Abstract
PURPOSE:
The aim of this study was to assess the potential risk factors and causes of infectious and sterile keratitis after accelerated collagen cross-linking.
METHODS:
Case records of 968 eyes that underwent accelerated corneal collagen cross-linking (ACXL) over the period of 4 years were reviewed retrospectively. ACXL was done using (Avedro KXL® system, Waltham, MA, USA) 9 mW/cm2 for 10 min protocol providing total energy of 5.4 J/cm2.
RESULTS:
Of 968 eyes, a total of three eyes developed infectious keratitis and seven eyes developed sterile infiltrates. Three of this infectious keratitis had two cases which were resistant to fourth-generation fluoroquinolones. Seven cases of sterile infiltrates had excellent resolution after treatment with topical steroids. Sterile infiltrates were common in corneas with thinnest pachymetry of <400 µm, except in one case of intra stromal corneal ring segments (INTACS) + ACXL.
CONCLUSION:
Judicious use of steroids in the initial postoperative period is recommended so as to prevent any form of microbial keratitis. Very steep corneas and too thin corneas should be looked with high index of suspicion in view of chances of developing sterile infiltrates.
Keywords: Accelerated collagen cross-linking, microbial keratitis, sterile infiltrates
Introduction
Since the advent of collagen cross-linking (CXL) after first published by Wollensak et al.,[1] it has become the gold standard in the treatment of keratoconus (KC), given its nature of minimally invasive modality along with safety. Conventionally, the older Dresden's[2] protocol for CXL has been followed for halting the progression of KC. Of late, the newer protocol with higher fluence and shorter duration also called accelerated corneal collagen cross-linking (ACXL) has been equally effective in the treatment of KC.[3,4] Although CXL is a safe procedure, infectious keratitis as a sight-threatening complication has been reported previously. Very recently, there had been isolated reports of infectious keratitis even after ACXL. Koller et al.[5] reported the largest case series of nine patients with peripheral sterile corneal infiltrates after conventional CXL. Even though sterile infiltrates following conventional CXL had been reported in the past, the literature on sterile infiltrates following ACXL is limited. After thorough literature review, we could find only one study by Çerman et al.[6] studying sterile infiltrates after ACXL protocol. The paucity of literature for this visually impairing complication and its noninfectious etiology following accelerated protocol prompted us to report this study.
In this study, we report a case series of infectious and sterile infiltrates after ACXL performed in our center over a period of 4 years.
Methods
Case records of 968 eyes that underwent ACXL (Avedro KXL® system, Waltham, MA, USA) for progressive KC over a period of 4 years (September 2013 to September 2017) at a tertiary eye care hospital in South India were analyzed retrospectively. Of these, 32 cases underwent INTACS + ACXL so as to improve the overall quality of vision along with strengthening procedure. Data collected included patient's age, sex, ocular medical and surgical history, Pentacam (Oculus Optikgeräte GmbH, Wetzlar, Germany), visual acuity before and after surgery, and complications. The study protocol adhered to the tenets of the Declaration of Helsinki. The study was approved by the institutional review board.
Surgical procedure
Iso-osmolar ACXL was performed for cases with thinnest pachymetry of >400 µm, whereas hypo-osmolar ACXL was performed for cases with thinnest pachymetry <400 µm. ACXL (Avedro KXL® system, Waltham, MA, USA) was performed under topical anesthesia 0.5% proparacaine HCl (Aurocaine, Aurolab, Tamil Nadu, India). The corneal epithelium was debrided manually using a 15 number blade in the central 8–9 mm zone. Riboflavin phosphate (0.1%; 10 mg of riboflavin-5-phosphate in 10 mL of dextran-T-500, 20% solution for iso-osmolar cases and 0.1% riboflavin in 0.9% saline instead of dextran for hypo-osmolar cases) was used for the procedure. After soaking time of 10 min with riboflavin, irradiation of 5.4 J/cm2 (9 mW/cm2 for 10 min was delivered) by ultraviolet A (UV-A) exposure (365-nm wavelength), from a distance of 5 cm. Following the procedure, a bandage contact lens (BCL) (O2 Optix contact lenses; Alcon, TX, USA) was placed and removed on the 4th postoperative day after the epithelium healed. Postoperatively, patients were given topical moxifloxacin hydrochloride 0.5% (Vigamox; Alcon, TX, USA) 4 times per day for 1 week. A low-potency topical steroid fluorometholone 0.1% 3 times per day (Allergan India Pvt. Ltd, Bengaluru, Karnataka, India) was administered after the epithelium healed and tapered over the next 3 weeks. All the cases were followed up at day 1, day 4, 1 month, 3 months, and 6 months. If there was any suspicion of keratitis, corneal scraping was done and the sample along with BCL was sent for microbiological examination (Gram stain and 10% KOH fresh mount), culture sensitivity testing (blood agar, Sabouraud dextrose agar, and nonnutrient agar with Escherichia coli overlay), and antibiotic sensitivity testing.
Results
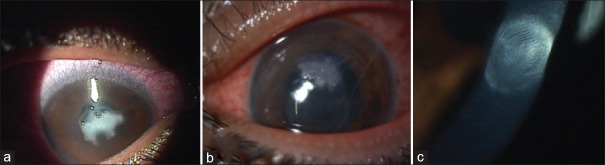
A total of 10 cases presented as postoperative keratitis after ACXL procedure. The baseline characteristics of these cases are shown in Table 1. Of the total, 3 cases were infectious keratitis revealing culture-positive Staphylococcus aureus. The minimum infiltrate size was 3 mm × 3 mm in the first case [Figure 1] and the maximum infiltrate size was 6 mm × 6 mm in the third case. Both these cases had hypopyon. The first case was treated with combination of 0.5% moxifloxacin and 1.3% tobramycin hourly and responded well with resolution over 3–4 weeks. The remaining two cases were started on hourly moxifloxacin empirically and shifted to fortified 5% cefazolin after the initial antibiotic sensitivity showed strains resistance to moxifloxacin. These two cases demonstrated resistance to fourth-generation fluoroquinolones (FQs) and responded well to cephalosporin. The first case healed well throughout 3–4 weeks with final best-corrected visual acuity (BCVA) of 6/12 at 6 months follow-up. The second and third case [Figures 2 and 3] also resolved over 3–4 weeks following treatment with fortified cefazolin.
Table 1.
Baseline demographics of cohort group
| Age (years) | Sex | Thinnest pachymetry (µm) | K-max (D)† | Type of keratitis | Ulcer location | Final BCVA | Sensitivity to fourth-generation FQs |
|---|---|---|---|---|---|---|---|
| 15 | Male | 456 | 49 | Infectious | Central | 6/12 | Sensitive |
| 18 | Female | 433 | 51 | Infectious | Central | 6/9 | Resistant |
| 22 | Male | 448 | 49 | Infectious | Central | 6/12 | Resistant |
| 24 | Male | 398 | 58 | Sterile | Mid-peripheral | 6/9 | Sensitive |
| 19 | Female | 390 | 55 | Sterile | Mid-peripheral | 6/12 | Sensitive |
| 14 | Male | 399 | 52 | Sterile | Mid-peripheral | 6/9 | Sensitive |
| 25 | Male | 385 | 60 | Sterile | Mid-peripheral | 6/9 | Sensitive |
| 21 | Male | 398 | 54 | Sterile | Mid-peripheral | 6/9 | Sensitive |
| 19 | Female | 392 | 49 | Sterile | Mid-peripheral | 6/9 | Sensitive |
| 24 | Male | 432 (INTACS) | 51 | Sterile | Mid-peripheral + central | 6/12 | Sensitive |
†Diopter. K-max: Maximum keratometry, BCVA: Best-corrected visual acuity, FQs: Fluoroquinolones, INTACS: Intra stromal corneal ring segments
Figure 1.
(a) Case of postaccelerated corneal collagen cross-linking microbial keratitis with central infiltrate, (b) after 3 weeks of treatment with moxifloxacin and tobramycin, (c) after 1 month of treatment, (d) at 6 months follow-up with nebular-macular scar
Figure 2.
(a) Case of postaccelerated corneal collagen cross-linking microbial keratitis with central infiltrate, (b) after 2 weeks of treatment with fortified 5% cefazolin, (c) after 1 month of treatment with nebular scar
Figure 3.
(a) Case of postaccelerated corneal collagen cross-linking microbial keratitis with central infiltrate and 3 mm hypopyon, (b) after 1 week of treatment with fortified 5% cefazolin, (c) after 1 month of treatment with nebular scar
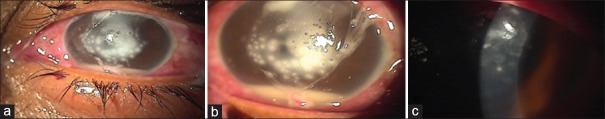
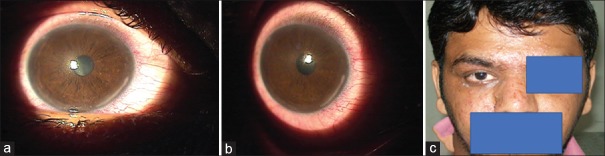
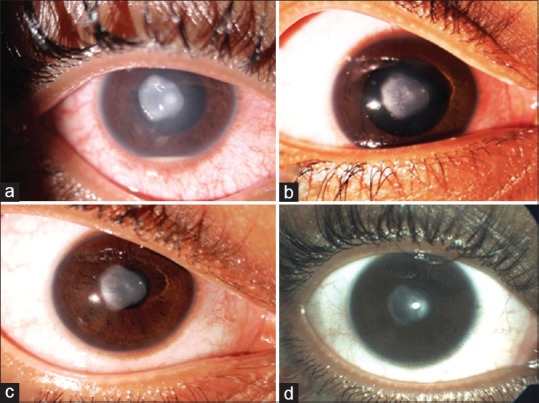
There were seven cases of sterile infiltrates of which one was after INTACS + ACXL. All seven cases were culture negative with no evidence of infectious etiology. Even though moxifloxacin was started empirically at the slightest suspicion of keratitis in all cases, it was stopped in these seven cases after initial culture report and patients were started only on topical steroids 0.1% prednisolone acetate eye drops (Pred Forte; Allergan, Irvine, CA, USA) hourly for first few days and then tapered gradually over 3–4 weeks depending on the response. Two patients with sterile infiltrates were operated on 1 day, while three others were operated on another day; 1 week apart with the same batch of riboflavin used, indicating a possible risk of contamination. The riboflavin sample was sent for culture and turned out to be negative for any infectious etiology. The concerned batch was replaced with fresh one and the company was informed. The six eyes with sterile infiltrates had a common presentation of mid-peripheral infiltrate occurring at the junction of epithelized and de-epithelized cornea on the 4th postoperative day, with no extension toward the center of the cornea [Figures 4-7]. The minimum infiltrate size was 1 mm × 1 mm and the maximum infiltrate size was 4 mm × 1 mm in the mid-peripheral region. One of these cases presented with peripheral infiltrate with marked skin rash and pustular lesions over the face [Figure 7c]. The seventh patient with INTACS + ACXL was normal on day 1 and presented with two focal infiltrates one at the junction of epithelized and de-epithelized cornea and other in the central area [Figure 8]. This infiltrate was again culture negative and responded well to regiment of topical steroids alone. The clinical features of lesions of each individual are shown in Table 2.
Figure 4.
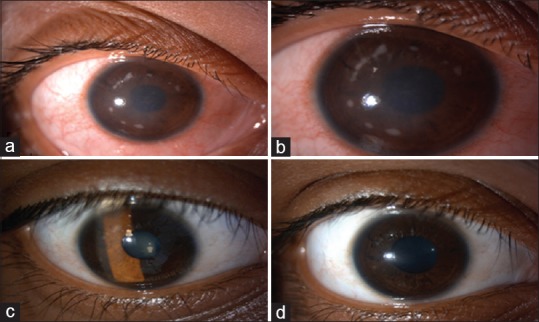
(a) Case of postaccelerated corneal collagen cross-linking sterile infiltrate in mid-periphery, (b) after 1 week of treatment with topical steroids, (c) after 3 weeks of treatment, (d) at 1-month follow-up with very faint scar in mid-periphery
Figure 7.
(a) Case of postaccelerated corneal collagen cross-linking sterile infiltrate in periphery involving 2 clock hours, (b) resolving infiltrate after 1 week of treatment with topical steroids, (c) the same patient presenting with pustular lesions on the face
Figure 8.
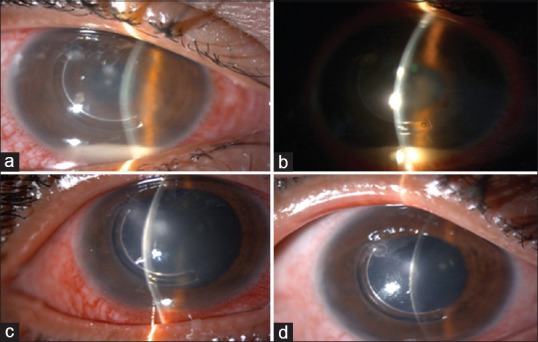
(a) Case of post-INTACS + accelerated corneal collagen cross-linking with central and paracentral sterile infiltrate along with 3 mm hypopyon, (b) after 1 week of treatment with topical steroids, (c) after 3 weeks of treatment, (d) at 6-week follow-up with nebular-macular scar
Table 2.
Clinical features of individual cases
| Type of keratitis | Location | Infiltrate size (mm) | Level of lesion | Focality | AC findings (hypopyon) | Suppuration | Conjunctival signs | VKC associated |
|---|---|---|---|---|---|---|---|---|
| Infectious | Central | 3×3 | Superficial stromal | Focal | Present | Absent | Moderate | No |
| Infectious | Central | 3.5×3 | Superficial stromal | Focal | Absent | Absent | Moderate | Yes |
| Infectious | Central | 6×6 | Deep stromal | Multiple foci | Present | Present | Marked | Yes |
| Sterile | Mid-peripheral | 1×1 | Superficial stromal | Multiple foci | Absent | Absent | Moderate | No |
| Sterile | Mid-peripheral | 2×1 | Superficial stromal | Multiple foci | Absent | Absent | Moderate | No |
| Sterile | Mid-peripheral | 3×2 | Superficial stromal | Multiple foci | Absent | Absent | Moderate | No |
| Sterile | Mid-peripheral | 2×1 | Superficial stromal | Multiple foci | Absent | Absent | Moderate | Yes |
| Sterile | Mid-peripheral | 2×1 | Superficial stromal | Multiple foci | Absent | Absent | Moderate | No |
| Sterile | Mid-peripheral | 4×1 | Superficial stromal | Focal | Absent | Absent | Moderate | Yes |
| Sterile (INTACS) | Mid-peripheral + central | 2×2 | Superficial stromal | 2 foci | Present | Absent | Marked | No |
AC: Anterior chamber, VKC: Vernal keratoconjunctivitis, INTACS: Intra stromal corneal ring segments
Figure 5.
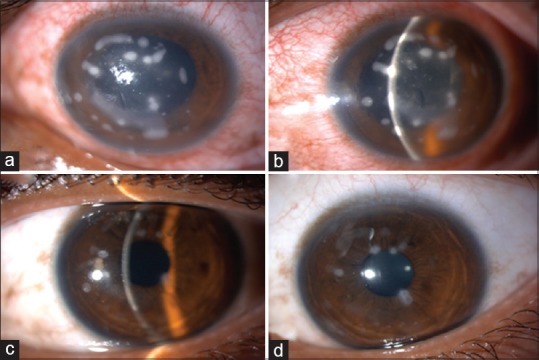
(a) Case of postaccelerated corneal collagen cross-linking sterile infiltrate in mid-periphery, (b) after 1 week of treatment with topical steroids, (c) after 3 weeks of treatment, (d) at 1-month follow-up with very faint scar in mid-periphery
Figure 6.
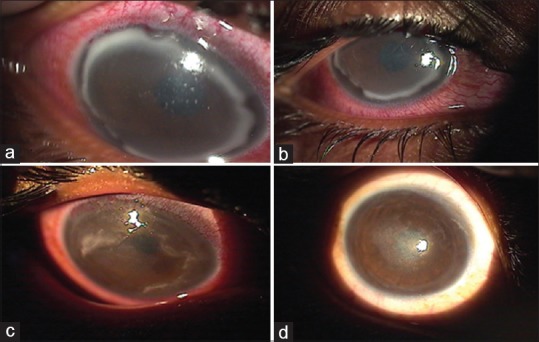
(a) Case of postaccelerated corneal collagen cross-linking sterile infiltrate in the periphery, (b) after 1 week of treatment with topical steroids, (c) after 2 weeks of treatment, (d) at 1-month follow-up with very faint scar in the periphery
Discussion
Postoperative keratitis following CXL is a dreadful sight-threatening complication. A lot of literature published previously had reported infectious keratitis after CXL, but very few information is available about complication after ACXL. Previous study by Kymionis et al.[7] described a patient who developed epithelial herpetic keratitis and iritis after CXL treatment. Zamora et al. reported culture-proven polymicrobial keratitis 3 days following CXL.[8] In our case series, we have reported not only cases of infectious keratitis (3 cases) after ACXL but also incidences of sterile infiltrates (7 cases) after ACXL.
As shown in Figure 1, the Kodavoor et al.[9] had already reported the first case of microbial keratitis after ACXL, where a 15-year-old male presented with a central deep stromal infiltrate and hypopyon on the 3rd day after the procedure. Complete resolution was noted at the end of 4 weeks with final BCVA of 6/12 at the end of 6 months of follow-up. The three cases of infectious keratitis in our series were culture positive for S. aureus; one of the probable explanation is that Staphylococcus spp. essentially being a part of normal microbial flora of ocular surface.[10] In the past, other reported organisms were Pseudomonas aeruginosa by Sharma et al.,[11] E. coli by Pollhammer and Cursiefen,[12] and Microsporidia by Gautam et al.[13] Shetty et al. in 2014 reported the case series of post-CXL microbial keratitis wherein four cases of moxifloxacin-resistant S. aureus strain were found.[14] All the cases were finally treated with fortified antibiotics, given the resistance of organism to fourth-generation FQs. In our series too, of the three cases, two cases of infectious keratitis were found to be resistant to fourth-generation FQs and responded well to fortified 5% cefazolin. Resistance to FQs could be explained due to rampant and irrational use of FQs in our country as an initial empirical treatment for any infectious keratitis, irrespective of its etiology. Maharana et al.[15] very recently reported seven cases of microbial keratitis after ACXL, three of which had mixed bacterial and fungal keratitis, two of which had infection with S. aureus, and coagulase-negative staphylococci and Alternaria spp. in one case each. They too reported resistance to fourth-generation FQs and patients were treated with fortified cefazolin and tobramycin. One hypothesis is that high power of UV-A radiation used in ACXL can induce mutations and increased drug resistance in Staphylococcus spp. The presence of associated vernal keratoconjunctivitis (VKC) was noted in 57.1% in this case series involving pediatric KC, hypothesizing that young age is itself a risk factor for post-CXL infection in developing countries where hygiene and environmental factors are of significant concern.[15] Furthermore, there is increased risk of postoperative keratitis due to chronic use of topical steroids in VKC cases presenting with KC later due to alteration in ocular flora.[16] However, in our case series, we found association of VKC in both infectious (two cases) and sterile keratitis (two cases).
We reported seven cases of sterile infiltrates of which one underwent INTACS + ACXL. All the cases presented with peripheral infiltrate on the 4th postoperative day. Gram stain and culture were negative in all seven cases, and they had excellent resolution over 3–4 weeks with topical steroid regiment alone. This could be probably due to the immunological and sterile nature of such a response pattern in the cornea after ACXL. After thorough literature review, we could not find any study that highlighted risk factors for the development of this sterile keratitis. One of the hypothesis is suggestive of antigenic alterations occurring in the native proteins after CXL, leading to patients recognizing the proteins as nonself and mounting an immune response.[17] The other hypothesis states that staphylococcal antigen deposition in areas of static tear pooling beneath the BCL can elicit a sterile immune response due to enhanced cell-mediated immunity.[18] Furthermore, one of the common findings in our case series of sterile keratitis was that the thinnest pachymetry was <400 µm except in one case with INTACS + ACXL where pachymetry was 432 µm. In addition, two cases of sterile keratitis had maximum keratometry >58 D. Lam et al.[19] concluded that individuals with thinner corneas and higher corneal curvatures appear to be at higher risk of developing a sterile infiltrate. A prospective study looking specifically at the complications of CXL found that no parameter was predictive for developing sterile infiltrates.[5]
Conclusion
Our study had certain limitations, being retrospective. It is probably the first largest reported case series of infectious and sterile keratitis combined after ACXL. Multiple risk factors such as a large epithelial defect and use of steroid in the immediate postoperative period may predispose the cornea to infectious keratitis in the postoperative period. In addition, a tailored approach of treating with fortified antibiotics rather than with FQs may help in early resolution of the infection. Despite developing sterile infiltrates, the prognosis for these patients is good. More number of prospective studies pertaining to complications of ACXL would throw light on mechanism of sterile infiltrates. We recommend that a very high index of suspicion should be maintained, especially when treating patients with thinner corneas and very steeper corneal curvatures.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 2.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–5. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 3.Wernli J, Schumacher S, Spoerl E, Mrochen M. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci. 2013;54:1176–80. doi: 10.1167/iovs.12-11409. [DOI] [PubMed] [Google Scholar]
- 4.Elbaz U, Shen C, Lichtinger A, Zauberman NA, Goldich Y, Chan CC, et al. Accelerated (9-mW/cm2) corneal collagen crosslinking for keratoconus – A 1-year follow-up. Cornea. 2014;33:769–73. doi: 10.1097/ICO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 5.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35:1358–62. doi: 10.1016/j.jcrs.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 6.Çerman E, Özcan DÖ, Toker E. Sterile corneal infiltrates after corneal collagen cross-linking: Evaluation of risk factors. Acta Ophthalmol. 2017;95:199–204. doi: 10.1111/aos.13218. [DOI] [PubMed] [Google Scholar]
- 7.Kymionis GD, Portaliou DM, Bouzoukis DI, Suh LH, Pallikaris AI, Markomanolakis M, et al. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. J Cataract Refract Surg. 2007;33:1982–4. doi: 10.1016/j.jcrs.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Zamora KV, Males JJ. Polymicrobial keratitis after a collagen cross-linking procedure with postoperative use of a contact lens: A case report. Cornea. 2009;28:474–6. doi: 10.1097/ICO.0b013e31818d381a. [DOI] [PubMed] [Google Scholar]
- 9.Kodavoor SK, Sarwate NJ, Ramamurhy D. Microbial keratitis following accelerated corneal collagen cross-linking. Oman J Ophthalmol. 2015;8:111–3. doi: 10.4103/0974-620X.159259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong RA. The microbiology of the eye. Ophthalmic Physiol Opt. 2000;20:429–41. [PubMed] [Google Scholar]
- 11.Sharma N, Maharana P, Singh G, Titiyal JS. Pseudomonas keratitis after collagen crosslinking for keratoconus: Case report and review of literature. J Cataract Refract Surg. 2010;36:517–20. doi: 10.1016/j.jcrs.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Pollhammer M, Cursiefen C. Bacterial keratitis early after corneal crosslinking with riboflavin and ultraviolet-A. J Cataract Refract Surg. 2009;35:588–9. doi: 10.1016/j.jcrs.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Gautam, Jhanji V, Satpathy G, Khokhar S, Agarwal T. Microsporidial keratitis after collagen cross-linking. Ocul Immunol Inflamm. 2013;21:495–7. doi: 10.3109/09273948.2013.824105. [DOI] [PubMed] [Google Scholar]
- 14.Shetty R, Kaweri L, Nuijts RM, Nagaraja H, Arora V, Kumar RS. Profile of microbial keratitis after corneal collagen cross-linking. Biomed Res Int. 2014;2014:340509. doi: 10.1155/2014/340509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maharana PK, Sahay P, Sujeeth M, Singhal D, Rathi A, Titiyal JS, et al. Microbial keratitis after accelerated corneal collagen cross-linking in keratoconus. Cornea. 2018;37:162–7. doi: 10.1097/ICO.0000000000001439. [DOI] [PubMed] [Google Scholar]
- 16.Ermis SS, Aktepe OC, Inan UU, Ozturk F, Altindis M. Effect of topical dexamethasone and ciprofloxacin on bacterial flora of healthy conjunctiva. Eye (Lond) 2004;18:249–52. doi: 10.1038/sj.eye.6700631. [DOI] [PubMed] [Google Scholar]
- 17.Ghanem RC, Netto MV, Ghanem VC, Santhiago MR, Wilson SE. Peripheral sterile corneal ring infiltrate after riboflavin-UVA collagen cross-linking in keratoconus. Cornea. 2012;31:702–5. doi: 10.1097/ICO.0b013e318226da53. [DOI] [PubMed] [Google Scholar]
- 18.Angunawela RI, Arnalich-Montiel F, Allan BD. Peripheral sterile corneal infiltrates and melting after collagen crosslinking for keratoconus. J Cataract Refract Surg. 2009;35:606–7. doi: 10.1016/j.jcrs.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 19.Lam FC, Georgoudis P, Nanavaty MA, Khan S, Lake D. Sterile keratitis after combined riboflavin-UVA corneal collagen cross-linking for keratoconus. Eye (Lond) 2014;28:1297–303. doi: 10.1038/eye.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]