RNA produced by flaviviruses is required for pathogenicity. Cell Host Microbe 4, 579–591 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Chapman EG, Moon SL, Wilusz J & Kieft JS RNA structures that resist degradation by Xrn1 produce a pathogenic Dengue virus RNA. Elite 3, e01892 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chapman EG et al. The Structural Basis of Pathogenic Subgenomic Flavivirus RNA (sfRNA) Production. Science 344, 307–310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schuessler A et al. West Nile virus noncoding subgenomic RNA contributes to viral evasion of the type I interferon-mediated antiviral response. J. Virol 86, 5708–5718 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bidet K, Dadlani D & Garcia-Blanco MA G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA. PLoS Pathog. 10, e1004242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manokaran G et al. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 350, 217–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu B et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat. Immunol 18, 499–508 (2017). [DOI] [PubMed] [Google Scholar]
- 84.Krawczyk M & Emerson BM p50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-kB complexes. Elite 3, e01776 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan J et al. Cutting Edge: A Natural Antisense Transcript, AS-IL1α, Controls Inducible Transcription of the Proinflammatory Cytokine IL-1α. J. Immunol 195, 1359–1363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu J, Wu X, Hong M, Tobias P & Han J A potential suppressive effect of natural antisense IL-1β RNA on lipopolysaccharide-induced IL-1β expression. J. Immunol 190, 6570–6578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castellanos-Rubio A et al. A long noncoding RNA associated with susceptibility to celiac disease. Science 352, 91–95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu Y et al. The NF-KB-Responsive Long Noncoding RNA FIRRE Regulates Posttranscriptional Regulation of Inflammatory Gene Expression through Interacting with hnRNPU. J. Immunol 199, 3571–3582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Penkala I et al. lncRHOXF1, a Long Noncoding RNA from the X Chromosome That Suppresses Viral Response Genes during Development of the Early Human Placenta. Mol. Cell. Biol 36, 1764–1775 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qian X, Xu C, Zhao P & Qi Z Long non-coding RNA GAS5 inhibited hepatitis C virus replication by binding viral NS3 protein. Virology 492, 155–165 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Xie Q et al. LncITPRIP-1 Positively Regulates Innate Immune Response through Promoting Oligomerization and Activation of MDA5. J. Virol JVI.00507–18 (2018). doi: 10.1128/JVI.00507-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Q-Y et al. Herpesviral latency-associated transcript gene promotes assembly of heterochromatin on viral lytic-gene promoters in latent infection. Proc. Natl. Acad. Sci. U.S.A 102, 16055–16059 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nicoll MP et al. The HSV-1 Latency-Associated Transcript Functions to Repress Latent Phase Lytic Gene Expression and Suppress Virus Reactivation from Latently Infected Neurons. PLoS Pathog. 12, e1005539 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reeves MB, Davies AA, McSharry BP, Wilkinson GW & Sinclair JH Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science 316, 1345–1348 (2007). [DOI] [PubMed] [Google Scholar]


