Abstract
Today, vaccinologists have come to understand that the hallmark of any protective immune response is the antigen. However, it is not the whole antigen that dictates the immune response, but rather the various parts comprising the whole that are capable of influencing immunogenicity. Protein-based antigens hold particular importance within this structural approach to understanding immunity because, though different molecules can serve as antigens, only proteins are capable of inducing both cellular and humoral immunity. This fact, coupled with the versatility and customizability of proteins when considering vaccine design applications, makes protein-based vaccines (PBVs) one of today's most promising technologies for artificially inducing immunity. In this review, we follow the development of PBV technologies through time and discuss the antigen-specific receptors that are most critical to any immune response: pattern recognition receptors, B cell receptors, and T cell receptors. Knowledge of these receptors and their ligands has become exceptionally valuable in the field of vaccinology, where today it is possible to make drastic modifications to PBV structure, from primary to quaternary, in order to promote recognition of target epitopes, potentiate vaccine immunogenicity, and prevent antigen-associated complications. Additionally, these modifications have made it possible to control immune responses by modulating stability and targeting PBV to key immune cells. Consequently, careful consideration should be given to protein structure when designing PBVs in the future in order to potentiate PBV efficacy.
Keywords: vaccine, immunity, antigen, epitope, modification, vaccine composition, vaccine structure
Introduction
Physicians have been aware of the effects of vaccination for over a millennium, though they had little understanding of the mechanisms through which immunity was achieved until the early nineteenth century. From the simple and dangerous live-pathogen variolation employed in ancient and medieval times to the recombinant protein and DNA vaccines we use today, vaccine development has followed a path of improving efficacy and safety due to an ever-increasing understanding of these mechanisms. The hallmarks of this understanding have been that an adaptive immune response cannot take place without an antigen and that the most effective antigens tend to be proteins. Ultimately, these two facts have led the field of vaccinology to focus on the research and development of protein-based vaccine (PBVs). As such, we now have a dense literature pool that can be used to recapitulate past and present PBV design concepts and illuminate fundamental structural vaccinology principles in PBV design.
Bacterial toxin vaccines were the first PBVs to be developed. They originally consisted of antitoxin isolated from animals inoculated with small quantities of unmodified toxin, but later it was discovered that active immunization could be safely achieved if toxin was either (1) co-administered with a sufficient amount of antitoxin (partial neutralization) or (2) treated chemically or thermally prior to vaccine administration (denaturation). Chemically inactivated toxin, dubbed toxoid, would go on to garner widespread celebrity due to its success in World War II and eventually become the primary means for immunization against Diphtheria and Tetanus (1). The development of these toxoid vaccines, along with the gradual acceptance of the side chain theory, the establishment of immunological proteomics, and the application of genetic engineering technologies, paved the way for the emergence of the PBVs we know today (2).
Recombinant vaccines, as their name suggests, are PBVs that are produced using recombinant DNA technology. The first recombinant PBV was produced in yeast in 1986 and targeted the surface antigen of the Hepatitis B virus (HBV) (3). Though it was one of the first recombinant proteins to be approved for use in humans, its discovery was largely overlooked due to perceived limitation in likely impact coupled with the fact that a serum-derived vaccine for HBV already existed (3). However, the success of this virus-like particle-based (VLP) PBV spurred an increased interest in whether the same recombinant strategy could be implemented with other viruses. This idea flourished over time, and today recombinant vaccine approaches have been attempted for nearly every viral structural protein identified. Results from this research have been considerable, with five VLP-based PBVs having been commercialized and dozens having reached clinical trials (4). It is important to note, however, that recombinant PBVs are not limited to VLP formulations, as many bacterial pathogen-derived and tumor-associated antigen-derived recombinant PBVs have also been developed (5, 6).
Conjugate vaccines, which consist of carrier protein—subunit complex, were conceived in the late 1980's in order to address a growing need for more effective vaccines against encapsulated bacterial pathogens from the Neisseria, Streptococcus, Staphylococcus, Haemophilus, and Pseudomonas generas. Before their discovery, vaccine formulations targeting these pathogens singularly consisted of polysaccharide (typically the exposed glycan from encapsulated bacterial surfaces). Although these polysaccharide vaccines were shown to elicit the production of protective antibodies, they proved to be tremendously ineffective at conferring protection in young and immunocompromised individuals and largely failed to elicit immunological memory (7). The limited success of the first subunit polysaccharide vaccines was eventually concomitant to the discovery that polysaccharide vaccines are unable to recruit the assistance of T helper cells and thus rely on T cell-independent activation alone (8). Protein-based, subunit vaccines, in contrast, were found to have all the components necessary to initiate T cell-dependent activation of B cells, a process characterized by a more robust immune response, affinity maturation, and immunological memory (9).
Toxoids have traditionally been used as carrier proteins in conjugate PBV formulations because of their excellent immunogenicity, availability, and simplicity (10). Many of the conjugate PBVs being developed today, however, use recombinantly produced carrier proteins that have been specifically designed to maximize efficacy while simultaneously maintaining a good safety profile (11). The first carrier protein of this type, cross-reactive material 197 (CRM197), was discovered upon the random, mutagenic conversion of glutamic acid to glycine at position 52 of diphtheria toxin (DT, Figure 1A). Though distal to the ADP-ribosyltransferase active site found on the A subunit of DT, this single point mutation on the B subunit was able to completely eliminate DT's toxicity without negatively impacting its ability to stimulate the immune system (19–21). The discovery of CRM197 ultimately led to the realization that the inherent toxicity of the antigens typically employed in conjugate PBV formulations could be modulated using precise structural modifications as opposed to broad-based chemical and thermal denaturation. Thus, the idea of structure-based vaccinology was born and a growing trend in research involving “designer vaccines” began. Since its conception, this concept has been applied to a plethora of pathogenic determinants, specifically toxins. It was observed that the use of cholera toxin B subunit (CTB) in PBV formulations, as opposed to complete toxin, could lead to improved safety profiles with little-to-no decline in overall immunogenicity (Figure 1B). The improved safety was attributed to the missing A1 domain, the portion of cholera toxin responsible for intracellular activity that leads to disease symptoms (22). A similar discovery was made for tetanus toxin when it was revealed that the heavy chain C fragment (TTc), when used as an immunogen, could confer protection upon toxin challenge in a mouse model without eliciting the neurotoxic effects of its parent protein (Figure 1C) (23). Unfortunately, the use of TTc in modern vaccines may be discouraged by its capacity to bind neurons, though researchers have undertaken structural and conformational approaches to the modulation of this interaction (23, 24). Similar methods to those outlined here have also be employed with other toxins, such as heat-liable enterotoxin (a close relative of cholera toxin) and botulinum toxin (a close relative of tetanus toxin) (12, 25).
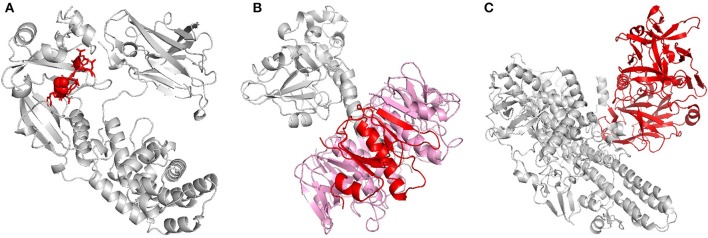
Figure 1.
Recombinant toxins. (A) Diphtheria toxin (DT), when replacing glycine with glutamic acid at position 52, loses its toxicity without affecting its antigenicity. The highlighted residues (red) indicate the exact residue (sphere) and area (licorice) where this substitution would occur on monomeric DT. (B) Cholera toxin (CT) is composed of six subunits; one A subunit and five B subunits. B subunit (monomer in red, remaining subunits in pink), which lacks the toxicity of its partner A subunit, has proven to be extremely immunogenic and is used as a carrier protein and adjuvant. B subunit of heat-labile enterotoxin, which shares much of the same homology as B subunit of cholera toxin, has been similarly investigated (12). (C) Tetanus toxin (TT) is comprised of two chains, a light chain and a heavy chain, of which the light chain is responsible for the protein's toxicity. In the past, proteolytic digestion of TT with papain yielded two fragments, a light chain-containing, toxic B fragment and a non-toxic C fragment (red). Vaccination with the non-toxic C fragment was found to be protective against lethal toxin dose in a mouse model, and today PBVs comprised of recombinant C fragment are being investigated as a potential replacement for TT vaccines. Botulinum toxin, which shares much of the same homology as tetanus toxin, has been similarly investigated (13). The 3D protein structures for DT, CT, and TT used in this image were rendered in PyMOL 2.3.0 and accessed via the Protein Data Bank (14–18).
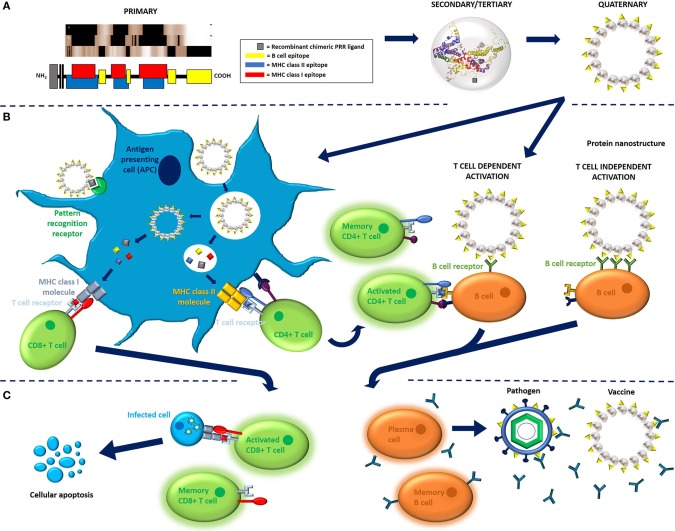
Today, interest in “designer vaccines” has been increasingly fueled by advancements in our understanding of the mechanisms behind innate and adaptive immunity, specifically the role of antigen composition in PBV immunogenicity (Figures 2A–C). X-ray crystallography, genetic sequencing, epitope prediction algorithms, and in vivo studies of adjuvant properties have all lead to a better understanding of why some proteins are simply more immunogenic than others. Ultimately, differences in protein structure can result in different capacities for antigen to interact with cells and receptors that are key to triggering an immune response (30). Knowledge of this phenomenon has led to a situation where vaccines are no longer conceptualized based on whole antigen, but rather on immune receptor epitopes/ligands and propensity of an antigen (based on structural motifs) for uptake by antigen presenting cells (APCs). As such, many of today's PBVs are engineered using structural vaccinology principles and rationally target APCs and the three receptor groups key to any adaptive immune response; the pattern recognition receptors (PRRs), the B cell receptors (BCRs), and the T cell receptors (Figures 3A–C).
Figure 2.
Immunological mechanisms of recombinant, protein-based vaccination. (A) PBV structure, as illustrated here for the model protein hepatitis B core antigen (HBcAg, 183 aa long, non-truncated form, Accession number P03146), is ultimately determined by primary sequence. Vaccine can comprise monomeric antigen (i.e., toxoid protein) or multimeric antigen (i.e., virus-like particles), though multimeric antigen is used for demonstration purposes here. T cell and B cell antigenic determinants can be identified in primary sequence using various in vitro and in silico methods. The linear MHC epitopes illustrated here were predicted using Epitope Analysis Resources on the Immune Epitope Database (IEDB) website. More specifically, MHC epitopes were predicted for HLA-A*02:01 (class I molecules) and all heterodimer combinations of DQB1*02:01 (class II molecules) using IEDB recommended methods. Linear B cell epitopes, on the other hand, were assigned using frequency analysis results from the IEDB website (26). PBV structures are color coded to represent epitope content. (B) Cell processing and activation in response to PBV is generally orchestrated by antigen presenting cells, of which the most important are dendritic cells. APCs sample their environment via endocytosis, specifically via receptor-mediated endocytosis when antigen presents glycan and/or protein pathogen-associated molecular patterns such as high mannose glycans or bacterial flagellin. Depending on the structural and compositional characteristics of the antigen, APCs will either process antigen via MHC class II pathway or MHC class I pathway using a mechanism known as cross-presentation, respectively, resulting in activation of either CD4+ (helper) or CD8+ (cytotoxic) T cell response. CD4+ T cell activation requires co-activating signals and results in the proliferation of effector and memory CD4+ T cell pools. Effector CD4+ T cells go on to assist with the activation of B cells (T cell dependent activation) and provide survival signals to activated CD8+ T cells, whereas CD8+ T cells have immediate effector functionality. B cells can also undergo T cell independent activation when antigen cross-links multiple BCRs on B cells surface (TI-2 activation) or co-signals via PRR (TI-1 activation) (27, 28). (C) Activation results in the proliferation of memory and effector cytotoxic T cell and B cell pools. Memory CD8+ cells remain dormant until they encounter cells presenting MHC class I molecules loaded with cognate epitope, upon which they begin mounting an effector response. Effector CD8+ T cells go on to instruct apoptosis in cells presenting MHC class I molecules loaded with cognate epitope. B cells activated via T cell independent pathway generally proliferate into short-lived plasmablasts that express low affinity IgM antibodies (not shown). B cells activated via T cell dependent pathway, on the other hand, result in the proliferation of memory B cells and long-lived plasma cells expressing high-affinity IgA, IgE, or IgG antibodies. Antibodies secreted by plasms cells (and plasmablasts) go on to bind vaccine and pathogen and initiate antibody effector functions. Memory B cells remain dormant until they encounter antigen presenting cognate epitope, upon which they rapidly proliferate and clones either class switch to become antibody secreting plasma cells or re-enter germinal centers and restart affinity maturation processes (27, 28). The 3D protein structure for HBcAg used in this image was rendered in PyMOL 2.3.0 and accessed via the Protein Data Bank (14, 18, 29).
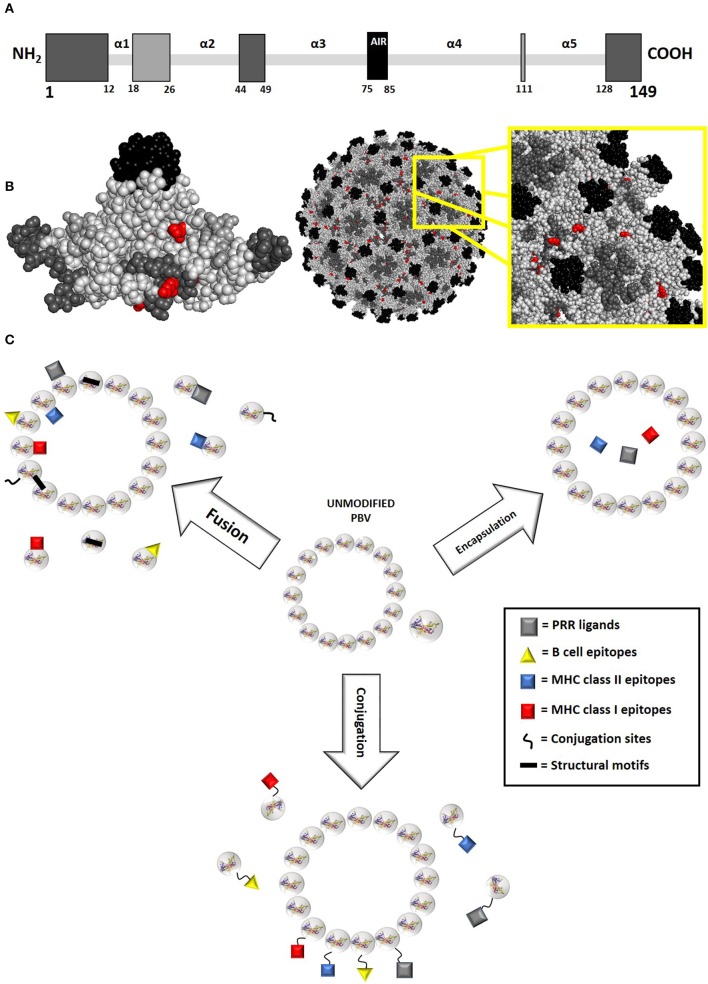
Figure 3.
PBV modification principles. (A) Potential fusion modifications sites for the model protein hepatitis B core antigen (HBcAg, 149 aa long, truncated form of AN P03146 used for vaccine purposes) as represented by primary structure. Residues are color-coded in gray scale, with darker residues indicating more exposed insertion locations. Polypeptide termini and random coil loop regions are primary targets for PBV fusion modification, as they generally have the least effect on protein structure. Within these considerations, surface regions that do not participate in intra- and intermolecular interactions are preferred. Specifically, most HBcAg fusion PBVs presenting foreign B cell epitopes have been modified within the α3α4 loop (AIR between amino acids 75 and 85), though modifications are also routinely made at the C and N termini. A different approach to insertion site selection should be taken when creating fusion PBVs targeting T cell immune responses, as antibody response to epitope becomes detrimental. Toward this goal, inserting epitopes within loop regions that are less exposed and less likely to negatively influence protein stability is optimal (31, 32). (B) Potential fusion modification and conjugation sites for truncated HBcAg model protein as represented by higher order folded and assembled structure. Residues are color-coded in gray scale, with darker residues indicating more exposed insertion locations. Natural conjugation sites (lysine and cysteine) have also been highlighted in red. (C) PBV modifications are generally orchestrated via fusion, conjugation, or encapsulation. Each type of modification occurs at a different level of protein structure, with fusion inserts occurring within primary structure, conjugated inserts occurring within secondary/tertiary structure, and encapsulated inserts occurring within the quaternary structure of proteins that form enclosed, organized matrixes. As such, both monomeric and multimeric protein can accommodate fusion and conjugation modifications, whereas only multimeric protein can accommodate encapsulation. The 3D protein structure for HBcAg used in this image was rendered in PyMOL 2.3.0 and accessed via the Protein Data Bank (14, 18, 29).
Targeting Pattern Recognition Receptors
PBVs, PRRs, and the Innate Immune System
PRRs are specific for highly conserved molecular signals indicating the presence of cell damage and/or pathogens. They activate the innate arm of the immune system, resulting in the production of pro-inflammatory cytokines and chemokines. This activation assists in the maturation of the adaptive immune response and can be critical to the success or failure of a PBV. There are a myriad of PRRs that have been discovered that recognize either pathogen associated molecular patterns (PAMPs) or damage associated molecular patterns (DAMPs) (33). Generally, it is the responsibility of the adjuvant in vaccine formulations to activate the innate immune system. To this effect, researchers usually co-administer free PRR ligand, such as Toll-like receptors (TLRs, intracellular and extracellular membrane-bound PRRs), NOD-like receptors (NLRs, intracellular cytosolic PRRs), C-type lectin receptors (CLRs, extracellular membrane-bound PRRs), and/or RIG-I-like receptor (RLRs, intracellular cytosolic PRRs) agonists with the target antigen when they want to potentiate PBV immunogenicity (34). This potentiation is crucial to PBV success, as choice of adjuvant has a profound influence on both the magnitude and type of immune response the vaccine ultimately elicits (for more on this, please refer to the excellent reviews of Del Giudice et al. and Bonam et al.) (35, 36). It is also possible to rationally incorporate PRR ligands with PBVs using various techniques, an approach that imparts a distinct advantage over simple co-formulation in that the immunogen maintains proximity to the antigen.
Rational Incorporation of PTMs as PAMPs
One strategy for the incorporation of PRR agonist is the manipulation of post translational modifications (PTMs) through the selection of the expression host (37). Since PTM types and sites vary widely between species, they are believed to direct the innate immune response against antigen (38). The PTM we know most about when concerning vaccine immunogenicity is glycosylation. With the exception of E. coli, all expression hosts that are commonly used for recombinant PBV production incorporate glycans on protein surfaces (39). These glycans can serve as ligands for many PRRs, specifically those of the CLR family (40). In fact, glycans have long been known to elicit immune responses to recombinant therapeutic proteins (41–43), and many of the potent adjuvants co-formulated with today's PBVs are polysaccharides (44–48). In one instance, glycosylation patterns were even shown to potentiate immune response to subunit vaccine (49). A thorough literature search, however, indicated that the modulation of PBV immunogenicity via the rational selection of the expression host has never been directly assessed. Considering that PTMs are incorporated in vivo on specific, surface-exposed amino acids found on most proteins, it should be possible to exploit expression host glycosylation patterns when designing PBVs. Likewise, the potentiation of post-translational modification through recombinant addition of these residues within protein primary structure where modification is likely to occur might prove to be an effective means of improving PBV immunogenicity. Detrimentally, however, PRR ligands also have the capacity to act as BCR and TCR epitopes (also known as antigenic determinants) and stereometrically crowd antigen surface. This can result in masked immunogenicity of and/or immune responses being redirected away from important epitopes contained within PBVs. A perfect example of this phenomenon was reported by Ansari et al. when they observed improved in vitro viral neutralization and in vivo epitope-specific antibody response to glycoprotein 5 (GP5) of the porcine reproductive and respiratory syndrome virus (PRRSV) when key N-linked glycosylation sites were eliminated from the immunodominant, N-terminal ectodomain (50).
Facilitating PAMP Proximity to PBV via Fusion
Another way to engineer PBVs that can directly engage a wide variety of PRRs is to covalently fuse PRR agonists to the PBV. This can be accomplished during protein synthesis via the inclusion of the gene for a protein adjuvant within the open reading frame of the target antigen (chimeric approach) or post synthesis by using a number of biochemical techniques (conjugate approach). Mechanistically, fusion of protein-based PRR ligands to PBVs ensures proximity of adjuvant to antigen, thus increasing the likelihood of antigen-adjuvant co-delivery to key immune cells. Ultimately, this approach to adjuvanting is intended to improve immune response profile and intensity while simultaneously minimizing off-target effects (51). It is important to note, however, that chimeric and conjugate modification of antigen with PRR ligand can have detrimental impacts similar to those mentioned previously for natural PRR ligand incorporation. For example, one study was able to show that the chemical conjugation of imidazoquinoline compound 3M-012, a TLR7/8 agonist, to HIV envelope glycoprotein gp120 results in improved in vitro expression of IFNα by peripheral dendritic cells (DCs) while simultaneously abrogating the binding of critical, broadly neutralizing antibodies (52).
Chemical Conjugation of PAMP to PBV
Chemical conjugation of PRR to PBV surface has been explored by many research groups. Chang et al. attached flagellin to the surface of ovalbumin (OVA) nanoparticles (NPs) and observed improved TLR5 activation in HeLa cells when compared with OVA NPs without adjuvant. However, there was no statistical difference when comparing OVA NPs that were conjugated to flagellin with those that were co-administered. These results indicated that conjugating adjuvant to antigen is a viable means of activating the innate immune system, but failed to implicate an effect of adjuvant proximity to antigen on PRR signaling (53). Alternatively, Kastenmüller et al. found that conjugation of TLR7/8 agonist to OVA improved DC uptake of antigen and subsequent innate immune activation when compared to co-administered antigen and adjuvant (54). A similar study targeted TLR7 by chemically conjugating the adenine-based adjuvant SA-26E to recombinantly expressed group 2 allergen from the house dust mite. Results indicated that conjugation not only improved innate immune response when compared to co-administered antigen and adjuvant, but also that conjugation was capable of redirecting immune response away from the Th2 cell subtype associated with hypersensitivity (55). Tighe et al. observed comparable results when conjugation, but not coadministration, of CpG oligonucleotide (CpG ODN, a TLR9 agonist) with the major short ragweed allergen Amb a 1 led to polarization of Th1 response in mice and higher IgG antibody titers in rabbits and monkeys (56). Schulke et al. observed enhanced secretion of all evaluated pro-inflammatory cytokines without bias toward the activation of any one T cell subtype when monophosphoryl lipid A (MPLA) was chemically conjugated to OVA and used to stimulate DC/T cell co-cultures (57). In one example of a PBV targeting a tumor associated antigen (TAA), McKee et al. observed increased activation of gp33-specific cells and prophylactic protection against tumor challenge in a mouse model when they conjugated α-galactosylceramide (a CD1d agonist) to a chimeric VLP consisting of gp33 MHC class I epitope and rabbit hemorrhagic disease virus protein (58). Finally, results from two additional studies indicate that adjuvant-mediated activation of the innate immune system is variable between species and that LPS and MPLA, two PRR ligands that signal through the same TLR4 receptor, has differing capacities to activate the innate immune system in vitro (59, 60). Together, these results indicate that PRR conjugation is a viable means of potentiating innate immune system activation, though it appears that there is still more to understand when considering the exact nature of the resulting immune response.
Genetic Fusion of PAMP to PBV
One example of recombinant fusion of PRR with PBVs are flagellin-fused antigens. These vaccines have intrinsic self-adjuvanting properties through their activation of TLR5 and, if the PBV is able to survive endocytosis, the NLRs NLRC4 and NAIP5 (61, 62). In one study assessing this approach, an 11.9-fold increase in hemagglutination inhibition assay titers was observed when a flagellin-fused hemagglutinin vaccine was compared with commercial influenza PBVs (63). Another promising study reported improved antibody titers (~60% increase), improved viral neutralization titers (~3-fold increase), and improved Th1 cytokine profile (~2-fold increase in IFNγ and TNFα expression) when comparing the effectiveness of flagellin-modified and wild-type porcine circovirus type 2 Cap protein (64). Additionally, many other studies evaluating the effectiveness of recombinantly fusing flagellin to PBV have shown promising results, with the majority reporting improved overall immunogenicity and/or survival when compared to antigen administered alone (65–71). Unsurprisingly, results like these have propelled multiple flagellin-fused PBVs targeting influenza to clinical trials (72–75). None of these candidates achieved commercialization, however, possibly due to the cytokine storm (a phenomenon that occurs when the immune system uncontrollably releases proinflammatory signals) observed upon vaccine administration in clinical trials (74). Nonetheless, structurally modified flagellin is still being investigated as a potential PRR fusion partner for antigen within recombinant PBV formulations (76, 77).
Another example of the PBV-PRR fusion approach to vaccination are recombinant and synthetic lipoprotein-based vaccines (LPBVs). These have become a popular research topic due to their ability to self-adjuvant via TLR2/1–6 heterodimers (78). LPBVs consist of a polypeptide sequence with a PRR-activating, N-terminal diacyl or triacyl lipid attachment (79). Incorporation of target epitopes using chimeric and conjugate approaches have been demonstrated in LPBVs targeting tuberculosis (TB), human papilloma virus, hepatitis C virus, influenza A virus, human immunodeficiency virus (HIV), and cancer (80–85). Generally, augmentation of immunogenicity has been reported in these studies, though it is important to note that prophylactic efficacy of the TB vaccine and therapeutic efficacy of the HIV vaccine was not observed (81, 84). Expression host may be an important factor in LPBV production as fatty acid incorporation in vivo not only varies among species but also results in a mixture of different lipoprotein structures that may have varying immunomodulating properties (86). This principle, however, has only been demonstrated when comparing the immunogenicity of naturally and synthetically produced LPBVs (87).
Facilitating PAMP Proximity to PBV via Encapsulation
PRR agonists can also be encapsulated by antigen when working with proteins that self-assemble into organized matrixes, as has been demonstrated by the “packaging” of nucleic acids and proteins within various VLPs (88–93). Post-expression encapsulation can be achieved via simple diffusion through vaccine matrix pores when working with nucleic acids, though assembly and disassembly cycling may be necessary to encapsulate larger particles, such as proteins. The efficiency of this process, at least when considering nucleic acids, is attributed to electrostatic interactions between PBV interior and PRR agonists (94). The natural encapsulation of ssRNA upon expression of recombinant, RNA-containing bacteriophage VLPs in E. coli has been observed, illuminating another mode in which choice of expression host can influence antigen composition (95). Encapsulation has been shown to improve the half-life of nucleic acids (over 9-fold in some instances), most likely due to the reduced access of endonucleases mediated by vaccine matrix (96, 97). Additionally, as vaccine matrix payload won't be accessible to PRRs until after cellular uptake of vaccine, this approach ensures that encapsulated PRR agonists are more effectively delivered to endosomal and/or cytosolic PRRs (4). This is especially important for nucleic acid PRR agonists, as all PRRs recognizing nucleic acids are intracellular (98). Finally, encapsulation of PRR agonist may also prove advantageous in the design of PBVs targeting TAAs, as the efficacy of these vaccines is generally reliant on the activation of cytotoxic lymphocytes (99). This may be the reason why the CpG-ODN loaded MelQbG10 VLP based cancer vaccine (Qbeta VLP covalently linked to TAA MART-116−35) developed by Speiser et al. was able to successfully reach Phase IIa in clinical trials (100).
Targeting B Cell Receptors
PBVs and the B Cell
BCRs (membrane bound immunoglobulin-CD79 protein complexes) and the B cell maturation process are used by the adaptive immune system to identify and neutralize linear and conformational epitopes exposed on the surface of antigens. Their activation eventually results in the proliferation of plasma cells and the subsequent secretion of antibodies that are highly specific for their target epitope. This, in turn, confers protection through various mechanisms that eventually result in antigen clearance and further stimulation of both the adaptive and innate immune system (27). Targeting the BCR in a vaccination strategy can therefore greatly impact the efficacy of a vaccine, especially if antibody-mediated neutralization or sequestration is required.
Mechanisms Behind BCR Recognition of PBV
Many structures can serve as BCR epitopes in PBV designs, though there are some limitations associated with BCR complimentary determining region (CDR, the portion of an immunoglobulin or TCR that interacts with antigen) binding (101). To start, initial BCR CDR binding is largely beyond the control of PBV design. This is because BCR CDR structure, and therefore binding, is randomly determined in pro-B cells via variable, diversity, and joining (VDJ) gene recombination (102). PBV design can influence BCR CDR structure during subsequent, somatic hypermutation rearrangements through many different mechanisms, though efficient control over these rearrangements is difficult to achieve (103). These mechanisms are mostly epitope-specific and include epitope localization within antigen, competition between epitopes, epitope shape, and epitope size. BCR epitope localization is an important consideration in PBV design because exposed regions on antigen surface will always have the highest probability of becoming antibody immunodominant regions (AIRs) due to increased BCR access (104). Epitope competition refers to situations in which a PBV has multiple AIRs that compete with each other for BCR recognition. This results in the generation of multiple, polyclonal memory B cell pools that compete with each other for PBV upon boosting, an effect that can be detrimental when attempting to target or avoid specific AIRs (105–107). Epitope shape refers to the inherent ability of a conformational epitope to fit within the CDR of a BCR. The sequence of a linear epitope similarly influences BCR recognition and can be included in this category. Prediction software has been developed for the purpose of recognizing both conformational and linear BCR epitopes, indicating that receptors do preferentially interact with epitopes displaying certain quantifiable properties (i.e., patterns in sequence) (108). However, the utility of these predictions when considering PBV design is limited due to small and inconsistent datasets, difficulties predicting antigen 3D structure, and the structural heterogeneity exhibited by some PBVs (108–111). The last consideration, epitope size, consists of constraints that are somewhat vague. Conceivably, any molecule or portion of a larger antigen that is soluble and large enough to initiate a BCR signaling cascade can be considered a BCR epitope. As such, there is no upper limit on antigen size outside of reasonable physiological constraints and any lower size limitation most likely coincides with major histocompatibility complex (MHC) binding limitations (where 10–20 mer polypeptides are necessary). BCR epitope size, on the other hand, is only limited by the diameter of recognition on a BCR paratope (~40 Å). A lower threshold for epitope size must also exist, but where this falls is subjectively based on the extent of non-covalent interaction between paratope and epitope.
Chemical Conjugation of BCR Epitope to PBV
Inherently non-immunogenic molecules that fall below the antigen size threshold but have the capacity to sufficiently interact with antibody paratope can be made immunogenic via covalent attachment to a larger immunogen. These molecules, dubbed haptens, are the premise behind conjugate PBVs. It is conceivable that any molecule could be forced into the role of a BCR epitope using this approach. This rationale is exemplified by conjugate PBVs that have successfully elicited humoral immune responses against glycans, self-antigens, and drugs of abuse (112–114). When designing a conjugate PBV, the most important considerations are choice of protein carrier and choice of hapten. Choice of protein carrier is crucial because it determines the number of potential conjugation sites and where they are located. These properties affect (1) conjugation efficiency, (2) conjugation number, and (3) masking of carrier protein AIRs. This, in turn, has a profound influence on the ability of conjugate PBVs to redirect the humoral immune response away from carrier protein and toward hapten (115). Additionally, the number of MHC class II epitopes found within carrier protein primary sequence and their affinity for receptors can potentiate humoral immune response through (1) the targeting of specific human leukocyte antigen (HLA) haplotypes within a population and (2) the activation of helper T (TH) cells (30, 116). This applies more to the targeting of TCR, however, and as such will be discussed in future sections. Choice of hapten has been shown to have a sizeable impact on the number and binding characteristics of antibodies elicited by conjugate PBVs (117, 118). This effect is exemplified in nicotine vaccines by the difference in efficacy observed when only the attachment position to nicotine is changed (119). Consequently, hapten design has been extensively investigated for PBV formulations targeting drugs of abuse, as these vaccines require large quantities of high affinity antibodies in order to effectively sequester drug in the blood and extracellular fluid (120, 121).
Genetic Fusion of BCR Epitope to PBV
A similar embodiment to the conjugate PBV is the chimeric, fusion PBV. By definition, the flagellin and lipoprotein fusion proteins described previously can be considered chimeric PBVs. However, within this context, the term most often refers to immunogens that have been rationally modified with recombinant epitopes. With this approach, instead of chemically attaching epitopes to immunogen surface, genes are recombined such that conformational or linear polypeptide epitopes are inserted within PBV immunodominant regions. These regions should be surface exposed if the intent is to activate a humoral immune response (i.e., AIRs). If epitope insertions can be made within AIRs without negatively influencing protein folding, it ultimately results in B cell responses being redirected away from the previous AIR and toward the introduced epitope. This concept was demonstrated by Gillam et al. when they reported increased antibody titers to recombinantly inserted YSNIGVCK epitope and decreased titers to hepatitis B core antigen (HBcAg) VLP when evaluating a porcine epidemic diarrhea PBV in mice (122). A maximal insert size exists for all AIRs in which proper protein folding can still be accommodated. Successful inserts within HBcAg have been reported at >200 residues, whereas inserts within HPV 16 L1 protein, even when at the C-terminus, rarely exceed 60 residues without negatively influencing PBV structure (123, 124). Additionally, Varsani et al. observed that epitopes of identical length had variable effects on the ability of chimeric HPV 16 L1 PBVs to form VLPs when they were inserted within different AIRs (125). Together, these results indicate that maximum insert size is variable and largely influenced by the properties of the protein, the insert, and the insert location. It also appears that larger epitopes may influence protein structure less than smaller ones in some cases, but this is not the norm (126).
The fusion approach to targeting epitopes is most commonly employed using VLPs as scaffolding, a technology which comprises the assemblage of multiple protein copies all containing the same immunodominant regions. In this way, high epitope densities per antigen can be achieved without sacrificing the intrinsic, immunogenic advantages provided by VLP shape, size, and structure (4). Chimeric VLP PBVs have been successfully developed to target a variety of infections that plague humans and livestock species. Examples include a HPV 16 L1-based vaccine targeting influenza A virus (126), a MS2-based vaccine targeting HIV (127), and a HBcAg-based vaccine targeting porcine reproductive and respiratory syndrome virus (128), though many other studies implementing this technology exist (129). Chimeric, fusion PBVs have also been specially designed to convey cross-protective immunity via the insertion of broadly neutralizing and/or multiple BCR epitopes (130). It is important to note, however, that VLP-forming proteins are not the only proteins that can support this approach. Alternatively, toxoid proteins have also been used in chimeric vaccines targeting insert-specific antibody production, though much less frequently than their VLP counterparts as they do not offer many of the same advantages mentioned earlier (131–134).
Carrier Induced Epitopic Suppression
Recent strides in vaccine development have been accompanied by increased interest in conjugate and chimeric BCR epitope PBVs. When combined, they present an outstanding opportunity for vaccinologists to generate vaccines that can target nearly all conceivable chemicals, biologicals, and polypeptides. In addition, they provide a means through which epitope densities can be increased on antigen surface such that BCR cross-linking and subsequent T cell-independent activation of B cells becomes more likely. However, the promise of a “vaccine for anything” provided by conjugate and chimeric PBVs has elicited various complications. Of these complications, carrier-induced epitopic suppression (CIES) is the most noteworthy issue. Both conjugate and chimeric PBVs require the use of existing immunogens as carrier proteins in order to elicit an immune response. This, along with the fact that there are a limited number of suitable immunogens available for use in vaccine formulations due to factors such as immunogenicity, toxicity, stability, and accessibility, leads to a situation where a select group of “preferred” immunogens are most often used. CIES refers to a phenomenon that occurs when the same immunogen is used in sequential, independent vaccine administrations targeting different epitopes, resulting in the sequestration, elimination, and/or inhibition of vaccine response to target epitope by pre-existing, immunogen specific antibodies and lymphocytes (135). As expected, this can lead to inhibition of hapten-specific lymphocyte recognition of vaccine and an ultimate reduction in vaccine efficacy (136).
Though CIES is likely to always occur to some extent when simply boosting a conjugate or chimeric PBV, prevention of antibody specificity for new vaccines is paramount to the creation of more successful immunizations (137). It has been shown that CIES can be overcome by increasing vaccine dosage and/or including more booster injections within a vaccine regimen. More interesting, however, is the positive effect that increasing hapten density has had on the occurrence of CIES, presumably through the crowding of carrier-specific BCR epitopes (138). Interruption, removal, or blocking of AIRs in chimeric VLP formulations via recombinant and/or chemical means has been shown to improve immunogenicity toward target epitopes and minimize immunogenicity toward carrier protein, further corroborating this presumption (122, 139, 140). However, chemical modification, such as PEGylation, is largely non-specific, thus resulting in unpredictable outcomes for vaccines. For this reason, it is mainly employed to reduce therapeutic protein antigenicity (141–144). Ideally, immunogen surface should be considered for each individual vaccine in order to simultaneously direct antibody-mediated immune response toward important epitopes and prevent pre-existing antibody recognition of carrier-specific immunodominant regions.
Targeting T Cell Receptors
PBVs and the T Cell
The most critical component in any adaptive immune response is arguably the T cell, as it serves as a key facilitator of both cell-mediated and humoral immunity. T cells assist with B cell maturation (TH cells), destroy infected and malfunctioning cells (cytotoxic T (TC) cells), prevent T cell autoreactivity and terminate T cell activity at the end of an immune response (regulatory T (TReg) cells), provide tissue, effector, central, and virtual antigen memory (memory T (TM) cells), in addition to assuming many other roles (natural killer T (NKT) cells, mucosal associated invariant T (MAIT) cells, and gamma delta T (Tγσ) cells) (145). At the heart of this broad functionality lies the TCR, the associated MHCs (class I and class II), and TCR epitopes. When a PBV is administered, cellular processing of antigen leads to MHC display of small, linear, antigen-derived peptides, also known as TCR epitopes, on cell surface. TCR CDR recognition of these MHC-peptide complexes, in turn, leads to T cell activation and proliferation. More specifically, activation of CD8+ TC cells is facilitated by MHC class I molecules whereas activation of CD4+ TH and TReg cells is facilitated by MHC class II molecules. CD4+ and CD8+ TM cells can be activated via either MHC class I or MHC class II pathway (28, 146). Many antigen-associated factors influence the type and magnitude of T cell response. The most important of these factors include antigen uptake, localization, processing, and T cell epitope content.
The Impact of APC Uptake of PBV on T Cell Activation
The first step in the T cell activation process is uptake of antigen by cells that express MHC molecules. For the purposes of PBVs, this process is generally orchestrated by specialized APCs, though many other cell types can participate in the event of cellular damage or infection. In fact, the MHC class I pathway can be initiated by any nucleated cell. Alternately, activation of the MHC class II pathway can only be achieved after phagocytosis of antigen by APCs (147). For this reason, fusion of PBVs to antibodies and antibody fragments specific for APC surface markers has been extensively investigated as means of potentiating both helper and cytotoxic T cell activation. This approach has routinely demonstrated success, with antibody-mediated, APC targeting generally resulting in a subsequent increase in cellular and humoral mediated immune response (148–156). PBVs have also aimed to rationally target T cell activation through the selective incorporation of ligands specific for specialized receptors found on APCs. These receptors include PRRs such as integrins and CLRs, MHC class II molecules, and Fcγ receptors, among others. However, most research has focused on CLRs such as DC-SIGN, langerin, and the DECTIN-1 subfamily (157, 158). This approach presents a major advantage over the old method of tethered antibody mediated targeting in that incorporation of glycans specific for CLRs do not require any additional steps when appropriate expression hosts are used.
The Impact of Cellular Localization of PBV Within APCs on T Cell Activation
Antigen localization is one of the major controlling factors behind the type of T cell response initiated by exogenously administered PBVs. MHC class I pathway requires internalization and processing of antigen into short peptide sequences (8–10 amino acids) within the cytoplasm. In contrast, MHC class II pathway requires phagocytosis of antigen by APCs and subsequent lysosomal processing into somewhat longer peptide sequences (15–24 amino acids) (147). As such, attempts have been made in the past to direct PBV to cytoplasm (to target MHC class I pathway) or lysosome (to target MHC class II pathway) using co-administration and fusion protein approaches. Results have been mixed, with some studies observing increases in pathway-specific immune response and others showing no improvement. Targeting lysosomal degradation by tethering antigen to lysosome-associated membrane protein (LAMP) generally has shown no significant increase in antigen-specific antibody population and only marginal increases in CD4+ T cell population (159, 160). Invariant chain (Ii) has also been fused to antigen in an attempt to target lysosome and subsequent MHC class II pathway. While fusion of full-length Ii to endogenous antigen results in inconsistent activation of CD4+ T cells, most likely due to the presence of class II associated Ii peptide (CLIP) on the C-terminus, fusion of Ii derivatives accounting for various lengths of N-terminal sequence have been shown to effectively direct antigen to lysosome (161, 162). Exogenously administered fusion proteins that have been tagged with an Ii portion thought to assist with MHC class II loading (LRMK, Ii-Key) have also been explored, resulting in potentiation of both CD4+ and CD8+ T cell immune response (163–168). Finally, since APCs are the only cell type capable of processing antigen via the MHC class II pathway, the targeting of APC uptake is also a viable means of targeting CD4+ T cell activation. This approach to controlling antigen localization has generally proven to be non-specific to humoral or cytotoxic pathway, however, as it dually potentiates activation of both CD4+ and CD8+ T cells (148–156).
The Impact of PBV Stability on APC Processing and T Cell Activation
APCs, specifically dendritic cells (DCs), are unique in their ability to activate TC cells after exposure to extracellular antigen. This cross-presentation (CP) of antigen is key to the initiation of cellular immunity to many cancers, viruses, and exogenously administered PBVs (169). The majority of CP in APCs is due to antigen stability and slowed lysosomal digestion, though other factors, such as the vacuolar pathway, cell maturation stage, and immunostimulatory environment, do play a role (170–177). PBVs can be engineered to improve stability, as has been demonstrated by the introduction of inter-capsomeric di-sulfide bonds within HBcAg VLPs and MS2 bacteriophage VLPs (122, 178–180). The effect that this engineered stability has on MHC pathway, however, has not been thoroughly investigated, though it appears that stabilization of antigen promotes CP and the activation of TC cells as destabilization has been shown to reduce cross-presentation efficiency (181). One study conducted by Schliehe et al. was able to observe that recombinant fusion of ubiquitin (Ub) or Ub-like modifier Fat 10 to vaccinia virus nucleoprotein (NP) resulted in abrogated protein stability and TC and TM cell response to select immunodominant NP epitopes (174). Another study conducted by Delamarre et al. showed that the slight structural difference between RNase-A and RNase-S (where RNase-S has a peptide bond cleaved between A 20 and S 21) caused RNase-S to be more susceptible to lysosomal proteolysis in vitro and that this susceptibility ultimately resulted in reduced ability for mice to mount a humoral immune response upon vaccination. The T cell activation upon incubation of RNase-A or RNase-S with splenocytes harvested from RNase-S vaccinated mice was near zero, however, which seems to indicate that RNase-S failed to reach APCs upon in vivo administration (182). When considering MHC class II pathway, increased antigen complexity has been shown to slow in vitro DC processing and decrease in vivo antibody response (183). For example, So et al. observed that the stability of two recombinantly modified hen-egg lysozyme proteins (one with a deleted di-sulfide bond and the other with a selectively added intramolecular ester bond) was inversely correlated with CD4+ T cell activation and both cytokine and antibody production in a mouse model (184). When paired with evidence suggesting that these observations are not due to differences in T cell epitope content, these results indicate that PBV stability plays a crucial role in managing the adaptive immune system during an immune response (173, 185). Whether or not the extent of PBV processing is orchestrated by the cell with the intent of modulating humoral and cellular immune responses, however, remains to be seen.
The Importance of the T Cell Epitopes in PBV Efficacy and Design
After cellular processing of antigen, presentation of MHC-peptide complex to TCR can't take place if there are no antigen-derived, T cell epitopes specific for the MHC molecules expressed by the cell, as only MHC-peptide complexes can bind TCRs and activate T cells (28). For this reason, toxoid proteins and toxoid protein derivatives have been used for many decades in the form of adjuvants and fusion proteins to supplement antigens that are lacking in T cell response. Eventually, the improved immunogenicity of these combinations was associated with the density and promiscuity of T cell epitopes contained within the toxoid proteins (186). Since this realization, scientists have searched for “universal” T cell epitopes (UTEs), or rather epitopes that can bind with a large portion of the MHC phenotypes found within the human population (187). This search has been rewarding, with UTEs having been discovered in a plethora of pathogen-associated proteins.
UTEs come in two forms; those that activate TH cells via MHC class II pathway (UhTEs) and those that active TC cells via MHC class I pathway (UcTEs). Synthetic UhTEs, such as the Pan DR Epitope (PADRE) peptides, have also been engineered. Their development has typically been based on the fact that properties of residues at certain positions within an epitope are more important for MHC binding than others (188, 189). This principle has also led to the construction of data-trained algorithms that can help predict MHC binding preferences (190, 191). As such, it is now possible to dissect an antigen in silico, identifying key sequences that are most likely to serve as epitopes for MHC molecules. Taking this approach to PBV design now makes it possible to direct the immune system toward either a cell-mediated or humoral response and increase in vivo T cell response to PBV by targeting more MHC phenotypes. Ultimately, the use of these algorithms is paving the way toward vaccine designs that target predominant HLA haplotypes, further broadening the range of subjects that respond to PBVs (192–195).
Though TCRs aren't subjected to the same post-activation, somatic hypermutation that BCRs are, they do undergo VDJ recombination and as such their affinity for MHC-peptide complex should theoretically be random (28). BCR specificity is not completely random, however, and considering the similarity between BCR and TCR CDRs, it is reasonable to assume that TCRs, like BCRs, may also preferentially bind polypeptide motifs displaying certain quantifiable properties. This hypothesis was recently supported by Chowell et al. when they observed that TCR contact residues exhibit a strong bias for hydrophobic amino acids contained within MHC class I epitopes (196). They postulated that this bias was due to the favorable thermodynamics associated with TCR covering hydrophobic residues on MHC-epitope complexes. Parrish et al. also presented evidence that TCRs are germline encoded to have intrinsic specificity for unloaded MHC molecules (largely independent of allele, class, and polypeptide sequence) (197). Furthermore, the existence of superantigens (SAgs), atypical immunogens that allosterically interact with MHC class II molecules, crosslink the variable region on TCR β-chain, and have the capacity to activate up to 20,000-fold more T cells than are activated by typical antigens, provides additional evidence for this principle. The binding of SAgs by TCR is largely non-specific when compared with interactions between TCR and peptide-loaded MHC molecules, indicating that, at least under certain circumstances, structurally distinct TCRs have a propensity to bind specific antigenic motifs (198). Together, these results strongly suggest that interactions between TCR and MHC-epitope complexes are not random and that there may be opportunities in the future to develop algorithms, like those modeling MHC-epitope interactions, that can predict TCR binding of MHC-epitope complexes.
Targeting TCR via Recombinant and Conjugate Approaches
Many groups have targeted T cell activation via chimeric or covalent attachment of naturally, synthetically, or computationally derived UTEs to protein and peptide-based vaccines. A recombinantly modified rabbit haemorrhagic disease virus-like particle-based vaccine developed by Jemon et al. that incorporated the universal T cell epitope PADRE and an MHC I-restricted epitope derived from the HPV 16 E6 protein (aa 48–57) showed promise as an anaphylactic HPV 16 vaccine when it reduced the tumor burden and improved the survival time of HPV tumor-bearing mice (199). In another study, Percival-Alwyn et al. observed that CD1 mice were able to mount an autoimmune response to self-protein ST2 only once it had been tethered to the A fragment of DT (DTA) or dual TH epitopes derived from TT. Interestingly, the dual epitope performed better than DTA at eliciting autoimmunity when comparing antibody titer differentials between ST2 and control fusion proteins. When considering the size of the two inserts, this result supports the earlier consideration that inserts have the capacity to redirect immune response away from target epitopes (in this case, antibody responses seem to have been redirected away from ST2 and toward DTA) (200). The epitome of the epitopic approach to vaccination was explored by Wu et al. when their group fused a single BCR epitope derived from epidermal growth factor receptor (EGFR, aa 237–267) to a UTE derived from measles virus fusion protein (MVF, aa 288–302) and saw a sizeable antibody response in a mouse model (201). A computational approach to vaccine design was evaluated by Hurtgen et al. when their group used software to predict the UTEs found within three Coccidioides posadasii antigens, Pep1, Amn1, and PIb. Using in vitro assays to screen for immunogenicity and MHC II affinity, five epitopes were selected for incorporation within a recombinant epitope-based vaccine (along with murine Ii-Key and spacer sequences) and subsequently evaluated in an HLA-DR4 transgenic mouse model. Recall epitope assay indicated that 4 of the 5 epitopes successfully activated T cells and challenge assay results showed early activation of TH cells, elevated interferon and interleukin expression, and prolonged survival rates in vaccinated mice (202). Other studies have observed similar, positive results when employing this approach to targeting T cell activation (203–206).
Limitations of Experimentally and Computationally Determined UTEs
UTEs provide a rational means of potentiating humoral and/or cytotoxic immunogenicity of both peptide and protein-based vaccines. Their incorporation into PBV designs could also provide a means of overcoming the effects T cell competition when common antigens are used as vaccine (207). Experimental discovery of UTEs, however, is made difficult by the fact that the HLA system is one of the most variable gene complexes found in humans. For example, at least 3.82e8 combinations exist for MHC class I-associated alleles, and that number is far greater for MHC class II-associated alleles (208, 209). Additionally, the HLA complex shows considerable difference between humans and common animal models such as mice (210). As such, computational approaches to predicting UTEs for specific MHC phenotypes are oftentimes plagued by insufficient amounts of raw data on MHC-epitope interactions, data that are needed in order to establish and validate prediction algorithms. As a result, most methods' predictions can establish what peptide stretches qualify as binders and non-binders, but for the most part these predictions fall short of the quality needed to become the basis for sizeable PBV decisions (185). Ultimately, however, predictions will improve as more data become available for algorithm training and calibration, making it likely that epitope prediction software will become a common tool in the vaccinology toolbox at some point in the future.
PBVs, T Cells, and Their Implications in Cancer Therapy
PBVs targeting cancer are particularly important when considering antigen processing and MHC epitope presentation. This is because, due to the intracellular nature of most TAAs, the activation of both CD4+ and CD8+ T cells are critical to their success. This makes targeting optimal antigen processing a difficult endeavor, as a balance between MHC class I and MHC class II epitope content must be considered. Additionally, since TAAs are generally endogenous, it is typical for the immune system to have already established a degree of central and peripheral tolerance toward them, making epitope-specific immunostimulation more difficult (211). Multi-epitope vaccines containing both MHC class I and MHC class II epitopes that have been defined using observation and/or predictions have been a promising development in the field of cancer immunotherapeutic. Xiang et al. explored the idea when their group strung together groups of UcTEs and UhTEs and chemically conjugated them to the surface of a polystyrene nanovaccine platform in their search for polypeptide-based, therapeutic HPV, survivin, and Wilms Tumor antigen 1 (WT1) vaccines (212). Results from this study indicated that incorporation of UhTEs with cancer-associated UcTEs can assist with the UcTEs' immunogenicity, though it is interesting that this result was not observed upon the incorporation of CpG adjuvant and that simpler epitope-based vaccines were more effective under certain conditions. In another example of this approach, Lin et al. evaluated the efficacy of a multi-epitope, cancer PBV consisting of predicted TH, TC, and B cell epitopes of the LMP2 protein (a TAA found in EBV-associated cancers) (213). Though therapeutic efficacy of this vaccine was not evaluated, inoculation of BALB/c mice resulted in sizeable PBV-specific IgG, IgA, and CTL response. Additional examples also exist, and it is likely that many more examples will arise in the years to come (214).
Additional Considerations
PBV Safety Considerations
One of the primary reasons PBVs were explored as an alternative to live attenuated and inactivated vaccines in the past was their improved safety profile. This, however, was before genetic modification of antigen had become feasible. Today, modifications made to antigen structure can influence PBV safety profile both positively and negatively. For example, attempts to improve PBV immunogenicity via the many fusion protein approaches previously described could backfire in the form of adverse events, such as cytokine storm and molecular mimicry (autoimmunity instigated by epitopic similarities between foreign and self-immunogens) (74, 215). On the opposite end of the spectrum, the need for safer carrier proteins in subunit vaccine formulations has led to modifications being made to many bacterial toxins with hopes of finding safer alternatives (19, 22). Ultimately, the extreme variations observed in PBV efficacy and safety when even the smallest changes are made in antigen structure leads to a situation where safety evaluation becomes all-the-more critical throughout the vaccine development process. This is compounded by the fact that vaccines are generally administered to healthy subjects, making adverse events even less acceptable when compared with other medicines being developed to maintain non-maleficence. As such, additional steps should be made beginning with the very first animal studies when assessing the safety and efficacy profiles of modified PBVs outside of the standard establishment of correlates of protection and the full characterization of vaccine formulation.
Animal Model Considerations
PBVs must first prove themselves in numerous animal studies before they can be used in humans. Unfortunately, however, immunological and physiological interspecies differences make it unwise to extrapolate pre-clinical results to human studies even when using the most optimal animal model for the PBV being developed (216–219). Outside of obvious issues with dose scaling, a perfect example of this is illustrated when one considers that the animal models typically used in vaccine research are inbred to the point of isogenecity. This can be considered determinantal when performing vaccine research because some of the most diverse genes in humans, such as those located on the HLA gene complex, code for important immune molecules that have a major impact on the nature and magnitude of immune responses to antigen and vaccine (220). Even when using humanized HLA transgenic (Tg) mice as an animal model in vaccine studies, cytotoxic T cell epitope recognition concordance rates with humans have only been reported at 47% for vaccinia virus (following immunization with full virus) and 68% for HIV (following immunization with peptide) (221, 222). Though the sample sizes for these studies were small, these results demonstrate that both epitope recognition and antigen processing are quite different between animal models and humans. The effectiveness of some adjuvants can also vary widely between species, making it difficult to ascertain the true value of vaccine efficacy studies done in animals. For example, the antibody response to HBcAg adjuvanted with oil-in-water MF59 adjuvant system is nearly 10 times more potent in humans than in mice and approximately 4 times more potent in humans than in baboons (223). Summarily, the correlative utility of potential animal models when assessing PBV efficacy, especially when investigating the effects of TCR epitopes and adjuvants, should be carefully considered before in vivo testing in order to insure the best translation from animal to human success. Only in this way can we hope to consistently elucidate the impact of PBV modification and formulation on vaccine safety and performance in humans. It is safe to assume, however, that there will always been some level of uncertainty prior to starting in vivo studies.
Additional Stability Considerations
As has already been mentioned, conformation and stability play an important role in the immunogenicity of PBVs. This importance, however, goes beyond the ability of APCs to cross-present antigen (Figure 4). Recombinant proteins, especially when expressed in lower-order systems and/or as inclusion bodies, are notorious for their inclination to misfold (224). This often results in unstable, heterogenous mixtures of protein-derived particles that may (1) inefficiently present BCR epitopes, (2) denature prior to encountering immune cells, or (3) fail to degrade within endosomal compartments (173). Many components commonly found in PBV formulations can also negatively affect conformation and stability. Specifically, alum, oil-in-water, and TLR agonist adjuvants have the potential to cause PBV structural changes due to electrostatic, hydrophobic, and/or coordination interactions with amino acid side chains (225). Since these immunostimulatory molecules are regularly necessary to improve PBV efficacy and help prevent peripheral tolerance, an event that occurs when the adaptive immune system encounters antigen in low doses or outside of an inflammatory setting, simple removal is not an option. Protein-protein interactions can also become a problem when PBVs are rich in reactive side chains. Stabilizing agents such as polysorbate 80 and sucrose can be employed to help prevent protein-protein and protein-adjuvant interactions in PBV formulations, but these molecules also have the capacity to detrimentally interact with certain amino acid side chains via glycation and oxidation (226). Protein aggregation, however, does not always have a negative impact on immunogenicity. Aggregation of therapeutic proteins has been shown to augment the formation of anti-drug antibodies in a phenomenon that is likely caused when immune epitopes/ligands become easier to access/process (227). Additionally, it seems that conjugate PBVs may benefit from some level of aggregation. This is because, while the conjugation approach to PBV design insures proper BCR epitope presentation, aggregation has also been shown to improve DC uptake by more than 3 × and facilitate DC drainage to lymph nodes (54). Of course, improving PBV immunogenicity via engineered aggregation would not be beneficial when employing a chimeric approach to PBV design as the aggregated vaccine structure would most likely present immunological determinants that are much different from those that were intended. Evidently, many important structural and formulative considerations must be made when designing modified PBVs.
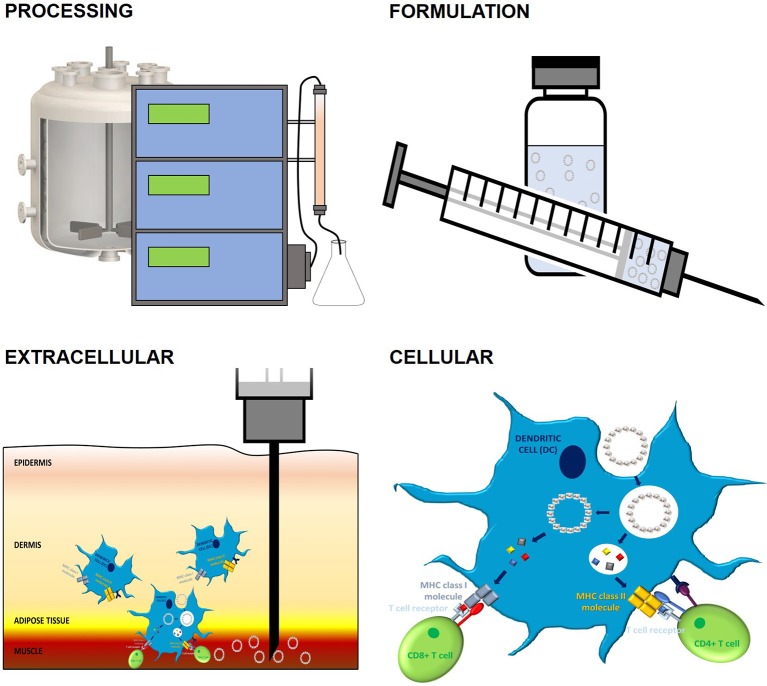
Figure 4.
The impact of PBV stability on immune response. PBV stability has a profound impact on conformation, immunogenicity, and vaccination outcome. Outside of inherent fold stability that's dictated by protein primary structure, many factors contribute to final PBV conformation and stability. Upstream and downstream processes, such as expression and purification, have a sizeable impact on the capacity of PBV to form higher-order structures and can ultimately lead to unintended PBV surface modification, aggregation, and/or decomposition. These issues can also present during formulation as a result of protein-protein, protein-adjuvant, and protein-container interactions. Incompatibilities between physiological conditions and PBV formulation can result in poor extracellular stability, a phenomenon that often presents as excessive local inflammation and poor transport of antigen to secondary lymphoid organs. Finally, cellular stability, which is a function of all the factors mentioned previously, largely dictates MHC processing and the nature of the immune response orchestrated by APCs (cellular vs. humoral, Th1 vs. Th2 vs. Th17, etc.). The 3D protein structure for HBcAg used in this image was rendered in PyMOL 2.3.0 and accessed via the Protein Data Bank (14, 18, 29).
PBV Targeting Strategies for Antigenically Variable Pathogens and Cancers
The development of PBVs targeting antigenically variable pathogens (AVPs) and immune-resistant cancers (ARCs) is complicated by the effects of epitope masking, antigenic drift (the natural accumulation of mutations within existing immunological determinants), antigenic shift (the natural recombination of immunological determinants between independent influenza A strains), and immunoediting (when the natural anti-tumor activity of the immune system puts pressure on oncogenic cells to resist/prevent immunorecognition). These events are characterized by mutations within antigenic determinants that make it difficult for an adaptive immune response to hone in on antigen (228). Multiple strategies to overcoming these complications have been investigated.
For PBVs targeting influenza, the most common approach is biannual reformulation of multivalent, recombinant hemagglutinin PBV such that the flu strains predicted to have the most impact on public health are targeted (229). Unfortunately, this approach to combating AVPs cannot be applied to HIV due to the severity of complications associated with and the chronic nature of HIV infection. One exceptionally promising vaccination approach to protecting against HIV, however, appears to be the reverse engineering of PBVs based on broadly neutralizing antibody (bNAb) populations either found in seropositive individuals or discovered by using massively parallel mapping techniques (230, 231). The premise behind this approach is simple. During the initial stages of HIV infection, the war between mutating antigen and adaptive immune response results in the generation of bNAbs that have been shown to confer powerful protection upon passive immunization in various animal models (232). Intuitively, it should be possible to use structural vaccinology principles and reverse engineer PBVs that can elicit protective antibodies with similar specificities to previously identified bNAbs using an active immunization approach. Scientists hoping to use this approach to develop an effective, prophylactic HIV PBV have identified five key sites associated with bNAbs and work is underway to construct PBVs that can target these vulnerable sites (233–235).
It is worth noting that any active vaccination attempt against HIV will most likely have to consider more than just BCR epitope presentation in order to be effective. This is because, in addition to masking and mutating BCR epitopes, HIV is notorious for its ability to escape immune recognition via alterations in TCR epitopes, specifically those that are recognized by MHC class I molecules (236). A post-hoc analysis of the most successful HIV vaccine study conducted to date, which used an RV144 pox virus prime, recombinant HIV-1 gp120 (rgp120) boosts, and had an estimated 31% overall protection rate, supports this conclusion. Within the vaccinated population, the HLA A*02 genotype was a marker for success, with A*02+ individuals showing significantly greater protection than A*02− individuals (54 vs. 3% effective). Other studies have made similar observations (237, 238). These results not only illuminate the importance of making HLA-targeting considerations in the PBV design process, but also indicate how impactful MHC escape mutations can be when using PBVs to protect against AVPs (even when only one set of HLA-specific epitopes is affected). In this same study there was also a significant increase in vaccine efficacy within the A*02+ population when the presence of a lysine at position 169 in the V2 region of rgp120 was factored in (74 vs. 15% effective) (239). Since this region of the HIV-1 proteome is known as one of the five key bNAb eliciting sites, this result further highlights the challenge associated with targeting AVPs via antibody-mediated approaches (240). Ultimately, it is very possible that the success of future HIV vaccines will be dictated by how well-antigenic determinants can be targeted and controlled using PBV formulations.
Much like viruses, cancers are subjected to selective pressures by the immune system. The majority of malignant cells are recognized and eliminated during immunosurveillance via a variety of effector mechanisms. However, these cells are continuously incentivized to alter and/or hide their antigenic determinants in order to survive and proliferate. As a result, malignant cells that survive immunosurveillance and establish tumors frequently express specific, targetable TAAs that have evolved to maximize survival and minimize immunorecognition (241). This makes the targeting of TAAs via PBVs a tangible option when conceptualizing cancer immunotherapeutics, especially when used in concert with therapies that abrogate the immunosuppressive nature of the tumor microenvironment (e.g., the immune checkpoint inhibitors (ICIs) anti-PD-1 and anti-CTLA-4 and the co-stimulatory molecules (CSMs) anti-CD137 and anti-OX40) (242, 243). Unfortunately, the combination of epitope and multi-epitope vaccines with ICIs has thus far produced largely unimpressive results (244). Additionally, little information could be found on the marriage of full-length PBVs and these immunomodulators (most cancer vaccines utilizing this approach are cellular, DNA, or viral vector-based). The lack of interest in applying this approach to PBVs could be due to the nature of the immune system (cellular, DNA, and viral vector-based vaccines are generally better than PBVs at eliciting CD8+ responses) and/or the failure of past PBV cancer vaccines to advance through clinical trials (245).
The Chimeric-Conjugate Approach
Residues commonly used for chemical crosslinking can be recombinantly incorporated onto protein surface in order to increase conjugation capacity of PBV and ultimately improve vaccine performance (chimeric-conjugate approach). In one study evaluating this approach, researchers were able to recombinantly substitute threonine-15 of the MS2 coat protein with a cysteine residue without influencing folding efficiency (246). Another study observed that replacement of proline-79 and alanine-80 with the peptide GGKGG within the immunodominant region of modified HBcAg resulted in increased titers against conjugated M2 and improved survival rates upon viral challenge when compared to WT HBcAg that had been recombinantly fused to the same protein (247). Similar research using lysine-modified tobacco mosaic virus (TMV) coat protein virus-like particles (VLPs) reported an increase in immunogenicity when compared to co-administered antigen and VLP (248) and another study that used lysine-modified and cysteine-modified TMV VLPs observed that coat protein assembly around RNA scaffold could be modulated by altering the ratio of the two mutant proteins during in vitro assembly (249). Together, these results indicate that a chimeric-conjugate approach could easily be employed to incorporate epitope and/or PAMP ligands with PBV, though more research will need to be done if we wish to fully understand the effect of this method on loading capacity and immunogenicity.
Additional PBV Modification Techniques
Additional PBV modification techniques exist that could be employed to modulate immune responses and target the key immune receptors discussed here. The incorporation of unnatural amino acids (UAAs), such as p-nitrophenylalanine, within PBV structure has been used to overcome autoimmune tolerance in vaccines against RANKL and TNFα (250, 251). Incorporation of the UAA azidohomoalanine have also been used to enable click chemistry on PBV surface (252). As opposed to traditional carbodiimide and maleimide conjugation techniques, click chemistry allows for rapid reaction kinetics, selective ligand attachment, and high yields of successfully utilized attachment sites (253). Sortase-mediated conjugation allows for the highly specific attachment of LPETG(G) tagged molecules to PBV bearing recombinantly inserted multi-glycine stretches, though coupling efficiencies (~30%) tend to be low when using this technique (254, 255). Polyhistidine (pHis) tags are well-known for ability to interact with immobilized metal ions in affinity chromatography applications. At least one research group has attempted to utilize this interaction when conjugating nickel-loaded, tris-nitrilotriacetic acid (tNTA) ligand to norovirus (NoV) VLPs that had been C-terminally modified with pHis tags of various lengths. Results confirmed the utility of the approach as a means of attaching ligand to PBV surface and suggested that the degree of tNTA loading correlated with the number of NoV VLP subunits that contained a pHis tag (256). It may be unwise to employ this conjugation technique when designing future vaccines, however, as nickel has been established as both an allergen and a carcinogen (257). Protein affinity-tag interactions have also been explored as an alternative means of tethering ligand to PBV (258). In one example of this approach, Thrane et al. screened multiple chimeric HPV 16 L1 vaccines that recombinantly displayed a biotin acceptor sequence on one of the protein's surface loops. Insertion did not prevent formation of VLPs in all but one construct, and biotinylation and subsequent attachment of cVLP (HI loop insert) with monovalent streptavidin (mSA)-fused VAR2CSA ligand (a portion of Plasmodium falciparum erythrocyte membrane protein 1 that can be implicated in malaria pathogenicity) showed significant improvement in antigenicity over free ligand early on in vaccination timeline and similar efficacy in final blood draws when the two PBVs were evaluated in a mouse model (259). Clearly, the vaccinologist toolbox is deep when it comes to ways of tethering ligand to PBV.
Conclusions
Modified PBVs present an effective means of overcoming many of the limitations encountered by today's vaccines. First, the addition of PAMP ligands to PBV structure insures that sufficient immune activation signals are co-delivered with vaccine upon administration. PBV modifications of this type have been shown to increase overall vaccine immunogenicity and should reduce the likelihood of initiating immune tolerance. Second, recombinant and/or chemical modification of PBVs with BCR epitopes can modulate humoral immune response to vaccine. Specifically, this type of modification makes it possible to (1) direct antibody specificity toward select epitopes, (2) design cross-reactive vaccines that can neutralize multiple epitopes, and (3) prevent CIES when the subject has previously been exposed to antigen. Third, the recombinant incorporation of TCR epitopes within PBV structure is useful in that it can (1) allow targeting of specific HLA genotypes/MHC phenotypes, (2) influence the type of cellular immune response that's initiated, and (3) overcome the effects of T cell antigenic competition. Finally, modifications can be made to PBV primary structure that influence stability and cellular uptake, both important factors when it comes to the magnitude and type of immune response initiated.
Limitations associated with PBVs also exist. These are generally structural in nature and can result in the negative modulation of both safety and efficacy. First, it is possible for modification and/or misfolding of PBV to result in the incorporation of unintended PAMPs and antigenemic determinants, an effect that could lead to issues with immunotoxicity and/or autoimmunity. Second, the extensive structural variability cognate with PBV production processes and the general weakness of animal models as success correlates also makes it difficult and expensive to quantify just how well PBVs will work prior to direct evaluation in their target species. Along these same lines, issues with protein stability and interactions between vaccine species when making modifications to PBV formulations can add an additional confounding element to the design process, as the margins between protective immunity and immune tolerance are often very fine. Lastly, AVPs present an additional challenge when concerning the utility of PBVs due to the difficulty of targeting ever-changing, antigenic determinants.
From prophylactic use aimed at preventing hard-to-treat diseases such as HIV to anaphylactic use aimed at alleviating chronic conditions such as addiction, allergies, and autoimmunity, the utility of PBVs has advanced well-beyond what was previously imagined possible. Here, we have described the mechanisms behind PBV immunogenicity and listed many structural modifications that have been explored in the past as a means of modulating and/or potentiating PBV in vivo effects. The results from these studies have shown promise on a case-by-case basis, but thus far we have yet to realize the dream of a silver bullet, modified, PBV vaccination approach that would allow the rational targeting of any epitope in any species with a high response rate and without complications. It is possible that we never will, due to the complexity of the immune system and the sheer number of interacting variables that influence the outcome of each immune response. Ultimately, however, the state of PBV research and the sizeable impact that even case-by-case breakthroughs have on the veterinary and medical worlds more than justify continued research into structural vaccinology and the design of modified PBVs.
Author Contributions
KS and FG initiated the review. All authors contributed to the writing and revision of the manuscript.
Conflict of Interest
FG is employed by Locus Biosciences. TL is employed by GlaxoSmithKline. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was partially supported by the National Institute on Drug Abuse (U01DA036850) and the American Association of Immunologists Careers in Immunology Fellowship Program, both of which provided support for KS. The authors declare that this study received funding from Smithfield Foods and Murphy-Brown LLC, which provided support for FG. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. The department of Biological Systems Engineering, which provided support for TL. Funds were also received from Virginia Tech Open Access Subvention Fund to cover the publication fee.
References
- 1.Centers for Disease Control and Prevention (U.S.), National Immunization Program (Centers for Disease Control and Prevention) Epidemiology and Prevention of Vaccine-Preventable Diseases. Atlanta, GA: Department of Health & Human Services, Public Health Service; (2015). p. 5. [Google Scholar]
- 2.Talmage DW. The acceptance and rejection of immunological concepts. Annu Rev Immunol. (1986) 4:1–11. 10.1146/annurev.iy.04.040186.000245 [DOI] [PubMed] [Google Scholar]
- 3.Huzair F, Sturdy S. Biotechnology and the transformation of vaccine innovation: the case of the hepatitis B vaccines 1968-2000. Stud Hist Philos Biol Biomed Sci. (2017) 64:11–21. 10.1016/j.shpsc.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohsen MO, Zha L, Cabral-Miranda G, Bachmann MF. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin Immunol. (2017) 34:123–32. 10.1016/j.smim.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 5.Lavelle EC, Leavy O, Mills KHG. Modified bacterial toxins. In Hackett CJ, Harn DA, editors. Vaccine Adjuvants: Immunological and Clinical Principles. Totowa, NJ: Humana Press; (2006). p. 111–53. 10.1007/978-1-59259-970-7_7 [DOI] [Google Scholar]
- 6.Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. (2013) 119:421–75. 10.1016/B978-0-12-407190-2.00007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ada G, Isaacs D. Carbohydrate-protein conjugate vaccines. Clin Microbiol Infect. (2003) 9:79–85. 10.1046/j.1469-0691.2003.00530.x [DOI] [PubMed] [Google Scholar]
- 8.Robbins JB. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. (1978) 15:839–54. 10.1016/0161-5890(78)90117-7 [DOI] [PubMed] [Google Scholar]
- 9.Katz DH, Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. (1972) 15:1–94. 10.1016/S0065-2776(08)60683-5 [DOI] [PubMed] [Google Scholar]
- 10.Pichichero ME. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother. (2013) 9:2505–23. 10.4161/hv.26109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vartak A, Sucheck SJ. Recent advances in subunit vaccine carriers. Vaccines. (2016) 4:E12. 10.3390/vaccines4020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millar DG, Hirst TR, Snider DP. Escherichia coli heat-labile enterotoxin B subunit is a more potent mucosal adjuvant than its vlosely related homologue, the B subunit of cholera toxin. Infect Immun. (2001) 69:3476–82. 10.1128/IAI.69.5.3476-3482.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellizzari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos Trans R Soc Lond B Biol Sci. (1999) 354:259–68. 10.1098/rstb.1999.0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrodinger LLC The PyMOL Molecular Graphics System, Version 2.3.0. (2019). [Google Scholar]
- 15.Bell CE, Eisenberg D. Crystal structure of nucleotide-free diphtheria toxin. Biochemistry. (1997) 36:481–8. 10.1021/bi962214s [DOI] [PubMed] [Google Scholar]
- 16.Zhang RG, Scott DL, Westbrook ML, Nance S, Spangler BD, Shipley GG, et al. The three-dimensional crystal structure of cholera toxin. J Mol Biol. (1995) 251:563–73. 10.1006/jmbi.1995.0456 [DOI] [PubMed] [Google Scholar]
- 17.Masuyer G, Conrad J, Stenmark P. The structure of the tetanus toxin reveals pH-mediated domain dynamics. EMBO Rep. (2017) 18:1306–17. 10.15252/embr.201744198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The protein data bank. Nucleic Acids Res. (2000) 28:235–42. 10.1093/nar/28.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannini G, Rappuoli R, Ratti G. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. (1984) 12:4063–9. 10.1093/nar/12.10.4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida T, Pappenheimer AM, Jr, Harper AA. Diphtheria toxin and related proteins. II. Kinetic studies on intoxication of HeLa cells by diphtheria toxin and related proteins. J Biol Chem. (1973) 248:3845–50. [PubMed] [Google Scholar]
- 21.Malito E, Bursulaya B, Chen C, Lo Surdo P, Picchianti M, Balducci E, et al. Structural basis for lack of toxicity of the diphtheria toxin mutant CRM197. Proc Natl Acad Sci USA. (2012) 109:5229–34. 10.1073/pnas.1201964109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldauf KJ, Royal JM, Hamorsky KT, Matoba N. Cholera toxin B: one subunit with many pharmaceutical applications. Toxins. (2015) 7:974–96. 10.3390/toxins7030974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figueiredo D, Turcotte C, Frankel G, Li Y, Dolly O, Wilkin G, et al. Characterization of recombinant tetanus toxin derivatives suitable for vaccine development. Infect Immun. (1995) 63:3218–21. 10.1128/IAI.63.8.3218-3221.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu R, Yi S, Yu C, Fang T, Liu S, Yu T, et al. A conformational change of C fragment of tetanus neurotoxin reduces its ganglioside-binding activity but does not destroy its immunogenicity. Clin Vaccine Immunol. (2011) 18:1668–72. 10.1128/CVI.05244-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith LA. Botulism and vaccines for its prevention. Vaccine. (2009) 27(Suppl. 4):D33–9. 10.1016/j.vaccine.2009.08.059 [DOI] [PubMed] [Google Scholar]
- 26.Vita R, Mahajan S, Overton JA, Dhanda SK, Martini S, Cantrell JR, et al. The immune epitope database (IEDB): 2018 update. Nucleic Acids Res. (2019) 47:D339–43. 10.1093/nar/gky1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. (2008) 112:1570–80. 10.1182/blood-2008-02-078071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. (2009) 27:591–619. 10.1146/annurev.immunol.021908.132706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bottcher B, Nassal M. Structure of Mutant hepatitis B Core protein capsids with premature secretion phenotype. J Mol Biol. (2018) 430:4941–54. 10.1016/j.jmb.2018.10.018 [DOI] [PubMed] [Google Scholar]
- 30.Tontini M, Romano MR, Proietti D, Balducci E, Micoli F, Balocchi C, et al. Preclinical studies on new proteins as carrier for glycoconjugate vaccines. Vaccine. (2016) 34:4235–42. 10.1016/j.vaccine.2016.06.039 [DOI] [PubMed] [Google Scholar]
- 31.Wynne SA, Crowther RA, Leslie AG. The crystal structure of the human hepatitis B virus capsid. Mol Cell. (1999) 3:771–80. 10.1016/S1097-2765(01)80009-5 [DOI] [PubMed] [Google Scholar]
- 32.Pumpens P, Ulrich RG, Sasnauskas K, Kazaks A, Ose V, Grens E. Construction of novel vaccines on the basis of virus-like particles: hepatitis B virus proteins as vaccine carriers. In Khudyakov YE, editor. Medicinal Protein Engineering. Boca Raton, FL: CRC Press; (2009). p. 563. [Google Scholar]
- 33.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. (2010) 140:805–20. 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- 34.Shah RR, Hassett KJ, Brito LA. Overview of Vaccine Adjuvants: Introduction, History, and Current Status. Methods Mol Biol. (2017) 1494:1–13. 10.1007/978-1-4939-6445-1_1 [DOI] [PubMed] [Google Scholar]
- 35.Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin Immunol. (2018) 39:14–21. 10.1016/j.smim.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 36.Bonam SR, Partidos CD, Halmuthur SKM, Muller S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol Sci. (2017) 38:771–93. 10.1016/j.tips.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 37.Karch CP, Burkhard P. Vaccine technologies: from whole organisms to rationally designed protein assemblies. Biochem Pharmacol. (2016) 120:1–14. 10.1016/j.bcp.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Qian C, Cao X. Post-translational modification control of innate immunity. Immunity. (2016) 45:15–30. 10.1016/j.immuni.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 39.Clausen H, Wandall HH, Steentoft C, Stanley P, Schnaar RL. Glycosylation engineering. In Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, editors. Essentials of Glycobiology. Cold Spring Harbor, NY: (2015). p. 713–28. [Google Scholar]
- 40.Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol. (2015) 32:21–7. 10.1016/j.coi.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuriakose A, Chirmule N, Nair P. Immunogenicity of biotherapeutics: causes and association with posttranslational modifications. J Immunol Res. (2016) 2016:1298473. 10.1155/2016/1298473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghaderi D, Taylor RE, Padler-Karavani V, Diaz S, Varki A. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol. (2010) 28:863–7. 10.1038/nbt.1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. (2008) 358:1109–17. 10.1056/NEJMoa074943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Min M, Du N, Gu Y, Hode T, Naylor M, et al. Chitin, chitosan, and glycated chitosan regulate immune responses: the novel adjuvants for cancer vaccine. Clin Dev Immunol. (2013) 2013:387023. 10.1155/2013/387023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin CC, Pan IH, Li YR, Pan YG, Lin MK, Lu YH, et al. The adjuvant effects of high-molecule-weight polysaccharides purified from antrodia cinnamomea on dendritic cell function and DNA vaccines. PLoS ONE. (2015) 10:e0116191. 10.1371/journal.pone.0116191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li P, Wang F. Polysaccharides: candidates of promising vaccine adjuvants. Drug Discov Ther. (2015) 9:88–93. 10.5582/ddt.2015.01025 [DOI] [PubMed] [Google Scholar]
- 47.Petrovsky N, Cooper PD. Carbohydrate-based immune adjuvants. Expert Rev Vaccines. (2011) 10:523–37. 10.1586/erv.11.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyake Y, Toyonaga K, Mori D, Kakuta S, Hoshino Y, Oyamada A, et al. C-type lectin MCL is an FcRgamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity. (2013) 38:1050–62. 10.1016/j.immuni.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 49.Li D, von Schaewen M, Wang X, Tao W, Zhang Y, Li L, et al. Altered glycosylation patterns increase immunogenicity of a subunit hepatitis C virus vaccine, inducing neutralizing antibodies which confer protection in mice. J Virol. (2016) 90:10486–98. 10.1128/JVI.01462-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ansari IH, Kwon B, Osorio FA, Pattnaik AK. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J Virol. (2006) 80:3994–4004. 10.1128/JVI.80.8.3994-4004.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Batista-Duharte A, Martinez DT, Carlos IZ. Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed Pharmacother. (2018) 105:616–24. 10.1016/j.biopha.2018.06.026 [DOI] [PubMed] [Google Scholar]
- 52.Feng Y, Forsell MN, Flynn B, Adams W, Lore K, Seder R, et al. Chemical cross-linking of HIV-1 Env for direct TLR7/8 ligand conjugation compromises recognition of conserved antigenic determinants. Virology. (2013) 446:56–65. 10.1016/j.virol.2013.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang TZ, Diambou I, Kim JR, Wang B, Champion JA. Host- and pathogen-derived adjuvant coatings on protein nanoparticle vaccines. Bioeng Transl Med. (2017) 2:120–30. 10.1002/btm2.10052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kastenmuller K, Wille-Reece U, Lindsay RW, Trager LR, Darrah PA, Flynn BJ, et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J Clin Invest. (2011) 121:1782–96. 10.1172/JCI45416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fili L, Vultaggio A, Cardilicchia E, Manuelli C, Casini A, Nencini F, et al. A novel allergen-adjuvant conjugate suitable for specific immunotherapy of respiratory allergy. J Allergy Clin Immunol. (2013) 132:84–92. 10.1016/j.jaci.2013.01.030 [DOI] [PubMed] [Google Scholar]
- 56.Tighe H, Takabayashi K, Schwartz D, Van Nest G, Tuck S, Eiden JJ, et al. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J Allergy Clin Immunol. (2000) 106:124–34. 10.1067/mai.2000.107927 [DOI] [PubMed] [Google Scholar]
- 57.Schulke S, Vogel L, Junker AC, Hanschmann KM, Flaczyk A, Vieths S, et al. A fusion protein consisting of the vaccine adjuvant monophosphoryl lipid a and the allergen ovalbumin boosts allergen-specific Th1, Th2, and Th17 responses In Vitro. J Immunol Res. (2016) 2016:4156456. 10.1155/2016/4156456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKee SJ, Young VL, Clow F, Hayman CM, Baird MA, Hermans IF, et al. Virus-like particles and alpha-galactosylceramide form a self-adjuvanting composite particle that elicits anti-tumor responses. J Control Release. (2012) 159:338–45. 10.1016/j.jconrel.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 59.Soudi S, Zavaran-Hosseini A, Muhammad Hassan Z, Soleimani M, Jamshidi Adegani F, Hashemi SM. Comparative study of the effect of LPS on the function of BALB/c and C57BL/6 peritoneal macrophages. Cell J. (2013) 15:45–54. [PMC free article] [PubMed] [Google Scholar]
- 60.Schulke S, Flaczyk A, Vogel L, Gaudenzio N, Angers I, Loschner B, et al. MPLA shows attenuated pro-inflammatory properties and diminished capacity to activate mast cells in comparison with LPS. Allergy. (2015) 70:1259–68. 10.1111/all.12675 [DOI] [PubMed] [Google Scholar]
- 61.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. (2014) 5:461. 10.3389/fimmu.2014.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gries CM, Mohan RR, Morikis D, Lo DD. Crosslinked flagella as a stabilized vaccine adjuvant scaffold. BMC Biotechnol. (2019) 19:48. 10.1186/s12896-019-0545-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu G, Tarbet B, Song L, Reiserova L, Weaver B, Chen Y, et al. Immunogenicity and efficacy of flagellin-fused vaccine candidates targeting 2009 pandemic H1N1 influenza in mice. PLoS ONE. (2011) 6:e20928. 10.1371/journal.pone.0020928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang C, Zhu S, Wei L, Yan X, Wang J, Quan R, et al. Recombinant flagellin-porcine circovirus type 2 cap fusion protein promotes protective immune responses in mice. PLoS ONE. (2015) 10:e0129617. 10.1371/journal.pone.0129617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu G, Song L, Beasley DW, Putnak R, Parent J, Misczak J, et al. Immunogenicity and efficacy of flagellin-envelope fusion dengue vaccines in mice and monkeys. Clin Vaccine Immunol. (2015) 22:516–25. 10.1128/CVI.00770-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mizel SB, Graff AH, Sriranganathan N, Ervin S, Lees CJ, Lively MO, et al. Flagellin-F1-V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin Vaccine Immunol. (2009) 16:21–8. 10.1128/CVI.00333-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitzmuller C, Kalser J, Mutschlechner S, Hauser M, Zlabinger GJ, Ferreira F, et al. Fusion proteins of flagellin and the major birch pollen allergen Bet v 1 show enhanced immunogenicity, reduced allergenicity, and intrinsic adjuvanticity. J Allergy Clin Immunol. (2018) 141:293–9 e6. 10.1016/j.jaci.2017.02.044 [DOI] [PubMed] [Google Scholar]
- 68.Cuadros C, Lopez-Hernandez FJ, Dominguez AL, McClelland M, Lustgarten J. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infect Immun. (2004) 72:2810–6. 10.1128/IAI.72.5.2810-2816.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonald WF, Huleatt JW, Foellmer HG, Hewitt D, Tang J, Desai P, et al. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J Infect Dis. (2007) 195:1607–17. 10.1086/517613 [DOI] [PubMed] [Google Scholar]
- 70.Farajnia S, Peerayeh SN, Tanomand A, Majidi J, Goudarzi G, Naghili B, et al. Protective efficacy of recombinant exotoxin a–flagellin fusion protein against Pseudomonas aeruginosa infection. Can J Microbiol. (2015) 61:60–4. 10.1139/cjm-2014-0501 [DOI] [PubMed] [Google Scholar]
- 71.Stepanova LA, Mardanova ES, Shuklina MA, Blokhina EA, Kotlyarov RY, Potapchuk MV, et al. Flagellin-fused protein targeting M2e and HA2 induces potent humoral and T-cell responses and protects mice against various influenza viruses a subtypes. J Biomed Sci. (2018) 25:33. 10.1186/s12929-018-0433-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Treanor JT, Albano FR, Sawlwin DC, Graves Jones A, Airey J, Formica N, et al. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine compared with two trivalent inactivated influenza vaccines containing alternate B strains in adults: a phase 3, randomized noninferiority study. Vaccine. (2017) 35:1856–64. 10.1016/j.vaccine.2017.02.066 [DOI] [PubMed] [Google Scholar]
- 73.Treanor JJ, Taylor DN, Tussey L, Hay C, Nolan C, Fitzgerald T, et al. Safety and immunogenicity of a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125) in healthy young adults. Vaccine. (2010) 28:8268–74. 10.1016/j.vaccine.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 74.Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. (2011) 29:5145–52. 10.1016/j.vaccine.2011.05.041 [DOI] [PubMed] [Google Scholar]
- 75.Taylor DN, Treanor JJ, Sheldon EA, Johnson C, Umlauf S, Song L, et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine. (2012) 30:5761–9. 10.1016/j.vaccine.2012.06.086 [DOI] [PubMed] [Google Scholar]
- 76.Yoon SI, Kurnasov O, Natarajan V, Hong M, Gudkov AV, Osterman AL, et al. Structural basis of TLR5-flagellin recognition and signaling. Science. (2012) 335:859–64. 10.1126/science.1215584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song WS, Jeon YJ, Namgung B, Hong M, Yoon SI. A conserved TLR5 binding and activation hot spot on flagellin. Sci Rep. (2017) 7:40878. 10.1038/srep40878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Neill LA, Golenbock D, Bowie AG. The history of toll-like receptors—redefining innate immunity. Nat Rev Immunol. (2013) 13:453–60. 10.1038/nri3446 [DOI] [PubMed] [Google Scholar]
- 79.Moyle PM. Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnol Adv. (2017) 35:375–89. 10.1016/j.biotechadv.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 80.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol. (2007) 3:663–7. 10.1038/nchembio.2007.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gartner T, Baeten M, Otieno S, Revets H, De Baetselier P, Huygen K. Mucosal prime-boost vaccination for tuberculosis based on TLR triggering opri lipoprotein from Pseudomonas aeruginosa fused to mycolyl-transferase Ag85A. Immunol Lett. (2007) 111:26–35. 10.1016/j.imlet.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 82.Chua BY, Eriksson EM, Brown LE, Zeng W, Gowans EJ, Torresi J, et al. A self-adjuvanting lipopeptide-based vaccine candidate for the treatment of hepatitis C virus infection. Vaccine. (2008) 26:4866–75. 10.1016/j.vaccine.2008.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeng W, Tan AC, Horrocks K, Jackson DC. A lipidated form of the extracellular domain of influenza M2 protein as a self-adjuvanting vaccine candidate. Vaccine. (2015) 33:3526–32. 10.1016/j.vaccine.2015.05.053 [DOI] [PubMed] [Google Scholar]
- 84.Seth A, Yasutomi Y, Jacoby H, Callery JC, Kaminsky SM, Koff WC, et al. Evaluation of a lipopeptide immunogen as a therapeutic in HIV type 1-seropositive individuals. AIDS Res Hum Retroviruses. (2000) 16:337–43. 10.1089/088922200309214 [DOI] [PubMed] [Google Scholar]
- 85.Huang CY, Chen JJ, Shen KY, Chang LS, Yeh YC, Chen IH, et al. Recombinant lipidated HPV E7 induces a Th-1-biased immune response and protective immunity against cervical cancer in a mouse model. PLoS ONE. (2012) 7:e40970. 10.1371/journal.pone.0040970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Basto AP, Piedade J, Ramalho R, Alves S, Soares H, Cornelis P, et al. A new cloning system based on the opri lipoprotein for the production of recombinant bacterial cell wall-derived immunogenic formulations. J Biotechnol. (2012) 157:50–63. 10.1016/j.jbiotec.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 87.Song YC, Liu HH, Chen IH, Chen HW, Chong P, Leng CH, et al. A purified recombinant lipopeptide as adjuvant for cancer immunotherapy. Biomed Res Int. (2014) 2014:349783. 10.1155/2014/349783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dhason MS, Wang JC, Hagan MF, Zlotnick A. Differential assembly of hepatitis B virus core protein on single- and double-stranded nucleic acid suggest the dsDNA-filled core is spring-loaded. Virology. (2012) 430:20–9. 10.1016/j.virol.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Porterfield JZ, Dhason MS, Loeb DD, Nassal M, Stray SJ, Zlotnick A. Full-length hepatitis B virus core protein packages viral and heterologous RNA with similarly high levels of cooperativity. J Virol. (2010) 84:7174–84. 10.1128/JVI.00586-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Strods A, Ose V, Bogans J, Cielens I, Kalnins G, Radovica I, et al. Preparation by alkaline treatment and detailed characterisation of empty hepatitis B virus core particles for vaccine and gene therapy applications. Sci Rep. (2015) 5:11639. 10.1038/srep11639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cadena-Nava RD, Comas-Garcia M, Garmann RF, Rao AL, Knobler CM, Gelbart WM. Self-assembly of viral capsid protein and RNA molecules of different sizes: requirement for a specific high protein/RNA mass ratio. J Virol. (2012) 86:3318–26. 10.1128/JVI.06566-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J Immunol. (2004) 172:1777–85. 10.4049/jimmunol.172.3.1777 [DOI] [PubMed] [Google Scholar]
- 93.Comellas-Aragones M, Engelkamp H, Claessen VI, Sommerdijk NA, Rowan AE, Christianen PC, et al. A virus-based single-enzyme nanoreactor. Nat Nanotechnol. (2007) 2:635–9. 10.1038/nnano.2007.299 [DOI] [PubMed] [Google Scholar]
- 94.Rohovie MJ, Nagasawa M, Swartz JR. Virus-like particles: next-generation nanoparticles for targeted therapeutic delivery. Bioeng Transl Med. (2017) 2:43–57. 10.1002/btm2.10049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pickett GG, Peabody DS. Encapsidation of heterologous RNAs by bacteriophage MS2 coat protein. Nucleic Acids Res. (1993) 21:4621–6. 10.1093/nar/21.19.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pasloske BL, Walkerpeach CR, Obermoeller RD, Winkler M, DuBois DB. Armored RNA technology for production of ribonuclease-resistant viral RNA controls and standards. J Clin Microbiol. (1998) 36:3590–4. 10.1128/JCM.36.12.3590-3594.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perrault SD, Shih WM. Virus-inspired membrane encapsulation of DNA nanostructures to achieve in vivo stability. ACS Nano. (2014) 8:5132–40. 10.1021/nn5011914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sparrer KM, Gack MU. Intracellular detection of viral nucleic acids. Curr Opin Microbiol. (2015) 26:1–9. 10.1016/j.mib.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ong HK, Tan WS, Ho KL. Virus like particles as a platform for cancer vaccine development. PeerJ. (2017) 5:e4053. 10.7717/peerj.4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Speiser DE, Schwarz K, Baumgaertner P, Manolova V, Devevre E, Sterry W, et al. Memory and effector CD8 T-cell responses after nanoparticle vaccination of melanoma patients. J Immunother. (2010) 33:848–58. 10.1097/CJI.0b013e3181f1d614 [DOI] [PubMed] [Google Scholar]
- 101.Sharon J, Rynkiewicz MJ, Lu Z, Yang CY. Discovery of protective B-cell epitopes for development of antimicrobial vaccines and antibody therapeutics. Immunology. (2014) 142:1–23. 10.1111/imm.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Z, Woo CJ, Iglesias-Ussel MD, Ronai D, Scharff MD. The generation of antibody diversity through somatic hypermutation and class switch recombination. Genes Dev. (2004) 18:1–11. 10.1101/gad.1161904 [DOI] [PubMed] [Google Scholar]
- 103.Doria-Rose NA, Joyce MG. Strategies to guide the antibody affinity maturation process. Curr Opin Virol. (2015) 11:137–47. 10.1016/j.coviro.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frank SA. Immunodominance Within Hosts. Immunology and Evolution of Infectious Disease. Princeton, NJ: Princeton University Press; (2002). 348 p. [PubMed] [Google Scholar]
- 105.Slifka MK, Amanna I. How advances in immunology provide insight into improving vaccine efficacy. Vaccine. (2014) 32:2948–57. 10.1016/j.vaccine.2014.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schutze MP, Deriaud E, Przewlocki G, LeClerc C. Carrier-induced epitopic suppression is initiated through clonal dominance. J Immunol. (1989) 142:2635–40. [PubMed] [Google Scholar]
- 107.Brauner S, Folkersen L, Kvarnstrom M, Meisgen S, Petersen S, Franzen-Malmros M, et al. H1N1 vaccination in sjogren's syndrome triggers polyclonal B cell activation and promotes autoantibody production. Ann Rheum Dis. (2017) 76:1755–63. 10.1136/annrheumdis-2016-210509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun P, Ju H, Liu Z, Ning Q, Zhang J, Zhao X, et al. Bioinformatics resources and tools for conformational B-cell epitope prediction. Comput Math Methods Med. (2013) 2013:943636. 10.1155/2013/943636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao Q, Li S, Yu H, Xia N, Modis Y. Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol. (2013) 31:654–63. 10.1016/j.tibtech.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 110.Gallagher JR, Torian U, McCraw DM, Harris AK. Characterization of the disassembly and reassembly of the HBV glycoprotein surface antigen, a pliable nanoparticle vaccine platform. Virology. (2017) 502:176–87. 10.1016/j.virol.2016.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCraw DM, Gallagher JR, Harris AK. Characterization of influenza vaccine hemagglutinin complexes by cryo-electron microscopy and image analyses reveals structural polymorphisms. Clin Vaccine Immunol. (2016) 23:483–95. 10.1128/CVI.00085-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krumm SA, Doores KJ. Targeting glycans on human pathogens for vaccine design. Curr Top Microbiol Immunol. (2018) 6:254ra129 10.1007/82_2018_103 [DOI] [PubMed] [Google Scholar]
- 113.Bremer PT, Janda KD. Conjugate vaccine immunotherapy for substance use disorder. Pharmacol Rev. (2017) 69:298–315. 10.1124/pr.117.013904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bot A, Berinstein EM, Berinstein NL. Cancer Vaccines. Plotkin's Vaccines. 7th ed Philadelphia, PA: Elsevier; (2018). p. xxi, 1691. [Google Scholar]
- 115.Garrity RR, Rimmelzwaan G, Minassian A, Tsai WP, Lin G, de Jong JJ, et al. Refocusing neutralizing antibody response by targeted dampening of an immunodominant epitope. J Immunol. (1997) 159:279–89. [PubMed] [Google Scholar]
- 116.Jorgensen KW, Buus S, Nielsen M. Structural properties of MHC class II ligands, implications for the prediction of MHC class II epitopes. PLoS ONE. (2010) 5:e15877. 10.1371/journal.pone.0015877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Collins KC, Janda KD. Hapten design for anti-addiction vaccine development. In: Ivan D, editor. Biologics to Treat Substance Use Disorders: Vaccines, Monoclonal Antibodies, and Enzymes. Cham: Springer: (2016). p. 327–65. [Google Scholar]
- 118.Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov. (2010) 9:308–24. 10.1038/nrd3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pryde DC, Jones LH, Gervais DP, Stead DR, Blakemore DC, Selby MD, et al. Selection of a novel anti-nicotine vaccine: influence of antigen design on antibody function in mice. PLoS ONE. (2013) 8:e76557. 10.1371/journal.pone.0076557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saylor K, Zhang C. A simple physiologically based pharmacokinetic model evaluating the effect of anti-nicotine antibodies on nicotine disposition in the brains of rats and humans. Toxicol Appl Pharmacol. (2016) 307:150–64. 10.1016/j.taap.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pentel PR, LeSage MG. New directions in nicotine vaccine design and use. Adv Pharmacol. (2014) 69:553–80. 10.1016/B978-0-12-420118-7.00014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gillam F, Zhang C. Epitope selection and their placement for increased virus neutralization in a novel vaccination strategy for porcine epidemic diarrhea virus utilizing the Hepatitis B virus core antigen. Vaccine. (2018) 36:4507–16. 10.1016/j.vaccine.2018.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kratz PA, Bottcher B, Nassal M. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc Natl Acad Sci USA. (1999) 96:1915–20. 10.1073/pnas.96.5.1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muller M, Zhou J, Reed TD, Rittmuller C, Burger A, Gabelsberger J, et al. Chimeric papillomavirus-like particles. Virology. (1997) 234:93–111. 10.1006/viro.1997.8591 [DOI] [PubMed] [Google Scholar]
- 125.Varsani A, Williamson AL, de Villiers D, Becker I, Christensen ND, Rybicki EP. Chimeric human papillomavirus type 16 (HPV-16) L1 particles presenting the common neutralizing epitope for the L2 minor capsid protein of HPV-6 and HPV-16. J Virol. (2003) 77:8386–93. 10.1128/JVI.77.15.8386-8393.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matic S, Rinaldi R, Masenga V, Noris E. Efficient production of chimeric human papillomavirus 16 L1 protein bearing the M2e influenza epitope in nicotiana benthamiana plants. BMC Biotechnol. (2011) 11:106. 10.1186/1472-6750-11-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peabody DS, Manifold-Wheeler B, Medford A, Jordan SK, do Carmo Caldeira J, Chackerian B. Immunogenic display of diverse peptides on virus-like particles of RNA phage MS2. J Mol Biol. (2008) 380:252–63. 10.1016/j.jmb.2008.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murthy AM, Ni Y, Meng X, Zhang C. Production and evaluation of virus-like particles displaying immunogenic epitopes of porcine reproductive and respiratory syndrome virus (PRRSV). Int J Mol Sci. (2015) 16:8382–96. 10.3390/ijms16048382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yan D, Wei YQ, Guo HC, Sun SQ. The application of virus-like particles as vaccines and biological vehicles. Appl Microbiol Biotechnol. (2015) 99:10415–32. 10.1007/s00253-015-7000-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gillam F, Zhang J, Zhang C. Hepatitis B core antigen based novel vaccine against porcine epidemic diarrhea virus. J Virol Methods. (2018) 253:61–9. 10.1016/j.jviromet.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 131.Zhang C, Knudsen DE, Liu M, Robertson DC, Zhang W, Group STTVC. Toxicity and immunogenicity of enterotoxigenic Escherichia coli heat-labile and heat-stable toxoid fusion 3xSTa(A14Q)-LT(S63K/R192G/L211A) in a murine model. PLoS ONE. (2013) 8:e77386. 10.1371/journal.pone.0077386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zeinalzadeh N, Salmanian AH, Goujani G, Amani J, Ahangari G, Akhavian A, et al. A chimeric protein of CFA/I, CS6 subunits and LTB/STa toxoid protects immunized mice against enterotoxigenic Escherichia coli. Microbiol Immunol. (2017) 61:272–9. 10.1111/1348-0421.12491 [DOI] [PubMed] [Google Scholar]
- 133.Gil LA, da Cunha CE, Moreira GM, Salvarani FM, Assis RA, Lobato FC, et al. Production and evaluation of a recombinant chimeric vaccine against clostridium botulinum neurotoxin types C and D. PLoS ONE. (2013) 8:e69692. 10.1371/journal.pone.0069692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hollingshead S, Jongerius I, Exley RM, Johnson S, Lea SM, Tang CM. Structure-based design of chimeric antigens for multivalent protein vaccines. Nat Commun. (2018) 9:1051. 10.1038/s41467-018-03146-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McCluskie MJ, Evans DM, Zhang N, Benoit M, McElhiney SP, Unnithan M, et al. The effect of preexisting anti-carrier immunity on subsequent responses to CRM197 or Qb-VLP conjugate vaccines. Immunopharmacol Immunotoxicol. (2016) 38:184–96. 10.3109/08923973.2016.1165246 [DOI] [PubMed] [Google Scholar]
- 136.Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: a review. Vaccine. (2010) 28:5513–23. 10.1016/j.vaccine.2010.06.026 [DOI] [PubMed] [Google Scholar]
- 137.Pecetta S, Tontini M, Faenzi E, Cioncada R, Proietti D, Seubert A, et al. Carrier priming effect of CRM197 is related to an enhanced B and T cell activation in meningococcal serogroup A conjugate vaccination. Immunological comparison between CRM197 and diphtheria toxoid. Vaccine. (2016) 34:2334–41. 10.1016/j.vaccine.2016.03.055 [DOI] [PubMed] [Google Scholar]
- 138.Jegerlehner A, Wiesel M, Dietmeier K, Zabel F, Gatto D, Saudan P, et al. Carrier induced epitopic suppression of antibody responses induced by virus-like particles is a dynamic phenomenon caused by carrier-specific antibodies. Vaccine. (2010) 28:5503–12. 10.1016/j.vaccine.2010.02.103 [DOI] [PubMed] [Google Scholar]
- 139.Sadeyen JR, Tourne S, Shkreli M, Sizaret PY, Coursaget P. Insertion of a foreign sequence on capsid surface loops of human papillomavirus type 16 virus-like particles reduces their capacity to induce neutralizing antibodies and delineates a conformational neutralizing epitope. Virology. (2003) 309:32–40. 10.1016/S0042-6822(02)00134-4 [DOI] [PubMed] [Google Scholar]
- 140.Sominskaya I, Skrastina D, Dislers A, Vasiljev D, Mihailova M, Ose V, et al. Construction and immunological evaluation of multivalent hepatitis B virus (HBV) core virus-like particles carrying HBV and HCV epitopes. Clin Vaccine Immunol. (2010) 17:1027–33. 10.1128/CVI.00468-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu Y, Shi Y, Zhou J, Yang W, Bai L, Wang S, et al. Structure-based antigenic epitope and PEGylation improve the efficacy of staphylokinase. Microb Cell Fact. (2017) 16:197. 10.1186/s12934-017-0801-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang T, Yu W, Wang Y, Hu T. Moderate PEGylation of the carrier protein improves the polysaccharide-specific immunogenicity of meningococcal group A polysaccharide conjugate vaccine. Vaccine. (2015) 33:3208–14. 10.1016/j.vaccine.2015.04.094 [DOI] [PubMed] [Google Scholar]
- 143.Gefen T, Vaya J, Khatib S, Harkevich N, Artoul F, Heller ED, et al. The impact of PEGylation on protein immunogenicity. Int Immunopharmacol. (2013) 15:254–9. 10.1016/j.intimp.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 144.Dozier JK, Distefano MD. Site-specific PEGylation of therapeutic proteins. Int J Mol Sci. (2015) 16:25831–64. 10.3390/ijms161025831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. (2018) 48:202–13. 10.1016/j.immuni.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Livingstone AM, Fathman CG. The structure of T-cell epitopes. Annu Rev Immunol. (1987) 5:477–501. 10.1146/annurev.iy.05.040187.002401 [DOI] [PubMed] [Google Scholar]
- 147.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. (2013) 31:443–73. 10.1146/annurev-immunol-032712-095910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grodeland G, Mjaaland S, Tunheim G, Fredriksen AB, Bogen B. The specificity of targeted vaccines for APC surface molecules influences the immune response phenotype. PLoS ONE. (2013) 8:e80008. 10.1371/journal.pone.0080008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kratzer R, Mauvais FX, Burgevin A, Barilleau E, van Endert P. Fusion proteins for versatile antigen targeting to cell surface receptors reveal differential capacity to prime immune responses. J Immunol. (2010) 184:6855–64. 10.4049/jimmunol.0902555 [DOI] [PubMed] [Google Scholar]
- 150.Lu L, Palaniyandi S, Zeng R, Bai Y, Liu X, Wang Y, et al. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. (2011) 85:10542–53. 10.1128/JVI.05441-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Loureiro S, Ren J, Phapugrangkul P, Colaco CA, Bailey CR, Shelton H, et al. Adjuvant-free immunization with hemagglutinin-Fc fusion proteins as an approach to influenza vaccines. J Virol. (2011) 85:3010–4. 10.1128/JVI.01241-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soleimanpour S, Farsiani H, Mosavat A, Ghazvini K, Eydgahi MR, Sankian M, et al. APC targeting enhances immunogenicity of a novel multistage Fc-fusion tuberculosis vaccine in mice. Appl Microbiol Biotechnol. (2015) 99:10467–80. 10.1007/s00253-015-6952-z [DOI] [PubMed] [Google Scholar]
- 153.Ooms K, Van Gorp H, Van Gaever T, Nauwynck HJ, Delputte PL. Development of a recombinant antibody to target peptides and proteins to sialoadhesin-expressing macrophages. BMC Biotechnol. (2013) 13:33. 10.1186/1472-6750-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tagliani E, Guermonprez P, Sepulveda J, Lopez-Bravo M, Ardavin C, Amigorena S, et al. Selection of an antibody library identifies a pathway to induce immunity by targeting CD36 on steady-state CD8 alpha+ dendritic cells. J Immunol. (2008) 180:3201–9. 10.4049/jimmunol.180.5.3201 [DOI] [PubMed] [Google Scholar]
- 155.Caminschi I, Maraskovsky E, Heath WR. Targeting dendritic cells in vivo for cancer therapy. Front Immunol. (2012) 3:13. 10.3389/fimmu.2012.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mi W, Wanjie S, Lo ST, Gan Z, Pickl-Herk B, Ober RJ, et al. Targeting the neonatal fc receptor for antigen delivery using engineered fc fragments. J Immunol. (2008) 181:7550–61. 10.4049/jimmunol.181.11.7550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Blander JM. Regulation of the cell biology of antigen cross-presentation. Annu Rev Immunol. (2018) 36:717–53. 10.1146/annurev-immunol-041015-055523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Plato A, Willment JA, Brown GD. C-type lectin-like receptors of the dectin-1 cluster: ligands and signaling pathways. Int Rev Immunol. (2013) 32:134–56. 10.3109/08830185.2013.777065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Simon GG, Hu Y, Khan AM, Zhou J, Salmon J, Chikhlikar PR, et al. Dendritic cell mediated delivery of plasmid DNA encoding LAMP/HIV-1 Gag fusion immunogen enhances T cell epitope responses in HLA DR4 transgenic mice. PLoS ONE. (2010) 5:e8574. 10.1371/journal.pone.0008574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dobano C, Rogers WO, Gowda K, Doolan DL. Targeting antigen to MHC class I and class II antigen presentation pathways for malaria DNA vaccines. Immunol Lett. (2007) 111:92–102. 10.1016/j.imlet.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 161.Fujii S, Senju S, Chen YZ, Ando M, Matsushita S, Nishimura Y. The CLIP-substituted invariant chain efficiently targets an antigenic peptide to HLA class II pathway in L cells. Hum Immunol. (1998) 59:607–14. 10.1016/S0198-8859(98)00058-5 [DOI] [PubMed] [Google Scholar]
- 162.Carstens C, Newman DK, Bohlen H, Konig A, Koch N. Invariant chains with the class II binding site replaced by a sequence from influenza virus matrix protein constrain low-affinity sequences to MHC II presentation. Int Immunol. (2000) 12:1561–8. 10.1093/intimm/12.11.1561 [DOI] [PubMed] [Google Scholar]
- 163.Wu C, Zhang DG, Chen FF, Liu XL, Liu SJ, Yu WY. N-terminal functional region of the invariant chain efficiently targets the binding of a CTL epitope to MHC class I molecules during cross-presentation. Genet Mol Res. (2014) 13:2438–50. 10.4238/2014.April.3.16 [DOI] [PubMed] [Google Scholar]
- 164.Capone S, Naddeo M, D'Alise AM, Abbate A, Grazioli F, Del Gaudio A, et al. Fusion of HCV nonstructural antigen to MHC class II-associated invariant chain enhances T-cell responses induced by vectored vaccines in nonhuman primates. Mol Ther. (2014) 22:1039–47. 10.1038/mt.2014.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Perez SA, Peoples GE, Papamichail M, Baxevanis CN. Invariant chain-peptide fusion vaccine using HER-2/neu. Methods Mol Biol. (2014) 1139:321–36. 10.1007/978-1-4939-0345-0_26 [DOI] [PubMed] [Google Scholar]
- 166.Kallinteris NL, Lu X, Blackwell CE, von Hofe E, Humphreys RE, Xu M. Ii-Key/MHC class II epitope hybrids: a strategy that enhances MHC class II epitope loading to create more potent peptide vaccines. Expert Opin Biol Ther. (2006) 6:1311–21. 10.1517/14712598.6.12.1311 [DOI] [PubMed] [Google Scholar]
- 167.Liu XL, Shan WJ, Xu SS, Zhang JJ, Xu FZ, Xia SL, et al. The efficacy of chimeric vaccines constructed with PEP-1 and Ii-Key linking to a hybrid epitope from heterologous viruses. Biologicals. (2015) 43:377–82. 10.1016/j.biologicals.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 168.Spencer AJ, Cottingham MG, Jenks JA, Longley RJ, Capone S, Colloca S, et al. Enhanced vaccine-induced CD8+ T cell responses to malaria antigen ME-TRAP by fusion to MHC class ii invariant chain. PLoS ONE. (2014) 9:e100538. 10.1371/journal.pone.0100538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moron G, Dadaglio G, Leclerc C. New tools for antigen delivery to the MHC class I pathway. Trends Immunol. (2004) 25:92–7. 10.1016/j.it.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 170.Belizaire R, Unanue ER. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proc Natl Acad Sci USA. (2009) 106:17463–8. 10.1073/pnas.0908583106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gutierrez-Martinez E, Planes R, Anselmi G, Reynolds M, Menezes S, Adiko AC, et al. Cross-presentation of cell-associated antigens by MHC class I in dendritic cell subsets. Front Immunol. (2015) 6:363. 10.3389/fimmu.2015.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Colbert JD, Farfan-Arribas DJ, Rock KL. Substrate-induced protein stabilization reveals a predominant contribution from mature proteins to peptides presented on MHC class I. J Immunol. (2013) 191:5410–9. 10.4049/jimmunol.1300078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Scheiblhofer S, Laimer J, Machado Y, Weiss R, Thalhamer J. Influence of protein fold stability on immunogenicity and its implications for vaccine design. Expert Rev Vaccines. (2017) 16:479–89. 10.1080/14760584.2017.1306441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schliehe C, Bitzer A, van den Broek M, Groettrup M. Stable antigen is most effective for eliciting CD8+ T-cell responses after DNA vaccination and infection with recombinant vaccinia virus in vivo. J Virol. (2012) 86:9782–93. 10.1128/JVI.00694-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thai R, Moine G, Desmadril M, Servent D, Tarride JL, Menez A, et al. Antigen stability controls antigen presentation. J Biol Chem. (2004) 279:50257–66. 10.1074/jbc.M405738200 [DOI] [PubMed] [Google Scholar]
- 176.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. (2003) 299:1400–3. 10.1126/science.1080106 [DOI] [PubMed] [Google Scholar]
- 177.Lennon-Dumenil AM, Bakker AH, Maehr R, Fiebiger E, Overkleeft HS, Rosemblatt M, et al. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J Exp Med. (2002) 196:529–40. 10.1084/jem.20020327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schumacher J, Bacic T, Staritzbichler R, Daneschdar M, Klamp T, Arnold P, et al. Enhanced stability of a chimeric hepatitis B core antigen virus-like-particle (HBcAg-VLP) by a C-terminal linker-hexahistidine-peptide. J Nanobiotechnology. (2018) 16:39. 10.1186/s12951-018-0363-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lu Y, Chan W, Ko BY, VanLang CC, Swartz JR. Assessing sequence plasticity of a virus-like nanoparticle by evolution toward a versatile scaffold for vaccines and drug delivery. Proc Natl Acad Sci USA. (2015) 112:12360–5. 10.1073/pnas.1510533112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ashcroft AE, Lago H, Macedo JM, Horn WT, Stonehouse NJ, Stockley PG. Engineering thermal stability in RNA phage capsids via disulphide bonds. J Nanosci Nanotechnol. (2005) 5:2034–41. 10.1166/jnn.2005.507 [DOI] [PubMed] [Google Scholar]
- 181.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. (2005) 307:1630–4. 10.1126/science.1108003 [DOI] [PubMed] [Google Scholar]
- 182.Delamarre L, Couture R, Mellman I, Trombetta ES. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J Exp Med. (2006) 203:2049–55. 10.1084/jem.20052442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhao Z, Hu Y, Harmon T, Pentel PR, Ehrich M, Zhang C. Hybrid nanoparticle-based nicotine nanovaccines: boosting the immunological efficacy by conjugation of potent carrier proteins. Nanomedicine. (2018) 14:1655–65. 10.1016/j.nano.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.So T, Ito H, Hirata M, Ueda T, Imoto T. Contribution of conformational stability of hen lysozyme to induction of type 2 T-helper immune responses. Immunology. (2001) 104:259–68. 10.1046/j.1365-2567.2001.01314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nielsen M, Lund O, Buus S, Lundegaard C. MHC class II epitope predictive algorithms. Immunology. (2010) 130:319–28. 10.1111/j.1365-2567.2010.03268.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.da Silva Antunes R, Paul S, Sidney J, Weiskopf D, Dan JM, Phillips E, et al. Correction: definition of human epitopes recognized in tetanus toxoid and development of an assay strategy to detect ex vivo tetanus CD4+ T cell responses. PLoS ONE. (2018) 13:e0193382. 10.1371/journal.pone.0193382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. (1989) 19:2237–42. 10.1002/eji.1830191209 [DOI] [PubMed] [Google Scholar]
- 188.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. (1994) 1:751–61. 10.1016/S1074-7613(94)80017-0 [DOI] [PubMed] [Google Scholar]
- 189.Jensen KK, Andreatta M, Marcatili P, Buus S, Greenbaum JA, Yan Z, et al. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology. (2018) 154:394–406. 10.1111/imm.12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol. (2008) 4:e1000048. 10.1371/journal.pcbi.1000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang P, Sidney J, Kim Y, Sette A, Lund O, Nielsen M, et al. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics. (2010) 11:568. 10.1186/1471-2105-11-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li Pira G, Ivaldi F, Moretti P, Manca F. High throughput T epitope mapping and vaccine development. J Biomed Biotechnol. (2010) 2010:325720. 10.1155/2010/325720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Patronov A, Doytchinova I. T-cell epitope vaccine design by immunoinformatics. Open Biol. (2013) 3:120139. 10.1098/rsob.120139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bui HH, Sidney J, Dinh K, Southwood S, Newman MJ, Sette A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinformatics. (2006) 7:153. 10.1186/1471-2105-7-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sanchez-Trincado JL, Gomez-Perosanz M, Reche PA. Fundamentals and methods for T- and B-cell epitope prediction. J Immunol Res. (2017) 2017:2680160. 10.1155/2017/2680160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chowell D, Krishna S, Becker PD, Cocita C, Shu J, Tan X, et al. TCR contact residue hydrophobicity is a hallmark of immunogenic CD8+ T cell epitopes. Proc Natl Acad Sci USA. (2015) 112:E1754–62. 10.1073/pnas.1500973112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Parrish HL, Deshpande NR, Vasic J, Kuhns MS. Functional evidence for TCR-intrinsic specificity for MHCII. Proc Natl Acad Sci USA. (2016) 113:3000–5. 10.1073/pnas.1518499113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Petersson K, Forsberg G, Walse B. Interplay between superantigens and immunoreceptors. Scand J Immunol. (2004) 59:345–55. 10.1111/j.0300-9475.2004.01404.x [DOI] [PubMed] [Google Scholar]
- 199.Jemon K, Young V, Wilson M, McKee S, Ward V, Baird M, et al. An enhanced heterologous virus-like particle for human papillomavirus type 16 tumour immunotherapy. PLoS ONE. (2013) 8:e66866. 10.1371/journal.pone.0066866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Percival-Alwyn JL, England E, Kemp B, Rapley L, Davis NH, McCarthy GR, et al. Generation of potent mouse monoclonal antibodies to self-proteins using T-cell epitope tags. MAbs. (2015) 7:129–37. 10.4161/19420862.2014.985489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wu M, Zhao L, Zhu L, Chen Z, Li H. Expression and purification of chimeric peptide comprising EGFR B-cell epitope and measles virus fusion protein T-cell epitope in Escherichia coli. Protein Expr Purif . (2013) 88:7–12. 10.1016/j.pep.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 202.Hurtgen BJ, Hung CY, Ostroff GR, Levitz SM, Cole GT. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against coccidioidomycosis. Infect Immun. (2012) 80:3960–74. 10.1128/IAI.00566-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang H, Su X, Zhang P, Liang J, Wei H, Wan M, et al. Recombinant heat shock protein 65 carrying PADRE and HBV epitopes activates dendritic cells and elicits HBV-specific CTL responses. Vaccine. (2011) 29:2328–35. 10.1016/j.vaccine.2010.12.124 [DOI] [PubMed] [Google Scholar]
- 204.Feng GD, Xue XC, Gao ML, Wang XF, Shu Z, Mu N, et al. Therapeutic effects of PADRE-BAFF autovaccine on rat adjuvant arthritis. Biomed Res Int. (2014) 2014:854954. 10.1155/2014/854954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ghaffari-Nazari H, Tavakkol-Afshari J, Jaafari MR, Tahaghoghi-Hajghorbani S, Masoumi E, Jalali SA. Improving multi-epitope long peptide vaccine potency by using a strategy that enhances CD4+ T help in BALB/c mice. PLoS ONE. (2015) 10:e0142563. 10.1371/journal.pone.0142563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bergmann-Leitner ES, Chaudhury S, Steers NJ, Sabato M, Delvecchio V, Wallqvist AS, et al. Computational and experimental validation of B and T-cell epitopes of the in vivo immune response to a novel malarial antigen. PLoS ONE. (2013) 8:e71610. 10.1371/journal.pone.0071610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. (2000) 192:1105–13. 10.1084/jem.192.8.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh SG. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. (2015) 43:D423–31. 10.1093/nar/gku1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Hum Immunol. (2007) 68:779–88. 10.1016/j.humimm.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 210.Shiina T, Blancher A, Inoko H, Kulski JK. Comparative genomics of the human, macaque and mouse major histocompatibility complex. Immunology. (2017) 150:127–38. 10.1111/imm.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. (2019) 4:7. 10.1038/s41541-019-0103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xiang SD, Wilson KL, Goubier A, Heyerick A, Plebanski M. Design of peptide-based nanovaccines targeting leading antigens from gynecological cancers to induce HLA-A2.1 restricted CD8(+) T cell responses. Front Immunol. (2018) 9:2968. 10.3389/fimmu.2018.02968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lin X, Chen S, Xue X, Lu L, Zhu S, Li W, et al. Chimerically fused antigen rich of overlapped epitopes from latent membrane protein 2 (LMP2) of epstein-barr virus as a potential vaccine and diagnostic agent. Cell Mol Immunol. (2016) 13:492–501. 10.1038/cmi.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang L. Multi-epitope vaccines: a promising strategy against tumors and viral infections. Cell Mol Immunol. (2018) 15:182–4. 10.1038/cmi.2017.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. (2018) 15:586–94. 10.1038/cmi.2017.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. (2004) 172:2731–8. 10.4049/jimmunol.172.5.2731 [DOI] [PubMed] [Google Scholar]
- 217.Huang M, Zhang W, Guo J, Wei X, Phiwpan K, Zhang J, et al. Improved transgenic mouse model for studying HLA class I antigen presentation. Sci Rep. (2016) 6:33612. 10.1038/srep33612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sesma L, Alvarez I, Marcilla M, Paradela A, Lopez de Castro JA. Species-specific differences in proteasomal processing and tapasin-mediated loading influence peptide presentation by HLA-B27 in murine cells. J Biol Chem. (2003) 278:46461–72. 10.1074/jbc.M308816200 [DOI] [PubMed] [Google Scholar]
- 219.Burgevin A, Saveanu L, Kim Y, Barilleau E, Kotturi M, Sette A, et al. A detailed analysis of the murine TAP transporter substrate specificity. PLoS ONE. (2008) 3:e2402. 10.1371/journal.pone.0002402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shiina T, Hosomichi K, Inoko H, Kulski JK. The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet. (2009) 54:15–39. 10.1038/jhg.2008.5 [DOI] [PubMed] [Google Scholar]
- 221.Kotturi MF, Assarsson E, Peters B, Grey H, Oseroff C, Pasquetto V, et al. Of mice and humans: how good are HLA transgenic mice as a model of human immune responses? Immunome Res. (2009) 5:3. 10.1186/1745-7580-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Corbet S, Nielsen HV, Vinner L, Lauemoller S, Therrien D, Tang S, et al. Optimization and immune recognition of multiple novel conserved HLA-A2, human immunodeficiency virus type 1-specific CTL epitopes. J Gen Virol. (2003) 84:2409–21. 10.1099/vir.0.19152-0 [DOI] [PubMed] [Google Scholar]
- 223.Davis HL. Novel vaccines and adjuvant systems: the utility of animal models for predicting immunogenicity in humans. Hum Vaccin. (2008) 4:246–50. 10.4161/hv.4.3.5318 [DOI] [PubMed] [Google Scholar]
- 224.Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol. (2004) 22:1399–408. 10.1038/nbt1029 [DOI] [PubMed] [Google Scholar]
- 225.Fox CB, Kramer RM, Barnes VL, Dowling QM, Vedvick TS. Working together: interactions between vaccine antigens and adjuvants. Ther Adv Vaccines. (2013) 1:7–20. 10.1177/2051013613480144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm Res. (2010) 27:544–75. 10.1007/s11095-009-0045-6 [DOI] [PubMed] [Google Scholar]
- 227.Ratanji KD, Derrick JP, Dearman RJ, Kimber I. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol. (2014) 11:99–109. 10.3109/1547691X.2013.821564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Servin-Blanco R, Zamora-Alvarado R, Gevorkian G, Manoutcharian K. Antigenic variability: obstacles on the road to vaccines against traditionally difficult targets. Hum Vaccin Immunother. (2016) 12:2640–8. 10.1080/21645515.2016.1191718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2018-2019 influenza season. MMWR Recomm Rep. (2018) 67:1–20. 10.15585/mmwr.rr6703a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gao Y, McKay PF, Mann JFS. Advances in HIV-1 vaccine development. Viruses. (2018) 10:E167. 10.3390/v10040167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dingens AS, Haddox HK, Overbaugh J, Bloom JD. Comprehensive mapping of HIV-1 escape from a broadly neutralizing antibody. Cell Host Microbe. (2017) 21:777–87 e4. 10.1016/j.chom.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pegu A, Hessell AJ, Mascola JR, Haigwood NL. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev. (2017) 275:296–312. 10.1111/imr.12511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Haynes BF, Mascola JR. The quest for an antibody-based HIV vaccine. Immunol Rev. (2017) 275:5–10. 10.1111/imr.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. (2013) 340:711–6. 10.1126/science.1234150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kwong PD, Mascola JR. HIV-1 Vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity. (2018) 48:855–71. 10.1016/j.immuni.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 236.Kloverpris HN, Leslie A, Goulder P. Role of HLA adaptation in HIV evolution. Front Immunol. (2015) 6:665. 10.3389/fimmu.2015.00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Crawford H, Lumm W, Leslie A, Schaefer M, Boeras D, Prado JG, et al. Evolution of HLA-B*5703 HIV-1 escape mutations in HLA-B*5703-positive individuals and their transmission recipients. J Exp Med. (2009) 206:909–21. 10.1084/jem.20081984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sun X, Shi Y, Akahoshi T, Fujiwara M, Gatanaga H, Schonbach C, et al. Effects of a single escape mutation on T cell and HIV-1 co-adaptation. Cell Rep. (2016) 15:2279–91. 10.1016/j.celrep.2016.05.017 [DOI] [PubMed] [Google Scholar]
- 239.Gartland AJ, Li S, McNevin J, Tomaras GD, Gottardo R, Janes H, et al. Analysis of HLA A*02 association with vaccine efficacy in the RV144 HIV-1 vaccine trial. J Virol. (2014) 88:8242–55. 10.1128/JVI.01164-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. (2014) 509:55–62. 10.1038/nature13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. (2014) 27:16–25. 10.1016/j.coi.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. (2018) 8:86. 10.3389/fonc.2018.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Attanasio J, Wherry EJ. Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity. (2016) 44:1052–68. 10.1016/j.immuni.2016.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhao J, Chen Y, Ding ZY, Liu JY. Safety and efficacy of therapeutic cancer vaccines alone or in combination with immune checkpoint inhibitors in cancer treatment. Front Pharmacol. (2019) 10:1184 10.3389/fphar.2019.01184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Vermaelen K. Vaccine strategies to improve anti-cancer cellular immune responses. Front Immunol. (2019) 10:8. 10.3389/fimmu.2019.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Peabody DS. A viral platform for chemical modification and multivalent display. J Nanobiotechnol. (2003) 1:5. 10.1186/1477-3155-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jegerlehner A, Tissot A, Lechner F, Sebbel P, Erdmann I, Kundig T, et al. A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses. Vaccine. (2002) 20:3104–12. 10.1016/S0264-410X(02)00266-9 [DOI] [PubMed] [Google Scholar]
- 248.Smith ML, Lindbo JA, Dillard-Telm S, Brosio PM, Lasnik AB, McCormick AA, et al. Modified tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications. Virology. (2006) 348:475–88. 10.1016/j.virol.2005.12.039 [DOI] [PubMed] [Google Scholar]
- 249.Geiger FC, Eber FJ, Eiben S, Mueller A, Jeske H, Spatz JP, et al. TMV nanorods with programmed longitudinal domains of differently addressable coat proteins. Nanoscale. (2013) 5:3808–16. 10.1039/c3nr33724c [DOI] [PubMed] [Google Scholar]
- 250.Wu T, Li F, Sha X, Li F, Zhang B, Ma W, et al. A novel recombinant RANKL vaccine prepared by incorporation of an unnatural amino acid into RANKL and its preventive effect in a murine model of collagen-induced arthritis. Int Immunopharmacol. (2018) 64:326–32. 10.1016/j.intimp.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 251.Grunewald J, Hunt GS, Dong L, Niessen F, Wen BG, Tsao ML, et al. Mechanistic studies of the immunochemical termination of self-tolerance with unnatural amino acids. Proc Natl Acad Sci USA. (2009) 106:4337–42. 10.1073/pnas.0900507106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Patel KG, Swartz JR. Surface functionalization of virus-like particles by direct conjugation using azide-alkyne click chemistry. Bioconjug Chem. (2011) 22:376–87. 10.1021/bc100367u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Brune KD, Howarth M. New routes and opportunities for modular construction of particulate vaccines: stick, click, and glue. Front Immunol. (2018) 9:1432. 10.3389/fimmu.2018.01432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Patterson D, Schwarz B, Avera J, Western B, Hicks M, Krugler P, et al. Sortase-mediated ligation as a modular approach for the covalent attachment of proteins to the exterior of the bacteriophage P22 virus-like particle. Bioconjug Chem. (2017) 28:2114–24. 10.1021/acs.bioconjchem.7b00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Therien A, Bedard M, Carignan D, Rioux G, Gauthier-Landry L, Laliberte-Gagne ME, et al. A versatile papaya mosaic virus (PapMV) vaccine platform based on sortase-mediated antigen coupling. J Nanobiotechnology. (2017) 15:54. 10.1186/s12951-017-0289-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Koho T, Ihalainen TO, Stark M, Uusi-Kerttula H, Wieneke R, Rahikainen R, et al. His-tagged norovirus-like particles: a versatile platform for cellular delivery and surface display. Eur J Pharm Biopharm. (2015) 96:22–31. 10.1016/j.ejpb.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 257.Clancy HA, Costa M. Nickel: a pervasive carcinogen. Future Oncol. (2012) 8:1507–9. 10.2217/fon.12.154 [DOI] [PubMed] [Google Scholar]
- 258.Frietze KM, Peabody DS, Chackerian B. Engineering virus-like particles as vaccine platforms. Curr Opin Virol. (2016) 18:44–9. 10.1016/j.coviro.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Thrane S, Janitzek CM, Agerbaek MO, Ditlev SB, Resende M, Nielsen MA, et al. A novel virus-like particle based vaccine platform displaying the placental malaria antigen VAR2CSA. PLoS ONE. (2015) 10:e0143071. 10.1371/journal.pone.0143071 [DOI] [PMC free article] [PubMed] [Google Scholar]