Abstract
Individuals with neuromuscular impairment from conditions like cerebral palsy face reduced quality of life due to diminishing mobility and independence. Lower-limb exoskeletons have potential to aid mobility, yet few studies have investigated their use during over-ground walking – an exercise that may contribute to our understanding of potential benefit in free-living settings. The goal of this study was to determine the potential for adaptive plantar-flexor assistance from an untethered ankle exoskeleton to improve over-ground walking economy and speed. Six individuals with cerebral palsy completed three consecutive daily over-ground training sessions to acclimate to, and tune, assistance. During a final assessment visit, metabolic cost, walking speed, and soleus electromyography were collected for baseline, unpowered, low, training-tuned, and high assistance conditions. Compared to each participant’s baseline condition, we observed a 3.9 ± 1.9% (p=0.050) increase in walking speed and a 22.0 ± 4.5% (p=0.002) reduction in soleus activity with training-tuned assistance; metabolic cost of transport was unchanged (p=0.130). High assistance resulted in an 8.5 ± 4.0% (p=0.042) reduction in metabolic cost of transport, a 6.3 ± 2.6% (p=0.029) increase in walking speed, and a 25.0 ± 4.0% (p<0.001) reduction in soleus activity. Improvement in exoskeleton-assisted walking economy was related to pre-training baseline walking speed (R2 = 0.94, p=0.001); the slower and more impaired participants improved the most. Energy cost and preferred walking speed remained generally unchanged for the faster and less impaired participants. These findings demonstrate that powered ankle exoskeletons have the potential to improve mobility-related outcomes for some people with cerebral palsy.
Keywords: Adaptive control, ankle assistance, cerebral palsy, exoskeleton, metabolic cost of transport, over-ground walking, plantarflexion, walking speed, soleus muscle activity
I. Introduction
CEREBRAL palsy (CP) is a set of child-onset neuromuscular disorders and is the most common motor disability in childhood [1]. Individuals with CP have abnormal and inefficient walking patterns [2] that can worsen with age until ambulatory ability is lost [3]. Surgical intervention for pathological walking patterns in CP, such as tendon lengthening, have proven to be only moderately effective in improving long-term mobility [4], [5]. Ankle-foot-orthoses (AFOs), commonly prescribed for ambulatory children with CP, also only have limited long-term benefits on gait and mobility [6], [7].
Pathological gait patterns associated with CP are characterized by significantly reduced positive ankle joint power during push-off [8], increased hip and knee joint flexion, and reduced ankle plantarflexion and plantar-flexor power [9]. These inefficient gait mechanics drastically decrease walking speed [10], increase the energy cost of walking [11], and reduce levels of physical activity [12]. There is clear need for effective interventions that can improve free-living mobility, walking speed, and efficiency.
Powered ankle exoskeletons have demonstrated potential to reduce metabolic cost of transport during controlled treadmill walking. Several studies utilizing different exoskeleton designs during treadmill walking have reduced metabolic cost of transport in unimpaired adults [13]–[16]. In a feasibility study with CP participants, powered plantarflexion assistance provided by an untethered exoskeleton during treadmill walking improved net metabolic cost of transport in CP participants by 19 ± 5% compared to walking without wearing the device [17].
To improve mobility, exoskeletons, by definition, must be able to effectively improve efficiency and speed while walking over-ground. Reducing metabolic cost via robotic assistance while walking over-ground is seemingly more challenging than during controlled treadmill walking. Very few exoskeleton studies have assessed energy cost during over-ground walking, and only in a limited number of unimpaired participants; the magnitudes of the reported reductions have been lower compared to treadmill studies. For example, in unimpaired individuals, untethered hip exoskeleton assistance reduced net metabolic cost by 2.7% during over-ground walking and by 3.9% during running compared to no exoskeleton [18]. A similar study with an untethered hip and ankle exoskeleton demonstrated an average 12.1% reduction in net metabolic cost of transport during assisted over-ground walking compared to no exoskeleton for two unimpaired participants [19]. Prior testing of tethered knee exoskeleton assistance demonstrated improved over-ground gait kinematics in children with CP [20]. However, to our knowledge, no published studies have reported the effects of untethered exoskeleton assistance on net metabolic cost of transport or walking speed during over-ground walking in the CP population.
The overarching goal of this study was to assess the potential for adaptive plantar-flexor assistance from an untethered ankle exoskeleton to improve over-ground mobility outcomes in individuals with CP. We hypothesized that acclimated over-ground walking with the exoskeleton would decrease net metabolic cost of transport, increase self-selected walking speed, and reduce soleus muscle activity compared to walking without the device and walking with the device unpowered.
II. Methods
This research was approved by Northern Arizona University’s Institutional Review Board under protocol #986744. Written informed consent for each adult participant, or written consent from a parent of each minor, was obtained prior to enrollment.
A. Recruitment
Six ambulatory individuals with CP of varying age, sex, disease severity, and prescription/use of walking aids (e.g. AFO) participated in the study (Table I). Inclusion criteria included diagnosis of CP, age between 5 and 75 years old, at least 10° of passive ankle plantarflexion, the ability to walk over-ground for at least 6 minutes with or without walking aids, and the ability to understand and follow simple directions. Exclusion criteria included orthopedic surgery within 6 months of participation and any health condition other than CP that could affect participant safety.
TABLE I.
Participant Information
| Participant | Age [Years] |
Sex | Height [m] |
Mass [kg] |
Baseline Condition |
GMFCSb Level |
Walking Preference |
|---|---|---|---|---|---|---|---|
| P1 | 9 | M | 1.37 | 30.7 | Shoe Inserts | I | Exoskeleton |
| P2 | 31 | M | 1.70 | 53.8 | Shod | II | None |
| P3 | 23 | F | 1.47 | 46.0 | AFOsa & Walker | III | Exoskeleton |
| P4 | 10 | M | 1.39 | 38.8 | Shod | I | Exoskeleton |
| P5 | 9 | M | 1.26 | 23.9 | Walker | III | Exoskeleton |
| P6 | 13 | M | 1.51 | 44.0 | Shod | I | Exoskeleton |
AFOs: Ankle-foot orthoses.
GMFCS: Gross Motor Function Classification System (ranges from I-V, from least to most impairment, with level III unable to walk without the use of a walker).
B. Mechanical System
We developed a battery-powered, lightweight ankle exoskeleton capable of providing bilateral plantar- and dorsiflexion assistance (Fig. 1A). A waist-mounted actuation assembly transmitted force through Bowden cables to pulleys at the ankle joints that rotated footplates relative to rigid shank cuffs (Fig. 1B). This exoskeleton was an improved version of the device used in our previous validation and pilot treadmill walking studies [17], [21], [22]. To minimize weight and improve long-term functionality, carbon fiber replaced aluminum components for the actuation housing, footplates, calf cuffs, and uprights (Fig. 1A). Reducing weight, particularly of the ankle assembly, is necessary as the metabolic detriment of increased distal leg mass is significant [23], [24] and could outweigh any benefit due to powered assistance. Exoskeleton mass, including electronics and battery, was 1.73 kg for a “small” assembly (12 Nm peak torque) and 2.07 kg for a “larger” assembly (18 Nm peak torque); mass was reduced by 6.5% and 5.9%, respectively, compared to the previous design [17]. Participants wore the lightest exoskeleton capable of providing the required personalized torque.
Fig. 1.
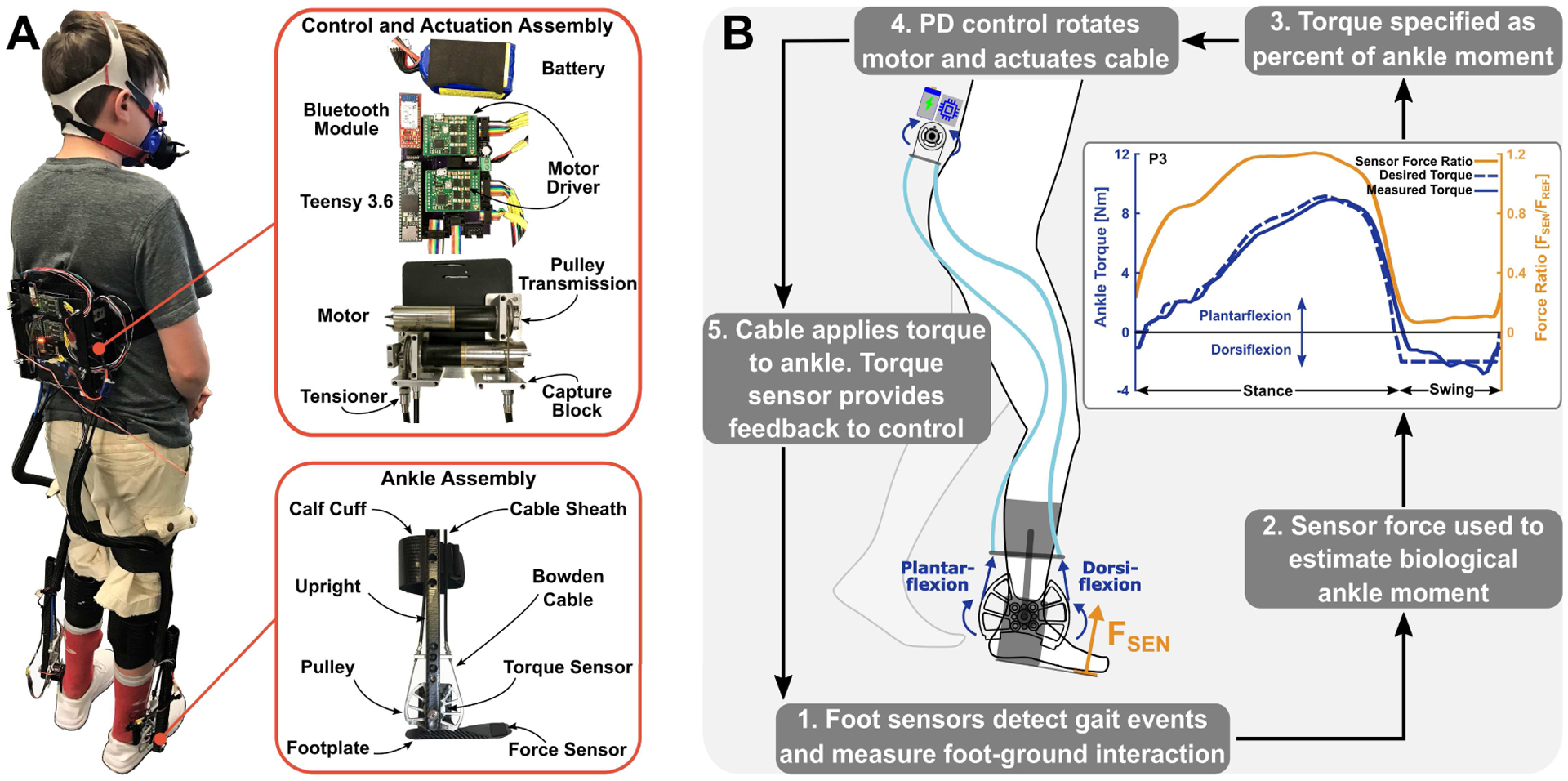
Mechanical and control system overview. (A) The exoskeleton consisted of the ankle, control, and actuation assemblies. (B) The actuation assembly transmitted force through Bowden cables to rotate the ankle pulley and footplate and provide plantar- or dorsi-flexion assistance. Custom force sensors controlled state transitions and served as input into the adaptive force controller. The proportional joint-moment control scheme adapted to each participant’s gait patterns as demonstrated by P3’s averaged experimental force sensor ratio, desired torque, and measured torque profiles.
Torque sensors (TRT-500, Transducer Techniques) at the ankle provided feedback to the control system to ensure proper tracking and force-sensitive resistors on the footplate detected transitions between stance and swing phases during gait (Fig. 1B). A custom printed circuit board housed motor drivers (Maxon), circuitry for sensor measurements, a Bluetooth module for communication with a MATLAB (R2018b) graphical user interface, and a 32-bit ARM microprocessor (Teensy 3.6, PJRC) for control implementation (Fig 1A).
C. Adaptive Exoskeleton Control System
There are several difficulties to address when transitioning from treadmill walking at constant, controlled speeds to over-ground walking, particularly step-to-step variability. Changes in terrain, walking speed, and the added complication of variable walking patterns amongst impaired individuals require an adaptive torque control scheme that parallels and supplements biological gait patterns. We recently developed an instantaneously adaptive proportional joint-moment control scheme (PJMC) that provides assistance proportional to a user’s estimated ankle joint moment [25].
To summarize Gasparri et al. 2019, the biological ankle joint moment, which can vary between steps, legs, and participants, can be estimated using readings from custom force sensor mechanisms on each footplate. Operation of the controller incorporated a calibration procedure that determined the peak sensor force (Fref) during walking at preferred speed. Forces measured during a step (Fsen) were normalized by the calibrated value, resulting in an in an instantaneous sensor force ratio (RF = Fsen/Fref). A regression equation used RF to estimate the instantaneous ankle joint moment ratio Mrel(t) used to calculate plantarflexion ankle assistance torque τ(t) = τ0Mrel(t), where τ0 is the prescribed torque set point (e.g. 10 Nm). The result is exoskeleton torque provided as a ratio of the biological ankle moment at the preferred walking speed. Provided assistance adapts to walking speed changes; can vary between steps, legs, and participants; and supplements biological ankle moment (Fig. 1B).
Heel and toe force sensors detected transitions between stance and swing phase of gait (Fig. 1B). Proportional plantarflexion assistance was provided during stance, whereas either dorsiflexion assistance or no assistance was provided during swing.
D. Experimental Data Collection
All walking trials took place on a 60.96 m (200 ft.) oval track. An operator controlled the exoskeleton through a MATLAB user interface. A trigger sent in MATLAB each time a participant passed through the starting line recorded the duration of the lap and initiated PJMC reference calibration. The operator provided 1–2 Nm dorsiflexion assistance to participants that exhibited foot-drop during swing.
A portable metabolic system (K5, COSMED, Rome, Italy) collected O2 and CO2 volume data during all walking trials (Fig. 1A). A technician, walking behind the participant, held the K5 transmitter unit to limit added mass to that of the exoskeleton and metabolic mask. At the start of each visit, prior to walking trials, we measured metabolic rate during quiet standing. Wireless electromyography (EMG) sensors (Trigno, Delsys) collected soleus muscle activity bilaterally.
We evaluated three general walking conditions. “Baseline”: each participant’s typical daily walking condition that included any physician-prescribed or required aids (e.g. AFOs, walkers); “Unpowered”: walking while wearing the exoskeleton with the footplates disconnected from the ankle assembly to isolate the effects of added mass; and “Assisted”: walking while the exoskeleton provided powered assistance.
On a pre-study screening visit, a licensed physical therapist completed a history and physical assessment and a technician took exoskeleton-fitting measurements.
On the first intervention visit, participants completed baseline, unpowered, and exoskeleton-assisted walking assessments and exoskeleton walking practice. Exoskeleton walking practice continued for two additional sessions, during which we tuned the torque set point in an attempt to maximize walking economy. The total exoskeleton acclimation time was 96.7 ± 8.2 minutes (mean ± standard error).
During the final visit, a comparison of baseline, unpowered, and three exoskeleton-assisted conditions took place in a randomized order (Supplemental Table I). The three assistance levels included “low”, “training-tuned”, and “high” torque magnitudes. The range spanning “low” to “high” assistance was informed from our prior treadmill study [17], and included to account for variation in walking speed on the final assessment. The average low assistance torque level was 0.225 ± 0.014 Nm/kg. The average training-tuned assistance torque level was 0.264 ± 0.012 Nm/kg. The high assistance torque level for all participants was 0.300 Nm/kg.
Metabolic measurement problems on the final visit for P1 (erroneous readings) and P4 (non-compliant caffeine intake) necessitated reassessment of net metabolic cost of transport on a supplemental visit for the final comparison of baseline, unpowered, and tuned assistance conditions, which happened to be 0.300 Nm/kg. Therefore, P1 and P4’s results were included in both the training-tuned and high assistance condition group comparisons, but not in the low condition group. Walking speed analysis was completed from the trials collected on the post-training visit for consistency in speed assessment immediately following the back-to-back acclimation/practice.
E. Data Analysis
Walking speed was calculated by dividing the lap distance in meters by the average lap time in seconds. Metabolic data were processed using Brockway’s standard equation [26] and averaged across the 6th minute of each walking condition. To calculate net metabolic cost of transport, we subtracted each participant’s standing metabolic rate from the metabolic rate of each walking condition, and normalized the result by the average walking speed of that condition.
We analyzed EMG data from an entire lap during the 6th minute of each walking condition. EMG data were band-pass filtered between 15 and 380 Hz, rectified and low-pass filtered at 7 Hz [27]. Filtered EMG data were normalized by the peak value of the baseline condition. The area under the averaged EMG - percent cycle curve during the stance phase was integrated for the baseline and best assistance conditions [27]. Integrated stance phase soleus EMG (iEMG) was averaged across both legs for each participant and normalized by the average walking speed of that condition.
We performed all calculations and statistical comparisons in MATLAB. Percent change in net metabolic cost of transport, walking speed, and soleus iEMG relative to baseline was calculated for the unpowered and all assisted conditions.
F. Statistics
Net metabolic cost of transport, walking speed, and soleus iEMG for each walking condition were checked for normality using Kolmogorov-Smirnov tests; all tests confirmed normality. To assess our hypothesis that walking with assistance would improve outcomes over baseline and unpowered conditions, one-tailed t-tests assessed significance of percent improvement in net metabolic cost of transport and walking speed between assisted vs. unpowered and baseline conditions. To assess our hypothesis that walking with assistance would reduce speed-normalized plantar-flexor muscle activity, a one-tailed t-test assessed significance of percent reduction in speed-normalized soleus iEMG for assisted vs. baseline conditions.
To assist with the design future intervention protocols, we used linear regression to investigate if a relationship existed between an easily assessed participant characteristic, normalized baseline walking speed, and the maximum observed change in net metabolic cost of transport during exoskeleton-assisted walking. We also used linear regression to assess the relationship between the anticipated detriment to net metabolic cost of transport due to wearing the exoskeleton (unpowered) and the anticipated improvement in net metabolic cost of transport during exoskeleton-assisted walking.
We performed statistical analyses for this feasibility study at 95% confidence; p≤0.05 indicates significance. Results are reported as mean ± standard error (SE). Data points 1.5 times beyond the inter-quartile range past the first quartile were considered outliers and excluded from the analysis.
III. Results
All participants had baseline metabolic costs of transport that were generally greater that what is typical for unimpaired individuals [28]. There was an 8.5 ± 4.0% (p=0.042) reduction in net metabolic cost of transport during high assistance compared to baseline (Fig. 2A), and a 17.6 ± 3.2% (p=0.001) reduction compared to the unpowered condition (Supplemental Fig. 1).
Fig. 2.
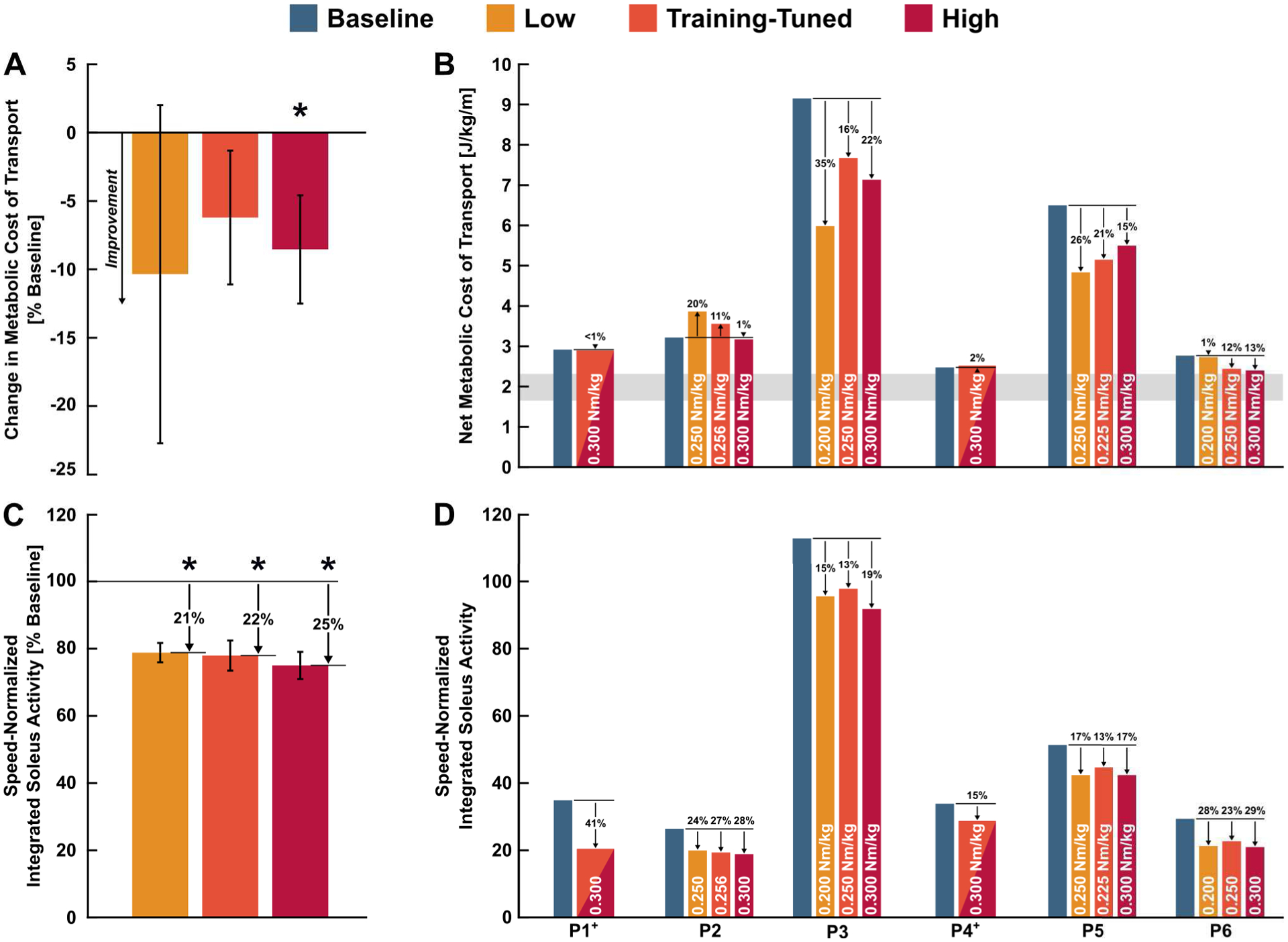
Comparison of net metabolic cost of transport (top) and soleus muscle activity (bottom) across baseline and assisted over-ground walking conditions. Average ± standard error. * indicates a significant reduction relative to baseline at 95% confidence. White text indicates torque level. (A) Change in group level metabolic cost of transport for low, training-tuned, and high assistance conditions relative to baseline. (B) Participant level net metabolic cost of transport. Gray bar indicates typical range of net metabolic cost of transport reported for unimpaired individuals from 9 years old to adulthood [28]. (C) Change in group level speed-normalized integrated soleus muscle activity for low, training-tuned, and high assistance conditions relative to baseline. (D) Participant level speed-normalized integrated soleus muscle activity. + For P1 and P4, the supplemental visit results were included in both training-tuned and high condition analyses, but not in the low condition analysis.
Net metabolic cost of transport did not change during the low (p=0.232) and training-tuned (p=0.130) exoskeleton-assisted walking conditions compared to baseline (Fig. 2A). Compared to the exoskeleton-unpowered condition, metabolic cost of transport did not change with low assistance (p=0.066) but decreased by 15.3 ± 4.6% (p=0.010) with training-tuned assistance.
Participants had increased net metabolic cost of transport when walking with the exoskeleton unpowered compared to baseline with the exception of P3, who wore prescribed AFOs that were heavier than the exoskeleton’s ankle assembly (Supplemental Fig. 1). Omitting P3, the metabolic detriment of walking with the unpowered exoskeleton was 18.0 ± 5.9% (p=0.019) compared to baseline.
Walking speed increased by 5.9 ± 2.5% (p=0.034) with low assistance, by 3.9 ± 1.9% (p=0.050) with training-tuned assistance, and by 6.9 ± 2.4% (p=0.018) with high assistance compared to baseline (Supplemental Fig. 2). Strong responders to ankle assistance had speed improvements of 17% (P3), 10% (P6), and 6% (P5) over baseline with high assistance. Walking with the exoskeleton unpowered did not significantly affect speed vs. baseline.
Speed-normalized soleus iEMG decreased by 21.2 ± 2.8% (p=0.003) with low assistance, by 22.0 ± 4.5% (p=0.002) with training-tuned assistance, and by 25.0 ± 4.0% (p<0.001) with high assistance compared to baseline walking (Fig. 2CD).
There was a significant relationship between the height-normalized first visit baseline walking speed and the change in final visit net metabolic cost of transport with high assistance compared to baseline walking (Fig. 3). Participants with slower pre-training baseline walking speeds had greater reductions in final visit net metabolic cost of transport during the high assistance condition (R2 = 0.94, p=0.001). There was a significant relationship (R2 = 0.75, p=0.025) between the percent change in high assistance vs. baseline net metabolic cost of transport and the percent change in unpowered vs. baseline metabolic cost of transport (Supplemental Fig. 3).
Fig. 3.
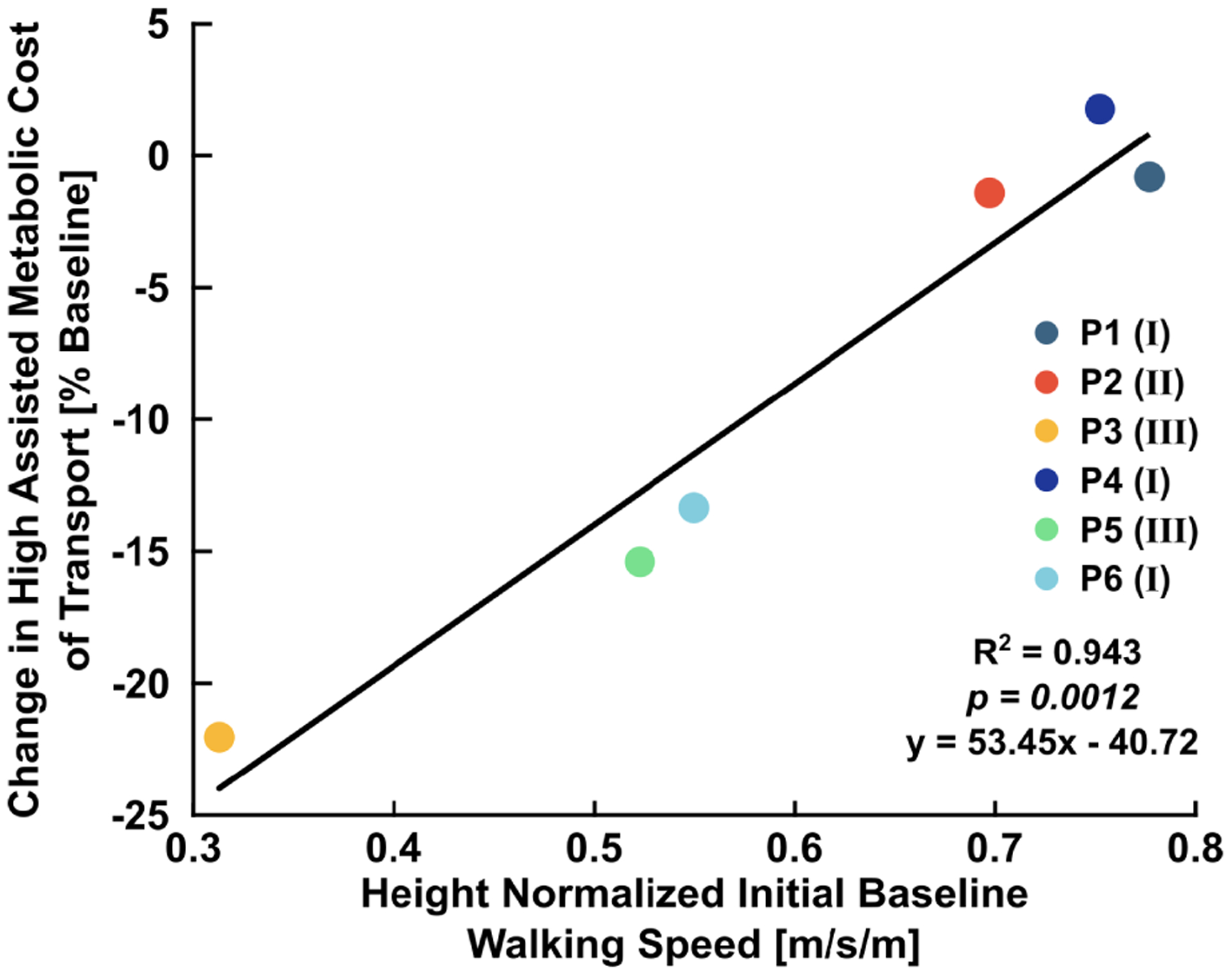
Relationship between the percent change in high assistance vs. baseline metabolic cost of transport and normalized pre-acclimation baseline walking speed. Normalized walking speed explained 94% of the variance in the change in metabolic cost of transport during walking with high assistance relative to baseline. Roman numerals in parentheses indicate participant GMFCS level.
IV. Discussion
We partially accept our primary hypothesis that net metabolic cost of transport would decrease, self-selected walking speed would increase, and soleus muscle activity would decrease during assisted walking compared to unpowered and baseline conditions. While reduction in metabolic cost of transport was significant for only the high assistance condition, all assisted conditions increased self-selected walking speed and reduced soleus muscle activity. Reduced soleus muscle activity appears to contribute to the improvement in exoskeleton-assisted walking economy. The results show that it is possible, but not guaranteed, to improve mobility-related outcomes with powered plantar-flexion assistance.
We asked our participants which general walking condition they preferred to assess whether participant perception supported our quantitative results. Five of the six participants conveyed a preference for walking with powered assistance to walking without an exoskeleton (Table I).
Our prior treadmill study demonstrated improved walking mechanics during walking with ankle exoskeleton assistance, including increased total ankle power and improved posture [17]. Anecdotal observation from the present over-ground study suggests that improved gait mechanics combined with our measurement of reduced muscle activity to facilitate the improvement in walking economy. In general, outcomes from this over-ground study were more variable than our prior treadmill study, and improvement in net metabolic cost of transport was more modest (8.5% vs. 19%) [17]. Plantar-flexor assistance during both treadmill and over-ground walking resulted in a reduction in soleus activity, which supports the idea that the neuromotor system “slacks” in an attempt to decrease muscle activation during repetitive tasks [29].
We anticipate that our findings of the potential for untethered ankle exoskeleton assistance to improve over-ground walking economy and speed have clinical relevance and justify continued exoskeleton intervention research in individuals with CP. Reduced energy expenditure during ambulation could facilitate greater accumulation of walking exercise and improve mobility, particularly for highly impaired individuals. Impairment-oriented therapy has proven to largely ineffective [30], [31] while modern, more successful approaches to improving motor learning involve task-oriented training specific to the desired outcome [32]–[34]. In short, gait-specific training has proven most successful in improving walking outcomes. The results of this study suggest that exoskeleton assistance may be a viable approach to maximizing walking exercise duration.
The presented results should be carefully interpreted. Improvements in over-ground walking economy and speed outcomes remained generally unchanged during walking with assistance for our less impaired participants. The potential for reducing net metabolic cost of transport during assisted walking compared to baseline depends on several device and participant characteristics. For example, finding an optimal magnitude of assistance for each participant remains challenging, particularly for over-ground walking. In most cases for this cohort, the training-tuned assisted condition did not reduce energy expenditure, but other improvements in mobility outcomes were detected. Human-in-the-loop optimization techniques may help address this challenge [13], [35].
The metabolic detriment of wearing the device unpowered accounted for 75% of the variance in the maximum change in net metabolic cost of transport with high assistance compared to baseline (Supplemental Fig. 3). A previous study demonstrated that the metabolic detriment due to wearing the device depends heavily on the relative body mass [17]. That is, if the exoskeleton is a small fraction of an individual’s mass the metabolic detriment of wearing the device is mitigated.
We found that baseline walking speed is a simple predictor of improvement in walking economy from ankle exoskeleton assistance for people with CP. Pre-acclimation baseline walking speed accounted for 94% of the variance in the potential benefit in net metabolic cost of transport with high assistance (Fig. 3). Slower walking speed is associated with greater level of neuromuscular impairment where there may be more capacity for improvement during walking with powered assistance [10], [11]. Improvements in propulsion symmetry were found during exoskeleton-assisted walking for stroke survivors, and, similar to our findings, individuals that walked more slowly benefitted the most from assistance [36]. It is possible that our more impaired participants exhibited the greatest reduction in metabolic cost of transport with assistance because they had the most to benefit in terms of posture and ankle function [17], [27].s
Participants with slow baseline walking speed, moderate-to-severe impairment, and reduced ankle joint function stand to benefit the most from powered assistance but only if the ratio of exoskeleton mass to participant mass is small. P3, P5, and P6 benefited the most from exoskeleton assistance because of slow baseline walking speeds and low metabolic detriments when wearing the device unpowered. More impaired participants could use an exoskeleton in conjunction with forearm support walkers for even greater improvement in metabolic cost of transport [37].
The nonlinear convex relationship between net metabolic cost of transport and walking speed may contribute to our understanding of the effect of exoskeleton assistance during over-ground walking at self-selected speeds. Ralston 1958 suggests a theoretical optimal speed at which energy expenditure is minimized [38]. The impaired participants from this study do not fall on the energy-speed curve proposed by Ralston, even during assisted walking, but assistance generally reduces deviation from metabolically optimal walking economy (Fig. 4).
Fig. 4.
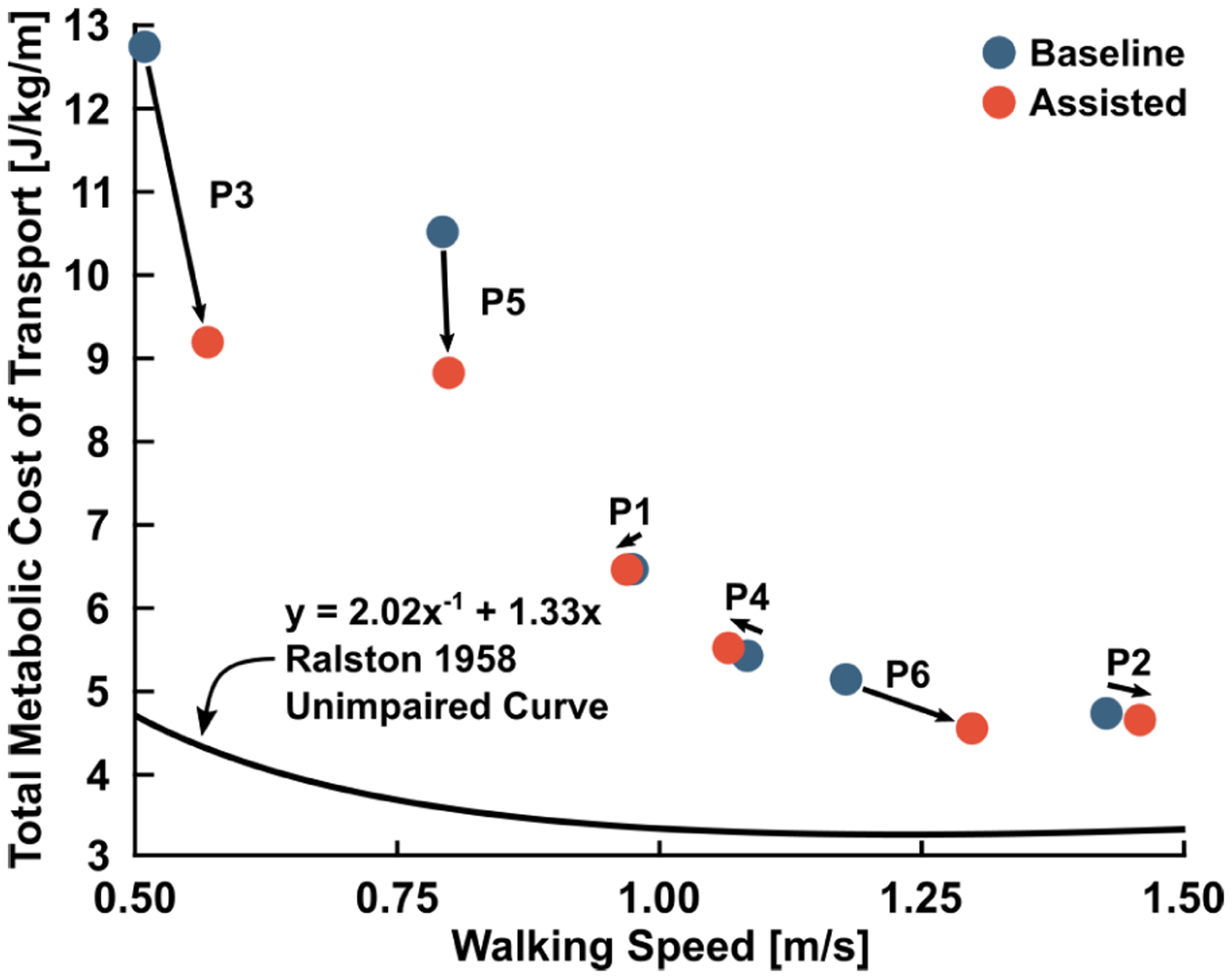
The effect of assistance on participant energy cost and speed. The Ralston 1958 curve is the energy-speed relationship for unimpaired participants [38]. Powered assistance generally increased walking speed and decreased net metabolic cost of transport in the cohort. The assistance condition that resulted in each individual’s greatest reduction in energy cost are depicted.
Consistent with prior research, we found that baseline metabolic cost of transport generally scaled with GMFCS level [39]. There is limited research on energy-speed relationships for individuals with CP, but one study found that energy expenditure index (EEI) for children with CP decreases with increasing walking speed [40]. However, the EEI-speed curve for CP children is incomplete due to the tendency of impaired individuals to transition to running when asked to walk at high speeds.
The primary limitation of this exploratory study was the small sample size and high variability of age, mass, walking aids, and impairment level, although the variability in participant characteristics may demonstrate translation of presented results to a wider population. Furthermore, we did not perform multiple comparisons correction with the intention that the actual p-values can be interpreted to determine relevance and significance of the presented results.
In conclusion, the presented results support continued research on the development and testing of ankle exoskeletons to improve over-ground mobility for individuals with gait disorders. Lightweight untethered ankle exoskeletons with adaptive control have the potential to reduce net metabolic cost of transport, increase over-ground walking speed, and reduce soleus muscle activity when an appropriate assistance level is used. Exoskeleton-assisted gait training may prove effective as task-specific exercise to improve mobility and motor skills in individuals with neuromuscular impairment. Future studies in this patient population should investigate the effects of long-term functional gait training with ankle exoskeleton assistance.
Supplementary Material
Acknowledgment
The authors thank Jenny L. Lawson and Thang Nguyen for their assistance with this study.
This work was supported by NSF grant #1756029 and NIH grant #1R15HD099664.
Footnotes
This paper has supplementary downloadable material available at link, provided by the author.
References
- [1].Boyle CA et al. , “Trends in the prevalence of developmental disabilities in US children, 1997–2008,” Pediatrics, vol. 127, no. 6, pp. 1034–1042, 2011. [DOI] [PubMed] [Google Scholar]
- [2].Armand S, Decoulon G, and Bonnefoy-Mazure A, “Gait analysis in children with cerebral palsy,” EFORT Open Rev, vol. 1, no. 12, pp. 448–460, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bjornson KF, Belza B, Kartin D, Logsdon R, and McLaughlin JF, “Ambulatory Physical Activity Performance in Youth With Cerebral Palsy and Youth Who Are Developing Typically,” Phys. Ther, vol. 87, no. 3, pp. 248–257, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].De Mattos C, Patrick Do K, Pierce R, Feng J, Aiona M, and Sussman M, “Comparison of hamstring transfer with hamstring lengthening in ambulatory children with cerebral palsy: further follow-up,” J. Child. Orthop, vol. 8, no. 6, pp. 513–520, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DahlbÄck GO and Norlin R, “The effect of corrective surgery on energy expenditure during ambulation in children with cerebral palsy,” Eur. J. Appl. Physiol. Occup. Physiol, 1985. [DOI] [PubMed] [Google Scholar]
- [6].Ries AJ, Novacheck TF, and Schwartz MH, “The Efficacy of Ankle-Foot Orthoses on Improving the Gait of Children With Diplegic Cerebral Palsy: A Multiple Outcome Analysis,” PM R, vol. 7, no. 9, pp. 922–929, 2015. [DOI] [PubMed] [Google Scholar]
- [7].Kerkum YL, Buizer AI, Van Den Noort JC, Becher JG, Harlaar J, and Brehm MA, “The effects of varying ankle foot orthosis stiffness on gait in children with spastic cerebral palsy who walk with excessive knee flexion,” PLoS One, vol. 10, no. 11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Romkes J and Brunner R, “Comparison of a dynamic and a hinged ankle-foot orthosis by gait analysis in patients with hemiplegic cerebral palsy,” Gait Posture, vol. 15, no. 1, pp. 18–24, 2002. [DOI] [PubMed] [Google Scholar]
- [9].McNee AE, Shortland AP, Eve LC, Robinson RO, and Gough M, “Lower limb extensor moments in children with spastic diplegic cerebral palsy,” Gait Posture, vol. 20, no. 2, pp. 171–176, 2004. [DOI] [PubMed] [Google Scholar]
- [10].Abel MF and Damiano DL, “Strategies for increasing walking speed in diplegic cerebral palsy,” J. Pediatr. Orthop, vol. 16, no. 6, pp. 753–758, 1996. [DOI] [PubMed] [Google Scholar]
- [11].Waters RL and Mulroy S, “The energy expenditure of normal and pathologic gait,” Gait and Posture, vol. 9, no. 3 pp. 207–231, 1999. [DOI] [PubMed] [Google Scholar]
- [12].Kerr C, Parkes J, Stevenson M, Cosgrove AP, and Mcdowell BC, “Energy efficiency in gait, activity, participation, and health status in children with cerebral palsy,” Dev. Med. Child Neurol, vol. 50, no. 3, pp. 204–210, 2008. [DOI] [PubMed] [Google Scholar]
- [13].Zhang J et al. , “Human-in-the-loop optimization of exoskeleton assistance during walking,” Science (80-.), vol. 356, no. 6344, pp. 1280–1283, 2017. [DOI] [PubMed] [Google Scholar]
- [14].Collins SH, Bruce Wiggin M, and Sawicki GS, “Reducing the energy cost of human walking using an unpowered exoskeleton,” Nature, vol. 522, no. 7555, pp. 212–215, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mooney LM, Rouse EJ, and Herr HM, “Autonomous exoskeleton reduces metabolic cost of walking,” in 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC 2014, 2014, pp. 3065–3068. [DOI] [PubMed] [Google Scholar]
- [16].Panizzolo FA et al. , “A biologically-inspired multi-joint soft exosuit that can reduce the energy cost of loaded walking,” J. Neuroeng. Rehabil, vol. 13, no. 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lerner ZF et al. , “An untethered ankle exoskeleton improves walking economy in a pilot study of individuals with cerebral palsy,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 26, no. 10, pp. 1985–1993, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim J et al. , “Autonomous and portable soft exosuit for hip extension assistance with online walking and running detection algorithm,” in Proceedings - IEEE International Conference on Robotics and Automation, 2018, pp. 5473–5480. [Google Scholar]
- [19].Lee S et al. , “Autonomous multi-joint soft exosuit for assistance with walking overground,” in Proceedings - IEEE International Conference on Robotics and Automation, 2018, pp. 2812–2819. [Google Scholar]
- [20].Lerner ZF, Damiano DL, and Bulea TC, “A lower-extremity exoskeleton improves knee extension in children with crouch gait from cerebral palsy,” Sci. Transl. Med, vol. 9, no. 404, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gasparri GM, Bair MO, Libby RP, and Lerner ZF, “Verification of a Robotic Ankle Exoskeleton Control Scheme for Gait Assistance in Individuals with Cerebral Palsy,” in IEEE International Conference on Intelligent Robots and Systems, 2018, pp. 4673–4678. [Google Scholar]
- [22].Lerner ZF, Harvey TA, and Lawson JL, “A Battery-Powered Ankle Exoskeleton Improves Gait Mechanics in a Feasibility Study of Individuals with Cerebral Palsy,” Ann. Biomed. Eng, vol. 47, no. 6, pp. 1345–1356, 2019. [DOI] [PubMed] [Google Scholar]
- [23].Soule RG and Goldman RF, “Energy cost of loads carried on the head, hands, or feet.,” J. Appl. Physiol, vol. 27, no. 5, pp. 687–690, 1969. [DOI] [PubMed] [Google Scholar]
- [24].Browning RC, Modica JR, Kram R, and Goswami A, “The effects of adding mass to the legs on the energetics and biomechanics of walking,” Med. Sci. Sports Exerc, vol. 39, no. 3, pp. 515–525, 2007. [DOI] [PubMed] [Google Scholar]
- [25].Gasparri GM, Luque J, and Lerner ZF, “Proportional Joint-Moment Control for Instantaneously Adaptive Ankle Exoskeleton Assistance,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 27, no. 4, pp. 751–759, 2019. [DOI] [PubMed] [Google Scholar]
- [26].Brockway JM, “Derivation of formulae used to calculate energy expenditure in man,” Hum. Nutr. Clin. Nutr, vol. 41, no. 6, pp. 463–471, 1987. [PubMed] [Google Scholar]
- [27].Lerner ZF, Damiano DL, and Bulea TC, “A robotic exoskeleton to treat crouch gait from cerebral palsy: Initial kinematic and neuromuscular evaluation,” in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, 2016. [DOI] [PubMed] [Google Scholar]
- [28].DeJaeger D, Willems PA, and Heglund NC, “The energy cost of walking in children,” Pflugers Arch. Eur. J. Physiol, vol. 441, no. 4, pp. 538–543, 2001. [DOI] [PubMed] [Google Scholar]
- [29].Reinkensmeyer DJ, Akoner OM, Ferris DP, and Gordon KE, “Slacking by the human motor system: Computational models and implic implications for robotic orthoses,” in Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, EMBC 2009, 2009, pp. 2129–2132. [DOI] [PubMed] [Google Scholar]
- [30].Damiano DL, “Activity, Activity, Activity: Rethinking Our Physical Therapy Approach to Cerebral Palsy,” Phys. Ther, vol. 86, no. 11, pp. 1534–1540, 2006. [DOI] [PubMed] [Google Scholar]
- [31].Ketelaar M, Vermeer A, Hart H‘T, Van Petegem-van Beek E, and Helders PJM, “Effects of a functional therapy program on motor abilities of children with cerebral palsy,” Phys. Ther, vol. 81, no. 9, pp. 1534–1545, 2001. [DOI] [PubMed] [Google Scholar]
- [32].Salem Y and Godwin EM, “Effects of task-oriented training on mobility function in children with cerebral palsy,” NeuroRehabilitation, vol. 24, no. 4, pp. 307–313, 2009. [DOI] [PubMed] [Google Scholar]
- [33].Salbach NM, Mayo NE, Wood-Dauphinee S, Hanley JA, Richards CL, and Côté R, “A task-orientated intervention enhances walking distance and speed in the first year post stroke: A randomized controlled trial,” Clin. Rehabil, vol. 18, no. 5, pp. 509–519, 2004. [DOI] [PubMed] [Google Scholar]
- [34].Ma HI, Trombly CA, and Robinson-Podolski C, “The effect of context on skill acquisition and transfer,” Am. J. Occup. Ther, vol. 53, no. 2, pp. 138–144, 1999. [DOI] [PubMed] [Google Scholar]
- [35].Felt W, Selinger JC, Donelan JM, and Remy CD, “‘Body-in-the-loop’: Optimizing device parameters using measures of instantaneous energetic cost,” PLoS One, vol. 10, no. 8, pp. 1–21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Awad LN et al. , “A soft robotic exosuit improves walking in patients after stroke,” Sci. Transl. Med, vol. 9, no. 400, pp. 1–12, 2017. [DOI] [PubMed] [Google Scholar]
- [37].Jayaraman C et al. , “Postural and Metabolic Benefits of Using a Forearm Support Walker in Older Adults With Impairments,” Arch. Phys. Med. Rehabil, vol. 100, no. 4, pp. 638–647, 2019. [DOI] [PubMed] [Google Scholar]
- [38].Ralston HJ, “Energy-speed relation and optimal speed during level walking,” Int. Zeitschrift für Angew. Physiol. Einschl. Arbeitsphysiologie, vol. 17, no. 4, pp. 277–283, 1958. [DOI] [PubMed] [Google Scholar]
- [39].Johnston TE, Moore SE, Quinn LT, and Smith BT, “Energy cost of walking in children with cerebral palsy: Relation to the Gross Motor Function Classification System,” Dev. Med. Child Neurol, vol. 47, no. 1, pp. 34–38, 2004. [DOI] [PubMed] [Google Scholar]
- [40].Rose J, Gamble JG, Burgos A, Medeiros J, and Haskell WL, “Energy Expenditure Index of Walking for Normal Children and for Children With Cerebral Palsy,” Dev. Med. Child Neurol, vol. 32, no. 4, pp. 333–340, 1990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


