Abstract
Background:
Transcranial direct current stimulation (tDCS) is investigated to modulate neuronal function by applying a fixed low-intensity direct current to scalp.
Objectives:
We critically discuss evidence for a monotonic response in effect size with increasing current intensity, with a specific focus on a question if increasing applied current enhance the efficacy of tDCS.
Methods:
We analyzed tDCS intensity does-response from different perspectives including biophysical modeling, animal modeling, human neurophysiology, neuroimaging and behavioral/clinical measures. Further, we discuss approaches to design dose-response trials.
Results:
Physical models predict electric field in the brain increases with applied tDCS intensity. Data from animal studies are lacking since a range of relevant low-intensities is rarely tested. Results from imaging studies are ambiguous while human neurophysiology, including using transcranial magnetic stimulation (TMS) as a probe, suggests a complex state-dependent non-monotonic dose response. The diffusivity of brain current flow produced by conventional tDCS montages complicates this analysis, with relatively few studies on focal High Definition (HD)-tDCS. In behavioral and clinical trials, only a limited range of intensities (1–2 mA), and typically just one intensity, are conventionally tested; moreover, outcomes are subject brain-state dependent. Measurements and models of current flow show that for the same applied current, substantial differences in brain current occur across individuals. Trials are thus subject to inter-individual differences that complicate consideration of population-level dose response.
Conclusion:
The presence or absence of simple dose response does not impact how efficacious a given tDCS dose is for a given indication. Understanding dose-response in human applications of tDCS is needed for protocol optimization including individualized dose to reduce outcome variability, which requires intelligent design of dose-response studies.
Keywords: Transcranial direct current stimulation (tDCS), Dose-response, Neuromodulation, Dose-control
Introduction
tDCS involves low-intensity direct currents (few mA) applied to the scalp via pad electrodes (typically 25–35 cm2) [1] or smaller electrodes in arrays (HD tDCS [2]). Encouraged by the general safety profile [3–5], low intensity tDCS has been broadly tested as a tool for cognitive research in healthy subjects [6] as well as to treat a broad range of neurological and psychiatric disorders and symptoms [7,8]. It is generally accepted that the physics of tDCS dictates that current flow intensity in the brain (electric field) will increase linearly with applied current (Fig. 1) [9]. Rather, our primary question is whether neurophysiological and behavioral responses also increase linearly, or at least monotonically, with applied current intensity. Specifically, does increasing the current of tDCS (e.g. from 1 to 2 mA) increase effects size for a given experiment and outcome measure? This question is relevant because any choice of stimulation protocol and comparison among studies with different protocols rests on the ability to relate the effects of one intensity to another in a rational way.
Fig. 1.
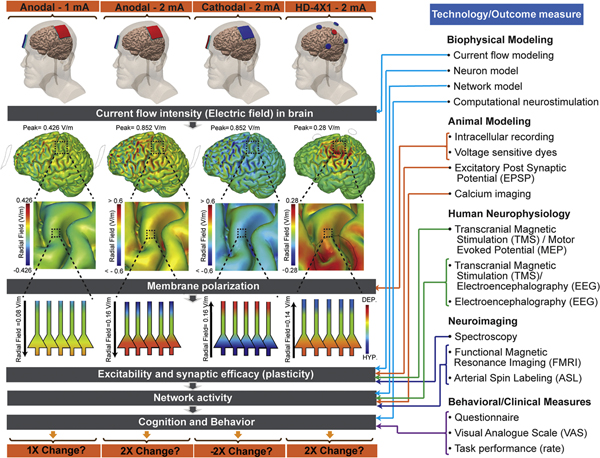
An aggregate linear tDCS intensity dose response requires linear input-output function in each scale from a single neuron to local neuronal circuits and plasticity, to large scale interconnected neuronal networks and ultimately behavior and task performance. Induced electric field (or current intensity) in the brain increases linearly with applied stimulation current. In well-controlled, in-vitro experiments, increased membrane polarization can be reasonably assumed with increasing tDCS intensity but in an active brain, nonlinear and complex behavior is more likely. Different experimental, modeling and imaging techniques assist to map tDCS modulation in specific scales.
However, the question is complicated because the complete dose of tDCS is defined by the applied current, the duration, and the electrode montage [10] which produce a complex pattern of current flow in the brain; nonetheless, we focus here on the role of applied current intensity while noting how other factors may influence (interact with) the current-intensity dose response. We discuss how individual anatomical differences in the amount of current density (electric field) to the brain vary for the same applied current, which may therefore lead to variations in individual intensity-response [11]. Moreover, we consider the extent to which tDCS responses vary with brain-state, magnifying individual- and task specific variations in dose-response.
In the last two decades, tDCS dose-response relationship has been evaluated from different perspectives ranging from single cells, to small local brain circuits and synapses, to large networks, to overall brain function and behavior. Assuming a causal chain across different scales (applied current first changes single cells, which alter local and large networks, which change behavior), the lack of a linear response at any of these scales may preclude an aggregate linear dose response at the behavioral level (Fig. 1). The organization of this document centers around measurement approaches (e.g. animal models, imaging) but specific techniques often map specific scales (Fig. 1; e.g. animal models measures small circuits, imaging measures large network). We discuss tDCS intensity dose response through these different perspectives.
Basic biophysics of intensity-response
Modeling studies relate the applied dose to the scalp [10], including current intensity, to the resulting electric field (or current density) in the brain [12]. While current (in units of mA) is the controllable stimulation parameter, electric field (in unit of V/m) reflects the local stimulation intensity each brain region is actually exposed to. It is generally accepted that the physics of tDCS dictates that current flow intensity in the brain (electric field) will increase linearly with applied current [9]. Therefore, the question is not if there is a linear response between increasing applied current and brain electric field but rather if the brain response to increasing electric field is itself linear. As noted above, in humans, the electric field varies with individual anatomy (the implications of which are discussed below), though in any case it tracks linearly for a given individual and scales across a population. In some animal models, notably in-vitro brain slices [13], the electric field intensity can be tightly controlled allowing direct testing of electric field dose-response.
Modeling studies predict that for low intensity range of applied current (i.e. 2 mA), induced electric field in the brain is less than 1 V/m (Fig. 1) [14]. These predictions have been directly [15–18] and indirectly validated [19,20]. Because of these low electric fields, it has been suggested that the primary effects of tDCS are due to changes in the membrane potentials of neurons with most attention paid to pyramidal grey matter neurons orientated orthogonal to the brain surface [13,21] or to synaptic terminals [22]. In this view, any effects of tDCS are secondary to changes in this polarization [23,24]. Even when other cell types may be implicated (e.g. glia [25], the primary mechanism of tDCS is speculated to act through polarization of these cell membranes [26].
Basic theory of tDCS suggests that membrane polarization would be polarity-specific and linear with applied current intensity (i.e. generated electric field (EF)). This is because tDCS is low intensity (few mA) and so considered to depend on subthreshold resting membrane potential changes rather than directly inducing neuronal firing (e.g., 700–1000 mA used in ECT). Thus, assuming membrane polarization is the key determinant for the effect of tDCS, it is reasonable to assume that increasing tDCS intensity will increase effects size in general (Fig. 1). However, this may strictly only apply in well-controlled preparations; in the brain, responses may be non-linear and occur in a complex (e.g. non-linear, homeostatic) manner. Therefore, a critical unanswered question is whether increasing current in the tDCS range applied in humans (4 mA or less) enhances neuromodulation and outcomes in a linear, or at least monotonic manner. This question of linear dose response for a given polarity can be distinct from whether there are any polarity specific effects. Notably, if one considers folding of the cortex and diffuse current flow, tDCS produces mixed polarity effects under each stimulation electrode [22,27]. This again emphasizes that extrapolation from well-controlled animal studies can be fraught with oversimplification.
Indications about intensity-response of tDCS from animal models
Quiescent neurons are those that are not spontaneously firing action potentials (which is an anomalous state because neurons in vivo are active); such neurons can be observed in brain slices with normal superfusate. Application of electric fields to such quiescent neurons suggest a linear correlation between induced membrane polarization and electric field intensity polarity (Fig. 1) (i.e. the more external electric field, the more neuronal polarization). However, this relationship has been thoroughly tested only for intensities above those applicable to studies in humans (>10 V/m). For example, Bikson et al. (2004) evaluated the effect of uniform DC electric field on neuronal excitability in a rat hippocampal slices using electric field between 10 and 100 V/m [13]. These authors reported membrane polarization was generally linear except when field intensities exceeded 80 V/m (equivalent to tens of mA for tDCS, which resulted in non-linear firing, [13]. While it is reasonable to assume this linear relationship continues with electric fields under 1 V/m (Fig. 1), this awaits empirical evidence. Other studies have reported a linear sensitivity of neurons to polarization with DC or low-frequency alternating current (AC), electric fields ranging from 2 −15 V/m DC [21,28] to 1–15 V/m AC [28–30]. We are not aware of any neurophysiological response directly demonstrating linear polarization effects with tDCS relevant fields intensities (<1 V/m). Theoretical neuron polarization models based on traditional electrical stimulation theory predict a linear polarization across all sub-threshold intensities in quiescent neurons including tDCS ranges of <1 V/m [31].
Membrane polarization is easiest to measure in quiescent neurons. However, neurons in vivo are active, not quiescent. Any dose response assessment should therefore be conducted in firing (non-quiescent) neurons. Assessing dose-response relationships is more complex in this case because: 1) the properties of the neuronal membrane changes with ongoing activity [31,32]; and 2) any targeted neuron is coupled with a larger population or entire network and its activity is presumably mediated by changes in network activity [28].
Animal studies have demonstrated changes in network activity (0.2 V/m, [28]; 0.5 V/m [30] and meta-plasticity (0.75 V/m [33]) for electric fields <1 V/m but have not systematically evaluated a dose-response within this range. We emphasize that showing an effect at one DCS intensity compared to no-stimulation does not establish a (linear) dose response. Another complication is that “classical” animal studies have applied electrodes on the surface of the brain with electric fields orders of magnitude above those generated by tDCS in humans [34,35]. Typically, these studies have used unit firing rate to measure response; here again caution is warranted in assuming any dose response at high DCS intensity applied to ranges below 1 V/m and in drawing direct comparison with measures obtained in humans, such as motor-evoked potentials (MEPs).
These considerations aside, animal studies, using both low and high-intensity DCS, have shown that the effects of DCS are activity (state) dependent, which indicates that the effects of any given DCS dose may vary depending on the outcome measure (experiment). For example, Bikson and colleagues (2013) showed that the direction of DCS modulation on synaptic efficacy depends on the afferent pathways; indeed, in the same columns (small network) one pathway may be enhanced even as another is inhibited [13,22]. Frohlich et al. [21] and Reato et al. [28] have shown that the variation of DCS effects can depend entirely on ongoing brain activity e evidently if tDCS modulates ongoing brain activity then the effect of tDCS entirely depends on what endogenous activity is present. Fritch et al. [33] and Kornberg et al. [36] showed pathway and activity state-dependent plasticity modulation by DCS. Although these findings do not in themselves indicate that the dose-response of any given activity is not monotonic, they show that the response to a given dose can categorically vary on different outcome measures (e.g. brain states). While, on the one hand, the ongoing activity in brain slices (“brain state”) is abstracted from the in vivo case, on the other hand, brain slices provide exquisite control and monitoring of brain state, supporting the testing of hypothesis on the role of brain state in DCS intensity dose response.
In summary, in both quiescent and active neurons of animal brains there is (remarkably) no comprehensive evidence for a linear dose-response relationship at electric field intensities below 1 V/m. There is, however, evidence of neurophysiological changes at specific low intensities supporting that tDCS can modulate brain function. Some dose response is expected in animal models (starting with no response for a no-stimulation condition of 0 V/m) but the absence of clear escalation in response with intensities up to 1 V/m (e.g. including 0.25 V/m, 0.5 V/m, 0.75 V/m, 1 V/m) is noteworthy and a critical area for future studies.
We note that evidence for dose response from other neuromodulation approaches using supra-threshold (high intensity) pulse approaches such as deep brain stimulation (DBS) [37,38], TMS [39] and transcranial electrical stimulation (TES) [19], do not establish a dose response for tDCS, which is sub-threshold. Within those supra-threshold techniques, more intensity simply results in a high-likelihood and/or number of recruited neurons. Evidence from low-intensity, sub-threshold, alternate waveforms such as transcranial alternate current stimulation (tACS) or transcranial random noise stimulation (tRNS) can also show non-linearity in dose-response as measured by TMS-MEP [40]. However, such data do not provide direct evidence in support of non-linear tDCS dose-response given the presumed unique mechanism of action when using a DC waveform. Finally, to foreshadow the following section, animal studies are anatomically constrained, and generally record from a very small section of cortex. Results from such preparation may not easily transfer to applications in humans, which lead to a much larger extent of stimulated cortex and thus are influenced by the complex interactions with the convoluted cortical structure.
Diffuse current flow in tDCS vs. HD-tDCS
Prior to expanding on dose-response data in humans, some comments on the relationship between applied current and resulting brain current flow patterns are critical. While dose of tDCS is defined by operator controlled factors including current intensity [10], the electric field generated in the brain will vary by individual and will fluctuate in space across the brain (Fig. 2). Intra-cranial recording [15,17], imaging [41], and current flow models [2] show that traditional pad-based tDCS montages deliver current flow across large brain areas including not just under, but in the brain regions between the electrodes (Fig. 2A and B). Many conventional tDCS montages can produce significant current flow through 30–70% of the brain including deep brain structures [42]. Moreover, peak current is often seen between, rather than under the electrodes [43]. The intensity and pattern of diffuse current flow and where peaks are generated reflect idiosyncratic anatomical differences and so there is variation across individuals (Fig. 2, Head #1 (A.1, B.1, C.1), Head #2 (A.2, B.2, C.2)) that is distinct from standard averaged head simulations (Fig. 2, Head #3 (A.3, B.3, C.3)) [44,45]. Attempts to develop a dose-response based on applied tDCS current (typically fixed across subjects) should be interpreted in this context. For example, the conventional “M1-SO” tDCS montage, used to probe the dose-response of M1 (see human neurophysiology below), is predicted to produce as high electric fields in many regions afferent to M1 [42] (Fig. 2,A). Therefore, the intensity-response may depend on how each area of the network responds to increased current density and then how the different brain areas interact. As we discuss later, one approach to account for this complexity is to use multiple tDCS montages along with models of current flow to regress dose-response in human studies.
Fig. 2.
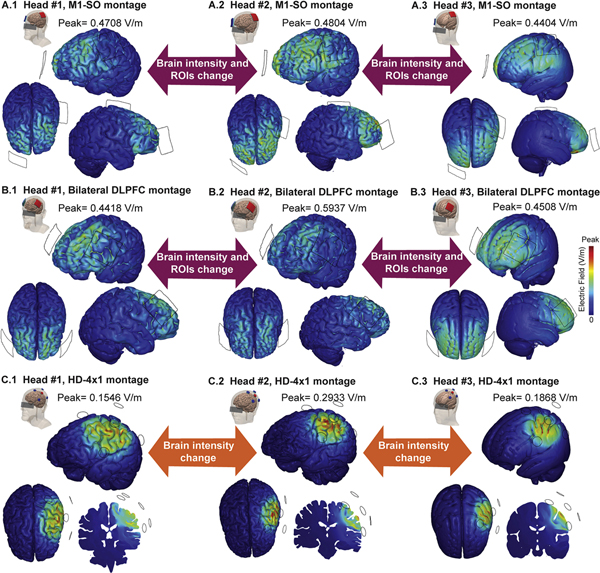
Cortical electric field intensity and pattern across two different subjects (Head #1, Head #2) and standard averaged head (Head #3) for 1 mA stimulation using different electrode montages. A: anode (red) over left M1 and cathode (blue) over contralateral-supraorbital across different heads (A.1, A.2, A.3). B: bilateral DLPFC, anode (red) over left DLPFC (F3, EEG standard system) and cathode (blue) over right DLPFC (F4, EEG standard system) across different heads (B.1, B.2, B.3). Conventional pad electrodes deliver current to multiple brain regions that varies across subjects. For HD-tDCS configuration, C: anode (red) over M1 and cathodes (blue) with 6 mm center to center distance from anode for three different heads (C.1, C.2, C.3). ROI, region of interest. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Since the spatial distribution of stimulation could impact dose response, a complimentary approach to conventional tDCS is the use of smaller HD electrodes. HD-tDCS electrode arrangements include concentric ring configurations (e.g. 4 × 1 HD-tDCS [46]) which applied to the (motor) cortex produce more focal simulation delivery (Fig. 2, C). This might reduce variability in targeting across subjects [19] compared to pad-based tDCS (Fig. 2A and B). HD montages can be designed to spare deep brain regions or maximize current to deep structures [47]. With the goal of understanding current intensity dose-response by stimulating a relatively smaller and more controlled area of cortex, concentric-ring HD-tDCS is especially a useful tool in addition to pad-based tDCS. However, direct comparisons are few [48–50].
Indications about intensity-response of tDCS from human neurophysiology
In exploring dose response in humans, tDCS studies have relied heavily on MEP changes in response to TMS to establish neurophysiological changes in motor regions by tDCS [4,51]. In the most basic experiments, the TMS-MEP threshold or MEP response to a fixed TMS intensity is measured before, during and/or after application of tDCS. A linear dose response would predict that increasing intensity (i.e., > mA) would proportionally increase the degree of modulation (i.e., > TMS-MEP). Indeed, early canonical studies in healthy subjects used a low-dose range (up to 1 mA for several minutes) and initially suggested a monotonic relationship between tDCS intensity and TMS-MEP size.
While several subsequent studies replicated the basic findings at 1 mA [52], a more complex dose response has emerged. Increasing stimulation intensity, increasing duration (in cases by > 10 min), and/or concurrent brain activation or pharmacological manipulation [53–58] can also change the extent and direction of excitability changes measured by TMS-MEP and, so, the dose-response [59]. For example, priming the motor region during “anodal” tDCS (asking subjects to activate hand muscles) can invert the direction of TMS-MEP modulation suggesting that the direction is state dependent. Increasing “cathodal” tDCS intensity to 2 mA can result in TMS-MEP enhancement [53]. Children also exhibited non-monotonic dose-response but over a different intensity range. Indeed, as compared to adults [54] “cathodal” stimulation became excitatory at only 1 mA. This difference in dose-response within children compared to adults was consistent with altered brain electric field for small head sizes [60,61].
As noted above, most of the extant clinical neurophysiology research has used conventional pad tDCS, where current may be delivered to diverse brain regions (Fig. 2A and B) [15,17]. To the extent that any given measured response is influenced by current from more than one region (e.g. TMS- MEP is influenced by current not only from the motor area but also from premotor regions and afferent deep brain structures), then dose response will be related to how increasing current to each of these regions in aggregate influences TMS response. Therefore, an important question is if using HD-tDCS, where more nuanced control of current flow is predicted, is useful in dissecting and clarifying dose response [62,63].
In summary, neurophysiological findings in humans indicate that tDCS outcomes are not necessarily linear, nor even monotonic, with increasing tDCS intensity (even in the limited range of 1–2 mA). Moreover, the nature of modulation is profoundly influenced by variations in brain state. TMS-MEP as a probe of brain function, represents a combination of complex measures itself influenced by several physiological factors including the excitability of neuronal circuits at both cortical and spinal level [3] and do not simply map to behavioral changes. In addition, TMS-MEP is typically measured after tDCS (i.e., offline) and thus may not always reflect the response to concurrent tDCS (i.e., online) effects, which presumably accumulate during the stimulation period (as reinforced by data on tDCS duration) [4,64]. Moreover, it is unclear whether non-motor cortex responds in a comparable manner following tDCS, which has profound implications for cognitive neuroscience and neuro-rehabilitative efforts. More generally, the notion that tDCS adjusts brain excitability and functions like a “sliding scale” (that is simply “measured” by TMS) is an oversimplification [65,66]. Rather, tDCS-induced excitability and plasticity changes may reflect a mixture of complex changes in a number of different sets of excitatory and inhibitory synapses [28,67]; a possibility supported by recent TMS-MEP work that provides some evidence in humans against a simple monotonic dose-response [53,59]. As recently pointed out by Bestmann and Ward (2017) [11], there is currently no data on the dose-response of tDCS that accounts for the current effectively delivered to the brain, recent computational neural network modeling studies aside [68]. This is an obvious caveat when interpreting the extant literature on non-linear effects of tDCS.
Indications about dose-response from imaging studies
PET, fMRI, and EEG studies in healthy populations corroborate the results from current flow models that tDCS has distributed effects [69–72]. For example, Clemens et al. (2014), applied tDCS over the right angular gyrus (AG) and induced large-scale changes in different resting state networks with significant changes at the ventral lateral thalamic nucleus despite the region not being nominally targeted. Hampstead and colleagues (2014) demonstrated polarity dependent BOLD signal change during task performance [73] and resting-state connectivity [74] in healthy young participants such that these measures were generally relatively enhanced with anodal stimulation but suppressed with cathodal stimulation. Arterial Spin Labeling (ASL), considered a direct measure of blood flow, suggests a monotonic correlation between tDCS dose (i.e., 0.8–2 mA) and regional cerebral blood flow underneath the anode [75]. Using changes in fMRI signal as an index of cortical recovery in a patient who received successful visual rehabilitation, Halko et al. (2011) reported correlations between the modeled electrical field and increased task-related fMRI activation in areas under the anode as well as in perilesional visual areas [76]. Broadly, these findings of a distributed effect of tDCS are not surprising when considering the diffuse current flow with conventional tDCS application (Fig. 2A and B). But currently there is scant evidence for the dose-response relationship of tDCS from neuroimaging. Future efforts should leverage different neuroimaging measures of distributed activity change to tDCS. However, we note that in some cases the transfer function between changes produced neural activity may itself not map linearly (Fig. 1) onto the measures obtained with neuroimaging [77–80], and tDCS may itself produce direct (e.g. changes in hemodynamics [75,81,82]; and indirect (artifact [20]) signal changes in imaging data.
Indications about dose-response from cognitive/behavioral outcomes in healthy population
A narrow range of intensities were tested in tDCS cognitive and behavioral studies (95% of trials used 1 or 2 mA) [83,84] with few exceptions [5]. However, even within this small range, there are limited data directly correlating effect size in tDCS human trials with current intensity [53]. For instance, the influence of current intensity (i.e. 1 mA, 2 mA) was investigated on a working memory task among healthy controls, indicating a non-monotonic current intensity dose-response [85]. In another study, none of the examined intensities (i.e. 1 mA, 2 mA) produced significant effects in a working memory task [86]. Cuypers and colleagues (2013) indicated a dose-response relationship in a motor learning task with significant enhancement in motor performance at 1.5 mA but not 1 mA [56]. We note the important statistical caveat that a significant response at one dose, but not another, does not itself establish a difference between two doses.
Most behavioral and cognitive studies have used large pad-sponge electrodes. Thus, any given response is influenced by stimulation of more than one cortical region, and dose-response is reflecting the amalgamation of current flow across many regions with varied intensity in brain areas (see below; Fig. 3). The use of HD-tDCS would significantly reduce current spread, but given current spread even under optimized HD-tDCS is greater than one gyri which is the size of a typical ROI. Use of HD-tDCS reduces but not remove this confound.
Fig. 3.
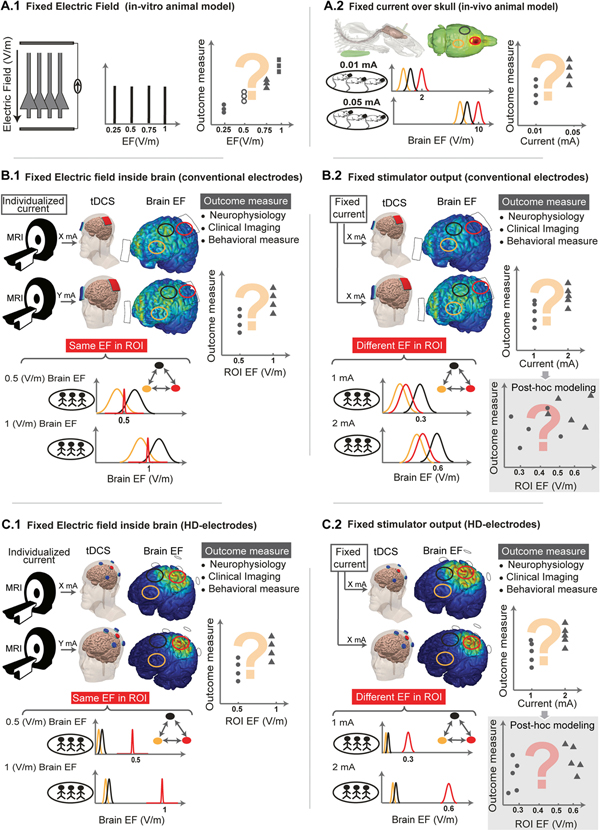
Experimental design of dose-response studies in animal and man. 6 experimental paradigms are illustrated, 2 in animal and 4 in human trials. Approaches where electric field is controlled (left column) are contrasted with approaches where applied current is fixed (right column). In human trial panels, the use of anatomical MRI scans is illustrated by a MRI cartoon. The use of a tDCS or HD-tDCS montage is illustrated on two semi-transparent head. Predicted electric field are shown in false color on the cortex. In each case, one or more outcome measures would be correlated against electric field in the ROI or the applied current, with the question-mark indicating a monotonic relationship is not necessarily established. The nominal ROI may be assumed to be “under” one electrode (red circle) with other brain region considered (yellow and black circles). In each panel, a simplified representation of the electric field distribution across a population (three stick figure cartoon) includes three brain regions (the nominal ROI in red, and other brain regions in yellow, black). These regions may be interconnected such that the outcome measure can reflect aggregate network stimulation. (A.1) In vitro animal brain slice models are stimulated with a uniform electric field. The electric field can be increased and an outcome measure recorded. Few in vitro studies applied several increments of electric magnitude in the tDCS range (<1 V/m). (A.2) In vivo animal models apply a fixed current with an epi-cranial electrode which results is animal-specific electric field in the ROI (red) and varied electric fields in other brain regions (Yellow, Black). Increasing the applied current increases all the electric field in each brain region proportionally. Electric field in animal models will be dramatically above the human case when comparable currents are applied. An outcome measures is recorded at varied applied current levels. (B.1) Using conventional electrode pads, controlled electric field intensity can be applied to a ROI in human trials by varying the applied current in each individual to generate a fixed electric field at the ROI. They require individual current flow modeling. The electric fields in other brain regions are not controlled and so vary across individuals and may be higher than in the ROI. An outcome measures is recorded at varied controlled ROI electric fields. (B.2) Using conventional electrode pads, a fixed current is applied across subjects for each dose, which results in variable electric field at the ROI as well as at other brain regions. For each subject, increasing the applied current increases all the electric field in each brain region proportionally. The electric field may be maximal outside the ROI. An outcome measures is recorded at varied applied current. [shaded inset] Post-hoc individual model may be used to reanalyze data based on predicted electric field in the ROI. This may result in some subjects in the lower-current group having a higher electric field at the ROI than some subjects in the low current group. (C.1) Using the high-definition 4 × 1 montage, controlled intensity electric field can be applied to a ROI in human trials by varying the applied current in each individual to generate a fixed electric field at the ROI. The require individual current flow modeling based on MRI. Across individuals, the electric field is predicted to be focal and maximal at the ROI across stimulation intensities. An outcome measures is recorded at varied controlled ROI electric fields. (B.2) Using the high-definition 4 × 1 montage, fixed currents are applied across, which results in variable electric field at the ROI at each current, however, the maximal electric field remains in the ROI across individuals. For each subject, increasing the applied current increases all the electric field in each brain region proportionally, but the electric field remains minimal outside the ROI. An outcome measures is recorded at varied applied current. [shaded inset] Post-hoc individual model may be used to reanalyze data based on predicted electric field in the ROI. This may result in some subjects in the lower-current group having a higher electric field at the ROI than some subjects in the low current group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Indications about dose-response from functional outcomes in medically ill populations
While tDCS is widely investigated as potential therapeutic tool to enhance cognitive rehabilitation in neuropsychiatric disorders [87,88], only a few studies explored dose-response and with a limited number and range of dose. Protocol variations limit generalization across studies (e.g. electrode montages, cognitive tasks, population inclusion criteria and type of disorder) on the role of intensity and there is a general consensus that other factors such as the number of tDCS sessions broadly enhance efficacy [89–91]. In a meta-analysis of tDCS trials for major depression, Brunoni et al. (2016) could not determine if current intensity (mA) was positively associated with tDCS efficacy [92]. However, within a crossover design trial, tinnitus relief was positively correlated with HD-tDCS current intensity [93]. Murry and colleges (2015) investigated tDCS current intensity in chronic spinal cord injury patient in a single blind, sham controlled, crossover study [94]; 2 mA, but not 1 mA, significantly enhanced TMS-MEP modulation. Boggio and colleagues explored effect of tDCS stimulation site (i.e. over DLPFC, over motor cortex) and intensity (i.e. 1 mA, 2 mA) in Parkinson’s disease [95]. Results indicated an intensity and montage-specific effect with only 2 mA anodal stimulation over DLPFC significantly improving accuracy of a working memory task. Optimization of stimulation parameters (i.e. current intensity [0.1–0.4 mA], duration [5–20 min] with respective steps of 0.1 mA and 5 min) for treatment of Parkinson’s disease in primates indicated that total charge (Σcurrent intensity × duration of stimulation) is correlated with treatment outcome instead of current intensity or stimulation duration [96]. In a case report, increasing current intensity (i.e. 1 mA to 3 mA) enhanced and accelerated benefits in a schizophrenia patient [97]. In another study, feasibility of tDCS for enhancing cognitive performance in schizophrenia using higher current intensity (i.e. 2 mAvs 1 mA) was shown [98]. We emphasize that demonstrating efficacy of increased current intensity compared to non-significant effect in commonly used tDCS current intensity (e.g.1 mA) do not stablish current intensity dose response. Investigation of dose response in patients require systematic escalation of current intensity (see below; Fig. 3), and would further benefits from an expanded current range - provided tolerability is controlled [99].
Use of current flow models to inform imaging, neurophysiological and behavioral studies of dose response
As noted above, intra-cranial measurements [15,17], imaging [41], and models of current flow show that conventional tDCS with large electrodes are placed “over” the target areas. In fact, they deliver current to brain regions between the anode and the cathode (Fig. 2A and B) [2,55]. This diffusivity and lack of clear targeting complicates the analysis of response intensity and, at the same time, it reinforces that computational models are needed to comprehensively investigate dose response. Using current flow modeling, there have been: 1) Retrospective attempts to correlate electric field intensity in regions of interest (ROI) with clinical outcomes using different montages (with fixed current), under the hypothesis that montages that enhance electric fields in ROIs (for a given current) will enhance outcomes; 2) Retrospective correlations of electric field intensity in ROIs with behavioral outcomes with a fixed montage and fixed current considering how individual head anatomy differences affect brain current intensity, and 3) Prospective attempts to optimize the tDCS montage to deliver electric fields to ROIs, in some cases accounting for individual anatomy, under the hypothesis that this would enhance outcomes compared to a uniform tDCS montage [100].
Using current flow modeling, retrospective efforts comparing montages have provided indirect evidence that electric field intensity produced by tDCS in a ROI correlates with enhanced clinical (e.g. pain [101], or neurophysiological outcomes (e.g. TMS MEP [102]). Kim et al. (2014) investigated the relationship between the behavioral outcomes in a verbal working memory task (WM) and variations in electric field intensity over the dorsolateral prefrontal cortex (DLPFC) based on subject specific anatomy. Participants who showed significant enhanced WM task performance (good responders) had significantly higher electric field intensity over the DLPFC than other participants (bad responders), suggesting that variability in behavioral outcomes of tDCS might be partly due to individual anatomical differences, consistent with a monotonic dose response.
In some cases, current flow models have been used to optimize response to tDCS, but in these cases an implicit assumption has been made about a local (tissue level) monotonic dose response, namely that designing approaches that deliver more electric field to a given brain region will increase effect size. Several attempts to individualize tDCS by using current flow models to optimize electric field to a target brain region have centered around stroke patients where individual lesions produce unique distortion of brain current flow patterns [103]. With the goal of optimizing the tDCS montage to maximize electric fields in specific anatomical regions implicated in neurorehabilitation (e.g. identified by fMRI), approaches to individualize HD-tDCS therapy were developed [28,47] but, even if these trials were conducted, the underlying assumption on dose response remains to be validated. Several studies have used current flow modeling to design an optimized HD-tDCS montage (across subjects) for specific ROIs. In applications including pain control [104], tinnitus [93], motion perception [105], verbal learning and memory function [63], such HD approaches have yielded encouraging effect sizes, often larger than those using conventional tDCS montages. However, these HD-tDCS montages typically reduce the spatial extent of current in the brain rather than electric field intensity [47] and reinforce the role of the spatial distribution of current flow in influencing dose response rather than providing support for a dose-response itself.
Methodology to systematically investigate dose-response
The thesis of this paper - that there is deficiency in the current knowledge on tDCS intensity dose response - in turn indicates a need for expanded and more rigorous current intensity dose response testing [106]. Approaches to experimental design of dose-response studies are discussed in this section (Fig. 3), two in animal (in-vitro and in-vivo) and four in human trials (fixed current with conventional pad electrodes, controlled electric field with conventional pad electrodes, fixed current with high-definition electrodes, and controlled electric field with high definition electrodes).
Animal studies provide special opportunities to explore dose-response relationships, but only if conducted in meaningful ways to the human stimulation [26]. In-vitro brain slice experiment uses an escalation of electric field intensity (Fig. 3 A.1), which is more meaningful to control than applied current. Using specific stimulation techniques (i.e. large parallel wires [13]), with a uniform electric field the entire tissue is exposed to a single magnitude and electric field direction (e.g.1 V/m normal to the cortical surface). An experimental measure from brain slices, which can be electrophysiological or molecular, can then be related to the applied electric field as a proxy for local tissue response to escalating electric field intensity. While some variability in response to a given electric field is expected in any experimental system, brain slices offer the possibility for high-throughput experimentation yielding results with high confidence.
In-vivo experiments involve non-invasive stimulation, under current control [26]. The animal anatomy will determine the resulting electric field in the ROI, and varied electric field across other brain regions which may influence outcomes (Fig. 3 A.2). Brain electric field distribution can be predicted using current flow models [5,107,108]. For any given applied current, significant interspecies variation and some inter-animal variation is expected in the resulting brain electric field. An experimental measure from animals, which can span electrophysiological, molecular, or behavioral, is correlated with the applied current. Variability in response across animals for a given dose, can be minimized through experimental design.
In a conventional tDCS current intensity dose response experiments, two or more stimulation currents (e.g. 1 mA and 2 mA) are applied across individuals using conventional sponge-pad electrodes (Figure B2). While straightforward from a design perspective, this approach has several methodological caveats. Applying fixed current in a population lead to significant inter-individual differences in brain EF that are a function of each subject’s head anatomy [11]. Considering a relatively wide distribution of brain EF in ROI, means that some subjects in the “low dose” (e.g. 1 mA) group may have a higher EF in the ROI than some subjects in the “high dose” (e.g. 2 mA) group. The range of doses typically explored (e.g. 2 × from 1 mA to 2 mA) is less than the range of sensitivity across subjects (e.g. 3–5x across healthy adults [44]). Use of a wider current range (if tolerated) mediates these overlaps, but does not mitigate the large variance in effective brain current with this approach.
Still more problematic is that with conventional tDCS montages, electric field is generated across wide regions of the brain, with the location of peak electric field varying across individuals, and often not occurring “under” the electrodes [2]. These issues compound such that the average electric field in a none ROI at the “low dose” can be higher than the electric field in the nominal ROI under high-dose. Ultimately, using this simplistic current intensity dose-response experimental design (Fig. 3 B.2), one must interpret the effects of tDCS, and so the dose response, as reflecting the amalgamation of current flow across many regions with varied intensity.
The above concerns can only be partially mitigated by normalizing electric field intensity to the ROI for each subject (Fig. 3 B.1). In a second experimental design for human trials, using individual MRI and modeling the individualized current needed for each subject is determined to produce a consistent electric field in a given ROI. Notably in this method each subject will receive a unique current for a given target electric field (e.g. 0.5 V/m) in the ROI, and this current may vary several-fold variation in current applied across individuals (e.g. 0.6 mA, 1.4 mA, 2.1 mA …) as a result of the aforementioned inter-individual anatomical differences [44]. Dose escalation therefore involved increased electric field in the ROI (e.g. 0.5 V/m, 1 V/m) not applying a multiple to the individual applied current for each subject. An experimental measure from the trial, which can span electrophysiological, imaging, or behavioral, is correlated with the electric field in the ROI. Because conventional tDCS pads are still used, current can flow through the brain with maximum electric field not necessarily in the ROI and not in a consistent location across subjects [44]. To the extent that current flow to other brain regions influences the outcome measure, it is a problem that the electric field intensity is not controlled outside the ROI.
An addition to the fixed-current approach (Fig. 3 B.2) is to retrospectively model individual current flow and then correlate with experimental measure with the post-hoc calculated electric field in the nominal ROI. This leads to a distribution of predicted electric fields with some subjects in the “low dose” current group (e.g. 1 mA) presenting a higher electric field in the ROI than some subjects in the “high dose” current group (e.g. 2 mA). This post-hoc modeling may not meaningfully mediate the concern with broad and varied brain current flow across subjects using pad montages, as the relative electric field distribution across individuals will vary.
Using High-Definition tDCS, and specifically the 4 × 1 montage, current is restricted to defined brain regions (ROI within the electrode ring); the peak electric field is within this brain region and thus consistent across subjects. In a third experimental design for human trials, a dose response trial design using 4 × 1 HD-tDCS and the fixed current escalation method (e.g. 1 mA, 2 mA) provides evidence at the population level if increased intensity at the ROI is correlated with an outcome measure (Fig. 3 C.2). Focal EF produced by HD montage provide a substrate for controlling impact of stimulating functional/structural connected areas outside the ROI (Fig. 3, C). Thus, an essential difference from conventional pad-tDCS is that electric fields outside the ROI are low enough that increasing applied current still does not result in significant current outside the ROI.
In a fourth experimental design for human trials, using the 4 × 1 High-Definition tDCS montage, individualized modeling based in subject-specific MRI can be used to normalize the electric field across individuals (Fig. 3 C.1). An experimental measure from the trial, which can span electrophysiological, imaging, or behavioral, can be meaningfully correlated with the electric field in the ROI. It is possible using the fixed current 4 × 1 High-Definition tDCS (Fig. 3 C.2) to use individual MRIs for post-hoc modeling of electric fields in the ROI, which in contrast to the fixed electric field approach leads a distribution of electric field values. This scattered representation of electric fields in the ROI is not deleterious to dose-response analysis and in fact may lead to a wider variation and range of electric fields in the ROI.
In all four experimental noted designs for human trial, variability in response for a given dose (fixed current or electric field controlled) is expected reflecting individual neurophysiological and brain state differences, which may be mitigate through rigorous experimental design (e.g. subject inclusion criteria, testing environment) but never eliminated. These physiological variations are compounded by any limitations in dose-response design described above which further emphasizes the need for careful consideration of dose-response experimental design. The four classifications described above by no means fully characterizes the diversity of approaches and issues which must be considered for meaningful tDCS dose-response experiments [106,109–112] and includes fundamental rigor in tDCS methodology [113]. For example, neural network modeling approaches can help generating hypotheses about the non-linear dynamics in neural activity under escalating tDCS dose [68].
Synopsis
Despite ongoing advances in the science of tDCS, we currently do not have a clear understanding of dose-response relationships in tDCS and principal open questions to be answered (Table 1). This limits empirical choice about the most efficacious stimulation protocol in a given context, renders inter-individual (and hence between study) comparison prone to complication, and hampers non-spurious assessment about the sources of tDCS response variability [114].
Table 1.
Open questions on dose-response.
| - Has the scale of research on tDCS efficacy outstripped understanding of dose response? |
| - To what extent could non-monotonic dose response, which is dependent on individual anatomy and subject to interactions with brain state (e.g. task engagement), lead to false-negatives? |
| The limited work on tDCS dose response had typically applied a straightforward model to measure a response with increased tDCS intensity (e.g. from 1 to 2 mA). |
| - To what extent is this approach subject to assumptions about the spatial extent of current flow? |
| - Could not accounting for inter-individual anatomical variability in such cases lead to false-negatives? |
| - Could inter-individual variations in the intensity of current delivered to the brain combined with a non-monotonic response of the brain lead to false-negatives? |
| - How can the assumptions, implicit in conventional dose-testing studies, be made more explicit? |
| - In dose response studies, can computational models be used to retrospectively predict brain current intensity across individuals for a fixed applied current ? |
| - Can the above retrospectively and prospective use of computational models reduce variability and/or increase effect size in tDCS efficacy trials? |
The biophysics of tDCS, namely the fact that increasing current produces a linear increase in brain electric field (Fig. 1) [9] and, then, presumably membrane polarization [13], is only a starting point and it does not allow conclusions that increasing tDCS intensity enhances a given neurophysiological, behavioral, or clinical outcome. A simplistic hypothesis on dose response emerged from classical animal studies (circa 1960) – anode/cathode increases/decreases excitability and plasticity - but modern efforts suggest a more nuanced dose-response. Investigations in animal studies provide a rich substrate for DCS mechanisms but are surprisingly lacking in electric fields relevant for humans (i.e. testing multiple intensities below 1 V/m). Studies using TMS-evoked potentials have also provided an extensive substrate to design and understand tDCS protocols [59], but challenges simple notions of linear dose-response of tDCS in humans on a group or individual level [52,53].
Canonical neurophysiological studies tested intensities only up to 1 mA in the absence of tasks [4,24] and suggested a simple polarity response consistent with classical animal studies. However, increasingly higher intensities are adopted (2 or 1.5 mA [95,115,116]; and tDCS is typically used in combination with training [117], where evidence suggest a multi-factorial dose response that is not necessarily monotonic with current intensity nor does it follow a simple excitability-change rule (anode/cathode, boost/suppress). Imaging studies support a complex response across brain regions. Computational models are a tool to normalize brain current intensity across individuals but are themselves subject to assumptions about local dose response (e.g. doubling local current intensity in a ROI increases its response) to current that remains to be validated.
In conclusion, extant data on tDCS mechanisms are inconclusive in regards to whether or not graded changes in applied current, and hence brain electric fields, enhance effect sizes in a linear or monotonic way. Put simply, we still do not know whether more intensity of electric field in a given brain area supports greater neurophysiological or behavioral outcomes [114]. We believe that this is a crucial point given extensive ongoing research on tDCS. Noting the heterogeneity of the literature on tDCS dose-response [118], we urgently need to understand how much current we should deliver and how different brain regions will respond. We suggest rigorous efforts to quantify dose-response in humans, regardless of approach and outcome measure, will benefit from including computational current flow models. Despite these conclusions, we emphasize that uncertainty about dose-response does not necessarily diminish the impact of exhaustive testing of tDCS effects, its potential utility, or the value of an extensive mechanistic analysis that already exists on tDCS.
Acknowledgement
This work was partially supported by VA Merit Review Award (RX001534) and NIH/NIA funded Michigan Alzheimer’s Disease Center (P30AG053760) and NIH (grants 1R01NS101362-01, 1R01MH111896-01, 1R01NS095123-01, 1R01MH109289-01).
References
- [1].Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Sci 2012;5(3):175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Sci 2009;2(4): 201–7. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Iyer M, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann E. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 2005;64(5):872–5. [DOI] [PubMed] [Google Scholar]
- [4].Nitsche M, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527(3):633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Sci 2016;9(5):641–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marshall L, Mölle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neurosci 2005;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, et al. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord 2006;21(10):1693e702. [DOI] [PubMed] [Google Scholar]
- [8].Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, et al. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia 2006;47(7):1216–24. [DOI] [PubMed] [Google Scholar]
- [9].Bikson M, Truong DQ, Mourdoukoutas AP, Aboseria M, Khadka N, Adair D, et al. Modeling sequence and quasi-uniform assumption in computational neurostimulation. Prog Brain Res 2015;222:1–23. [DOI] [PubMed] [Google Scholar]
- [10].Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Sci 2012;5(4):435–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bestmann S, Ward N. Are current flow models for transcranial electrical stimulation fit for purpose? Brain Sci 2017;10(4):865–6. [DOI] [PubMed] [Google Scholar]
- [12].Bikson M, Rahman A, Datta A. Computational models of transcranial direct current stimulation. Clin EEG Neurosci 2012;43(3):176–83. [DOI] [PubMed] [Google Scholar]
- [13].Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol 2004;557(1):175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Truong DQ, Magerowski G, Blackburn GL, Bikson M, Alonso-Alonso M. Computational modeling of transcranial direct current stimulation (tDCS) in obesity: impact of head fat and dose guidelines. Neuroimage Clin 2013;2: 759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang Y, Liu AA, Lafon B, Friedman D, Dayan M, Wang X, et al. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Elife 2017;6, e18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jog MV, Smith RX, Jann K, Dunn W, Lafon B, Truong D, et al. In-vivo imaging of magnetic fields induced by transcranial direct current stimulation (tDCS) in human brain using MRI. Sci Rep 2016:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Opitz A, Falchier A, Yan C-G, Yeagle EM, Linn GS, Megevand P, et al. Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Sci Rep 2016:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Esmaeilpour Z, Milosevic M, Azevedo K, Khadka N, Navarro J, Brunoni A, et al. Proceedings# 21. Intracranial voltage recording during transcranial direct current stimulation (tDCS) in human subjects with validation of a standard model. Brain Sci 2017;10(4):e72–5. [Google Scholar]
- [19].Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage 2013;74:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Antal A, Bikson M, Datta A, Lafon B, Dechent P, Parra LC, et al. Imaging artifacts induced by electrical stimulation during conventional fMRI of the brain. Neuroimage 2014;85:1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fröhlich F, McCormick DA. Endogenous electric fields may guide neocortical network activity. Neuron 2010;67(1):129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, et al. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 2013;591(10):2563–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sun Y, Lipton JO, Boyle LM, Madsen JR, Goldenberg MC, Pascual-Leone A, et al. Direct current stimulation induces mGluR5-dependent neocortical plasticity. Ann Neurol 2016;80(2):233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nitsche M, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 2003;553(1): 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Monai H, Ohkura M, Tanaka M, Oe Y, Konno A, Hirai H, et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun 2016:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jackson MP, Rahman A, Lafon B, Kronberg G, Ling D, Parra LC, et al. Animal models of transcranial direct current stimulation: methods and mechanisms. Clin Neurophysiol 2016;127(11):3425–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lafon B, Rahman A, Bikson M, Parra LC. Direct current stimulation alters neuronal input/output function. Brain Sci 2017;10(1):36e–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J Neurosci 2010;30(45):15067–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chan C, Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol 1986;371(1): 89–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Deans JK, Powell AD, Jefferys JG. Sensitivity of coherent oscillations in rat hippocampus to AC electric fields. J Physiol 2007;583(2):555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Sci 2009;2(4):215–28. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Paulus W, Rothwell JC. Membrane resistance and shunting inhibition: where biophysics meets state-dependent human neurophysiology. J Physiol 2016;594(10):2719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 2010;66(2):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bindman LJ, Lippold O, Redfearn J. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol 1964;172(3):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gartside IB. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: role of protein synthesis. Nature 1968;220(5165):383–4. [DOI] [PubMed] [Google Scholar]
- [36].Kronberg G, Bridi M, Abel T, Bikson M, Parra LC. Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Sci 2017;10(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yamamoto T, Katayama Y, Kano T, Kobayashi K, Oshima H, Fukaya C. Deep brain stimulation for the treatment of parkinsonian, essential, and poststroke tremor: a suitable stimulation method and changes in effective stimulation intensity. J Neurosurg 2004;101(2):201–9. [DOI] [PubMed] [Google Scholar]
- [38].Cif L, Ruge D, Gonzalez V, Limousin P, Vasques X, Hariz MI, et al. The influence of deep brain stimulation intensity and duration on symptoms evolution in an OFF stimulation dystonia study. Brain Sci 2013;6(4):500–5. [DOI] [PubMed] [Google Scholar]
- [39].Padberg F, Zwanzger P, Keck ME, Kathmann N, Mikhaiel P, Ella R, et al. Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology 2002;27(4):638–45. [DOI] [PubMed] [Google Scholar]
- [40].Moliadze V, Atalay D, Antal A, Paulus W. Close to threshold transcranial electrical stimulation preferentially activates inhibitory networks before switching to excitation with higher intensities. Brain Sci 2012;5(4):505–11. [DOI] [PubMed] [Google Scholar]
- [41].Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci 2005;22(2):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].DaSilva AF, Mendonca ME, Zaghi S, Lopes M, DosSantos MF, Spierings EL, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache 2012;52(8):1283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seibt O, Brunoni AR, Huang Y, Bikson M. The pursuit of DLPFC: non-neuronavigated methods to target the left dorsolateral pre-frontal cortex with symmetric bicephalic transcranial direct current stimulation (tDCS). Brain Sci 2015;8(3):590–602. [DOI] [PubMed] [Google Scholar]
- [44].Datta A, Truong D, Minhas P, Parra LC, Bikson M. Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Front Psychiatry 2012;3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Huang Y, Parra LC, Haufe S. The New York Headda precise standardized volume conductor model for EEG source localization and tES targeting. Neuroimage 2016;140:150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Alam M, Truong DQ, Khadka N, Bikson M. Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Phys Med Biol 2016;61(12):4506. [DOI] [PubMed] [Google Scholar]
- [47].Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng 2011;(4):8 046011. [DOI] [PubMed] [Google Scholar]
- [48].Gbadeyan O, Steinhauser M, McMahon K, Meinzer M. Safety, tolerability, blinding efficacy and behavioural effects of a novel MRI-compatible, high-definition tDCS set-up. Brain Sci 2016;9(4):545–52. [DOI] [PubMed] [Google Scholar]
- [49].Kuo H-I, Bikson M, Datta A, Minhas P, Paulus W, Kuo M- F, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Sci 2013;6(4):644–8. [DOI] [PubMed] [Google Scholar]
- [50].Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. Effects of prefrontal bipolar and high-definition transcranial direct current stimulation on cortical reactivity and working memory in healthy adults. Neuroimage 2017;152:142–57. [DOI] [PubMed] [Google Scholar]
- [51].Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001;57(10): 1899–901. [DOI] [PubMed] [Google Scholar]
- [52].Strube W, Bunse T, Nitsche MA, Nikolaeva A, Palm U, Padberg F, et al. Bidirectional variability in motor cortex excitability modulation following 1 mA transcranial direct current stimulation in healthy participants. Physiol Rep 2016;4(15), e12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche M. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 2013;591(7):1987–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Moliadze V, Schmanke T, Andreas S, Lyzhko E, Freitag CM, Siniatchkin M. Stimulation intensities of transcranial direct current stimulation have to be adjusted in children and adolescents. Clin Neurophysiol 2015;126(7): 1392–9. [DOI] [PubMed] [Google Scholar]
- [55].Kidgell DJ, Daly RM, Young K, Lum J, Tooley G, Jaberzadeh S, et al. Different current intensities of anodal transcranial direct current stimulation do not differentially modulate motor cortex plasticity. Neural Plast 2013:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cuypers K, Leenus DJ, van den Berg FE, Nitsche MA, Thijs H, Wenderoth N, et al. Is motor learning mediated by tDCS intensity? PLos One 2013;8(6): e67344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ho K-A, Taylor JL, Chew T, Galvez V, Alonzo A, Bai S, et al. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: evidence from single and repeated sessions. Brain Sci 2016;9(1):1–7. [DOI] [PubMed] [Google Scholar]
- [58].Ammann C, Lindquist MA, Celnik PA. Response variability of different anodal transcranial direct current stimulation intensities across multiple sessions. Brain Sci 2017;10(4):757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jamil A, Batsikadze G, Kuo HI, Labruna L, Hasan A, Paulus W, et al. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J Physiol 2017;595(4): 1273–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Minhas P, Bikson M, Woods AJ, Rosen AR, Kessler SK, editors. Transcranial direct current stimulation in pediatric brain: a computational modeling study; 2012. p. 859–62. Conf Proc IEEE Eng Med Biol Soc 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PLos One 2013;8(9), e76112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Caparelli-Daquer EM, Zimmermann TJ, Mooshagian E, Parra LC, Rice JK, Datta A, et al. , editors. A pilot study on effects of 4 × 1 high-definition tDCS on motor cortex excitability; 2012. p. 735–8. Conf Proc IEEE Eng Med Biol Soc 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nikolin S, Loo CK, Bai S, Dokos S, Martin DM. Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. Neuroimage 2015;117: 11–9. [DOI] [PubMed] [Google Scholar]
- [64].Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol 2003;114(4):600–4. [DOI] [PubMed] [Google Scholar]
- [65].Hämmerer D, Bonaiuto J, Klein-Flügge M, Bikson M, Bestmann S. Selective alteration of human value decisions with medial frontal tDCS is predicted by changes in attractor dynamics. Sci Rep 2016;6:25160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Weiss M, Lavidor M. When less is more: evidence for a facilitative cathodal tDCS effect in attentional abilities. J Cogn Neurosci 2012;24(9):1826–33. [DOI] [PubMed] [Google Scholar]
- [67].Rahman A, Lafon B, Parra LC, Bikson M. Direct current stimulation boosts synaptic gain and cooperativity in vitro. J Physiol 2017595(11):3535–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bonaiuto JJ, Bestmann S. Understanding the nonlinear physiological and behavioral effects of tDCS through computational neurostimulation. Prog Brain Res 2015;222:75–103. [DOI] [PubMed] [Google Scholar]
- [69].Meinzer M, Antonenko D, Lindenberg R, Hetzer S, Ulm L, Avirame K, et al. Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. J Neurosci 2012;32(5): 1859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Antal A, Polania R, Schmidt-Samoa C, Dechent P, Paulus W. Transcranial direct current stimulation over the primary motor cortex during fMRI. Neuroimage 2011;55(2):590–6. [DOI] [PubMed] [Google Scholar]
- [71].Mangia AL, Pirini M, Cappello A. Transcranial direct current stimulation and power spectral parameters: a tDCS/EEG co-registration study. Front Hum Neurosci 2014;8:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kunze T, Hunold A, Haueisen J, Jirsa V, Spiegler A. Transcranial direct current stimulation changes resting state functional connectivity: a large-scale brain network modeling study. Neuroimage 2016;140:174–87. [DOI] [PubMed] [Google Scholar]
- [73].Hampstead BM, Brown GS, Hartley JF. Transcranial direct current stimulation modulates activation and effective connectivity during spatial navigation. Brain Stimulation 2014;7(2):314–24. [DOI] [PubMed] [Google Scholar]
- [74].Krishnamurthy V, Gopinath K, Brown GS, Hampstead BM. Resting-state fMRI reveals enhanced functional connectivity in spatial navigation networks after transcranial direct current stimulation. Neurosci Lett 2015;604:80–5. [DOI] [PubMed] [Google Scholar]
- [75].Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage 2011;58(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Halko M, Datta A, Plow EB, Scaturro J, Bikson M, Merabet LB. Neuroplastic changes following rehabilitative training correlate with regional electrical field induced with tDCS. Neuroimage 2011;57(3):885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Heeger DJ, Huk AC, Geisler WS, Albrecht DG. Spikes versus BOLD: what does neuroimaging tell us about neuronal activity? Nat Neurosci 2000;3:631–2. [DOI] [PubMed] [Google Scholar]
- [78].Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci 2002;25(1):27–31. [DOI] [PubMed] [Google Scholar]
- [79].Boussida S, Traoré AS, Durif F. Mapping of the brain hemodynamic responses to sensorimotor stimulation in a rodent model: a BOLD fMRI study. PLos One 2017;12(4), e0176512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Horwitz B Relating fMRI and PET signals to neural activity by means of large-scale neural models. Neuroinformatics 2004;2(2):251–66. [DOI] [PubMed] [Google Scholar]
- [81].Homan P, Kindler J, Federspiel A, Flury R, Hubl D, Hauf M, et al. Muting the voice: a case of arterial spin labeling-monitored transcranial direct current stimulation treatment of auditory verbal hallucinations. Am J Psychiatry 2011;168(8):853e–4. [DOI] [PubMed] [Google Scholar]
- [82].Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, et al. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci 2013;33(28):11425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimulation 2016;10(5):983–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Stephens JA, Jones KT, Berryhill ME. Task demands, tDCS intensity, and the COMT val 158 met polymorphism impact tDCS-linked working memory training gains. Sci Rep 2017;7(1):13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hoy KE, Emonson MR, Arnold SL, Thomson RH, Daskalakis ZJ, Fitzgerald PB. Testing the limits: investigating the effect of tDCS dose on working memory enhancement in healthy controls. Neuropsychologia 2013;51(9):1777–84. [DOI] [PubMed] [Google Scholar]
- [86].Teo F, Hoy KE, Daskalakis ZJ, Fitzgerald PB. Investigating the role of current strength in tDCS modulation of working memory performance in healthy controls. Front Psychiatry 2011;2:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cappon D, Jahanshahi M, Bisiacchi P. Value and efficacy of transcranial direct current stimulation in the cognitive rehabilitation: a critical review since 2000. Front Neurosci 2016;10:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hampstead BM, Sathian K, Bikson M, Stringer AY. Combined mnemonic strategy training and high-definition transcranial direct current stimulation for memory deficits in mild cognitive impairment. Alzheimers Dement (N Y) 2017;3(3):459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fiori V, Coccia M, Marinelli CV, Vecchi V, Bonifazi S, Ceravolo MG, et al. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. J Cogn Neurosci 2011;23(9):2309–23. [DOI] [PubMed] [Google Scholar]
- [90].Jung I-Y, Lim JY, Kang EK, Sohn HM, Paik N- J. The factors associated with good responses to speech therapy combined with transcranial direct current stimulation in post-stroke aphasic patients. Ann Rehabil Med 2011;35(4): 460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Marangolo P, Fiori V, Campana S, Calpagnano MA, Razzano C, Caltagirone C, et al. Something to talk about: enhancement of linguistic cohesion through tdCS in chronic non fluent aphasia. Neuropsychologia 2014;53:246–56. [DOI] [PubMed] [Google Scholar]
- [92].Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. BJPsych Open 2016;208(6):522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shekhawat GS, Sundram F, Bikson M, Truong D, De Ridder D, Stinear CM, et al. Intensity, duration, and location of high-definition transcranial direct current stimulation for tinnitus relief. Neurorehabilitation Neural Repair 2016;30(4):349–59. [DOI] [PubMed] [Google Scholar]
- [94].Murray LM, Edwards DJ, Ruffini G, Labar D, Stampas A, Pascual-Leone A, et al. Intensity dependent effects of transcranial direct current stimulation on corticospinal excitability in chronic spinal cord injury. Arch Phys Med Rehabil 2015;96(4):S114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci 2006;249(1):31–8. [DOI] [PubMed] [Google Scholar]
- [96].Li H, Lei X, Yan T, Li H, Huang B, Li L, et al. The temporary and accumulated effects of transcranial direct current stimulation for the treatment of advanced Parkinson’s disease monkeys. Sci Rep 2015;5:12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Andrade C Once-to twice-daily, 3-year domiciliary maintenance transcranial direct current stimulation for severe, disabling, clozapine-refractory continuous auditory hallucinations in schizophrenia. J ECT 2013;29(3): 239–42. [DOI] [PubMed] [Google Scholar]
- [98].Hoy KE, Arnold SL, Emonson MR, Daskalakis ZJ, Fitzgerald PB. An investigation into the effects of tDCS dose on cognitive performance over time in patients with schizophrenia. Schizophr Res 2014;155(1):96–100. [DOI] [PubMed] [Google Scholar]
- [99].Nitsche MA, Bikson M. Extending the parameter range for tDCS: safety and tolerability of 4 mA stimulation. Brain Sci 2017;10(3):541–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bikson M, Rahman A, Datta A, Fregni F, Merabet L. High-Resolution modeling assisted design of customized and individualized transcranial direct current stimulation protocols. Neuromodulation 2012;15(4):306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mendonca ME, Santana MB, Baptista AF, Datta A, Bikson M, Fregni F, et al. Transcranial DC stimulation in fibromyalgia: optimized cortical target supported by high-resolution computational models. J Pain 2011;12(5):610–7. [DOI] [PubMed] [Google Scholar]
- [102].Bikson M, Datta A, Rahman A, Scaturro J . Electrode montages for tDCS and weak transcranial electrical stimulation: role of “return” electrode’s position and size. Clin Neurophysiol 2010;121(12):1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Datta A, Baker JM, Bikson M, Fridriksson J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Sci 2011;4(3):169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Castillo-Saavedra L, Gebodh N, Bikson M, Diaz-Cruz C, Brandao R, Coutinho L, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J Pain 2016;17(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zito GA, Senti T, Cazzoli D, Müri RM, Mosimann UP, Nyffeler T, et al. Cathodal HD-tDCS on the right V5 improves motion perception in humans. Front Behav Neurosci 2015:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Giordano J, Bikson M, Kappenman ES, Clark VP, Coslett HB, Hamblin MR, et al. Mechanisms and effects of transcranial direct current stimulation. Dose-Response 2017;15(1). 1559325816685467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Jackson MP, Truong D, Brownlow ML, Wagner JA, McKinley RA, Bikson M, et al. Safety parameter considerations of anodal transcranial Direct Current Stimulation in rats. Brain Behav Immun 2017;64:152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Márquez-Ruiz J, Leal-Campanario R, Sánchez-Campusano R, Molaee-Ardekani B, Wendling F, Miranda PC, et al. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proc Natl Acad Sci U S A 2012;109(17):6710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni A, Chen R, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol 2017;128(9):1774–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sánchez-Kuhn A, Pérez-Fernández C, Cánovas R, Flores P, Sánchez-Santed F. Transcranial direct current stimulation as a motor neurorehabilitation tool: an empirical review. Biomed Eng Online 2017;16(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J, et al. Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin Neurophysiol 2017;128(4):589–603. [DOI] [PubMed] [Google Scholar]
- [112].Weinberger AB, Green AE, Chrysikou EG. Using transcranial direct current stimulation to enhance creative cognition: interactions between task, polarity, and stimulation site. Front Hum Neurosci 2017:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Woods A, Antal A, Bikson M, Boggio PS, Brunoni A, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016;127(2):1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bestmann S, de Berker AO, Bonaiuto J. Understanding the behavioural consequences of noninvasive brain stimulation. Trends Cognit Sci 2015;19(1): 13–20. [DOI] [PubMed] [Google Scholar]
- [115].Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006;122(1):197–209. [DOI] [PubMed] [Google Scholar]
- [116].Shah-Basak PP, Norise C, Garcia G, Torres J, Faseyitan O, Hamilton RH. Individualized treatment with transcranial direct current stimulation in patients with chronic non-fluent aphasia due to stroke. Front Hum Neurosci 2015;9:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bikson M, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci 2013;7:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Sci 2014;7(3):372e80. [DOI] [PubMed] [Google Scholar]


