Abstract
Introduction:
The neuropeptide calcitonin gene-related peptide (CGRP) is recognized as a critical player in migraine pathophysiology. Excitement has grown regarding CGRP because of the development and clinical testing of drugs targeting CGRP or its receptor. While these drugs alleviate migraine symptoms in half of patients, the remaining unresponsive half of this population creates an impetus to address unanswered questions that exist in this field.
Areas covered:
We describe the role of CGRP in migraine pathophysiology and CGRP-targeted therapeutics currently under development and in use. We also discuss how a second CGRP receptor may provide a new therapeutic target.
Expert opinion:
CGRP targeting drugs have shown a remarkable safety profile. We speculate that this may reflect the redundancy of peptides within the CGRP family and a second CGRP receptor that may compensate for reduced CGRP activity. Furthermore, we propose that an inherent safety feature of peptide-blocking antibodies is attributed to the fundamental nature of peptide release, which occurs as a large bolus in short bursts of volume transmission. These facts support the development of more refined CGRP therapeutic drugs, as well as drugs that target other neuropeptides. We believe that the future of migraine research is bright with exciting advances on the horizon.
Keywords: migraine, CGRP, gepants, monoclonal antibodies, trigeminovascular system
1. Introduction
Migraine is a complex neurovascular disorder. The International Headache Society defines migraine as a headache lasting 4–72 hours that presents with multiple sensory abnormalities such as pain and photophobia (Table 1) [1–4]. Affecting 15% of all people, migraine is more common in women relative to men and is the second most disabling disease in the world according to the World Health Organization [5]. Globally, over 1 billion people are thought to suffer from migraine [5, 6]. As a result, headache is one of the top 5 reasons people visit the emergency room [6].
Table 1:
Diagnostic criteria for migraine from the International Classification of Headache Disorders, 3rd edition (beta version) 4.
| A. At least 5 attacks fulfilling criteria B-D; |
| B. Headache attacks lasting 4–72 hours (untreated or unsuccessfully treated); |
| C. Headache has at least two of the following four characteristics: |
| a. unilateral location, |
| b. pulsating quality, |
| c. moderate or severe pain intensity, |
| d. aggravation by or causing avoidance of routine physical activity (e.g. walking or climbing stairs) |
| D. During headache at least one of the following: |
| a. Nausea and/or vomiting |
| b. Photophobia (light-induced discomfort) and phonophobia (sound-induced discomfort) |
Over the past 30 years, both clinical and preclinical studies have documented the role of the neuropeptide calcitonin gene-related peptide (CGRP), the most potent vasodilatory peptide known, in migraine pathophysiology [7]. While there are two forms of CGRP, αCGRP and βCGRP, they have similar activity, so we will simply use the term CGRP [7]. CGRP is widely expressed through both the peripheral and central nervous system (CNS) [8]. In the trigeminovascular system, CGRP is released from nerve fibers running along meningeal and cerebral arteries [9] and blood vessels [10]. CGRP can also be released in the trigeminal ganglion where about half the neurons have CGRP immunoreactivity [11]. In the CNS, CGRP-containing neurons can be found in the superficial layers of the spinal trigeminal nucleus, the locus coeruleus, the raphe nuclei, some nuclei of the thalamus and the cerebellum, to name a few [11–14]. It is important to note that CGRP does not apparently cross the blood brain barrier (BBB) [8].
Clinical studies provided evidence that, at least in some patients, CGRP was both necessary and sufficient to induce migraine. CGRP levels are elevated in the plasma [15, 16], saliva [17] and tear fluid [18] of patients during spontaneous migraine attacks and in the plasma during nitroglycerin-evoked migraine attacks [19]. However, not all studies demonstrated elevated plasma levels during attacks [20]. Interictal elevation of CGRP has also been reported in the tears of episodic and chronic migraine patients [18], and in the plasma [21] and cerebrospinal fluid [22] of chronic migraine patients. Not only are CGRP levels elevated during and between migraine but, when CGRP was infused into migraine patients, most developed a delayed migraine-like headache, whereas patients who do not get migraine only got a mild headache [23–26]. Sumatriptan can reverse some of these CGRP induced phenotypes. For example, elevated CGRP levels are normalized after sumatriptan administration in migraine patients [15], and following CGRP infusion, the resulting increased vasodilation and delayed headaches were treated with sumatriptan. This suggests that at least some of the anti-migraine effects of sumatriptan are through a CGRP-dependent pathway [27, 28]. Other agents can induce migraine-like headaches including pituitary adenylate cyclase-activating polypeptide (PACAP) [29] and nitric oxide donors such as nitroglycerin [19] or sodium nitroprusside [30]. Interestingly these substances all have strong vasodilatory effects. However, not all vasodilators can induce migraine headache. Specifically, vasoactive intestinal peptide (VIP) induces vasodilation but does not induce migraine, suggesting that the vasodilation induced by CGRP is by itself not solely responsible for migraine [31]. However, as discussed later, this could be due to the rapid break down of VIP relative to CGRP and PACAP [32].
The need for this review is driven by the recent approvals of CGRP-targeted drugs that has occurred with relatively little understanding of how the drugs are working or why they have been safe so far. Thus, it is important to stay current in findings on how CGRP is involved in migraine pathophysiology and potential adverse effects of those drugs. In this review, we will briefly cover the current theories of where and how CGRP may be acting in migraine, discuss CGRP receptors, and update the growing list of current CGRP targeted therapeutics. We will finish with our opinion on why these CGRP antagonist drugs appear safe so far and where the field goes from here.
2. CGRP and migraine
2.1. Where is CGRP acting in migraine?
CGRP has been shown to act both in the peripheral and CNS in preclinical studies and both sites are likely important in migraine pathophysiology (Figure 1). In the periphery, CGRP targets mast cells, blood vessels, glial cells, trigeminal afferents in the meninges, and neural cell bodies and satellite glia in the trigeminal ganglia (for review see Messlinger and Russo 2018) [33]. In the meninges, CGRP likely contributes to neurogenic inflammation by triggering the release of neuron sensitizing agents from mast cells, which in turn leads to increased vasodilation in the dura [34]. The modulation of neural activity in the meninges could trigger a feedback loop that ultimately results in peripheral sensitization of nociceptors [33]. The efficacy of systemically administered CGRP targeting monoclonal antibodies strongly supports a peripheral role of CGRP in migraine since the antibodies have very poor BBB permeability [35]. Overall, peripheral sensitization is critical for CGRP’s actions and likely sets the stage for CGRP actions in the CNS.
Figure 1.
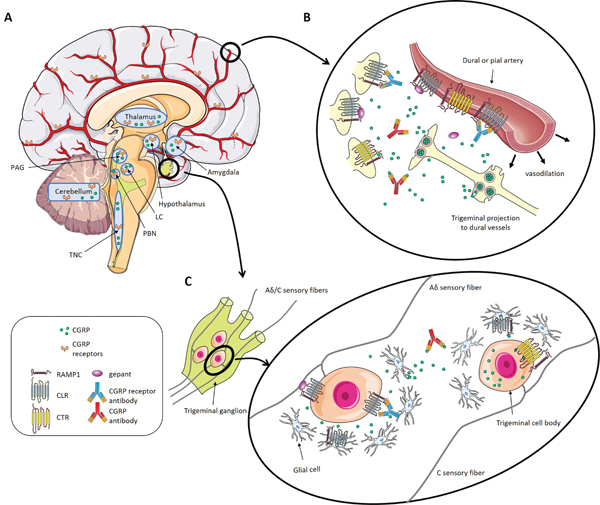
CGRP and CGRP receptor distribution in the peripheral and central nervous systems. A) In the brain, CGRP and its receptors are present in the thalamus, the amygdala, periaqueductal grey, locus coeruleus, trigeminal nucleus caudalis, parabrachial nucleus, hypothalamus, and the cerebellum. The receptors can also be found at the meningeal vasculature. B) CGRP is released from trigeminal axons onto blood vessels in the meninges where it is involved in vasodilation and activation of trigeminal neurons. CGRP targeting drugs likely act at this site to treat migraine. C) CGRP is released in the trigeminal ganglion by small C fibers and binds to its receptors on other neurons (Aδ fibers) and glial cells triggering neurogenic inflammation. CGRP targeting drugs likely act at this site to treat migraine since the trigeminal ganglion is located outside of the BBB. While the AMY1 receptor can also be found in the trigeminal ganglion, the CGRP receptor and the AMY1 receptor do not seem to colocalize on the same neuron at this site [87]. CGRP and the CGRP receptor are rarely co-localized on the same neuron. At this point, it is still unknown if AMY1 can be expressed on CGRP containing cells and our schematic representing both on the C fiber is still speculation. It is also unknown if the glial cells only express the CGRP receptor or also express AMY1. The schematic art used in this figure were provided by Servier Medical art (http://servier.com/Powerpoint-image-bank).
Within the CNS, CGRP and its receptor are present in multiple pathways believed to play a role in migraine pathophysiology [8]. The trigeminal ganglion (located outside of the BBB) projects to the trigeminal nucleus caudalis (TNC) where second order neurons carry the signals to the posterior thalamic area (PTA). We use PTA as a term to encompass all nuclei in the posterior thalamic area. The PTA appears to be a sensory integration center that is abnormal during migraine. Neurons in the thalamus receive input from the TNC and retinal ganglion cells [36], and several key studies have demonstrated in rodents the importance of the PTA in the development of photophobia and highlight the PTA as a possible center for the integration of light and pain [36–38]. CGRP likely contributes to this pathway as both CGRP and its receptors are present in discrete nuclei of the PTA. This is further supported by a study showing that injection of CGRP into the PTA facilitates neuronal firing [38]. Moreover, somatosensory and nociceptive stimuli from ascending pathways converge on the CGRP producing neurons of the subparafasicular and intralaminar nuclei [39]. In humans, the posterior thalamus is known to be activated during migraine attacks and has altered functional connectivity with multiple brain regions [40, 41]. Taken together, these data suggest that pre- and post-synaptic neuromodulatory actions of CGRP that have been reported in other neural circuits [42], could contribute to a state where the PTA is hypersensitive to sensory stimuli.
Pathways contributing to this sensory sensitization are likely to involve the “general alarm” system of the parabrachial nucleus (PBN) [43, 44]. The PBN is a relay station for pain and other sensory input as it travels to the forebrain [43]. The PBN receives direct projections from the trigeminal nucleus, leading to the affective component of pain [45]. CGRP is abundant in the PBN and the PBN projects to multiple brain areas that are thought to be involved in migraine pathophysiology [43]. It is possible that altered signaling in this pathway could underly some of the hypersensitivity experienced during migraine. Peripheral and central actions of CGRP probably work in concert to induce migraine, and the complex nature of the disorder makes it improbable that just one of CGRP’s action is solely responsible for inducing migraine.
2.2. How are the migraine actions of CGRP assessed?
Most of the mechanistic evidence concerning the involvement of CGRP in migraine comes from preclinical studies. CGRP is one of the most well-defined migraine triggers and can induce multiple migraine-like symptoms in animals that mimic CGRP in infusion in humans, including pain-like symptoms. Mechanical hypersensitivity is a common symptom of migraine [46] and can be reproduced in rodents after CGRP administration [47]. Dural administration of CGRP in mice induced periorbital touch hypersensitivity [48]. Intrathecal injection of CGRP induced hyperalgesia in the hindpaw in response to pinch in rats [49] and increased mechanical allodynia in mice [50]. While evoked pain phenotypes are well described preclinically, for many years there was no assay to measure spontaneous pain in animals. In 2010 Mogil and colleagues demonstrated that some types of pain could be assessed through facial grimace without having to evoke a response [51]. We used that assay to demonstrate that peripheral CGRP injection in mice could produce spontaneous pain that could be partially blocked with sumatriptan, suggesting the pain was migraine-like [52]. The eye-opening action unit proved to carry most of the weight for the grimace assay, and we were able to use a continuous objective measurement of eye closure to measure CGRP-induced grimace [52]. With this set of experiments, we also demonstrated that CGRP induced pain was light independent, which suggests that pain and light-aversion (another CGRP induced phenotype) are independent of each other.
Photophobia is one of the diagnostic criteria of migraine (Table 1). Photophobia occurs when ordinarily non-painful light becomes uncomfortable or painful. Patients with migraine are bothered by even dim light and want to escape bright environments [53]. This idea of using increased light sensitivity has translated well into a mouse model. Light aversion can be induced by both central and peripheral CGRP injection in wild-type mice, and it can be treated by triptans suggesting that light-aversive behavior in mice is migraine-like. This behavior requires a bright light stimulus (~25,000 lux) in wild-type mice. In a CGRP-sensitized mouse model that overexpresses human RAMP1 (the rate limiting component of the CGRP receptor) in the nervous system, only a low light stimulus of 55 lux is needed to induce light aversion after central administration of CGRP [54]. However, peripheral administration of CGRP in those mice didn’t induce any light aversion with dim light, suggesting that neural CGRP receptors are not the rate-limiting site of action outside the CNS. Of importance, this light aversion behavior is not due to anxiety as mice show no difference in a light independent anxiety test (open field assay) [54–57]. One interesting feature of the light aversion assay is that mice do not move as much when injected with CGRP, but this is only seen in the dark side of the box [54–57]. This suggests that the mice are not sedated or that their motor activity is not impaired, since they move just as much as vehicle-injected animals in the light. The desire to go into a dark area and rest is consistent with human behavior.
2.3. CGRP-based treatments
In the past years, different molecules have been developed to block CGRP signaling in order to treat migraine symptoms. The first molecules to show potential were the CGRP receptor antagonists called “gepants”. These molecules have a high affinity for the canonical CGRP receptor and prevent CGRP binding and signal transduction. Gepants do not cause direct vasoconstriction [58–61], which is an advantage compared to triptans since the migraine population is known to have an increased prevalence of cardiovascular diseases [62]. Multiple clinical trials showed that intravenous and oral gepants alleviated migraine symptoms acutely (Table 2). In contrast, the prophylactic efficacy of gepants are still debatable as some clinical trials had to be interrupted because of adverse effects, and others are still ongoing (for review see Negro and Martelletti, 2019 [63] and Table 2). The development of several gepants was stopped for different reasons: olcegepant has a low oral bioavailability and telcagepant and MK-3207 were discontinued because of liver toxicity after frequent use [63].
Table 2:
CGRP targeting drugs: completed and ongoing trials.
| Drug name, type of molecule | Indication (acute or prophylactic) | Development stage | References for clinical trials (type of migraine, number of treated subjects) |
|---|---|---|---|
| Olcegepant (BIBN 4096 BS), CGRP antagonist |
Acute | Terminated for lack of oral availability | 112 (episodic, 126 subjects) |
| Telcagepant (MK-0974), CGRP antagonist |
Acute and prophylactic | Terminated for liver toxicity | 113 (episodic, 1068 subjects) 114 (episodic, 1294 subjects) 115 (episodic, 683 subjects) 116 (episodic, 660 subjects) 117 (episodic, 1380 subjects) 118 (episodic perimenstrual, 3960 subjects) 119 (episodic, 330 subjects) 120 (episodic, 1677 subjects) |
| MK-3207, CGRP antagonist |
Acute | Terminated for liver toxicity | 121 (episodic, 547 subjects) |
| BI 44370 TA, CGRP antagonist |
Acute | Terminated | 122 (episodic, 341 subjects) |
| Rimegepant (BMS-927711), CGRP antagonist |
Acute | Ongoing | 64 (episodic, 799 subjects) 65 (episodic, not available at this time) 66 (episodic, not available at this time) 67 (episodic, 1375 subjects) 123 (episodic, 1186 subjects) 124 (episodic, not available at this time) |
| Ubrogepant (MK-1602), CGRP antagonist |
Acute | FDA-approved | 60 (healthy, 518 subjects) 68 (episodic, 640 subjects) 69 (episodic, 1672 subjects) 70 (episodic, 1254 subjects) 125 (episodic, 1672 subject) 126 (episodic, 1465 subjects) |
| Atogepant (AGN-241689), CGRP antagonist |
Prophylactic | Ongoing | 72 (episodic, 825 subjects) 127 (episodic, not available at this time) 128 (episodic, not available at this time) 129 (chronic, not available at this time) 130 (episodic, not available at this time) |
| Fremanezumab (TEV48125), CGRP monoclonal antibody |
Prophylactic | FDA-approved | 74 (high frequency episodic, 296 subjects) 131 (chronic, 264 subjects) 132 (healthy, 139 subjects) 133 (episodic and chronic, 133 subjects) 134 (healthy, 64 subjects) 135 (episodic, 874 subjects) 136 (high frequency episodic and chronic, 297 subjects) 137 (chronic, 1130 subjects) |
| Galcanezumab (LY2951742), CGRP monoclonal antibody |
Prophylactic | FDA-approved | 76 (episodic, 217 subjects) 138 (healthy, 63 subjects) 139 (episodic, 410 subjects) 140 (episodic, 915 subjects) 141 (episodic, 410 subjects) 142 (episodic, 862 subjects) |
| Eptinezumab (ALD403), CGRP monoclonal antibody |
Prophylactic | Ongoing | 75 (frequent episodic, 174 subjects) 143 (chronic, 588 subjects) |
| Erenumab (AMG 334), CGRP receptor monoclonal antibody |
Prophylactic | FDA-approved | 77 (episodic, 483 subjects) 144 (episodic, 383 subjects) 145 (chronic, 667 subjects) 146 (healthy and episodic, 108 subjects) 147 (episodic, 577 subjects) 148 (episodic, 965 subjects) 149 (chronic, 667 subjects) |
Despite those initial safety concerns, the efficacy of gepants led to further efforts to develop safe CGRP blocking molecules. Three gepants remain in clinical development: rimegepant, ubrogepant and atogepant. In phase 2b clinical trials, efficacy of rimegepant to treat acute migraine was assessed with different endpoints such as pain free, migraine free, photophobia and phonophobia free, and nausea remission [64]. Intermediate doses of rimegepant (75, 150 and 300 mg) were found to be significantly more efficient than placebo. In addition, rimegepant did not show any effect on liver function, suggesting that it is safer than previously terminated gepants. Intriguingly, a higher dose of rimegepant (600 mg) did not significantly differ from placebo [64], and authors hypothesized that it was due to inherent variability present in the patients randomized to this dose group. Following this study, three phase 3, double-blind, randomized, placebo-controlled studies (NCT03235479, NCT03237845, NCT03461757), and a safety study (NCT03266588) [65–67] were started, for which the results are still awaited. Similarly, ubrogepant demonstrated a positive dose-response outcome in the treatment of acute migraine in a phase 2b, double-blind, randomized, placebo-controlled study [68], with minimum adverse effects. However, results from this study are dampened by the high response rate of the placebo group, and the limited number of patients included. Two phase 3, double-blind, randomized, placebo-controlled studies (NCT02867709, NCT02828020) [69, 70] were completed in December 2017 and February 2018. Preliminary results confirm the findings of the phase 2b study. Atogepant has a different chemical structure than other gepants and is currently tested for migraine prophylaxis [71]. The very first results from a phase 2b/3 study (NCT02848326 [72]) show that adults treated with atogepant had greater reduction from baseline in monthly migraine days on average, compared with those treated with placebo [73]. No serious adverse events related to treatment occurred. As we are writing this review, the first gepant (ubrogepant) was approved for acute treatment of migraine with and without aura by the FDA.
Monoclonal antibodies against CGRP (fremanezumab galcanezumab, eptinezumab) and against CGRP receptor (erenumab) are another class of molecules able to block CGRP signaling. Three of these antibodies (fremanezumab, galcanezumab, and erenumab) have recently been approved by the FDA for the prophylactic treatment of migraine and a decision on a fourth (eptinezumab) is expected in 2020. About half of the patients receiving these antibodies experience a 50% reduction in migraine days (Table 2) [74–78]. Interestingly there has been no difference in efficacy between the antibodies, whether they sequester CGRP or bind to the receptor. Moreover, the antibodies still show efficacy for over a month after administration and therefore can be used as prophylactic drugs administered monthly or even quarterly to patients, which presents an advantage compared to daily oral administration for the gepants.
It appears that the newly designed CGRP-blocking drugs are quite safe so far. These safety concerns are serious since the vasodilatory action of CGRP could be critical during certain pathological states like stroke [79, 80]. From the many clinical trials and being on the market for almost a year, it appears that CGRP and CGRP receptor antibodies are safe and well tolerated. What is not known are the long-term effects of CGRP blockade, although it is encouraging that Amgen/Novartis found their antibody to be safe so far at the three-plus year point from an ongoing five-year open label study [81]. In addition, a study with patients with angina found no detrimental effect of the antibody [82]. This study however presents several limitations such as (1) the inclusion of 78% of male subjects in a predominantly female disorder, (2) the inclusion of patients suffering from stable angina pectoris, when patients suffering from a microvascular disease would better represent the population at risk, and (3) assessing the effects of the drug at an early time-point at which it is unsure if the receptor antibody has had time to affix the receptor [79]. Cardiovascular concerns are paramount since migraine patients are known to be at a high risk of stroke and cardiovascular disease (for review see Sacco and Kurth, 2014 [62]). Could blockade of CGRP increase the severity of stroke? In one recent case report, one patient developed an ischemic event following treatment with a CGRP antagonist drug [83]. However, no conclusion should be made from a single patient report. Long term studies on cardiovascular health are warranted, beginning with animal studies investigating the role of CGRP blockade on ischemia.
2.4. CGRP receptors, signaling, and regulation
CGRP belongs to a family of structurally related peptides among which two other major members are amylin and adrenomedullin. Amylin and CGRP have a close molecular composition which explains their overlapping activity at different receptors [84]. Structure, activity and trafficking of those peptides at their receptors have been extensively reviewed by the Hay and Walker labs [84–87]. Briefly, all receptors for this family are composed of a G-protein coupled receptor (calcitonin receptor-like receptor (CLR) or calcitonin receptor (CTR)) associated to an accessory protein (receptor activity-modifying protein (RAMP) 1, 2, or 3). A recent finding is that CGRP can bind and activate both CLR/RAMP1 (canonical CGRP receptor) and CTR/RAMP1 (amylin receptor 1 (AMY1)) equally. As a result of the late discovery of CGRP binding AMY1, the majority of migraine research has focused on the canonical CGRP receptor. This problem was exacerbated by the lack of properly validated antibodies for the detection of the different components involved in these pathways. It is therefore a common assumption that CGRP-blocking drugs only block signaling through the canonical CGRP receptor. Even antagonists designed to selectively bind to CLR/RAMP1 are at best “relatively selective”, and this selectivity depends on which agonist is used, and some molecules have not yet been properly investigated [86].
The signaling of CGRP receptors at the plasma membrane generally involves cAMP pathways, but MAP kinase and calcium pathways can also be recruited [88]. However, signaling does not end at the cell surface. The complex CLR/RAMP1-CGRP is internalized into endosomes in response to CGRP binding [89, 90]. A recent study demonstrated that the CGRP receptor can signal from endosomes and that blocking this signaling can have antinociceptive effects [91]. It is unknown whether this signaling is involved in migraine pathogenesis. While the complex CTR/RAMP1-CGRP is also internalized, this phenomenon does not always depend on activation by an agonist [92] and the possible endosomal signaling for this receptor has not yet been investigated.
2.5. Conclusion
Even after the approval of the first molecules targeting CGRP or its receptor, research is still ongoing to elucidate where and how CGRP is involved in migraine pathophysiology. Deepening our knowledge of CGRP signaling in both physiological and pathophysiological conditions is crucial to optimize current and future treatments for migraine.
3. Expert opinion
To date, the key findings in the field are that CGRP is necessary and sufficient to induce migraine in many patients and that drugs targeting CGRP or its receptor are effective in migraine treatment. In this section, we will speculate first on the safety issue of the monoclonal antibodies and then on where the field might now be headed.
How is it possible for a drug that blocks such a potent cardioprotective molecule to be safe? We think that the answer to this safety question is that there likely only a partial, not a full, blockade of CGRP function. The CGRP receptor antibody was designed to target the canonical CGRP receptor. However, as described above, CGRP can also bind a second receptor, AMY1, with equal affinity and based on pharmacological studies the CGRP receptor antibody is predicted to only bind the canonical receptor and not AMY1 [93]. Likewise, the most studied small molecule receptor antagonists, olcegepant and telcagepant, preferentially bind the canonical CGRP receptor over AMY1 [94]. Thus, AMY1 might provide a “safety valve” to maintain some CGRP activity, for example on the vasculature, even in the presence of the receptor antibody or antagonists. Moreover, if the CGRP receptor antibody had equal binding to the AMY1 and CGRP receptors, there might be greater efficacy from targeting both receptors. However, the additional blockade of these receptors might also contribute to a decreased safety profile.
What about the apparently equally safe ligand antibodies? For the ligand antibodies, one reason may be due to the basic biology of peptide release and action by volume transmission [95]. When a neuron releases CGRP, it is released in a large bolus with an estimated 10,000 peptides from a single dense core vesicle. With an estimated release of thousands of vesicles from a single neuron, there could be millions of peptides released in single burst. Hence, it seems likely that antibodies could blunt the spread or volume of CGRP from the release sites, but not completely block it.
For the receptor-targeted and ligand-targeted drugs, an additional safety valve could be redundancy within the CGRP family. Adrenomedullin also has vasodilatory activity [96]. Perhaps adrenomedullin, as well as other family members, including amylin, step up to help compensate for the reduced levels of CGRP signaling. Therefore, it is possible that the reassuring safety profile seen to date from CGRP receptor and ligand targeting drugs may be due to those drugs only partially blocking the actions of CGRP coupled with compensation from other CGRP family members.
There is plenty of need for additional migraine therapeutics since only about 50% of patients positively respond to CGRP blocking molecules [97], and on average, the patients who respond have only a two-fold reduction in headache days. Given that 57–77% of migraine patients get a migraine-like headache upon CGRP infusion [23–26, 98], it seems likely that there is still a large pool of patients who could benefit from improved CGRP therapeutics. Despite making great strides in our understanding of how CGRP contributes to migraine pathophysiology, we still do not fully understand where CGRP is being blocked to reduce migraine symptoms. The evidence to date is on the side of the peripheral action, since neither the small molecule antagonists or antibodies easily cross the blood brain barrier [76, 99–103]. In addition, some of the triptans (which can lower CGRP levels during a migraine episode) do not easily cross the BBB and the amount that does is not likely to be sufficient to induce a therapeutic effect [104, 105]. While there is a debate regarding a possible BBB break down during migraine attacks, recent evidence suggests this does not occur [104]. One interesting study done with a PET tracer (MK-4232, which is a validated ligand for the CGRP receptor) demonstrated that telcagepant could block less than 10% of the CNS receptor binding at therapeutic doses [106]. Besides the peripheral sites of action, is the small amount of drug penetrating the CNS enough to induce an effect? Some patients seem to respond to a single dose of antibody for a longer period than others. The possibility that those patients have a greater central penetration of the drug should be studied. If this was the case, then it would warrant the design and testing of molecules blocking CGRP signaling in the CNS. Admittedly, centrally-acting CGRP drugs might have greater side-effects, but they also might be more effective and may be helpful for other pain states involving central sensitization.
In addition to targeting central CGRP actions, a second and not mutually exclusive direction to take is to investigate the role of the AMY1 receptor in migraine? It is possible that blocking AMY1 may be more effective than blocking the canonical receptor for some patients. AMY1 is expressed in the trigeminovascular system and CNS, but there is a dearth of knowledge surrounding its role in migraine pathophysiology. Likewise, if the AMY1 receptor is involved in migraine, this raises the question whether the peptide amylin can also induce migraine symptoms similar to CGRP. Preclinical and clinical studies on a possible role of amylin are currently being pursued.
In addition to CGRP, there is an abundance of other neuropeptides that could possibly play a similar role in migraine [107]. In particular, PACAP and its receptors represent promising targets as PACAP displays many of the same properties as CGRP. PACAP injection into humans can induce migraine-like headaches [108, 109]. PACAP antibodies are already in the pipeline and seem to have a promising future. However, the development of a PACAP receptor (PAC1) antibody was stopped because it was not efficacious for the treatment of migraine. While this is disappointing, it is possible that other receptors play a role in PACAP signaling. For instance, PACAP can bind and signal through the VIP/PACAP receptors. If PACAP signaling through those receptors contributes to migraine, it would suggest that the short acting nature of VIP is a likely explanation as to why VIP fails to induce migraine-like symptoms after infusion, and highlights the possibility that prolonged stimulation of receptors could contribute to migraine pathophysiology. Beyond PACAP, there are hundreds of neuropeptides that could act as neuromodulators [95]. Furthermore, pro-inflammatory cytokines such as interleukin-1β, interleukin-6, and tumor necrosis factor-α have also been shown to be elevated in the serum of migraine patients [110, 111]. Delineating the role of these other peptides and pro-inflammatory cytokines and how their altered activities contribute to heightened sensory states will likely be a fruitful endeavor, undoubtingly leading to a more thorough understanding of migraine pathogenesis, and hopefully to new efficacious drugs.
In conclusion, the success of CGRP-targeting drugs as migraine therapeutics is an exciting story, especially for the patients. Yet, this represents not the end, but rather the beginning of the story. CGRP has set the precedent for looking deeper at how it acts and at other neuropeptides that help modulate bidirectional information flow between the senses of the body and the brain.
Article highlights.
Preclinical and clinical studies have shown that calcitonin gene-related peptide (CGRP) is a critical neuropeptide in migraine pathophysiology, but the mechanisms by which it is involved remain to be elucidated.
Targeting CGRP or its receptor with antibodies or small molecules is very effective at alleviating migraine symptoms in about half of the patients.
CGRP antagonizing drugs appear to be safe so far, however long-term open label studies are needed to confirm this safety profile.
Better knowledge of the sites of CGRP action and signaling pathways is likely to lead to improved treatments for patients.
A second CGRP receptor known as the amylin 1 receptor could be a novel target for migraine treatment.
Targeting other neuropeptides, such as PACAP and amylin that have similar functions as CGRP, represents an additional avenue for alternative and/or complementary therapeutics.
Acknowledgments
Funding
This paper was not funded.
Abbreviations
- CGRP
calcitonin gene-related peptide
- FDA
Food and Drug Administration
- CNS
central nervous system
- BBB
blood brain barrier
- PACAP
pituitary adenylate cyclase-activating polypeptide
- TNC
trigeminal nucleus caudalis
- PTA
posterior thalamic area
- PBN
parabrachial nucleus
- CLR
calcitonin receptor-like receptor
- CTR
calcitonin receptor
- AMY1
amylin receptor 1
- RAMP
receptor activity-modifying protein
Footnotes
Declaration of interest
AS Wattiez and LP Sowers are consultants for Pieris Pharmaceuticals. AF Russo serves as a consultant for Alder Biopharmaceuticals, Amgen, Novartis, Lilly, Pharmnovo, Schedule One Therapeutics and receives grant support from Alder Biopharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Charles A. The evolution of a migraine attack - a review of recent evidence. Headache 2013. February;53(2):413–9. [DOI] [PubMed] [Google Scholar]
- 2.de Tommaso M, Ambrosini A, Brighina F, Coppola G, Perrotta A, Pierelli F, et al. Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol 2014. March;10(3):144–55. [DOI] [PubMed] [Google Scholar]
- 3.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev 2017. April;97(2):553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Headache Classification Committee of the International Headache S. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013. July;33(9):629–808. [DOI] [PubMed] [Google Scholar]
- 5.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017. September 16;390(10100):1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burch R, Rizzoli P, Loder E. The Prevalence and Impact of Migraine and Severe Headache in the United States: Figures and Trends From Government Health Studies. Headache 2018. April;58(4):496–505. [DOI] [PubMed] [Google Scholar]
- 7.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 2014. October;94(4):1099–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edvinsson L, Warfvinge K. Recognizing the role of CGRP and CGRP receptors in migraine and its treatment. Cephalalgia 2019. March;39(3):366–73. [DOI] [PubMed] [Google Scholar]
- 9.Edvinsson L, Ekman R, Jansen I, McCulloch J, Uddman R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blood Flow Metab 1987. December;7(6):720–8. [DOI] [PubMed] [Google Scholar]
- 10.Messlinger K, Hanesch U, Baumgartel M, Trost B, Schmidt RF. Innervation of the dura mater encephali of cat and rat: ultrastructure and calcitonin gene-related peptide-like and substance P-like immunoreactivity. Anat Embryol (Berl) 1993. September;188(3):219–37. [DOI] [PubMed] [Google Scholar]
- 11.Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 2010. August 25;169(2):683–96. [DOI] [PubMed] [Google Scholar]
- 12.Edvinsson L, Eftekhari S, Salvatore CA, Warfvinge K. Cerebellar distribution of calcitonin gene-related peptide (CGRP) and its receptor components calcitonin receptor-like receptor (CLR) and receptor activity modifying protein 1 (RAMP1) in rat. Mol Cell Neurosci 2011. January;46(1):333–9. [DOI] [PubMed] [Google Scholar]
- 13.Eftekhari S, Edvinsson L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci 2011. November 10;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messlinger K. The big CGRP flood - sources, sinks and signalling sites in the trigeminovascular system. J Headache Pain 2018. March 12;19(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993. January;33(1):48–56. [DOI] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990. August;28(2):183–7. [DOI] [PubMed] [Google Scholar]
- 17.Cady RK, Vause CV, Ho TW, Bigal ME, Durham PL. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache 2009. October;49(9):1258–66. [DOI] [PubMed] [Google Scholar]
- 18.Kamm K, Straube A, Ruscheweyh R. Calcitonin gene-related peptide levels in tear fluid are elevated in migraine patients compared to healthy controls. Cephalalgia 2019. October;39(12):1535–43. [DOI] [PubMed] [Google Scholar]
- 19.Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia 2005. March;25(3):179–83. [DOI] [PubMed] [Google Scholar]
- 20.Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol 2005. October;58(4):561–8. [DOI] [PubMed] [Google Scholar]
- 21.Cernuda-Morollon E, Larrosa D, Ramon C, Vega J, Martinez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013. October 1;81(14):1191–6. [DOI] [PubMed] [Google Scholar]
- 22.van Dongen RM, Zielman R, Noga M, Dekkers OM, Hankemeier T, van den Maagdenberg AM, et al. Migraine biomarkers in cerebrospinal fluid: A systematic review and meta-analysis. Cephalalgia 2017. January;37(1):49–63. [DOI] [PubMed] [Google Scholar]
- 23.Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, et al. Evidence for a vascular factor in migraine. Ann Neurol 2011. April;69(4):635–45. [DOI] [PubMed] [Google Scholar]
- 24.Guo S, Vollesen AL, Olesen J, Ashina M. Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain 2016. December;157(12):2773–81. [DOI] [PubMed] [Google Scholar]
- 25.Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 2010. October;30(10):1179–86. [DOI] [PubMed] [Google Scholar]
- 26.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia 2002. February;22(1):54–61. [DOI] [PubMed] [Google Scholar]
- 27.Amin FM, Asghar MS, Guo S, Hougaard A, Hansen AE, Schytz HW, et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia 2012. January;32(2):140–9. [DOI] [PubMed] [Google Scholar]
- 28.Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther 2005. March;77(3):202–13. [DOI] [PubMed] [Google Scholar]
- 29.Vecsei L, Tuka B, Tajti J. Role of PACAP in migraine headaches. Brain 2014. March;137(Pt 3):650–1. [DOI] [PubMed] [Google Scholar]
- 30.Guo S, Ashina M, Olesen J, Birk S. The effect of sodium nitroprusside on cerebral hemodynamics and headache in healthy subjects. Cephalalgia 2013. April;33(5):301–7. [DOI] [PubMed] [Google Scholar]
- 31.Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia 2008. March;28(3):226–36. [DOI] [PubMed] [Google Scholar]
- 32.Caughey GH, Leidig F, Viro NF, Nadel JA. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J Pharmacol Exp Ther 1988. January;244(1):133–7. [PubMed] [Google Scholar]
- 33.Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia 2018. January 1:333102418786261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med 2011. November 29;13:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edvinsson JCA, Warfvinge K, Krause DN, Blixt FW, Sheykhzade M, Edvinsson L, et al. C-fibers may modulate adjacent Adelta-fibers through axon-axon CGRP signaling at nodes of Ranvier in the trigeminal system. J Headache Pain 2019. November 12;20(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010. February;13(2):239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delwig A, Logan AM, Copenhagen DR, Ahn AH. Light evokes melanopsin-dependent vocalization and neural activation associated with aversive experience in neonatal mice. PLoS One 2012;7(9):e43787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Summ O, Charbit AR, Andreou AP, Goadsby PJ. Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain 2010. September;133(9):2540–8. [DOI] [PubMed] [Google Scholar]
- 39.de Lacalle S, Saper CB. Calcitonin gene-related peptide-like immunoreactivity marks putative visceral sensory pathways in human brain. Neuroscience 2000;100(1):115–30. [DOI] [PubMed] [Google Scholar]
- 40.Mehnert J, May A. Functional and structural alterations in the migraine cerebellum. J Cereb Blood Flow Metab 2019. April;39(4):730–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehnert J, Schulte L, Timmann D, May A. Activity and connectivity of the cerebellum in trigeminal nociception. Neuroimage 2017. April 15;150:112–18. [DOI] [PubMed] [Google Scholar]
- 42.Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol 2015;55:533–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmiter RD. The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends Neurosci 2018. May;41(5):280–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campos CA, Bowen AJ, Roman CW, Palmiter RD. Encoding of danger by parabrachial CGRP neurons. Nature 2018. March 29;555(7698):617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez E, Sakurai K, Xu J, Chen Y, Toda K, Zhao S, et al. A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat Neurosci 2017. December;20(12):1734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathew NT, Kailasam J, Seifert T. Clinical recognition of allodynia in migraine. Neurology 2004. September 14;63(5):848–52. [DOI] [PubMed] [Google Scholar]
- 47.De Logu F, Landini L, Janal MN, Li Puma S, De Cesaris F, Geppetti P, et al. Migraine-provoking substances evoke periorbital allodynia in mice. J Headache Pain 2019. February 14;20(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. Dural Calcitonin Gene-Related Peptide Produces Female-Specific Responses in Rodent Migraine Models. J Neurosci 2019. May 29;39(22):4323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oku R, Satoh M, Fujii N, Otaka A, Yajima H, Takagi H. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res 1987. February 17;403(2):350–4. [DOI] [PubMed] [Google Scholar]
- 50.Marquez de Prado B, Hammond DL, Russo AF. Genetic enhancement of calcitonin gene-related Peptide-induced central sensitization to mechanical stimuli in mice. J Pain 2009. September;10(9):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 2010. June;7(6):447–9. [DOI] [PubMed] [Google Scholar]
- 52.Rea BJ, Wattiez AS, Waite JS, Castonguay WC, Schmidt CM, Fairbanks AM, et al. Peripherally administered calcitonin gene-related peptide induces spontaneous pain in mice: implications for migraine. Pain 2018. November;159(11):2306–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russo AF, Recober A. Unanswered questions in headache: so what is photophobia, anyway? Headache 2013. Nov-Dec;53(10):1677–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci 2009. July 8;29(27):8798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci 2012. October 31;32(44):15439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mason BN, Kaiser EA, Kuburas A, Loomis MM, Latham JA, Garcia-Martinez LF, et al. Induction of Migraine-Like Photophobic Behavior in Mice by Both Peripheral and Central CGRP Mechanisms. J Neurosci 2017. January 4;37(1):204–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology 2010. January;58(1):156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan KY, Edvinsson L, Eftekhari S, Kimblad PO, Kane SA, Lynch J, et al. Characterization of the calcitonin gene-related peptide receptor antagonist telcagepant (MK-0974) in human isolated coronary arteries. J Pharmacol Exp Ther 2010. September 1;334(3):746–52. [DOI] [PubMed] [Google Scholar]
- 59.Petersen KA, Birk S, Lassen LH, Kruuse C, Jonassen O, Lesko L, et al. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia 2005. February;25(2):139–47. [DOI] [PubMed] [Google Scholar]
- 60.Goadsby PJ, Tepper SJ, Watkins PB, Ayele G, Miceli R, Butler M, et al. Safety and tolerability of ubrogepant following intermittent, high-frequency dosing: Randomized, placebo-controlled trial in healthy adults. Cephalalgia 2019. December;39(14):1753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conway CM, Croop R, Dubowchik GM, Coric V, Lipton RB. Cardiovascular safery of rimegepant 75 mg in 3 randomized clinical trials and systematic evaluations from in vitro ex vivo and in vivo nonclinical assays. American Headache Society Annual Scientific Meeting Philadelphia, PA 2019. [Google Scholar]
- 62.Sacco S, Kurth T. Migraine and the risk for stroke and cardiovascular disease. Curr Cardiol Rep 2014. September;16(9):524. [DOI] [PubMed] [Google Scholar]
- 63.Negro A, Martelletti P. Gepants for the treatment of migraine. Expert Opin Investig Drugs 2019. June;28(6):555–67. [DOI] [PubMed] [Google Scholar]
- 64.Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ. BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia 2014. February;34(2):114–25. [DOI] [PubMed] [Google Scholar]
- 65.Safety and efficacy study in adult subjects with acute migraines. [cited 2019. October 29th]; Available from: https://clinicaltrials.gov/show/NCT03235479
- 66.Open label safety study in acute treatment of migraine. [cited 2019. October 29th]; Available from: https://clinicaltrials.gov/show/NCT03266588
- 67.Croop R, Goadsby PJ, Stock DA, Conway CM, Forshaw M, Stock EG, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet 2019. August 31;394(10200):737–45. [DOI] [PubMed] [Google Scholar]
- 68.Voss T, Lipton RB, Dodick DW, Dupre N, Ge JY, Bachman R, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia 2016. August;36(9):887–98. [DOI] [PubMed] [Google Scholar]
- 69.Efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. [cited 2019. October 29th]; Available from: https://clinicaltrials.gov/show/NCT02828020
- 70.An extension study to evaluate the long-term safety and tolerability of ubrogepant in the treatment of migraine. [cited 2019. October 29th]; Available from: https://clinicaltrials.gov/show/NCT02873221
- 71.Schuster NM, Rapoport AM. Calcitonin Gene-Related Peptide-Targeted Therapies for Migraine and Cluster Headache: A Review. Clin Neuropharmacol 2017. Jul/August;40(4):169–74. [DOI] [PubMed] [Google Scholar]
- 72.Efficacy, Safety, and Tolerability of Multiple Dosing Regimens of Oral Atogepant (AGN-241689) in Episodic Migraine Prevention. [cited 2019. October 29th]; Available from: https://clinicaltrials.gov/ct2/show/NCT02848326
- 73.Goadsby PJ, Dodick DW, Trugman JM, Finnegan M, Lakkis H, Kaifeng L, et al. Orally Administered Atogepant Was Efficacious, Safe, and Tolerable for the Prevention of Migraine: Results From a Phase 2b/3 Study (S17.001). Neurology 2019;92(15 Supplement). [Google Scholar]
- 74.Bigal ME, Dodick DW, Rapoport AM, Silberstein SD, Ma Y, Yang R, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015. November;14(11):1081–90. [DOI] [PubMed] [Google Scholar]
- 75.Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014. November;13(11):1100–07. [DOI] [PubMed] [Google Scholar]
- 76.Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 2014. September;13(9):885–92. [DOI] [PubMed] [Google Scholar]
- 77.Sun H, Dodick DW, Silberstein S, Goadsby PJ, Reuter U, Ashina M, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016. April;15(4):382–90. [DOI] [PubMed] [Google Scholar]
- 78.Lionetto L, Curto M, Cisale GY, Capi M, Cipolla F, Guglielmetti M, et al. Fremanezumab for the preventive treatment of migraine in adults. Expert Rev Clin Pharmacol 2019. August;12(8):741–48. [DOI] [PubMed] [Google Scholar]
- 79.Maassen van den Brink A, Rubio-Beltran E, Duncker D, Villalon CM. Is CGRP Receptor Blockade Cardiovascularly Safe? Appropriate Studies Are Needed. Headache 2018. September;58(8):1257–58. [DOI] [PubMed] [Google Scholar]
- 80.MaassenVanDenBrink A, Meijer J, Villalon CM, Ferrari MD. Wiping Out CGRP: Potential Cardiovascular Risks. Trends Pharmacol Sci 2016. September;37(9):779–88. [DOI] [PubMed] [Google Scholar]
- 81.Ashina M, Goadsby PJ, Reuter U, Silberstein S, Dodick D, Rippon GA, et al. Long-term safety and tolerability of erenumab: Three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia 2019. October;39(11):1455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Depre C, Antalik L, Starling A, Koren M, Eisele O, Lenz RA, et al. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effect of Erenumab on Exercise Time During a Treadmill Test in Patients With Stable Angina. Headache 2018. May;58(5):715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aradi S, Kaiser E, Cucchiara B. Ischemic Stroke Associated With Calcitonin Gene-Related Peptide Inhibitor Therapy for Migraine: A Case Report. J Stroke Cerebrovasc Dis 2019. October;28(10):104286. [DOI] [PubMed] [Google Scholar]
- 84.Hendrikse ER, Bower RL, Hay DL, Walker CS. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia 2019. March;39(3):403–19. [DOI] [PubMed] [Google Scholar]
- 85.Hay DL. CGRP Receptor Biology: Is There More Than One Receptor? Handb Exp Pharmacol 2019;255:13–22. [DOI] [PubMed] [Google Scholar]
- 86.Hay DL, Walker CS. CGRP and its receptors. Headache 2017. April;57(4):625–36. [DOI] [PubMed] [Google Scholar]
- 87.Walker CS, Eftekhari S, Bower RL, Wilderman A, Insel PA, Edvinsson L, et al. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann Clin Transl Neurol 2015. June;2(6):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walker CS, Conner AC, Poyner DR, Hay DL. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol Sci 2010. October;31(10):476–83. [DOI] [PubMed] [Google Scholar]
- 89.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Grady EF, et al. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J Biol Chem 2007. April 20;282(16):12260–71. [DOI] [PubMed] [Google Scholar]
- 90.Kuwasako K, Shimekake Y, Masuda M, Nakahara K, Yoshida T, Kitaura M, et al. Visualization of the calcitonin receptor-like receptor and its receptor activity-modifying proteins during internalization and recycling. J Biol Chem 2000. September 22;275(38):29602–9. [DOI] [PubMed] [Google Scholar]
- 91.Yarwood RE, Imlach WL, Lieu T, Veldhuis NA, Jensen DD, Klein Herenbrink C, et al. Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc Natl Acad Sci U S A 2017. November 14;114(46):12309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seck T, Baron R, Horne WC. Binding of filamin to the C-terminal tail of the calcitonin receptor controls recycling. J Biol Chem 2003. March 21;278(12):10408–16. [DOI] [PubMed] [Google Scholar]
- 93.Shi L, Lehto SG, Zhu DX, Sun H, Zhang J, Smith BP, et al. Pharmacologic Characterization of AMG 334, a Potent and Selective Human Monoclonal Antibody against the Calcitonin Gene-Related Peptide Receptor. J Pharmacol Exp Ther 2016. January;356(1):223–31. [DOI] [PubMed] [Google Scholar]
- 94.Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Determinants of 1-piperidinecarboxamide, N-[2-[[5-amino-l-[[4-(4-pyridinyl)-l-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-d ibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazoliny l) (BIBN4096BS) affinity for calcitonin gene-related peptide and amylin receptors--the role of receptor activity modifying protein 1. Mol Pharmacol 2006. December;70(6):1984–91. [DOI] [PubMed] [Google Scholar]
- 95.Russo AF. Overview of Neuropeptides: Awakening the Senses? Headache 2017. May;57 Suppl 2:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 1993. April 30;192(2):553–60. [DOI] [PubMed] [Google Scholar]
- 97.Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol 2018. June;14(6):338–50. [DOI] [PubMed] [Google Scholar]
- 98.Christensen CE, Younis S, Deen M, Khan S, Ghanizada H, Ashina M. Migraine induction with calcitonin gene-related peptide in patients from erenumab trials. J Headache Pain 2018. November 8;19(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DosSantos MF, Holanda-Afonso RC, Lima RL, DaSilva AF, Moura-Neto V. The role of the blood-brain barrier in the development and treatment of migraine and other pain disorders. Front Cell Neurosci 2014;8:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edvinsson L. CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol 2015. August;80(2):193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edvinsson L, Ho TW. CGRP receptor antagonism and migraine. Neurotherapeutics 2010. April;7(2):164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, CGRP(8–37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol 2007. March;150(5):633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Edvinsson L, Tfelt-Hansen P. The blood-brain barrier in migraine treatment. Cephalalgia 2008. December;28(12):1245–58. [DOI] [PubMed] [Google Scholar]
- 104.Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain 2005. May;115(1–2):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia 2002. October;22(8):633–58. [DOI] [PubMed] [Google Scholar]
- 106.Hostetler ED, Joshi AD, Sanabria-Bohorquez S, Fan H, Zeng Z, Purcell M, et al. In vivo quantification of calcitonin gene-related peptide receptor occupancy by telcagepant in rhesus monkey and human brain using the positron emission tomography tracer [11C]MK-4232. J Pharmacol Exp Ther 2013. November;347(2):478–86. [DOI] [PubMed] [Google Scholar]
- 107.Russo AF. Overview of Neuropeptides: Awakening the Senses? 2017;57(S2):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Edvinsson L, Tajti J, Szalardy L, Vecsei L. PACAP and its role in primary headaches. J Headache Pain 2018. March 9;19(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaiser EA, Russo AF. CGRP and migraine: could PACAP play a role too? Neuropeptides 2013. December;47(6):451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uzar E, Evliyaoglu O, Toprak G, Acar A, Yucel Y, Calisir T, et al. Increased asymmetric dimethylarginine and nitric oxide levels in patients with migraine. J Headache Pain 2011. April;12(2):239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martami F, Razeghi Jahromi S, Togha M, Ghorbani Z, Seifishahpar M, Saidpour A. The serum level of inflammatory markers in chronic and episodic migraine: a case-control study. Neurol Sci 2018. October;39(10):1741–49. [DOI] [PubMed] [Google Scholar]
- 112.Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 2004. March 11;350(11):1104–10. [DOI] [PubMed] [Google Scholar]
- 113.Connor KM, Aurora SK, Loeys T, Ashina M, Jones C, Giezek H, et al. Long-term tolerability of telcagepant for acute treatment of migraine in a randomized trial. Headache 2011. January;51(1):73–84. [DOI] [PubMed] [Google Scholar]
- 114.Connor KM, Shapiro RE, Diener HC, Lucas S, Kost J, Fan X, et al. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology 2009. September 22;73(12):970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hewitt DJ, Martin V, Lipton RB, Brandes J, Ceesay P, Gottwald R, et al. Randomized controlled study of telcagepant plus ibuprofen or acetaminophen in migraine. Headache 2011. April;51(4):533–43. [DOI] [PubMed] [Google Scholar]
- 116.Ho TW, Connor KM, Zhang Y, Pearlman E, Koppenhaver J, Fan X, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology 2014. September 9;83(11):958–66. [DOI] [PubMed] [Google Scholar]
- 117.Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 2008. December 20;372(9656):2115–23. [DOI] [PubMed] [Google Scholar]
- 118.Ho TW, Ho AP, Ge YJ, Assaid C, Gottwald R, MacGregor EA, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for prevention of headache in women with perimenstrual migraine. Cephalalgia 2016. February;36(2):148–61. [DOI] [PubMed] [Google Scholar]
- 119.Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology 2008. April 15;70(16):1304–12. [DOI] [PubMed] [Google Scholar]
- 120.Ho AP, Dahlof CG, Silberstein SD, Saper JR, Ashina M, Kost JT, et al. Randomized, controlled trial of telcagepant over four migraine attacks. Cephalalgia 2010. December;30(12):1443–57. [DOI] [PubMed] [Google Scholar]
- 121.Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, et al. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia 2011. April;31(6):712–22. [DOI] [PubMed] [Google Scholar]
- 122.Diener HC, Barbanti P, Dahlof C, Reuter U, Habeck J, Podhorna J. BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia 2011. April;31(5):573–84. [DOI] [PubMed] [Google Scholar]
- 123.Lipton RB, Croop R, Stock EG, Stock DA, Morris BA, Frost M, et al. Rimegepant, an Oral Calcitonin Gene-Related Peptide Receptor Antagonist, for Migraine. N Engl J Med 2019. July 11;381(2):142–49. [DOI] [PubMed] [Google Scholar]
- 124.Study to Evaluate the PK of BMS-927711 in Patient With Migraine During Acute Migraine and Non-migraine Condition. [cited 2019. October 29th]; Available from: https://clinicaltrials.gov/ct2/show/NCT01445067
- 125.Dodick DW, Lipton RB, Ailani J, Lu K, Finnegan M, Trugman JM, et al. Ubrogepant for the Treatment of Migraine. N Engl J Med 2019. December 5;381(23):2230–41. [DOI] [PubMed] [Google Scholar]
- 126.Lipton RB, Dodick DW, Ailani J, Lu K, Finnegan M, Szegedi A, et al. Effect of Ubrogepant vs Placebo on Pain and the Most Bothersome Associated Symptom in the Acute Treatment of Migraine: The ACHIEVE II Randomized Clinical Trial. JAMA 2019. November 19;322(19):1887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Extension Study to Evaluate the Long-Term Safety and Tolerability of Oral Atogepant for the Prevention of Migraine in Participants With Episodic Migraine. [cited 2019. October 29th]; Available from: https://clinicaltrials.gov/ct2/show/NCT03939312
- 128.To Evaluate the Safety and Tolerability of Treatment With Atogepant 60 mg Daily for the Prevention of Migraine in Participants With Episodic Migraine. [cited 2019. October 29th]; Available from: https://clinicaltrials.gov/ct2/show/NCT03700320
- 129.Efficacy, Safety, and Tolerability of Atogepant for the Prevention of Chronic Migraine. [cited 2019. October 29th]; Available from: https://clinicaltrials.gov/ct2/show/NCT03855137
- 130.To Evaluate the Safety and Tolerability of Atogepant 10mg, 30 mg and 60 mg Once a Day for the Prevention of Migraine in Participants With Episodic Migraine. [cited 2019. October 29th]; Available from: https://clinicaltrials.gov/ct2/show/NCT03777059
- 131.Bigal ME, Dodick DW, Krymchantowski AV, VanderPluym JH, Tepper SJ, Aycardi E, et al. TEV-48125 for the preventive treatment of chronic migraine: Efficacy at early time points. Neurology 2016. July 5;87(1):41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bigal ME, Escandon R, Bronson M, Walter S, Sudworth M, Huggins JP, et al. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: Results of the Phase 1 program. Cephalalgia 2014. June;34(7):483–92. [DOI] [PubMed] [Google Scholar]
- 133.Cohen JM, Dodick DW, Yang R, Newman LC, Li T, Aycardi E, et al. Fremanezumab as Add-On Treatment for Patients Treated With Other Migraine Preventive Medicines. Headache 2017. October;57(9):1375–84. [DOI] [PubMed] [Google Scholar]
- 134.Cohen-Barak O, Weiss S, Rasamoelisolo M, Faulhaber N, Yeung PP, Loupe PS, et al. A phase 1 study to assess the pharmacokinetics, safety, and tolerability of fremanezumab doses (225 mg, 675 mg and 900 mg) in Japanese and Caucasian healthy subjects. Cephalalgia 2018. November;38(13):1960–71. [DOI] [PubMed] [Google Scholar]
- 135.Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Effect of Fremanezumab Compared With Placebo for Prevention of Episodic Migraine: A Randomized Clinical Trial. JAMA 2018. May 15;319(19):1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Halker Singh RB, Aycardi E, Bigal ME, Loupe PS, McDonald M, Dodick DW. Sustained reductions in migraine days, moderate-to-severe headache days and days with acute medication use for HFEM and CM patients taking fremanezumab: Post-hoc analyses from phase 2 trials. Cephalalgia 2019. January;39(1):52–60. [DOI] [PubMed] [Google Scholar]
- 137.Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Fremanezumab for the Preventive Treatment of Chronic Migraine. N Engl J Med 2017. November 30;377(22):2113–22. [DOI] [PubMed] [Google Scholar]
- 138.Monteith D, Collins EC, Vandermeulen C, Van Hecken A, Raddad E, Scherer JC, et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the CGRP Binding Monoclonal Antibody LY2951742 (Galcanezumab) in Healthy Volunteers. Front Pharmacol 2017;8:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oakes TMM, Skljarevski V, Zhang Q, Kielbasa W, Hodsdon ME, Detke HC, et al. Safety of galcanezumab in patients with episodic migraine: A randomized placebo-controlled dose-ranging Phase 2b study. Cephalalgia 2018. May;38(6):1015–25. [DOI] [PubMed] [Google Scholar]
- 140.Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia 2018. July;38(8):1442–54. [DOI] [PubMed] [Google Scholar]
- 141.Skljarevski V, Oakes TM, Zhang Q, Ferguson MB, Martinez J, Camporeale A, et al. Effect of Different Doses of Galcanezumab vs Placebo for Episodic Migraine Prevention: A Randomized Clinical Trial. JAMA Neurol 2018. February 1;75(2):187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial. JAMA Neurol 2018. September 1;75(9):1080–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dodick DW, Lipton RB, Silberstein S, Goadsby PJ, Biondi D, Hirman J, et al. Eptinezumab for prevention of chronic migraine: A randomized phase 2b clinical trial. Cephalalgia 2019. August;39(9):1075–85. [DOI] [PubMed] [Google Scholar]
- 144.Ashina M, Dodick D, Goadsby PJ, Reuter U, Silberstein S, Zhang F, et al. Erenumab (AMG 334) in episodic migraine: Interim analysis of an ongoing open-label study. Neurology 2017. September 19;89(12):1237–43. [DOI] [PubMed] [Google Scholar]
- 145.Ashina M, Tepper S, Brandes JL, Reuter U, Boudreau G, Dolezil D, et al. Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: A subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia 2018. September;38(10):1611–21. [DOI] [PubMed] [Google Scholar]
- 146.de Hoon J, Van Hecken A, Vandermeulen C, Yan L, Smith B, Chen JS, et al. Phase I, Randomized, Double-blind, Placebo-controlled, Single-dose, and Multiple-dose Studies of Erenumab in Healthy Subjects and Patients With Migraine. Clin Pharmacol Ther 2018. May;103(5):815–25. [DOI] [PubMed] [Google Scholar]
- 147.Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V, et al. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018. May;38(6):1026–37. [DOI] [PubMed] [Google Scholar]
- 148.Goadsby PJ, Reuter U, Hallstrom Y, Broessner G, Bonner JH, Zhang F, et al. A Controlled Trial of Erenumab for Episodic Migraine. N Engl J Med 2017. November 30;377(22):2123–32. [DOI] [PubMed] [Google Scholar]
- 149.Tepper S, Ashina M, Reuter U, Brandes JL, Dolezil D, Silberstein S, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017. June;16(6):425–34. [DOI] [PubMed] [Google Scholar]


