Abstract
Rotator cuff tears are common and often repaired surgically, but post-operative repair tissue healing, and shoulder function can be unpredictable. Tear chronicity is believed to influence clinical outcomes, but conventional clinical approaches for assessing tear chronicity are subjective. Shear wave elastography (SWE) is a promising technique for assessing soft tissue via estimates of shear wave speed (SWS), but this technique has not been used extensively on the rotator cuff. Specifically, the effects of age and pathology on rotator cuff SWS are not well known. The objectives of this study were to assess the association between SWS and age in healthy, asymptomatic subjects, and to compare measures of SWS between patients with a rotator cuff tear and healthy, asymptomatic subjects. SWE images of the supraspinatus muscle and intramuscular tendon were acquired from 19 asymptomatic subjects and 11 patients with a rotator cuff tear. Images were acquired with the supraspinatus under passive and active (i.e., minimal activation) conditions. Mean SWS was positively associated with age in the supraspinatus muscle and tendon under passive and active conditions (p ≤ 0.049). Compared to asymptomatic subjects, patients had a lower mean SWS in their muscle and tendon under active conditions (p ≤ 0.024), but no differences were detected under passive conditions (p ≥ 0.783). These findings identify the influences of age and pathology on SWS in the rotator cuff. These preliminary findings are an important step toward evaluating the clinical utility of SWE for assessing rotator cuff pathology.
Keywords: muscle, tendon, rotator cuff, shear wave elastography, pathology
Rotator cuff tears are common, affecting 40% (or more) of individuals over age 601,2 and resulting in about 250,000 rotator cuff repairs performed annually in the United States.3 This procedure provides short/medium-term pain relief for many patients, but healing after rotator cuff repair is a significant clinical problem (20–70% of rotator cuff repairs fail4–10) and post-operative shoulder function is unpredictable. Clinical studies indicate that patient age, tear size, and tear retraction contribute to a higher incidence of repair failure and poor clinical outcomes,11–13 whereas lab-based studies suggest that tendon degeneration (e.g.,14) and muscle changes—specifically, fatty degeneration, fibrosis, and atrophy15–18—also contribute to poor healing and worse clinical outcomes. Taken together, these factors implicate tear chronicity (i.e., the extent of muscle/tendon degeneration) as a critical factor in determining post-op repair tissue healing and clinical outcomes.
The chronicity of rotator cuff pathology is assessed in animal studies by measuring the mechanical properties (i.e., stiffness and/or modulus) of the muscle/tendon tissues (e.g.,19), but it is currently impossible to directly measure these quantities in humans. In the absence of direct information on tissue stiffness or modulus, patients with rotator cuff tears are typically described using estimates of tear dimensions, muscle condition, joint function, and/or pain. For example, a patient may be described as having a 2 cm wide, moderately retracted, grade two tear with deficits in external rotation strength, and pain of 7/10. Unfortunately, this clinical description provides little insight into the chronicity of the tear (i.e., the extent of muscle/tendon degeneration) which likely plays a critical role in healing and clinical outcomes after surgery.
Shear wave elastography (SWE) is an ultrasound-based imaging modality that provides a non-invasive estimate of tissue mechanical properties. To accomplish this, the technique involves mechanically perturbing the tissue with an acoustic radiation force impulse to generate a shear wave, imaging the resultant tissue displacements, and then computing the local shear wave speed (SWS) by estimating the time of flight. Previous research has demonstrated that measures of SWS in soft tissues can be interpreted as an indirect assessment of shear modulus.20 For tendons, the relationship between SWS and shear modulus is more complex,21 but previous studies have still interpreted SWS as an indication of shear modulus (e.g.,22). More recently, this understanding has been further advanced by Cortes et al.23 who examined how SWS frequency can provide additional information on dispersion and viscosity. Taken together, fundamental studies such as these have formed the basis for understanding the interaction between shear wave propagation and soft tissue mechanical properties. One issue that potentially confounds the interpretation of SWS is that tissue tension influences SWS, with increasing tissue tension associated with increasing SWS.24–27 Consequently, care must be taken to insure that SWE imaging is performed in a state of no/low tissue tension if the data are to be interpreted solely as an indication of shear modulus. Alternatively, if the state of tissue tension is not fully known, then reporting the data in the native units of SWS (typically meters/second) is a responsible and prudent approach.
SWE has been used clinically for breast and liver imaging where pathology is diagnosed via changes in tissue stiffness (e.g.,28,29), and has been used increasingly in musculoskeletal applications. In recent years, SWE has been used to evaluate the rotator cuff’s supraspinatus muscle and tendon.30–38 Specifically, research efforts investigating the feasibility and reliability of SWE for evaluating the rotator cuff31,34,36,38 have provided preliminary assessments of modulus, strain ratios, and shear wave velocity30,32,37,38 and have used SWE in conjunction with cadaveric experiments to evaluate the influence of surgical techniques on rotator cuff tissue stiffness.33,35 However, relatively little information exists on the influence of rotator cuff pathology on measures of shear wave speed (SWS). Furthermore, although previous research has shown that SWS is influenced by age in the biceps muscle39 and Achilles tendon,40 the extent to which SWS is influenced by age in the rotator cuff is not known. Thus, the objectives of this study were to assess the association between SWS and age in healthy, asymptomatic subjects, and to compare measures of SWS between patients with a rotator cuff tear and healthy, asymptomatic subjects. We hypothesized that SWS would significantly increase with age and that SWS would be significantly lower in rotator cuff tear patients than in control subjects.
METHODS
This was a retrospective case-control study (level of evidence 3), investigating the effects of pathology on SWS in rotator cuff tear subjects, and asymptomatic shoulders.
Following IRB approval, SWE images (Siemens ACUSON S3000, 9L4 probe, Erlangen, Germany) of the supraspinatus tendon and muscle were acquired from 30 subjects. These subjects consisted of asymptomatic control subjects with no history of injury or pain in their dominant shoulder (CTL, n = 19, age: 42.4 ± 18.9, range: 18–73, 2 males 17 females) and patients who had been diagnosed with a full-thickness rotator cuff tear of the supraspinatus tendon (RCT, n = 11, age: 60.0 ± 6.1, range: 53–73, 7 males, 4 females). Long-axis tendon images were acquired with the probe positioned medial to the acromion, aligned parallel to the intramuscular portion of the tendon (Fig. 1). Supraspinatus muscle images were acquired anterior to the tendon with the image plane aligned parallel to the muscle fibers as described by Itoigawa et al.36 Probe positioning was facilitated by real-time brightness mode (B-mode) images. Our research team has recently reported that SWE imaging of the supraspinatus muscle and tendon have high repeatability, with intra-and inter-user ICC values of greater than 0.87 and 0.73, respectively.41
Figure 1.
Photograph showing the scanning position for passive trials. The transducer was oriented parallel to the muscle fibers (as shown) or intramuscular tendon fibers. The shoulder was positioned in 30 degrees of scapular-plane abduction.
The muscle and tendon SWE images were acquired under both passive and active conditions. For the passive condition, subjects were seated with their elbow resting against a 30° abduction pillow (Bledsoe Arc 2.0, Carlsbad, CA) and their forearm pronated and resting on their thigh. The active condition involved the subjects lifting their forearm off their thigh and abducting their shoulder in the scapular plane only enough to remove contact with the abduction pillow, resulting in the patient holding their arm at approximately 30° scapular plane abduction. For each active trial, the tendon or muscle body were first identified in the B-mode ultrasound image with the subject’s arm in the passive position. The subjects were then instructed to move their shoulder into the active position, as described above. Minor adjustments to the probe position were made as needed to accommodate any changes in orientation of the tissues under active conditions. Image acquisition was initiated as soon as probe realignment was completed and subjects remained in the active position for the entirety of image acquisition. Upon completion of image acquisition, subjects returned to the passive position. For each subject, five trials of SWE images were acquired for each tissue (muscle, tendon) and condition (passive, active) with at least 15 s between trials. Subjects were all tested in the same order: passive muscle, active muscle, passive tendon, active tendon. None of the subjects reported any difficulty or discomfort during any trial.
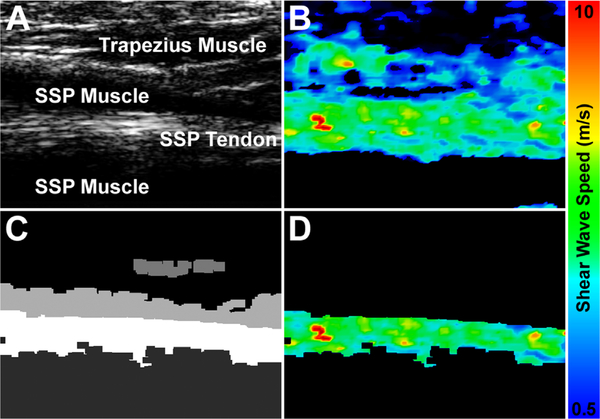
Each trial resulted in a brightness mode (B-mode) image (Fig. 2A) and a corresponding SWE image (Fig. 2B) where proprietary software calculated SWS at each pixel within a rectangular region of approximately, 2.5 × 2.5 cm. To isolate soft tissue structures, a semi-automated thresholding algorithm was applied to the B-mode image, segmenting the image into soft tissue regions based on an echogenic threshold value (Fig. 2C). The region corresponding to the tissue of interest (i.e., muscle or tendon) was then manually selected as the ROI for each trial, and this ROI was used to select the data from the corresponding SWE image (Fig. 2D). For each trial, the central 90th percentile of SWS values within the ROI were used for further analysis. The ROI consisted of an average of 2948 ± 2239 individual SWS values for the tendon trials, and an average of 16,324 ± 6406 individual SWS values for the muscle trials (resolution ~0.25 mm/pixel). For each trial, the mean, median, and standard deviation of SWS within the ROI were calculated. These outcome measures were then averaged across the five trials for each testing condition. In addition to SWE imaging, shoulder function, and pain were assessed for each participant using a visual analog scale for pain and the Western Ontario Rotator Cuff (WORC) score.
Figure 2.
To calculate mean shear wave speed, B-mode (A) and shear wave elastography (B) images were simultaneously acquired. The B-mode image was segmented based on echogenic thresholds to create a segmentation mask (C), and then this segmentation mask was applied to the shear wave elastography image to retain only the shear wave speed values of interest (D).
Linear regression was used to assess the association between age and mean SWS for each testing condition (passive muscle, active muscle, passive tendon, active tendon) in the asymptomatic subjects. In order to provide the appropriate age-matched comparison, unpaired t-tests were used to compare the mean, median, and standard deviation of SWS from the RCT patients to the 10 asymptomatic control subjects over age 50 (age: 58.7 ± 6.3, range: 50–70, 2 males, 8 females). Unpaired t-tests were used to compare the patient-reported measures with SWS. The sensitivity and specificity of SWS for distinguishing between patients with a rotator cuff tear and asymptomatic control subjects was assessed using a logistic regression model. A p-value of ≤0.05 was considered statistically significant for all tests.
RESULTS
The RCT patients’ average age (60.0 ± 6.1, range: 53–73) was not significantly different than that of the age-matched CTL subjects (58.7 ± 6.3, range: 50–70, p = 0.635). The rotator cuff tear was in the dominant shoulder in 82% (9 of 11) of the RCT patients. Gender was not found to have a significant effect (p > 0.317) on mean SWS for any of the four combinations of tissue (muscle, intramuscular tendon) and condition (active, passive). No significant association was found between the patient-reported outcomes (pain, WORC) and mean SWS for any tissue/condition (p > 0.645).
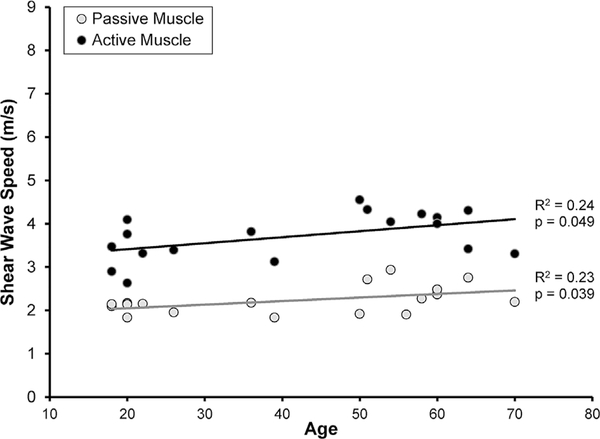
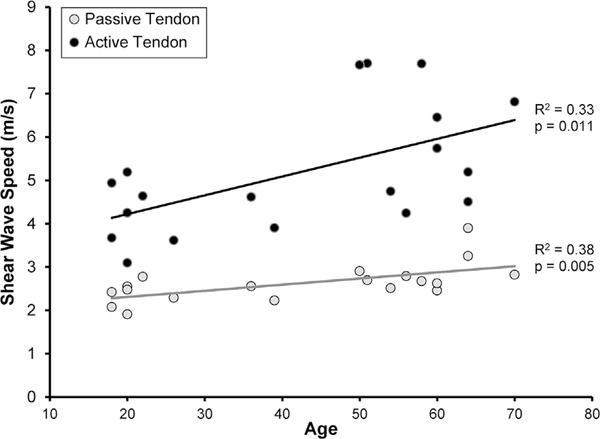
For the CTL subjects, mean SWS was significantly associated with age in active muscle (p = 0.049, R2 = 0.24, Fig. 3), passive muscle (p = 0.039, R2 = 0.23, Fig. 3), active tendon (p = 0.011, R2 = 0.33, Fig. 4), and passive tendon (p = 0.005, R2 = 0.38, Fig. 4).
Figure 3.
In asymptomatic subjects, mean SWS of the supraspinatus muscle increased significantly with age under both active (●p = 0.049) and passive (●p = 0.039) testing conditions.
Figure 4.
In asymptomatic subjects, mean SWS of the supraspinatus intramuscular tendon increased significantly with age under both active (●p = 0.011) and passive (●p = 0.005) testing conditions.
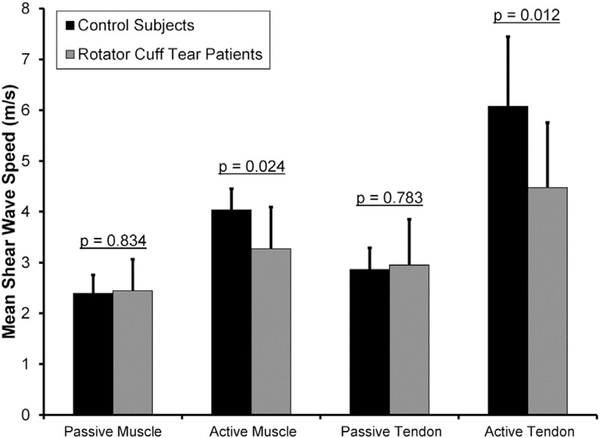
For SWE images acquired from passive muscle, no significant differences were detected between the RCT patients and age-matched CTL subjects in the mean (RCT: 2.4 ± 0.6 m/s, range: 1.9–3.5 m/s; age-matched CTL: 2.4 ± 0.4 m/s, range: 1.9–2.9 m/s; p = 0.834, Fig. 5), median (RCT: 2.2 ± 0.5 m/s; age-matched CTL: 2.3 ± 0.3 m/s; p = 0.678), or standard deviation (RCT: 0.7 ± 0.8 m/s; age-matched CTL: 0.6 ± 0.5 m/s; p = 0.829) of SWS.
Figure 5.
Compared to asymptomatic control subjects, patients with a rotator cuff tear had a significantly lower mean SWS in the supraspinatus muscle (p = 0.024) and intramuscular tendon (p = 0.012) when shear wave elastography images were acquired under active testing conditions.
For SWE images acquired from passive tendon, no significant differences were detected between the RCT patients and the age-matched CTL subjects in the mean (RCT: 3.0 ± 0.9 m/s, range: 2.0–4.5 m/s; age-matched CTL: 2.9 ± 0.4 m/s, range: 2.5–3.9 m/s; p = 0.783, Fig. 5), median (RCT: 2.6 ± 0.8 m/s; age-matched CTL: 2.8 ± 0.3 m/s; p = 0.558), or standard deviation (RCT: 0.7 ± 0.9 m/s; age-matched CTL: 0.7 ± 0.5 m/s; p = 0.967) of SWS.
For active muscle measurements, significant differences were detected between the RCT patients and the age-matched CTL subjects in the mean (RCT: 3.3 ± 0.8 m/s, range: 2.2–4.7 m/s; age-matched CTL: 4.0 ± 0.4 m/s, range: 3.3–4.6 m/s; p = 0.024, Fig. 5) and median (RCT: 3.2 ± 0.8 m/s; age-matched CTL: 4.0 ± 0.4; p = 0.013) SWS, but not in the standard deviation of SWS (RCT: 0.9 ± 0.4 m/s; age-matched CTL: 1.0 ± 0.2 m/s; p = 0.336).
For active tendon measurements, significant differences were detected between the RCT patients and age-matched CTL subjects in the mean (RCT: 4.5 ± 1.3 m/s, range: 2.5–7.3 m/s; age-matched CTL: 6.1 ± 1.4 m/s, range: 4.2–7.7 m/s; p = 0.012, Fig. 5), median (RCT: 4.3 ± 1.3 m/s; age-matched CTL: 5.9 ± 1.5 m/s; p = 0.017), and standard deviation (RCT: 1.1 ± 0.5 m/s; age-matched CTL: 1.7 ± 0.6 m/s; p = 0.029) of SWS.
The sensitivity/specificity in distinguishing between asymptomatic controls and pathologic subjects was 0.67/0.63 for passive muscle, 0.78/0.75 for active muscle, 0.73/0.71 for passive tendon, and 0.78/0.67 for active tendon.
DISCUSSION
The first objective of this study was to assess the association between SWS and age in healthy, asymptomatic subjects. As hypothesized, mean SWS was found to increase significantly with age in the supraspinatus muscle (Fig. 3) and tendon (Fig. 4). The study’s second objective was to compare measures of SWS between patients with a rotator cuff tear and healthy, asymptomatic subjects. No differences in SWS were detected between the RCT patients and the age-matched CTL subjects in SWE images acquired under passive conditions. However, under active conditions, the RCT patients had significantly lower mean and median SWS in both the supraspinatus muscle and tendon than the age-matched CTL subjects (Fig. 5).
The findings reported here are in good agreement with previous research and, in particular, the findings by Rosskopf et al.38 provide the most direct comparison with the current study. Specifically, Rosskopf et al.38 acquired SWE images of the supraspinatus muscle under passive conditions and reported a SWS of 3.0 m/s in healthy volunteers. By comparison, the current study reported a mean SWS of passive supraspinatus muscle in healthy CTL subjects of 2.4 m/s. One might expect the findings from these two studies to be in closer agreement since the data were acquired using SWE technology from the same manufacturer (Siemens). However, there are at least two potential explanations for the discrepancy in reported values between these studies. First, Rosskopf et al.38 acquired SWE images with the volunteers’ shoulders at their side, whereas the current study acquired images at 30° of abduction. Passive tension on the supraspinatus decreases with increasing abduction angle as the muscle-tendon unit become shorter, so SWS should decrease with increasing abduction angle. Consequently, the difference in arm position between these two studies may alone explain the difference in reported SWS values. Another potential explanation for the difference in SWS values between these two studies is that the current study acquired longitudinal SWE images (i.e., parallel to the long axis of the supraspinatus), whereas the Rosskopf et al.38 study acquired transverse SWE images (i.e., perpendicular to the long axis of the supraspinatus). While it may be expected that SWS would be higher along the fiber direction,42,43 Chino et al.44 have reported that the orientation of acquired SWE images can affect the reported SWS, but that SWS values from longitudinal images may be higher, lower, or no different than SWS values from transverse images, depending on which muscle is being evaluated.44 Our preliminary testing suggested that SWS of the supraspinatus is similar from images acquired in longitudinal and transverse directions, but to our knowledge the effect of image orientation on supraspinatus SWS has not been rigorously evaluated. Consequently, it is speculative to suggest that differences in SWE image orientation may contribute to the difference in findings between the current study and the Rosskopf et al.38 study.
Previous research has reported that patients with tendon pathology have lower SWS values when compared to healthy subjects.25,38 The current study reports similar findings, but only for SWE images acquired under active conditions (Fig. 5). Specifically, no significant difference was detected in the mean, median, or standard deviation of SWS for the supraspinatus muscle or tendon when the SWE images were acquired under passive conditions. There are several potential explanations for why differences were detected under active, but not passive, conditions. First, the testing position of 30° shoulder abduction may be a position of low/no supraspinatus tension. This is relevant because SWS is influenced not only by the tissue’s modulus,21,22 but also by tissue tension, with SWS increasing as tissue tension increases.24,33,39 Consequently, if 30° abduction is indeed a position of low/no supraspinatus tension, then the lack of differences in SWS for data acquired under passive conditions may suggest that differences in muscle and tendon modulus between the two subject populations were relatively minor. This explanation is further supported given that some of the age-matched CTL subjects may have had asymptomatic rotator cuff pathology, thus lowering the mean SWS in what otherwise appeared to be a healthy subject population. However, this explanation is speculative since no clinical imaging was performed on the CTL subjects to evaluate the underlying condition of their rotator cuff. Lastly, the differences in SWS values observed during the active condition may reflect changes in neuromuscular firing patterns associated with pathology. Previous research has reported that patients with a rotator cuff tear have less EMG activity in their supraspinatus than healthy control subjects,45 so it is possible that RCT patients may have had altered glenohumeral control (i.e., compensation for a torn tendon by using other muscles), thus resulting in lower SWS values under active conditions. However, in the current study no obvious compensation mechanics (e.g., shrugging during the active tests) were observed in any subject.
SWS increased with age in the CTL subjects (Figs. 3–4), and these findings both agree with and contradict previous research. For example, Eby et al.39 used SWE to demonstrate that shear modulus of the biceps muscle increases with age, but only in volunteers over the age of 60.39 Interestingly, Slane et al.40 reported that SWS increased with age in the Achilles tendon with the ankle in a plantarflexed position, but that SWS decreased with age for the soleus aponeurosis and gastrocnemius aponeurosis when the ankle was in a dorsiflexed position. The Achilles/soleus/gastrocnemius complex is believed to be slack when the ankle is plantarflexed and under passive tension when the ankle is dorsiflexed, so these findings reported by Slane et al.40 suggest that aging may be associated with a complex interaction between changes in modulus and changes in specific muscle-tendon function. The findings in the current study—that is, that supraspinatus muscle and tendon SWS under both active and passive conditions increase with age—suggest an increase in supraspinatus muscle-tendon shear modulus with age in asymptomatic volunteers. Previous research on the human supraspinatus has demonstrated age-related decreases in muscle cross-sectional area,46 increases in fatty infiltration,46 and increases in passive stiffness.47 In terms of the supraspinatus tendon, previous studies have reported changes in the composition and structure with age, but many of these age-related changes appear to be indicative of changes associated with tissue maturation. In contrast, age-related changes that occur after skeletal maturity has been reached appear to be more subtle and/or less well understood.48 Perhaps the only age-related change in tendon composition that occurs after skeletal maturity is the accumulation of advanced glycation endproduct (AGE) crosslinks, which have been shown to increase tendon modulus.49 It is possible that the increases in SWS with age may be related to an increase in AGE crosslinks. Alternatively, given that the control population included seven subjects whose age ranged from 18 to 24, the age-related changes in SWS may simply be an indication of the effects of skeletal maturation. However, to our knowledge, no study has reported the effect of age on stiffness or modulus of the human supraspinatus muscle or tendon. Consequently, in the absence of corroborating mechanical property data from human samples, it is difficult to discern the extent to which these changes in SWS with age represent age-related changes in shear modulus and/or muscle-tendon function.
The clinical utility of SWE is not yet fully apparent, but this study has shown that SWE can identify differences between asymptomatic control subjects and patients with a rotator cuff tear with a sensitivity of 0.67 or higher and a specificity of 0.63 or higher. Detecting differences between normal and pathologic subject populations is, in our opinion, an import first step toward establishing the clinical utility of SWE. Although this study identified a significant difference in mean SWS between the two subject populations, it is perhaps the range of SWS values that may be of greater clinical interest. Specifically, it is plausible that a patient whose SWS is near the lower end of SWS values may have a worse outcome after clinical intervention (i.e., physical therapy or surgical repair) than a patient whose SWS is considerably higher. Furthermore, if SWS is shown to be predictive of clinical outcome, then it may be possible to improve outcomes by using measures of SWS to inform patient-specific physical therapy, surgical repair, and/or post-operative rehabilitation treatment protocols. However, these hypotheses will remain speculative until further research evaluates the extent to which pre-intervention measures of SWS predict post-intervention measures of clinical outcome.
There are several limitations with this study. One potential concern is that we assessed the intramuscular portion of the supraspinatus tendon, which is medial to the portion of the tendon involved in rotator cuff tears and/or healing. However, this approach was necessary due to the presence of aberrant SWE signals (i.e., image noise) when assessing the supraspinatus tendon at its insertion site, which are likely caused by reflections from the humeral head at that location. Another limitation is that no clinical imaging (e.g., MRI) was performed on the CTL subjects to determine the integrity of their rotator cuff. Consequently, we cannot eliminate the possibility of asymptomatic rotator cuff pathology in some of these asymptomatic subjects. The study also did not measure intramuscular tendon cross-sectional dimensions, which could potentially influence SWS measurements. Lastly, the study was not designed to assess the relationship between shear wave speed and any particular mechanical property, in part because it is currently not possible to directly measure tissue mechanical properties in living humans.
In conclusion, this study has demonstrated that SWS of the supraspinatus muscle and tendon increases with age under both active and passive conditions in asymptomatic subjects. In addition, SWS of the active supraspinatus muscle and tendon of patients with a rotator cuff tear were significantly lower than asymptomatic volunteers. These preliminary findings are an important step toward establishing the clinical utility of SWE. However, further research is necessary to establish the relationships between SWS, pathology, healing, and post-surgical pain and function.
ACKNOWLEDGMENTS
This study was funded by Henry Ford Health Systems. The ultrasound system was provided by Siemens Medical Solutions USA.
Grant sponsor: Henry Ford Health System.
REFERENCES
- 1.Milgrom C, Schaffler M, Gilbert S, et al. 1995. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br 77:296–298. [PubMed] [Google Scholar]
- 2.Yamamoto A, Takagishi K, Osawa T, et al. 2010. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 19:116–120. [DOI] [PubMed] [Google Scholar]
- 3.Colvin AC, Egorova N, Harrison AK, et al. 2012. National trends in rotator cuff repair. J Bone Joint Surg Am 94: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berth A, Neumann W, Awiszus F, et al. 2010. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthop Traumatol 11:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung SW, Kim JY, Kim MH, et al. 2013. Arthroscopic repair of massive rotator cuff tears: outcome and analysis of factors associated with healing failure or poor postoperative function. Am J Sports Med 41:1674–1683. [DOI] [PubMed] [Google Scholar]
- 6.Djahangiri A, Cozzolino A, Zanetti M, et al. 2013. Outcome of single-tendon rotator cuff repair in patients aged older than 65 years. J Shoulder Elbow Surg 22:45–51. [DOI] [PubMed] [Google Scholar]
- 7.Kluger R, Bock P, Mittlbock M, et al. 2011. Long-term survivorship of rotator cuff repairs using ultrasound and magnetic resonance imaging analysis. Am J Sports Med 39: 2071–2081. [DOI] [PubMed] [Google Scholar]
- 8.Randelli P, Spennacchio P, Ragone V, et al. 2012. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg 96:9–16. [DOI] [PubMed] [Google Scholar]
- 9.Tashjian RZ, Hollins AM, Kim HM, et al. 2010. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med 38:2435–2442. [DOI] [PubMed] [Google Scholar]
- 10.Wu XL, Briggs L, Murrell GA. 2012. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med 40:2771–2776. [DOI] [PubMed] [Google Scholar]
- 11.Thomazeau H, Boukobza E, Morcet N, et al. 1997. Prediction of rotator cuff repair results by magnetic resonance imaging. Clin Orthop Rel Res 344:275–283. [PubMed] [Google Scholar]
- 12.McElvany MD, McGoldrick E, Gee AO, et al. 2015. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med 43: 491–500. [DOI] [PubMed] [Google Scholar]
- 13.Cho NS, Lee BG, Rhee YG. 2011. Arthroscopic rotator cuff repair using a suture bridge technique: is the repair integrity actually maintained? Am J Sports Med 39: 2108–2116. [DOI] [PubMed] [Google Scholar]
- 14.Killian ML, Cavinatto LM, Ward SR, et al. 2015. Chronic degeneration leads to poor healing of repaired massive rotator cuff tears in rats. Am J Sports Med 43:2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuzel BR, Grindel S, Papandrea R, et al. 2013. Fatty infiltration and rotator cuff atrophy. J Am Acad Orthop Surg 21:613–623. [DOI] [PubMed] [Google Scholar]
- 16.Gumucio JP, Korn MA, Saripalli AL, et al. 2014. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg 23: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laron D, Samagh SP, Liu X, et al. 2012. Muscle degeneration in rotator cuff tears. J Shoulder Elbow Surg 21: 164–174. [DOI] [PubMed] [Google Scholar]
- 18.Sato EJ, Killian ML, Choi AJ, et al. 2014. Skeletal muscle fibrosis and stiffness increase after rotator cuff tendon injury and neuromuscular compromise in a rat model. J Orthop Res 32:1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimbel JA, Van Kleunen JP, Mehta S, et al. 2004. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech 37: 739–749. [DOI] [PubMed] [Google Scholar]
- 20.Bercoff J, Tanter M, Fink M. 2004. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq Control 51: 396–409. [DOI] [PubMed] [Google Scholar]
- 21.Royer D, Gennisson JL, Deffieux T, et al. 2011. On the elasticity of transverse isotropic soft tissues (L). J Acoustic Soc Amer 129:2757–2760. [DOI] [PubMed] [Google Scholar]
- 22.Brum J, Bernal M, Gennisson JL, et al. 2014. In vivo evaluation of the elastic anisotropy of the human Achilles tendon using shear wave dispersion analysis. Phys Med Biol 59:505–523. [DOI] [PubMed] [Google Scholar]
- 23.Cortes DH, Suydam SM, Silbernagel KG, et al. 2015. Continuous shear wave elastography: a new method to measure viscoelastic properties of tendons in vivo. Ultrasound Med Biol 41:1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ates F, Hug F, Bouillard K, et al. 2015. Muscle shear elastic modulus is linearly related to muscle torque over the entire range of isometric contraction intensity. J Electromyogr Kinesiol 25:703–708. [DOI] [PubMed] [Google Scholar]
- 25.Aubry S, Nueffer JP, Tanter M, et al. 2015. Viscoelasticity in Achilles tendonopathy: quantitative assessment by using real-time shear-wave elastography. Radiology 274: 821–829. [DOI] [PubMed] [Google Scholar]
- 26.Dubois G, Kheireddine W, Vergari C, et al. 2015. Reliable protocol for shear wave elastography of lower limb muscles at rest and during passive stretching. Ultrasound Med Biol 41:2284–2291. [DOI] [PubMed] [Google Scholar]
- 27.Yavuz A, Bora A, Bulut MD, et al. 2015. Acoustic Radiation Force Impulse (ARFI) elastography quantification of muscle stiffness over a course of gradual isometric contractions: a preliminary study. Med Ultrason 17:49–57. [DOI] [PubMed] [Google Scholar]
- 28.Berg WA, Mendelson EB, Cosgrove DO, et al. 2015. Quantitative maximum shear-Wave stiffness of Breast masses as a predictor of histopathologic severity. AJR Am J Roentgenol 205:448–455. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Lee HK, Cho JH, et al. 2015. Quantitative comparison of transient elastography (TE), shear wave elastography (SWE) and liver biopsy results of patients with chronic liver disease. J Phys Ther Sci 27:2465–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arda K, Ciledag N, Aktas E, et al. 2011. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol 197: 532–536. [DOI] [PubMed] [Google Scholar]
- 31.Krepkin K, Bruno M, Raya JG, et al. 2017. Quantitative assessment of the supraspinatus tendon on MRI using T2/T2* mapping and shear-wave ultrasound elastography: a pilot study. Skeletal Radiol 46:191–199. [DOI] [PubMed] [Google Scholar]
- 32.Muraki T, Ishikawa H, Morise S, et al. 2015. Ultrasound elastography-based assessment of the elasticity of the supraspinatus muscle and tendon during muscle contraction. J Shoulder Elbow Surg 24:120–126. [DOI] [PubMed] [Google Scholar]
- 33.Hatta T, Giambini H, Hooke AW, et al. 2016. Comparison of passive stiffness changes in the supraspinatus muscle after double-Row and knotless transosseous-equivalent rotator cuff repair techniques: a cadaveric study. Arthroscopy 32:1973–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatta T, Giambini H, Uehara K, et al. 2015. Quantitative assessment of rotator cuff muscle elasticity: reliability and feasibility of shear wave elastography. J Biomech 48:3853–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatta T, Giambini H, Zhao C, et al. 2016. Biomechanical effect of margin convergence techniques: quantitative assessment of supraspinatus muscle stiffness. PLoS ONE 11: e0162110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itoigawa Y, Sperling JW, Steinmann SP, et al. 2015. Feasibility assessment of shear wave elastography to rotator cuff muscle. Clin Anat (New York, NY) 28:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joy J, McLeod G, Lee N, et al. 2015. Quantitative assessment of Thiel soft-embalmed human cadavers using shear wave elastography. Ann Anat 202:52–56. [DOI] [PubMed] [Google Scholar]
- 38.Rosskopf AB, Ehrmann C, Buck FM, et al. 2016. Quantitative shear-Wave US elastography of the supraspinatus muscle: reliability of the method and relation to tendon integrity and muscle quality. Radiology 278:465–474. [DOI] [PubMed] [Google Scholar]
- 39.Eby SF, Cloud BA, Brandenburg JE, et al. 2015. Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clin Biomech (Bristol, Avon) 30:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slane LC, Martin J, DeWall R, et al. 2017. Quantitative ultrasound mapping of regional variations in shear wave speeds of the aging Achilles tendon. Eur Radiol 27:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahrmann SA. 2002. Diagnosis and treatment of movement impairment syndromes. St. Louis, MO: Mosby. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luttgens K, Wells KF. 1989. Kinesiology: scientific Basis of Human Motion. Dubuque, IA: Wm. C. Brown Publishers. [Google Scholar]
- 43.Gennisson JL, Deffieux T, Mace E, et al. 2010. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol 36:789–801. [DOI] [PubMed] [Google Scholar]
- 44.Chino K, Kawakami Y, Takahashi H. 2015. Tissue elasticity of in vivo skeletal muscles measured in the transverse and longitudinal planes using shear wave elastography. Clin Physiol Funct Imaging 37:394–399. [DOI] [PubMed] [Google Scholar]
- 45.Reddy AS, Mohr KJ, Pink MM, et al. 2000. Electromyographic analysis of the deltoid and rotator cuff muscles in persons with subacromial impingement. J Shoulder Elbow Surg 9:519–523. [DOI] [PubMed] [Google Scholar]
- 46.Raz Y, Henseler JF, Kolk A, et al. 2015. Patterns of age-associated degeneration differ in shoulder muscles. Front Aging Neurosci 7:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer TB, Thompson BJ. 2017. Influence of age on passive stiffness and size, quality, and strength characteristics. Muscle Nerve 55:305–315. [DOI] [PubMed] [Google Scholar]
- 48.Svensson RB, Heinemeier KM, Couppe C, et al. 2016. Effect of aging and exercise on the tendon. J Appl Physiol (Bethesda, Md: 1985) 121:1353–1362. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Fessel G, Georgiadis M, et al. 2013. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol 32:169–177. [DOI] [PubMed] [Google Scholar]