Abstract
Background
Being woman is associated with higher survival rates after transcatheter aortic valve replacement (TAVR) despite the increase in periprocedural complications. The left ventricle (LV) remodelling process that follows TAVR is considered to play an important role. We aim to investigate whether gender difference affects the process of LV remodelling after TAVR.
Materials and Methods
A total of 100 patients (50 men and 50 women) after TAVR were enrolled. Echocardiography was performed at baseline before the TAVR procedure and repeated upon discharge, and at three, nine and 12 months post‐TAVR.
Results
Women exhibited an early regression of LV mass and the LV mass index (LVMi) decreased 12.0% from 148.3 ± 48.0 to 130.5 ± 43.7 g/m2 at just a median of 17 days after the procedure (P < .001). Almost one‐half of the LVMi regression occurred by 17 days post‐TAVR and the LVMi regressed 22.0% by 12 months post‐TAVR. In contrast, the regression of LVMi in men seemed to be more gradual and the significant regression of LVMi from baseline began to be observed since three months later after TAVR. The LVMi reduction at nine months was 11.5% and achieved 15.4% over one year. Multivariable logistic regression analysis showed only the female sex, better LVEF and greater baseline LVMi were independently associated with greater LVMi regression after TAVR, indicating female gender is an independent predictor for favourable LV remodelling after TAVR.
Conclusion
In conclusion, female patients with AS had favourable reverse remodelling with greater and earlier LV mass regression post‐TAVR compared with the male patients.
Keywords: aortic stenosis, LV remodelling, transcatheter aortic valve replacement
1. INTRODUCTION
With a reported prevalence of 12.4% among elderly patients, aortic stenosis (AS) is the most common form of valvular heart disease in the ageing population.1 Without valve replacement, the prognosis for hemodynamically severe and symptomatic AS is poor. Transcatheter aortic valve replacement (TAVR) has become the gold standard of care for patients with hemodynamically severe AS who are symptomatic but deemed to be too high‐risk for surgery. Recent reports suggest that sex‐based differences exist in terms of post‐TAVR clinical outcome. Being woman is associated with better mid‐ and long‐term survival following TAVR despite increased periprocedural complications, particularly more vascular complications (6%‐20% vs 2%‐14%) and higher bleeding rates (10%‐44% vs 8%‐25%) at 30 days post‐TAVI.2, 3, 4, 5, 6
Why female patients experience better outcomes remains poorly understood. The process of LV remodelling post‐TAVI likely contributes to the sex‐based differences in outcome after TAVI. The pressure overload in the LV caused by AS induces cardiac muscle hypertrophy and interstitial collagen deposition, leading to structural changes in the LV (ie LV remodelling). Sex appears to exert a strong influence on LV remodelling in surgical aortic valve replacement.7, 8, 9, 10 Women with severe AS typically manifest more concentric LV geometry, less myocardial fibrosis and better systolic function compared with their male counterparts. Surgical studies of patients with AS undergoing AVR have found less fibrosis on the myocardium biopsies from women, and the regression of the myocardial hypertrophy also occurs more rapidly in female patients.11 The goal of the present study was to investigate the timeline of LV remodelling in both sexes towards establishing whether women have more favourable outcomes relative to men and identifying independent predictors for early LV mass regression following TAVR.
2. METHODS
2.1. Patients
From May 2010 to March 2016, a total of 130 consecutive patients with severe, symptomatic AS (valve area ≤ 1.0 cm2) were evaluated. All patients had New York Heart Association (NYHA) symptoms exceeding class II and were slated to undergo TAVR. These patients were selected for TAVR after a heart team discussion deemed them either unsuitable or too high‐risk for surgical aortic valve replacement. Their individual operative risk was calculated using the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) system. Patient selection was based on the approved indication for TAVR in Taiwan using either of the following criteria: (a) patients considered to be at high surgical risk with logistic EuroSCOREs ≥ 20%; (b) patients aged over 80 years old; (c) a history or the presence of previous cardiac surgery, porcelain aorta, thoracic burning sequelae contraindicating open heart surgery, mediastinum radiotherapy, severe connective tissue disease contraindicative to surgery, cirrhosis of the liver (child class A or B) or severe pulmonary insufficiency with forced expiratory volume in one second (FEV1) under one litre. The selection of patients for whom TAVR was determined to be the most suitable treatment approach required the clinical consensus of a multidisciplinary team comprised of cardiac surgeons, interventional cardiologists, anaesthetists and imaging specialists. The main exclusion criteria were a native aortic valve annulus of less than 18 mm or more than 29 mm, acute myocardial infarction for under 14 days, a left ventricular ejection fraction below 20%, active infection, hemodynamic instability or a life expectancy under 12 months. This study was approved by the institutional research board in Taipei Veterans General Hospital and followed STROBE statement and the broader EQUATOR guidelines.12
2.2. TAVR procedure
All TAVR procedures were performed in a specially‐equipped hybrid operating suite. At the beginning of this study, the TAVR procedures were performed under general anaesthesia. Since December 2013, local anaesthesia with conscious sedation has been used exclusively for transfemoral TAVR at our institution. The standard approach was through the transfemoral route, when feasible. For patients without the adequate anatomy required to allow for safe transfemoral access, alternative access routes were employed. Adjunct pharmacologic therapy included heparin treatment during the procedure and aspirin (100 mg/d) indefinitely and clopidogrel (75 mg/d) for three to six months following the procedure.
2.3. Echocardiography
Echocardiography was performed using a GE Vivid E9 system (GE Healthcare) with a 4‐MHz transducer. An independent core laboratory analysed all of the patient echocardiograms. The mass of the LV was calculated using the formula put forth by the American Society of Echocardiography and indexed to body surface area (termed LV mass index).13 The presence and severity of post‐procedural aortic regurgitation were determined according to the Valve Academic Research Consortium‐2 (VARC‐2) criteria.14 As described in their protocol, echocardiograms were obtained at baseline (within 45 days of the TAVR) and post‐TAVR at discharge (or 7 days), three months, nine months and one year.13
2.4. Clinical endpoints
The 30‐day combined safety endpoint is defined by the VARC‐2 [13] as a composite of all‐causes of mortality, major stroke, life‐threatening or disabling bleeding, acute stage 2 or 3 kidney injury including renal replacement therapy, major vascular complications and repeat procedure for valve‐related dysfunction. Stroke was defined as a focal neurologic deficit lasting over 24 hours or a focal neurologic deficit lasting under 24 hours with imaging findings of acute infarction or haemorrhage. The VARC‐2 proposed using the AKIN system for reporting acute kidney injury (AKI).
2.5. Statistical analysis
Data were expressed as the mean ± standard deviation for the numeric variables and as a per cent for the categorical variables. Continuous variable comparisons between groups were achieved using a one‐way analysis of variance (ANOVA). Subgroup comparisons among the categorical variables were assessed using the Chi‐square and Fisher's exact tests. Serial changes in LV mass index (LVMi) post‐TAVR were analysed using a paired t test, which enabled evaluation of the differences at each LVMi follow‐up. A logistic regression analysis was performed to investigate the independent factors potentially contributing to LV remodelling after the TAVR procedure.
3. RESULTS
A total of 100 severe, symptomatic AS patients (n = 50 men and 50 women) received echocardiograms measured at baseline, discharge, and three and nine months post‐TAVR were enrolled in this study. Echocardiograms were not obtained post‐TAVR for 29 patients after one year for the following reasons: noncardiac‐related deaths (n = 13); cardiac‐related deaths (n = 8); underwent follow‐up at a referral hospital (n = 7) and poor image quality (n = 2). The study flow and baseline characteristics are listed in Table 1 and Figure 1.
Table 1.
Baseline characteristics of study population
| Male (n = 50) | Female (n = 50) | P | |
|---|---|---|---|
| Age, y | 80.96 ± 8.79 | 79.46 ± 9.95 | .371 |
| Height, mm Hg | 164.5 ± 6.2 | 151.3 ± 4.5 | <.001 |
| Weight, kg | 65.3 ± 11.6 | 56.0 ± 10.5 | <.001 |
| BMI, kg/m2 | 24.0 ± 3.5 | 24.62 ± 4.1 | .431 |
| Hypertension, n (%) | 38 (76) | 34 (68) | .504 |
| BSA | 1.72 ± 0.17 | 1.53 ± 0.17 | <.001 |
| DM, n (%) | 19 (38) | 17 (34) | .835 |
| CABG, n (%) | 1 (2) | 3 (6) | .307 |
| PCI, n (%) | 19 (38) | 11 (22) | .081 |
| PVD, n (%) | 12 (24) | 7 (14) | .202 |
| Hyperlipidemia, n (%) | 22 (44) | 22 (44) | 1.000 |
| Euroscore | 16.48 ± 12.65 | 20.94 ± 18.35 | .004 |
| CHF, III‐IV | 29 (58) | 30 (60) | .839 |
| Bicuspid, n (%) | 6 (12) | 6 (12) | 1.000 |
| PLF‐LG AOST, n (%) | 16 (32) | 17 (34.7) | .872 |
| AF, n (%) | 7 (14) | 13 (26) | .211 |
| Stroke/TIA, n (%) | 13 (26) | 7 (14) | .084 |
| Hyperlipidemia, n (%) | 22 (44) | 22 (44) | 1.000 |
| Pulmonary disease | 15 (30) | 14 (28) | .826 |
| Prior BAV | 2 (4) | 5 (10) | .436 |
| Prior MI | 2 (4) | 3 (6) | 1.000 |
| Prior CABG, n (%) | 1 (2) | 3 (6) | .307 |
| Prior PCI, n (%) | 19 (38) | 11 (22) | .081 |
| Prior BAV | 2 (4) | 5 (10) | .436 |
| Prior AF | 7 (14) | 13 (26) | .211 |
| Valve type | |||
| Edward | 10 (20) | 6 (12) | <.001 |
| 23 mm | 3 (6) | 5 (10) | |
| 26 mm | 6 (12) | 1 (2) | |
| 29 mm | 1 (2) | 0 | |
| Metronic | 40 (80) | 44 (88) | |
| 26 mm | 11 (22) | 28 (56) | |
| 29 mm | 22 (44) | 15 (30) | |
| 31 mm | 7 (14) | 1 (2) | |
| Vascular access | |||
| Transfemoral, n (%) | 42 (84) | 45 (89) | .631 |
| Transapical, n (%) | 1 (2) | 1 (2) | |
| Direct aortic, n (%) | 7 (14) | 4 (9) | |
Values data are n (%) or mean ± SD.
Abbreviations: AF, atrial fibrillation; AVA, aortic valve area; BAV, balloon aortic valvuloplasty; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHF, congestive heart failure; DM, diabetes; LAD, left atrium diameter; LVEF, left ventricular ejection fraction; LVMi, left ventricle mass index; MPG, mean pressure gradient; PCI, percutaneous coronary intervention; PLF‐LG AOST, paradoxical low‐flow low gradient aortic stenosis; PVD, peripheral vascular disease.
Figure 1.
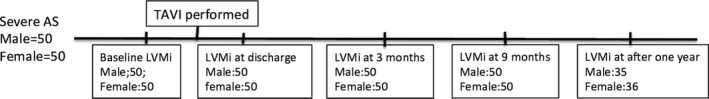
Study flow of LVMi change after TAVI for severe AS patients
The study population had a mean age of 80.21 ± 8.37 years and an average aortic valve area of 0.70 ± 0.23 cm2. The female subjects had higher EuroSCOREs (20.94 ± 18.35 vs 16.48 ± 12.65, P = .004) and shorter statures (151.3 ± 4.5 vs 164.5 ± 6.2 cm) and weighed less (56 ± 10.5 vs 65.3 ± 11.6 kg) than the men, though both groups had similar body mass indexes (BMIs). The prevalence of co‐morbidities including hypertension, diabetes, bicuspid aortic valve, history of CAD, previous coronary intervention, peripheral vascular disorder, atrial fibrillation, previous stroke and percentage of stage III‐VI congestive heart failure (CHF) were comparable for both sexes. Although there is a slightly higher percentage of low‐flow low gradient aortic stenosis (PLF‐LG AOST) in woman, their difference is insignificant (34.7% vs 32%, P = .872). Echocardiographic analysis revealed narrower aortic roots (30.2 ± 3.5 mm vs 33.3 ± 3.4 mm, P < .001) and smaller aortic valve areas in women (0.64 ± 0.21 cm2 vs 0.76 ± 0.23 mm2, P = .007), but indexed aortic valve area, mean pressure gradient, peak pressure gradient, ejection fraction, LV mass and indexed LV mass were similar in both sexes (Table 2). In addition, the valvulo‐arterial impedance (Zva)15, 16 was estimated and female patients have significant higher (Zva) at baseline before TAVI procedure than male patients (6.4 ± 2.1 vs 5.6 ± 1.7 mm Hg/mL/m2, P = .039).
Table 2.
Serial change of parameters of echocardiography after TAVI
| Male, N = 50 | Female, N = 50 | P | |
|---|---|---|---|
| Baseline | |||
| Aortic root, mm | 33.3 ± 3.4 | 30.2 ± 3.5 | <.001 |
| LAD, mm | 43.8 ± 7.6 | 45.36 ± 9.5 | .378 |
| AVA, cm2 | 0.76 ± 0.23 | 0.64 ± 0.21 | .007 |
| AVA/BSA | 0.45 ± 0.14 | 0.41 ± 0.14 | .29 |
| MPG, mm Hg | 43.0 ± 17.6 | 49.1 ± 22.5 | .136 |
| PPG, mm Hg | 72.5 ± 28.8 | 79.7 ± 35.1 | .268 |
| Septum, mm | 12.5 ± 1.7 | 12.4 ± 2.0 | .745 |
| PW, mm | 12.4 ± 1.7 | 12.2 ± 1.8 | .415 |
| LVEDV, mL | 89.6 ± 29.5 | 67.7 ± 29.2 | .001 |
| LVESV, mL | 43.3 ± 24.0 | 32.74 ± 23.5 | .037 |
| LVIDd, mm | 49.2 ± 7.9 | 47.3 ± 7.7 | .56 |
| LVIDs, mm | 31.1 ± 8.8 | 30.0 ± 9.1 | .22 |
| LVEF, % | 54.7 ± 11.5 | 55.2 ± 10.9 | .823 |
| LV mass | 245.8 ± 75.7 | 227.3 ± 76.5 | .227 |
| LVMi | 143.1 ± 39.6 | 148.3 ± 48.0 | .552 |
| Zva, mm Hg/mL/m2 | 5.6 ± 1.7 | 6.4 ± 2.1 | .039 |
| E/E′ | 22.1 ± 9.6 | 26.2 ± 11.3 | .065 |
| LA volume, mL | 76.4 ± 44.9 | 74.5 ± 54.9 | .847 |
| PA pressure, mm Hg | 42.4 ± 15.2 | 48.2 ± 16.8 | .098 |
| Discharge | |||
| Aortic root, mm | 30.3 ± 4.6 | 27.1 ± 4.4 | .001 |
| LAD, mm | 44.8 ± 8.4 | 45.1 ± 9.6 | .858 |
| AVA, cm2 | 1.57 ± 0.39 | 1.51 ± 0.40 | .580 |
| MPG, mm Hg | 10.1 ± 4.3 | 9.5 ± 4.9 | .502 |
| PPG, mm Hg | 18.6 ± 8.2 | 17.6 ± 8.6 | .572 |
| Septum, mm | 12.2 ± 1.9 | 11.5 ± 1.9 | .09 |
| PW, mm | 12.2 ± 1.8 | 11.2 ± 2.0 | .013 |
| LVEDV, mL | 85.1 ± 32.4 | 68.2 ± 28.2 | .009 |
| LVESV, mL | 39.6 ± 20.5 | 31.6 ± 22.4 | .080 |
| LVIDd, mm | 49.1 ± 6.9 | 46.8 ± 7.7 | .12 |
| LVIDs, mm | 31.6 ± 7.0 | 28.2 ± 8.8 | .035 |
| LVEF, % | 55.1 ± 8.8 | 56.9 ± 9.6 | .338 |
| LV mass | 237.3 ± 69.3 | 199.7 ± 68.7 | .008 |
| LVMi | 138.4 ± 36.9 | 130.5 ± 43.7 | .335 |
| Zva, mm Hg/mL/m2 | 4.5 ± 2.1 | 4.8 ± 1.7 | .434 |
| E/E′ | 18.6 ± 7.3 | 24.1 ± 8.6 | .003 |
| LA volume, mL | 72.6 ± 43.5 | 70.4 ± 42.9 | .799 |
| PA pressure, mm Hg | 35.9 ± 9.7 | 43.7 ± 15.2 | .005 |
| 2nd follow‐up | |||
| Aortic root, mm | 30.0 ± 4.2 | 27.9 ± 4.7 | .016 |
| LAD, mm | 42.9 ± 7.5 | 45.5 ± 9.1 | .127 |
| AVA, cm2 | 1.9 ± 0.8 | 1.6 ± 0.4 | .148 |
| MPG, mm Hg | 10.1 ± 4.7 | 9.7 ± 4.9 | .691 |
| PPG, mm Hg | 18.5 ± 8.9 | 18.1 ± 9.2 | .828 |
| Septum, mm | 12.0 ± 2.5 | 11.1 ± 2.1 | .003 |
| PW, mm | 11.5 ± 1.8 | 10.9 ± 1.5 | .057 |
| LVEDV, mL | 81.3 ± 28.3 | 67.4 ± 30.8 | .027 |
| LVESV, mL | 37.7 ± 17.0 | 31.4 ± 24.0 | .152 |
| LVIDd, mm | 49.9 ± 6.8 | 45.5 ± 7.6 | .09 |
| LVIDs, mm | 31.5 ± 6.7 | 29.0 ± 8.1 | .003 |
| LVEF, % | 55.1 ± 7.4 | 56.3 ± 10.0 | .498 |
| LV mass | 231.4 ± 72.3 | 182.8 ± 69.5 | .001 |
| LVMi | 133.9 ± 37.9 | 119.1 ± 42.9 | .062 |
| Zva, mm Hg/mL/m2 | 4.0.7 ± 1.9 | 4.2 ± 1.5 | .147 |
| E/E′ | 19.9 ± 8.8 | 25.3 ± 10.9 | .016 |
| LA volume, mL | 68.9 ± 37.3 | 68.4 ± 52.1 | .956 |
| PA pressure, mm Hg | 36.2 ± 10.1 | 44.3 ± 16.6 | .005 |
| 3rd follow‐up | |||
| Aortic root, mm | 30.8 ± 4.9 | 27.2 ± 4.7 | <.001 |
| LAD, mm | 42.7 ± 9.0 | 45.5 ± 9.1 | .125 |
| AVA, cm2 | 1.7 ± 0.6 | 1.7 ± 0.6 | .629 |
| MPG, mm Hg | 9.5 ± 4.6 | 9.4 ± 4.8 | .845 |
| PPG, mm Hg | 17.8 ± 7.9 | 17.5 ± 8.8 | .873 |
| Septum, mm | 11.7 ± 2.2 | 10.8 ± 1.9 | .021 |
| PW, mm | 11.2 ± 1.7 | 10.7 ± 1.5 | .172 |
| LVEDV, mL | 83.9 ± 33.0 | 64.0 ± 32.0 | .005 |
| LVESV, mL | 38.1 ± 17.9 | 28.9 ± 23.2 | .039 |
| LVIDd, mm | 49.0 ± 7.3 | 45.7 ± 6.6 | .03 |
| LVIDs, mm | 31.3 ± 7.0 | 28.2 ± 7.2 | .02 |
| LVEF, % | 55.5 ± 8.3 | 57.8 ± 9.2 | .211 |
| LV mass | 218.0 ± 70.0 | 178.4 ± 51.5 | .002 |
| LVMi | 126.7 ± 36.1 | 116.8 ± 33.5 | .171 |
| Zva, mm Hg/mL/m2 | 4.7 ± 1.5 | 4.3 ± 1.2 | .144 |
| E/E′ | 20.1 ± 11.1 | 25.2 ± 14.2 | .07 |
| LA volume, mL | 62.2 ± 39.2 | 66.1 ± 57.4 | .692 |
| PA pressure, mm Hg | 35.4 ± 10.1 | 42.2 ± 15.8 | .017 |
| 4th follow‐up | |||
| Aortic root, mm | 31.6 ± 4.6 | 26.5 ± 3.8 | <.001 |
| LAD, mm | 42.0 ± 6.2 | 47.4 ± 11.6 | .018 |
| AVA, cm2 | 1.59 ± 0.48 | 1.58 ± 0.44 | .902 |
| MPG, mm Hg | 10.1 ± 6.1 | 10.1 ± 5.0 | .973 |
| PPG, mm Hg | 19.0 ± 10.9 | 18.5 ± 9.3 | .844 |
| Septum, mm | 11.4 ± 1.8 | 11.2 ± 2.1 | .649 |
| PW, mm | 11.5 ± 1.5 | 10.7 ± 1.4 | .207 |
| LVEDV, mL | 83.6 ± 34.4 | 62.6 ± 29.1 | .008 |
| LVESV, mL | 38.2 ± 22.5 | 28.7 ± 23.2 | .090 |
| LVIDd, mm | 48.8 ± 8.1 | 44.4 ± 7.8 | .024 |
| LVIDs, mm | 31.2 ± 8.0 | 28.4 ± 8.3 | .17 |
| LVEF, % | 56.4 ± 9.1 | 56.9 ± 9.3 | .839 |
| LV mass | 210.3 ± 66.7 | 174.4 ± 47.9 | .012 |
| LVMi | 121.0 ± 32.1 | 115.7 ± 30.8 | .487 |
| Zva, mm Hg/mL/m2 | 4.5 ± 1.9 | 4.2 ± 1.8 | .497 |
| E/E′ | 21.1 ± 9.3 | 23.6 ± 10.5 | .326 |
| LA volume, mL | 48.9 ± 39.2 | 54.0 ± 49.1 | .626 |
| PA pressure, mm Hg | 36.4 ± 9.2 | 43.5 ± 15.6 | .025 |
Values data are mean ± SD.
Abbreviations: AVA, aortic valve area; LAD, left atrium diameter; LVEF, left ventricular ejection fraction; LVMi, left ventricle mass index; MPG, mean pressure gradient; PW, posterior wall; Zva, valvulo‐arterial impedance.
The procedural details are listed in Table 1. The Edwards SAPIEN valve or SAPIEN XT valve was implanted into 16 patients and the Medtronic CoreValve into 84 patients. In accordance with their narrower aortic annuli, significantly smaller valves were implanted into women. The transfemoral approach was mostly used with 87 patients. The VARC‐2–defined combined safety endpoint at 30 days was comparable for both sexes (Table 3). Moderate paravalvular aortic regurgitation (PVL) occurred in two female patients (4%) and none of the male patients. Of the entire patient population, 5 (10%) required permanent pacemaker implants and 16 (32%) developed new‐onset left bundle branch blocks (LBBB) after the procedure without significant sex‐specific differences.
Table 3.
VARC‐2 outcomes at 30 d
| Male, N = 50 | Female, N = 50 | P | |
|---|---|---|---|
| ARC‐2 defined outcomes at 30 d after TAVI | |||
| CV death, n (%) | 0 (0) | 0 (0) | ns |
| Ischaemic stroke, n (%) | 1 (2) | 0 (0) | ns |
| Major bleeding, n (%) | 3 (6) | 1 (2) | .31 |
| Acute kidney injury, n (%) | 3 (6) | 2 (4) | .65 |
| Coronary obstruction, n (%) | 1 (2) | 1 (2) | 1.0 |
| Myocardial infarction, n (%) | 2 (4) | 1 (2) | .56 |
| Major vascular complication, n (%) | 2 (4) | 1 (2) | .56 |
| New permanent pacemaker implantation, n (%) | 3 (6) | 2 (4) | .64 |
| Valve‐related dysfunction, n (%) | 0 (0) | 0 (0) | ns |
| Post‐procedure moderate‐to‐severe PVL, n (%) | 0 (0) | 2 (4) | ns |
| New‐onset LBBB, n (%) | 7 (14) | 9 (18) | .58 |
Abbreviations: CV, cardiovascular; LBBB, left bundle branch block; PVL, paravalvular leak.
Table 2 shows the sequential change in the echocardiographic parameters of the LV remodelling after the TAVR procedure. Echocardiography was initially performed just prior to the TAVR procedure, the second time echocardiography at discharge performed at a mean of 16.7 days after the TAVR, the third echocardiography at a mean of 98 days and the fourth a mean of 240.9 days after the procedure (Figure 1). Predictably, the parameters describing AS severity changed profoundly after the TAVR procedure, with a statistically significant increase in AVA and a significant decrease in the transvalvular gradients, which remained consistently stable across the follow‐up period for both sexes (Figure 2A,B). In addition to changes of AVA and pressure gradients, significant reductions in Zva were observed after TAVI as well as left atrium (LA) volume and pulmonary artery (PA) pressure, and Zva remained consistent during the follow‐up period. As shown in Figure 3, the female patients exhibited an early pronounced regression of the LV mass after TAVR and their LVMi decreased from 148.3 ± 48.0 to 130.5 ± 43.7 g/m2 (mean change, −17.8 g/m2) at only a median of 16.7 days after the procedure (P < .001). Significant LVMi regression was observed at the time of discharge in the female patients: the mean fractional LVMi regressed by 12.0% at 16.7 days after TAVR and achieved 22.0% after one year (Figure 3). In contrast, the regression of the LVMi from baseline in men was seen at three months post‐TAVR, decreasing from 143.1 ± 39.6 to 133.9 ± 37.9 g/m2 (mean change, −9.2 g/m2). The mean fractional decrease of the LVMi was 11.5% at the nine‐month follow‐up and reached 15.4% after one year (Figure 3), suggesting female patients had significantly faster and greater LVMi regression after TAVR (P < .05). Table 4 presents the results of the logistic regression analysis, which evaluated the independent predictors of greater early LV mass regression after TAVI. After adjusting for age, sex, BMI, echocardiographic parameters, history of hypertension, diabetes, LV ejection fraction, new‐onset LBBB, moderate‐to‐severe PVL post‐TAVI and the need for permanent pacemaker implantation, only the female sex, LVEF and baseline LVMi were independently associated with greater LVMi regression after TAVR.
Figure 2.
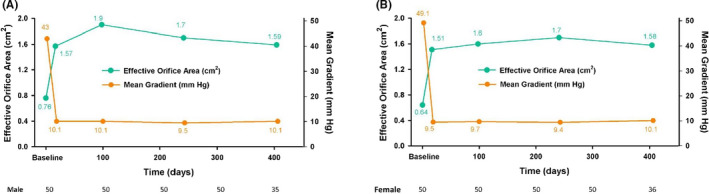
Serial follow‐up of artic aortic valve area (AVA) and mean pressure gradient (MPG) in man (A) and woman (B) after TAVI
Figure 3.
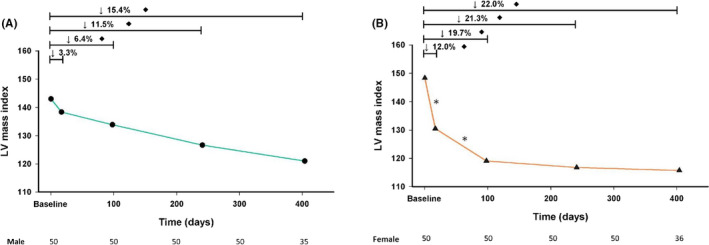
Serial LVMi change after TAVI in man (A) and woman (B). *P < .05 between two LVMi measurements; ♦ P < .05 compared with baseline LVMi
Table 4.
Factors associated with early regression of LV after TAVI within 3 mo
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P value | β | 95% CI | P value | |
| Age | −.001 | −0.006 to 0.005 | .801 | −.002 | −0.008 to 0.003 | .388 |
| Female | −.125 | −0.213 to −0.037 | .006 | −.113 | −0.203 to −0.023 | .025 |
| BMI | .002 | −0.01 to 0.014 | .72 | .005 | −0.007 to 0.018 | .388 |
| Hypertension | −.001 | −0.11 to 0.124 | .744 | .003 | 0.097‐0.104 | .946 |
| Diabetes | −.01 | −0.111 to 0.091 | .85 | .024 | 0.075‐0.122 | .63 |
| Baseline AVA | .087 | −0.114 to 0.288 | .393 | −.002 | −0.246 to 0.242 | .987 |
| Mean PPG | −.002 | −0.004 to 0 | .094 | −.001 | −0.004 to 0.002 | .586 |
| LVEF | −.007 | −0.011 to −0.004 | <.001 | −.007 | −0.011 to −0.002 | .003 |
| LVMI | −.002 | −0.003 to −0.001 | <.001 | −.003 | −0.004 to −0.001 | .001 |
| Moderate‐to‐severe PLV | .028 | −0.288 to 0.344 | .859 | .155 | −0.135 to 0.444 | .29 |
| New‐onset LBBB | −.006 | −0.186 to 0.006 | .347 | −.046 | −0.158 to 0.066 | .417 |
Model 1. Crude analysis. Model 2. Adjust with age, female, BMI, history of hypertension, diabetes, baseline AVA, mean PPG, LVEF and LVMI.
4. DISCUSSION
Our current result demonstrated that women had favourable reverse remodelling with significant LV mass regression occurring within the first two weeks after the procedure. In contrast, the regression of the LVMi in men seemed to be more gradual. The overall percentage of LVMi regression was greater among women. Additionally, the female sex, better LVEF and baseline LVMi were independently associated with greater early LV remodelling process, indicating that these could be used as indicators for early LV mass regression post‐TAVR in the Asian population.
The pressure overload in the LV caused by AS induces cardiac muscle hypertrophy and interstitial collagen deposition, leading to structural changes in the LV (LV remodelling). LV remodelling alone can greatly impact morbidity in this patient population.17, 18 TAVR causes an acute decrease in the transvalvular gradient that could lead to unloading the LV and, thus reverses LV remodelling and improves clinical outcomes.18
Previous studies have suggested that female sex is associated with better mid‐ and long‐term survival post‐TAVR despite the increased rate of periprocedural complications.2, 3, 4, 5, 6 There is currently no clear‐cut explanation for why women fare better than men after TAVR. However, the interplay of several risk factors and co‐morbidities, as well as different sex‐related cardiac pathophysiological responses to pressure afterload, are likely involved. Multiple studies have shown that sex‐specific differences exist in terms of the LV response to chronic afterload.5, 19 Women with severe AS typically manifest more concentric LV geometry, less myocardial fibrosis and better systolic function compared with men.7, 8, 9, 10 Surgical studies of patients undergoing AVR have revealed reduced levels of fibrosis in the myocardium biopsied from women and the regression of the myocardial hypertrophy is also more rapid in women.11 Stangl et al20 showed that, while regression of hypertrophy occurred in both men and women post‐TAVR, the improvement in the ejection fraction was significant only in female patients, potentially reflecting a lower burden of irreversible myocardial damage prior TAVR. Myocardial fibrosis can be identified and quantified pre‐TAVR by cardiac magnetic resonance (CMR). Mid‐wall late gadolinium enhancement (LGE) is predictive of patient outcome and also of the likely improvement in LV remodelling following AVR or TAVR.21, 22, 23 Treibel et al observed that myocardial fibrosis and extracellular matrix expansion were elevated in male patients with severe AS.23
Cellular, molecular and neurohormonal mechanisms underlying the differential response of the sexes have been proposed and include increased interstitial fibrosis, greater activation of pro‐fibrotic and pro‐inflammatory pathways, and the differential expression of the androgen and oestrogen receptors, respectively.10, 11, 24, 25 There is a large body of evidence linking abnormal myocardial hypertrophy and fibrosis at the molecular level in male patients with AS to the dysregulation of extracellular matrix turnover. Other studies have indicated that the increase in cardiac fibrosis observed in men with AS is associated with increased TGF‐β1 protein expression and SMAD2 phosphorylation.11, 26, 27
The present study provides additional insight into the timeline of LV mass regression after TAVR. It has been well documents in the literature that most of the mass regression occurs within 30 days post‐TAVI, at rates of 3%‐10%.28, 29 In our study, men and women showed a similar degree of indexed aortic valve area restriction and transvalvular gradients prior to TAVR. While both sexes exhibited increases in the thicknesses of IVS and PW and in the indexed LVH, only women exhibited signs of reverse remodelling almost immediately after the TAVR procedure, with significant LV mass regression occurring within the first two weeks after the procedure. In contrast, the regression of the LVMi in male patients appeared to be more gradual. The significant regression of the LVMi was only observable starting at three months post‐TAVR. Using echocardiography‐based approaches to explore sexual dimorphisms in cardiac remodelling in patients with AS has not been extensively described in the literature. Lindman et al showed that LVMi regression over the first year post‐TAVR occurred in both men and women (P < .001 for both) with similar patterns and incremental regressions; however, the overall percentage of LVMi regression was greater in women (P = .004). In contrast, Stangl et al found that the LVM and the LVM index had both decreased significantly at the three‐month post‐TAVR follow‐up without relevant differences between the sexes. Furthermore, although there is no difference in paradoxical low‐flow low gradient aortic stenosis (PLF‐LG AOST) percentage between genders, the female patients have higher baseline valvulo‐arterial impedance (Zva). The valvulo‐arterial impedance (Zva) provides an estimate of the global left ventricle (LV) hemodynamic load that results from the summation of the valvular and vascular loads.15, 16 Additionally, it incorporates stenosis severity, volume flow rate, body size and systemic vascular resistance. High Zva was observed in severe AS and was associated with poor prognosis. It has been reported TAVI cause acute declines in Zva15 and the reduction in Zva after TAVI was shown to persist during a 2‐year follow‐up.16 In our current study, female patients have significantly higher (Zva) at baseline before TAVI procedure and the Zva significantly decreased after TAVl and remained consistent during follow‐up period, which is in concordance with previous observations. Greater early change of Zva in female gender may contribute to the LV regression after TAVI procedure and further study is needed to confirm this hypothesis.
4.1. Study limitations
While our study did not use a matched‐control group design, the baseline risk factors, severity of the AS, included valve area, and pressure gradient were similar between the sexes. Second, most of the cases in this study used self‐expandable valves, but the percentage of balloon‐expendable valves and self‐expandable valves was nonsignificant difference in both groups. Third, in order to evaluate serial LVMi changes after TAVI, only these patients who received regular echocardiography at least nine months in the hospital were analysed in this study, the possible existence of selection bias cannot be excluded. Furthermore, some useful information such as global longitudinal strain by STE or mitral annulus displacement by MAPSE was not recorded in our current study. Finally, longer follow‐up periods will be useful for determining the long‐term impact of LVMi regression and any associated clinical outcomes of the TAVI procedure.
5. CONCLUSIONS
In conclusion, our current results demonstrated that female patients with AS had favourable reverse remodelling with greater and earlier LV mass regression relative to the males one. The favourable LV reverse remodelling observed in women might provide a mechanistic explanation for their survival advantage. Furthermore, better LVEF and higher baseline LVMi were associated with earlier LV remodelling post‐TAVR.
CONFLICT OF INTEREST
There is no conflict of interest.
ACKNOWLEDGEMENTS
This work is funded by Taiwan Association of integration of Cardiology and Surgery (TAICS‐107‐2‐Y2).
Chen S‐C, Leu H‐B, Chang H‐H, et al. Women had favourable reverse left ventricle remodelling after TAVR. Eur J Clin Invest. 2020;50:e13183 10.1111/eci.13183
Hsin‐Bang Leu and Ying‐Hwa Chen contribute equally to this manuscript and both are the corresponding authors.
Contributor Information
Hsin‐Bang Leu, Email: hsinbangleu@gmail.com.
Ying‐Hwa Chen, Email: yhchen@vghtpe.gov.tw.
REFERENCES
- 1. Osnabrugge RLJ, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta‐analysis and modeling study. J Am Coll Cardiol. 2013;62:1002‐1012. [DOI] [PubMed] [Google Scholar]
- 2. O'Connor SA, Morice M‐C, Gilard M, et al. Revisiting sex equality with transcatheter aortic valve replacement outcomes: a collaborative, patient‐level meta‐analysis of 11,310 patients. J Am Coll Cardiol. 2015;66:221‐228. [DOI] [PubMed] [Google Scholar]
- 3. Kodali S, Williams MR, Doshi D, et al. Sex‐specific differences at presentation and outcomes among patients undergoing transcatheter aortic valve replacement: a cohort study. Ann Intern Med. 2016;164:377‐384. [DOI] [PubMed] [Google Scholar]
- 4. Chandrasekhar J, Dangas G, Yu J, et al. Sex‐based differences in outcomes with transcatheter aortic valve therapy: TVT registry from 2011 to 2014. J Am Coll Cardiol. 2016;68:2733‐2744. [DOI] [PubMed] [Google Scholar]
- 5. Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex‐related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55:1057‐1065. [DOI] [PubMed] [Google Scholar]
- 6. Azarbaijani Y, O'Callaghan K, Sanders WE, et al. Sex‐specific outcomes after transcatheter aortic valve replacement: a review of the literature. Cardiol Rev. 2018;26:73‐81. [DOI] [PubMed] [Google Scholar]
- 7. Carroll JD, Carroll EP, Feldman T, et al. Sex‐associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86:1099‐1107. [DOI] [PubMed] [Google Scholar]
- 8. Douglas PS, Otto CM, Mickel MC, Labovitz A, Reid CL, Davis KB. Gender differences in left ventricle geometry and function in patients undergoing balloon dilatation of the aortic valve for isolated aortic stenosis. NHLBI Balloon Valvuloplasty Registry. Br Heart J. 1995;73:548‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aurigemma GP, Gaasch WH. Gender differences in older patients with pressure‐overload hypertrophy of the left ventricle. Cardiology. 1995;86:310‐317. [DOI] [PubMed] [Google Scholar]
- 10. Kararigas G, Dworatzek E, Petrov G, et al. Sex‐dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. 2014;16:1160‐1167. [DOI] [PubMed] [Google Scholar]
- 11. Petrov G, Regitz‐Zagrosek V, Lehmkuhl E, et al. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation. 2010;122:S23‐S28. [DOI] [PubMed] [Google Scholar]
- 12. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 13. Douglas PS, Waugh RA, Bloomfield G, et al. Implementation of echocardiography core laboratory best practices: a case study of the PARTNER I trial. J Am Soc Echocardiogr. 2013;26:348‐358.e3. [DOI] [PubMed] [Google Scholar]
- 14. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6‐23. [DOI] [PubMed] [Google Scholar]
- 15. Jang JY, Seo J‐S, Sun BJ, et al. Impact of valvuloarterial impedance on concentric remodeling in aortic stenosis and its regression after valve replacement. J Cardiovasc Ultrasound. 2016;24:2071‐2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katsanos S, Yiu KH, Clavel M‐A, et al. Impact of valvuloarterial impedance on 2‐year outcome of patients undergoing transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2013;26:691‐698. [DOI] [PubMed] [Google Scholar]
- 17. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561‐1566. [DOI] [PubMed] [Google Scholar]
- 18. Une D, Mesana L, Chan V, et al. Clinical impact of changes in left ventricular function after aortic valve replacement: analysis from 3112 patients. Circulation. 2015;132:741‐747. [DOI] [PubMed] [Google Scholar]
- 19. Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26:562‐568. [DOI] [PubMed] [Google Scholar]
- 20. Stangl V, Baldenhofer G, Knebel F, et al. Impact of gender on three‐month outcome and left ventricular remodeling after transfemoral transcatheter aortic valve implantation. Am J Cardiol. 2012;110:884‐890. [DOI] [PubMed] [Google Scholar]
- 21. Dweck MR, Joshi S, Murigu T, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271‐1279. [DOI] [PubMed] [Google Scholar]
- 22. Barone‐Rochette G, Piérard S, De Meester de Ravenstein C, et al. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol. 2014;64:144‐154. [DOI] [PubMed] [Google Scholar]
- 23. Treibel TA, Kozor R, Fontana M, et al. Sex dimorphism in the myocardial response to aortic stenosis. JACC Cardiovasc Imaging. 2018;11:962‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Villari B, Campbell SE, Schneider J, Vassalli G, Chiariello M, Hess OM. Sex‐dependent differences in left ventricular function and structure in chronic pressure overload. Eur Heart J. 1995;16:1410‐1419. [DOI] [PubMed] [Google Scholar]
- 25. Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation. 1998;98:256‐261. [DOI] [PubMed] [Google Scholar]
- 26. Villar AV, Llano M, Cobo M, et al. Gender differences of echocardiographic and gene expression patterns in human pressure overload left ventricular hypertrophy. J Mol Cell Cardiol. 2009;46:526‐535. [DOI] [PubMed] [Google Scholar]
- 27. Petrov G, Dworatzek E, Schulze TM, et al. Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc Imaging. 2014;7:1073‐1080. [DOI] [PubMed] [Google Scholar]
- 28. Lindman BR, Stewart WJ, Pibarot P, et al. Early regression of severe left ventricular hypertrophy after transcatheter aortic valve replacement is associated with decreased hospitalizations. JACC Cardiovasc Interv. 2014;7:662‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Douglas PS, Hahn RT, Pibarot P, et al. Hemodynamic outcomes of transcatheter aortic valve replacement and medical management in severe, inoperable aortic stenosis: a longitudinal echocardiographic study of cohort B of the PARTNER trial. J Am Soc Echocardiogr. 2015;28:210‐217. [DOI] [PubMed] [Google Scholar]


