Abstract
This meta-analysis was designed to compare the effectiveness of two cognitive training modules, single-component training, which targets one specific cognitive ability, vs. multi-component training, which trains multiple cognitive abilities, on both trained abilities (near transfer) and untrained abilities (far transfer) in older adults. The meta-analysis also assessed whether individual differences in mental status interacted with the extent of transfer. Eligible randomized controlled trials (215 training studies) examined the immediate effects of cognitive training in either healthy aging or mild cognitive impairment (MCI). Results yielded an overall net-gain effect size (g) for the cognitive training, of 0.28 (p<0.001). These effects were similar across mental status and training modules, and were significant for both near (g=0.37) and far (g=0.22) transfer. Although all training modules yielded significant near transfer, only a few yielded significant far transfer. Single-component training of executive functions was most effective on near and far transfer, with processing speed training improving everyday functioning. All modules of multi-component training (specific and non-specific) yielded significant near and far transfer, including everyday functioning. Training effects on cognition were moderated by educational attainment and number of cognitive outcomes, but only in healthy aging. These findings suggest that, in older adults, all modules of multi-component training are more effective in engendering near and far transfer, including everyday functioning, when compared to single-component training modules.
Keywords: Cognitive Training, MCI, Healthy Aging, Far Transfer, Everyday functioning
Although the average life expectancy is increasing in the United States of America (Beller, 2013), there has been little change in the average age of onset for age-related neuropathological illnesses (Sperling et al., 2011). Therefore, there is a great interest in developing behavioral interventions that can delay the onset of age-related neuropathological illnesses in our lifespan by preserving our cognitive skills into late adulthood (Hertzog, Kramer, Wilson & Lindenberger, 2008). One such promising behavioral intervention is cognitive training, which, in healthy aging, has been argued to not only preserve but even enhance cognitive functions that typically decline in late adulthood (e.g., Anguera et al., 2013; Basak, Boot, Voss, & Kramer, 2008; Borella et al., 2014; Cavallini et al., 2015; Park et al., 2014; for a review, see Stine-Morrow & Basak, 2011). We argue that effective cognitive training during late adulthood builds resistance to the age-related neuropathological illnesses through cognitive plasticity, defined by recovery of declining age-sensitive cognitive abilities (“fluid” cognitive ability). Some of these declining fluid abilities are processing speed, episodic memory, reasoning, and executive functions (Cerella, 1990; Park et al., 2002; Salthouse, 1996; Verhaeghen, 2011).
Researchers have argued that enhancing basic fluid abilities can improve everyday functioning in older adults, which in turn can provide additional years of independence and hopefully delay the onset of dementia (Hall et al., 2009; Wilson et al., 2010). At meta-analysis level, cognitive training has been observed to induce such improvements on the trained basic cognitive abilities (e.g., Karbach & Verhaeghen, 2014). The debate in the field is whether such improvements from cognitive training are also induced on untrained cognitive skills in older adults, including everyday functioning (Simons et al., 2016). One promising cognitive training approach is processing speed training. Processing speed training studies have reported not only immediate and long-term improvements in processing speed, the trained cognitive domain (Ball et al., 2002; Rebok et al., 2013; 2014), but also long-term gains in a subjective self-reported measure of everyday functioning (e.g., Rebok et al., 2013). Based on such promising findings on both basic cognition and everyday functioning, and with a rise in the use of accessible technological devices (Gatto & Tak, 2008; Selwyn, 2004; Wagner, Hassanein, & Head, 2010) that can impart these cognitive trainings from the comfort of participants’ home (Basak & Qin, 2018), cognitive training in older adults has recently gained widespread popularity.
However, it is not clear when in late adulthood is cognitive training most beneficial? Is cognitive training most beneficial during healthy aging, when we have the most robust cognitive health, or can it be equally beneficial when our mental health status is somewhat compromised, such as in patients with mild cognitive impairment (MCI)? It is also not clear whether the cognitive components (e.g., processing speed, memory, executive functions, etc.) should be trained individually or be trained in tandem to improve not only the trained cognitive abilities (near transfer), but also to other untrained cognitive abilities (far transfer)? Moreover, it is important to know how individual differences in participant’s characteristics (e.g., education, gender, and age) or training characteristics (e.g., duration, location, and frequency) can interact with the extent of cognitive plasticity. Such a research agenda can help us tailor individualized cognitive training in older adults to boost their basic cognitive abilities and everyday functioning.
Although we hypothesize that effective cognitive training will assist in recovery of declining age-sensitive basic cognitive abilities, there are certain cognitive abilities (“crystallized” cognitive ability) that remain relatively stable in middle-aged and healthy older adults (Bischof & Park, 2010). This crystallized ability, consisting of measures of semantic knowledge, phonemic knowledge and vocabulary, is related to educational attainment. Both crystallized ability and educational attainment are often used as a proxy of cognitive reserve. Cognitive reserve is defined as the cumulative lifelong improvement of crystallized ability through environmental factors (such as, educational attainment) that mitigate the rate of decline in fluid cognitive abilities and ultimately the clinical expression of dementia (Cizginer et al., 2017). The current study was therefore also designed to investigate how individual differences in cognitive reserve, built by cumulative lifelong experiences and proxied by educational attainment, may interact with cognitive plasticity, a recovery of cognitive abilities that declines in both healthy older adults and MCI.
Cognitive Training and Mental Status
Many cognitive training studies have found that individuals with better cognitive health (indexed by cognitive ability or educational attainment) show greater transfer effects from cognitive training (e.g., memory training, Rebok et al., 2013; videogame training, Basak, Voss, Erickson, Boot, & Kramer, 2011; strategy videogame training in young, Lee et al., 2012). For example, higher executive functions, and its related fronto-parietal grey matter volumes (Basak et al., 2011), predict improvements not only in near transfer, but also in far transfer (Basak & O’Connell, 2016; Whitlock, McLaughlin, & Allaire, 2012). Moreover, healthy older adults who have more rapid skill acquisition (determined by individual’s learning curve) on the trained cognitive task, also show greater far transfer (Basak et al., 2008; Basak & O’Connell, 2016). Therefore, cognitive training may induce more cognitive plasticity and greater transfer for healthy aging, when compared to MCI, because cognitive plasticity is argued to be compromised by lower mental status (Calero & Navarro, 2004; Fernández-Ballesteros et al., 2012). Some studies however have reported in greater cognitive gains from cognitive training in individuals with lower cognitive ability (e.g., processing speed training, Ball et al., 2013; imagery training in healthy aging, da Silva et al., 2009; strategy videogame training using videogames in young, Boot et al., 2017). Since adults with MCI have lower cognitive ability compared to healthy older adults, and many cognitive training studies have reported significant effects in MCI (for a review, see Li et al., 2011), it is plausible that cognitive training may be more effective in MCI participants. Given these mixed findings regarding who benefits more from cognitive training, it is important that we determine how individual differences in cognitive ability (mental status: healthy aging vs. MCI) interacts with cognitive plasticity in older adults, and whether educational attainment and age influence these relationships.
It is also possible that the effectiveness of cognitive interventions is similar across healthy aging and MCI populations, because intervention studies in healthy aging may inadvertently include some MCI adults. Most intervention studies on healthy aging determine their participants’ mental status through a general, short cognitive assessment (e.g., Mini -Mental Status Examination), which can mask the early stages of the pathophysiological disease processes evidenced only through imaging techniques (Sperling et al., 2011). Intervention studies on healthy aging also do not typically include any clinical diagnosis of MCI. Such clinical diagnosis however may not be very helpful in screening MCI, because it has been shown that the pathological processes of MCI begin at least 3 to 4 years before its clinical diagnosis when individuals appear to be cognitively healthy (Howieson et al., 2008; Morris, 2005).
Single-component vs. Multi-component Training: Extant of Transfer
Cognitive interventions typically focus training on either one specific cognitive ability (single-component training) or multiple cognitive abilities (multi-component training). Although many cognitive training studies aim to improve performance on different measures of the trained ability (near transfer), others are aimed at improving performance on cognitive abilities that are different from the trained ability (far transfer). Certain basic cognitive abilities (e.g., attention, executive functions, episodic memory), which decline rapidly in late adulthood (Bopp & Verhaeghen, 2018; Nyberg, Lövdén, Riklund, Lindenberger, & Bäckman, 2012; Park & Bischof, 2011; Salthouse, 2010), subserve everyday functioning and complex skills (Allaire & Marsiske, 1999; Baniqued et al., 2013; Miyake et al., 2010; Ray et al., 2017). Therefore, it is imperative to understand which cognitive abilities, when optimized individually, will not only engender near transfer, but also far transfer (Stine-Morrow & Basak, 2011). Hence, a goal of this current study was to determine which specific cognitive abilities, when trained singularly, can induce far transfer to untrained cognitive skills in older adults, given the lack of support for far transfer from single-component training in both young and old adults (e.g., Redick et al., 2013; Simons et al., 2016).
Cognitive interventions typically focus training on either one specific cognitive ability (single-component training) or multiple cognitive abilities (multi-component training). We hypothesized that training-related benefits to near transfer ability will be greater than far transfer abilities, particularly for single-component training. A meta-analysis on single-component, executive functions training (Karbach & Verhaeghen, 2014) reported a slightly higher effect size for near transfer (0.5; k=9) than far transfer (0.4; k=4) for older adults, which is line with this hypothesis. These effects were combined across task-switching training and working memory training studies, because task-switching and working memory updating are considered to be inter-related aspects of executive functions (Miyake et al., 2000). It is important to note that although the results of Karbach and Verhaeghen are in line with our hypothesis, the number of reported studies was quite small (k ranging from 4 to 9). Moreover, no statistical tests were reported that contrasted the effect sizes of near transfer with that of far transfer.
It is also not known which single cognitive ability is the best basic ability to train to engender both near and far transfer in older adults. Although past meta-analyses in older adults have investigated which type of single-component training (e.g., working memory, processing speed, episodic memory, reasoning) may be the best approach to improve overall cognition (Hill et al., 2016; Lampit et al., 2013; Mewborn et al., 2017), no systematic investigation has yet contrasted different types of single-component training to determine the best single basic ability to train to engender far transfer to other basic cognitive abilities and far transfer to everyday functioning. However, a recent review reported that processing speed training engenders far transfer to everyday functioning immediately after training (Cohen’s d=0.27; nine studies; Edwards et al., 2018). The authors claimed that these effects on everyday functioning were sustained over longer retention periods (from 1 to 10 years), but no effect size was reported. Everyday functioning in these nine studies was assessed by a subjective report on a wide variety of daily behavioral activities using Instrumental Activities of Daily Living scale (IADL) or by a timed IADL test. It is important to note that everyday functioning construct includes a mix of both objective and subjective measures, ranging from those assessing a specific aspect of cognition (e.g., Rivermead Behavioral Memory Test, RBMT) to those broadly requesting report of daily behavioral activities (e.g., IADL). A more recent meta-analysis has found that executive functions training (working memory, inhibition, flexibility, or their combinations) is effective both immediately and at long-term on overall cognition, but everyday functioning was not assessed (Nguyen, Murphy & Andrews, 2019). To date no meta-analysis has examined the other types of single component training to evaluate not just the overall effect, but also the differential effects, of single-component training on everyday functioning. Such an examination is of great importance for cognitive optimization, because by improving our everyday functioning, we can extend our functional independence.
In addition to single-component training, researchers have also employed multi-component training, where multiple cognitive components are trained, either simultaneously or sequentially, to improve a broad range of cognitive functions. These multi-component training modules can be either class-room based, where each class is focused on training one type of cognition (Kinsella et al., 2009), or based on laboratory-tasks, where cognition is trained sequentially or simultaneously using computerized paradigms (Pereira-Morales, Cruz-Salinas, Aponte, & Pereira-Manrique, 2018), or simulation based, where people engage in learning complex real-world tasks (Park et al., 2014) or learning simulation games (Basak et al., 2008). However, it is possible that even for multi-component training, effective gains are limited to the trained cognitive skills (near transfer), irrespective of how broad those skills may be. It is also not known whether training multiple cognitive components improves untrained cognitive abilities (far transfer) more than training a single cognitive component. The current meta-analysis is designed to investigate not only the effects on overall cognition from both single- and multi-component training, but also to evaluate the near transfer and the far transfer effects of various types of single-component and multi-component training modules. Moreover, we examined the effectiveness on everyday functioning from both single- and multi-component training – a domain that has been overlooked in previous systematic reviews.
The Current Study
Despite the large volume of research on cognitive training in healthy aging and MCI, its effectiveness remains uncertain, partly because of the difficulty in interpreting one randomized control trial at a time or interpreting qualitative reviews (Park & Reuter-Lorenz, 2012; Rebok et al., 2014; Simons et al., 2016; Stine-Morrow & Basak, 2011). At least three meta-analyses (Lampit, Hallock, & Valenzuela, 2014; Li et al., 2011; Mewborn, Lindbergh, & Stephen Miller, 2017) have attempted to synthesize the results, but each had some limitations. These limitations include narrow inclusion criteria of mental status (Lampit et al., 2014; Li et al., 2011) and cognitive outcomes (Karr, Areshenkoff, Rast, & Garcia-Barrera, 2014), an incomplete corpus of existing studies (Mewborn et al., 2017), lack of specificity of near and far transfer (Lampit et al., 2014), or no quantitative contrasts between the best estimates of cognitive gains in the healthy aging to that of the MCI (Mewborn et al., 2017), or of the training group to that of the control group.
We, therefore, sought to identify and quantitatively summarize all cognitive training studies involving either healthy aging or MCI, and to answer the following three questions. First, who would benefit most from cognitive training in augmenting a broad-range of cognitive abilities in comparison to a control group, healthy aging or MCI?
Second, what is the extent of benefits from cognitive training? Many researchers have hypothesized that cognitive training is limited to improvements in trained abilities (i.e., near transfer; Simons et al., 2016). Based on the strength of the theoretical foundations and the currency in the field, we chose two types of cognitive training modules (single-component vs. multi-component) for our main analyses and studied the effects of these training modules on both near transfer (trained cognitive skills) and far transfer (untrained cognitive skills) tasks. Furthermore, in a subsequent analysis we compared different types of single-component training in order to identify “core” single abilities that engendered not only near transfer, but also far transfer.
Third, which cognitive training approach would be most effective in engendering broader cognitive transfer? We hypothesized that single-component training may engender near transfer, with some “core” trained abilities also improving untrained, far abilities. However, multi-component training is hypothesized to engender transfer to both near and far cognition, particularly to tasks of everyday functioning, which require integration of multiple cognitive abilities, such as attention, memory, executive functions, and reasoning.
This study also evaluated the effects of moderators (such as, age and training duration) on gains from cognitive training. Effectiveness of training could also vary with age, because cognitive plasticity has been shown to diminish with increasing age in older adults (Baltes & Kliegl, 1992; Hertzog et al., 1996; Kliegl et al., 1989, 1990). Duration of training is important to understand the dose-responsiveness of cognitive training (e.g., Basak et al., 2008).
Method
Protocol and Registration
The review was planned, conducted and reported in line with PRISMA (Liberati et al., 2009) standards of quality for reporting meta-analysis. The review protocol (#42017078569) was pre-registered with PROSPERO International Prospective Register of Systematic Reviews. The only difference between the registered protocol and the current protocol is the separate analysis of everyday functioning as a construct. Everyday functioning, a measure of functional independence, is widely touted as a far transfer task for interventions by most clinicians, yet is vastly understudied. Therefore, we deemed that effects of cognitive training on everyday functioning is warranted to better understand the extant of transfer from cognitive training.
Eligibility Criteria
To provide a comprehensive overview of cognitive gains from cognitive training in late adulthood, we utilized the following inclusion criteria: 1. randomized controlled trials (RCTs), 2. human participants over the age of 60, 3. patients with mild cognitive impairment, 4. cognitive intervention or training focusing on one or more cognitive domains, and 5. included at least one cognitive outcome. List of inclusion and exclusion criteria are detailed in supplementary materials.
Search Strategy
Systematic searches were conducted in the PsychInfo, Google Scholar, and Medline/PubMed databases. The final literature search cut-off date was set at November 30, 2018. Search keywords, associated with each inclusion criterion, were developed by the first and second authors and are reported in Table 1. The second and third authors performed the search independently, and then screened the reference lists of selected articles and related review articles. The search was limited to publications written in English. Reference lists of past meta-analyses (Hill et al., 2017; Karbach & Verhaeghen, 2014; Karr et al., 2014; Li et al., 2011; Mewborn et al., 2017; Toril, Reales, & Ballesteros, 2014) were also examined to achieve maximal inclusion of relevant training studies.
Table 1.
List of Inclusion Criteria, Search Terms Associated with Each Criterion, and Corresponding Exclusion Criteria
| Inclusion Criteria | Search Terms | Exclusion Criteria |
|---|---|---|
| Randomized controlled trials | Clinical trial or randomized trial or controlled trial or longitudinal or treatment or rehabilitation | No control group. Lack of age-matched and mental status matched control group. Participants were not randomly assigned to each group. |
| Human participants with mean age over 60 | Age or aged or ageing or aging or age difference or older adults or elderly or seniors | Non-human animal studies. Training studies focusing on participants from other stages of life-span, such as younger adults and children. |
| Patients with mild cognitive impairment | Mild cognitive impairment or MCI or memory complains or memory loss | Inclusion of participants with other neuropsychological disorders, such as possible Alzheimer’s disease or schizophrenia. |
| Cognitive intervention or training focusing on one or more cognitive domains | Cognitive intervention or cognitive training or rehabilitation or cognitive stimulation or mnemonic training or memory training or executive functions training or computerized training or video game training or speed of processing training or working memory training or engagement | Intervention effects could not be attributed just to cognitive training, such as training that combined physical or pharmacological interventions with cognitive training. |
Study Selection
Titles and abstracts were first reviewed on the inclusion and exclusion criteria (Table 1). Articles deemed eligible were then retrieved and read thoroughly by the second author to make a final determination. When eligibility determinations could not be made directly by the second author, the first author was consulted. The first author approved the final list of eligible studies.
Data Collection Process
Data necessary to calculate effect sizes were extracted by the second author. For most studies, the data entered were outcome means and standard deviations both before and after training for all groups, i.e., training and control. Some studies reported pre-post changes in outcome means and standard deviations; whereas others reported standardized effect size measures for the outcomes (Table 2). When studies did not report sufficient data to calculate effect sizes, corresponding authors on these publications were emailed.
Table 2.
Characteristics of Cognitive Training Studies in Healthy Aging (HA) and MCI
| Citation | Training Type | Ctrl (A/P) | % F | Edu | Age | Tot. Hr | Hr per Wk | Location (H/L) | Adapt (Y/N) | # Cog. Outcomes | PEDro Score | g (95%CI) | NT, NC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HA: Single-Component Training | |||||||||||||
| Borella et al., 2014 | EF | A | - | 8.8 | 69 | 5 | 2.5 | L | N | 10 | 8 | 1.23 (0.56, 1.91) | 20, 20 |
| Cantarella et al., 2017 | EF | A | 50 | 10.5 | 69.34 | 3 | 1 | L | Y | 4 | 7 | 1.18 (0.47, 1.88) | 18, 18 |
| Borella, Carretti, Riboldi, & De Beni, 2010 | EF | A | 65 | 9.3 | 69 | 10 | 5 | L | N | 10 | 7 | 1.17 (0.5, 1.84) | 20, 20 |
| Carretti, Borella, Zavagnin, & De Beni, 2013 | EF | A | 57 | 8.5 | 70 | 3 | 1.5 | L | Y | 6 | 7 | 0.96 (0.27, 1.66) | 17, 19 |
| Bier, De Boysson, & Belleille, 2014 | Speed | P | - | 15 | 69.12 | 6 | 3 | L | N | 4 | 8 | 0.94 (0.11, 1.77) | 13, 12 |
| Xin, Lai, Li, & Maes, 2014 | EF | A | 53 | - | 69.53 | 10 | 3.5 | L | Y | 4 | 6 | 0.87 (0.11, 1.63) | 15, 14 |
| Chen, Wei, Deng, & Sun, 2018 | Memory | P | 58 | - | 68.6 | 10 | 1 | L | N | 3 | 6 | 0.82 (0.53, 1.1) | 15, 20 |
| Stepankova et al., 2014 | EF | P | 72 | 15 | 68.3 | 6.6 | 1.4 | H | Y | 7 | 7 | 0.81 (0.2, 1.42) | 20, 25 |
| Garcia-Campuzano, Virues-Ortega, Smith, & Moussavi, 2013 | EF | P | 79 | - | 78.3 | 12 | 1.5 | L | N | 1 | 7 | 0.78 (−0.05, 1.61) | 13, 11 |
| Chen et al., 2018 | Memory | P | 58 | - | 68.6 | 10 | 1 | L | N | 3 | 6 | 0.73 (0.42, 1.03) | 19, 20 |
| Woolverton, Scogin, Shackelford, Black, & Duke, 2001 | Memory | P | 65 | 15.7 | 71.3 | 13 | 7 | H | N | 6 | 6 | 0.72 (0.04, 1.41) | 14, 23 |
| Margrett & Willis, 2006 | Reason | P | 50 | 15.9 | 71.3 | 10 | 2 | H | N | 3 | 6 | 0.64 (0.15, 1.13) | 34, 34 |
| Margrett & Willis, 2006 | Reason | P | 50 | 15.9 | 71.3 | 10 | 2 | H | N | 3 | 6 | 0.6 (0.1, 1.1) | 30, 34 |
| Heinzel, Rimpel, Stelzel, & Rapp, 2017 | EF | P | 79 | 16 | 65.39 | 9 | 2.25 | L | Y | 8 | 6 | 0.59 (−0.1, 1.28) | 18, 16 |
| Hill, Storandt, & Simeone, 1990 | Memory | A | 60 | 14 | 70.33 | - | - | L | N | 3 | 7 | 0.59 (0.01, 1.17) | 16, 16 |
| Chen et al., 2018 | Memory | P | 58 | - | 68.6 | 10 | 1 | L | N | 3 | 6 | 0.58 (0.28, 0.87) | 17, 20 |
| Stepankova et al., 2014 | EF | P | 72 | 15 | 68.3 | 3.3 | 0.7 | H | Y | 7 | 7 | 0.57 (−0.03, 1.17) | 20, 25 |
| Lussier, Bugaiska, & Bherer, 2016 | EF | A | 68 | 14 | 61.2 | 5 | 2.5 | L | Y | 6 | 7 | 0.56 (−0.01, 1.13) | 18, 38 |
| ǂAnguera et al., 2013 | EF | P | 87 | 16.5 | 67 | 12 | 3 | H | Y | 11 | 7 | 0.56 (−0.17, 1.29) | 15, 15 |
| Gao, Peng, & Wen, 2014 | EF | P | 53 | 6.9 | 84.5 | 0.5 | 0.5 | L | N | 2 | 5 | 0.55 (−0.06, 1.16) | 20, 23 |
| Bailey, Dagenbach, & Jennings, 2011 | EF | A | - | 16 | 69.5 | 6 | 3 | L | Y | 3 | 6 | 0.53 (−0.39, 1.44) | 10, 9 |
| Lussier, Gagnon, & Bherer, 2012 | EF | P | 78 | 14 | 68.5 | 5 | 2.5 | L | Y | 9 | 8 | 0.53 (−0.31, 1.36) | 13, 10 |
| Murphy, O’Sullivan, & Kelleher, 2014 | Sem. | A | 73 | - | 71.5 | - | - | H | N | 4 | 7 | 0.52 (−0.14, 1.17) | 19, 18 |
| Engvig et al., 2010 | Memory | P | 55 | 15.5 | 61 | 8 | 1 | L | N | 4 | 6 | 0.51 (−0.12, 1.15) | 21, 19 |
| Chen et al., 2018 | Reason | P | 58 | - | 68.6 | 10 | 1 | L | N | 3 | 6 | 0.49 (0.210.78) | 15, 20 |
| Buschkuehl et al., 2008 | EF | A | 59 | - | 80 | 18 | 1.5 | L | Y | 4 | 6 | 0.48 (−0.24, 1.19) | 13, 19 |
| McAvinue et al., 2013 | EF | A | 64 | - | 70 | 12. 5 | 2.5 | H | Y | 7 | 5 | 0.48 (−0.19, 1.14) | 19, 17 |
| Eramudugolla, Kiely, Chopra, & Anstey, 2017 | Speed | P | 47 | - | 71.9 | 10 | 1 | H | Y | 6 | 7 | 0.47 (0.18, 0.75) | 24, 24 |
| Payne & Stine-Morrow, 2017 | EF | A | 73 | 17 | 67.9 | 7.5 | 2.5 | H | Y | 8 | 8 | 0.45 (−0.17, 1.08) | 22, 19 |
| Dustman et al., 1992 | Speed | A | 62 | 14.5 | 65 | 33 | 3 | L | Y | 7 | 5 | 0.44 (0.13, 0.75) | 20, 20 |
| Caprio-Prevette & Fry, 1996 | Memory | A | 71 | - | 69.2 | 20 | 2 | L | N | 9 | 8 | 0.43 (0.06, 0.8) | 54, 61 |
| Dunlosky, Kubat-Silman, & Hertzog, 2003 | Memory | P | - | - | 67.73 | 4 | 2 | L | N | 4 | 8 | 0.41 (−0.09, 0.9) | 33, 31 |
| Brehmer, Westerberg, & Bäckman, 2012 | EF | A | 60 | 15.3 | 63.7 | 8.67 | 1.73 | H | Y | 9 | 9 | 0.4 (−0.19, 1) | 26, 19 |
| Ball et al., 2002 | Speed | P | 75 | 16 | 73.6 | 10 | 2 | H | Y | 3 | 7 | 0.37 (0.01, 0.72) | 712, 704 |
| Belchior et al., 2013 | Speed | P | - | 13 | 74 | - | - | L | Y | 10 | 7 | 0.37 (−0.37, 1.11) | 16, 13 |
| Cuenen et al., 2016 | EF | P | - | 71.25 | - | - | H | Y | 3 | 7 | 0.36 (−0.29, 1) | 19, 18 | |
| Zinke et al., 2014 | EF | P | 74 | 14 | 77.2 | 4.5 | 1.5 | L | Y | 4 | 7 | 0.35 (−0.09, 0.79) | 40, 40 |
| ǂAnguera et al., 2013 | EF | P | 87 | 67 | 12 | 3 | L | N | 4 | 7 | 0.34 (−0.37, 1.05) | 16, 15 | |
| Cuenen et al., 2016 | Speed | P | - | 16.5 | 71.25 | - | - | H | Y | 11 | 7 | 0.34 (−0.31, 0.98) | 19, 18 |
| Dunlosky et al., 2003 | EF | A | - | 67.73 | 4 | 2 | H | N | 3 | 8 | 0.34 (−0.16, 0.83) | 33, 31 | |
| Borella et al., 2014 | EF | A | - | 7 | 79 | 5 | 2.5 | L | Y | 12 | 8 | 0.33 (−0.3, 0.95) | 20, 20 |
| Kim, Chey, & Lee, 2017 | EF | P | 96 | 8.8 | 71.44 | 24 | 3 | L | N | 10 | 6 | 0.33 (−0.43, 1.09) | 14, 13 |
| Chan, Wu, Liang, & Yan, 2015 | EF | A | 54 | - | 70.6 | 10 | 1 | L | Y | 12 | 7 | 0.32 (−0.52, 1.17) | 12, 10 |
| Hayslip, 1989 | Reason | P | - | - | 70.58 | 5 | - | L | N | 2 | 8 | 0.31 (0, 0.62) | 76, 88 |
| Borella, Carretti, Zanoni, Zavagnin, & De Beni, 2013 | EF | A | 61 | - | 79 | 3 | 1 | L | Y | 11 | 8 | 0.3 (−0.36, 0.95) | 18, 18 |
| Salminen, Frensch, Strobach, & Schubert, 2016 | EF | P | 59 | 11 | 65 | 14 | 3 | L | Y | 13 | 7 | 0.29 (−0.29, 0.87) | 25, 21 |
| Lange & SuB, 2015 | EF | A | 68 | 14 | 67.97 | 12 | 2 | L | Y | 18 | 8 | 0.27 (−0.24, 0.78) | 31, 29 |
| †Nouchi, Saito, Nouchi, & Kawashima, 2016 | Speed | A | 61. 11 | 12 | 68.92 | 5 | 1.25 | H | Y | 11 | 8 | 0.26 (−0.21, 0.72) | 36, 36 |
| Dustman et al., 1992 | Speed | P | 62 | 14.5 | 65 | 33 | 3 | L | Y | 7 | 5 | 0.25 (−0.060.56) | 20, 20 |
| Woolverton et al., 2001 | Memory | P | 65 | 15.7 | 71.3 | 24 | 7 | H | N | 6 | 6 | 0.25 (−0.4, 0.89) | 16, 23 |
| ǂCheng et al., 2012 | Reason | P | 68 | 9.5 | 70.2 | 24 | 2 | L | N | 6 | 10 | 0.24 (−0.12, 0.6) | 59, 60 |
| Ji, Wang, Chen, Du, & Zhan, 2016 | EF | A | 14.2 | 70.06 | 12 | 3 | L | Y | 18 | 7 | 0.23 (−0.44, 0.91) | 18, 16 | |
| Hayslip, 1989 | Reason | P | - | - | 70.58 | 5 | - | L | N | 2 | 8 | 0.22 (−0.07, 0.51) | 92, 88 |
| Hill, Allen, & McWhorter, 1991 | Mem. | A | 39 | 14 | 70.4 | - | - | L | N | 3 | 7 | 0.21 (−0.38, 0.8) | 23, 21 |
| Lange & SuB, 2015 | EF | P | 68 | 14 | 67.97 | 12 | 2 | L | Y | 18 | 8 | 0.21 (−0.29, 0.71) | 31, 31 |
| Bellander et al., 2017 | Memory | A | 43 | 14.7 | 68.38 | 13. 2 | 2.25 | H | Y | 12 | 9 | 0.19 (−0.44, 0.82) | 19, 20 |
| Ball et al., 2002 | Reas. | P | 75 | 13 | 73.6 | 10 | 2 | L | N | 10 | 8 | 0.18 (−0.15, 0.51) | 705, 704 |
| Edwards et al., 2005 | Speed | P | 58 | - | 73.57 | 10 | 2 | L | Y | 1 | 6 | 0.18 (−0.17, 0.53) | 63, 63 |
| Scogin & Prohaska, 1992 | Memory | A | 70 | - | 68.4 | 24 | 7 | H | N | 8 | 7 | 0.16 (−0.53, 0.84) | 16, 17 |
| Baltes, Kliegl, & Dittmann-Kohli, 1988 | Reason | P | 68 | - | 73 | 10 | H | N | 8 | 6 | 0.15 (−0.36, 0.67) | 29, 29 | |
| Dahlin, Nyberg, Bäckman, & Neely, 2008 | EF | P | 62 | 12.5 | 68 | 11. 3 | 2.25 | L | N | 7 | 7 | 0.15 (−0.59, 0.88) | 13, 16 |
| Scogin & Prohaska, 1992 | Memory | P | 70 | 13 | 68.4 | 24 | 7 | L | Y | 10 | 7 | 0.15 (−0.49, 0.79) | 16, 23 |
| Sandberg, Rönnlund, Nyberg, & Stigsdotter Neely, 2014 | EF | P | 57 | 12 | 69.3 | 11. 3 | 2.25 | L | N | 17 | 7 | 0.14 (−0.57, 0.86) | 15, 15 |
| Basak & O’Connell, 2016 | EF | A | 67 | 14 | 68.8 | 5 | 2.5 | L | Y | 6 | 9 | 0.12 (−0.48, 0.72) | 22, 21 |
| Theill, Schumacher, Adelsberger, Martin, & Jäncke, 2013 | EF | P | 73 | 15 | 71.8 | 10 | 1 | L | N | 9 | 5 | 0.12 (−0.59, 0.83) | 12, 21 |
| Flegal & Lustig, 2015 | EF | A | - | 16.5 | 75 | - | - | L | Y | 8 | 7 | 0.09 (−0.41, 0.6) | 30, 30 |
| Wilkinson & Yang, 2012 | EF | A | - | 16 | 71.05 | 3 | 1.5 | L | N | 7 | 7 | 0.08 (−0.52, 0.69) | 42, 14 |
| †Li et al., 2016 | Memory | P | 76 | 13 | 68.3 | 16 | 3 | L | N | 13 | 7 | 0.07 (−1.01, 1.15) | 19, 4 |
| Zimmermann, von Bastian, Röcke, Martin, & Eschen, 2016 | Memory | A | 61 | 14.5 | 67.49 | 75 | 12. 5 | H | Y | 13 | 10 | 0.07 (−0.42, 0.55) | 36, 31 |
| Weicker et al., 2018 | EF | P | 50 | 15.8 | 67 | 9 | 2.25 | L | N | 18 | 8 | 0.06 (−0.25, 0.37) | 20, 20 |
| Ball et al., 2002 | Memory | P | 75 | 73.6 | 10 | 2 | L | Y | 1 | 8 | 0.06 (−0.28, 0.39) | 711, 704 | |
| Mohs et al., 1998 | Memory | A | 77 | 13 | 78 | 13. 5 | 1.5 | L | N | 10 | 8 | 0.06 (−0.28, 0.4) | 64, 69 |
| Sutter, Zöllig, & Martin, 2013 | Sem. | A | 55 | 10 | 72.27 | 1.5 | 0.5 | H | N | 5 | 6 | 0.05 (−0.57, 0.67) | 19, 21 |
| Bürki, Ludwig, Chicherio, & de Ribaupierre, 2014 | EF | P | 69 | 14.3 | 68 | 5 | 2.5 | L | N | 3 | 6 | 0.04 (−0.54, 0.62) | 22, 23 |
| Hill et al., 1991 | Memory | A | 39 | 15 | 70.4 | - | - | L | Y | 11 | 7 | 0.04 (−0.65, 0.73) | 27, 21 |
| Bailey et al., 2011 | EF | A | - | 14.5 | 70.5 | 6 | 3 | L | Y | 11 | 6 | 0.03 (−0.85, 0.91) | 10, 10 |
| Edwards et al., 2002 | Speed | A | 63 | 16 | 76 | 10 | 2 | L | Y | 3 | 7 | 0.03 (−0.38, 0.44) | 44, 47 |
| Weicker et al., 2018 | EF | A | 50 | 16.3 | 67 | 9 | 2.25 | L | Y | 18 | 8 | 0.02 (−0.29, 0.33) | 20, 20 |
| Hampstead et al., 2012 | Memory | A | - | - | 72 | 3 | 3 | L | Y | 2 | 9 | 0.02 (−0.84, 0.87) | 11, 10 |
| Richmond, Morrison, Chein, & Olson, 2011 | EF | A | 80 | - | 66 | 10 | 2.5 | L | Y | 8 | 7 | 0.02 (−0.6, 0.64) | 21, 19 |
| G. H. Kim et al., 2015 | Speed | A | - | - | 72.3 | 16 | 2 | L | N | 9 | 6 | 0.01 (−0.4–0.04) | 14, 14 |
| Bürki et al., 2014 | EF | P | 69 | 15 | 68 | 5 | 2.5 | L | Y | 11 | 6 | 0.01 (−0.59, 0.61) | 20, 23 |
| Lussier, Brouillard, & Bherer, 2015 | EF | A | 72 | 14.5 | 72.6 | 12 | 1 | L | Y | 6 | 9 | −0.01 (−0.66, 0.64) | 13, 31 |
| von Bastian, Langer, Jäncke, & Oberauer, 2013 | EF | A | 40 | - | 68 | 10 | 2.5 | H | Y | 6 | 9 | −0.01 (−0.53, 0.51) | 27, 30 |
| Bailey et al., 2011 | EF | A | - | 16 | 69.5 | 6 | 3 | L | Y | 3 | 6 | −0.07 (−0.93, 0.78) | 11, 10 |
| Flegal & Lustig, 2015 | EF | A | - | 14.5 | 75 | - | - | L | Y | 6 | 7 | −0.08 (−0.58, 0.43) | 30, 30 |
| Boot et al., 2013 | Speed | P | 53 | 16.5 | 70 | 60 | 5 | L | Y | 26 | 5 | −0.08 (−0.41, 0.22) | 21, 20 |
| Lussier et al., 2015 | EF | A | 72 | - | 72.6 | 12 | 1 | H | Y | 8 | 9 | −0.08 (−0.71, 0.56) | 14, 31 |
| Goghari & Lawlor-Savage, 2017 | EF | P | 67 | 13.3 | 70.43 | 20 | 2.5 | L | N | 8 | 7 | −0.11 (−0.58, 0.35) | 36, 36 |
| N. Zimmermann, Netto, Amodeo, Ska, & Fonseca, 2014 | EF | A | - | 15.4 | 68.19 | 24 | 4 | H | Y | 40 | 6 | −0.11 (−1.17, 0.95) | 8, 6 |
| Bier et al., 2014 | Speed | P | - | 15 | 69.12 | 6 | 3 | L | N | 4 | 8 | −0.12 (−0.92, 0.68) | 12, 12 |
| Goghari & Lawlor-Savage, 2017 | Reason | P | 67 | 15.4 | 70.43 | 20 | 2.5 | H | Y | 8 | 7 | −0.13 (−0.59, 0.33) | 36, 36 |
| Edwards et al., 2013 | Speed | P | 69 | - | 73.99 | 20 | 2.5 | L | Y | 16 | 6 | −0.26 (−0.77, 0.25) | 27, 33 |
| Hill et al., 1990 | Engage | A | 60 | 14.3 | 70.33 | - | - | L | N | 3 | 7 | −0.78 (−1.5, − 0.06) | 16, 16 |
| Average g = 0.3, k = 94 | |||||||||||||
| HA: Multi-Component Training | |||||||||||||
| Cavallini et al., 2015 | Class | A | - | 8.5 | 85.13 | 6 | 1 | H | -- | 8 | 7 | 1.88 (1.07, 2.69) | 16, 18 |
| Postigo, Javier, & Trives, 2010 | Class | A | 64 | - | 66.9 | 15 | 3 | L | -- | 2 | 9 | 1.47 (0.66, 2.28) | 15, 15 |
| Postigo et al., 2010 | Class | P | 64 | - | 66.9 | 15 | 3 | L | -- | 2 | 9 | 1.08 (0.32, 1.85) | 15, 15 |
| McDougall & House, 2012 | Lab | P | 51 | - | 74.6 | - | - | H | Y | 4 | 5 | 1 (0.35, 1.65) | 21, 20 |
| Pereira-Morales, Cruz-Salinas, Aponte, & Pereira-Manrique, 2018 | Lab | P | 90 | 12 | 67 | 36 | 4.5 | H | Y | 5 | 7 | 0.78 (0.23,1.4) | 17,11 |
| Fairchild & Scogin, 2010 | Class | A | 81 | - | 72.4 | 4.5 | 0.75 | L | -- | 9 | 7 | 0.66 (0.1, 1.21) | 28, 25 |
| Pereira-Morales et al., 2018 | Lab | P | 90 | 12 | 67 | 32 | 4 | H | Y | 5 | 7 | 0.58 (0.02,1.03) | 12,11 |
| †Nouchi et al., 2012 | Lab | A | 53 | 13.3 | 68 | 5 | 1.25 | H | Y | 8 | 10 | 0.56 (−0.2, 1.31) | 14, 14 |
| Cantarella, Borella, Marigo, & De Beni, 2017 | Class | A | 50 | 8 | 69.34 | 3 | 1 | L | -- | 3 | 7 | 0.48 (−0.22, 1.18) | 16, 16 |
| Uchida & Kawashima, 2008 | Class | P | 44 | 12.5 | 75 | 40. 3 | 1.75 | H | -- | 9 | 9 | 0.44 (0.03, 0.84) | 49, 46 |
| Mahncke et al., 2006 | Lab | A | 50 | 16.3 | 70.9 | 45 | 5 | H | Y | 2 | 6 | 0.41 (0.02, 0.81) | 50, 51 |
| Craik et al., 2007 | Class | A | 55 | - | 78.7 | 36 | 3 | L | -- | 22 | 6 | 0.38 (−0.2, 0.95) | 29, 20 |
| Tranter & Koutstaal, 2008 | Engage | P | 75 | 14 | 67.8 | 9 | 1.5 | H | -- | 2 | 7 | 0.38 (−0.22, 0.97) | 22, 22 |
| Kwok et al., 2013 | Class | P | 85 | 3.56 | 75.4 | 8 | 1 | L | -- | 9 | 9 | 0.37 (0.08, 0.67) | 86, 90 |
| †Cheng et al., 2012 | Class | A | 57 | 9.5 | 70 | 3 | 1.5 | L | -- | 6 | 10 | 0.36 (−0.02, 0.73) | 54, 60 |
| Shatil, 2013 | Lab | A | 68 | 15 | 76.8 | 32 | 2 | L | Y | 13 | 5 | 0.34 (−0.16, 0.84) | 33, 29 |
| Shatil, Mikulecká, Bellotti, & Bureš, 2014 | Lab | A | 63 | 18 | 68 | 8 | 1 | L | Y | 6 | 8 | 0.33 (−0.03, 0.69) | 60, 59 |
| Golino, Flores Mendoza, & Golino, 2017 | Lab | P | 77 | 7.25 | 69.69 | 15 | 1.25 | L | N | 8 | 8 | 0.28 (−0.16, 0.73) | 47, 33 |
| †Lee et al., 2013 | Lab | P | - | - | 65 | 12 | 0.5 | L | N | 6 | 7 | 0.28 (−0.43, 0.99) | 15, 16 |
| Mahncke et al., 2006 | Lab | P | 50 | 16.3 | 70.9 | 45 | 5 | H | Y | 2 | 6 | 0.28 (−0.11, 0.66) | 50, 54 |
| Gajewski & Falkenstein, 2012 | Lab | P | 59 | - | 70.9 | 48 | 3 | L | Y | 3 | 7 | 0.24 (−0.23, 0.71) | 32, 40 |
| †Li et al., 2016 | Lab | P | 62 | 15 | 68.3 | 16 | 3 | L | Y | 6 | 7 | 0.23 (−0.46, 0.91) | 20, 14 |
| Rasmusson, Rebok, Bylsma, & Brandt, 1999 | Lab | P | 59 | 13 | 78 | 13. 5 | 1.5 | L | N | 13 | 7 | 0.23 (−0.57, 1.04) | 13, 11 |
| Lima-Silva et al., 2010 | Class | P | 81 | 16.2 | 64.69 | 7.5 | 7.5 | L | -- | 4 | 7 | 0.22 (−0.26, 0.69) | 37, 32 |
| Park et al., 2014 | Engage | A | 73 | 8.38 | 71.67 | 198 | 16. 51 | L | -- | 12 | 9 | 0.22 (−0.26, 0.7) | 29, 39 |
| Kalbe et al., 2018 | Lab | A | 77 | 15 | 67 | 13. 5 | 2.25 | L | Y | 13 | 9 | 0.2 (0.01, 0.4) | 21, 18 |
| ǂSmith et al., 2009 | Lab | A | 52 | - | 75 | 40 | 5 | L | N | 9 | 9 | 0.2 (0.02, 0.38) | 242, 245 |
| van Zon, Kirby, & Anderson, 2016 | Class | A | 69 | 15.6 | 83.3 | 8 | 1 | H | -- | 8 | 7 | 0.2 (−0.46, 0.85) | 18, 18 |
| Belchior et al., 2013 | Engage | P | - | 16 | 74 | - | - | H | Y | 3 | 7 | 0.19 (−0.53, 0.92) | 17, 13 |
| Sutter et al., 2013 | Lab | P | 55 | 10 | 72.27 | 1.5 | 0.5 | H | N | 5 | 6 | 0.19 (−0.42, 0.8) | 21, 21 |
| van Muijden et al., 2012 | Lab | A | 44 | 12 | 67 | 24. 5 | 3.5 | H | Y | 10 | 7 | 0.19 (−0.34, 0.72) | 44, 20 |
| Basak et al., 2008 | Engage | A | 74 | 13.5 | 69 | 23. 5 | 4.5 | L | N | 9 | 6 | 0.18 (−0.46, 0.82) | 19, 19 |
| Belchior et al., 2013 | Engage | P | - | 16 | 74 | - | - | H | Y | 3 | 7 | 0.18 (−0.58, 0.94) | 14, 13 |
| Gajewski & Falkenstein, 2012 | Lab | A | 59 | 16.2 | 70.9 | 48 | 3 | L | Y | 17 | 7 | 0.18 (−0.3, 0.66) | 32, 34 |
| Buitenweg, van de Ven, Prinssen, Murre, & Ridderinkhof, 2017 | Lab | A | 60 | - | 67.5 | 30 | 2.5 | L | Y | 25 | 7 | 0.18 (0.004, 0.35) | 33, 50 |
| G. H. Kim et al., 2015 | Lab | P | 71 | 13.7 | 67.5 | 90 | 7.5 | L | Y | 3 | 9 | 0.18 (−0.37, 0.73) | 23, 28 |
| Simpson, Camfield, Pipingas, Macpherson, & Stough, 2012 | Lab | A | 53 | - | 62.3 | 7 | 2.3 | H | Y | 10 | 9 | 0.17 (−0.54, 0.87) | 17, 14 |
| Ordonez, Yassuda, & Cachioni, 2011 | Engage | P | 71 | 9.3 | 67.4 | 30 | 2 | L | -- | 7 | 6 | 0.16 (−0.45, 0.76) | 22, 20 |
| Stine-Morrow, Parisi, Morrow, & Park, 2008 | Engage | P | - | 16 | 72.5 | - | - | L | -- | 13 | 5 | 0.15 (−0.18, 0.47) | 87, 63 |
| Park et al., 2014 | Engage | A | 73 | 16.2 | 71.67 | 198 | 16. 51 | L | -- | 4 | 9 | 0.14 (−0.29, 0.58) | 42, 39 |
| Ballesteros et al., 2014 | Lab | P | - | 74 | - | - | H | N | 18 | 7 | 0.13 (−0.59, 0.86) | 17, 13 | |
| G. H. Kim et al., 2015 | Lab | P | 71 | 13.5 | 67.5 | 90 | 7.5 | L | N | 15 | 9 | 0.12 (−0.45, 0.7) | 20, 28 |
| Banducci et al., 2017 | Engage | A | 63 | 14.5 | 69.46 | 10 | 2.5 | H | - | 8 | 8 | 0.11 (−0.19, 0.41) | 86, 82 |
| Lopes & Argimon, 2016 | Lab | P | 80 | 12.5 | 68.75 | 12 | 1.5 | L | N | 17 | 5 | 0.11 (−0.32, 0.54) | 45, 38 |
| Park et al., 2014 | Engage | A | 73 | 12.7 | 71.67 | 198 | 16. 51 | L | - | 6 | 9 | 0.11 (−0.35, 0.56) | 35, 39 |
| Peretz et al., 2011 | Lab | A | 63 | 16.2 | 68 | 15 | 1.25 | L | N | 4 | 9 | 0.11 (−0.25, 0.47) | 66, 55 |
| Stern et al., 2011 | Engage | A | 57 | 16 | 66 | 36 | 3 | L | N | 14 | 7 | 0.11 (−0.56, 0.77) | 17, 18 |
| Hars, Herrmann, Gold, Rizzoli, & Trombetti, 2014 | Engage | P | 96 | - | 75.5 | 25 | 1 | L | -- | 5 | 8 | 0.1 (−0.24, 0.44) | 66, 68 |
| Oh, Seo, Lee, Song, & Shin, 2017 | Lab | P | 53 | 13.9 | 59.3 | 13. 3 | 1.3 | L | Y | 10 | 6 | 0.1 (−0.44, 0.34) | 18, 16 |
| Ballesteros et al., 2017 | Lab | A | 79 | 16 | 65 | 8 | 4 | H | Y | 7 | 7 | 0.097 (−0.2, 0.38) | 30, 25 |
| Souders et al., 2017 | Lab | A | 56 | 16 | 72.35 | 22. 5 | 5.25 | H | Y | 8 | 7 | 0.09 (−0.42, 0.59) | 30, 30 |
| Oh et al., 2017 | Lab | P | 53 | - | 59.3 | 13. 3 | 1.3 | H | Y | 10 | 6 | 0.07 (−0.74, 0.68) | 19, 16 |
| Reijnders, Geusgens, Ponds, & van Boxtel, 2017 | Class | P | 72 | - | 56.35 | 7 | 2.5 | H | -- | 6 | 7 | 0.07 (−0.14, 0.28) | 228, 148 |
| Whitlock, McLaughlin, & Allaire, 2012 | Engage | P | 51 | - | 65 | 14 | 7 | L | Y | 7 | 4 | 0.07 (−0.55, 0.7) | 19, 20 |
| Miller et al., 2013 | Lab | P | 67 | 13.9 | 81 | 15 | 1.88 | L | Y | 9 | 7 | 0.06 (−0.39, 0.52) | 38, 36 |
| G. J. McDougall et al., 2010 | Class | A | 79 | 16 | 74 | 8 | 4 | L | -- | 5 | 7 | 0.05 (−0.2, 0.3) | 127, 117 |
| Bozoki, Radovanovic, Winn, Heeter, & Anthony, 2013 | Lab | A | 58 | 13.5 | 69 | 27 | 2.5 | H | N | 9 | 7 | 0.04 (−0.47, 0.55) | 32, 28 |
| Stern et al., 2011 | Engage | P | 57 | 16.8 | 65 | 36 | 3 | L | N | 14 | 7 | 0.01 (−0.64, 0.66) | 17, 19 |
| Buiza et al., 2008 | Lab | P | 73 | - | 74 | 270 | 3 | L | N | 9 | 5 | 0.001 (−0.31, 0.32) | 68, 85 |
| Hynes, 2016 | Lab | P | 75 | 16 | 70.99 | 6.25 | 1.75 | H | Y | 3 | 5 | 0.001 (−0.79, 0.78) | 13, 12 |
| Legault et al., 2011 | Lab | A | 51 | - | 76.4 | 16 | 1 | L | Y | 10 | 8 | −0.01 (−0.7, 0.67) | 16, 17 |
| Buitenweg et al., 2017 | Lab | A | 60 | - | 67.5 | 30 | 2.5 | L | Y | 25 | 7 | −0.04 (−0.25, 0.16) | 56, 50 |
| Buiza et al., 2008 | Lab | P | 73 | - | 74 | 270 | 3 | L | N | 9 | 5 | −0.04 (−0.35, 0.26) | 85, 85 |
| Rasmusson et al., 1999 | Class | P | 59 | 13.7 | 78 | 13. 5 | 1.5 | L | -- | 6 | 7 | −0.1 (−0.96, 0.76) | 10, 11 |
| Rasmusson et al., 1999 | Class | P | 59 | - | 78 | 13. 5 | 1.5 | H | -- | 6 | 7 | −0.12 (−0.94, 0.7) | 12, 11 |
| Boot et al., 2013 | Lab | P | 58 | 15 | 70 | 60 | 5 | H | Y | 26 | 5 | −0.36 (−0.99, 0.26) | 20, 20 |
| Giuli, Papa, Lattanzio, & Postacchini, 2016 | Lab | P | 80 | 15 | 72.45 | 15 | 1.5 | L | N | 6 | 7 | −0.49 (−0.89, − 0.09) | 47, 53 |
| Average g = 0.24, k = 67 | |||||||||||||
| MCI: Single-Component Training | |||||||||||||
| Mudar et al., 2017 | Memory | A | 4 | 16.5 | 73 | 8 | 2 | H | N | 9 | 8 | 0.98 (0.72, 1.23) | 23, 27 |
| Savulich et al., 2017 | Memory | A | 40 | 10 | 75.5 | 8 | 2 | L | Y | 4 | 8 | 0.68 (0.05, 1.2) | 21, 21 |
| Calero & Navarro, 2007 | Memory | P | - | - | 14 | 2 | L | Y | 3 | 6 | 0.56 (0.02, 1.1) | 27, 28 | |
| Mansbach, Mace, & Clark, 2017 | Memory | P | 58 | 78.08 | 4.5 | 1.5 | L | N | 1 | 9 | 0.52 (−0.39, 1.42) | 12, 8 | |
| Carretti et al., 2013 | EF | A | 50 | 7.3 | 70 | 4.5 | 2 | L | Y | 8 | 7 | 0.51 (−0.38, 1.4) | 10, 10 |
| Vance et al., 2007 | Speed | A | 48 | 14 | 75.2 | 10 | 1 | L | Y | 8 | 7 | 0.31 (0, 0.62) | 82, 77 |
| F Scogin, Storandt, & Lott, 1985 | Memory | P | 79 | 66 | 16 | 7 | H | N | 8 | 6 | 0.28 (−0.3, 0.86) | 20, 27 | |
| Giuli et al., 2016 | Memory | P | 63 | 11.5 | 76.25 | 7.5 | 0.75 | L | N | 13 | 7 | 0.26 (−0.14, 0.65) | 48, 49 |
| †Konsztowicz, Anton, Crane, Moafmashhadi, & Koski, 2013 | Memory | P | 54 | 6 | 77.5 | 10. 5 | 1.5 | L | N | 12 | 6 | 0.26 (−0.98, 1.49) | 7, 4 |
| Hyer et al., 2016 | EF | A | 53 | 75 | 16. 7 | 3.3 | L | N | 5 | 8 | 0.2 (−0.32, 0.71) | 29, 30 | |
| Hampstead, Stringer, Stilla, Giddens, & Sathian, 2012 | Memory | A | - | 17.5 | 72 | 3 | 3 | L | Y | 2 | 9 | 0.16 (−0.58, 0.9) | 14, 14 |
| Finn & McDonald, 2015 | EF | P | 29 | 13.7 | 75.2 | - | - | L | Y | 6 | 7 | 0.14 (−0.66, 0.94) | 12, 12 |
| Emsaki, NeshatDoost, Tavakoli, & Barekatain, 2017 | Memory | P | - | 14.1 | 63.4 | 6.7 | 1.3 | L | N | 8 | 6 | 0.13 (−0.62, 0.88) | 10, 10 |
| Olchik, Farina, Steibel, Teixeira, & Yassuda, 2013 | Memory | P | 80 | 13.2 | 70.3 | 12 | 3 | L | N | 8 | 8 | 0.13 (−0.59, 0.85) | 16, 14 |
| Jean, Bergeron, Thivierge, & Simard, 2010 | Memory | A | 59 | 14.5 | 68.5 | 4.5 | 1.5 | L | N | 20 | 8 | 0.05 (−0.79, 0.88) | 11, 11 |
| Gagnon & Belleville, 2012 | Speed | A | - | 14 | 67 | 6 | 3 | L | Y | 8 | 9 | 0.04 (−0.76, 0.84) | 12, 12 |
| Cahn-Weiner, Malloy, Rebok, & Ott, 2003 | Memory | A | 59 | 12.9 | 76.9 | 6 | 1 | L | N | 13 | 6 | 0.01 (−0.67, 0.68) | 17, 17 |
| Cohen-Mansfield et al., 2015b | Memory | P | 72 | 15 | 73.5 | 10 | 1 | L | N | 5 | 9 | 0.001 (−0.8, 0.8) | 12, 12 |
| Average g = 0.27, k = 18 | |||||||||||||
| MCI: Multi-Component Training | |||||||||||||
| Herrera, Chambon, Michel, Paban, & Alescio-Lautier, 2012 | Lab | A | 50 | - | 76.7 | 24 | 2 | L | Y | 9 | 8 | 1.14 (0.24, 2.04) | 11, 11 |
| Vranić, Španić, Carretti, & Borella, 2013 | Class | A | 80 | 13 | 73 | 13. 5 | 1.5 | L | -- | 14 | 7 | 0.92 (0.33, 1.51) | 31, 20 |
| †Gooding et al., 2016 | Engage | A | 42 | 15.1 | 75.57 | 30 | 2 | L | Y | 6 | 7 | 0.82 (0.2, 1.45) | 23, 20 |
| Man, Lee, Yu, & Yip, 2013 | Lab | P | 68 | - | 77.7 | 6 | 1 | L | N | 6 | 8 | 0.63 (−0.49, 1.75) | 7, 6 |
| †Gooding et al., 2016 | Lab | A | 42. 9 | 15.1 | 75.57 | 30 | 2 | L | Y | 6 | 7 | 0.6 (0.03, 1.17) | 31, 20 |
| Buschert et al., 2011 | Lab | A | 50 | 12.5 | 71.2 | 48 | 2 | L | N | 7 | 9 | 0.58 (−0.28, 1.44) | 10, 12 |
| Kinsella et al., 2009 | Class | P | 54 | 10.5 | 76.8 | 6.5 | 1.5 | L | -- | 6 | 8 | 0.55 (−0.04, 1.13) | 22, 24 |
| Rojas et al., 2013 | Class | P | 43 | 12 | 74 | 96 | 4 | L | -- | 3 | 6 | 0.55 (−0.17, 1.28) | 15, 15 |
| †Ceccato et al., 2012 | Lab | P | 80 | - | 86.4 | 18 | 1.5 | L | N | 2 | 9 | 0.51 (−0.05, 1.08) | 27, 23 |
| Hwang et al., 2012 | Lab | P | 70 | - | 65 | 18 | 1 | L | Y | 9 | 6 | 0.51 (−0.75, 1.77) | 5, 5 |
| Kawashima, 2013 | Lab | P | - | 6.7 | 85 | 35 | 1.5 | L | N | 15 | 5 | 0.51 (−0.19, 1.22) | 16, 16 |
| Moro et al., 2015 | Lab | P | - | 9.5 | 75.5 | - | - | L | N | 11 | 6 | 0.49 (−0.23, 1.22) | 15, 15 |
| ǂLampit et al., 2014 | Lab | A | 68. 8 | 72 | 24 | 2 | L | Y | 6 | 9 | 0.42 (−0.03, 0.87) | 39, 38 | |
| Moro et al., 2012 | Class | P | - | 9.5 | 70.9 | - | - | L | -- | 16 | 6 | 0.41 (−0.31, 1.14) | 15, 15 |
| Boripuntakul, Kothan, Methapatara, Munkhetvit, & Sungkarat, 2012 | Lab | P | 60 | 10 | 78 | 18 | 3 | L | N | 4 | 6 | 0.36 (−0.89, 1.61) | 5, 5 |
| Kurz, Pohl, Ramsenthaler, & Sorg, 2009 | Engage | P | 57 | 11 | 70.6 | 88 | 22 | L | -- | 4 | 4 | 0.36 (−0.42, 1.14) | 18, 10 |
| Belleville et al., 2018 | Lab | P | 55 | 14.5 | 72.2 | 16 | 2 | L | N | 4 | 7 | 0.28 (0.23,0.47) | 40, 44 |
| Regan, Wells, Farrow, O’Halloran, & Workman, 2017 | Engage | P | 55 | - | 77.2 | 8 | 4 | H | -- | 9 | 7 | 0.25 (−0.39, 0.89) | 25, 15 |
| Man et al., 2013 | Lab | P | 68 | - | 77.7 | 6 | 1 | L | N | 6 | 8 | 0.21 (−0.93, 1.34) | 6, 6 |
| Belleville et al., 2006 | Lab | P | - | - | 66 | 16 | 2 | L | N | 7 | 5 | 0.2 (−0.64, 1.04) | 17, 8 |
| Zhuang et al., 2013 | Lab | P | 76 | 7 | 83 | 90 | 3.75 | L | Y | 6 | 7 | 0.19 (−0.5, 0.88) | 19, 14 |
| †Konsztowicz et al., 2013 b | Engage | P | 50 | 11.5 | 78 | 10. 5 | 1.5 | L | -- | 13 | 6 | 0.18 (−1.02, 1.38) | 8, 4 |
| Diamond et al., 2015 | Lab | P | 67 | 14 | 66.5 | 28 | 4 | L | Y | 12 | 9 | 0.15 (−0.35, 0.64) | 36, 28 |
| Mowszowski et al., 2014 | Lab | P | 62 | - | 66.6 | 14 | 2 | L | N | 6 | 8 | 0.13 (−0.51, 0.77) | 25, 15 |
| ǂBarnes et al., 2009 | Lab | A | 40 | 17 | 74 | 50 | 8.3 | H | Y | 13 | 9 | 0.11 (−0.46, 0.68) | 22, 25 |
| Rapp, Brenes, Marsh, Brenes, & Marsh, 2002 | Class | P | 58 | - | 75.1 | 12 | 2 | L | -- | 8 | 7 | 0.11 (−0.79, 1.01) | 9, 10 |
| Finn & McDonald, 2011 | Lab | P | 50 | 10 | 74.2 | 10 | 1 | L | N | 8 | 8 | 0.08 (−0.9, 1.06) | 8, 8 |
| †Jeong et al., 2016 | Lab | P | 50 | 10 | 70.3 | 36 | 3 | H | N | 8 | 7 | 0.08 (−0.24, 0.41) | 71, 77 |
| †Jeong et al., 2016 | Lab | P | 50 | 12.5 | 70.3 | 30 | 2.5 | H | Y | 6 | 7 | 0.08 (−0.23, 0.4) | 76, 77 |
| Leung et al., 2015 | Lab | A | 78 | 9 | 70.1 | 39 | 3 | L | Y | 8 | 8 | 0.07 (−0.2, 0.34) | 109, 100 |
| †Vidovich et al., 2015 | Class | A | 54 | - | 75 | 15 | 3 | L | -- | 6 | 9 | 0.03 (−0.28, 0.35) | 77, 78 |
| †Küster et al., 2016 | Lab | P | 55 | 14 | 71.4 | 50 | 5 | H | Y | 22 | 6 | −0.06 (−0.72, 0.59) | 16, 20 |
| Greenaway, Duncan, & Smith, 2013 | Engage | P | 60 | 16.4 | 72.5 | 12 | 2 | L | -- | 3 | 7 | −0.1 (−0.75, 0.54) | 18, 19 |
| Cohen-Mansfield et al., 2015b | Class | P | 72 | 15 | 73 | 10 | 1 | L | -- | 5 | 9 | −0.17 (−1.04, 0.69) | 9, 12 |
| Belleville et al., 2018 | Engage | P | 55 | 14.5 | 72.2 | 16 | 2 | L | -- | 4 | 7 | −0.22 (−1.05, 0.37) | 43, 44 |
| Olchik et al., 2013 | Class | P | 80 | 13.2 | 70.3 | 12 | 3 | L | -- | 8 | 8 | −0.33 (−1.04, 0.39) | 17, 14 |
| Average g = 0.29, k = 36 | |||||||||||||
Note. Studies are ordered based on their individual g, from largest to smallest in their respective subgroups. Missing values are denoted by “-”. Publications with either multiple training groups or multiple control groups are marked by letters (a, b, c, or d) behind the first author’s last name.
denotes studies that reported pre-post mean difference instead of raw means.
denotes studies that reported standardized effect sizes. Training Type = different types of training.. EF=Executive Functions. Reason= Reasoning. Sem.=Semantic. Engage=Engagement-based. Lab=Lab-based. Class=Classroom-based. Ctrl= Control. A=Active. P=Passive. % F= percent of female participants. Edu = years of formal education. Tot. Hr = total training hours. Hr. per Wk.= training hours per week. Adapt = adaptiveness.
# cog. outcomes = number of cognitive outcomes. For training location, “H”= home-based training, “L” = Lab-based training. For adaptiveness, “Y” = individualized adaptive, “N” = not individualized adaptive or not adaptive, “--” = not applicable. MCI=Mild cognitive impairment. g=net-gain effect size, NT=sample size in the training group, NC=sample size in the control group. g denotes net-gain effect size. k denotes sample size for g.
Included studies were categorized into two cognitive training modules: single-component or multi-component training. Single-component training studies trained only one cognitive function (memory, executive functions, processing speed, reasoning, or language, See Table 2).
Single-component executive functions training included training on one of the three components of executive functions, as defined in Miyake et al. (2000) and McCabe et al. (2010), viz. updating (the constant monitoring and rapid addition/deletion of working memory contents, e.g., N-back task), shifting (switching flexibly between tasks or mental sets, e.g., task-switching), inhibition (deliberate overriding of dominant or prepotent responses, e.g., go-no-go task) and working memory capacity. If the training module involved two or more components of executive functions, it was not considered as a single-component training study.
Studies were considered under memory training, if they trained either mnemonic abilities (Verhaeghen, Marcoen, & Goossens, 1992) or episodic memory (e.g., associative learning, Naveh-Benjamin, 2000). Studies were considered under processing speed training, if they trained on perceptual discrimination, speed of processing, or attentional abilities (Ball, Edwards, & Ross, 2007; Bier, de Boysson, & Belleville, 2014; Strauss, Sherman & Spreech, 2006).
Studies were considered under reasoning training if they trained logical reasoning or progressive matrices (Ball et al., 2002; Willis & Schaie, 1986, 1994). Lastly, studies were considered language training if they trained on verbal fluency or crossword puzzles (Miller, 1984; Strauss, Sherman & Spreech, 2006).
In contrast, multi-component studies trained two or more cognitive abilities, by either targeting certain specific cognitive abilities sequentially or by training individuals on multiple cognitive abilities non-specifically and simultaneously, such as video game training (Basak et al., 2008) and engagement training (Park et al., 2014; Stine-Morrow et al., 2008). Training that targeted multiple specific cognitive abilities were either conducted in the lab (lab-based training; e.g. Souders et al., 2017), where participants were typically trained individually, or were conducted in classroom settings (class-based training; Cantarella, Borella, Carretti, Kliegel, & de Beni, 2017), where the participants did the training in groups. Class-based training studies, compared to lab-based training studies, were more socially interactive. Given that social interaction can interact with cognitive gains, we considered lab-based and class-based training as two different modules of targeted multi-component training.
Training studies using video games as training tools were categorized based on game type. For example, a video game training study (Dustman, Emmerson, Steinhaus, Shearer, & Dustman, 1992) that used only speed-based, short-playing ATARI games (e.g., Pacman) was categorized as a single-component training. All commercially available or in-lab “brain training” games, such as Lumosity (Ballesteros et al., 2017) and Nintendo Brain Fit (van Muijden, Band, & Hommel, 2012), were categorized as lab-based multi-component training studies. Training module categorization was performed independently by the second and third author (Cohen’s kappa=0.94), and the classification was determined by first author.
Seven cognitive constructs were created based on the reported cognitive outcomes and their classification according to the following references: 1. episodic memory (e.g., subsequent memory or associative learning; Brewer et al., 1998; Wagner et al., 1998; Naveh-Benjamin, 2000; Strauss, Sherman & Spreech, 2006), 2. executive functions (e.g., shifting, working memory capacity, updating or inhibition; Miyake et al., 2000; McCabe et al., 2010), 3. processing speed (perceptual discrimination, attention or visual perception; Salthouse, 1990; Stauss, Sherman & Spreech, 2006) 4. short term memory (Baddeley, Eysenck & Anderson, 2015), 5. everyday functioning, encompassing both subjective measures (e.g., Everyday Memory Questionnaire; Royle & Lincoln, 2008) and objective tests (e.g., Everyday Cognition Battery; Allaire et al., 2013), 6. Reasoning (e.g. progressive matrices; Raven, 2003), and 7. language and semantic knowledge (Strauss, Sherman & Spreech, 2006; Park et al., 2002). A list of outcome tasks and their assigned cognitive constructs are provided in Supp. Table 1.
Coding of these seven cognitive constructs was independently conducted by the first and second author (Cohen’s kappa=0.95) using the above-mentioned references as a guide. Moreover, two types of transfer (near and far) were determined independently by both first and second authors using the following approach. For single-component training, near transfer constituted the cognitive construct that was the same as the cognitive component trained (e.g., episodic memory was a near construct for memory training). Far transfer included any of the other six untrained cognitive constructs that were reported. For multi-component training, near transfer constituted outcome measures from the trained cognitive components.
In class- and lab-based training, the trained cognitive components were specified and targeted during the training. For example, in a class-based training study (Cantarella, Borella, Carretti, et al., 2017), which used mnemonic techniques and memory for grocery lists as training tools, episodic memory was considered as near transfer. Far transfer included the remaining cognitive outcomes that were reported by the authors.
For engagement training approaches, we read each paper in detail to determine what the cognitive domains that were directly trained. For example, in engaging video game training, we reviewed the video games online as well as read the authors’ description, to determine which of the seven cognitive constructs were trained in the video game. All time-based video games were assumed to target speed of processing, given the nature of the game. Moreover, many video games also encompassed one or more components of executive functions, and reasoning. These abilities were coded as near-transfer. Other abilities, such as language or everyday functioning were considered to be far transfer. For engaging cognitively stimulating activities, near transfer typically included a combination of reasoning and episodic memory. Specific studies may also include executive functions or processing speed or everyday functioning. For example, Senior Odyssey, a creative problem-solving program, involved group-based discussions to reason and solve novel complex problems within a determined timeline, reasoning, episodic memory and processing speed were considered as near abilities.
Risk of Bias in Individual Studies
Risk of bias in individual studies was assessed using the Physiotherapy Evidence Database (PEDro) scale. The PEDro scale is an 11-item scale designed to assess the quality and reporting of RCTs (Maher, Sherrington, Herbert, Moseley, & Elkins, 2003), and has been used extensively in past reviews of cognitive training (Gates, Sachdev, Fiatarone Singh, & Valenzuela, 2011; Li et al., 2011; Mewborn et al., 2017; Toril et al., 2014). PEDro was chosen over an alternate tool for assessing risk of bias in individual studies, the Cochrane Collaboration’s tool (Higgins et al., 2011), because the latter is more subjective and may be more affected by the coder’s bias. The PEDro scores were entered as a publication characteristic measure in the moderator analyses. The second and third author coded the PEDro scale independently.
Data Items
For each study, a series of variables was coded for subsequent moderator analyses: 1. participant characteristics (percent female; average age of participants; years of formal education received by participants), 2. training characteristics (total hours of training; hours of training per week), 3. control characteristics (no-contact passive vs. active control group), 4. publication characteristics (PEDro scores, with higher numbers indicating higher quality; numbers of cognitive outcomes reported in the study), 5. individual adaptiveness (adaptive: yes vs. no), and 6. training location (in lab vs. at home training). For training that was administered individually to one person at a time, it was considered to be individually adaptive if the task difficulty was updated continuously based on an individual’s performance (e.g., reach a certain threshold of performance/score before the next level is unlocked; Belchior et al., 2013); otherwise it was considered not to be individually adaptive. For training that was administered in group settings, there was typically a fixed schedule for the group, and individualized adaptiveness was not implemented. Therefore, these studies were excluded from the individualized adaptiveness analysis. Such group-based training studies included class-based instructional training (Cantarella, Borella, Carretti, Kliegel, & de Beni, 2017) as well as engagement training, (e.g., Park et al., 2014, Stine-Morrow et al., 2008). See Table 2 for all coded moderator variables. The second and third author coded these variables (Cohen’s kappa=0.9).
Summary Measures
All the analyses reported in the current meta-analysis were conducted using net-gain effect sizes (g), based on the recommended pretest-posttest-control formula by Morris (2008). The effect sizes were calculated such that positive numbers indicated greater (pre-training to post-training) gains for the training group, compared to the controls.
For every cognitive outcome in every study, Cohen’s d was calculated as follows.
The pooled pre-training standard deviation was calculated as shown below.
NT and NC represent the sample sizes of the training and control groups, respectively. To correct for small sample size bias, the final effect sizes used in analyses were the Hedges’ g (Hedges & Olkin, 1985) calculated as follows:
Net gain effect sizes were then weighted by their inversed variance (wnet) to calculate Q and I2 statistics in the mixed-effects modeling (Cooper H, Hedges LV, 2009).
Synthesis of Results
To evaluate overall gains in cognition, an overall g and an overall wnet were calculated, averaging across all the cognitive outcomes, in each study, using the standard procedure (Borenstein, Hedges, Higgins, & Rothstein, 2009). For studies with multiple training conditions but only one control group, each training condition was considered a separate trial against the same control group. For studies with both passive and active control groups, each control condition was considered a separate trial against the same training group. To control for the inter-correlation produced in the studies that had multiple training or control groups, a multi-level model analysis with robust maximum likelihood estimation was conducted in R (www.R-project.org) using the “Metafor” package (Viechtbauer, 2010). Metafor uses a multivariate (mixed-effects) analysis to model correlations that occur within studies.
Heterogeneity of g was assessed using the Q statistic (Higgins, Thompson, Deeks, & Altman, 2003) and I2 statistic (Hedges & Olkin, 1985). A significant within-group Qw would indicate a significant amount of heterogeneity that could not be attributed to sampling error alone. I2 was calculated as the percentage of variance between effect sizes that are attributable to true variation rather than sampling error. I2 values of 75%, 50%, and 25% are considered to indicate high, moderate, and low heterogeneity, respectively.
Our analyses were conducted in three stages. The first stage determined main effects for the following three variables -- Mental Status, Training Module, and Transfer -- by conducting between-group heterogeneity (Qb) tests that contrasted the two groups for Mental Status (healthy aging, HA, vs. MCI), Training Modules (Single-component vs. Multi-component), and Transfer (Near vs. Far). Significant Qb indicates that an observed group difference is not merely due to sampling error. The second stage determined the effects of cognitive training on the seven cognitive constructs across HA and MCI. The third, exploratory stage further investigated whether the different types of Single-component (i.e., Processing speed, Executive functions, Reasoning, and Memory) and Multi-component (i.e., Laboratory tasks, Class-based, and Entertainment/engagement) training modules differentially influenced near and far transfer.
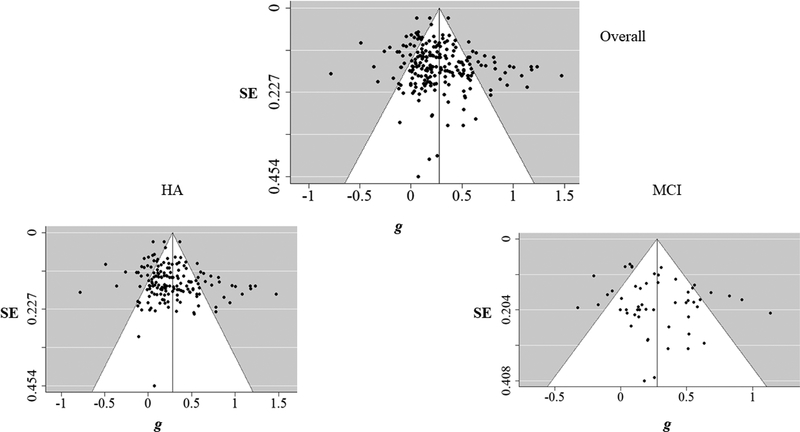
Risk of Bias Across Studies
To visualize potential publication bias, funnel plots were constructed based on g for the overall sample, as well as separate plots for HA and MCI participants. Weighted regression analyses (Egger, Davey Smith, Schneider, & Minder, 1997) were conducted to examine the asymmetry of the funnel plots. Significant asymmetry in funnel plots is diagnostic of possible publication bias. Finally, the Fail-safe N (Rosenberg, 2005) was calculated to find out the number of null results needed to cancel the effect of the current meta-analysis. Fail-safe N was calculated for the overall sample, as well as for HA and MCI participants.
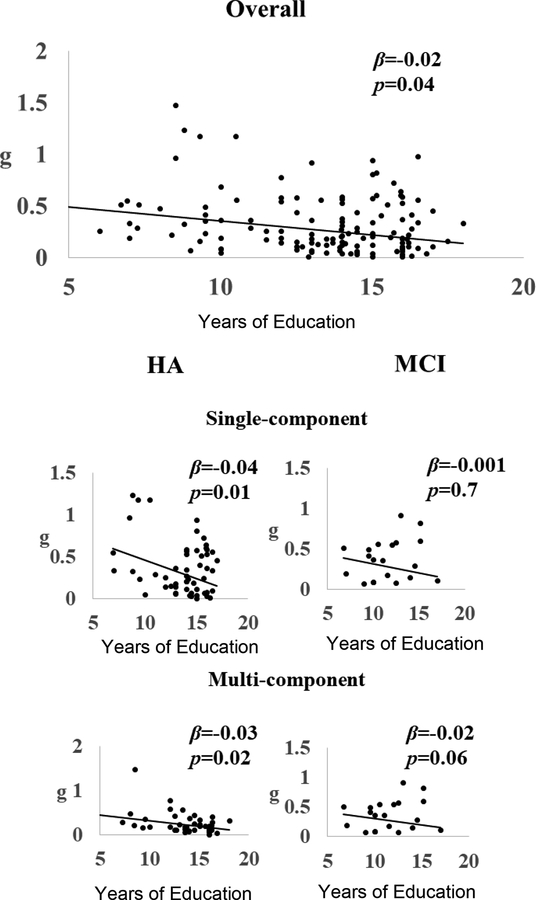
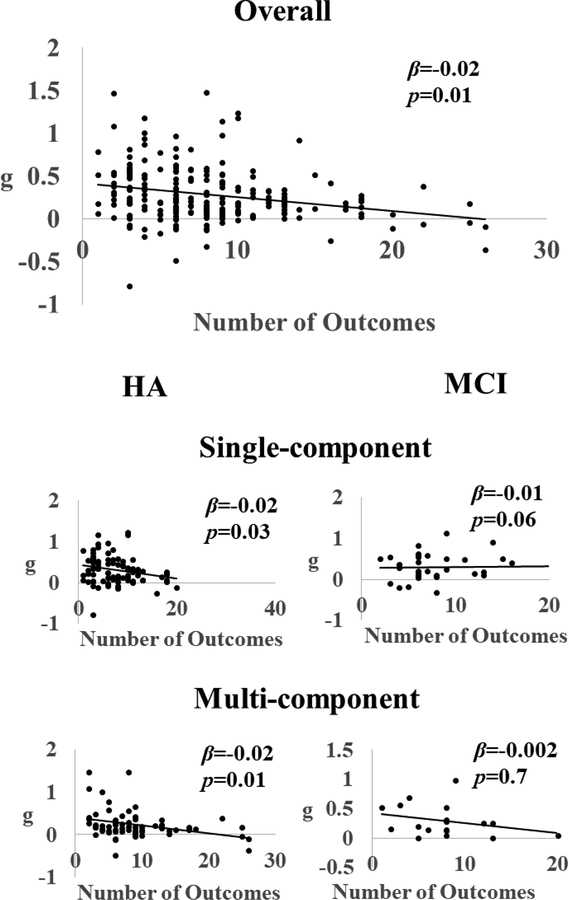
Additional Analyses: Moderator Analyses
To determine the effects of moderators on the observed heterogeneity on the overall g, modified weighted least-square regression analyses were conducting using the Metafor package. A-priori specified moderators included two categorical moderators (control characteristics and individual adaptiveness) and three sets of continuous moderators. Two of these continuous moderators were from data items (participant characteristics, training characteristics) and the third was publication characteristics (PEDro score and the number of cognitive outcomes reported). The categorical moderators were analyzed via subgroup analysis, while the continuous moderators were subjected to meta-regression analyses.
Results
Study Selection
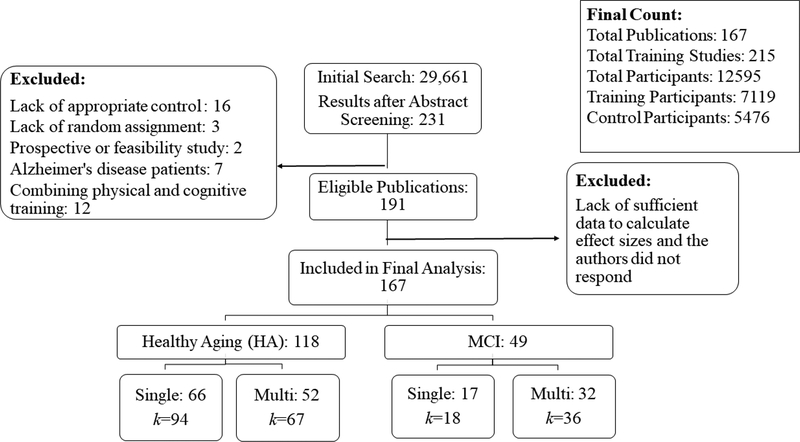
The initial search from PubMed, PsychInfo and Google Scholar resulted in a total of 29,661 publications. After screening the titles and abstracts, as well as removing any duplicates, 234 full texts were retrieved and read thoroughly. After a full-text review and contacting corresponding authors for missing data, a total of 167 publications remained eligible. From the 167 publications, results of 215 training studies were reported, based on 12,595 older participants (Figure 1). Publications excluded from the meta-analyses and the reasons for their exclusion are provided in Supp. Table 2.
Figure 1.
Flowchart summary of literature search. For details of included publication, see Table 1.
Study Characteristics
Characteristics of included training studies (N=215) are reported in Table 2, which reports the training module used (single- or multi-component), the mental status of the study sample (HA or MCI), the type of control group used (active or passive), the mean age of the study sample, the percent of females in the study (%), years of education (edu), mean age of participants, overall duration of training (total hours), weekly training dosage (hours/week), location of training (at home or in lab), whether the training is individually adaptive or not, number of cognitive outcomes assessed, the PEDro score, the individual g with 95% confidence interval, and the sample size of training and control groups. Table 2 was organized in descending order of g to simulate a detailed forest plot.
One hundred and sixty-one studies targeted HA, with 94 using single-component and 67 using multi-component training modules. Fifty-four studies targeted MCI, with 18 using single-component training and 36 multi-component training modules. Most of the studies on MCI (i.e., 47 out of 54 studies) recruited patients from clinics or hospitals, where Peterson criteria (Petersen et al., 2001) were used to diagnose MCI status in 30 studies; the other 17 studies either used a different criterion (e.g., Winblad et al., 2004) or did not report the diagnostic criteria used. The remaining seven studies used a combination of neuropsychological tests as a diagnostic criteria (e.g., Mini Mental State Examination and Montreal Cognitive Assessment in Cohen-Mansfield et al., 2015a).
There was a total of 112 single-component training studies included in this meta-analysis that considered episodic memory (34), executive functions (51), reasoning (10), processing speed (15), and semantic/language processing (2). Since there were only two studies targeted language functions, we did not analyze transfer effects of language training further. There was a total of 103 multi-component training studies included in this meta-analysis, where 60 studies utilized lab-based sequential training, 23 studies utilized class-based sequential training, and 20 studies utilized entertainment/engagement training methods. See Table 2 for details of each included study.
Risk of Bias Within Studies
The average PEDro score was MPEDro=7.13 with a range from 4 to 10. PEDro score was used as one of the variables of publication characteristic in the moderator analyses (Table 5).
Table 5.
Comparing the Continuous Moderator Variables, Using t-statistic, Across A) Single- vs. Multi-Component Training Studies, and B) HA vs. MCI
| Single-Component | Multi-Component | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean (SD) | Median | k | Mean (SD) | Median | k | t (df) | p |
| % Female | 63.89 (1.99) | 64 | 81 | 62.61 (12.18) | 59 | 97 | −0.7 (176) | 0.48 |
| Hours Per Week | 2.55 (1.75) | 2.25 | 91 | 3.46 (3.15) | 2 | 100 | 1.5 (189) | 0.14 |
| Total Hours | 12.17 (10.84) | 10 | 96 | 33.43 (49.68) | 15 | 100 | 4.1 (194) | 0.001 |
| Total Hours (without outliers) | 12.17 (10.84) | 10 | 96 | 19.50 (13.59) | 15 | 90 | 4.09 (184) | 0.001 |
| Age | 70.63 (4.01) | 70.2 | 105 | 71.56 (5.35) | 71.1 | 104 | 1.4 (207) | 0.16 |
| PEDrO | 7.10 (1.15) | 7 | 98 | 7.19 (1.36) | 7 | 100 | 0.49 (196) | 0.63 |
| Education | 13.73 (2.55) | 14.3 | 82 | 12.94 (3.06) | 13.5 | 69 | −1.7 (149) | 0.1 |
| Number of Outcomes | 7.41 (4.44) | 7 | 108 | 8.59 (5.34) | 8 | 107 | 1.56 (213) | 0.12 |
| HA | MCI | |||||||
| Variable | Mean (SD) | Median | k | Mean (SD) | Median | k | t (df) | p |
| % Female | 64.64 (11.61) | 63 | 133 | 58.91 (12.55) | 57 | 45 | 2.8 (176) | 0.01 |
| Hours Per Week | 2.90 (2.66) | 2.25 | 142 | 3.17 (2.77) | 2 | 49 | 0.27 (189) | 0.79 |
| Total Hours | 23.53 (41.84) | 12 | 146 | 21.51 (21.51) | 14 | 50 | 0.33 (194) | 0.74 |
| Total Hours (without outliers) | 15.26 (12.8) | 11.25 | 139 | 17.05 (12.4) | 13.5 | 47 | −0.8 (184) | 0.41 |
| Age | 70.31 (4.49) | 70 | 157 | 73.45 (4.72) | 73.75 | 52 | −4.3 (207) | 0.01 |
| PEDrO | 7.09 (1.25) | 7 | 147 | 7.31 (1.27) | 7 | 51 | −1.1 (196) | 0.27 |
| Education | 13.71 (2.7) | 14.5 | 112 | 12.41 (2.93) | 13 | 39 | 2.53 (149) | 0.01 |
| Number of Outcomes | 8.02 (5.25) | 7 | 161 | 7.94 (3.86) | 7 | 54 | 0.25 (213) | 0.8 |
Synthesis of Results
Post-intervention Effects of Cognitive Training versus Control Group
As shown in Table 3 and explained in the Methods section, only the net-gain effect sizes of cognitive training, where positive numbers indicate greater pre-training to post-training gains for the cognitive training group, compared to the controls, are reported. The net-gain effect of cognitive training on overall cognition was positive and statistically significant (g=0.28, 95% CI=0.23–0.33, p<0.01). There was significant heterogeneity across the studies (Qw=1062.4, I2=79.86%).
Table 3.
Results for Overall Effect Size, and Effect Size Separated by Mental status, Training Modules and Transfer
| g | 95% CI | k | p | Qw (df) | I2 | |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Overall Net Gain (g) | 0.28 | 0.23–0.33 | 215 | <0.01 | 1062.4 (214) | 79.86% |
| Net Gain Effects Separated by Mental Status and Training Modules | ||||||
| Mental Status | ||||||
| HA | 0.28 | 0.22–0.34 | 161 | <0.01 | 903 (160) | 82.28% |
| MCI | 0.27 | 0.18–0.37 | 54 | <0.01 | 159.4 (53) | 66.75% |
| Training Modules | ||||||
| Single-component | 0.29 | 0.23–0.36 | 112 | <0.01 | 465.86 (111) | 76.17% |
| Multi-component | 0.26 | 0.18–0.33 | 103 | <0.01 | 507.9 (102) | 79.92% |
| Transfer | ||||||
| Near | 0.37 | 0.3–0.44 | 173 | <0.01 | 1929.1 (172) | 91.08% |
| Far | 0.22 | 0.16–0.27 | 141 | <0.01 | 795.77 (140) | 82.41% |
| Transfer by Training Modules | ||||||
| Near: Single-component | 0.36 | 0.27–0.45 | 90 | <0.01 | 1045.7 (89) | 91.49% |
| Far: Single-component | 0.2 | 0.12–0.29 | 70 | <0.01 | 377.81 (69) | 81.74% |
| Near: Multi-component | 0.38 | 0.26–0.51 | 83 | <0.01 | 867.6 (82) | 90.55% |
| Far: Multi-component | 0.23 | 0.15–0.30 | 71 | <0.01 | 407.94 (70) | 82.84% |
| Mental Status by Training Modules | ||||||
| HA: Single-component | 0.30.23–0.37 | 94 | <0.01 | 519.86 (93) | 84.03% | |
| HA: Multi-component | 0.24 | 0.15–0.34 | 67 | <0.01 | 373.3 (66) | 82.32% |
| MCI: Single-component | 0.27 | 0.17–0.36 | 18 | <0.05 | 22.61 (17) ** | 24.81% |
| MCI: Multi-component | 0.29 | 0.18–0.4 | 36 | <0.01 | 134.33 (35) | 73.94% |
| Transfer by Mental Status | ||||||
| Near: HA | 0.38 | 0.14–0.47 | 139 | <0.01 | 420.96 (138) | 85.44% |
| Far: HA | 0.22 | 0.15–0.34 | 115 | <0.01 | 402.88 (114) | 82.34% |
| Near: MCI | 0.27 | 0.07–0.46 | 38 | <0.05 | 166.23 (37) | 76.92% |
| Far: MCI | 0.18 | 0.04–0.45 | 26 | <0.01 | 134.33 (25) | 73.94% |
Note:
indicates significant between-group heterogeneity test at p<.05.
indicates non-significant within-group heterogeneity test at p<.05. HA=Healthy Aging. MCI=Mild Cognitive Impairment.
When this overall net-gain effect was separately investigated for the two different populations of mental status, healthy aging (HA) and MCI, these effects were positive and significant for both HA and MCI (Figure 2). Heterogeneity tests within the HA and within the MCI were significant (see Table 3).
Figure 2.
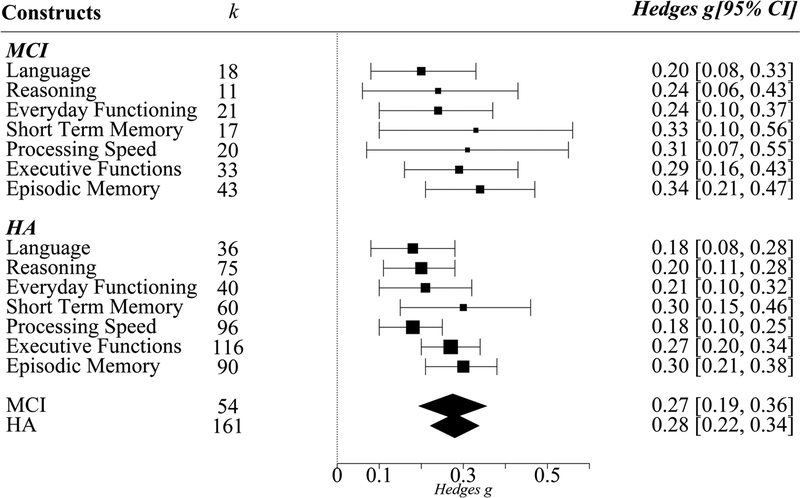
Effects of cognitive training (g) on the seven cognitive constructs in HA and MCI separately. Cognitive training had significant g’s on all cognitive constructs in HA and MCI. Error bars are 95% confidence intervals. K indicates number of studies. Point sizes indicate precision of g, such that larger points have smaller variance and greater precision.
When the overall net-gain effect was separately investigated for the two types of training modules, both single- and multi-component training modules were effective in improving overall cognition (single-component: g=0.29, 95% CI=0.23–0.36, p<0.01; multi-component: g=0.26, 95% CI=0.16–0.27, p<0.01). As shown in Table 3, these two types of training modules remained significant in both HA (single-component: g=0.3, 95% CI=0.23–0.37, p<0.01; multi-component: g=0.24, 95% CI=0.15–0.34, p<0.01) and MCI (single-component: g=0.27, 95% CI=0.17–0.36, p<0.05; multi-component: g=0.29, 95% CI=0.18–0.4, p<0.01) groups. For single-component training in the MCI, heterogeneity level was low, indicated by a non-significant Qw (22.61) and a low I2 (24.81%), suggesting that the differences among the studies within this group might be the result of sampling variance due to its small sample size (k=18). Subgroup analyses revealed no significant differences in overall net-gain effect sizes (g) between HA and MCI (Qb=0.003, df=1), and between the two types of training modules, single vs. multi-component (Qb=0.71, df=1).
Transfer effect analyses to various cognitive outcomes with respect to the mental status of the participants are presented in Figure 2. Cognitive training resulted in significant improvements to all cognitive outcomes (viz., Short-term memory, Processing Speed, Executive Functions, Episodic Memory, Reasoning, Language, and Everyday Functioning) in both HA and MCI. However, visual inspection of Figure 2 suggests that improvements in HA had greater precision than that in MCI, indicated by the spatial size of the effect size points. Although the net-gain effect of cognitive training on various cognitive outcomes is interesting, it does not inform us about whether these gains are limited to near abilities or are extended to far, untrained abilities. Therefore, in subsequent analyses, we investigated the effects of cognitive training on near and far transfer, and whether these effects varied by mental status and training modules.
Near vs. Far Transfer Effects of Cognitive Training Versus Control Group
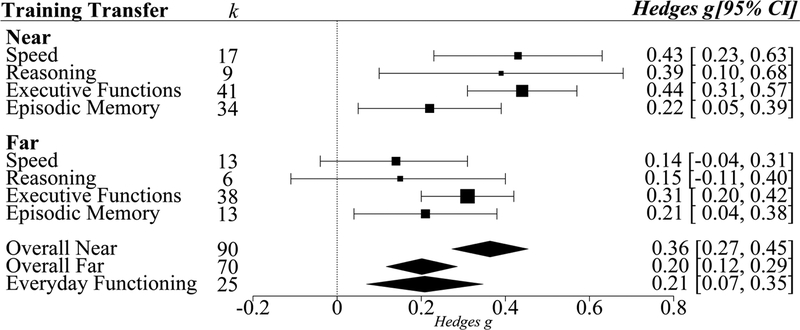
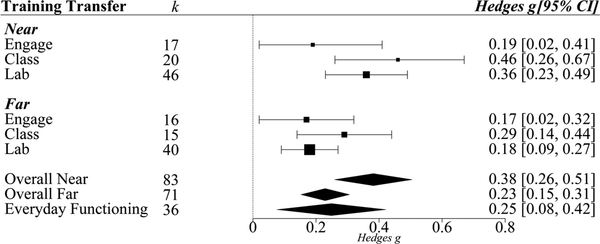
Overall, net-gain effects of cognitive training versus control group on near transfer, g=0.37, was significantly larger than that on far transfer, g=0.22, Qb(3)=10.59, p<0.01 (Table 3). When separated by training modules (single- vs. multi-component), near transfer net-gain effects were always larger than far transfer net-gain effects; single-component training Qb(1)=6.47, p<0.01; multi-component training Qb(1)=4.2, p<0.01. However, there was no difference in near transfer net-gain effects between single-component training and multi-component training, Qb(1)=0.06, p=0.82. There was also no difference in far transfer net-gain effects between single-component training versus multi-component training, Qb(1)=0.22, p=0.64.
To identify the specific single abilities that can engender not only near transfer, but also far transfer, assessments of near and far transfer for each specific training method in the single-component module (i.e., speed training, reasoning training, episodic memory training, and executive functions training) were conducted; see Figure 3. Language training was not considered in this subgroup analyses because of the lack of number of sufficient studies (k=2) required to draw meaningful conclusions. All four types of single-component training that were investigated resulted in significant near transfer effects (g ranging from 0.22 to 0.44).
Figure 3.
Transfer effects of single-component cognitive training. Effects from the four different types of single-component cognitive training (targeting either Processing Speed, Reasoning, Executive Functions, or Episodic Memory) on Overall Near and Overall Far transfer are shown. Transfer effects from single-component cognitive training on Overall Near, Overall Far and Everyday Functioning are also depicted. Error bars are 95% confidence intervals. K indicates number of studies. Point sizes indicate precision of g, such that larger points have smaller variance and greater precision.
Significant far transfer effects from single-component training, however, were observed only for executive functions and episodic memory. Executive functions, however, yielded the largest and most precise (depicted by the spatial size of the effect size points) effects for near and far transfer. Post-hoc tests were therefore conducted to evaluate whether executive functions training had a larger effect than other three training modules. For far transfer, executive functions training had a significantly larger effect than speed of processing training (Qb(1)=2.52, p=0.05), but not from reasoning training (Qb(1)=0.62, p=0.22) or episodic memory training (Qb(1)=0.4, p=0.26). For near transfer, executive functions training had a significantly larger effect only compared to episodic memory training (Qb(1)=3.43, p=0.03).
To summarize, both executive functions and episodic memory training produced significant near and far transfer effects. However, executive functions training not only had the largest and most robust effect sizes for both types of transfer, it was also the only single-component training module that had significantly larger effects than other training modules for both near and far transfer.
We also conducted assessments of near and far transfer effects for the different training methods within the multi-component training (i.e., lab-based, class-based, and engagement); the results are presented in Figure 4. All three training methods of the multi-component training resulted in significant near transfer (g ranging from 0.19 to 0.46) as well as far transfer (g ranging from 0.17 to 0.29). However no module was both largest and most robust regarding near and far transfer.
Figure 4.
Transfer effects of multi-component cognitive training. Effects from the three different types of multi-component cognitive training (Engagement-based, Classroom-based, Laboratory-based) on Overall Near and Overall Far transfer are shown. Transfer effects from multi-component cognitive training on Overall Near, Overall Far and Everyday Functioning are also depicted. Error bars are 95% confidence intervals. K indicates number of studies. Point sizes indicate precision of g, such that larger points have smaller variance and greater precision.
Effects of Cognitive Training on Everyday Functioning
Overall net-gain effects of cognitive training versus control group on everyday functioning were significant (g=0.22, p=0.01, Table 4). When separated by mental status of participants, training in both HA and MCI resulted in significant effects on everyday functioning. Both single-component training and multi-component training resulted in significant transfer to everyday functioning. To follow up with results from previous meta-analysis (Edwards et al., 2018) that showed significant transfer to everyday functioning by processing speed training, we also examined the effects of different types of single-component and multi-component training on everyday functioning. Results from the follow up analyses showed that in single-component training studies, only processing speed training resulted in significant transfer to everyday functioning (g=0.25, p=0.05; Table 4). For multi-component training studies, both specific (lab- and class-based) and non-specific (engagement) trainings resulted in significant transfer to everyday functioning (Table 4). There was no significant difference in g between training types.
Table 4.
Results for Transfer to Everyday Functioning
| g | 95% CI | k | p | |
|---|---|---|---|---|
| Overall Effect (g) | 0.22 | 0.12–0.32 | 61 | <0.01 |
| Mental Status | ||||
| HA | 0.19 | 0.08–0.32 | 40 | <0.01 |
| MCI | 0.26 | 0.08–0.44 | 21 | <0.01 |
| Training Modules | ||||
| Single-component | 0.21 | 0.02–0.39 | 25 | <0.05 |
| Multi-component | 0.25 | 0.09–0.40 | 36 | <0.01 |
| Single-component Training Types | ||||
| EF | 0.18 | −0.07–0.42 | 8 | n.s. |
| Memory | 0.24 | −0.04–0.53 | 7 | n.s. |
| Speed | 0.25 | 0.03–0.46 | 10 | <0.05 |
| Multi-component Training Types | ||||
| Specific | 0.18 | 0.02–0.34 | 24 | <0.05 |
| Non-Specific | 0.36 | 0.06–0.67 | 12 | <0.05 |
Risk of Bias Across Studies
Visual inspection of the funnel plot (Figure 5) for the overall g (k=215) revealed no evidence of asymmetry. A weighted regression test, with standard error as a predictor, also showed non-significant asymmetry (z=2.01, p=0.07), with random effect Fail-safe N of 45,944. Funnel plots for HA and MCI also did not show significant asymmetry (Figure 5). Weighted regression tests, with standard error as a predictor, showed non-significant asymmetry in both HA (z=1.9, p=0.06) and MCI (z=1.14, p=0.25). The random effect Fail-safe N for HA is 29,351 and for MCI is 1,664.
Figure 5.
Funnel plots with all included studies (Top), HA (Bottom Left) and MCI (Bottom Right).
Effects of Moderators on Overall Cognitive Gain
The first set of moderator analyses examined the effects of categorical moderators on overall g. The three categorical moderators were 1) control characteristics (active vs. passive control groups), 2) individualized adaptiveness (adaptive vs. non-adaptive training studies) and 3) training location (at-home vs. in-lab training studies).
The second set of moderator analyses examined the effects of continuous moderators on overall g, which were 1) participant characteristics (percent female, average age of participants, years of education), 2) training characteristics (total hours, hours/week, training location: at home or in lab), and 3) publication quality (PEDro scores, numbers of cognitive outcomes reported)
Categorical Moderator: Control Characteristics
There was no significant difference in overall g, indexed by Qb (1) =2.12, p=0.12, between the studies that used active control (g=0.31, 95% CI=0.25–0.38, p<0.01) versus those that used passive control (g=0.25, 95% CI=0.19–0.31, p<0.01). Seventy-seven out of 118 studies (i.e., 65% of studies) on HA used active control, but only 17 out of 49 studies (i.e., 35% of studies) on MCI used active control1. Therefore, separate comparison of control characteristics for each mental status is warranted. In HA, studies that used active control (g=0.29, 95% CI=0.22–0.37, p<0.01) did not differ significantly (Qb (1) =0.33, p=0.57) from studies that used passive control (g=0.26, 95% CI=0.19–0.33, p<0.01). In MCI, however, there was a marginal difference (Qb (1) =3.38, p=0.06) between studies that used active control (g=0.37, 95% CI=0.24–0.5, p<0.01) versus those that used passive control (g=0.22, 95% CI=0.12–0.31, p<0.01).
To ensure that the comparison of active and passive control groups is controlled for study quality, we focused on studies that included both active and passive control groups, in addition to their training group(s). This resulted in 20 studies for HA (twelve single- and eight multi-component training), but none for MCI. We then compared the net-gain effects for training on overall cognition (g) based on active control groups (g=0.46, 95% CI=0.25–0.68, p<0.01) to the g based on passive control groups (g=0.31, 95% CI=0.07–0.55, p<0.01) in these 20 HA studies. The two g’s were again statistically indistinguishable, Qb (1)=0.14, p=0.64. For the 12 single-component training studies, there was no difference between the two g’s (active: g=0.27, 95% CI=0.19–0.35, p<0.01; passive: g=0.28, 95% CI=0.19–0.37, p<0.01], Qb (1) =0.18, p = 0.89). For the eight multi-component training studies, there was also no difference between the two g’s (active: g=0.24, 95% CI=0.18–0.33, p<0.01; passive: g=0.22, 95% CI=0.14–0.30, p<0.01), Qb (1) =0.24, p = 0.56.
Categorical Moderator: Individualized Adaptiveness
After excluding engagement training programs (see Method), the adaptiveness analysis included 178 studies, with 101 adaptive and 77 non-adaptive studies. There was no significant difference in overall g, Qb (1)=0.36, p=0.54, between the studies that were adaptive (g=0.26, 95% CI=0.2–0.31, p<0.01) versus those that were non-adaptive (g=0.28, 95% CI=0.22–0.35, p<0.01). These differences were not significant either for single-component (Qb (1)=0.5, p=0.48) or for multi-component (Qb (1)=0.01, p=0.92) training module. They were also not significant for HA (Qb (1)=0.38, p=0.43) or MCI (Qb (1)=0.27, p=0.39).
Categorical Moderator: Location
The location analysis included 210 studies, with 54 at-home and 156 in-lab training studies. There was no significant difference in overall g, Qb (1)=0.26, p=0.6, between the training studies that were conducted in-lab (g=0.27, 95% CI=0.22–0.32, p<0.01) versus those conducted at-home (g=0.29, 95% CI=0.21–0.38, p<0.01). These differences were not significant either for single-component (Qb (1)=0.29, p=0.58) or for multi-component (Qb (1)=0.3, p=0.43) training module. They were also not significant for HA (Qb (1)=0.91, p=0.34) or MCI (Qb (1)=1.29, p=0.25).
Continuous Moderators: Participant Characteristics, Training Characteristics and Publication Quality
Means, standard deviations, and medians of all continuous moderators are reported in Table 5. It also reports results from independent samples t-tests on these moderators that compared a) single- and multi-component training modules, and b) HA and MCI. In general, multi-component training studies had significantly longer training duration (33.4 hours on average) compared to single-component training studies (12.2 hours on average; t(194)=4.1, p<0.01). Closer examination of the mean and median of training duration, using box-plots, for multi-component training studies indicated possible outliers (see Supp. Figure 1). After removing the 10 outlier studies, multi-component training still showed longer training duration (19.5 hours on average) compared to single-component training, t(184)=4, p<0.01 (see Table 5). Comparison between HA and MCI participants showed that MCI participants had a more balanced gender distribution, t(176)=2.8, p=0.01, larger average age, t(207)=−4.3, p=0.01, and less years of formal education, t(149)=2.53, p=0.01.
The next sets of analyses examined the effects of the continuous moderators (participant characteristics, training characteristics and publication quality) on overall g, using three separate meta-regression models, one for each moderator (Table 6). Results from these meta-regressions showed that participant characteristics (QM =7.39, p=0.05) and publication quality (QM =8.96, p<0.01) significantly influenced overall g. Training characteristics, which included training duration, training hours per week, however, was not a significant moderator (QM=3.16, p=0.08) in predicting overall g.
Table 6.
Results from the Meta-regressions of the Continuous Moderators on Overall Effect (g)
| Moderator | Model Q | Beta | 95% CI | p |
|---|---|---|---|---|
| Participant Characteristics | ||||
| 7.39 | 0.05 | |||
| Gender (% Female) | −0.002 | −0.006 to 0.0018 | 0.32 | |
| Age | 0.005 | −0.005 to 0.01 | 0.33 | |
| Education | −0.02 | −0.037 to −0.009 | <0.01 | |
| Training Characteristics | ||||
| 4.87 | 0.09 | |||
| Total hours | −0.0013 | −0.003 to 0.0001 | 0.08 | |
| Hours per week | −0.01 | −0.03 to 0.008 | 0.24 | |
| Publication Quality | ||||
| 8.96 | <0.01 | |||
| PEDro | −0.009 | −0.05 to 0.03 | 0.63 | |
| Number of Cognitive Outcomes | −0.017 | −0.02 to −0.005 | <0.01 | |
We then examined which individual variables for the two significant moderators -- participant characteristics and publication quality -- contributed significantly to the model. Years of education and the number of cognitive outcomes were driving the significant effects for participant characteristics and publication quality, respectively. Specifically, years of education negatively predicted overall g (β=−0.019, p<0.01; Figure 6), suggesting that participants with less formal education benefitted more from cognitive training. Close examination of the number of cognitive outcomes, the significant variable for publication quality, showed that one study reported 40 cognitive outcomes (K. Zimmermann, von Bastian, Röcke, Martin, & Eschen, 2016), a significant outlier compared to the other studies. Removal of this outlier study did not change the direction or significance of the effect of number of outcomes on g (β=−0.017, p<0.01, Table 6). This result suggests that studies with larger number of outcomes tended to have a relatively smaller effect (Figure 7).
Figure 6.
Scatter plot of g’s as a function of years of formal education for Overall g (Top Center), and separated by Mental Status and Training Modules (Bottom).
Figure 7.
Scatter plot of g’s as a function of the number of cognitive outcomes for Overall g (Top Center), and separated by Mental Status and Training Modules (Bottom).
Residual heterogeneity from both participant characteristics (Qw=782.43, df=149) and publication quality (Qw=1048.63, df=213) models were significant, indicating the presence of other (unaccounted for) variables that were influencing the overall g. These unaccounted variables could include training module or mental status. Therefore, post-hoc analyses were conducted on training modules (single-component, multi-component) separated by mental status for the two significant variables: years of education (Figure 6) and number of cognitive outcomes (Figure 7). Years of education was a significant moderator for both single-component (β=−0.03, p=0.02) and multi-component (β=−0.026, p<0.01) modules in HA, but not in MCI. We further separated years of education in HA studies into two bins (< 12 years versus > 12 years) and found that g was 0.42 for years of education < 12 years compared to g of 0.22 for years of education > 12 years (QB=12.74; p<.01).
Number of cognitive outcomes was also a significant moderator for both single-component (β=−0.021, p<0.01) and multi-component (β=−0.024, p<0.01) training modules in HA, but not in MCI.
Discussion
To our knowledge, this is the first meta-analysis that compared the cognitive training effects between healthy aging and MCI on various cognitive outcomes, investigated the possible interaction between cognitive training module and mental status on cognition (by directly contrasting the four groups: single-component HA, single-component MCI, multi-component HA, multi-component MCI); and investigated the possible interaction between cognitive training module and extent of transfer (by directly contrasting four groups: single-component near, single-component far, multi-component near, multi-component far). Furthermore, this meta-analysis included not only experimenter-developed cognitive training modules, but also engagement-based cognitive training modules. A recent meta-analysis did compare the effects of experimenter-developed cognitive training on overall cognition in healthy aging and MCI (Mewborn et al., 2017). However, this study only condidered the main effects of training domain and transfer on overall cognition, combined across the two mental statuses. Moreover, it did not evaluate the effects of engagement-based cognitive training, an increasingly popular approach in older adults (Park et al., 2014; Stine-Morrow, Parisi, Morrow, & Park, 2008), or the effects of cognitive training on everyday functioning.
By synthesizing across 161 cognitively healthy aging studies and 54 MCI studies, we first compared the immediate effects of cognitive intervention on cognitive function to that of control group. A significant net-gain effect size on overall cognition was found for cognitive intervention versus control group. This net-gain effect size of 0.28, obtained from a larger corpus of 215 studies, is comparable to the moderate net-gain effects observed in a past meta-analysis on cognitive training that included both healthy aging and those with MCI (g=0.29 from 97 studies, Mewborn et al., 2017).
In this meta-analysis, we primarily wanted to address three main questions regarding gains from cognitive intervention: 1) Who would benefit the most from cognitive training not only on overall cognition, but also on a variety of cognitive outcomes – healthy aging or MCI? 2) What is the extent of this benefit from cognitive training – near or far transfer? 3) Which type of cognitive training module (single- or multi-component) would be the most effective in engendering not only near, but, importantly, far transfer? We also wanted to investigate effects of cognitive training on everyday functioning –which training would be most beneficial and who would benefit most.
Comparing the Effects on Healthy Aging and MCI
Regarding who would benefit most from cognitive training (cognitively healthy or MCI), no significant difference in the net-gain effect size for overall cognition was observed between the two groups. Our net-gain effect sizes are more conservative estimates than the effect size statistics reported in the previous meta-analyses on healthy aging and MCI, where the reported effect sizes were either gain scores of the training group (calculated from the difference between post- and pre-training cognitive scores) or post-test difference scores between training and control groups that did not account for baseline differences between the groups (gain score d = 0.86 for executive functions training in healthy aging, Karbach & Verhaeghen, 2014; gain score d = 0.41 for cognitive training in MCI, Li et al., 2011; post training difference g= 0.28 for computer-based cognitive training in healthy aging, Lampit et al., 2014). Net gain effect size, in contrast, compares the cognitive gains in the training group with the cognitive gains in the control group, and therefore can only be calculated in intervention studies that have a control group and yields a more conservative estimate (Morris, 2008). The net-gain effect sizes from the current study on healthy aging and MCI are comparable with that from a meta-analysis that focused on experimenter-developed cognitive paradigms as interventions (Mewborn et al., 2017).
In the current study, the overall cognitive benefits, averaged across many cognitive constructs, were found to be similar across healthy aging and MCI. These cognitive constructs ranged from basic, cognitive abilities (processing speed, short-term memory, executive functions and episodic memory) to complex cognition (reasoning, language, and everyday functioning). It is however plausible that the pattern of training effects across the different cognitive constructs were distributed differently between healthy aging and MCI. We therefore investigated how mental status interacted with the training-related benefits on seven cognitive constructs under investigation. Both healthy aging and MCI groups showed transfer to all seven cognitive constructs. These results suggest that cognitive training is not only effective in improving overall cognition in older adultys with MCI, but the extent and breadth of improvement is similar to that observed in healthy older adults. Importantly, our results supported the hypothesis from Sperling et al. (2011) that behavioral interventions may be effective in improving cognition at the preclinical stages of Alzheimer’s disease (AD).
It is important to note the physical exercise training has been touted to be superior to cognitive training to improve cognition and well-being (Simons et al., 2016), with early meta-analyses reporting significant effects on overall cognition from physical fitness training in both healthy aging (Colcombe & Kramer, 2003), and MCI and related dementia (Heyn, Abreu & Ottenbacher, 2004). However, these analyses only reported pre-post gains from the fitness training and included studies with no control groups. By not comparing gains from the fitness training to that of controls, their reported of effect sizes are exaggerated. There are, however, two recent meta-analyses on healthy aging that have compared physical fitness training directly to either cognitive training (Karr, Areshenkoff, Rast & Garcia-Barrera, 2014) or training that combines physical exercise and cognition (Zhu et al., 2016). Both meta-analyses found physical fitness training to be significantly less effective than cognitive training or combination training, by calculating net-gain effects. Importantly, Karr et al. specifically investigated training effects on executive functions, an ability has been shown to benefit most from fitness training in healthy aging (Colcombe & Kramer, 2003). These net-gain effects were very small (0.12) and are less than half the effect of cognitive training on executive functions from the current meta-analysis.
Comparing the Effects on Near and Far Transfer
We had hypothesized that cognitive interventions would have a larger effect on near transfer than far transfer. The important question was whether the far transfer effects, though small, were significant. Our results show that cognitive training had a moderately large net-gain effect on near transfer and a smaller, but significant, net-gain effect on far transfer. Both single and multi-component training modules had similar net-gain effects on both near and far transfer. In addition to these far transfer effects, we specifically investigated transfer to everyday functioning. Everyday functioning, an index of functional independence, is considered to be the far transfer task in most targeted cognitive interventions. We found that cognitive interventions indeed had a significant effect on everyday functioning, suggesting that cognitive training also has the potential to enhance functional independence in older adults.
Comparing the Effects of Different Training Modules
Regarding which training module was more effective in improving overall cognition, both single- and multi-component training modules benefited healthy aging and MCI, with net-gain effect sizes ranging from small to medium. However, there was significant heterogeneity in both modules. Much of this heterogeneity could be due to the heterogeneity of cognitive components trained in the single-component training studies or that multi-component training either specifically target cognitive skills or are non-specific in nature. It is possible that only certain “core” cognitive abilities, when trained in relative isolation from other abilities, may engender far transfer for single-component training. In contrast, we hypothesized that all multi-component training modules may engender significant far transfer, irrespective of the type of training approach used, because of plausible inclusion of many of these core abilities. We, therefore, investigated the cognitive benefits from different types of single- and multi-component training modules on both near and far transfer, in comparison to the control group.
All single-component and multi-component training modules improved trained, near abilities, with net-gain effect sizes ranging from small to moderate (0.19 to 0.46). For near transfer from single-component training modules, the largest (and the most precise) net-gain effect size was from executive functions training (moderate effect of 0.44), whereas the smallest effect was from episodic memory training, which was half of the effect from executive functions training. For near transfer from multi-component training modules, the largest (class-based training) and the most precise (laboratory-based training) estimates of net-gain effect sizes were from modules that targeted specific cognitive skills. Therefore, these results suggest that training targeting specific cognitive skills engender significant and moderate near transfer.
Regarding far transfer, only two single-component training modules were found to be effective on the far transfer composite score-- episodic memory, which had a small net-gain effect, and executive functions training, which had a moderate net-gain effect. Overall, executive functions training was the most effective single-component training module in engendering transfer to both trained cognitive abilities and untrained cognitive abilities. It not only had the largest effect size, but also had the most precise estimate for both near and far transfer. Furthermore, it yielded a significantly higher far transfer than processing speed training and significantly higher near transfer than episodic memory training. These results are in line with other meta-analyses which have not only found executive functions training to be effective in both younger and older adults (Karbach & Verhaeghen, 2014; Nguyen et al., 2019), but that this training is more effective on overall cognition than other cognitive training modules (Lampit et al., 2014; Mewborn et al., 2017).
Importantly, we specifically assessed far transfer from different types of single-component training modules to everyday functioning and found only processing speed training to be significant. This result is in line with Edwards et al. (2018), where processing speed training was found to be significant on everyday functioning across eight studies that covered a more heterogenous population (including, younger adults, Parkinson’s patients). It is important to note that everyday functioning was the only cognitive construct in this meta-analysis that included subjective reports in addition to objective measures. Given the paucity of data, we cannot currently determine if the gains in everyday functioning from processing speed training are due changes in the objective measures or changes in the subjective reports.
In contrast to single-component training modules, all multi-component training modules (specific: class-based, laboratory-based; non-specific: engagement-based) showed significant net-gain effects on far transfer. All of these different types of multi-component training were significant on everyday functioning. Importantly, non-specific engagement training has significant, medium net-gain effects on everyday functioning. This effect was largest amongst all training types, including processing speed training. We therefore conclude that all types of multi-component training modules were effective in improving both near and far abilities, including everyday functioning, unlike the limited effects of different single-component training modules on far transfer. This finding is novel and together with the findings from single-component training, suggests that future studies on multi-component training that combine “core” cognitive abilities (e.g., executive functions, processing speed) in an engaging manner (such as, gamifying approach, group settings) may be most promising for inducing robust far transfer, especially to everyday functioning.
Effects of Moderators on Overall Cognition
Although we investigated a series of variables as potential moderators, such as participant characteristics (percent female, average age of participants, years of education), training characteristics (total hours, hours/week, training location: at home or in lab), control characteristics (passive vs. active, adaptive vs. non-adaptive), and publication characteristics (PEDro scores, numbers of cognitive outcomes reported), only years of education and the number of cognitive outcomes reported by each study reduced the effect sizes. Given the significant heterogeneity of these effects, we further tested the effects of these two variables for the two types of training modules separated by mental status. That is, we investigated the effects of these variables in four separate groups: single-component studies in healthy aging, single-component studies in MCI, multi-component studies in healthy aging, and multi-component studies in MCI. We found significant effects of both of these variables for both single- and multi-component training modules in healthy aging, but not in MCI.
Although results regarding effects of educational attainment on gains from cognitive training have been mixed across different training studies (Rebok et al., 2013; Ball et al., 2013), past meta-analyses on cognitive training have not explored the influence of educational attainment on cognitive gains. Our results suggest that educational attainment, a common proxy for cognitive reserve, that has been argued by researchers to allow for cumulative accumulation of crystallized intellectual resources (Cizginer et al., 2017), can interact with recovery of fluid cognitive abilities (such as, processing speed, executive functions, reasoning and episodic memory) in healthy older adults. In particular, healthy older adults with lower educational attainment show greater cognitive plasticity resulting from cognitive training, irrespective of the type of training module. The g for studies where participants had an average of high school education or less was 0.42; this value is significantly higher than the g (=0.22) for those with more than high school education. This result is very significant for the scientific community, because building cognitive reserve has been argued to mitigate the rate of cognitive decline in fluid abilities, and ultimately the clinical expression of dementia or AD-related pathologies (Roe, Xiong, Miller, & Morris, 2007;Stern, Albert, Tang, & Tsai, 1999; Tucker & Stern, 2011). However, higher education may not have been a viable option during youth for many older adults for numerous reasons (e.g., gender disparity, socio-economic status, proximity to educational institutions, etc.). Our results suggest that healthy older adults with lesser cognitive reserve (proxied by educational attainment), and therefore are at greater risk of conversion to MCI or dementia, may be able to recover their fluid cognitive abilities through cognitive training.
Another moderator that had a significant effect on healthy aging was the number of cognitive outcomes, such that the greater the number of cognitive outcomes, the lesser was the effect of cognitive training. This effect did not vary across the two training modules: multi-component training studies (Mcognitive_outcomes=8.46) vs. single-component training studies (Mcognitive_outcomes =7.57). We recommend that future studies are designed with planned cognitive outcomes for both single- and multi-component training studies, particularly addressing the extant of transfer, from nearer abilities to farther abilities. Particularly, abilities such as everyday functioning should be explored in future studies as improvements in everyday functioning has the potential to extend the independence in older adults. However, many past training studies have focused on subjective reports of everyday functioning that focus on a wide variety of daily activities (e.g., IADL), of which not all activities show significant declines in healthy aging. We propose that future studies on healthy aging as well as MCI should also focus on objective measures of specific everyday cognitive functions that show age-related declines or are early markers of MCI.
For single-component studies and laboratory-based directed multi-component training studies, it is easier to hypothesize what the near and far abilities are prior to starting the study, based on the cognitive component(s) targeted during the training. For non-specific multi-component training studies, such as the engagement-based studies, it is much harder to hypothesize a priori what the near and far abilities should be. One approach of establishing which cognitive components are being trained in a multi-component training is to elicit tacit knowledge of participants regarding the training task using either machine learning techniques (e.g., intrinsic personalization neural networks models in Ross et al., 2017) or determine the cognitive predictors of the training protocol by using exploratory factor analysis (e.g., Young adults: Baniqued et al., 2013) or determine both neural and cognitive predictors of learning the training protocol (Young and Old adults: Multi-Factor Analysis in Ray et al., 2017; Old adults: Basak et al., 2011). These studies should precede the RCT, and would identify the potential cognitive constructs that may be affected by that particular training module. This will therefore help reduce the number of potential cognitive outcomes in a future randomized controlled trial.
In the moderator analyses, interestingly, we found no differences in control characteristics. That is, g based on passive control was not greater than g based on active control. Many researchers have recommended that cognitive training should do away with passive control, in favor of active control, because active control matches most closely the training group on motivation, social contact and expected cognitive gains from the intervention (Simons et al., 2016). Although at least one meta-analysis on cognitive training supports this recommendation by finding that use of active controls resulted in significantly smaller net-gain effects on cognition than use of passive controls (e.g., N-Back training in young and middle-aged adults, Au et al., 2014), other meta-analyses have found no significant difference between studies using active control vs. or passive control (e.g., executive functions training across younger and older adults, Karbach & Verhaeghen, 2014; computerized cognitive training in healthy aging, Lampit et al., 2014; video game training in healthy aging, Toril et al., 2014).
Surprisingly, a meta-analysis found that studies using passive control groups have significantly smaller effects than those using active control groups (cognitive training collapsed across healthy aging and MCI, Mewborn et al., 2017), but this meta-analysis failed to support that finding. One reason could be the larger sample size of the current study. However, we also noted a discrepancy in study quality between healthy aging and MCI groups. Only 35% of MCI studies used active control; in contrast, nearly twice the number of healthy aging studies (65%, to be exact) used active control. When the control characteristics were examined separately in healthy aging and MCI, we only found difference in MCI favoring active control. It is therefore possible that the effects found in Mewborn et al. are driven by the studies of individuals with MCI.
It is possible that there are significant differences in study quality that use active control versus passive control (Boot, Simons, Stothart, & Stutts, 2013; Simons et al., 2016). To account for such potential differences in study quality, we specifically conducted analyses on those studies where both types of control groups were used. This resulted in 20 healthy aging studies; a number large enough to conduct a meta-analysis to explore the effect of control characteristics on overall cognition. No discernable difference was observed between the g obtained from two control groups. Moreover when we conducted separate sub-group analyses on single-component training modules and on multi-component training modules we still failed to find g for passive control to be larger than g for active control – a result that would have provided evidence supporting the inferiority of passive control group in cognitive training studies in healthy aging.
We could not conduct such analysis in MCI, where we could control for study quality, due to lack of any such study. We therefore recommend that future cognitive training studies of individuals with MCI (and in healthy aging) should include both passive and active control groups, particularly active controls that have the same expectancy of cognitive improvements as the training group. Such studies will help determine the specificity of cognitive training, beyond the effects of motivation, social contact and expected cognitive gains from the intervention (Simons et al., 2016).
Limitations and Recommendations
One limitation of the current meta-analysis is the lack of long-term follow-up data in most of the included studies. While training participants show cognitive gains at immediate post-training sessions, the lengths of retention of these gains are important in evaluating the long-term effectiveness of any training program. Out of the 215 studies, only 48 reported some follow-up data, ranging from 1 month to 10 years post-training. Additionally, some included booster training, therefore separating the long-term gains from continued training effects are difficult to discern. Future meta-analyses could examine the long-term effects of cognitive training in older adults to determine if cognitive training is effective in delaying the onset of AD.
Another limitation that the meta-analysis could not address, given the paucity of data, is the effect of cognitive training on objective vs. subjective measures of everyday functioning. Everyday functioning, an index of functional independence, is argued by many as an important outcome variable in clinical trials, but many researchers use objective measures (e.g., RBMT) while others use subjective reports (e.g., IADL) to assess this construct. It is important to determine whether the gains in everyday functioning are not just due to the perceived changes in the participants’ ability or function. Moreover, RCTs typically use only one task or measure to assess the everyday functioning construct. In contrast, the other six constructs under investigation comprised of objective measures and, in many studies, were measured by more than one task.
Based on current findings, we recommend that future cognitive training on older adults that aim to improve a broad range of both near and far abilities, including everyday functioning, should train multiple cognitive abilities. Maybe, these multi-component trainings could combine the effective cognitive abilities of executive functions, episodic memory and processing speed in an engaging and stimulating manner (example, gamified approaches in group settings).
We also recommend future studies to investigate different training strategies, particularly in the modules that resulted in robust far transfer, which may induce even greater far transfer. There are some promising strategies for multi-component and executive function training in younger adults (e.g., variable priority training, Boot et al., 2017; dual vs. single n-back training, Jaeggi et al., 2010), but these studies are relatively sparse in older adults.
We recommend cognitive training to healthy older adults with lower cognitive reserve, and encourage future training studies to investigate how cognitive reserve or mental status interacts with cognitive plasticity. We need more large-scale studies to investigate individual differences in cognitive health, such as education and cognitive abilities, to understand who would benefit more from a particular training module. Such investigations can help us tailor cognitive training to specific individuals.
Given how cognitive training compares favorably to much-touted physical fitness training, particularly to executive functions in healthy aging, where current net-gain effects were twice to that reported from a physical fitness training meta-analysis (Karr et al., 2014), we recommend cognitive training to older adults if the interested outcome is cognitive plasticity.
Conclusions
The current comprehensive meta-analysis shows that cognitive training, compared to the control group, is modestly effective in improving cognition not only in healthy older adults, but also in adults with MCI. Our results suggest that cognitive plasticity extends into late adulthood, even when our mental status is compromised by cognitive impairments. The effect size estimates from the current meta-analysis on the seven different cognitive outcomes were more robust for healthy aging than for MCI. Such a difference in robustness suggests a need for more cognitive training studies on individuals with MCI.
The current meta-analysis also examined the extent of transfer from single- and multi-component training modules. We observed that all types of training modules were effective on near transfer, and that these effects were larger than those on far transfer. However, only a few modules of cognitive training induced significant far transfer. These effective training modules either specifically targeted only one cognitive ability, viz. executive functions or episodic memory, or targeted multiple cognitive abilities during training.
For single-component training, executive functions training was the most effective approach in engendering both near transfer to the trained cognitive abilities and far transfer to untrained cognitive abilities. It was the only single-component training module that had significantly larger effects than other training modules for both near transfer (compared to episodic memory training) and far transfer (compared to processing speed training). For far transfer to everyday functioning, only processing speed training had significant effects, but it is not known to what extent this significance is driven by subjective measures.
In contrast to the single-component training modules, all types of multi-component training modules yielded significant far transfer, including transfer to everyday functioning. Moreover, the effects of multi-component training on everyday functioning was the largest.
The effect size was significantly related to only two moderators, years of education and number of cognitive outcomes. However, these relationships were limited to healthy aging studies. Cognitive training was most effective in studies with lower educational attainment, on avererage, of their participants and for studies that employed less cognitive outcomes. Having more cognitive outcomes may result in the inclusion of outcome measures that have little overlap with the trained skills, thus minimizing the overall effect size. We can, in future, design studies with planned cognitive outcomes, after first establishing the relationship between various cognitive outcomes and the cognitive training protocol, and conducting a cognitive component analysis (such as, factor analysis or machine-learning approaches). Such an approach could be particularly useful for engagement-based multi-component training studies, where it is not certain what cognitive abilities are being targeted.
Supplementary Material
Acknowledgments
This research was supported by a grant from National Institute on Aging (titled “Strategic Training to Optimize Neurocognitive Functions in Older Adults” under award number R56AG060052) to Chandramallika Basak. Preliminary results from this meta-analyses on a reduced dataset were presented at the Cognitive Aging Conference, Atlanta, 2018 and Annual Meeting for Psychonomic Society, 2017.
Footnotes
A chi-square test of independence was performed to examine the relation between control characteristics and mental status. The relation between these variables was significant, X2 (2, N = 215) = 4.4, p =0.03. There are more studies using passive control group for MCI than for HA.
References
* in front of last name of the first author indicates publication included in the current meta-analysis.
- Allaire JC, & Marsiske M (1999). Everyday cognition: Age and intellectual ability correlates. Psychology and Aging, 14(4), 627–644. 10.1037/0882-7974.14.4.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire JC, Gamaldo A, Ayotte BJ, Sims R, & Whitfield K (2009). Mild cognitive impairment and objective instrumental everyday functioning: the everyday cognition battery memory test. Journal of the American Geriatrics Society, 57(1), 120–125. doi: 10.1111/j.1532-5415.2008.02054.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Anguera J. a., Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J & Gazzaley A (2013). Video game training enhances cognitive control in older adults. Nature, 501(7465), 97–101. doi: 10.1038/nature12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Eysenck MW, & Anderson MC (2015). Memory. 2nd edition New York, USA: Psychology Press; ISBN: 978-1-84872-183-5 (pbk) [Google Scholar]
- *.Bailey H, Dagenbach D, & Jennings JM (2011). The locus of the benefits of repetition-lag memory training. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 18(5), 577–593. doi: 10.1080/13825585.2011.591921 [DOI] [PubMed] [Google Scholar]
- *.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, & S W (2002). Effects of cognitive training interventions with older adults. JAMA, 288(18), 2271. doi: 10.1001/jama.288.18.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Edwards JD, & Ross LA (2007). The impact of speed of processing training on cognitive and everyday functions. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences, 62B, 19–31. doi: 10.1093/geronb/62.special_issue_1.19 [DOI] [PubMed] [Google Scholar]
- *.Ballesteros S, Mayas J, Prieto A, Ruiz-Marquez E, Toril P, & Reales JM (2017). Effects of video game training on measures of selective attention and working memory in older adults: Results from a randomized controlled trial. Frontiers in Aging Neuroscience, 9, 354. doi: 10.3389/fnagi.2017.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, & Kliegl R (1992). Further testing of limits of cognitive plasticity: Negative age differences in a mnemonic skill are robust. Developmental Psychology, 28(1), 121–125. doi: 10.1037/0012-1649.28.1.121 [DOI] [Google Scholar]
- *.Baltes PB, Kliegl R, & Dittmann-Kohli F (1988). On the locus of training gains in research on the plasticity of fluid intelligence in old age. Journal of Educational Psychology, 80(3), 392–400. doi: 10.1037/0022-0663.80.3.392 [DOI] [Google Scholar]
- *.Banducci SE, Daugherty AM, Biggan JR, Cooke GE, Michelle W, Noice T, & Kramer AF (2017). Active experiencing training improves episodic memory recall in older adults. Frontiers in Aging Neuroscience, 9, 1–11. doi: 10.3389/fnagi.2017.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniqued PL, Lee H, Voss MW, Basak C, Cosman JD, DeSouza S, & Kramer AF (2013). Selling points: What cognitive abilities are tapped by casual video games? Acta Psychologica, 142(1), 74–86. doi: 10.1016/j.actpsy.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR, & Kramer JH (2009). Computer-based cognitive training for mild cognitive impairment. Alzheimer Disease & Associated Disorders, 23(3), 205–210. doi: 10.1097/WAD.0b013e31819c6137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Basak C, Boot WR, Voss MW, & Kramer AF (2008). Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychology and Aging, 23(4), 765–777. doi: 10.1037/a0013494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Basak C, & O’Connell MA (2016). To switch or not to switch: role of cognitive control in working memory training in older adults. Frontiers in Psychology, 7, 230. doi: 10.3389/fpsyg.2016.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak C, & Qin S (2018). Virtual cognitive training in healthy aging and mild cognitive impairment In Aging, Technology and Health (pp. 215–235). Elsevier. doi: 10.1016/B978-0-12-811272-4.00009-9 [DOI] [Google Scholar]
- Basak C, Voss MW, Erickson KI, Boot WR, & Kramer AF (2011). Regional differences in brain volume predict the acquisition of skill in a complex real-time strategy videogame. Brain and Cognition, 76(3), 407–414. doi: 10.1016/j.bandc.2011.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak C, & Zelinski EM (2012). A hierarchical model of working memory and its change in healthy older adults In Working Memory (pp. 83–106). Routledge. doi: 10.4324/9780203094600 [DOI] [Google Scholar]
- *.Belchior P, Marsiske M, Sisco SM, Yam A, Bavelier D, Ball K, & Mann WC (2013). Video game training to improve selective visual attention in older adults. Computers in Human Behavior, 29(4), 1318–1324. doi: 10.1016/j.chb.2013.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bellander M, Eschen A, Lövdén M, Martin M, Bäckman L, & Brehmer Y (2017). No evidence for improved associative memory performance following process-based associative memory training in older adults. Frontiers in Aging Neuroscience, 8(1), 1–11. doi: 10.3389/fnagi.2016.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller GA (2013). The United States has lower life expectancy than other developed nations, despite having highest health care costs. Journal of Nuclear Cardiology, 20(2), 177–178. doi: 10.1007/s12350-013-9692-4 [DOI] [PubMed] [Google Scholar]
- *.Belleville S, Clément F, Mellah S, Gilbert B, Fontaine F, & Gauthier S (2011). Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain : A Journal of Neurology, 134(6), 1623–1634. doi: 10.1093/brain/awr037 [DOI] [PubMed] [Google Scholar]
- *.Belleville S, Gilbert B, Fontaine F, Gagnon L, Ménard É, & Gauthier S (2006). Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: Evidence from a cognitive intervention program. Dementia and Geriatric Cognitive Disorders, 22, 486–499. doi: 10.1159/000096316 [DOI] [PubMed] [Google Scholar]
- *.Belleville S, Hudon C, Bier N, Brodeur C, Gilbert B, Grenier S, & Gauthier S (2018). MEMO+: efficacy, durability and effect of cognitive training and psychosocial intervention in individuals with mild cognitive impairment. Journal of the American Geriatrics Society, 66(4), 655–663. doi: 10.1111/jgs.15192 [DOI] [PubMed] [Google Scholar]
- *.Bier B, De Boysson C, & Belleille S (2014). Identifying training modalities to improve multitasking in older adults. Age, 36(4). doi: 10.1007/s11357-014-9688-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Boot WR, Champion M, Blakely DP, Wright T, Souders DJ, & Charness N (2013). Video games as a means to reduce age-related cognitive decline: attitudes, compliance, and effectiveness. Frontiers in Psychology, 4(2), 31. doi: 10.3389/fpsyg.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot WR, Simons DJ, Stothart C, & Stutts C (2013). The pervasive problem with placebos in psychology: why active control groups are not sufficient to rule out placebo effects. Perspectives on Psychological Science, 8(4), 445–454. doi: 10.1177/1745691613491271 [DOI] [PubMed] [Google Scholar]
- Boot WR, Sumner A, Towne TJ, Rodriguez P, & Ericsson AK (2017). Applying aspects of the expert performance approach to better understand the structure of skill and mechanisms of skill acquisition in video games. Top Cogn Sci, 9, 413–436. doi: 10.1111/tops.12230 [DOI] [PubMed] [Google Scholar]
- Bopp KL, & Verhaeghen P (2018). Aging and n-back performance: A meta-analysis. The Journals of Gerontology: Series B, gby024, 1–12. doi: 10.1093/geronb/gby024 [DOI] [PubMed] [Google Scholar]
- *.Borella E, Carretti B, Cantarella A, Riboldi F, Zavagnin M, & De Beni R (2014). Benefits of training visuospatial working memory in young–old and old–old. Developmental Psychology, 50(3), 714–727. doi: 10.1037/a0034293 [DOI] [PubMed] [Google Scholar]
- *.Borella E, Carretti B, Riboldi F, & De Beni R (2010). Working memory training in older adults: evidence of transfer and maintenance effects. Psychology and Aging, 25(4), 767–778. doi: 10.1037/a0020683 [DOI] [PubMed] [Google Scholar]
- *.Borella E, Carretti B, Zanoni G, Zavagnin M, & De Beni R (2013). Working memory training in old age: an examination of transfer and maintenance effects. Archives of Clinical Neuropsychology : The Official Journal of the National Academy of Neuropsychologists, 28(4), 331–347. doi: 10.1093/arclin/act020 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JT, Rothstein HR (2009). Introduction to Meta-Analysis. Elsevier. [Google Scholar]
- *.Boripuntakul S, Kothan S, Methapatara P, Munkhetvit P, & Sungkarat S (2012). Short-term effects of cognitive training program for individuals with amnestic mild cognitive impairment: a pilot study. Physical & Occupational Therapy in Geriatrics, 30(2), 138–149. doi: 10.3109/02703181.2012.657822 [DOI] [Google Scholar]
- *.Bozoki A, Radovanovic M, Winn B, Heeter C, & Anthony JC (2013). Effects of a computer-based cognitive exercise program on age-related cognitive decline. Archives of Gerontology and Geriatrics, 57(1), 1–7. doi: 10.1016/j.archger.2013.02.009 [DOI] [PubMed] [Google Scholar]
- *.Brehmer Y, Westerberg H, & Bäckman L (2012). Working-memory training in younger and older adults: training gains, transfer, and maintenance. Frontiers in Human Neuroscience, 6, 63. doi: 10.3389/fnhum.2012.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Buitenweg JIV, van de Ven RM, Prinssen S, Murre JMJ, & Ridderinkhof KR (2017). Cognitive flexibility training: a large-scale multimodal adaptive active-control intervention study in healthy older adults. Frontiers in Human Neuroscience, 11, 529. doi: 10.3389/fnhum.2017.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Buiza C, Etxeberria I, Galdona N, González MF, Arriola E, de Munain AL, & Yanguas JJ (2008). A randomized, two-year study of the efficacy of cognitive intervention on elderly people: the Donostia Longitudinal Study. International Journal of Geriatric Psychiatry, 23(1), 85–94. doi: 10.1002/gps.1846 [DOI] [PubMed] [Google Scholar]
- *.Bürki CN, Ludwig C, Chicherio C, & de Ribaupierre A (2014). Individual differences in cognitive plasticity: an investigation of training curves in younger and older adults. Psychological Research, 78(6), 821–835. doi: 10.1007/s00426-014-0559-3 [DOI] [PubMed] [Google Scholar]
- *.Buschert VC, Friese U, Teipel SJ, Schneider P, Merensky W, Rujescu D, & Buerger K (2011). Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild alzheimer’s disease: A pilot study. Journal of Alzheimer’s Disease, 25(4), 679–694. doi: 10.3233/JAD-2011-100999 [DOI] [PubMed] [Google Scholar]
- *.Buschkuehl M, Jaeggi SM, Hutchison S, Perrig-Chiello P, Däpp C, Müller M, & Perrig WJ (2008). Impact of working memory training on memory performance in old-old adults. Psychology and Aging, 23(4), 743–753. doi: 10.1037/a0014342 [DOI] [PubMed] [Google Scholar]
- *.Cahn-Weiner D. a, Malloy PF, Rebok GW, & Ott BR (2003). Results of a randomized placebo-controlled study of memory training for mildly impaired Alzheimer’s disease patients. Applied Neuropsychology, 10(4), 215–223. doi: 10.1207/s15324826an1004_3 [DOI] [PubMed] [Google Scholar]
- *.Calero MD, & Navarro E (2004). Relationship between plasticity, mild cognitive impairment and cognitive decline. Archives of Clinical Neuropsychology, 19(5), 653–660. doi: 10.1016/j.acn.2003.08.008 [DOI] [PubMed] [Google Scholar]
- *.Calero MD, & Navarro E (2007). Cognitive plasticity as a modulating variable on the effects of memory training in elderly persons. Archives of Clinical Neuropsychology, 22(1), 63–72. doi: 10.1016/j.acn.2006.06.020 [DOI] [PubMed] [Google Scholar]
- *.Cantarella A, Borella E, Carretti B, Kliegel M, & de Beni R (2017). Benefits in tasks related to everyday life competences after a working memory training in older adults. International Journal of Geriatric Psychiatry, 32(1), 86–93. doi: 10.1002/gps.4448 [DOI] [PubMed] [Google Scholar]
- *.Cantarella A, Borella E, Marigo C, & De Beni R (2017). Benefits of well-being training in healthy older adults. Applied Psychology: Health and Well-Being, 9(3), 261–284. doi: 10.1111/aphw.12091 [DOI] [PubMed] [Google Scholar]
- *.Caprio-Prevette MD, & Fry PS (1996). Memory enhancement program for community-based older adults: Development and evaluation. Experimental Aging Research, 22(3), 281–303. doi: 10.1080/03610739608254012 [DOI] [PubMed] [Google Scholar]
- *.Carretti B, Borella E, Zavagnin M, & De Beni R (2013). Gains in language comprehension relating to working memory training in healthy older adults. International Journal of Geriatric Psychiatry, 28(5), 539–546. doi: 10.1002/gps.3859 [DOI] [PubMed] [Google Scholar]
- *.Cavallini E, Bottiroli S, Capotosto E, De Beni R, Pavan G, Vecchi T, & Borella E (2015). Self-help memory training for healthy older adults in a residential care center: Specific and transfer effects on performance and beliefs. International Journal of Geriatric Psychiatry, 30(8), 870–880. doi: 10.1002/gps.4230 [DOI] [PubMed] [Google Scholar]
- *.Ceccato E, Vigato G, Bonetto C, Bevilacqua A, Pizziolo P, Crociani S, & Barchi E (2012). STAM protocol in dementia. American Journal of Alzheimer’s Disease & Other Dementia, 27(5), 301–310. doi: 10.1177/1533317512452038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerella J (1990). Aging and information-processing rate. Handbook of the Psychology of Aging, 201–221. doi: 10.1016/B978-0-12-101280-9.50018-8 [DOI] [Google Scholar]
- *.Chan JSY, Wu Q, Liang D, & Yan JH (2015). Visuospatial working memory training facilitates visually-aided explicit sequence learning. Acta Psychologica, 161, 145–153. doi: 10.1016/j.actpsy.2015.09.008 [DOI] [PubMed] [Google Scholar]
- *.Chen B, Wei Y, Deng W, & Sun S (2018). The effects of cognitive training on cognitive abilities and everyday function: a 10-week randomized controlled trial. The International Journal of Aging and Human Development, 86(1), 69–81. doi: 10.1177/0091415017697725 [DOI] [PubMed] [Google Scholar]
- *.Cheng Y, Wu W, Feng W, Wang J, Chen Y, Shen Y, & Li C (2012). The effects of multi-domain versus single-domain cognitive training in non-demented older people: a randomized controlled trial. BMC Medicine, 10(1), 30. doi: 10.1186/1741-7015-10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cohen-Mansfield J, Cohen R, Buettner L, Eyal N, Jakobovits H, Rebok G, & Sternberg S (2015). Interventions for older persons reporting memory difficulties: a randomized controlled pilot study. International Journal of Geriatric Psychiatry, 30(5), 478–486. doi: 10.1002/gps.4164 [DOI] [PubMed] [Google Scholar]
- Colcombe S, & Kramer AF (2003). Fitness Effects on the Cognitive Function of Older Adults. Psychological Science, 14(2), 125–130. doi: 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- Cooper H, Hedges LV, Valentine JC (2009). The handbook of research synthesis and meta-analysis. Russell Sage Foundation [Google Scholar]
- *.Craik FIM, Winocur G, Palmer H, Binns MA, Edwards M, Bridges K, & Stuss DT (2007). Cognitive rehabilitation in the elderly: effects on memory. Journal of the International Neuropsychological Society, 13(01), 132–142. doi: 10.1017/S1355617707070166 [DOI] [PubMed] [Google Scholar]
- *.Cuenen A, Jongen EMM, Brijs T, Brijs K, Houben K, & Wets G (2016). Effect of a working memory training on aspects of cognitive ability and driving ability of older drivers: Merits of an adaptive training over a non-adaptive training. Transportation Research Part F: Traffic Psychology and Behavior, 42, 15–27. doi: 10.1016/j.trf.2016.06.012 [DOI] [Google Scholar]
- *.Dahlin E, Nyberg L, Bäckman L, & Neely AS (2008). Plasticity of executive functioning in young and older adults: immediate training gains, transfer, and long-term maintenance. Psychology and Aging, 23(4), 720–730. doi: 10.1037/a0014296 [DOI] [PubMed] [Google Scholar]
- *.Diamond K, Mowszowski L, Cockayne N, Norrie L, Paradise M, Hermens DF, & Naismith SL (2015). Randomized controlled trial of a healthy brain ageing cognitive training program: effects on memory, mood, and sleep. Journal of Alzheimer’s Disease, 44(4), 1181–1191. doi: 10.3233/JAD-142061 [DOI] [PubMed] [Google Scholar]
- *.Dunlosky J, Kubat-Silman AK, & Hertzog C (2003). Training monitoring skills improves older adults’ self-paced associative learning. Psychology and Aging, 18(2), 340–345. doi: 10.1037/0882-7974.18.2.340 [DOI] [PubMed] [Google Scholar]
- *.Dustman RE, Emmerson RY, Steinhaus LA, Shearer DE, & Dustman TJ (1992). The effects of videogame playing on neuropsychological performance of elderly individuals. Journal of Gerontology: Psychological Sciences, 47(3), 168–171. [DOI] [PubMed] [Google Scholar]
- *.Edwards JD, Wadley VG, Vance DE, Wood K, Roenker DL, & Ball KK (2005). The impact of speed of processing training on cognitive and everyday performance. Aging & Mental Health, 9(3), 262–271. doi: 10.1080/13607860412331336788 [DOI] [PubMed] [Google Scholar]
- *.Edwards Jerri D., Wadley VG, Myers RS, Roenker DL, Cissell GM, & Ball KK (2002). Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology, 48(5), 329–340. doi: 10.1159/000065259 [DOI] [PubMed] [Google Scholar]
- *.Edwards JD, Valdés EG, Peronto C, Castora-Binkley M, Alwerdt J, Andel R, & Lister JJ (2015). The efficacy of insight cognitive training to improve useful field of view performance: a brief report. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 70(3), 417–422. doi: 10.1093/geronb/gbt113 [DOI] [PubMed] [Google Scholar]
- Edwards J, Fausto BA, Tetlow A & Corona RT, & Valdés E (2018). Systematic review and meta-analyses of useful field of view cognitive training. Neuroscience & Biobehavioral Reviews. 84, 72–91. 10.1016/j.neubiorev.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, & Minder C (1997). Bias in meta-analysis detected by a simple, graphical test. British Medical Journal, 315(7109), 629–634. doi: 10.1136/bmj.316.7129.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Emsaki G, NeshatDoost HT, Tavakoli M, & Barekatain M (2017). Memory specificity training can improve working and prospective memory in amnestic mild cognitive impairment. Dementia & Neuropsychologia, 11(3), 255–261. doi: 10.1590/1980-57642016dn11-030007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, & Walhovd KB (2010). Effects of memory training on cortical thickness in the elderly. NeuroImage, 52(4), 1667–1676. doi: 10.1016/j.neuroimage.2010.05.041 [DOI] [PubMed] [Google Scholar]
- *.Eramudugolla R, Kiely KM, Chopra S, & Anstey KJ (2017). Effect of speed of processing training on older driver screening measures. Frontiers in Aging Neuroscience, 9, 338. doi: 10.3389/fnagi.2017.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Fairchild JK, & Scogin FR (2010). Training to Enhance Adult Memory (TEAM): An investigation of the effectiveness of a memory training program with older adults. Aging & Mental Health, 14(3), 364–373. doi: 10.1080/13607860903311733 [DOI] [PubMed] [Google Scholar]
- Fernández-Ballesteros R, Botella J, Zamarrón MD, Molina MÁ, Cabras E, Schettini R, & Tárraga L (2012). Cognitive plasticity in normal and pathological aging. Clinical Interventions in Aging, 7, 15–25. doi: 10.2147/CIA.S27008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Finn M, & McDonald S (2011). Computerized cognitive training for older persons with mild cognitive impairment: a pilot study using a randomized controlled trial design. Brain Impairment, 12(3), 187–199. doi: 10.1375/brim.12.3.187 [DOI] [Google Scholar]
- *.Finn M, & McDonald S (2015). Repetition-lag training to improve recollection memory in older people with amnestic mild cognitive impairment. A randomized controlled trial. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 22(2), 244–258. doi: 10.1080/13825585.2014.915918 [DOI] [PubMed] [Google Scholar]
- *.Flegal KE, & Lustig C (2015). You can go your own way: effectiveness of participant-driven versus experimenter-driven processing strategies in memory training and transfer. Aging, Neuropsychology, and Cognition, 5585(11), 1–29. doi: 10.1080/13825585.2015.1108386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gagnon LG, & Belleville S (2012). Training of attentional control in mild cognitive impairment with executive deficits: results from a double-blind randomized controlled study. Neuropsychological Rehabilitation, 22(6), 809–835. doi: 10.1080/09602011.2012.691044 [DOI] [PubMed] [Google Scholar]
- *.Gajewski PD, & Falkenstein M (2012). Training-induced improvement of response selection and error detection in aging assessed by task switching: effects of cognitive, physical, and relaxation training. Frontiers in Human Neuroscience, 6(5), 130. doi: 10.3389/fnhum.2012.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gao Y, Peng H, & Wen J (2014). The training effect of working memory based on central executive system intervention in older adults: a randomized controlled study. Journal of Adult Development, 21(2), 80–88. doi: 10.1007/s10804-013-9181-7 [DOI] [Google Scholar]
- *.Garcia-Campuzano MT, Virues-Ortega J, Smith S, & Moussavi Z (2013). Effect of cognitive training targeting associative memory in the elderly: a small randomized trial and a longitudinal evaluation. Journal of the American Geriatrics Society, 61(12), 2252–2254. doi: 10.1111/jgs.12574 [DOI] [PubMed] [Google Scholar]
- Gates NJ, Sachdev PS, Fiatarone Singh MA, & Valenzuela M (2011). Cognitive and memory training in adults at risk of dementia: a systematic review. BMC Geriatrics, 11(1), 55. doi: 10.1186/1471-2318-11-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto SL, & Tak SH (2008). Computer, internet, and e-mail use among older adults: benefits and barriers. Educational Gerontology, 34(9), 800–811. doi: 10.1080/03601270802243697 [DOI] [Google Scholar]
- *.Giuli C, Papa R, Lattanzio F, & Postacchini D (2016). The effects of cognitive training for elderly: results from my mind project. Rejuvenation Research, 19(6), 485–494. doi: 10.1089/rej.2015.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Goghari VM, & Lawlor-Savage L (2017). Comparison of cognitive change after working memory training and logic and planning training in healthy older adults. Frontiers in Aging Neuroscience, 9(2), 1–12. doi: 10.3389/fnagi.2017.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Golino MTS, Flores Mendoza C, & Golino HF (2017). Effects of cognitive training on cognitive performance of healthy older adults. The Spanish Journal of Psychology, 20, E39. doi: 10.1017/sjp.2017.38 [DOI] [PubMed] [Google Scholar]
- *.Gooding AL, Choi J, Fiszdon JM, Wilkins K, Kirwin PD, van Dyck CH, & Rivera Mindt M (2016). Comparing three methods of computerized cognitive training for older adults with subclinical cognitive decline. Neuropsychological Rehabilitation, 26(5–6), 810–821. doi: 10.1080/09602011.2015.1118389 [DOI] [PubMed] [Google Scholar]
- *.Greenaway MC, Duncan NL, & Smith GE (2013). The memory support system for mild cognitive impairment: randomized trial of a cognitive rehabilitation intervention. International Journal of Geriatric Psychiatry, 28(4), 402–409. doi: 10.1002/gps.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, & Verghese J (2009). Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology, 73(5), 356–361. doi: 10.1212/WNL.0b013e3181b04ae3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hars M, Herrmann FR, Gold G, Rizzoli R, & Trombetti A (2014). Effect of music-based multitask training on cognition and mood in older adults. Age and Ageing, 43(2), 196–200. doi: 10.1093/ageing/aft163 [DOI] [PubMed] [Google Scholar]
- *.Hayslip BJ (1989). Alternative mechanisms for improvements in fluid ability performance among older adults. Psychology and Aging, 4(1), 122–124. [DOI] [PubMed] [Google Scholar]
- Hedges LV, & Olkin I (1985). Statistical methods for meta-analysis. Elsevier [Google Scholar]
- *.Heinzel S, Rimpel J, Stelzel C, & Rapp MA (2017). Transfer effects to a multimodal dual-task after working memory training and associated neural correlates in older adults – a pilot study. Frontiers in Human Neuroscience, 11(2), 1–15. doi: 10.3389/fnhum.2017.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Herrera C, Chambon C, Michel BF, Paban V, & Alescio-Lautier B (2012). Positive effects of computer-based cognitive training in adults with mild cognitive impairment. Neuropsychologia, 50(8), 1871–1881. doi: 10.1016/j.neuropsychologia.2012.04.012 [DOI] [PubMed] [Google Scholar]
- Hertzog C, Cooper BP, & Fisk AD (1996). Aging and individual differences in the development of skilled memory search performance. Psychology and Aging, 11(3), 497–520. [DOI] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, & Ottenbacher KJ (2004). The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Archives of Physical Medicine and Rehabilitation, 85(10), 1694–1704. doi: 10.1016/j.apmr.2004.03.019 [DOI] [PubMed] [Google Scholar]
- Higgins, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, & Sterne JAC (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ, 343, d5928–d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NTM, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, & Lampit A (2017). Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. American Journal of Psychiatry, 174(4), 329–340. doi: 10.1176/appi.ajp.2016.16030360 [DOI] [PubMed] [Google Scholar]
- *.Hill RD, Allen C, & McWhorter P (1991). Stories as a mnemonic aid for older learners. Psychology and Aging, 6(3), 484–486. doi: 10.1037/0882-7974.6.3.484 [DOI] [PubMed] [Google Scholar]
- *.Hill RD, Storandt M, & Simeone C (1990). The effects of memory skills training and incentives on free recall in older learners. Journal of Gerontology, 45(6), 227–232. doi: 10.1093/geronj/45.6.P227 [DOI] [PubMed] [Google Scholar]
- Howieson DB, Carlson NE, Moore MM, Wasserman D, Abendroth CD, Payne-Murphy J, & Kaye JA (2008). Trajectory of mild cognitive impairment onset. Journal of the International Neuropsychological Society : JINS, 14(2), 192–198. doi: 10.1017/S1355617708080375 [DOI] [PubMed] [Google Scholar]
- *.Hwang HR, Choi SH, Yoon DH, Yoon BN, Suh YJ, Lee D, & Hong CG (2012). The effect of cognitive training in patients with mild cognitive impairment and early Alzheimer’s disease: a preliminary study. Journal of Clinical Neurology (Seoul, Korea), 8(3), 190–197. doi: 10.3988/jcn.2012.8.3.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hyer L, Scott C, Atkinson MM, Mullen CM, Lee A, Johnson A, & Mckenzie LC (2016). Cognitive training program to improve working memory in older adults with MCI. Clinical Gerontologist, 39(5), 410–427. doi: 10.1080/07317115.2015.1120257 [DOI] [PubMed] [Google Scholar]
- *.Hynes SM (2016). Internet, home-based cognitive and strategy training with older adults: a study to assess gains to daily life. Aging Clinical and Experimental Research, 28(5), 1003–1008. doi: 10.1007/s40520-015-0496-z [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Studer-Luethi B, Buschkuehl M, Su Y, Jonides J, & Perrig WJ (2010). The relationship between n-back performance and matrix reasoning - implications for training and transfer. Intelligence. 38(6), 625–635. doi: 10.1016/j.intell.2010.09.001 [DOI] [Google Scholar]
- *.Jean L, Bergeron M-E, Thivierge S, & Simard M (2010). Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. The American Journal of Geriatric, 18(4), 281–296. doi: 10.1097/JGP.0b013e3181c37ce9 [DOI] [PubMed] [Google Scholar]
- *.Jeong JH, Na HR, Choi SH, Kim J, Na DL, Seo SW, & Kim JY (2016). Group- and home-based cognitive intervention for patients with mild cognitive impairment: A randomized controlled trial. Psychotherapy and Psychosomatics, 85(4), 198–207. doi: 10.1159/000442261 [DOI] [PubMed] [Google Scholar]
- *.Ji Y, Wang J, Chen T, Du X, & Zhan Y (2016). Plasticity of inhibitory processes and associated far-transfer effects in older adults. Psychology and Aging, 31(5), 415–429. doi: 10.1037/pag0000102 [DOI] [PubMed] [Google Scholar]
- *.Kalbe E, Bintener C, Ophey A, Reuter C, Göbel S, Klöters S, & Kalbe E (2018). Computerized cognitive training in healthy older adults: baseline cognitive level and subjective cognitive concerns predict training outcome. Health, 10(10), 20–55. doi: 10.4236/health.2018.101003 [DOI] [Google Scholar]
- Karbach J, & Verhaeghen P (2014). Making working memory work: a meta-analysis of executive-control and working memory training in older adults. Psychological Science, 25(11), 2027–2037. doi: 10.1177/0956797614548725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr JE, Areshenkoff CN, Rast P, & Garcia-Barrera MA (2014). An empirical comparison of the therapeutic benefits of physical exercise and cognitive training on the executive functions of older adults: A meta-analysis of controlled trials. Neuropsychology, 28(6), 829–845. doi: 10.1037/neu0000101 [DOI] [PubMed] [Google Scholar]
- *.Kawashima R (2013). Mental exercises for cognitive function: clinical evidence. Journal of Preventive Medicine & Public Health, 46(Suppl 1), S22–S27. doi: 10.3961/jpmph.2013.46.S.S22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kim GH, Jeon S, Im K, Kwon H, Lee BH, Kim GY, & Na DL (2015). Structural brain changes after traditional and robot-assisted multi-domain cognitive training in community-dwelling healthy elderly. PLoS ONE, 10(4), 1–20. doi: 10.1371/journal.pone.0123251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kim H, Chey J, & Lee S (2017). Effects of multicomponent training of cognitive control on cognitive function and brain activation in older adults. Neuroscience Research, 124, 8–15. doi: 10.1016/j.neures.2017.05.004 [DOI] [PubMed] [Google Scholar]
- *.Kinsella GJ, Mullaly E, Rand E, Ong B, Burton C, Price S, & Storey E (2009). Early intervention for mild cognitive impairment: a randomised controlled trial. Journal of Neurology, Neurosurgery, and Psychiatry, 80(7), 730–736. doi: 10.1136/jnnp.2008.148346 [DOI] [PubMed] [Google Scholar]
- Kliegl R, Smith J, & Baltes PB (1989). Testing-the-limits and the study of adult age differences in cognitive plasticity of a mnemonic skill. Developmental Psychology, 25, 247–256. doi: 10.1037/0012-1649.25.2.247 [DOI] [Google Scholar]
- Kliegl R, Smith J, & Baltes PB (1990). On the locus and process of magnification of age differences during mnemonic training. Developmental Psychology, 26(6), 894–904. doi: 10.1037/0012-1649.26.6.894 [DOI] [Google Scholar]
- *.Konsztowicz S, Anton J, Crane J, Moafmashhadi P, & Koski L (2013). A pilot study of training and compensation interventions for mild cognitive impairment. Dementia and Geriatric Cognitive Disorder, 3, 192–201. doi: 10.1159/000350026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Larish JF, & Strayer DL (1995). Training for attentional control in dual task settings: A comparison of young and old adults. Journal of Experimental Psychology: Applied, 1(1), 50–76. doi: 10.1037//1076-898X.1.1.50 [DOI] [Google Scholar]
- *.Kurz A, Pohl C, Ramsenthaler M, & Sorg C (2009). Cognitive rehabilitation in patients with mild cognitive impairment. International Journal of Geriatric Psychiatry, 24(2), 163–168. doi: 10.1002/gps.2086 [DOI] [PubMed] [Google Scholar]
- *.Küster OC, Fissler P, Laptinskaya D, Thurm F, Scharpf A, Woll A, & Kolassa IT (2016). Cognitive change is more positively associated with an active lifestyle than with training interventions in older adults at risk of dementia: a controlled interventional clinical trial. BMC Psychiatry, 16(1), 315. doi: 10.1186/s12888-016-1018-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kwok T, Wong, Chan, Shiu, Lam KC, Young, … Ho F (2013). Effectiveness of cognitive training for Chinese elderly in Hong Kong. Clinical Interventions in Aging, 13(8), 213–219. doi: 10.2147/CIA.S38070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lampit A, Hallock H, Moss R, Kwok S, Rosser M, Lukjanenko M, & Valenzuela M (2014). The time course of global cognitive gains from supervised computer-assisted cognitive training: a randomised, active-controlled trial in elderly with multiple dementia risk factors. The Journal of Prevention of Alzheimer’s Disease, 1(1), 33–39. doi: 10.14283/jpad.2014.18 [DOI] [PubMed] [Google Scholar]
- Lampit Amit, Hallock H, & Valenzuela M (2014). Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Medicine, 11(11), e1001756. doi: 10.1371/journal.pmed.1001756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lange S, & Süß HM (2015). Experimental evaluation of near- and far-transfer effects of an adaptive multicomponent working memory training. Applied Cognitive Psychology, 29(4), 502–514. doi: 10.1002/acp.3126 [DOI] [Google Scholar]
- *.Lee T-S, Goh SJA, Quek SY, Phillips R, Guan C, Cheung YB, & Krishnan KRR (2013). A brain-computer interface based cognitive training system for healthy elderly: a randomized control pilot study for usability and preliminary efficacy. PloS One, 8(11), e79419. doi: 10.1371/journal.pone.0079419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Legault C, Jennings JM, Katula J. a, Dagenbach D, Gaussoin S. a, Sink KM, & Espeland M. a. (2011). Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: The Seniors Health and Activity Research Program Pilot (SHARP-P) study, a randomized controlled trial. BMC Geriatrics, 11(1), 27. doi: 10.1186/1471-2318-11-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Leung NTY, Tam HMK, Chu LW, Kwok TCY, Chan F, Lam LCW, & Lee TMC (2015). Neural plastic effects of cognitive training on aging brain. Neural Plasticity, 2015. 535618. doi: 10.1155/2015/535618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Li B, Zhu X, Hou J, Chen T, Wang P, & Li J (2016). Combined cognitive training vs. memory strategy training in healthy older adults. Frontiers in Psychology, 7, 1–11. doi: 10.3389/fpsyg.2016.00834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li J, Li N, Li B, Wang P, & Zhou T (2011). Cognitive intervention for persons with mild cognitive impairment: A meta-analysis. Ageing Research Reviews, 10(2), 285–296. doi: 10.1016/j.arr.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, & Moher D (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology, 62(10), e1–e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- *.Lima-Silva TB, Ordonez TN, Santos GD dos, Fabrício AT, Aramaki FO, B. De Almeida, E., & Yassuda MS (2010). Effects of cognitive training based on metamemory and mental images. Dementia & Neuropsychologia, 4(2), 114–119. doi: 10.1590/S1980-57642010DN40200007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes RMF, & Argimon I. I. de L. (2016). Cognitive training in the elderly and its effect on the executive functions. Acta Colombiana de Psicología, 19(2), 159–176. doi: 10.14718/ACP.2016.19.2.8 [DOI] [Google Scholar]
- *.Lussier M, Brouillard P, & Bherer L (2015). Limited benefits of heterogeneous dual-task training on transfer effects in older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 00(00), gbv105. doi: 10.1093/geronb/gbv105 [DOI] [PubMed] [Google Scholar]
- *.Lussier M, Bugaiska A, & Bherer L (2016). Specific transfer effects following variable priority dual-task training in older adults. Restorative Neurology and Neuroscience, 1–14. doi: 10.3233/RNN-150581 [DOI] [PubMed] [Google Scholar]
- *.Lussier M, Gagnon C, & Bherer L (2012). An investigation of response and stimulus modality transfer effects after dual-task training in younger and older. Frontiers in Human Neuroscience, 6(5), 129. doi: 10.3389/fnhum.2012.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher CG, Sherrington C, Herbert RD, Moseley AM, & Elkins M (2003). Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical Therapy, 83(8), 713–721. doi: 10.1016/s0895-4356(00)00360-7 [DOI] [PubMed] [Google Scholar]
- *.Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, & Merzenich MM (2006). Memory enhancement in healthy older adults using a brain plasticity-based training program: A randomized, controlled study. Proceedings of the National Academy of Sciences, 103(33), 12523–12528. doi: 10.1073/pnas.0605194103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Man D, Lee G, Yu E, & Yip. (2013). Evaluation of a computer-assisted errorless learning-based memory training program for patients with early Alzheimer’s disease in Hong Kong: a pilot study. Clinical Interventions in Aging, 623. doi: 10.2147/CIA.S45726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mansbach WE, Mace RA, & Clark KM (2017). The efficacy of a computer-assisted cognitive rehabilitation program for patients with mild cognitive deficits: a pilot study. Experimental Aging Research, 43(1), 94–104. doi: 10.1080/0361073X.2017.1258256 [DOI] [PubMed] [Google Scholar]
- *.Margrett JA, & Willis SL (2006). In-home cognitive training with older married couples: individual versus collaborative learning. Aging, Neuropsychology, and Cognition, 13(2), 173–195. doi: 10.1080/138255890969285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.McAvinue LP, Golemme M, Castorina M, Tatti E, Pigni FM, Salomone S, & Robertson IH (2013). An evaluation of a working memory training scheme in older adults. Frontiers in Aging Neuroscience, 5(5), 20. doi: 10.3389/fnagi.2013.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.McDougall GJ, Becker H, Pituch K, Acee TW, Vaughan PW, & Delville CL (2010). Differential benefits of memory training for minority older adults in the SeniorWISE study. The Gerontologist, 50(5), 632–645. doi: 10.1093/geront/gnq017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.McDougall S, & House B (2012). Brain training in older adults: evidence of transfer to memory span performance and pseudo-Matthew effects. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 19(1–2), 195–221. doi: 10.1080/13825585.2011.640656 [DOI] [PubMed] [Google Scholar]
- Mewborn CM, Lindbergh CA, & Stephen Miller L (2017). Cognitive interventions for cognitively healthy, mildly impaired, and mixed samples of older adults: a systematic review and meta-analysis of randomized-controlled trials. Neuropsychology Review, 27(4), 403–439. doi: 10.1007/s11065-017-9350-8 [DOI] [PubMed] [Google Scholar]
- *.Miller KJ, Dye RV, Kim J, Jennings JL, O’Toole E, Wong J, & Siddarth P (2013). Effect of a computerized brain exercise program on cognitive performance in older adults. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry, 21(7), 655–663. doi: 10.1016/j.jagp.2013.01.077 [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognitive Psychology, 41(1), 49–100. doi: 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- *.Mohs RC, Ashman T. a, Jantzen K, Albert M, Brandt J, Gordon B, & Stern Y (1998). A study of the efficacy of a comprehensive memory enhancement program in healthy elderly persons. Psychiatry Research, 77(3), 183–195. doi: 10.1016/S0165-1781(98)00003-1 [DOI] [PubMed] [Google Scholar]
- *.Moro V, Condoleo MT, Sala F, Pernigo S, Moretto G, & Gambina G (2012). Cognitive stimulation in a-MCI: an experimental study. American Journal of Alzheimer’s Disease and Other Dementias, 27(2), 121–130. doi: 10.1177/1533317512441386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Moro V, Condoleo MT, Valbusa V, Broggio E, Moretto G, & Gambina G (2015). Cognitive stimulation of executive functions in mild cognitive impairment. American Journal of Alzheimer’s Disease & Other Dementias, 30(2), 153–164. doi: 10.1177/1533317514539542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC (2005). Early-stage and preclinical Alzheimer disease. Alzheimer Disease and Associated Disorders, 19(3), 163–165. doi: 10.1097/01.wad.0000184005.22611.cc [DOI] [PubMed] [Google Scholar]
- Morris SB (2008). Estimating effect sizes from pretest-posttest-control group designs. Organizational Research Methods, 11(2), 364–386. doi: 10.1177/1094428106291059 [DOI] [Google Scholar]
- *.Mowszowski L, Hermens DF, Diamond K, Norrie L, Cockayne N, Ward PB, & Naismith SL (2014). Cognitive training enhances pre-attentive neurophysiological responses in older adults “at risk” of dementia. Journal of Alzheimer’s Disease, 41(4), 1095–1108. doi: 10.3233/JAD-131985 [DOI] [PubMed] [Google Scholar]
- *.Mudar RA, Chapman SB, Rackley A, Eroh J, Chiang H-S, Perez A, & Spence JS (2017). Enhancing latent cognitive capacity in mild cognitive impairment with gist reasoning training: a pilot study. International Journal of Geriatric Psychiatry, 32(5), 548–555. doi: 10.1002/gps.4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Murphy M, O’Sullivan K, & Kelleher KG (2014). Daily crosswords improve verbal fluency: a brief intervention study. International Journal of Geriatric Psychiatry, 29(9), 915–919. doi: 10.1002/gps.4079 [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M (2000). Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition, 26, 1170–1187. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Murphy K, & Andrews G (2019). Immediate and long-term efficacy of executive functions cognitive training in older adults: a systematic review and meta-analysis. Psychological Bulletin. 10.1037/bul0000196 [DOI] [PubMed] [Google Scholar]
- *.Nouchi R, Saito T, Nouchi H, & Kawashima R (2016). Small acute benefits of 4 weeks processing speed training games on processing speed and inhibition performance and depressive mood in the healthy elderly people: Evidence from a randomized control trial. Frontiers in Aging Neuroscience, 8(12), 1–12. doi: 10.3389/fnagi.2016.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Nouchi R, Taki Y, Takeuchi H, Hashizume H, Akitsuki Y, Shigemune Y, & Kawashima R (2012). Brain training game improves executive functions and processing speed in the elderly: a randomized controlled trial. PloS One, 7(1), e29676. doi: 10.1371/journal.pone.0029676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, & Bäckman L (2012). Memory aging and brain maintenance. Trends in Cognitive Sciences, 16(5), 292–305. doi: 10.1016/J.TICS.2012.04.005 [DOI] [PubMed] [Google Scholar]
- *.Oh SJ, Seo S, Lee JH, Song MJ, & Shin M-S (2017). Effects of smartphone-based memory training for older adults with subjective memory complaints: a randomized controlled trial. Aging & Mental Health, 7863, 1–9. doi: 10.1080/13607863.2016.1274373 [DOI] [PubMed] [Google Scholar]
- *.Olchik MR, Farina J, Steibel N, Teixeira AR, & Yassuda MS (2013). Memory training (MT) in mild cognitive impairment (MCI) generates change in cognitive performance. Archives of Gerontology and Geriatrics, 56(3), 442–447. doi: 10.1016/j.archger.2012.11.007 [DOI] [PubMed] [Google Scholar]
- *.Ordonez TN, Yassuda MS, & Cachioni M (2011). Elderly online: effects of a digital inclusion program in cognitive performance. Archives of Gerontology and Geriatrics, 53(2), 216–219. doi: 10.1016/j.archger.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Park DC, & Reuter-Lorenz P (2009). The adaptive brain: aging and neurocognitive scaffolding. Annual Review of Psychology, 60(1), 173–196. doi: 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, & Bischof GN (2011). Neuroplasticity, aging, and cognitive function In Handbook of the Psychology of Aging (pp. 109–119). Elsevier. doi: 10.1016/B978-0-12-380882-0.00007-3 [DOI] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, & Smith PK (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17(2), 299–320. doi: 10.1037/0882-7974.17.2.299 [DOI] [PubMed] [Google Scholar]
- *.Park DC, Lodi-Smith J, Drew L, Haber S, Hebrank A, Bischof GN, & Aamodt W (2014). The impact of sustained engagement on cognitive function in older adults: the Synapse Project. Psychological Science, 25(1), 103–112. doi: 10.1177/0956797613499592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Payne BR, & Stine-Morrow EAL (2017). The effects of home-based cognitive training on verbal working memory and language comprehension in older adulthood. Frontiers in Aging Neuroscience, 9(8), 1–20. doi: 10.3389/fnagi.2017.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Pereira-Morales AJ, Cruz-Salinas AF, Aponte J, & Pereira-Manrique F (2018). Efficacy of a computer-based cognitive training program in older people with subjective memory complaints: a randomized study. International Journal of Neuroscience, 128(1), 1–9. doi: 10.1080/00207454.2017.1308930 [DOI] [PubMed] [Google Scholar]
- *.Peretz C, Korczyn AD, Shatil E, Aharonson V, Birnboim S, & Giladi N (2011). Computer-based, personalized cognitive training versus classical computer games: a randomized double-blind prospective trial of cognitive stimulation. Neuroepidemiology, 36(2), 91–99. doi: 10.1159/000323950 [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, & Winblad B (2001). Current concepts in mild cognitive impairment. Archives of Neurology, 58(12), 1985. doi: 10.1001/archneur.58.12.1985 [DOI] [PubMed] [Google Scholar]
- *.Latorre Postigo JM, Hernández-Viadel JV, & Trives JJR (2010). Efficacy of a group memory training method for older adults based on visualization and association techniques: A randomized, controlled trial with a placebo group. Applied Cognitive Psychology, 24(7), 956–968. doi: 10.1002/acp.1596 [DOI] [Google Scholar]
- *.Rapp S, Brenes G, Marsh AP, Brenes G, & Marsh AP (2002). Memory enhancement training for older adults with mild cognitive impairment: a preliminary study. Aging & Mental Health, 6(1), 5–11. doi: 10.1080/1360786012010107 [DOI] [PubMed] [Google Scholar]
- *.Rasmusson DX, Rebok GW, Bylsma FW, & Brandt J (1999). Effects of three types of memory training in normal elderly. Aging, Neuropsychology, and Cognition, 6(1), 56–66. doi: 10.1076/anec.6.1.56.790 [DOI] [Google Scholar]
- Raven J (2003) Raven Progressive Matrices In: McCallum RS (eds) Handbook of Nonverbal Assessment. Springer, Boston, MA [Google Scholar]
- Ray NR, O’connell MA, Nashiro K, Smith ET, Qin S, & Basak C (2017). Evaluating the relationship between white matter integrity, cognition, and varieties of video game learning. Restorative Neurology and Neuroscience, 35, 437–456. doi: 10.3233/RNN-160716 [DOI] [PubMed] [Google Scholar]
- Rebok GW, Langbaum JBS, Jones RN, Gross AL, Parisi JM, Spira AP, & Brandt J (2013). Memory training in the active study. Journal of Aging and Health, 25(8), 21S–42S. doi: 10.1177/0898264312461937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redick TS, Shipstead Z, Harrison TL, Hicks KL, Fried DE, Hambrick DZ, & Engle RW (2013). No evidence of intelligence improvement after working memory training: A randomized, placebo-controlled study. Journal of Experimental Psychology-General, 142(2), 359–379. doi: 10.1037/a0029082 [DOI] [PubMed] [Google Scholar]
- *.Regan B, Wells Y, Farrow M, O’Halloran P, & Workman B (2017). MAXCOG—maximizing cognition: a randomized controlled trial of the efficacy of goal-oriented cognitive rehabilitation for people with mild cognitive impairment and early Alzheimer disease. The American Journal of Geriatric Psychiatry, 25(3), 258–269. doi: 10.1016/j.jagp.2016.11.008 [DOI] [PubMed] [Google Scholar]
- *.Reijnders JSAM, Geusgens CAV, Ponds RWHM, & van Boxtel MPJ (2017). “Keep your brain fit!” Effectiveness of a psychoeducational intervention on cognitive functioning in healthy adults: A randomised controlled trial. Neuropsychological Rehabilitation, 27(4), 455–471. doi: 10.1080/09602011.2015.1090458 [DOI] [PubMed] [Google Scholar]
- *.Richmond LL, Morrison AB, Chein JM, & Olson IR (2011). Working memory training and transfer in older adults. Psychology and Aging, 26(4), 813–822. doi: 10.1037/a0023631 [DOI] [PubMed] [Google Scholar]
- Roe CM, Xiong C, Miller JP, & Morris JC (2007). Education and Alzheimer disease without dementia: Support for the cognitive reserve hypothesis. Neurology, 68(3), 223–228. doi: 10.1212/01.wnl.0000251303.50459.8a [DOI] [PubMed] [Google Scholar]
- *.Rojas GJ, Villar V, Iturry M, Harris P, Serrano CM, Herrera J. a, & Allegri RF (2013). Efficacy of a cognitive intervention program in patients with mild cognitive impairment. International Psychogeriatrics, 25(5), 825–831. doi: 10.1017/S1041610213000045 [DOI] [PubMed] [Google Scholar]
- Rosenberg MS (2005). The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution, 59(2), 464–468. doi: 10.1111/j.0014-3820.2005.tb01004.x [DOI] [PubMed] [Google Scholar]
- Ross AS, Hughes MC, and Doshi-Velez F (2017). Right for the right reasons: training differentiable models by constraining their explanations. In Proceedings of the 26th International Joint Conference on Artificial Intelligence(pp. 2662–2670). [Google Scholar]
- Royle J, & Lincoln N,B, (2008). The everyday memory questionnaire – revised: development of a 13-item scale, Disability and Rehabilitation, 30 (2), 114–121, doi: 10.1080/09638280701223876 [DOI] [PubMed] [Google Scholar]
- *.Salminen T, Frensch P, Strobach T, & Schubert T (2016). Age-specific differences of dual n-back training. Neuropsychology, Development, and Cognition B Aging Neuropsychol Cognition, 23(1), 18–39. doi: 10.1080/13825585.2015.1031723 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103(3), 403–428. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2010). Selective review of cognitive aging. Journal of the International Neuropsychological Society, 16(05), 754–760. doi: 10.1017/S1355617710000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Savulich G, Piercy T, Fox C, Suckling J, Rowe JB, O’brien JT, & Sahakian BJ (2017). Cognitive training using a novel memory game on an iPad in patients with amnestic mild cognitive impairment (aMCI). International Journal of Neuropsychopharmacology, 20(8), 624–633. doi: 10.1093/ijnp/pyx040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Scogin F, Storandt M, & Lott L (1985). Memory-Skills training, memory complaints, and depression in older adults. Journal of Gerontology, 40(5), 562–568. doi: 10.1093/geronj/40.5.562 [DOI] [PubMed] [Google Scholar]
- *.Scogin F, & Prohaska M (1992). The efficacy of self‐taught memory training for community-dwelling older adults. Educational Gerontology, 18(8), 751–766. doi: 10.1080/0360127920180801 [DOI] [Google Scholar]
- Selwyn N (2004). The information aged: A qualitative study of older adults’ use of information and communications technology. Journal of Aging Studies, 18(4), 369–384. doi: 10.1016/J.JAGING.2004.06.008 [DOI] [Google Scholar]
- *.Shatil E (2013). Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Frontiers in Aging Neuroscience, 5(3), 8. doi: 10.3389/fnagi.2013.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Shatil E, Mikulecká J, Bellotti F, & Bureš V (2014). Novel television-based cognitive training improves working memory and executive function. PloS One, 9(7), e101472. doi: 10.1371/journal.pone.0101472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Boot WR, Charness N, Gathercole SE, Chabris CF, Hambrick DZ, & Stine-Morrow EAL (2016). Do brain training programs work? Psychological Science in the Public Interest, 17(3), 103–186. doi: 10.1177/1529100616661983 [DOI] [PubMed] [Google Scholar]
- *.Simpson T, Camfield D, Pipingas A, Macpherson H, & Stough C (2012). Improved processing speed: online computer-based cognitive training in older adults. Educational Gerontology, 38(7), 445–458. doi: 10.1080/03601277.2011.559858 [DOI] [Google Scholar]
- *.Smith GE, Housen ÃP, Yaffe K, Ruff R, Kennison RF, Mahncke HW, & Zelinski EM (2009). A cognitive training program based on principles of brain plasticity: Results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. Journal of the American Geriatrics Society, 57(4), 594–603. doi: 10.1111/j.1532-5415.2008.02167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Souders DJ, Boot WR, Blocker K, Vitale T, Roque NA, & Charness N (2017). Evidence for narrow transfer after short-term cognitive training in older adults. Frontiers in Aging Neuroscience, 9(2), 1–10. doi: 10.3389/fnagi.2017.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … Phelps CH (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 280–292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Stepankova H, Lukavsky J, Buschkuehl M, Kopecek M, Ripova D, & Jaeggi SM (2014). The malleability of working memory and visuospatial skills: A randomized controlled study in older adults. Developmental Psychology, 50(4), 1049–1059. doi: 10.1037/a0034913 [DOI] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, & Tsai WY (1999). Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology, 53(9), 1942–1942. doi: 10.1212/WNL.53.9.1942 [DOI] [PubMed] [Google Scholar]
- *.Stern Y, Blumen HM, Rich LW, Richards A, Herzberg G, & Gopher D (2011). Space Fortress game training and executive control in older adults: a pilot intervention. Neuropsychology, Development, and Cognition. 18(6), 653–677. doi: 10.1080/13825585.2011.613450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow EAL, & Basak C (2011). Cognitive interventions In Handbook of the Psychology of Aging (pp. 153–171). Elsevier. doi: 10.1016/B978-0-12-380882-0.00010-3 [DOI] [Google Scholar]
- *.Stine-Morrow EAL, Parisi JM, Morrow DG, & Park DC (2008). The effects of an engaged lifestyle on cognitive vitality: A field experiment. Psychology and Aging, 23(4), 778–786. doi: 10.1037/a0014341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, & Spreen O (2006). A compendium of neuropsychological tests: Administration, norms, and commentary (3rd ed.). New York, NY, US: Oxford University Press. [Google Scholar]
- *.Sutter C, Zöllig J, & Martin M (2013). Plasticity of verbal fluency in older adults: a 90-minute telephone-based intervention. Gerontology, 59(1), 53–63. doi: 10.1159/000342199 [DOI] [PubMed] [Google Scholar]
- *.Theill N, Schumacher V, Adelsberger R, Martin M, & Jäncke L (2013). Effects of simultaneously performed cognitive and physical training in older adults. BMC Neuroscience, 14, 103. doi: 10.1186/1471-2202-14-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toril P, Reales JM, & Ballesteros S (2014). Video game training enhances cognition of older adults: A meta-analytic study. Psychology and Aging, 29(3), 706–716. doi: 10.1037/a0037507 [DOI] [PubMed] [Google Scholar]
- *.Tranter LJ, & Koutstaal W (2008). Age and flexible thinking: an experimental demonstration of the beneficial effects of increased cognitively stimulating activity on fluid intelligence in healthy older adults. Neuropsychology, Development, and Cognition, 15(2), 184–207. doi: 10.1080/13825580701322163 [DOI] [PubMed] [Google Scholar]
- Tucker A, & Stern Y (2011). Cognitive reserve in aging. Current Alzheimer Research, 8(4), 354–360. doi: 10.2174/156720511795745320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Uchida S, & Kawashima R (2008). Reading and solving arithmetic problems improves cognitive functions of normal aged people: a randomized controlled study. Age, 30(1), 21–29. doi: 10.1007/s11357-007-9044-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.van Muijden J, Band GPH, & Hommel B (2012). Online games training aging brains: limited transfer to cognitive control functions. Frontiers in Human Neuroscience, 6(8), 221. doi: 10.3389/fnhum.2012.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.van Zon L, Kirby JR, & Anderson N (2016). The efficacy of a volunteer-administered cognitive stimulation program in long-term care homes. International Psychogeriatrics, 28(6), 995–1004. doi: 10.1017/S1041610215002392 [DOI] [PubMed] [Google Scholar]
- *.Vance D, Dawson J, Wadley V, Edwards J, Roenker D, Rizzo M, & Ball K (2007). The accelerate study: The longitudinal effect of speed of processing training on cognitive performance of older adults. Rehabilitation Psychology, 52(1), 89–96. doi: 10.1037/0090-5550.52.1.89 [DOI] [Google Scholar]
- Verhaeghen P (2011). Aging and executive control: reports of a demise greatly exaggerated. Current Directions in Psychological Science, 20(3), 174–180. doi: 10.1177/0963721411408772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Vidovich MR, Lautenschlager NT, Flicker L, Clare L, McCaul K, & Almeida OP (2015). The PACE Study: A randomized clinical trial of cognitive activity strategy training for older people with mild cognitive impairment. The American Journal of Geriatric Psychiatry, 23(4), 360–372. doi: 10.1016/j.jagp.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Viechtbauer W (2010). Conducting meta-analyses in r with the metafor package. Journal of Statistical Software, 36(3), 1–48. doi: 10.1103/PhysRevB.91.121108 [DOI] [Google Scholar]
- *.von Bastian CC, Langer N, Jäncke L, & Oberauer K (2013). Effects of working memory training in young and old adults. Memory & Cognition, 41(4), 611–624. doi: 10.3758/s13421-012-0280-7 [DOI] [PubMed] [Google Scholar]
- *.Vranić A, Španić AM, Carretti B, & Borella E (2013). The efficacy of a multifactorial memory training in older adults living in residential care settings. International Psychogeriatrics, 25(11), 1885–1897. doi: 10.1017/S1041610213001154 [DOI] [PubMed] [Google Scholar]
- Wagner N, Hassanein K, & Head M (2010). Computer use by older adults: A multi-disciplinary review. Computers in Human Behavior, 26(5), 870–882. doi: 10.1016/J.CHB.2010.03.029 [DOI] [Google Scholar]
- *.Weicker J, Hudl N, Frisch S, Lepsien J, Mueller K, Villringer A, & Thöne-Otto A (2018). WOME: theory-based working memory training — a placebo-controlled, double-blind evaluation in older adults. Frontiers in Aging Neuroscience, 10, 247. doi: 10.3389/fnagi.2018.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Whitlock L. a., McLaughlin AC, & Allaire JC (2012). Individual differences in response to cognitive training: Using a multi-modal, attentionally demanding game-based intervention for older adults. Computers in Human Behavior, 28(4), 1091–1096. doi: 10.1016/j.chb.2012.01.012 [DOI] [Google Scholar]
- *.Wilkinson AJ, & Yang L (2012). Plasticity of inhibition in older adults: retest practice and transfer effects. Psychology and Aging, 27(3), 606–615. doi: 10.1037/a0025926 [DOI] [PubMed] [Google Scholar]
- Willis SL, Schaie KW. (1986) Can decline in adult intellectual functioning be reversed? Developmental Psychology, 22(2), 223–232. [Google Scholar]
- Willis SL, & Schaie KW (1994). Cognitive training in the normal elderly In Forette F, Christen Y, & Boller F (Eds.), Plasticité Cérébrale et Stimulation Cognitive Paris: Foundation Nationale de Gérontologie. [Google Scholar]
- Wilson BA, Cockburn J, Baddeley AD (1985) The rivermead behavioral memory test. Suffolk: Thames Valley. [Google Scholar]
- Wilson RS, Barnes LL, Aggarwal NT, Boyle PA, Hebert LE, Mendes de Leon CF, & Evans DA (2010). Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology, 75(11), 990–996. doi: 10.1212/WNL.0b013e3181f25b5e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L-O, & Petersen RC (2004). Mild cognitive impairment - beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256(3), 240–246. doi: 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- *.Woolverton M, Scogin F, Shackelford J, Black S, & Duke L (2001). Problem-targeted memory training for older adults. Aging, Neuropsychology, and Cognition, 8(4), 241–255. doi: 10.1076/anec.8.4.241.5637 [DOI] [Google Scholar]
- *.Xin Z, Lai Z, Li F, & Maes JHR (2014). Near- and far-transfer effects of working memory updating training in elderly adults. Applied Cognitive Psychology, 28(3), 403–408. doi: 10.1002/acp.3011 [DOI] [Google Scholar]
- Zhu X, Yun S, Lang M, He R, Li J (2016). The more the better? A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res Rev., 31, 67–79. doi: 10.1016/j.arr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- *.Zhuang JP, Fang R, Feng X, Xu XH, Liu LH, Bai QK, & Chen SD (2013). The impact of human-computer interaction-based comprehensive training on the cognitive functions of cognitive impairment elderly individuals in a nursing home. Journal of Alzheimer’s Disease , 36(2), 245–251. doi: 10.3233/JAD-130158 [DOI] [PubMed] [Google Scholar]
- *.Zimmermann K, von Bastian CC, Röcke C, Martin M, & Eschen A (2016). Transfer after process-based object-location memory training in healthy older adults. Psychology and Aging, 31(7), 798–814. doi: 10.1037/pag0000123 [DOI] [PubMed] [Google Scholar]
- *.Zimmermann N, Netto TM, Amodeo MT, Ska B, & Fonseca RP (2014). Working memory training and poetry-based stimulation programs: are there differences in cognitive outcome in healthy older adults? NeuroRehabilitation, 35(1), 159–170. doi: 10.3233/NRE-141104 [DOI] [PubMed] [Google Scholar]
- *.Zinke K, Zeintl M, Rose NS, Putzmann J, Pydde A, & Kliegel M (2014). Working memory training and transfer in older adults: effects of age, baseline performance, and training gains. Developmental Psychology, 50(1), 304–315. doi: 10.1037/a0032982 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.