Abstract
Background
While the use of supplemental oxygen has a long history in neonatal care, resulting in both significant health care benefits and harms, uncertainty remains as to the most appropriate range to target blood oxygen levels in preterm and low birth weight infants. Potential benefits of higher oxygen targeting may include more stable sleep patterns and improved long‐term growth and development. However, there may be significant deleterious pulmonary effects and health service use implications resulting from such a policy.
Objectives
To determine whether targeting ambient oxygen concentration to achieve a lower vs. higher blood oxygen range, or administering restricted vs. liberal supplemental oxygen, effects mortality, retinopathy of prematurity, lung function, growth or development in preterm or low birth weight infants.
Search methods
The standard search strategy of the Neonatal Review Group was used. An additional literature search was conducted of the MEDLINE and CINAHL databases in order to locate any trials in addition to those provided by the Cochrane Controlled Trials Register (CENTRAL/CCTR). Search updated to week two July 2008.
Selection criteria
All trials in preterm or low birth weight infants utilising random or quasi‐random patient allocation in which ambient oxygen concentrations were targeted to achieve a lower vs. higher blood oxygen range, or restricted vs. liberal oxygen was administered were eligible for inclusion.
Data collection and analysis
The methodological quality of the eligible trials was assessed independently by each review author for the degree of selection, performance, attrition and detection bias. Data were extracted and reviewed independently by the each author. Data analysis was conducted according to the standards of the Cochrane Neonatal Review Group.
Main results
In the meta‐analysis of the five trials included in this review, the restriction of oxygen significantly reduced the incidence and severity of retinopathy of prematurity without unduly increasing death rates The one prospective, multicenter, double‐blind, randomized trial investigating lower vs. higher blood oxygen levels from 32 weeks postmenstrual age showed no significant differences in the rates of ROP, mortality or growth and development between the two groups. However, this study did show increased rates of chronic lung disease and home oxygen use.
Authors' conclusions
The results of this systematic review confirm that (the now historical) policy of unrestricted, unmonitored oxygen therapy has potential harms without clear benefits. However, the question of what is the optimal target range for maintaining blood oxygen levels in preterm/LBW infants was not answered by the data available for inclusion in this review.
Plain language summary
Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants
Restricting oxygen supplementation significantly reduces the rate and severity of vision problems (retinopathy) in premature and low birth weight babies. Babies born either prematurely (before 37 weeks) or with a low birth weight often have breathing problems and need extra oxygen. Oxygen supplementation has provided many benefits for these babies but can cause damage to the eyes (retinopathy) and lungs. The review of trials found that unrestricted oxygen supplementation has these potential adverse effects without any clear benefits. Restricted oxygen significantly reduces these risks. More research is needed to find the best level of oxygen supplementation.
Background
The administration of supplemental oxygen has a long history in neonatal care (Wilson 1942). The use of oxygen in preterm and low birth weight infants suffering respiratory insufficiency has resulted in significant health care benefits, such as reduced mortality and spastic diplegia (Avery 1960; McDonald 1963), but has also been associated with significant deleterious effects such as retinopathy of prematurity and lung toxicity (Duc 1992).
Improvements in technology in the past few decades have led to both the increased survival of preterm and low birth weight infants and an ability to measure their oxygen levels more accurately. Despite the exceedingly common use of supplemental oxygen in this population of infants, there is little consensus as to the optimal mode of administration and appropriate levels of oxygen for maximising short or long‐term growth and development, while minimising harmful effects (Poets 1998; McIntosh 2001).
Uncertainty remains as to the most appropriate range to target blood oxygen levels in preterm and low birth weight infants. Usher (Usher 1973) examined the effect of targeting a lower vs. higher range of PaO2 on death, the need for mechanical ventilation and other clinical outcomes and concluded there was no benefit in targeting a higher range, and there may in fact be deleterious respiratory effects (Coates 1982). A cohort study by Tin et al (Tin 2001) also suggested an increase in adverse respiratory outcomes and a significant increase in the incidence of ROP occurred when higher oxygen ranges were targeted in preterm infants. However, Phelps and Rosenbaum (Phelps 1984) demonstrated significantly more severe retinopathy in kittens recovering from hyperoxic‐induced disease when allowed to recover in lower levels of ambient oxygen, suggesting that targeting higher blood oxygen levels may be beneficial to visual outcomes. The STOP‐ROP trial (STOP‐ROP 2000) found that higher oxygen targeting did not significantly decrease the incidence of pre‐threshold ROP progression, but did cause an exacerbation of adverse pulmonary events. The results of this trial are included in a separate Cochrane review entitled: "Supplemental oxygen for the treatment of pre‐threshold retinopathy of prematurity" (Lloyd 2003). The effects of either policy of oxygen administration on long‐term growth and development in preterm or low birth weight infants remains uncertain.
Two related Cochrane reviews have summarised the findings on gradual vs. abrupt (Askie 2001a) and early vs. late discontinuation of oxygen therapy (Askie 2001b) in preterm or low birth weight infants.
Objectives
To determine whether targeting ambient oxygen concentration to achieve a lower vs. higher blood oxygen range, or administering restricted vs. liberal supplemental oxygen effects mortality, retinopathy of prematurity, lung function, growth or development in preterm or low birth weight infants.
A priori sub‐group analyses:
Method of oxygen monitoring. Infants born at different gestational age and birth weight subgroups: as there are differing baseline risks of the outcome measures in these subgroups. Time of discontinuation: early vs. late discontinuation as this is hypothesized to influence outcome measures (Gunn 1980). Method of discontinuation: gradual vs. abrupt discontinuation as this is hypothesized to influence outcome measures (Chan‐Ling 1995)
Methods
Criteria for considering studies for this review
Types of studies
Trials utilising random or quasi‐random patient allocation were eligible for inclusion.
Types of participants
Preterm (< 37 weeks gestation) or low birth weight (< 2500 g) infants receiving supplemental oxygen.
Types of interventions
Restricted vs. liberal administration of supplemental oxygen; or targeting a lower vs. higher range of blood oxygen levels.
Types of outcome measures
Retinopathy of prematurity (ROP) ‐ any, severe (stage 3 or greater)
Mortality ‐ any, early neonatal period (< 1 week postnatal age), later neonatal period (≥ 3 weeks postnatal age)
ROP (severe) or death (any)
Apnea of prematurity
Chronic lung disease/bronchopulmonary dysplasia
Growth ‐ neonatal period and long‐term
Neurodevelopment ‐ long‐term
Visual function ‐ long‐term
Outcome data with attrition rates greater than 20% were not included in analyses.
Search methods for identification of studies
The standard search strategy of the Cochrane Neonatal Review Group was used. This includes searches of the Cochrane Controlled Trials Register (CENTRAL/CCTR, The Cochrane Library, Issue 2, 2008), the Oxford Database of Perinatal trials, MEDLINE, previous reviews including cross references, abstracts, conferences and symposia proceedings, expert informants, journal hand searching mainly in the English language.
An additional literature search, using OVID software, was conducted of the MEDLINE (1996 ‐ June, Week 2, 2008), Maternity and Infant Care (1971 ‐ June 2008), and CINAHL (1982 ‐ June 2008) databases in order to locate any trials in addition to those provided by the Cochrane Controlled Trials Register (CENTRAL/CCTR, The Cochrane Library, Issue 2, 2008). The search strategy involved various combinations of the following keywords, using the search fields of abstract, MeSH subject heading, exploded subject heading, floating subject heading, publication type, registry number word, subject heading word, text word, and title: oxygen, preterm, premature, neonate, newborn, infant, oxygen saturation, hypoxia, retinopathy of prematurity, retrolental fibroplasia, low birth weight, very low birth weight, extremely low birth weight, randomized controlled trial, controlled clinical trial, clinical trial, random allocation, placebo.
Data collection and analysis
The standard methods of the Cochrane Collaboration and its Neonatal Review Group were used to select trials, assess quality and extract and synthesise data. For each trial, each author independently assessed the methodological quality and extracted the data from the report. Results were compared and differences resolved as required. Level of agreement between the two authors was greater than 90% in all cases. Eligible trials were assessed for the degree of selection, performance, attrition and detection bias. Additional information was requested from authors to clarify methodology or results as necessary.
Meta‐analyses were carried out with use of relative risk (RR) and risk difference (RD). When appropriate, number needed to treat (1/RD) was calculated. The fixed effects "assumption free" model was used. Evaluation of heterogeneity, subgroup and sensitivity analyses were undertaken as appropriate.
Results
Description of studies
The systematic review located six trials that addressed the question of targeting oxygen administration in preterm/LBW infants. Fourteen other studies were excluded from the analysis as they either did not address this particular question or did not involve random allocation of one of the interventions under review.
Participants:
The enrolment period for five included studies was between 1951 ‐ 1969 (referred to as "pre‐1990" trials or studies hereon in) and one (Askie 2003) included study was conducted between 1996 ‐2000 (referred to as "post‐1990" trials or studies hereon in). The five pre‐1990 studies were done during an early era of neonatal care, with therapies and practices quite different from modern "intensive" care. These studies included only small numbers of survivors with birth weights under 1000 g, the infants who carry the greatest mortality and morbidity burden today. There was a wide range of birth weights among trial participants, from less than 1000 to 2500 g. The largest pre‐1990 era trial (Kinsey 1956) only enrolled infants who survived beyond 48 hours, while the other four trials randomized infants on admission to the neonatal nursery anywhere from two hrs (Usher 1973) to > 48 hours (Kinsey 1956). Infants from these five trials have been categorised as belonging to the early neonatal period (< 1 week postnatal age), which was defined as treatments starting at < 1 week of age. The only post‐1990 trial (Askie 2003) enrolled infants < 30 weeks gestation who remained dependent on supplemental oxygen at 32 weeks postmenstrual age; therefore, infants in this trial were at least three weeks postnatal age at randomization. Infants in this trial have been defined in this review as belonging to the later neonatal period (≥ 3 weeks postnatal age). The five pre‐1990 trials used birth weight as inclusion criteria, with the most recent trial using gestational and postmenstrual age as inclusion criteria. Three trials also selected infants for inclusion based on a diagnosis of respiratory distress syndrome (Usher 1973) or hypoxia/acidemia (Sinclair 1968) or continued dependence on supplemental oxygen more than three weeks after birth (Askie 2003).
Intervention:
Three trials (Askie 2003; Usher 1973; Sinclair 1968) administered oxygen based on actual arterial, saturation or capillary blood oxygen levels. The other three trials were conducted in an era before accurate blood oxygen monitoring in infants was possible. As such, these trials could only test the effects of cruder measures of oxygenation, such as ambient oxygen concentration, and even these in only general terms, labelled "liberal" and "restricted" oxygen administration in this review. For the included studies, due to the variation in measurement methods, restricted oxygen ranged from values of Fn SpO2 91‐94% (Askie 2003), either 0.4 or 0.5 maximum FiO2 (Kinsey 1956; Lanman 1954; Patz 1954), or PaO2<45mmHg (PcapO2<35mmHg) (Usher 1973) or maximum O2 of 35% (in a headbox, PaO2 50‐120mmHg) (Sinclair 1968). Liberal O2 ranged from values of Fn SpO2 95‐98% (Askie 2003), O2 levels at 50% (Kinsey 1956), 60‐70% (Patz 1954), or 100% (in a headbox; PaO2 50‐120mmHg) (Sinclair 1968), FiO2 69% (Lanman 1954), or minimum O2 40% (PaO2 80‐120mmHg or PcapO2 50‐60mmHg) (Usher 1973).
Five of the included trials started the intervention in the early neonatal period (< 1 week postnatal age), but continued it for a wide range of time; from one day to seven weeks. Of these, four studies randomized infants from birth (defined as < 48 hours after birth) (Lanman 1954; Patz 1954; Sinclair 1968; Usher 1973), while one study did not randomize infants until > 48 hours after birth (Kinsey 1956). One trial started the intervention in the later neonatal period (from 32 weeks postmenstrual age) (Askie 2003), and continued for a median of 17.5 days (IQR 7.0 to 41.0 days) for lower oxygen targeting and a median of 40.0 days (IQR 20.5 to 73.0 days) for the higher oxygen targeting group. When oxygen weaning was indicated, it was done so gradually in two trials, abruptly in one trial, and the method not specified in the remaining three trials.
Outcomes:
Outcome measures were assessed at time periods ranging from two days to 12 months. Only Askie 2003 reported the longer term (12 months corrected age) effects of the interventions on growth, neurodevelopment, lung function, or chronic lung disease. Coates 1982 reported some long‐term outcomes on infants from Usher's 1973 study (Usher 1973). Unfortunately, he was only able to obtain outcome data for 23% of survivors, and in keeping with our a priori specification of only including outcome measures with 80% or greater ascertainment, these data are not included in the review.
Only one study (Askie 2003) reporting eye outcome data used the International Classification of Retinopathy of Prematurity grading system (ROP Committee 1984). This was assessed by routine ophthalmic examinations at two‐week intervals from enrolment until the resolution of retinopathy.
The only other study to report eye outcome data used the retrolental fibroplasia (RLF) classifications (Reese 1953). Vascular RLF grade 1, vascular RLF grade 2, and cicatricial RLF / RLF grade 3 correspond approximately with retinopathy of prematurity (ROP) stage 3, ROP stage 4, and ROP stage 5 / blindness respectively. These inferred classifications were gathered from references to RLF/ROP cross‐classification from the International Classification of Retinopathy of Prematurity system (ROP Committee 1984; ROP Committee 1987; Garner 1985; Hindle 1986; Hindle 1990; Sira 1988; Szewcyk 1953). Ascertainment of RLF in the five trials from 1951 ‐ 1969 was by direct ophthalmoscope, visualising the posterior pole only. The only findings that could be identified using this method were dilation and tortuosity of the retinal vessels ("plus disease", using the 1984 and 1987 classifications, as above). The more common findings in the anterior pole that can today be identified with indirect ophthalmoscopy were unable to be identified. Hence, even the least severe eye outcomes reported in this review equate with what today would be described as "threshold" ROP.
The largest trial (Askie 2003, n = 358) only enrolled infants who survived and were oxygen dependent beyond three weeks postnatal age. The second largest trial (Kinsey 1956, n = 212) only enrolled infants who survived beyond 48 hours. Unfortunately, the third largest trial (Patz 1954, n = unknown, but greater than 120) did not report any mortality data and these data are not retrievable (Duc 1992).
Risk of bias in included studies
All included trials used either quasi‐random or random patient allocation, had at least one clinically meaningful outcome, and were thus included in the analyses. The overall methodological quality of the included trials was fair. Askie 2003 stratified the randomization with the use of a dynamic balancing method to ensure a balance of treatment group assignment within each stratum defined according to hospital, singleton or multiple birth, and gestational age.
Three of the trials had adequate allocation concealment: Askie 2003 and Kinsey 1956 used central telephone randomization, and Sinclair 1968 used a method of sealed envelopes. Allocation concealment is unclear in the other three trials. Patz 1954 used quasi‐random patient allocation, while the remaining five trials were truly randomized. Askie 2003 was the only trial to employ masking with families, clinicians and outcome assessors in this trial unaware of treatment allocation. Askie 2003 and Kinsey 1956 were the only two trials to report power calculations a priori. Five of the included studies had adequate short‐term outcome measure ascertainment. The Patz 1954 trial did not report deaths or losses to follow‐up, but it is assumed that outcome data were reported only on survivors and assessed by six months age.
Effects of interventions
RESTRICTED VS. LIBERAL OXYGEN THERAPY (ALL PRETERM/LBW INFANTS) IN EARLY NEONATAL PERIOD (Comparison 1):
In this meta‐analysis, restricted compared with liberal oxygen administration when started during the early neonatal period did not have any statistically significant effect on the incidence of death. It should be noted that there were a range of times for enrolment in this early period from two hrs (Usher 1973) to > 48 hrs (Kinsey 1956). However, restricted oxygen administration did significantly reduce the incidence of all forms of retrolental fibroplasia (RLF) in survivors. Cicatricial RLF (any grade) was significantly reduced in surviving infants who were exposed to a restricted oxygen regime (summary RR 0.26, 95% CI 0.11‐0.58). There was also a significant reduction in the precursor, vascular RLF (any stage), in surviving infants exposed to restricted oxygen (summary RR 0.34, 95% CI CI 0.25‐0.46).
During the early neonatal period, neither restricted compared with liberal oxygen administration nor lower vs. higher blood oxygen levels (where blood oxygen was directly measured) had significant independent effects on death rates, either in all preterm/LBW infants or in a sub‐group of infants with birth weights < 1250 g. However, restricted compared with liberal oxygen administration did significantly reduce a combined measure of adverse outcome, death or RLF (vascular, any stage) (summary RR 0.59, 95% CI 0.48‐0.72). Thus, one would need to treat only three infants with restricted oxygen to prevent one infant from having the adverse outcome of death or RLF (NNT = 1/RD = 1/0.310 = 3.2). Restricted compared with liberal oxygen administration also reduced the more severe measure of adverse outcome, death or RLF (cicatricial, any grade) (summary RR 0.77, 95% CI 0.56‐1.07) for the trial where the intervention was used in the early neonatal period, although this result was not statistically significant.
No other outcome measures specified a priori as clinically meaningful were reported in enough detail or with satisfactory follow‐up rates to be included in the analysis (chronic lung disease; long‐term growth, development, lung or visual function).
SUBGROUP ANALYSIS FOR THE EARLY NEONATAL PERIOD (Comparisons 2‐4):
Only one of the a priori stated subgroup analyses was possible with the available data for the early neonatal period.
Subgroup analysis of lower vs. higher blood oxygen levels in the early neonatal period showed that for infants with BW < 1250 g weeks gestational age, there was no significant difference in the incidence of death. However, it should be noted that this trial (Usher 1973) only enrolled 45 infants.The only reported effect of restricted vs. liberal oxygen saturation targeting on infants with birth weight less than 1000g was a non‐significant decrease in RLF (cicatricial, severe) in the Patz 1954 trial. The analysis was based on very small numbers, with uneven denominators in each group. This may reflect a difference in the number of survivors in the two groups resulting from deaths which were not accounted for by Patz 1954. This result should thus be interpreted with caution as the small numbers in this subgroup (as reflected in the wide confidence intervals) and non‐reported deaths make any meaningful interpretation of these data difficult.
It was not possible to undertake any of the other a priori specified subgroup analyses such as time or method of oxygen weaning, or a comprehensive analysis of the method of oxygen monitoring due to insufficient data.
LOWER VS. HIGHER BLOOD OXYGEN LEVELS (ALL PRETERM/LBW INFANTS) IN THE LATER NEONATAL PERIOD (Comparison 5):
Only one study (Askie 2003), with 358 infants, contributed to the results in the later neonatal period. There was no significant difference in the incidence of death between lower or higher oxygen saturation targeting when started in the later neonatal period. There were no statistically significant differences in the incidence of ROP (any stage) in survivors, the incidence of ROP > Stage 2 nor ROP Stage 4 or 5 or blindness between the infants receiving lower or higher oxygen saturation targeting. There were no statistically significant differences between intervention strategies for the combined outcomes of death or ROP > Stage 2, nor with death or ROP Stage 4 or 5 or blindness.
Some outcome measures specified a priori as clinically meaningful were reported. There was no statistically significant difference in the incidence of major developmental abnormality at 12 months corrected age between lower or higher oxygen saturation targeting. In relation to lung function, there was a significant reduction on the dependence of supplemental oxygen at 36 weeks of postmenstrual age with using a lower oxygen saturation target (RR 0.71, 95% CI 0.59‐0.87). There was no statistically significant difference between interventions for the incidence of use of postnatal corticosteroids and diuretics for chronic lung disease with the use of either a lower or higher oxygen saturation targeting.
Some outcomes were either not reported at all or not reported in enough detail or with satisfactory follow‐up rates to be included in the analysis (long‐term lung or visual function).
SUBGROUP ANALYSIS FOR THE LATER NEONATAL PERIOD (Comparison 6):
Only one of the a priori stated subgroup analysis could be undertaken with the available data for the later neonatal period.
Comparison of lower vs. higher oxygen saturation targeting when started in the later neonatal period in infants < 28 weeks gestational age revealed no statistically significant difference in the incidence of death, ROP Stage 3 or 4, nor in the incidence of blindness.
Evaluation of heterogeneity:
No statistical heterogeneity was demonstrated in any of the outcome measures analysed that included more than one trial.
There was considerable clinical heterogeneity amongst the six trials included in this review. All included trials contained a wide range of birth weights, followed infants for a relatively wide ranging period (and all but one in the short‐term only), used different definitions of outcome measures (five trials used RLF and one trial used ROP eye outcome definitions), and implemented the interventions in either an early or later neonatal period. There was a very wide range of exposure to the interventions under review (1 day to >10 weeks). Moreover, there were three distinct intervention comparisons included in the review (hence the division of comparisons into restricted vs. liberal oxygen administration in the early neonatal period, lower vs. higher blood oxygen levels in the early neonatal period, and lower vs. higher blood oxygen levels in the later neonatal period). The Kinsey 1956, Lanman 1954 and Patz 1954 trials were conducted in an early (pre‐1990) era of neonatal care where methods of oxygen monitoring and administration were crude in comparison to today's techniques and thus only restricted vs. liberal oxygen administration could be compared in these trials. The Usher 1973 and Sinclair 1968 trials used more modern techniques (including umbilical artery catheterization, arterialised capillary sampling, micromethods for blood gases and acid‐base), so comparison of lower vs. higher blood oxygen levels were possible with these data. The Askie 2003 trial used pulse oximeters whose algorithm assessed functional oxygen saturation, and thus comparisons of lower vs. higher blood oxygen level via oxygen saturation targets were possible with data from these trials.
Sensitivity analyses:
The results of the meta‐analyses were tested for robustness with regard to study quality. We had stated a priori that trials containing outcome measures with greater than 20% attrition would not be included in the analysis. In one trial, Patz 1954, it was unclear whether outcome ascertainment was complete as attrition due to losses to follow‐up and deaths were not reported. This, plus the fact that it was the only trial using a quasi‐random method of patient allocation, led us to test the results without the inclusion of this trial.
There were two outcome measures for the early neonatal period analysis that included data from the Patz 1954 trial. The outcomes to which the Patz trial contributed were RLF (vascular, any stage) and RLF (cicatricial, severe grades). The results for neither of these outcome measures were significantly affected by the exclusion of the Patz trial. Hence, the results of these meta‐analyses were not sensitive to the effect of study quality.
Discussion
The answer to the question of what is the optimal therapeutic range of blood oxygen level for preterm/LBW infants to maximise benefits, while minimising harms, remains uncertain.
To date only two randomized trials (Askie 2003; Usher 1973) have attempted to address this question directly. Sinclair 1968 assessed the effects of lower vs. higher blood oxygen levels and other co‐interventions in a group of hypoxic, acidaemic low birth weight infants. The related, but now historic, question of restricted vs. liberal oxygen administration was addressed by three randomized trials (Kinsey 1956; Lanman 1954; Patz 1954) in an era before accurate and/or continuous monitoring of infant blood oxygen levels was possible. Both interventions were included in this review, which addresses the general question of the effect of oxygen dose on outcomes for preterm/LBW infants.
In this analysis, restricting oxygen exposure in the early neonatal period significantly reduced the incidence and severity of RLF without unduly increasing death rates. The results of the largest trial contributing to these outcomes (Kinsey 1956) have often been misinterpreted, with the resulting extrapolation of aggressive restriction of oxygen from birth leading to a substantial increase in mortality rates among preterm/LBW infants in the years following its publication (Cross 1973). This trial did not enrol infants until at least 48 hours of age. It should also be noted that the second largest trial, Patz 1954, did not report any mortality data and this information is not retrievable (Duc 1992). Unfortunately, the confidence intervals around the point estimate for this outcome are quite wide (RR 1.20, 95% CI 0.80‐1.80), and the addition of the Patz 1954 mortality data would have been helpful in resolving this issue. It is possible that the difference in RLF rates seen in survivors may be influenced by the trend toward excess deaths caused by the restricted oxygen policy.
Since the publication of these earlier era trials, other authors have attempted to further investigate the association between RLF/ROP and blood oxygen levels. A large, prospective, non‐randomized study (Kinsey 1977) involving a detailed survey across five collaborating centres in the USA was undertaken between 1969 and 1972. No definitive relationship between blood oxygen levels and the occurrence of RLF could be established. It should be noted that this analysis was undertaken using the limited information available from intermittent blood gas sampling. The study did find an association between susceptibility to RLF and decreasing birth weight and increasing time in oxygen. However, no guidelines for the optimal range of blood oxygen level were suggested by this study.
Two trials (Sinclair 1968; Usher 1973) that addressed the question of low vs. higher blood oxygen levels in the early neonatal period (< 1 week postnatal age) found no significant effect on death in the early neonatal period, but did not report (in sufficient detail to warrant inclusion) the effect of this intervention on eye or other outcomes. The effects of either of these oxygen administration policies on other clinically meaningful outcomes, including chronic lung disease, long‐term growth, neurodevelopment, lung or visual function were not reported.
No further trials were undertaken until a prospective, multicenter, double‐blind, randomized, controlled trial (Askie 2003) involving eight collaborating centres in Australia was conducted between 1996 and 2000. There were no significant differences in the rates of ROP at any stage between the lower and higher oxygen saturation target groups in the later neonatal period (≥ 3 weeks postnatal age). There were no significant differences between the groups in mortality rates either. However, this study noted that there was a disadvantage to using higher oxygen saturation targeting because of the increase in the proportion of infants needing oxygen therapy for longer, as well as supplemental oxygen after discharge. Again, this study made no recommendations for an optimal blood oxygen level, but suggested that targeting higher blood oxygen levels may increase the burden of health services for these infants. This trial enrolled oxygen‐dependent infants at 32 weeks postmenstrual age who were at least 3 weeks of age. There is therefore a need to evaluate this therapy when commenced soon after birth as this may alter the rates of ROP or death. A number of trials currently underway are examining this (BOOST NZ (NZ); BOOSTII (Australia); BOOSTII (UK); COT (Canada); SUPPORT (USA)).
All studies included in this review measured eye outcomes. Unlike the pre‐1990 studies, the Askie 2003 trial also reported the effect of interventions on growth and development. However, these outcomes were not measured beyond 12 months corrected age and thus studies with longer term outcomes will need to be conducted. Since 2001, several observational studies (Tin 2001; Anderson 2004; Sun 2002; Chow 2003) have been published that have suggested short‐term ophthalmic and respiratory outcomes might be significantly improved by a policy of lower oxygen range targeting without causing increases in mortality or long‐term morbidity. However, these non‐randomized studies lack adequate statistical power to exclude possible small, but important, increases in death and disability that could have major implications if a policy of lower oxygen targeting was implemented worldwide. Currently, there are five ongoing randomized trials being conducted to assess the effects of lower vs. higher oxygen saturation levels in extremely preterm infants from birth. The individual patient data from these trials will be combined in a prospective meta‐analysis to help resolve this remaining question.
The role of careful, continuous monitoring of oxygen levels on the incidence of retinopathy of prematurity has also been investigated by several authors since the publication of the earlier studies included in this review. Bancalari and co‐workers (Bancalari 1987a; Bancalari 1987b; Flynn 1987) conducted the only large randomized trial of continuous transcutaneous PO2 monitoring to date. This study showed no significant difference in the incidence or severity of ROP, mortality or chronic lung disease in the continuously monitored infants compared with those who received standard (intermittent) monitoring of PO2 levels. The utility of pulse oximetry monitoring in preventing adverse neonatal outcomes remains largely untested. The value of pulse oximetry in reducing major hypoxic events during anaesthesia among 152 children undergoing surgery has been assessed in one study (Cote 1988). Another trial (Watkin 1999) compared near infrared spectroscopy and pulse oximetry in the detection of hypoxaemia in neonates with pauses in nasal airflow. Roemer and colleagues (Roemer 2005) examined the diagnostic power of pulse oximetry, other blood oxygen measures and acid‐base measurements for hypoxia in term fetuses. However, randomized controlled trial evidence for the effectiveness of pulse oximetry monitoring in the early neonatal period is still unavailable.
Authors' conclusions
Implications for practice.
The results of this systematic review confirm that (the now historical) policy of unrestricted, unmonitored oxygen therapy has potential harms without clear benefits. However, the question of what is the optimal target range for maintaining blood oxygen levels in preterm/LBW infants in the modern clinical setting from birth or soon thereafter was not answered by the data available for inclusion in this review.
Implications for research.
As the question of what is the optimal target range for maintaining blood oxygen levels remains unclear, further research should be undertaken to resolve this important clinical question. An ongoing international collaboration is attempting to address this issue. The BOOSTII trials (BOOST NZ (NZ); BOOSTII (Australia); BOOSTII (UK); COT (Canada); SUPPORT (USA)) are all assessing the effects of higher oxygen levels on infants 27 weeks or less gestational age in terms of both short and long‐term outcomes. Results from these trials will be combined in a prospective meta‐analysis (known as the NeOProM Collaboration) and will be incorporated into this systematic review as they become available. The STOP‐ROP trial (STOP‐ROP 2000) assessed the effect of higher oxygen levels on the progression of pre‐threshold ROP. The results of this trial are included in a separate Cochrane review entitled: "Supplemental oxygen for the treatment of pre‐threshold retinopathy of prematurity" (Lloyd J, Askie LM, Smith J, Tarnow‐Mordi WO). It should be noted that this trial did not address the effect of oxygen levels administered in the early neonatal period either as infants were 35.6 weeks postmenstrual age at enrolment into this trial.
What's new
| Date | Event | Description |
|---|---|---|
| 13 May 2009 | Amended | Minor amendment ‐ References Watkin and Roemer added |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 14 August 2008 | New search has been performed | This review updates the existing review "Restricted versus liberal oxygen for preventing morbidity and mortality in preterm or low birth weight infants" published in the Cochrane Database of Systematic Reviews. This update includes an updated literature search, revised Background section including RLF/ROP cross‐classification information and references, revised data analysis with a new included study, updated Discussion and conclusion sections, updated information regarding ongoing clinical trials. |
| 14 August 2008 | New citation required but conclusions have not changed | Substantive amendment |
| 25 January 2008 | Amended | Converted to new review format. |
| 1 October 2003 | New search has been performed | This review updates the existing review "Restricted versus liberal oxygen for preventing morbidity and mortality in preterm or low birth weight infants" which was published in the Cochrane Library Issue 2, 2001. The background section has additional references; The STOP‐ROP 2000, trial previously listed as ongoing, has now been listed as an excluded trial and will be included in another Cochrane systematic review entitled "Supplemental oxygen in the treatment of pre‐threshold retinopathy of prematurity" (Lloyd J, Askie LM, Smith J, Tarnow‐Mordi WO); a synopsis and a background section to the abstract have also been added. No new trials were identified as a result of the most recent search, and hence no substantive changes have been made to either the results or conclusions of the review. |
Acknowledgements
None.
Data and analyses
Comparison 1. Restricted versus liberal oxygen therapy (all preterm/LBW infants) in early neonatal period.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (any) | 2 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.80, 1.90] |
| 2 Cicatricial RLF (any grade) in survivors | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.58] |
| 3 Vascular RLF (any stage) in survivors | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.25, 0.46] |
| 4 Vascular RLF (severe stages) in survivors | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.17, 0.85] |
| 5 Cicatricial RLF (severe grades) in survivors | 2 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.50] |
| 6 Death or vascular (RLF (any stage) | 2 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.48, 0.72] |
| 7 Death or cicatricial RLF (any grade) | 2 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.07] |
| 8 Cicatricial RLF (severe grades) in survivors (excluding Patz 1954) | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.11, 0.93] |
| 9 Vascular RLF (any stage) in survivors (excluding Patz 1954) | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.26, 0.51] |
1.1. Analysis.
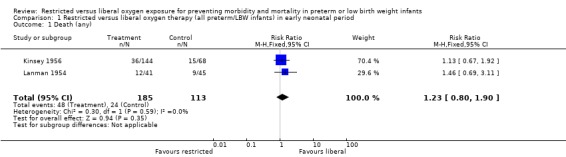
Comparison 1 Restricted versus liberal oxygen therapy (all preterm/LBW infants) in early neonatal period, Outcome 1 Death (any).
1.2. Analysis.
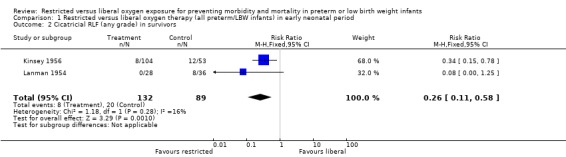
Comparison 1 Restricted versus liberal oxygen therapy (all preterm/LBW infants) in early neonatal period, Outcome 2 Cicatricial RLF (any grade) in survivors.
1.3. Analysis.
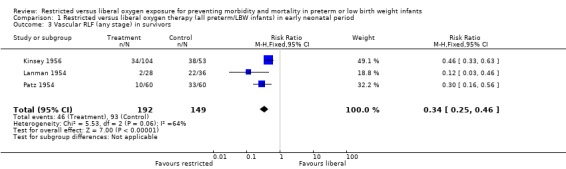
Comparison 1 Restricted versus liberal oxygen therapy (all preterm/LBW infants) in early neonatal period, Outcome 3 Vascular RLF (any stage) in survivors.
1.4. Analysis.
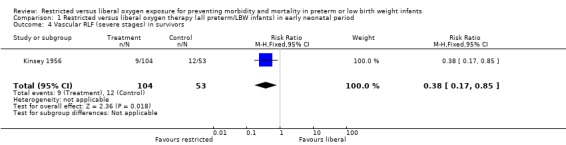
Comparison 1 Restricted versus liberal oxygen therapy (all preterm/LBW infants) in early neonatal period, Outcome 4 Vascular RLF (severe stages) in survivors.
1.5. Analysis.
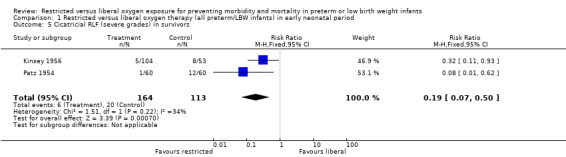
Comparison 1 Restricted versus liberal oxygen therapy (all preterm/LBW infants) in early neonatal period, Outcome 5 Cicatricial RLF (severe grades) in survivors.
1.6. Analysis.
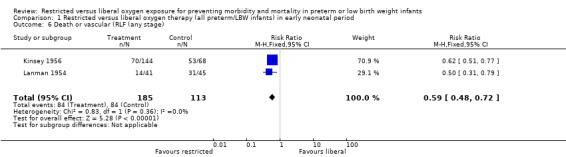
Comparison 1 Restricted versus liberal oxygen therapy (all preterm/LBW infants) in early neonatal period, Outcome 6 Death or vascular (RLF (any stage).
1.7. Analysis.
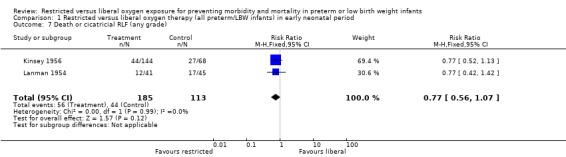
Comparison 1 Restricted versus liberal oxygen therapy (all preterm/LBW infants) in early neonatal period, Outcome 7 Death or cicatricial RLF (any grade).
1.8. Analysis.
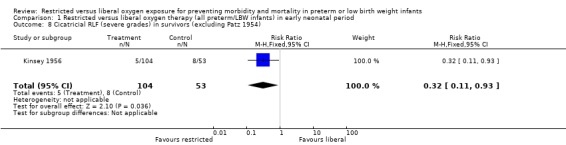
Comparison 1 Restricted versus liberal oxygen therapy (all preterm/LBW infants) in early neonatal period, Outcome 8 Cicatricial RLF (severe grades) in survivors (excluding Patz 1954).
1.9. Analysis.
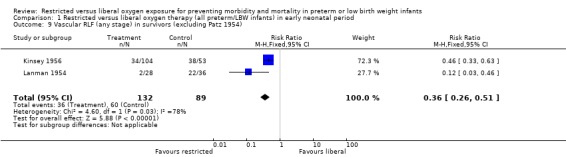
Comparison 1 Restricted versus liberal oxygen therapy (all preterm/LBW infants) in early neonatal period, Outcome 9 Vascular RLF (any stage) in survivors (excluding Patz 1954).
Comparison 2. Restricted versus liberal oxygen therapy (BW<1000g) in early neonatal period.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cicatricial RLF (severe grades) in survivors | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.02, 3.79] |
2.1. Analysis.
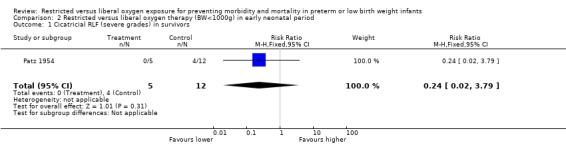
Comparison 2 Restricted versus liberal oxygen therapy (BW<1000g) in early neonatal period, Outcome 1 Cicatricial RLF (severe grades) in survivors.
Comparison 3. Lower versus higher blood oxygen levels (all preterm/LBW infants) in early neonatal period.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (any) | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.57, 1.44] |
3.1. Analysis.
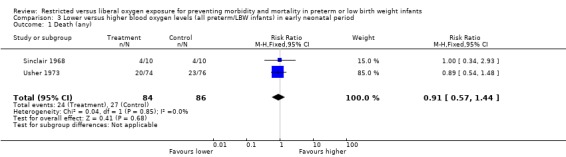
Comparison 3 Lower versus higher blood oxygen levels (all preterm/LBW infants) in early neonatal period, Outcome 1 Death (any).
Comparison 4. Lower versus higher blood oxygen levels (BW<1250g) in early neonatal period.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (any) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.75, 1.58] |
4.1. Analysis.
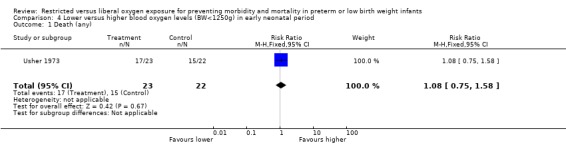
Comparison 4 Lower versus higher blood oxygen levels (BW<1250g) in early neonatal period, Outcome 1 Death (any).
Comparison 5. Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.64] |
| 2 ROP (any stage) in survivors | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.19] |
| 3 ROP >Stage 2 in survivors | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.77, 2.16] |
| 4 ROP Stage 4 or 5 or blindness in survivors | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.06 [0.60, 42.85] |
| 5 Death or ROP >Stage 2 | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.69, 1.68] |
| 6 Death or ROP Stage 4 or 5 or blindness | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.43, 2.37] |
| 7 Dependence on supplemental oxygen at 36 weeks of postmenstrual age | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.59, 0.87] |
| 8 Postnatal corticosteroids | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.05] |
| 9 Diuretics for chronic lung disease | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.68, 1.05] |
| 10 Major developmental abnormality at 12 months corrected age | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.53] |
5.1. Analysis.
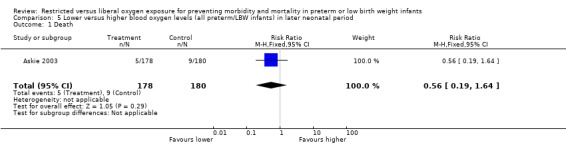
Comparison 5 Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period, Outcome 1 Death.
5.2. Analysis.
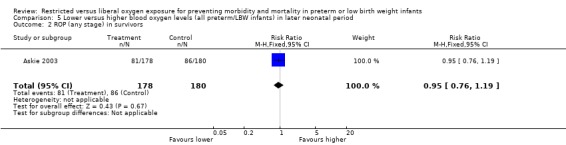
Comparison 5 Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period, Outcome 2 ROP (any stage) in survivors.
5.3. Analysis.
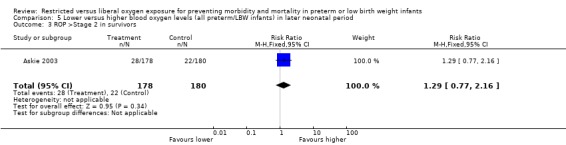
Comparison 5 Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period, Outcome 3 ROP >Stage 2 in survivors.
5.4. Analysis.
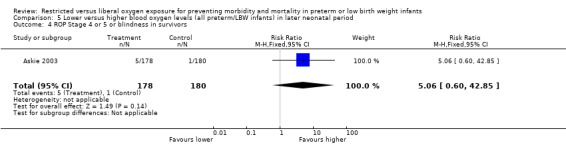
Comparison 5 Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period, Outcome 4 ROP Stage 4 or 5 or blindness in survivors.
5.5. Analysis.
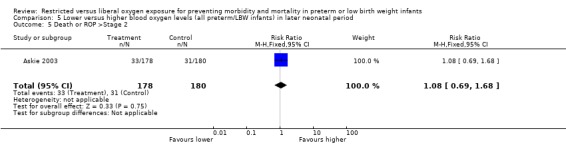
Comparison 5 Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period, Outcome 5 Death or ROP >Stage 2.
5.6. Analysis.
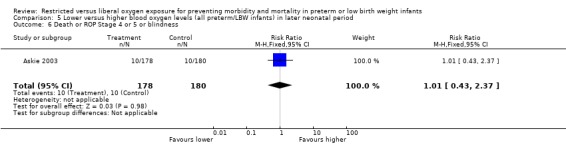
Comparison 5 Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period, Outcome 6 Death or ROP Stage 4 or 5 or blindness.
5.7. Analysis.
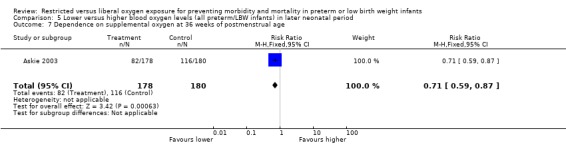
Comparison 5 Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period, Outcome 7 Dependence on supplemental oxygen at 36 weeks of postmenstrual age.
5.8. Analysis.
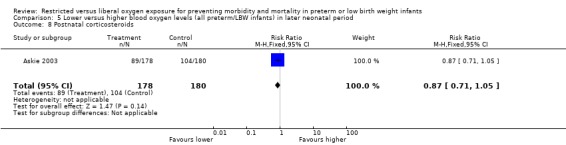
Comparison 5 Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period, Outcome 8 Postnatal corticosteroids.
5.9. Analysis.
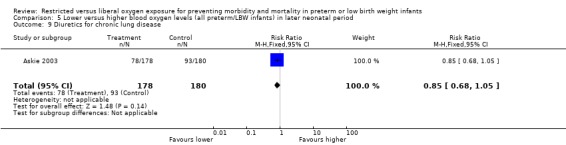
Comparison 5 Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period, Outcome 9 Diuretics for chronic lung disease.
5.10. Analysis.
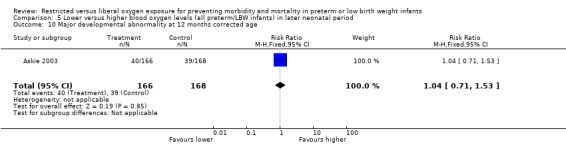
Comparison 5 Lower versus higher blood oxygen levels (all preterm/LBW infants) in later neonatal period, Outcome 10 Major developmental abnormality at 12 months corrected age.
Comparison 6. Lower versus higher blood oxygen levels (<28 weeks GA) in later neonatal period.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ROP Stage 3 or 4 | 1 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.85, 2.36] |
| 2 Blindness | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [0.47, 36.46] |
6.1. Analysis.
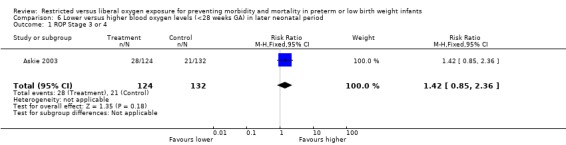
Comparison 6 Lower versus higher blood oxygen levels (<28 weeks GA) in later neonatal period, Outcome 1 ROP Stage 3 or 4.
6.2. Analysis.
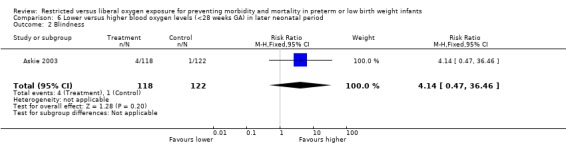
Comparison 6 Lower versus higher blood oxygen levels (<28 weeks GA) in later neonatal period, Outcome 2 Blindness.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Askie 2003.
| Methods | Randomization was stratified with the use of a dynamic balancing method to ensure a balance of treatment‐group assignment within each stratum defined according to hospital, singleton or multiple birth, and gestational age. Central telephone randomization ensured adequate allocation concealment. The intervention group (standard oxygen) received oxygen to achieve Fn SpO2 91‐94%, while the control group (high oxygen) received oxygen to achieve Fn SpO2 95‐98%. Masking of all interventions was achieved by using oximeters designed to display levels either 2% higher or lower than what it really was, thereby giving readings between 93‐96%. Caregivers were not aware of the offset level (double‐blinding). There were no losses in follow‐up. There were detailed power calculations. |
|
| Participants | 358 infants < 30wks gestation who remained dependent on supplemental oxygen at 32 wks of postmenstrual age. The mean birth weight for standard saturation group was 918g and for high saturation group 916g. Infants were followed and measured at 12 months corrected age. | |
| Interventions | Experimental group (standard oxygen): received oxygen to achieve Fn SpO2 91‐94%. Intervention treatment applied at 32wks postmenstrual age and maintained for the duration of the supplemental oxygen therapy. Control group (high oxygen): received oxygen to achieve Fn SpO2 95‐98%. Intervention treatment applied at 32 wks postmenstrual age and maintained for the duration of the supplemental‐oxygen therapy. |
|
| Outcomes | Worst retinopathy of prematurity (< stage 3) Worst retinopathy of prematurity (stage 3 or 4) Ablative retinal surgery for severe retinopathy of prematurity Death (after randomization) Growth measures: ‐ weight ‐ length ‐ head circumference Major developmental abnormality Dependence on supplemental oxygen at 36 wks of postmenstrual age Home‐based oxygen therapy & duration of oxygen therapy after randomization Postmenstrual age at cessation of oxygen therapy Duration of assisted ventilation after randomization Postnatal corticosteroids Diuretics for chronic lung disease Length of stay after randomization Postmenstrual age at discharge from hospital Postmenstrual age at time of fully oral feeding Infant rehospitalized Number of health service visits per infant Scores on psychological measures ‐Edinburgh postnatal depression scale (mother) ‐infant temperature scale ‐toddler temperment scale ‐parenting stress index, short form ‐impact‐on‐family scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization was stratified with the use of a dynamic balancing method. |
| Allocation concealment? | Low risk | Central telephone randomization. |
| Blinding? All outcomes | Low risk | Oxygen saturation levels were adjusted to display a value 2% higher than the actual saturation in infants in the standard O2 group or 2% lower than the actual saturation in infants in the high‐saturation group. |
| Incomplete outcome data addressed? All outcomes | Low risk | There was complete follow‐up for outcome data. |
| Free of selective reporting? | Low risk | All outcomes were reported. |
Kinsey 1956.
| Methods | Central telephone randomization ensured adequate allocation concealment. The ratio of experimental group : control group was 2:1 in first 3 months of enrolment. Following that, 574 infants were consecutively allocated to the experimental group and had no concurrent controls. These infants are not included in this review. The number of infants excluded before randomization is not known. Randomization was stratified by birth weight categories and institution. The intervention was not blinded and the blinding of outcome assessments is unclear. The follow‐up rate for outcome measures was 97%. There were detailed power calculations. | |
| Participants | 212 infants with BW <1500g who survived to 48 hours. Enrolment commenced in July 1953. The mean BW in the two groups was 1242g (restricted) and 1234g (liberal) respectively. Infants were followed until 2.5 months of age. | |
| Interventions | Experimental group (restricted oxygen): received oxygen only if clinical condition indicated and maximum FiO2 permitted was 0.5. Control group (liberal oxygen): received supplemental oxygen in excess of 50% for a minimum of 28 days and were then weaned over 3 days. | |
| Outcomes | Vascular RLF (any stage) in survivors
Vascular RLF (severe stages) in survivors
Cicatricial RLF (any grade) in survivors
Cicatricial RLF (severe grades) in survivors
Mortality (48 hours‐40 days) Of the 144 infants assigned to the restricted oxygen group, 36 died before 40 days and 4 were lost to follow‐up. There were 15 deaths and no losses to follow‐up among the 68 infants allocated to the liberal oxygen group. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Block and stratified randomization. |
| Allocation concealment? | Low risk | Central telephone randomization. |
| Blinding? All outcomes | High risk | Blinding not stated. |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow‐up was 97%. Reasons were given for loss to follow‐up (e.g. death) |
| Free of selective reporting? | Low risk | There were 21 tables and 8 appendices tables of results and measured data reporting various analyses of the outcome data and breakdown of the characteristics of the populations from all the participating centres. |
Lanman 1954.
| Methods | Infants were randomized by random numbers, method unspecified, and thus allocation concealment is unclear. There was no blinding of the intervention and it is unknown if outcome assessments were done blinded to treatment allocation. There was only one loss to follow‐up of the 86 infants enrolled. Power calculations were inadequate with the completion of the study being determine by a date specified one year in advance. | |
| Participants | 86 infants with BW 1000‐1850g admitted within 12 hours of birth. Infants were followed until 3 months age. | |
| Interventions | Experimental group (restricted oxygen): only received oxygen when cyanosed, at a maximum FiO2 of 0.5. The mean FiO2 received by this group was 0.38. Control group (liberal oxygen): received supplemental oxygen for a minimum of 2 weeks or until reaching 1500g, and were then weaned abruptly. The mean FiO2 received by this group was 0.69. | |
| Outcomes | Vascular RLF (any stage) in survivors Cicatricial RLF (any grade) in survivors Mortality (12 hours‐3 months) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random numbers, but method was not specified. |
| Allocation concealment? | Unclear risk | Allocation was in order of admission by random numbers but method was not specified. Allocated to one of 4 groups: high oxygen, high oxygen + estrogen given orally, low oxygen, & low oxygen + estrogen given orally. |
| Blinding? All outcomes | High risk | For restricted oxygen intervention, oxygen was given only when infants were cyanosed, so blinding would not have been easily done. |
| Incomplete outcome data addressed? All outcomes | Low risk | There was complete follow‐up, and infants who were lost lost to follow‐up were accounted for. |
| Free of selective reporting? | Low risk | All participants and outcomes were reported, even those that were lost to follow‐up were reported. |
Patz 1954.
| Methods | Quasi‐random treatment allocation, based on alternate admission basis. Allocation concealment was thus inadequate. There was no blinding of the intervention and it is unclear whether outcome assessments were blinded to treatment allocation. Attrition due to deaths or losses to follow‐up are not reported, so it is unclear whether there was complete outcome measure ascertainment. No power calculations were reported. | |
| Participants | An unknown number of very low birthweight infants (</= 1500g) were enrolled from Jan 1951 to May 1953. 120 infants survived and had eye outcome assessments completed by 6 months age and were included in the analysis. | |
| Interventions | Experimental group (restricted oxygen): infants received oxygen only for clinical indications, and to a maximum FiO2 of 0.4. The range of duration of oxygen in this group was 1 day ‐ 2 weeks. Once weaning was indicated, it proceeded over 1‐3 days. Control group (liberal oxygen): infants were placed in supplemental oxygen of 60‐70% for 4‐7 weeks, then weaned over one week. | |
| Outcomes | Vascular RLF (any stage) in survivors
Cicatricial RLF (severe grades) in survivors
Cicatricial RLF (severe grades), BW <1000g, in survivors There are no data available, either published or unpublished, on mortality rates. The number of infants allocated to each group was not reported, hence outcome data can only be expressed in relation to the surviving infants presenting for follow‐up assessment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Quasi‐random allocation based on alternate admission basis. |
| Allocation concealment? | High risk | Quasi‐random allocation based on alternate admission basis. |
| Blinding? All outcomes | High risk | Blinding not stated. Also, the experimental and control interventions were applied for different lengths of time, so treatment differences would have been obvious. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | The focus of results seemed to be on qualitative histological data. The quantitative results seemed to report on all outcomes. |
Sinclair 1968.
| Methods | Randomized to one of 4 treatment groups, using sealed envelopes and thus allocation concealment was adequate. There was no blinding of treatment intervention, and it is unclear whether there was blinding of outcome assessments. No power calculations were reported. Short‐term follow up was complete. | |
| Participants | 20 infants with BW 1000‐2500g less than 24 hours age who were hypoxic and acidemic were included. | |
| Interventions | Infants were randomized to one of four treatment groups including combinations of the following treatments: restricted vs. liberal ambient oxygen, rapid vs. slow alkali infusion, assisted vs. spontaneous ventilation. There was random allocation of the other two treatments within the two oxygen therapy groups, hence the data from this trial were included in the review. Experimental group (restricted oxygen): supplemental oxygen, to a maximum of 35%, to keep PaO2 50‐120 mmHg. If PaO2 fell below 40 mmHg or infant became bradycardic, could give unlimited oxygen and would be considered as a treatment failure. Control group (liberal oxygen): received 100% headbox oxygen for first 2 hours, then aimed to maintain PaO2 at 50‐120 mmHg using any FiO2 needed. | |
| Outcomes | Mortality (any) Physiological measures including: ‐ acid‐base balance ‐ PaO2 levels ‐ percentage right‐left shunt ‐ serum electrolytes, blood urea nitrogen, serum lactate ‐ urinary net acid excretion ‐ plasma bicarbonate ‐ "apparent" bicarbonate space Long‐term neurological assessments reported as "in progress" in the paper were never completed (personal communication J. Sinclair, July 1998). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Sealed envelopes & stratified |
| Allocation concealment? | Low risk | Random allocation to 1 of 4 treatment groups, using sealed envelopes; stratified by severity of A (severe vs. moderate). |
| Blinding? All outcomes | High risk | Blinding not stated. |
| Incomplete outcome data addressed? All outcomes | Low risk | There was complete follow‐up (but not specified). Short‐term follow‐up was complete. |
| Free of selective reporting? | Low risk | There were 14 tables and 15 figures of results and analysed data reporting various analyses of the outcome data. It seemed all outcomes were reported. |
Usher 1973.
| Methods | Infants were randomized by a stratified random sampling technique. Allocation concealment is unclear. There was no blinding of the intervention. One author was unblinded to the treatment allocation, but is unclear whether this author was involved in outcome assessments. No power calculations were reported. Early outcome data were reported completely. However, long‐term outcome data included only 15% of the enrolled infants and thus have not been included in this review. | |
| Participants | 150 infants with a diagnosis of respiratory distress syndrome or BW <1000g were eligible for inclusion. The numbers excluded prior to randomization are not reported. | |
| Interventions | Experimental group (low PaO2): infants received oxygen only if their PaO2 fell below 40 mmHg or PcapO2 fell below 35 mmHg. Sufficient oxygen was used to maintain these tensions. Control group (high PaO2): infants were kept in a minimum of 40% oxygen for 72 hours. Aim was to maintain PaO2 80‐120 mmHg or PcapO2 50‐60 mmHg. Mechanical ventilation was not available to either group. | |
| Outcomes | Mortality (any) Mortality (respiratory) Descriptive results of respiratory failure measures were reported (such as retractions, grunting, respiratory pattern and rate, chest Xray changes). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Stratified random sampling technique. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Blinding not stated. |
| Incomplete outcome data addressed? All outcomes | Low risk | There was complete follow‐up for early outcomes, but not for late outcomes. Only 15% follow‐up at 10yrs (Coates). |
| Free of selective reporting? | Low risk | There were 10 tables and 16 figures of results and analysed data reporting various analyses of the outcome data. It seemed that all outcomes were reported. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bard 1996 | Infants were not randomly assigned to target two different arterial blood oxygen saturations (90% and 95%). Infants acted as their own controls. This was not a random or quasi‐random trial and was thus excluded from the review. |
| Cunningham 1995 | This non‐randomized, retrospective study assessed the effects of variability of oxygen levels, as measured by transcutaneous oxygen monitoring, on the incidence of retinopathy of prematurity. Patient allocation was not randomized, and thus the study was excluded from the review. |
| Deulofeut 2007 | This was a non‐randomized study of infants from January 2000 to December 2004, where there was a change from SpO2 92‐100% to SpO2 85‐93% from January 2003. Since allocation of treatment was non‐randomized, this study was excluded from the review. |
| Engleson 1958 | This non‐randomized trial addressed a different question from that under review. It examined the effects of keeping preterm infants at oxygen concentrations below that of room air, and was thus not included in the review. |
| Fitzgerald 1998 | Infants in this study were randomized to receive either air/usual supplementary oxygen (to maintain SpO2 >93%) or increased supplementary oxygen (to maintain SpO2 >97%) only for one night whilst the sleep study was done. Included trials randomized infants to an ongoing policy of higher / lower SpO2. Infants also already had CLD at the start of the study (which was one of this study's population inclusion criteria). |
| Gaynon 1997 | The study was a retrospective analysis of different target ranges of oxygen saturation on the incidence of ROP. There was no random allocation of patients to different treatment groups, thus the trial was excluded from the review. |
| Kitchen 1978 | This study was a randomized trial of a "package" of intensive care, including intravenous glucose, umbilical arterial catheterisation, bicarbonate infusion, and high PaO2 levels, vs. the standard neonatal care regimen of the late 1960s. The trial was excluded from the review because the entire "package" of interventions, rather than the separate elements within it, was the randomized intervention. Thus, other interventions that could affect clinical outcomes were unbalanced between oxygen exposure groups. |
| Lundstrom 1995 | This randomized trial addressed a different question from that under review. It compared the use of atmospheric air vs. 80% oxygen for preterm infants during initial stabilization in the delivery room, and was thus excluded from the review. |
| Mendicini 1971 | This study was a randomized trial of a "package" of intensive care, including intravenous glucose, bicarbonate infusion, and high PaO2 levels, vs. the standard neonatal care regimen of the late 1960s. The trial was excluded from the review because the entire "package" of interventions, rather than the separate elements within it, was the randomized intervention. Thus, other interventions that could affect clinical outcomes were unbalanced between oxygen exposure groups. |
| Schulze 1995 | This was a non‐randomized, crossover trial comparing the effects of two different oxygen saturation target ranges on cardiac output, oxygen extraction, and oxygen consumption in mechanically ventilated, low birth weight infants. As treatment allocation was not random or quasi‐random, the trial was excluded from the review. |
| STOP‐ROP 2000 | This trial included preterm/LBW infants with pre‐threshold ROP. The intervention tested was supplemental oxygen for the treatment of pre‐threshold ROP, not a preventative strategy. The results of this trial will be included in a separate Cochrane review entitled: "Supplemental oxygen in the treatment of pre‐threshold retinopathy of prematurity" (Lloyd J, Askie LM, Smith J, Tarnow‐Mordi WO). |
| Wallace 2007 | This was a non‐randomized retrospective cohort study of infants. Eligible infants born between October 1, 2002, and July 31, 2003, were given SpO2 98‐100%. Eligible infants born between January 1, 2004, and April 30, 2005, were given SpO2 90‐96%. Since allocation of treatment was non‐randomized, this study was excluded from the review. |
| Weintraub 1956 | The planned scheme of quasi‐random, alternate allocation was not adhered to, resulting in the possibility of substantial selection bias, and the study was thus excluded from the review. |
| Wright 2006 | This was a non‐randomized prospective observational study of infants from 3 centres where there was a change in SpO2 from >90%, 89‐94% or 90‐95% to 83‐93% for all centres. Eligible infants born after the transition year were given the lower SpO2 treatment. Since allocation of treatment was non‐randomized, this study was excluded from the review. |
Characteristics of ongoing studies [ordered by study ID]
BOOST NZ (NZ).
| Trial name or title | Benefits of oxygen saturation targeting trial (NZ) |
| Methods | Infants are randomized centrally by telephone, using a computerized interactive voice response system. Randomization is stratified by site, sex, gestation and inborn and outborn. Computer‐generated randomization lists are prepared by an independent statistician and not accessible to staff involved in the daily care of infants. The intervention monitored through Masimo Radical SET pulse oximeters are masked by offsetting the assigned SpO2 by +/‐3% points. Staff will (a) target SpO2 88‐92% and (b) aim to maximize time spent with SpO2 between 85‐95%. From 85‐95%, the offset will be 3% above or below the actual SpO2. Outside 85‐95%, study oximeters read actual SpO2. 320 infants will be enrolled. This data will be analysed with the data from the Australian BOOST‐II trial. A sample size of 1200 infants has 80% power (2p=0.05) to detect an absolute 8% increase or decrease in the composite outcome of death or major disability at 2 years. This would mean one less infant who died or was disabled for every 12 infants managed in the optimal range. This would have similar power to detect a reduction in severe ROP from 10% to 7.8% and in CLD from 40% to 32%. |
| Participants | Infants <27 weeks' gestation at birth and <24 hours old |
| Interventions | Lower (Fn SpO2 85‐89%) vs higher (Fn SpO2 91‐95%) O2 targeting |
| Outcomes | Survival and major disability at 2 years corrected age, other secondary outcomes |
| Starting date | 2006 |
| Contact information | Professor Brian Darlow; Email: brian.darlow@chmeds.ac.nz |
| Notes |
BOOSTII (Australia).
| Trial name or title | Benefits of oxygen saturation targeting trial 2 (Australia) |
| Methods | Infants are randomized centrally by telephone, using a computerized interactive voice response system. Randomization is stratified by site, sex, gestation and inborn and outborn. Computer‐generated randomization lists are prepared by an independent statistician and not accessible to staff involved in the daily care of infants. The intervention monitored through Masimo Radical SET pulse oximeters are masked by offsetting the assigned SpO2 by +/‐3% points. Staff will (a) target SpO2 88‐92% and (b) aim to maximize time spent with SpO2 between 85‐95%. From 85‐95%, the offset will be 3% above or below the actual SpO2. Outside 85‐95%, study oximeters read actual SpO2. A sample size of 1200 infants has 80% power (2p=0.05) to detect an absolute 8% increase or decrease in the composite outcome of death or major disability at 2 years. This would mean one less infant who died or was disabled for every 12 infants managed in the optimal range. This would have similar power to detect a reduction in severe ROP from 10% to 7.8% and in CLD from 40% to 32%. |
| Participants | Infants <27 weeks' gestation at birth and <24 hours old |
| Interventions | Lower (Fn SpO2 85‐89%) vs higher (Fn SpO2 91‐95%) O2 targeting |
| Outcomes | Death or major disability at 2 years corrected age, other secondary outcomes |
| Starting date | 2006 |
| Contact information | Alpana Ghadge; Tel: +61 2 9562 5000; Fax: +61 2 9562 5094 |
| Notes |
BOOSTII (UK).
| Trial name or title | Benefits of oxygen saturation targeting trial 2 (UK) |
| Methods | Infants are randomized centrally by a secure website at the National Perinatal Epidemiology Unit (NPEU) in Oxford. A computer‐generated program that used minimization will be used to ensure balanced allocation to the two arms of the trials in each recruiting unit from a knowledge of weight, gestation and sex at birth. The NPEU is write the randomization program and hold the code. The intervention monitored through Masimo Radical SET pulse oximeters are masked by offsetting the assigned SpO2 by +/‐3% points. Staff will (a) target SpO2 88‐92% and (b) aim to maximize time spent with SpO2 between 85‐95%. From 85‐95%, the offset will be 3% above or below the actual SpO2. Outside 85‐95%, study oximeters read actual SpO2. A sample size of 1200 infants has 80% power (2p=0.05) to detect an absolute 8% increase or decrease in the composite outcome of death or major disability at 2 years. This would mean one less infant who died or was disabled for every 12 infants managed in the optimal range. This would have similar power to detect a reduction in severe ROP from 10% to 7.8% and in CLD from 40% to 32%. Data analysis will be intention to treat. |
| Participants | Infants <28 weeks’ gestation at birth and <12 hours old (24 hours old if the baby is outborn) |
| Interventions | Lower (Fn SpO2 85‐89%) vs higher (Fn SpO2 91‐95%) O2 targeting |
| Outcomes | Death or serious neurosensory disability at 2 years corrected age, other secondary outcomes |
| Starting date | 2007 |
| Contact information | Professor Peter Brocklehurst; Email: peter.brocklehurst@npeu.ox.ac.uk |
| Notes |
COT (Canada).
| Trial name or title | Canadian oxygen trial |
| Methods | Infants are randomized centrally by telephone. Randomization is stratified by gestational age (23‐25 and 26‐27 weeks) and by study centre. Alocation will incorporate variable block sizes. The concealed study allocation will be determined, in advance, using a computer‐based random number generator. The intervention monitored through Masimo Radical SET pulse oximeters are masked by offsetting the assigned SpO2 by +/‐3% points. Staff will (a) target SpO2 88‐92% and (b) aim to maximize time spent with SpO2 between 85‐95%. From 85‐95%, the offset will be 3% above or below the actual SpO2. Outside 85‐95%, study oximeters read actual SpO2. A sample size of 1200 infants has 80% power (2p=0.05) to detect an absolute 8% increase or decrease in the composite outcome of death or major disability at 2 years. This would mean one less infant who died or was disabled for every 12 infants managed in the optimal range. This would have similar power to detect a reduction in severe ROP from 10% to 7.8% and in CLD from 40% to 32%. |
| Participants | Infants <27 weeks' gestation at birth and <24 hours old |
| Interventions | Lower (Fn SpO2 85‐89%) vs higher (Fn SpO2 91‐95%) O2 targeting |
| Outcomes | Death or major disability (cognition, neuromotor function, vision, hearing) at 2 years corrected age, other secondary outcomes |
| Starting date | October 2006 |
| Contact information | Dr Barbara Schmidt; Email: schmidt@mcmaster.ca |
| Notes |
SUPPORT (USA).
| Trial name or title | The surfactant positive airway pressure and pulse oximetry trial in extremely low birth weight infants |
| Methods | This is a prospective, randomized, factorial 2x2 design multi‐centre trial. Randomization will be stratified by gestational age, and will be done utilizing double‐sealed envelopes. The individual factors to be tested will be: 1) A prospective comparison of CPAP and a permissive ventilatory strategy begun in the delivery room and continuing in the NICU with early (<1 hour) surfactant and mechanical ventilation; 2) A prospective comparison of a lower SpO2 range (85% to 89%) with a higher more conventional SpO2 range (91% to 95%) until the infant is no longer requiring ventilatory support or oxygen. The intervention monitored through Masimo Radical SET pulse oximeters are masked by offsetting the assigned SpO2 by +/‐3% points. Staff will (a) target SpO2 88‐92% and (b) aim to maximize time spent with SpO2 between 85‐95%. From 85‐95%, the offset will be 3% above or below the actual SpO2. Outside 85‐95%, study oximeters read actual SpO2. Power has been calculated to be 80% for detecting an absolute difference of 10% in the primary and secondary outcomes, with a sample size of 1310. |
| Participants | Infants <27 weeks' gestation at birth and <24 hours old |
| Interventions | Lower (Fn SpO2 85‐89%) vs higher (Fn SpO2 91‐95%) O2 targeting |
| Outcomes | Death or major disability at 2 years corrected age, survival without BPD at 36 weeks, survival without ROP, other secondary outcomes |
| Starting date | February 2005 |
| Contact information | Dr Neil Finer; Email: nfiner@ucsd.edu |
| Notes |
Differences between protocol and review
In the original protocol, visual function was to be recorded only in the first year of life, but in the review it was expanded to measure long‐term visual function. The outcomes from long‐term visual function observations fit well with the original protocol and subsequent review outcome measures of long‐term growth and neurodevelopment.
In the current review, results have been split into observations made in the early neonatal period of life and the later neonatal period of life. This differentiation was not stated in the protocol. The splitting of the observations was due to the large time gap between when infants commenced the different oxygen strategies: either early in the neonatal period (< 1 week) and later in the neonatal period (> 3 weeks postnatal age).
As stated in the review, some long‐term growth and development measures could not be measured due to no data being available for those outcomes.
Contributions of authors
Askie and Henderson‐Smart developed the original protocol for this review, as well as doing the original literature searching, background, data analysis, discussion and conclusions. Askie and Henderson‐Smart also contributed to the updated version of the review. Ko updated the review with an updated literature search, background with RLF/ROP cross‐classification information and references, data analysis with the new included trial, updated the discussion and conclusions, updated the information on the ongoing clinical trials, and created the GRADE summary of findings tables which will be included at a later date. Askie and Henderson‐Smart reviewed this.
Sources of support
Internal sources
NHMRC Clinical Trials Centre, University of Sydney, Australia.
NSW Centre for Perinatal Health Services Research, University of Sydney, Australia.
External sources
Department of Public Health and Community Medicine, University of Sydney, Australia.
Declarations of interest
Askie and Henderson‐Smart have conducted and published a randomized, controlled trial of the effect of higher vs. standard oxygen saturation targeting on long‐term growth and development of preterm infants.
Edited (no change to conclusions)
References
References to studies included in this review
Askie 2003 {published and unpublished data}
- Askie LM, Henderson‐Smart DJ, Irwig, L, Simpson JM. Oxygen‐saturation targets and outcomes in extremely preterm infants. NEJM 2003;349(10):959‐67. [DOI] [PubMed] [Google Scholar]
Kinsey 1956 {published data only}
- Kinsey VE. Cooperative study of retrolental fibroplasia and the use of oxygen. Archives of Ophthalmology 1956;56:481‐543. [PubMed] [Google Scholar]
Lanman 1954 {published data only}
- Lanman JT, Guy LP, Dancis J. Retrolental fibroplasia and oxygen therapy. JAMA 1954;155:223‐6. [DOI] [PubMed] [Google Scholar]
Patz 1954 {published data only}
- Patz A. Oxygen studies in retrolental fibroplasia. IV. Clinical and experimental observations. American Journal of Ophthalmology 1954;38:291‐308. [DOI] [PubMed] [Google Scholar]
- Patz A. The role of oxygen in retrolental fibroplasia. Pediatrics 1957;19:504‐24. [PubMed] [Google Scholar]
- Patz A, Hoeck LE, Cruz E De La. Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations. American Journal of Ophthalmology 1952;36:1248‐53. [DOI] [PubMed] [Google Scholar]
Sinclair 1968 {published data only}
- Sinclair JC, Engel K, Silverman WA. Early correction of hypoxemia and acidemia in infants of low birth weight. A controlled trial of oxygen breathing, rapid alkali infusion and assisted ventilation. Pediatrics 1968;42:565‐89. [PubMed] [Google Scholar]
Usher 1973 {published data only}
- Coates AL, Desmond K, Willis D, Nogrady MB. Oxygen therapy and long‐term pulmonary outcome of respiratory distress syndrome in newborns. Am J Dis Child 1982;136:892‐895. [DOI] [PubMed] [Google Scholar]
- Usher RH. Treatment of respiratory distress syndrome. In: Winters RW editor(s). The Body Fluids in Pediatrics. Boston & Toronto: Little, Brown and Company, 1974:303‐337. [Google Scholar]
References to studies excluded from this review
Bard 1996 {published data only}
- Bard H, Belanger S, Fouron J. Comparisons of effects of 95% and 90% oxygen saturations in respiratory distress syndrome. Archives of Disease in Childhood 1996;75:F94‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunningham 1995 {published data only}
- Cunningham S, Fleck BW, Elton RA, McIntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet 1995;346:1464‐5. [DOI] [PubMed] [Google Scholar]
Deulofeut 2007 {published data only}
- Deulofeut R, Golde D, Augusto S. Treatment‐by‐gender effect when aiming to avoid hyperoxia in preterm infants in the NICU. Acta Paediatrica 2007;96(7):990‐4. [DOI] [PubMed] [Google Scholar]
Engleson 1958 {published data only}
- Engleson, G, Rooth G, Sjostedt S. Treatment of premature infants with 15% oxygen. Acta Paediatrica Scandinavica 1958;118 Suppl:47‐9. [DOI] [PubMed] [Google Scholar]
- Rooth G, EnglesonG, Tornblom M. A follow‐up study of premature infants treated with low oxygen tension. Acta Paediatrica Scandinavica 1966;55:85‐7. [DOI] [PubMed] [Google Scholar]
- Sjostedt S, Rooth G. Low oxygen tension in the management of newborn infants. Archives of Disease in Childhood 1957;32:397‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 1998 {published data only}
- Fitzgerald D, Asperen PV, Leslie G, Arnold J, Sullivan C. Higher SaO2 in chronic neonatal lung disease: Does it improve sleep?. Pediatric Pulmonology 1998;26:235‐40. [DOI] [PubMed] [Google Scholar]
Gaynon 1997 {published data only}
- Gaynon, MW, Stevenson DK, Sunshine P, Fleisher BE, Landers MB. Supplemental oxygen may decrease progression of prethreshold disease to threshold retinopathy of prematurity. Journal of Perinatology 1997;17:434‐8. [PubMed] [Google Scholar]
Kitchen 1978 {published data only}
- Kitchen WH, Campbell DG. Controlled trial of intensive care for very low birth weight infants. Pediatrics 1971;48:711‐4. [PubMed] [Google Scholar]
- Kitchen WH, Rickards A, Ryan MM, McDougall AB, Billson FA, Keir EH, Naylor FD. A longitudinal study of very low‐birthweight infants. II. Results of controlled trial of intensive care and incidence of handicaps. Developmental Medicine and Child Neurology 1979;21:582‐9. [DOI] [PubMed] [Google Scholar]
- Kitchen WH, Ryan MM, Rickards A, Gaudry E, Brenton AM, Billson FA, Fortune DW, Keir EH, Lundahl‐Hegedus EE. A longitudinal study of very low birthweight infants. I. Study design and mortality rates. Developmental Medicine and Child Neurology 1978;20:605‐18. [DOI] [PubMed] [Google Scholar]
Lundstrom 1995 {published data only}
- Lundstrom KE, Larsen PB, Brendstrup L, Skov L, Greisen G. Cerebral blood flow and left ventricular output in spontaneously breathing, newborn preterm infants treated with caffeine or aminophylline. Acta Paediatrica 1995;84:6‐9. [DOI] [PubMed] [Google Scholar]
- Lundstrom KE, Pryds O, Greisen G. 80% oxygen administration to newborn premature infants causes prolonged cerebral vasoconstriction [abstract]. Pediatric Research 1994;35:275. [Google Scholar]
- Lundstrom KE, Pryds O, Greisen G. Oxygen at birth and prolonged cerebral vasoconstriction in preterm infants. Archives of Disease in Childhood 1995;73:F81‐F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendicini 1971 {published data only}
- Mendicini M, Scalamandre A, Savignoni PG, Picece‐Bucci S, Esuperanzi R, Bucci G. A controlled trial on therapy for newborns weighing 750‐1250g. I. Clinical findings and mortality in the newborn period. Acta Paediatrica Scandinavica 1971;60:407‐16. [DOI] [PubMed] [Google Scholar]
Schulze 1995 {published data only}
- Schulze A, Whyte RK, Way RC, Sinclair JC. Effect of the arterial oxygenation level on cardiac output, oxygen extraction, and oxygen consumption in low birth weight infants receiving mechanical ventilation. Journal of Pediatrics 1995;126:777‐84. [DOI] [PubMed] [Google Scholar]
STOP‐ROP 2000 {published data only}
- The STOP‐ROP Multicenter Study Group. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP‐ROP), a randomized, controlled trial. I: Primary outcomes. Pediatrics 2000;105:295‐310. [DOI] [PubMed] [Google Scholar]
Wallace 2007 {published data only}
- Wallace DK, Veness‐Meehan KA, Miller WC. Incidence of severe retinopathy of prematurity before and after a modest reduction in target oxygen saturation levels. J AAPOS 2007;11(2):170‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weintraub 1956 {published data only}
- Weintraub DH, Tabankin A. Relationship of retrolental fibroplasia to oxygen concentration. Journal of Pediatrics 1956;49:75‐9. [DOI] [PubMed] [Google Scholar]
Wright 2006 {published data only}
- Wright KW, Sami D, Thompson L, Ramanathan R, Joseph R, Farzavandi S. A physiologic reduced oxygen protocol decreases the incidence of threshold retinopathy of prematurity. Transactions of the American Ophthalmological Society 2006;104:78‐84. [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
BOOST NZ (NZ) {unpublished data only}
- Benefits of oxygen saturation targeting trial (NZ). Ongoing study 2006.
BOOSTII (Australia) {unpublished data only}
- Benefits of oxygen saturation targeting trial 2 (Australia). Ongoing study 2006.
BOOSTII (UK) {unpublished data only}
- Benefits of oxygen saturation targeting trial 2 (UK). Ongoing study 2007.
COT (Canada) {unpublished data only}
- Canadian oxygen trial. Ongoing study October 2006.
SUPPORT (USA) {unpublished data only}
- The surfactant positive airway pressure and pulse oximetry trial in extremely low birth weight infants. Ongoing study February 2005.
Additional references
Anderson 2004
- Anderson CG, Benitz WE, Madan A. Retinopathy of prematurity and pulse oximetry: a national survey of recent practices. Journal of Perinatology 2004;24(3):164‐8. [DOI] [PubMed] [Google Scholar]
Askie 2001a
- Askie LM, Henderson‐Smart DJ. Gradual versus abrupt discontinuation of oxygen in preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001075] [DOI] [PMC free article] [PubMed] [Google Scholar]
Askie 2001b
- Askie LM, Henderson‐Smart DJ. Early versus late discontinuation of oxygen in preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001076] [DOI] [PubMed] [Google Scholar]
Avery 1960
- Avery ME, Oppenheimer EH. Recent increase in mortality from hyaline membrane disease. Journal of Pediatrics 1960;57:553. [DOI] [PubMed] [Google Scholar]
Bancalari 1987a
- Bancalari E, Flynn J, Goldberg RN, Bawol R, Cassady J, Schiffman J, Feuer W, Roberts J, Gillings D, Sim E. Influence of transcutaneous oxygen monitoring on the incidence of retinopathy of prematurity. Pediatrics 1987;79:663‐9. [PubMed] [Google Scholar]
Bancalari 1987b
- Bancalari E, Flynn J, Goldberg RN, Bawol R, Cassady J, Schiffman J, Feuer W, Roberts J, Gillings D, Sim E. Transcutaneous oxygen monitoring and retinopathy of prematurity. Adv Exper Med Biol 1987;220:109‐113. [DOI] [PubMed] [Google Scholar]
Baraldi 2007
- Baraldi E, Filippone M. Chronic lung disease after premature birth. New England Journal of Medicine 2007;357(19):1946‐55. [DOI] [PubMed] [Google Scholar]
Chan‐Ling 1995
- Chan‐Ling T, Gock B, Stone J. Supplemental oxygen therapy. Basis for noninvasive treatment of retinopathy of prematurity. Investigative Ophthalmology and Visual Science 1995;36:1215‐30. [PubMed] [Google Scholar]
Chow 2003
- Chow LC, Wright KW, Sola S. Can changes in clinical practice decrease the incidence of severe retinopathy in very low birth weight infants?. Pediatrics 2003;111(2):339‐45. [DOI] [PubMed] [Google Scholar]
Coates 1982
- Coates AL, Desmond K, Willis D, Nogrady B. Oxygen therapy and long‐term pulmonary outcome of respiratory distress syndrome in newborns. American Journal of Diseases of Children 1982;136:892‐5. [DOI] [PubMed] [Google Scholar]
Cote 1988
- Cote CJ, Goldstein EA, Cote MA, Hoaglin DC, Ryan JF. A single‐blind study of pulse oximetry in children. Anesthesiology 1988;68:184‐8. [DOI] [PubMed] [Google Scholar]
Cross 1973
- Cross KW. Cost of preventing retrolental fibroplasia?. Lancet 1973;2:954‐6. [DOI] [PubMed] [Google Scholar]
Duc 1992
- Duc G, Sinclair JC. Oxygen Administration. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:178‐98. [Google Scholar]
Flynn 1987
- Flynn J, Bancalari E, Bawol R, Goldberg R, Cassady MA, Schiffman J, Feuer W, Roberts J, Gillings D, Sim E, Buckley E, Bachynski BN. Retinopathy of prematurity. A randomized prospective trial of transcutaneous oxygen monitoring. Ophthalmology 1987;94:630‐637. [DOI] [PubMed] [Google Scholar]
Garner 1985
- Garner A. The pathology of retinopathy of prematurity. In: Silverman WA, Flynn JT editor(s). Retinopathy of prematurity. Boston: Blackwell Scientific Publications, 1985:19‐52. [Google Scholar]
Gunn 1980
- Gunn TR, Easdown J, Outerbridge EW, Aranda JV. Risk factors in retrolental fibroplasia. Pediatrics 1980;65:1096‐100. [PubMed] [Google Scholar]
Hindle 1986
- Hindle NW. Cryotherapy for retinopathy of prematurity: timing of intervention. British Journal of Ophthalmology 1986;1986:269‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hindle 1990
- Hindle NW. Critical mass retinopathy of prematurity: What is it and what can you do about it?. Documenta Ophthalmologica 1990;74:253‐62. [DOI] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163:1723‐9. [DOI] [PubMed] [Google Scholar]
Kinsey 1977
- Kinsey VE, Arnold HJ, Kalina RE, Stern L, Stahlman M, Odell G, Driscoll JM, Elliott JH, Payne J, Patz A. PaO2 levels and retrolental fibroplasia: a report of the cooperative study. Pediatrics 1977;60:655‐68. [PubMed] [Google Scholar]
Lloyd 2003
- Lloyd J, Askie L, Smith J, Tarnow‐Mordi W. Supplemental oxygen for the treatment of prethreshold retinopathy of prematurity. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003482] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 1963
- McDonald AD. Cerebral palsy in children of low birth weight. Archives of Disease in Childhood 1963;38:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntosh 2001
- McIntosh N, Marlow N. High or low oxygen saturation for the preterm baby. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;84:F149‐50. [PubMed] [Google Scholar]
Payne 1979
- Payne JW, Patz A. Current status of retrolental fibroplasia: the retinopathy of prematurity. Ann Clin Res 1979;11:205‐21. [PubMed] [Google Scholar]
Phelps 1984
- Phelps DL, Rosenbaum AL. Effects of marginal hypoxemia on recovery from oxygen‐induced retinopathy in the kitten model. Pediatrics 1984;73:1‐6. [PubMed] [Google Scholar]
Poets 1998
- Poets CF. When do infants need additional inspired oxygen? A review of the current literature. Pediatric Pulmonology 1998;26:424‐8. [DOI] [PubMed] [Google Scholar]
Reese 1953
- Reese AB, King M, Owens WC. A classification of retrolental fibroplasia. American Journal of Ophthalmology 1953;36:1333‐5. [PubMed] [Google Scholar]
Roemer 2005
- Roemer VM. Outcome measures in perinatal medicine‐‐p02 and S02. With remarks on pulse oximetry. Zeitschrift fur Geburtshilfe und Neonatologie 2005;209:173‐85. [DOI] [PubMed] [Google Scholar]
ROP Committee 1984
- Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. British Journal of Ophthalmology 1984;68:690‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
ROP Committee 1987
- Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. II The classification of retinal detachment. Archives of Ophthalmology 1987;105:906‐12. [PubMed] [Google Scholar]
Sira 1988
- Sira IB, Nissenkorn I, Kremer I. Retinopathy of prematurity. Survey of Ophthalmology 1988;33(1):1‐16. [DOI] [PubMed] [Google Scholar]
Sun 2002
- Sun SC. Relation of target SpO2 levels and clinical outcome in ELBW infants on supplemental oxygen. Pediatric Research 2002;51:350A. [Google Scholar]
Szewcyk 1953
- Szewcyk TS. Retrolental fibroplasia and related ocular diseases. American Journal of Ophthalmology 1953;36(10):1333‐61. [DOI] [PubMed] [Google Scholar]
Tin 2001
- Tin W, Milligan DWA, Pennefather P, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Archives of Disease in Childhood Fetal Neonatal Edition 2001;84:F106‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watkin 1999
- Watkin SL, Spencer SA, Dimmock PW, Wickramasinghe YA, Rolfe P. A comparison of pulse oximetry and near infrared spectroscopy (NIRS) in the detection of hypoxaemia occurring with pauses in nasal airflow in neonates. Journal of Clinical Monitoring and Computing 1999;15:441‐7. [DOI] [PubMed] [Google Scholar]
Wilson 1942
- Wilson JL, Long SB, Howard PJ. Respiration of premature infants: response to variations of oxygen and to increased carbon dioxide in inspired air.. American Journal of Diseases of Children 1942:1080‐5. [Google Scholar]
References to other published versions of this review
Askie 2001c
- Askie LM, Henderson‐Smart DJ. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants (Cochrane Review). Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001077] [DOI] [PubMed] [Google Scholar]


