Abstract
Ambidoxin is a designed, minimal dodecapeptide consisting of alternating L and D amino acids that binds a 4Fe–4S cluster through ligand–metal interactions and an extensive network of second-shell hydrogen bonds. The peptide can withstand hundreds of oxidation–reduction cycles at room temperature. Ambidoxin suggests how simple, prebiotic peptides may have achieved robust redox catalysis on the early Earth.
The 4Fe–4S cubane cluster is a ubiquitous inorganic cofactor mediating a wide variety of electron transfer reactions across the tree of life.1 The structurally conserved ferredoxin fold binds a 4Fe–4S cubane cluster with four cysteine residues in a common sequence motif (Cys-X1-X2-Cys-X3-X4-Cys-(X)n-Cys).2–4 The ferredoxin α/β topology is one of a small number of folds proposed to have given rise to myriad proteins.5 The origin and evolution of the ferredoxin fold family was likely a key evolutionary milestone in origins of biological electron transfer.6 Although the structures of ferredoxins are well studied, it has proven challenging to design simpler, artificial peptides that stably bind iron–sulfur complexes and are capable of reversible electron transfer reactions. De novo designs based on intrinsic symmetry of iron–sulfur clusters have produced artificial folds that bind metal and exhibit reversible redox stability for a few dozen cycles.7–9 Here, we present a minimal dodecapeptide ferredoxin, consisting of alternating L and D amino acid residues, comprised only of cysteine with arginine or lysine. This peptide binds an 4Fe–4S cluster through ligand–metal interactions and an extensive network of second-shell hydrogen bonds. The resulting “ambidoxin” peptide has robust redox stability; the holo-peptide can undergo hundreds of oxidation–reduction cycles at room temperature.
Analyses of backbone dihedral angles of iron–sulfur cluster binding heptapeptide (CXXCXXC) fragments from non-redundant structures in the Protein Data Bank (Figure 1a,b) revealed a five-residue alternating alpha-left and alpha-right structural motif. This motif is similar to the alpha-pleated sheet postulated by Pauling,10 and nest motifs observed by Watson and Milner-White.11,12 Extending this pattern to 12 residues produces a unique curved backbone that encircles the iron sulfur cluster (Figure 1c,d, S1). This unusual conformation could be enforced with a sequence of alternating L and D amino acids that stabilize alpha-right and alpha-left backbone conformations respectively (Figure S1g). Molecular dynamics simulations, starting from geometrically and energetically idealized conformations, converged on a unique stable structure that closely aligns with natural ferredoxin (Figure 1e, S1). Because of the equal contributions of L and D amino acids in the design, we refer to this class of molecules as “ambidoxins”.
Figure 1.
Design of ambidoxin. (a) Bacterial ferredoxin has a conserved CXXCXXC motif near iron sulfur cluster structures. (b) Ramachandran plot of the X1-X2-C-X3-X4 sequence motif surrounding the central cysteine reveals conserved alpha-right conformation (R) in X1 and X4 positions (green) and alpha-left conformation (L) in X2 an X3 positions (orange). (c) Ferredoxin loop was built by extending the original XXCXX peptide (αRαLαRαLαR conformation) with its mirror image (αLαRαLαRαL conformation), maintaining an alternating right/left conformation throughout. (d) Sequences and computationally determined structural models of ambidoxin peptides. (e) Portion of ambidoxin model and natural ferredoxin structures are super-imposable.
Given the stability of the 4Fe–4S cubane cluster in the absence of oxygen,13,14 we expected that reconstitution of peptides in the presence of iron and sulfur salts would assemble as a cluster fully coordinated by a single peptide. To test this, we synthesized three ambidoxin variants that included either basic or acidic amino acids at the positions between cysteines, and a tryptophan at the third position to allow quantification by absorption at 280 nm. Upon iron–sulfur cluster assembly, K and R ambidoxin peptides showed a 420 nm charge-transfer absorption band prior to reduction with sodium dithionite (Figure S6),15 and rhombic EPR signatures consistent with a bound 4Fe–4S cluster in the reduced state after treatment with sodium dithionite (Figure 2a, S8).16–18 Holo-peptide yields of 80–94% were determined by spin counting experiments. Dipole–dipole interactions between clusters were not observed by EPR, supporting the presence of a monomeric holo-peptide. After reconstitution, holo-peptides were stable for days under anaerobic conditions at room temperature from pH 5 to 8 (Figure S9).
Figure 2.
Structural characterization of ambidoxin. (a) X-band EPR spectra of a dithionite-reduced [4Fe–4S]+ cluster reconstituted in K and R peptides in HEPES/pH 5.0 buffer, measured at 9.48 GHz and 10 K (blue traces). The simulated spectra (red dashed traces) used three rhombic g-factor values (g1 = 2.0445, g2 = 1.9365, g3 = 1.903) as marked by vertical lines. (b) Cosine HYSCORE spectrum for [4Fe–4S]+ cluster with K peptide in deuterated HEPES buffer at pH 5.0, measured at magnetic field 340 mT (cp. the g1 field position in panel a) and temperature 12 K. Cross-peaks from three nonexchangeable cysteine protons (H1–H3) were resolved. (c) 600 MHz 1H NMR spectrum of a dithionite-reduced [4Fe–4S]+ cluster reconstituted in K peptide in 95% D2O HEPES-d18/pH 8.5 buffer measured at 278 K. Hyperfine shifted lines (labeled A–E) are from β-CH2 protons of four cysteines ligating to paramagnetic [4Fe–4S]+ cluster. Weak resonances marked with asterisks are from unknown minor (<10%) species. (d) Temperature dependence of the chemical shifts: Lines A–C with Curie temperature dependence are from four β-CH2 protons of two cysteine ligands bound to mixed-valence Fe2.5+−Fe2.5+ pair, and lines D–E with anti-Curie dependence are from two β-CH2 protons of two cysteine ligands bound to ferrous Fe2+−Fe2+ pair.
We did not observe cluster binding to the negatively charged E peptide (Figure S7). Assembled 4Fe–4S–4(Cys) complexes would have a −2 or −3 charge depending on the oxidation state. It is plausible that the significant charge associated with seven surrounding glutamates electrostatically destabilizes the holo-peptide, preventing assembly.
To validate the modeled structure of the paramagnetic peptide-cluster complex, we examined proton hyperfine interactions within 4 Å of the cluster by pulsed-EPR methods. Orientation-selective ESEEM and HYSCORE experiments were performed at several magnetic field positions across the rhombic EPR line-shape (Figures S10, S12–S17).19,20 Both techniques consistently identified several protons strongly coupled to an unpaired electron spin residing on the 4Fe–4S cluster. Experiments in deuterated buffer revealed that four protons (labeled H1–H4 in Figure 2b, S16–S17) were nonexchangeable and likely belong to β-protons of the four cysteine ligands. From the HYSCORE cross-peak analysis (Figure S18), we were able to extract isotropic and anisotropic hyperfine coupling constants of the four protons, including relative orientations of their anisotropic hyperfine tensors with respect to the g1 axis of electron g-factor (Table S2). These hyperfine parameters provided both Fe–H distances and angles between Fe–H vectors and the g1 axis, providing eight constraints that were consistent with models from molecular mechanics simulations of ambidoxin (Figure S19 and Table S2).
To resolve the structure and electronic state of the cluster at ambient temperature, we performed paramagnetic NMR experiments21 from 5 to 32 °C on holo-K peptide prepared to near-stoichiometric yield. At all temperatures, large chemical shifts were observed, with Curie and anti-Curie temperature dependencies indicative of strong hyperfine coupling between cysteine β-protons and mixed- or ferrous-valence iron, similar to those observed in natural protein environments22 (Figure 2c,d, Figure S20). Combining the HYSCORE-based analysis, the computational ambidoxin model, and the temperature dependencies of NMR derived chemical shifts, we assigned three contact-shifted 1H resonances to D-Cys2 and L-Cys5 Hβ atoms, ligating a mixed-valence Fe2.5+ pair, and two other 1H resonances (Figure 2c) to D-Cys8 and L-Cys11 Hβ atoms, ligating a ferrous Fe2+ pair (Figures S19 and S20). The temperature-dependent shifts were fully reversible and persistent at both pH 5 and pH 8.5 (Figure S20). These results demonstrate a stable peptide-cluster coordination structure around the bound paramagnetic 4Fe–4S center.
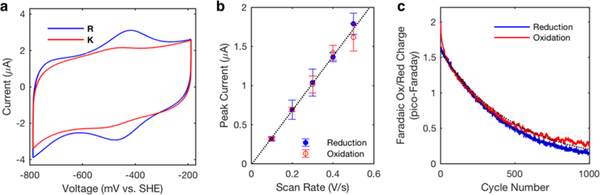
To test redox turnover stability, we performed cyclic voltammetry on a pyrolytic graphite edge electrode under anoxic conditions.23 The midpoint potential of 4Fe–4S bound to K and R peptides at p H 8.5 (Figure 3a) was approximately −450 mV, which is within the range for extant bacterial ferredoxins.24 One would expect the extensive hydrogen bonding and solvent exposure to reduce the Fe–S bond covalency and produce a species with a less reductive potential than bacterial ferredoxin,25 but electrostatic contributions from K and R ambidoxin side chains appear to compensate, leading to a more reductive midpoint potential. Measurement of peak currents at different scan rates confirmed that current was not diffusion limited, and was due to direct electron transfer between the working electrode a metallo-peptide film26 (Figure 3b). The holo-peptide endures hundreds of redox turnovers at room temperature, decaying with a rate constant of around 0.2% per cycle (Figure 3c). This robust redox stability exceeds that of many previously designed artificial metallo-peptides by ten to a hundred-fold. As in natural iron–sulfur proteins such as ferredoxin and rubredoxin, ambidoxin redox stability likely originates from the extensive network of second-shell hydrogen bonds from backbone amides to cysteine thiols and cluster μ-sulfurs.27,28
Figure 3.
Electrochemical characterization of ambidoxin. (a) Cyclic voltammogram of R peptide (blue trace) and K peptide (red trace) at room temperature, pH 8.5. (b) Peak faradaic current of R peptide measured at different scan rates. (c) Measured faradaic charge from oxidation (red) and reduction (blue) of R peptide repeated over 1000 reduction–oxidation cycles. The rate decay constant estimated from exponential fits (dotted lines) is approximately 0.002 per cycle.
On the basis of analysis of a protein sequence in 1966, Eck and Dayhoff proposed that the ancestral ferredoxin might have had a repeating four-amino-acid pattern.29 Since then, there have been many attempts to make minimal ferredoxin-like peptides based on similar sequence repeats.30–33 The ambidoxin design, based on repeating structural features rather than sequence, produced a simple, artificial ferredoxin that closely mimics redox behavior of its biologically evolved counterparts. Such structural and sequence simplicity greatly increases the likelihood of prebiotic evolution of catalytic redox-active peptides on early Earth.34 Molecules much like the ambidoxins may have served as soluble electron carriers prior to the emergence of homochirality required for protein translation and folding.35 In this scenario, ambidoxin would have assembled from racemic sources of amino acids present in the environment, with peptide bonds formed abiologically, near hydrothermal vents36 or on mineral surfaces.37
Wächtershäuser and others proposed short heterochiral peptides with catalytic metal centers were critical intermediates in the earliest stages of chemolithotrophic origins of life.38,39 The robust redox stability of ambidoxins is a clear demonstration in the laboratory that early peptide catalysts could have been functionally on par or even superior to modern proteins in some respects. Without chaperones, metal cluster biogenesis and homeostasis mechanisms present in a cellular milieu, early peptides had to specifically and stably coordinate metals in order to be effective catalysts.40 This is in contrast to the view of early enzymes as poorly functioning ancestors of modern catalysts.41,42 Geometric calculations indicate multiple solutions for short heterochiral molecules that form rings of different lengths (Supporting Information). Ambidoxins may be just one case in a larger family of metalchelating peptides that could have served as prebiotic molecular ancestors for redox catalysts that sparked the emergence of modern oxidoreductases.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Andrew Mutter, Antonio Rosato and Lucia Banci for helpful discussions and Dr. Kevin Wyman for technical assistance. This work was supported by a grant from the Gordon and Betty Moore Foundation on “Design and Construction of Life’s Transistors” (GBMF-4742) to V.N. and P.G.F. and by NIH shared instrumentation grant (1S10OD018207–01) to G.T.M.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b07553. Experimental details (PDF)
Notes
The authors declare the following competing financial interest(s): J.D.K., D.H.P, V.N. and P.G.F. are inventors on a patent application submitted by Rutgers, the State University of New Jersey. G.T.M. is founder of Nexomics Bioscience, Inc.
REFERENCES
- (1).Johnson DC; Dean DR; Smith AD; Johnson MK Annu. Rev. Biochem 2005, 74, 247–281. [DOI] [PubMed] [Google Scholar]
- (2).Raanan H; Pike DH; Moore EK; Falkowski PG; Nanda V. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (6), 1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Chandonia J-M; Fox NK; Brenner SE J. Mol. Biol 2017, 429 (3), 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Adman ET; Sieker LC; Jensen LH J. Biol. Chem 1973, 248 (11), 3987–3996. [PubMed] [Google Scholar]
- (5).Orengo CA; Thornton JM Annu. Rev. Biochem 2005, 74, 867–900. [DOI] [PubMed] [Google Scholar]
- (6).Kim JD; Rodriguez-Granillo A; Case DA; Nanda V; Falkowski PG PLoS Comput. Biol 2012, 8 (4), e1002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Nanda V; Senn S; Pike DH; Rodriguez-Granillo A; Hansen WA; Khare SD; Noy D. Biochim. Biophys. Acta, Bioenerg 2016, 1857 (5), 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Grzyb J; Xu F; Weiner L; Reijerse EJ; Lubitz W; Nanda V; Noy D. Biochim. Biophys. Acta, Bioenerg 2010, 1797 (3), 406–413. [DOI] [PubMed] [Google Scholar]
- (9).Roy A; Sarrou I; Vaughn MD; Astashkin AV; Ghirlanda G. Biochemistry 2013, 52 (43), 7586–7594. [DOI] [PubMed] [Google Scholar]
- (10).Pauling L; Corey RB Proc. Natl. Acad. Sci. U. S. A 1951, 37 (5), 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Watson JD; Milner-White EJ; Thornton JJ Mol. Biol 2002, 315 (2), 183–191. [DOI] [PubMed] [Google Scholar]
- (12).Watson JD; Milner-White EJ J. Mol. Biol 2002, 315 (2), 171–182. [DOI] [PubMed] [Google Scholar]
- (13).Rao PV; Holm RH Chem. Rev 2004, 104 (2), 527–560. [DOI] [PubMed] [Google Scholar]
- (14).Bruice TC; Maskiewicz R; Job R. Proc. Natl. Acad. Sci. U. S. A 1975, 72 (1), 231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Tagawa K; Arnon DI Nature 1962, 195, 537. [DOI] [PubMed] [Google Scholar]
- (16).Bertrand P; Gayda JP; Rao KK J. Chem. Phys 1982, 76 (10), 4715–4719. [Google Scholar]
- (17).Guigliarelli B; Bertrand P. Adv. Inorg. Chem 1999, 47, 421–497. [Google Scholar]
- (18).Ohnishi T; Ragan CI; Hatefi YJ Biol. Chem 1985, 260 (5), 2782–2788. [PubMed] [Google Scholar]
- (19).Hofer P; Grupp A; Nebenfuhr H; Mehring M. Chem. Phys. Lett 1986, 132 (3), 279–282. [Google Scholar]
- (20).Reijerse EJ; Dikanov SA J. Chem. Phys 1991, 95 (2), 836–845. [Google Scholar]
- (21).Brancaccio D; Gallo A; Mikolajczyk M; Zovo K; Palumaa P; Novellino E; Piccioli M; Ciofi-Baffoni S; Banci LJ Am. Chem. Soc 2014, 136 (46), 16240–16250. [DOI] [PubMed] [Google Scholar]
- (22).Antonkine ML; Bentrop D; Bertini I; Luchinat C; Shen G; Bryant DA; Stehlik D; Golbeck JH JBIC, J. Biol. Inorg. Chem 2000, 5 (3), 381–392. [DOI] [PubMed] [Google Scholar]
- (23).Armstrong FA; Heering HA; Hirst J. Chem. Soc. Rev 1997, 26 (3), 169–179. [Google Scholar]
- (24).Liu J; Chakraborty S; Hosseinzadeh P; Yu Y; Tian S; Petrik I; Bhagi A; Lu Y. Chem. Rev 2014, 114 (8), 4366–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Dey ,A; Jenney ,FE; Adams ,MW; Babini ,E; Takahashi ,Y; Fukuyama ,K; Hodgson ,KO; Hedman ,B; Solomon ,EI Science 2007, 318 (5855), 1464–1468. [DOI] [PubMed] [Google Scholar]
- (26).Léger C; Elliott SJ; Hoke KR; Jeuken LJ; Jones AK; Armstrong FA Biochemistry 2003, 42 (29), 8653–8662. [DOI] [PubMed] [Google Scholar]
- (27).Blake PR; Park JB; Adams MWW; Summers MF J. Am. Chem. Soc 1992, 114 (12), 4931–4933. [Google Scholar]
- (28).Backes G; Mino Y; Loehr TM; Meyer TE; Cusanovich MA; Sweeney WV; Adman ET; Sanders-Loehr JJ Am. Chem. Soc 1991, 113 (6), 2055–2064. [Google Scholar]
- (29).Eck RV; Dayhoff MO Science 1966, 152 (3720), 363–366. [DOI] [PubMed] [Google Scholar]
- (30).Gibney BR; Mulholland SE; Rabanal F; Dutton PL Proc. Natl. Acad. Sci. U. S. A 1996, 93 (26), 15041–15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Nanda V; Rosenblatt MM; Osyczka A; Kono H; Getahun Z; Dutton PL; Saven JG; DeGrado WF J. Am. Chem. Soc 2005, 127 (16), 5804–5805. [DOI] [PubMed] [Google Scholar]
- (32).Benson DE; Wisz MS; Hellinga HW Proc. Natl. Acad. Sci. U. S. A 2000, 97 (12), 6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Grzyb J; Xu F; Nanda V; Łuczkowska R; Reijerse E; Lubitz W; Noy D. Biochim. Biophys. Acta, Bioenerg 2012, 1817 (8), 1256–1262. [DOI] [PubMed] [Google Scholar]
- (34).Jelen BI; Giovannelli D; Falkowski PG Annu. Rev. Microbiol 2016, 70 (1), 45–62. [DOI] [PubMed] [Google Scholar]
- (35).Nanda V; DeGrado WF J. Am. Chem. Soc 2004, 126 (44), 14459–14467. [DOI] [PubMed] [Google Scholar]
- (36).Cleaves HJ; Aubrey AD; Bada JL Origins Life Evol. Biospheres 2009, 39 (2), 109–126. [DOI] [PubMed] [Google Scholar]
- (37).Ferris JP; Hill AR Jr, ; Liu R; Orgel LE Nature 1996, 381, 59. [DOI] [PubMed] [Google Scholar]
- (38).Huber C; Wachtershauser G. Science 1998, 281 (5377), 670–672. [DOI] [PubMed] [Google Scholar]
- (39).Milner-White EJ; Russell MJ Biol. Direct 2008, 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Dupont CL; Butcher A; Valas RE; Bourne PE; Caetano-Anollés G. Proc. Natl. Acad. Sci. U. S. A 2010, 107 (23), 10567–10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Johnsson K; Allemann RK; Widmer H; Benner SA Nature 1993, 365, 530–532. [DOI] [PubMed] [Google Scholar]
- (42).Jiang L; Althoff EA; Clemente FR; Doyle L; Röthlisberger D; Zanghellini A; Gallaher JL; Betker JL; Tanaka F; Barbas CF; Hilvert D; Houk KN; Stoddard BL; Baker D. Science 2008, 319 (5868), 1387–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.