Abstract
Tacrolimus is the cornerstone of immunosuppressive therapy after kidney transplantation. Its narrow therapeutic window mandates serum level strict monitoring and dose adjustments to ensure the optimal risk‐benefit balance. This observational retrospective study analyzed the effectiveness and safety of conversion from twice‐daily immediate‐release tacrolimus (IR‐Tac) or once‐daily prolonged‐release tacrolimus (PR‐Tac) to the recent formulation once‐daily MeltDose® extended‐release tacrolimus (LCP‐Tac) in 365 stable kidney transplant recipients. We compared kidney function three months before and three months after the conversion. Three months after conversion, the total daily dose was reduced ~35% (P < .0001), and improved bioavailability and stable serum LCP‐Tac concentrations were observed. There was no increase in the number of patients requiring tacrolimus dose adjustments after conversion. Renal function was unaltered, and no cases of BPAR were reported. Reports of tremors, as collected in the clinical histories for each patient, decreased from pre‐conversion (20.8%) to post‐conversion (11.8%, P < .0001). LCP‐Tac generated a cost reduction of 63% compared with PR‐Tac. In conclusion, the conversion strategy to LCP‐Tac from other tacrolimus formulations in stable kidney transplant patients showed safety and effectiveness in a real‐world setting, confirming the data from RCTs. The specific pharmacokinetic properties of LCP‐Tac could be potentially advantageous in patients with tacrolimus‐related adverse events.
Keywords: extended‐release tacrolimus, immediate‐release tacrolimus, kidney transplant, LCPT, prolonged‐release tacrolimus, tacrolimus
1. INTRODUCTION
Calcineurin inhibitors such as tacrolimus are the cornerstone of immunosuppressive therapy for kidney transplantation. Tacrolimus acts at different levels of T lymphocyte activity and proliferation, leading to a general reduction in the T lymphocyte–mediated cytotoxicity.1 The most widely used maintenance immunosuppressive treatment is a combination of tacrolimus and an antiproliferative drug (such as mycophenolate mofetil), with or without corticosteroids.2, 3 However, previous studies have shown, for kidney and liver transplant patients, significant correlation of low tacrolimus concentrations with rejection and of high concentrations with nephrotoxicity.4, 5 Tacrolimus is a Narrow Therapeutic Index drug that requires individual dose titration to achieve a correct balance between maximizing efficacy and minimizing dose‐related toxicity.6
In Spain, there are currently three available formulations of tacrolimus: immediate‐release twice‐daily tacrolimus (IR‐Tac: Prograf®, Astellas Pharma and generics); prolonged‐release once‐daily tacrolimus (PR‐Tac, Advagraf®, Astellas Pharma); and MeltDose® extended‐release once‐daily tacrolimus (LCP‐Tac, Envarsus®, Chiesi). Several clinical and nonclinical studies have shown the pharmacokinetics of twice‐daily tacrolimus, and the two formulations of once‐daily tacrolimus are significantly different.7, 8, 9 LCP‐Tac, the most recent formulation, is based on the MeltDose® technology, which improves the solubility of tacrolimus and, thereby, its bioavailability, by dispersing tacrolimus in a polymeric matrix. The result is a progressive release of the drug to the distal part of the large intestine, a part of the gut where first‐pass metabolism is minimal due to lower CYP3A activity.10 Pharmacokinetic studies of LCP‐Tac have shown gradual absorption, rapid reach of therapeutic concentrations, and longer time needed to reach maximum blood concentration and less fluctuation between maximum and minimum concentrations. Oral bioavailability in kidney transplant patients was approximately 40% higher with LCP‐Tac than with IR‐Tac or PR‐Tac.11, 12
The efficacy and safety of LCP‐Tac were studied in controlled clinical trials both in patients‐recipients of de novo renal transplants and in conversion patients.13, 14 These pivotal studies demonstrated that LCP‐Tac has a similar safety profile and an efficacy not inferior to IR‐Tac. The STRATO clinical trial also showed that the use of LCP‐Tac may be associated with less neurotoxicity compared with twice‐daily tacrolimus formulations.15 In a pooled analysis of over 800 kidney transplant recipients, it has been observed that LCPT was at least as effective as tacrolimus twice daily in the overall target population and was associated with improved efficacy in high‐risk groups, including black and older‐age recipients.16
Although controlled clinical trials offer high‐quality data with great internal validity, they need to be complemented with data from observational studies to confirm and better define the effectiveness, safety, and tolerability in real clinical practice and in a broad patient population. The aim of this retrospective observational study was to evaluate the effectiveness and safety of the conversion from other formulations of tacrolimus to LCP‐Tac in stable kidney transplant recipients in routine clinical practice conditions.
2. PATIENTS AND METHODS
2.1. Study design
This multicenter, retrospective, single‐cohort conversion study was performed from January to May 2017 in 18 Nephrology Departments of Spanish hospitals. The inclusion criteria were as follows: age ≥18 years; recipients of a kidney transplant; treated (≥6 months) with tacrolimus formulations different from LCP‐Tac before conversion; treatment with LCP‐Tac initiated ≥3 months before inclusion in the study; and having signed the informed consent form. Patients with at least one episode of biopsy‐proven acute rejections (BPAR), of any severity, or significant decline of renal function (>10% increase in serum creatinine) in the 3 months before the conversion to LCP‐Tac were excluded from the study.
Although the inclusion criteria specified that patients had to be on treatment with tacrolimus ≥6 months, an exception was made for 10 patients who had received tacrolimus for <6 months (≥4.6 months) before conversion. Given that these patients represented only 2.7% of the total, no changes in the overall results were expected.
The data were retrieved from patients’ medical records. For all patients were collected demographic and anthropometric data, information on the donor, patient's medical history, including history of allograft rejections, and initial post‐transplantation immunosuppression regimen. The following data were collected for both periods, the 3 months before the conversion and 3 months after the conversion to LCP‐Tac: tacrolimus regimen and concomitant immunosuppression drugs, tacrolimus serum levels, renal function, analytical values obtained in routine clinical practice, vital signs and physical examination, concomitant antidiabetic and antihypertensive medication, registered signs of neurotoxicity (tremors, headache, concentration problems, insomnia), and tacrolimus‐related adverse reactions. Reasons for conversion to LCP‐Tac were also collected when available. In addition, data on treatment failures and treatment discontinuation were collected for a maximum of 12 months of follow‐up, when available.
The study was carried out in agreement with the Declaration of Helsinki,17 Good Clinical Practices, and applicable Spanish legislation. The study was approved by the Ethics committee of Hospital Clinic, Barcelona, Spain. All patients signed a written informed consent before being included in the study. The data were entered by the investigators into anonymized online formularies designed ad hoc for the study.
The administration of tacrolimus formulations to the patients followed clinical criteria and did not depend on their participation in this study. The initial doses used in the conversion were at the investigator's discretion. The patients received concomitant medication following usual clinical practice.
2.2. Study Outcomes
The primary outcome was the change in kidney function 3 months after the conversion to LCP‐Tac, compared with 3 months before the conversion, using the estimated glomerular filtration rate (eGFR) as calculated with the CKD‐EPI formula.18
The secondary outcomes were as follows: blood concentrations (C min, actual trough drawn clinically), total daily dose (TDD), and the need for dose adjustments of Tac; renal function parameters (creatinine, Mg2+); arterial pressure, weight, vital signs, and laboratory parameters (total cholesterol, low‐density lipoprotein (LDL), high‐density lipoprotein (HDL), triglycerides, glucose, glycosylated hemoglobin); information on adverse drug reactions; and serious adverse reactions to tacrolimus. Rate of BPAR, graft failure, and mortality after the conversion to LCP‐Tac and determination of the rate and reasons of LCP‐Tac discontinuation were also considered.
2.3. Sample size calculation
The sample size calculation was performed considering that the main objective of the study was to compare renal function (eGFR), before and after the conversion to LCP‐Tac. We therefore used the eGFR data obtained in the phase II conversion study from twice daily to once daily (LCP‐Tac).8 The eGFR in patients with stable kidney transplants (with the IR‐Tac formulation) was 58.67 ± 16.85. After 21 days of conversion to LCP‐Tac, the eGFR was 59.41 ± 15.81. A total of 350 patients were necessary to confirm the non‐inferiority of LCP‐Tac treatment compared with other tacrolimus formulations with 90% power and a confidence level of 0.025, applying a non‐inferiority margin of 5% (2.93 mL/min/1.73 m2). Considering 10% of patients with non‐evaluable data, the sample size was adjusted to 389 patients.
2.4. Statistical methods
The categorical variables were described using absolute and relative frequencies, and continuous variables were described using the mean with its 95% confidence interval (95% CI), the standard deviation (SD), the median, the 25th and 75th percentiles, and the minimum and maximum values.
For continuous variables, subgroups of patients were compared using parametric tests (Student's t test or ANOVA) or nonparametric tests (Mann‐Whitney U test), according to the characteristics of the study variables (assumption of normality) and the number of groups to compare. For the comparisons before and after the conversion, parametric tests (paired test of Student's t tests) or nonparametric tests (Wilcoxon tests) were used for continuous data, and McNemar tests were used for categorical data. A level of statistical significance of 0.05 has been applied in all statistical tests. There have been no adjustments for multiplicity in the evaluation of statistical significance. The data were analyzed using the statistical package SAS 9.4.
3. RESULTS
3.1. Patient disposition and baseline characteristics
Patient disposition is summarized in Figure 1. Out of the 389 enrolled patients, 365 met the selection criteria, had enough data for the primary end point evaluation, and were included in the effectiveness analysis; 384 were included in the safety analysis. The patients’ baseline characteristics are shown in Table 1. The median time between the transplant and conversion to LCP‐Tac was 49.1 months (IQR: 21.7‐109.3). The main causes of end‐stage renal disease (ESRD) were glomerulonephritis (23.6%) and polycystic kidney disease or hereditary nephropathies (20.3%). Most patients (86.3%) had no history of kidney transplant rejection.
Figure 1.
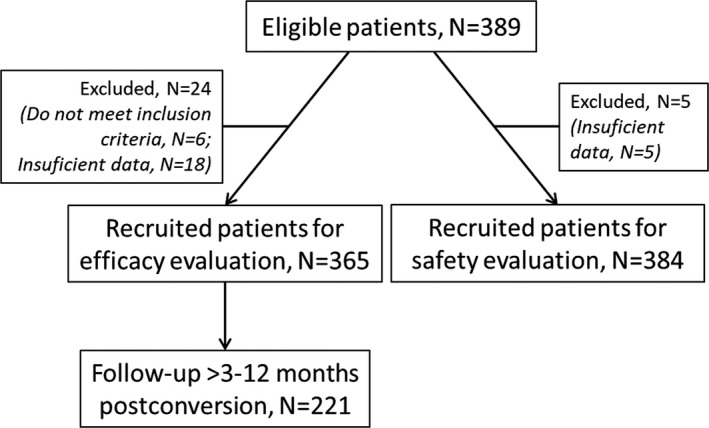
Patient disposition
Table 1.
Baseline characteristics of the patients
| N | 365 |
| Age (years), mean (SD) | 56.6 (13.6) |
| Male gender, N (%) | 226 (61.9) |
| Ethnic group, Caucasian, N (%) | 342 (93.7) |
| BMI (kg/m2), mean (SD) | 27.0 (4.9) |
| SBP, mean (SD) | 136.2 (14.6) |
| DBP, mean (SD) | 78.6 (9.7) |
| Total cholesterol mmol/L, mean (SD) | 4.5 ± 1.1 |
| Diabetes, N (%) | 83 (22.7) |
| Diabetes (post‐transplant)a, N (%) | 39 (47.0) |
| History of previous transplants, N (%) | 38 (10.4) |
| Time from transplant to conversion (months), median (range) | 49.1 (4.6‐367.3) |
| Induction treatment (thymoglobulin or anti‐IL‐2R antibodies), N (%) | 166 (45.5) |
| Initial tacrolimus, N (%) | 332 (91.0) |
| History of pre‐acute rejection, N (%) | 50 (13.7) |
| Donors | |
| Age (years), mean (SD) | 51.1 (15.5) |
| Living donor, N (%) | 56 (15.4) |
| Deceased donor, N (%) | 307 (84.6) |
| After brain death, N (%) | 280 (91.2) |
| After cardiac death, N (%) | 27 (8.8) |
| Primary diagnosis of renal failure | |
| Glomerulonephritis | 86 (23.6) |
| Polycystosis, hereditary nephropathies | 74 (20.3) |
| Nephroangiosclerosis | 44 (12.1) |
| Chronic interstitial nephritis | 30 (8.2) |
| Diabetes | 28 (7.7) |
| Otherb | 30 (8.2) |
| Unknown | 73 (20.0) |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; N, number; SBP, systolic blood pressure.
Of the 39 post‐transplant cases of diabetes, 28 cases were before LCP‐Tac conversion, 1 case was after conversion, and 8 were not specified.
Includes urologic causes (N = 14), systemic diseases (N = 9), and vascular diseases (N = 7).
Immunosuppressive therapy at the time of conversion consisted of IR‐Tac (4.1 ± 3.7 mg/d) for 168 patients (46.0%) and PR‐Tac (4.6 ± 3.1 mg/d) for 197 patients (54.0%) (Table 2). Most patients (87.6%) were also receiving prednisone, mycophenolate mofetil, or both at the time of conversion.
Table 2.
Immunosuppressive treatment, N (%)
| Pre‐conversion | Post‐conversion | |
|---|---|---|
| Tac | 365 (100) | 365 (100) |
| 12 h (Prograf®) | 142 (38.9) | |
| 12 h (Adoport®, Modigraf®, Tacrolimus Mylan®) | 26 (7.1) | |
| 24 h (Advagraf®) | 197 (54) | |
| Tac + prednisone +mycophenolate | 164 (44.9) | 163 (44.7) |
| Tac + mycophenolate | 95 (26.0) | 92 (25.2) |
| Tac + prednisone | 49 (13.4) | 48 (13.2) |
| Tac + prednisone +m‐TOR inhibitors | 12 (3.3) | 12 (3.3) |
| Tac + m‐TOR inhibitors | 8 (2.2) | 8 (2.2) |
| Tac only | 36 (9.9) | 40 (11) |
| Other | 1 (0.3) | 2 (0.6) |
Abbreviations: m‐TOR, mechanistic target of rapamycin; Tac, tacrolimus.
Data on reasons for conversion were available from 209 patients. For 84 patients (40.2%), the reason for conversion to LCP‐Tac was toxicity attributable to TAC that investigators considered might improve after conversion. In the remaining cases, conversion was not triggered by any adverse event and it was aimed to facilitate the dosing regimen and adherence (28.7%), optimize tacrolimus levels (13.9%), physician's decision (13.4%), or other reasons (3.8%).
3.2. Clinical end points
The analysis of the estimated glomerular filtration rate (eGFR) revealed no significant differences when comparing values 3 months pre‐ and post‐conversion (Table 3). The mean absolute difference before and after the conversion was 0.8 (CI95 = −0.2‐0.8), P = .076, suggesting that for eGFR the conversion to LCP‐Tac was non‐inferior to the other two formulations. None of the analyzed vital signs or metabolic parameters measured showed significant changes after conversion to LCP‐Tac (Table 3). Only 31 (8.5%) patients had adjustments in their antidiabetic or antihypertensive medication during the three months after conversion, and adjustments in the concomitant immunosuppressive therapy were reported in 23 (6.3%) patients.
Table 3.
Clinical and analytical parameters 3 months pre‐ and post‐conversion
| Pre‐conversion (mean ± SD) | Post‐conversion (mean ± SD) | P a | |
|---|---|---|---|
| eGFR (CKD‐EPI), mL/min/1.73 m2 | 52.3 ± 21.3 | 51.5 ± 21.6 | .14 |
| Creatinine, mg/dL | 1.56 ± 0.64 | 1.61 ± 0.76 | .049 |
| Weight, Kg | 73.8 ± 14.5 | 73.8 ± 14.3 | .72 |
| SBP, mm Hg | 136.4 ± 14.2 | 137.0 ± 15.1 | .48 |
| DBP, mm Hg | 78.4 ± 9.3 | 78.0 ± 10.0 | .41 |
| Total cholesterol, mmol/L | 4.5 ± 1.1 | 4.5 ± 1.0 | .53 |
| LDL cholesterol, mmol/L | 2.5 ± 0.9 | 2.5 ± 0.9 | .39 |
| HDL cholesterol, mmol/L | 1.3 ± 0.4 | 1.4 ± 0.5 | .06 |
| Triglycerides, mmol/L | 1.5 ± 0.7 | 1.6 ± 0.9 | .14 |
| Glucose, mmol/L | 5.8 ± 1.8 | 5.9 ± 1.9 | .23 |
| HbA1c, % | 6.1 ± 1.1 | 6.0 ± 1.3 | .41 |
| Mg2+, mmol/L | 0.7 ± 0.1 | 0.7 ± 0.1 | .28 |
Abbreviations: CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration equation; DBP, diastolic blood pressure; eGRF, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
Student's t test, Wilcoxon test
Overall, there were five cases of treatment failure during the follow‐up, all reported between 3 and 12 months after conversion to LCP‐Tac. One was an unrelated death (hemorrhagic stroke), and four were cases of graft failure (two due to chronic fibrosis and tubulointerstitial atrophy, one due to chronic rejection, and one due to de novo glomerulopathy; in all cases with a poor eGFR of ≤20 mL/min/1.73 m2 pre‐conversion). There were no cases of acute rejection during the follow‐up. Additionally, there were two cases of treatment discontinuation during the 3 months after conversion due to lack of adherence.
3.3. Conversion to MeltDose® extended‐release Tac (LCP‐Tac)
The minimal concentration levels in blood (C min) and total daily dose (TDD) of Tac in the three months before conversion and at the time of conversion were similar for patients receiving IR‐Tac and PR‐Tac, suggesting that the tacrolimus treatment was stable. The evolution of the C min and TDD of Tac before, during, and after the conversion of patients from IR‐Tac or PR‐Tac to LCP‐Tac is shown in Figure 2.
Figure 2.
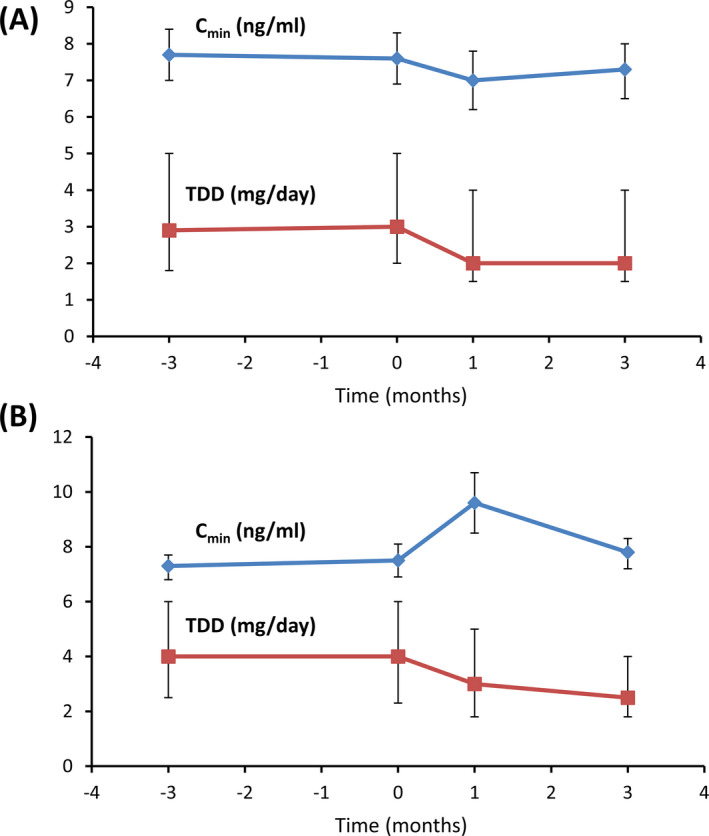
Evolution of C min and TDD in the conversion from IR‐Tac to LCP‐Tac (A) and from PR‐Tac to LCP‐Tac (B). The plots show values at 3 months pre‐conversion (t = −3), at conversion (T = 0), in early post‐conversion (t = 1), and at 3 months post‐conversion (t = 3). C min (blue lines) is shown as mean ± CI95, and TDD (red lines) is shown as median ± P25‐P75
For the patients treated with IR‐Tac, the C min [mean (CI95)] in the 3 months before conversion was 7.7 (7.0‐8.4) ng/mL and 3 months after conversion remained unchanged at 7.3 (6.6‐8.1) ng/mL. Before conversion, the median TDD [median (IQR)] was 2.9 (1.8‐5.0) mg/d, and after conversion, the TDD was reduced to 2.0 (1.5‐3.0).
For the patients treated with PR‐Tac, the C min (mean [CI95]) 3 months before conversion was 7.3 (6.8‐7.7) ng/mL. In this group, the C min increased initially but stabilized by the third month after the conversion (P < .05) at 7.8 (7.2‐8.3) ng/mL. Before the conversion, the TDD (median [IQR]) was 4.0 (2.5‐6.0) mg/d and after the conversion was reduced to 3.0 (2.0‐5.0) mg/d. However, 3 months post‐conversion the TDD had to be further reduced to 2.5 (1.8‐4.0) mg/d in this group of patients.
Overall, there were no differences 3 months after conversion for the mean C min (7.4 ± 2.5 vs 7.6 ± 2.6 ng/mL; P = .95), but the mean TDD decreased from 4.3 ± 3.3 to 3.1 ± 2.3 mg/d (P < .0001). Conversion ratios to LCP‐Tac were 0.91 from IR‐Tac and 0.70 from PR‐Tac. Adjustments of the tacrolimus dose were recorded; 94 patients (25.8%, 29 patients with IR‐Tac, 64 with PR‐Tac, 1 with other) needed dose adjustment in the 3 months before the conversion and 91 patients (24.9%) after the conversion (P = .740). Of the patients requiring dose adjustment after conversion, 63.3% required one adjustment, 19.4% required two adjustments, and 17.3% required three or more adjustments.
The ratio C min /TDD increased significantly for both conversions, 16% in the case of IR‐Tac to LCP‐Tac and 52% in the case of PR‐Tac to LCP‐Tac (P = .0250 and P < .0001, respectively), confirming the higher LCP‐Tac bioavailability (Figure 3).
Figure 3.
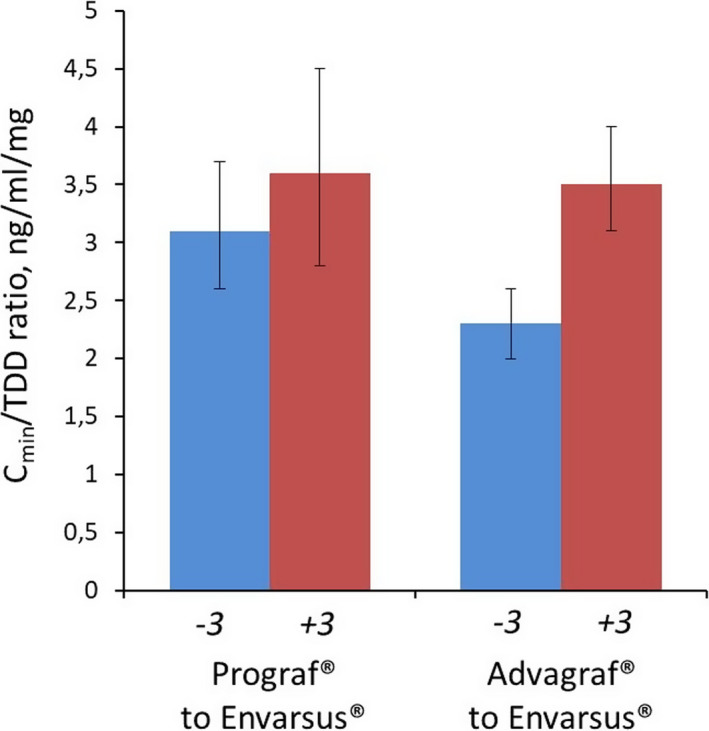
Bioavailability of Tac 3 months before and 3 months after conversion to LCP‐Tac. For IR‐Tac to LCP‐Tac, P = .0250; for PR‐Tac to LCP‐Tac, P < .0001 (Wilcoxon test)
For 221 patients (60.5%), data were available for longer than 3 months after the conversion; the median length of follow‐up in these patients was 8.9 months (Figure 1). For those patients, the last available C min (mean ± SD) was 7.0 ± 2.3 ng/mL, and TDD was 2.7 ± 2.0 mg/d.
3.4. Safety
Tacrolimus‐related adverse reactions (ARs) were recorded for all the patients included in the population evaluated for safety (N = 384) for the 3 months before and the 3 months after conversion to LCP‐Tac. As shown in Table 4, a total of 59 ARs in 46 patients were observed in the 3‐month period prior to conversion, of which 4 were serious ARs in two patients. The most common ARs were neurological (61%) and psychiatric (8.6%). Of these ARs, 41 occurred in patients treated with PR‐Tac and 18 in patients treated with IR‐Tac. During the 3 months after conversion, 7 new ARs in six patients related to the treatment with LCP‐Tac were reported, of which 1 was a serious AR.
Table 4.
Adverse reactions (ARs), N (%), N = 384
| Pre‐conversion | Post‐conversion | |
|---|---|---|
| Infections | 2 (3.4) | 2 (28.6) |
| Cardiovascular | 1 (1.7) | |
| Skin and mucosa | 2 (3.4) | |
| Ear | 1 (1.7) | |
| Neurological | 36 (61) | 2 (28.6) |
| Gastrointestinal | 1 (1.7) | 1 (14.3) |
| Overdose | 1 (1.7) | |
| Edema | 1 (1.7) | |
| Psychiatric | 11 (18.6) | 1 (14.3) |
| Renal and urinary tract | 2 (3.4) | |
| Musculoskeletal | 1 (1.7) | |
| Neoplasia | 1 (14.3) | |
| Total AEs | 59 | 7 |
| Prograf® | 16 | |
| Advagraf® | 41 | |
| Not specified | 2 | |
| Total serious AEs | 4 | 1 |
| Prograf® | 1 | |
| Advagraf® | 4 |
Abbreviation: Tac, tacrolimus.
Data on neurotoxicity, including tremor, were extracted from the patient's medical records. In the three months before conversion, 84 (23%) patients presented neurotoxicity, with tremor reported in 76 patients (20.8%). In the three months after the conversion, signs of neurotoxicity were reported by 48 (13.2%) patients, including tremor in 43 (11.8%) patients (P < .0001).
Overall, six cases of treatment discontinuation were recorded in the 12 months of follow‐up, and the reasons were clinical criteria (three cases), anxiety (one case), request by the patient (one case), or unknown (one case). In all cases, the patients were converted to LCP‐Tac.
3.5. Costs
We performed a post hoc analysis of relative costs of tacrolimus therapies. To estimate costs, we used Spanish official prices as of September 2017 (IR‐Tac = 1.2 €/mg; PR‐Tac = 2.07 €/mg; LCP‐Tac = 1.2 €/mg). In Spain, the cost of the new formulation is by law similar to the cost of generic formulations. For patients treated with IR‐Tac (median dose = 3 mg/d), the cost was 1.314 €/year and when converted to LCP‐Tac (median dose = 2 mg/d) the cost was 876 €/year, which generated overall savings of 438 €/year (−33%). For patients treated with PR‐Tac (median dose = 4 mg/d), the cost was 3.022 €/year and when converted to LCP‐Tac (median dose = 2.5 mg/d) the cost was 1.095 €/year, generating overall savings of 1.927 €/year (−63%).
4. DISCUSSION
This study, carried out in conditions of current clinical practice in Spanish hospitals, evaluated the effectiveness and safety of the conversion to LCP‐Tac from other formulations of tacrolimus in stable kidney transplant recipients. The primary end point of the study, renal function, as a determined by eGFR, did not present statistically significant differences in the periods pre‐ and post‐conversion to LCP‐Tac, suggesting that LCP‐Tac is non‐inferior to the other formulations. Also, the conversion did not increase nephrotoxicity, a common adverse effect. We found that generally the TDD of tacrolimus was significantly lower after the conversion, and especially, the conversion from PR‐Tac may require lower doses. Additionally, the mean blood tacrolimus levels were optimal, and no increase in the number of dose adjustments was observed when compared with the pre‐conversion. Adverse reactions that emerged after the conversion were few, and the number of patients reporting signs of neurological toxicity, especially tremor, decreased after the conversion. Finally, we observed a reduction in pharmaceutical costs from the conversion to LCP‐Tac.
The current 1‐year and 5‐year allograft survival rates for kidney transplants in Europe are 90.7% and 77.8%, respectively.19 Although intensive research is being carried out on immunosuppressive treatments, the acute and chronic organ rejection still remains an issue for 10%‐20% of patients. Lack of adherence with immunosuppressive treatment has been associated with poor outcomes of long‐term transplantation.20, 21 In this regard, the use of tacrolimus once‐a‐day formulations such as PR‐Tac or LCP‐Tac instead of twice‐a‐day formulations could significantly improve adherence.22
Tacrolimus is a drug with a narrow therapeutic margin that requires customized adjustments of doses to achieve a correct balance between maximum efficacy and minimal toxicity.1, 23, 24 Due to these characteristics, it is necessary to control blood concentrations of the drug to ensure correct dose adjustment, even though in some patients issues can arise independently from trough levels (eg, fast metabolizers). The use of conventional tacrolimus formulations often leads to a wide inter‐ and intrapatient variability and high fluctuations between the maximum and minimum concentrations. The bioavailability of tacrolimus in its traditional formulation is low and variable (between 17% and 23%).12, 24 It is believed that the low bioavailability of tacrolimus is multifactorial and is related to its poor solubility in water, fast metabolism, the interaction with the P‐glycoprotein transporter, and food intake.25 In this regard, the MeltDose® technology used in LCP‐Tac achieves the following goals, all observed in our study: improved bioavailability (observed through a proxy variable, ratio C min/TDD), convenient regimen (once‐daily administration), and overall lower doses of tacrolimus. By enhancing gradual absorption and avoiding concentration peaks, this technology could help prevent the neurotoxicity associated with tacrolimus regimens.15 A recent comparative study has shown that LCP‐Tac has about 30% greater relative bioavailability, about 30% lower peak‐to‐trough fluctuation, and a consistently lower daily dose compared with PR‐Tac.26 These results are very similar to ours for the change in bioavailability (34%) after the switch from PR‐Tac to LCP‐Tac (Figure 3).
This real‐world study has helped analyze the extent to which the instructions for treatment change are followed in clinical practice and whether there were changes in treatment dose adjustments. It should be noted that only in 43.9% of the patients, the conversion was carried out, as specified in the summary of product characteristics, with a dose reduction of 30% (conversion ratio of 1:0.70), which could explain the transient increment in C min observed in the first month after conversion. We recommend that clinicians carefully follow recommendations for conversion dose ratios when converting to LCP‐Tac.
The MeltDose® formulation of LCP‐Tac could help to reduce peak‐to‐trough fluctuations and high peaks that may be the cause of toxicities. Tremor is one of the most common Tac‐associated adverse effects reported by kidney transplant recipients, severely affecting their quality of life. In this regard, the STRATO phase 3b clinical trial showed that LCP‐Tac was associated with clinically meaningful improvements of hand tremor symptoms after switching from twice‐daily tacrolimus.15 Here, although the recording and evaluation of adverse events was not performed in systematic and standardized manner due to the retrospective nature of the study, we observed that one of the consequences of switching to LCP‐Tac was a strong reduction in reported signs of neurotoxicity. We found a significant decrease in the number of patients reporting tremor and other symptoms such as difficulty in concentration, headache, and insomnia of about 50%. No cases of biopsy‐proven acute rejections were reported in our 365 patients, and there were only five cases of treatment discontinuation.
Finally, although we did not aim to perform a full pharmacoeconomic analysis, in our study we found that the costs of immunosuppressive treatment decreased substantially after the conversion. In Spain, the costs of IR‐Tac and generics are the same of LCP‐Tac, but we observed savings of 438 €/year, a 33% reduction. PR‐Tac is more costly in Spain, and savings after conversion to LCP‐Tac were of 1,927 €/year, a 63% reduction. Reductions in costs after conversion to LCP‐Tac have been observed in other studies in the context of kidney or liver transplantation.27, 28
A major limitation of this study was its retrospective nature, which restricted us to variables that are used in routine clinical practice. It also led to missing data from some patients. Further, it limited the number of observations available for each patient and caused a lack of timepoint standardization. The causes for conversion, mostly related to toxicity, could be biased toward certain groups of patients, and there was limited information on adherence, which could affect the conclusions of bioavailability. For these reasons, it was difficult to reach robust general conclusions on safety and the impact of the conversion on the reduction in neurotoxic reactions or the overall quality of life of the patient. It should also be noted that adverse events were documented by clinicians treating the patients and could not be recorded in a fully systematic or standardized manner. The clinicians determined retrospectively whether a given adverse event was mild, moderate, or serious, and whether it was the result of the study drug. Another limitation is the lack in ethnic diversity in the study population, which was 93.7% Caucasian. This fact could limit the generalizability of the results presented here, as some studies have shown that ethnicity could play a relevant role in tacrolimus dosing.29 Finally, the lack of a control group limits the conclusions derived from our study.
The major strength of this study was that it was a large‐scale (N = 365) observational analysis of real clinical practice, which is especially relevant in the field of transplants due to the complexity of the disease and its treatment. The close monitoring of these patients in real clinical practice in Spain allowed for the assessment of a large number of variables. It should also be noted that, although data from all patients were collected 3 months after conversion, for 60.5% of them the median follow‐up was 8.9 months.
In summary, our study suggests that in real clinical practice the results are consistent with the evidence from the clinical trials. (Budde, 2014 #5;Bunnapradist, 2013 #6;Bunnapradist, 2016 #21;Gaber, 2013 #7; Tremblay, 2017 #20) This suggests that MeltDose® extended‐release tacrolimus, due to its unique pharmacokinetic characteristics compared with other tacrolimus formulations, has a better bioavailability, a non‐inferior efficacy, and probably a reduced neurotoxicity profile with a lower total daily dose. It could be potentially advantageous in treating patients keen to develop tacrolimus‐related adverse events in a highly cost‐effective way.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHORS' CONTRIBUTIONS
All authors performed research and collected and analyzed data. Juan Carlos Ruiz wrote the manuscript.
ACKNOWLEDGEMENTS
We gratefully acknowledge the collaboration of investigators and patients from the following hospitals: University Hospital Clínico San Carlos (Madrid, Spain), University Hospital Marqués de Valdecilla (Santander, Spain), University Hospital General (Alicante, Spain), University Hospital del Mar (Barcelona, Spain), University Hospital Clinic (Barcelona, Spain), Hospital Lucus Augusti (Lugo, Spain), Fundación Puigvert (Barcelona, Spain), Hospital Miguel Servet, (Zaragoza, Spain), Hospital da Costa (Burela, Spain), University Hospital Bellvitge (Hospitalet de Llobregat, Spain), Fundación Jiménez Díaz (Madrid, Spain), University Hospital son Espases (Palma de Mallorca, Spain), University Hospital Nuestra Señora de la Candelaria (Santa Cruz de Tenerife, Spain), University Hospital Doce de Octubre (Madrid, Spain), University Hospital La Fe (Valencia, Spain), University Hospital A Coruña (A Coruña, Spain), University Hospital Vall de Hebrón (Barcelona, Spain), University Hospital of Vigo (Vigo, Spain), and Hospital Can Ruti (Badalona, Spain). The authors thank Francisco López de Saro (Trialance SCCL) for medical writing support.
Sánchez Fructuoso A, Ruiz JC, Franco A, et al. Effectiveness and safety of the conversion to MeltDose® extended‐release tacrolimus from other formulations of tacrolimus in stable kidney transplant patients: A retrospective study. Clin Transplant. 2020;34:e13767 10.1111/ctr.13767
Funding information
This study was supported by Chiesi, Spain.
REFERENCES
- 1. Allison AC. Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacology. 2000;47(2‐3):63‐83. [DOI] [PubMed] [Google Scholar]
- 2. Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta‐analysis and meta‐regression of randomised trial data. BMJ. 2005;331(7520):810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim MA, Kohli J, Bloom RD. Immunosuppression for kidney transplantation: where are we now and where are we going? Transplant Rev (Orlando). 2017;31(1):10‐17. [DOI] [PubMed] [Google Scholar]
- 4. Hedayat S, Kershner RP, Su G. Relationship of whole‐blood FK506 concentrations to rejection and toxicity in liver and kidney transplants. J Biopharm Stat. 1996;6(4):411‐424. [DOI] [PubMed] [Google Scholar]
- 5. Kahan BD, Keown P, Levy GA, Johnston A. Therapeutic drug monitoring of immunosuppressant drugs in clinical practice. Clin Ther. 2002;24(3):330–350. discussion 329. [DOI] [PubMed] [Google Scholar]
- 6. Federal Drug Administration . https://www.accessdata.fda.gov/drugsatfda_docs/psg/Tacrolimus_ERcap_204096_RC07-14.pdf. Accessed May 14, 2019.
- 7. Alloway RR, Eckhoff DE, Washburn WK, Teperman LW. Conversion from twice daily tacrolimus capsules to once daily extended‐release tacrolimus (LCP‐Tacro): phase 2 trial of stable liver transplant recipients. Liver Transpl. 2014;20(5):564‐575. [DOI] [PubMed] [Google Scholar]
- 8. Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S. Conversion from twice‐daily tacrolimus capsules to once‐daily extended‐release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation. 2013;96(2):191‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanko‐Resmer J, Boillot O, Wolf P, Thorburn D. Renal function, efficacy and safety postconversion from twice‐ to once‐daily tacrolimus in stable liver recipients: an open‐label multicenter study. Transpl Int. 2012;25(3):283‐293. [DOI] [PubMed] [Google Scholar]
- 10. Baraldo M. Meltdose tacrolimus pharmacokinetics. Transplant Proc. 2016;48(2):420‐423. [DOI] [PubMed] [Google Scholar]
- 11. Grinyo JM, Petruzzelli S. Once‐daily LCP‐Tacro MeltDose tacrolimus for the prophylaxis of organ rejection in kidney and liver transplantations. Expert Rev Clin Immunol. 2014;10(12):1567‐1579. [DOI] [PubMed] [Google Scholar]
- 12. Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady‐state head‐to‐head pharmacokinetic comparison of All FK‐506 (Tacrolimus) formulations (ASTCOFF): an open‐label, prospective, randomized, two‐arm three‐period crossover study. Am J Transplant. 2017;17(2):432‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Budde K, Bunnapradist S, Grinyo JM, et al. Novel once‐daily extended‐release tacrolimus (LCPT) versus twice‐daily tacrolimus in de novo kidney transplants: one‐year results of Phase III, double‐blind, randomized trial. Am J Transplant. 2014;14(12):2796‐2806. [DOI] [PubMed] [Google Scholar]
- 14. Bunnapradist S, Ciechanowski K, West‐Thielke P, et al. Conversion from twice‐daily tacrolimus to once‐daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. Am J Transplant. 2013;13(3):760‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Langone A, Steinberg SM, Gedaly R, et al. Switching STudy of Kidney TRansplant PAtients with Tremor to LCP‐TacrO (STRATO): an open‐label, multicenter, prospective phase 3b study. Clin Transplant. 2015;29(9):796‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bunnapradist S, Rostaing L, Alloway RR, et al. LCPT once‐daily extended‐release tacrolimus tablets versus twice‐daily capsules: a pooled analysis of two phase 3 trials in important de novo and stable kidney transplant recipient subgroups. Transpl Int. 2016;29(5):603‐611. [DOI] [PubMed] [Google Scholar]
- 17. World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang JH, Skeans MA, Israni AK. Current status of kidney transplant outcomes: dying to survive. Adv Chronic Kidney Dis. 2016;23(5):281‐286. [DOI] [PubMed] [Google Scholar]
- 20. Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77(5):769‐776. [DOI] [PubMed] [Google Scholar]
- 21. Dew MA, DiMartini AF, De Vito DA, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858‐873. [DOI] [PubMed] [Google Scholar]
- 22. Kuypers DR, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus once‐daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95(2):333‐340. [DOI] [PubMed] [Google Scholar]
- 23. Garnock‐Jones KP. Tacrolimus prolonged release (Envarsus(R)): a review of its use in kidney and liver transplant recipients. Drugs. 2015;75(3):309‐320. [DOI] [PubMed] [Google Scholar]
- 24. Staatz CE, Tett SE. Clinical pharmacokinetics of once‐daily tacrolimus in solid‐organ transplant patients. Clin Pharmacokinet. 2015;54(10):993‐1025. [DOI] [PubMed] [Google Scholar]
- 25. Provenzani A, Santeusanio A, Mathis E, et al. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J Gastroenterol. 2013;19(48):9156‐9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kamar N, Cassuto E, Piotti G, et al. Pharmacokinetics of prolonged‐release once‐daily formulations of tacrolimus in de novo kidney transplant recipients: a randomized, parallel‐group, open‐label, multicenter study. Adv Ther. 2019;36(2):462‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altieri M, Delaval G, Kimmoun E, Allaire M, Salame E, Dumortier J. Conversion from once‐daily prolonged‐release tacrolimus to once‐daily extended‐release tacrolimus in stable liver transplant recipients. Exp Clin Transplant. 2018;16:321‐325. [DOI] [PubMed] [Google Scholar]
- 28. Glander P, Waiser J, Kasbohm S, et al. Bioavailability and costs of once‐daily and twice‐daily tacrolimus formulations in de novo kidney transplantation. Clin Transplant. 2018;32(8):e13311. [DOI] [PubMed] [Google Scholar]
- 29. Glick L, Shamy F, Nash M, et al. A prospective cohort conversion study of twice‐daily to once‐daily extended‐release tacrolimus: role of ethnicity. Transplant Res. 2014;3(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]


