Abstract
The liver is the largest organ in the human body and is prone for cancer metastasis. Although the metastatic pattern can differ depending on the cancer type, the liver is the organ to which cancer cells most frequently metastasize for the majority of prevalent malignancies. The liver is unique in several aspects: the vascular structure is highly permeable and has unparalleled dual blood connectivity, and the hepatic tissue microenvironment presents a natural soil for the seeding of disseminated tumor cells. Although 70% of the liver is composed of the parenchymal hepatocytes, the remaining 30% is composed of nonparenchymal cells including Kupffer cells, liver sinusoidal endothelial cells, and hepatic stellate cells. Recent discoveries show that both the parenchymal and the nonparenchymal cells can modulate each step of the hepatic metastatic cascade, including the initial seeding and colonization as well as the decision to undergo dormancy versus outgrowth. Thus, a better understanding of the molecular mechanisms orchestrating the formation of a hospitable hepatic metastatic niche and the identification of the drivers supporting this process is critical for the development of better therapies to stop or at least decrease liver metastasis. The focus of this perspective is on the bidirectional interactions between the disseminated cancer cells and the unique hepatic metastatic niche.
Metastasis is the spreading of cancer cells from the primary tumor site to secondary distant sites in the human body, and it is estimated that metastasis accounts for about 90% of cancer related deaths (Chaffer and Weinberg 2011). The liver is a highly metastasis-permissive organ and the majority of the most common solid cancers, namely lung, pancreas, breast, colorectal, prostate, gastric, esophagus, cervix uteri, thyroid, and bladder cancer frequently metastases to the liver (Budczies et al. 2015). As a result, the liver represents the organ with the highest metastatic incidence (Hess et al. 2006). In fact, liver metastases are even more common than primary liver tumors (Bosch et al. 2004). It is estimated that 30%–70% of cancer patients die with liver metastasis (Pickren et al. 1982) and most patients with liver metastasis will die of their disease (Gilbert et al. 1982).
Clinical observations show that different cancer types display dramatic variations in their metastatic pattern. Some tumors mainly disseminate to only one organ (e.g., ocular melanoma to liver, prostate to bones), whereas others metastasize to multiple organs (e.g., skin melanoma, breast, and lung cancer) (Vanharanta and Massagué 2013). The cancers with high-hepatic metastatic prevalence include uveal melanoma (>90%) (Amaro et al. 2017) and gastrointestinal cancers, namely, pancreas (75%–80%) (Ryan et al. 2014) and colorectal (50%) (Chow and Chok 2019).
The concept of organ selectivity of metastases, or tissue tropism, was first introduced in 1889 by the English surgeon Stephen Paget (Paget 1989). He proposed that tumor cells have an affinity to certain organs, where they seed into a friendly “soil,” which facilitates their initial survival and their later outgrowth. Because this original work, significant advances have been made in our understanding of both the cell-autonomous mechanisms that drive metastasis, and alterations in the “soil” at the secondary site that allow efficient metastatic colonization and outgrowth leading to clinically relevant metastatic lesions (Minn et al. 2005; Lu et al. 2011; Qian et al. 2011; Sevenich et al. 2014; Kitamura et al. 2015; Nielsen et al. 2016; Roe et al. 2017; Quaranta et al. 2018). Systemic effects from the primary tumor that occur before metastasis have also been shown to affect the tropism and efficiency of disseminated cancer cells to colonize the secondary site (Kaplan et al. 2005; Hoshino et al. 2015). This precondition of the future metastatic niche, known as the premetastatic niche, has also been described for liver and enhances the engraftment, survival, and outgrowth of arrested disseminated tumor cells (DTCs) (Costa-Silva et al. 2015; Lee et al. 2019).
In general, the metastatic colonization of the liver is divided into five phases: (1) adhesion and arrest phase within the sinusoidal lumen, (2) extravasation phase into the space of Disse, (3) hepatic niche activation phase, (4) latency and resistance, and (5) outgrowth phase (expansion of metastasis). The first four phases do not require angiogenesis and likely are the entirety of the process for dormant metastases; these could remain as such for years to decades or proceed because of unknown stimuli to emergent masses as noted in the last phase. In addition, the premetastatic phase could set the stage for efficient liver colonization by DTCs.
This perspective aims to discuss our emerging understanding of the bidirectional interactions between the disseminated tumor cells and the hepatic metastatic niche, and to highlight potential therapeutic opportunities to develop better treatments for liver metastasis.
LIVER ARCHITECTURE AND HEMODYNAMIC FLOW
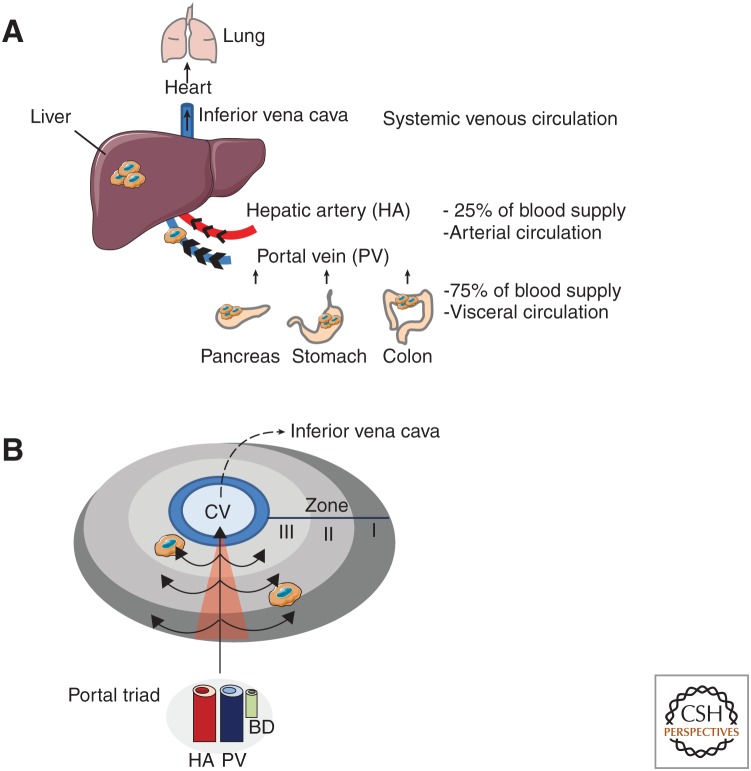
The liver is the largest organ in the human body and is composed of smaller functional units called lobules. The main components of each lobule are the parenchymal hepatocytes and they are aligned in a sheet-like structure. These structures are surrounded by branches of the hepatic artery, portal vein, and bile duct, which build together the portal triad. The liver is the only organ that has a dual blood connectivity receiving blood from both the hepatic artery and the portal vein. The hepatic artery provides the oxygenated blood and contributes to ∼25% of the blood influx. The portal vein brings nutrient-rich blood from visceral circulation, which is connected to the intestine, pancreas, and spleen and contributes to ∼75% of the blood supply to the liver (Fig. 1A). At the cellular level, the liver is composed of 70% parenchymal hepatocytes and cholangiocytes, and 30% nonparenchymal cells. Parenchymal cells are responsible for the metabolic, detoxification, and glandular functions. The nonparenchymal cells are represented by a mixture of highly specialized cells, including Kupffer cells (KC), liver sinusoidal endothelial cells (LSEC), and hepatic stellate cells (HSC) (Kmiec 2001; Heymann and Tacke 2016). Finally, the liver can be functionally further classified into zones (I, II, III) depending on the level of oxygen, with zone I being proximate to the portal triad and thus the most oxygenated and zone III being located close to the central vein and thus the most hypoxic (Fig. 1B; Kmiec 2001).
Figure 1.
Anatomy of the liver and hemodynamic flow. (A) The liver is the largest organ of the human body and is the only organ which is connected to two blood circulation systems. Disseminated tumor cells (DTCs) in the arterial and visceral circulation are drained to the liver by the hepatic artery and the portal vein, respectively. Gastrointestinal cancers (pancreas, colon, stomach) are directly connected to the visceral circulation and show high-liver tropism for metastases. (B) General microanatomy of the liver showing the location of the portal triads [consisting of the hepatic artery (HA), portal vein (PV), bile duct (BD)], the central vein (CV), and the direction of the blood flow across the three different zones (I, II, III). Owing to extensive branching of portal vessels into liver sinusoids, and the accompanying increase of vascularization, the hepatic microcirculation is characterized by low pressure and slow blood flow.
The liver has several unique features which are necessary for its normal physiological functions, but make the liver intrinsically susceptible for blood borne metastasis: (1) the liver is a highly vascularized organ, (2) has an exceptional low-blood flow rate, and (3) the LSEC are highly fenestrated and lack a subendothelial basement membrane making them the most permeable endothelial cells of the mammalian body (Poisson et al. 2017). These organ-specific features not only facilitate the exchange of larger molecules necessary for the blood detoxification, as part of the liver's homeostatic function, but also allow the extravasation of DTC, as shown in quantitative cell-tracking studies in mice (MacDonald et al. 2002).
Whereas the close proximity and direct connection of the gastrointestinal track to the liver might in part explain the high-hepatic metastatic prevalence of gastrointestinal carcinomas, including pancreas and colorectal cancer (Ryan et al. 2014; Chow and Chok 2019), it does not explain the high hepatic metastatic rate for other cancer types such as breast, lung, and uveal melanoma. Thus, there is an emerging interest to better understand the cellular and molecular processes responsible for the high hepatic metastatic rate.
METASTATIC STEPS TO THE LIVER
The final step of cancer progression is the development of distant metastatic tumors, also known as secondary tumor sites. During the metastatic cascade, neoplastic cells need to undergo a series of steps before they are able to generate clinically detectable metastases. To start the metastatic cascade, at the primary site, cancer cells must invade from the confined primary tumor into the adjacent parenchyma and must intravasate into the circulation. Once tumor cells are in the circulation they must survive until they reach a potential secondary site. Survival of tumor cells in the circulating blood depends on their interaction with platelets and platelet-derived factors such as transforming growth factor β (TGFβ) and fibrin that promote a mesenchymal phenotype in the DTCs and protect them from natural killer (NK) cell-mediated elimination (Palumbo et al. 2005; Labelle et al. 2011). In breast cancer, DTCs have also been found within the blood stream in association with neutrophils, which enhanced cell cycle progression of DTCs and increased their metastasis to the lungs (Szczerba et al. 2019). Whether a similar survival mechanism exists for DTCs targeting the liver is currently unknown.
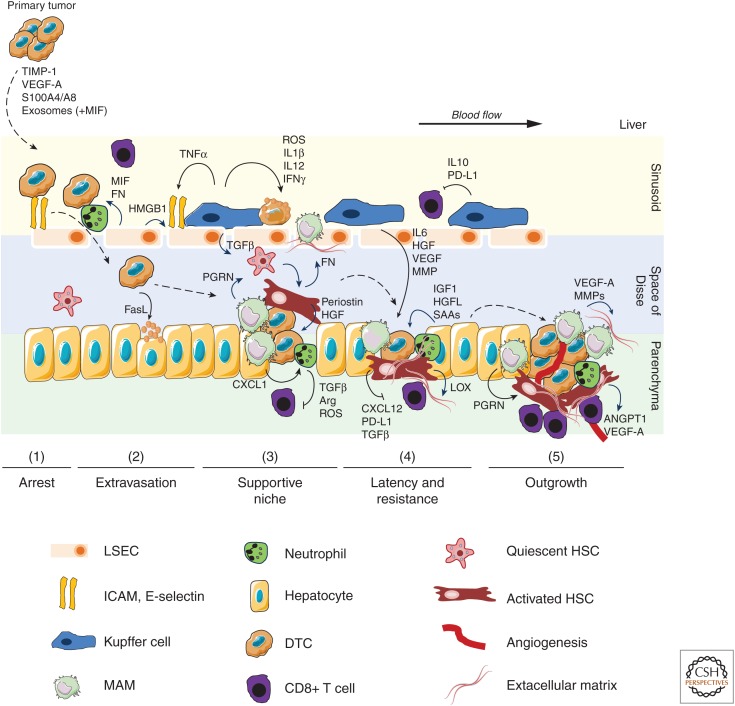
The main rate-limiting step for metastasis formation occurs during the colonization of distant organs (Vanharanta and Massagué 2013). DTC reaching the new microenvironment of the distant organ are vulnerable to immune surveillance and host-tissue defense. In the liver, initial immune surveillance is mediated by tissue resident KCs and NK cells (Heymann and Tacke 2016). In general, on entering the liver via either the hepatic artery or the portal vein, DTCs become arrested and trapped in the sinusoidal capillaries of the liver whereby LSEC play a key function (phase 1). At this stage, DTCs are either able to extravasate or they die. On arrest, DTCs access the perisinusoidal space (space of Disse) by endothelial transmigration (phase 2). The up-regulation of cell adhesion molecules by LSEC promotes the arrest, retention, and transendothelial migration of DTCs. Even after successful extravasation, the vast majority of cancer cells die, but a minority of these cells may remain in the preangiogenic phases in dormancy (phase 3 and 4) or start their metastatic expansion (phase 5) (Fig. 2; Massagué and Obenauf 2016). Noteworthy, the colonization of the hostage environment represents a bottleneck in the metastatic cascade and can be critically facilitated by a fine-tuned bidirectional interaction between cancer cells and the hepatic microenvironment. The hepatic metastatic niche can facilitate the metastatic colonization in many different ways, including protecting of tumor cells from immune surveillance, providing growth and survival signals, and promoting the formation of intratumoral stroma and blood vessels as described in more detail in the following sections.
Figure 2.
Tumor cell interactions with parenchymal and nonparenchymal cells during liver colonization by disseminated tumor cells. The colonization of the liver is a multistep process including arrest (1), extravasation (2), supportive niche formation (3), latency and resistance (4), and finally outgrowth (5). Major intercellular interactions and factors involved in this process are depicted. Primary tumors release factors involved in the generation of a premetastatic niche. On entry of disseminated tumor cells (DTC) into the sinusoidal vessels, DTCs first interact with LSEC and KC. Arrest of DTC is increased by cell adhesion molecules expressed by inflamed LSEC and via neutrophil interaction. Depending on the activation state, KCs release tumoricidal factors, suppress cytotoxic CD8+ T cells, or secrete growth and survival factors for DTCs. On extravasation, tumor secreted FasL induces apoptosis of hepatocytes, which facilitates colonization of the parenchyma. Metastasis-associated macrophages (MAMs), mainly monocyte-derived, rapidly accumulate in high numbers and MAM-released factors, including PGRN, CXCL1, MMPs, TGFβ, and VEGF-A promote the generation of a hospitable hepatic niche. Key events are activation of hepatic stellate cells (HSC), recruitment of immunosuppressive neutrophils, and remodeling of extracellular matrix (ECM). Hepatocyte-derived factors such as IGF1, HGFL, and SAAs contribute to the generation of a supportive niche. The supportive niche also protects neoplastic cells during potential latency and against anticancer therapies. Metastatic expansion and outgrowth requires the formation of new blood vessels (angiogenesis), sustained suppression of an antitumoral immune response, and continuous ECM remodeling. (ARG) arginase, (ANGPT1) angiopoietin 1, (FN) fibronectin, (HGF) hepatocyte growth factor, (HMGB1) high mobility group box 1, (IGF1) insulin-like growth factor 1, (IL) interleukin, (LOX) lysil oxidase, (MIF) macrophage migration inhibitory factor, (MMP) matrix metalloproteinase, (PD-L1) programmed death ligand 1, (PGRN) progranulin, (ROS) reactive oxygen species, (SAA) serum amyloid A1/2, (TGFβ) transforming growth factor β, (TNFα) tumor necrosis factor α, (VEGF-A) vascular endothelial growth factor A.
THE PREMETASTATIC NICHE
A growing body of research has shown that organs of future metastasis are selectively and actively modified by the primary tumor before metastatic spread occurs. Thus, tumors can induce the formation of a susceptible metastatic microenvironment before their arrival at these sites. These microenvironments are termed premetastatic niches (PMN) (Kaplan et al. 2005; Hoshino et al. 2015). Although the dependency of metastasis on these PMN formation remains controversial and difficult to verify in patients, numerous preclinical studies have identified various molecular and cellular changes that occur in the PMN, including the liver, to support future metastatic tumor growth (Psaila and Lyden 2009). Tumor-secrete factors and tumor-shed extracellular vehicles, called exosomes, have been identified to orchestrate step by step the formation of the PMN. Enhanced vascular leakage is the earliest event in this sequence, followed by the recruitment of bone marrow-derived cells and the local activation of resident stroma cells, such as fibroblasts, which all aim to better attract, arrest, and retain DTCs (Joyce and Pollard 2009; Becker et al. 2016). Tumor-derived tissue metallopeptidase 1 (TIMP1) has been linked to PMN formation in the liver in colorectal cancer (CRC). CRC patients showed increased TIMP1 levels, which correlated with liver metastasis. TIMP-1 led to increased stromal-derived factor (SDF) 1α levels, which in turn promoted recruitment of neutrophils to the liver (Seubert et al. 2015). VEGF-A has been identified as another CRC-derived factor linked to hepatic PMN formation. VEGF-A expressed by CRC induces the secretion of CXCL-1 in macrophages, which lead to the accumulation of CXCR2+ MDSC in the liver and the formation of a hepatic PMN (Wang et al. 2017). S100 family proteins A8, A9, and P were also identified to drive liver PMN formation in preclinical CRC models (Zhang et al. 2013; Weidle et al. 2015). More recently, the role of exosomes in hepatic PMN formation attracted major attention. Macrophage migration inhibitory factor-1 (MIF-1) was identified as main cargo of PDAC-derived exosomes, able to induce a hepatic PMN. MIF containing exosomes taken up by KCs, up-regulated their expression of TGFβ which led to the activation of resident HSC. Activated HST formed a fibronectin-rich niche, thereby facilitating the adhesion of DTCs and the infiltration of bone marrow-derived cells via their fibronectin-binding surface receptors α4β7 and α4β1, respectively (Costa-Silva et al. 2015). Interestingly, high plasma exosomal MIF-1 levels were also detected in early stage PDAC patients, suggesting that a PMN could be formed at very early stages of PDAC development. Indeed, an early metastatic spreading during tumor progression has been reported in a genetically engineered mouse model of pancreatic cancer (Rhim et al. 2012). The molecular characterization of pancreatic cancer-derived exosomes found in the circulation of PDAC patients and tumor-bearing mice also provides an explanation of how cancers determine organ tropism. Integrin αvβ5 expression on PDAC-derived exosomes was identified as a key adhesion receptor that specifically binds to KC and is necessary for the update of exosomes into KC, leading to the subsequent formation of the hepatic PMN (Hoshino et al. 2015). In colorectal cancer, microRNA-21-5p-rich exosomes released by tumor cells induce the formation of a proinflammatory, premetastatic niche in the liver by binding to TLR7 on macrophages leading to the release of interleukin 6 (IL-6) (Shao et al. 2018). Although proteoglycan Glypican-1-positive exosomes have been described to specifically accumulate in PDAC patients, their role in hepatic PMN formation has not been reported (Melo et al. 2015). Likewise, the contribution of circulating tumor cells, present early in tumor development and after resection of the primary tumor, to hepatic PMN formation remains unknown (Bork et al. 2015; Tsai et al. 2016).
THE HEPATIC METASTATIC NICHE
Role of Tissue Resident Cells
Liver Sinusoidal Endothelial Cells
DTCs entering through the blood circulation first encounter the liver sinusoidal endothelial cells (LSEC), which cover the luminal side of the sinusoids. LSEC are a heterogeneous cell population (Strauss et al. 2017) that, similarly to the KC, function as scavengers, clearing macromolecular waste molecules from the circulation. LSEC express different scavenger receptors, including stabilin 1 and 2 allowing a high endocytic capacity (Sorensen et al. 2012). LSEC can regulate the arrest and adhesion of DTCs by expressing of cell adhesion molecules, including E-selectin, vascular cell adhesion molecule 1(VCAM-1), and intercellular cell adhesion molecule 1 (ICAM-1). The induction of cell adhesion molecules on LSECs can be triggered by an inflammatory response mediated by KC and NK cells in response to the arriving DTCs. Although this immune response was initially thought to be a tumoricidal response, the inflammatory response and the activation of LSEC facilitates cancer cells adhesion and endothelial transmigration into the presinusoidal space, where cancer cells are protected from KC and NK cells (Glinskii et al. 2005). In pancreatic cancer, IL-35 is highly expressed by cancer cells and induces ICAM-1 expression on LSEC, thereby increasing adhesion of DTC to the endothelial wall and enhancing liver metastasis (Huang et al. 2017). Inhibition of integrin β2 expression, a ligand of ICAM-1, on the C26 CRC cell line led to reduced retention of DTCs in the liver in a preclinical mouse model of colon cancer (Benedicto et al. 2017), whereas blockade of adhesion molecules or inhibition of the inflammatory TNFα/TNFR2 signaling axis reduced CRC liver metastasis (Khatib et al. 2002, 2005; Yoshimoto et al. 2012; Ham et al. 2015). LSEC-mediated activation of Notch signaling also increased metastasis of melanoma and colorectal cancer cells to the liver. In this study, reduced liver metastasis was linked to a reduction of the cell adhesion molecule ICAM-1 expressed by LSEC, resulting in impaired adhesion and retention of tumor cells in sinusoids. Similarly, anti-ICAM-1 antibody treatment significantly reduced tumor cell adhesion to LSEC under normal Notch expression levels suggesting that Notch signaling controls liver metastasis via modulation of adhesion molecule expression on LSEC (Wohlfeil et al. 2019).
The interaction of DTCs with LSECs not only facilitates the extravasation of DTCs into a more protected environment, but also triggers the activation of signaling pathways in cancer cells enhancing their survival and growth potential. Namely, the interaction of ligands sLewA, sLewX, and CD44 isoforms expressed on cancer cells with E-selectin expressed on inflamed LSEC enhances liver metastasis in CRC (Witz 2008; Elliott et al. 2014) and induces the release of the pro-inflammatory signal factor high-mobility group box 1 (HMBG1), thereby further fueling the expression of adhesion molecules on LSEC (Aychek et al. 2008).
LSEC also secrete factors that enhance the metastatic potential of cancer cells. LSEC-derived fibronectin and macrophage migration inhibitory factor (MIF) induced EMT in CRC cells resulting in increased invasion and migration of CRC cells into the liver parenchyma (Ou et al. 2014; Hu et al. 2015).
LSEC are a key component of tumor angiogenesis, which is a critical step in tumor expansion. Although LSEC under homeostatic conditions are fenestrated, fibrosis and the growth of neoplastic cells in the liver can lead to LSEC trans-differentiation with loss of LSEC markers and sinusoidal fenestrae, a process known as capillarization, during which LSECs lose their protective properties and promote angiogenesis and vasoconstriction (Ding et al. 2014; DeLeve 2015). In contrast, non-angiogenic-dependent expansion of metastatic lesions in the liver has also been described (Vermeulen et al. 2001; Stessels et al. 2004). In the liver, tumors cells have been shown to hijack the existing dense vascular network by migrating along LSECs instead of inducing angiogenesis. This mechanism is called vessel cooption and in patients with colorectal cancer liver metastases, vessel cooption is associated with poor response to the antiangiogenic agent bevacizumab (Frentzas et al. 2016)
Taken together, LSEC can play multiple roles in liver metastasis. Although they present a natural barrier for DTCs to access the liver parenchyma, in response to local inflammation, activated LSEC can help DTCs enter the liver. The increased expression of adhesion molecules on LSEC not only helps the DTCs to arrest in the sinusoids, but can also trigger prometastatic functions in cancer cells on ligation of cancer cell-specific receptors. Cooption of the dense LSEC network in the liver further promotes metastatic tumor growth by providing tumors with access to a preexisting blood supply system.
Kupffer Cells
The liver parenchyma is rich in cells of the innate immune system, potentially posing an obstacle to cancer cells. Particularly macrophages are highly abundant. In rodent livers, every 100 hepatocytes are accompanied by 10–20 macrophages (Lopez et al. 2011). These constitutively resident hepatic macrophages are known as Kupffer cells (KC) and are seeded along the LESC (Tacke and Zimmermann 2014). KC originate from fetal liver-derived erythromyeloid progenitors and they rely on self-renewal rather than on infiltrating monocytes for their maintenance (Gomez Perdiguero et al. 2015). Because in human and mouse the expression of KC surface markers largely overlaps with monocyte-derived macrophages and other phagocytes, a combination of several surface markers is needed to identify KCs. Murine KC stain positive for F4/80+, CD11b+/−low, CD68+, and C-type lectin domain family 4-member F (CLEC4F), whereas they are negative for CX3CR1 because of their nonmonocytic origin (Heymann et al. 2015). Human KC are less well characterized and are commonly identified by CD14+, CD68+, TLR4+, CX3CR1neg expression (Krenkel and Tacke 2017).
KCs are highly specialized macrophages and act as a prime sensor for immune surveillance during homeostasis and disease. The constant low-level exposure to bacterial components such as lipopolysaccharide (LPS) renders the cells refractory to LPS stimulation. The tolerogenic potential of KCs in homeostasis is reflected by their constitutive ability to secrete IL-10 on LPS stimulation, as well as by expression of the T-cell inhibitory molecule programmed cell death 1 ligand (PD-L1) and the induction of regulatory T (Treg) cells under homeostatic conditions in vivo (Heymann et al. 2015). In their interaction with invading cancer cells, KCs can play diverse and opposing roles that depend on several factors including the stage of the metastatic process, tumor antigen load, and interactions with other immune cells. KCs can exert cytotoxic activity toward DTCs by releasing oxygen metabolites, phagocyte tumor cells, release cytotoxic cytokines, and secrete proteases (Gardner et al. 1991; Wang et al. 2000; Seki et al. 2011). DTC adhesion to KCs can induce a rapid phagocytosis of tumor cells and their removal from the liver (Bayon et al. 1996). This antitumoral activity of KCs may require the recruitment of other inflammatory cells, such as NK cells, which promote the tumoricidal effect of KCs by secreting inflammatory factors such as granulocyte macrophage colony stimulating factor (GM-CSF) and interferon γ (IFNγ) (Timmers et al. 2004). KC can decrease hepatic metastatic tumor burden of CRC during early stages and this was associated with increased TNFα and IL-1β levels (Khatib et al. 2005; Matsumura et al. 2014). Extensive phagocytosis and elimination of tumor cells is attributed to the first 24 h of tumor cell entry, suggesting that the tumoricidal activity of KC is limited to the early events of metastatic colonization of the liver (Matsumura et al. 2014). Cancer cells, which survive the initial insult of KC can later benefit from the KC tumor promoting functions. The switch is determined predominantly by tumor cell burden. KCs show a capacity for immune-surveillance when tumor cell numbers are low. However, KCs switch to promote liver colonization and metastatic progression when their phagocytic capacity is overwhelmed because of excessive number of DTCs invading the liver (Bayon et al. 1996).
KC can increase the adhesion of DTCs to the endothelium by inducing the expression of vascular endothelial cell adhesion molecules (Khatib et al. 1999, 2002). Moreover, KC can produce a plethora of factors including IL-6, hepatocyte growth factor (HGF), VEGF, and matrix metalloproteinases (MMPs) 9 and 14 that can accelerate tumor invasion into and within the parenchymal space as well as promote tumor cell proliferation and angiogenesis, thereby enhancing liver metastasis.
Hepatic Stellate Cells
In normal liver, hepatic stellate cells (HSC) maintain a quiescent, nonproliferative phenotype and are located in the peri-sinusoidal space (space of Disse), interposed between the basolateral surface of hepatocytes and LSEC. The storage of retinyl esters in cytoplasmic lipid droplets is the most distinctive feature of this cell type and enables their isolation by density gradient centrifugation (Friedman and Roll 1987). HSC can be further characterized by the expression of platelet-derived growth factors receptor (PDGFR)-β, enzyme lecithin retinol acyltransferase (LRAT), the cytoskeletal proteins desmin and glial fibrillary acidic protein (GFAP) (Mederacke et al. 2013, 2015; Tsuchida and Friedman 2017). In response to inflammatory stimuli, triggered by liver damage or the presence of neoplastic cells, quiescent HSC transdifferentiate from vitamin A-storing cells to myofibroblasts, which are proliferative, migratory, and contractile. In addition, activated HSC secrete a variety of chemokines and cytokines, which can shape the immune response and the tumor microenvironment. HSC are characterized by enhanced ECM production, which makes them a major driver for liver fibrosis and cancer-associated desmoplasia (Tsuchida and Friedman 2017).
HSC have been shown to promote the formation of the premetastatic hepatic niche as introduced before (Kaplan et al. 2006). Lyden and colleagues showed that in pancreatic cancer models, resident KC are initially activated by PDAC-derived exosome released by the primary tumor. These exosomes contain MIF, and are taken up by KCs, triggering their activation. Activated KCs release the HSC-activating factor TGFβ, which leads to the transactivation of HSC and increased deposition of fibronectin at the future metastatic site. Lyden and colleagues further showed that a FN-rich hepatic niche facilitates the recruitment of bone-marrow-derived myeloid cells. Most likely, extracellular deposited FN within the perisinusoidal space serves as a docking site for circulating myeloid immune cells, which characteristically express high levels of FN-binding integrins, including α4β1 and αvβ3 (Costa-Silva et al. 2015).
Tissue fibrosis can enhance cancer metastasis by creating a growth-permissive fibrotic microenvironment capable of supporting metastatic growth by enhancing tumor cell survival. Treatment of mice with the fibrosis inducing chemical dimethylnitrosamine (DMN) increased hepatic metastatic frequency of orthotopically implanted 4T1 breast cancer carcinoma cells. The enhanced hepatic spreading was linked to the presence of lysyl oxidase (LOX), secreted by activated (αSMA+) HSCs (Cox et al. 2013). LOX is an extracellular amine oxidase whose primary function is to posttranslationally modify collagens and elastins, which is a critical step in organ fibrosis and in the formation of a desmoplastic tumor stroma (Barker et al. 2012).
In pancreatic cancer metastasis, the transactivation of resident HSC by immune cells has been identified to be critical for efficient hepatic metastatic outgrowth. On initial seeding of the liver by disseminated pancreatic cancer cells, bone marrow-derived inflammatory monocytes are rapidly recruited to the metastatic niche, where they differentiate into metastasis associated macrophages (MAMs). The accumulation of inflammatory monocytes and MAMs preceded the aberrant activation of HSC and the genetic and/or pharmacological targeting of MAMs abolished the formation of a desmoplastic hepatic niche (Nielsen et al. 2016). MAMs promote the activation of resident HSC by secreting progranulin, a glycoprotein and a potent activator of fibroblasts (He et al. 2003; Elkabets et al. 2011). Activated HSC secrete a plethora of ECM proteins, including periostin, which enhances the metastatic outgrowth of disseminated PDAC and CRC cells by increasing cell survival via the AKT pathway (Bao et al. 2004; Nielsen et al. 2016).
A proangiogenic role of HSC was also described. In a metastatic B16 melanoma model, activated HSC secrete VEGF-A and angiopoietin 1 to initiate angiogenesis (Olaso et al. 2003; Taura et al. 2008; Copple et al. 2011). In hepatic CRC metastasis, TIMP-1 is highly expressed in HSC which are in close proximity to CD34+ endothelial cells, suggesting a vascular remodeling function of HSC (Illemann et al. 2016). Isolated HSC secrete laminin and show enhanced endothelial cell network formation in matrigel assays. Coinjection of HSC together with colorectal cancer cells enhanced the metastatic process to the liver by supporting angiogenesis (Eveno et al. 2015). Activated HSCs can also directly promote tumor growth by secreting HGF and TGFβ (Thompson et al. 2015). Less well understood is the function of HSCs in controlling the immune response in liver metastasis. Skin cancer associated fibroblasts regulate the recruitment of immune cells and their functions by releasing cytokines/chemokines (Erez et al. 2010). Activated HSC secrete a plethora of cytokines and chemokines with well-known effects on immune cell recruitment and functions (Nielsen et al. 2016), suggesting that HSC also shape the immune response during liver metastasis. In primary HCC, HSC-derived factors promote immune suppression by promoting the expansion of immunosuppressive regulatory T cells (Tregs) and the induction of myeloid-derived suppressor cells (Zhao et al. 2012; Xu et al. 2016). However, whether or how HSC influence immune cell recruitment during liver metastasis requires further investigation. Myofibroblasts have also been linked to the adaptive immune response, particularly regulating T cell infiltration and survival. In pancreatic cancer and melanoma models, cancer associated fibroblasts at the primary tumor site have been shown to impair CD8+ T cell infiltration (Kraman et al. 2010; Feig et al. 2013) or induce their depletion (Lakins et al. 2018), although the exact mechanism(s) by which pancreatic tumors prevent CD8+ T cell recruitment is not fully understood yet. In regard to the hepatic niche, in liver biopsies of metastatic PDAC patients, CD8+ T cells were found in αSMA+ myofibroblast-rich regions. In a metastatic mouse model of PDAC, activation of HSC led to CD8+ T cell exclusion and resistance to immune checkpoint therapy (αPD-1), which could be reversed by reducing the fibrotic stroma (Quaranta et al. 2018). Taken together, these results suggest that liver fibrosis driven by HSC enhances hepatic metastasis growth and further showcases a bidirectional cross talk between immune cells and HSC in the hepatic metastatic niche.
Hepatocytes
The role of the parenchymal hepatocytes in liver metastasis is less well understood. In CRC, the interaction of tumor cells with hepatocytes increased their metastatic potential. Integrin αv, α6, and β1, desmosomes, as well as osteopontin enhanced the interaction of cancer cells with the hepatocyte ECM (Shimizu et al. 2000; Mook et al. 2008; Huang et al. 2012; Zvibel et al. 2013). The interaction of CRC with ECM induced the induction of gene signatures related to tumor cell survival and stemness, suggesting that tumor cells—hepatocyte ECM interaction promotes the adaptation of DTCs to the hepatic niche. Disseminated CRC expressing Fas ligand (FasL) induce apoptosis of Fas receptor bearing hepatocytes on arrival in the liver. The induction of hepatocyte apoptosis creates a niche in the liver where blood borne DTC can settle and propagate (Li et al. 2009).
Hepatocytes release several growth factors, including insulin-like growth factor 1 (IGF-1) and inhibition of IGF-1 reduces liver metastasis of colon and lung carcinoma cells (Wang et al. 2015). Overexpression of the Ron receptor, a member of the Met family of surface receptor tyrosine kinases, has been reported in several human cancers including breast, pancreas, colon, liver and bladder. Ron is activated by the hepatocyte growth factor-like protein/macrophage stimulating-protein (HGFL) which is primarily secreted by hepatocytes. Activation of RON leads to the induction of signaling pathways related to cellular growth, motility, invasion, and metastasis (Wagh et al. 2008). Hepatocyte-derived heregulin (HRG) phosphorylates erbB3 and erbB2 in CRC cells, promoting integrin αvβ5 depended migration. Depletion of integrin αv or erbB3 reduced liver metastasis of CRC cells (Yoshioka et al. 2010).
Parenchymal hepatocytes promote hepatic metastasis for different cancer types, including pancreatic and colorectal carcinomas, by coordinating the formation of a pro-metastatic niche. In response to primary tumor-derived factors, particularly IL-6, hepatocytes up-regulate the release of serum amyloid A1 and A2 (SAAs), which increases myeloid cell recruitment and liver fibrosis. The secretion of SAAs by hepatocytes occurs in response to IL-6 stimulation and is STAT3 dependent. IL-6 is mainly produced by nonmalignant stroma cells at the distant primary tumor site, including αSMA+ myofibroblasts, but not at the metastatic site, thereby controlling hepatocyte activation in a systemic manner. The identified IL-6–STAT3-SAA signaling axis is responsible for the formation of a pro-metastatic niche in the liver, but not in the lung (Lee et al. 2019). Thus, this study provides an example of how parenchymal cells might steer metastatic organ tropism.
Role of Recruited Inflammatory Cells
Monocyte-Derived Macrophages
In addition to the presence of tissue resident KCs, liver metastasis is accompanied by the accumulation of blood-derived myeloid cells, including monocytes and neutrophils. Monocytes in the circulation can be differentiated into two subsets based on cell surface expression of different markers. Inflammatory monocytes are characterized by Ly6Chigh CX3CR1mid CCR2+ CD62L+ CD43low expression, whereas patrolling monocytes are characterized by Ly6Clow CX3CR1high CCR2− CD62L− CD43high expression (Geissmann et al. 2010). The recruitment of inflammatory monocytes is necessary for efficient hepatic metastasis for several cancer types with liver tropism. In colorectal and Lewis lung carcinoma models, inflammatory monocyte accumulation is mediated by the CCL2/CCR2 axis. Tumor-secreted CCL2 attracts CCR2+ myeloid cells to the liver and genetic and/or pharmacological ablation of CCL2/CCR2 signaling reduced myeloid cells recruitment and metastatic tumor burden. In pancreatic cancer, inhibition of CCR2 reduced primary tumor formation and metastatic spreading to the liver (Mitchem et al. 2013).
On infiltration of the hepatic niche, monocytes differentiate into monocyte-derived macrophages (Nielsen et al. 2016). Macrophages are highly plastic and depending on their activation status, macrophages can execute tumoricidal or protumorigenic functions (Biswas et al. 2013; Mantovani et al. 2017). On infiltration of the hepatic niche, monocytes differentiate into monocyte-derived macrophages (Nielsen et al. 2016). MAMs play a significant role in pancreatic cancer metastasis. During metastatic tumor growth in pancreatic cancer, MAMs rapidly accumulate at the metastatic site and represent the most abundant immune cell population. Bone marrow chimera studies revealed that MAMs mainly originated from bone marrow-derived monocytes (Nielsen et al. 2016). Genetic inhibition of MAM accumulation by depleting PI3Kγ reduced MAM accumulation and PDAC metastasis. Similarly, chemical depletion of macrophages by clodronate containing liposomes or pharmacological targeting of macrophages by anti-CSF-1/anti-CSF-1R ablated MAM numbers and impaired hepatic metastatic growth of disseminated PDAC cells (Nielsen et al. 2016; Quaranta et al. 2018). In regard of MAM activation, smaller, micrometastatic lesions were rich in macrophage expressing proinflammatory markers including COX, NOS2, and MHC class II, whereas established, large metastatic lesions were highly infiltrated by immunosuppressive macrophages, expressing elevated levels of Arginase, Tgfb, and Il10. The phenotype of macrophage polarization directly affected the activation state of metastasis infiltrating CD8+ T cells. Increased abundance of immune-suppressive MAMs reduced CD8+ T cell activation, whereas MAM-targeted therapies restored cytotoxic CD8+ T cell functions (Quaranta et al. 2018). A clinical study showed that high numbers of circulating inflammatory monocytes correlates with shortened survival in pancreatic cancer, a disease which primarily metastasizes to the liver (Sanford et al. 2013). In a preclinical colorectal cancer model, the genetic depletion of the N-myc downstream-regulated gene 2 (NDRG2) reduced metastatic tumor burden, which correlated with an increased percentage of M1-like MAMs within the hepatic metastatic niche. Mechanistically, NDRG2-deficiency induced nuclear factor (NF)-κB pathway activation in macrophages, thereby promoting the expression of M1-like inflammatory cytokines including Il12, Il1b, and Tnfa (Li et al. 2018).
Neutrophils
Neutrophils are part of the innate immune response and are also rapidly recruited to site of tumor formation, including the liver. Similar to macrophages, neutrophils can acquire opposing roles in cancer depending on the environmental context (Fridlender et al. 2009; Shaul and Fridlender 2017). Neutrophils can release cytolytic factors and produce high levels of TNFα, FasL, reactive oxygen species (ROS), thereby executing tumorical activity. In contrast, it has been shown that, particularly in cancer, neutrophils can acquire an immunosuppressive phenotype and express high levels of arginase, MMP-9, and VEGF-A (Fridlender et al. 2009). In mice, independent of their phenotype, neutrophils are identified by their expression of CD11b and Ly6G. The same combination of markers is also used to identify granulocytic myeloid-derived suppressor cells (gMDSC), which are defined by their potent immunosuppressive activity (Talmadge and Gabrilovich 2013; Coffelt et al. 2016). It has recently become apparent that neutrophils represent a heterogeneous population of cells with significant functional plasticity and that immune suppressive functions associated to MDSC might have been performed by immunosuppressive neutrophils sharing the same surface markers and vice versa.
Several factors have been reported to promote the recruitment and accumulation of neutrophils/MDSC to metastatic liver tumors including CXCL1, CXCL2, CXCL5, and SDF-1α (also known as CXCL12), which bind to their cognate receptors CXCR2 or CXCR4 expressed on neutrophils (Zhao et al. 2013; Seubert et al. 2015; Steele et al. 2016). Immunosuppressive functions of neutrophils/MDSC promote liver metastasis. In a pancreatic cancer model, primary and hepatic metastatic tumors are infiltrated by Ly6G+ neutrophils. Neutrophil depletion resulted in increased T cell infiltrating into the pancreas and the liver. Inhibition of neutrophil recruitment by blocking CXCR2 ablated hepatic metastatic spreading (Steele et al. 2016). This study suggests that immunosuppressive neutrophils play an important role in metastatic spreading of pancreatic cancer to the liver, at least during the initial steps of the pre- and/or early metastatic niche formation. In CRC models, neoplastic cell-derived VEGF-A induced the secretion of CXCL1, a CXCR2 ligand for neutrophils/MDSC at the primary tumor site. Elevated CXCL1 levels promoted the accumulation of neutrophils/MDSC in the premetastatic niche that ultimately promoted liver metastasis (Wang et al. 2017). Increased circulating MDSC levels correlate with advanced clinical cancer stage and metastatic tumor burden. Although the analysis included a limited number (n = 56) of patients with different types of cancer (colon, pancreas, gastric, breast among others), patients with extensive metastatic involvement tended to have the highest number of circulating MDSC, suggesting their important role in promoting metastasis (Diaz-Montero et al. 2009).
Neutrophils promote hepatic metastasis independent of their immunosuppressive capacity. In a murine lung carcinoma model, neutrophils promote cancer cell adhesion within the liver sinusoids by providing a docking site for cancer cells to arrest. Intravital microscopy revealed that DTC adhere directly on top of neutrophils arrested on LSEC within the liver sinusoids. Cancer cell–neutrophil interaction was dependent on CD11b expression by neutrophils and ICAM by cancer cells (Spicer et al. 2012). Moreover, neutrophils can arrest DTC in the liver by neutrophil extracellular trap (NET) formation. NETs are extracellular structures composed of chromatin coated with histones, proteases, and granular and cytosolic proteins that help catch and kill microorganisms. In the presence of circulating tumor cells, NET formation in the liver sinusoids results in increased retention of DTC and enhanced tumor cells adhesion, proliferation, migration, and increased liver metastasis (Cools-Lartigue et al. 2013; Tohme et al. 2016).
Tumor promoting functions of neutrophils have been attributed to the release of extracellular matrix-degrading proteinases, including MMP-8, MMP-9, elastases, and cathepsin G, thereby increasing tumor invasion (Sionov et al. 2015), and by promoting angiogenesis through the release of VEGF and Bv8 (Coffelt et al. 2016). However, these studies focused on the TME at the primary tumor site and pulmonary metastatic site, and to understand whether these mechanisms also play a role in the formation of a hepatic metastatic niche requires further investigation (Jablonska et al. 2017).
Role of Liver Infiltrating Lymphocytes
CD8+ T cells and NK cells are the main effector cells of the immune system that kill cancer cells. CD8+ cytotoxic T cells are part of the adaptive immune system and recognize tumor antigens presented in the context of MHC class I and deliver cytolytic factors including granzymes, perforin, and FasL (Hadrup et al. 2013). CD8+ T cells also release the proapoptotic cytokines IFNγ and TNFα to suppress tumor growth (Barth et al. 1991; Detjen et al. 2001). NK cells belong to the innate immune system and do not need MHC class I mediated antigen presentation to recognize and kill cancer cells. Similar to CD8+ T cells, NK deliver cytotoxic hits to cancer cells through the release of perforin and granzymes (Lowry and Zehring 2017). The inhibition and evasion of a specific tumoricidal T cell and/or NK cell mediated immune response is critical for neoplastic cells to survive and grow in the liver. In contrast, the infiltration of other lymphocytes, namely regulatory T cells which suppress effector T cells, favors liver metastasis. Tregs are a subset of immunosuppressive CD4+ T cells and are defined by their expression of the FoxP3 transcription factor. Although immunosuppressive Tregs have a critical role during tumor development and in response to therapy (Takeuchi and Nishikawa 2016; Oweida et al. 2018; Shabaneh et al. 2018), their role in the formation of a hepatic metastatic niche is less well understood. Efficient liver metastasis of colon and lung carcinoma correlates with not only the increase of MDSC, but also with the recruitment and accumulation of CD4+ FoxP3+ Tregs. In this report, the recruitment of Tregs was dependent on TNFR2 signaling, because TNFR2 depleted animals showed a marked decrease in Treg accumulation (Ham et al. 2015). In a retrospective analysis for FoxP3+ Treg and CD8+ cytotoxic T cell infiltration, using tissue sections from resected colorectal cancer liver metastases, a high ratio of FoxP3+: CD8+ cells correlated with shorter overall survival of patients after surgery (Katz et al. 2013), suggesting a potential pro-metastatic role for Tregs at the hepatic niche.
CANCER CELL INTRINSIC GENETIC PROGRAMS DRIVING LIVER METASTASIS
Unlike the deep understanding of mutational mechanisms that initiate cancer progression, the genetic basis for liver metastasis is less well understood. General metastasis promoting transcriptional programs regulate pathways including self-renewal and EMT (Kong et al. 2011; Neureiter et al. 2014; Krebs et al. 2017). A few studies have focused on cell intrinsic programs that specifically drive liver metastasis. Pro-metastatic programs driving liver metastasis have been identified in cancer from the gastrointestinal tract. In CRC, miR-551a and miR-483 suppress liver colonization and metastasis by inhibiting creatine kinase, brain-type (CKB) in CRC cells. The release of CKB by metastasized cancer cells generate phosphocreatine in the extracellular space, which was imported back into CRC cells and used for ATP generation and thereby enhanced metastatic survival (Loo et al. 2015). In pancreatic cancer, the expression of the pioneer factor FOX1 increases anchorage-independent growth of PDAC cells in vitro, and invasion and liver metastasis in vivo (Roe et al. 2017). Claudin-2 has been shown to mediate breast cancer liver metastasis and was identified to be specifically expressed by liver-metastatic breast cancer cells compared with populations derived from bone or lung metastasis. The extracellular loop of claudin-2 mediated tumor-hepatocyte interaction and thereby increased metastatic tropism to the liver (Tabaries et al. 2012).
CONCLUDING REMARKS
The papers discussed here, and many other reports that because of word limitation we could unfortunately not cite, provide evidence that the hepatic metastatic niche is critical for promoting liver metastases and that inhibition of key effector proteins and/or cells within the hepatic niche could open new avenues for better therapies against liver metastasis. Inhibition of the various prosurvival cues provided by the hepatic environment could improve the response to anticancer treatments. However, a major challenge that requires further investigation is that the type of interactions DTC establish with the different components of the hepatic niche constantly change during the metastatic steps. This might explain why targeting specific cellular interactions and effectors in the TME have markedly reduced, but not yet completely eradicated, metastatic disease progression.
Although it has been shown that stromal cells promote liver metastasis, an additional layer of complexity that also deserves further investigation is their potential heterogeneity and their distinct contribution to the formation (or inhibition) of a hepatic niche. However, the recent technical advances in single cell analysis now allows to deconvolute the complex structure of the hepatic niche to a single cell level and such studies will most likely shed light on the cellular heterogeneity of hepatic metastatic lesions.
The cellular and molecular structure of the hepatic metastatic niche is not only forged by DTC, but is also influenced by cues from the primary tumor site. The importance of the cross-talk between the primary and secondary tumor site has been clearly shown by the importance of the premetastatic niche formation in the metastatic process. However, whether and how the genomic profile of cancer cells orchestrates the formation of a cancer cell “personalized” hepatic niche is a question that remains to be addressed. Thus, to better understand how the hepatic niche supports metastasis, future studies should use preclinical (mouse) models that faithfully recapitulate the primary–secondary tumor interaction, and also provide the diversity of all stromal/immune partners. Based on the overall literature herein presented, we would like to reinforce the importance of further investigating the hepatic niche and help translate the exciting preclinical observations into the clinics.
COMPETING INTEREST STATEMENT
The authors declare no potential conflicts of interest.
ACKNOWLEDGMENTS
We thank all investigators who, because of space limitations, despite not having their studies cited in this particular review have contributed to this field of research. This work was made possible thanks to the support from Cancer Research UK (M.C.S., grant number C48719/A25607) and Medical Research Council (M.C.S., grant number MR/P018920/1) and the Wellcome Trust and Royal Society (A.M., grant number 102521/Z/13/Z).
Footnotes
Editors: Jeffrey W. Pollard and Yibin Kang
Additional Perspectives on Metastasis: Mechanism to Therapy available at www.perspectivesinmedicine.org
REFERENCES
- Amaro A, Gangemi R, Piaggio F, Angelini G, Barisione G, Ferrini S, Pfeffer U. 2017. The biology of uveal melanoma. Cancer Metastasis Rev 36: 109–140. 10.1007/s10555-017-9663-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aychek T, Miller K, Sagi-Assif O, Levy-Nissenbaum O, Israeli-Amit M, Pasmanik-Chor M, Jacob-Hirsch J, Amariglio N, Rechavi G, Witz IP. 2008. E-selectin regulates gene expression in metastatic colorectal carcinoma cells and enhances HMGB1 release. Int J Cancer 123: 1741–1750. 10.1002/ijc.23375 [DOI] [PubMed] [Google Scholar]
- Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. 2004. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 5: 329–339. 10.1016/S1535-6108(04)00081-9 [DOI] [PubMed] [Google Scholar]
- Barker HE, Cox TR, Erler JT. 2012. The rationale for targeting the LOX family in cancer. Nat Rev Cancer 12: 540–552. 10.1038/nrc3319 [DOI] [PubMed] [Google Scholar]
- Barth RJ Jr, Mule JJ, Spiess PJ, Rosenberg SA. 1991. Interferon γ and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor-infiltrating lymphocytes. J Exp Med 173: 647–658. 10.1084/jem.173.3.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayon LG, Izquierdo MA, Sirovich I, van Rooijen N, Beelen RH, Meijer S. 1996. Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology 23: 1224–1231. 10.1002/hep.510230542 [DOI] [PubMed] [Google Scholar]
- Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. 2016. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30: 836–848. 10.1016/j.ccell.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedicto A, Marquez J, Herrero A, Olaso E, Kolaczkowska E, Arteta B. 2017. Decreased expression of the β2 integrin on tumor cells is associated with a reduction in liver metastasis of colorectal cancer in mice. BMC Cancer 17: 827 10.1186/s12885-017-3823-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Allavena P, Mantovani A. 2013. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol 35: 585–600. 10.1007/s00281-013-0367-7 [DOI] [PubMed] [Google Scholar]
- Bork U, Rahbari NN, Schölch S, Reissfelder C, Kahlert C, Büchler MW, Weitz J, Koch M. 2015. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer 112: 1306–1313. 10.1038/bjc.2015.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch FX, Ribes J, Díaz M, Clèries R. 2004. Primary liver cancer: worldwide incidence and trends. Gastroenterology 127: S5–S16. 10.1053/j.gastro.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Budczies J, von Winterfeld M, Klauschen F, Bockmayr M, Lennerz JK, Denkert C, Wolf T, Warth A, Dietel M, Anagnostopoulos I, et al. 2015. The landscape of metastatic progression patterns across major human cancers. Oncotarget 6: 570–583. 10.18632/oncotarget.2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA. 2011. A perspective on cancer cell metastasis. Science 331: 1559–1564. 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. 2002. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2: 563–572. 10.1038/nrc865 [DOI] [PubMed] [Google Scholar]
- Chow FC, Chok KS. 2019. Colorectal liver metastases: an update on multidisciplinary approach. World J Hepatol 11: 150–172. 10.4254/wjh.v11.i2.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Wellenstein MD, de Visser KE. 2016. Neutrophils in cancer: neutral no more. Nat Rev Cancer 16: 431–446. 10.1038/nrc.2016.52 [DOI] [PubMed] [Google Scholar]
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. 2013. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 123: 3446–3458. 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple BL, Bai S, Burgoon LD, Moon JO. 2011. Hypoxia-inducible factor-1α regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver Int 31: 230–244. 10.1111/j.1478-3231.2010.02347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. 2015. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17: 816–826. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT. 2013. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 73: 1721–1732. 10.1158/0008-5472.CAN-12-2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeve LD. 2015. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology 61: 1740–1746. 10.1002/hep.27376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detjen KM, Farwig K, Welzel M, Wiedenmann B, Rosewicz S. 2001. Interferon γ inhibits growth of human pancreatic carcinoma cells via caspase-1 dependent induction of apoptosis. Gut 49: 251–262. 10.1136/gut.49.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. 2009. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58: 49–59. 10.1007/s00262-008-0523-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. 2014. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505: 97–102. 10.1038/nature12681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabets M, Gifford AM, Scheel C, Nilsson B, Reinhardt F, Bray MA, Carpenter AE, Jirstrom K, Magnusson K, Ebert BL, et al. 2011. Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J Clin Invest 121: 784–799. 10.1172/JCI43757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott VA, Rychahou P, Zaytseva YY, Evers BM. 2014. Activation of c-Met and upregulation of CD44 expression are associated with the metastatic phenotype in the colorectal cancer liver metastasis model. PLoS ONE 9: e97432 10.1371/journal.pone.0097432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D. 2010. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17: 135–147. 10.1016/j.ccr.2009.12.041 [DOI] [PubMed] [Google Scholar]
- Eveno C, Hainaud P, Rampanou A, Bonnin P, Bakhouche S, Dupuy E, Contreres JO, Pocard M. 2015. Proof of prometastatic niche induction by hepatic stellate cells. J Surg Res 194: 496–504. 10.1016/j.jss.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. 2013. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci 110: 20212–20217. 10.1073/pnas.1320318110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, Nathan M, Wotherspoon A, Gao ZH, Shi Y, et al. 2016. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med 22: 1294–1302. 10.1038/nm.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. 2009. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16: 183–194. 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Roll FJ. 1987. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem 161: 207–218. 10.1016/0003-2697(87)90673-7 [DOI] [PubMed] [Google Scholar]
- Gardner CR, Wasserman AJ, Laskin DL. 1991. Liver macrophage-mediated cytotoxicity toward mastocytoma cells involves phagocytosis of tumor targets. Hepatology 14: 318–324. 10.1002/hep.1840140219 [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. 2010. Development of monocytes, macrophages, and dendritic cells. Science 327: 656–661. 10.1126/science.1178331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert HA, Kagan AR, Hintz BL, Rao AR, Nussbaum H. 1982. Patterns of metastases. In Liver metastases (ed. Weiss LHG), pp. 19–39, GK Hall Medical Publishers, Boston. [Google Scholar]
- Glinskii OV, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. 2005. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia 7: 522–527. 10.1593/neo.04646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, et al. 2015. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518: 547–551. 10.1038/nature13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup S, Donia M, Thor Straten P. 2013. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron 6: 123–133. 10.1007/s12307-012-0127-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham B, Wang N, D'Costa Z, Fernandez MC, Bourdeau F, Auguste P, Illemann M, Eefsen RL, Hoyer-Hansen G, Vainer B, et al. 2015. TNF receptor-2 facilitates an immunosuppressive microenvironment in the liver to promote the colonization and growth of hepatic metastases. Cancer Res 75: 5235–5247. 10.1158/0008-5472.CAN-14-3173 [DOI] [PubMed] [Google Scholar]
- He Z, Ong CH, Halper J, Bateman A. 2003. Progranulin is a mediator of the wound response. Nat Med 9: 225–229. 10.1038/nm816 [DOI] [PubMed] [Google Scholar]
- Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. 2006. Metastatic patterns in adenocarcinoma. Cancer 106: 1624–1633. 10.1002/cncr.21778 [DOI] [PubMed] [Google Scholar]
- Heymann F, Tacke F. 2016. Immunology in the liver—from homeostasis to disease. Nat Rev Gastroenterol Hepatol 13: 88–110. 10.1038/nrgastro.2015.200 [DOI] [PubMed] [Google Scholar]
- Heymann F, Peusquens J, Ludwig-Portugall I, Kohlhepp M, Ergen C, Niemietz P, Martin C, van Rooijen N, Ochando JC, Randolph GJ, et al. 2015. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 62: 279–291. 10.1002/hep.27793 [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. 2015. Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CT, Guo LL, Feng N, Zhang L, Zhou N, Ma LL, Shen L, Tong GH, Yan QW, Zhu SJ, et al. 2015. MIF, secreted by human hepatic sinusoidal endothelial cells, promotes chemotaxis and outgrowth of colorectal cancer in liver prometastasis. Oncotarget 6: 22410–22423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Pan C, Hu H, Zheng S, Ding L. 2012. Osteopontin-enhanced hepatic metastasis of colorectal cancer cells. PLoS ONE 7: e47901 10.1371/journal.pone.0047901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Li N, Li Z, Chang A, Chen Y, Zhao T, Li Y, Wang X, Zhang W, Wang Z, et al. 2017. Tumour-derived interleukin 35 promotes pancreatic ductal adenocarcinoma cell extravasation and metastasis by inducing ICAM1 expression. Nat Commun 8: 14035 10.1038/ncomms14035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illemann M, Eefsen RH, Bird NC, Majeed A, Osterlind K, Laerum OD, Alpizar-Alpizar W, Lund IK, Hoyer-Hansen G. 2016. Tissue inhibitor of matrix metalloproteinase-1 expression in colorectal cancer liver metastases is associated with vascular structures. Mol Carcinog 55: 193–208. 10.1002/mc.22269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska J, Lang S, Sionov RV, Granot Z. 2017. The regulation of pre-metastatic niche formation by neutrophils. Oncotarget 8: 112132–112144. 10.18632/oncotarget.22792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. 2009. Microenvironmental regulation of metastasis. Nat Rev Cancer 9: 239–252. 10.1038/nrc2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. 2005. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438: 820–827. 10.1038/nature04186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Rafii S, Lyden D. 2006. Preparing the “soil”: the premetastatic niche. Cancer Res 66: 11089–11093. 10.1158/0008-5472.CAN-06-2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz SC, Bamboat ZM, Maker AV, Shia J, Pillarisetty VG, Yopp AC, Hedvat CV, Gonen M, Jarnagin WR, Fong Y, et al. 2013. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann Surg Oncol 20: 946–955. 10.1245/s10434-012-2668-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib AM, Kontogiannea M, Fallavollita L, Jamison B, Meterissian S, Brodt P. 1999. Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells. Cancer Res 59: 1356–1361. [PubMed] [Google Scholar]
- Khatib AM, Fallavollita L, Wancewicz EV, Monia BP, Brodt P. 2002. Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res 62: 5393–5398. [PubMed] [Google Scholar]
- Khatib AM, Auguste P, Fallavollita L, Wang N, Samani A, Kontogiannea M, Meterissian S, Brodt P. 2005. Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am J Pathol 167: 749–759. 10.1016/S0002-9440(10)62048-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW. 2015. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med 212: 1043–1059. 10.1084/jem.20141836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec Z. 2001. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol 161: III–XIII, 1–151. [DOI] [PubMed] [Google Scholar]
- Kong SL, Li G, Loh SL, Sung WK, Liu ET. 2011. Cellular reprogramming by the conjoint action of ERα, FOXA1, and GATA3 to a ligand-inducible growth state. Mol Syst Biol 7: 526 10.1038/msb.2011.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. 2010. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science 330: 827–830. 10.1126/science.1195300 [DOI] [PubMed] [Google Scholar]
- Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, et al. 2017. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol 19: 518–529. 10.1038/ncb3513 [DOI] [PubMed] [Google Scholar]
- Krenkel O, Tacke F. 2017. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol 17: 306–321. 10.1038/nri.2017.11 [DOI] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. 2011. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20: 576–590. 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. 2018. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T cells to protect tumour cells. Nat Commun 9: 948 10.1038/s41467-018-03347-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, Delman D, Graham K, Gladney WL, Hua X, et al. 2019. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 567: 249–252. 10.1038/s41586-019-1004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Fan X, Stoicov C, Liu JH, Zubair S, Tsai E, Ste Marie R, Wang TC, Lyle S, Kurt-Jones E, et al. 2009. Human and mouse colon cancer utilizes CD95 signaling for local growth and metastatic spread to liver. Gastroenterology 137: 934–944. 944 e931–e934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Lai X, Zhao Y, Zhang Y, Li M, Li D, Kong J, Zhang Y, Jing P, Li H, et al. 2018. Loss of NDRG2 in liver microenvironment inhibits cancer liver metastasis by regulating tumor associate macrophages polarization. Cell Death Dis 9: 248 10.1038/s41419-018-0284-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo JM, Scherl A, Nguyen A, Man FY, Weinberg E, Zeng Z, Saltz L, Paty PB, Tavazoie SF. 2015. Extracellular metabolic energetics can promote cancer progression. Cell 160: 393–406. 10.1016/j.cell.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez BG, Tsai MS, Baratta JL, Longmuir KJ, Robertson RT. 2011. Characterization of Kupffer cells in livers of developing mice. Comp Hepatol 10: 2 10.1186/1476-5926-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry LE, Zehring WA. 2017. Potentiation of natural killer cells for cancer immunotherapy: a review of literature. Front Immunol 8: 1061 10.3389/fimmu.2017.01061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede BJ, Haffty BG, et al. 2011. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 20: 701–714. 10.1016/j.ccr.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald IC, Groom AC, Chambers AF. 2002. Cancer spread and micrometastasis development: Quantitative approaches for in vivo models. Bioessays 24: 885–893. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. 2017. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14: 399–416. 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Obenauf AC. 2016. Metastatic colonization by circulating tumour cells. Nature 529: 298–306. 10.1038/nature17038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Kondo T, Ogawa K, Tamura T, Fukunaga K, Murata S, Ohkohchi N. 2014. Kupffer cells decrease metastasis of colon cancer cells to the liver in the early stage. Int J Oncol 45: 2303–2310. 10.3892/ijo.2014.2662 [DOI] [PubMed] [Google Scholar]
- Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. 2013. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 4: 2823 10.1038/ncomms3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederacke I, Dapito DH, Affò S, Uchinami H, Schwabe RF. 2015. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat Protoc 10: 305–315. 10.1038/nprot.2015.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. 2015. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523: 177–182. 10.1038/nature14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, Ponomarev V, Gerald WL, Blasberg R, Massagué J. 2005. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest 115: 44–55. 10.1172/JCI22320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, et al. 2013. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 73: 1128–1141. 10.1158/0008-5472.CAN-12-2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook OR, van Marle J, Jonges R, Vreeling-Sindelarova H, Frederiks WM, Van Noorden CJ. 2008. Interactions between colon cancer cells and hepatocytes in rats in relation to metastasis. J Cell Mol Med 12: 2052–2061. 10.1111/j.1582-4934.2008.00242.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neureiter D, Jager T, Ocker M, Kiesslich T. 2014. Epigenetics and pancreatic cancer: pathophysiology and novel treatment aspects. World J Gastroenterol 20: 7830–7848. 10.3748/wjg.v20.i24.7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, et al. 2016. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol 18: 549–560. 10.1038/ncb3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaso E, Salado C, Egilegor E, Gutierrez V, Santisteban A, Sancho-Bru P, Friedman SL, Vidal-Vanaclocha F. 2003. Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology 37: 674–685. 10.1053/jhep.2003.50068 [DOI] [PubMed] [Google Scholar]
- Ou J, Peng Y, Deng J, Miao H, Zhou J, Zha L, Zhou R, Yu L, Shi H, Liang H. 2014. Endothelial cell-derived fibronectin extra domain A promotes colorectal cancer metastasis via inducing epithelial-mesenchymal transition. Carcinogenesis 35: 1661–1670. 10.1093/carcin/bgu090 [DOI] [PubMed] [Google Scholar]
- Oweida A, Hararah MK, Phan A, Binder D, Bhatia S, Lennon S, Bukkapatnam S, Van Court B, Uyanga N, Darragh L, et al. 2018. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res 24: 5368–5380. 10.1158/1078-0432.CCR-18-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. 1989. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8: 98–101. [PubMed] [Google Scholar]
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. 2005. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105: 178–185. 10.1182/blood-2004-06-2272 [DOI] [PubMed] [Google Scholar]
- Pickren JW, Tsukada Y, Lane WW. 1982. Liver metastasis: analysis of autopsy data. In Liver metastasis (ed. Weiss L, Gilbert HA), pp. 2–18, GK Hall Medical Publishers, Boston. [Google Scholar]
- Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou PE. 2017. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol 66: 212–227. 10.1016/j.jhep.2016.07.009 [DOI] [PubMed] [Google Scholar]
- Psaila B, Lyden D. 2009. The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9: 285–293. 10.1038/nrc2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. 2011. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475: 222–225. 10.1038/nature10138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta V, Rainer C, Nielsen SR, Raymant ML, Ahmed MS, Engle DD, Taylor A, Murray T, Campbell F, Palmer DH, et al. 2018. Macrophage-derived granulin drives resistance to immune checkpoint inhibition in metastatic pancreatic cancer. Cancer Res 78: 4253–4269. 10.1158/0008-5472.CAN-17-3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, et al. 2012. EMT and dissemination precede pancreatic tumor formation. Cell 148: 349–361. 10.1016/j.cell.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe JS, Hwang CI, Somerville TDD, Milazzo JP, Lee EJ, Da Silva B, Maiorino L, Tiriac H, Young CM, Miyabayashi K, et al. 2017. Enhancer reprogramming promotes pancreatic cancer metastasis. Cell 170: 875–888.e20. 10.1016/j.cell.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DP, Hong TS, Bardeesy N. 2014. Pancreatic adenocarcinoma. N Engl J Med 371: 2140–2141. [DOI] [PubMed] [Google Scholar]
- Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, et al. 2013. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 19: 3404–3415. 10.1158/1078-0432.CCR-13-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, Nakashima H, Nakashima M, Kinoshita M. 2011. Antitumor immunity produced by the liver Kupffer cells, NK cells, NKT cells, and CD8+ CD122+ T cells. Clin Dev Immunol 2011: 868345 10.1155/2011/868345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert B, Grunwald B, Kobuch J, Cui H, Schelter F, Schaten S, Siveke JT, Lim NH, Nagase H, Simonavicius N, et al. 2015. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 61: 238–248. 10.1002/hep.27378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ, et al. 2014. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol 16: 876–888. 10.1038/ncb3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabaneh TB, Molodtsov AK, Steinberg SM, Zhang P, Torres GM, Mohamed GA, Boni A, Curiel TJ, Angeles CV, Turk MJ. 2018. Oncogenic BRAFV600E governs regulatory T-cell recruitment during melanoma tumorigenesis. Cancer Res 78: 5038–5049. 10.1158/0008-5472.CAN-18-0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Chen T, Zheng X, Yang S, Xu K, Chen X, Xu F, Wang L, Shen Y, Wang T, et al. 2018. Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis 39: 1368–1379. 10.1093/carcin/bgy115 [DOI] [PubMed] [Google Scholar]
- Shaul ME, Fridlender ZG. 2017. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol 102: 343–349. 10.1189/jlb.5MR1216-508R [DOI] [PubMed] [Google Scholar]
- Shimizu S, Yamada N, Sawada T, Ikeda K, Nakatani K, Seki S, Kaneda K, Hirakawa K. 2000. Ultrastructure of early phase hepatic metastasis of human colon carcinoma cells with special reference to desmosomal junctions with hepatocytes. Pathol Int 50: 953–959. 10.1046/j.1440-1827.2000.01153.x [DOI] [PubMed] [Google Scholar]
- Sionov RV, Fridlender ZG, Granot Z. 2015. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron 8: 125–158. 10.1007/s12307-014-0147-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen KK, McCourt P, Berg T, Crossley C, Le Couteur D, Wake K, Smedsrod B. 2012. The scavenger endothelial cell: a new player in homeostasis and immunity. Am J Physiol Regul Integr Comp Physiol 303: R1217–R1230. 10.1152/ajpregu.00686.2011 [DOI] [PubMed] [Google Scholar]
- Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, Ferri LE. 2012. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 72: 3919–3927. 10.1158/0008-5472.CAN-11-2393 [DOI] [PubMed] [Google Scholar]
- Steele CW, Karim SA, Leach JD, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, et al. 2016. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 29: 832–845. 10.1016/j.ccell.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stessels F, Van den Eynden G, Van der Auwera I, Salgado R, Van den Heuvel E, Harris AL, Jackson DG, Colpaert CG, Van Marck EA, Dirix LY, et al. 2004. Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer 90: 1429–1436. 10.1038/sj.bjc.6601727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O, Phillips A, Ruggiero K, Bartlett A, Dunbar PR. 2017. Immunofluorescence identifies distinct subsets of endothelial cells in the human liver. Sci Rep 7: 44356 10.1038/srep44356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. 2019. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566: 553–557. 10.1038/s41586-019-0915-y [DOI] [PubMed] [Google Scholar]
- Tabaries S, Dupuy F, Dong Z, Monast A, Annis MG, Spicer J, Ferri LE, Omeroglu A, Basik M, Amir E, et al. 2012. Claudin-2 promotes breast cancer liver metastasis by facilitating tumor cell interactions with hepatocytes. Mol Cell Biol 32: 2979–2991. 10.1128/MCB.00299-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Zimmermann HW. 2014. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 60: 1090–1096. 10.1016/j.jhep.2013.12.025 [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Nishikawa H. 2016. Roles of regulatory T cells in cancer immunity. Int Immunol 28: 401–409. 10.1093/intimm/dxw025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE, Gabrilovich DI. 2013. History of myeloid-derived suppressor cells. Nat Rev Cancer 13: 739–752. 10.1038/nrc3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, Kodama Y, Miura K, Ikai I, Uemoto S, et al. 2008. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology 135: 1729–1738. 10.1053/j.gastro.2008.07.065 [DOI] [PubMed] [Google Scholar]
- Thompson AI, Conroy KP, Henderson NC. 2015. Hepatic stellate cells: central modulators of hepatic carcinogenesis. BMC Gastroenterol 15: 63 10.1186/s12876-015-0291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers M, Vekemans K, Vermijlen D, Asosingh K, Kuppen P, Bouwens L, Wisse E, Braet F. 2004. Interactions between rat colon carcinoma cells and Kupffer cells during the onset of hepatic metastasis. Int J Cancer 112: 793–802. 10.1002/ijc.20481 [DOI] [PubMed] [Google Scholar]
- Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H, Tsung A. 2016. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res 76: 1367–1380. 10.1158/0008-5472.CAN-15-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WS, Chen JS, Shao HJ, Wu JC, Lai JM, Lu SH, Hung TF, Chiu YC, You JF, Hsieh PS, et al. 2016. Circulating tumor cell count correlates with colorectal neoplasm progression and is a prognostic marker for distant metastasis in non-metastatic patients. Sci Rep 6: 24517 10.1038/srep24517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, Friedman SL. 2017. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 14: 397–411. 10.1038/nrgastro.2017.38 [DOI] [PubMed] [Google Scholar]
- Vanharanta S, Massagué J. 2013. Origins of metastatic traits. Cancer Cell 24: 410–421. 10.1016/j.ccr.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen PB, Colpaert C, Salgado R, Royers R, Hellemans H, Van Den Heuvel E, Goovaerts G, Dirix LY, Van Marck E. 2001. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol 195: 336–342. 10.1002/path.966 [DOI] [PubMed] [Google Scholar]
- Wagh PK, Peace BE, Waltz SE. 2008. Met-related receptor tyrosine kinase Ron in tumor growth and metastasis. Adv Cancer Res 100: 1–33. 10.1016/S0065-230X(08)00001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, McIntosh AR, Hasinoff BB, Rector ES, Ahmed N, Nance DM, Orr FW. 2000. B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res 60: 5862–5869. [PubMed] [Google Scholar]
- Wang N, Rayes RF, Elahi SM, Lu Y, Hancock MA, Massie B, Rowe GE, Aomari H, Hossain S, Durocher Y, et al. 2015. The IGF-trap: novel inhibitor of carcinoma growth and metastasis. Mol Cancer Ther 14: 982–993. 10.1158/1535-7163.MCT-14-0751 [DOI] [PubMed] [Google Scholar]
- Wang D, Sun H, Wei J, Cen B, DuBois RN. 2017. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res 77: 3655–3665. 10.1158/0008-5472.CAN-16-3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidle UH, Birzele F, Krüger A. 2015. Molecular targets and pathways involved in liver metastasis of colorectal cancer. Clin Exp Metastasis 32: 623–635. 10.1007/s10585-015-9732-3 [DOI] [PubMed] [Google Scholar]
- Witz IP. 2008. The selectin-selectin ligand axis in tumor progression. Cancer Metastasis Rev 27: 19–30. 10.1007/s10555-007-9101-z [DOI] [PubMed] [Google Scholar]
- Wohlfeil SA, Häfele V, Dietsch B, Schledzewski K, Winkler M, Zierow J, Leibing T, Mohammadi MM, Heineke J, Sticht C, et al. 2019. Hepatic endothelial notch activation protects against liver metastasis by regulating endothelial-tumor cell adhesion independent of angiocrine signaling. Cancer Res 79: 598–610. 10.1158/0008-5472.CAN-18-1752 [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhao W, Xu J, Li J, Hong Z, Yin Z, Wang X. 2016. Activated hepatic stellate cells promote liver cancer by induction of myeloid-derived suppressor cells through cyclooxygenase-2. Oncotarget 7: 8866–8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K, Tajima H, Ohta T, Okamoto K, Sakai S, Kinoshita J, Furukawa H, Makino I, Hayashi H, Nakamura K, et al. 2012. Increased E-selectin in hepatic ischemia-reperfusion injury mediates liver metastasis of pancreatic cancer. Oncol Rep 28: 791–796. 10.3892/or.2012.1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T, Nishikawa Y, Ito R, Kawamata M, Doi Y, Yamamoto Y, Yoshida M, Omori Y, Kotanagi H, Masuko T, et al. 2010. Significance of integrin αvβ5 and erbB3 in enhanced cell migration and liver metastasis of colon carcinomas stimulated by hepatocyte-derived heregulin. Cancer Sci 101: 2011–2018. 10.1111/j.1349-7006.2010.01640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Davis C, Ryan J, Janney C, Peña MM. 2013. Development and characterization of a reliable mouse model of colorectal cancer metastasis to the liver. Clin Exp Metastasis 30: 903–918. 10.1007/s10585-013-9591-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Su W, Kuang P, Zhang L, Liu J, Yin Z, Wang X. 2012. The role of hepatic stellate cells in the regulation of T-cell function and the promotion of hepatocellular carcinoma. Int J Oncol 41: 457–464. 10.3892/ijo.2012.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier TT, Im JH, Cao Y, Beech J, Allen D, Smart S, Muschel RJ. 2013. Recruitment of a myeloid cell subset (CD11b/Gr1mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 57: 829–839. 10.1002/hep.26094 [DOI] [PubMed] [Google Scholar]
- Zvibel I, Wagner A, Pasmanik-Chor M, Varol C, Oron-Karni V, Santo EM, Halpern Z, Kariv R. 2013. Transcriptional profiling identifies genes induced by hepatocyte-derived extracellular matrix in metastatic human colorectal cancer cell lines. Clin Exp Metastasis 30: 189–200. 10.1007/s10585-012-9527-8 [DOI] [PubMed] [Google Scholar]