Abstract
Background
Postpartum endometritis occurs when vaginal organisms invade the endometrial cavity during the labor process and cause infection. This is more common following cesarean birth. The condition warrants antibiotic treatment.
Objectives
Systematically, to review treatment failure and other complications of different antibiotic regimens for postpartum endometritis.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 November 2014) and reference lists of retrieved studies.
Selection criteria
We included randomized trials of different antibiotic regimens after cesarean birth or vaginal birth; no quasi‐randomized trials were included.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
The review includes a total of 42 trials, and 40 of these trials contributed data on 4240 participants.
Twenty studies, involving 1918 women, compared clindamycin plus an aminoglycoside (gentamicin for all studies except for one that used tobramycin) with another regimen.
When assessing the individual subgroups of other antibiotic regimens (i.e. cephalosporins, monobactams, penicillins, and quinolones), there were fewer treatment failures in those treated with clindamycin plus an aminoglycoside as compared to those treated with cephalosporins (RR 0.69, 95% CI 0.49 to 0.99; participants = 872; studies = 8; low quality evidence) or penicillins (RR 0.65, 95% CI 0.46 to 0.90; participants = 689; studies = 7, low quality evidence). For the remaining subgroups for the primary analysis, the differences were not significant. There were significantly fewer wound infections in those treated with clindamycin plus aminoglycoside versus cephalosporins (RR 0.53, 95% CI 0.30 to 0.93; participants = 500; studies = 4; low quality evidence). Similarly, there were more treatment failures in those treated with an gentamicin/penicillin when compared to those treated with gentamIcin/clindamycin (RR 2.57, 95% CI 1.48 to 4.46; participants = 200; studies = 1).
There were fewer treatment failures when an agent with a longer half‐life that is administered less frequently was used (RR 0.61, 95% CI 0.40 to 0.92; participants = 484; studies = 2) as compared to using cefoxitin. There were more treatment failures (RR 1.94, 95% CI 1.38 to 2.72; participants = 774; studies = 7) and wound infections (RR 1.88, 95% CI 1.17 to 3.02; participants = 740; studies = 6) in those treated with a regimen with poor activity against penicillin‐resistant anaerobic bacteria as compared to those treated with a regimen with good activity against penicillin‐resistant anaerobic bacteria. Once‐daily dosing was associated with a shorter length of hospital stay (MD ‐0.73, 95% CI ‐1.27 to ‐0.20; participants = 322; studies = 3).
There were no differences between groups with respect to severe complications and no trials reported any maternal deaths.
Regarding the secondary outcomes, three studies that compared continued oral antibiotic therapy after intravenous therapy with no oral therapy, found no differences in recurrent endometritis or other outcomes. There were no differences between groups for the outcomes of allergic reactions.
The overall risk of bias was unclear in the most of the studies. The quality of the evidence using GRADE comparing clindamycin and an aminoglycoside with another regimen (compared with cephalosporins or penicillins) was low to very low for therapeutic failure, severe complications, wound infection and allergic reaction.
Authors' conclusions
The combination of clindamycin and gentamicin is appropriate for the treatment of endometritis. Regimens with good activity against penicillin‐resistant anaerobic bacteria are better than those with poor activity against penicillin‐resistant anaerobic bacteria. There is no evidence that any one regimen is associated with fewer side‐effects. Following clinical improvement of uncomplicated endometritis which has been treated with intravenous therapy, the use of additional oral therapy has not been proven to be beneficial.
Plain language summary
Antibiotic regimens for postpartum endometritis
Intravenous clindamycin plus gentamicin is more effective than other antibiotics or combinations of antibiotics for treatment of womb infection after childbirth.
Inflammation of the lining of the womb (endometritis) can be caused by vaginal bacteria entering the womb (uterus) during childbirth and causing infection within six weeks of the birth (postpartum endometritis). Postpartum endometritis occurs after about 1% to 3% of vaginal births, and up to 27% of cesarean births. Prolonged rupture of the membranes (breaking the bag of water that surrounds the baby) and multiple vaginal examinations during birth also appear to increase the risk.
Endometritis causes fever, tenderness in the pelvic region and unpleasant‐smelling vaginal discharge after the birth. It can have serious complications such as the formation of pelvic abscesses, blood clots, infection of the thin layer of tissue that covers the inside of the abdomen and abdominal organs (peritonitis), and whole body inflammation (sepsis). It is also an important cause of maternal deaths worldwide, although with the use of antibiotics, this is very rare in high‐income countries.
There are many antibiotic treatments currently in use. This review compared different antibiotics, routes of administration and dosages for endometritis. The review identified 42 relevant randomised controlled studies, which are the most reliable type of medical trial for this type of investigation; 40 of these (involving 4240 women) contributed data for analysis.
The results showed that the combination of intravenous gentamicin and clindamycin, and drugs with a broad range of activity against the relevant penicillin‐resistant bacterial strains, are the most effective for treating endometritis after childbirth. Women treated with clindamycin plus an aminoglycoside (gentamicin) showed fewer treatment failures than those treated with penicillin, but this difference was not evident when women treated with clindamycin plus an aminoglycoside were compared to women who received other antibiotic treatments.
There were more treatment failures in women treated with an penicillin plus gentamicin (one study) compared with those treated with clindamycin plus gentamicin. Seven trials showed that an antibiotic treatment that had poor activity against bacteria resistant to penicillin had a higher failure rate and more wound infections than an antibiotic treatment that had good activity against these bacteria.
There was no evidence that any of the antibiotic combinations had fewer adverse effects ‐ including allergic reaction ‐ than other antibiotic combinations. If the endometritis was uncomplicated and improved with intravenous antibiotics, there did not appear to be a need to follow the intravenous antibiotics with a course of oral antibiotics.
Overall the reliability of the studies' results was unclear, the numbers of women studied were often small and data on other outcomes were limited; furthermore, a number of the studies had been funded by drug companies that conceivably would have had a vested interest in the results.
Summary of findings
Summary of findings for the main comparison. Clindamycin plus aminoglycoside versus any other regimen for postpartum endometritis.
| Clindamycin plus aminoglycoside versus cephalosporins or penicillins for postpartum endometritis | ||||||
| Population: women with postpartum endometritis Settings: hospitals in US, France, Mexico, Colombia, Peru, Italy (most studies from USA) Intervention: clindamycin plus aminoglycoside versus any other regimen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Clindamycin plus aminoglycoside versus any other regimen | |||||
| Treatment failure ‐ lincosamides versus cephalosporins | Study population | RR 0.69 (0.49 to 0.99) | 872 (8 studies) | ⊕⊕⊝⊝ low1,3 | ||
| 148 per 1000 | 102 per 1000 (73 to 147) | |||||
| Moderate | ||||||
| 237 per 1000 | 164 per 1000 (116 to 235) | |||||
| Treatment failure ‐ lincosamides versus penicillins | Study population | RR 0.65 (0.46 to 0.90) | 689 (7 studies) | ⊕⊕⊝⊝ low1,3 | ||
| 209 per 1000 | 136 per 1000 (96 to 188) | |||||
| Moderate | ||||||
| 189 per 1000 | 123 per 1000 (87 to 170) | |||||
| Severe complication ‐ lincosamides versus cephalosporins | Study population | RR 2.40 (0.30 to 19.19) | 476 (4 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 4 per 1000 | 10 per 1000 (1 to 77) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Severe complication ‐ lincosamides versus penicillins | Study population | RR 0.33 (0.09 to 1.18) | 422 (5 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 38 per 1000 | 13 per 1000 (3 to 45) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Wound infection ‐ lincosamides versus cephalosporins | Study population | RR 0.53 (0.3 to 0.93) | 500 (4 studies) | ⊕⊕⊝⊝ low1,3 | ||
| 114 per 1000 | 60 per 1000 (34 to 106) | |||||
| Moderate | ||||||
| 121 per 1000 | 64 per 1000 (36 to 113) | |||||
| Wound infection ‐ lincosamides versus penicillins | Study population | RR 0.46 (0.21 to 1) | 339 (3 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 107 per 1000 | 49 per 1000 (22 to 107) | |||||
| Moderate | ||||||
| 63 per 1000 | 29 per 1000 (13 to 63) | |||||
| Allergic reaction ‐ lincosamides versus cephalosporins | Study population | RR 1.36 (0.44 to 4.21) | 680 (6 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 12 per 1000 | 17 per 1000 (5 to 51) | |||||
| Moderate | ||||||
| 14 per 1000 | 19 per 1000 (6 to 59) | |||||
| Allergic reaction ‐ lincosamides versus penicillins | Study population | RR 1 (0.14 to 6.96) | 247 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 16 per 1000 | 16 per 1000 (2 to 113) | |||||
| Moderate | ||||||
| 10 per 1000 | 10 per 1000 (1 to 70) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 Most studies contributing data had design limitations 2 Small sample size with confidence interval crossing the line of no effect 3 Estimate based on small sample size
Background
Description of the condition
The diagnosis of postpartum endometritis is based on the presence of fever in the absence of any other cause. Uterine tenderness, purulent or foul‐smelling lochia and leukocytosis are common clinical findings used to support the diagnosis of endometritis. The American Committe of Maternal Welfare's standard definition for reporting rates of puerperal morbidity is an "oral temperature of 38.0 degrees centigrade or more on any two of the first 10 days postpartum or 38.7 degrees centigrade or higher during the first 24 hours postpartum". Alternatively, postpartum endometritis has been divided into early‐onset disease occurring within 48 hours postpartum, and late‐onset disease presenting up to six weeks postpartum (Wager 1980; Williams 1995). Endometritis is diagnosed after 1% to 3% of vaginal births; and it is up to 10 times more common after cesarean birth (Calhoun 1995).
Description of the intervention
The pathogenesis of endometritis is related to contamination of the uterine cavity with vaginal organisms during labor and birth and invasion of the myometrium. The presence of certain bacteria (e.g. groups A and B streptococci, aerobic Gram‐negative rods, Neisseria gonorrhoeae, Mycoplasma hominis and certain anaerobic bacteria) in amniotic fluid cultures at the time of cesarean birth is associated with an increased risk of postpartum endometritis (Newton 1990). For vaginal births, the presence of the organisms associated with bacterial vaginosis (e.g. certain anaerobic bacteria and Gardnerella vaginalis) or genital cultures positive for aerobic Gram‐negative organisms is associated with an increased risk for endometritis (Newton 1990). Prolonged rupture of membranes and multiple vaginal examinations have also been identified as potential risk factors (Gibbs 1980). Women with bacterial vaginosis in early pregnancy have three times significantly higher risk of postpartum endometritis (Jacobsson 2002).
Endometritis is usually a polymicrobial infection associated with mixed aerobic and anaerobic flora. Bacteremia may be present in 10% to 20% of cases. Unless a specimen is obtained from the upper genital tract without contamination from the vagina, or blood cultures are positive, there is seldom laboratory confirmation of the microbiological etiology of endometritis.
Complications of endometritis include extension of infection to involve the peritoneal cavity with peritonitis, intra‐abdominal abscess, or sepsis. Septic pelvic thrombophlebitis, which can be associated with septic pulmonary emboli, can occur rarely as a complication of postpartum endometritis.
How the intervention might work
Before the advent of the antibiotic era, puerperal fever was an important cause of maternal death. With the use of antibiotics, a sharp decrease in maternal morbidity has been observed, and it is now accepted that antibiotic treatment for postpartum endometritis is warranted.
There are many antibiotic treatment regimens currently in use. An empiric regimen active against the mixed aerobic and anaerobic organisms likely to be causing infection is generally selected. Treatment is usually considered successful after the woman is afebrile for 24 to 48 hours. The spectrum of activity of clindamycin with gentamicin makes these antibiotics a popular choice for initial therapy and this combination is widely considered as the gold standard (Monga 1993). However, alternative treatment regimens for endometritis with different antimicrobial activity or pharmacokinetic profiles may be associated with differences in clinical effectiveness, side‐effects or cost.
Why it is important to do this review
Determination of the appropriate antibiotic regimen to treat postpartum endometritis has multiple short and long term ramifications. Appropriate initial treatment may not only decrease maternal morbidity but may also improve antibiotic stewardship.
Objectives
The objective of this review was to determine, from the best evidence available, the effect of different antibiotic regimens for the treatment of postpartum endometritis on the rate of therapeutic failure, the duration of fever, the rates of complications, and the rates of side‐effects of treatment. The effects of different drugs, routes of administration, and duration of therapy were sought. In addition, we sought to compare the effectiveness of regimens known to be active against the penicillin‐resistant Bacteroides fragilis group of anaerobic organisms compared with those that are not.
Methods
Criteria for considering studies for this review
Types of studies
All trials in which the authors described random allocation (by any method) of participants to different treatment regimens for postpartum endometritis were considered. Cluster‐randomized trials are eligible for inclusion, but we did not consider cross‐over trials suitable for inclusion. We excluded quasi‐randomized and pseudo‐randomized studies.
Types of participants
Women who were diagnosed with endometritis (as defined by the authors of the individual studies) during the first six weeks of the postpartum period.
Types of interventions
We considered trials if a comparison was made between different antibiotic regimens (including, but not limited to, different drug/drugs, different routes of administration, and different durations of therapy). Our main comparison was between clindamycin plus an aminoglycoside (usually gentamicin) versus another regimen. Where appropriate, we grouped different antibiotics with a similar antimicrobial spectrum of activity (e.g. lincosamides plus aminoglycoside versus cephalosporins, monobactams, quinolones, and penicillins).
Types of outcome measures
Primary outcomes
Treatment failure (as defined by the individual trials);
severe complications (including pelvic abscess and septic pelvic vein thrombophlebitis);
maternal death.
Secondary outcomes
We collected data (where available) on the following additional outcome measures:
any change made to the initial antibiotic regimen;
duration of fever;
wound infection (not prespecified);
allergic reactions;
diarrhoea;
superinfection or colonization with resistant organisms;
quantity of resources (e.g. length of stay, amount of drug) utilized;
treatment failure despite administration of prophylactic antibiotics for cesarean (not prespecified);
financial costs;
recurrent endometritis (not prespecified)*;
nephrotoxicity (not prespecified)**.
*For the analysis of continued oral therapy versus no additional therapy after intravenous treatment, we also assessed the outcome of recurrent endometritis. **For the analysis of daily versus thrice‐daily gentamicin, we also assessed the outcome of nephrotoxicity.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 November 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review see French 2004.
For this update we used the following methods when assessing the reports identified by the updated search. These methods are based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed all the potential additional studies we identified as a result of the search strategy for inclusion. We resolved any disagreement through discussion or, if required, we consulted a third person.
Data extraction and management
We designed a form to abstract data. For eligible studies, two review authors abstracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software and checked for accuracy (RevMan 2014).
When information regarding any of the above was unclear, we attempted to contact authors of the original reports for further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
For each included study we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included the missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any additional concerns regarding other possible sources of bias. For example, a potential source of bias related to the specific study design, or the trial stopped early due to some data‐dependent process, or extreme baseline imbalance, or claimed to be fraudulent.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence using GRADE
For this update we used the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following primary and secondary outcomes for the main comparison (i.e. clindamycin plus aminoglycoside versus cephalosporins or penicillins):
treatment failures;
severe complications (including pelvic abscess and septic pelvic vein thrombophlebitis);
wound infections;
allergic reactions.
We used GRADEpro Guideline Development Tool to import data from Review Manager 5.3 in order to create 'Summary of findings’ tables (RevMan 2014). We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference where outcomes are measured in the same way between trials. We will use the standardized mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomized trials
In future updates, we will include cluster‐randomized trials in the analyses along with individually randomized trials. We will adjust their sample size using the methods described in the Cochrane Handbook for Systematic Review of Interventions using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomized trials and individually‐randomized trials, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
Other unit of analysis issues
We did not include cross‐over trials. We did not use any special methods for trials with more than one treatment group.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomized to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
As there are more than 10 studies in the meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry had been suggested by a visual assessment, we would have performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful we did not combine trials.
If we used random‐effects analyses, we presented the results as the average treatment effect with 95% confidence intervals, with the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did subgroup analyses for class of antibiotics for any other regimens in the comparison group. We classified the class of antibiotics according to the classification in the Gyte 2014 Cochrane review. When referring to penicillins, we included penicillin, ampicillin and extended spectrum penicillins. Monobactam refers to aztreonam. Aminoglycosides typically refer to gentamicin with the exception of one study that used tobramycin (Pastorek 1987). Lincosamides refer to clindamycin. For Analysis 1.1, the regimen typically included clindamycin plus gentamicin. A priori, we had planned subgroup analyses based on the presence of risk factors such as mode of delivery or genital tract infections, if an adequate number of studies were available. We planned a separate sub analysis including only those studies in which all participants had received prophylactic antibiotic treatment during cesarean birth, if an adequate number of studies were available. However, there were not enough studies available to perform the planned subgroup analyses. We also planned to perform sensitivity analyses based on methodological quality if necessary. Given that in all but five of the studies, treatment allocation was inadequately described, we did not perform a sensitivity analysis incorporating allocation concealment as a measure of study quality as this was not appropriate.
1.1. Analysis.
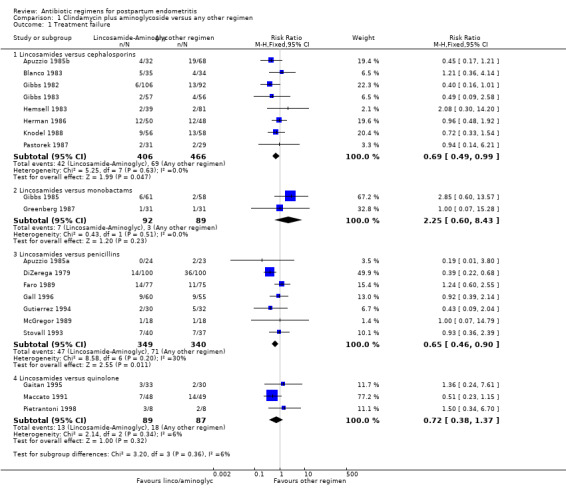
Comparison 1 Clindamycin plus aminoglycoside versus any other regimen, Outcome 1 Treatment failure.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We excluded studies from the analysis when more than 20% of participants dropped out or were excluded after randomization. In future updates, we will carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
The clinical criteria listed to define endometritis were consistent across trials. Febrile morbidity is a standard obstetrical outcome and was generally consistently reported, although there was some variation in the exact criteria used for height of fever, interval between febrile episodes and interval from the operative procedure. Urinary tract infection was usually defined as a positive urine culture; symptoms related to the urinary tract were rarely required to be present. Wound infection was diagnosed clinically and generally included induration, erythema, cellulitis or drainage. A positive microbiological diagnosis was rarely required for the diagnosis of either wound infection or endometritis. There was no consistent approach to the definition of serious morbidity. For this review, all episodes of bacteremia have been classified as serious, as have complications such as pelvic thrombophlebitis, pelvic abscess, and peritonitis. Some studies included other outcomes, for example the need for additional antibiotic use and other infections such as pneumonia. Some provided a measure of the fever as a 'fever index' which incorporated both the height of the fever and its duration.
Results of the search
We identified 72 trials. We included 42 (40 of these trials contributed data on 4240 participants), and excluded 30.
Included studies
For a detailed description of included studies, see the table of Characteristics of included studies.
All, but seven studies were conducted in the United States: one was conducted in France, two in Mexico, and one each in Italy, Peru, and Colombia. One study was a multicenter study conducted in many countries, including the United States.
The studies that contributed data to this meta‐analysis compared several different antibiotic regimens. Twenty studies compared clindamycin plus an aminoglycoside (typically gentamicin) with another regimen. Other comparisons included:
an aminoglycoside (gentamicin) plus penicillin or ampicillin versus any other regimen;
a beta‐lactamase inhibitor combination versus any other regimen;
the combination of aztreonam plus clindamycin versus any other regimen;
agents with a long half‐life versus those with a short half‐life;
the combination of metronidazole plus gentamicin versus any other regimen;
once daily versus thrice‐daily dosing of gentamicin;
continued oral therapy versus no therapy after an intravenous antibiotic course;
regimens with good activity against penicillin‐resistant anaerobes versus regimens with poor activity (e.g. ciprofloxacin, ampicillin, penicillin or ampicillin and an aminoglycoside, and certain cephalosporins such as cefamandole and ceftazidime) against such organisms;
oral ofloxacin plus intravenous clindamycin versus intravenous clindamycin and intravenous gentamicin.
Twenty studies enrolled only postpartum women who developed endometritis after cesarean birth; in four studies, the mode of delivery was not reported. In the remainder, a variable proportion of cases followed cesarean birth. In women who developed endometritis postcesarean birth there was no consistent approach to the use of prophylactic antibiotics. While four studies excluded women who had received prophylaxis, five others stated that all women had received prophylaxis. Cefazolin was the agent selected when prophylaxis was given except in one study in which cefoxitin was used (Tuomala 1989). Although women who developed endometritis during the first six weeks of the postpartum period were eligible for inclusion in this review, the vast majority appeared to have been enrolled within 48 hours of birth.
Excluded studies
We excluded 30 studies identified in the search from the analysis for the following reasons:
more than 20% exclusions after randomization (n = 7);
not a study of postpartum endometritis (n = 7);
study not randomized or the method of allocation to treatment was inadequate, e.g. alternation (n = 6);
no clinical outcomes on postpartum women reported or postpartum endometritis not defined (n = 4);
actual numbers not provided (n = 4);
different antibiotic regimens not compared (n = 1); or
antibiotic regimen dosing and frequency not described (n = 1).
None of the five studies we identified that compared an extended spectrum penicillin with any other regimen met the methodological criteria for inclusion in this review. See Characteristics of excluded studies.
Risk of bias in included studies
For risk of bias for included studies, see the risk of bias tables, Figure 1; and Figure 2. The risk of bias information below pertains only to those studies that contributed data to this meta‐analysis.
1.
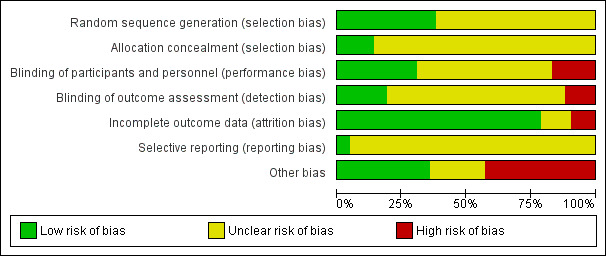
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
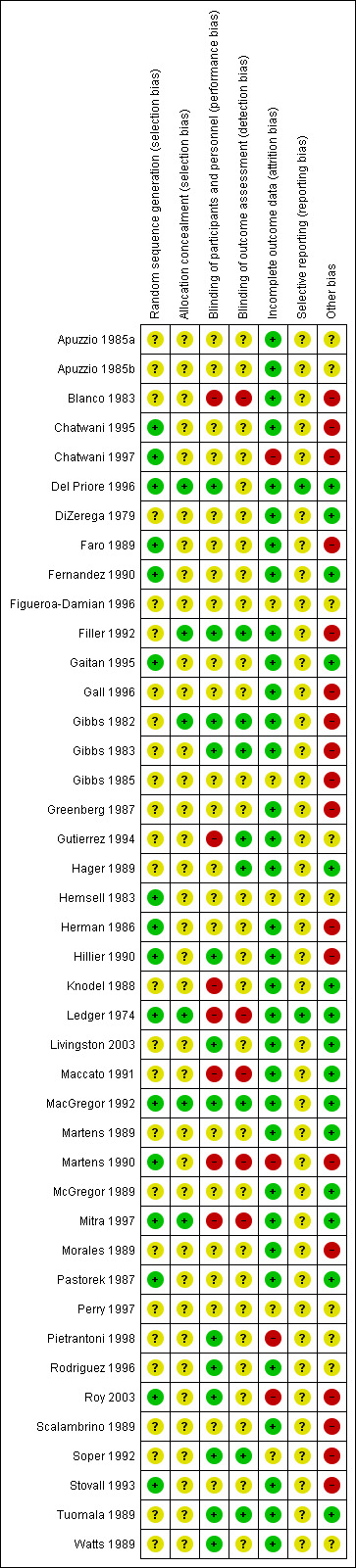
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In all of the studies, women were randomly allocated to treatment groups as per the inclusion criteria. Allocation concealment was sufficiently described and considered to be adequate in only five studies (Del Priore 1996; Filler 1992; Gibbs 1982; MacGregor 1992; Mitra 1997). For the remaining studies, the adequacy of allocation of participants to treatment groups was unclear. Although many of these studies did report that a computerized randomization schedule was used, it was unclear how the randomization schedule was actually administered.
Blinding
Blinding was described in only a few studies. Only four studies used placebo doses and, although nine studies reported a 'double‐blind' design, only three studies described how they attempted to ensure the medications appeared similar in appearance (Gibbs 1982; Gibbs 1983; Hillier 1990). One other study stated that interventions were similar in appearance without describing how this was accomplished (MacGregor 1992). Three studies were described as 'single‐blind'. In most trials there was no description of blinding.
Incomplete outcome data
Since women were usually hospitalized, loss to follow‐up was not a significant problem. When drop‐outs were reported, the reasons why women who had initially been randomized were eventually excluded from the analysis were usually explained. Frequently, however, the number corresponding to each arm of the study was not given. The most frequent reasons given for drop‐outs were protocol violations of various descriptions. For this reason we have provided analysis of available cases (rather than intention‐to‐treat). To reduce the likelihood of bias, we excluded studies in which more than 20% of participants had dropped out or been excluded form the analysis after randomization.
Selective reporting
Only one study had its protocol available, and all of the pre‐specified outcomes were reported (Del Priore 1996). Most of the study protocols were not available and therefore risk of bias was judged to be unclear, due to insufficient information.
Other potential sources of bias
Pharmaceutical sponsorship was evident in 18 studies, which were therefore judged as being at high risk of bias. We judged eight studies to have an unclear additional bias. For three studies we had truncated versions of the original publication that were only partially translated from the initial language (Figueroa‐Damian 1996; Gutierrez 1994; Rodriguez 1996). We suspected that three studies had pharmaceutical sponsorship, but this was not overtly reported (Apuzzio 1985a; Apuzzio 1985b; Hemsell 1983). One study had only an abstract that was available, which could lead to information about potential biases being missed (Perry 1997). One study published what appeared to be preliminary data; neither finalized data nor reasons for failing to complete the study were discovered (Pietrantoni 1998).
Effects of interventions
See: Table 1
Among all the comparisons reported, there was no evidence that any particular regimen was associated with a different rate of allergic reactions. Despite the large number of trials and different antibiotic regimens, only one comparison revealed statistical heterogeneity (Analysis 2.1); therefore we applied random‐effects analyses. Given that in all but five of the studies, treatment allocation was inadequately described, we did not perform a sensitivity analysis incorporating allocation concealment as a measure of study quality as this was not appropriate. As there were more than 10 trials in certain analyses, we conducted visual inspection of the funnel plots to assess reporting bias. There was no funnel plot asymmetry found in the following analyses: Analysis 1.1; Analysis 1.2; Analysis 1.4; Analysis 1.5; Analysis 3.1 (Figure 3; Figure 4; Figure 5; Figure 6; Figure 7).
2.1. Analysis.
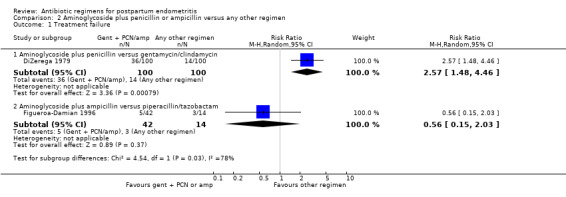
Comparison 2 Aminoglycoside plus penicillin or ampicillin versus any other regimen, Outcome 1 Treatment failure.
1.2. Analysis.
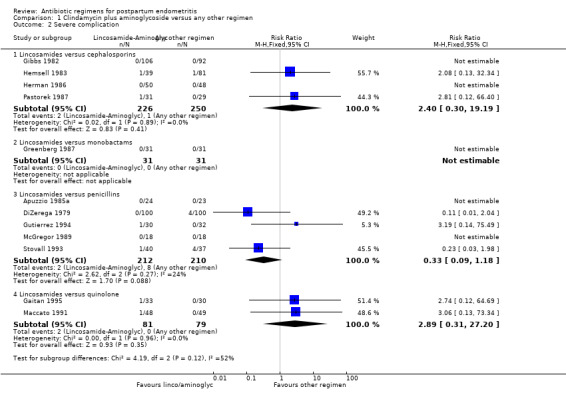
Comparison 1 Clindamycin plus aminoglycoside versus any other regimen, Outcome 2 Severe complication.
1.4. Analysis.
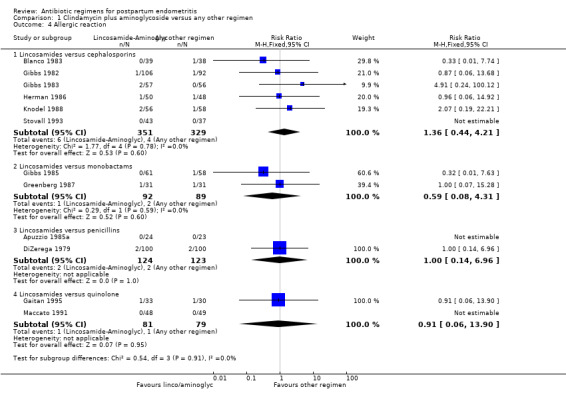
Comparison 1 Clindamycin plus aminoglycoside versus any other regimen, Outcome 4 Allergic reaction.
1.5. Analysis.
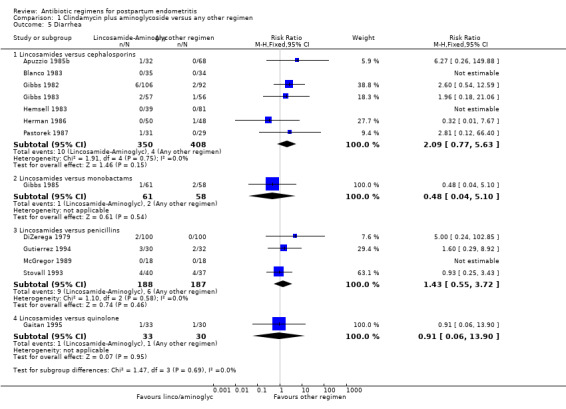
Comparison 1 Clindamycin plus aminoglycoside versus any other regimen, Outcome 5 Diarrhea.
3.1. Analysis.
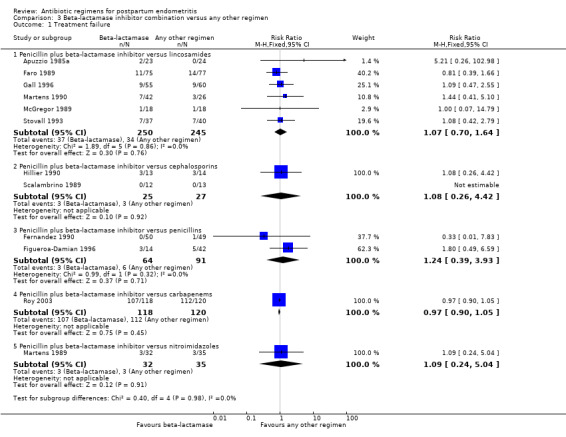
Comparison 3 Beta‐lactamase inhibitor combination versus any other regimen, Outcome 1 Treatment failure.
3.
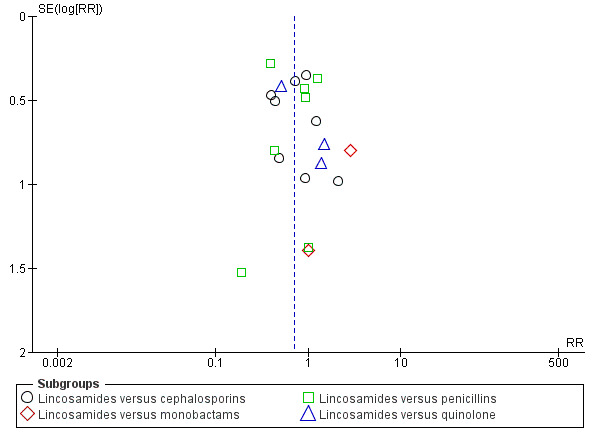
Funnel plot of comparison: 1 Clindamycin plus aminoglycoside versus any other regimen, outcome: 1.1 Treatment failure.
4.
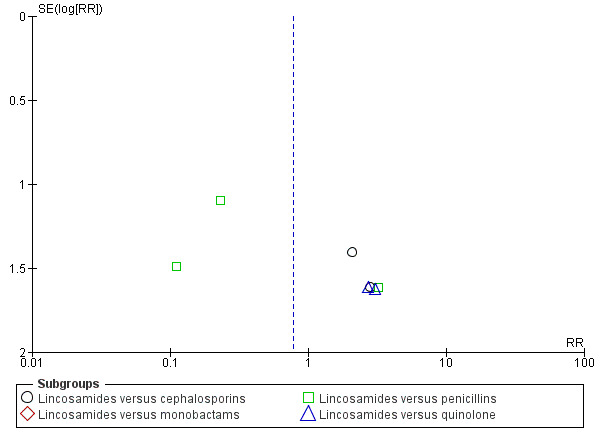
Funnel plot of comparison: 1 Clindamycin plus aminoglycoside versus any other regimen, outcome: 1.2 Severe complication.
5.
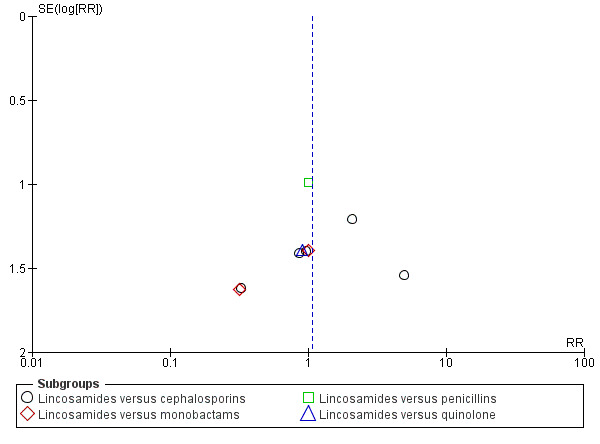
Funnel plot of comparison: 1 Clindamycin plus aminoglycoside versus any other regimen, outcome: 1.4 Allergic reaction.
6.
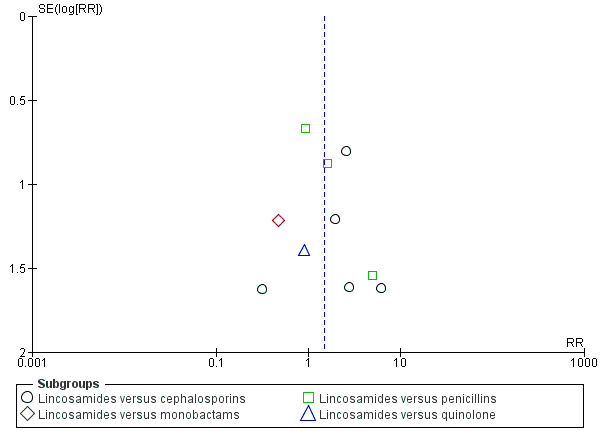
Funnel plot of comparison: 1 Clindamycin plus aminoglycoside versus any other regimen, outcome: 1.5 Diarrhea.
7.
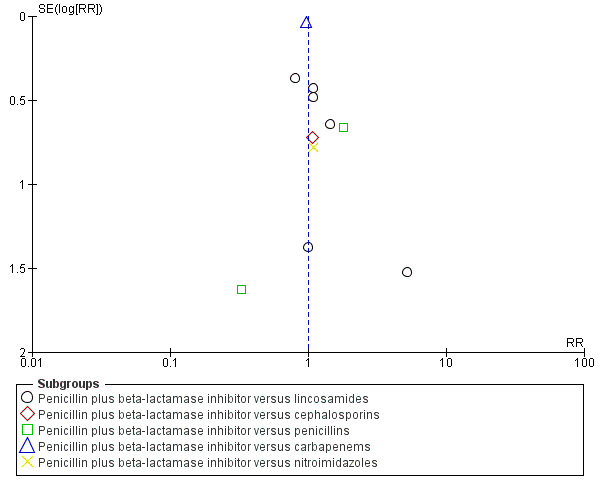
Funnel plot of comparison: 3 Beta‐lactamase inhibitor combination versus any other regimen, outcome: 3.1 Treatment failure.
1. Clindamycin plus aminoglycoside versus any other regimen ‐ 20 studies, 1918 women
Twenty studies, involving 1918 women, compared clindamycin plus an aminoglycoside (gentamicin used for all studies except for Pastorek 1987 that used tobramycin) with another regimen (Apuzzio 1985a; Apuzzio 1985b; Blanco 1983; DiZerega 1979; Faro 1989; Gaitan 1995; Gall 1996; Gibbs 1982; Gibbs 1983; Gibbs 1985; Greenberg 1987; Gutierrez 1994; Hemsell 1983; Herman 1986; Knodel 1988; Maccato 1991; McGregor 1989; Pastorek 1987; Pietrantoni 1998; Stovall 1993).
Primary outcomes
When assessing the individual subgroups of other antibiotic regimens (i.e. cephalosporins, monobactams, penicillins, and quinolones), there were fewer treatment failures in those treated with clindamycin plus an aminoglycoside as compared to those treated with cephalosporins (RR 0.69, 95% CI 0.49 to 0.99; participants = 872; studies = 8; Analysis 1.1.1) or penicillins (RR 0.65, 95% CI 0.46 to 0.90; participants = 689; studies = 7, Analysis 1.1.3). For the remaining subgroups, the differences were not significant.
There were no significant differences between groups with respect to severe complications (Analysis 1.2): lincosamides versus cephalosporins (RR 2.40, 95% CI 0.30 to 19.19; 476 participants; 4 studies; I² 0%, Analysis 1.2.1), lincosamides versus monobactams had only one study with no events (Analysis 1.2.2), lincosamides versus penicillins (RR 0.33, 95% CI 0.09 to 1.18; 422 participants; 5 studies; I² 24%, Analysis 1.2.3), lincosamides versus quinolone (RR 2.89, 95% CI 0.31 to 27.20; participants = 160; studies = 2; Analysis 1.2.4).
Secondary outcomes
There were significantly fewer wound infections with clindamycin plus aminoglycoside versus cephalosporins (RR 0.53, 95% CI 0.30 to 0.93; 500 participants; 4 studies, I² 0%, Analysis 1.3,1). There was no statistically significant difference with other comparison subgroup analysis for wound infections with clindamycin plus aminoglycoside versus monobactams (RR 0.95, 95% CI 0.06 to 14.85; 119 participants; 1 study, Analysis 1.3.2) or penicillins (RR 0.46, 95% CI 0.21 to 1.00; 339 participants; 3 studies, Analysis 1.3.3) or quinolone ((RR 0.51, 95% CI 0.05 to 5.45; participants = 97; studies = 1, Analysis 1.3.4). There were no significant differences between lincosamides versus other regimen subgroups with the outcomes of allergic reactions (Analysis 1.4), diarrhea (Analysis 1.5), length of stay (Analysis 1.6) or treatment failure post cesarean with prophylaxis (Analysis 1.7).
1.3. Analysis.
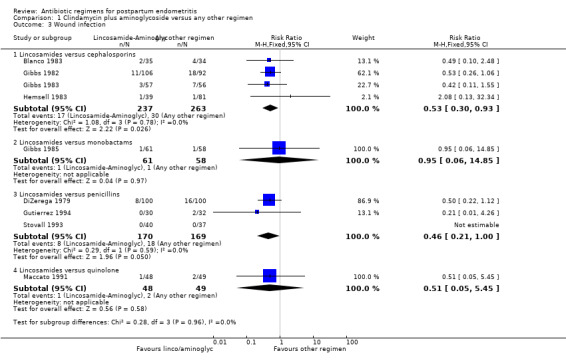
Comparison 1 Clindamycin plus aminoglycoside versus any other regimen, Outcome 3 Wound infection.
1.6. Analysis.
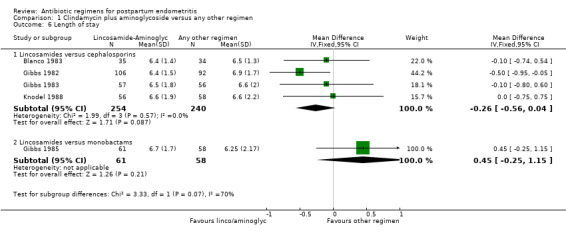
Comparison 1 Clindamycin plus aminoglycoside versus any other regimen, Outcome 6 Length of stay.
1.7. Analysis.
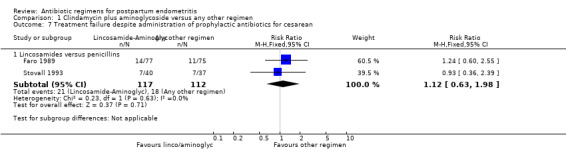
Comparison 1 Clindamycin plus aminoglycoside versus any other regimen, Outcome 7 Treatment failure despite administration of prophylactic antibiotics for cesarean.
2. Aminoglycoside (specifically gentamicin) plus penicillin or ampicillin versus any other regimen ‐ two studies, 256 women
Two trials compared gentamicin plus penicillin or ampicillin with other regimens (DiZerega 1979; Figueroa‐Damian 1996): gentamicin/penicillin versus gentamicin/clindamycin (DiZerega 1979), and gentamicin/ampicillin versus piperacillin/tazobactam (Figueroa‐Damian 1996).
Primary outcomes
There were no significant differences in treatment failures (RR 0.56, 95% CI 0.15 to 2.03; 56 participants, Analysis 2.1.2) or wound infection (RR 2.44, 95% CI 0.13 to 44.57; 56 participants; 1 study, Analysis 2.3.2) when comparing gentamicin plus ampicillin versus piperacillin/tazobactam. However, there were significantly more treatment failures for those treated with gentamicin plus penicillin compared to gentamicin plus clindamycin (RR 2.57, 95% CI 1.48 to 4.46; 200 participants, Analysis 2.1).
2.3. Analysis.
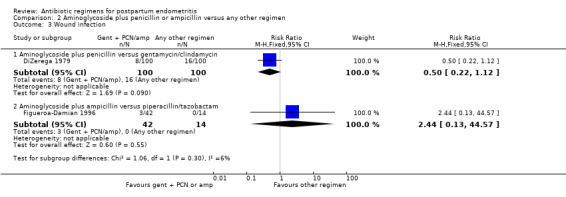
Comparison 2 Aminoglycoside plus penicillin or ampicillin versus any other regimen, Outcome 3 Wound infection.
There were no significant differences in gentamicin/penicillin versus gentamicin/clindamycin with respect to severe complications (RR 0.11, 95% CI 0.01 to 2.04; 200 participants; 1 study; Analysis 2.2.1).
2.2. Analysis.
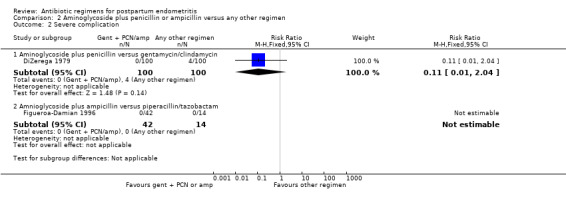
Comparison 2 Aminoglycoside plus penicillin or ampicillin versus any other regimen, Outcome 2 Severe complication.
Secondary outcomes
There were no significant differences in wound infections (RR 0.50, 95% CI 0.22 to 1.12; 200 participants; 1 study, Analysis 2.3.1), allergic reactions (RR 1.00, 95% CI 0.14 to 6.96; 200 participants; 1 study, Analysis 2.4.1) or diarrhea (RR 5.00, 95% CI 0.24 to 102.85; 200 participants; 1 study, Analysis 2.5.1).
2.4. Analysis.
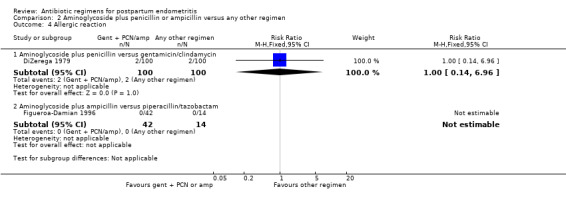
Comparison 2 Aminoglycoside plus penicillin or ampicillin versus any other regimen, Outcome 4 Allergic reaction.
2.5. Analysis.
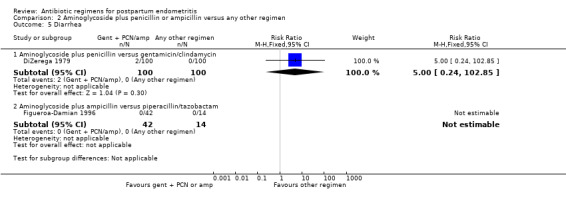
Comparison 2 Aminoglycoside plus penicillin or ampicillin versus any other regimen, Outcome 5 Diarrhea.
3. Beta‐lactamase inhibitor combination versus any other regimen ‐ 12 studies, 1007 women
Twelve trials (1007 participants) compared a beta‐lactam/beta‐lactamase inhibitor combination with another regimen.
Primary outcomes
There were no differences in treatment failures in any subgroup; e.g. penicillin plus beta‐lactamase inhibitor versus lincosamides (RR 1.07, 95% CI 0.70 to 1.64; participants = 495; studies = 6; I² = 0%, , Analysis 3.1) as well as no difference in severe complication (RR 0.11, 95% CI 0.01 to 2.04, Analysis 3.2).
3.2. Analysis.
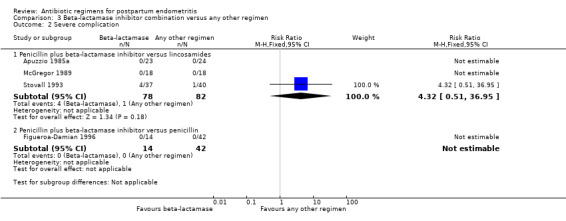
Comparison 3 Beta‐lactamase inhibitor combination versus any other regimen, Outcome 2 Severe complication.
Secondary outcomes
There were no statistically significant differences for any other outcome (Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6). CIs were wide for other outcomes due to the low number of participants with those outcomes.
3.3. Analysis.
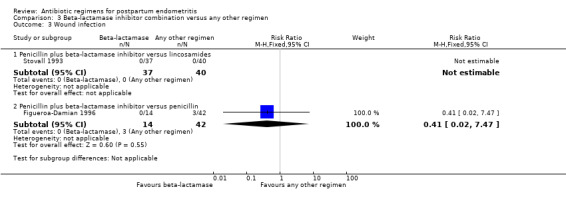
Comparison 3 Beta‐lactamase inhibitor combination versus any other regimen, Outcome 3 Wound infection.
3.4. Analysis.
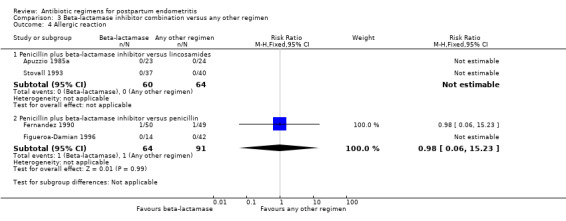
Comparison 3 Beta‐lactamase inhibitor combination versus any other regimen, Outcome 4 Allergic reaction.
3.5. Analysis.
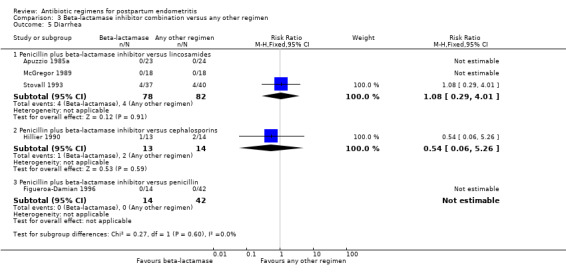
Comparison 3 Beta‐lactamase inhibitor combination versus any other regimen, Outcome 5 Diarrhea.
3.6. Analysis.
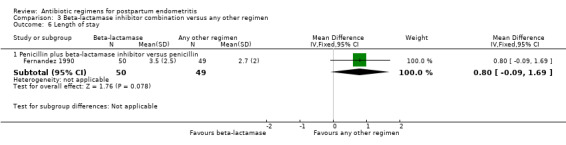
Comparison 3 Beta‐lactamase inhibitor combination versus any other regimen, Outcome 6 Length of stay.
4 Aztreonam plus clindamycin versus any other regimen ‐ four studies, 603 women
Four trials (603 participants) compared aztreonam plus clindamycin with other regimens. Two of these were comparisons with clindamycin plus aztreonam versus clindamycin plus gentamicin (Gibbs 1985; Greenberg 1987). The other two trials compared clindamycin and aztreonam with trospectomycin (Chatwani 1997; Filler 1992).
Primary outcomes
There was no difference between these regimens for any of the outcomes (Analysis 4.1; Analysis 4.2).
4.1. Analysis.
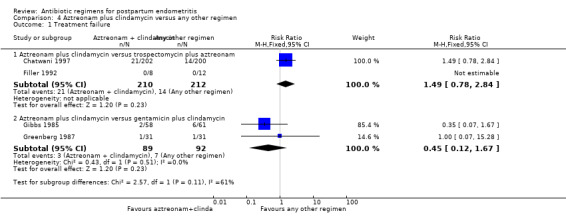
Comparison 4 Aztreonam plus clindamycin versus any other regimen, Outcome 1 Treatment failure.
4.2. Analysis.
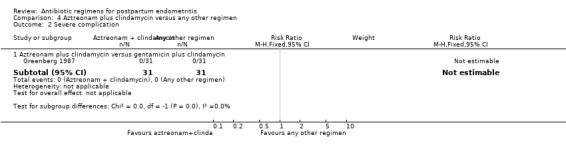
Comparison 4 Aztreonam plus clindamycin versus any other regimen, Outcome 2 Severe complication.
Secondary outcomes
There was no difference between these regimens for any of the outcomes (Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6).
4.3. Analysis.
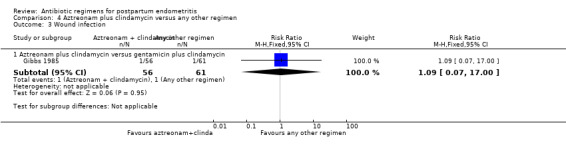
Comparison 4 Aztreonam plus clindamycin versus any other regimen, Outcome 3 Wound infection.
4.4. Analysis.
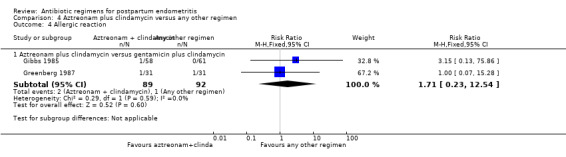
Comparison 4 Aztreonam plus clindamycin versus any other regimen, Outcome 4 Allergic reaction.
4.5. Analysis.
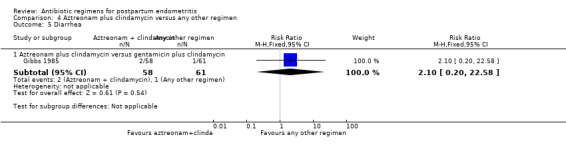
Comparison 4 Aztreonam plus clindamycin versus any other regimen, Outcome 5 Diarrhea.
4.6. Analysis.
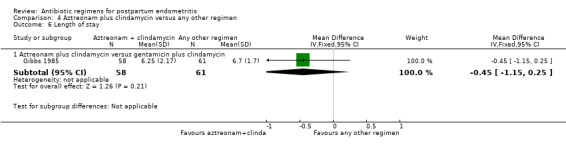
Comparison 4 Aztreonam plus clindamycin versus any other regimen, Outcome 6 Length of stay.
5. Agent with longer half‐life versus similar agent with shorter half‐life ‐ two studies, 484 women
Two trials (484 participants) compared agents with a longer half‐life to a drug in the same class with a shorter half‐life. All regimens were cephalosporins: cefoxitin administered every six hours was compared with either cefmetazole administered every eight hours (Chatwani 1995) or cefotetan administered every 12 hours (MacGregor 1992).
Primary outcomes
Treatment with an agent with a longer half life that is administered less frequently was associated with fewer treatment failures (RR 0.61, 95% CI 0.40 to 0.92; 484 participants; 2 studies; I² 0%, Analysis 5.1) than cefoxitin. No significant differences were found for severe complications (Analysis 5.2)
5.1. Analysis.
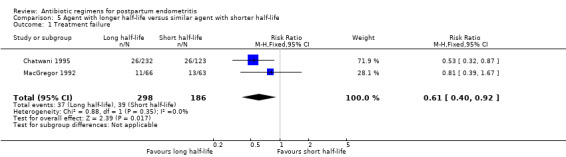
Comparison 5 Agent with longer half‐life versus similar agent with shorter half‐life, Outcome 1 Treatment failure.
5.2. Analysis.
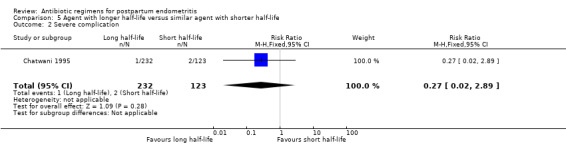
Comparison 5 Agent with longer half‐life versus similar agent with shorter half‐life, Outcome 2 Severe complication.
Secondary outcomes
No significant differences were found for the other outcomes (Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6).
5.3. Analysis.
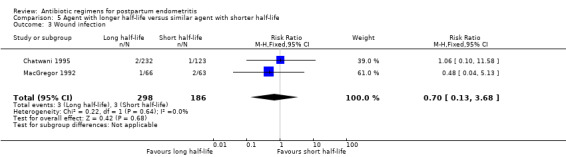
Comparison 5 Agent with longer half‐life versus similar agent with shorter half‐life, Outcome 3 Wound infection.
5.4. Analysis.
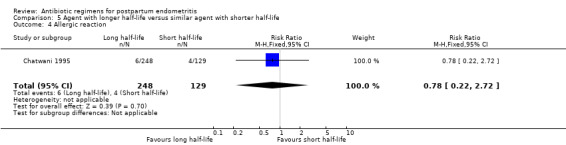
Comparison 5 Agent with longer half‐life versus similar agent with shorter half‐life, Outcome 4 Allergic reaction.
5.5. Analysis.
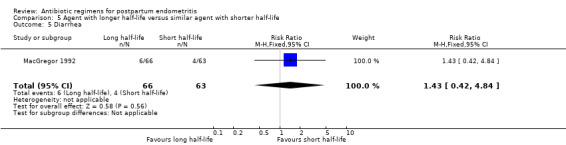
Comparison 5 Agent with longer half‐life versus similar agent with shorter half‐life, Outcome 5 Diarrhea.
5.6. Analysis.
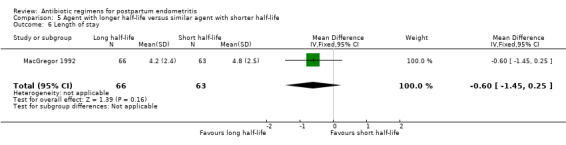
Comparison 5 Agent with longer half‐life versus similar agent with shorter half‐life, Outcome 6 Length of stay.
6. Metronidazole plus gentamicin versus any other regimen ‐ one study, 67 women
One small trial (Martens 1989, 67 participants) compared metronidazole and gentamicin with ampicillin/sulbactam.
Primary outcomes
There was no difference in treatment failures between the two regimens (RR 0.91, 95% CI 0.20 to 4.21, Analysis 6.1).
6.1. Analysis.
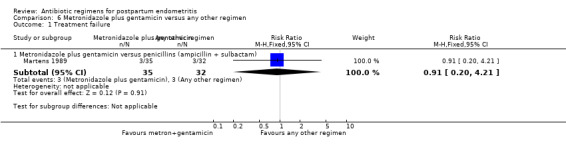
Comparison 6 Metronidazole plus gentamicin versus any other regimen, Outcome 1 Treatment failure.
Secondary outcomes
There were no secondary outcomes reported for this analysis.
7. Once‐daily versus thrice‐daily gentamicin dosing ‐ four studies, 463 women
Four trials (463 participants) compared once‐daily versus thrice‐daily (i.e. eight‐hourly) administration of gentamicin (Del Priore 1996; Livingston 2003; Mitra 1997; Perry 1997).
Primary outcomes
There was a non‐significant trend toward fewer treatment failures with once‐daily dosing (RR 0.70, 95% CI 0.49 to 1.00; 463 participants; 4 studies; I² 29%, Analysis 7.1).
7.1. Analysis.
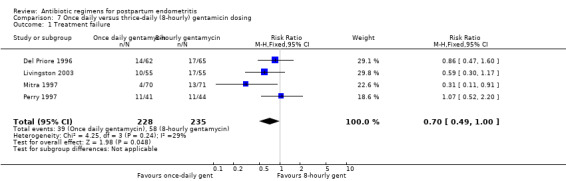
Comparison 7 Once daily versus thrice‐daily (8‐hourly) gentamicin dosing, Outcome 1 Treatment failure.
Secondary outcomes
There was no difference in the incidence of nephrotoxicity between regimens (Analysis 7.2). Once‐daily dosing was associated with a shorter length of hospital stay (MD ‐0.73, 95% CI ‐1.27 to ‐0.20; 322 participants; 3 studies; I² 0%, Analysis 7.3).
7.2. Analysis.
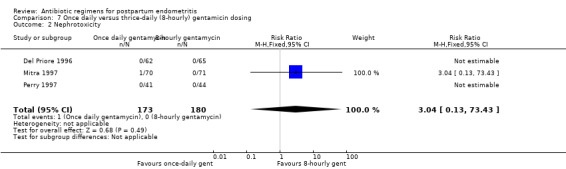
Comparison 7 Once daily versus thrice‐daily (8‐hourly) gentamicin dosing, Outcome 2 Nephrotoxicity.
7.3. Analysis.
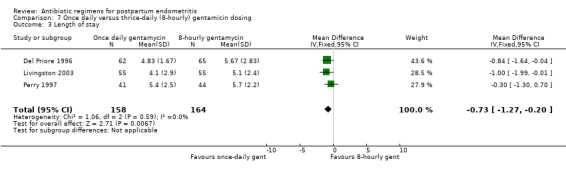
Comparison 7 Once daily versus thrice‐daily (8‐hourly) gentamicin dosing, Outcome 3 Length of stay.
8. Continued oral versus no treatment after intravenous antibiotic course ‐ three studies, 253 women
Three trials (253 participants) compared continued oral antibiotic therapy with no treatment after intravenous therapy (Hager 1989; Morales 1989; Rodriguez 1996). The incidence of recurrent endometritis was exceptionally low in both groups (only one episode in 253 women).
Primary outcomes
No differences were found in treatment failure (Analysis 8.1). There were no severe complications in the studies (Analysis 8.2).
8.1. Analysis.
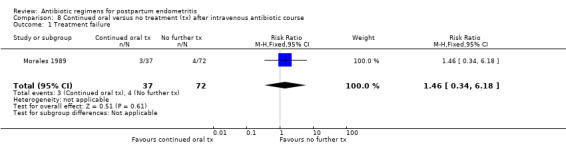
Comparison 8 Continued oral versus no treatment (tx) after intravenous antibiotic course, Outcome 1 Treatment failure.
8.2. Analysis.
Comparison 8 Continued oral versus no treatment (tx) after intravenous antibiotic course, Outcome 2 Severe complication.
Secondary outcomes
No differences were found in wound infection (Analysis 8.3), urinary tract infection (Analysis 8.4), recurrence of endometritis (Analysis 8.5), or length of stay (Analysis 8.6).
8.3. Analysis.
Comparison 8 Continued oral versus no treatment (tx) after intravenous antibiotic course, Outcome 3 Wound infection.
8.4. Analysis.
Comparison 8 Continued oral versus no treatment (tx) after intravenous antibiotic course, Outcome 4 Urinary tract infection.
8.5. Analysis.
Comparison 8 Continued oral versus no treatment (tx) after intravenous antibiotic course, Outcome 5 Recurrent endometritis.
8.6. Analysis.
Comparison 8 Continued oral versus no treatment (tx) after intravenous antibiotic course, Outcome 6 Length of stay.
9. Poor activity against penicillin‐resistant anaerobic bacteria versus good activity ‐ seven studies, 774 women
Seven trials (774 participants) compared a regimen of antibiotics with poor activity against penicillin‐resistant anaerobic bacteria (e.g. the Bacteroides fragilis group) with a regimen with good activity.
Primary outcomes
Antibiotics with poor activity against penicillin‐resistant anaerobes were associated with higher failure rates of the regimen (RR 1.94, 95% CI 1.38 to 2.72; 774 participants; 7 studies; I² 23%, Analysis 9.1). There were no significant differences in severe complications (Analysis 9.2).
9.1. Analysis.
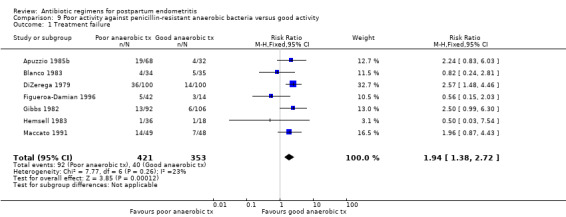
Comparison 9 Poor activity against penicillin‐resistant anaerobic bacteria versus good activity, Outcome 1 Treatment failure.
9.2. Analysis.
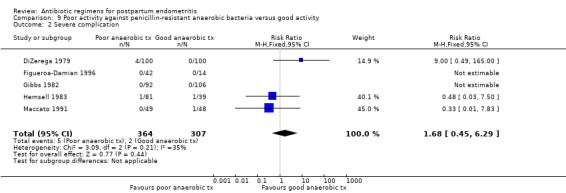
Comparison 9 Poor activity against penicillin‐resistant anaerobic bacteria versus good activity, Outcome 2 Severe complication.
Secondary outcomes
Antibiotics with poor activity against penicillin resistant anaerobes were associated with more wound infections (RR 1.88, 95% CI 1.17 to 3.02; 740 participants; 6 studies; I² 0%, Analysis 9.3).
9.3. Analysis.
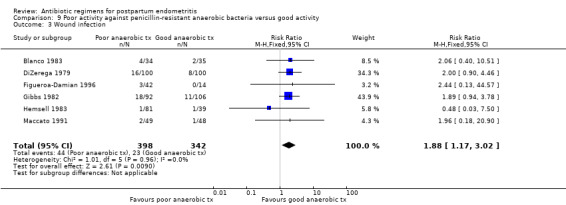
Comparison 9 Poor activity against penicillin‐resistant anaerobic bacteria versus good activity, Outcome 3 Wound infection.
There were no significant differences between the groups for the other outcomes (Analysis 9.2; Analysis 9.4; Analysis 9.5; Analysis 9.6).
9.4. Analysis.
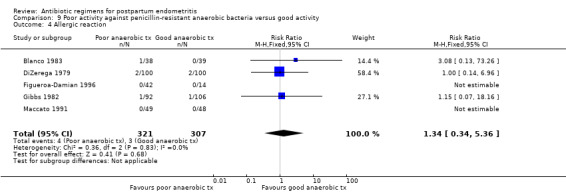
Comparison 9 Poor activity against penicillin‐resistant anaerobic bacteria versus good activity, Outcome 4 Allergic reaction.
9.5. Analysis.
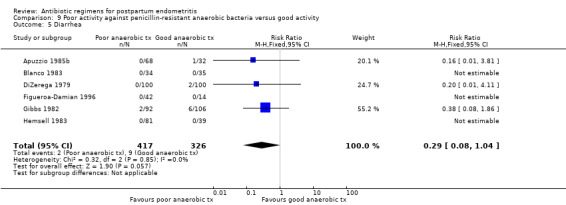
Comparison 9 Poor activity against penicillin‐resistant anaerobic bacteria versus good activity, Outcome 5 Diarrhea.
9.6. Analysis.
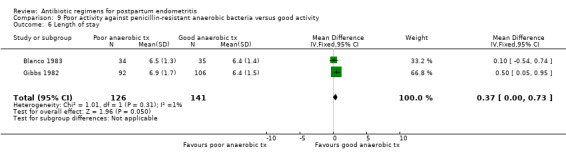
Comparison 9 Poor activity against penicillin‐resistant anaerobic bacteria versus good activity, Outcome 6 Length of stay.
10. Oral ofloxacin/clindamycin versus intravenous clindamycin/gentamicin ‐ one study, 16 women
One small trial (16 participants) compared oral ofloxacin/intravenous clindamycin versus intravenous clindamycin/gentamicin.
Primary outcomes
Primary outcomes showed no significant differences for treatment failures (RR 0.67, 95% CI 0.15 to 2.98; I² 0%, Analysis 10.1).
10.1. Analysis.
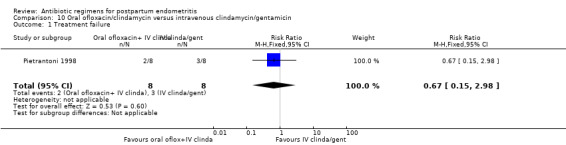
Comparison 10 Oral ofloxacin/clindamycin versus intravenous clindamycin/gentamicin, Outcome 1 Treatment failure.
Secondary outcomes
No secondary outcomes were reported.
Discussion
Summary of main results
The combination of clindamycin and an aminoglycoside was more effective than treatment with cephalosporins or penicillins as evidenced by fewer treatment failures. There were also fewer wound infections with clindamycin and an aminoglycoside as compared to cephalosporins. There were more treatment failures in women receiving gentamicin/penicillin compared with gentamicin/clindamycin. There is evidence that cefoxitin with a shorter half‐life is less effective than the cephamycins with a longer half‐life that are administered less frequently. Once‐daily dosing of gentamicin was associated with shorter hospital stays than thrice‐daily dosing. Regimens with poor activity against penicillin‐resistant anaerobic bacteria had higher failure rates and more wound infections than regimens with good activity against these organisms. For all the other outcomes, there were no differences between treatment regimens. However, for many of these comparisons the numbers studied were small and, although unlikely, significant differences may not have been detected.
If the improved response with clindamycin and gentamicin compared with any other regimen is expressed as the number needed to treat for an additional beneficial outcome (NNTB), 20 women (95% confidence interval (CI) 12 to 56) would need to be treated with clindamycin and gentamicin, rather than any other regimen, to prevent one additional treatment failure. What is missing from these studies, however, and what is needed to use the NNTB to help make treatment decisions, is a better assessment of side‐effects of the regimens and reporting of the cost of the different therapies. No study looked at the effect of treatment on the infant of a breastfeeding mother and any maternal renal toxicity was not described systematically. Very rarely were drug costs collected and overall no attempt was made to collect and compare all costs of treatment, including length of stay.
For the other regimens that were compared, where there were no differences in treatment failures, it is unfortunate that there were so few data on other outcomes. These factors might determine whether a regimen, albeit equally effective, had some other advantage. As a minimum, drug costs should have been reported consistently.
Overall completeness and applicability of evidence
Overall the studies were at an unclear risk of bias. There were opportunities for systematic bias: allocation concealment was usually inadequately described and only rarely was there any attempt at 'blinding'. Often the study was sponsored by the manufacturer of a new drug and this drug was compared with the control regimen, typically clindamycin plus gentamicin. But despite all these potential biases, which would most likely work against the control arm, the combination of clindamycin and an aminoglycoside was more effective than other regimens with fewer treatment failures and wound infections. However, for many of these comparisons the numbers studied were small and, although unlikely, significant differences may not have been detected.
Although there may be differences in the expected response of women who developed endometritis after cesarean birth compared with those who developed infection after a vaginal birth, insufficient data were provided to allow us to perform a subgroup analysis. We could not perform subgroup analyses based on the presence of bacterial vaginosis or genital tract cultures positive for virulent organisms, as the data were not available. There were too few studies to detect whether there are differences in outcomes between regimens when prophylactic antibiotics have been given for cesarean births. Many of the studies performed extensive bacteriological work‐up on endometrial cultures, but this could not be approached systematically nor incorporated into this review.
Quality of the evidence
The overall risk of bias was unclear in the most of the studies. We assessed the quality of the evidence using GRADE and judged the evidence for an aminoglycoside plus clindamycin with another regimen compared with cephalosporins or penicillins as low to very low quality for therapeutic failure, severe complications, wound infection and allergic reaction (Table 1). We downgraded scores as most studies had design limitations, few events, and wide confidence intervals crossing the line of no effect. Though drop‐outs were reported with reasons explained, frequently, the number corresponding to each arm of a study was not given. For this reason we have provided analysis of available cases (rather than intention‐to‐treat). Many of the studies date back to the 1970s and 1980s. Since then there may have been changes in the causative organisms, as well as in the antimicrobial resistance profile.
Potential biases in the review process
We tried to minimize potential biases in the review process by having at least two review authors independently assess the eligibility for inclusion and exclusion, perform data abstraction and assess the risk of bias.
Agreements and disagreements with other studies or reviews
Very few studies have been conducted outside of the USA, with only four studies (from Central and South America) performed in the developing world. Since postpartum endometritis is an important cause of maternal morbidity and mortality in low‐income countries, the lack of studies conducted in such environments leaves a gap in our knowledge.
Barza 1996 performed a meta‐analysis of single versus multiple doses of aminoglycoside for the treatment of various infections, and the conclusions support a once‐daily regimen.
Any study of a new drug for the treatment of endometritis should, rather than have as its only objective the demonstration of equivalence between the regimens, be designed to incorporate other relevant outcomes in the analyses, and ideally should incorporate some form of cost‐benefit analysis. While concern about ototoxicity and nephrotoxicity are identified as contraindications to the routine use of an aminoglycoside in community‐acquired intra‐abdominal infections (Solomkin 2003), healthy women with postpartum endometritis, whose treatment course is usually short, could be assumed to suffer from less toxicity from aminoglycosides compared with other women who are more likely to have significant co‐morbid illness. Although the studies included in this review did not collect information systematically on renal toxicity, there is no evidence that using an aminoglycoside in the clinical setting of postpartum endometritis should not be recommended because of toxicity. It is, however, important that any new regimen that is compared with clindamycin and an aminoglycoside should include ototoxicity and nephrotoxicity as outcomes.
There is evidence of increasing resistance in the Bacteroides fragilis group of organisms to clindamycin (Aldridge 2002). While there are no data to suggest that this is having an impact on treatment outcome in women with endometritis, whose infections are generally uncomplicated, there should be ongoing surveillance of the effect of changing antibiotic resistance patterns. Although overall a regimen with activity against the B fragilis group is better than one without, 80% of women treated with a regimen without that activity were cured, raising questions about the type of woman in which a broad spectrum regimen is necessary.
Authors' conclusions
Implications for practice.
It can be concluded from this review that the combination of clindamycin plus an aminoglycoside (such as gentamicin) is appropriate for the treatment of endometritis and that a regimen with activity against the Bacteroides fragilis group and other penicillin resistant anaerobic bacteria is better than one without. There is no good evidence that any one regimen is associated with fewer side‐effects. No specific recommendations can be made for the treatment of women who develop endometritis after receiving antibiotic prophylaxis for cesarean birth as we were unable to specifically study that population in this review. Also, it should be noted that none of the trials' regimens included ampicillin plus clindamycin plus an aminoglycoside, so we cannot make a recommendation as to whether these three antibiotics are superior to clindamycin plus gentamicin alone.
Implications for research.
The majority of these studies took a traditional approach to the treatment of endometritis and compared new regimens to the standard of care in North America. Any further studies that compare clindamycin and an aminoglycoside with an alternative regimen, with efficacy as the primary outcome, should include regimens that are routinely used outside of North America and consider alternatives suitable for use in low‐income countries.
With the availability of new antibiotics with improved oral bioavailability, novel ways of managing endometritis should be explored and more creative study designs should evaluate early switching to the oral route. Although the new quinolones have a broader spectrum of activity than ciprofloxacin and excellent oral bioavailability, and are used widely to treat intra‐abdominal infections, it is generally recommended that they be avoided if a woman is breastfeeding, because their safety in breastfeeding has not been established. However, as more information on the safety of these agents in infants and children becomes available, their usefulness in treating women with endometritis should be studied. Any study of a new drug for the treatment of endometritis should, rather than have as its only objective the demonstration of equivalence between the regimens, be designed to incorporate other relevant outcomes in the analyses, and ideally should incorporate some form of cost‐benefit analysis.
Traditionally an empiric regimen active against the mixed aerobic and anaerobic organisms likely to be causing infection is selected, but with increasing concern about the appropriate utilization of antibiotics and developing antimicrobial resistance, this approach may no longer be appropriate. We should ask whether the use of endometrial cultures, collected under conditions where contamination is avoided, has a role for targeting antibiotic therapy more specifically to individual women. Studies may be designed that compare different strategies for selecting an antibiotic regimen.
What's new
| Date | Event | Description |
|---|---|---|
| 22 June 2015 | Amended | Corrected errors. Three trials (Gibbs 1983; Knodel 1988; Pastorek 1987) were inadvertently misclassified as quinolones. They belong with cephalosporins. Additionally, we removed two analyses (cephalosporins and cephamycins) as the initial analysis 1.1 included both of these medications and all applicable studies, so these analyses were redundant. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 30 November 2014 | New search has been performed | Search updated and two trials identified. Methods and risk of bias tables have been updated. A 'Summary of findings' table incorporated for this update |
| 30 November 2014 | New citation required but conclusions have not changed | Review updated. One trial included, one trial excluded. Two trials previously excluded are now included (Ledger 1974; Watts 1989) |
| 8 June 2012 | Amended | Search updated. Two reports added to Studies awaiting classification (Pietrantoni 1998a; Sweet 1988a). |
| 12 May 2008 | Amended | Converted to new review format. |
| 25 January 2007 | New search has been performed | Search updated. One new study included (Roy 2003). The conclusions have not changed. |
| 31 January 2004 | New search has been performed | Two new studies have been included (Hemsell 1997; Livingston 2003) and one has been excluded (Pastorek 1987b). |
| 30 October 2001 | New search has been performed | Eight additional studies were evaluated for inclusion in the review. Six were added to the review and two were excluded. The conclusions drawn from the meta‐analysis were not changed. |
Acknowledgements
We are grateful to Fiona M Smaill who developed the original review (French 2004).
Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
We would like to thank Becky Davie and Sally Reynolds, student medical statisticians, who helped with the risk of bias assessments of some included studies.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Clindamycin plus aminoglycoside versus any other regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Lincosamides versus cephalosporins | 8 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.49, 0.99] |
| 1.2 Lincosamides versus monobactams | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.60, 8.43] |
| 1.3 Lincosamides versus penicillins | 7 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.90] |
| 1.4 Lincosamides versus quinolone | 3 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.38, 1.37] |
| 2 Severe complication | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Lincosamides versus cephalosporins | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.30, 19.19] |
| 2.2 Lincosamides versus monobactams | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Lincosamides versus penicillins | 5 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.18] |
| 2.4 Lincosamides versus quinolone | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.31, 27.20] |
| 3 Wound infection | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Lincosamides versus cephalosporins | 4 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.30, 0.93] |
| 3.2 Lincosamides versus monobactams | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.06, 14.85] |
| 3.3 Lincosamides versus penicillins | 3 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.21, 1.00] |
| 3.4 Lincosamides versus quinolone | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.45] |
| 4 Allergic reaction | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Lincosamides versus cephalosporins | 6 | 680 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.44, 4.21] |
| 4.2 Lincosamides versus monobactams | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.08, 4.31] |
| 4.3 Lincosamides versus penicillins | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.96] |
| 4.4 Lincosamides versus quinolone | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.06, 13.90] |
| 5 Diarrhea | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Lincosamides versus cephalosporins | 7 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.77, 5.63] |
| 5.2 Lincosamides versus monobactams | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.10] |
| 5.3 Lincosamides versus penicillins | 4 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.55, 3.72] |
| 5.4 Lincosamides versus quinolone | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.06, 13.90] |
| 6 Length of stay | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Lincosamides versus cephalosporins | 4 | 494 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.56, 0.04] |
| 6.2 Lincosamides versus monobactams | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐0.25, 1.15] |
| 7 Treatment failure despite administration of prophylactic antibiotics for cesarean | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Lincosamides versus penicillins | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.63, 1.98] |
Comparison 2. Aminoglycoside plus penicillin or ampicillin versus any other regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Aminoglycoside plus penicillin versus gentamycin/clindamycin | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [1.48, 4.46] |
| 1.2 Aminoglycoside plus ampicillin versus piperacillin/tazobactam | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.15, 2.03] |
| 2 Severe complication | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Aminoglycoside plus penicillin versus gentamycin/clindamycin | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.04] |
| 2.2 Amnioglycoside plus ampicillin versus piperacillin/tazobactam | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Wound infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Aminoglycoside plus penicillin versus gentamycin/clindamycin | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.22, 1.12] |
| 3.2 Aminoglycoside plus ampicillin versus piperacillin/tazobactam | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.13, 44.57] |
| 4 Allergic reaction | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Aminoglycoside plus penicillin versus gentamicin/clindamycin | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.96] |
| 4.2 Aminoglycoside plus ampicillin versus piperacillin/tazobactam | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Diarrhea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Aminoglycoside plus penicillin versus gentamicin/clindamycin | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.85] |
| 5.2 Aminoglycoside plus ampicillin versus piperacillin/tazobactam | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Beta‐lactamase inhibitor combination versus any other regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Penicillin plus beta‐lactamase inhibitor versus lincosamides | 6 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.70, 1.64] |
| 1.2 Penicillin plus beta‐lactamase inhibitor versus cephalosporins | 2 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.26, 4.42] |
| 1.3 Penicillin plus beta‐lactamase inhibitor versus penicillins | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.39, 3.93] |
| 1.4 Penicillin plus beta‐lactamase inhibitor versus carbapenems | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.05] |
| 1.5 Penicillin plus beta‐lactamase inhibitor versus nitroimidazoles | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.24, 5.04] |
| 2 Severe complication | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Penicillin plus beta‐lactamase inhibitor versus lincosamides | 3 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.32 [0.51, 36.95] |
| 2.2 Penicillin plus beta‐lactamase inhibitor versus penicillin | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Wound infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Penicillin plus beta‐lactamase inhibitor versus lincosamides | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Penicillin plus beta‐lactamase inhibitor versus penicillin | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.02, 7.47] |
| 4 Allergic reaction | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Penicillin plus beta‐lactamase inhibitor versus lincosamides | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Penicillin plus beta‐lactamase inhibitor versus penicillin | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.23] |
| 5 Diarrhea | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Penicillin plus beta‐lactamase inhibitor versus lincosamides | 3 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.29, 4.01] |
| 5.2 Penicillin plus beta‐lactamase inhibitor versus cephalosporins | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.06, 5.26] |
| 5.3 Penicillin plus beta‐lactamase inhibitor versus penicillin | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Length of stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Penicillin plus beta‐lactamase inhibitor versus penicillin | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.09, 1.69] |
Comparison 4. Aztreonam plus clindamycin versus any other regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Aztreonam plus clindamycin versus trospectomycin plus aztreonam | 2 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.78, 2.84] |
| 1.2 Aztreonam plus clindamycin versus gentamicin plus clindamycin | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.12, 1.67] |
| 2 Severe complication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Aztreonam plus clindamycin versus gentamicin plus clindamycin | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Aztreonam plus clindamycin versus gentamicin plus clindamycin | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 17.00] |
| 4 Allergic reaction | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Aztreonam plus clindamycin versus gentamicin plus clindamycin | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.23, 12.54] |
| 5 Diarrhea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Aztreonam plus clindamycin versus gentamicin plus clindamycin | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.20, 22.58] |
| 6 Length of stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Aztreonam plus clindamycin versus gentamicin plus clindamycin | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.15, 0.25] |
Comparison 5. Agent with longer half‐life versus similar agent with shorter half‐life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 2 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.40, 0.92] |
| 2 Severe complication | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.02, 2.89] |
| 3 Wound infection | 2 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.68] |
| 4 Allergic reaction | 1 | 377 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.22, 2.72] |
| 5 Diarrhea | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.42, 4.84] |
| 6 Length of stay | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.45, 0.25] |
Comparison 6. Metronidazole plus gentamicin versus any other regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Metronidazole plus gentamicin versus penicillins (ampicillin + sulbactam) | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.20, 4.21] |
Comparison 7. Once daily versus thrice‐daily (8‐hourly) gentamicin dosing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 4 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.49, 1.00] |
| 2 Nephrotoxicity | 3 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.13, 73.43] |
| 3 Length of stay | 3 | 322 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.27, ‐0.20] |
Comparison 8. Continued oral versus no treatment (tx) after intravenous antibiotic course.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.34, 6.18] |
| 2 Severe complication | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Wound infection | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [0.14, 80.70] |
| 4 Urinary tract infection | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.07, 17.48] |
| 5 Recurrent endometritis | 3 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.12, 68.81] |
| 6 Length of stay | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐1.44, 1.02] |
Comparison 9. Poor activity against penicillin‐resistant anaerobic bacteria versus good activity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 7 | 774 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.38, 2.72] |
| 2 Severe complication | 5 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.45, 6.29] |
| 3 Wound infection | 6 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.17, 3.02] |
| 4 Allergic reaction | 5 | 628 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.34, 5.36] |
| 5 Diarrhea | 6 | 743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.04] |
| 6 Length of stay | 2 | 267 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.00, 0.73] |
Comparison 10. Oral ofloxacin/clindamycin versus intravenous clindamycin/gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.15, 2.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Apuzzio 1985a.
| Methods | Randomized trial Study period: March 1983 through January 1984 | |
| Participants | Inclusion criteria: postcesarean birth with temperature of 100.4 °F (38 °C) or higher on 2 occasions after the first 24 hours after delivery, with uterine tenderness and no other foci of infection Setting: urban university hospital, New Jersey, USA Number of participants: n = 47 | |
| Interventions | Ticarcillin/clavulanic acid 3 g/100 g iv every 4 hours (n = 23) vs clindamycin 600 mg iv every 6 hours with gentamicin 60 mg ‐ 80 mg IM every 8 hours (n = 24) | |
| Outcomes | Treatment failure Allergic reactions Diarrhea | |
| Notes | Participants receiving antibiotic prophylaxis were excluded Pharmaceutical sponsorship ‐ probable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation: "randomly assigned" without further description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 (8.8%) were excluded from analysis as important demographic or laboratory data were not obtained |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Unclear risk | The study appears to be free of other sources of bias, but pharmaceutical support is suspected |
Apuzzio 1985b.
| Methods | Randomized trial Study period: February 1981 through December 1982 | |
| Participants | Inclusion criteria: diagnosis of postcesarean endometritis based on oral temperature of at least 100.4 °F (38 °C) after the first 24 hours postpartum, uterine tenderness and absence of other foci of infection Setting: urban university hospital, New Jersey, USA Number of participants: n = 124 | |
| Interventions | Ceftizoxime 2 g‐3 g iv every 8‐12 hours (n = 68) vs cefoxitin 2 g every 12 hours iv (n = 24) vs clindamycin 600 mg iv every 6 hours with gentamicin 60 mg‐80 mg iv every 8 hours (n = 32) | |
| Outcomes | Treatment failure Diarrhea Septic pelvic thrombophlebitis Thrombophlebitis | |
| Notes | It is not stated whether any of these women received prophylactic antibiotic treatment during surgery Pharmaceutical sponsorship ‐ probable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly assigned" without further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 women initially randomized excluded from analysis with excessive loss (10 women) in cefoxitin group. Cefoxitin group not included in analysis |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, insufficient information to permit judgement |
| Other bias | Unclear risk | The study appears to be free of other sources of bias, but pharmaceutical support is suspected |
Blanco 1983.
| Methods | Randomized trial Study period: April through October 1982 | |
| Participants | Inclusion criteria: clinical diagnosis of postpartum endometritis, salpingitis, or pelvic cellulitis after hysterectomy, all with oral temperature of 38 °C or higher, leukocytosis, and local tenderness Setting: county hospital, San Antonio, Texas, USA Number of participants: n = 77 (69 postcesarean birth) | |
| Interventions | Ceftazidime 2 g iv every 8 hours vs clindamycin 600 mg iv every 8 hours plus gentamicin 1.5 mg/kg iv every 8 hours | |
| Outcomes | Treatment failure Complications including wound infections, allergic reactions, and diarrhea Mean length of stay | |
| Notes | For the outcome of allergy, postcesarean birth participants were not analyzed separately Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random schedule provided by pharmaceutical sponsor. No specific methods described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Not blinded” in the methods |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgement |
| Other bias | High risk | Sponsored by pharmaceutical company, Glaxo |
Chatwani 1995.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: women with postcesarean endometritis defined as temperature of at least 38.3 °C during the first 24 hours after surgery or at least 38 °C after 24 hours with fundal tenderness, adnexal tenderness, and purulent lochia, and no other evident focus of infection. Initially women with other gynecologic infections were to be included. There were 22 women randomized, but later excluded because they were not postcesarean birth Setting: multicenter, USA Number of participants: n = 382 | |
| Interventions | Cefmetazole 2 g iv every 8 hours (n = 232) vs cefoxitin 2 g iv every 6 hours (n = 123) | |
| Outcomes | Treatment failure Septic thrombophlebitis (serious complication) Wound infections Allergic reactions Mean length of stay. Standard deviation for mean length of stay was not given (5.0 days for cefmetazole; 5.4 days for cefoxitin) | |
| Notes | 5 women initially randomized did not receive medication. Drop‐outs were otherwise adequately explained, most were excluded due to protocol change that excluded women who were not postcesarean. These 22 participants are included in the analysis of allergic reactions Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table provided by pharmaceutical sponsor |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States "single‐blind" without further explanation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 382 women initially enrolled, 377 women received medication were evaluated for safety. The initial protocol for enrolment was restricted, excluding a further 22 women – all eligible women with complete data. Drop‐outs were otherwise adequately explained, most were excluded due to protocol change that excluded women who were not postcesarean. These 22 participants are included in the analysis of allergic reactions |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgement |
| Other bias | High risk | Sponsored by pharmaceutical company, the Upjohn company |
Chatwani 1997.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: women with pelvic cellulitis after hysterectomy or postpartum endometritis (defined as temperature of at least 38.3 °C after the first 24 hours and after cesarean birth and fundal tenderness, parametrial tenderness, and purulent lochia) Setting: multicenter, USA Number of participants: n = 579 (404 with postpartum endometritis) | |
| Interventions | Clindamycin 900 mg iv every 8 hours (n = 242; 202 postcesarean birth) plus aztreonam 1 g iv every 8 hours vs trospectomycin 500 mg iv every 8 hours (n = 243; 200 postcesarean birth) plus aztreonam 1 g iv every 8 hours | |
| Outcomes | Treatment failure (postcesarean birth women with endometritis provided separately) For other outcomes (wound infection, serious complications, diarrhea) the results for endometritis postcesarean birth were not reported separately and have not been included The 1 serious complication observed was septic thrombophlebitis in the trospectomycin group | |
| Notes | Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table by pharmaceutical sponsor |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States “double‐blinded” without further description |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Forty‐nine patients from the trospectomycin group and 45 from the clindamycin group were excluded for the efficacy evaluation. The reasons for exclusion included protocol violations as well as use of concomitant antibiotic, and other foci of infection”‐ balanced in numbers over both arms but numbers are high (30%) and the reasons for exclusion given could be related to true outcome |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ explicit |
Del Priore 1996.
| Methods | Randomized trial Study period: February 1991 through March 1993 | |
| Participants | Inclusion criteria: clinical diagnosis of postpartum endometritis (defined as temperature of at least 38 °C orally on 2 occasions or at least 39 °C on 1 occasion, uterine tenderness, absence of any other source of infection), serum creatinine less than 1.4 mg/dL Setting: Chicago, Illinois, USA Number of participants: n = 142 | |
| Interventions | Gentamicin 5 mg/kg of body weight iv once daily (n = 62) vs gentamicin every 8 hours with dosing adjustments based on peak and trough blood levels (n = 65) Other antibiotics allowed | |
| Outcomes | Duration of fever (20.8 hours vs 23.7 hours); post‐treatment serum creatinine levels; nephrotoxicity (not defined further) Change of initial regimen (14/62 vs 17/65) Pharmacy (USD 16.12 vs USD 41.75) and nurse labor costs; length of stay | |
| Notes | 15 of the women enrolled were excluded for protocol violations; administrative errors, misdiagnosis, concomitant infection; no data on treatment allocation to include in intention‐to‐treat analysis Cesarean births = 78 Pharmaceutical sponsorship ‐ none apparent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table via sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Used sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The managing clinical service and the subjects were blinded to the gentamicin treatment regimen.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “A total of 142 subjects were randomized. Fifteen were excluded from analysis because of study‐protocol violations: three had concomitant infections, three had a misdiagnosis of endometritis, and nine had administrative errors. A total of 127 subjects remained for analysis: 62 in the study group and 65 in the control group” – about 89% of data available, well‐balanced between the 2 arms and the majority of reasons for missing data are unlikely to be related to true outcome (apart from possibly 6 patients (4%) with concomitant infections or with a misdiagnosis) |
| Selective reporting (reporting bias) | Low risk | Protocol available and all of the study’s pre‐specified outcomes have been reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
DiZerega 1979.
| Methods | Randomized trial Study period: February 1976 through October 1977 | |
| Participants | Inclusion criteria: women with diagnosis of postpartum endometritis based on fever and uterine tenderness Setting: urban county hospital, Los Angeles, California, USA Number of participants: n = 200 | |
| Interventions | Clindamycin 600 mg iv every 6 hours plus gentamicin 80 mg iv every 8 hours (n = 100) vs penicillin 5 million units iv every 6 hours plus gentamicin 80 mg iv every 8 hours (n = 100) | |
| Outcomes | Treatment failure (defined as those women whose therapy was not completed without problems) Serious complications including pelvic abscess and need for addition of heparin Wound infections Rash (allergic reaction) Diarrhea Mean length of stay 7.4 days for clindamycin‐gentamicin vs 8.7 days for penicillin‐gentamicin (variance not given) | |
| Notes | All participants were postcesarean birth without prophylactic antibiotic treatment Endometritis was defined vaguely Pharmaceutical sponsorship ‐ none apparent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "random basis" not further described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs ‐ none |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgement |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
Faro 1989.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: women with a diagnosis of postpartum endometritis defined as temperature of at least 38.3 °C occurring 24 hours after the administration the last dose of cefazolin, tachycardia, a white blood count of at least 14,000 or immature polymorphonuclear leukocytes, and marked uterine tenderness Setting: Houston, Texas, USA Number of participants: n = 170 | |
| Interventions | Ticarcillin/clavulanic acid 3.1 g iv every 6 hours (n = 85) vs clindamycin 900 mg iv every 8 hours and gentamicin iv dosed by body weight every 8 hours (n = 85) | |
| Outcomes | Therapeutic failure (lack of resolution of all signs and symptoms of infection resolved within 72 hours) Length of hospital stay | |
| Notes | All participants had cesarean births with 3 doses of prophylactic cefazolin 18 women were excluded after enrolment for protocol violations Pharmaceutical sponsorship ‐ explicit All participants without clinical cure at 72 hours responded with the addition of ampicillin iv Bacteriologic studies were performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States “a computer‐generated randomisation schedule provided by Miles Pharmaceuticals.” |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Different treatments had different intervals between treatments suggesting the personnel would have known which treatment plan each patient was on. Insufficient information to make judgment for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 women (10.6%) were excluded after enrolment for protocol violations “Ten women who received ticarcillin/clavulanic acid and eight in the clindamycin‐gentamicin group were disqualified for not fulfilling the criteria of the study protocol” – incomplete data are well‐balanced, small amount compared with the amount of data, and the reason for missing data is unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgement |
| Other bias | High risk | Pharmaceutical sponsorship ‐ explicit ‐ Miles Pharmaceuticals |
Fernandez 1990.
| Methods | Randomized trial Study period: March 1985 through March 1986 | |
| Participants | Inclusion criteria: fever (defined as more than 37.8 °C in the first 24 hours postpartum), with pelvic tenderness or malodorous lochia, or both, without other obvious diagnosis. Participants were classified as having mild (temperature 37.8 °C‐38.4 °C) or severe (temperature greater than 38.4 °C) forms Setting: Clamart, France Number of participants: n = 101 ("severe form": n = 26, "mild form": n = 73) | |
| Interventions | 'Severe' disease: amoxicillin/clavulanic acid 1.2 g iv every 8 hours (n = 14) versus ampicillin 2 g iv every 8 hours and gentamicin iv by body weight every 12 hours (n = 12) changing to oral amoxicillin/clavulanic acid or amoxicillin to complete 8 days treatment once afebrile "Mild" disease: oral treatment only amoxicillin/clavulanate (n = 36) vs ampicillin/metronidazole (n = 37) |
|
| Outcomes | Treatment failure Mean time to defervescence (3.5 vs 2.7 days, not significant) Mean time to resolution of clinical signs of endometritis (2.3 vs 1.7 days, P value < 0.05) Duration of treatment Incidence of urticaria (allergic reaction) | |
| Notes | 2 women were excluded after enrolment (1 in each group) with culture demonstrating resistant Staphylococcus aureus Vaginal births = 62 Participants receiving both the iv and oral form of amoxicillin/clavulanic acid (Augmentin) have been combined Pharmaceutical sponsorship ‐ probable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number tables |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs < 5% |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
Figueroa‐Damian 1996.
| Methods | Randomized trial Study period: March 1993 through May 1994 | |
| Participants | Inclusion criteria: women with postcesarean endometritis defined as fever, presence of foul smelling lochia, and pain on fundal palpation Setting: Mexico Number of participants: n = 56 | |
| Interventions | Piperacillin/tazobactam 500 mg iv every 6 hours for 5 days vs Ampicillin 1 g iv every 6 hours plus gentamicin 80 mg iv every 8 hours for 5 days followed by oral ampicillin and IM gentamicin for 5 additional days |
|
| Outcomes | Therapeutic failure Wound infection Mean length of stay 7 days vs 6 days (standard deviations not given) | |
| Notes | All postcesarean births Pharmaceutical sponsorship ‐ none apparent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "random" 3:1, without further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Unclear risk | Only a reduced translated copy of the review is available, which could result in information about potential biases being missed |
Filler 1992.
| Methods | Randomized trial Study period: not given | |
| Participants | Inclusion criteria: postcesarean birth with endometritis diagnosed based on elevated temperatures and white count and abnormal uterine tenderness Setting: South Carolina, USA Number of participants: n = 21 | |
| Interventions | Trospectomycin 500 mg iv every 8 hours plus aztreonam 1 g iv every 8 hours (n = 12) vs clindamycin 900 g iv every 8 hours plus aztreonam 1 g iv every 8 hours (n = 8) | |
| Outcomes | Therapeutic failure (defined as lack of resolution of fever, uterine tenderness, and high white blood count) | |
| Notes | All participants were postcesarean birth Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Code prepared by pharmaceutical company and carried out by hospital pharmacy. Did not state specific methods they used |
| Allocation concealment (selection bias) | Low risk | “Randomization code was prepared by the Upjohn (pharmaceutical) Company and was carried out by the (hospital) pharmacy.” It was a pharmacy controlled randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The hospital pharmacy dispensed [antibiotics] to the floor in a double blind fashion.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States they “undertook a small double blind study” with no further description given, indicating the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 (95.2%) patients completed treatment out of 21 patients enrolled. 1 patient “left against medical advice to be treated with oral antibiotics and was withdrawn from the study” |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgement |
| Other bias | High risk | Pharmaceutical sponsorship explicit – “The Upjohn Company prepared the randomised code” |
Gaitan 1995.
| Methods | Randomized trial Study period: September 1993 through August 1994. | |
| Participants | Inclusion criteria: women with postpartum endometritis after emergency cesarean birth Setting: tertiary care centre, Bogota, Colombia Number of participants: n = 71 | |
| Interventions | Pefloxacine 400 mg iv every 12 hours plus metronidazole 500 mg iv every 8 hours (n = 35) vs clindamycin 600 mg iv every 6 hours plus gentamicin 2 mg/kg/day iv divided into doses every 12 hours (n = 36) | |
| Outcomes | Clinical cure or improvement Allergic reactions Antibiotic associated diarrhea | |
| Notes | All women had undergone emergency cesarean births Use of prophylactic antibiotics not described. Women with cultures demonstrating microorganisms resistant to the antibiotics used were excluded from the study Pharmaceutical sponsorship ‐ probable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization was done in blocks utilizing a table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States treatment was blinded but no further description, insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States treatment was blinded but no further description, insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs < 5%: 3 from Group A and 2 from Group B |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
Gall 1996.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: women with a diagnosis of postpartum endometritis by temperature elevation to 39 °C on 1 occasion or 38.5 °C on 2 occasions after delivery Setting: Louisville, Kentucky, USA Number of participants: n = 129 | |
| Interventions | Ampicillin 2 g plus sulbactam 1 g iv (n = 64) every 6 hours vs clindamycin 900 mg plus gentamicin by body weight iv every 8 hours (n = 65) | |
| Outcomes | Cure (disappearance of presenting signs and symptoms) Improvement (partial alleviation of presenting signs and symptoms) Failure (no significant effect of study drug therapy on presenting signs and symptoms) Indeterminate (does not fit into any other category or unable to evaluate (n = 1 in clindamycin/gentamicin group) Diarrhea (9 vs 8) Length of hospital stay (9 vs 10 days; no variance given) | |
| Notes | 13 women were excluded after enrolment for numerous reasons, generally protocol violations The number of women who underwent cesarean birth versus vaginal birth is not described Endometritis was poorly defined Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised" without further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 116 women (89.9%) out of 129 women included in the analysis. 9 lost to follow up in the ampicillin/sulbactam group and 4 in the clindamycin/gentamicin group |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ explicit |
Gibbs 1982.
| Methods | Randomized trial Study period: January 1980 through June 1981 | |
| Participants | Inclusion criteria: women who had undergone cesarean birth with clinical diagnosis of postpartum endometritis (based on fever > 101 °F (38.3 °C), uterine tenderness, and leukocytosis) Setting: San Antonio, Texas, USA Number of participants: n = 198 | |
| Interventions | Clindamycin 600 g every 6 hours plus gentamicin by body weight every 8 hours both iv (n = 106) vs cefamandole 2 g iv every 6 hours plus placebo doses every 8 hours (n = 92) | |
| Outcomes | Therapeutic failure (persistent fever > 3 days), wound infection, serious complication Complications including rash (allergic reaction) and diarrhea Mean length of stay Culture results | |
| Notes | All cesarean births, without antibiotic prophylaxis Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “randomisation provided by the sponsor” without further description |
| Allocation concealment (selection bias) | Low risk | “The solutions were prepared by the pharmacy as each patient was enrolled.” The pharmacy controlled randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “bottles were wrapped in dark plastic bags. In the intravenous tubing, these two solutions could not be distinguished from one another” Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States “double‐blind” without further description, indicates that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 women (5.2%) randomized but excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ explicit |
Gibbs 1983.
| Methods | Randomized trial Study period: July 1981 through March 1982 | |
| Participants | Inclusion criteria: women with postcesarean endomyometritis defined as oral temperature of at least 38.4 °C, uterine tenderness, and leukocytosis Setting: urban medical centre hospital, San Antonio, Texas, USA Number of participants: n = 113 | |
| Interventions | Moxalactam 2 g iv every 8 hours (n = 56) vs clindamycin 600 mg iv every 8 hours and gentamicin 1 mg/kg iv every 8 hours (n = 57) | |
| Outcomes | Treatment failure Wound infection Allergic reactions Diarrhea Length of stay | |
| Notes | All participants were postcesarean birth Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomization provided by the sponsor” without further description of the sequence generation, insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Solutions were prepared by the pharmacy.” “To obscure the very light amber colour of the moxalactam solution, both the clindamycin and the moxalactam bottle were wrapped in dark plastic bags. In the intravenous tubing, these two solutions could not be distinguished from one another.” Blinding ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “A double‐blind comparison” indicates outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 113 enrolled and analyzed, no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ explicit |
Gibbs 1985.
| Methods | Randomized trial Study period: November 1982 through December 1983 | |
| Participants | Inclusion criteria: women with postcesarean birth endometritis defined as an oral temperature of at least 38 °C, uterine tenderness and without other sources of fever Setting: San Antonio, Texas, USA Number of participants: n = 119 | |
| Interventions | Aztreonam 2 g every 8 hours plus clindamycin 600 mg iv every 6 hours (n = 58) vs gentamicin iv dosed by body weight every 8 hours plus clindamycin 600 mg iv every 6 hours (n = 61) | |
| Outcomes | Therapeutic failure (lack of resolution of signs and symptoms within 72 hours) Side‐effects (diarrhea, allergy) leading to discontinuation of treatment Length of hospital stay | |
| Notes | All participants had cesarean births Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization schedule provided by pharmaceutical sponsor |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States “119 patients were evaluated”, but number randomized not stated, so insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ explicit |
Greenberg 1987.
| Methods | Randomized trial Study period: December 1984 through April 1986 | |
| Participants | Inclusion criteria: postpartum women with temperature of 100.4 °F (38 °C) or greater, uterine tenderness, no other source of fever identified Setting: St Louis, Missouri, USA Number of participants: n = 62 | |
| Interventions | Aztreonam 1 g‐2 g iv every 8 hours plus clindamycin 900 mg iv every 8 hours (n = 31) vs gentamicin ("per manufacturer's instructions") every 8 hours plus clindamycin 900 mg iv every 8 hours (n = 31) | |
| Outcomes | Cure (defined as defervescence and complete resolution of signs and symptoms) or partial response (defined as "substantial or temporary improvement") or therapeutic failure Mortality Side‐effects including abnormal laboratory findings, pruritus following drug administration, pain and phlebitis at infusion site | |
| Notes | 45 women had cesarean births and 17 had vaginal births All women given oral antibiotics to complete a 10‐14 day course Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly assigned" according to a schedule provided by the sponsoring company, not further described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs ‐ none |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ explicit |
Gutierrez 1994.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: diagnosis of postpartum endometritis (temperature at least 38 °C, uterine tenderness, and leukocytosis) Setting: Lima, Peru Number of participants: n = 65 | |
| Interventions | Penicillin 3 million units iv every 4 hours plus gentamicin 1.5 mg/kg iv every 8 hours plus chloramphenicol 1 g iv every 8 hours (n = 33) vs clindamycin 600 mg iv every 8 hours plus gentamicin 1.5 mg/kg every 8 hours (n = 32) | |
| Outcomes | Clinical cure or improvement Abscess Antibiotic associated diarrhea Phlebitis, anemia and wound infections | |
| Notes | Mode of delivery not provided. 1 woman from each group withdrew from the study 1 exclusion for wrong diagnosis. Pharmaceutical sponsorship not apparent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "random", not further described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “The trial was single‐blinded.” It specifies that the people giving the intervention (the gynecologists) were blinded. Nothing is mentioned regarding the participants or other people involved. Incomplete blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The trial was single‐blinded.” Report specifies that the people giving the intervention (the gynecologists) were blinded. Nothing is mentioned regarding the participants or other people involved. Outcome assessors (gynecologists) were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs < 5%. 1 woman from each group withdrew from the study. 1 exclusion for wrong diagnosis |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgement |
| Other bias | Unclear risk | The article is non‐English and a full translation is not available, so insufficient information to permit judgement |
Hager 1989.
| Methods | Randomized trial Study period: not given | |
| Participants | Inclusion criteria: women treated for chorioamnionitis, postpartum endometritis (defined as temperature of at least 38.1 °C, leukocytosis 15,000/mL, and uterine tenderness), or post hysterectomy cellulitis. All had received standard parenteral antibiotics until 48‐72 hours afebrile and clinically well Setting: Lexington, Kentucky, USA Number of participants: n = 163 evaluated, n = 81 with postpartum endometritis | |
| Interventions | Oral ampicillin 500 mg every 6 hours or tetracycline 500 mg every 6 hours (if penicillin allergic) to complete 10 days total of antibiotic therapy (n = 38) vs no treatment after iv antibiotics (n = 43) | |
| Outcomes | Further treatment with antibiotics by the time of follow up at 2‐4 weeks after hospital discharge Postdischarge infections (wound or urinary tract infection) classified as failures | |
| Notes | Information on route of delivery was not given Pharmaceutical sponsorship ‐ none apparent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “random” without further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States “single‐blinded study” in abstract but no further description |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The physician evaluating the patient at the follow‐up examination [..] was blinded as to whether they had taken the oral antibiotic or not:” Outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 163 (80%) out of 204 women were evaluated. 31 had no follow‐up visits, 10 had infection at other organ sites |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgement |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
Hemsell 1983.
| Methods | Randomized trial Study period: May 1980 through March 1981 | |
| Participants | Inclusion criteria: women with postcesarean birth endometritis defined as temperature of at least 38.3 °C on 2 occasions 4 hours or more apart, abdominal pain with abdominal, uterine and perhaps parametrial tenderness Setting: university hospital, Dallas, Texas, USA Number of participants: n = 120 | |
| Interventions | Cefotaxime 2 g iv every 8 hours (n = 81) vs clindamycin 600 mg iv every 6 hours plus gentamicin 1 mg/kg every 8 hours (n = 39) | |
| Outcomes | Treatment failure Complications including pelvic abscess (severe complication), wound infection, and diarrhea Length of treatment was 5.5 +/‐ SD 2.1 days versus 5.6 +/‐ SD 1.9 days | |
| Notes | All participants were postcesarean births Although not specifically stated, the earlier citation appears to include women included in the later citation Pharmaceutical sponsorship ‐ probable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by computer‐generated list 2:1 |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In dose‐finding study 117(99.2%) of 118 patients completed treatment. “Two women treated early in the study were excluded from group 1. One woman [..] was included in group 2. The second woman was excluded when she developed cellulitis at the site of intramuscular injection and cefotaxime therapy was discontinued.” In comparative phase, 120 women treated, but number randomized is not stated Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgement |
| Other bias | Unclear risk | Study appeared to be free of other sources of bias, however pharmaceutical sponsorship is suspected |
Herman 1986.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: postpartum endometritis defined as postoperative fever of 38.3 °C orally or higher, uterine tenderness, and absence of other infectious foci Setting: University hospital, Philadelphia, Pennsylvania, USA Number of participants: n = 98 | |
| Interventions | Cefoxitin 2 g iv every 6 hours (n = 48) vs clindamycin 600 mg iv every 8 hours plus gentamicin 1.5 mg/kg iv every 8 hours (n = 50) | |
| Outcomes | Therapeutic failure, serious complication, diarrhea, rash Follow up at 6 weeks included skin wound breakdown, pelvic infection and urinary tract infection | |
| Notes | All participants were postcesarean birth. Women with and without antibiotic prophylaxis were included Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “The nurse and pharmacist both functioned autonomously to maintain the blinding of the study.” Unclear for patients, low risk for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 98 (89.9%) out of 109 women enrolled were evaluated; insufficient information provided on drop‐outs to include in intent‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available but all expected outcomes appear to be reported |
| Other bias | High risk | Pharmaceutical sponsorship ‐ explicit |
Hillier 1990.
| Methods | Randomized trial Study period: August 1986 through August 1989 | |
| Participants | Inclusion criteria: women with a temperature elevation of at least 38.5 °C within 24 hours after cesarean birth or at least 38 °C for 4 consecutive hours more than 24 hours postoperatively, uterine tenderness, and no other apparent source of fever Setting: Seattle, Washington, USA Number of participants: 27 | |
| Interventions | Ticarcillin/clavulanic acid 3/1 g iv every 8 hours 9 (n = 13) vs cefoxitin 2 g iv every 8 hours (n = 14) | |
| Outcomes | Cure (defined as resolution of fever and tenderness and no further signs of infection during follow‐up period) Therapeutic failure (defined as fever after 48 hours of antibiotic therapy) | |
| Notes | All but 1 woman received antibiotic prophylaxis with a cephalosporin at the time of surgery Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated randomization schedule |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States “single‐blinded study”; “Each antibiotic was reconstituted according to the directions provided and 0.1 ml of multivitamin was added to each bag to endure a uniform colour.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs are described |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ explicit |
Knodel 1988.
| Methods | Randomized trial Study period: January through December 1984 | |
| Participants | Inclusion criteria: postcesarean birth endometritis (oral temperature at least 38 °C and uterine tenderness) Setting: Bethesda, Maryland, USA Number of participants: n = 114 | |
| Interventions | Moxalactam 2 g iv every 8 hours (n = 58) vs clindamycin 600 mg every 6 hours plus gentamicin 1.5 mg/kg iv every 8 hours (n = 56) | |
| Outcomes | Clinical cure or improvement Allergic reactions Length of stay | |
| Notes | All postcesarean births with or without antibiotic prophylaxis at surgery Pharmaceutical sponsorship ‐ probable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation by randomization schedule without further detail |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | States "an open randomised prospective trial.” Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Note stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported and data is balanced in numbers across groups; drop outs not explicitly stated |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ledger 1974.
| Methods | Randomized trial Time period: 28 Oct 1971 through 28 July 1972 |
|
| Participants | Inclusion criteria: clinically "severe" obstetric/gynecologic infections that required parenteral antibiotics as deemed by attending or resident physician Site: University of Michigan Hospital, USA Number of participants: n = 44 |
|
| Interventions | Clindamycin varied dosage every 6‐8 hours with kanamycin 0.5 mg every 12 hours (n = 21) vs penicillin G 5‐10 million units every 6‐8 hours with kanamycin every 12 hours (n = 23) | |
| Outcomes | Treatment failure, additional therapy required (antibiotic usage or surgical intervention), bacterial species identified | |
| Notes | Pharamceutical sponsorship: Upjohn Did not provide data separately for those patients with endometritis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized blocks of 10 envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed numbered envelopes utilized |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Initial selection was blinded, but both physician and patient were privy to the treatment at the time of administration |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding for outcomes noted |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop outs |
| Selective reporting (reporting bias) | Low risk | It appears as though all outcomes were reported |
| Other bias | Low risk | ‐ |
Livingston 2003.
| Methods | Randomized trial Study period: December 1998 through December 2000 | |
| Participants | Inclusion criteria: temperature of at least 100.4 °F (38 °C) on at least 2 occasions 6 hours apart after the first 12 hours postpartum or greater than 101.5 °F (38.05 °C) at any time, no other evident source of infection, uterine tenderness or diagnosis of chorioamnionitis before birth thought to require antibiotics postpartum Setting: University of Tennessee Health Science Center, USA Number of participants: n = 112 | |
| Interventions | Gentamicin 5 mg/kg plus clindamycin 2700 mg iv once daily (n = 55) vs gentamicin 1.5 mg/kg plus clindamycin 900 mg every 8 hours (n = 55) | |
| Outcomes | Treatment failure Length of hospital stay | |
| Notes | 40 women undergoing cesarean were in the thrice‐daily dosing group and 46 in the once‐daily group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Random” without further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Double‐blinded study” in methods but no further description. Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 110 (98%) were evaluated |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
Maccato 1991.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: postpartum women with oral temperature > 38 °C, tachycardia, uterine tenderness, and white blood count > 14,000 or an increase > 10% in immature leukocytes Setting: Houston, Texas, USA Number of subjects: n = 99 | |
| Interventions | Ciprofloxacin 200 mg iv every 12 hours (n = 50) vs clindamycin 900 mg iv every 8 hours and gentamicin 120 mg iv loading followed by dosage adjustment based on peak and trough blood levels (n = 49) | |
| Outcomes | Therapeutic failure (defined as persistence of fever, elevated white blood count, lack of bowel sounds, signs of peritonitis, wound tenderness or infection leading to wound breakdown after 48 hours of therapy) Complications (abscess, septic pelvic thrombophlebitis) | |
| Notes | 2 women (1 from each group) were not evaluated due to administration of other antibiotics < 48 hours after enrolment Only 3 women had vaginal births Pharmaceutical sponsorship ‐ probable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “randomized” without further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | States “open”, not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | States “open”, not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 women excluded from analysis with reasons |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Low risk | The study appears to be free of other sources of bias |
MacGregor 1992.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: postcesarean at least 12 hours postoperative who had received 3 doses of cefazolin as prophylaxis, and who presented with uterine tenderness, temperature at least 38.3 °C on 1 occasion or at least 38 °C on 2 occasions at least 6 hours apart, and no other obvious source of infection Setting: Philadelphia, Pennsylvania, USA Number of participants: n = 140 | |
| Interventions | Cefotetan 2 g iv every 12 hours (plus placebo doses; n = 66) vs cefoxitin 2 g iv every 6 hours (n = 63) | |
| Outcomes | Therapeutic failure (defined as a lack of decrease in temperature and uterine tenderness within 48 hours of therapy) Incidence of enterococcal bacteremia (considered automatically as a treatment failure): cefotetan n = 3; cefoxitin n = 1 Relapse (defined as those women meeting criteria for cure with subsequent wound infection, abscess, recurrent endometritis within 6 weeks) ‐ 1 in each group Complications (wound infection) Diarrhea | |
| Notes | 11 women were excluded due to protocol violations (4 from cefotetan group, 7 from the cefoxitin group) Pharmaceutical sponsorship ‐ probable All participants were postcesarean birth | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization schedule |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded. All doses were identical in appearance and were dispensed from the pharmacy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All clinical decisions made by the primary physicians, not by the study team |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 (7.8%) women were excluded due to protocol violations (4 from cefotetan group, 7 from the cefoxitin group) |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Martens 1989.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: women postcesarean who had received prophylactic cefazolin (3 doses) with temperature of at least 38.3 °C that occurred 24 hours after the last dose of cefazolin, marked uterine tenderness, and at least 1 of the following; tachycardia, white blood count of at least 14,000 or at least 10% increase in immature polymorphonuclear leukocytes Setting: Houston, Texas, USA Number of participants: n = 70 | |
| Interventions | Sulbactam 1 g with ampicillin 2 g iv every 6 hours (n = 34) vs metronidazole 500 mg iv every 6 hours with gentamicin every 8 hours adjusted by peak and trough levels (n = 36) | |
| Outcomes | Therapeutic failure (defined as lack of resolution of all signs and symptoms of infection within 72 hours) | |
| Notes | All participants were women postcesarean who had received 3 doses of cefazolin as prophylaxis Oral antibiotics were not given Pharmaceutical sponsorship ‐ probable 3 women excluded because they had vaginal births (2 in sulbactam/ampicillin group; 1 in metronidazole/gentamicin group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “randomized” without further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women excluded because they had vaginal births (2 in sulbactam/ampicillin group; 1 in metronidazole/gentamicin group) Drop‐outs < 5% |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Martens 1990.
| Methods | Randomized trial Allocation: 2:1 computer‐generated randomization provided by the pharmaceutical sponsor Blinding: not stated Study period: not stated |
|
| Participants | Inclusion criteria: women with diagnosis of postpartum endomyometritis defined as temperature of at least 38.3 °C within 24 hours after the last dose of prophylactic antibiotic, tachycardia, white blood cell count of at least 14,000/mL or at least 10% increase in immature polymorphonuclear leukocytes, and marked uterine tenderness Study setting: Houston, Texas, USA Number of participants: n = 68 (75 with 7 excluded due to protocol violations) | |
| Interventions | Ampicillin/sulbactam 2 g/1 g iv every 6 hours (n = 42) vs clindamycin 900 mg iv every 8 hours (n = 26) | |
| Outcomes | Treatment failure | |
| Notes | All participants were postcesarean birth Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2:1 computer‐generated randomization provided by the pharmaceutical sponsor |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open comparative study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open comparative study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 75 participants were enrolled but 4 cases were declared invalid, organisms from pretreatment samples from 10 participants failed to grow and 3 participants who had vaginal birth were excluded. Thus 68 clinically diagnosed but lacking specific pathogens were evaluated along with 58 patients who fulfilled all protocol criteria – 90.6% of the data evaluated but only 77.3% fulfilled protocol criteria. All 10 excluded from evaluation came from the ampicillin arm (2:1) – because of this imbalance, the attrition bias is at high risk. Drop‐outs > 5%; insufficient information provided on women excluded to include in intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ explicit |
McGregor 1989.
| Methods | Randomized trial Study period: September 1987 through July 1988 | |
| Participants | Inclusion criteria: women with clinical findings of upper genital tract infection in the puerperium Setting: university hospital, Denver, Colorado, USA Number of participants: n = 36 | |
| Interventions | Ampicillin/sulbactam 2 g/1 g iv every 6 hours (n = 18) vs clindamycin 900 mg iv every 8 hours and gentamicin 1.5 mg/kg every 8 hours (n = 18) | |
| Outcomes | Therapeutic failure Adverse reactions Calculated daily costs (drug and pharmacy). Sulbactam/ampicillin USD 91.20 vs clindamycin/gentamicin USD 116.97 | |
| Notes | Included 23 participants with endometritis following cesarean birth and 13 with endometritis following vaginal birth Pharmaceutical sponsorship ‐ probable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “randomised” without further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs described |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mitra 1997.
| Methods | Randomized trial Study period: July 1994 through July 1996 | |
| Participants | Inclusion criteria: women with 1 of the following; (1) 2 temperatures of at least 100.4 °F (38 °C) more than 12 hours postpartum, (2) a single temperature of at least 102 °F (38.9 °C) in the first 12 postpartum hours, (3) diagnosis of chorioamnionitis in labor thought to require prophylactic antibiotic therapy, (4) diagnosis of postpartum endometritis after initial discharge from the hospital. Women with criteria 1 or 4 were considered to have endometritis Setting: Charlotte, North Carolina, USA Number of participants: n = 299 (endometritis participants only n = 141) | |
| Interventions | Clindamycin 800 mg iv plus gentamicin 1.33 mg/kg body weight iv every 8 hours (n = 71) vs clindamycin 1200 mg iv every 12 hours and gentamicin 4 mg/kg body weight every iv 24 hours (n = 70) | |
| Outcomes | Cure (average temperature not more than 99 °F (37.2 °C) and resolution of symptoms) Failure (elevated temperature after 72 hours of treatment, clinical deterioration, or the need for additional antibiotic or heparin treatment) Relapse (cure with subsequent wound infection, abscess or endometritis up to 6 weeks postpartum) Time to resolution of infection (time from first dose to last dose of antibiotic administered). This was 2.8 +/‐ 2.4 days versus 2.3 +/‐ 2.0 days for the conventional thrice‐daily vs once‐daily gentamicin groups respectively, P value 0.02 Patient charges for antibiotic treatment (medication and administration): total charges for antibiotic treatment was USD 442.49 per patient in the conventional thrice‐daily dosing group and USD 250.79 for the once‐daily gentamicin group Nephrotoxicity (0.5 mg/dL increase in serum creatinine over the baseline). 1 participant (once‐daily group) had a serum creatinine level of 2.3 after therapy that resolved spontaneously | |
| Notes | 27 women were excluded after enrolment for protocol violations; insufficient information on drop‐outs to include study in intention‐to‐treat analysis There were 102 cesarean birth and 39 vaginal birth participants in the endometritis categories The conventional thrice‐daily dosing treatment group had more cesarean births (56/71) than the once‐daily gentamicin treatment group (46/70), which could confound results such as length of treatment, which favored the once‐daily group Multiple logistic regression demonstrated that the experimental dosing was not more efficacious when mode of delivery was accounted for Pharmaceutical sponsorship ‐ none apparent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | States “Physicians were not blinded with respect to the dosing regimen.” Blinding not done, allocation evident |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not done for physicians, allocation evident |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 27 (10%) excluded for protocol violations with reasons. All the women who randomized were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgement |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
Morales 1989.
| Methods | Randomized trial Study period: July 1987 through April 1988 | |
| Participants | Inclusion criteria: women with diagnosis of postpartum endomyometritis defined as temperature greater than 100.4 °F (38 °C) on 2 occasions at least 6 hours apart or 101 °F (38.3 °C) once excluding the first postpartum day, uterine tenderness, leukocytosis, and absence of other foci of infection. Women with bacteremia were excluded Setting: urban hospital, Tampa, Florida, USA Number of participants: n = 109 | |
| Interventions | Oral ampicillin/clavulanic acid for 7 days following iv antibiotic therapy (clindamycin/tobramycin until afebrile for at least 24 hours; n = 37) vs no treatment following iv antibiotics (n = 72) | |
| Outcomes | Treatment failure Need for additional antibiotic treatment (recurrent endometritis) Costs were calculated was also evaluated and was a mean of USD 412 more in the oral antibiotic group | |
| Notes | There were 81 postcesarean births in this study There were 2 control groups, 1 receiving iv antibiotics until 24 hours afebrile, the other receiving them until 48 hours afebrile. There was no difference between these 2 groups, and they are combined in this analysis Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized" 2:1, not further described |
| Allocation concealment (selection bias) | Unclear risk | No discussion of method of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding/masking the participants, physicians or abstractors was noted |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding/masking the participants, physicians or abstractors was noted |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19 (14.8%) excluded from analysis with reasons shown in Table 1 of the manuscript |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ evident |
Pastorek 1987.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: women with puerperal infection based on standard febrile morbidity; uterine, parametrial, or vaginal cuff tenderness; and leukocytosis Setting: New Orleans, Louisiana, USA Number of participants: n = 60 | |
| Interventions | Moxalactam 2 g iv every 8 hours (n = 29) vs clindamycin 600 mg iv every 6 hours plus tobramycin 1 mg/kg‐1.5 mg/kg iv every 8 hours (n = 31) | |
| Outcomes | Treatment failure Pelvic abscess (severe complication) Wound abscess Diarrhea | |
| Notes | Diarrhea was a complication regarded as clinical failure with change of antibiotic regimen. This case not included in our analysis of therapeutic failure Pharmaceutical sponsorship ‐ probable Information on number of cesarean or vaginal births was not provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | No discussion of method for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No discussion of blinding was reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No discussion of blinding was reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
Perry 1997.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: women with clinical diagnosis of postcesarean endometritis Setting: not stated (presumably university hospital Jackson, Mississippi, USA) Number of participants: n = 100 | |
| Interventions | Gentamicin 1.5 mg/kg iv every 8 hours plus clindamycin 900 mg iv every 8 hours (n = 44) vs gentamicin 5 mg/kg iv every 24 hours plus clindamycin 900 mg iv every 8 hours (n = 41) | |
| Outcomes | Therapeutic failure Nephrotoxicity Mean length of stay | |
| Notes | All participants were postcesarean births This is a published abstract; insufficient information provided on excluded women to perform intention‐to‐treat analysis Pharmaceutical sponsorship ‐ probable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized" without further explanation |
| Allocation concealment (selection bias) | Unclear risk | No allocation procedure was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No discussion of blinding patients, physicians or outcome abstractors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No discussion of blinding patients, physicians or outcome abstractors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 85 out of 100 (85%) remained in the analysis . Reasons for drop out were not stated |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Unclear risk | Only abstract available for assessment |
Pietrantoni 1998.
| Methods | Randomized trial Study period: not stated | |
| Participants | Inclusion criteria: women clinically diagnosed as having postpartum endomyometritis; the presence of fever (102.2 °F/39 °C), pelvic pain, and foul lochia Setting: Lousiville, USA Number of participants: n = 19 | |
| Interventions | Oral therapy using ofloxacin 400 mg every 12 hours plus clindamycin 900 mg every 8 hours until 24 hours afebrile vs clindamycin 900 mg iv every 8 hours plus gentamicin iv 5mg/kg/day every 8 hours until afebrile "Antibiotic therapy was continued for at least 48 hours unless significant clinical deterioration occurred necessitating the withdrawal of the patient from the study." |
|
| Outcomes | Treatment failure | |
| Notes | This study was a preliminary study that enrolled 19 women towards the overall enrolment of 60 women for statistical significance There is no publication or report found after this publication Pharmaceutical sponsorship ‐ none apparent Information on number of cesarean births and vaginal births were not given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised" in the title and objective without further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The interventions were evident, although the outcomes would not have been affected by a lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was no reason stated for 3 (16%) women loss to follow‐up; since n = 19, the loss of 3 women could certainly affect the outcome |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Only 1 outcome exists in the results |
| Other bias | Unclear risk | This study was a preliminary study that enrolled 19 women towards the overall enrolment of 60 women for statistical significance. However, we could not find any relevant reports or publications that followed this publication. There is a possibility that the trial stopped early due to some data‐dependent process |
Rodriguez 1996.
| Methods | Randomized trial Study period: November 1993 through May 1994 | |
| Participants | Inclusion criteria: women with postpartum endometritis defined as temperature of at least 38 °C on 2 occasions separated by at least 4 hours after the first 24 hours postpartum without evidence of other foci of infection. All were postcesarean birth Setting: military hospital, Mexico Number of participants: n = 77 | |
| Interventions | Penicillin 10 million units iv every 4 hours plus amikacin 500 mg iv every 12 hours until afebrile for 24 hours then oral and IM to complete 10 days (n = 31) vs same iv regimen until afebrile 48 hours with no further treatment (n = 32) | |
| Outcomes | Therapeutic failure Mean length of stay Amount of drug utilized | |
| Notes | All participants were postcesarean birth Pharmaceutical sponsorship ‐ none apparent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization reported, no method specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation method reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding here due to length of treatment, unlikely effecting outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding data abstractor in the English translation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 patients in group A and 7 in group B were excluded. Would have been better to maintain in the study for intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Specific outcomes not reported in translated article |
| Other bias | Unclear risk | Only a reduced translated copy of the review is available which could lead to information about potential biases being missed |
Roy 2003.
| Methods | Computer‐generated randomization Blinding: double‐blinded | |
| Participants | Inclusion criteria: women with acute pelvic infection including postpartum endometritis defined as temperature > 38 °C, white blood cell count > 10,500/microliter or > 10% immature granulocytes, and at least 1 of the following: pelvic pain or tenderness or imaging suggesting infection Setting: 47 sites in multiple countries Number of participants: n = 412, of which 238 had postpartum endometritis | |
| Interventions | Ertapenem 1 g iv daily (n = 216) and 3 placebo doses daily for blinding vs piperacillin‐tazobactam 3.375 g iv every 6 hours (n = 196) | |
| Outcomes | Clinical cure or improvement | |
| Notes | 128 women had cesarean births and 110 vaginal births | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers to make allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States “double‐blind (with sponsor blinding)”, “To ensure blinding, patients in the ertapenem group also received subsequent matching placebo infusions of 50ml of normal saline every 6 hours.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 163 patients in the ertapenem group (75%) and 153 patients in the piperacillin‐tazobactam group (78%) were evaluated as the remainder had assessments outside the protocol‐defined follow‐up period or had inappropriate or inadequate courses of study therapy ‐ though specific numbers for each of these were not provided |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ evident |
Scalambrino 1989.
| Methods | Randomized trial Study period: January through December 1987 | |
| Participants | Inclusion criteria: women with infections or febrile morbidity defined as temperature of at least 38 °C on 2 successive measurements 24 hours apart after abortion or delivery for postpartum endometritis participants Setting: Italy (at least 2 sites) Number of participants: n = 95, of which 25 were cases of postpartum endometritis | |
| Interventions | Sulbactam/ampicillin 1 g/2 g iv every 8 hours (n = 12) vs cefotetan 2 g iv every 12 hours (n = 13) | |
| Outcomes | Therapeutic failure | |
| Notes | Outcomes for postpartum women were identified. There were 19 vaginal births and 6 cesarean births Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “according to a random schedule” without further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ evident |
Soper 1992.
| Methods | Randomized trial Study period: not given | |
| Participants | Inclusion criteria: women with postpartum endometritis based on 2 temperatures of more than 38.6 °C at least 4 hours apart or a single temperature of more than 38.6 °C during the first 24 hours after delivery; uterine tenderness; and no other apparent source of fever Setting: Medical College of VIrgina Hospitals, Richmond, Virginia, USA Number of participants: n = 81 | |
| Interventions | Ceftizoxime 2 g iv every 12 hours (n = 43) vs cefoxitin 2 g every 6 hours (n = 38) | |
| Outcomes | Treatment failure Complications including phlebitis, wound infection, allergic reactions, and diarrhea | |
| Notes | Cesarean births could have received cefazolin antibiotic prophylaxis during surgery (n = 73) Vaginal births (n = 8) Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “Patients were randomly assigned” no further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States “Double blinded” ”physicians did not know which antibiotic was being used.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States ”physicians did not know which antibiotic was being used.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs unclear |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ evident |
Stovall 1993.
| Methods | Randomized trial Study period: January 1989 through November 1989 | |
| Participants | Inclusion criteria: women postcesarean birth who had received a single 1 g dose of cefazolin during surgery with diagnosis of postpartum endometritis (defined as oral temperature of at least 101 °F (38.3 °C) > 24 postoperative hours and concomitant tachycardia, white blood count of at least 14,000 or a > 10% increase in immature leukocytes, and abnormal uterine tenderness) Setting: Winston‐Salem, North Carolina, USA Number of participants: n = 77 | |
| Interventions | Ampicillin 2 g plus sulbactam 1 g iv every 6 hours (n = 37) vs clindamycin 900 mg plus gentamicin 80 mg iv every 8 hours (n = 40) | |
| Outcomes | Therapeutic failure (defined as fever and no improvement in uterine tenderness after 72 hours treatment) Diarrhea Severe complications (septic pelvic thrombophlebitis, abscess) | |
| Notes | No oral antibiotics were given after discharge There was a 6 week follow‐up period All women were postcesarean birth with prophylactic antibiotics Pharmaceutical sponsorship ‐ explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization schedule |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs ‐ none |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | High risk | Pharmaceutical sponsorship ‐ evident |
Tuomala 1989.
| Methods | Randomized trial Study period: January 1982 through November 1984 | |
| Participants | Inclusion criteria: women with postpartum endometritis (meeting 2 of the following criteria: temperature at least 101 °F (38.3 °C), uterine tenderness, foul‐smelling lochia) Setting: Boston, Massachusetts, USA Number of participants: n = 50 | |
| Interventions | Ampicillin 3 g iv every (n = 25) vs cefotaxime 2 g iv every 6 hours (n = 25) | |
| Outcomes | Clinical cure or improvement Pelvic abscess Length of stay | |
| Notes | 13 vaginal births evenly distributed between groups 5 of the 7 women who failed treatment had received cefoxitin prophylaxis at the time of cesarean birth Pharmaceutical sponsorship ‐ probable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “randomly assigned” in abstract but no further detail description available |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States “All doses were blinded to participants, study personnel, physicians, and nursing staff.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States “All doses were blinded to participants, study personnel, physicians, and nursing staff.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 45 (90%) analyzed |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available, insufficient information to permit judgment |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
Watts 1989.
| Methods | Randomized trial Study period: February 1980 through February 1983 |
|
| Participants | Inclusion criteria: patients with early postpartum endometritis with elevated temperature (≥ 38.5 °C); abdominal pain; abdominal, uterine and adnexal tenderness and no other apparent source of fever outside of the genital tract Setting: University of Washington Hospital, Seattle, Washington, USA |
|
| Interventions | Intravenous beta‐lactam (n not stated) vs clindamycin‐gentamicin (n not stated) | |
| Outcomes | Presence of bacteria and site of isolation | |
| Notes | This study did not address the effect of antibiotic regimen in reference to treatment failure, rather it focused on the isolated microbes as well as associated complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated, but not likely to change microbial isolate |
| Allocation concealment (selection bias) | Unclear risk | Method not stated, but not likely to change microbial isolate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinding noted, but not likely to change outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs noted |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes are prespecified in the materials and methods section, although this would not have an effect on this systematic review as the outcomes are not consistent with other studies |
| Other bias | Unclear risk | Not clear |
IM: intramuscular iv: intravenous SD: standard deviation vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 1988 | Pseudorandomization methodology based on odd or even year of birth |
| Berkeley 1986 | Postpartum women not identified, postpartum endometritis not defined |
| Briggs 1989 | This study compared 2 approaches to thrice‐daily dosing for gentamicin, based on calculated body mass versus adjustments based on peak and trough serum measurements, and compared 2 different dosing regimens. Although outcomes measured included nephrotoxicity, hospital stay, duration of treatment and costs, treatment failures were not reported |
| Crombleholme 1987 | Of the 44 women enrolled in this study, only 5 women had endomyometritis; the results for this group were not given separately |
| Cunningham 1978 | Pseudorandomization methodology based on last digit of medical record number |
| Dinsmoor 1991 | Exclusions after randomization were more than 20% |
| Duff 1982 | Pseudorandomization methodology based on odd or even medical record number |
| Faro 1987a | Exclusions after randomization were more than 20% |
| Faro 1987b | Exclusions after randomization were more than 20% in the control group |
| Fernandez 1993 | This was not a study of treatment of postpartum endometritis, but a study of antibiotic prophylaxis for vaginal birth to prevent postpartum endometritis |
| Gall 1981 | Eligible participant included women with postpartum endometritis (31/47) as well as pelvic inflammatory disease and postoperative infection; outcomes, however, were not given for the endometritis group separately |
| Gonik 1992 | Antibiotic regimens' dose and frequency were not described |
| Hemsell 1988 | This study included postpartum women. However, endometritis was not defined, and women treated for endometritis were not analyzed separately |
| Hemsell 1997 | Exclusions were more than 20% after randomization |
| Knuppel 1988 | Participants not identified as postpartum. Postpartum endometritis not defined |
| Kreutner 1979 | Study of prophylaxis rather than treatment of postpartum endometritis |
| Lancheros 1997 | This is a published abstract. The number of women in each treatment group was not given |
| Malik 1996 | This study looked at rates of endometritis in women with premature rupture of membranes, rather than treatment of postpartum endometritis |
| Marshall 1982 | Postpartum women not identified |
| Pastorek 1987a | Observational study |
| Pastorfide 1987 | Not a study of treatment of postpartum endometritis |
| Perry 1999 | Participants were randomized to receive either high‐ or low‐dose ampicillin/sulbactam; this study has not been included because of the similarity of these regimens |
| Pond 1979 | Pseudorandomization methodology based on odd or even medical record number |
| Resnik 1994 | Exclusions after randomization were more than 20% in the control group |
| Rosene 1986 | Actual numbers not provided |
| Sen 1980 | Exclusions after randomization were more than 20% |
| Sorrell 1981 | Exclusions after randomization were more than 20% |
| Sweet 1988 | Participants not identified as postpartum. Postpartum endometritis not defined |
| Turnquest 1998 | Study of prevention (prophylaxis) rather than treatment |
| Wager 1980 | Not randomized |
Differences between protocol and review
Four outcomes, not previously specified, were added for this 2015 update:
wound infection;
recurrent endometritis;
nephrotoxicity;
treatment failure despite administration of prophylactic antibiotics for cesarean.
Contributions of authors
Erika Ota and Roger E Packard independently rated all the included studies for the risk of bias tables from the previous review and also applied the study selection criteria and abstracted data from the included studies for updates. A Dhanya Mackeen, Roger E Packard and Erika Ota revised the manuscript. A Dhanya Mackeen, Roger E Packard and Erika Ota reconfirmed that previously entered data had been correctly abstracted and changed data entry as necessary. Linda Speer developed the original review (French 2004). All the authors read and approved the final version to be published. A Dhanya Mackeen is the guarantor of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
A Dhanya Mackeen: none known. Roger E Packard: none known. Erika Ota: none known. Linda Speer: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Apuzzio 1985a {published data only}
- Apuzzio JJ, Kaminski Z, Gamesh V, Louria DB. Comparative clinical evaluation of ticarcillin plus clavulanic acid versus clindamycin plus gentamicin in treatment of post‐cesarean endomyometritis. American Journal of Medicine 1985;79(Suppl 5B):164‐7. [DOI] [PubMed] [Google Scholar]
Apuzzio 1985b {published data only}
- Apuzzio JJ, Ganesh V, Pelosi MA, Landau I, Kaminetzky HA, Kaminski Z, et al. Comparative clinical evaluation of ceftizoxime with clindamycin and gentamicin and cefoxitin in the treatment of postcesarean endomyometritis. Surgery, Gynecology and Obstetrics 1985;161:518‐22. [PubMed] [Google Scholar]
Blanco 1983 {published data only}
- Blanco JD, Gibbs RS, Duff P, Casteneda YS, Clair PJ. Randomized comparison of ceftazadime versus clindamycin ‐tobramycin in the treatment of obstetrical and gynecological infections. Antimicrobial Agents and Chemotherapy 1983;24:500‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatwani 1995 {published data only}
- Chatwani A, Martens M, Grimes DA, Chatterjee M, Noah M, Stamp‐Cole MM, et al. Single‐blind, prospective, randomized study of cefmetazole and cefoxitin in the treatment of postcesarean endometritis. Infectious Diseases in Obstetrics and Gynecology 1995;3:28‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatwani 1997 {published data only}
- Chatwani A, Martens M, Blanco J, Gall S, Przybylko K, Wajszczuk CP, et al. Double‐blind, multicenter, prospective randomized study of trospectomycin versus clindamycin, both with aztreonam, in non‐community acquired obstetric and gynecologic infections. Infectious Diseases in Obstetrics and Gynecology 1997;5:280‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Priore 1996 {published data only}
- Priore G, Jackson‐Stone M, Shim EK, Garfinkel J, Eichmann MA, Frederiksen MC. A comparison of once‐daily and 8‐hour gentamicin dosing in the treatment of postpartum endometritis. Obstetrics & Gynecology 1996;87(6):994‐1000. [DOI] [PubMed] [Google Scholar]
DiZerega 1979 {published data only}
- diZerega G, Yonekura L, Roy S, Nakamura RM, Ledger WJ. A comparison of clindamycin‐gentamicin and penicillin‐gentamicin in the treatment of post‐cesarean section endomyometritis. American Journal of Obstetrics and Gynecology 1979;134:238‐41. [DOI] [PubMed] [Google Scholar]
Faro 1989 {published data only}
- Faro S, Martens M, Hammill H, Phillips LE, Smith D, Riddle G. Ticarcillin/clavulanic acid versus clindamycin and gentamicin in the treatment of post‐cesarean endometritis following antibiotic prophylaxis. Obstetrics & Gynecology 1989;73(5):808‐12. [PubMed] [Google Scholar]
- Faro S, Martens M, Phillips LE, Hammill H, Smith D, Riddle G. Ticarcillin disodium/clavulanate potassium versus clindamycin/gentamycin in the treatment of postpartum endometritis. Journal of Reproductive Medicine 1988;33(6):603‐6. [PubMed] [Google Scholar]
Fernandez 1990 {published data only}
- Fernandez H, Claquin C, Guibert M, Papiernik E. Suspected postpartum endometritis: a controlled clinical trial of single‐agent therapy with amox‐CA (Augmentin) versus ampicillin‐metronidazole +/‐ aminoglycoside. European Journal of Obstetrics & Gynecology and Reproductive Biology 1990;36:69‐74. [DOI] [PubMed] [Google Scholar]
Figueroa‐Damian 1996 {published data only}
- Figueroa‐Damian R, Villagrana‐Zesati R, San Martin‐Herrasti JM, Arredondo‐Garcia JL. Comparison of therapeutic efficacy of piperacillin/tazaobactam versus ampicillin plus gentamicin in the treatment of postcesarean endometritis [Comparacion de la eficacia terapeutica de piperacilina/tazobactam versus ampicilina mas gentamicina en el tratamiento de endometritis poscesarea]. Ginecologia y Obstetricia de Mexico 1996;64:214‐8. [PubMed] [Google Scholar]
Filler 1992 {published data only}
- Filler L, Shipley CF, Dennis EJ, Nelson GH. Postcesarean endometritis: a brief review and comparison of three antibiotic regimens. Journal of the South Carolina Medical Association 1992;88:291‐5. [PubMed] [Google Scholar]
Gaitan 1995 {published data only}
- Gaitan H, Troisi T, Lancheros S, Saravia J, Pardo G. Open randomized controlled clinical study of the efficacy of gentamycin‐clindamycin vs. pefloxacin‐metronidazole in the treatment of post‐cesarean section endometritis. Revista Colombiana de Obstetricia y Ginecologia 1995;46:165‐72. [Google Scholar]
Gall 1996 {published data only}
- Gall S, Koukol DH. Ampicillin/sulbactam versus clindamycin/gentamicin in the treatment of postpartum endometritis. Journal of Reproductive Medicine 1996;41:575‐80. [PubMed] [Google Scholar]
Gibbs 1982 {published data only}
- Gibbs RS, Blanco JD, Casteneda YS, Clair PJ. A double‐blind, randomized comparison of clindamycin‐gentamicin versus cefamandole for treatment of post‐cesarean section endometritis. American Journal of Obstetrics and Gynecology 1982;144:261‐7. [DOI] [PubMed] [Google Scholar]
- Gibbs RS, Blanco JD, Clair PJ, Castaneda YS. Vaginal colonization with resistant aerobic bacteria after antibiotic therapy for endometritis. American Journal of Obstetrics and Gynecology 1982;142:130‐4. [DOI] [PubMed] [Google Scholar]
Gibbs 1983 {published data only}
- Gibbs RS, Blanco JD, Duff P, Casteneda YS, Clair PJ. A double‐blind, randomized comparison of moxalactam vs clindamycin‐gentamicin in treatment of endomyometritis after cesarean section delivery. American Journal of Obstetrics and Gynecology 1983;146:769‐72. [DOI] [PubMed] [Google Scholar]
Gibbs 1985 {published data only}
- Gibbs RS, Blanco JD, Lipscomb KA, Clair PJ. Aztreonam versus gentamicin, each with clindamycin, in the treatment of endometritis. Obstetrics & Gynecology 1985;65(6):825‐9. [PubMed] [Google Scholar]
Greenberg 1987 {published data only}
- Greenberg RN, Reilly PM, Weinandt WJ, Wilson KM, Bollinger M, Ojile JM. Comparison trial of clindamycin with aztreonam or gentamicin in the treatment of postpartum endometritis. Clinical Therapeutics 1987;10(1):36‐9. [PubMed] [Google Scholar]
Gutierrez 1994 {published data only}
- Gutierrez C, Carrillo C, Escudero F, Caciano S, Hjarles MG. Treatment of puerperal endometritis: Evaluation of efficacy and safety of clindamycin + gentamicin vs. penicillin + chloramphenicol + gentamicin. Ginecologia y Obstetricia de Mexico 1994;62:345‐53. [PubMed] [Google Scholar]
Hager 1989 {published data only}
- Hager WD, Pascuzzi M, Vernon M. Efficacy of oral antibiotics for serious infections in obstetrics and gynecology. Obstetrics & Gynecology 1989;73:326‐9. [PubMed] [Google Scholar]
Hemsell 1983 {published data only}
- Hemsell DL, Cunningham G, Nolan CM, Miller TT. Clinical experience with cefotaxime in obstetric and gynecologic infections. Reviews of Infectious Diseases 1982;4:432‐8. [DOI] [PubMed] [Google Scholar]
- Hemsell DL, Cunnningham FG, DePalma RT, Nobles BJ, Heard M, Hemsell PG. Cefotaxime sodium therapy for endomyometritis following cesarean section: dose‐finding and comparative studies. Obstetrics & Gynecology 1983;62:489‐97. [PubMed] [Google Scholar]
Herman 1986 {published data only}
- Herman G, Cohen AW, Talbot GH, Coghlan R, Faidley‐Mangen P, MacGregor RR. Cefoxitin versus clindamycin and gentamicin in the treatment of postcesarean section infections. Obstetrics & Gynecology 1986;67:371‐6. [PubMed] [Google Scholar]
Hillier 1990 {published data only}
- Hillier S, Watts DH, Lee MF, Eschenbach DA. Etiology and treatment of post‐cesarean section endometritis after cephalosporin prophylaxis. Journal of Reproductive Medicine 1990;35(3 Suppl):322‐8. [PubMed] [Google Scholar]
Knodel 1988 {published data only}
- Knodel LC, Goldspiel BR, Gibbs RS. Prospective cost analysis of moxalactam versus clindamycin plus gentamicin for endomyometritis after cesarean section. Antimicrobial Agents and Chemotherapy 1988;32:853‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ledger 1974 {published data only}
- Ledger WJ, Kriewall TJ, Sweet RL, Fekety FR. The use of parenteral clindamycin in the treatment of obstetric‐gynecologic patients with severe infections. Obstetrics & Gynecology 1974;43:490‐7. [PubMed] [Google Scholar]
Livingston 2003 {published data only}
- Livingston J, Llata E, Rinehart E, Leidwnager C, Mabie B, Haddad B, et al. Gentamicin and clindamycin for postpartum endometritis: the efficacy of daily dosing versus dosing every 8 hours. American Journal of Obstetrics and Gynecology 2003;188:149‐52. [DOI] [PubMed] [Google Scholar]
- Livingston JC, Llata E, Rinehart E, Leidwanger C, Mabie B, Haddad B, et al. Gentamicin and clindamycin therapy in postpartum endometritis: the efficacy of daily dosing versus dosing every 8 hours [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S169. [DOI] [PubMed] [Google Scholar]
Maccato 1991 {published data only}
- Maccato ML, Faro S, Martens MG, Hammill HA. Ciprofloxacin versus gentamicin/clindamycin for postpartum endometritis. Journal of Reproductive Medicine 1991;36(12):857‐61. [PubMed] [Google Scholar]
MacGregor 1992 {published data only}
- MacGregor RR, Graziani AL, Samuels P. Randomized, double‐blind study of cefotetan and cefoxitin in post‐cesarean section endometritis. American Journal of Obstetrics and Gynecology 1992;167(1):139‐43. [DOI] [PubMed] [Google Scholar]
Martens 1989 {published data only}
- Martens MK, Faro S, Hammill HA, Smith D, Riddle G, Maccato M. Sulbactam/ampicillin versus metronidazole/gentamicin in the treatment of post‐cesarean section endometritis. Diagnostic Microbiology and Infectious Diseases 1989;12:189S‐94S. [DOI] [PubMed] [Google Scholar]
Martens 1990 {published data only}
- Martens MG, Faro S, Hammill HA, Maccato M, Riddle G, Smith D. Ampicillin/sulbactam versus clindamycin in the treatment of postpartum endomyometritis. Southern Medical Journal 1989;82:39. [DOI] [PubMed] [Google Scholar]
- Martens MG, Faro S, Hammill HA, Smith D, Riddle G, Maccato M. Ampicillin/sulbactam versus clindamycin in the treatment of postpartum endomyometritis. Southern Medical Journal 1990;83:408‐13. [DOI] [PubMed] [Google Scholar]
McGregor 1989 {published data only}
- McGregor JA, Christensen FB. A comparison of ampicillin plus sulbactam versus clindamycin and gentamicin for treatment of postpartum infection. International Journal of Obstetrics & Gynecology 1989;2 Suppl:35‐9. [DOI] [PubMed] [Google Scholar]
Mitra 1997 {published data only}
- Mitra AG, Whitten MK, Laurent SL, Anderson WE. A randomized prospective study comparing once‐daily gentamicin versus thrice‐daily gentamicin in the treatment of puerperal infection. American Journal of Obstetrics and Gynecology 1997;177:786‐92. [DOI] [PubMed] [Google Scholar]
- Whitten K, Mitra A, Laurent S, Anderson B. A randomized, prospective study comparing once daily gentamycin with thrice daily gentamycin in the treatment of puerperal endometritis. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S32. [DOI] [PubMed] [Google Scholar]
Morales 1989 {published data only}
- Morales WJ, Collins EM, Angel JL, Knuppel RA. Short course of antibiotic therapy in treatment of postpartum endomyometritis. American Journal of Obstetrics and Gynecology 1989;161:568‐72. [DOI] [PubMed] [Google Scholar]
Pastorek 1987 {published data only}
- Pastorek JG, Faro S, Aldridge KE, Nicaud SK. Moxalactam versus clindamycin plus tobramycin for the treatment of puerperal infections. Southern Medical Journal 1987;80:1116‐9. [DOI] [PubMed] [Google Scholar]
Perry 1997 {published data only}
- Perry KJ, Theilman GD, Coker KA, Martin JN. A prospective randomized trial comparing gentamicin administered every 24 hours for the treatment of postcesarean section endometritis. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S59. [Google Scholar]
Pietrantoni 1998 {published data only}
- Pietrantoni M, Goss S, Gall SA. A prospective, randomized, clinical study to compare the clinical safety, effectiveness, and cost of oral ofloxacin/clindamycin vs intravenous clindamycin/gentamicin for the treatment of postpartum endomyometritis [abstract]. Primary Care Update for Ob/Gyns 1998;5(4):146‐7. [DOI] [PubMed] [Google Scholar]
Rodriguez 1996 {published data only}
- Rodriguez‐Ballesteros R, Alarcon‐Aburto VM, Madrigal‐de la Campa MLA, Valerio‐Castro E. Short‐term antibiotic therapy of post‐cesarean section endometritis [Esquema corto de antibioticos en el tratamiento de endometritis posterior a cesarea]. Ginecologia y Obstetricia de Mexico 1996;64:359‐62. [PubMed] [Google Scholar]
Roy 2003 {published data only}
- Roy S, Higareda I, Angel‐Muller E, Ismail M, Hague C, Adeyi B, et al. Ertapenem once a day versus piperacillin‐tazobactam every 6 hours for treatment of acute pelvic infections: a prospective, multicenter, randomized, double‐blind study. Infectious Diseases in Obstetrics and Gynecology 2003;11(1):27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scalambrino 1989 {published data only}
- Scalambrino S, Mangioni C, Milani R, Regallo M, Norchi S, Negri L, et al. Sulbactam/ampicillin versus cefotetan in the treatment of obstetric and gynecologic infections. International Journal of Obstetrics & Gynecology 1989;2 Suppl:21‐7. [DOI] [PubMed] [Google Scholar]
Soper 1992 {published data only}
- Soper DE, Brockwell NJ, Dalton HP. The importance of wound infection in antibiotic failures in the therapy of postpartum endometritis. Surgery, Gynecology and Obstetrics 1992;174:265‐9. [PubMed] [Google Scholar]
Stovall 1993 {published data only}
- Stovall TG, Thorpe EM, Ling FW. Treatment of post‐cesarean section endometritis with ampicillin and sulbactam or clindamycin and gentamicin. Journal of Reproductive Medicine 1993;38(11):843‐8. [PubMed] [Google Scholar]
Tuomala 1989 {published data only}
- Tuomala RE, Stoukides CA, Fischer S, Souney PF, Steele L, Mutnick AH. Comparison of the relative efficacy and hospital charges in patients treated with ampicillin or cefotaxime for postpartum endometritis. Current Therapeutic Research, Clinical and Experimental 1989;46:292‐302. [Google Scholar]
Watts 1989 {published data only}
- Watts DH, Eschenbach DA, Kenny G. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstetrics & Gynecology 1989;73(1):52‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez 1988 {published data only}
- Alvarez RD, Kilgore LC, Huddleston JF. A comparison of mezlocillin versus clindamycin/gentamicin for the treatment of postcesarean endomyometritis. American Journal of Obstetrics and Gynecology 1988;158:425‐9. [DOI] [PubMed] [Google Scholar]
Berkeley 1986 {published data only}
- Berkeley AS, Freedman KS, Hirsch JC, Ledger WJ. Randomized, comparative trial of imipenem/cilastatin and moxalactam in the treatment of serious obstetric and gynecologic infections. Surgery, Gynecology and Obstetrics 1986;162:204‐8. [PubMed] [Google Scholar]
Briggs 1989 {published data only}
- Briggs GG, Ambrose P, Nageotte MP. Gentamicin dosing in postpartum women with endometritis. American Journal of Obstetrics and Gynecology 1989;160(2):309‐13. [DOI] [PubMed] [Google Scholar]
Crombleholme 1987 {published data only}
- Crombleholme WR, Landers D, Ohm‐Smith M, Robbie MO, Hadley WK, DeKay V. Sulbactam‐ampicillin versus metronidazole‐gentamicin in the treatment of obstetric and gynecologic infections. Archives of Gynecology 1985;237(Suppl 1):44. [Google Scholar]
- Crombleholme WR, Ohm‐Smith M, Robbie MO, DeKay V, Sweet RL. Ampicillin/sulbactam versus metronidazole‐gentamicin in the treatment of soft tissue pelvic infections. American Journal of Obstetrics and Gynecology 1987;156:507‐12. [DOI] [PubMed] [Google Scholar]
Cunningham 1978 {published data only}
- Cunningham FG, Hauth JC, Strong JD, Kappus SS. Infectious morbidity following cesarean section. Comparison of two treatment regimens. Obstetrics & Gynecology 1978;52:656‐61. [PubMed] [Google Scholar]
Dinsmoor 1991 {published data only}
- Dinsmoor MJ, Newton ER, Gibbs RS. A randomized, double‐blind, placebo‐controlled trial of oral antibiotic therapy following intravenous antibiotic therapy for postpartum endometritis. Obstetrics & Gynecology 1991;77(1):60‐2. [PubMed] [Google Scholar]
Duff 1982 {published data only}
- Duff P, Keiser JF, Strong SL. A comparative study of two antibiotic regimens for the treatment of operative site infections. American Journal of Obstetrics and Gynecology 1982;142:996‐1003. [DOI] [PubMed] [Google Scholar]
Faro 1987a {published data only}
- Faro S, Phillips LE, Baker JL, Goodrich KH, Turner RM, Riddle G. Comparative efficacy and safety of mezlocillin, cefoxitin, and clindamycin plus gentamicin in postpartum endometritis. Obstetrics & Gynecology 1987;69(5):760‐6. [PubMed] [Google Scholar]
Faro 1987b {published data only}
- Faro S, Pastorek JG, Aldridge KE, Martens M, Phillips LE, Nicaud S, et al. Piperacillin versus clindamycin plus gentamicin in the treatment of postpartum endometritis. Current Therapeutic Research, Clinical and Experimental 1987;42:995‐1002. [Google Scholar]
Fernandez 1993 {published data only}
- Fernandez H, Gagnepain A, Bourget P, Peray P, Frydman R, Paiernik E, et al. Antibiotic prophylaxis against postpartum endometritis after vaginal delivery: a prospective randomized comparison between amox‐CA (Augmentin) and abstention. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;50:169‐75. [DOI] [PubMed] [Google Scholar]
Gall 1981 {published data only}
- Gall SA, Kohan AP, Ayers OM, Hughes CE, Addison WA, Hill GB. Intravenous metronidazole or clindamycin with tobramycin for therapy of pelvic infections. Obstetrics & Gynecology 1981;57:51‐8. [PubMed] [Google Scholar]
Gonik 1992 {published data only}
- Gonik B. Postpartum endometritis: efficacy and tolerability of two antibiotic regimens. Clinical Therapeutics 1992;14:83‐9. [PubMed] [Google Scholar]
Hemsell 1988 {published data only}
- Hemsell DL, Wendel GD, Gall SA, Newton ER, Gibbs RS, Knuppel RA, et al. Multicenter comparison of cefotetan and cefoxitin in the treatment of acute obstetric and gynecologic infections. American Journal of Obstetrics and Gynecology 1988;158:722‐7. [DOI] [PubMed] [Google Scholar]
Hemsell 1997 {published data only}
- Hemsell DL, Martens MG, Faro S, Gall S, McGregor JA. A multicenter study comparing intravenous meropenem with clindamycin plus gentamicin for the treatment of acute gynecologic and obstetric infections in hospitalized women. Clinical Infectious Diseases 1997;24(Suppl 2):S222‐S230. [DOI] [PubMed] [Google Scholar]
Knuppel 1988 {published data only}
- Knuppel RA, O'Bryan D, Lake M. Cefotetan: comparative and noncomparative studies in obstetric and gynecologic infections. Southern Medical Journal 1988;81:185‐8. [PubMed] [Google Scholar]
Kreutner 1979 {published data only}
- Kruetner AK, Bene VE, Delamar D, Bodden JL, Loadholt CB. Perioperative cephalosporin prophylaxis in cesarean section: effect of endometritis in the high‐risk patient. American Journal of Obstetrics and Gynecology 1979;134(8):925‐35. [DOI] [PubMed] [Google Scholar]
Lancheros 1997 {published data only}
- Lancheros S, Gaitan H. Comparison between two therapeutic schedules: gentamicin plus clindamicin and pefloxacin plus metronidazole in post‐cesarean endometritis treatment. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167 Suppl):28. [Google Scholar]
Malik 1996 {published data only}
- Malik N, Gittens L, Gonzalez D, Bardeguez A, Ganesh V, Apuzzio J. Clinical amnionitis and endometritis in patients with premature rupture of membranes: endocervical prostaglandin E2 gel versus oxytocin for induction of labor. Obstetrics & Gynecology 1996;88(4):540‐3. [DOI] [PubMed] [Google Scholar]
Marshall 1982 {published data only}
- Marshall JR, Chow AW, Sorrell TC. Effectiveness of mezlocillin in female genital tract infections. Journal of Antimicrobal Chemotherapy 1982;9(Suppl A):149‐58. [DOI] [PubMed] [Google Scholar]
Pastorek 1987a {published data only}
- Pastorek JG, Ragan FA, Phelan M. Tobramycin dosing in the puerperal patient. Journal of Reproductive Medicine 1987;32:343‐6. [PubMed] [Google Scholar]
Pastorfide 1987 {published data only}
- Pastorfide GB, Gorgonio NM, Ganzon AR, Alberto RMN. Zinc chloride spray‐magnesium hydroxide ointment dual topical regimen in the treatment of obstetric and gynecologic incisional wounds. Clinical Therapeutics 1989;11:258‐63. [PubMed] [Google Scholar]
Perry 1999 {published data only}
- Perry KG, Larmon JE, Cadle JF, Isler CM, Martin RW. A randomized, double‐blind comparison of ampicillin/sulbactam at 1.5g and 3.0g doses for the treatment of postpartum endometritis. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S76. [Google Scholar]
Pond 1979 {published data only}
- Pond DG, Bernstein PE, Love KR, Morgan JR, Velland H, Smith JA. Comparison of ampicillin with clindamycin plus gentamicin in the treatment of postpartum uterine infection. Canadian Medical Association Journal 1979;120:533‐7. [PMC free article] [PubMed] [Google Scholar]
Resnik 1994 {published data only}
- Resnik E, Harger JH, Kuller JA. Early postpartum endometritis. Randomized comparison of ampicillin/sulbactam vs. ampicillin, gentamicin and clindamycin. Journal of Reproductive Medicine 1994;39:467‐72. [PubMed] [Google Scholar]
Rosene 1986 {published data only}
- Rosene K, Eschenbach DA, Tompkins LS, Kenny GE, Watkins H. Polymicrobial early postpartum endometritis with facultative and anaerobic bacteria, genital mycoplasmas, and chlamydia trachomatis: treatment with piperacillin or cefoxitin. Journal of Infectious Diseases 1986;153:1028‐37. [DOI] [PubMed] [Google Scholar]
Sen 1980 {published data only}
- Sen P, Apuzzio J, Reyelt C, Kaminski T, Levy F, Kapila R, et al. Prospective evaluation of combinations of antimicrobial agents for endometritis after cesarean section. Surgery, Gynecology and Obstetrics 1980;151:89‐92. [PubMed] [Google Scholar]
Sorrell 1981 {published data only}
- Sorrell TC, Marshall JR, Yoshimori R, Chow AW. Antimicrobial therapy of postpartum endometritis. II. Prospective randomized trial of mezlocillin vs ampicillin. American Journal of Obstetrics and Gynecology 1981;141:246‐51. [DOI] [PubMed] [Google Scholar]
Sweet 1988 {published data only}
- Sweet RL, Gall SA, Gibbs RS, Hemsell DL, Knuppel RA, Lane TW, et al. Multicenter clinical trials comparing cefotetan with moxalactam or cefoxitin as therapy for obstetric and gynecologic infections. American Journal of Surgery 1988;155(5A):56‐60. [DOI] [PubMed] [Google Scholar]
Turnquest 1998 {published data only}
- Turnquest MA, How HY, Cook CR, O'Rourke P, Cureton AC, Spinnato JA, et al. Chorioamnionitis: is continuation of antibiotic therapy necessary after cesarean section?. Obstetrics & Gynecology 1998;179:1261‐6. [DOI] [PubMed] [Google Scholar]
Wager 1980 {published data only}
- Wager GP, Martin DH, Koutsky L, Eschenbach DA, Daling JR, Chiang WT, et al. Puerperal infectious morbidity: relationship to route of delivery and to antepartum chlamydia trachomatis infection. American Journal of Obstetrics and Gynecology 1980;138(7):1028‐33. [DOI] [PubMed] [Google Scholar]
Additional references
Aldridge 2002
- Aldridge KE, O'Brien M. In vitro susceptibilities of the Bacteroides fragilis group species: change in isolation rates significantly affects overall susceptibility data. Journal of Clinical Microbiology 2002;40(11):4349‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barza 1996
- Barza M, Ioannidis JP, Cappelleri JC, Lau J. Single or multiple daily doses of aminoglycosides: a meta‐analysis. BMJ 1996;312:338‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calhoun 1995
- Calhoun B, Brost B. Emergency management of sudden puerperal fever. Obstetrics and Gynecology Clinics of North America 1995;22:357‐67. [PubMed] [Google Scholar]
Gibbs 1980
- Gibbs RS. Clinical risk factors for puerperal infection. Obstetrics and Gynecology 1980;55(5 Suppl):178S‐184S. [PUBMED: 6990333] [DOI] [PubMed] [Google Scholar]
Gyte 2014
- Gyte GM, Dou L, Vazquez JC. Different classes of antibiotics given to women routinely for preventing infection at caesarean section. The Cochrane database of systematic reviews 2014;11:CD008726. [PUBMED: 25402227] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jacobsson 2002
- Jacobsson B, Pernevi P, Chidekel L, Jorgen Platz‐Christensen J. Bacterial vaginosis in early pregnancy may predispose for preterm birth and postpartum endometritis. Acta Obstetricia et Gynecologica Scandinavica 2002;81(11):1006‐10. [PUBMED: 12421167] [DOI] [PubMed] [Google Scholar]
Monga 1993
- Monga M, Oshiro B. Puerperal infections. Seminars in Perinatology 1993;17:426‐31. [PubMed] [Google Scholar]
Newton 1990
- Newton ER, Prihoda TJ, Gibbs RS. A clinical and microbiologic analysis of risk factors for puerperal endometritis. Obstetrics & Gynecology 1990;75(3):402‐6. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Solomkin 2003
- Solomkin JS, Mazuski JE, Baron EJ, Sawyer RG, Nathens AB, DiPiro JT, et al. Guidelines for the selection of anti‐infective agents for complicated intra‐abdominal infections. Clinical Infectious Diseases 2003;37:997‐1005. [DOI] [PubMed] [Google Scholar]
Williams 1995
- Williams KL, Pastorek JG. Postpartum endometritis. Infectious Diseases in Obstetrics and Gynecology 1995;3:210‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
French 2004
- French L, Smaill FM. Antibiotic regimens for endometritis after delivery. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD001067.pub2] [DOI] [PubMed] [Google Scholar]


