Abstract
Rhythmic increases in intracellular Ca2+ concentration underlie the contractile function of the heart. These heart muscle-wide changes in intracellular Ca2+ are induced and coordinated by electrical depolarization of the cardiomyocyte sarcolemma by the action potential. Originating at the sinoatrial node, conduction of this electrical signal throughout the heart ensures synchronization of individual myocytes into an effective cardiac pump. Ca2+ signaling pathways also regulate gene expression and cardiomyocyte growth during development and in pathology. These fundamental roles of Ca2+ in the heart are illustrated by the prevalence of altered Ca2+ homeostasis in cardiovascular diseases. Indeed, heart failure (an inability of the heart to support hemodynamic needs), rhythmic disturbances, and inappropriate cardiac growth all share an involvement of altered Ca2+ handling. The prevalence of these pathologies, contributing to a third of all deaths in the developed world as well as to substantial morbidity makes understanding the mechanisms of Ca2+ handling and dysregulation in cardiomyocytes of great importance.
THE PHYSIOLOGY OF CALCIUM SIGNALING IN CARDIOMYOCYTES
The Heart and Circulation
The pump function of the heart is mediated by the synchronous contraction of its constituent muscle cells, the cardiomyocytes. Cardiomyocyte contraction is triggered by an electrical stimulus, the action potential (AP), which induces transient depolarization of the cardiomyocyte plasma membrane (the sarcolemma). The AP is generated by specialized cells known as pacemaker cells located at the sinoatrial node (SAN), which set the frequency of contraction of the heart. This electrical signal propagates through specialized conduction pathways and via myocyte-to-myocyte transfer through gap junctions, across the atria until it reaches the atrioventricular node (Rohr 2004). After a short delay to allow atrial systole, the impulse propagates through the ventricles via the bundle of His and Purkinje fibers to induce ventricular systole (Stephenson et al. 2012). Opening and closing of valves further defines the filling and ejection of blood from the chambers. Eventually, blood is propelled from the left ventricle into the aorta to the systemic circulation and from the right ventricle into the pulmonary artery to the lungs. The AP is a transient phenomenon and repolarization of the cardiomyocytes is associated with relaxation, again in a sequential manner for each chamber of the heart. This relaxation phase (diastole) is important for the filling of the chambers, and is an essential part of the cardiac cycle (Fukuta and Little 2008). This highly coordinated contraction and relaxation of the four chambers of the heart is necessary for optimal support of the circulation and ensures a supply of nutrients and signals throughout the body.
ECC and Ca2+: Generation of the Ca2+ Transient and Induction of Contraction
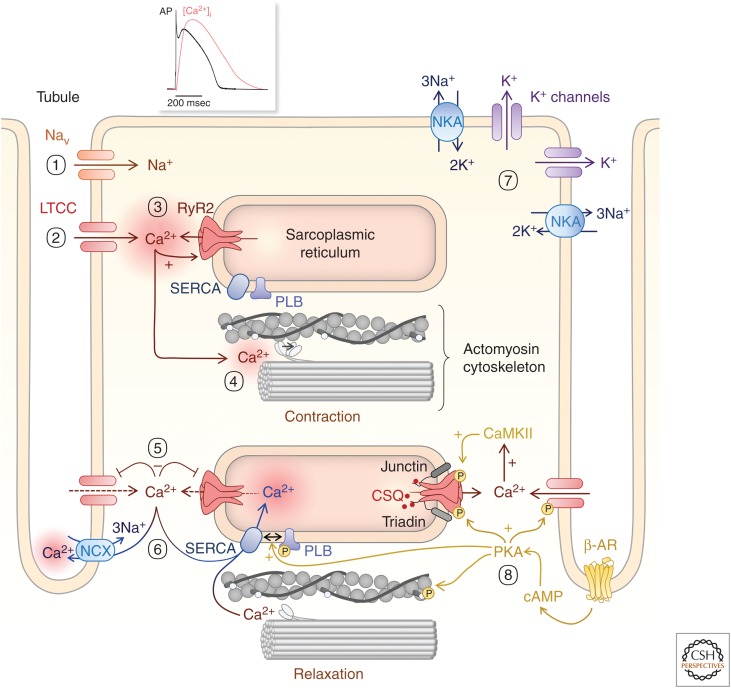
The mechanism by which the AP is transduced into the contractile response is termed “excitation–contraction coupling” (ECC) (Fozzard 1977; Bers 2002; Fearnley et al. 2011; Eisner 2014). Changes in intracellular Ca2+ concentration are central to this mechanism and couple (within a few msec) membrane depolarization with the engagement of the actomyosin cytoskeleton (via the energy of ATP hydrolysis) and thus sarcomere contraction (Fig. 1). During diastole (when the heart is relaxed), the cells are in their most extended state; their membrane potential (i.e., difference between inside and out) lies between −70 mV and −90 mV for atrial and ventricular cardiomyocytes and −60 mV for nodal cells. This negative resting membrane potential is close to the equilibrium potential for K+ channels, which are the dominant conductance (through Kir2.x channels) at rest (Miake et al. 2003; Zobel et al. 2003; Cordeiro et al. 2015). The negative equilibrium potential results from the large K+ gradient across the sarcolemma. This ionic gradient is generated and maintained by the Na+K+ATPase pumping three Na+ ions out of the cell in exchange for two K+ ions (Glitsch 1979; Gadsby 1984; Shattock et al. 2015). This pump also maintains the Na+ gradient across the cell membrane, compensating for the Na+ influx during the upstroke of the AP. Arrival of the AP results in an increase in membrane potential (depolarization) to the activation threshold of sodium channels (NaV; pore-forming subunit NaV1.5), −40 mV, and induces a rapid influx of Na+ (Fig. 1(1)). This increase in intracellular Na+ further depolarizes the cell, to an extent that the threshold for activation of voltage-gated L-type Ca2+ channels (LTCCs) is crossed (comprising its pore-forming subunit, CaV1.2). The activation of these channels and ensuing Ca2+ influx underlies the plateau phase of the AP (Fig. 1(2)). Although the magnitude of this Ca2+ influx is not by itself sufficient to induce effective contraction, it represents a key triggering step in ECC. Specifically, Ca2+ entering the cell is amplified by Ca2+ release from the sarcoplasmic reticulum (SR) Ca2+ store through a mechanism called Ca2+-induced Ca2+ release (CICR) (Fabiato and Fabiato 1977; Fabiato 1983; Roderick et al. 2003). Through this mechanism, the Ca2+ entry signal arising via LTCC stimulates the opening of ryanodine receptor Ca2+ channels (RyR; type 2 isoform) located on the SR (Cannell et al. 1987; Beuckelmann and Wier 1988; Stern 1992; Wang et al. 2001). The RyR channel is a multimeric protein complex that is comprised of an RyR tetramer as well as a number of accessory proteins that contribute to modulating its activity under basal conditions as well as in response to physiological agonists and altering cell conditions. One of the first such proteins to be described is the archetypal Ca2+ sensor CaM, which acts to inhibit the channel activity (Xu and Meissner 2004). An interaction with the immunophilin FKBP12.6 is also well established. Although this interaction was at one time considered dynamic (Wehrens et al. 2003), evidence now supports a model in which the interaction is highly stable and serves to maintain the closed state of RyRs (Guo et al. 2010). Other interacting proteins include protein phosphatases and kinases that may act to couple kinase signaling cascades with RyR function (Zalk et al. 2007). In the SR, Ca2+ is primarily stored bound to the low-affinity Ca2+-binding protein calsequestrin (Cala et al. 1990). Notably, calsequestrin associates with the RyR through the proteins junctin and triadin (Györke et al. 2004). This efficient coupling between plasmalemma Ca2+ influx and Ca2+ release from the SR is ensured through a close apposition (10–15 nm) of the associated channels (LTCC and RyR2) and their host membranes in a confined space called the dyadic cleft (Fig. 1(3); Hayashi et al. 2009; Scriven et al. 2010; Pinali et al. 2013). This architecture ensures that RyRs are exposed to high levels of Ca2+ entering the cardiomyocyte during the AP, which are necessary for their opening and subsequent Ca2+ release.
Figure 1.
Excitation–contraction coupling (ECC). (1) An action potential depolarizes the cardiomyocyte and induces Na+ influx through voltage-gated Na+ channels (Nav). (2) This further depolarizes the cell membrane and induces Ca2+ influx through voltage-gated L-type Ca2+ channels (LTCCs). (3) This Ca2+ entry stimulates Ca2+ release via dyadic RyR2 on the SR (4), which in turn triggers cell contraction through activating myofilament crossbridges. (5) LTCC inactivate and RyR close. (6) Cytosolic Ca2+ is then moved out of the cell by the Na+/Ca2+ exchanger (NCX) and pumped back into the SR by SERCA2a, thereby decreasing cytosolic Ca2+ concentration and bringing about relaxation. (7) A family of K+ channels participate in cell repolarization with K+ efflux as a last step for returning membrane potential to its resting value before a new cycle starts. (8) Tuning ECC to meet cardiovascular demands involves β-adrenergic pathways that induce the activation of CaMKII and of cAMP/PKA, which phosphorylates voltage-gated LTCCs and RyRs to enhance their activity and phospholamban (PLB) to remove its inhibition of SERCA activity.
However, these couplons do not provide a sufficient Ca2+ influx to overcome the high-buffering capacity of the cardiomyocyte cytosol to induce RyR-mediated Ca2+ release throughout the volume of large ventricular cardiomyocytes (150 µm long × 20 µm deep on average) and induce contraction (Hüser et al. 1996; Smyrnias et al. 2010). To overcome this problem, and convey the AP to the entire cell volume, ventricular cardiomyocytes are endowed with a highly elaborate network of membrane tubules extending from invaginations of the sarcolemma (Smyrnias et al. 2010; Scardigli et al. 2018a). This network comprises transverse tubules (T-tubules [TTs]), which are distributed along the Z-lines of the sarcomere and axial tubules that are arranged along the long axis of the cardiomyocyte (together, the transverse and axial tubule system [TATS]). Further, TATS brings the sarcolemma and SR and its associated LTCCs and RyRs, respectively, into close proximity, forming couplons to generate a homogenous cell-wide Ca2+ transient (Scriven et al. 2010; Pinali et al. 2013). At this location, RyRs are organized in clusters, thereby optimizing release at each site (Walker et al. 2014; Shen et al. 2019). Although clusters of more than 100 RyRs have been reported, recent superresolution imaging experiments describe average cluster sizes ranging between 9 and 23 RyRs (Jayasinghe et al. 2018b; Shen et al. 2019). The localization of dyads to the Z-line of the sarcomere ensures exposure of contractile machinery to maximal Ca2+ elevation and flux (Fig. 1(4)). In atrial cardiomyocytes or ventricular cardiomyocytes from fetal and neonatal animals (Mackenzie et al. 2004; Zima and Blatter 2004; Bootman et al. 2006), TATS are however either absent or at a very low density (Brandenburg et al. 2016). In these cells, Ca2+ entry across the sarcolemma and its coupling with subsarcolemmal RyRs is sufficient to support their relatively lower contraction. Through the action of a number of players, such as kinases (e.g., calmodulin kinase II [CaMKII] and protein kinase A [PKA]), phosphatases, and reactive/nitric oxygen species, Ca2+ release in the dyad is regulated by many signal transduction cascades (for review, see Zalk et al. 2007).
Cardiomyocyte Relaxation and Ca2+ Clearance
Depending on species, size of animal, and level of activity, the heart beats between 1 and 12 times per second. Thus, subsequent to systole and to allow the next round of contraction to occur, efficient mechanisms are required to bring about relaxation (diastole), and allow refilling of the chambers with blood. An initial step in this relaxation phase is the termination of mechanisms responsible for raising cytosolic Ca2+. RyR2 release channels behave stochastically and their opening terminates rapidly through a combination of attrition, store depletion, regulation of their gating by luminal Ca2+, and inactivation at the cytosolic side (Cannell and Kong 2017). LTCC also rapidly inactivates by a Ca2+- and voltage-dependent mechanism (Cens et al. 2006; Tadross et al. 2008). Repolarization of the cell membrane potential is the result of an outward current of K+ via different voltage-activated K+ channels (IKr, IKs) and, finally, opening of K+ channels that maintain the resting membrane potential (IK1-Kir2.1, 2.2, and 2.3) (Fig. 1(6); Cordeiro et al. 2015). Overall, these channel activities determine the time course and phases of the AP (Carmeliet 1999; Chiamvimonvat et al. 2017). It is also important to note that it is not possible to trigger a new AP during the plateau phase of the ventricular cardiomyocyte AP. This refractory period protects the heart from prematurely unwanted contractions (Burton and Cobbe 2001).
Active mechanisms substantially contribute to return intracellular cytosolic Ca2+ concentration to its resting levels and to bring about relaxation. These mechanisms also serve to replenish intracellular Ca2+ stores to prepare the cell for its next contraction. The primary contributor to cytosolic Ca2+ clearance as well as SR refilling is the sarco/endoplasmic reticulum Ca2+ ATPases (SERCA) pump. The SERCA 2a isoform is primarily expressed in the heart where it is localized to the SR membrane proximal to RyR2 and the contractile machinery (Dally et al. 2010). Subsequent to its Ca2+-dependent activation (K1/2 0.4 µm) (Lytton et al. 1992), via ATP hydrolysis, SERCA actively pumps Ca2+ from the cytosol into the SR (Fig. 1(5)). Ca2+ is also extruded out of the cell by a Na+/Ca2+ exchanger (NCX) in its forward mode to exchange one Ca2+ out of the cell and three Na+ into the cell (Fig. 1(5); Shattock et al. 2015). Although expressed in cardiomyocytes, the plasma membrane Ca2+ ATPase does not make a major contribution to Ca2+ extrusion during ECC (Hammes et al. 1998; Bers 2002). Although SERCA dominates in cytosolic Ca2+ clearance, its contribution varies according to species ranging between 70% and 90% of all Ca2+ removed from the cytosol (Bassani et al. 1994; Piacentino et al. 2003). The remainder is predominantly via NCX. In steady state, extrusion via NCX equals influx via LTCC and Ca2+ release and reuptake into the SR are in equilibrium at a set level of Ca2+ in the store (Eisner 2014). Dysregulation of any of these transport mechanisms will lead to a new equilibrium, with a new filling level of the store (e.g., an increase in intracellular Na+ will raise the Ca2+ store filling by reducing NCX-mediated Ca2+ extrusion) (Eisner 2014).
Tuning ECC to Modulate Cardiac Function
Heart function is dynamically regulated to meet the changing needs of the organism during alterations in the body activity, by the autonomic nervous system (Lymperopoulos et al. 2013; Eisner 2014; Wang et al. 2018). For example, during the fight-or-flight response, cardiomyocytes show inotropic (increased force of contraction) and lusitropic (increased relaxation) responses (Lymperopoulos et al. 2013). An increase in heart rate is also observed, although this is mediated by an effect on the spontaneous generation of AP at the SAN. This fight-or-flight response is primarily mediated by the adrenergic system and its hormone mediators noradrenaline and adrenaline. In altering cardiomyocyte function, the latter acts through β-adrenergic receptors (primarily β1) expressed on the cardiomyocyte plasma membrane. β-Adrenergic receptors are 7-transmembrane G-protein-coupled receptors (GPCRs) that couple via the stimulatory G-protein (Gs) to activate adenylate cyclases and increase levels of the intracellular messenger cyclic AMP (cAMP) (Wang et al. 2018). Increased cAMP activates protein kinase A (PKA), which then phosphorylates multiple targets involved in ECC to augment cardiomyocyte contraction, and in turn cardiac function. Indeed, PKA phosphorylation targets each of the key functional components of ECC. Specifically, PKA phosphorylation of LTCC results in increased Ca2+ current, and phosphorylation of RyR leads to greater sensitivity to Ca2+ (Valdivia et al. 1995; van der Heyden et al. 2005). PKA also phosphorylates myofilament proteins (troponin C, myosin-binding protein C [MyBPC] and troponin I), most notably troponin I, decreasing the affinity of the myofilaments for Ca2+ and thereby enhancing crossbridge cycling (Fig. 1(8); Metzger and Westfall 2004). In bringing about the effects of PKA, and modulation of Ca2+ handling, phospholamban (PLB) is an important target. PLB is a small 5 kDa protein that interacts with SERCA, inhibiting its activity. On phosphorylation by PKA, PLB dissociates from SERCA resulting in its disinhibition (Fig. 1; for review, see MacLennan and Kranias 2003). As well as bringing about the cardiomyocyte lusitropic response via more rapid clearance of cytosolic Ca2+, enhancing of SERCA activity results in an elevated SR Ca2+ load. The latter in turn increases Ca2+ availability in the SR for release, also sensitizing the open probability of RyRs. Many of the proteins targeted by PKA, most notably RyR and PLB, are also phosphorylated during β-adrenergic stimulation by CaMKII with similar consequences for cardiomyocyte function (Ullrich et al. 2012; Grimm et al. 2015; Uchinoumi et al. 2016; Potenza et al. 2018). Recently, a number of new micropeptide regulators of SERCA such as DWORF have been described (Nelson et al. 2016). Although these proteins are not modulated by PKA, their interaction with SERCA appears to act in competition with PLB. A direct regulation of SERCA by a long noncoding RNA has also been reported (Vervliet et al. 2018; Zhang et al. 2018). β-Adrenergic stimulation also shortens the AP duration by enhancing K+ channel activity (Hegyi et al. 2018). Together, these combined actions of β-adrenergic stimulation facilitates the increased heart rate and cardiac output required under times of stress.
To balance the sympathetic system in the heart, the parasympathetic system with its principal mediator acetylcholine (ACh) lower the heart rate. ACh acts on the M2 muscarinic GPCRs within the heart. On activation of M2 ACh receptors Gβγ subunits are liberated, and through activation of GIRK channels (also known as IKACh) result in a more negative resting membrane potential and slower heart rate (for review, see Saw et al. 2018).
Multiple circulating hormones act on cardiomyocyte activity to modify contractility, and in so doing, couple other organ systems and cell types with cardiomyocyte function (Drawnel et al. 2013; Mayourian et al. 2018; Smyrnias et al. 2018). Those modulations act through altered Ca2+ signals as well as through changes in myofilament response to Ca2+ (Kobayashi and Solaro 2005). Notable cardioactive hormones include angiotensin II, which is produced in the kidney, endothelin-1, which is released locally and systemically by the endothelium, and by ATP. These hormones/mediators are also produced by cardiomyocytes themselves, thus eliciting an autocrine action (Dostal and Baker 1999; Drawnel et al. 2013). Of these hormones, many engage GPCRs, but in contrast to the β-adrenergic receptors, act via Gαq to promote phospholipase C–dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate into inositol 1,4,5-trisphosphate (InsP3) and diacyglycerol (DAG). DAG acts through protein kinase C (PKC) and InsP3 acts on InsP3 receptors (InsP3R) to modify ECC (Kockskämper et al. 2008b; Drawnel et al. 2013; Smyrnias et al. 2018). In particular, PKC phosphorylates myofilament proteins to alter contractility, and on the Na+/H+ exchanger to modify intracellular pH and Na+ (Russell and Molenaar 2000). In so doing, myofilament sensitivity to Ca2+ is altered and Ca2+ balance further modulated through NCX consequent to changes in Na+ (Despa and Bers 2013; Garciarena et al. 2013). The role of InsP3 in regulation of ECC is discussed below.
Membrane Tubules and Microdomain Signaling in Cardiomyocytes
Cell-wide increases in Ca2+ and efficient contraction rely on TATS in adult ventricular cardiomyocytes (Heinzel et al. 2002, 2011; Smyrnias et al. 2010; Crocini et al. 2014, 2016). Owing to its essential role in ECC, the complexity of TATS has gained great interest over recent years, with detailed knowledge arising from the advances in superresolution and electron microscopical imaging technologies that are able to visualize TATS in all their detail (Ferrantini et al. 2013; Soeller and Baddeley 2013; Crocini et al. 2014; Crossman et al. 2017). The density of TATS varies substantially through development, across the chambers of the heart, between species and during pathology (Song et al. 2006; Jayasinghe et al. 2012; Frisk et al. 2016; Manfra et al. 2017; Jones et al. 2018). During early postnatal development, the organization of TATS in rat cardiomyocytes is completed by 10–12 days postbirth (Ziman et al. 2010), although at this stage, very few LTCCs lie in close proximity to RyRs (Franzini-Armstrong et al. 2005). With development, the colocalization of LTCC with RyR increases (Sedarat et al. 2000), constructing the mature couplon in the dyad to enable efficient CICR. The structure and organization of TATS shows species-dependent differences, with mice having narrower tubules (∼0.17 µm) (Kong et al. 2017) compared with humans, pigs, and rats (∼0.25 µm) (Soeller and Cannell 1999) or rabbits (∼0.45 µm) (Savio-Galimberti et al. 2008; Kong et al. 2017; Rog-Zielinska et al. 2018). TATS are also more densely distributed in small rodents compared with large mammals including human, with TT flanking each sarcomere (Fig. 2; Manfra et al. 2017). The density of TATS in ventricular cardiomyocytes seems to associate with a high heart rate and the need for a very fast contraction and relaxation (e.g., in mice with a heart rate of 600 bpm). In atrial cardiomyocytes, TATS have been generally considered to be absent or rudimentary in nature. Recent data, however, shows that the nature of TATS in atrial cardiomyocytes is more diverse, with observed differences in right versus left atria, and between species. This diversity is possibly associated with different atrial workloads and cardiomyocyte width (Frisk et al. 2014; Brandenburg et al. 2016, 2018; Gadeberg et al. 2016; Denham et al. 2018). High-resolution microscopy studies in small and large mammals showed distinct populations of atrial cardiomyocytes that possess different configurations of TATS (i.e., organized, disorganized, and empty cells) (Frisk et al. 2014; Glukhov et al. 2015; Yue et al. 2017).
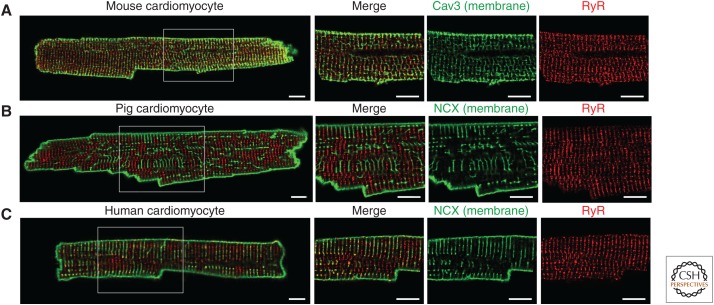
Figure 2.
Difference in the TATS in isolated cardiomyocytes from mouse (A), pig (B), and human (C). The TATS is stained in green (Caveolin-3 [Cav3] or NCX) and RyRs are stained in red. Scale bar, 10 µm. Images were acquired with a Nikon A1R confocal microscope using a 60× oil immersion objective. A4× zoom of the white square is shown (unpubl.).
Structural differences in TATS have an impact on cardiomyocyte physiology and function. With a high TATS density, the majority of RyRs reside within couplons (Fig. 2A; Louch et al. 2006; Scriven et al. 2010; Wong et al. 2013). In this situation, for example in small rodents including mice and rats, AP-depolarization and RyR-mediated Ca2+ release show high-temporal fidelity. However, a low TATS density (e.g., humans and pigs) results in a significant number of RyR that lie outside of couplons/dyad cleft (i.e., noncoupled) and are therefore likely not exposed to the same Ca2+ concentration directly following the AP (Fig. 2B,C; Heinzel et al. 2002; Louch et al. 2004; Dries et al. 2013). As a consequence, this fraction of RyRs is activated with a delay, resulting in inhomogeneous Ca2+ release during a cell transient/contraction (Heinzel et al. 2002). This role of TATS in Ca2+ transient generation is further illustrated in cells in which they are normally absent or sparse, such as atrial cells (Bootman et al. 2011; Brandenburg et al. 2016). On electrical stimulation, these cells show an increase in intracellular Ca2+ concentration ([Ca2+]i) that initially occurs at the cell periphery and, which then propagates to the center of the cell via a combination of CICR and Ca2+ diffusion (Mackenzie et al. 2004; Sheehan et al. 2006). Conversely, disruption of TATS in ventricular cells by osmotic shock or through maintenance in culture for 6 days leads to inhomogeneous Ca2+ release during transients (Lipp et al. 1996; Brette et al. 2005; Smyrnias et al. 2010). The efficiency of CICR is similarly low in ventricular cardiomyocytes isolated from neonatal hearts, which show little evidence of TATS (Haddock et al. 1999). The mechanisms underlying the expression and maintenance of TATS is not yet fully resolved. Titin cap protein (telethonin), junctophilin 2, and the Bin/Amphiphysin/Rvs domain protein amphiphysin II (AmpII or BIN-1) have all been shown, however, to modulate the biogenesis and maintenance of TATS (Chen et al. 2013; Ibrahim et al. 2013; Reynolds et al. 2013; Hong et al. 2014; Fu and Hong 2016).
NON-CANONICAL Ca2+ SIGNAL GENERATION IN THE HEART
In addition to the Ca2+ signaling mechanisms underlying ECC (described above), other Ca2+ transport pathways are expressed in the heart contributing to a more complex picture of the role of Ca2+ in cardiomyocyte function. These additional Ca2+ signaling pathways interact with the canonical ECC signaling Ca2+ mechanism to selectively and simultaneously control ECC as well as other cellular processes (Fig. 3).
Figure 3.
Noncanonical Ca2+ channels. (1) G-protein-coupled receptors activated by endothelin-1 (ET-1) or angiotensin-II (Ang-II), produce InsP3 that activates Ca2+ release via InsP3 receptors (IP3RII), which cross talks with RyR2 to modify Ca2+-induced Ca2+ release (CICR). (2) Ca2+ release from InsP3R2 present at the perinuclear SR and the nuclear envelope stimulates nuclear Ca2+ signaling pathways. (3) β-Adrenergic receptor (β-AR) activation leads to the activation of protein kinase A (PKA), which in turn enhances the activity of the CD38 enzyme. (4) CD38 produces nicotinic acid adenine dinucleotide phosphate (NAADP) that promotes Ca2+ leak via endolysosomal two-pore channels (TPCs). (5) Activation of CD38 also generates cyclic ADP-ribose (cADPR), which acts on the RyR2 to enhance its activity. (6) To refill the SR as well as affect cytosolic Ca2+ levels, the STIM/ORAI/TRP system may be engaged.
InsP3 and InsP3 Receptors
Inositol 1,4,5-trisphosphate receptor (InsP3R) intracellular Ca2+ release channels are expressed in cardiomyocytes (Lipp et al. 2000; Kockskämper et al. 2008b; Garcia et al. 2017). Although all three isoforms of InsP3Rs have been identified in the heart, the type 2 InsP3R is predominant (Perez et al. 1997; Lipp et al. 2000). InsP3R expression is required in cardiac development and contributes to heart function during the embryonic and neonatal stages (Rosemblit et al. 1999; Sasse et al. 2007; Kockskämper et al. 2008b). InsP3R expression level declines during postnatal heart maturation, as the number of RyRs increases. Ca2+ release via InsP3R is initiated by InsP3 generated downstream to the activation of Gαq-coupled GPCR signaling cascades, for example, by endothelin-1 and angiotensin II (Fig. 3; Berridge 1993). As for all InsP3R isoforms, InsP3R2 activity shows a bell-shaped dependency for Ca2+, with high activity occurring at Ca2+ levels present at diastole (Foskett et al. 2007). Perhaps making this isoform most appropriate for its function in the heart, it shows the highest sensitivity to InsP3 (Fig. 3(1); Mak and Foskett 2015; Vervloessem et al. 2015).
InsP3R expression varies substantially across the chambers of the heart, with ∼10-fold higher expression in the atria than ventricle (Lipp et al. 2000). Greater expression levels are also observed in cardiomyocytes of the SAN (Kapoor et al. 2015) and the Purkinje conduction fibers (Gorza et al. 1993; Hirose et al. 2008) in which ECC is not the primary function. In the adult ventricle, InsP3R is reported to be expressed at a substantially lower level than RyRs (10- to 50-fold) (Moschella and Marks 1993) and owing to this lower expression, as well their lower conductance compared with RyRs, have not been considered to play a substantial role in regulation of ECC in this heart region. Despite this low expression, roles for InsP3R in regulation of ECC, hypertrophic gene expression, Purkinje fiber function, and SAN activity are reported (Domeier et al. 2008; Hirose et al. 2008; Higazi et al. 2009; Nakayama et al. 2010; Kapoor et al. 2015). The ability of InsP3Rs to influence cardiomyocyte function despite low expression is made possible through their localization to functionally relevant cellular subcompartments and local signaling events (Fig. 3(2); Wu et al. 2006; Higazi et al. 2009; Ljubojevic et al. 2014; Hohendanner et al. 2015a; Wullschleger et al. 2017). In atrial and ventricular cardiomyocytes, InsP3Rs are colocalized with RyRs in the subsarcolemmal space or at dyads, respectively (Lipp et al. 2000; Harzheim et al. 2009). At these locations, Ca2+ released via InsP3Rs modulates ECC by bringing proximal RyRs closer to the threshold for activation (Harzheim et al. 2009; Horn et al. 2013; Hohendanner et al. 2015b; Wullschleger et al. 2017) leading to sensitization of Ca2+ release during the transient. Depending on the model studied, this channel cross talk induces SR Ca2+ leak and elicits inotropic and/or pro-arrhythmic effects (Mackenzie et al. 2002; Zima and Blatter 2004; Proven et al. 2006; Bootman et al. 2007; Domeier et al. 2008; Kockskämper et al. 2008a; Namekata et al. 2008; Signore et al. 2013; Blanch and Egger 2018). Although positive inotropic effects of InsP3Rs are observed in atrial cardiomyocytes, the impact of InsP3 signaling on contractility in ventricular cardiomyocytes is less obvious with effects on cellular automaticity more often reported (Harzheim et al. 2009; Blanch and Egger 2018).
Two-Pore Channels and Nicotinic Acid Adenine Dinucleotide Phosphate
Nicotinic acid adenine dinucleotide phosphate (NAADP) is an intracellular signaling messenger that stimulates Ca2+ release via transient receptor potential (TRP) channels and two-pore channels (TPCs) from the endo/lysosome (Calcraft et al. 2009; Pitt et al. 2010; Grimm et al. 2012). NAADP is a potent second messenger, which acts at nanomolar levels to trigger micromolar release of Ca2+ from TPCs. Several lines of evidence support a role for NAADP signaling in cardiomyocyte ECC. Specifically, NAADP triggers Ca2+ release from microsomes prepared from SR; it activates single RyR2 incorporated into bilayer lipid membranes and direct application of NAADP increases the amplitude of Ca2+ transient and frequency/amplitude of Ca2+ sparks in intact guinea pig cardiomyocytes (Bak et al. 2001; Mojzisova et al. 2001; Macgregor et al. 2007a; Collins et al. 2011). Although a role for NAADP as a physiologically relevant messenger in the heart was for a long time not clear, the identification of its production downstream of β-adrenergic stimulation makes it an important regulator of cardiomyocyte function (Macgregor et al. 2007a; Lewis et al. 2012). NAADP is produced following β-adrenergic stimulation through activation of the enzyme CD38, an ADP-ribosyl cyclase, and is thereby proposed to contribute to both the inotropic and arrhythmogenic effects of this signaling pathway (Macgregor et al. 2007a; Lewis et al. 2012; Nebel et al. 2013; Warszta et al. 2014; Lin et al. 2017). To elicit its inotropic effect, NAADP is proposed to enhance Ca2+ loading of the SR (Macgregor et al. 2007a; Collins et al. 2011). A requirement for CaMKII in mediating this effect of NAADP has been shown (Capel et al. 2015). Reminiscent of the cross talk between InsP3Rs and RyRs, Ca2+ release from the NAADP-sensitive stores sensitizes RyR2 at lysosomal–endoplasmic reticulum nanojunctions leading to spontaneous diastolic Ca2+ release events (Fig. 2(4); Macgregor et al. 2007a; Nebel et al. 2013; Warszta et al. 2014).
RyRs and cADPR
Cyclic ADP-ribose (cADPR) is a second messenger that is also produced by ADP-ribosyl cyclase (CD38) in mammalian cardiomyocytes after sympathetic or angiotensin II stimulation (Galione et al. 1998; Higashida et al. 1999, 2000; Xie et al. 2005). cADPR modulates the peak amplitude of Ca2+ transients and hence cardiomyocyte contractility (Rakovic et al. 1996; Cui et al. 1999; Macgregor et al. 2007a). Mechanisms involved in mediating this action of cADPR include increased RyR activity and greater SR Ca2+ load (Rakovic et al. 1999; Lukyanenko et al. 2001; Macgregor et al. 2007b; Zhang et al. 2009). Consistent with this hypothesis, a higher frequency of Ca2+ sparks is observed in response to increased cADPR. However, the relatively slow kinetics for the development of the full effect of cADPR on Ca2+ release, and evidence for a cADPR-responsive component independent of RyR2 is suggestive of a more complex mechanism (Cui et al. 1999; Prakash et al. 2000). Apart from the role in Ca2+ homeostasis, the ADPR-cyclase and hence cADPR are implicated in the development of angiotensin II–induced hypertrophic responses (Gul et al. 2008, 2009) and in cellular toxicity following ischemia/reperfusion injury (Fig. 3(5); Xie et al. 2005).
Ca2+ Influx and STIM/Orai
A functional store-operated Ca2+ entry (SOCE) pathway is described in cardiomyocytes (Voelkers et al. 2010; Bootman and Rietdorf 2017). As in other cellular systems, in cardiomyocytes this pathway is activated in response to Ca2+ depletion of the SR, for example by treatment with SERCA inhibitors (cyclopiazonic acid or thapsigargin) and/or GPCR agonists (for review, see Hunton et al. 2002, 2004; Uehara et al. 2002; Kojima et al. 2012; Luo et al. 2012; Avila-Medina et al. 2018). The molecular components of SOCE, including the ER/SR Ca2+ sensors STIM1 and STIM2 and the plasma membrane channel ORAI are also all expressed in cardiomyocytes, further supporting the presence of this pathway in this cell type (Correll et al. 2015; Zhao et al. 2015). Although the role of SOCE in cardiomyocytes is not fully resolved, it has been shown to participate in regulation of gene transcription and Ca2+ homeostasis (Ohba et al. 2007; Voelkers et al. 2010; Hulot et al. 2011). SOCE activity is developmentally regulated, being readily detectible in fetal and neonatal cardiomyocytes, but is thereafter down-regulated, with low-to-nondetectable activity in healthy adult cardiomyocytes (Uehara et al. 2002). As such, it does not significantly contribute to ECC in healthy adult cardiomyocytes (Fig. 3(6)).
Mitochondrial Calcium in Cardiomyocytes
Cardiomyocyte Ca2+ dynamics are also influenced by mitochondria. They constitute about one-third of the volume of cardiomyocytes being arranged as bricks spanning the sarcomere (Bossen et al. 1978). Via the tricarboxylic acid (TCA) cycle and oxidative phosphorylation, mitochondria are responsible for ∼90% of ATP generated in cardiomyocytes. Although mitochondria take up Ca2+ from the cytosol during ECC and show a high capacity for Ca2+ accumulation (Wei et al. 2012, 2015), their influence on Ca2+ dynamics is more subtle, especially under physiological conditions (Drago et al. 2012; Williams et al. 2013; Boyman et al. 2014). Ca2+ uptake into mitochondria is, however, an important regulator of mitochondrial metabolism. It augments the activity of pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase, which are key enzymes of the TCA cycle as well as the activity of cytochrome c oxidase and F0-F1-ATPase, which are components of the electron transport chain (ETC) (Denton et al. 1988; Balaban 2009). By augmenting mitochondrial ATP generation, Ca2+ thus coordinates cellular metabolism, and the dynamic changes in the energy requirements of the cardiac myocyte, including for Ca2+ clearance mechanisms and actomyosin-mediated contraction that are enhanced during the fight-or-flight response (Balaban 2009; Kwong et al. 2015). During pathology such as following ischemia, excessive mitochondrial Ca2+ uptake is toxic, resulting in increased reactive oxygen species generation and permeability transition pore opening associated with cell death mechanisms (Santulli et al. 2015).
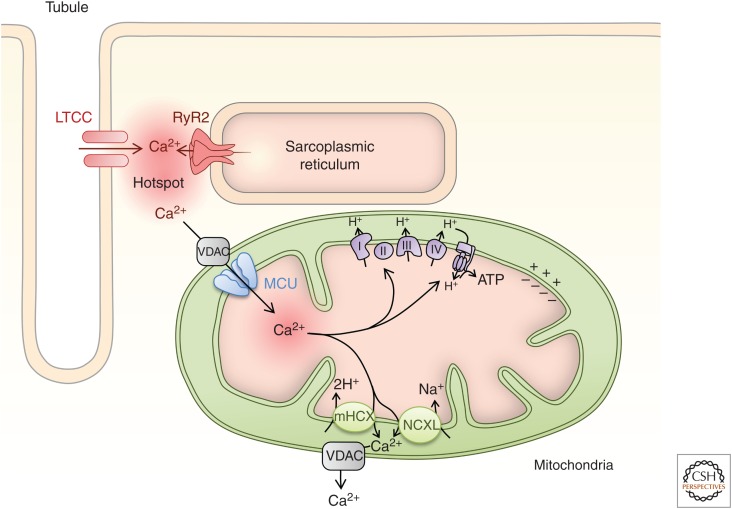
Cardiomyocyte mitochondrial Ca2+ has been reported to either mirror changes in cytosolic Ca2+ showing short-lived transients (Robert et al. 2001; Kettlewell et al. 2009) or to show steady-state Ca2+ changes that increase according to the frequency of cytosolic Ca2+ transients (Paillard et al. 2017). Reasons for these different models are not clear but are in part proposed to be methodological (Dedkova and Blatter 2013). The mitochondrial Ca2+ uptake mechanism has a low affinity, in the tens of µm, precluding its activation by bulk cytosolic Ca2+ elevations generated during ECC and in response to hormonal stimulation that do not reach this level (Collins et al. 2002; Kirichok et al. 2004; Kettlewell et al. 2009; Dedkova and Blatter 2013). Mitochondrial Ca2+ uptake sites are, however, localized to within tens of nm of Ca2+ release channels on the SR/ER, exposing them to a microdomain of Ca2+ in the tens of µm, which is sufficiently high to activate the low-affinity Ca2+ uniporter (Hajnóczky et al. 1995; Csordás et al. 1999, 2010). These ER/SR mitochondrial uptake sites are known as “hotspots” and structurally as mitochondrial-associated membranes (MAMs) (Rizzuto et al. 2004). Supporting colocalization of sites for mitochondrial Ca2+ uptake and SR Ca2+ release in cardiomyocytes, cardiomyocyte mitochondria show a gradient in Ca2+ from the Ca2+ release sites at the junctional SR to the center of the sarcomere (Lu et al. 2013).
Mitochondrial Ca2+ uptake primarily occurs via the voltage-dependent anion channel (VDAC) of the outer mitochondrial membrane (OMM) (Shimizu et al. 2015) and a low affinity mitochondrial Ca2+ uniporter (MCU) of the inner mitochondrial membrane (IMM) (Kirichok et al. 2004). Other mechanisms of mitochondrial Ca2+ uptake have been described, including a rapid mode of mitochondrial uptake (RaM) (Buntinas et al. 2001) via mitochondrial RyR1 (Beutner et al. 2005) and connexin 43 (Gadicherla et al. 2017). The molecular identification of the MCU in the last decade (Baughman et al. 2011; De Stefani et al. 2011) has led to new insights into its role in mitochondrial Ca2+ sequestration in the heart (Pan et al. 2013; Kwong et al. 2015; Wu et al. 2015). A common finding of these studies is that MCU function is not required for baseline function but is necessary for adaptive responses of the heart to stress or work. This is illustrated by results of loss-of-function studies, which showed that MCU was required for heart rate acceleration at the level of pacemaker cells and inotropy of ventricular cardiomyocytes (Kwong et al. 2015; Rasmussen et al. 2015; Wu et al. 2015). The greatest contribution of these effects of loss of MCU function was to limit increases in ATP generation during periods of greater stress/workload and not on Ca2+ buffering (Drago et al. 2012). As a consequence of insufficient ATP, the increase in SERCA function required to bring about chronotropic and inotropic responses is prevented (Kwong et al. 2015; Rasmussen et al. 2015; Wu et al. 2015). MCU is associated with a number of regulatory proteins, including MICU1 and MICU2 and EMRE (Perocchi et al. 2010; Vais et al. 2016). Of particular importance in the context of the cardiomyocyte is MICU1, which sets the threshold of activation and properties of Ca2+ uptake via MCU (Csordás et al. 2013). Notably, whereas MCU and MICU1 interact in a stoichiometric manner in liver, a reduced proportion of MCU is associated with MICU1 in cardiomyocytes (Paillard et al. 2017). Through this decreased interaction with MICU1, cardiomyocyte mitochondrial Ca2+ uptake shows a higher affinity and lower cooperativity than in nonmuscle tissues such as liver (Paillard et al. 2017). Whereas MCU is positioned close to RyR to facilitate mitochondrial Ca2+ uptake (De La Fuente et al. 2016), Ca2+ extrusion mechanisms, including the Na+/Ca2+/Li+ exchanger NCXL (Palty et al. 2010; Luongo et al. 2017), are localized to regions of the mitochondria distinct from the Ca2+ uptake route (De La Fuente et al. 2018). Furthermore, an electrogenic Ca2+/2H+ exchanger (potentially the leucine zipper EF-hand-containing transmembrane protein 1 [LETM1]) has also been proposed to extrude Ca2+ out of the mitochondria (Jiang et al. 2009; Haumann et al. 2018).
REMODELING OF ECC IN DISEASE
Given its fundamental role in regulation of cardiac function, it is not surprising that disturbances in the organization and regulation of Ca2+ signals result in cardiac dysfunction (Hasenfuss and Pieske 2002; Wehrens et al. 2005; Luo and Anderson 2013; Marks 2013). Indeed, altered Ca2+ handling is observed during hypertrophic remodeling and heart failure, as well as during diseases associated with rhythmic disturbances, including atrial fibrillation (Berridge et al. 2003; Fearnley et al. 2011; Mozaffarian et al. 2015; Denham et al. 2018). Perturbations in Ca2+ handling are also apparent in hearts remodeled because of mutations in myofilament proteins (hypertrophic as well as dilated cardiomyopathies). Further, mutations in Ca2+-handling proteins, including RyRs (e.g., during catecholaminergic polymorphic ventricular tachycardia [CPVT]) and PLB are described to have a direct effect on cardiomyocyte function (Wehrens et al. 2005; Liu et al. 2015).
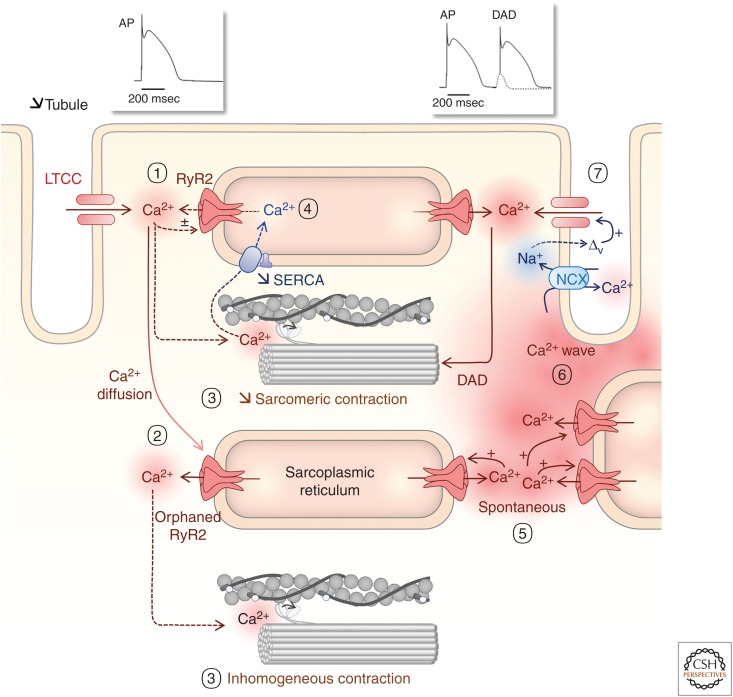
Cardiac remodeling (i.e., changes in structure and function), including altered Ca2+ handling, is seen in diseases with different etiologies, and is often the first step in progression to frank heart failure (Marks 2013; Schirone et al. 2017). Despite variability in the disease phenotype, some general features and patterns are common. They have been characterized in detail in animal models (e.g., during pressure overload). In the early stages of pathological remodeling, the change of ECC is adaptive, with augmented ECC observed (Ohkusa et al. 1997; Lymperopoulos et al. 2013). This is characterized by enhanced Ca2+ transient amplitude, greater contraction, and more effective relaxation (SR Ca2+ sequestration). As remodeling progresses to failure, ECC capacity decreases, eventually reaching a point that effective contraction is no longer possible (Gomez et al. 1997; Marks 2013; Høydal et al. 2018; Munro et al. 2018). Under these conditions, intracellular Ca2+ release becomes less homogenous, the kinetics of Ca2+ release and reuptake are slower, Ca2+ transient amplitude is lower, and ultimately these maladaptations combine to decrease contractile function (Fig. 4; Gomez et al. 1997; Mozaffarian et al. 2015; Høydal et al. 2018). This decline in ECC is brought about through a combined alteration in membrane architecture and Ca2+-handling machinery. The Ca2+ influx through LTCC is generally unchanged, but because of changes in cytoarchitecture, it may have a different microdistribution (Sanchez-Alonso et al. 2016). A particular feature of the remodeling of membrane architecture is alteration in TATS (Song et al. 2006; Heinzel et al. 2008; Lenaerts et al. 2009; Lyon et al. 2009; Crossman et al. 2015, 2017; Crocini et al. 2016; Seidel et al. 2017; Dries et al. 2018; Høydal et al. 2018). During the early stages of pathological remodeling, the TATS becomes disorganized and membrane/SR distances increased (Xu et al. 2007; Wu et al. 2012; Jones et al. 2018). As disease progresses, together with cardiomyocyte hypertrophy, a further disorganization (seen in rodent models) and decrease in density (recorded in large mammals) of TATS is observed, resulting in an increase in the fraction of noncoupled RyRs (Song et al. 2006; Sachse et al. 2012; Seidel et al. 2017; Dries et al. 2018; Munro et al. 2018). Consequently, a lower proportion of RyRs is directly activated by Ca2+ influx during the initial phase of the AP. The noncoupled RyRs are activated, albeit with a delay, by Ca2+ diffusion from the coupled RyRs (Dries et al. 2018). Owing to decreased synchrony in RyR activation, the Ca2+ flux from the SR is lower, consequently reducing Ca2+ transient amplitude and contraction. The rate of Ca2+ clearance is also substantially reduced in disease (Fig. 4). This is manifest as a slower rate of decline of the Ca2+ transient/contraction and an increase in cytosolic Ca2+ during relaxation (Kubalova et al. 2005; Hohendanner et al. 2013). Poor cardiomyocyte relaxation contributes to the overall decrease in diastolic function and consequently poor filling of the ventricle. A reduction in SERCA expression and/or activity is primarily responsible for this reduced Ca2+ clearance (Røe et al. 2018). Notably, in vivo re-expression of SERCA reverses many of the features of disease-associated remodeling of Ca2+ handling raising its potential as a therapy (Lyon et al. 2011). Disappointingly, clinical trials have not shown a similar benefit, which is possibly related to methodological hurdles to reach the necessary increase in SERCA expression within failing cardiomyocytes. Further advances in this area may yet provide benefit (Greenberg et al. 2016).
Figure 4.
Mitochondrial Ca2+. Mitochondria are densely packed in the cardiomyocyte being aligned along the myofilaments as bricks. Their Ca2+ uptake sites are closely localized to the junctional sarcoplasmic reticulum (SR) and a microdomain of elevated Ca2+ generated by SR Ca2+ release via RyRs. Ca2+ enters the mitochondria through the voltage-gated anion channel (VDAC) of the outer mitochondrial membrane and the mitochondrial Ca2+ uniporter (MCU) of the inner mitochondrial membrane. Ca2+ acts at multiple sites in the electron transport chain and tricarboxylic acid (TCA) cycle to modulate mitochondrial metabolism and ATP production. Ca2+ is extruded from the mitochondria through a Na+/Li+/Ca2+ exchanger (NCXL) and/or a Ca2+/H+ exchanger (mHCX).
Further adding to the perturbation of Ca2+ handling in disease is a dysregulation of SR Ca2+ release. At the nanoscale, RyR clusters are reorganized in heart failure, which is associated with an increase in their activity (Kolstad et al. 2018). Atrial fibrillation is also associated with changes in RyR cluster size, distribution, and activity (Macquaide et al. 2015). Increased RyR channel activity in diastole, observed as increased spark frequency, is very often seen, including in late-stage human heart failure (Fischer et al. 2014; Dries et al. 2018). Although RyR expression is not substantially altered in disease, posttranslational modifications including phosphorylation by PKA and CaMKII, oxidation, as well as remodeling of RyR clusters, contribute to the increase in spontaneous Ca2+ release events (Fischer et al. 2014; Ho et al. 2014; Grimm et al. 2015; Fu et al. 2016; Uchinoumi et al. 2016; Walweel et al. 2017; Dries et al. 2018). The greater frequency of these spontaneous Ca2+ release events can lead to a leak of Ca2+ from the SR as well as an increase in the propensity of Ca2+ waves (Cheng et al. 1996; Venetucci et al. 2008; Curran et al. 2010; Dries et al. 2018). These Ca2+ waves stimulate NCX activity, which because of its electrogenic nature, leads to the generation of delayed after depolarizations (DADs), triggering APs that may propagate to neighboring cardiomyocytes and induce arrhythmia (Venetucci et al. 2008). A similar proarrhythmic phenotype arises because of CPVT-associated mutations in RyR2 (Priori and Chen 2011). These mutated forms of RyRs show enhanced sensitivity to luminal and/or cytosolic Ca2+ and show increased spontaneous openings. Although the heart can accommodate for these more active RyRs under normal conditions, during periods of increased catecholamine stimulation and associated increased SR store loading, large spontaneous Ca2+ release events induce NCX activity and AP generation. This can trigger arrhythmogenic events, ventricular tachycardia, and eventually sudden cardiac death. The identification of small molecules such as K201 and EL0 that stabilize the closed state of the RyR may reverse the pathological SR Ca2+ leak observed in heart failure as well as in CPVT (Li et al. 2017).
A re-expression of a gene program expressed in the fetus/neonate (the fetal gene program) characterized by atrial and brain natriuretic factors (NPPA and NPPB) is often described during hypertrophy. Notably this gene program includes a number of proteins involved in Ca2+ handling. Indeed, increases in expression of InsP3R (Harzheim et al. 2010), proteins involved in SOCE (Correll et al. 2015), and of T-type Ca2+ channels are observed during cardiac pathology (Izumi et al. 2003). Moreover, the increase in the expression of these proteins contributes to pathological alterations of cardiomyocyte physiology (Izumi et al. 2003; Harzheim et al. 2010; Correll et al. 2015).
Increased InsP3R expression, as well as the mechanisms responsible for InsP3 generation, are observed in hypertrophy and heart failure, as well as in atrial fibrillation (Yamda et al. 2001; Harzheim et al. 2010; Drawnel et al. 2012; Signore et al. 2013). As a consequence, Ca2+ release independent of ECC is enhanced (Harzheim et al. 2009; Nakayama et al. 2010; Hohendanner et al. 2015b). Although InsP3-mediated Ca2+ release remains of a low magnitude, through promoting Ca2+ release via neighboring Ca2+-sensitive RyRs, it leverages increased Ca2+ spark events that may lead to arrhythmogenic cell-wide Ca2+ activity (Hohendanner et al. 2015b). The summation of increased RyR sensitivity and greater InsP3-mediated Ca2+ release may create a perfect storm in which the cardiomyocyte is sensitized to spontaneous release that, in turn, via activation of NCX, induces the generation of APs and tissue-wide arrhythmia.
SOCE is also increased in hypertrophied cardiomyocytes (Hulot et al. 2011; Luo et al. 2012). Remarkably, the overwhelming majority of studies showed that stress-induced overexpression of SOCE-associated proteins and, hence, the enhanced activity of SOCE in cardiomyocytes contribute to, and sustain, hypertrophic remodeling (Hunton et al. 2002, 2004; Ohba et al. 2007; Voelkers et al. 2010; Hulot et al. 2011). Attenuation of SOCE components suppresses this induction of hypertrophy in neonatal cardiac myocytes (Voelkers et al. 2010; Luo et al. 2012). Increased SOCE through transgenic STIM1 overexpression is also sufficient to induce hypertrophic remodeling of the heart, promoting enhanced CnA/NFAT and CaMKII signaling. Moreover, in advance of disease development, STIM1 overexpression induces spontaneous Ca2+ transients, increased LTCC current and Ca2+ sparks—effects that may underlie the sudden death observed in these mice (Correll et al. 2015). Together these combined roles of SOCE in cardiomyocytes would suggest that SOCE inhibition may be of therapeutic benefit (Kojima et al. 2012).
Ca2+ Triggering of Hypertrophic Remodeling
Alterations in Ca2+ handling are a cue for cardiac remodeling during disease. The connection between Ca2+ and cardiomyocyte growth—hypertrophy associated with disease—was first uncovered by examining the responses of neonatal cardiomyocytes to altered frequency of electrical stimulation (McDonough et al. 1994; Tavi et al. 2004). In vivo, tachypacing or catecholamine infusion is also sufficient to elicit a hypertrophic response (Kubalova et al. 2005). The absence of hypertrophy in mice deficient for PLB that show a constitutive inotropic state is not sufficient to trigger hypertrophy, and suggests that enhanced Ca2+ cycling per se is not, however, sufficient for the induction of hypertrophic gene expression (Kiriazis and Kranias 2000). Indeed, increases in nuclear Ca2+ are now considered to play an important role in activation/modulation of cardiomyocyte gene expression (Fig. 5; Wu et al. 2006; Guatimosim et al. 2008; Higazi et al. 2009; Arantes et al. 2012). Moreover, in the absence of these nuclear Ca2+ changes, altered frequency of cytosolic Ca2+ oscillations may not be sufficient to increase hypertrophic gene expression (Higazi et al. 2009). In generating nuclear-specific Ca2+ changes, InsP3Rs have been proposed to play a role (Wu et al. 2006; Guatimosim et al. 2008; Higazi et al. 2009; Arantes et al. 2012). Since InsP3Rs are activated downstream of Gαq-coupled receptors by circulating hormones or phospholipase Cε (Zhang et al. 2013), this mechanism can be engaged independent of the Ca2+ changes associated with ECC (Fig. 5).
Figure 5.
Remodeling of excitation–contraction coupling (ECC) in disease. (1) The coupling between Ca2+ influx and Ca2+ release is compromised because of a loss of tight connections between the transverse and axial tubule system (TATS) and the sarcoplasmic reticulum (SR) with an atrophy and remodeling of the tubular network. (2) RyRs thus become uncoupled and are then activated with a delay by Ca2+ diffusion across the cell from coupled RyRs. (3) Consequently, activation of myofilaments is less homogeneous and contraction impaired. (4) SERCA expression is reduced leading to an elevation in diastolic Ca2+ and reduced SR Ca2+, which in turn leads to a decrease in Ca2+ release via RyRs during each ECC cycle, thereby reducing contraction amplitude. (5) RyRs display spontaneous releases of Ca2+, (6) generating Ca2+ waves. (7) This cytosolic Ca2+ overload leads to NCX activation, triggering after-depolarizations and potentially a spontaneous AP.
Figure 6.
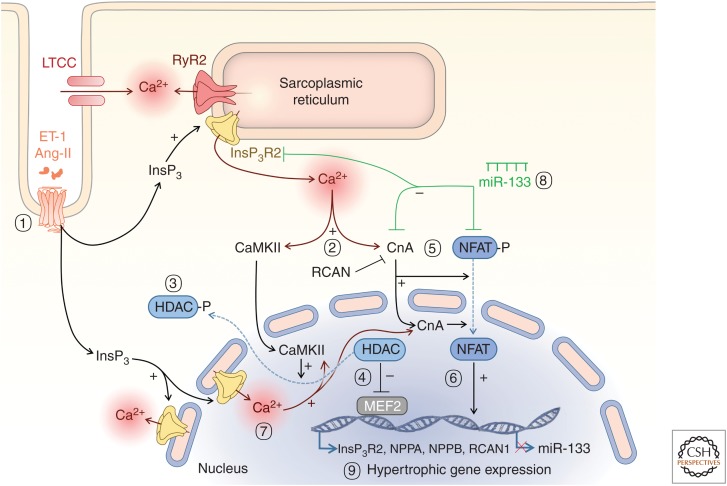
Excitation–transcription coupling. (1) InsP3R-mediated cytosolic Ca2+ increase induces activation of different signaling pathways: (2) Ca2+ activates cytosolic and nuclear CaMKII that phosphorylates the inhibitory transcription factor histone deacetylase (HDAC) and (3) induces its export out of the nucleus. (4) MEF2 is activated and this triggers hypertrophic genes expression. (5) Ca2+ increases, including via InsP3Rs, activates calcineurin (CnA), leading to dephosphorylation of nuclear factor of activated T-cells (NFAT) and its translocation to the nucleus. (6) NFAT then activates the transcription of hypertrophic genes including fetal genes (InsP3R, NPPA, NPPB, RCAN1) and can also repress miR-133. (7) Nuclear Ca2+ signals arising from InsP3R can contribute to activation of nuclear CnA and trigger pathways for hypertrophy (9). (7) miR-133 suppresses expression of NFAT, CnA, and InsP3R, which will inhibit the hypertrophic responses previously described (8).
The role of Ca2+ in hypertrophic gene expression is further substantiated by gain- and loss-of-function studies involving downstream sensors and mediators of Ca2+. Through a number of studies, key roles for a pathway involving calmodulin (CaM) (Obata et al. 2005), the archetypal Ca2+ sensor, calcineurin (CnA) a calcium-dependent phosphatase and its downstream transcription factor—the nuclear factor of activated T-cells (NFAT) in hypertrophic gene expression has been identified (Fig. 5(5) and 5(6); Molkentin et al. 1998; Sussman et al. 1998; Bourajjaj et al. 2008). Nuclear Ca2+ changes have been invoked in regulation of this pathway through maintaining CnA in the nucleus and prolonging its dephosphorylation of NFAT (Higazi et al. 2009; Olivares-Florez et al. 2018). Indeed, nuclear translocation of CaM is important in the activity of nuclear CnA (Zhu and McKeon 1999; Oda et al. 2018). The involvement of this CnA/NFAT pathway in hypertrophy induced by a pathological tropomyosin mutation suggests a more general role for this pathway in cardiac pathology (Sussman et al. 1998). Ca2+ also elicits its hypertrophic effects via CaMKII. This kinase phosphorylates the type II histone deacetylase (HDAC) causing its translocation out of the nucleus and loss of inhibition of MEF2-dependent gene expression (Fig. 5(7) and 5(8); Zhang et al. 2002; Wu et al. 2006; Backs et al. 2008).
Ca2+ Handling and Hypertrophic Remodeling Are Interdependent
Notably, while ECC and the gene expression underlying hypertrophic remodeling are selectively controlled by Ca2+, these cell mechanisms are intimately linked. Indeed, at the same time that Ca2+ stimulates the induction of hypertrophy, the expression of Ca2+-handling proteins involved in ECC is altered during disease. Thus, the Ca2+ signals that stimulate hypertrophy are modified. As a consequence, the hypertrophic response is augmented and sustained. Clear examples of this feedback/feedforward system are illustrated by the roles of InsP3Rs and SERCA2a. As indicated above, SERCA activity/expression is often decreased during heart failure contributing to the associated diminished contractility. Re-expression of SERCA leads, however, to a reversal of the hypertrophic phenotype: SR Ca2+ leak, β-adrenergic receptor distribution and ECC return to a healthy state (Lyon et al. 2011). InsP3R expression is increased and the nuclear membrane invaginations where it is localized decreased during disease (Ljubojevic et al. 2014). Nuclear InsP3 signaling is also a cue for the activation of CnA/NFAT and CaMKII/HDAC pathways and the induction of the hypertrophic response (Wu et al. 2006; Higazi et al. 2009; Plačkić et al. 2016). Notably, InsP3 signaling suppresses the expression of microRNA miR-133 (Figs. 5(2), 5(3), and 6; Drawnel et al. 2012), which is not only an inhibitor of hypertrophy-related genes but also of InsP3R expression (Li et al. 2018). Thus, enhanced InsP3 signaling that may initiate hypertrophy, is self-augmenting and sustaining. Further, InsP3R expression has also been reported to be under the control of NFAT (Sankar et al. 2014; Olivares-Florez et al. 2018), which is again sensitive to InsP3 signaling independent of Ca2+ changes associated with ECC (Higazi et al. 2009). CnA/NFAT signaling also shows feedback regulation. In particular, NFAT stimulates induction of RCAN1.4 (regulator of CnA) also known as DCSR1 and calcipressin), which then directly inhibits the activity of CnA (Oh et al. 2010). These links between modifications in expression of certain Ca2+-handling proteins, in particular those that show disease-dependent changes, are particularly interesting targets for therapeutic intervention.
CONCLUSIONS AND PERSPECTIVES
Cardiomyocytes rely on Ca2+ signaling for their core task as contractile units in the heart, and Ca2+ dysregulation is one of the main contributors to failure of the heart as pump. Yet, Ca2+ signaling in cardiomyocytes is equally pivotal as a mechanism for regulating cardiomyocyte growth and physiological remodeling. Further, Ca2+-handling mechanisms are remodeled in disease, generating a unique link with contraction and arrhythmias in heart disease (Denham et al. 2018). Such feedback loops involving these dual roles of Ca2+ in the cardiomyocyte may represent ideal targets for therapy (Hulot et al. 2012; Gabisonia and Recchia 2018). To gain greater insight into these mechanisms and to develop strategies for their exploitation for therapeutic ends, novel approaches and methodologies are required. Further insights into cardiomyocyte physiology and mechanisms underlying ECC are expected to emerge with the development of new technologies including live cell imaging, superresolution microscopy, and in particular automated image analysis and artificial intelligence for high throughput and discovery (Sacconi et al. 2006; Jayasinghe et al. 2018a). Using multiomics approaches and single-cell analysis, new molecular targets and their interactions that determine the physiological status of the cardiomyocyte will be discovered (Barwari et al. 2016; Perrino et al. 2017; See et al. 2017; Thienpont et al. 2017; Gilsbach et al. 2018), for example, microRNAs, long noncoding RNAs, and epigenetic modifications that govern the expression of Ca2+-handling proteins. Notably, some of these microRNAs are found in the circulation in plasma (sometimes in exosomes) and act on the cardiomyocyte compartment as well as being biomarkers of disease progression (Hoefer et al. 2015; Devaux et al. 2017). As our understanding and methodology for physiological analysis of intact tissue develops, analysis of the interactions of the multiple cell types in the heart (e.g., fibroblasts with cardiac myocytes) and their heterogeneity will be made possible (Perbellini et al. 2018; Scardigli et al. 2018b). Through these means, a true picture of how Ca2+ signaling at the cellular level is regulated to control cardiac function will emerge.
COMPETING INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
Work in the authors’ laboratories is supported by grants from the Research Foundation Flanders (FWO). Specifically, Odysseus grant (90663) and Project Grant (G08861N) to H.L.R., Project Grant (G091815N) to K.S., and Post-doctoral Fellowships to G.G. (12W6218N) and E.D. (12Q1117N).
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Arantes LA, Aguiar CJ, Amaya MJ, Figueiró NC, Andrade LM, Rocha-Resende C, Resende RR, Franchini KG, Guatimosim S, Leite MF. 2012. Nuclear inositol 1,4,5-trisphosphate is a necessary and conserved signal for the induction of both pathological and physiological cardiomyocyte hypertrophy. J Mol Cell Cardiol 53: 475–486. 10.1016/j.yjmcc.2012.06.017 [DOI] [PubMed] [Google Scholar]
- Avila-Medina J, Mayoral-Gonzalez I, Dominguez-Rodriguez A, Gallardo-Castillo I, Ribas J, Ordoñez A, Rosado JA, Smani T. 2018. The complex role of store operated calcium entry pathways and related proteins in the function of cardiac, skeletal and vascular smooth muscle cells. Front Physiol 9: 257 10.3389/fphys.2018.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. 2008. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol 28: 3437–3445. 10.1128/MCB.01611-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak J, Billington RA, Timar G, Dutton AC, Genazzani AA. 2001. NAADP receptors are present and functional in the heart. Curr Biol 11: 987–990. 10.1016/S0960-9822(01)00269-X [DOI] [PubMed] [Google Scholar]
- Balaban RS. 2009. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochem Biophys Acta 1787: 1334–1341. 10.1016/j.bbabio.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwari T, Joshi A, Mayr M. 2016. MicroRNAs in cardiovascular disease. J Am Coll Cardiol 68: 2577–2584. 10.1016/j.jacc.2016.09.945 [DOI] [PubMed] [Google Scholar]
- Bassani JW, Bassani RA, Bers DM. 1994. Relaxation in rabbit and rat cardiac cells: Species-dependent differences in cellular mechanisms. J Physiol 476: 279–293. 10.1113/jphysiol.1994.sp020130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. 2011. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345. 10.1038/nature10234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. 1993. Inositol trisphosphate and calcium signalling. Nature 361: 315–325. 10.1038/361315a0 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Bers DM. 2002. Cardiac excitation–contraction coupling. Nature 415: 198–205. 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- Beuckelmann DJ, Wier WG. 1988. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol 405: 233–255. 10.1113/jphysiol.1988.sp017331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. 2005. Type 1 ryanodine receptor in cardiac mitochondria: Transducer of excitation-metabolism coupling. Biochem Biophys Acta 1717: 1–10. 10.1016/j.bbamem.2005.09.016 [DOI] [PubMed] [Google Scholar]
- Blanch ISJ, Egger M. 2018. Obstruction of ventricular Ca2+-dependent arrhythmogenicity by inositol 1,4,5-trisphosphate-triggered sarcoplasmic reticulum Ca2+ release. J Physiol 596: 4323–4340. 10.1113/JP276319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Rietdorf K. 2017. Tissue specificity: Store-operated Ca2+ entry in cardiac myocytes. Adv Exp Med Biol 993: 363–387. 10.1007/978-3-319-57732-6_19 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Higazi DR, Coombes S, Roderick HL. 2006. Calcium signalling during excitation-contraction coupling in mammalian atrial myocytes. J Cell Sci 119: 3915–3925. 10.1242/jcs.03223 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Harzheim D, Smyrnias I, Conway SJ, Roderick HL. 2007. Temporal changes in atrial EC-coupling during prolonged stimulation with endothelin-1. Cell Calcium 42: 489–501. 10.1016/j.ceca.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Smyrnias I, Thul R, Coombes S, Roderick HL. 2011. Atrial cardiomyocyte calcium signalling. Biochem Biophys Acta 1813: 922–934. 10.1016/j.bbamcr.2011.01.030 [DOI] [PubMed] [Google Scholar]
- Bossen EH, Sommer JR, Waugh RA. 1978. Comparative stereology of the mouse and finch left ventricle. Tissue Cell 10: 773–784. 10.1016/0040-8166(78)90062-9 [DOI] [PubMed] [Google Scholar]
- Bourajjaj M, Armand AS, da Costa Martins PA, Weijts B, van der Nagel R, Heeneman S, Wehrens XH, De Windt LJ. 2008. NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J Biol Chem 283: 22295–22303. 10.1074/jbc.M801296200 [DOI] [PubMed] [Google Scholar]
- Boyman L, Chikando AC, Williams GS, Khairallah RJ, Kettlewell S, Ward CW, Smith GL, Kao JP, Lederer WJ. 2014. Calcium movement in cardiac mitochondria. Biophys J 107: 1289–1301. 10.1016/j.bpj.2014.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg S, Kohl T, Williams GS, Gusev K, Wagner E, Rog-Zielinska EA, Hebisch E, Dura M, Didié M, Gotthardt M, et al. 2016. Axial tubule junctions control rapid calcium signaling in atria. J Clin Invest 126: 3999–4015. 10.1172/JCI88241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg S, Pawlowitz J, Fakuade FE, Kownatzki-Danger D, Kohl T, Mitronova GY, Scardigli M, Neef J, Schmidt C, Wiedmann F, et al. 2018. Axial tubule junctions activate atrial Ca2+ release across species. Front Physiol 9: 1227 10.3389/fphys.2018.01227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brette F, Despa S, Bers DM, Orchard CH. 2005. Spatiotemporal characteristics of SR Ca2+ uptake and release in detubulated rat ventricular myocytes. J Mol Cell Cardiol 39: 804–812. 10.1016/j.yjmcc.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Buntinas L, Gunter KK, Sparagna GC, Gunter TE. 2001. The rapid mode of calcium uptake into heart mitochondria (RaM): Comparison to RaM in liver mitochondria. Biochem Biophys Acta 1504: 248–261. 10.1016/S0005-2728(00)00254-1 [DOI] [PubMed] [Google Scholar]
- Burton FL, Cobbe SM. 2001. Dispersion of ventricular repolarization and refractory period. Cardiovasc Res 50: 10–23. 10.1016/S0008-6363(01)00197-3 [DOI] [PubMed] [Google Scholar]
- Cala SE, Scott BT, Jones LR. 1990. Intralumenal sarcoplasmic reticulum Ca2+-binding proteins. Semin Cell Biol 1: 265–275. [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. 2009. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459: 596–600. 10.1038/nature08030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Kong CHT. 2017. Quenching the spark: Termination of CICR in the submicroscopic space of the dyad. J Gen Physiol 149: 837–845. 10.1085/jgp.201711807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Berlin JR, Lederer WJ. 1987. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science 238: 1419–1423. 10.1126/science.2446391 [DOI] [PubMed] [Google Scholar]
- Capel RA, Bolton EL, Lin WK, Aston D, Wang Y, Liu W, Wang X, Burton RA, Bloor-Young D, Shade KT, et al. 2015. Two-pore channels (TPC2s) and nicotinic acid adenine dinucleotide phosphate (NAADP) at lysosomal-sarcoplasmic reticular junctions contribute to acute and chronic β-adrenoceptor signaling in the heart. J Biol Chem 290: 30087–30098. 10.1074/jbc.M115.684076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. 1999. Cardiac ionic currents and acute ischemia: From channels to arrhythmias. Physiol Rev 79: 917–1017. 10.1152/physrev.1999.79.3.917 [DOI] [PubMed] [Google Scholar]
- Cens T, Rousset M, Leyris JP, Fesquet P, Charnet P. 2006. Voltage- and calcium-dependent inactivation in high voltage-gated Ca2+ channels. Prog Biophys Mol Biol 90: 104–117. 10.1016/j.pbiomolbio.2005.05.013 [DOI] [PubMed] [Google Scholar]
- Chen B, Guo A, Zhang C, Chen R, Zhu Y, Hong J, Kutschke W, Zimmerman K, Weiss RM, Zingman L, et al. 2013. Critical roles of junctophilin-2 in T-tubule and excitation–contraction coupling maturation during postnatal development. Cardiovasc Res 100: 54–62. 10.1093/cvr/cvt180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer MR, Lederer WJ, Cannell MB. 1996. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol 270: C148–C159. 10.1152/ajpcell.1996.270.1.C148 [DOI] [PubMed] [Google Scholar]
- Chiamvimonvat N, Chen-Izu Y, Clancy CE, Deschenes I, Dobrev D, Heijman J, Izu L, Qu Z, Ripplinger CM, Vandenberg JI, et al. 2017. Potassium currents in the heart: Functional roles in repolarization, arrhythmia and therapeutics. J Physiol 595: 2229–2252. 10.1113/JP272883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins TJ, Berridge MJ, Lipp P, Bootman MD. 2002. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J 21: 1616–1627. 10.1093/emboj/21.7.1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins TP, Bayliss R, Churchill GC, Galione A, Terrar DA. 2011. NAADP influences excitation–contraction coupling by releasing calcium from lysosomes in atrial myocytes. Cell Calcium 50: 449–458. 10.1016/j.ceca.2011.07.007 [DOI] [PubMed] [Google Scholar]
- Cordeiro JM, Zeina T, Goodrow R, Kaplan AD, Thomas LM, Nesterenko VV, Treat JA, Hawel L III, Byus C, Bett GC, et al. 2015. Regional variation of the inwardly rectifying potassium current in the canine heart and the contributions to differences in action potential repolarization. J Mol Cell Cardiol 84: 52–60. 10.1016/j.yjmcc.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll RN, Goonasekera SA, van Berlo JH, Burr AR, Accornero F, Zhang H, Makarewich CA, York AJ, Sargent MA, Chen X, et al. 2015. STIM1 elevation in the heart results in aberrant Ca2+ handling and cardiomyopathy. J Mol Cell Cardiol 87: 38–47. 10.1016/j.yjmcc.2015.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocini C, Coppini R, Ferrantini C, Yan P, Loew LM, Tesi C, Cerbai E, Poggesi C, Pavone FS, Sacconi L. 2014. Defects in T-tubular electrical activity underlie local alterations of calcium release in heart failure. Proc Natl Acad Sci 111: 15196–15201. 10.1073/pnas.1411557111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocini C, Ferrantini C, Scardigli M, Coppini R, Mazzoni L, Lazzeri E, Pioner JM, Scellini B, Guo A, Song LS, et al. 2016. Novel insights on the relationship between T-tubular defects and contractile dysfunction in a mouse model of hypertrophic cardiomyopathy. J Mol Cell Cardiol 91: 42–51. 10.1016/j.yjmcc.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman DJ, Young AA, Ruygrok PN, Nason GP, Baddelely D, Soeller C, Cannell MB. 2015. T-tubule disease: Relationship between T-tubule organization and regional contractile performance in human dilated cardiomyopathy. J Mol Cell Cardiol 84: 170–178. 10.1016/j.yjmcc.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman DJ, Shen X, Jüllig M, Munro M, Hou Y, Middleditch M, Shrestha D, Li A, Lal S, Dos Remedios CG, et al. 2017. Increased collagen within the transverse tubules in human heart failure. Cardiovasc Res 113: 879–891. 10.1093/cvr/cvx055 [DOI] [PubMed] [Google Scholar]
- Csordás G, Thomas AP, Hajnóczky G. 1999. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J 18: 96–108. 10.1093/emboj/18.1.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG, Balla T, Hajnóczky G. 2010. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39: 121–132. 10.1016/j.molcel.2010.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Golenár T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, et al. 2013. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab 17: 976–987. 10.1016/j.cmet.2013.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Galione A, Terrar DA. 1999. Effects of photoreleased cADP-ribose on calcium transients and calcium sparks in myocytes isolated from guinea-pig and rat ventricle. Biochem J 342: 269–273. 10.1042/bj3420269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. 2010. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca2+-calmodulin-dependent protein kinase II. J Mol Cell Cardiol 49: 25–32. 10.1016/j.yjmcc.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dally S, Corvazier E, Bredoux R, Bobe R, Enouf J. 2010. Multiple and diverse coexpression, location, and regulation of additional SERCA2 and SERCA3 isoforms in nonfailing and failing human heart. J Mol Cell Cardiol 48: 633–644. 10.1016/j.yjmcc.2009.11.012 [DOI] [PubMed] [Google Scholar]
- Dedkova EN, Blatter LA. 2013. Calcium signaling in cardiac mitochondria. J Mol Cell Cardiol 58: 125–133. 10.1016/j.yjmcc.2012.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente S, Fernandez-Sanz C, Vail C, Agra EJ, Holmstrom K, Sun J, Mishra J, Williams D, Finkel T, Murphy E, et al. 2016. Strategic positioning and biased activity of the mitochondrial calcium uniporter in cardiac muscle. J Biol Chem 291: 23343–23362. 10.1074/jbc.M116.755496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente S, Lambert JP, Nichtova Z, Fernandez Sanz C, Elrod JW, Sheu SS, Csordás G. 2018. Spatial separation of mitochondrial calcium uptake and extrusion for energy-efficient mitochondrial calcium signaling in the heart. Cell Rep 24: 3099–3107.e4. 10.1016/j.celrep.2018.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham NC, Pearman CM, Caldwell JL, Madders GWP, Eisner DA, Trafford AW, Dibb KM. 2018. Calcium in the pathophysiology of atrial fibrillation and heart failure. Front Physiol 9: 1380 10.3389/fphys.2018.01380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RM, McCormack JG, Midgley PJ, Rutter GA, Thomas AP. 1988. The role of Ca2+ in the hormonal control of intramitochondrial metabolism in heart, liver, and adipose tissue. Adv Second Messenger Phosphoprotein Res 21: 157–164. [PubMed] [Google Scholar]
- Despa S, Bers DM. 2013. Na+ transport in the normal and failing heart—Remember the balance. J Mol Cell Cardiol 61: 2–10. 10.1016/j.yjmcc.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340. 10.1038/nature10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux Y, Creemers EE, Boon RA, Werfel S, Thum T, Engelhardt S, Dimmeler S, Squire I. 2017. Circular RNAs in heart failure. Eur J Heart Fail 19: 701–709. 10.1002/ejhf.801 [DOI] [PubMed] [Google Scholar]
- Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. 2008. IP3 receptor-dependent Ca2+ release modulates excitation–contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circulatory Physiol 294: H596–H604. 10.1152/ajpheart.01155.2007 [DOI] [PubMed] [Google Scholar]
- Dostal DE, Baker KM. 1999. The cardiac renin-angiotensin system: Conceptual, or a regulator of cardiac function? Circ Res 85: 643–650. 10.1161/01.RES.85.7.643 [DOI] [PubMed] [Google Scholar]
- Drago I, De Stefani D, Rizzuto R, Pozzan T. 2012. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci 109: 12986–12991. 10.1073/pnas.1210718109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drawnel FM, Wachten D, Molkentin JD, Maillet M, Aronsen JM, Swift F, Sjaastad I, Liu N, Catalucci D, Mikoshiba K, et al. 2012. Mutual antagonism between IP3RII and miRNA-133a regulates calcium signals and cardiac hypertrophy. J Cell Biol 199: 783–798. 10.1083/jcb.201111095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drawnel FM, Archer CR, Roderick HL. 2013. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. Br J Pharmacol 168: 296–317. 10.1111/j.1476-5381.2012.02195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dries E, Bito V, Lenaerts I, Antoons G, Sipido KR, Macquaide N. 2013. Selective modulation of coupled ryanodine receptors during microdomain activation of calcium/calmodulin-dependent kinase II in the dyadic cleft. Circ Res 113: 1242–1252. 10.1161/circresaha.113.301896 [DOI] [PubMed] [Google Scholar]
- Dries E, Santiago DJ, Gilbert G, Lenaerts I, Vandenberk B, Nagaraju CK, Johnson DM, Holemans P, Roderick HL, Macquaide N, et al. 2018. Hyperactive ryanodine receptors in human heart failure and ischaemic cardiomyopathy reside outside of couplons. Cardiovasc Res 114: 1512–1524. 10.1093/cvr/cvy088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. 2014. Calcium in the heart: From physiology to disease. Exp Physiol 99: 1273–1282. 10.1113/expphysiol.2013.077305 [DOI] [PubMed] [Google Scholar]
- Fabiato A. 1983. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol 245: C1–C14. 10.1152/ajpcell.1983.245.1.C1 [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. 1977. Calcium release from the sarcoplasmic reticulum. Circ Res 40: 119–129. 10.1161/01.RES.40.2.119 [DOI] [PubMed] [Google Scholar]
- Fearnley CJ, Roderick HL, Bootman MD. 2011. Calcium signaling in cardiac myocytes. Cold Spring Harb Perspect Biol 3: a004242 10.1101/cshperspect.a004242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrantini C, Crocini C, Coppini R, Vanzi F, Tesi C, Cerbai E, Poggesi C, Pavone FS, Sacconi L. 2013. The transverse-axial tubular system of cardiomyocytes. Cell Mol Life Sci 70: 4695–4710. 10.1007/s00018-013-1410-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TH, Eiringhaus J, Dybkova N, Förster A, Herting J, Kleinwächter A, Ljubojevic S, Schmitto JD, Streckfuss-Bömeke K, Renner A, et al. 2014. Ca2+/calmodulin-dependent protein kinase II equally induces sarcoplasmic reticulum Ca2+ leak in human ischaemic and dilated cardiomyopathy. Eur J Heart Failure 16: 1292–1300. 10.1002/ejhf.163 [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO. 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658. 10.1152/physrev.00035.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard HA. 1977. Heart: Excitation-contraction coupling. Ann Rev Physiol 39: 201–220. 10.1146/annurev.ph.39.030177.001221 [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F, Tijskens P. 2005. The assembly of calcium release units in cardiac muscle. Ann NY Acad Sci 1047: 76–85. 10.1196/annals.1341.007 [DOI] [PubMed] [Google Scholar]
- Frisk M, Koivumäki JT, Norseng PA, Maleckar MM, Sejersted OM, Louch WE. 2014. Variable t-tubule organization and Ca2+ homeostasis across the atria. Am J Physiol Heart Circ Physiol 307: H609–H620. 10.1152/ajpheart.00295.2014 [DOI] [PubMed] [Google Scholar]
- Frisk M, Ruud M, Espe EK, Aronsen JM, Røe AT, Zhang L, Norseng PA, Sejersted OM, Christensen GA, Sjaastad I, et al. 2016. Elevated ventricular wall stress disrupts cardiomyocyte t-tubule structure and calcium homeostasis. Cardiovasc Res 112: 443–451. 10.1093/cvr/cvw111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Hong T. 2016. BIN1 regulates dynamic t-tubule membrane. Biochim Biophys Acta 1863: 1839–1847. 10.1016/j.bbamcr.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Shaw SA, Naami R, Vuong CL, Basheer WA, Guo X, Hong T. 2016. Isoproterenol promotes rapid ryanodine receptor movement to bridging integrator 1 (BIN1)-organized dyads. Circulation 133: 388–397. 10.1161/circulationaha.115.018535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta H, Little WC. 2008. The cardiac cycle and the physiologic basis of left ventricular contraction, ejection, relaxation, and filling. Heart Fail Clin 4: 1–11. 10.1016/j.hfc.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabisonia K, Recchia FA. 2018. Gene therapy for heart failure: New perspectives. Curr Heart Fail Rep 15: 340–349. 10.1007/s11897-018-0410-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeberg HC, Bond RC, Kong CH, Chanoit GP, Ascione R, Cannell MB, James AF. 2016. Heterogeneity of T-tubules in pig hearts. PLoS ONE 11: e0156862 10.1371/journal.pone.0156862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadicherla AK, Wang N, Bulic M, Agullo-Pascual E, Lissoni A, De Smet M, Delmar M, Bultynck G, Krysko DV, Camara A, et al. 2017. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res Cardiol 112: 27 10.1007/s00395-017-0618-1 [DOI] [PubMed] [Google Scholar]
- Gadsby DC. 1984. The Na/K pump of cardiac cells. Annu Rev Biophys Bioeng 13: 373–398. 10.1146/annurev.bb.13.060184.002105 [DOI] [PubMed] [Google Scholar]
- Galione A, Cui Y, Empson R, Iino S, Wilson H, Terrar D. 1998. Cyclic ADP-ribose and the regulation of calcium-induced calcium release in eggs and cardiac myocytes. Cell Biochem Biophys 28: 19–30. 10.1007/BF02738307 [DOI] [PubMed] [Google Scholar]
- Garcia MI, Karlstaedt A, Chen JJ, Amione-Guerra J, Youker KA, Taegtmeyer H, Boehning D. 2017. Functionally redundant control of cardiac hypertrophic signaling by inositol 1,4,5-trisphosphate receptors. J Mol Cell Cardiol 112: 95–103. 10.1016/j.yjmcc.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciarena CD, Youm JB, Swietach P, Vaughan-Jones RD. 2013. H+-activated Na+ influx in the ventricular myocyte couples Ca2+-signalling to intracellular pH. J Mol Cell Cardiol 61: 51–59. 10.1016/j.yjmcc.2013.04.008 [DOI] [PubMed] [Google Scholar]
- Gilsbach R, Schwaderer M, Preissl S, Grüning BA, Kranzhöfer D, Schneider P, Nührenberg TG, Mulero-Navarro S, Weichenhan D, Braun C, et al. 2018. Distinct epigenetic programs regulate cardiac myocyte development and disease in the human heart in vivo. Nat Commun 9: 391 10.1038/s41467-017-02762-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch HG. 1979. Characteristics of active Na transport in intact cardiac cells. Am J Physiol 236: H189–H199. 10.1152/ajpheart.1979.236.2.H189 [DOI] [PubMed] [Google Scholar]
- Glukhov AV, Balycheva M, Sanchez-Alonso JL, Ilkan Z, Alvarez-Laviada A, Bhogal N, Diakonov I, Schobesberger S, Sikkel MB, Bhargava A, et al. 2015. Direct evidence for microdomain-specific localization and remodeling of functional L-type calcium channels in rat and human atrial myocytes. Circulation 132: 2372–2384. 10.1161/circulationaha.115.018131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. 1997. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276: 800–806. 10.1126/science.276.5313.800 [DOI] [PubMed] [Google Scholar]
- Gorza L, Schiaffino S, Volpe P. 1993. Inositol 1,4,5-trisphosphate receptor in heart: Evidence for its concentration in Purkinje myocytes of the conduction system. J Cell Biol 121: 345–353. 10.1083/jcb.121.2.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR, et al. 2016. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): A randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 387: 1178–1186. 10.1016/S0140-6736(16)00082-9 [DOI] [PubMed] [Google Scholar]
- Grimm C, Hassan S, Wahl-Schott C, Biel M. 2012. Role of TRPML and two-pore channels in endolysosomal cation homeostasis. J Pharmacol Exp Ther 342: 236–244. 10.1124/jpet.112.192880 [DOI] [PubMed] [Google Scholar]
- Grimm M, Ling H, Willeford A, Pereira L, Gray CB, Erickson JR, Sarma S, Respress JL, Wehrens XH, Bers DM, et al. 2015. CaMKIIδ mediates β-adrenergic effects on RyR2 phosphorylation and SR Ca2+ leak and the pathophysiological response to chronic β-adrenergic stimulation. J Mol Cell Cardiol 85: 282–291. 10.1016/j.yjmcc.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatimosim S, Amaya MJ, Guerra MT, Aguiar CJ, Goes AM, Gómez-Viquez NL, Rodrigues MA, Gomes DA, Martins-Cruz J, Lederer WJ, et al. 2008. Nuclear Ca2+ regulates cardiomyocyte function. Cell Calcium 44: 230–242. 10.1016/j.ceca.2007.11.016 [DOI] [PubMed] [Google Scholar]
- Gul R, Kim SY, Park KH, Kim BJ, Kim SJ, Im MJ, Kim UH. 2008. A novel signaling pathway of ADP-ribosyl cyclase activation by angiotensin II in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol 295: H77–H88. 10.1152/ajpheart.01355.2007 [DOI] [PubMed] [Google Scholar]
- Gul R, Park JH, Kim SY, Jang KY, Chae JK, Ko JK, Kim UH. 2009. Inhibition of ADP-ribosyl cyclase attenuates angiotensin II-induced cardiac hypertrophy. Cardiovasc Res 81: 582–591. 10.1093/cvr/cvn232 [DOI] [PubMed] [Google Scholar]
- Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, Fruen BR, Bers DM. 2010. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res 106: 1743–1752. 10.1161/circresaha.110.219816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke I, Hester N, Jones LR, Györke S. 2004. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J 86: 2121–2128. 10.1016/S0006-3495(04)74271-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock PS, Coetzee WA, Cho E, Porter L, Katoh H, Bers DM, Jafri MS, Artman M. 1999. Subcellular [Ca2+]i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. Circ Res 85: 415–427. 10.1161/01.RES.85.5.415 [DOI] [PubMed] [Google Scholar]
- Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. 1995. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82: 415–424. 10.1016/0092-8674(95)90430-1 [DOI] [PubMed] [Google Scholar]
- Hammes A, Oberdorf-Maass S, Rother T, Nething K, Gollnick F, Linz KW, Meyer R, Hu K, Han H, Gaudron P, et al. 1998. Overexpression of the sarcolemmal calcium pump in the myocardium of transgenic rats. Circ Res 83: 877–888. 10.1161/01.RES.83.9.877 [DOI] [PubMed] [Google Scholar]
- Harzheim D, Movassagh M, Foo RS, Ritter O, Tashfeen A, Conway SJ, Bootman MD, Roderick HL. 2009. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc Natl Acad Sci 106: 11406–11411. 10.1073/pnas.0905485106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzheim D, Talasila A, Movassagh M, Foo RS, Figg N, Bootman MD, Roderick HL. 2010. Elevated InsP3R expression underlies enhanced calcium fluxes and spontaneous extra-systolic calcium release events in hypertrophic cardiac myocytes. Channels (Austin) 4: 67–71. 10.4161/chan.4.1.10531 [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Pieske B. 2002. Calcium cycling in congestive heart failure. J Mol Cell Cardiol 34: 951–969. 10.1006/jmcc.2002.2037 [DOI] [PubMed] [Google Scholar]
- Haumann J, Camara AKS, Gadicherla AK, Navarro CD, Boelens AD, Blomeyer CA, Dash RK, Boswell MR, Kwok WM, Stowe DF. 2018. Slow Ca2+ efflux by Ca2+/H+ exchange in cardiac mitochondria is modulated by Ca2+ re-uptake via MCU, extra-mitochondrial pH, and H+ pumping by FOF1-ATPase. Front Physiol 9: 1914 10.3389/fphys.2018.01914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Martone ME, Yu Z, Thor A, Doi M, Holst MJ, Ellisman MH, Hoshijima M. 2009. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J Cell Sci 122: 1005–1013. 10.1242/jcs.028175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi B, Bossuyt J, Ginsburg KS, Mendoza LM, Talken L, Ferrier WT, Pogwizd SM, Izu LT, Chen-Izu Y, Bers DM. 2018. Altered repolarization reserve in failing rabbit ventricular myocytes: Calcium and β-adrenergic effects on delayed- and inward-rectifier potassium currents. Circ Arrhythm Electrophysiol 11: e005852 10.1161/CIRCEP.117.005852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel FR, Bito V, Volders PG, Antoons G, Mubagwa K, Sipido KR. 2002. Spatial and temporal inhomogeneities during Ca2+ release from the sarcoplasmic reticulum in pig ventricular myocytes. Circ Res 91: 1023–1030. 10.1161/01.RES.0000045940.67060.DD [DOI] [PubMed] [Google Scholar]
- Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, Claus P, Dymarkowski S, Maes F, Bogaert J, et al. 2008. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res 102: 338–346. 10.1161/circresaha.107.160085 [DOI] [PubMed] [Google Scholar]
- Heinzel FR, MacQuaide N, Biesmans L, Sipido K. 2011. Dyssynchrony of Ca2+ release from the sarcoplasmic reticulum as subcellular mechanism of cardiac contractile dysfunction. J Mol Cell Cardiol 50: 390–400. 10.1016/j.yjmcc.2010.11.008 [DOI] [PubMed] [Google Scholar]
- Higashida H, Egorova A, Higashida C, Zhong ZG, Yokoyama S, Noda M, Zhang JS. 1999. Sympathetic potentiation of cyclic ADP-ribose formation in rat cardiac myocytes. J Biol Chem 274: 33348–33354. 10.1074/jbc.274.47.33348 [DOI] [PubMed] [Google Scholar]
- Higashida H, Zhang J, Hashii M, Shintaku M, Higashida C, Takeda Y. 2000. Angiotensin II stimulates cyclic ADP-ribose formation in neonatal rat cardiac myocytes. Biochem J 352: 197–202. 10.1042/bj3520197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higazi DR, Fearnley CJ, Drawnel FM, Talasila A, Corps EM, Ritter O, McDonald F, Mikoshiba K, Bootman MD, Roderick HL. 2009. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol Cell 33: 472–482. 10.1016/j.molcel.2009.02.005 [DOI] [PubMed] [Google Scholar]
- Hirose M, Stuyvers B, Dun W, ter Keurs H, Boyden PA. 2008. Wide long lasting perinuclear Ca2+ release events generated by an interaction between ryanodine and IP3 receptors in canine Purkinje cells. J Mol Cell Cardiol 45: 176–184. 10.1016/j.yjmcc.2008.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HT, Liu B, Snyder JS, Lou Q, Brundage EA, Velez-Cortes F, Wang H, Ziolo MT, Anderson ME, Sen CK, et al. 2014. Ryanodine receptor phosphorylation by oxidized CaMKII contributes to the cardiotoxic effects of cardiac glycosides. Cardiovasc Res 101: 165–174. 10.1093/cvr/cvt233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer IE, Steffens S, Ala-Korpela M, Bäck M, Badimon L, Bochaton-Piallat ML, Boulanger CM, Caligiuri G, Dimmeler S, Egido J, et al. 2015. Novel methodologies for biomarker discovery in atherosclerosis. Eur Heart J 36: 2635–2642. 10.1093/eurheartj/ehv236 [DOI] [PubMed] [Google Scholar]
- Hohendanner F, Ljubojević S, MacQuaide N, Sacherer M, Sedej S, Biesmans L, Wakula P, Platzer D, Sokolow S, Herchuelz A, et al. 2013. Intracellular dyssynchrony of diastolic cytosolic [Ca2+] decay in ventricular cardiomyocytes in cardiac remodeling and human heart failure. Circ Res 113: 527–538. 10.1161/circresaha.113.300895 [DOI] [PubMed] [Google Scholar]
- Hohendanner F, Maxwell JT, Blatter LA. 2015a. Cytosolic and nuclear calcium signaling in atrial myocytes: IP3-mediated calcium release and the role of mitochondria. Channels 9: 129–138. 10.1080/19336950.2015.1040966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohendanner F, Walther S, Maxwell JT, Kettlewell S, Awad S, Smith GL, Lonchyna VA, Blatter LA. 2015b. Inositol-1,4,5-trisphosphate induced Ca2+ release and excitation-contraction coupling in atrial myocytes from normal and failing hearts. J Physiol 593: 1459–1477. 10.1113/jphysiol.2014.283226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T, Yang H, Zhang SS, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, et al. 2014. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med 20: 624–632. 10.1038/nm.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn T, Ullrich ND, Egger M. 2013. “Eventless” InsP3-dependent SR-Ca2+ release affecting atrial Ca2+ sparks. J Physiol 591: 2103–2111. 10.1113/jphysiol.2012.247288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høydal MA, Kirkeby-Garstad I, Karevold A, Wiseth R, Haaverstad R, Wahba A, Stølen TL, Contu R, Condorelli G, Ellingsen O, et al. 2018. Human cardiomyocyte calcium handling and transverse tubules in mid-stage of post-myocardial-infarction heart failure. ESC Heart Fail 5: 332–342. 10.1002/ehf2.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulot JS, Fauconnier J, Ramanujam D, Chaanine A, Aubart F, Sassi Y, Merkle S, Cazorla O, Ouillé A, Dupuis M, et al. 2011. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation 124: 796–805. 10.1161/circulationaha.111.031229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulot JS, Senyei G, Hajjar RJ. 2012. Sarcoplasmic reticulum and calcium cycling targeting by gene therapy. Gene Ther 19: 596–599. 10.1038/gt.2012.34 [DOI] [PubMed] [Google Scholar]
- Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell'Italia LJ, Marchase RB. 2002. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J Biol Chem 277: 14266–14273. 10.1074/jbc.M107167200 [DOI] [PubMed] [Google Scholar]
- Hunton DL, Zou L, Pang Y, Marchase RB. 2004. Adult rat cardiomyocytes exhibit capacitative calcium entry. Am J Heart Physiol Circ Physiol 286: H1124–H1132. 10.1152/ajpheart.00162.2003 [DOI] [PubMed] [Google Scholar]
- Hüser J, Lipsius SL, Blatter LA. 1996. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol 494: 641–651. 10.1113/jphysiol.1996.sp021521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M, Siedlecka U, Buyandelger B, Harada M, Rao C, Moshkov A, Bhargava A, Schneider M, Yacoub MH, Gorelik J, et al. 2013. A critical role for Telethonin in regulating t-tubule structure and function in the mammalian heart. Hum Mol Genet 22: 372–383. 10.1093/hmg/dds434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Kihara Y, Sarai N, Yoneda T, Iwanaga Y, Inagaki K, Onozawa Y, Takenaka H, Kita T, Noma A. 2003. Reinduction of T-type calcium channels by endothelin-1 in failing hearts in vivo and in adult rat ventricular myocytes in vitro. Circulation 108: 2530–2535. 10.1161/01.CIR.0000096484.03318.AB [DOI] [PubMed] [Google Scholar]
- Jayasinghe I, Crossman D, Soeller C, Cannell M. 2012. Comparison of the organization of T-tubules, sarcoplasmic reticulum and ryanodine receptors in rat and human ventricular myocardium. Clin Exp Pharmacol Physiol 39: 469–476. 10.1111/j.1440-1681.2011.05578.x [DOI] [PubMed] [Google Scholar]
- Jayasinghe I, Clowsley AH, de Langen O, Sali SS, Crossman DJ, Soeller C. 2018a. Shining new light on the structural determinants of cardiac couplon function: Insights from ten years of nanoscale microscopy. Front Physiol 9: 1472 10.3389/fphys.2018.01472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasinghe I, Clowsley AH, Lin R, Lutz T, Harrison C, Green E, Baddeley D, Di Michele L, Soeller C. 2018b. True molecular scale visualization of variable clustering properties of ryanodine receptors. Cell Rep 22: 557–567. 10.1016/j.celrep.2017.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clapham DE. 2009. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326: 144–147. 10.1126/science.1175145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PP, MacQuaide N, Louch WE. 2018. Dyadic plasticity in cardiomyocytes. Front Physiol 9: 1773 10.3389/fphys.2018.01773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor N, Tran A, Kang J, Zhang R, Philipson KD, Goldhaber JI. 2015. Regulation of calcium clock-mediated pacemaking by inositol-1,4,5-trisphosphate receptors in mouse sinoatrial nodal cells. J Physiol 593: 2649–2663. 10.1113/JP270082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettlewell S, Cabrero P, Nicklin SA, Dow JA, Davies S, Smith GL. 2009. Changes of intra-mitochondrial Ca2+ in adult ventricular cardiomyocytes examined using a novel fluorescent Ca2+ indicator targeted to mitochondria. J Mol Cell Cardiol 46: 891–901. 10.1016/j.yjmcc.2009.02.016 [DOI] [PubMed] [Google Scholar]
- Kiriazis H, Kranias EG. 2000. Genetically engineered models with alterations in cardiac membrane calcium-handling proteins. Annu Rev Physiol 62: 321–351. 10.1146/annurev.physiol.62.1.321 [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364. 10.1038/nature02246 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Solaro RJ. 2005. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol 67: 39–67. 10.1146/annurev.physiol.67.040403.114025 [DOI] [PubMed] [Google Scholar]
- Kockskämper J, Seidlmayer L, Walther S, Hellenkamp K, Maier LS, Pieske B. 2008a. Endothelin-1 enhances nuclear Ca2+ transients in atrial myocytes through Ins(1,4,5)P3-dependent Ca2+ release from perinuclear Ca2+ stores. J Cell Sci 121: 186–195. 10.1242/jcs.021386 [DOI] [PubMed] [Google Scholar]
- Kockskämper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. 2008b. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol 45: 128–147. 10.1016/j.yjmcc.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima A, Kitagawa H, Omatsu-Kanbe M, Matsuura H, Nosaka S. 2012. Presence of store-operated Ca2+ entry in C57BL/6J mouse ventricular myocytes and its suppression by sevoflurane. Br J Anaesth 109: 352–360. 10.1093/bja/aes212 [DOI] [PubMed] [Google Scholar]
- Kolstad TR, van den Brink J, MacQuaide N, Lunde PK, Frisk M, Aronsen JM, Norden ES, Cataliotti A, Sjaastad I, Sejersted OM, et al. 2018. Ryanodine receptor dispersion disrupts Ca2+ release in failing cardiac myocytes. eLife 7: e39427 10.7554/eLife.39427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong CHT, Rog-Zielinska EA, Orchard CH, Kohl P, Cannell MB. 2017. Sub-microscopic analysis of t-tubule geometry in living cardiac ventricular myocytes using a shape-based analysis method. J Mol Cell Cardiol 108: 1–7. 10.1016/j.yjmcc.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, da Cunha DN, Sridhar A, Feldman DS, Hamlin RL, et al. 2005. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci 102: 14104–14109. 10.1073/pnas.0504298102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD. 2015. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep 12: 15–22. 10.1016/j.celrep.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D'Hooge J, Heidbüchel H, Sipido KR, Willems R. 2009. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ Res 105: 876–885. 10.1161/circresaha.109.206276 [DOI] [PubMed] [Google Scholar]
- Lewis AM, Aley PK, Roomi A, Thomas JM, Masgrau R, Garnham C, Shipman K, Paramore C, Bloor-Young D, Sanders LE, et al. 2012. β-Adrenergic receptor signaling increases NAADP and cADPR levels in the heart. Biochem Biophys Res Commun 427: 326–329. 10.1016/j.bbrc.2012.09.054 [DOI] [PubMed] [Google Scholar]
- Li N, Wang Q, Sibrian-Vazquez M, Klipp RC, Reynolds JO, Word TA, Scott L, Salama G, Strongin RM, Abramson JJ, et al. 2017. Treatment of catecholaminergic polymorphic ventricular tachycardia in mice using novel RyR2-modifying drugs. Int J Cardiol 227: 668–673. 10.1016/j.ijcard.2016.10.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhou H, Tang Q. 2018. miR-133: A suppressor of cardiac remodeling? Front Pharmacol 9: 903 10.3389/fphar.2018.00903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WK, Bolton EL, Cortopassi WA, Wang Y, O'Brien F, Maciejewska M, Jacobson MP, Garnham C, Ruas M, Parrington J, et al. 2017. Synthesis of the Ca2+-mobilizing messengers NAADP and cADPR by intracellular CD38 enzyme in the mouse heart: Role in β-adrenoceptor signaling. J Biol Chem 292: 13243–13257. 10.1074/jbc.M117.789347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Hüser J, Pott L, Niggli E. 1996. Spatially non-uniform Ca2+ signals induced by the reduction of transverse tubules in citrate-loaded guinea-pig ventricular myocytes in culture. J Physiol 497: 589–597. 10.1113/jphysiol.1996.sp021792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. 2000. Functional InsP3 receptors that may modulate excitation–contraction coupling in the heart. Curr Biol 10: 939–942. 10.1016/S0960-9822(00)00624-2 [DOI] [PubMed] [Google Scholar]
- Liu GS, Morales A, Vafiadaki E, Lam CK, Cai WF, Haghighi K, Adly G, Hershberger RE, Kranias EG. 2015. A novel human R25C-phospholamban mutation is associated with super-inhibition of calcium cycling and ventricular arrhythmia. Cardiovasc Res 107: 164–174. 10.1093/cvr/cvv127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubojevic S, Radulovic S, Leitinger G, Sedej S, Sacherer M, Holzer M, Winkler C, Pritz E, Mittler T, Schmidt A, et al. 2014. Early remodeling of perinuclear Ca2+ stores and nucleoplasmic Ca2+ signaling during the development of hypertrophy and heart failure. Circulation 130: 244–255. 10.1161/circulationaha.114.008927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. 2004. Reduced synchrony of Ca2+ release with loss of T-tubules—A comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res 62: 63–73. 10.1016/j.cardiores.2003.12.031 [DOI] [PubMed] [Google Scholar]
- Louch WE, Mørk HK, Sexton J, Strømme TA, Laake P, Sjaastad I, Sejersted OM. 2006. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol 574: 519–533. 10.1113/jphysiol.2006.107227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Ginsburg KS, Kettlewell S, Bossuyt J, Smith GL, Bers DM. 2013. Measuring local gradients of intramitochondrial [Ca2+] in cardiac myocytes during sarcoplasmic reticulum Ca2+ release. Circ Res 112: 424–431. 10.1161/circresaha.111.300501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Györke I, Wiesner TF, Györke S. 2001. Potentiation of Ca2+ release by cADP-ribose in the heart is mediated by enhanced SR Ca2+ uptake into the sarcoplasmic reticulum. Circ Res 89: 614–622. 10.1161/hh1901.098066 [DOI] [PubMed] [Google Scholar]
- Luo M, Anderson ME. 2013. Mechanisms of altered Ca2+ handling in heart failure. Circ Res 113: 690–708. 10.1161/circresaha.113.301651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA. 2012. STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol 52: 136–147. 10.1016/j.yjmcc.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, Carpenter AC, Kolmetzky D, Gao E, van Berlo JH, et al. 2017. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature 545: 93–97. 10.1038/nature22082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G, Koch WJ. 2013. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ Res 113: 739–753. 10.1161/circresaha.113.300308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J. 2009. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci 106: 6854–6859. 10.1073/pnas.0809777106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, O'Gara P, Liang L, Kohlbrenner E, et al. 2011. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol 4: 362–372. 10.1161/CIRCEP.110.961615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH. 1992. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem 267: 14483–14489. [PubMed] [Google Scholar]
- Macgregor A, Yamasaki M, Rakovic S, Sanders L, Parkesh R, Churchill GC, Galione A, Terrar DA. 2007a. NAADP controls cross-talk between distinct Ca2+ stores in the heart. J Biol Chem 282: 15302–15311. 10.1074/jbc.M611167200 [DOI] [PubMed] [Google Scholar]
- Macgregor AT, Rakovic S, Galione A, Terrar DA. 2007b. Dual effects of cyclic ADP-ribose on sarcoplasmic reticulum Ca2+ release and storage in cardiac myocytes isolated from guinea-pig and rat ventricle. Cell Calcium 41: 537–546. 10.1016/j.ceca.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Mackenzie L, Bootman MD, Laine M, Berridge MJ, Thuring J, Holmes A, Li WH, Lipp P. 2002. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol 541: 395–409. 10.1113/jphysiol.2001.013411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. 2004. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci 117: 6327–6337. 10.1242/jcs.01559 [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Kranias EG. 2003. Phospholamban: A crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4: 566–577. 10.1038/nrm1151 [DOI] [PubMed] [Google Scholar]
- Macquaide N, Tuan HT, Hotta J, Sempels W, Lenaerts I, Holemans P, Hofkens J, Jafri MS, Willems R, Sipido KR. 2015. Ryanodine receptor cluster fragmentation and redistribution in persistent atrial fibrillation enhance calcium release. Cardiovasc Res 108: 387–398. 10.1093/cvr/cvv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, Foskett JK. 2015. Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: A single-channel point of view. Cell Calcium 58: 67–78. 10.1016/j.ceca.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfra O, Frisk M, Louch WE. 2017. Regulation of cardiomyocyte T-tubular structure: Opportunities for therapy. Curr Heart Fail Rep 14: 167–178. 10.1007/s11897-017-0329-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR. 2013. Calcium cycling proteins and heart failure: Mechanisms and therapeutics. J Clin Invest 123: 46–52. 10.1172/JCI62834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayourian J, Ceholski DK, Gonzalez DM, Cashman TJ, Sahoo S, Hajjar RJ, Costa KD. 2018. Physiologic, pathologic, and therapeutic paracrine modulation of cardiac excitation-contraction coupling. Circ Res 122: 167–183. 10.1161/circresaha.117.311589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough PM, Stella SL, Glembotski CC. 1994. Involvement of cytoplasmic calcium and protein kinases in the regulation of atrial natriuretic factor secretion by contraction rate and endothelin. J Biol Chem 269: 9466–9472. [PubMed] [Google Scholar]
- Metzger JM, Westfall MV. 2004. Covalent and noncovalent modification of thin filament action: The essential role of troponin in cardiac muscle regulation. Circ Res 94: 146–158. 10.1161/01.RES.0000110083.17024.60 [DOI] [PubMed] [Google Scholar]
- Miake J, Marbán E, Nuss HB. 2003. Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant-negative suppression. J Clin Invest 111: 1529–1536. 10.1172/JCI200317959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojžišová A, Križanová O, Žáčiková L, Komínková V, Ondriaš K. 2001. Effect of nicotinic acid adenine dinucleotide phosphate on ryanodine calcium release channel in heart. Pflugers Arch 441: 674–677. 10.1007/s004240000465 [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228. 10.1016/S0092-8674(00)81573-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschella MC, Marks AR. 1993. Inositol 1,4,5-trisphosphate receptor expression in cardiac myocytes. J Cell Biol 120: 1137–1146. 10.1083/jcb.120.5.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. 2015. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 131: e29–e322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- Munro ML, Shen X, Ward M, Ruygrok PN, Crossman DJ, Soeller C. 2018. Highly variable contractile performance correlates with myocyte content in trabeculae from failing human hearts. Sci Rep 8: 2957 10.1038/s41598-018-21199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Bodi I, Maillet M, DeSantiago J, Domeier TL, Mikoshiba K, Lorenz JN, Blatter LA, Bers DM, Molkentin JD. 2010. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ Res 107: 659–666. 10.1161/circresaha.110.220038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namekata I, Fujiki S, Kawakami Y, Moriwaki R, Takeda K, Kawanishi T, Takahara A, Shigenobu K, Tanaka H. 2008. Intracellular mechanisms and receptor types for endothelin-1-induced positive and negative inotropy in mouse ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol 376: 385–395. 10.1007/s00210-007-0228-9 [DOI] [PubMed] [Google Scholar]
- Nebel M, Schwoerer AP, Warszta D, Siebrands CC, Limbrock AC, Swarbrick JM, Fliegert R, Weber K, Bruhn S, Hohenegger M, et al. 2013. Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling and arrhythmias in the heart evoked by β-adrenergic stimulation. J Biol Chem 288: 16017–16030. 10.1074/jbc.M112.441246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BR, Makarewich CA, Anderson DM, Winders BR, Troupes CD, Wu F, Reese AL, McAnally JR, Chen X, Kavalali ET, et al. 2016. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 351: 271–275. 10.1126/science.aad4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Nagata K, Iwase M, Odashima M, Nagasaka T, Izawa H, Murohara T, Yamada Y, Yokota M. 2005. Overexpression of calmodulin induces cardiac hypertrophy by a calcineurin-dependent pathway. Biochem Biophys Res Commun 338: 1299–1305. 10.1016/j.bbrc.2005.10.083 [DOI] [PubMed] [Google Scholar]
- Oda T, Yamamoto T, Kato T, Uchinoumi H, Fukui G, Hamada Y, Nanno T, Ishiguchi H, Nakamura Y, Okamoto Y, et al. 2018. Nuclear translocation of calmodulin in pathological cardiac hypertrophy originates from ryanodine receptor bound calmodulin. J Mol Cell Cardiol 125: 87–97. 10.1016/j.yjmcc.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M, Dey A, Gerard RD, Hill JA, Rothermel BA. 2010. The CCAAT/enhancer binding protein β (C/EBPβ) cooperates with NFAT to control expression of the calcineurin regulatory protein RCAN1–4. J Biol Chem 285: 16623–16631. 10.1074/jbc.M109.098236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. 2007. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol 42: 498–507. 10.1016/j.yjmcc.2006.10.020 [DOI] [PubMed] [Google Scholar]
- Ohkusa T, Hisamatsu Y, Yano M, Kobayashi S, Tatsuno H, Saiki Y, Kohno M, Matsuzaki M. 1997. Altered cardiac mechanism and sarcoplasmic reticulum function in pressure overload-induced cardiac hypertrophy in rats. J Mol Cell Cardiol 29: 45–54. 10.1006/jmcc.1996.0250 [DOI] [PubMed] [Google Scholar]
- Olivares-Florez S, Czolbe M, Riediger F, Seidlmayer L, Williams T, Nordbeck P, Strasen J, Glocker C, Jansch M, Eder-Negrin P, et al. 2018. Nuclear calcineurin is a sensor for detecting Ca2+ release from the nuclear envelope via IP3R. J Mol Med 96: 1239–1249. 10.1007/s00109-018-1701-2 [DOI] [PubMed] [Google Scholar]
- Paillard M, Csordas G, Szanda G, Golenár T, Debattisti V, Bartok A, Wang N, Moffat C, Seifert EL, Spat A, et al. 2017. Tissue-specific mitochondrial decoding of cytoplasmic Ca2+ signals is controlled by the stoichiometry of MICU1/2 and MCU. Cell Rep 18: 2291–2300. 10.1016/j.celrep.2017.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, et al. 2010. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci 107: 436–441. 10.1073/pnas.0908099107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, et al. 2013. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 15: 1464–1472. 10.1038/ncb2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbellini F, Watson SA, Bardi I, Terracciano CM. 2018. Heterocellularity and cellular cross-talk in the cardiovascular system. Front Cardiovasc Med 5: 143 10.3389/fcvm.2018.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez PJ, Ramos-Franco J, Fill M, Mignery GA. 1997. Identification and functional reconstitution of the type 2 inositol 1,4,5-trisphosphate receptor from ventricular cardiac myocytes. J Biol Chem 272: 23961–23969. 10.1074/jbc.272.38.23961 [DOI] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. 2010. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467: 291–296. 10.1038/nature09358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino C, Barabási AL, Condorelli G, Davidson SM, De Windt L, Dimmeler S, Engel FB, Hausenloy DJ, Hill JA, Van Laake LW, et al. 2017. Epigenomic and transcriptomic approaches in the post-genomic era: Path to novel targets for diagnosis and therapy of the ischaemic heart? Position paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 113: 725–736. 10.1093/cvr/cvx070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentino V III, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. 2003. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res 92: 651–658. 10.1161/01.RES.0000062469.83985.9B [DOI] [PubMed] [Google Scholar]
- Pinali C, Bennett H, Davenport JB, Trafford AW, Kitmitto A. 2013. Three-dimensional reconstruction of cardiac sarcoplasmic reticulum reveals a continuous network linking transverse-tubules: This organization is perturbed in heart failure. Circ Res 113: 1219–1230. 10.1161/circresaha.113.301348 [DOI] [PubMed] [Google Scholar]
- Pitt SJ, Funnell TM, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, et al. 2010. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+. J Biol Chem 285: 35039–35046. 10.1074/jbc.M110.156927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plačkić J, Preissl S, Nikonova Y, Pluteanu F, Hein L, Kockskamper J. 2016. Enhanced nucleoplasmic Ca2+ signaling in ventricular myocytes from young hypertensive rats. J Mol Cell Cardiol 101: 58–68. 10.1016/j.yjmcc.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Potenza DM, Janicek R, Fernandez-Tenorio M, Camors E, Ramos-Mondragon R, Valdivia HH, Niggli E. 2018. Phosphorylation of the ryanodine receptor 2 at serine 2030 is required for a complete beta-adrenergic response. J Gen Physiol 151: 131–145. 10.1085/jgp.201812155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Kannan MS, Walseth TF, Sieck GC. 2000. cADP ribose and [Ca2+]i regulation in rat cardiac myocytes. Am J Physiol Heart Circ Physiol 279: H1482–H1489. 10.1152/ajpheart.2000.279.4.H1482 [DOI] [PubMed] [Google Scholar]
- Priori SG, Chen SR. 2011. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res 108: 871–883. 10.1161/circresaha.110.226845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proven A, Roderick HL, Conway SJ, Berridge MJ, Horton JK, Capper SJ, Bootman MD. 2006. Inositol 1,4,5-trisphosphate supports the arrhythmogenic action of endothelin-1 on ventricular cardiac myocytes. J Cell Sci 119: 3363–3375. 10.1242/jcs.03073 [DOI] [PubMed] [Google Scholar]
- Rakovic S, Galione A, Ashamu GA, Potter BV, Terrar DA. 1996. A specific cyclic ADP-ribose antagonist inhibits cardiac excitation-contraction coupling. Curr Biol 6: 989–996. 10.1016/S0960-9822(02)00643-7 [DOI] [PubMed] [Google Scholar]
- Rakovic S, Cui Y, Iino S, Galione A, Ashamu GA, Potter BV, Terrar DA. 1999. An antagonist of cADP-ribose inhibits arrhythmogenic oscillations of intracellular Ca2+ in heart cells. J Biol Chem 274: 17820–17827. 10.1074/jbc.274.25.17820 [DOI] [PubMed] [Google Scholar]
- Rasmussen TP, Wu Y, Joiner ML, Koval OM, Wilson NR, Luczak ED, Wang Q, Chen B, Gao Z, Zhu Z, et al. 2015. Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart. Proc Natl Acad Sci 112: 9129–9134. 10.1073/pnas.1504705112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JO, Chiang DY, Wang W, Beavers DL, Dixit SS, Skapura DG, Landstrom AP, Song LS, Ackerman MJ, Wehrens XH. 2013. Junctophilin-2 is necessary for T-tubule maturation during mouse heart development. Cardiovasc Res 100: 44–53. 10.1093/cvr/cvt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Duchen MR, Pozzan T. 2004. Flirting in little space: The ER/mitochondria Ca2+ liaison. Sci STKE 2004: re1 10.1126/stke.2152004re1 [DOI] [PubMed] [Google Scholar]
- Robert V, Gurlini P, Tosello V, Nagai T, Miyawaki A, Di Lisa F, Pozzan T. 2001. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. EMBO J 20: 4998–5007. 10.1093/emboj/20.17.4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick HL, Berridge MJ, Bootman MD. 2003. Calcium-induced calcium release. Curr Biol 13: R425 10.1016/S0960-9822(03)00358-0 [DOI] [PubMed] [Google Scholar]
- Røe AT, Ruud M, Espe EK, Manfra O, Longobardi S, Aronsen JM, Norden ES, Husebye T, Kolstad TRS, Cataliotti A, et al. 2018. Regional diastolic dysfunction in post-infarction heart failure: Role of local mechanical load and SERCA expression. Cardiovasc Res 115: 752–764. 10.1093/cvr/cvy257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rog-Zielinska EA, Kong CHT, Zgierski-Johnston CM, Verkade P, Mantell J, Cannell MB, Kohl P. 2018. Species differences in the morphology of transverse tubule openings in cardiomyocytes. Europace 20: iii120–iii124. 10.1093/europace/euy245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S. 2004. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res 62: 309–322. 10.1016/j.cardiores.2003.11.035 [DOI] [PubMed] [Google Scholar]
- Rosemblit N, Moschella MC, Ondrias˘ova E, Gutstein DE, Ondrias˘ K, Marks AR. 1999. Intracellular calcium release channel expression during embryogenesis. Dev Biol 206: 163–177. 10.1006/dbio.1998.9120 [DOI] [PubMed] [Google Scholar]
- Russell FD, Molenaar P. 2000. The human heart endothelin system: ET-1 synthesis, storage, release and effect. Trends Pharmacol Sci 21: 353–359. 10.1016/S0165-6147(00)01524-8 [DOI] [PubMed] [Google Scholar]
- Sacconi L, Tolic-Nørrelykke IM, D'Amico M, Vanzi F, Olivotto M, Antolini R, Pavone FS. 2006. Cell imaging and manipulation by nonlinear optical microscopy. Cell Biochem Biophys 45: 289–302. 10.1385/CBB:45:3:289 [DOI] [PubMed] [Google Scholar]
- Sachse FB, Torres NS, Savio-Galimberti E, Aiba T, Kass DA, Tomaselli GF, Bridge JH. 2012. Subcellular structures and function of myocytes impaired during heart failure are restored by cardiac resynchronization therapy. Circ Res 110: 588–597. 10.1161/circresaha.111.257428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alonso JL, Bhargava A, O'Hara T, Glukhov AV, Schobesberger S, Bhogal N, Sikkel MB, Mansfield C, Korchev YE, Lyon AR, et al. 2016. Microdomain-specific modulation of L-type calcium channels leads to triggered ventricular arrhythmia in heart failure. Circ Res 119: 944–955. 10.1161/circresaha.116.308698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar N, deTombe PP, Mignery GA. 2014. Calcineurin-NFATc regulates type 2 inositol 1,4,5-trisphosphate receptor (InsP3R2) expression during cardiac remodeling. J Biol Chem 289: 6188–6198. 10.1074/jbc.M113.495242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G, Xie W, Reiken SR, Marks AR. 2015. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci 112: 11389–11394. 10.1073/pnas.1513047112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse P, Zhang J, Cleemann L, Morad M, Hescheler J, Fleischmann BK. 2007. Intracellular Ca2+ oscillations, a potential pacemaking mechanism in early embryonic heart cells. J Gen Physiol 130: 133–144. 10.1085/jgp.200609575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savio-Galimberti E, Frank J, Inoue M, Goldhaber JI, Cannell MB, Bridge JH, Sachse FB. 2008. Novel features of the rabbit transverse tubular system revealed by quantitative analysis of three-dimensional reconstructions from confocal images. Biophys J 95: 2053–2062. 10.1529/biophysj.108.130617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw EL, Kakinuma Y, Fronius M, Katare R. 2018. The non-neuronal cholinergic system in the heart: A comprehensive review. J Mol Cell Cardiol 125: 129–139. 10.1016/j.yjmcc.2018.10.013 [DOI] [PubMed] [Google Scholar]
- Scardigli M, Ferrantini C, Crocini C, Pavone FS, Sacconi L. 2018a. Interplay between sub-cellular alterations of calcium release and T-tubular defects in cardiac diseases. Front Physiol 9: 1474 10.3389/fphys.2018.01474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scardigli M, Müllenbroich C, Margoni E, Cannazzaro S, Crocini C, Ferrantini C, Coppini R, Yan P, Loew LM, Campione M, et al. 2018b. Real-time optical manipulation of cardiac conduction in intact hearts. J Physiol 596: 3841–3858. 10.1113/JP276283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirone L, Forte M, Palmerio S, Yee D, Nocella C, Angelini F, Pagano F, Schiavon S, Bordin A, Carrizzo A, et al. 2017. A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxid Med Cell Longev 2017: 3920195 10.1155/2017/3920195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriven DR, Asghari P, Schulson MN, Moore ED. 2010. Analysis of Cav1.2 and ryanodine receptor clusters in rat ventricular myocytes. Biophys J 99: 3923–3929. 10.1016/j.bpj.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedarat F, Xu L, Moore ED, Tibbits GF. 2000. Colocalization of dihydropyridine and ryanodine receptors in neonate rabbit heart using confocal microscopy. Am J Physiol Heart Circ Physiol 279: H202–H209. 10.1152/ajpheart.2000.279.1.H202 [DOI] [PubMed] [Google Scholar]
- See K, Tan WLW, Lim EH, Tiang Z, Lee LT, Li PYQ, Luu TDA, Ackers-Johnson M, Foo RS. 2017. Single cardiomyocyte nuclear transcriptomes reveal a lincRNA-regulated de-differentiation and cell cycle stress-response in vivo. Nat Commun 8: 225 10.1038/s41467-017-00319-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel T, Navankasattusas S, Ahmad A, Diakos NA, Xu WD, Tristani-Firouzi M, Bonios MJ, Taleb I, Li DY, Selzman CH, et al. 2017. Sheet-like remodeling of the transverse tubular system in human heart failure impairs excitation-contraction coupling and functional recovery by mechanical unloading. Circulation 135: 1632–1645. 10.1161/circulationaha.116.024470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattock MJ, Ottolia M, Bers DM, Blaustein MP, Boguslavskyi A, Bossuyt J, Bridge JH, Chen-Izu Y, Clancy CE, Edwards A, et al. 2015. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J Physiol 593: 1361–1382. 10.1113/jphysiol.2014.282319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan KA, Zima AV, Blatter LA. 2006. Regional differences in spontaneous Ca2+ spark activity and regulation in cat atrial myocytes. J Physiol 572: 799–809. 10.1113/jphysiol.2005.103267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, van den Brink J, Hou Y, Colli D, Le C, Kolstad TR, MacQuaide N, Carlson CR, Kekenes-Huskey PM, Edwards AG, et al. 2019. 3D dSTORM imaging reveals novel detail of ryanodine receptor localization in rat cardiac myocytes. J Physiol 597: 399–418. 10.1113/JP277360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Schredelseker J, Huang J, Lu K, Naghdi S, Lu F, Franklin S, Fiji HD, Wang K, Zhu H, et al. 2015. Mitochondrial Ca2+ uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. eLife 4 10.7554/eLife.04801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signore S, Sorrentino A, Ferreira-Martins J, Kannappan R, Shafaie M, Del Ben F, Isobe K, Arranto C, Wybieralska E, Webster A, et al. 2013. Inositol 1,4,5-trisphosphate receptors and human left ventricular myocytes. Circulation 128: 1286–1297. 10.1161/circulationaha.113.002764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyrnias I, Mair W, Harzheim D, Walker SA, Roderick HL, Bootman MD. 2010. Comparison of the T-tubule system in adult rat ventricular and atrial myocytes, and its role in excitation–contraction coupling and inotropic stimulation. Cell Calcium 47: 210–223. 10.1016/j.ceca.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Smyrnias I, Goodwin N, Wachten D, Skogestad J, Aronsen JM, Robinson EL, Demydenko K, Segonds-Pichon A, Oxley D, Sadayappan S, et al. 2018. Contractile responses to endothelin-1 are regulated by PKC phosphorylation of cardiac myosin binding protein-C in rat ventricular myocytes. J Mol Cell Cardiol 117: 1–18. 10.1016/j.yjmcc.2018.02.012 [DOI] [PubMed] [Google Scholar]
- Soeller C, Baddeley D. 2013. Super-resolution imaging of EC coupling protein distribution in the heart. J Mol Cell Cardiol 58: 32–40. 10.1016/j.yjmcc.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Soeller C, Cannell MB. 1999. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res 84: 266–275. 10.1161/01.RES.84.3.266 [DOI] [PubMed] [Google Scholar]
- Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. 2006. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci 103: 4305–4310. 10.1073/pnas.0509324103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson RS, Boyett MR, Hart G, Nikolaidou T, Cai X, Corno AF, Alphonso N, Jeffery N, Jarvis JC. 2012. Contrast enhanced micro-computed tomography resolves the 3-dimensional morphology of the cardiac conduction system in mammalian hearts. PLoS ONE 7: e35299 10.1371/journal.pone.0035299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MD. 1992. Theory of excitation-contraction coupling in cardiac muscle. Biophys J 63: 497–517. 10.1016/S0006-3495(92)81615-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD. 1998. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science 281: 1690–1693. 10.1126/science.281.5383.1690 [DOI] [PubMed] [Google Scholar]
- Tadross MR, Dick IE, Yue DT. 2008. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell 133: 1228–1240. 10.1016/j.cell.2008.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavi P, Pikkarainen S, Ronkainen J, Niemela P, Ilves M, Weckström M, Vuolteenaho O, Bruton J, Westerblad H, Ruskoaho H. 2004. Pacing-induced calcineurin activation controls cardiac Ca2+ signalling and gene expression. J Physiol 554: 309–320. 10.1113/jphysiol.2003.053579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thienpont B, Aronsen JM, Robinson EL, Okkenhaug H, Loche E, Ferrini A, Brien P, Alkass K, Tomasso A, Agrawal A, et al. 2017. The H3K9 dimethyltransferases EHMT1/2 protect against pathological cardiac hypertrophy. J Clin Invest 127: 335–348. 10.1172/JCI88353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchinoumi H, Yang Y, Oda T, Li N, Alsina KM, Puglisi JL, Chen-Izu Y, Cornea RL, Wehrens XHT, Bers DM. 2016. CaMKII-dependent phosphorylation of RyR2 promotes targetable pathological RyR2 conformational shift. J Mol Cell Cardiol 98: 62–72. 10.1016/j.yjmcc.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara A, Yasukochi M, Imanaga I, Nishi M, Takeshima H. 2002. Store-operated Ca2+ entry uncoupled with ryanodine receptor and junctional membrane complex in heart muscle cells. Cell Calcium 31: 89–96. 10.1054/ceca.2001.0257 [DOI] [PubMed] [Google Scholar]
- Ullrich ND, Valdivia HH, Niggli E. 2012. PKA phosphorylation of cardiac ryanodine receptor modulates SR luminal Ca2+ sensitivity. J Mol Cell Cardiol 53: 33–42. 10.1016/j.yjmcc.2012.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais H, Mallilankaraman K, Mak DD, Hoff H, Payne R, Tanis JE, Foskett JK. 2016. EMRE is a matrix Ca2+ sensor that governs gatekeeping of the mitochondrial Ca2+ uniporter. Cell Rep 14: 403–410. 10.1016/j.celrep.2015.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. 1995. Rapid adaptation of cardiac ryanodine receptors: Modulation by Mg2+ and phosphorylation. Science 267: 1997–2000. 10.1126/science.7701323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heyden MA, Wijnhoven TJ, Opthof T. 2005. Molecular aspects of adrenergic modulation of cardiac L-type Ca2+ channels. Cardiovasc Res 65: 28–39. 10.1016/j.cardiores.2004.09.028 [DOI] [PubMed] [Google Scholar]
- Venetucci LA, Trafford AW, O'Neill SC, Eisner DA. 2008. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res 77: 285–292. 10.1093/cvr/cvm009 [DOI] [PubMed] [Google Scholar]
- Vervliet T, Robinson EL, Roderick HL. 2018. Lnc'ing Ca2+, SERCA and cardiac disease. Cell Calcium 72: 132–134. 10.1016/j.ceca.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Vervloessem T, Yule DI, Bultynck G, Parys JB. 2015. The type 2 inositol 1,4,5-trisphosphate receptor, emerging functions for an intriguing Ca2+-release channel. Biochem Biophys Acta 1853: 1992–2005. 10.1016/j.bbamcr.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA, et al. 2010. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol 48: 1329–1334. 10.1016/j.yjmcc.2010.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MA, Williams GSB, Kohl T, Lehnart SE, Jafri MS, Greenstein JL, Lederer WJ, Winslow RL. 2014. Superresolution modeling of calcium release in the heart. Biophys J 107: 3018–3029. 10.1016/j.bpj.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walweel K, Molenaar P, Imtiaz MS, Denniss A, Dos Remedios C, van Helden DF, Dulhunty AF, Laver DR, Beard NA. 2017. Ryanodine receptor modification and regulation by intracellular Ca2+ and Mg2+ in healthy and failing human hearts. J Mol Cell Cardiol 104: 53–62. 10.1016/j.yjmcc.2017.01.016 [DOI] [PubMed] [Google Scholar]
- Wang SQ, Song LS, Lakatta EG, Cheng H. 2001. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 410: 592–596. 10.1038/35069083 [DOI] [PubMed] [Google Scholar]
- Wang J, Gareri C, Rockman HA. 2018. G-protein-coupled receptors in heart disease. Circ Res 123: 716–735. 10.1161/circresaha.118.311403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warszta D, Nebel M, Fliegert R, Guse AH. 2014. NAD derived second messengers: Role in spontaneous diastolic Ca2+ transients in murine cardiac myocytes. DNA Repair (Amst) 23: 69–78. 10.1016/j.dnarep.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, et al. 2003. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113: 829–840. 10.1016/S0092-8674(03)00434-3 [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Marks AR. 2005. Intracellular calcium release and cardiac disease. Annu Rev Physiol 67: 69–98. 10.1146/annurev.physiol.67.040403.114521 [DOI] [PubMed] [Google Scholar]
- Wei AC, Liu T, Winslow RL, O'Rourke B. 2012. Dynamics of matrix-free Ca2+ in cardiac mitochondria: Two components of Ca2+ uptake and role of phosphate buffering. J Gen Physiol 139: 465–478. 10.1085/jgp.201210784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei AC, Liu T, O'Rourke B. 2015. Dual effect of phosphate transport on mitochondrial Ca2+ dynamics. J Biol Chem 290: 16088–16098. 10.1074/jbc.M114.628446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GS, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. 2013. Mitochondrial calcium uptake. Proc Natl Acad Sci 110: 10479–10486. 10.1073/pnas.1300410110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Baddeley D, Bushong EA, Yu Z, Ellisman MH, Hoshijima M, Soeller C. 2013. Nanoscale distribution of ryanodine receptors and caveolin-3 in mouse ventricular myocytes: Dilation of t-tubules near junctions. Biophys J 104: L22–L24. 10.1016/j.bpj.2013.02.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. 2006. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest 116: 675–682. 10.1172/JCI27374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HD, Xu M, Li RC, Guo L, Lai YS, Xu SM, Li SF, Lü QL, Li LL, Zhang HB, et al. 2012. Ultrastructural remodelling of Ca2+ signalling apparatus in failing heart cells. Cardiovasc Res 95: 430–438. 10.1093/cvr/cvs195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Rasmussen TP, Koval OM, Joiner ML, Hall DD, Chen B, Luczak ED, Wang Q, Rokita AG, Wehrens XH, et al. 2015. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun 6: 6081 10.1038/ncomms7081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger M, Blanch J, Egger M. 2017. Functional local crosstalk of inositol 1,4,5-trisphosphate receptor- and ryanodine receptor-dependent Ca2+ release in atrial cardiomyocytes. Cardiovasc Res 113: 542–552. 10.1093/cvr/cvx020 [DOI] [PubMed] [Google Scholar]
- Xie GH, Rah SY, Kim SJ, Nam TS, Ha KC, Chae SW, Im MJ, Kim UH. 2005. ADP-ribosyl cyclase couples to cyclic AMP signaling in the cardiomyocytes. Biochem Biophys Res Commun 330: 1290–1298. 10.1016/j.bbrc.2005.03.114 [DOI] [PubMed] [Google Scholar]
- Xu L, Meissner G. 2004. Mechanism of calmodulin inhibition of cardiac sarcoplasmic reticulum Ca2+ release channel (ryanodine receptor). Biophys J 86: 797–804. 10.1016/S0006-3495(04)74155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Zhou P, Xu SM, Liu Y, Feng X, Bai SH, Bai Y, Hao XM, Han Q, Zhang Y, et al. 2007. Intermolecular failure of L-type Ca2+ channel and ryanodine receptor signaling in hypertrophy. PLoS Biol 5: e21 10.1371/journal.pbio.0050021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamda J, Ohkusa T, Nao T, Ueyama T, Yano M, Kobayashi S, Hamano K, Esato K, Matsuzaki M. 2001. Up-regulation of inositol 1,4,5 trisphosphate receptor expression in atrial tissue in patients with chronic atrial fibrillation. J Am Coll Cardiol 37: 1111–1119. 10.1016/S0735-1097(01)01144-5 [DOI] [PubMed] [Google Scholar]
- Yue X, Zhang R, Kim B, Ma A, Philipson KD, Goldhaber JI. 2017. Heterogeneity of transverse-axial tubule system in mouse atria: Remodeling in atrial-specific Na+–Ca2+ exchanger knockout mice. J Mol Cell Cardiol 108: 50–60. 10.1016/j.yjmcc.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalk R, Lehnart SE, Marks AR. 2007. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem 76: 367–385. 10.1146/annurev.biochem.76.053105.094237 [DOI] [PubMed] [Google Scholar]
- Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. 2002. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110: 479–488. 10.1016/S0092-8674(02)00861-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tallini YN, Chen Z, Gan L, Wei B, Doran R, Miao L, Xin HB, Kotlikoff MI, Ji G. 2009. Dissociation of FKBP12.6 from ryanodine receptor type 2 is regulated by cyclic ADP-ribose but not β-adrenergic stimulation in mouse cardiomyocytes. Cardiovasc Res 84: 253–262. 10.1093/cvr/cvp212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Malik S, Pang J, Wang H, Park KM, Yule DI, Blaxall BC, Smrcka AV. 2013. Phospholipase Cε hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell 153: 216–227. 10.1016/j.cell.2013.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jiao L, Sun L, Li Y, Gao Y, Xu C, Shao Y, Li M, Li C, Lu Y, et al. 2018. LncRNA ZFAS1 as a SERCA2a inhibitor to cause intracellular Ca2+ overload and contractile dysfunction in a mouse model of myocardial infarction. Circ Res 122: 1354–1368. 10.1161/circresaha.117.312117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Li T, Brochet DX, Rosenberg PB, Lederer WJ. 2015. STIM1 enhances SR Ca2+ content through binding phospholamban in rat ventricular myocytes. Proc Natl Acad Sci 112: E4792–E4801. 10.1073/pnas.1423295112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, McKeon F. 1999. NF-AT activation requires suppression of Crm1-dependent export by calcineurin. Nature 398: 256–260. 10.1038/18473 [DOI] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. 2004. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol 555: 607–615. 10.1113/jphysiol.2003.058529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman AP, Gómez-Viquez NL, Bloch RJ, Lederer WJ. 2010. Excitation–contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol 48: 379–386. 10.1016/j.yjmcc.2009.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel C, Cho HC, Nguyen TT, Pekhletski R, Diaz RJ, Wilson GJ, Backx PH. 2003. Molecular dissection of the inward rectifier potassium current (IK1) in rabbit cardiomyocytes: Evidence for heteromeric co-assembly of Kir2.1 and Kir2.2. J Physiol 550: 365–372. 10.1113/jphysiol.2002.036400 [DOI] [PMC free article] [PubMed] [Google Scholar]