Abstract
Background/Objectives:
Impaired insulin-mediated glucose partitioning is an intrinsic metabolic defect in skeletal muscle from severely obese humans (BMI ≥ 40 kg/m2). Roux-en-Y gastric bypass (RYGB) surgery has been shown to improve glucose metabolism in severely obese humans. The purpose of the study was to determine the effects of RYGB surgery on glucose partitioning, mitochondrial network morphology, and markers of mitochondrial dynamics skeletal muscle from severely obese humans.
Subject/Methods:
Human skeletal muscle cells were isolated from muscle biopsies obtained from RYGB patients (BMI = 48.0 ± 2.1, n=7) prior to, 1-month and 7-months following surgery and lean control subjects (BMI = 22.4 ± 1.1, n=7). Complete glucose oxidation, non-oxidized glycolysis rates, mitochondrial respiratory capacity, mitochondrial network morphology and regulatory proteins of mitochondrial dynamics were determined in differentiated human myotubes.
Results:
Myotubes derived from severely obese humans exhibited enhanced glucose oxidation (13.5%; 95%CI [7.6, 19.4], P = 0.043) and reduced non-oxidized glycolysis (−1.3%; 95%CI [−11.1, 8.6]) in response to insulin stimulation at 7-months after RYGB when compared to the pre-surgery state (−0.6%; 95%CI [−5.2, 4.0] and 19.5%; 95%CI [4.0, 35.0], P =0.006), and were not different from the lean controls (16.7%; 95%CI [11.8, 21.5] and 1.9%; 95%CI [−1.6, 5.4], respectively). Further, number of fragmented mitochondria and Drp1(Ser616) phosphorylation and were trended to reduced/reduced (0.0104, 95%CI [0.0085, 0.0126], P = 0.091 and 0.0085, 95%CI [0.0068, 0.0102], P = 0.05) in myotubes derived from severely obese humans at 7-months after RYGB surgery in comparison to the pre-surgery state. Finally, Drp1(Ser616) phosphorylation was negatively correlated with insulin-stimulated glucose oxidation (r = −0.49, P = 0.037).
Conclusion/Interpretation:
These data indicate that an intrinsic metabolic defect of glucose partitioning in skeletal muscle from severely obese humans is restored by RYGB surgery. The restoration of glucose partitioning may be regulated through reduced mitochondrial fission protein Drp1 phosphorylation.
Keywords: Glucose Metabolism, Mitochondrial Dynamics, Severe Obesity, Skeletal Muscle
INTRODUCTION
Severe obesity (BMI ≥ 40 kg/m2) is accompanied with an increased risk for metabolic diseases (e.g., insulin resistance and type 2 diabetes). One of the metabolic perturbation in severe obesity is the impaired intracellular glucose partitioning in skeletal muscle where glucose is partitioned towards non-oxidized glycolysis products (i.e., lactate production) rather than complete glucose oxidation 1. Such derangement in glucose partitioning can have serious consequences on whole-body metabolism since glycolytic products can serve as gluconeogenic substrates in liver to increase blood glucose production and subsequently hyperglycemia with type 2 diabetes 2. Roux-en-Y gastric bypass (RYGB) surgery can effectively improve glucose metabolism and reverse type 2 diabetes 3–5. Specifically, whole body glycemic control is normalized as early as 1-week after surgery 6, whereas insulin-stimulated glucose oxidation and peripheral insulin sensitivity improve more slowly 4–6. Although the metabolic alterations of glucose metabolism at various phases after RYGB surgery have been well studied 7–9, the intrinsic (i.e., skeletal muscle-specific) changes in skeletal muscle, via studying primary skeletal muscle cells raised in culture, just been explored recently 10,11. Hinkley et al., recently reported that insulin action was enhanced in primary skeletal muscle cells as early as 1-month and retained at chronic phase (7-months) after RYGB surgery 10, suggesting intrinsic remodeling in skeletal muscle occurs following RYGB surgery. However, how RYGB surgery regulates intracellular glucose partitioning intrinsically in skeletal muscle with severe obesity is still unclear.
Mitochondria are core organelles responsible for substrate oxidation in skeletal muscle 12. These highly dynamic organelles continually fuse and divide to maintain the interconnected network structure and their overall quality and function. The machinery of mitochondrial dynamics is finely regulated by mitochondrial fusion proteins; Mitofusin 1 (Mfn1), Mitofusin 2 (Mfn2), and Optic Atrophy 1 (Opa1) and fission proteins Dynamin-Related Protein 1 (Drp1) and Fission 1 (Fis1) 13. There is emerging evidence that obesity impairs mitochondrial dynamics with upregulation of mitochondrial fission that lead to fragmented mitochondrial networks, mitochondrial dysfunction and impaired glucose oxidation 14–17 and weight loss surgery improves these defects in skeletal muscle 15,18. In addition, the imbalance of mitochondrial dynamics towards fission has been linked to increased glycolysis 19 and impaired glucose oxidation 14. However, whether the changes in the mitochondrial dynamics machinery after RYGB surgery is imprinted in skeletal muscle cells derived from severely obese patients and whether the changes are associated with insulin-mediated glucose partitioning following RYGB surgery are largely unknown.
In the present study, primary human skeletal muscle cell raised in culture (HSkMC) were utilized to investigate the intrinsic (i.e., skeletal-muscle specific) alterations in skeletal muscle following RYGB surgery. This in vitro model eliminates the acute influence of in vivo systemic factors (i.e., hormones) during prolonged cell culture process. Thus, it has been widely used to study skeletal muscle intrinsic metabolic phenotypes that are changed after RYGB surgery. 20. The purpose of the present study was to examine the effects of RYGB surgery on glucose partitioning, mitochondrial respiratory capacity, network morphology, and markers of mitochondrial dynamics at two different time points (1 and 7-months) after surgery in primary myotubes established from severely obese humans. We hypothesized that RYGB would progressively improve glucose partitioning, mitochondrial network morphology and expression of mitochondrial dynamics proteins in myotubes from severely obese humans after surgery.
MATERIALS/SUBJECTS AND METHODS
Human Subjects
Severely obese women (body mass index [BMI] ≥ 40 kg/m2, n = 7) undergoing RYGB (detail of surgery can be found in 21) at the East Carolina University bariatric surgery center were recruited. Participants were excluded if they were taking medication known to alter metabolism, smokers, involved in regular exercise, or if they had been previously diagnosed with heart disease, diabetes, or cancer. The metabolic data from some of the severely obese participants have been presented in previous studies 10,22. Additionally, lean healthy women (BMI < 25 kg/m2; n = 7) was included in the current study. Skeletal muscles biopsies from the vastus lateralis using percutaneous needle biopsy and fasting blood samples (glucose and insulin) were obtained from lean and severely obese subjects prior to (Pre), 1-month (1-Month), and 7-months following surgery (7-Months). All procedures were approved by the East Carolina University and the University of Massachusetts Boston Institutional Review Boards, and informed consent was obtained from all subjects.
Human Skeletal Muscle Cell Culture
Immediately after the muscle biopsy, satellite cells were isolated and cultured into myoblasts as previously described 23. Briefly, human skeletal muscle cells (HSkMCs) were thawed and grown in a humidified environment with 5% CO2 at 37°C on collagen I TC flask (Greiner Bio-one, Monroe, NC) 23. Myoblasts were sub-cultured onto a 12-well type I collagen-coated plate, 6-well type I collagen-coated plate (Corning, Corning, NY), 35 mm poly-d-lysine coated glass bottom dish (MatTek, Ashland, MA) or a seahorse XFp cell culture miniplate (Agilent Technologies, Santa Clara, CA). At ~80–90% confluence, myoblasts were differentiated into myotubes. All experimental procedures were performed at 7 days of differentiation.
Glucose Oxidation and Non-Oxidized Glycolysis
Glucose oxidation and non-oxidized glycolysis were determined as previously described 1. Briefly, myotubes were serum starved for 3-hr and then incubated in a sealed 12-well plate containing radioactive media (1.5 μCi/ml D-[1-14C] glucose (American Radiolabeled Chemicals, St. Louis, MO)) in the presence or absence of 100 nM insulin for 2-hr at 37°C. After incubation, media was transferred to a microtiter plate. 14CO2 was liberated via acidification from media and collected by 1M NaOH. Radioactivity was counted in a liquid scintillation counter, as glucose oxidation was determined through the incorporation of 14C-labeled glucose into CO2. Glucose oxidation data from the participants included in the previous study10 were collected from independent experiments using new vials of muscle cells.
Non-oxidized glycolytic metabolites (e.g., lactate, pyruvate, and alanine) were measured with the remaining incubation media1. Briefly, 100 μL of the remaining incubation media was centrifuged for 5 min at 10,000 rpm. Supernatant was then added onto a Whatman, Grade DE81 ion-exchange cellulose paper (GE Healthcare Life Sciences, Marlborough, MA), followed by 30 min of drying and washed four times with dH2O. Ion-exchange cellulose paper was then collected in liquid scintillation fluid. Glycolytic metabolites were quantified by liquid scintillation as 14C-labeled glucose was converted into glycolytic metabolites. Water after each wash was also collected and analyzed to verify that there were no unionized products left on papers after washes. All data were normalized to total protein content. Relative change (%) in glucose oxidation and non-oxidized glycolysis in response to insulin was calculated as [(insulin-stimulated conditions – basal conditions)/basal condition]*100.
Cellular Respiration
Seahorse XFp (Agilent Technologies, Santa Clara, CA) Analyzer was used to determine cellular respiratory rates by measuring oxygen consumption rates (OCR). On the day of the assay, media was changed to XF Assay Medium containing modified DMEM (no phenol red and glucose) supplemented with 5 mM glucose and 2 mM glutamine and placed in the non-CO2 37°C incubator for 30 minutes prior to start of the assay. Oligomycin (1 μM), carbonyl cyanide-4 phenylhydrazone (FCCP) (0.75 μM), and rotenone/antimycin A (0.5 μM) were successively injected to measure OCR for different respiratory states. All data were analyzed using Agilent Seahorse Wave software and values were normalized to protein concentrations for each well.
Immunocytochemistry
Following differentiation, myotubes cultured on a 35 mm Collagen-I coated glass bottom dish were stained with MitoTracker™ RedFM (Thermo Fisher Scientific, Waltham, MA) according to the manufacture’s instructions. Briefly, MitoTracker™ RedFM was diluted to a stock concentration of 1mM and then further diluted in differentiation media to a final concentration of 100 nM. Myotubes were incubated for 15 minutes with prewarmed (37°C) MitoTracker working solution. Myotubes were then imaged via a Zeiss confocal microscopy (Zeiss, Germany) using 64x 1.4NA oil objective. Fifteen images per subject were taken for quantification.
Quantification of Mitochondrial Morphology
To evaluate mitochondrial network morphology in primary myotubes, images were taken from differentiated myotubes and analyzed using mitochondrial network analysis macro (MiNA) tool developed for use on the FIJI distribution of ImageJ as previously described 24. The tool and its related documentations are available at https://github.com/ScienceToolkit/MiNA. For image analysis, images were all uploaded to ImageJ software and preprocessed via unsharp mask with a two-pixel radius and enhancement of local contrast (CLAHE) before analysis. A filter was then utilized to remove background, and images were binarized and skeletonized for further analysis. Individual non-networked mitochondria, number of mitochondrial networks, branch length per network and number of branches per network (markers of mitochondrial network size) were quantified using a previously developed protocol 24. Data were normalized by total mitotracker intensity.
Immunoblot analyses
Myotubes were harvested for protein extraction as previously described1. Myotubes were harvested in lysis buffer and lysates were sonicated, centrifuged and collected. Protein concentration was determined by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). Equal amount of protein was subjected to SDS-PAGE using 4–20% gradient polyacrylamide gels (Bio-Rad, Hercules, CA) and transferred to a nitrocellulose membrane using a Trans-Blot Transfer system (Bio-Rad, Hercules, CA). Membranes were probed with antibodies recognizing Phospho-Drp1 (Ser616) (cat# 3455), Drp1 (cat# 8570), Opa1 (cat# 67589), Citrate Synthase (cat# 14309), Voltage-dependent anion channel (VDAC, cat# 4661) (Cell Signaling, Danvers, MA), Mfn2 (cat# 515647), GAPDH (cat# 47724) (Santa Cruz Biotechnology, Dallas, TX), Mfn1 (cat# H00055669-M04), Fis1 (H00051024-M01) (Abnova, Walnut, CA), and OXPHOS Cocktail (cat# ab110411, Abcam, MA). Membranes were probed with an IRDye secondary antibody (Li-Cor, Lincoln, NE) and quantified using Odyssey CLx software (Li-Cor, Lincoln, NE). Data were normalized to GAPDH protein expression.
Quantitative RT-PCR
Total RNA was extracted using an RNeasy kit (Qiagen, Hilden, Germany). Concentrations and purity of RNA samples were assessed on a NanoDrop One Microvolume UV-Vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). cDNA was reverse transcribed from 100ng of RNA using a High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA). cDNA was amplified in a 0.2 mL reaction containing TaqMan Universal PCR Master Mix, TaqMan Gene Expression Assay and RNase-free water. RT-PCR was performed using a QuantStudio Real-Time PCR (Thermo Fisher Scientific, Waltham, MA). Gene expression was quantified for all genes of interest (Supplementary Table 1) using the ΔΔCT method. Expression of GAPDH was used as the housekeeping gene.
Statistical analyses
Power analysis was performed to calculate the sample size necessary for our design based on a previous study10. We achieved a power of greater than 90% for detecting the effects that we anticipate at a significance level of P < 0.05 with 6 subjects. All values are expressed as mean ± SEM. To account for small sample size, statistical comparison was performed between the lean group and pre, 1-month and 7-month individually using a Mann-Whitney U test. A Wilcoxon signed-rank test was used to compare differences between timepoints. Spearman correlation analysis was used to assess linear relationships. All calculations were performed with SPSS statistical software (24.0; SPSS, Inc., Chicago, IL). Significance was set at P < 0.05.
RESULTS
RYGB surgery induces weight loss and improves insulin sensitivity.
Subject characteristics are presented in Table 1. The RYGB patients prior to surgery had a higher body mass, BMI, fasting plasma insulin level, and HOMA-IR compared to lean subjects (P < 0.05). RYGB surgery resulted in significant weight loss at 1-month (~15 kg, P < 0.05) and a further reduction at 7-months (~22 kg, P <0.05). Fasting glucose level, fasting insulin level and HOMA-IR were all significantly reduced at 7-months following RYGB surgery (P <0.05).
Table 1:
Subject Characteristics
| Lean (N=7) | Pre-surgery (N=7) | 1-Month (N=6) | 7-Months (N=7) | |
|---|---|---|---|---|
| Ethnicity | 3C/4AA | 3C/4AA | - | - |
| Age | 25.0 ± 3.6 | 39.6 ± 3.3* | - | - |
| Weight (kg) | 60.9 ± 2.3 | 136.3 ± 5.9 | 121.7 ± 7.3 | 99.7 ± 5.9 |
| BMI (kg/m2) | 22.4 ± 1.1 | 48.0 ± 2.1* | 43.6 ± 2.4*‡ | 35.5 ± 2.1*#Δ |
| Glucose (mg/dL) | 85.1 ± 1.9 | 92.9 ± 2.6 | 85.7 ± 3.2 | 75.2 ± 4.2 #Δ |
| Insulin (uIu/mL) | 5.7 ± 0.8 | 16.2 ± 1.0* | 13.2 ± 3.3* | 9.5 ± 1.4*# |
| HOMA-IR | 1.2 ± 0.2 | 3.7 ± 0.3* | 2.9 ± 0.8* | 1.7 ± 0.2 *# |
Data are presented as mean ± SEM. C, Caucasian; AA, African American; BMI, body mass index.
P < 0.05 vs lean;
P < 0.05 vs pre;
P <0.05 vs 1-month post-RYGB.
RYGB surgery restores insulin-mediated glucose partitioning in myotubes derived from severely obese humans.
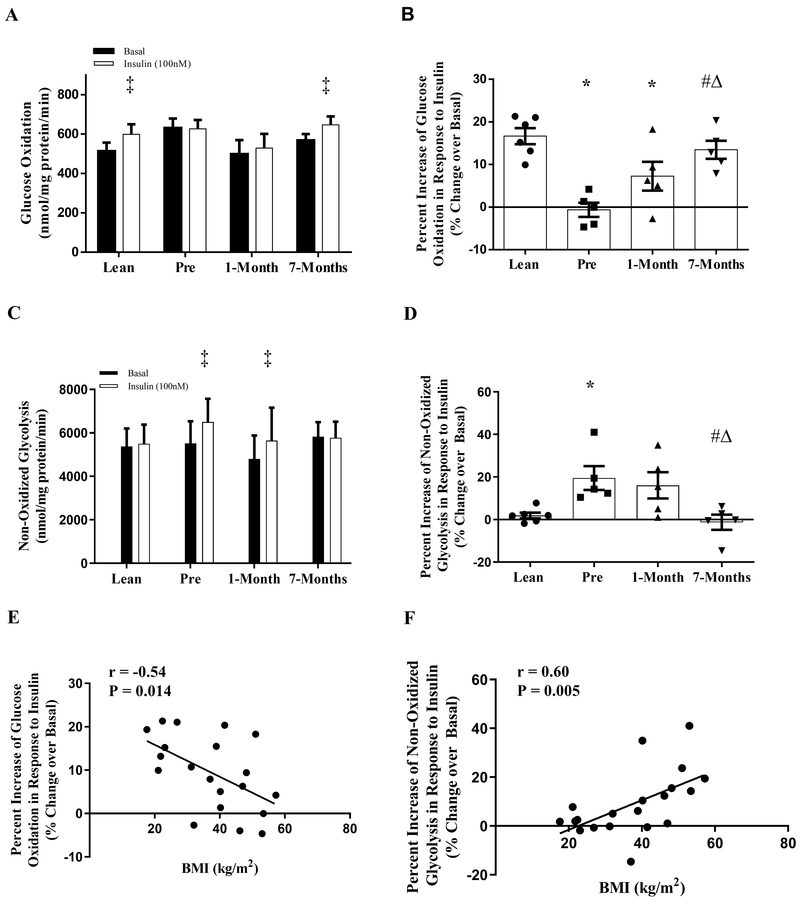
Myotubes from the lean subjects had a significant increase in glucose oxidation rate (16.7%, Fig. 1A, P < 0.05) without an increase in non-oxidized glycolysis rate in response to insulin stimulation (Figs. 1A, 1C); however, myotubes from the severely obese humans before RYGB surgery had the opposite response with a significant increase in non-oxidized glycolysis (19.5%, Fig. 1C, P < 0.05) and no increase in glucose oxidation rate (Fig. 1A) with insulin stimulation. Interestingly, myotubes from the same severely obese patients exhibited a trend of improvement in the relative change of glucose oxidation rate in response to insulin stimulation at 1-month following RYGB surgery when compared to pre-surgery (Fig 1B, P = 0.08). This improvement was further enhanced at 7-months following RYGB surgery (Fig. 1B, P < 0.05) with a concomitant reduction in relative non-oxidized glycolysis rate in response to insulin stimulation (Fig. 1D, P < 0.05). There was no difference in insulin-stimulated glucose partitioning in myotubes derived severely obese humans at 7-months following RYGB surgery in comparison to the lean controls (Fig 1B and D). In addition, glucose oxidation and non-oxidized glycolysis are negatively and positively correlated with BMI, respectively (Fig. 1E and F, r = −0.54 and 0.60 respectively, P < 0.05)
Figure 1:
Glucose partitioning in primary human myotubes derived from lean and severely obese humans before (Pre), 1-month, and 7-months after RYGB surgery. (A) Glucose oxidation under basal and insulin-stimulated conditions. (B) Percentage change of glucose oxidation in response to insulin stimulation. (C) Non-oxidized glycolysis under basal and insulin-stimulated conditions. (D) Percentage change of non-oxidized glycolysis in response to insulin stimulation. (E) Correlation BMI and percentage change of glucose oxidation in response to insulin stimulation. (F) Correlation between BMI and percentage change of non-oxidized glycolysis in response to insulin stimulation. Data are presented as mean ± SEM. n = 6, Lean; n = 5, Pre, 1-month, and 7-months post-RYGB. ‡ P < 0.05 vs. Basal; * P < 0.05 vs. Lean; # P < 0.05 vs. Pre; Δ P < 0.05 vs. 1-month.
RYGB surgery did not alter mitochondrial respiratory capacity or content in myotubes derived from severely obese humans.
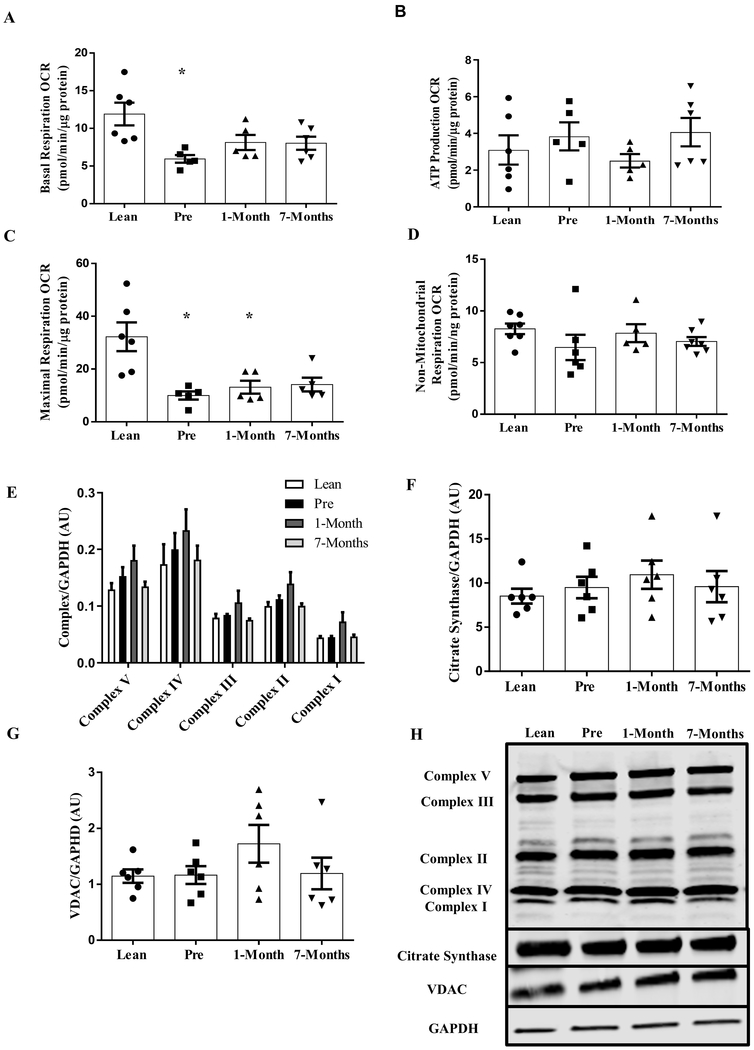
Both basal and FCCP-induced maximal respiratory rates were significantly lower in myotubes derived from severely obese patients before RYGB surgery than the lean controls (Fig. 2A and C, P <0.05). Myotubes from the same severely obese patients following RYGB surgery exhibited no differences in basal and FCCP-induced maximal respiratory rates when compared to the pre-surgery state (Fig. 2A and C). There were no differences in oligomycin-induced and non-mitochondrial respiration among any of the groups (Fig. 2B and D). The protein content of various complexes of the oxidative phosphorylation system (OXPHOS), citrate synthase and VDAC in myotubes established from the severely obese humans were not different from the lean controls, and RYGB surgery did not change any of these after surgery (Fig. 2E–G).
Figure 2:
Mitochondrial respiration in primary human myotubes derived from lean and severely obese humans before (Pre), 1-month, and 7-months after RYGB surgery. (A) Basal respiration. (B) ATP production. (C) FCCP-induced maximal respiration. (D) Non-mitochondrial respiration. (E) OXPHOS protein content. (F) Citrate Synthase protein expression. (G) VDAC protein expression. (H) Representative immunoblots for OXPHOS, Citrate Synthase, VDAC and GAPDH. Data are presented as mean ± SEM. n = 6, Lean; n = 6, Pre, 1-month, and 7-months post-RYGB. * P < 0.05 vs. Lean.
RYGB surgery improves mitochondrial network morphology in myotubes derived from severely obese humans that exhibit fragmented mitochondrial network.
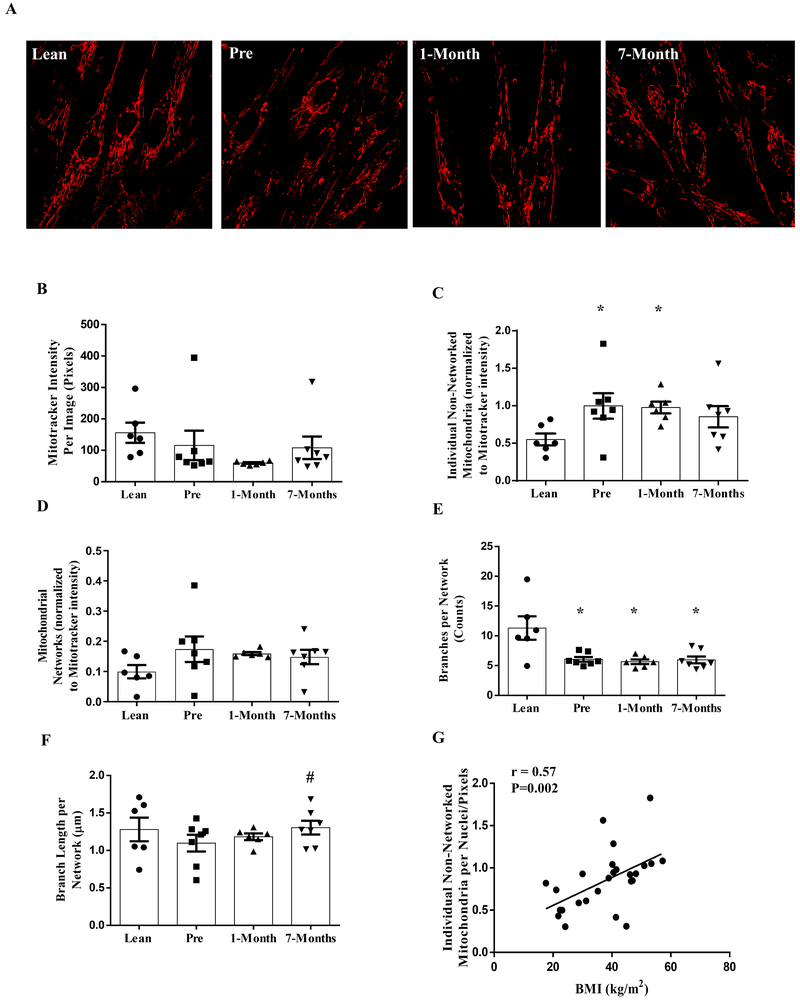
Representative images of the mitochondrial network morphology from myotubes from each group are presented (Fig. 3A). Total intensity of MitoTracker was not different in myotubes derived from severely obese and lean control humans, and RYGB surgery did not alter the intensity (Fig. 3B). However, myotubes derived from severely obese individuals before RYGB surgery exhibited an increase in the number of non-networked individual mitochondria and a reduced mitochondrial network size as measured by the number of interconnecting branches in each mitochondrial network (Fig. 3C and E, P <0.05) which is an indication of mitochondrial fragmentation. Although there were no changes in the number of mitochondrial network and interconnecting branches in each network following RYGB surgery in comparison to pre-surgery state (Fig. 3D and E), myotubes derived from these severely obese individuals exhibited a significant enhancement in mitochondrial branch length (Fig. 3F, P < 0.05) and a trend in the reduction of non-networked individual mitochondria (Fig. 3C, P = 0.091) at 7-months following RYGB surgery. This suggests that RYGB surgery reduces mitochondrial fragmentation in myotubes derived from severely obese humans. Furthermore, the number of non-networked individual mitochondria was positively correlated with BMI (Fig. 3G, r = 0.57, P < 0.05).
Figure 3:
Mitochondrial morphology in primary human myotubes derived from lean and severely obese humans before (Pre), 1-month, and 7-months after RYGB surgery. (A) Representative images of myotubes stained with MitoTracker™ RedFM. (B) Mitotracker intensity. (C) Number of individual non-networked mitochondria (D) Number of mitochondrial networks. (E) Number of branches per network (network size). (F) Average branch length per network (network size). (G) Correlation between body mass index (BMI) and the number of individual non-networked mitochondria. Data are presented as mean ± SEM. n = 6, Lean and 1-month post-RYGB; n = 7, Pre and 7-months post-RYGB. * P < 0.05 vs. Lean; # P < 0.05 vs. Pre.
RYGB surgery reduces mitochondrial fission marker Drp1 phosphorylation in myotubes derived from severely obese humans.
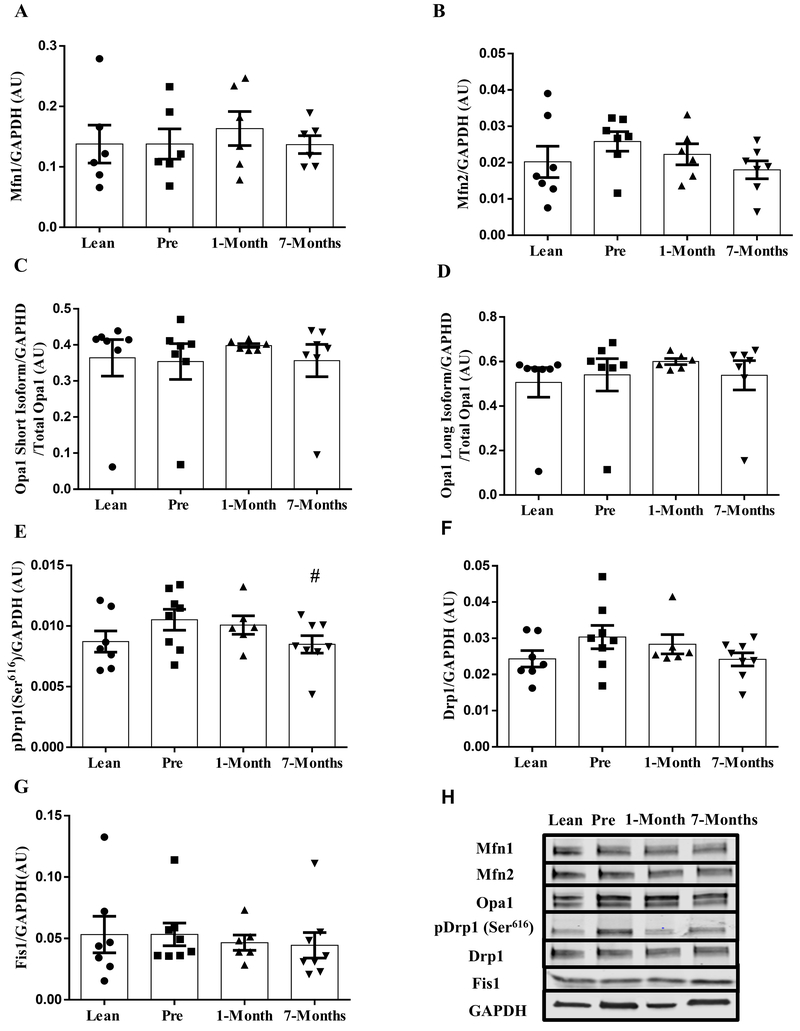
There were no differences in mitochondrial fusion protein expression among any groups (Fig. 4A–D). However, myotubes derived from severely obese humans had a reduction in Drp1(Ser616) phosphorylation at 7-months following RYGB surgery in comparison to the pre-surgery time point (Fig. 4E, P = 0.05). No differences were found in total Drp1 and Fis1 protein expressions among any groups (Fig. 4F and G).
Figure 4:
Protein expression of mitochondrial dynamics markers in primary human myotubes derived from lean and severely obese humans before (Pre), 1-month, and 7-months after RYGB surgery. (A) MFN1. (B) MFN2. (C) OPA1 short isoform. (D) OPA1 long isoform. (E) pDrp1(ser616). (F) Drp1. (G) Fis1. (H) Representative immunoblots for mitochondrial dynamics markers. Data are presented as mean ± SEM. n = 7, Lean, Pre, and 7-months post-RYGB; n = 6, 1-month post-RYGB. # P < 0.05 vs. Pre.
In addition, DNM1L (Gene coding for Drp1) mRNA expression was significantly reduced at 7-months following RYGB surgery in comparison to the lean control (Supplement Fig. 1D, P < 0.05). Other markers involved in mitochondrial dynamics (fission and fusion) exhibited no significant difference between any groups (Supplementary Fig. 1A, B, C and E)
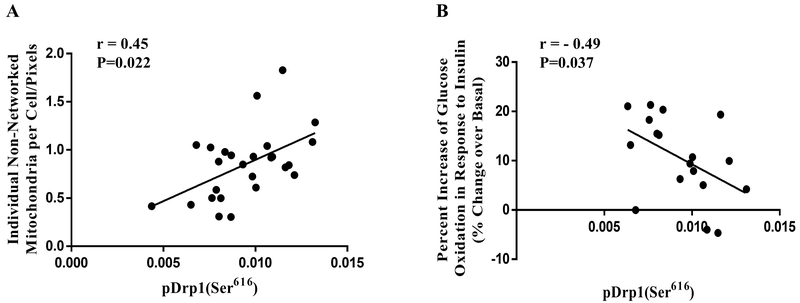
Drp1(Ser616) phosphorylation is negatively correlated with insulin-stimulated glucose oxidation.
There was a positive correlation between Drp1(Ser616) phosphorylation and individual non-networked mitochondria (Fig. 5A; r = 0.45, P = 0.022). Furthermore, Drp1(Ser616) phosphorylation was negativity correlated with relative change of glucose oxidation in response to insulin stimulation (Fig 5B; r = −0.49, P = 0.037).
Figure 5:
(A) Correlation between Drp1(ser616) phosphorylation and individual non-networked mitochondria (r=0.45, P = 0.022). (B) Correlation between Drp1(ser616) phosphorylation and percentage change of glucose oxidation in response to insulin stimulation (r=−0.49, P = 0.037).
DISCUSSION
It has been well-documented that RYGB surgery improves glucose metabolism in severely obese humans at both whole body and skeletal muscle levels 4,8,9,25. By using the human skeletal muscle cell culture model, our study found that impaired glucose partitioning, an intrinsic metabolic defect in skeletal muscle with severe obesity 1, was significantly improved at 7-months (chronic phase), but not 1-month (acute phase) after surgery with concomitant improvement in mitochondrial network morphology and reduction in mitochondrial fission protein Drp1 activation. In addition, our study added novel knowledge that RYGB surgery not only reprograms intrinsic metabolic characteristics in skeletal muscle from severely obese humans, but more impressively, restores some measures (i.e., glucose partitioning and mitochondrial dynamics) to levels in lean healthy individuals at chronic phase after surgery despite the fact these individuals are still obese (BMI > 30 kg/m2).
We recently reported that the intracellular fates of glucose were impaired in a manner that favors partitioning to non-oxidized glycolysis products over complete glucose oxidation under insulin stimulation 1. Such derangement in glucose partitioning can have potential severe consequences on whole-body glucose homeostasis because lactate is a precursor of hepatic gluconeogenesis and excessive lactate production from skeletal muscle could potentially increase glucose production by the liver and contribute to hyperglycemia 2. In the present study, we observed that the derangement in glucose partitioning in response to insulin stimulation was restored to the level of lean controls after RYGB surgery. Our findings are consistent with previous studies and expand the growing evidence that RYGB surgery can remodel the metabolic characteristics related to glucose metabolism in human skeletal muscle 10,11. In relation to the whole-body metabolic improvements after RYGB surgery, it can be postulated that the reduced production of glycolytic products (e.g., lactate) may possibly lead to the decrease of supplying glyconeogenic substrates to the liver, hence reducing glyconeogenesis and re-establishing whole body glycemic control after RYGB surgery. Findings of decreased blood levels of gluconeogenic substrates after RYGB surgery from previous studies 26,27 are in support of this hypothesis. Lastly, the strong correlation between insulin-mediated glucose partitioning and BMI suggests that such improvement may be due to the significant weight and/or fat mass loss after RYGB. Gaster et. al., reported that insulin-mediated glucose oxidation rates were significantly dampened in primary myotubes when exposure to free fatty acids 28, suggesting obesity-induced dyslipidemia may impair the intrinsic ability of skeletal muscle to oxidize glucose and RYGB-induced fat mass loss may have corrected this intrinsic metabolic defect.
In the present study, myotubes derived from severely obese individuals after RYGB surgery exhibited more interconnected networks with less fragmented individual mitochondria. Our observation is in agreement with a previous study showing improved mitochondrial network morphology in skeletal muscle tissue after RYGB surgery 15, but adds novel information that such remodeling of mitochondrial structure was “imprinted” in skeletal muscle cells after RYGB surgery. Given that systemic factors are eliminated in the human muscle cell culture system, our results indicate that the improvement in the mitochondrial network in skeletal muscle after RYGB surgery is likely due to the intrinsic remodeling of muscle cells. Furthermore, the positive correlation between the number of fragmented individual mitochondria and BMI suggests that the improvement of mitochondrial network morphology after RYGB surgery may be due to RYGB-induced weight loss. Multiple previous studies have shown that energy deficit (occurs during weight loss) leads to a more connected mitochondrial network 29–31, which corroborates our hypothesis that RYGB-induced weight loss may lead to the improvement of mitochondrial network morphology in skeletal muscle.
We next investigated the expression of regulatory proteins in mitochondrial dynamics and found a reduction in Drp1(ser616) phosphorylation without any change of the mitochondrial fusion machinery following RYGB surgery, suggesting the alleviation of mitochondrial fission may contribute to the improved balance of mitochondrial dynamics and mitochondrial network morphology. In contrast to a previous study 15, we did not find any change of MFN1 or MFN2 protein expression after RYGB. One possible explanation for this discrepancy is the gender differences in study population. The subjects in the current study were exclusively females while previous studies included both males and females. Sexual dimorphism has been well documented in mitochondrial alterations in response to various signals 32. Therefore, future studies should consider gender differences in mitochondrial adaptations following RYGB surgery.
In this study, we observed that FCCP-induced maximal respiration was compromised in myotubes established from severely obese humans but did not improve following RYGB surgery. These data are in agreement with previous reports showing that reduced mitochondrial respiratory capacity was imprinted in skeletal muscle cells derived from severely obese humans 33–37 and RYGB surgery was not able to reverse it 34,38–40. This observation, along with improvements in mitochondrial network morphology and dynamics protein Drp1 activation at 7-months after RYGB, suggests that alterations in mitochondrial dynamics may precede improvements in mitochondrial respiratory capacity. Although mitochondrial uncoupled maximal respiration was not changed after RYGB surgery, it is important to point out that the results from our study does not rule out other improvements related to mitochondrial function. It has been reported that ADP-stimulated State III respiration was improved after weight loss surgery 34,38,39. Thus, future studies should seek to use more comprehensive approaches to assess mitochondrial function in primary myotubes from RYGB patients.
Drp1-mediated mitochondrial fission has been linked to metabolic derangements in skeletal muscle 14–17,41. Our finding of a positive correlation between Drp1(Ser616) phosphorylation and insulin-stimulated glucose oxidation provides more evidence to support this notion. In addition, our data also suggests that reduced Drp1 phosphorylation may be a contributor to the improvement of insulin-stimulated glucose partitioning observed in myotubes from severely obese humans after RYGB surgery. The precise mechanism by which reduced Drp1 activation may improve intracellular glucose partitioning is still unknown. However, a recent study reported that Drp1 activation markedly increased expressions of pyruvate dehydrogenase kinase (PDK) and lactate dehydrogenase A (LDH-A), the key proteins involved in glucose partitioning that shift glucose towards glycolysis over complete glucose oxidation, while inactivation of Drp1 inhibited both gene expressions in tumor cells 42. Therefore, it is conceivable that reduced activity of Drp1 in skeletal muscle after RYGB surgery may alter the expression of PDK4 and LDH-A, which then shift intracellular glucose metabolism towards glucose oxidation. This postulation is supported by a recent study conducted by Barres et.al, who reported that PDK4 promoter methylation and mRNA expression were normalized to the lean control level in skeletal muscle after RYGB surgery 43. In order to mechanistically understand the role of Drp1 in regulating glucose partitioning in skeletal muscle, future studies should evaluate glycolytic pathway and glucose partitioning in skeletal muscle cells from severely obese humans by inhibiting Drp1 activation using genetic or pharmacological approaches.
It is noteworthy that physical activity level was not measured before or after RYGB surgery in the current study, which is a potential limitation. The findings of physical activity level in patients after RYGB surgery are mixed 44–46. A recent study reported that physical activity was moderately increased after RYGB surgery 44. It was also recently reported that exercise training reduced pDrp1616 and improved insulin sensitivity in skeletal muscle from obese humans. 29 Therefore, we cannot exclude the possible effects of increased physical activity on the improvements in mitochondrial dynamics and glucose metabolism in primary myotubes raised from RYGB patients. In fact, the similar improvements in myotubes derived from obese humans at the chronic stage after RYGB surgery (7-months) 10 and after exercise training 47,48 suggests that RYGB surgery-induced weight loss and exercise training may share the same mechanisms for improving intrinsic phenotypes of mitochondrial dynamics and glucose metabolism in skeletal muscle from obese humans.
In summary, shunting glucose towards glycolysis rather than oxidation, a metabolic defect with severe obesity, was restored to lean, healthy levels at 7 months after RYGB. This intrinsic improvement in skeletal muscle occurs concomitantly with improved mitochondrial network morphology and reduced activation of the mitochondrial fission protein Drp1that are imprinted in skeletal muscle from severely obese humans after RYGB. Future studies targeting Drp1will provide more mechanistic insights into how Drp1 regulates insulin-mediated glucose metabolism and Drp1 may emerge as a novel target for developing therapeutics to treat obesity-related metabolic disorders.
Supplementary Material
ACKNOWLEDGMENTS
Funding
This study was supported by the University of Massachusetts Boston (startup fund to K.Z.) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK-56112 to J.A.H.)
Sources of Support: This study was supported by the University of Massachusetts Boston (startup fund to K.Z.) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK-56112 to J.A.H.)
Footnotes
CONFLICTS OF INTEREST: No potential conflicts of interest were reported.
CONFLICT OF INTEREST
No potential conflicts of interest relevant to this article were reported.
Supplementary information is available at International Journal of Obesity’s website
References:
- 1.Zou K, Hinkley JM, Park S, Zheng D, Jones TE, Pories WJ et al. Altered tricarboxylic acid cycle flux in primary myotubes from severely obese humans. Int J Obes 2018; : 1. [DOI] [PubMed] [Google Scholar]
- 2.Consoli A, Nurjhan N, Reilly JJ, Bier DM, Gerich JE. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabilism. J Clin Invest 1990. doi: 10.1172/JCI114940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ et al. Weight and Type 2 Diabetes after Bariatric Surgery: Systematic Review and Meta-analysis. Am J Med 2009; 122: 248–256. [DOI] [PubMed] [Google Scholar]
- 4.Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011; 54: 2093–2102. [DOI] [PubMed] [Google Scholar]
- 5.Albers PH, Bojsen-Møller KN, Dirksen C, Serup AK, Kristensen DE, Frystyk J et al. Enhanced insulin signaling in human skeletal muscle and adipose tissue following gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 2015; 309: R510–24. [DOI] [PubMed] [Google Scholar]
- 6.Bojsen-Møller KN, Dirksen C, Jørgensen NB, Jacobsen SH, Serup AK, Albers PH et al. Early Enhancements of Hepatic and Later of Peripheral Insulin Sensitivity Combined With Increased Postprandial Insulin Secretion Contribute to Improved Glycemic Control After Roux-en-Y Gastric Bypass. Diabetes 2014; 63: 1725–1737. [DOI] [PubMed] [Google Scholar]
- 7.Dirksen C, Jørgensen NB, Bojsen-Møller KN, Jacobsen SH, Hansen DL, Worm D et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia 2012; 55: 1890–1901. [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen NB, Jacobsen SH, Dirksen C, Bojsen-Moller KN, Naver L, Hvolris L et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. AJP Endocrinol Metab 2012. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen NB, Dirksen C, Bojsen-Møller KN, Kristiansen VB, Wulff BS, Rainteau D et al. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J Clin Endocrinol Metab 2015. doi: 10.1210/jc.2014-1658. [DOI] [PubMed] [Google Scholar]
- 10.Hinkley JM, Zou K, Park S, Zheng D, Dohm GL, Houmard JA. Differential acute and chronic responses in insulin action in cultured myotubes following from nondiabetic severely obese humans following gastric bypass surgery. Surg Obes Relat Dis 2017. doi: 10.1016/j.soard.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Nascimento EBM, Riedl I, Jiang LQ, Kulkarni SS, Näslund E, Krook A. Enhanced glucose metabolism in cultured human skeletal muscle after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 2015. doi: 10.1016/j.soard.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Martin SD, McGee SL. The role of mitochondria in the aetiology of insulin resistance and type 2 diabetes. Biochim Biophys Acta 2014; 1840: 1303–12. [DOI] [PubMed] [Google Scholar]
- 13.Fealy CE, Mulya A, Axelrod CL, Kirwan JP. Mitochondrial dynamics in skeletal muscle insulin resistance and type 2 diabetes. Transl Res 2018; 202: 69–82. [DOI] [PubMed] [Google Scholar]
- 14.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J et al. Mitofusin-2 Determines Mitochondrial Network Architecture and Mitochondrial Metabolism. J Biol Chem 2003; 278: 17190–17197. [DOI] [PubMed] [Google Scholar]
- 15.Kristensen MD, Petersen SM, Møller KE, Lund MT, Hansen M, Hansen CN et al. Obesity leads to impairments in the morphology and organization of human skeletal muscle lipid droplets and mitochondrial networks, which are resolved with gastric bypass surgery-induced improvements in insulin sensitivity. Acta Physiol 2018; : e13100. [DOI] [PubMed] [Google Scholar]
- 16.Greene NP, Lee DE, Brown JL, Rosa ME, Brown LA, Perry RA et al. Mitochondrial quality control, promoted by PGC-1α, is dysregulated by Western diet-induced obesity and partially restored by moderate physical activity in mice. Physiol Rep 2015. doi: 10.14814/phy2.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jheng H-F, Tsai P-J, Guo S-M, Kuo L-H, Chang C-S, Su I-J et al. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol 2012; 32: 309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: Effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor and interleukin-6. Diabetes 2005; 54: 2685–2693. [DOI] [PubMed] [Google Scholar]
- 19.Prieto J, León M, Ponsoda X, Sendra R, Bort R, Ferrer-Lorente R et al. Early ERK1/2 activation promotes DRP1-dependent mitochondrial fission necessary for cell reprogramming. Nat Commun 2016; 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aas V, Bakke SS, Feng YZ, Kase ET, Jensen J, Bajpeyi S et al. Are cultured human myotubes far from home? Cell Tissue Res 2013; 354: 671–682. [DOI] [PubMed] [Google Scholar]
- 21.Pories WJ, MacDonald KG, Flickinger EG, Dohm GL, Sinha MK, Barakat HA et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg 1992; 215: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinkley JM, Zou K, Park S, Turner K, Zheng D, Houmard JA. Roux-en-Y gastric bypass surgery enhances contraction-mediated glucose metabolism in primary human myotubes. Am J Physiol - Endocrinol Metab 2017. doi: 10.1152/ajpendo.00413.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muoio D, Way J, Tanner C, Winegar D, Houmard J, Kraus W et al. Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes 2002; 51: 901–909. [DOI] [PubMed] [Google Scholar]
- 24.Valente AJ, Maddalena LA, Robb EL, Moradi F, Stuart JA. A simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture. Acta Histochem 2017; 119: 315–326. [DOI] [PubMed] [Google Scholar]
- 25.Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: Role of gut peptides. J Clin Endocrinol Metab 2011. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 26.Lopes TIB, Geloneze B, Pareja JC, Calixto AR, Ferreira MMC, Marsaioli AJ. “Omics” Prospective Monitoring of Bariatric Surgery: Roux-En-Y Gastric Bypass Outcomes Using Mixed-Meal Tolerance Test and Time-Resolved 1 H NMR-Based Metabolomics. Omi A J Integr Biol 2016. doi: 10.1089/omi.2016.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoo CM, Muehlbauer MJ, Stevens RD, Pamuklar Z, Chen J, Newgard CB et al. Postprandial metabolite profiles reveal differential nutrient handling after bariatric surgery compared with matched caloric restriction. Ann Surg 2014. doi: 10.1097/SLA.0b013e318296633f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaster M, Beck-Nielsen H. The reduced insulin-mediated glucose oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin - Evidence from cultured myotubes. Biochim Biophys Acta - Mol Basis Dis 2004. doi: 10.1016/j.bbadis.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Fealy CE, Mulya A, Lai N, Kirwan JP. Exercise training decreases activation of the mitochondrial fission protein dynamin-related protein-1 in insulin-resistant human skeletal muscle. J Appl Physiol 2014; 117: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci 2011. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toledo FGS, Watkins S, Kelley DE. Changes induced by physical activity and weight loss in the morphology of intermyofibrillar mitochondria in obese men and women. J Clin Endocrinol Metab 2006. doi: 10.1210/jc.2006-0002. [DOI] [PubMed] [Google Scholar]
- 32.Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V et al. Mitochondria: a central target for sex differences in pathologies. Clin Sci 2017. doi: 10.1042/CS20160485. [DOI] [PubMed] [Google Scholar]
- 33.Paran CW, Verkerke ARP, Heden TD, Park S, Zou K, Lawson HA et al. Reduced efficiency of sarcolipin-dependent respiration in myocytes from humans with severe obesity. Obesity 2015. doi: 10.1002/oby.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vijgen GHEJ Bouvy ND, Hoeks J, Wijers S, Schrauwen P, Van Marken Lichtenbelt WD. Impaired skeletal muscle mitochondrial function in morbidly obese patients is normalized one year after bariatric surgery. Surg Obes Relat Dis 2013; 9: 936–941. [DOI] [PubMed] [Google Scholar]
- 35.Bakkman L, Fernström M, Loogna P, Rooyackers O, Brandt L, Lagerros YT. Reduced respiratory capacity in muscle mitochondria of obese subjects. Obes Facts 2010; 3: 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritov VB, Menshikova E V., Azuma K, Wood R, Toledo FGS, Goodpaster BH et al. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. AJP Endocrinol Metab 2010; 298: E49–E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyle KE, Zheng D, Anderson EJ, Darrell Neufer P, Houmard JA. Mitochondrial Lipid Oxidation is Impaired in Cultured Myotubes from Obese Humans. Int J Obes 2012; 36: 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernström M, Bakkman L, Loogna P, Rooyackers O, Svensson M, Jakobsson T et al. Improved Muscle Mitochondrial Capacity Following Gastric Bypass Surgery in Obese Subjects. Obes Surg 2016; 26: 1391–1397. [DOI] [PubMed] [Google Scholar]
- 39.Coen PM, Menshikova E V, Distefano G, Zheng D, Tanner CJ, Standley RA et al. Exercise and Weight Loss Improve Muscle Mitochondrial Respiration, Lipid Partitioning, and Insulin Sensitivity After Gastric Bypass Surgery. Diabetes 2015; 64: 3737–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nijhawan S, Richards W, O’Hea MF, Audia JP, Alvarez DF. Bariatric surgery rapidly improves mitochondrial respiration in morbidly obese patients. Surg Endosc 2013; 27: 4569–4573. [DOI] [PubMed] [Google Scholar]
- 41.Chen CCW, Erlich AT, Hood DA. Role of Parkin and endurance training on mitochondrial turnover in skeletal muscle. Skelet Muscle 2018; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Huang Q, Long X, Guo X, Sun X, Jin X et al. Mitochondrial elongation-mediated glucose metabolism reprogramming is essential for tumour cell survival during energy stress. Oncogene 2017. doi: 10.1038/onc.2017.98. [DOI] [PubMed] [Google Scholar]
- 43.Barres R, Kirchner H, Rasmussen M, Yan J, Kantor FR, Krook A et al. Weight Loss after Gastric Bypass Surgery in Human Obesity Remodels Promoter Methylation. Cell Rep 2013. doi: 10.1016/j.celrep.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Wefers JF, Woodlief TL, Carnero EA, Helbling NL, Anthony SJ, Dubis GS et al. Relationship among physical activity, sedentary behaviors, and cardiometabolic risk factors during gastric bypass surgery–induced weight loss. Surg Obes Relat Dis 2017. doi: 10.1016/j.soard.2016.08.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berglind D, Willmer M, Eriksson U, Thorell A, Sundbom M, Uddén J et al. Longitudinal Assessment of Physical Activity in Women Undergoing Roux-en-Y Gastric Bypass. Obes Surg 2015. doi: 10.1007/s11695-014-1331-x. [DOI] [PubMed] [Google Scholar]
- 46.Berglind D, Willmer M, Tynelius P, Ghaderi A, Näslund E, Rasmussen F. Accelerometer-Measured Versus Self-Reported Physical Activity Levels and Sedentary Behavior in Women Before and 9 Months After Roux-en-Y Gastric Bypass. Obes Surg 2016. doi: 10.1007/s11695-015-1971-5. [DOI] [PubMed] [Google Scholar]
- 47.Bourlier V, Saint-Laurent C, Louche K, Badin PM, Thalamas C, Glisezinski I De et al. Enhanced glucose metabolism is preserved in cultured primary Myotubes from obese donors in response to exercise training. J Clin Endocrinol Metab 2013; 98: 3739–3747. [DOI] [PubMed] [Google Scholar]
- 48.Park S, Turner KD, Zheng D, Brault JJ, Zou K, Chaves AB et al. Electrical pulse stimulation induces differential responses in insulin action in myotubes from severely obese individuals. J Physiol 2019. doi: 10.1113/JP276990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.