Abstract
Although high-resolution single-particle electron cryo-microscopy (cryo-EM) is now producing a rapid stream of breakthroughs in structural biology, it nevertheless remains the case that the preparation of suitable frozen-hydrated samples on electron microscopy grids is often quite challenging. Purified samples that are intact and structurally homogeneous – while still in the test tube – may not necessarily survive the standard methods of making extremely thin, aqueous films on grids. As a result, it is often necessary to try a variety of experimental conditions before finally finding an approach that is optimal for the specimen at hand. Here, we summarize some of our collective experiences to date in optimizing sample preparation, in the hope that doing so will be useful to others, especially those new to the field. We also hope that an open discussion of these common challenges will encourage the development of more generally applicable methodology. Our collective experiences span a diverse range of biochemical samples and most of the commonly used variations in how grids are currently prepared. Unfortunately, none of the currently used optimization methods can be said, in advance, to be the one that ultimately will work when a project first begins. Nevertheless, there are some preferred first steps to explore when facing specific problems that can be more generally recommended, based on our experience and that of many others in the cryo-EM field.
INTRODUCTION
When prepared as well-dispersed particles for high-resolution electron cryo-microscopy (cryo-EM), biological macromolecules are ideally embedded in a film of vitrified buffer that is not much thicker than the particle itself. The standard method for preparing such specimens is to apply excess sample to a holey-carbon support film, blot away most of the excess sample, and vitrify the remaining thin film by plunging it into liquid ethane. As diagrammed in Figure 1, this idealized picture implies that the condition of particles within the thin layer of vitrified buffer is almost identical to what it was within the test tube. This assumption, in turn, implies that the preparation of electron microscope grids of frozen-hydrated specimens (cryo-EM grids) should be successful every time, for every specimen, at least in those areas of the EM grid where the vitrified ice is thin enough, but not too thin.
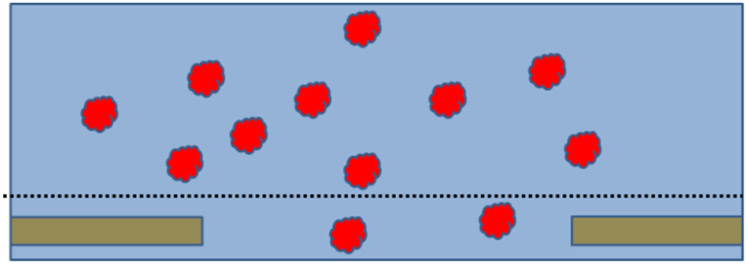
Figure 1.
Cartoon of what an ideal cryo-EM specimen might be like. Depicted here is just a single hole in a thin, holey-carbon support film, which itself is supported by an EM grid (not shown). An excess amount of sample is first applied to the carbon film, after which everything above an imaginary dotted line is blotted away with filter paper. Before blotting, the biological macromolecules – represented by the red particles – are distributed randomly in suspension, and their distribution within the remaining sample, after blotting, is not imagined to be affected by removal of material above the dotted line. After freezing, the particles are embedded in vitreous ice, thus providing a sample whose structure is nearly identical to what it was in bulk solution. This is what had long been thought to be true, but we now know that it frequently is not what really happens.
The reality is that preparation of samples for cryo-EM can fail in at least 4 generic ways (1), even when the condition of macromolecules within the test tube is excellent. The problems encountered in practice include: (1) preferential orientation of particles may occur within thin films (2-4); (2) unexpectedly low numbers of particles may be found within holes, i.e. many fewer than is expected from their concentration in bulk solution (5, 6); (3) particles may disintegrate within thin aqueous films; and (4) unexplained aggregation of sample material may be observed. It is evident that the idealized cartoon of what happens when cryo-grids are made, shown in Figure 1, is not the complete story.
Interaction of particles with the air-water interface is the most likely cause of problems that emerge when making extremely thin films of sample on grids, but not otherwise, i.e. not in bulk solution (7). The denaturation of proteins at gas-liquid interfaces was observed many decades ago (8), and it is common advice that air bubbles should be avoided even when handling proteins in bulk solution. These denaturing effects must also be present when making thin films of sample on EM grids. Cautions about interactions with the air-water interface were already mentioned in the earliest papers describing how to vitrify thin films (9). The issue was raised again in the context of estimating that diffusion will result in approximately 1000 or more collisions per second with the air-water interface when the sample thickness is 100 nm thick or less (10). Nevertheless, it is only recently that electron cryo-tomography has been used to show that adsorption to the air-water interface often leads to preferential orientation of particles (2, 11), and that partial (4) or full disruption of particles can occur.
As has recently been reviewed (12), denaturation of proteins at the air-water interface has long been studied in other fields, and thus it is not surprising that it also causes problems when making cryo-EM samples. Indeed, what is perhaps surprising, is the fact that many proteins seem to survive when they are adsorbed to the air-water interface, even when they are preferentially oriented (2), although that is not always the case (4). In general, large, symmetric structures such as virus particles and filamentous assemblies often appear to be more resilient, and bacterial proteins and complexes are also generally more robust when prepared as cryo-EM specimens than are their eukaryotic counterparts.
Several ideas and approaches have already been developed to optimize the outcome when the preparation of cryo-grids proves to be difficult (1). But while any given method, such as adding detergent to the sample, or chemically crosslinking the particles, may work for some “difficult” macromolecular complexes, the same method may not work for others. As a result, the current situation in the field is that one must empirically try a number of such methods, one after the other, without knowing in advance which, if any, will succeed.
We here present a set of examples that reflects our experience with preparing cryo-grids, which covers work spanning a diverse range of biological macromolecules. Our goals in presenting these are (1) to share our collective estimates regarding how frequently the preparation of cryo-grids actually proves to be quite difficult and (2) to gather, in one place, a number of examples in which a given optimization method worked well for one type of specimen but not for another. In addition, we recommend that the previous critique of outcomes published by (1) be read together with what we add here.
Not surprisingly, efforts are currently under way to develop better solutions for preparing cryo-grids, some of which are being pursued in our own respective laboratories (13-16). In the interim, while these efforts are under way, this compendium may make it easier for others to get a broader view about the all-too-frequent number of cases where preparing cryo-grids proves to be difficult.
Our experiences are presented in three sections. The first section consists of a narrative synthesis of the responses of the participating authors to a survey-questionnaire. This questionnaire covered issues such as the frequency with which preparing grids did or did not require optimization; the nature of the challenges that had to be overcome for samples that proved to be difficult; and examples in which a given optimization approach did – or did not – succeed. The second section presents representative images that show examples of unsatisfactory results obtained when preparing grids for cryo-EM, and the third section presents examples in which a given optimization approach finally did produce the desired improvement.
SYNTHESIS OF SURVEY RESULTS
A multiple-choice questionnaire, covering five topics and three classes of specimen, was first circulated to participating authors. They were asked to individually identify, for each question, the one response that most closely matched their own experiences, rather than what they had heard colleagues say. The full questionnaire, and the tabulated results, are included as part of Supplemental Material.
Although responses to this questionnaire are necessarily based on imprecise estimates, and to some extent they may be considered to be anecdotal, the premise behind this methodology is that the combination of many such estimates is more likely to describe what can be expected to happen, than does an estimate made by any single individual – see, for example, https://en.wikipedia.org/wiki/The_Wisdom_of_Crowds.
In brief, there is overwhelming consensus that (1) optimization is required, much more often than not, for the way in which each kind of sample is prepared for cryo-EM, and (2) none of the currently used methods can be identified in advance as being one that will work. Although we expect that few will disagree with this consensus, we nevertheless believe that it is valuable to further elaborate on these two points.
Success on the first few attempts is rare; optimization is needed more often than not
It was generally felt that successful preparation of cryo-EM grids, during the first few attempts, happens less often than 25% of the time. Three authors actually felt that the success rate may be less than 10% of the time for soluble macromolecules, while one author felt that the success rate for icosahedral particles and helices might be as high as 50%. The consensus opinion is that the chance of success (without extensive optimization) is perhaps somewhat better for solubilized membrane proteins than it is for soluble macromolecules.
All participants responded that all four types of unwanted behavior enumerated in the Introduction can be expected both for soluble macromolecular complexes and for detergent-solubilized membrane proteins. The four problems of preferential orientation, too few particles, disintegration of particles, and aggregation of particles were mentioned less often for icosahedral particles and helices, however.
The optimization methods that are currently used within the cryo-EM community include varying the buffer composition (pH, ionic strength, etc.) (17); adding small-molecule ligands, substrate molecules or inhibitors; adding macromolecular binding partners or antibodies; creating intramolecular crosslinks with glutaraldehyde (18, 19) or BS3 (20, 21); adding detergents or other surfactants; and adsorption to a support film such as graphene oxide (22) or even evaporated carbon. Less commonly used optimization methods include applying the sample to holey grids two or more times (with washes in between separate applications) (5); exposing grids to a glow discharge in vapor of amylamine (23, 24); eliminating the step of treating grids with a glow discharge (25); optimizing the blotting conditions such as the time, blotting force, pause between blotting and plunging, ambient temperature, and relative humidity; and use of manual blotting (26, 27) rather than an automated machine.
When optimization is required, the consensus opinion is that approximately 10 different methods, or combinations of methods, have to be tried for soluble macromolecular complexes before one is found that works well. Responses on this point were more varied for detergent-solubilized membrane proteins and for icosahedra and helices, however.
None of the existing optimization methods works consistently well for different kinds of specimens
The addition of detergent or, in the case of membrane proteins, another surfactant such as nanodiscs (28) or amphipol (29) was the most frequently mentioned method that resulted in successful preparation of cryo-grids. However, because trying different surfactants is a common strategy, it was also the most frequently mentioned method that did NOT produce a successful result.
While optimizing the buffer conditions was reported to be successful for some particles, it was mentioned even more frequently as being something that did not help. Adding a substrate, an inhibitor or another ligand was mentioned as being successful about as often as it was said to not result in success. Crosslinking with glutaraldehyde or with BS3 was mentioned more frequently as being successful than otherwise. Including an additional macromolecular binding partner was also mentioned more frequently as a method leading to success than it was said to have led to no improvement. Although applying sample two or more times, the use of evaporated carbon as a support film, and the use of graphene oxide as a support film are all methods that did work well for some specimens, these were the most frequent ones to be mentioned as not giving a successful result. Similarly, not exposing the grids to a glow discharge was mentioned quite frequently as not resulting in improvement.
The participating authors were next asked to identify up to 5 cases in which some of the above methods either succeeded or failed. A subset of examples, both successes and failures, were selected from the many responses. The number selected for publication was limited to 2 for each lab, so as to not put too heavy a burden on the preparation of Figures for publication by the students or postdocs involved in the original work, who are acknowledged in the Figure legends.
EXAMPLES OF CASES IN WHICH A PARTICULAR OPTIMIZATION METHOD WAS NOT EFFECTIVE
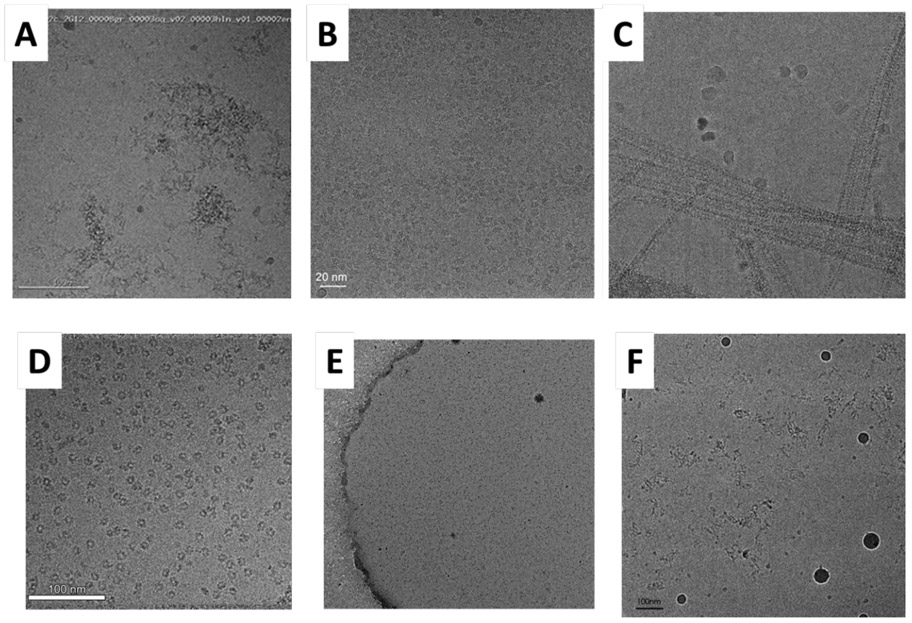
Figure 2 shows six examples of what various problematic samples look like when the results after the first few trials to prepare cryo-grids failed, and it was recognized that further optimization is needed. Problems encountered in the initial screens included: extensive particle-aggregation when thin films were prepared on EM grids (Fig. 2A), preferential orientation of nicely dispersed particles that had otherwise looked very promising (Fig. 2B and 2D), clumping of filamentous particles (Fig. 2C), and disintegration of particles into small pieces (Fig. 2E and 2F). Results of initial optimization attempts included binding IgG antibodies or IgG-derived Fab fragments (Fig. 2A), having detergent present in the sample-buffer (Fig. 2B and 2C), and chemical crosslinking with either glutaraldehyde or BS3 (Fig. 2E and 2F).
Figure 2.
Six examples of what various samples look like when the first few trials were not good, and it is recognized that further optimization is needed. (A) HIV-1 envelope trimers in complex with a monoclonal IgG antibody, unpublished work; scale bar indicates 100 nm. This is an example in which extensive aggregation of the sample is seen on the cryo-EM grid. Figure provided by Dr. Priyamvada Acharya. (B) TMEM16A, a calcium-activated chloride channel, purified in lauryl maltose neopentyl glycol (LMNG) (31); scale bar indicates 20 nm. This an example in which particles and class averages look promising, but the resolution of the 3-D reconstruction obtained for these particles was limited in one direction to about 12Å because of preferential orientation. Figure provided by Dr. Shangyu Dang. (C) Filaments of a complex formed between Dynamin-Related Protein 1 (DRP1) and Mitochondrial Dynamics Protein 49 (MID49) (32); scale bar indicates 50 nm. Unwanted clumping of filaments is seen in negatively stained samples as well as in the cryo-EM sample shown here. Figure provided by Dr. Raghav Kalia. (D) The type I-F CRISPR RNA-guided surveillance complex (Csy complex) in a buffer containing 0.05% (v/v) LMNG (unpublished results); scale bar indicates 100 nm. This is a case in which the particles looked promising in the raw micrographs, but subsequent data processing showed that they exhibited stubborn preferred orientation, which persisted in the presence of detergent. Figure provided by Dr. Saikat Chowdhury. (E) Polycomb Repressive Complex 2 (PRC2) (33); scale bar indicates 200 nm. Many particles are present, but they are much smaller than the intact complex. Similar results were obtained for samples crosslinked with either glutaraldehyde or with BS3, and such crosslinked samples did not go into holes with the addition of 0.01% NP40 detergent. Other conditions that also failed included the use of continuous, glow-discharge treated carbon film and graphene oxide support films. Figure provided by Dr. Vignesh Kasinath. (F) Exocyst complex(unpublished results); scale bar indicates 100 nm. This is a case in which crosslinking with 0.1% glutaraldehyde was not effective in solving the problem of disintegration and possible aggregation of particles on the grid. Figure provided by Yan Li.
Turning to the complete survey results reported in Table S1 of the Supplemental Material, 5 responses cited cases in which either using graphene oxide support films or adding detergent or another surfactant did not improve the situation; 4 reported cases in which optimizing the buffer, optimizing the type and concentration of small-molecule ligand, performing chemical crosslinking, applying sample two or more times, or using continuous carbon as the support film each did not improve the situation; and 2 cited cases in which adding a macromolecular binding partner did not solve the problem. Although each of these methods failed for some of the samples, they nevertheless were effective for others, as will be discussed in the following section.
EXAMPLES OF CASES IN WHICH OPTIMIZATION METHODS LED TO SIGNIFICANT IMPROVEMENT
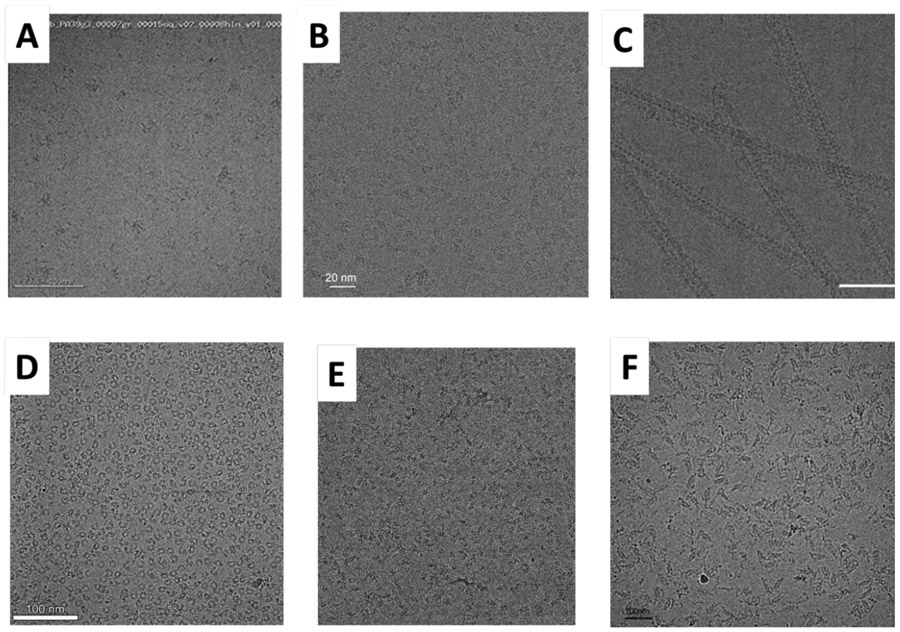
Figure 3 shows six examples of what some samples looked like after non-trivial optimization that was at least partially successful. Methods include binding of Fab fragments (Fig. 3A and 3B), addition of detergent (Fig. 3C), addition of a macromolecular binding partner (Fig. 3D), a combination of binding to continuous carbon film, chemical crosslinking, and addition of detergent (Fig. 3E), and using less aggressive chemical crosslinking (Fig. 3F).
Figure 3.
Six examples of what various samples look like after non-trivial optimization has led to improvement. (A) HIV-1 envelope trimers in complex with monoclonal Fab fragments at two separate antigenic sites (34); scale bar indicates 100 nm. This is an example in which the use of additional macromolecular binding partners not only overcame the problem of aggregation that occurred on the grid, but, in addition, this made it possible to obtain a 3-D reconstruction at sub-nm resolution. Figure provided by Dr. Priyamvada Acharya. (B) The calcium-activated chloride channel, TMEM16A, again purified in LMNG, but now Fab fragments have been bound at two independent sites; scale bar indicates 20 nm. The addition of antibodies improved the distribution of Euler angles, but the average resolution of the 3-D map obtained with these particles was worse than without the antibodies. Nevertheless, a better, more interpretable map resulted when data were combined for particles with and without bound antibodies, Figure provided by Dr. Shangyu Dang. (C) Filaments of a complex formed between Dynamin-Related Protein 1 (DRP1) and Mitochondrial Dynamics Protein 49 (MDP49) (32); scale bar indicates 50 nm. The addition of 0.2% octyl glucoside detergent substantially relieved the clumping seen in Figure 2C. Figure provided by Dr. Raghav Kalia. (D) The Csy complex shown in Figure 2D was subsequently bound to a double-stranded DNA oligomer target and vitrified in a buffer containing 0.05% LMNG; scale bar indicates 100 nm. The addition of a macromolecular cofactor, DNA in this case, overcame the problem of preferential orientation. Figure provided by Dr. Saikat Chowdhury (35). (E) Chemically crosslinked Polycomb Repressive Complex 2 (PRC2) on continuous carbon film, with 0.01% NP40 detergent added (20); scale bar indicates 200 nm. While these particles now look good, the sample still suffers from preferential orientation. Figure provided by Dr. Vignesh Kasinath. (F) Exocyst complex (19); scale bar indicates 100 nm. Less aggressive crosslinking than that used for Figure 2F, in this case using 0.0025% glutaraldehyde, was effective in preserving particles when on the grid. Figure provided byYan Li.
Turning again to the complete survey results reported in Table S1 of the Supplemental Material, 5 responses cited success in optimizing grid preparation by adding some type of detergent or other surfactant and 5 improved the grids by adding a small-molecule inhibitor, substrate, or other ligand; 4 improved their results by using some form of chemical crosslinking; 3 had success after adding a macromolecular binding partner and 3 had success after applying sample to the grid two or more times; while only 2 found that further optimization of the pH, ionic strength, etc. was effective. Although each of these methods were effective for some proteins, they had nevertheless failed for some other proteins, as was described in the previous section.
Based on our combined experience at this point, we suggest a few common-sense actions as the ones to consider taking first, should optimization of grid preparation be required. The suggestions given below assume that the particles appear to be homogeneous in size and shape when in negative stain, to a resolution of perhaps 15 Å or 20 Å. If that is not the case, one may have to reconsider whether the particles are, in fact, well behaved in the test tube, i.e. before the step of making cryo-EM grids.
PREFERENTIAL ORIENTATION.
Try any, or even a combination of, the following: (1) adding detergent, (2) adding Fab fragments or IgG antibodies, or (3) adding an additional macromolecular binding partner. It is also worthwhile to try binding the particle to a very thin, continuous support film such as glow-discharge treated, evaporated-carbon films or perhaps graphene oxide. Finally, it is worthwhile to try using holey-gold on gold grids (30), rather than holey carbon on copper grids. The use of holey-gold support films makes it practical to record images at moderate tilt angles, thereby increasing the angular distribution of particles with respect to the incident beam (3).
PARTICLES DO NOT GO INTO HOLES.
One recommended action is to again try binding the particles to very thin, glow-discharge treated evaporated-carbon films or perhaps to graphene oxide. Because adsorption to a continuous support film is likely to result in concentrating the particles on the grid, such measures may be necessary if only very small amounts of protein are available. When the amount of sample is not a limitation, however, one can try to apply sample two or more times (5).
PARTICLES ARE BROKEN OR DISINTIGRATED.
Crosslinking with glutaraldehyde or BS3 is the best option to try in this case.
PARTICLES ARE BADLY AGGREGATED.
Try adding detergent or another surfactant. This is especially recommended if aggregation is already seen in negatively stained samples.
SUMMARY AND CONCLUSIONS
It is not uncommon to get poor results when preparing grids for single-particle cryo-EM; only rarely does grid preparation succeed during the first few tries. This commonly occurring issue need not be because the biochemistry has not yet been optimized, or because the investigator did not know how to make grids (although either could contribute to the problem). As a result, extensive optimization is often required, even by those who have had considerable previous success with other samples.
Several orthogonal optimization methods, enumerated here, have each proved to be effective for at least some particles. Any one method nevertheless does not work equally well for all macromolecular particles. As a result, optimization of the method used to prepare cryo-grids requires the empirical testing of many different ideas, without knowing in advance which, if any, is likely to succeed.
This compendium presents examples of both successes and failures for some of the more commonly used optimization methods. The goal is that our experiences, as well as those of other work cited here, may serve as a starting point for others, should the preparation of cryo-grids prove to be difficult for a new particle of interest.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by the following: (BC) Simons Foundation (SF349247) and NIH grant GM103310; (YC) NIH grants GM098672, HL134183, P01GM111126, S100D020054 and S100D021741; (AF) American Asthma Foundation, Chan Zuckerberg Biohub, HHMI, and NIH grants S100D020054, 1S100D021741, GM127673, and DP2GM 110772-02; (RMG) NIH grant P01GM051487; (GCL) young investigator award from Amgen and NIH grant DP2EB020402 ; (EN) NIH grants GM051487 and GM127018, and with work on PRC2 supported by Eli Lilly; and (HW) grant 2016YFA0501100 from the Ministry of Science and Technology of China. YC and EN are Investigators of the Howard Hughes Medical Institute. BC has a commercial relationship with TTP Labtech, a company that will produce a commercially available vitrification instrument called Chameleon. All authors declare that they have no financial conflict of interest that might be construed to influence the results or interpretation of the manuscript.
Contributor Information
Bridget Carragher, Simon Electron Microscopy Center, New York Structural Biology Center, New York, NY 10027.
Yifan Cheng, HHMI and Department of Biochemistry and Biophysics, University of California, San Francisco, CA 94158.
Adam Frost, Department of Biochemistry and Biophysics, University of California San Francisco, San Francisco, CA 94158.
Robert M. Glaeser, Lawrence Berkeley National Laboratory, University of California, Berkeley, CA 94720
Gabriel C. Lander, The Scripps Research Institute, La Jolla, CA 92037
Eva Nogales, Molecular and Cell Biology Department, University of California Berkeley, Berkeley, CA 94720: MBIB Division, Lawrence Berkeley National Laboratory, University of California, Berkeley, CA 94720; Howard Hughes Medical Institute, University of California Berkeley, Berkeley, CA 94720.
Hong-Wei Wang, Ministry of Education Key Laboratory of Protein Sciences, Beijing Advanced Innovation Center for Structural Biology, Beijing Frontier Research Center of Biological structures, School of Life Sciences, Tsinghua University, Beijing 100084, China.
REFERENCES
- 1.Drulyte I, Johnson RM, Hesketh EL, Hurdiss DL, Scarff CA, Porav SA, Ranson NA, Muench SP, and Thompson RF. 2018. Approaches to altering particle distributions in cryo-electron microscopy sample preparation. Acta Crystallogr. Sect. D-Struct. Biol 74:560–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noble AJ, Dandey VP, Wei H, Braschi J, Chase J, Acharya P, Tan YZ, Zhang ZN, Kim LY, Scapin G, Rapp M, Eng ET, Rice WJ, Cheng AC, Negro CJ, Shapiro L, Kwong PD, Jeruzalmi D, des Georges A, Potter CS, and Carragher B 2018. Routine single particle cryoEM sample and grid characterization by tomography. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan YZ, Baldwin PR, Davis JH, Williamson JR, Potter CS, Carragher B, and Lyumkis D 2017. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nature Methods 14:793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Imprima E, Floris D, Joppe M, Sänchez R, Grininger M, and Kühlbrandt W. 2019. Protein denaturation at the air-water interface and how to prevent it. Elife 8:e42747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snijder J, Borst AJ, Dosey A, Walls AC, Burrell A, Reddy VS, Kollman JM, and Veesler D. 2017. Vitrification after multiple rounds of sample application and blotting improves particle density on cryo-electron microscopy grids. Journal of Structural Biology 198:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyerson JR, Rao P, Kumar J, Chittori S, Banerjee S, Pierson J, Mayer ML, and Subramaniam S. 2014. Self-assembled monolayers improve protein distribution on holey carbon cryo-EM supports. Scientific Reports 4:7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaeser RM, and Han B-G. 2017. Opinion: hazards faced by macromolecules when confined to thin aqueous films. Biophysics Reports 3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donaldson TL, Boonstra EF, and Hammond JM. 1980. Kinetics of protein denaturation at gas-liauqid interfaces. Journal of Colloid and Interface Science 74:441–450. [Google Scholar]
- 9.Dubochet J, Adrian M, Chang JJ, Homo JC, Lepault J, McDowall AW, and Schultz P. 1988. Cryo-Electron Microscopy of Vitrified Specimens. Quarterly Reviews of Biophysics 21:129–228. [DOI] [PubMed] [Google Scholar]
- 10.Taylor KA, and Glaeser RM. 2008. Retrospective on the early development of cryoelectron microscopy of macromolecules and a prospective on opportunities for the future. Journal of Structural Biology 163:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Noble AJ, Kang JY, and Darst SA. 2019. Eliminating effects of particle adsorption to the air/water interface in single-particle cryo-electron microscopy: Bacterial RNA polymerase and CHAPSO. Journal of Structural Biology: X:100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaeser RM 2018. Proteins, interfaces, and cryo-EM Grids. Current Opinion in Colloid & Interface Science 34:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razinkov I, Dandey VP, Wei H, Zhang Z, Melnekoff D, Rice WJ, Wigge C, Potter CS, and Carragher B. 2016. A new method for vitrifying samples for cryoEM. Journal of Structural Biology 195:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han B-G, Watson Z, Kang H, Pulk A, Downing KH, Cate J, and Glaeser RM. 2016. Long shelf-life streptavidin support-films suitable for electron microscopy of biological macromolecules. Journal of Structural Biology 195:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dandey VP, Wei H, Zhang ZN, Tan YZ, Acharya P, Eng ET, Rice WJ, Kahn PA, Potter CS, and Carragher B. 2018. Spotiton: New features and applications. Journal of Structural Biology 202:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu N, Zhang J, Chen Y, Liu C, Zhang X, Xu K, Wen J, Luo Z, Chen S, Gao P, Jia K, Liu Z, Peng H, and Wang H-W. 2019. Bioactive Functionalized Monolayer Graphene for High-Resolution Cryo-Electron Microscopy. Journal of the American Chemical Society 141:4016–4025. [DOI] [PubMed] [Google Scholar]
- 17.Chari A, Haselbach D, Kirves J-M, Ohmer J, Paknia E, Fischer N, Ganichkin O, Moller V, Frye JJ, Petzold G, Jarvis M, Tietzel M, Grimm C, Peters J-M, Schulman BA, Tittmann K, Markl J, Fischer U, and Stark H. 2015. ProteoPlex: stability optimization of macromolecular complexes by sparse-matrix screening of chemical space. Nat Meth 12:859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kastner B, Fischer N, Golas MM, Sander B, Dube P, Boehringer D, Hartmuth K, Deckert J, Hauer F, Wolf E, Uchtenhagen H, Urlaub H, Herzog F, Peters JM, Poerschke D, Hrmann RL, and Stark H. 2008. GraFix: sample preparation for single-particle electron cryomicroscopy. Nature Methods 5:53–55. [DOI] [PubMed] [Google Scholar]
- 19.Mei K, Li Y, Wang S, Shao G, Wang J, Ding Y, Luo G, Yue P, Liu J-J, Wang X, Dong M-Q, Wang H-W, and Guo W. 2018. Cryo-EM structure of the exocyst complex. Nature Structural & Molecular Biology 25:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasinath V, Faini M, Poepsel S, Reif D, Feng XA, Stjepanovic G, Aebersold R, and Nogales E. 2018. Structures of human PRC2 with its cofactors AEBP2 and JARID2. Science 359:940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenner LR, Anand AA, Nguyen HC, Myasnikov AG, Klose CJ, McGeever LA, Tsai JC, Miller-Vedam LE, Walter P, and Frost A. 2019. eIF2B-catalyzed nucleotide exchange and phosphoregulation by the integrated stress response. Science 364:491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantelic RS, Meyer JC, Kaiser U, Baumeister W, and Plitzko JM. 2010. Graphene oxide: A substrate for optimizing preparations of frozen-hydrated samples. Journal of Structural Biology 170:152–156. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen THD, Tam J, Wu RA, Greber BJ, Toso D, Nogales E, and Collins K. 2018. Cryo-EM structure of substrate-bound human telomerase holoenzyme. Nature 557:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Fonseca PCA, and Morris EP. 2015. Cryo-EM reveals the conformation of a substrate analogue in the human 20S proteasome core. Nature Communications 6:7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen PS, Park J, Qin Y, Li X, Parsawar K, Larson MH, Cox J, Cheng Y, Lambowitz AM, Weissman JS, Brandman O, and Frost A. 2015. Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains. Science 347:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herzik MA, Wu M, and Lander GC. 2019. High-resolution structure determination of sub-100 kDa complexes using conventional cryo-EM. Nature Communications 10:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzik MA Jr, Wu M, and Lander GC. 2017. Achieving better-than-3-Å resolution by single-particle cryo-EM at 200 keV. Nature Methods 14:1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y, Cao E, Julius D, and Cheng Y. 2016. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao MF, Cao EH, Julius D, and Cheng YF. 2013. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russo CJ, and Passmore LA. 2014. Ultrastable gold substrates for electron cryomicroscopy. Science 346:1377–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dang SY, Feng SJ, Tien J, Peters CJ, Bulkley D, Lolicato M, Zhao JH, Zuberbuhler K, Ye WL, Qi LJ, Chen TX, Craik CS, Jan YN, Minor DL, Cheng YF, and Jan LY. 2017. Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 552:426–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalia R, Wang RYR, Yusuf A, Thomas PV, Agard DA, Shaw JM, and Frost A. 2018. Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature 558:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasinath V, Poepsel S, and Nogales E. 2019. Recent Structural Insights into Polycomb Repressive Complex 2 Regulation and Substrate Binding. Biochemistry 58:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuang G-Y, Zhou J, Acharya P, Rawi R, Shen C-H, Sheng Z, Zhang B, Zhou T, Bailer RT, Dandey VP, Doria-Rose NA, Louder MK, McKee K, Mascola JR, Shapiro L, and Kwong PD. 2019. Structural Survey of Broadly Neutralizing Antibodies Targeting the HIV-1 Env Trimer Delineates Epitope Categories and Characteristics of Recognition. Structure 27:196–206.e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rollins MF, Chowdhury S, Carter J, Golden SM, Miettinen HM, Santiago-Frangos A, Faith D, Lawrence CM, Lander GC, and Wiedenheft B. 2019. Structure Reveals a Mechanism of CRISPR-RNA-Guided Nuclease Recruitment and Anti-CRISPR Viral Mimicry. Molecular Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.