Abstract
Inflammation is important for antimicrobial defense and for tissue repair after trauma. The inflammatory response and its resolution are both active processes that must be tightly regulated to maintain homeostasis. Excessive inflammation and nonresolving inflammation cause tissue damage and chronic disease, including autoinflammatory and cardiovascular diseases. An improved understanding of the cellular and molecular mechanisms that regulate inflammation has supported development of novel therapies for several inflammatory diseases, including rheumatoid arthritis and inflammatory bowel disease. Many of the specific anticytokine therapies carry a risk for excessive immunosuppression and serious side effects. The discovery of the inflammatory reflex and the increasingly detailed understanding of the molecular interactions between homeostatic neural reflexes and the immune system have laid the foundation for bioelectronic medicine in the field of inflammatory diseases. Neural interfaces and nerve stimulators are now being tested in human clinical trials and may, as the technology develops further, have advantages over conventional drugs in terms of better compliance, continuously adaptable control of dosing, better monitoring, and reduced risks for unwanted side effects. Here, we review the current mechanistic understanding of common autoinflammatory conditions, consider available therapies, and discuss the potential use of increasingly capable devices in the treatment of inflammatory disease.
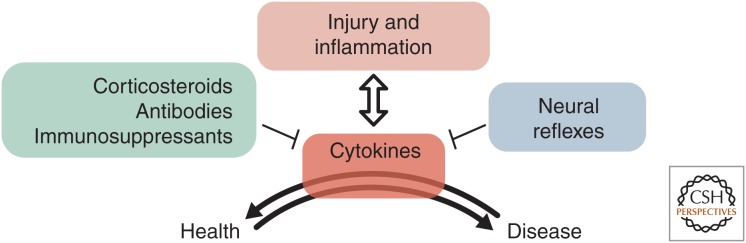
The treatment approach to chronic inflammatory diseases, such as inflammatory bowel disease (IBD), rheumatoid arthritis (RA), and atherosclerosis, has primarily focused on immunosuppression (Fig. 1). The therapeutic options for IBD include glucocorticosteroids, sulphasalazine/5-aminosalicylates, thiopurines, methotrexate, JAK-inhibitors, and antibodies directed against tumor necrosis factor α (TNF-α), the integrin α4β7, and interleukin (IL)-12/IL-23, also called biologicals (Gomollon et al. 2017; Eberhardson et al. 2018). Many of these treatments also demonstrate efficacy in RA with the addition of antibodies against IL-1, IL-6, and IL-17 (Guo et al. 2018). The antibody against IL-1β, canakinumab, has recently proven efficacious in a large clinical study of cardiovascular disease (Ridker et al. 2017). However, all these treatments may also cause severe side effects such as (opportunistic) infections or paradoxical inflammation with treatment-induced autoimmunity in noninflamed organs, such as autoimmune hepatitis and dermatitis (Gomollon et al. 2017). An increased risk of malignancy development has also been established for immunosuppressants in large epidemiological studies, and thus a history of malignancy becomes a relative contraindication for immunosuppressive therapy (Gomollon et al. 2017). Accordingly, there is a yet unmet need for functional therapeutics capable of regulating inflammation in a precise and personalized way. This would ideally be achieved by continuous monitoring of treatment efficacy coupled to adaptable dosing in a closed-loop fashion. Currently available treatments are not well suited to provide this flexibility and functionality. A novel approach is found in the field of bioelectronic medicine, where combining neuroscience, immunology, engineering, and clinical medicine aims for individualized targeted control of inflammation through neural stimulation and monitoring. This research field is at the forefront of scientific discoveries, particularly with regard to the technology development for deciphering nervous electrical signals. Important milestones have been reached in the clinical trials of treatment of RA and Crohn's disease (CD), providing patients with new hope for sustainable and effective treatments. In this review, we highlight the current understanding of chronic inflammation, neural control of the immune system, and the application of bioelectronic medicine in the context of IBD, RA, and atherosclerosis.
Figure 1.
Regulation of the inflammatory response. Cytokines are key mediators of inflammation and their production and release is tightly regulated by a number of mechanisms that can be utilized for therapeutic intervention in excessive and nonresolving inflammation.
THE INFLAMMATORY RESPONSE
The human body continuously upholds integrity and homeostasis by immune surveillance. Any breach of mucosal or epithelial barriers by injury and invasion of pathogens triggers an acute inflammatory response. This reaction aims to constrain and eradicate the threat, clear dead cells, and heal the tissue (Sethi et al. 2012; Fullerton and Gilroy 2016; Chen et al. 2018; Rajendran et al. 2018). Local inflammation commonly engages both tissue-resident cells and immune cells recruited from blood and the lymphatic circulation (Rogler 2017; Chen et al. 2018).
The first immune cells on site at the injury or invasion are the neutrophils, ready to release antimicrobial peptides, tissue-degrading enzymes, chemokines, and cytokines and promote local containment (Amulic et al. 2012; Kastenmuller et al. 2012; Rogler 2017; Stephens and Liao 2018). Monocytes, macrophages, and dendritic cells play a major role in recognizing danger signals, such as damage- and pathogen-associated molecular patterns (DAMPs and PAMPs) and promoting a local milieu conducive to leukocyte recruitment, purging of microbes, and, eventually, tissue repair (Takeuchi and Akira 2010).
The innate cells also attract additional immune cells to the injury and present foreign peptides trapped in the antigen-binding cleft of major histocompatibility complex class II (MHCII) to the T cells (Brusselle and Bracke 2014; Chen et al. 2018). In contrast to the innate cells, which recognize general “danger signals,” the adaptive immune system has the capacity to launch a high-precision response against invading pathogens by an elaborate setup of antigen-specific T-cell receptors (TCRs) as well as antibody release from B cells.
B cells play multiple roles in inflammatory conditions. Antibody responses as well as cytokine production are a double-edged sword. The humoral antibody defense against invading pathogens is aimed at neutralization and elimination of the microbe or parasite. Contrarily, the excessive response that occurs, for instance, in autoimmune diseases upon break of the tolerance, is characterized by a shift of the humoral repertoire toward self-epitopes. In fact, in autoimmune diseases, autoantibodies evoked upon T-dependent stimulation recognize self-epitopes and trigger a sterile proinflammatory reaction. Therefore, B-cell-derived antibodies have the potential to both protect and harm the host (Silverstein 2001).
The intricate interaction between the innate and adaptive immune systems through an antigen-presentation link is a crucial step in the evolution from an acute innate inflammation to a more prolonged antigen-specific response (Duffney et al. 2018). The quickly initiated general defense response and the subsequent specific eradication of the threat must be localized and tightly regulated to minimize host damage and promote homeostasis. Gradually, the initial inflammatory response is shifted toward a well-orchestrated process to terminate the inflammatory response, limit tissue damage, and promote resolution and repair.
RESOLUTION OF INFLAMMATION
Specialized Pro-Resolving Mediators (SPMs)
Leukotrienes are major lipid mediators produced by neutrophils to enhance the immune response. Over the course of an inflammatory response, there is a class switch from production of leukotriene B4 (LTB4) to the SPM lipoxin. Additional SPMs are resolvins, protectins, and maresins, all with potent pro-resolving capabilities. SPMs represent a family of central “immunoresolvent” agents without apparent immunosuppressive function, and they promote active resolution of innate as well as adaptive inflammatory responses (Serhan and Levy 2018). SPMs reduce neutrophil chemotaxis and induce macrophage phagocytosis of apoptotic neutrophils and other cellular remains (Dalli et al. 2013). In addition, they regulate activity of innate lymphoid cells (ILCs), natural killer cells (NK cells), and T and B cells to reduce production of, for example, IL-2, TNF-α, interferon γ (IFN-γ), and IL-17 (Serhan and Levy 2018).
Inflammation is tightly controlled by negative feedback loops that regulate the process from eradication of pathogens to limitation of collateral damage and initiation of tissue repair (Alivernini et al. 2018). Thus, resolution is an active process of the inflammatory response with highly coordinated interactions between the innate and adaptive immunity (Buckley et al. 2013; Serhan et al. 2015; Fullerton and Gilroy 2016; Rogler 2017). The eradication of the inflammatory stimulus is likely a major trigger for the initiation of resolution. The gradual clearance of proinflammatory cytokines, chemokines, and adhesion molecules, followed by reduced recruitment of immune cells, are additional factors (Fullerton and Gilroy 2016). Neutrophils, key players in the acute phase response, are loaded with antimicrobial peptides and other reactive molecules that also may cause tissue damage at the site of inflammation. Therefore, tight and timely regulation of leukocyte activity is vital for the resolution of inflammation (Amulic et al. 2012). This is promoted by programmed cell death after completion of the neutrophilic antimicrobial agenda. The apoptosis is triggered by lipid mediators and reactive oxygen species from activated neutrophils and macrophages, and will lead to the release of inhibitory signals directed against continued neutrophil recruitment (Rogler 2017). In addition, neutrophils that reach the lymph nodes exert an inhibitory effect on dendritic cells and thereby counteract the antigen-presenting process and promote resolution (Amulic et al. 2012).
Activated macrophages may return to a quiescent phenotype by accumulation of endogenous anti-inflammatory signals such as adenosine, prostaglandin E2, resolvin, and lipoxin, many of which are released by the macrophages themselves (Hamidzadeh et al. 2017; Serhan and Levy 2018). Moreover, when apoptotic neutrophils are phagocytosed by macrophages, the latter start producing anti-inflammatory cytokines such as transforming growth factor β (TGF-β) (Hamidzadeh et al. 2017). Experimental observations suggest that proinflammatory macrophages (M1) are activated by infectious antigens and processes in tissue necrosis, while apoptotic cells induce tissue-healing M2 macrophages (Anders and Ryu 2011). The M2 phenotype is also promoted by resolvin and the anti-inflammatory cytokines IL-10 as well as TGF-β (Titos et al. 2011; Rogler 2017).
An excessive inflammatory response to trauma or infection, or failure to resolve an inflammatory response properly, may result in chronic conditions such as autoinflammatory and cardiovascular diseases (Nathan and Ding 2010).
CHRONIC INFLAMMATION AND HUMAN DISEASES
Inflammatory Bowel Disease
The pathogenesis behind IBD (e.g., CD and ulcerative colitis [UC]) is not fully elucidated. The two conditions share the features of chronic inflammatory activity in the intestinal mucosa reflected by loose stools with blood, abdominal pain, and, many times, also fatigue. CD and UC differ in the range of cytokines driving the inflammation as well as subsequent activation of diverse innate and adaptive immune cells. IFN-γ, IL-17, IL-12, and IL-23 are abundant in CD inflammation, while UC is associated with IL-13. TNF-α plays a central role in both diagnoses (Fuss et al. 2004; Brown and Mayer 2007; Strober et al. 2007; Brand 2009). CD is often regarded as a microbiota-driven inflammation and with granuloma as a hallmark, reflecting the innate inflammation, while UC exhibits more features of autoimmunity.
The antigens causing the initial activation of the intestinal immune system are still unknown, probably because there are several potential triggers that, in conjunction with a breach of the host integrity, set off the excessive and prolonged inflammatory activity. The roles of commensal or pathogenic bacteria have been studied, especially in CD (Xavier and Podolsky 2007; Alexander et al. 2014). Beside the hypothesis that the commensal bacteria fuel the inflammation once the integrity of the mucosal lining is destroyed, other studies have identified specific pathogenic species such as adhesive invading Escherichia coli (AIEC) and Mycobacterium tuberculosis avium. A reduced number of Faecalibacterium prausnitzii has also been proposed to promote ileocecal CD (Greenstein 2003; Barnich and Darfeuille-Michaud 2007; Sokol et al. 2008). Especially the finding of bacteria adherent to the epithelium beneath the mucus layer in IBD patients, an aseptic region in healthy individuals, has corroborated the importance of microorganisms in IBD pathogenesis. In addition, the efficacy of antibiotics in certain studies have further supported the role of microorganisms in the intestinal inflammation (Barbosa and Rescigno 2010; Khan et al. 2011).
A leaky mucosal barrier has also been suggested to trigger the inflammation by exposing the lamina propria immune cells to commensal bacteria. Indeed, significantly increased intestinal permeability has been observed in first-degree relatives of IBD patients. Genetic variants associated with dysfunctional epithelium has been observed in IBD, for example, mdr-1 in UC and reduced expression of defensins in CD (Söderholm et al. 1999; Dessein et al. 2008; Wehkamp et al. 2008). The third hypothesis behind IBD is a primary dysfunction in the mucosal immunity. The first gene polymorphism associated with CD, NOD-2, suggests a dysfunction in an intracellular pathogen-associated molecular pattern receptor for a bacterial antigen, muramyl dipeptide. In addition, macrophages from CD patients show decreased cytokine release upon activation, which can be correlated to polymorphisms in the IRGM and ATG16L1 genes. This defect is caused by dysfunctional posttranslational modifications resulting in transport of the cytokines to lysosomes followed by degradation instead of extracellular release (autophagia) (Cho 2008; Dessein et al. 2008; Fava and Danese 2010; Dupaul-Chicoine et al. 2013). Accordingly, treatment of IBD should address the local excess of proinflammatory cytokines, tissue swelling with infiltration of leukocytes into intestinal tissue, and ideally promote a normal intestinal microflora.
Rheumatoid Arthritis
RA is the leading chronic inflammatory disease-causing major disability worldwide. The articular inflammation is driven by a loss of tolerance to self, and RA patients produce autoantibodies to posttranslationally modified proteins, including fibrin, vimentin, fibronectin, and type II collagen, via citrullination and carbamylation (so-called antimodified protein autoantibodies [AMPAs]) (Alivernini et al. 2018). RA is a polygenetic disease with a multitude of alleles associated with different immunological aberrations, and HLA-DR1 and HLA-DR4 represent the strongest genetic risk factors (Okada et al. 2014; Guo et al. 2018). Environmental factors such as infections, intestinal microbiota, and cigarette smoking appear to play an additional role in triggering arthritis. Periodontal infections with Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans may also contribute. (Konig et al. 2016; Alivernini et al. 2018; Guo et al. 2018). Studies also point to articular injury or transient local infection to be a possible initial catalyst. These factors will promote immune complex formation with synovial antigens and fuel the inflammatory response further by activating macrophages, stromal cells, mast cells, and osteoclasts (Smolen et al. 2016; Malmström et al. 2017). The antigen-presenting cells recognize citrullinated peptides and subsequently activate T helper cells type 1 (Th1) and T helper cells type 17 (Th17). These T cells produce IFN-γ and IL-17, respectively, together with granulocyte macrophage colony-stimulating factor (GM-CSF), and facilitate production of autoantibodies, such as anticitrullinated protein antibody (ACPA) from B cells (Smolen et al. 2016; Malmström et al. 2017). This immune response to self-antigens may go on for years before symptoms occur (van der Woude et al. 2010).
TNF-α and IL-6 are central proinflammatory cytokines and reach significant levels in the synovial fluids of inflamed joints (Rajendran et al. 2018). The chronic inflammation affects the synovial membrane and leads to synovitis, characterized by joint swelling (Alivernini et al. 2018; Guo et al. 2018). The imbalance between proinflammatory M1 and anti-inflammatory M2 macrophages promotes osteoclastogenesis (Fukui et al. 2018). Ultimately, the accumulation of T cells, B cells, and neutrophils, promoted by the local synthesis of cytokines and matrix metalloproteinases (MMPs), leads to the synovium becoming hyperplastic (Filer et al. 2006). The process eventually results in irreversible tissue destruction in terms of cartilage damage because of apoptotic chondrocytes followed by localized bone erosion (Guo et al. 2018).
Inflammation and Cardiovascular Disease
Cardiovascular disease is the most common cause of death worldwide, and inflammation plays an important role in the development of the key underlying causes, including atherosclerosis and hypertension (Hansson and Hermansson 2011; McMaster et al. 2015; Söderström et al. 2018). In support of this notion, commonly used experimental models for atherosclerosis and hypertension fail to develop the disease in the absence of T cells. Proinflammatory cytokines, including TNF-α and IL-17, promote development of experimental hypertension, and treatment with anti-TNF-α antibodies reduces inflammation and hypertension (Harrison et al. 2011; Norlander et al. 2018). In atherosclerotic disease, rupture of lesions may initiate coagulation that results in intra-arterial thrombus formation, disruption of blood flow, organ ischemia, and tissue damage. Inflammation localized to the arterial wall and atherosclerotic lesions can promote thinning of the stabilizing fibrous cap and render lesions prone to rupture, as evidenced by numerous experimental studies (Hansson and Hermansson 2011; Libby et al. 2013; Hansson et al. 2015). The underlying mechanisms are complex and involve infiltration of lipoproteins as well as adaptive and innate immune cells into the arterial wall, which will lead to local and systemic release of proinflammatory as well as pro-resolving mediators (Libby et al. 2013; Bäck and Hansson 2019). In a recent clinical trial, treatment with canakinumab, an antibody directed against the proinflammatory cytokine IL-1β, reduced cardiovascular risk in patients with a history of myocardial infarction (Ridker et al. 2017). Hence, improved control of inflammatory activity in the vasculature may likely be beneficial in treatment of cardiovascular disease. The vasculature and lymph nodes are innervated, and accordingly potentially accessible for bioelectronic medicine intervention.
FAILED RESOLUTION AND CHRONIC INFLAMMATORY DISEASES
When an inflammatory process fails to properly resolve, chronic inflammation will develop in organs such as joints, intestine, or vascular endothelium, with consequential damage, leading to disability and risk of a reduced life span (Nathan and Ding 2010). In IBD, T-regulatory (Treg) cells seem to be an important player in the resolution of inflammation. The activated intestinal T-cell population gradually shifts to a regulatory phenotype during inflammation and acquires suppressive characteristics, characterized by the production of TGF-β, IL-10, and IL-35. The importance of Tregs in the healing process of experimental colitis was established by the classical adoptive transfer model, where intestinal repair was achieved by Tregs transferred to FOXP3-deficient mice with colitis. Therefore, the central role of Tregs has been highlighted in the search of mechanisms behind failed resolution in the gut (Fantini et al. 2006; Wirtz et al. 2011; Gagliani et al. 2015). Other candidates include regulatory ILCs (ILCregs), releasing IL-10 and TGF-β in parallel with efficient inhibition of IFN-γ and IL-17 in the intestinal lamina propria (Wang et al. 2017). IL-22 produced by ILCs type 3 (ILC3s) induces epithelial healing and restores mucosal integrity. This observation renders IL-22 a central role in resolution and is exemplified by IL-17 blocking agents that deteriorate colitis presumably by parallel inhibition of IL-22 production (Schett and Neurath 2018).
In RA, IL-9 is highlighted as a central pro-resolving cytokine, based on animal arthritis models where IL-9 ameliorates the chronic inflammation (Rauber et al. 2017). Pro-resolving ILC2s appear to be the target of IL-9 in an autocrine fashion, and the subsequent activation of Tregs mediates suppression by cell-to-cell contact through GITRL and ICOSL. The IL-9-mediated resolution appears to be limited to arthritis, since the interleukin promotes inflammation in gut and lungs (Gerlach et al. 2014; Koch et al. 2017; Rauber et al. 2017). An additional important pro-resolving mechanism is the aggregation of neutrophil extracellular traps (aggNETs), where neutrophils during apoptosis expel their chromatin. These large agglomerates of dead neutrophils trap and degrade local cytokines and chemokines (Schauer et al. 2014; Schett and Neurath 2018).
Inflammation resolution likely plays an important role also in cardiovascular disease, such as atherosclerosis, as evidenced by studies in genetically modified mice, human epidemiological studies, and clinical trials (Merched et al. 2008; Bäck and Hansson 2019). Treatment with precursors of pro-resolving mediators, such as icosapent ethyl, reduced adverse cardiovascular events in the REDUCE-IT trial, and experimental studies show that other mediators derived from omega-3 fatty acids reduce atherosclerosis development (Bäck and Hansson 2019). Chronic inflammation and failed resolution is also associated with blood vessel changes that promote hypertension development.
NEURAL CONTROL OF CHRONIC INFLAMMATION
The Inflammatory Reflex
Neural reflexes regulate blood pressure, body temperature, and gastrointestinal activity. Sensory nerves monitor organ function, including immune system activity, and motor nerves send signals that promote preservation of homeostasis (Chiu et al. 2012; Steinberg et al. 2016a,b; Caravaca et al. 2017b; Pinho-Ribeiro et al. 2018; Zanos et al. 2018). The “inflammatory reflex” regulates cytokine release (Figs. 1 and 2). Cytokines activate sensory vagus nerve fibers that trigger motor activity in the vagus nerve and splenic nerve (Niijima 1996). Nerve endings of the adrenergic splenic nerve are found in close proximity of choline acetyltransferase (ChAT)-expressing, acetylcholine (ACh)-producing T cells in spleen. Norepinephrine (NE) promotes ChAT+ T-cell release of ACh, a ligand for α7 nicotinic ACh receptor (α7nAChR) found on innate immune cells in spleen (Olofsson et al. 2012a,b; Pavlov and Tracey 2012). Activation of α7nAChR cholinergic receptors has long-term effects on cytokine release from innate immune cells (Wang et al. 2003; Huston et al. 2006; Parrish et al. 2008; Rosas-Ballina et al. 2009; Olofsson et al. 2012b; Tarnawski et al. 2018). Based on these and other discoveries on neural control of inflammation, pharmacological as well as electrical activation of the inflammatory reflex have been used to reduce inflammation and inflammatory disease in experimental models and in clinical trials (Olofsson et al. 2012a; Rosas-Ballina et al. 2015; Koopman et al. 2016; Consolim-Colombo et al. 2017). An interesting observation is that a minute-long—or even shorter—electrical stimulation of the cervical vagus nerve has effects on release of proinflammatory cytokines and inflammation for days, as shown in Figure 3. This mechanism involves neural signals as well as specific intracellular signaling in monocytes/macrophages and provides a possible explanation for how a daily, minute-long, unilateral electrical activation of the cervical vagus nerve can be sufficient to reduce patients’ clinical scores in RA (Tarnawski et al. 2018).
Figure 2.
Neural circuits regulate key mechanisms in experimental inflammation. Examples include: (A–C) The efferent vagus nerve connects with the myenteric plexus in the gut, as well as with the celiac ganglion, through the splenic nerve and to the spleen, where the signals are relayed by acetylcholine (ACh)-releasing T cells. Subsequent activation of cholinergic receptors may, for example, attenuate macrophage cytokine release or promote other physiological events. (D) Electrical vagus nerve stimulation is implicated in the reduction of antibody secretion and B-cell migration in the spleen. (E) Challenge with an antigen triggers a response in TRPV1− NaV1.8+ sensory neurons, which leads to restriction of antigen transit from local lymph node A to B. TNF-α, Tumor necrosis factor α; L-Arg, L-arginine; eNOS, endothelial nitric oxide synthase; EC, endothelial cell; SMC, smooth muscle cell; α7nAChR, α7 nicotinic acetylcholine receptor.
Figure 3.
VNS suppresses endotoxin-induced serum tumor necrosis factor (TNF) levels for over 24 h. Rats were subjected to 60 sec of VNS or sham surgery, followed by intraperitoneal endotoxin injection at a specified time after VNS. Serum was collected and analyzed for TNF by ELISA. Open squares, mean TNF ± SEM in sham animals; filled diamonds, mean TNF ± SEM in vagus nerve–stimulated animals (reproduced from Tarnawski et al. 2018).
The development and resolution of inflammation, and the clinical manifestations of inflammatory diseases, are complex processes, which commonly involves interactions between affected organs, the immune system, peripheral nerves, and the central nervous system. Interestingly, a substantial fraction of monocytes recruited to sites of inflammation come from the spleen (Swirski et al. 2009). The spleen is a target organ of efferent signals in the inflammatory reflex in which electrical stimulation of the vagus nerve reduces cytokine release to proinflammatory agents for days (Parrish et al. 2008; Rosas-Ballina et al. 2008; Tarnawski et al. 2018). Therefore, it is conceivable that even a short activation of the inflammatory reflex can attenuate cytokine release in immune cells recruited to ongoing inflammation. The neural control of leukocyte recruitment in chronic inflammatory diseases is not well understood, but it is tempting to speculate that activation of the inflammatory reflex may result in a shift toward a less proinflammatory phenotype of leukocytes available for recruitment to inflammatory foci (Swirski et al. 2009; Tarnawski et al. 2018). In this way, vagus nerve stimulation (VNS) may convey a systemic attenuating effect on inflammation by bringing about a phenotypic change of leukocytes in the spleen (Saeed et al. 2005; Huston et al. 2006).
Observations from clinical studies of biological treatment suggest that the clinical symptoms of inflammatory diseases are strongly influenced by an intricate interplay between mechanisms in the central nervous system, the peripheral nervous system, the immune system, and the affected tissue and organs. For example, pain attenuation after treatment with anticytokine therapy in patients with RA happens more rapidly than resolution of inflammation in the joints is expected (Diamond and Tracey 2011; Hess et al. 2011). This clinical observation illustrates the complexity of pain perception and suggests that direct effects on the central nervous system are at play, in addition to the intricate interactions between peripheral nerves and immune cells. Clearly, interactions between mechanisms for pain and inflammation have significant implications for homeostasis and health (Chiu et al. 2013, 2016; Pinho-Ribeiro et al. 2018).
The vagus nerve also directly innervates the gastrointestinal tract by functionally connecting with postganglionic neurons in the myenteric plexus (Fig. 2; Berthoud et al. 1990, 1991; Prechtl and Powley 1990). However, the further relay of immune-regulating signals from the vagus nerve to macrophages and dendritic cells in the intestinal wall has not been mapped in detail. Data from distinct experimental models of intestinal inflammation suggest that signals in the inflammatory reflex (i.e., requiring involvement of the spleen) regulate intestinal inflammation under certain conditions, while the vagus nerve directly regulates intestinal inflammatory processes in other experimental settings (Ji et al. 2014; Matteoli et al. 2014). Based on these findings, the current view is that efferent vagus nerve signals control gut inflammation through at least two different pathways. One involves efferent vagus neurons that directly target the myenteric plexus. Another pathway travels through efferent vagus neurons that functionally synapse in the celiac ganglion. These signals are further conveyed through the adrenergic splenic nerve, and splenic ChAT+ T cells release ACh, the ligand for α7nAChR, expressed by innate immune cells (Matteoli and Boeckxstaens 2013). Both pathways are likely to reduce release of proinflammatory cytokines through activation of α7nAChR in monocytes and macrophages (Borovikova et al. 2000; Wang et al. 2003; de Jonge et al. 2005; Huston et al. 2006; Rosas-Ballina et al. 2008, 2011; Tarnawski et al. 2018). Moreover, the ChAT+ ACh-producing T cells, which relay neural signals in the inflammatory reflex, are also involved in regulation of blood pressure, vascular contractility, tissue immune cell infiltration in chronic infection, and antiviral defense (e.g., as evidenced by the significantly reduced defense against chronic viral infection and a different intestinal microbial flora in TChAT-deficient mice) (Rosas-Ballina et al. 2011; Dhawan et al. 2016; Olofsson et al. 2016; Cox et al. 2019). These observations open for speculation on another route for information transfer between inflammation-regulating neural signals and target organs in which circulating ChAT+ T cells are possibly “primed” by neural signals in spleen.
Other Neural Circuits that Control Inflammation and Immunity
In addition to the mechanisms related to the inflammatory reflex outlined above, additional neural reflex circuits that regulate inflammation and immunity have been described. An interesting example includes observations of neural control of lymph flow. Recent findings indicate that movement of antigen through the lymphatic system is different depending on the immunization status of experimental animals and that this mechanism at least partly involves both sensory and motor signals (Hanes et al. 2016). In this study, regulation of antigen flow involved nociceptive neurons expressing NAV1.8+ and neuronal Fc receptors, and possibly binding of immune complexes to Fc receptors on neurons. It is well established that adrenergic innervation regulate vasculature tone and, consequently, impacts the rate of systemic and local blood and lymph flow. Furthermore, vagus nerve signals may control coagulation (Czura et al. 2010) and tissue perfusion. In infections, this contributes to regulation of fluid shifts and immune cell trafficking. Components in the inflammatory reflex (e.g., ACh-producing T cells) also contribute to regulation of vascular tone and immunity (Olofsson et al. 2016; Cox et al. 2019).
Neural Control of B-Cell Function
Lymphoid organs, including lymph nodes, thymus, and Peyer's patches are innervated (Williams et al. 1981; Panuncio et al. 1999). In the intestinal epithelium, neural signals are transduced by Toll-like receptor (TLR) ligands. Adrenergic nerves innervate the spleen, in particular the marginal zone (Felten et al. 1985). The neural control of lymphocyte traffic has been known for years, as exemplified by the finding that nerve stimulation of the popliteal lymph node in sheep induces an increase in lymph flow and white blood cell output from the lymph node (McGale 1990). Moreover, splenic follicle expansion and germinal center formation were reduced after norepinephrine depletion in both the primary and secondary response to immunization, possibly because norepinephrine is required for up-regulation of B-cell activating and costimulatory molecules (such as B7), which drive the germinal center reaction (Han et al. 1995; Kohm and Sanders 2001). Considering these findings, the mechanism of neural control of immune cell migration in the periphery and, in particular, of B-cell localization in the spleen and antibody production in response to infection, was revealed in mice immunized with the T-independent antigen Streptococcus pneumoniae (SPn). The induced CD138+ antibody-secreting cells (ASCs) were specific for the immunizing antigen, possibly of B1 cell or marginal zone origin, and shortly after activation migrated to the red pulp and colocalized with synaptophysin-positive conduits. In the same SPn immunization model, in response to cholinergic stimulation or VNS, activated B cells arrested their migration in the marginal zone and reduced their antibody secretion rates (Mina-Osorio et al. 2012; Nakai et al. 2014; Wulfing and Gunther 2015).
Furthermore, neural regulation of splenic antibody responses by adrenergic signals is known. For example, chemical sympathectomy in mice increased antibody production after immunization with keyhole limpet hemocyanin (KLH) (Kruszewska et al. 1995).
NEURAL CONTROL OF RESOLUTION OF INFLAMMATION
Inflammation is important in the normal antimicrobial defense and tissue repair, but it is key that inflammation resolves as the threat is removed and the tissue can heal. Resolution of inflammation is an active process that is regulated by specific molecular mediators promoting tissue repair and homeostasis (Serhan 2014). Neural signals regulate inflammation as outlined in Figures 1 and 2, and disruption of the inflammatory reflex reduces levels of pro-resolving mediators in peritoneal inflammation and significantly slows resolution (Mirakaj et al. 2014). Signals through the vagus nerve regulate infection resolution by a mechanism that involves group 3 ILCs and immunoresolvents (Dalli et al. 2017). Importantly, recent observations suggest that electrical activation of the cervical vagus nerve and the inflammatory reflex reduces resolution time in experimental peritonitis, suggesting that activation of components in the inflammatory reflex not only reduces activity of proinflammatory mechanisms, but also actively promotes resolution, healing, and homeostasis (Caravaca et al. 2017a; Serhan et al. 2018). These preliminary experimental findings remain to be corroborated in other models and eventually evaluated in clinical studies.
TREATMENT BASED ON NEURAL REFLEX CONTROL OF INFLAMMATION
Electrical VNS, implemented using a pacemaker-like stimulator device connected to an electrode around the left vagus nerve, is being evaluated in clinical trials for treatment of both RA and CD (Bonaz et al. 2016; Koopman et al. 2016; D'Haens et al. 2018). Results show promise in RA, with an observed reduction of clinical symptoms, corroborating the data from several experimental studies where VNS reduced cytokine release, inflammation, and potentially promoted inflammation resolution and homeostasis (Koopman et al. 2016; Steinberg et al. 2016a).
The first clinical trial in IBD included seven patients with CD in a 6-month-long study. Five of these patients were treated with VNS as monotherapy, and two patients had concomitant immunosuppression with azathioprine (Bonaz et al. 2016). At inclusion, all patients had a Crohn's disease endoscopic inflammation score (CDEIS) >7 points. The patients were stimulated by an electrical current of 1.25 mA at 10 Hz and pulse width 500 µs, for 30 sec ON and 5 min OFF continuously for 6 months. Crohn's disease activity index (CDAI) and CDEIS were evaluated at the end of the study. Two patients were excluded before end of trial due to exacerbation of the disease. The disease activity decreased in all five remaining patients and four of these reached clinical remission (CDAI <150). All patients had reached endoscopic remission (CDEIS <6) at 6 months and they reported reduced abdominal pain with mean VAS score reduced from 3.5 to 1.6. No device-related severe adverse events were observed.
In an additional CD trial, 16 CD patients with partial or no response to biological treatment (antibodies against the cytokine TNF-α or the integrin α4β4) were treated with a vagus nerve stimulator in a 16-week-long trial (D'Haens et al. 2018). Eight stayed on biological treatment during the study and eight patients stopped biologics before inclusion. The electrical stimulation was divided into minutes-long episodes 1–4 times daily. The VNS-stimulated patients demonstrated clinical improvement in an interim analysis, but the final report has not yet been published.
The promising observations in these clinical trials of VNS are important, because the currently available therapies are insufficient for many, and the need for further innovations in therapeutic options is great. Much work remains however, before VNS is ready for implementation in clinical routines for a wider range of inflammatory conditions. Optimal stimulation parameters are not known and long-term effects of VNS in inflammatory disease have not been comprehensively studied. Based on available reports on individuals with implanted vagus nerve stimulators, relatively few serious adverse events have been reported. However, there is a lack of long-term VNS studies on individuals with excessive inflammation. Importantly, the available data on VNS regulation of inflammation certainly warrant further clinical trials with VNS as a therapeutic option in RA, IBD, and other chronic inflammatory diseases.
The clinical course of chronic inflammatory disease commonly involves “flares” with intensified symptoms, followed by periods with relatively mild disease. Sometimes, disease symptoms can change quickly over the course of days, or even hours. It would, therefore, be valuable to individualize treatment and optimize the dosing to keep the disease at bay while minimizing side effects. Current technologies have a limited ability to adapt to variations in disease severity. Recent data indicate that specific electrical activity in the vagus nerve can be deconvoluted and provide information on tissue levels of inflammatory cytokines and even distinguish between different cytokines (Caravaca et al. 2017b; Zanos et al. 2018). Development of methods for real-time monitoring of disease that can continuously inform patients, physicians, and—perhaps—devices, about the need for anti-inflammatory therapy with good resolution in time would potentially provide a significant step toward better individualization of treatment of inflammatory diseases (Olofsson and Tracey 2017).
CONCLUSION
Inflammation onset and resolution are both active processes (Buckley et al. 2013; Fullerton and Gilroy 2016; Rogler 2017). Incomplete resolution of inflammation has been implicated in the pathogenesis of numerous diseases, including IBD, RA, and cardiovascular diseases (Nathan and Ding 2010). Current therapies focus mainly on the inhibition of the proinflammatory mediators. This approach has multiple caveats as the purpose of inflammation is primarily protective and life preserving, as exemplified by the devastating effects of immunodeficiency disorders (Raje and Dinakar 2015). By also adding emphasis to a pro-resolution approach for the restoration of immune homeostasis, a new avenue for targeting uncontrolled inflammation is unfolding. As the inflammatory reflex is involved in regulating both inflammation onset and resolution, harnessing neural control of inflammation by applying bioelectronic medicine in chronic inflammatory diseases holds great promise. The potential efficacy of VNS has been demonstrated in both CD and RA, and nerve stimulation for treatment of other chronic inflammatory diseases is being considered (Bonaz et al. 2016; Koopman et al. 2016; D'Haens et al. 2018; Eberhardson et al. 2018). As our understanding of the neural reflex control of inflammation and inflammation resolution improves, it is likely that improved treatment options that incorporate more sophisticated information on inflammation physiology in individual patients and real-time adjustment of anti-inflammatory and pro-resolution therapies will become available through concerted multidisciplinary efforts in immunology, neuroscience, engineering, data science, and clinical medicine.
Footnotes
Editors: Valentin A. Pavlov and Kevin J. Tracey
Additional Perspectives on Bioelectronic Medicine available at www.perspectivesinmedicine.org
REFERENCES
- Alexander KL, Targan SR, Elson CO III. 2014. Microbiota activation and regulation of innate and adaptive immunity. Immunol Rev 260: 206–220. 10.1111/imr.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alivernini S, Tolusso B, Ferraccioli G, Gremese E, Kurowska-Stolarska M, McInnes IB. 2018. Driving chronicity in rheumatoid arthritis: Perpetuating role of myeloid cells. Clin Exp Immunol 193: 13–23. 10.1111/cei.13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. 2012. Neutrophil function: From mechanisms to disease. Annu Rev Immunol 30: 459–489. 10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- Anders HJ, Ryu M. 2011. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 80: 915–925. 10.1038/ki.2011.217 [DOI] [PubMed] [Google Scholar]
- Bäck M, Hansson GK. 2019. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. FASEB J 33: 1536–1539. 10.1096/fj.201802445R [DOI] [PubMed] [Google Scholar]
- Barbosa T, Rescigno M. 2010. Host-bacteria interactions in the intestine: Homeostasis to chronic inflammation. Wiley Interdiscip Rev Syst Biol Med 2: 80–97. 10.1002/wsbm.48 [DOI] [PubMed] [Google Scholar]
- Barnich N, Darfeuille-Michaud A. 2007. Role of bacteria in the etiopathogenesis of inflammatory bowel disease. World J Gastroenterol 13: 5571 10.3748/wjg.v13.i42.5571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Jedrzejewska A, Powley TL. 1990. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with Dil injected into the dorsal vagal complex in the rat. J Comp Neurol 301: 65–79. 10.1002/cne.903010107 [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL. 1991. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol 260: R200–R207. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Sinniger V, Hoffmann D, Clarençon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocmé C, Faure P, et al. 2016. Chronic vagus nerve stimulation in Crohn's disease: A 6-month follow-up pilot study. Neurogastroenterol Motil 28: 948–953. 10.1111/nmo.12792 [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462. 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- Brand S. 2009. Crohn's disease: Th1, Th17 or both? The change of a paradigm: New immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut 58: 1152–1167. 10.1136/gut.2008.163667 [DOI] [PubMed] [Google Scholar]
- Brown AJ, Mayer L. 2007. The immune response in inflammatory bowel disease. Am J Gastroenterol 102: 2058–2069. 10.1111/j.1572-0241.2007.01343.x [DOI] [PubMed] [Google Scholar]
- Brusselle G, Bracke K. 2014. Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Ann Am Thorac Soc 11: S322–S328. 10.1513/AnnalsATS.201403-118AW [DOI] [PubMed] [Google Scholar]
- Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. 2013. The resolution of inflammation. Nat Rev Immunol 13: 59–66. 10.1038/nri3362 [DOI] [PubMed] [Google Scholar]
- Caravaca AS, Tarnawski L, Arnardottir H, Bäck M, Olofsson PS. 2017a. Electrical vagus nerve stimulation accelerates resolution of inflammation in experimental peritonitis. Scand J Immunol 86: A-31270 10.1111/sji.12587 [DOI] [Google Scholar]
- Caravaca AS, Tsaava T, Goldman L, Silverman H, Riggott G, Chavan SS, Bouton C, Tracey KJ, Desimone R, Boyden ES, et al. 2017b. A novel flexible cuff-like microelectrode for dual purpose, acute and chronic electrical interfacing with the mouse cervical vagus nerve. J Neural Eng 14: 066005 10.1088/1741-2552/aa7a42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. 2018. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9: 7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, von Hehn CA, Woolf CJ. 2012. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15: 1063–1067. 10.1038/nn.3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, et al. 2013. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501: 52–57. 10.1038/nature12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Pinho-Ribeiro FA, Woolf CJ. 2016. Pain and infection: Pathogen detection by nociceptors. Pain 157: 1192–1193. 10.1097/j.pain.0000000000000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH. 2008. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 8: 458–466. 10.1038/nri2340 [DOI] [PubMed] [Google Scholar]
- Consolim-Colombo FM, Sangaleti CT, Costa FO, Morais TL, Lopes HF, Motta JM, Irigoyen MC, Bortoloto LA, Rochitte CE, Harris YT, et al. 2017. Galantamine alleviates inflammation and insulin resistance in patients with metabolic syndrome in a randomized trial. JCI insight 2: 93340 10.1172/jci.insight.93340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MA, Duncan GS, Lin GHY, Steinberg BE, Yu LX, Brenner D, Buckler LN, Elia AJ, Wakeham AC, Nieman B, et al. 2019. Choline acetyltransferase-expressing T cells are required to control chronic viral infection. Science 363: 639–644. 10.1126/science.aau9072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czura CJ, Schultz A, Kaipel M, Khadem A, Huston JM, Pavlov VA, Redl H, Tracey KJ. 2010. Vagus nerve stimulation regulates hemostasis in swine. Shock 33: 608–613. 10.1097/SHK.0b013e3181cc0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Serhan CN. 2013. Novel n-3 immunoresolvents: Structures and actions. Sci Rep 3: 1940 10.1038/srep01940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Arnardottir H, Serhan CN. 2017. Vagal regulation of group 3 innate lymphoid cells and the immunoresolvent PCTR1 controls infection resolution. Immunity 46: 92–105. 10.1016/j.immuni.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, et al. 2005. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6: 844–851. 10.1038/ni1229 [DOI] [PubMed] [Google Scholar]
- Dessein R, Chamaillard M, Danese S. 2008. Innate immunity in Crohn's disease: The reverse side of the medal. J Clin Gastroenterol 42: S144–S147. 10.1097/MCG.0b013e3181662c90 [DOI] [PubMed] [Google Scholar]
- D'Haens GR, Cabrijan Z, Eberhardson M, van den Berg RM, Löwengert M, Fiorino G, Danese S, Levine YA, Chernoff D. 2018. Mo1906—The effects of vagus nerve stimulation in biologicrefractory Crohn's disease: A prospective clinical trial. Gastroenterology 154: S-847 10.1016/S0016-5085(18)32870-1 [DOI] [Google Scholar]
- Dhawan S, De Palma G, Willemze RA, Hilbers FW, Verseijden C, Luyer MD, Nuding S, Wehkamp J, Souwer Y, de Jong EC, et al. 2016. Acetylcholine-producing T cells in the intestine regulate antimicrobial peptide expression and microbial diversity. Am J Physiol Gastrointest Liver Physiol 311: G920–G933. 10.1152/ajpgi.00114.2016 [DOI] [PubMed] [Google Scholar]
- Diamond B, Tracey KJ. 2011. Mapping the immunological homunculus. Proc Natl Acad Sci 108: 3461–3462. 10.1073/pnas.1100329108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffney PF, Falsetta ML, Rackow AR, Thatcher TH, Phipps RP, Sime PJ. 2018. Key roles for lipid mediators in the adaptive immune response. J Clin Invest 128: 2724–2731. 10.1172/JCI97951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaul-Chicoine J, Dagenais M, Saleh M. 2013. Crosstalk between the intestinal microbiota and the innate immune system in intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis 19: 2227–2237. 10.1097/MIB.0b013e31828dcac7 [DOI] [PubMed] [Google Scholar]
- Eberhardson M, Hedin C, Carlson M, Tarnawski L, Levine YA, Olofsson PS. 2018. Toward improved control of inflammatory bowel disease. Scand J Immunol 23: e12745. [DOI] [PubMed] [Google Scholar]
- Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P, Neurath MF. 2006. Transforming growth factor β induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut 55: 671–680. 10.1136/gut.2005.072801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava F, Danese S. 2010. Crohn's disease: Bacterial clearance in Crohn's disease pathogenesis. Nat Rev Gastroenterol Hepatol 7: 126–128. 10.1038/nrgastro.2010.1 [DOI] [PubMed] [Google Scholar]
- Felten D, Felten SY, Carlson S, Olschowka J, Livnat S. 1985. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol 135: 755s–765s. [PubMed] [Google Scholar]
- Filer A, Parsonage G, Smith E, Osborne C, Thomas AM, Curnow SJ, Rainger GE, Raza K, Nash GB, Lord J, et al. 2006. Differential survival of leukocyte subsets mediated by synovial, bone marrow, and skin fibroblasts: Site-specific versus activation-dependent survival of T cells and neutrophils. Arthritis Rheum 54: 2096–2108. 10.1002/art.21930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Iwamoto N, Takatani A, Igawa T, Shimizu T, Umeda M, Nishino A, Horai Y, Hirai Y, Koga T, et al. 2018. M1 and M2 monocytes in rheumatoid arthritis: A contribution of imbalance of M1/M2 monocytes to osteoclastogenesis. Front Immunol 8: 1958 10.3389/fimmu.2017.01958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton JN, Gilroy DW. 2016. Resolution of inflammation: A new therapeutic frontier. Nat Rev Drug Discov 15: 551–567. 10.1038/nrd.2016.39 [DOI] [PubMed] [Google Scholar]
- Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, et al. 2004. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest 113: 1490–1497. 10.1172/JCI19836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, et al. 2015. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523: 221–225. 10.1038/nature14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, Steiner S, Lehr HA, Wirtz S, Vieth M, Waisman A, et al. 2014. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol 15: 676–686. 10.1038/ni.2920 [DOI] [PubMed] [Google Scholar]
- Gomollon F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, et al. 2017. 3rd European Evidence-Based Consensus on the Diagnosis and Management of Crohn's Disease 2016. Part 1: Diagnosis and medical management. J Crohns Colitis 11: 3–25. 10.1093/ecco-jcc/jjw168 [DOI] [PubMed] [Google Scholar]
- Greenstein RJ. 2003. Is Crohn's disease caused by a mycobacterium? Comparisons with leprosy, tuberculosis, and Johne's disease. Lancet Infect Dis 3: 507–514. 10.1016/S1473-3099(03)00724-2 [DOI] [PubMed] [Google Scholar]
- Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. 2018. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res 6: 15 10.1038/s41413-018-0016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidzadeh K, Christensen SM, Dalby E, Chandrasekaran P, Mosser DM. 2017. Macrophages and the recovery from acute and chronic inflammation. Annu Rev Physiol 79: 567–592. 10.1146/annurev-physiol-022516-034348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. 1995. Cellular Interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol 155: 556–567. [PubMed] [Google Scholar]
- Hanes WM, Olofsson PS, Talbot S, Tsaava T, Ochani M, Imperato GH, Levine YA, Roth J, Pascal MA, Foster SL, et al. 2016. Neuronal circuits modulate antigen flow through lymph nodes. Bioelectron Med 3: 18–28. 10.15424/bioelectronmed.2016.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, Hermansson A. 2011. The immune system in atherosclerosis. Nat Immunol 12: 204–212. 10.1038/ni.2001 [DOI] [PubMed] [Google Scholar]
- Hansson GK, Libby P, Tabas I. 2015. Inflammation and plaque vulnerability. J Int Med 278: 483–493. 10.1111/joim.12406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. 2011. Inflammation, immunity, and hypertension. Hypertension 57: 132–140. 10.1161/HYPERTENSIONAHA.110.163576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S, Sergeeva M, Saake M, Garcia M, Kollias G, et al. 2011. Blockade of TNF-α rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci 108: 3731–3736. 10.1073/pnas.1011774108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, et al. 2006. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203: 1623–1628. 10.1084/jem.20052362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. 2014. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunology 7: 335–347. 10.1038/mi.2013.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. 2012. A spatially organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 150: 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, Talley NJ, Moayyedi P. 2011. Antibiotic therapy in inflammatory bowel disease: A systematic review and meta-analysis. Am J Gastroenterol 106: 661–673. 10.1038/ajg.2011.72 [DOI] [PubMed] [Google Scholar]
- Koch S, Sopel N, Finotto S. 2017. Th9 and other IL-9-producing cells in allergic asthma. Semin Immunopathol 39: 55–68. 10.1007/s00281-016-0601-1 [DOI] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. 2001. Norepinephrine and β2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev 53: 487–525. [PubMed] [Google Scholar]
- Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, Rosen A, Nigrovic PA, Sokolove J, Giles JT, et al. 2016. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med 8: 369ra176 10.1126/scitranslmed.aaj1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, et al. 2016. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci 113: 8284–8289. 10.1073/pnas.1605635113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszewska BB, Felten S, Moynihan J. 1995. Alterations in cytokine and antibody production following chemical sympathectomy in two strains of mice. J Immunol 155: 4613–4620. [PubMed] [Google Scholar]
- Libby P, Lichtman AH, Hansson GK. 2013. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity 38: 1092–1104. 10.1016/j.immuni.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström V, Catrina AI, Klareskog L. 2017. The immunopathogenesis of seropositive rheumatoid arthritis: From triggering to targeting. Nat Rev Immunol 17: 60–75. 10.1038/nri.2016.124 [DOI] [PubMed] [Google Scholar]
- Matteoli G, Boeckxstaens GE. 2013. The vagal innervation of the gut and immune homeostasis. Gut 62: 1214–1222. 10.1136/gutjnl-2012-302550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, et al. 2014. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63: 938–948. 10.1136/gutjnl-2013-304676 [DOI] [PubMed] [Google Scholar]
- McGale MG. 1990. Sympathetic stimulation causes increased output of lymphocytes from the popliteal node in anaesthetized sheep. Exp Physiol 75: 847–850. 10.1113/expphysiol.1990.sp003467 [DOI] [PubMed] [Google Scholar]
- McMaster WG, Kirabo A, Madhur MS, Harrison DG. 2015. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033. 10.1161/CIRCRESAHA.116.303697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. 2008. Atherosclerosis: Evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 22: 3595–3606. 10.1096/fj.08-112201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina-Osorio P, Rosas-Ballina M, Valdes-Ferrer SI, Al-Abed Y, Tracey KJ, Diamond B. 2012. Neural signaling in the spleen controls B-cell responses to blood-borne antigen. Mol Med 18: 618–627. 10.2119/molmed.2012.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. 2014. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med 211: 1037–1048. 10.1084/jem.20132103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. 2014. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J Exp Med 211: 2583 10.1084/jem.20141132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A. 2010. Nonresolving inflammation. Cell 140: 871–882. 10.1016/j.cell.2010.02.029 [DOI] [PubMed] [Google Scholar]
- Niijima A. 1996. The afferent discharges from sensors for interleukin 1β in the hepatoportal system in the anesthetized rat. J Auton Nerv Syst 61: 287–291. 10.1016/S0165-1838(96)00098-7 [DOI] [PubMed] [Google Scholar]
- Norlander AE, Madhur MS, Harrison DG. 2018. The immunology of hypertension. J Exp Med 215: 21–33. 10.1084/jem.20171773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, Kochi Y, Ohmura K, Suzuki A, Yoshida S, et al. 2014. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506: 376–381. 10.1038/nature12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson PS, Tracey KJ. 2017. Bioelectronic medicine: Technology targeting molecular mechanisms for therapy. J Int Med 282: 3–4. 10.1111/joim.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. 2012a. Rethinking inflammation: Neural circuits in the regulation of immunity. Immunol Rev 248: 188–204. 10.1111/j.1600-065X.2012.01138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson PS, Katz DA, Rosas-Ballina M, Levine YA, Ochani M, Valdés-Ferrer SI, Pavlov VA, Tracey KJ, Chavan SS. 2012b. α7 nicotinic acetylcholine receptor (α7nAChR) expression in bone marrow–derived non-T cells is required for the inflammatory reflex. Mol Med 18: 539–543. 10.2119/molmed.2011.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson PS, Steinberg BE, Sobbi R, Cox MA, Ahmed MN, Oswald M, Szekeres F, Hanes WM, Introini A, Liu SF, et al. 2016. Blood pressure regulation by CD4+ lymphocytes expressing choline acetyltransferase. Nat Biotechnol 34: 1066–1071. 10.1038/nbt.3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuncio AL, De La Peña S, Gualco G, Reissenweber N. 1999. Adrenergic innervation in reactive human lymph nodes. J Anat 194: 143–146. 10.1046/j.1469-7580.1999.19410143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, et al. 2008. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med 14: 567–574. 10.2119/2008-00079.Parrish [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. 2012. The vagus nerve and the inflammatory reflex—Linking immunity and metabolism. Nat Rev Endocrinol 8: 743–754. 10.1038/nrendo.2012.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho-Ribeiro FA, Baddal B, Haarsma R, O'Seaghdha M, Yang NJ, Blake KJ, Portley M, Verri WA, Dale JB, Wessels MR, et al. 2018. Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell 173: 1083–1097.e22. 10.1016/j.cell.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prechtl JC, Powley TL. 1990. The fiber composition of the abdominal vagus of the rat. Anat Embryol (Berl) 181: 101–115. 10.1007/BF00198950 [DOI] [PubMed] [Google Scholar]
- Raje N, Dinakar C. 2015. Overview of immunodeficiency disorders. Immunol Allergy Clin North Am 35: 599–623. 10.1016/j.iac.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P, Chen YF, Chen YF, Chung LC, Tamilselvi S, Shen CY, Day CH, Chen RJ, Viswanadha VP, Kuo WW, et al. 2018. The multifaceted link between inflammation and human diseases. J Cell Physiol 233: 6458–6471. 10.1002/jcp.26479 [DOI] [PubMed] [Google Scholar]
- Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, Lin NY, Dietel K, Bozec A, Herrmann M, et al. 2017. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med 23: 938–944. 10.1038/nm.4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. 2017. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- Rogler G. 2017. Resolution of inflammation in inflammatory bowel disease. Lancet Gastroenterol Hepatol 2: 521–530. 10.1016/S2468-1253(17)30031-6 [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. 2008. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci 105: 11008–11013. 10.1073/pnas.0803237105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdés-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. 2009. The selective α7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med 15: 195–202. 10.2119/molmed.2009.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, et al. 2011. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101. 10.1126/science.1209985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Valdés-Ferrer SI, Dancho ME, Ochani M, Katz D, Cheng KF, Olofsson PS, Chavan SS, Al-Abed Y, Tracey KJ, et al. 2015. Xanomeline suppresses excessive pro-inflammatory cytokine responses through neural signal-mediated pathways and improves survival in lethal inflammation. Brain Behav Immun 44: 19–27. 10.1016/j.bbi.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. 2005. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med 201: 1113–1123. 10.1084/jem.20040463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, Lell M, Manger B, Rech J, Holmdahl R, et al. 2014. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 20: 511–517. 10.1038/nm.3547 [DOI] [PubMed] [Google Scholar]
- Schett G, Neurath MF. 2018. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat Commun 9: 3261 10.1038/s41467-018-05800-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510: 92–101. 10.1038/nature13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Levy BD. 2018. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J Clin Invest 128: 2657–2669. 10.1172/JCI97943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Dalli J. 2015. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol 27: 200–215. 10.1016/j.smim.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, de la Rosa X, Jouvene C. 2018. Novel mediators and mechanisms in the resolution of infectious inflammation: Evidence for vagus regulation. J Int Med 10.111/jlim.12871 [DOI] [PubMed] [Google Scholar]
- Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. 2012. Multifaceted link between cancer and inflammation. Biosci Rep 32: 1–15. 10.1042/BSR20100136 [DOI] [PubMed] [Google Scholar]
- Silverstein AM. 2001. Autoimmunity versus horror autotoxicus: The struggle for recognition. Nat Immunol 2: 279–281. 10.1038/86280 [DOI] [PubMed] [Google Scholar]
- Smolen JS, Aletaha D, McInnes IB. 2016. Rheumatoid arthritis. Lancet 388: 2023–2038. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- Söderholm JD, Olaison G, Lindberg E, Hannestad U, Vindels A, Tysk C, Järnerot G, Sjödahl R. 1999. Different intestinal permeability patterns in relatives and spouses of patients with Crohn's disease: An inherited defect in mucosal defence? Gut 44: 96–100. 10.1136/gut.44.1.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström LA, Tarnawski L, Olofsson PS. 2018. CD137: A checkpoint regulator involved in atherosclerosis. Atherosclerosis 272: 66–72. 10.1016/j.atherosclerosis.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, et al. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci 105: 16731–16736. 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg BE, Sundman E, Terrando N, Eriksson LI, Olofsson PS. 2016a. Neural control of inflammation: Implications for perioperative and critical care. Anesthesiology 124: 1174–1189. 10.1097/ALN.0000000000001083 [DOI] [PubMed] [Google Scholar]
- Steinberg BE, Silverman HA, Robbiati S, Gunasekaran MK, Tsaava T, Battinelli E, Stiegler A, Bouton CE, Chavan SS, Tracey KJ, et al. 2016b. Cytokine-specific neurograms in the sensory vagus nerve. Bioelectron Med 3: 7–17. 10.15424/bioelectronmed.2016.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Liao S. 2018. Neutrophil-lymphatic interactions during acute and chronic disease. Cell Tissue Res 371: 599–606. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss I, Mannon P. 2007. The fundamental basis of inflammatory bowel disease. J Clin Invest 117: 514–521. 10.1172/JCI30587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. 2009. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616. 10.1126/science.1175202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140: 805–820. 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- Tarnawski L, Reardon C, Caravaca AS, Rosas-Ballina M, Tusche MW, Drake AR, Hudson LK, Hanes WM, Li JH, Parrish WR, et al. 2018. Adenylyl cyclase 6 mediates inhibition of TNF in the inflammatory reflex. Front Immunol 9: 2648 10.3389/fimmu.2018.02648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titos E, Rius B, González-Périz A, López-Vicario C, Morán-Salvador E, Martínez-Clemente M, Arroyo V, Clària J. 2011. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol 187: 5408–5418. 10.4049/jimmunol.1100225 [DOI] [PubMed] [Google Scholar]
- van der Woude D, Rantapää-Dahlqvist S, Ioan-Facsinay A, Onnekink C, Schwarte CM, Verpoort KN, Drijfhout JW, Huizinga TW, Toes RE, Pruijn GJ. 2010. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis 69: 1554–1561. 10.1136/ard.2009.124537 [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. 2003. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421: 384–388. 10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- Wang S, Xia P, Chen Y, Qu Y, Xiong Z, Ye B, Du Y, Tian Y, Yin Z, Xu Z, et al. 2017. Regulatory innate lymphoid cells control innate intestinal inflammation. Cell 171: 201–216.e18. 10.1016/j.cell.2017.07.027 [DOI] [PubMed] [Google Scholar]
- Wehkamp J, Koslowski M, Wang G, Stange EF. 2008. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn's disease. Mucosal Immunol 1: S67–S74. 10.1038/mi.2008.48 [DOI] [PubMed] [Google Scholar]
- Williams JM, Peterson R, Shea PA, Schmedtje JF, Bauer DC, Felten DL. 1981. Sympathetic innervation of murine thymus and spleen: Evidence for a functional link between the nervous and immune systems. Brain Res Bull 6: 83–94. 10.1016/S0361-9230(81)80072-X [DOI] [PubMed] [Google Scholar]
- Wirtz S, Billmeier U, McHedlidze T, Blumberg RS, Neurath MF. 2011. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology 141: 1875–1886. 10.1053/j.gastro.2011.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfing C, Gunther HS. 2015. Dendritic cells and macrophages neurally hard-wired in the lymph node. Sci Rep 5: 16866 10.1038/srep16866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434. 10.1038/nature06005 [DOI] [PubMed] [Google Scholar]
- Zanos TP, Silverman HA, Levy T, Tsaava T, Battinelli E, Lorraine PW, Ashe JM, Chavan SS, Tracey KJ, Bouton CE. 2018. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc Natl Acad Sci 115: E4843–E4852. 10.1073/pnas.1719083115 [DOI] [PMC free article] [PubMed] [Google Scholar]