Abstract
The innate immune system senses and responds to pathogens and endogenous damage through supramolecular protein complexes known as inflammasomes. Cytosolic inflammasome sensor proteins trigger inflammasome assembly on detection of infection and danger. Assembled inflammasomes activate a cascade of inflammatory caspases, which process procytokines and gasdermin D (GSDMD). Cleaved GSDMD forms membrane pores that lead to cytokine release and/or programmed lytic cell death, called pyroptosis. In this review, we provide a primer on pyroptosis and focus on its executioner, the GSDM protein family. In addition to inflammasome-mediated GSDMD pore formation, we describe recently discovered GSDMD activation by caspase-8 and elastase in Yersinia-infected macrophages and aging neutrophils, respectively, and GSDME activation by apoptotic caspases. Finally, we discuss strategies that host cells and pathogens use to restrict GSDMD pore formation, in addition to therapeutics targeting the GSDM family.
PYROPTOSIS AND GASDERMIN D ACTIVATION
Canonical and Noncanonical Inflammasome Pathways
The innate immune system recognizes a broad array of exogenous pathogens and endogenous damage to elicit an inflammatory response. To accomplish this, cytosolic sensor proteins (de Vasconcelos and Lamkanfi 2019) rapidly detect a diverse set of pathogen- and danger-associated molecular patterns, PAMPs and DAMPs, respectively (Bergsbaken et al. 2009). In one pathway, PAMPs and DAMPs such as bacterial flagellin, lipopolysaccharides (LPSs) of Gram-negative bacteria, bacterial toxins, uric acid crystals, cytosolic double-stranded DNA (dsDNA), and many others trigger the assembly of supramolecular signaling complexes called inflammasomes (Fig. 1A; Martinon et al. 2006; Pétrilli et al. 2007; Hornung et al. 2009; Zhao et al. 2011; Zhou et al. 2011; Hagar et al. 2013; Kayagaki et al. 2013; de Vasconcelos and Lamkanfi 2019). Inflammasomes activate inflammatory caspases, which include human and mouse caspase-1, human caspase-4 and -5, and mouse caspase-11.
Figure 1.
Biological pathways of gasdermin D (GSDMD) activation. (A) Canonical inflammasomes triggered by a wide array of pathogen- and danger-associated molecular patterns (DAMPs and PAMPs) activate caspase-1, which cleaves GSDMD and liberates GSDMD-NT for pore formation, cell content release, and pyroptosis. Caspase-1 also cleaves certain interleukin 1 (IL-1) family cytokines into their mature forms. (B) A complex of cytosolic lipopolysaccharides (LPSs) and caspase-11 known as the noncanonical inflammasome cleaves GSDMD to generate the pore-forming GSDMD-NT. (C) YopJ of Yersinia pestis inhibits transforming growth factor β–activated kinase 1 (TAK1) for caspase-8-mediated cleavage of GSDMD and pyroptosis. (D) In aging neutrophils, an elastase cleaves and activates GSDMD.
Canonical inflammasomes are comprised of a pattern recognition receptor (PRR) such as NLRP3 or AIM2, the adaptor protein ASC, and the effector caspase-1 (Martinon et al. 2002; Lamkanfi and Dixit 2014; Man and Kanneganti 2015). Different PAMPs and DAMPs activate unique sensor proteins, leading to their homo-oligomerization. Sensor protein oligomers recruit ASC, which scaffolds procaspase-1 polymerization (Lu et al. 2014). The locally high concentration of procaspase-1 at the inflammasome facilitates its homodimerization, autoprocessing, and activation (Lamkanfi and Dixit 2014; Lu et al. 2014; Man and Kanneganti 2015). Activated caspase-1 subsequently cleaves the procytokines pro-IL-1β and pro-IL-18 into their bioactive forms, IL-1β and IL-18, respectively (Martinon et al. 2002; Faustin et al. 2007), and leads to pyroptosis, a lytic form of programmed cell death (Fink and Cookson 2005; Chen et al. 2016; Zhang et al. 2018).
The molecular platform that leads to the activation of murine caspase-11 or its human homologs caspases-4 and -5 is known as the noncanonical inflammasome (Fig. 1B; Kayagaki et al. 2011; Broz et al. 2012). Intracellular LPSs from Gram-negative bacteria and self-encoded oxidized phospholipids directly bind these caspases, resulting in their oligomerization, autoproteolysis, and activation (Hagar et al. 2013; Kayagaki et al. 2013; Shi et al. 2014; Zanoni et al. 2016; Lee et al. 2018). Although all inflammatory caspases can lead to pyroptosis, the molecular player that mediates this effector function and the mechanism for inflammasome-induced cytokine release were largely unknown until recently.
GSDMD Identification, Autoinhibition, and Activation by Inflammasomes
To identify a molecule that is responsible for cytokine secretion and pyroptosis, Kayagaki et al. (2015) conducted an N-ethyl-N-nitrosourea (ENU)-based forward genetic screen in mice to identify mutations that impaired IL-1β secretion in bone marrow macrophages following LPS challenge. Simultaneously, Shi et al. (2015) performed a genome-wide CRISPR-Cas9 knockout screen in murine macrophages for genes involved in caspase-1- and caspase-11-dependent pyroptosis, and He et al. (2015) used quantitative mass spectrometry to discover new inflammasome component proteins. All studies identified gasdermin D (GSDMD) as a direct substrate of the inflammatory caspases downstream from canonical and noncanonical inflammasomes, which is required for both cytokine release and pyroptosis.
GSDMD features a two-domain architecture, with a pore-forming amino-terminal fragment (GSDMD-NT) of ∼30 kDa (p30 fragment) and a repressive carboxy-terminal fragment (GSDMD-CT) of ∼20 kDa (p20 fragment). These domains are separated by a long disordered interdomain linker cleavable by the inflammatory caspases after Asp276 (D276) (Kayagaki et al. 2015; Shi et al. 2015). On cleavage, GSDMD-NTs translocate to the plasma and mitochondrial membranes to bind acidic lipids, such as phosphatidylinositol phosphates (PIPs), phosphatidylserine (PS), and cardiolipin (CL), oligomerize into a ring-like structure, and insert into the lipid bilayer to form large transmembrane (TM) pores (Fig. 1; Aglietti et al. 2016; Chen et al. 2016; Ding et al. 2016; Liu et al. 2016; Sborgi et al. 2016; Platnich et al. 2018). These GSDMD pores release cytosolic contents, including the inflammatory cytokines IL-1β and IL-18, cause membrane rupture by disrupting osmotic potentials, and eventually result in pyroptotic cell death (Fink and Cookson 2006; Evavold et al. 2018; Heilig et al. 2018). Cleaved GSDMD can also form pores on bacterial membranes, directly killing intracellular bacteria (Liu et al. 2016). Thus, GSDMD facilitates cytokine release, pyroptotic cell death, and pathogen destruction in innate immunity.
Because of its inflammatory role, pyroptosis was long regarded as exclusive to monocytes, macrophages, and dendritic cells. Recently, however, pyroptosis was shown to occur in various other cell types including epithelial cells, keratinocytes, and peripheral blood mononuclear cells (Shi et al. 2014; Zhong et al. 2016). GSDMD and caspase-4/5/11 are expressed in a wide range of tissues and cell types (Kayagaki et al. 2011; Broz et al. 2012; Saeki and Sasaki 2012; Hagar et al. 2013; Uhlen et al. 2015). These data are consistent with the finding that pyroptosis is not limited to a few subgroups of immune cells.
PORE-FORMING MECHANISM OF THE GSDM FAMILY
GSDMD belongs to the GSDM family, which is comprised of five other members in humans including GSDMA, GSDMB, GSDMC, DFNA5/GSDME, and DFNB59/GSDMF. Mice have three GSDMAs (GSDMA1–3) and four GSDMCs (GSDMC1–4) generated by gene duplication events, and they lack GSDMB (Tamura et al. 2007; Tanaka et al. 2013). Human GSDMB has multiple transcript variants that might show differences in their mechanisms of autoinhibition (Ding et al. 2016; Chao et al. 2017; Chen et al. 2018b; Panganiban et al. 2018). Because the GSDM family shares the common pore-forming activity of GSDMD, the Nomenclature Committee on Cell Death recently redefined pyroptosis as a type of regulated lytic cell death mediated by GSDM pore formation on the plasma membrane, often but not always as a result of inflammatory caspase activation (Kovacs and Miao 2017; Galluzzi et al. 2018).
Mechanism of GSDM Autoinhibition
GSDMs contain an amino-terminal pore-forming domain (GSDM-NT) and a carboxy-terminal autoinhibitory domain (GSDM-CT), with the exception of GSDMF, which lacks homology in the GSDM-CT and might have different regulatory mechanisms (Feng et al. 2018). In the autoinhibited state, GSDM-CT folds back onto GSDM-NT to repress its activity. The basis for this autoinhibitory mechanism was elucidated by the X-ray crystal structures of full-length (FL) mouse GSDMA3 as well as both human and mouse GSDMD (Ding et al. 2016; Kuang et al. 2017; Liu et al. 2018, 2019). GSDM-NT contains both α-helices and extended β-strands, whereas GSDM-CT is almost exclusively α-helical. The long linker between these domains is disordered in these crystal structures, indicating flexibility that underlies its accessibility to caspases and perhaps other unknown activating proteases (Fig. 2A,B). The α1 and α4 helices of GSDM-NT closely interact with GSDM-CT in the autoinhibited state via electrostatic and hydrophobic interactions (Fig. 2A). GSDM-NT and GSDM-CT remain noncovalently associated in vitro following cleavage of the interdomain linker, suggesting that a lipidic environment drives complex separation, GSDM-NT oligomerization, and pore formation (Ding et al. 2016; Ruan et al. 2018).
Figure 2.
Mechanisms of gasdermin (GSDM) autoinhibition and pore formation. (A) Crystal structure of full-length GSDMA3 (PDB ID: 5B5R) shows a two-domain organization of the GSDM family. GSDMA3-CT is colored in orange and key secondary elements in GSDMA3-NT are labeled. (B) Structural overlay of mouse GSDMD-CT (PDB ID: 6AO3) and GSDMA3-CT suggests a conserved mechanism of autoinhibition in the GSDM family. (C) Cryo-electron microspcopic (cryo-EM) structure of the GSDMA3 pore (PDB ID: 6CB8) shows a large 27-subunit ring with a 108-strand β-barrel as the TM region. Each subunit contributes an α1 helix that directly interacts with acidic lipid heads and two β-hairpins that line the β-barrel. (D) Structural overlay of GSDMA3-NT in autoinhibited and membrane-inserted conformations reveals drastic conformational changes in the β3-β4-β5 and β7-α4-β8 regions, which form the two TM β-hairpins. (PyMOL session files are available at osf.io/x7dv8.)
Mechanism of GSDM Pore Formation
Several GSDMs have been shown to form large TM pores following proteolytic activation of their NTs, including GSDMD, GSDMA3, and GSDME (Aglietti et al. 2016; Chen et al. 2016; Ding et al. 2016; Liu et al. 2016; Wang et al. 2017). The cryo-electron microscopic (cryo-EM) structure of the mouse GSDMA3 pore elucidated the mechanism of pore formation in the GSDM family (Ruan et al. 2018). The pore is a 27-fold symmetric, ∼0.8-MDa oligomeric assembly, spanning 18 nm in inner diameter (Fig. 2C). The size of the inner diameter permits the passage of many soluble cytosolic contents to the extracellular space including proinflammatory cytokines IL-1β and IL-18 (Fig. 2C). However, lactate dehydrogenase (LDH), whose release is often used as an indication of lytic cell death, is too large to pass through the GSDMD pore, consistent with the use of LDH release as a hallmark of membrane rupture and cell death. In addition to the 27-subunit structure, 26- and 28-fold symmetric GSDMA3 pores were also reported, indicating heterogeneity and plasticity in GSDM-NT oligomerization (Mulvihill et al. 2018; Ruan et al. 2018).
The 27-subunit GSDMA3 pore features a complete 108-strand antiparallel β-barrel formed by two β-hairpins from each GSDMA3-NT subunit and a rim adjacent to the cytoplasmic side of the lipid bilayer (Fig. 2C). Whereas the β-barrel with a height of ∼7 nm serves as the TM region, the rim is soluble and does not integrate into the membrane. In the cryo-EM structure, the positively charged α1 helix lies in close proximity to the negative lipid headgroup of CL, consistent with the observation that GSDMs bind acidic lipids (Ding et al. 2016; Liu et al. 2016). It should be noted that acidic lipids are not exclusive to the plasma membrane, and the localization of CL in mitochondrial and bacterial membranes raises the possibility of GSDM pore formation elsewhere. The α1 helix is occluded by GSDM-CT in the autoinhibited conformation, and therefore FL GSDMs do not associate with lipids. Superimposition of the GSDMA3 autoinhibited NT and pore structures reveal that large conformational changes occur at the TM β-strands, whereas the globular domain that constitutes the soluble rim remains relatively unaltered (Fig. 2D). On membrane insertion, the entire β3-β4-β5 region of GSDMA3-NT reaches into the membrane to form the first β-hairpin, and the β7-α4-β8 region straightens into the second β-hairpin.
Although other protein families are known to form large β-barrel TM pores, including the membrane attack complex component/perforin (MACPF) and cholesterol-dependent cytolysin (CDC) superfamily, which include pneumolysin and hemolysin (Song et al. 1996; van Pee et al. 2017), GSDMs likely represent a novel class of pore-forming proteins given their unique membrane insertion mechanism and divergence at the amino acid sequence level (Ruan et al. 2018).
ADDITIONAL PATHWAYS REGULATING GSDM ACTIVATION
GSDMD Activation by Other Proteases
In addition to the inflammasome pathways, several other mechanisms for GSDMD activation have recently been elucidated. One such activation pathway occurs through transforming growth factor β–activated kinase 1 (TAK1) and caspase-8 in response to Yersinia pestis infection (Fig. 1C). Under normal conditions, TAK1 restricts the NLRP3 inflammasome and phosphorylation of receptor-interacting protein 1 (RIPK1) (Geng et al. 2017; Malireddi et al. 2018). YopJ, a Y. pestis effector molecule, binds TAK1 to abolish its repression of RIPK1 (Orning et al. 2018; Sarhan et al. 2018). Active RIPK1 then recruits FADD and procaspase-8 to form a structure called the RIPoptosome, which activates caspase-8 through autoprocessing. Active caspase-8 then cleaves GSDMD to promote pyroptosis (Fig. 1C; Orning et al. 2018; Sarhan et al. 2018).
Although the majority of GSDMD-activating enzymes are caspases, a GSDMD activator in aging neutrophils, an inflammatory cell type (Mayadas et al. 2014), is the neutrophil elastase (ELANE). ELANE cleaves GSDMD at a location upstream of the caspase cleavage site to generate functional GSDMD-NTs that form pores and result in neutrophil death (Fig. 1D; Kambara et al. 2018). ELANE-mediated cleavage of GSDMD also facilitates the formation of neutrophil extracellular traps (NETs) in response to noncanonical inflammasome stimuli (Sollberger et al. 2018; Chen et al. 2018a). Neutrophils expel these NETs, antimicrobial weblike structures composed of DNA and proteins, under certain pathogenic conditions. GSDMD facilitates the formation of NETs by forming pores in organelles, as well as their extrusion into the surrounding extracellular milieu by causing cell rupture (Chen et al. 2018a; Sollberger et al. 2018). Thus, the function of the GSDM family likely extends beyond pyroptosis and can be modulated through different upstream activating enzymes.
GSDME Activation by Caspase-3
GSDME was recently shown to form pores to mediate pyroptosis following its cleavage and activation by caspase-3 (Wang et al. 2017). Interestingly, caspase-3 is traditionally defined as an apoptotic caspase, highlighting a role of GSDME in cross talk between multiple cell death pathways. Additionally, GSDME functions as a tumor suppressor by potentiating pyroptosis in response to chemotherapeutics that activate caspase-3 and becomes silenced in many tumors. Cancer cells that express high levels of GSDME undergo pyroptosis in response to chemotherapies that generally result in apoptosis (Rogers et al. 2017; Wang et al. 2018). Further research into the regulation of GSDME expression and activation will elucidate the complex circuitry underlying its cross talk among multiple cell death pathways.
Proteolytic Restriction of GSDMD by Caspase-3/7 and Enterovirus71
Before pore formation, cleavage of GSDMD at noncanonical amino-terminal sites (amino-terminal to the caspase-1/11 cleavage site) can abolish its ability to form functional pores. One such mechanism involves proteolysis by apoptotic caspases (MacFarlane 2019), particularly caspases-3 and -7 (Fig. 3B; Taabazuing et al. 2017). In murine monocytes, multiple apoptotic stimuli resulted in a restricted “p43” GSDMD fragment, rather than the canonical p30 (GSDMD-NT) and p20 (GSDMD-CT) fragments, suggesting that apoptotic caspases might inactivate GSDMD. In vitro cleavage reactions showed that caspase-3 and caspase-7 indeed cleaved GSDMD at a different site than inflammatory caspases, and the cleavage site was mapped to aspartate 87 (D87). Neither the 1–87 nor 88–484 GSDMD fragment was toxic to HEK293T cells (Taabazuing et al. 2017), confirming that caspase-3 and caspase-7 inhibit pore formation by cleaving GSDMD within its NT domain. Conversely, however, a related GSDM family member, GSDME, can be activated by caspase-3 (Rogers et al. 2017; Wang et al. 2017). Cross talk between apoptosis and pyroptosis likely prevents an inflammatory response during apoptosis, which could otherwise lead to chronic inflammatory diseases (Nagata 2018). Failure to execute apoptosis might necessitate other pathways such as pyroptosis to ensure cell death.
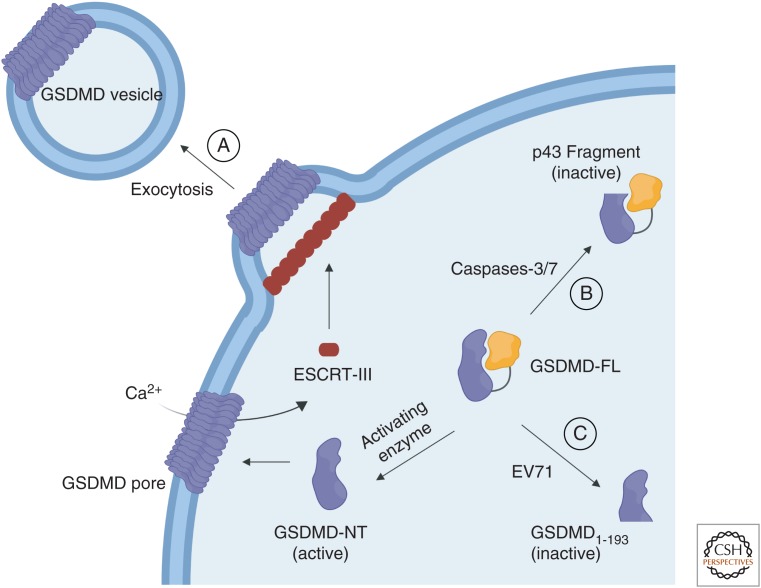
Figure 3.
Pyroptotic death evasion through gasdermin D (GSDMD). (A) GSDMD pores are exocytosed from the cell through the endosomal sorting complexes required for transport (ESCRT)-III machinery. (B) Caspase-3 and caspase-7 cleave the GSDMD-NT after D87, generating a nonfunctional protein fragment. (C) The 3C protease of enterovirus 71 cleaves the GSDMD-NT at a noncanonical site, generating an inactive NT fragment.
Pathogens would also benefit from preventing pyroptosis by silencing pore formation, thus protecting their niche and avoiding the innate immune response. One such case is enterovirus 71 (EV71), the causative agent of hand-foot-and-mouth disease. The 3C protease of EV71 cleaves GSDMD at an amino-terminal site, inhibiting GSDMD (Fig. 3C; Lei et al. 2017). EV71 infection resulted in this GSDMD cleavage in multiple cell lines (Lei et al. 2017). The same GSDMD cleavage bands were observed by co-expressing EV71 3C protease with GSDMD, and its catalytic activity was necessary for GSDMD cleavage. By mutating a 3C-like consensus site, glutamate 193 (Q193) of GSDMD was identified as the EV71 3C protease cleavage site, which is amino-terminal to the conventional caspase cleavage site (D276). The truncated GSDMD-NT, residues 1–193, could not cause cell death (Lei et al. 2017). Although EV71 remains the only known pathogen to cleave GSDMD directly, other pathogens have likely evolved such mechanisms.
Mechanisms of Cytokine Release without Pyroptosis
Although GSDMD pore formation generally leads to pyroptotic cell death and membrane rupture, recent discoveries call into question the finality of eliciting pyroptosis. Several studies showed uncoupling of inflammasome activation and IL-1β release from cell death in multiple cell types, suggesting that live cells might release cytokines through GSDMD pores and perhaps even survive pore formation. The first line of such evidence showed that murine neutrophils undergo NLRC4-dependent cytokine maturation in response to Salmonella stimulus but not concomitant loss of membrane integrity and cell death (Chen et al. 2014). Macrophages underwent cell death under the same stimuli (Chen et al. 2014) but were also recently shown capable of releasing cytokines while maintaining membrane integrity when buffered with glycine, an osmoprotectant (Evavold et al. 2018). Murine macrophages evade cell death in the presence of Gram-negative peptidoglycan, which results in cytokine release with unconventional signaling through the metabolic enzyme hexokinase and the NLRP3 inflammasome (Wolf et al. 2016). In dendritic cells, NLRP3 stimuli such as LPS and ATP resulted in pyroptotic cell death, whereas oxidized phospholipids, which often accompany tissue cell death (Chang et al. 2004), triggered cytokine release without eliciting pyroptotic cell death (Zanoni et al. 2016). The NLRP3 inflammasome also mediates living IL-1β release in peripheral blood mononuclear cells (Gaidt et al. 2016). Thus, several cell types were shown capable of releasing inflammatory cytokines through GSDMD pores without concomitant cell death.
Of note, many of these studies used bulk cell assays, in which it is difficult to distinguish cytokine release from a small population of dying cells if IL-1β effect sizes are not sufficiently large. Furthermore, cell death (cessation of cell movement/mitochondrial integrity) likely precedes GSDMD-mediated cell lysis (DiPeso et al. 2017), confounding the ability to experimentally decouple pore formation (PI uptake), cell death, and cell lysis (LDH release). Nonetheless, prolonged cytokine maturation and release in living cells would be a reasonable mechanism for a more robust recruitment of immune effector cells. Indeed, several mechanisms to evade GSDMD-mediated cell death have been discovered, such as the exocytosis of GSDMD pore-containing membrane vesicles (Rühl et al. 2018) as described below.
GSDMD cleavage leads to its oligomerization and membrane insertion, which is likely an energetically irreversible process. How, then, can cells repair these GSDMD pores once they have been inserted into the plasma membrane? The first and only known mechanism of such repair involves exocytosis of vesicles containing GSDMD pores (Fig. 3A). Removal of GSDMD pores from the cell membrane prevents or prolongs membrane lysis (Rühl et al. 2018). This pathway occurs through the endosomal sorting complexes required for transport (ESCRT)-III machinery (Rühl et al. 2018), which mediates membrane fission and subsequent exocytosis of membrane vesicles in many cellular processes (McCullough et al. 2018), including exocytosis of mixed lineage kinase domain like pseudokinase (MLKL) pores in necroptosis (Gong et al. 2017).
Similar to MLKL pores (Gong et al. 2017), GSDMD pores cause rapid calcium ion (Ca2+) influx (Rühl et al. 2018), which is known to activate ESCRT-III (Scheffer et al. 2014). Inhibition of ESCRT-III through Ca2+ chelation or knockdowns of ESCRT-III components led to increased cell death in response to several inflammasome stimuli, including those that activate NLRC4, AIM2, and noncanonical activation of NLRP3 through caspase-11. Additionally, fluorescently labeled CHMP4, a component of the ESCRT-III machinery, formed membrane-localized puncta in response to inflammasome stimulus (Rühl et al. 2018), consistent with other ESCRT-III-mediated repair processes (Jimenez et al. 2014). Thus, cells can shed GSDMD pores to prevent or prolong pyroptotic cell death, enabling survival and/or increased cytokine maturation (Fig. 3). It remains unclear whether additional cellular mechanisms disassemble or destroy GSDMD pores, and the functional outcomes of these mechanisms in vivo.
Pyroptosis in Disease
Precise discrimination between healthy and infected host cells is an important challenge of innate immunity, as unchecked activity can lead to autoimmune disorders. Thus, although phagocytes, including neutrophils and macrophages, efficiently detect pathogens in the extracellular space, pathogens that enter host cells might escape surveillance. Pyroptosis serves a crucial protective function of alerting the immune system to intracellular pathogenic insult. Pyroptosis directly kills compromised host cells, removing the niche for invading pathogens. Additionally, release of cytokines through GSDMD pores establishes a cytokine gradient that directs phagocytes to the site of infection. Moreover, tears on the membranes of postlytic pyroptotic cells are small enough to retain large organelles and bacteria by forming an intracellular trap, driving the recruitment of phagocytes to engulf the trapped bacteria and cell debris through a process termed efferocytosis (Jorgensen et al. 2016; Davidson and Wood 2019; Moldoveanu and Czabotar 2019).
Despite its importance to pathogen defense, inappropriate or hyperactive pyroptosis can be highly pathological, causing damaging inflammation that contributes to diseases such as arthritis, inflammatory bowel disease, Alzheimer's, and sepsis (Lamkanfi and Dixit 2012). Thus, inhibiting pyroptosis might improve autoimmune and autoinflammatory disease prognoses.
Inhibiting Pyroptosis with Small Molecules
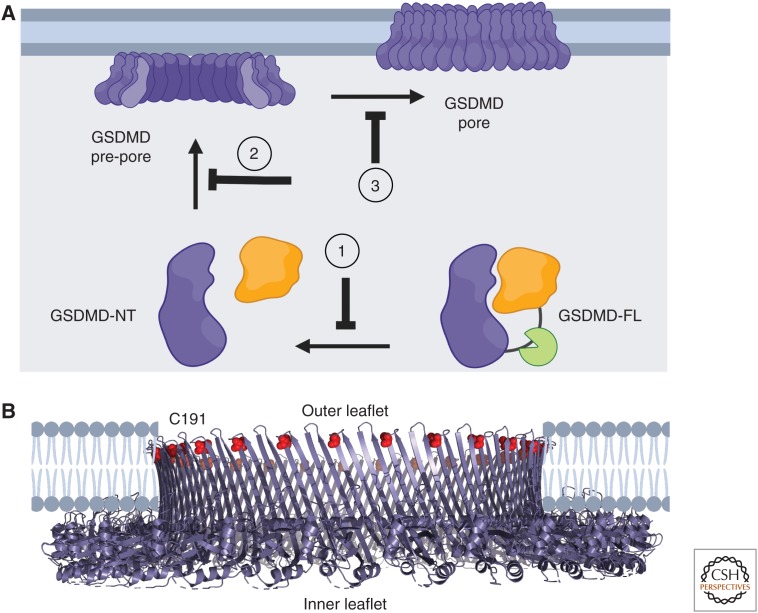
Because GSDMD integrates a multitude of endogenous and exogenous upstream signals (de Vasconcelos and Lamkanfi 2019) by executing pyroptosis, it presents a highly desirable molecular target for therapeutic intervention. Mechanistically, strategies to directly inhibit GSDMD could involve preventing its cleavage, lipid binding, oligomerization, or membrane insertion (Fig. 4A). Recently, four small molecules were identified as targeting GSDMD: necrosulfonamide (Rathkey et al. 2018), LDC7559 (Sollberger et al. 2018), Bay 11-7082, and disulfiram (Hu et al. 2018). Necrosulfonamide was originally identified as a cysteine reactive drug that targeted MLKL to inhibit necroptosis (Sun et al. 2012) and might also inhibit multiple steps of the pyroptotic pathway (Rathkey et al. 2018). LDC7559 inhibited NET formation, presumably by binding and suppressing GSDMD pore formation (Sollberger et al. 2018). Bay 11-7082 and disulfiram were discovered through drug screening efforts using an in vitro assay for GSDMD pore formation and disulfiram was shown to suppress pyroptosis in vitro and in vivo (Hu et al. 2018).
Figure 4.
Inhibiting gasdermin D (GSDMD) with small molecules. (A) Potential routes of small-molecule intervention include (1) cleavage of GSDMD and release of GSDMD-NT, (2) lipid binding and oligomerization of GSDMD-NT, and (3) membrane insertion of GSDMD-NT. (B) Small-molecule drugs disulfiram, necrosulfonamide, and Bay 11-702 target C191 of GSDMD and therefore might disrupt membrane insertion and/or oligomerization of GSDMD-NT. C191, highlighted in red, is located at the tip of a β-hairpin according to a model of the GSDMD pore, generated based on the structure of the GSDMA3 pore (PDB ID: 6CB8). (PyMOL session files are available at osf.io/x7dv8.)
In characterizing these drug candidates, a reactive cysteine on GSDMD, C191 (C192 in mouse), was identified as the target for covalent modification by necrosulfonamide, disulfiram, and Bay 11-7082. Based on the structure of the homologous GSDMA3 pore (Ruan et al. 2018), C191 likely lies on the tip of a β-hairpin that sits at the membrane interface (Fig. 4B), and modification of C191 by drug candidates could prevent its membrane insertion and/or oligomerization (Liu et al. 2016). Ongoing efforts to target GSDMD will benefit from a more complete knowledge of its membrane insertion mechanism. Conversely, cellular studies will benefit from potent and specific small-molecule inhibitors of GSDMD.
CONCLUSIONS AND FUTURE PERSPECTIVES
During the last several years, a rich body of research has established the critical function of the GSDM family in programmed cell death. Inflammatory caspases are activated upon sensing PAMPs and DAMPs by inflammasomes, resulting in the cleavage of autoinhibited GSDMD and liberation of GSDMD-NT to form membrane pores. In addition to inflammasome activation, Yersenia infection and ELANE trigger cleavage of GSDMD. GSDMD-NT pore formation on the plasma membrane facilitates the release of soluble cytosolic contents including mature IL-1 family cytokines and often leads to lytic, pyroptotic cell death. To prevent pyroptosis, host cells and pathogens have evolved mechanisms for repairing GSDMD-perforated membranes and inactivating GSDMD.
GSDME was also shown to be an executioner of pyroptosis following cleavage by active caspase-3 (Rogers et al. 2017; Wang et al. 2017). Despite our increasing knowledge of GSDMD and GSDME, the biological functions remain elusive for most GSDMs. In fact, although conserved in domain organization, many GSDM family members mediate cellular processes beyond pyroptosis. For example, a main function of murine GSDMA3 is to regulate the production of reactive oxygen species by forming pores on mitochondrial membranes (Lin et al. 2015), and GSDMD functions in NETosis (Chen et al. 2018a; Sollberger et al. 2018). Underlying the diverse functions of the GSDM family are intricate signaling networks that might have been previously underappreciated. GSDM-activating enzymes are not strictly inflammatory caspases, and increasing evidence indicates that cell death pathways are regulated by multiple cross-talking activating enzymes (Vince and Silke 2016; Mascarenhas et al. 2017; Rauch et al. 2017; Taabazuing et al. 2017). A more comprehensive investigation into the mechanisms and regulation of the GSDM family will benefit future understanding of GSDM-mediated diseases and the development of GSDM-targeting therapeutics.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grants DP1HD087988 and R01Al124491 to H.W. and Albert J. Ryan Fellowships to S.X. and L.R.H. We thank J. Ruan, C. Shen, J. Hu, and A. Brown for discussion and critical reading of the manuscript, and BioRender.com for figure design. The authors apologize for incomplete citations due to space limitations.
Footnotes
Editors: Kim Newton, James M. Murphy, and Edward A. Miao
Additional Perspectives on Cell Survival and Cell Death available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC. 2016. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci 113: 7858–7863. 10.1073/pnas.1607769113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. 2009. Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol 7: 99–109. 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. 2012. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490: 288–291. 10.1038/nature11419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M-K, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. 2004. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med 200: 1359–1370. 10.1084/jem.20031763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao KL, Kulakova L, Herzberg O. 2017. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc Natl Acad Sci 114: E1128–E1137. 10.1073/pnas.1616783114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Groß CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, Schroder K. 2014. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep 8: 570–582. 10.1016/j.celrep.2014.06.028 [DOI] [PubMed] [Google Scholar]
- Chen X, He WT, Hu L, Li J, Fang Y, Wang X, Xu X, Wang Z, Huang K, Han J. 2016. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res 26: 1007–1020. 10.1038/cr.2016.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K. 2018a. Noncanonical inflammasome signaling elicits gasdermin D–dependent neutrophil extracellular traps. Sci Immunol 3: eaar6676 10.1126/sciimmunol.aar6676 [DOI] [PubMed] [Google Scholar]
- Chen Q, Shi P, Wang Y, Zou D, Wu X, Wang D, Hu Q, Zou Y, Huang Z, Ren J, et al. 2018b. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J Mol Cell Biol 11: 496–508. 10.1093/jmcb/mjy056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Davidson AJ, Wood W. 2019. Phagocyte responses to cell death in flies. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a036350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.de Vasconcelos NM, Lamkanfi M. 2019. Recent insights on inflammasomes, gasdermin pores, and pryroptosis. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a036392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. 2016. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535: 111–116. 10.1038/nature18590 [DOI] [PubMed] [Google Scholar]
- DiPeso L, Ji DX, Vance RE, Price JV. 2017. Cell death and cell lysis are separable events during pyroptosis. Cell Death Discov 3: 17070 10.1038/cddiscovery.2017.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. 2018. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48: 35–44.e6. 10.1016/j.immuni.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. 2007. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell 25: 713–724. 10.1016/j.molcel.2007.01.032 [DOI] [PubMed] [Google Scholar]
- Feng S, Fox D, Man SM. 2018. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J Mol Biol 430: 3068–3080. 10.1016/j.jmb.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. 2005. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun 73: 1907–1916. 10.1128/IAI.73.4.1907-1916.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. 2006. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 8: 1812–1825. 10.1111/j.1462-5822.2006.00751.x [DOI] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson Avril AB, Cooper MA, Graf T, Hornung V. 2016. Human monocytes engage an alternative inflammasome pathway. Immunity 44: 833–846. 10.1016/j.immuni.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. 2018. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25: 486–541. 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Ito Y, Shi L, Amin P, Chu J, Ouchida AT, Mookhtiar AK, Zhao H, Xu D, Shan B, et al. 2017. Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun 8: 359 10.1038/s41467-017-00406-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, Linkermann A, Green DR. 2017. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169: 286–300.e16. 10.1016/j.cell.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. 2013. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 341: 1250–1253. 10.1126/science.1240988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. 2015. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res 25: 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, Broz P. 2018. The gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur J Immunol 48: 584–592. 10.1002/eji.201747404 [DOI] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458: 514–518. 10.1038/nature07725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JJ, Liu X, Zhao J, Xia S, Ruan J, Luo X, Kim J, Lieberman J, Wu H. 2018. Identification of pyroptosis inhibitors that target a reactive cysteine in gasdermin D. bioRxiv 10.1101/365908. [DOI] [Google Scholar]
- Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F. 2014. ESCRT machinery is required for plasma membrane repair. Science 343: 1247136 10.1126/science.1247136 [DOI] [PubMed] [Google Scholar]
- Jorgensen I, Zhang Y, Krantz BA, Miao EA. 2016. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med 213: 2113–2128. 10.1084/jem.20151613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, Zhao L, Zhou S, Yu H, Zhou W, et al. 2018. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep 22: 2924–2936. 10.1016/j.celrep.2018.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121. 10.1038/nature10558 [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, et al. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341: 1246–1249. 10.1126/science.1240248 [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526: 666–671. 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- Kovacs SB, Miao EA. 2017. Gasdermins: Effectors of pyroptosis. Trends Cell Biol 27: 673–684. 10.1016/j.tcb.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Zheng J, Yang H, Li S, Duan S, Shen Y, Ji C, Gan J, Xu XW, Li J. 2017. Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc Natl Acad Sci 114: 10642–10647. 10.1073/pnas.1708194114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. 2012. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 28: 137–161. 10.1146/annurev-cellbio-101011-155745 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. 2014. Mechanisms and functions of inflammasomes. Cell 157: 1013–1022. 10.1016/j.cell.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Lee BL, Stowe IB, Gupta A, Kornfeld OS, Roose-Girma M, Anderson K, Warming S, Zhang J, Lee WP, Kayagaki N. 2018. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J Exp Med 215: 2279–2288. 10.1084/jem.20180589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Zhang Z, Xiao X, Qi J, He B, Wang J. 2017. Enterovirus 71 inhibits pyroptosis through cleavage of gasdermin D. J Virol 91: e01069 10.1128/JVI.01069-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PH, Lin HY, Kuo CC, Yang LT. 2015. N-terminal functional domain of gasdermin A3 regulates mitochondrial homeostasis via mitochondrial targeting. J Biomed Sci 22: 44 10.1186/s12929-015-0152-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. 2016. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535: 153–158. 10.1038/nature18629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang C, Rathkey JK, Yang J, Dubyak GR, Abbott DW, Xiao TS. 2018. Structures of the gasdermin D C-terminal domains reveal mechanisms of autoinhibition. Structure 26: 778–784.e3. 10.1016/j.str.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang C, Yang J, Zhou B, Yang R, Ramachandran R, Abbott DW, Xiao TS. 2019. Crystal structures of the full-length murine and human gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity 51: 43–49.e44. 10.1016/j.immuni.2019.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H, Egelman EH. 2014. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156: 1193–1206. 10.1016/j.cell.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi RKS, Gurung P, Mavuluri J, Dasari TK, Klco JM, Chi H, Kanneganti TD. 2018. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J Exp Med 215: 1023–1034. 10.1084/jem.20171922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Kanneganti TD. 2015. Regulation of inflammasome activation. Immunol Rev 265: 6–21. 10.1111/imr.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. 2002. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10: 417–426. 10.1016/S1097-2765(02)00599-3 [DOI] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241. 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- Mascarenhas DPA, Cerqueira DM, Pereira MSF, Castanheira FVS, Fernandes TD, Manin GZ, Cunha LD, Zamboni DS. 2017. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog 13: e1006502 10.1371/journal.ppat.1006502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadas TN, Cullere X, Lowell CA. 2014. The multifaceted functions of neutrophils. Annu Rev Pathol 9: 181–218. 10.1146/annurev-pathol-020712-164023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough J, Frost A, Sundquist WI. 2018. Structures, functions, and dynamics of ESCRT-III/Vps4 membrane remodeling and fission complexes. Annu Rev Cell Dev Biol 34: 85–109. 10.1146/annurev-cellbio-100616-060600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.MacFarlane M. 2019. Mechanisms of caspase activation. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a036335 [DOI] [Google Scholar]
- *.Moldoveanu T, Czabotar PE. 2019. BAX, BAK, and BOK; a coming of age for the BCL-2 family effector proteins. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a036319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E, Sborgi L, Mari SA, Pfreundschuh M, Hiller S, Müller DJ. 2018. Mechanism of membrane pore formation by human gasdermin-D. EMBO J 37: e98321. 10.15252/embj.201798321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S. 2018. Apoptosis and clearance of apoptotic cells. Annu Rev Immunol 36: 489–517. 10.1146/annurev-immunol-042617-053010 [DOI] [PubMed] [Google Scholar]
- Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, et al. 2018. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362: 1064–1069. 10.1126/science.aau2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban RA, Sun M, Dahlin A, Park HR, Kan M, Himes BE, Mitchel JA, Iribarren C, Jorgenson E, Randell SH, et al. 2018. A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. J Allergy Clin Immunol 142: 1469–1478.e2. 10.1016/j.jaci.2017.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. 2007. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14: 1583–1589. 10.1038/sj.cdd.4402195 [DOI] [PubMed] [Google Scholar]
- Platnich JM, Chung H, Lau A, Sandall CF, Bondzi-Simpson A, Chen HM, Komada T, Trotman-Grant AC, Brandelli JR, Chun J, et al. 2018. Shiga toxin/lipopolysaccharide activates caspase-4 and gasdermin D to trigger mitochondrial reactive oxygen species upstream of the NLRP3 inflammasome. Cell Rep 25: 1525–1536.e7. 10.1016/j.celrep.2018.09.071 [DOI] [PubMed] [Google Scholar]
- Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, Benson BL, Chirieleison SM, Huang AY, Dubyak GR, et al. 2018. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol 3: eaat2738 10.1126/sciimmunol.aat2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K, et al. 2017. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 46: 649–659. 10.1016/j.immuni.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. 2017. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 8: 14128 10.1038/ncomms14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Xia S, Liu X, Lieberman J, Wu H. 2018. Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557: 62–67. 10.1038/s41586-018-0058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. 2018. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362: 956–960. 10.1126/science.aar7607 [DOI] [PubMed] [Google Scholar]
- Saeki N, Sasaki H. 2012. Gasdermin superfamily: A novel gene family functioning in epithelial cells. In Endothelium and epithelium: Composition, functions and pathology (ed. Carrasco J, Mot M), pp. 193–211. Nova Science, New York. [Google Scholar]
- Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, et al. 2018. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci 115: E10888 10.1073/pnas.1809548115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Müller DJ, Broz P, Hiller S. 2016. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 35: 1766–1778. 10.15252/embj.201694696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer LL, Sreetama SC, Sharma N, Medikayala S, Brown KJ, Defour A, Jaiswal JK. 2014. Mechanism of Ca2+-triggered ESCRT assembly and regulation of cell membrane repair. Nat Commun 5: 5646 10.1038/ncomms6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514: 187–192. 10.1038/nature13683 [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665. 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, et al. 2018. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 3: eaar6689 10.1126/sciimmunol.aar6689 [DOI] [PubMed] [Google Scholar]
- Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. 1996. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 274: 1859–1865. 10.1126/science.274.5294.1859 [DOI] [PubMed] [Google Scholar]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. 2012. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148: 213–227. 10.1016/j.cell.2011.11.031 [DOI] [PubMed] [Google Scholar]
- Taabazuing CY, Okondo MC, Bachovchin DA. 2017. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol 24: 507–514.e4. 10.1016/j.chembiol.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Tanaka S, Fujii T, Aoki A, Komiyama H, Ezawa K, Sumiyama K, Sagai T, Shiroishi T. 2007. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics 89: 618–629. 10.1016/j.ygeno.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Mizushina Y, Kato Y, Tamura M, Shiroishi T. 2013. Functional conservation of Gsdma cluster genes specifically duplicated in the mouse genome. G3 (Bethesda) 3: 1843–1850. 10.1534/g3.113.007393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, et al. 2015. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- van Pee K, Neuhaus A, D'Imprima E, Mills DJ, Kühlbrandt W, Yildiz Ö. 2017. CryoEM structures of membrane pore and prepore complex reveal cytolytic mechanism of Pneumolysin. eLife 6: e23644 10.7554/eLife.23644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Silke J. 2016. The intersection of cell death and inflammasome activation. Cell Mol Life Sci 73: 2349–2367. 10.1007/s00018-016-2205-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. 2017. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547: 99–103. 10.1038/nature22393 [DOI] [PubMed] [Google Scholar]
- Wang Y, Yin B, Li D, Wang G, Han X, Sun X. 2018. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun 495: 1418–1425. 10.1016/j.bbrc.2017.11.156 [DOI] [PubMed] [Google Scholar]
- Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML, Cho HC, Popescu NI, Coggeshall KM, Arditi M, et al. 2016. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166: 624–636. 10.1016/j.cell.2016.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, et al. 2016. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352: 1232–1236. 10.1126/science.aaf3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen X, Gueydan C, Han J. 2018. Plasma membrane changes during programmed cell deaths. Cell Res 28: 9–21. 10.1038/cr.2017.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477: 596–600. 10.1038/nature10510 [DOI] [PubMed] [Google Scholar]
- Zhong FL, Mamaï O, Sborgi L, Boussofara L, Hopkins R, Robinson K, Szeverényi I, Takeichi T, Balaji R, Lau A, et al. 2016. Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell 167: 187–202.e17. 10.1016/j.cell.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225. 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]