Abstract
Since the inception of mass spectrometry more than a century ago, the field has matured as analytical capabilities have progressed, instrument configurations multiplied, and applications proliferated. Modern systems are able to characterize volatile and nonvolatile sample materials, quantitatively measure abundances of molecular and elemental species with low limits of detection, and determine isotopic compositions with high degrees of precision and accuracy. Consequently, mass spectrometers have a rich history and promising future in planetary exploration. Here, we provide a short review on the development of mass analyzers and supporting subsystems (eg, ionization sources and detector assemblies) that have significant heritage in spaceflight applications, and we introduce a selection of emerging technologies that may enable new and/or augmented mission concepts in the coming decades.
Keywords: ion trap, mass analyzer, Orbitrap, quadrupole, sector field, spaceflight, spectrometry, time‐of‐flight
1. INTRODUCTION TO APPLICATIONS OF MASS SPECTROMETRY
Since the formulation of the first prototype system more than 100 years ago,1 the field of mass spectrometry has expanded (eg, grown its user base), diversified (eg, broadened its applicability), and matured (eg, ruggedized its technology) to the point where modern instruments have become indispensable in science and industry. Today, the most conventional applications of mass spectrometry include proteomics; metabolomics; drug discovery; disease profiling; chemical synthesis confirmation; materials analysis; environmental monitoring; and basic research, such as in the geological sciences. However, technological innovations have enabled instruments formerly relegated to the laboratory (because of their size, weight, and power, or SWaP requirements) to be miniaturized and mobilized for in situ field deployment, thereby extending operations into the realms of chemical/biological/nuclear forensics, luggage and package screening, and the exploration of remote and/or dangerous environments, to name a few. The development of novel ionization sources, and the design of adaptive interfaces that allow multiple sources to be integrated with the same analyzer, facilitate quantitative assays of solid, liquid, gas, and even plasma phases via mass spectrometry. Emerging fields of research, such as the identification of nucleosynthetic isotope anomalies (eg, Yokoyama and Walker2), in situ planetary geochronology (eg, Farley et al3), and the detection of agnostic biosignatures (eg, Johnson et al4), are empowered by the progressive analytical capabilities offered by contemporary instruments. The analytical specificity offered by the different flavors of mass filters available to users, such as those described below, supports focused investigations with maximum return on investment (eg, data products).
The capabilities and resulting applications outlined above serve to highlight the versatility of mass spectrometers and indicate why these tools have enjoyed a long and successful history as mission‐enabling payload instruments for the in situ investigation of planetary bodies. Space represents the ultimate frontier in the exploration of “the unknown,” and mission concepts prioritized by the scientific community are growing in scope and ambition,5, 6 targeting planets and moons farther and farther out in the solar system with only limited reconnaissance (largely because of cost constraints). Consequently, payload instruments need to offer broad analytical capabilities in order to avoid implicit assumptions, especially in the search for living systems (extant or extinct) that may or may not follow the same “rules” as terrestrial biology. Although custom‐tailored systems can be configured to focus on a specific set of measurement objectives, mass spectrometry as a whole supports a comprehensive approach to the unbiased characterization of planetary materials, providing sensitive and quantitative measurements of chemical composition, including isotopic, elemental, and molecular abundances. Recent examples of the paradigm‐shifting findings enabled by highly capable mass spectrometers flown on planetary missions include constraints on the dynamic water content of the lunar surface7; evidence for hydrothermal activity in the subsurface ocean on Enceladus8; and the detection of indigenous organic compounds preserved in Martian surface materials (eg, Eigenbrode et al9 and Freissinet et al10).
However, compared with state‐of‐the‐art commercial systems, spaceflight instruments are necessarily constrained in analytical capability because of a trade‐off in performance versus resource requirements; even the most advanced orbiters and landed platforms offer only limited SWaP for scientific instruments. For example, the car‐sized Curiosity rover (899 kg) constituted only a fraction of the mass of the Mars Science Laboratory spacecraft (MSL; 3893 kg), and the rover's entire analytical payload is only a small slice of that fraction (75 kg), meaning less than 2% of the mass of the total mission was dedicated to scientific instrumentation.11 The balance of the spacecraft's mass consisted primarily of the fueled cruise stage (539 kg) and entry, descent, and landing system (2401 kg). This illustration is not unique, though it may serve as an extreme example because of technical challenges associated with safely deploying a massive rover onto the surface of a planet with a tenuous atmosphere, which limits the effectiveness of parachute technologies, thus requiring additional resources for a more elaborate landing system. For comparison, the scientific payloads of the MAVEN,12 Galileo,13 and Phoenix14 missions comprised 3%, 5%, and 8% the total mass of their respective spacecraft (not including launch vehicles). These case studies underscore the critical need for spaceflight mass spectrometers to be miniaturized by orders of magnitude in “scale” relative to laboratory instruments.
Further, all spaceflight hardware (not just analytical systems) must be qualified to survive launch, cruise, and deployment phases of the mission; validation normally occurs as structural analysis via finite element modeling, shock and random vibration testing, and demonstrated operations across a representative range of environmental conditions (temperature, pressure, radiation, etc). For these reasons, a mass spectrometer that may be sent into space, and deployed to any number of planetary bodies, is inherently different from an analogous instrument that may be used in the laboratory, even if the fundamental operating principles are the same. All spaceflight systems are custom “one of a kind” units, and thus significantly more costly to design and develop. Depending on the type of mass analyzer and its required specifications, as well as the complexity of the ionization source(s) and other essential subsystems, a mass spectrometer adapted for spaceflight applications may require more than a decade and tens of millions of dollars to build and qualify for a targeted mission opportunity. Instruments that incorporate heritage (ie, space‐proven or “build to print”) components may be developed on accelerated timelines with tighter budgets. For example, the neutral mass spectrometer (NMS) onboard the LADEE spacecraft, which leveraged the legacy quadrupole analyzer and electron ionization (EI) source designs flown on Cassini‐Huygens, required less than 3 years to deliver. In contrast, the linear ion trap developed for the Mars Organic Molecule Analyzer (MOMA) onboard the ExoMars rover was designed with far fewer heritage components, requiring more than 10 years to deliver.
The main objective of this paper is to provide historical perspectives and future insights into mass spectrometry as an invaluable tool for planetary exploration, enabling investigations that have probed the chemistry of inner (eg, Venus and Mars) and outer solar system bodies (eg, Jupiter and Saturn), as well as future missions to Europa and Titan, ocean worlds that may harbor alien life. The evolution of mass analyzers from prototype systems to commercial instruments to flight models that have been adapted and qualified for specific mission concepts will be described, and particularly impactful and/or highly visible examples will be summarized. However, this is not meant to be an exhaustive presentation of all applications of mass spectrometry in space, nor a complete historical review of the field. Rather, this synopsis is intended as an educational background and prospective starting point for future research endeavors as the reader sees fit. An extension of the materials introduced in this review may be found in articles that focus more exclusively on historical missions and legacy instrumentation (eg, Palmer and Limero15 and Ren et al16) and/or those that cover complementary topics, such as the development of ambient ionization sources and microfabrication techniques (eg, Snyder et al17).
2. CONVENTIONAL INSTRUMENT ARCHITECTURES
At the most fundamental level, mass spectrometers may be divided into three key subsystems: (a) ionization source; (b) mass analyzer; and (c) detector assembly (Figure 1). The ionization source converts neutral analyte into charged particles (ie, ions) that can be separated by the mass analyzer according to their respective mass/charge ratios (m/z). In the laboratory, a wide variety of ionization sources have been demonstrated, each with distinct advantages that enable the effective analysis of specific types of sample media; a subset of these front ends have been qualified for spaceflight. By far, the most common source for a spaceflight mass spectrometer is the EI source because of its simplicity, robustness, and proficient characterization of gas phases. With this type of source, volatile compounds are bombarded by a beam of thermionic or field‐emitted electrons, often accelerated to a potential of 70 eV, resulting in molecular ionization via the removal of one or more electrons from the highest occupied molecular orbitals. The recent desire to look for larger organic compounds as potential biosignatures has shifted the interest from “hard ionization” techniques (such as EI), which incur significant molecular fragmentation and/or atomization, to “soft ionization” techniques that preserve many species as intact molecular ions. Laser desorption (LD) is one such technique that can form molecular ions through low photon fluences at specific wavelengths (corresponding to specific photon energies). Although many other ionization sources are available in the laboratory, such as high‐temperature filaments, high‐voltage electrosprays, and inductively coupled plasmas, such techniques have yet to be fully qualified for spaceflight, largely because of resource requirements (eg, power/energy needs) and/or inherent risk (eg, liquid handling).
Figure 1.
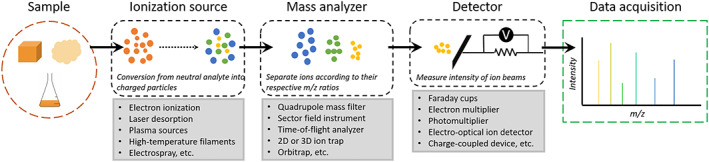
The modularity of key subsystems allows for a number of permutations of mass spectrometer designs, defining unique combinations of hardware capable of targeted applications/chemical measurements
All mass spectrometers are also equipped with some kind of a detector assembly that converts ion intensities (total counts or fluxes) into electrical signals that can be digitized and read out by the data acquisition system. The first mass spectrographs relied on photographic plates, but today, the Faraday cup is the simplest ion detector. Incoming ions collide with a conductive metal collector that is connected to ground through a high‐ohmage resistor. The current passing through the resistor, which is proportional to the number of ions hitting the Faraday cup, causes a voltage drop that can be measured to quantify the signal (per Ohm's Law: V = IR). Faraday cups are still used in many instruments today because of their mechanical robustness and quantitative accuracy (ie, linearity between ion flux and measured current). Further, because detection is based solely on the accumulation of charge (as opposed to incident ion energy, mass, etc), Faraday cups do not exhibit mass discrimination, the preferential detection of high or low mass ions. However, these detectors are limited by low sensitivity (eg, no gain without augmented electronics, and high noise levels versus low ion currents), slow response times, and multiple sources of error, including the emission of low‐energy secondary electrons from the surface of the collector, backscattering of incident ions, and sputtering.
The most common type of detector found in commercial and spaceflight systems, the electron multiplier, uses a series of dynodes to convert an incoming ion into a cascade of secondary electrons that are measured at the final dynode stage, resulting in signal amplification (up to 108 gain), and by extension high sensitivity. Other operational advantages include a linear response to ion beams at limited fluxes and adjustable gain based on the voltage applied to the initial dynode stage (or cathode). Continuous dynode multipliers (or channeltrons) represent a subset of electron multipliers that replace the array of dynodes with a continuously resistive surface, often plated inside a glass tube to allow cascading secondary ion generation and amplification along the entire surface. Microchannel plates (MCPs) are a cousin to channeltrons but consist of a 2D array of channels, each allowing amplification of incident ion signal. Therefore MCPs can provide large areal coverage of ion beams and enable 2D ion imaging. However, electron multipliers are not without limitations. At high gain and/or elevated count rates, the detector response deviates from linearity because of saturation effects and/or dead time violations, and the devices themselves are intolerant to high operating pressures because of the bias voltage required to generate the electron cascade. Further, the production rate of electrons at the first dynode stage is sensitive to incident ion energy, resulting in mass discrimination effects.
Other types of detectors include (but are not limited to) photomultipliers; hybridized electro‐optical ion detectors (EOIDs); charge‐coupled device (CCD) cameras; and image current amplifiers. Regardless of the specific detection scheme, ions generated at the source are sorted according to their m/z by the mass analyzer, the heart of any mass spectrometer. Because of the critical importance and the rapid evolution of these subsystems, the many flavors of analyzer that have been (or soon will be) exploited for planetary exploration are reviewed below.
3. SPACEFLIGHT MASS SPECTROMETRY: A HISTORICAL PERSPECTIVE
Mass spectrometers have been employed as key payload instruments for planetary exploration since the 1970s (Figure 2), and extend even earlier for the investigation of the Earth's upper atmosphere. Although the evolution of mass spectrometry as a field is often described in a chronological arc, an alternative historical perspective may be provided by reviewing the original inception, technological progression, and current state of mass analyzers that have been deployed successfully to one or more extraterrestrial environments, thus identifying them as heritage planetary instruments. Recent advances in the development of innovative mass analyzers and/or other strategic subsystems that could define planetary exploration in the coming decades are described in Section 4.
Figure 2.
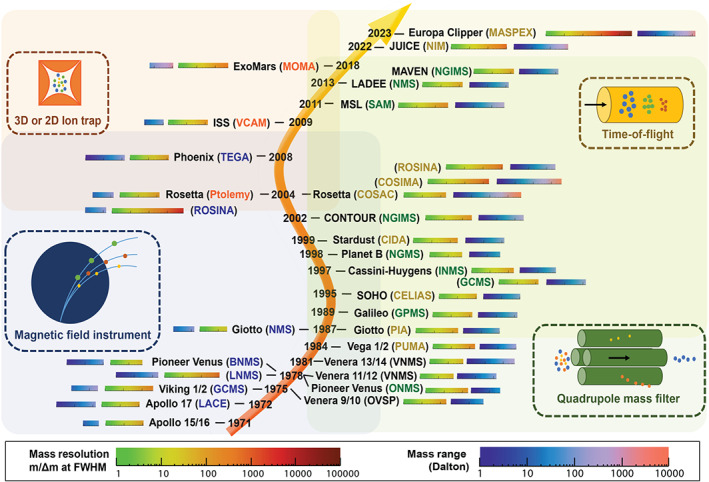
Major milestones in the evolutionary history of mass spectrometry, applied for planetary exploration, show the variety of mass analyzers exploited to date and the progression from instruments offering limited analytical performance to those capable of enhanced science return (expanded mass ranges, higher mass resolving powers, etc). For an even more detailed history than that provided here, the reader is referred to Ren et al16
3.1. Sector field instruments
Sir Joseph John Thomson, Nobel Laureate in Physics (1906), first demonstrated the separation of ions according to their respective m/z by applying external magnetic and electric fields.1 Francis William Aston, Thomson's student and Nobel Laureate in Chemistry (1922), applied these foundational principles to build the first dual sector mass spectrograph18 capable of sequentially scanning across a range of m/z and consisting of
two planar electrodes, held at at nonzero potentials (and opposite polarities) and arranged in parallel to deflect the incident ion beam;
a magnetic coil to focus the deflected beam to a point; and
a photographic plate to detect the focused ion beam.
With the two sector fields (electric and magnetic) arranged in series, this revolutionary instrument design served as a forerunner to modern double‐focusing instruments (Figure 3), using homogeneous electromagnetic fields to effectively separate ions of different m/z according to their respective momenta. The magnitude of ion deflection is described by the Lorentz Force law: F = q(E + v × B), where q is the charge of the ion (in coulombs), E the electric field strength (in volts per meter), v the ion velocity (in meters per second), and B the magnetic flux density (in tesla).
Figure 3.
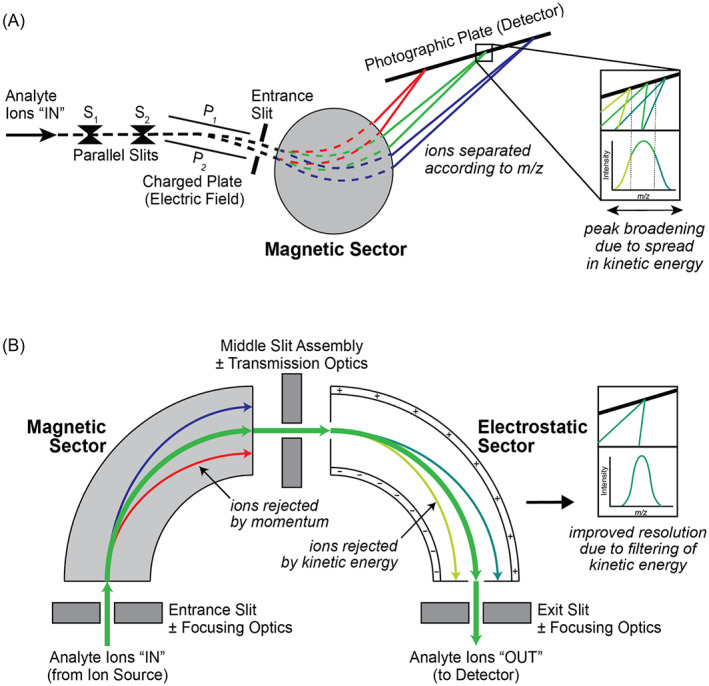
(A) The mass spectrograph invented by Francis William Aston18 provided the cornerstone by which (B) contemporary double‐focusing sector fields instruments are designed
Sector field mass spectrometers (SFMSs) were first deployed on Earth‐orbiting satellites (eg, Explorer 17)19 prior to exploitation as planetary payload instruments. The first mass spectrometer deployed beyond Earth's atmosphere was a single‐focusing magnetic sector instrument flown on a boom extended from the Apollo 15 service module; an identical SFMS was also launched on the following Apollo 16 mission. Both instruments enabled scanning across a mass range of 12 to 67 Da with a mass resolving power of m/Δm ≈ 120 (full width at half maximum [FWHM]20). However, perhaps the most iconic sector field instruments were the twin gas chromatograph mass spectrometers (GCMSs) on the Viking 1 and Viking 2 missions to Mars, both launched in 1975. Equipped with these two double‐focusing SFMSs, each capable of measuring ions up to 200 Da (nearly three times the mass range of the Apollo instruments) with a mass resolving of m/Δm ≈ 360 (FWHM21), the primary science objectives of the Viking Program were to determine if the Martian subsurface contained organic compounds, and to characterize the quantity and complexity of organic materials if detected. Despite exploring two distinct landing sites on opposite sides of the planet, neither Viking GCMS instruments initially reported the detection of organic molecules (eg, Biemann et al22); however, these results continue to be interpreted to this day (eg, Guzman23). For example, oxychlorine species observed across the Martian surface24 may have caused organics in the Viking experiments to combust during pyrolysis,25 potentially masking ppmw levels of endogenous organic carbon and indicating a false negative in the original Viking conclusions.
Since the Viking era, SFMSs have been sent to
characterize the composition of the lower and upper atmosphere of Venus via neutral mass spectrometers deployed on the Pioneer Venus Sounder Probe and Multiprobe Bus26;
determine the molecular, elemental, and isotopic composition of gases and low‐energy ions derived from the coma of comet Halley via the Giotto NMS27; and
identify local mineralogy,28 detect low‐ and high‐temperature release of H2O in surface soils,29 and measure the stable isotopic composition of atmospheric CO2 on Mars30 via the thermal and evolved gas analyzer (TEGA) on the Phoenix Lander.
Debatably the most advanced planetary sector field instrument, the double focusing mass spectrometer (DFMS) was launched in 2004 as part of the Rosetta Orbiter Sensor for Ion and Neutral Analysis (ROSINA; Figure 4) experiment, which arrived at comet 67P/Churyumov‐Gerasimenko in 2014 after a 10‐year journey. The primary science objectives of the ROSINA investigation were to determine the isotopic, elemental, and molecular composition of the comet's dusty atmosphere and ionosphere and characterize the temperature, bulk velocity, and reactivity of local gases and ions. To meet these demanding requirements, the ROSINA investigation integrated three individual sensors into a cohesive instrument suite: the comet pressure sensor (COPS); reflectron time‐of‐flight (RTOF) mass spectrometer; and the DFMS sector field instrument. With a mass on the order of 16 kg and a mean operating power of 19 W, the DFMS flight model offered a mass range of 12 to 150 Da with a mass resolution of m/Δm ≈ 8000 (FWHM, or 0.01 Da peak widths up to mass 80 Da) and a total dynamic range of 1010 (albeit with an intrascan dynamic range closer to 106).31 Although the DFMS engenders technological advances, resulting in improved analytical performance relative to its predecessors from the Apollo Program, sector field instruments are challenged by next generation analyzers that offer higher scanning speeds, expanded mass ranges, and reduced resource requirements.
Figure 4.
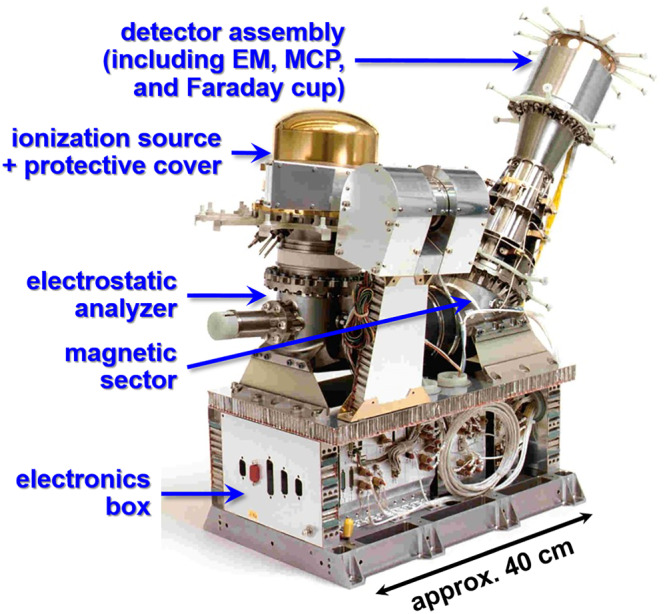
The analyzer module of the double focusing mass spectrometer (DFMS) instrument, part of the Rosetta Orbiter Sensor for Ion and Neutral Analysis (ROSINA) experiment onboard the Rosetta spacecraft, consists of high‐ and low‐resolution entrance slits, an electrostatic analyzer followed by a magnetic sector, and a suite of “zoom” ion optics that together enable the instrument to achieve a mass resolving power higher than previous sector field mass spectrometer (SFMS) spaceflight instruments. Three distinct detectors support a 1010 total dynamic range. Image courtesy of the University of Bern
3.2. Quadrupole mass filters
The basic blueprint of a quadrupole mass filter, such as those used in commercial and academic laboratories for more than half a century and patented by Wolfgang Paul (Nobel Laureate in Physics, 1989), consists of four matching cylindrical or hyperbolic rod electrodes positioned in parallel to one another, equidistant from a central axis extending in the z‐direction.32, 33 Each metal rod has a DC offset (U) and AC potential (with amplitude V and radio frequency f = ω/2π) applied to it with pairs of opposing rods connected electrically. Ions with a specific m/z are transmitted through the rod assembly on the basis of the forces generated by the combined DC and radio frequency (RF) voltages; other ions will have unstable trajectories and will collide with the electrodes (Figure 5). A quadrupole mass spectrometer (QMS) scans across a range of m/z ratios by ramping the DC and AC potentials at a constant ratio. Because the fundamental principles of operation depend upon the secular frequencies of ion motion rather than the incident energy of the ion beam, QMS instruments may be adapted to interface with multiple ion sources (eg, EI and plasma), making them versatile options for spaceflight applications.
Figure 5.
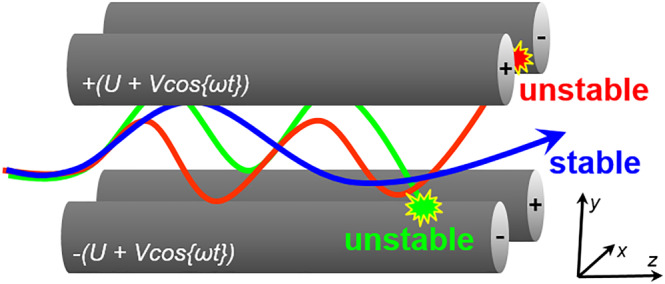
Quadrupole mass filters use an oscillating electric field, including DC and radio frequency (RF) voltages, to permit selected ions with a specific m/z ratio to pass through the rod assembly
The landmark Pioneer Venus Orbiter launched the first planetary QMS in 1978 to measure spatially and temporally resolved neutral gas densities in the upper atmosphere of Venus.34, 35 The mass analyzer consisted of four 7.5‐cm long hyperbolic rods precision ground with a mechanical tolerance of only 0.0002 cm and a field radius of 0.2 cm (r0, the minimum distance between each rod and the z‐axis), leveraging the design flown previously on the Earth orbiting satellites: the Atmospheric Explorer (AE) missions (eg, Spencer et al36). The instrument incorporated an EI source comprising redundant filaments capable of generating either 27 or 70 eV electrons (via software control), a simple series of focusing electrodes, and an ion repeller grid that served to reject positively charged atmospheric particles from entering the source region. Ion detection was performed by a grid and box electron multiplier with 14 discrete dynodes.37
Together, these components defined the Pioneer Venus Orbiter Neutral Mass Spectrometer (ONMS), a 3.8‐kg instrument that successfully measured atomic and molecular ions between 1 and 46 Da with a mass resolving power of m/Δm ≈ 50 (FWHM or unit mass resolution38) and an intrascan dynamic range of 106, requiring an average of 12 W during science operations.37 Planetary QMS instruments launched in later decades, such as those highlighted below, made only incremental changes to the original ONMS analyzer design, such as lengthening the hyperbolic rod assembly in order to improve the instrument's mass resolution at a single radio frequency. However, technological advancements related to other key subsystems augmented future QMS designs, including the development of drive electronics offering multiple radio frequencies, the integration of two or more distinct ion sources configured for “open” or “closed” operations, and the addition of complex gas processing systems suitable for use in deep planetary atmospheres. Consequently, generations of planetary quadrupole mass filters derived from the ONMS instrument have revolutionized our understanding of inner and outer solar system dynamics through transformative measurements of an array of planetary environments, including (but not limited to)
quantification of gas mixing ratios and isotopic abundances in the Jovian atmosphere via the Galileo Probe Mass Spectrometer (GPMS; eg, Niemann et al39);
profiles of the composition (including organic content) of Titan's atmosphere via the Huygens GCMS40;
analysis of the chemistry and structure of the Enceladus plume via the Cassini Ion Neutral Mass Spectrometer (INMS; eg, Waite et al41);
measurements of exospheric ions, noble gases, and water eroded from the lunar surface via the LADEE NMS (eg, Benna et al7);
characterization of atmospheric gravity waves in the Martian thermosphere via the MAVEN Neutral Gas and Ion Mass Spectrometer (NGIMS; eg, England et al42); and
detection of endogenous organics on the surface of Mars9, 10 and in situ determination of radiometric and exposure ages of a Martian mudstone3 via the Sample Analysis at Mars (SAM) instrument suite on the Mars Science Laboratory (MSL).
The most contemporary QMS flown to date, the MSL SAM investigation onboard the Curiosity rover, employs 15 cm long rods (two times those of the Pioneer Venus ONMS; Figure 6) with r0 = 0.5 cm and three fixed radio frequencies to achieve an extended mass range of 2 to 535 Da with a mass resolving power up to m/Δm = 500 (FWHM) and 109 total dynamic range.43 Small corrections may be applied to the RF to compensate for thermal drift, ensuring reliable performance across a temperature range of −40°C to +50°C. Although the SAM QMS is comparable in mass with its ancestors (on the order of 20 kg), the instrument's supporting infrastructure is significantly more complex. For example, the SAM gas processing system includes two wide‐range pumps, six gas chromatograph columns, 16 manifolds, 52 microvalves, and 60 individual heaters, requiring more power during operations (50 W or greater, depending on the experiment) than earlier QMS generations, reinforcing the trade‐off between science return and resource requirements. Today, quadrupole mass filters remain highly competitive sensors in the formulation of new mission concepts, such as the DAVINCI Venus probe44 that was a finalist in the NASA Discovery 13 mission competition. However, other types of mass analyzers and next generation technologies (both discussed further below) are beginning to challenge the dominion of QMS instruments by offering higher precision/accuracy and more advanced analytical modes (eg, tandem mass spectrometry, or MSn).
Figure 6.

Since the Pioneer Venus Orbiter Neutral Mass Spectrometer (ONMS), more recent quadrupole mass spectrometer (QMS) instruments have implemented longer hyperbolic rods in order to expand mass range and maintain or improve mass resolution. The hyperbolic rods manufactured for the Mars Organic Molecule Analyzer (MOMA) linear ion trap, onboard the ExoMars rover, leverage the same mechanical design as heritage QMS systems. Image modified from Arevalo Jr. et al45
3.3. Time‐of‐flight analyzers
Although their origins extend to the 1940s, time‐of‐flight mass spectrometers (TOFMSs) are significantly younger in both commercial and planetary realms compared with quadrupole and sector field instruments.46, 47, 48 A TOF analyzer operates on the simple principle that ions accelerated to the same kinetic energy (E = ½mv 2) will separate according to their respective m/z in a field‐free drift region; lower m/z ions travel at greater velocities than higher m/z ions, resulting in separation based on the time it takes for each to reach the detector. Unlike quadrupole and sector field instruments, TOFMSs do not scan across a mass range to collect a mass spectrum but rather collect an entire spectrum via pulsed operations. Ideally, given the same extraction or acceleration potential and a fixed length of field‐free drift path, ions of different m/z derived from the same pulsed source will arrive at the detector at distinct but predictable times. However, in reality, ion arrival times at the detector also contain variances stemming from the exact timing and positioning of individual ion formation, as well as divergent initial velocity vectors (direction and magnitude) with respect to the detector (ie, nonuniform temporal, spatial, and initial kinetic energy distributions, respectively; eg, Wiley and McLaren48). The influence of each of these deviations may be minimized through specific combinations of hardware (Figure 7), but the cumulative effect of these delays contributes to the width of spectral peaks, thereby controlling the mass resolving power of the analyzer.
Figure 7.
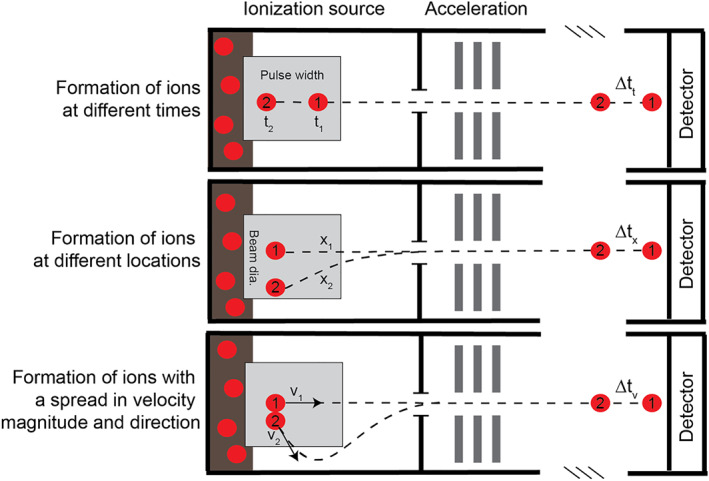
The mass resolving power of a linear time‐of‐flight (TOF) mass analyzer is controlled by variances in the timing, spatial distribution, and kinetic energy spread of ions formed within the source region and transmitted into the analyzer. However, the implementation of an ionization source capable of ultrafast pulsing (eg, femtosecond laser) can attenuate temporal variances in ion formation, and one or more ion mirrors (eg, reflectrons) can normalize the spread in kinetic energies, together improving the achievable mass resolving power of advanced time‐of‐flight mass spectrometer (TOFMS) systems
Although TOFMS offer many analytical advantages, such as fast scanning rates (up to kilohertz), broad mass ranges (up to kilodaltons), exceptional total dynamic ranges (up to 1010 when equipped with a pulsed ion gate), and spectral continuity, some performance metrics (eg, mass resolution) lagged behind competing analyzer designs until the advent of the reflectron in the 1970s.49 A reflectron is an ion mirror comprised of a series of grids/rings with retarding electric field potentials that serve to reverse the direction of travel of the incoming ion packet. Ions of a given m/z with high initial kinetic energies (derived from the initial velocity component in the direction of the flight tube) penetrate more deeply into the reflectron, thereby spending more time in the reflecting field and slightly extending their path length, than those with lower initial kinetic energies. Thus, ions with the same m/z but a distribution of initial kinetic energies will be reflected and refocused to the detection plane, improving mass resolving power. Reflectrons also provide an extended path length in a given overall instrument size, further improving ion separation.
The first interplanetary reflectron TOFMS instruments were launched on the Vega 1 and 2 spacecrafts in December of 1984, followed closely by the Giotto mission in July 1985; all three investigations targeted the capture and chemical analysis of dust derived from comet Halley. Prior to their respective cometary encounters, both Vega spacecraft first flew by Venus and deployed identical 1500‐kg descent modules consisting of a balloon gondola, advanced lander platform, and suite of analytical instrumentation. In all three TOFMS systems (Vega 1, Vega 2, and Giotto), captured dust particles collided with a silver target, releasing molecular and atomic ions sourced from both the projectile and the target. Positive ions created during each event were accelerated through a drift tube, reflected by uniform electrostatic field, and detected via electron multiplier with a mass resolving power up to m/Δm ≈ 200 (FWHM50, 51). Consequently, the Vega and Giotto missions are credited with collecting the first direct (in situ) measurements of the physicochemical properties of cometary dust particles, including organic and inorganic components.52, 53, 54
The launch of the Rosetta mission in 2004 provided transportation for three more planetary reflectron TOFMSs to comet 67P/Churyumov–Gerasimenko. In order to expand the mass range of the investigation, the ROSINA experiment included a dual sector instrument (ie, DFMS described above) as well as an RTOF mass spectrometer designed to detect ions up to 2000 Da with a mass resolving power up to m/Δm = 5000 (FWHM55). However, a failure in the high‐voltage converter during the commissioning phase in 2004 limited the flight model to a mass range of 1 to 1150 Da at m/Δm ≈ 500 (FWHM) during mission operations.56 The COmetary Secondary Ion Mass Analyser (COSIMA57), which targeted the chemical analysis (organic and inorganic) of cometary grains, offered a mass range stretching up to 3500 Da and a mass resolving power of m/Δm = 2000 (FWHM). The COmetary Sampling And Composition (COSAC, Figure 8, 58) instrument, which was deployed to the surface of the comet via the Philae lander module, offered an even higher mass range up to 5000 Da with a comparable mass resolving power m/Δm = 2000 (FWHM).
Figure 8.
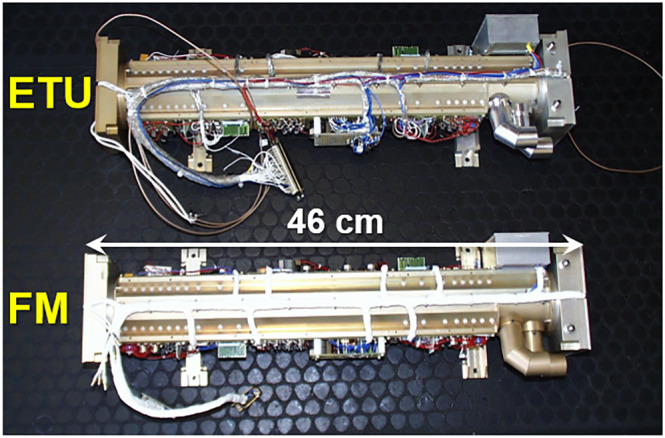
The COmetary Sampling And Composition (COSAC) instrument deployed on Rosetta's Philae lander was a high‐resolution multipass time‐of‐flight mass spectrometer (TOFMS) that centered on a linear reflectron, with the electron ionization (EI) source (to the right as depicted) and a multi‐sphere‐plate secondary electron multiplier (to the left) on opposite ends, supporting a mass resolving power of m/Δm = 2000 (FWHM). The Engineering Test Unit (ETU) and Flight Model (FM), shown above, conform to the same design with the exception of the electrical harnessing. Image courtesy of Fred Goesmann (principal investigator of COSAC)
The Europa Clipper mission, due to launch around 2023, selected the MAss Spectrometer for Planetary EXploration (MASPEX) multibounce TOFMS instrument for its payload, which will represent the highest resolution planetary mass spectrometer to be deployed to space. In multibounce mode, the ion flight path is lengthened via multiple reflections between two opposing electrostatic reflectrons (separated by a drift tube) that serve to reverse the direction of the ions while maintaining temporal and spatial focusing. Once the ions have been sufficiently separated in time, the potential on one of the reflectrons is disabled, and the ions are directed to an MCP for detection.59 Because ion separation is proportional to flight time (t) but inversely proportional to ion packet width (Δt), mass resolution increases with more bounces within the mass analyzer (m/Δm = t/2Δt). Consequently, multibounce operations are becoming increasingly popular in TOFMS as they effectively fold the flight path, enabling longer ion travel times (and by extension, higher resolving powers) to be accessed by smaller instrument configurations. The MASPEX instrument is capable of achieving mass resolving powers up to m/Δm ≈ 46,000 (FWHM), but such high resolution spectra come at the expense of the mass range (due to greater ion dispersion over time), sensitivity (due to the probability of collisions), and the cadence of sequential measurements.59
3.4. Ion trap sensors
The original concept for the ion trap was described in the same patent that first detailed the operating principles and fundamental design of the quadrupole mass filter, filed by Wolfgang Paul and colleagues,32 though other groups were conducting similar lines of research around the same time.60, 61, 62 The most conventional type of ion trap, commonly referred to as the Paul trap, comes in two different geometries: quadrupole/cylindrical (or 3D) and linear (or 2D). The 3D quadrupole trap consists of three electrodes with hyperbolic surfaces, namely, a central ring electrode and two adjacent (and electrically isolated) endcap electrodes (Figure 9). Traditionally, one endcap incorporates a small central aperture through which electrons (eg, EI source) and/or ions can be pulsed or gated into the trap, and the second endcap one or more apertures that enable ions to be ejected towards a detector (eg, channeltron). The sensor is radially symmetric about the y‐axis, and the capacity to store ions is controlled primarily by dimensions r0 (inner radius of the ring electrode) and z0 (radius between the two end plates). The shape and orientation of the hyperbolic electrodes support a near ideal quadrupolar field when an RF potential is applied to the ring electrode and the two endcaps are held near electrical ground (ie, 0 V).
Figure 9.
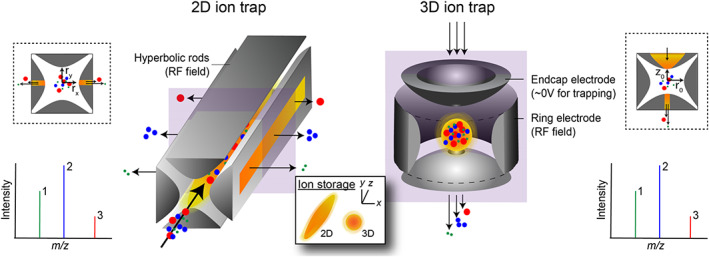
Because 2D ion traps focus ions along a line (providing ions one degree of freedom along the z‐axis) while 3D traps compress ions to a single point (no degrees of freedom), 2D traps offer comparatively greater ion storage capacities
The 2D trap comprises two planar endcaps and four hyperbolic (or cylindrical) rods situated in parallel to one another, much like a quadrupole mass filter (though significantly shorter in the z‐dimension). Although there are operational schemes that allow axial mass selective ion ejection,63 most 2D traps contain one pair of opposing rods with slits cut along the lengths of their vertices to enable radial ion ejection, support redundant detection pathways, and provide symmetrical interfaces for two distinct ion sources. Unlike the 3D trap, the 2D geometry is not radially symmetric, with rx defining the radius in the x‐direction and ry in the y‐direction. Ions are confined radially by an RF field (orthogonal to the z‐axis of the rod assembly) and axially by potentials applied to the endcap electrodes. In both 3D and 2D designs, collisions with a buffer gas (often He) dampen the kinetic energies of incoming ions, focusing trajectories towards the center of the ion volume and enabling more efficient trapping and more accurate mass analysis. By adjusting RF and/or DC potentials, Paul traps can modify the radial and axial frequencies of trapped ions, enabling sequential ejection as a function of their respective m/z. This ejection scheme is commonly aided by a secondary AC frequency, applied to the endcap(s) of the 3D trap or the slit rods of the 2D trap, that resonates with a given m/z frequency, promoting prompt ejection and improving mass resolution.64
Ion traps offer many analytical advantages relative to competing sensors. For example, they offer versatile operations as ion storage devices and/or standalone mass analyzers, and they may be united with other types of mass filters to create highly capable hybridized instruments (eg, linear ion trap/TOFMS65). Paul traps also operate at pressures (eg, 102 Pa) orders of magnitude higher than traditional QMS, SFMS, and/or TOFMS (typically <10−1 Pa), relaxing pumping requirements. Other benefits include: (a) compact footprints and robust mechanical designs; (b) highly sensitive measurements of trace chemical species; (c) empirical observations of ion/molecule reactions; (d) selective (targeted) ion isolation and enrichment experiments; and (e) tandem mass spectrometry (ie, MSn), supporting the derivation of molecular structure. Drawbacks include finite ion volume/storage (and by extension, restricted intrascan dynamic range), limited mass resolving powers, and only semiquantitative measurements of atomic/molecular abundances. In comparison with the 3D geometry, 2D traps offer higher injection efficiencies (due to minimized potential barriers to injection along the z‐axis), greater ion volume/storage per unit volume, and as described earlier, the option to eject ions radially (as opposed to axial ejection) and implement redundant detector assemblies (improving hardware lifetime) and multiple distinct ion sources via injection through either endcap aperture.
Other types of traps include the Penning Trap,66 invented by Hans George Dehmelt (Nobel Laureate in Physics in 1989) but named in honor of the early work of Frans Michel Penning (experimental physicist) in the 1930s, and the Orbitrap,67 invented and patented by Alexander Makarov less than 20 years ago. The Penning Trap uses a strong homogenous magnetic field (B) to confine ions radially, and a weak electrostatic field (ideally quadrupolar) to confine ions axially. Together, these potentials constrain ion motion to three independent modes; the observed frequencies of these motions/oscillations are controlled by the magnitude of B, the shape of the quadrupolar potential, and importantly, the m/z of the ions. In contrast, the Orbitrap relies solely on electrostatic fields to enable ion separation via a combination of quadrupolar and logarithmic potentials, as described further in Section 4.1.
The first ion trap mass spectrometer (ITMS) sent into space was carried on Rosetta's Philae lander, targeting the in situ exploration of the surface of comet 67P/Churyumov‐Gerasimenko. The instrument was an evolved gas analyzer named Ptolemy, comprising a gas chromatograph and 3D Paul trap capable of providing precise measurements of elemental/molecular abundances and stable isotope ratios of light elements H, C, N, and O.68 Another miniaturized 3D Paul trap with gas chromatograph was employed in the Vehicle Cabin Atmosphere Monitor (VCAM), which was launched on shuttle mission STS‐131 and brought onboard the International Space Station in 2010. To protect astronaut safety, VCAM was tasked with identifying and quantifying a range of chemical compounds in the cabin air supply and indicating when filters needed replacement; the instrument was broadly capable of detecting ions across a mass range of 15 to 100 Da with a mass resolving power of m/∆m ≈ of 220 (FWHM69).
The MOMA instrument onboard the ExoMars rover, set to launch in 2020, is equipped with a dual source 2D ITMS (Figure 10) capable of investigating the composition of materials collected during multiple vertical surveys, extending as deep as 2 m below the surface.45 The MOMA ion trap supports two ionization sources, and by extension, two distinct modes of analysis: (a) EI of volatile compounds derived from a pyrolysis oven and gas chromatograph (pyr/GCMS mapping to Viking GCMS and MSL SAM investigations); and (b) laser desorption mass spectrometry (LDMS) to characterize local mineralogy and detect refractory organic materials in situ, a first in planetary exploration.70 To further analyze large organic compounds that are desorbed from the sample matrix and ionized by the pulsed UV laser source, the MOMA instrument supports selective ion excitation and MSn operations to isolate and disambiguate complex molecular signatures up to 1000 Da with a mass resolving power of m/Δm ≈ 500 (FWHM). The Dragonfly mission to Saturn's moon Titan, which was recently selected as NASA's fourth New Frontiers mission, will also have a 2D ion trap based on the fundamental MOMA design, albeit a modified version offering dual polarity ion detection. The Dragonfly Mass Spectrometer (DraMS) will utilize a similar dual ionization source design to enable diverse analyses of drilled samples collected at the base of the rotorcraft skids.71
Figure 10.
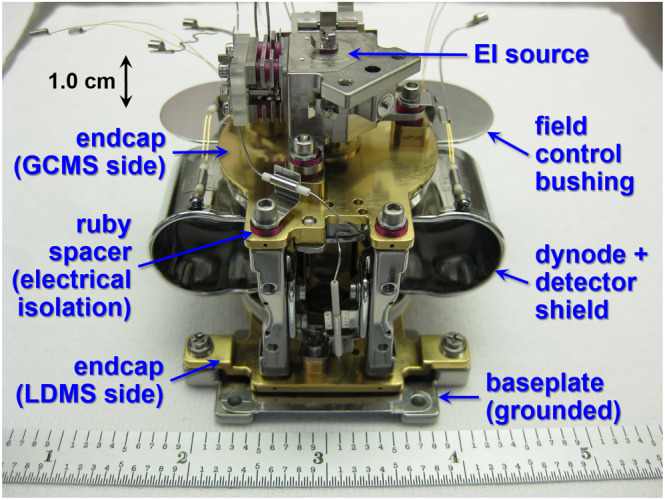
The symmetry of the Mars Organic Molecule Analyzer (MOMA) linear ion trap supports two distinct ionization sources (a heritage‐derived electron ionization [EI] source and miniaturized pulsed UV laser) and redundant shielded detector assemblies for mass scanning via radial ion ejection
4. NEXT GENERATION SPACEFLIGHT TECHNOLOGIES
For nearly 50 years, heritage mass analyzers (ie, SFMS, QMS, TOFMS, and ITMS) have helped shed light on some of the most compelling science questions our solar system has posed. However, innovation and strategic investments over that same period of time have empowered the development of an array of potentially game‐changing technologies, including next generation sensors, novel ionization sources, and the integration of formerly independent subsystems to define powerful hybridized instrument suites. Here, we review a selection of advanced technologies that, on their current trajectories, could shape the future of spaceflight mass spectrometry for decades to come.
4.1. Instruments capable of ultrahigh mass resolving powers
With few exceptions, legacy mass spectrometers from the Apollo SFMS instruments of the 1960s to the MSL SAM investigation onboard the Curiosity rover (still in operation on Mars today) are commonly limited to mass resolving powers of m/Δm ≤ 500 (FWHM), leading to tenuous peak assignments and uncertainty in the identification of molecular signals without additional subsystems (eg, chromatographs) and/or MSn techniques. The inclusion of “high‐resolution” instruments on the Rosetta spacecraft (launched in 2004) and the selection of the MASPEX instrument on the Europa Clipper mission (launch scheduled for 2023) indicate a transition towards analyzers that enable the effective separation of isobaric species, and by extension, progressive atomic/molecular disambiguation. These analytical capabilities are vital for the separation of monatomic isobars, such as 54Cr+ versus 54Fe+ (requiring m/Δm > 70 000), and polyatomic interferences, such as the variety of metabolites within tens of ppm of the monoisotopic mass of protonated aspartic acid (C4H7NO4H+), as identified by the METLIN database,72 requiring m/Δm > 100 000 to resolve all species.
One advanced technology that has emerged as a viable candidate for spaceflight applications is the Orbitrap, a mass analyzer that delivers ultrahigh mass resolving powers up to m/Δm ≥ 1 000 000 (FWHM73) and ppm‐level mass accuracy74 by using a quadro‐logarithmic potentials to trap ions radially about a central spindle electrode.67 The harmonic oscillations of the orbiting ions, whose frequencies are proportional to (m/z)−½, are detected as transient image currents that may be transformed into mass spectra via fast Fourier transform (FFT). Hence, no heavy magnets, sensitive RF electronics, or consumable detector assemblies are required, reducing technical risk and the mechanical footprint of the instrument. A prototype system that maps to a spaceflight design and employs a 266‐nm pulsed laser source (similar to the LDMS mode of MOMA) has demonstrated successful detection of refractory organic compounds and mineralogical signals in a suite of Mars and Europa analog samples while maintaining mass resolution and accuracy requirements.75 A higher fidelity engineering test unit that will be qualified for the rigors of spaceflight is currently in development for the Europa Lander mission concept.
Another innovative instrument that promises ultrahigh mass resolution (up to m/Δm ≥ 350 000; FWHM) is the multiturn TOF mass spectrometer (MULTUM), which relies on a symmetrical arrangement of electric sector fields to achieve “perfect space and time focusing”76; this investigation has been proposed for the OKEANOS mission to a Jupiter Trojan asteroid. Laser‐based resonance ionization mass spectrometers, which can selectively ionize specific isotopes of atomic and/or molecular compounds (circumventing the risk for isobaric interferences), are also in development for spaceflight applications.77 These next generation instruments will likely come to the forefront of planetary exploration in the coming years.
4.2. Plasma mass spectrometers to determine trace element chemistry
Unlike major elements (weight percent levels), trace element abundances (micrograms per gram levels) can vary by more than three orders of magnitude in geological samples, and thus serve as extraordinarily sensitive tracers of a variety of events that shape planetary bodies, including (but not limited to): (a) atmospheric evolution (eg, outgassing); (b) surface processes (eg, erosion); and (c) interior dynamics (eg, volcanism). Pyrolysis ovens, coupled with traditional EI sources, have served as benchmark front end subsystems for multiple mass spectrometers deployed on planetary surfaces (eg, Viking GCMS and MSL SAM); however, such hardware has difficulties breaking down many refractory phases, including primary minerals derived from magmatic activity (eg, olivine). High‐power laser sources, such as those that support LDMS applications (eg, MOMA78) and laser‐induced breakdown spectroscopy (LIBS; eg, ChemCam79), deposit a higher concentration of energy on the sample surface (ie, fluence), effectively supporting the ablation of otherwise unprocessed solid samples. Several investigations have coupled a pulsed laser system with a TOF mass analyzer and reported limits of detections between 1 and 100 μg/g,80, 81, 82, with some observing isotopic signals at or below 100 ppb atom levels.83, 84, 85 However, such techniques are challenged to quantify trace element abundances with sufficient accuracy and precision at these detection limits. Plasmas, on the other hand, may be designed specifically to generate high electron densities and hot source regions, thereby enabling efficient atomization and ionization of most geological phases.86
In the commercial realm, trace elements are measured routinely via plasma mass spectrometry using sources ranging from microwave and inductively coupled plasmas to glow discharges and spark sources; these front‐end systems are commonly interfaced to a quadrupole or sector field instrument. Most prevalent in the geological sciences are inductively coupled plasma mass spectrometers (ICPMSs) that rely on high‐temperature plasmas (~104 K), energized by spark electrons colliding with initially neutral gas (typically Ar) in a rapidly oscillating RF magnetic field, to atomize and ionize solid aerosols and/or aspirated liquids. Reactions between the spark electrons and the incoming stream of plasma gas effectively “strip” valence electrons off the gas atoms (eg, Ar ➔Ar+ + e−). Both the ionized gas and newly liberated valence electrons are mobilized by the RF magnetic field, promoting further ionization and resulting in a “cascade effect” until the rate of release of new valence electrons via collisional interactions is balanced by the rate of recombination of electrons with gas ions (eg, Ar+ + e− ➔ Ar).
State‐of‐the‐art ICPMS instruments offer high intrascan dynamic ranges (up to 1012 if equipped with an electron multiplier with attenuation grid plus a Faraday cup) and minimal instrumental backgrounds (other than ionized plasma gas), contributing to highly sensitive and quantitative measurements of nearly any element in the periodic table. When coupled with a commercial laser ablation system, laboratory ICPMS routinely deliver percent‐level accuracy/precision with detection limits at or below picograms per gram (eg, Gonzalez et al87), though single particle detection enabled by ICPMS has emerged as an exciting new avenue in nanomaterial research (eg, Lee et al88). Investments in the miniaturization of plasma sources (eg, Franzke et al89), including plasmas designed for operations at ambient pressures (eg, Tendero et al90) and low‐power/self‐sustaining ICP sources adapted for specific planetary environments (eg, Taghioskoui and Zaghloul91), suggest that a spaceflight ICPMS will become a reality in the next decade. Such an instrument may be combined with a pulsed laser source, enabling in situ chemical imaging without requiring sample contact (see Section 4.4 below).
4.3. Molecular separation and ion mobility spectrometry
Molecular separation techniques enable the isolation of isobaric interferences, provide a diagnostic measure of composition, and extend the total dynamic range of mass analyzers, defining a collection of highly coveted analytical capabilities for planetary exploration. Gas chromatography, which separates volatile compounds via respective chemical affinities for a specific stationary phase, has been used successfully on a number of QMS instruments extending from the Viking GCMS investigations to MSL SAM (described earlier). More recently, a gas chromatograph has been integrated with a compact TOFMS and baselined for the Russian Luna‐Resurs mission to the Moon.92, 93 However, traditional GCMS methods are generally incapable of detecting refractory macromolecular carbon that may represent partially preserved biomass (eg, kerogen). In contrast, liquid chromatography and ion mobility spectrometry (IMS) support the separation of molecular species with a wider range of physicochemical properties (such as nonvolatile peptides or oligonucleotides). In particular, IMS facilitates the identification of competing isomers and conformers at fast scan rates (eg, 10 to 100 ms), including chiral amino acids (eg, Mie et al94), supporting life detection objectives (eg, Dwivedi et al95).
Although many system designs are available, the simplest IMS instruments are composed of an ionization source, followed by an ion gate that pulses ions into a drift tube that acts as the mobility analyzer, and finally a detector that measures ion current as a function of drift time. A technique commonly referred to as drift tube IMS (DTIMS) enables the compositional analysis of gas phase samples by exposing each pulse of ions to a uniform electric field in addition to a counterflow of buffer gas (typically N2 and/or He); ions of different m/z and cross‐sectional areas are separated effectively on the basis of ion‐neutral dynamics within the drift tube. Specifically, ions with higher charges (z) will be accelerated more strongly towards the detector because of a greater response to the electric field, while ions of higher mass (m) and/or reaction cross sections will be slowed because of more frequent collisions with the buffer gas (eg, May and McLean96).
Standalone IMS systems cannot compete with the mass resolution and/or accuracy realized by the pioneering mass analyzers discussed above, but they may be considered as add‐ons for hybrid instrument configurations, enabling multidimensional separations of ions based on chemical affinity, atomic/molecular size, charge state, and mass.96 A variety of integrative instrument designs have been implemented successfully and found wide ranging adoption in the commercial sector. For example, an IMS front‐end system has been successfully interfaced to an Orbitrap analyzer and used to characterize a suite of proteomic and petroleum samples.97 However, one of the primary obstacles to qualifying IMS instruments for spaceflight, particularly hybrid instruments, is the pressure gradient between the IMS (commonly operated at 105 Pa) and mass analyzer (eg, ≤10−6 Pa for full Orbitrap performance), which would require significant mass/volume/power resources to support a multistage vacuum system. A standalone IMS, coupled with a gas chromatograph, was deployed on the International Space Station in 2001 as part of the Volatile Organic Analyzer (VOA) to monitor for permanent gases and volatile organic contaminants in the cabin atmosphere and safeguard crew health.98 In 2009, the aging VOA was replaced by the first Air Quality Monitor (AQM), a GC/differential mobility spectrometer (GC/DMS) that uses a combination of RF and electrostatic potentials to separate ions at higher resolutions.99 A number of IMS instruments have been developed and proposed in response to astrobiology mission opportunities, including concepts to explore small bodies, Mars, and Titan, but as of yet none have been selected.
4.4. Spatially resolved analyses and chemical imaging
Microbeam techniques are ideally suited for the in situ chemical analysis of precious planetary materials, as these methods enable the characterization of unprocessed samples without physical contact, reducing the risk for cross‐contamination and relaxing Planetary Protection requirements. In particular, laser desorption and ablation microprocessing provides spatially resolved measurements of elemental and/or molecular composition, delivering 2D (or 3D with depth profiling) chemical imaging capabilities when united with an appropriate analyzer. The spatial resolution of laser desorption/ablation mass spectrometry is limited only by the size of the beam at the sample surface, allowing for compositional measurements of individual geological phases, melt/fluid inclusions, and microfossils/biofabrics at the micron scale. Challenges associated with disequilibrium mineral compositions (eg, concentric zoning), grain boundary dissolution (eg, thermal alteration), and potentially contaminated surface materials (eg, spacecraft outgassing) may be circumvented by precision targeting and/or pre‐ablating the sample substrate. Moreover, laser sampling applied to planetary investigations requires orders‐of‐magnitude less sample mass (ie, nanograms) compared with traditional pyrolysis techniques (ie, milligrams).
Laser desorption/ablation mass spectrometry techniques have a long history of use in molecular and elemental analysis of solid samples. High peak fluences (greater than 1 J/cm2) achieved by many modern pulsed laser systems permit even the most refractory mineral phases to be sampled, while lower fluences (less than 0.1 J/cm2) can serve to liberate and ionize organic compounds without incurring excessive molecular fragmentation. Solid‐state laser systems that generate nanosecond pulses have served as benchmarks for laser‐based mass spectrometry for decades, largely because of their ability to generate tens of millions of pulses at high energies in robust and economical packages. The MOMA instrument onboard the ExoMars rover100 includes an ultra‐compact Nd:YAG laser capable of generating more than 135 μJ energy per pulse at 266 nm; such UV wavelengths couple well with many geological phases,101 as well as aromatic hydrocarbons that may be adsorbed onto mineral surfaces.102 Higher power systems that can access deeper UV wavelengths are currently in development for the next generation of laser‐based mass spectrometers, but an even more innovative direction is the miniaturization of laser sources capable of generating femtosecond pulses. Femtosecond laser pulses reduce heating, melting, vaporization, and recondensation of the sample, resulting in the attenuation of laser‐induced elemental fractionation (eg, Russo et al103). Ruggedized femtosecond laser sources have already been integrated with prospective spaceflight mass analyzers and shown to deliver high‐resolution chemical depth profiles of solid substrates104; maps of local mineralogy in meteoritic samples105; chemical identification of putative microfossils106; detection of an array of complex organic molecules107; and improvements in the precision of Rb‐Sr isochrons.108 Because of its unique sampling capabilities, laser desorption/ablation mass spectrometry will likely be an analytical technique applied on future in situ planetary exploration missions.
4.5. Multisource/hybrid instruments for crosscutting investigations
The modularity of conventional mass spectrometer designs supports the definition of a multitude of distinct instrument configurations, each capable of conducting focused and broadband investigations into the chemical composition of planetary samples. However, depending on the planetary body, targeted environment, sample type/phase, and prioritized measurement objectives, the optimal ionization technique may not be an obvious choice. Likewise, standard mass spectra (peak intensity versus m/z) may not be enough to meet the scientific goals of the mission; instead, information such as fragmentation patterns (eg, via MSn), stoichiometric formula (eg, via highly accurate mass determinations), and/or molecular cross sections (eg, via ion‐neutral dynamics) may be needed. Just as multiple techniques would be employed in a terrestrial laboratory, including redundant (but independent) measures of the same geochemical or biological proxies, they are similarly desired for space applications when available resources permit multiple lines of sample interrogation. In such cases, hybrid instruments that unite two (or more) sensors and/or ionization sources are essential, as they can provide enhanced science return without requiring multiple individual instruments that otherwise may not share resource allocations.
Examples of heritage instruments that have coupled two distinct ionization sources to a single mass analyzer include LADEE NMS, MAVEN NGIMS, and the Neutral gas and Ion Mass spectrometer (NIM) on the JUpiter ICy moons Explorer (JUICE) mission, each of which employs discrete “closed” and “open” EI sources to expand their respective analytical capabilities.109, 110, 111 As mentioned previously, the MOMA instrument centers around a single analyzer with two fundamentally different but analytically complementary ionization sources, namely, a closed EI source and pulsed UV laser system that target volatile and refractory organics, respectively.70 Thus, the two modes of operation may be considered as completely different instruments, greatly increasing the quantity and quality of data return without the proportional increase in resources (ie, SWaP). Hybrid instrumentation is not, however, limited to single analyzer instruments with multiple sources. Instrumentation with multiple mass analyzers (including ion mobility analyzers) integrated together can provide benefits for space applications, just as these designs have achieved success in commercial laboratories. While no hybrid analyzer instruments have flown yet, several are in development, like the AROMA investigation that combines a 2D ion trap (eg, MOMA) and an Orbitrap system, mimicking the popular Thermo LTQ Orbitrap XL system.112 The ability to triage a sample with various efficient but low bandwidth modes, and then employ more advanced mode(s) once the sample has been shown to be of strategic value, is potentially transformative for remote and/or autonomous in situ missions.
4.6. Vacuum pumps
Despite the emergence of ambient ionization sources, such as desorption electrospray ionization (DESI113), and pulsed ion injection schemes like the discontinuous atmospheric pressure interface (DAPI114), mass analyzers and detector assemblies typically need to be maintained at lower pressures for high voltage stability, longer mean free path lengths, and other practical reasons. As a result, gas processing systems, and vacuum pumps in particular, are critical for ground‐based laboratory instruments as well as spaceflight hardware. For the exploration of planetary environments with negligible atmospheric pressure (eg, less than 10−1 Pa), such as small bodies (eg, asteroids) and moons with only limited exospheres (eg, Europa), venting to space with a high‐conductance valve (such as those flown on the SAM instrument on MSL43) can provide access to vacuum for payload instruments that require low pressures. However, substantial atmospheric pressures (eg, greater than or equal to 10−1 Pa) require pumping solutions to evacuate mechanical housings that support high‐voltage analyzers and detectors. Getter pumps, which have no moving parts but rather promote chemisorption and/or physisorption between a variety of gases and an adsorbent phase (eg, sintered porous alloy), provide passive pumping of vacuum chambers; power/energy may be required to maximize “sticking probabilities” and to recondition the adsorbent. Chemical getters have extensive flight heritage, ranging from the mass spectrometers flown on the Pioneer Venus and Venera missions to the MSL SAM investigation, but such solutions are inherently limited by finite pumping capacity. The miniaturization of mechanical pumping systems represents a key milestone in the viability of high‐priority mission concepts targeting surface operations on Mars (eg, MSL), Titan (eg, Dragonfly), and Venus (eg, DAVINCI). Small‐scale vacuum pumps are not without drawbacks, though, as they are often limited in pumping speed, compression ratios, and operational lifetimes (or on/off cycles).
To enable surface operations on Mars, Creare, Inc (in collaboration with NASA Goddard Space Flight Center) developed a 0.6 kg/9 W wide range pump consisting of a molecular drag stage in series with a turbomolecular pump, together providing 4 L/s pumping speeds (N2) and exhausting directly to the Mars ambient atmosphere (approximately 103 Pa).115 Two of these pumps, which spin at 100 k revolutions per minute and achieve a compression ratio on the order of 108 for N2, support the ongoing MSL SAM investigation,43 and a single unit will enable operations of the MOMA instrument onboard the ExoMars rover.70 The recently selected Dragonfly mission relies on two “upgraded” versions of these pumps, which spin at twice the speed but require less than half the mass.116 Although Creare, Inc has a number of cutting‐edge pumps in development, other commercial vendors are also innovating small but highly capable pumping options. Pfeiffer Vacuum GmbH manufactures a HiPace10 turbomolecular pump that offers more than twice the pumping speed of the Creare wide range pumps but at significantly higher mass (1.5 kg) and power requirements (29 W) and a lower compression ratio (106 for N2). The KNF Neuberger, Inc N84.3 diaphragm pump, with a pumping speed of 5 L/min, base pressure of 700 Pa, and mass of only 0.9 kg, represents a promising low‐power (18 W) backing pump. SynSysco offers an even smaller option with their 0.5 kg SSC05‐075 scroll pump, which requires only 15 W to reach a pumping speed of 5 L/min and a base pressure of 10 Pa. Originally designed to pressurize CO2 over the triple point on Mars (enabling CO2 liquefaction for in situ resource utilization), Air Squared has developed a miniature (multistage) scroll compressor that may also be exploited as a planetary backing pump. For small volumes (eg, lab‐on‐a‐chip systems), a variety of MEMS pumping systems also show promise for spaceflight applications (eg, Grzebyk117). These offerings, and the continued investment in the development of smaller and more capable solutions, illustrate the importance of pumping elements to future planetary endeavors.
4.7. Quantitative performance
The translation of raw signal intensities into high‐fidelity elemental/molecular abundances and isotopic ratios is quickly evolving from an ancillary science goal to a central investigation requirement. For life detection missions, population distributions of organic materials, including relative abundances of amino acids and left‐handed (l‐) and right‐handed (d‐) chiral forms, have emerged as important biological indices.118, 119, 120 Geochronology objectives, including the derivation of formation and exposure ages, require highly precise and accurate measurements of isotopic compositions to reduce uncertainties in the timing of climactic events in solar system history (eg, Cohen et al121). Progressive quantitative performance could augment other chemical lines of inquiry, too, such as tracing material origins (eg, endogenous versus exogeneous sources) and constructing models of bulk planetary composition. Exhaustive calibration campaigns that characterize instrument responses through the analysis of reference materials, including those carried with the instrument to the planetary target (eg, perfluorotributylamine for the SAM and MOMA instruments), have become essential activities in the development of spaceflight mass spectrometers. The analysis of representative planetary analog samples adds fidelity to measurement capabilities and informs more directly on spectra acquired in situ during mission operations. The reproducibility of mass spectra collected on replicate analyses, particularly the fractionation of elements and molecules with different electronic structures and chemical affinities, bounds the precision of the system and feeds into the achievable accuracy of inferred abundances. Quantitative performance metrics will only grow in importance, particularly for ambitious mission concepts to challenging and previously unexplored destinations.
4.8. Radiation tolerance
Exposure to high fluences of ionizing radiation, from solar energetic particles (eg, high‐energy protons and electrons) to galactic cosmic rays (eg, high‐energy atomic nuclei), can compromise instrument measurements, alter material properties, and permanently damage electronic modules via single event effects and/or accumulated doses. Specific to mass spectrometry, radiation threats include
excessive dark counts and/or current induction on the detector assembly, compromising detection limits and spectral reproducibility, and risking false positive signals;
deterioration of polymers, including those commonly used to seal hermetic interfaces, and the depression of laser damage thresholds of optical components; and
irreparable damage to semiconductor electronic components, including non‐volatile memory caches, via gate ruptures, burnouts, and other failure types.
These problems may be alleviated to some degree through the implementation of heavy shielding (eg, instrument vaults composed of layered metal alloys), materials selection (eg, metal gaskets in place of Viton seals), and designed redundancies (eg, tripling memory requirements). However, sensitive electrical components need to be radiation hardened for missions to high‐risk environments, such as the magnetosphere of Jupiter, which traps and accelerates charged particles akin to the Van Allen belts (but with orders of magnitude higher fluences). The Europa Clipper and JUICE missions are taking steps to maximize spacecraft and instrument lifetimes, and future missions (such as the Europa Lander mission concept) will need to implement similar measures.
5. CONCLUSIONS: CONTEMPORARY CHALLENGES AND FORWARD OUTLOOK
Mass spectrometry has advanced significantly since the earliest prototype systems were built and tested in the early 20th century. Since that time, the number of distinct types of mass analyzers has expanded, performance capabilities have progressed, and the design of modern instruments has transitioned towards modularity, together supporting analytical specificity and selectivity through custom system configurations. Innovations in the field, including the development of novel ionization sources, demonstration of advanced chemical separation techniques, and further miniaturization/ruggedization of critical hardware components, provide access to new planetary environments and address deeper scientific inquiries. With the maturation of key technologies already in the pipeline, such as ultrahigh resolution sensors, sources that enable trace element measurements, and lasers that promote spatially‐resolved chemical imaging, mission concepts have (and will continue to) become more ambitious. A focus on quantitative performance and radiation hardening of sensitive instrument components will ultimately serve to extend the reach of future mission targets farther out into the solar system, promising even greater scientific discovery.
ACKNOWLEDGEMENTS
We would like to thank Paul Mahaffy and an anonymous reviewer for editorial comments and insights into how to make this article more inclusive, impactful, and accurate. This research was supported by NASA grants 80NSSC18K0932 and 80NSSC19K0610.
Arevalo R Jr, Ni Z, Danell RM. Mass spectrometry and planetary exploration: A brief review and future projection. J Mass Spectrom. 2020;55:e4454 10.1002/jms.4454
REFERENCES
- 1. Thompson JJ. Positive Rays and Their Application to Chemical Analysis. London, UK: Longmans Green and Co; 1913. [Google Scholar]
- 2. Yokoyama T, Walker RJ. Nucleosynthetic isotope variations of siderophile and chalcophile elements in the solar system. Rev Mineral Geochem. 2016;81(1):107‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farley KA, Malespin C, Mahaffy P, et al. In situ radiometric and exposure age dating of the Martian surface. Science. 2014;343(6169):1247166. [DOI] [PubMed] [Google Scholar]
- 4. Johnson SS, Anslyn EV, Graham HV, Mahaffy PR, Ellington AD. Fingerprinting non‐terran biosignatures. Astrobiology. 2018;18(7):915‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Research Council . Vision and Voyages for Planetary Science in the Decade 2013‐2022. Washington, DC: The National Academies Press; 2011:398. [Google Scholar]
- 6. European Space Agency . Cosmic Vision: Space Science for Europe 2015‐2025. Noordwijk, The Netherlands: ESA Publications Division; 2005:109. [Google Scholar]
- 7. Benna M, Hurley DM, Stubbs TJ, Mahaffy PR, Elphic RC. Lunar soil hydration constrained by exospheric water liberated by meteoroid impacts. Nat Geosci. 2019;12(5):333‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waite JH, Glein CR, Perryman RS, et al. Cassini finds molecular hydrogen in the Enceladus plume: evidence for hydrothermal processes. Science. 2017;356(6334):155‐159. [DOI] [PubMed] [Google Scholar]
- 9. Eigenbrode JL, Summons RE, Steele A, et al. Organic matter preserved in 3‐billion‐year‐old mudstones at Gale Crater, Mars. Science. 2018;360(6393):1096‐1101. [DOI] [PubMed] [Google Scholar]
- 10. Freissinet C, Glavin DP, Mahaffy PR, et al. Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars. J Geophys Res‐Planet. 2015;120(3):495‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. JPL, N . Mars Science Laboratory Launch (Press Kit). 2011, NASA.
- 12. NASA . MAVEN: Mars Atmosphere and Volatile EvolutioN Mission (Press Kit). 2013, NASA.
- 13. JPL, N . Galileo Jupiter Arrival. 1995, NASA.
- 14. JPL, N . Phoenix Launch: Mission to the Martian North Pole (Press Kit). 2007, NASA.
- 15. Palmer PT, Limero TF. Mass spectrometry in the U.S. space program: past, present, and future. J Am Soc Mass Spectrom. 2001;12(6):656‐675. [DOI] [PubMed] [Google Scholar]
- 16. Ren Z, Guo M, Cheng Y, et al. A review of the development and application of space miniature mass spectrometers. Vacuum. 2018;155:108‐117. [Google Scholar]
- 17. Snyder DT, Pulliam CJ, Ouyang Z, Cooks RG. Miniature and fieldable mass spectrometers: recent advances. Anal Chem. 2016;88(1):2‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aston FW. LXXIV. A positive ray spectrograph. Lond Edinb Dubl Phil Mag J Sci. 1919;38(228):707‐714. [Google Scholar]
- 19. Reber C. Data from Explorer 17 on composition of the upper atmosphere. J Geophys Res. 1964;69(21):4681‐4685. [Google Scholar]
- 20. Hoffman JH, Hodges RR Jr, Evans DE. Lunar orbital mass spectrometer experiment. Proc Third Lunar Sci Conf. 1972;3:2205‐2216. [Google Scholar]
- 21. Anderson DM, Biemann K, Orgel LE, et al. Mass spectrometric analysis of organic compounds, water and volatile constituents in the atmosphere and surface of Mars: the Viking Mars Lander. Icarus. 1972;16(1):111‐138. [Google Scholar]
- 22. Biemann K, Oro JI, Toulmin P III, et al. The search for organic substances and inorganic volatile compounds in the surface of Mars. J Geophys Res (1896‐1977). 1977;82(28):4641‐4658. [Google Scholar]
- 23. Guzman M, McKay CP, Quinn RC, et al. Identification of chlorobenzene in the Viking gas chromatograph‐mass spectrometer data sets: reanalysis of Viking mission data consistent with aromatic organic compounds on Mars. J Geophys Res‐Planet. 2018;123(7):1674‐1683. [Google Scholar]
- 24. Hecht MH, Kounaves SP, Quinn RC, et al. Detection of perchlorate and the soluble chemistry of Martian soil at the Phoenix lander site. Science. 2009;325(5936):64‐67. [DOI] [PubMed] [Google Scholar]
- 25. Navarro‐González R, Vargas E, de la Rosa J, Raga AC, McKay CP. Reanalysis of the Viking results suggests perchlorate and organics at midlatitudes on Mars. J Geophys Res‐Planet. 2010;115(E12):E12010. [Google Scholar]
- 26. Hoffman JH, Oyama VI, von Zahn U. Measurements of the Venus lower atmosphere composition: a comparison of results. J Geophys Res Space Phys. 1980;85(A13):7871‐7881. [Google Scholar]
- 27. Krankowsky D, Lämmerzahl P, Herrwerth I, et al. In situ gas and ion measurements at comet Halley. Nature. 1986;321(6067):326‐329. [Google Scholar]
- 28. Boynton WV, Ming DW, Kounaves SP, et al. Evidence for calcium carbonate at the Mars Phoenix landing site. Science. 2009;325(5936):61‐64. [DOI] [PubMed] [Google Scholar]
- 29. Smith PH, Tamppari LK, Arvidson RE, et al. H2O at the Phoenix landing site. Science. 2009;325(5936):58‐61. [DOI] [PubMed] [Google Scholar]
- 30. Niles PB, Boynton WV, Hoffman JH, Ming DW, Hamara D. Stable isotope measurements of Martian atmospheric CO2 at the Phoenix landing site. Science. 2010;329(5997):1334‐1337. [DOI] [PubMed] [Google Scholar]
- 31. Balsiger H, Altwegg K, Bochsler P, et al. Rosina – Rosetta Orbiter Spectrometer for ion and neutral analysis. Space Sci Rev. 2007;128(1):745‐801. [Google Scholar]
- 32. Paul W, Steinwedel H. In German Patent DE944900C . 1956.
- 33. Paul W, Raether M. Das elektrische Massenfilter. Z Phys. 1955;140(3):262‐273. [Google Scholar]
- 34. Niemann HB, Hartle RE, Hedin AE, et al. Venus upper atmosphere neutral gas composition: first observations of the diurnal variations. Science. 1979;205(4401):54‐56. [DOI] [PubMed] [Google Scholar]
- 35. Niemann HB, Hartle RE, Kasprzak WT, Spencer NW, Hunten DM, Carignan GR. Venus upper atmosphere neutral composition: preliminary results from the Pioneer Venus Orbiter. Science. 1979;203(4382):770‐772. [DOI] [PubMed] [Google Scholar]
- 36. Spencer NW, Niemann HB, Carignan GR. The neutral‐atmosphere temperature instrument. Radio Sci. 1973;8(4):287‐296. [Google Scholar]
- 37. Niemann HB, Booth JR, Cooley JE, et al. Pioneer Venus Orbiter neutral gas mass spectrometer experiment. IEEE Trans Geosci Remote Sens. 1980;GE‐18(1):60‐65. [Google Scholar]
- 38. Kasprzak WT, Niemann HB, Mahaffy P. Observations of energetic ions on the nightside of Venus. J Geophys Res Space Phys. 1987;92(A1):291‐298. [Google Scholar]
- 39. Niemann HB, Atreya SK, Carignan GR, et al. The Galileo Probe mass spectrometer: composition of Jupiter's atmosphere. Science. 1996;272(5263):846‐849. [DOI] [PubMed] [Google Scholar]
- 40. Niemann HB, Atreya SK, Bauer SJ, et al. The abundances of constituents of Titan's atmosphere from the GCMS instrument on the Huygens probe. Nature. 2005;438(7069):779‐784. [DOI] [PubMed] [Google Scholar]
- 41. Waite JH, Combi MR, Ip WH, et al. Cassini ion and neutral mass spectrometer: Enceladus plume composition and structure. Science. 2006;311(5766):1419‐1422. [DOI] [PubMed] [Google Scholar]
- 42. England SL, Liu G, Yiğit E, et al. MAVEN NGIMS observations of atmospheric gravity waves in the Martian thermosphere. J Geophys Res Space Phys. 2017;122(2):2310‐2335. [Google Scholar]
- 43. Mahaffy PR, Webster CR, Cabane M, et al. The sample analysis at Mars Investigation and Instrument Suite. Space Sci Rev. 2012;170(1):401‐478. [Google Scholar]
- 44. Glaze LS, Garvin JB, Robertson B, et al. DAVINCI: Deep atmosphere venus investigation of noble gases, chemistry, and imaging. In 2017 IEEE Aerospace Conference 2017.
- 45. Arevalo R Jr, Brinckerhoff W, van Amerom F, et al. Design and demonstration of the Mars Organic Molecule Analyzer (MOMA) on the ExoMars 2018 rover. In 2015 IEEE Aerospace Conference 2015.
- 46. Stephens WE. Proceedings of the American Physical Society. Phys Rev. 1946;69(11‐12):674‐674. [Google Scholar]
- 47. Cameron AE, Eggers DF. An Ion "Velocitron". Rev Sci Instrum. 1948;19(9):605‐607. [Google Scholar]
- 48. Wiley WC, McLaren IH. Time‐of‐flight mass spectrometer with improved resolution. Rev Sci Instrum. 1955;26(12):1150‐1157. [Google Scholar]
- 49. Mamyrin BA, Karataev VI, Shmikk DV, Zagulin VA. The mass‐reflectron, a new nonmagnetic time‐of‐flight mass spectrometer with high resolution. Zh Eksp Teor Fiz. 1973;64(1):82‐89. [Google Scholar]
- 50. Kissel J. Particle impact analyser (PIA). 1985, NASA Space Science Data Coordinated Archive. p. NSSDCA ID: 1985‐056A‐04.
- 51. Kissel J, Sagdeev RZ, Bertaux J‐LC. Dust mass spectrometer (PUMA). 1984, NASA Space Science Data Coordinated Archive. p. NSSDCA ID: 1984‐128A‐04.
- 52. Kissel J, Krueger FR. The organic component in dust from comet Halley as measured by the PUMA mass spectrometer on board Vega 1. Nature. 1987;326(6115):755‐760. [Google Scholar]
- 53. Kissel J, Sagdeev RZ, Bertaux JL, et al. Composition of comet Halley dust particles from Vega observations. Nature. 1986;321(6067):280‐282. [Google Scholar]
- 54. Kissel J, Brownlee DE, Büchler K, et al. Composition of comet Halley dust particles from Giotto observations. Nature. 1986;321(6067):336‐337. [Google Scholar]
- 55. Scherer S, Altwegg K, Balsiger H, et al. A novel principle for an ion mirror design in time‐of‐flight mass spectrometry. Int J Mass Spectrom. 2006;251(1):73‐81. [Google Scholar]
- 56. Gasc S, Altwegg K, Fiethe B, et al. Sensitivity and fragmentation calibration of the time‐of‐flight mass spectrometer RTOF on board ESA's Rosetta mission. Planet Space Sci. 2017;135:64‐73. [Google Scholar]
- 57. Kissel J, Altwegg K, Clark BC, et al. Cosima – High resolution time‐of‐flight secondary ion mass spectrometer for the analysis of cometary dust particles onboard Rosetta. Space Sci Rev. 2007;128(1):823‐867. [Google Scholar]
- 58. Goesmann F, Rosenbauer H, Roll R, et al. COSAC, The Cometary Sampling and Composition Experiment on Philae. Space Sci Rev. 2007;128(1):257‐280. [Google Scholar]
- 59. Brockwell TG, Meech KJ, Pickens K, et al. The mass spectrometer for planetary exploration (MASPEX). In 2016 IEEE Aerospace Conference 2016.
- 60. Courant ED, Livingston MS, Snyder HS. The Strong‐Focusing Synchroton—a new high energy accelerator. Phys Rev. 1952;88(5):1190‐1196. [Google Scholar]
- 61. Good ML. University of California Radiation Laboratory Report (S. Shewchuck), in UCRL 2209 . 1953: Berkeley, CA.
- 62. Post RF, Heinrich L. University of California Radiation Laboratory Report (S. Shewchuck), in UCRL 2209 . 1953: Berkeley, CA.
- 63. Hager JW. Axial ejection in a multipole mass spectrometer, U.P. Office, Editor. 2001.
- 64. Stafford GC Jr, Kelley PE, Syka JE, Reynolds WE, Todd JF. Recent improvements in and analytical applications of advanced ion trap technology. Int J Mass Spectrom Ion Processes. 1984;60(1):85‐98. [Google Scholar]
- 65. Campbell JM, Collings BA, Douglas DJ. A new linear ion trap time‐of‐flight system with tandem mass spectrometry capabilities. Rapid Commun Mass Spectrom. 1998;12(20):1463‐1474. [DOI] [PubMed] [Google Scholar]
- 66. Dehmelt HG. Radiofrequency spectroscopy of stored ions I: storage**Part II: spectroscopy is now scheduled to appear in Volume V of this series In: Bates DR, Estermann I, eds. Advances in Atomic and Molecular Physics. New York: Academic Press Inc; 1968:53‐72. [Google Scholar]
- 67. Makarov A. Electrostatic axially harmonic orbital trapping: a high‐performance technique of mass analysis. Anal Chem. 2000;72(6):1156‐1162. [DOI] [PubMed] [Google Scholar]
- 68. Wright IP, Barber SJ, Morgan GH, et al. Ptolemy—an instrument to measure stable isotopic ratios of key volatiles on a cometary nucleus. Space Sci Rev. 2007;128(1):363‐381. [Google Scholar]
- 69. Shortt BJ, Darrach MR, Holland PM, Chutjian A. Miniaturized system of a gas chromatograph coupled with a Paul ion trap mass spectrometer. J Mass Spectrom. 2005;40(1):36‐42. [DOI] [PubMed] [Google Scholar]
- 70. Goesmann F, Brinckerhoff WB, Raulin F, et al. The Mars Organic Molecule Analyzer (MOMA) instrument: characterization of organic material in Martian sediments. Astrobiology. 2017;17(6‐7):655‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lorenz RD, Turtle EP, Barnes JW, et al. Dragonfly: a rotorcraft lander concept for scientific exploration at Titan. Johns Hopkins APL Tech Dig (Applied Physics Laboratory). 2018;34(3):374‐387. [Google Scholar]
- 72. Guijas C, Montenegro‐Burke JR, Domingo‐Almenara X, et al. METLIN: A technology platform for identifying knowns and unknowns. Anal Chem. 2018;90(5):3156‐3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Denisov E, Damoc E, Lange O, Makarov A. Orbitrap mass spectrometry with resolving powers above 1,000,000. Int J Mass Spectrom. 2012;325‐327:80‐85. [Google Scholar]
- 74. Olsen JV, de Godoy LMF, Li G, et al. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C‐trap. Mol Cell Proteomics. 2005;4(12):2010‐2021. [DOI] [PubMed] [Google Scholar]
- 75. Arevalo R Jr, Selliez L, Briois C, et al. An Orbitrap‐based laser desorption/ablation mass spectrometer designed for spaceflight. Rapid Commun Mass Spectrom. 2018;32(21):1875‐1886. [DOI] [PubMed] [Google Scholar]
- 76. Toyoda M, Okumura D, Ishihara M, Katakuse I. Multi‐turn time‐of‐flight mass spectrometers with electrostatic sectors. J Mass Spectrom. 2003;38(11):1125‐1142. [DOI] [PubMed] [Google Scholar]
- 77. Scott Anderson F, Levine J, Whitaker TJ. Dating the Martian meteorite Zagami by the 87Rb‐87Sr isochron method with a prototype in situ resonance ionization mass spectrometer. Rapid Commun Mass Spectrom. 2015;29(2):191‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kolleck C, Büttner A, Ernst M, et al. Enhancement of the design of a pulsed UV laser system for a laser‐desorption mass spectrometer on Mars. In International Conference on Space Optics (ICSO) 2012. Ajaccio, Corsica, France.
- 79. Le Roch N, Dalmau J, Pares L, et al. ChemCam on the next NASA mission to Mars (MSL‐2011): measured performances of the high power LIBS laser beam. In International Conference on Space Optics (ICSO) 2010. Rhodes, Greece.
- 80. Brinckerhoff WB, Managadze GG, McEntire RW, Cheng AF, Green WJ. Laser time‐of‐flight mass spectrometry for space. Rev Sci Instrum. 2000;71(2):536‐545. [Google Scholar]
- 81. Managadze GG, Wurz P, Sagdeev RZ, et al. Study of the main geochemical characteristics of Phobos' regolith using laser time‐of‐flight mass spectrometry. Solar Syst Res. 2010;44(5):376‐384. [Google Scholar]
- 82. Tulej M, Iakovleva M, Leya I, Wurz P. A miniature mass analyser for in‐situ elemental analysis of planetary material—performance studies. Anal Bioanal Chem. 2011;399(6):2185‐2200. [DOI] [PubMed] [Google Scholar]
- 83. Riedo A, Bieler A, Neuland M, Tulej M, Wurz P. Performance evaluation of a miniature laser ablation time‐of‐flight mass spectrometer designed for in situ investigations in planetary space research. J Mass Spectrom. 2013;48(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 84. Riedo A, Meyer S, Heredia B, et al. Highly accurate isotope composition measurements by a miniature laser ablation mass spectrometer designed for in situ investigations on planetary surfaces. Planet Space Sci. 2013;87:1‐13. [Google Scholar]
- 85. Tulej M, Riedo A, Neuland MB, et al. CAMAM: a miniature laser ablation ionisation mass spectrometer and microscope‐camera system for in situ investigation of the composition and morphology of extraterrestrial materials. Geostand Geoanal Res. 2014;38(4):441‐466. [Google Scholar]
- 86. Houk RS, Fassel VA, Flesch GD, Svec HJ, Gray AL, Taylor CE. Inductively coupled argon plasma as an ion source for mass spectrometric determination of trace elements. Anal Chem. 1980;52(14):2283‐2289. [Google Scholar]
- 87. Gonzalez JJ, Oropeza D, Mao X, Russo RE. Assessment of the precision and accuracy of thorium (232Th) and uranium (238 U) measured by quadrupole based inductively coupled plasma‐mass spectrometry using liquid nebulization, nanosecond and femtosecond laser ablation. J Anal At Spectrom. 2008;23(2):229‐234. [Google Scholar]
- 88. Lee S, Bi X, Reed RB, Ranville JF, Herckes P, Westerhoff P. Nanoparticle size detection limits by single particle ICP‐MS for 40 elements. Environ Sci Technol. 2014;48(17):10291‐10300. [DOI] [PubMed] [Google Scholar]
- 89. Franzke J, Kunze K, Miclea M, Niemax K. Microplasmas for analytical spectrometry. J Anal At Spectrom. 2003;18(7):802‐807. [Google Scholar]
- 90. Tendero C, Tixier C, Tristant P, Desmaison J, Leprince P. Atmospheric pressure plasmas: a review. Spectroch Acta B: At Spectrosc. 2006;61(1):2‐30. [Google Scholar]
- 91. Taghioskoui M, Zaghloul M. Plasma ionization under simulated ambient Mars conditions for quantification of methane by mass spectrometry. Analyst. 2016;141(7):2270‐2277. [DOI] [PubMed] [Google Scholar]
- 92. Fausch RG, Wurz P, Tulej M, et al. Flight electronics of GC‐mass spectrometer for investigation of volatiles in the lunar regolith. In 2018 IEEE Aerospace Conference 2018.
- 93. Hofer L, Wurz P, Buch A, et al. Prototype of the gas chromatograph–mass spectrometer to investigate volatile species in the lunar soil for the Luna‐Resurs mission. Planet Space Sci. 2015;111:126‐133. [Google Scholar]
- 94. Mie A, Jörntén‐Karlsson M, Axelsson BO, Ray A, Reimann CT. Enantiomer separation of amino acids by complexation with chiral reference compounds and high‐field asymmetric waveform ion mobility spectrometry: preliminary results and possible limitations. Anal Chem. 2007;79(7):2850‐2858. [DOI] [PubMed] [Google Scholar]
- 95. Dwivedi P, Wu C, Matz LM, Clowers BH, Siems WF, Hill HH Jr. Gas‐phase chiral separations by ion mobility spectrometry. Anal Chem. 2006;78(24):8200‐8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. May JC, McLean JA. Ion mobility‐mass spectrometry: time‐dispersive instrumentation. Anal Chem. 2015;87(3):1422‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ibrahim YM, Garimella SVB, Prost SA, et al. Development of an ion mobility spectrometry‐Orbitrap mass spectrometer platform. Anal Chem. 2016;88(24):12152‐12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Limero T, Reese E, Trowbridge J, Hohman R, James JT. The volatile organic analyzer (VOA) aboard the International Space Station. 2002, SAE International.
- 99. Limero T, Reese E, Wallace WT, Cheng P, Trowbridge J. Results from the air quality monitor (gas chromatograph‐differential mobility spectrometer) experiment on board the International Space Station. Int J Ion Mobil Spectrom. 2012;15(3):189‐198. [Google Scholar]
- 100. Kalms R, Büttner A, Ernst M, et al. Opto‐mechanical design and verification of the MOMA UV laser source for the ExoMars 2020 mission. SPIE LASE. Vol. 10896. 2019: SPIE.
- 101. Guillong M, Horn I, Günther D. A comparison of 266 nm, 213 nm and 193 nm produced from a single solid state Nd:YAG laser for laser ablation ICP‐MS. J Anal At Spectrom. 2003;18(10):1224‐1230. [Google Scholar]
- 102. Steglich M, Bouwman J, Huisken F, Henning T. Can neutral and ionized PAHs be carriers of the UV extinction bump and the diffuse interstellar bands? Astrophys J. 2011;742(1):2. [Google Scholar]
- 103. Russo RE, Mao X, Gonzalez JJ, Zorba V, Yoo J. Laser ablation in analytical chemistry. Anal Chem. 2013;85(13):6162‐6177. [DOI] [PubMed] [Google Scholar]
- 104. Grimaudo V, Moreno‐García P, Riedo A, et al. High‐resolution chemical depth profiling of solid material using a miniature laser ablation/ionization mass spectrometer. Anal Chem. 2015;87(4):2037‐2041. [DOI] [PubMed] [Google Scholar]
- 105. Neuland MB, Meyer S, Mezger K, Riedo A, Tulej M, Wurz P. Probing the Allende meteorite with a miniature laser‐ablation mass analyser for space application. Planet Space Sci. 2014;101:196‐209. [Google Scholar]
- 106. Wiesendanger R, Wacey D, Tulej M, et al. Chemical and optical identification of micrometer‐sized 1.9 billion‐year‐old fossils by combining a miniature laser ablation ionization mass spectrometry system with an optical microscope. Astrobiology. 2018;18(8):1071‐1080. [DOI] [PubMed] [Google Scholar]
- 107. Moreno‐García P, Grimaudo V, Riedo A, Tulej M, Wurz P, Broekmann P. Towards matrix‐free femtosecond‐laser desorption mass spectrometry for in situ space research. Rapid Commun Mass Spectrom. 2016;30(8):1031‐1036. [DOI] [PubMed] [Google Scholar]
- 108. Anderson FS, Alexander A, Crow C, Whitaker TJ, Levine J. Improved precision for the chemistry and dating experiment using fs‐laser ablation. In 82nd Annual Meeting of The Meteoritical Society 2019. Hokkaido, Japan.
- 109. Mahaffy PR, Benna M, King T, et al. The neutral gas and ion mass spectrometer on the Mars Atmosphere and Volatile Evolution Mission. Space Science Reviews. 2015;195(1):49‐73. [Google Scholar]
- 110. Mahaffy PR, Hodges RR, Benna M, et al. The neutral mass spectrometer on the Lunar Atmosphere and Dust Environment Explorer Mission In: Elphic RC, Russell CT, eds. The Lunar Atmosphere and Dust Environment Explorer Mission (LADEE). Cham: Springer International Publishing; 2015:27‐61. [Google Scholar]
- 111. Meyer S, Tulej M, Wurz P. Mass spectrometry of planetary exospheres at high relative velocity: direct comparison of open‐ and closed‐source measurements. Geosci Instrum Method Data Syst. 2017;6(1):1‐8. [Google Scholar]
- 112. Arevalo R, Danell RM, Gundersen C, et al. Advanced Resolution Organic Molecule Analyzer (AROMA): simulations, development and initial testing of a linear ion trap‐Orbitrap instrument for space. In 3rd International Workshop on Instrumentation for Planetary Mission 2016. Pasadena, CA: LPI Contribution No. 1980.
- 113. Takáts Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306(5695):471‐473. [DOI] [PubMed] [Google Scholar]
- 114. Gao L, Cooks RG, Ouyang Z. Breaking the pumping speed barrier in mass spectrometry: discontinuous atmospheric pressure interface. Anal Chem. 2008;80(11):4026‐4032. [DOI] [PubMed] [Google Scholar]
- 115. Sorenson P, Kline‐Schoder R, Farley R. Wide range vacuum pumps for the SAM instrument on the MSL Curiosity Rover. In 42nd Aerospace Mechanisms Symposium 2014. NASA Goddard Space Flight Center, Greenbelt, MD.
- 116. Chen C‐H, Chen TC, Zhou X, et al. Design of portable mass spectrometers with handheld probes: aspects of the sampling and miniature pumping systems. J Am Soc Mass Spectrom. 2015;26(2):240‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Grzebyk T. MEMS vacuum pumps. J Microelectromech Syst. 2017;26(4):705‐717. [Google Scholar]
- 118. Hand KP, Murray AE, Garvin JB, et al., Report of the Europa Lander Science Definition Team. 2017, NASA HQ: Washington, DC.
- 119. Hendrix AR, Hurford TA, Barge LM, et al. The NASA roadmap to ocean worlds. Astrobiology. 2018;19(1):1‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Neveu M, Hays LE, Voytek MA, New MH, Schulte MD. The ladder of life detection. Astrobiology. 2018;18(11):1375‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cohen BA, Malespin CA, Farley KA, Martin PE, Cho Y, Mahaffy PR. In situ geochronology on Mars and the development of future instrumentation. Astrobiology. 2019;19:1‐12. 10.1089/ast.2018.1871 [DOI] [PMC free article] [PubMed] [Google Scholar]


