Abstract
Nasal high-flow therapy (NHFT) is an upcoming treatment for chronic obstructive pulmonary disease (COPD) patients. It supplies heated, humidified, and, desirably, oxygen-enriched air through a nasal cannula at flow rates up to 60 L/min. Several studies examined the effect of NHFT in COPD patients, but a clear overview is lacking. The present review aimed to give an overview of the clinical evidence of NHFT in 3 aspects of COPD care: long-term use in stable COPD patients, use for treatment of COPD exacerbations, and use during exercise therapy in COPD. For each topic, a specific literature search was performed up to December 9, 2019. Studies show promising results, with most evidence for its long-term use in hypoxemic COPD patients that frequently exacerbate, and very limited evidence for its use during COPD exacerbations or as a worthwhile adjunct to exercise training. More evidence is therefore needed to know how to incorporate NHFT in standard clinical practice.
Keywords: Nasal high-flow therapy, Chronic obstructive pulmonary disease, Long-term treatment, Exacerbation, Exercise training
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of mortality worldwide [1, 2]. The disease is characterized by airflow limitation leading to dyspnea, cough, and sputum production. Patients with severe COPD may suffer from respiratory failure. At this stage, long-term oxygen therapy (LTOT) and/or noninvasive ventilation (NIV) may offer respiratory support. However, both therapies are indicated in particular subgroups and side effects may hinder proper treatment and good patient compliance [3, 4].
Nasal high-flow therapy (NHFT) is an upcoming treatment for COPD patients. It supplies heated, humidified, and, desirably, oxygen-enriched air through a nasal cannula at flow rates up to 60 L/min. Several studies examined the physiological effect of NHFT in stable COPD patients and showed that it leads to an improvement in gas exchange and a reduction in the work of breathing [5, 6, 7]. The physiological effects accountable for these benefits are hypothesized to be quite extensive and are reviewed in the paper by Pisani and Vega [8]. Shortly summarized, among others, important mechanisms described are the flow induced increase in airway pressure, washout of CO2, and increased mucociliary function due to humidification and heating of the inspired air. The increase in airway pressure may counteract the intrinsic positive end-expiratory pressure of COPD patients, thereby leading to a decrease in work of breathing [8]. Second, NHFT washes the upper (and lower) airways; that is, by the continuous high flow the exhaled air in the upper airways is replaced continuously by fresh air with low CO2 levels thereby leading to less CO2 rebreathing [5]. The physiological effects of NHFT, combined with its simplicity and comfortability, make NHFT not only an ideal candidate for long-term use but also for ventilatory assistance during acute exacerbations of COPD (AECOPD) or exercise training during pulmonary rehabilitation.
The present review aims to give practical advice about the use of NHFT in COPD patients based on available clinical evidence. Three aspects of NHFT in COPD will be discussed: long-term domiciliary use of NHFT in in stable COPD patients, NHFT use for the treatment of COPD exacerbations, and NHFT during exercise in COPD patients. As it is a clinical review. We do not consider the short-term physiological studies in detail but refer to the dedicated literature [8, 9].
Methods
Search Strategy
Three separate literature searches were performed using the databases of PubMed and Embase. The following keywords were used in the search “long-term use of NHFT in stable COPD”: pulmonary disease, chronic obstructive, copd, chronic obstructing lung disease(s), COPD(s), high flow, optiflow, treatment outcome, long-term care, long term, domiciliary, stable and outcome(s). The following keywords were used in the search strategy for “use of NHFT during AECOPD”: pulmonary disease, chronic obstructive, copd, chronic obstructing lung disease(s), COPD(s), high flow, optiflow, acute disease, acute, exacerbat(e/ion/ions). Keywords used for the search “NHFT use during exercise in COPD” were as follows: pulmonary disease, chronic obstructive, copd, chronic obstructing lung disease(s), COPD(s), high flow, optiflow, exercise, exercise therapy and rehabilitation. The exact search strategies can be found in Appendix 1. Only papers written in English were selected. The last update of the search was performed on December 9, 2019.
Inclusion Criteria
A first selection was based on the title and abstract. The relevance of the remaining articles was assessed by reading the full text versions. Case reports and abstracts were excluded. All articles had to meet one of the following criteria: (1) long-term use of NHFT in stable COPD patients, (2) NHFT use during an AECOPD or acute respiratory failure (ARF) in a cohort of solely COPD patients, or (3) NHFT use during exercise therapy in COPD patients. Studies regarding extubation or weaning were discarded.
Results
Long-Term Use of NHFT in Stable COPD Patients
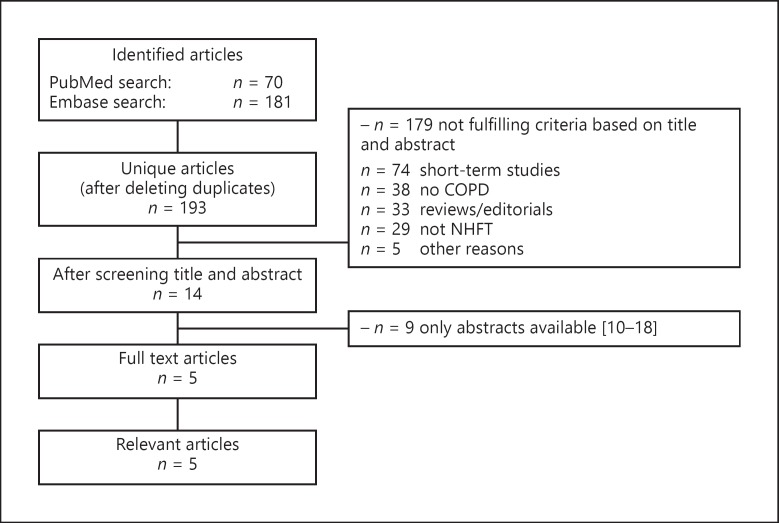
The searches in PubMed and Embase yielded, respectively, 70 and 181 publications. After deleting duplicates, 193 unique publications remained. Fourteen articles were considered relevant for full reading. Of these publications, 9 publications were abstracts [10, 11, 12, 13, 14, 15, 16, 17, 18]. The remaining 5 publications studied long-term NHFT use varying from 6 weeks to 1 year and will be discussed separately (Fig. 1). Of these 5 studies, 1 was performed in COPD or bronchiectasis patients, 1 was performed in chronic hypoxemic COPD patients, and 3 were performed in chronic hypercapnic COPD patients.
Fig. 1.
Study selection procedure for long-term studies. COPD, chronic obstructive pulmonary disease; NHFT, nasal high-flow therapy.
COPD or Bronchiectasis
Rea et al. [19] performed a randomized controlled trial (RCT) in 108 patients with COPD or bronchiectasis (mean FEV1 45% of pred.; mean FEV1/FVC 61.3 [18.8]%). NHFT with a temperature of 37°C and a flow of 20–25 L/min was compared to standard care for the duration of 1 year. Although no difference in exacerbation frequency and hospital admissions was observed between both groups, the number of exacerbation days was significantly lower (18.2 vs. 33.5/patient, p = 0.045). and the median time to first exacerbation (52 vs. 27 days, p = 0.0495) was significantly higher in the NHFT group compared to the control group. Furthermore, differences were observed in the lung function and in quality of life (QoL), all in favor to the NHFT group. However, daily use of NHFT was very limited with a mean use of only 1.6 h per day. Long-term use of NHFT was well tolerated, which is confirmed by the fact that 77% of the remaining patients wished to continue NHFT at study completion. Because the study included a mixed patient group consisting of both COPD and bronchiectasis patients without COPD, the study does not provide suitable data to draw conclusions about the usefulness of NHFT in pure COPD.
Chronic Hypoxemic Failure
A RCT in COPD patients with chronic hypoxemic respiratory failure was performed by Storgaard et al. [20]. Two hundred moderately severe COPD patients (FEV1 ± 31% of pred.) already on LTOT (PaO2 9.9 kPa while supplemented with oxygen at a mean flow rate of 1.6 L/min) were equally randomized between NHFT in addition to LTOT or LTOT alone. Mean adherence to NHFT was 6 h per day with a recommended flow setting of 20 L/min. Compared to the previous year, patient-reported exacerbations increased from 2.90 to 4.95/patient/year in the control group, while in the HFNC group, exacerbation rate slightly decreased from 3.23 to 3.12/patient/year. Therefore, lower rates of patient-reported acute COPD exacerbation were found in the NHFT group during the study period (p < 0.001). No differences in hospital admissions and all-cause mortality were observed between the groups after 1 year. However, it seemed that patients that used the NHFT more hours per day had a reduction in hospital admissions, although these benefits were shown by modeling of the results and do not represent the intention to treat principle. Furthermore, dyspnea symptoms, health-related QoL (HRQoL), PaCO2, and exercise capacity improved in the NHFT group compared to the LTOT group (p < 0.001). This study shows that domiciliary use of NHFT may be a beneficial add-on to LTOT in COPD patients with chronic hypoxemic respiratory failure in terms of improvement in QoL, symptoms, number of exacerbations, and exercise capacity.
Chronic Hypercapnic Failure
Nagata et al. [21] performed a multicenter cross-over study in which 30 COPD patients with stable hypercapnia (mean PaCO2 52 mm Hg [6.9 kPa] and mean FEV1 29% of pred.) already on LTOT were randomized between 2 groups: (A) 6 weeks of NHFT/LTOT followed by 6 weeks of LTOT alone or (B) 6 weeks of LTOT followed by 6 weeks of NHFT/LTOT. The mean use of NHFT per night was, respectively, 7.1 and 8.6 h in groups A and B, with a mean flow rate of approximately 30 L/min in both groups. The Saint George Respiratory Questionnaire, a measure of HRQoL, improved above the minimum clinically important difference (MCID = 4 Saint George Respiratory Questionnaire points) in the NHFT/LTOT groups compared to LTOT alone with a treatment effect of 7.8 points. Additionally, significant improvements were seen in the NHFT/LTOT group in the PaCO2 (reduction of 4.1 mm Hg [0.5 kPa], p < 0.01), pH (increase of 0.02, p = 0.01), and nocturnal transcutaneous CO2 levels (reduction of 4.8 mm Hg [0.6 kPa], p < 0.01) in comparison to the LTOT group. No differences were seen in PaO2, lung function, exercise capacity, and physical activity. NHFT was well tolerated, despite some mild adverse events like night sweating, and no patient discontinued the treatment during the study period. This may indicate that NHFT may be a potential therapy in hypercapnic COPD patients on LTOT. However, only a small number of mildly hypercapnic COPD patients were included. Furthermore, hypercapnia persisted after treatment with NHFT which arises the question whether LTOT and/or NHFT is the appropriate therapy for these patients, since the gold standard for chronic hypercapnic patients is nowadays chronic nocturnal NIV [22, 23].
To investigate whether NHFT could be an alternative treatment to NIV in COPD patients with chronic hypercapnic respiratory failure, Bräunlich et al. [24] performed a study in 11 hypercapnic COPD patients (mean PaCO2 of 53.7 mm Hg [7.2 kPa] and mean FEV1 30% of pred.). The patients were first treated with NHFT for 6 weeks using a flow of 20 L/min after which they switched to NIV for 6 weeks. Patients were instructed to use the device for >5 h daily, but the exact adherence data are unknown. A reduction in capillary pCO2 was observed after 6 weeks of NHFT (reduction of 8.2 mm Hg [1.1 kPa], p < 0.05). This pCO2 level was preserved after switching to NIV with no difference between the pCO2 levels after NHFT and NIV (45.5 vs. 46.4 mm Hg [6.1 vs. 6.2 kPa], p > 0.05). This indicates that NHFT may be an alternative therapy in hypercapnic COPD patients. However, this is a small, nonrandomized and monocenter study in which moderate hypercapnic COPD patients were included. Furthermore, all participants started with NHFT. More recently, the results of a larger multicenter RCT performed by Bräunlich et al. [25] were presented comparing NHFT to NIV in chronic hypercapnic COPD patients (mean pCO2 of 56.5 mm Hg [7.5 kPa] and mean FEV1 29% of pred.). In this cross-over study, 6 weeks of NHFT were followed by 6 weeks of NIV or vice-versa for the advised duration of 6 h per day. A total of 102 patients were randomized of which 67 patients completed the entire protocol. NHFT and NIV led to a pCO2 reduction of, respectively, 2.8 [0.4 kPa] and 4.2 mm Hg [0.6 kPa] from baseline. The difference in pCO2 change between both devices was −1.4 mm Hg [0.2 kPa, 95% CI −3.1 to −0.4 mm Hg]. A noninferiority analysis was performed with a rather large margin of 5 mm Hg [0.7 kPa], and NHFT was found to be noninferior to NIV with respect to pCO2 decrease. The authors state that NHFT can therefore be used as an alternative to NIV, especially because it is generally assumed that NHFT is more easy to use. However, the amount of drop-outs was comparable between groups, just like the assessment of the devices and QoL scores, indicating no increase in comfort using NHFT. An important remark is that the application of both treatments was probably not optimal. The authors state that NIV pressure settings were adjusted to optimal tolerability and pCO2 reduction. However, compliance during NIV was very limited with an average of 3.9 ± 2.5 h/day and the effect in pCO2 reduction was moderate. Also the administration of NHFT was not optimal [26] since the flow rate of NHFT was limited to 20 L/min due to technical aspects, but CO2 washout is flow dependent and higher flows could thus lead to more CO2 washout.
To conclude, for long-term use, most evidence exists with regard to hypoxemic COPD patients as an adjunct to LTOT. In this group, NHFT may reduce COPD exacerbation frequency, especially in patients with frequent prior exacerbations. In hypercapnic COPD patients, NHFT may increase HRQoL and reduce PaCO2, although it is not clear how the effects compare to effects of optimal high-intensity NIV. An overview of all discussed studies regarding the long-term use of NHFT in COPD patients can be found in Table 1.
Table 1.
Overview of long-term studies with nasal high-flow therapy in COPD patients
| Ref. | Study design | NHFT settings | Mean daily NHFT use, h | Population | Patients, n | Main results |
|---|---|---|---|---|---|---|
| COPD or bronchiectasis | ||||||
| [19] | RCT: NHFT vs. standard care (ambient air or LTOT) for 1 year | Flow 20–25 L/min, temp 37° C, FiO2 based on SaO2 | 1.6±0.67 | COPD or bronchiectasis (mean FEV1 45% pred.) | 108 | No difference in exacerbation frequency and hospital admission. Reduced number of exacerbation days and increased time to first exacerbation. Improved lung function and quality of life |
| Hypoxemic failure | ||||||
| [20] | RCT: NHFT/LTOT vs. LTOT for 1 year | Flow 20 L/min, oxygen supply 1.75±0.8 L/min | 6 | Hypoxemic COPD (PaO2 9.9 kPa during 1.6 L/min O2 and FEV1 31% pred.) | 200 | No difference in mortality or hospital admission. Reduced exacerbation rate and improvement in dyspnea symptoms, quality of life, PaCO2, and exercise capacity |
| Hypercapnic failure | ||||||
| [21] | Cross-over RCT: 6 weeks LTOT/NHFT vs. 6 weeks LTOT | Flow 29.2±1.9 L/min and 30.3±4.6 L/min | 7.1±1.5 and 8.6±2.9 | Stable hypercapnic COPD on LTOT (PaCO2 6.9 kPa, FEV1 29% pred.) | 30 | Improved quality of life, PaCO2, pH and nocturnal PtCO2. No differences in PaO2, lung function, exercise capacity, and physical activity |
| [24] | Cross-over: 6 weeks NHFT, thereafter 6 weeks NIV | Flow 20 L/min | Unknown (advice of 5 h) | Stable hypercapnic COPD (PaCO2 7.2 kPa, FEV1 30% pred.) | 11 | Reduction of capillary pCO2 compared to baseline, no difference in capillary pCO2 reduction between NHFT and NIV |
| [25] | Cross-over RCT: 6 weeks NHFT vs. 6 weeks NIV | Flow 19.8±0.6 L/min, O2 supply 2.2±0.9 L/min | 5.2±3.3 | Stable hypercapnic COPD (PaCO2 7.5 kPa, FEV1 29% pred.) | 102 | Reduction of pCO2 compared to baseline, no difference in pCO2 reduction and quality of life between NHFT and NIV |
COPD, chronic obstructive pulmonary disease; RCT, randomized controlled trial; NHFT, nasal high-flow therapy; LTOT, long-term oxygen therapy; FiO2, fraction of inspired oxygen; SaO2, oxygen saturation; FEV1, forced expiratory volume in 1 second; PaO2, partial arterial oxygen pressure; PaCO2, partial arterial carbon dioxide pressure; PtCO2, transcutaneous carbon dioxide pressure; NIV, noninvasive ventilation; pCO2, partial carbon dioxide pressure.
Use of NHFT during COPD Exacerbations
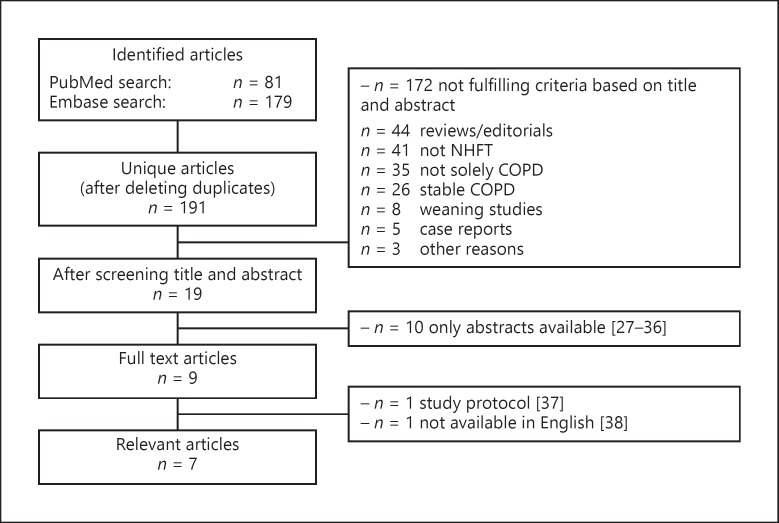
The searches in PubMed and Embase yielded 81 and 179 publications, respectively. After deleting duplicates, 190 unique publications remained. Eighteen articles were considered relevant for full reading. Of these publications, 9 were abstracts, 1 was a study protocol description, and 1 publication was only available in Chinese[27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37]. The remaining 7 publications will be discussed separately (Fig. 2). Of these studies, 2 studies compared NHFT with oxygen therapy, 3 studies compared NHFT with NIV, 1 study compared NHFT with NIV in patients already on NIV, and 1 study performed NHFT in patients who did not tolerate NIV.
Fig. 2.
Study selection procedure for exacerbation studies. COPD, chronic obstructive pulmonary disease; NHFT, nasal high-flow therapy.
NHFT Compared to Oxygen Therapy
Pilcher et al. [38] performed a short randomized controlled cross-over physiological trial to investigate whether giving oxygen using NHFT would facilitate CO2 removal compared to supplementing oxygen with standard nasal prongs (SNP) for AECOPD. Twenty-four patients (mean FEV1 27% of pred.) admitted in hospital due to AECOPD and on oxygen therapy were randomized to 30 min of NHFT at 35 L/min at 37°C followed by 30 min of SNP or vice versa at a median of 1 day after admission, with oxygen flow rates/fraction of inspired oxygen (FiO2) titrated to maintain a stable oxygen saturation (SpO2). A washout period of at least 15 min was performed between interventions during which SNP at baseline flow rate (mean flow of 1.6 L/min) was provided. Fourteen of the 24 patients were hypercapnic, and the mean baseline transcutaneous carbon dioxide tension (PtCO2) was 49 ± 10 mm Hg [6.5 ± 1.3 kPa]. Mean FiO2 during NHFT was 27 ± 4%, while flow rate with SNP was 1.5 ± 0.7 L/min. PtCO2, adjusted for baseline PtCO2, decreased significantly more with NHFT compared to SNP (−1.4 mm Hg [0.2 kPa], p = 0.001), but this difference is unlikely to be clinically significant. Respiratory rate, recorded manually by the investigator, was significantly lower during NHFT compared to SNP at 10, 20, and 25 min, but not 30 min after the start of the therapy. NHFT was well tolerated, but participants found it noisier than SNP. Due to the short administration time of NHFT, this study does not answer the question whether NHFT could be used as a therapy for AECOPD. This study indicates that delivering oxygen via NHFT may reduce PtCO2 levels in comparison with SNP, but a larger trial with longer administration periods is needed to prove clinical significance.
Longhini et al. [39] compared the short-term effect of NHFT with standard oxygen supplementation during NIV interruption periods in patients recovering from AECOPD. Thirty nonacidotic patients who were on NIV for a minimum of 24 h with an improvement of their condition (i.e., no dyspnea symptoms) with a mean PaCO2 of 54.1 mm Hg [7.2 kPa] and a mean pH of 7.39 during NIV participated in the study. Five 30-min trials were performed; the first, third, and fifth trial were on NIV, while randomization determined the order of the second and fourth trial: spontaneous breathing with standard oxygen or NHFT at a flow rate of 50 L/min and a temperature of 37°C. FiO2 was constant during all trials. Eventually, PaCO2 and pH were not different among trials, while PaO2 was significantly higher during the NIV trials compared to the NHFT (p ≤ 0.05) and standard oxygen trials (p ≤ 0.001). Diaphragm thickening, as measured by sonographic evaluation of the right hemidiaphragm, and considered to be an indirect measure of inspiratory effort, was significantly higher in the standard oxygen trial, compared to both NIV and NHFT trials (p < 0.001). This was confirmed by a higher respiratory rate during the oxygen trial. NHFT was considered to be the most comfortable therapy. This study suggests that NHFT is preferred over standard oxygen during intervals of NIV interruption in patients recovering from AECOPD, since inspiratory effort was reduced and comfort was improved in comparison with oxygen therapy.
NHFT Compared to NIV
Sun et al. [40] performed an retrospective observational cohort study comparing the effectiveness of NHFT and NIV in COPD patients treated on the ICU for AECOPD. They included patients with moderate hypercapnia (PaCO2 ≥50 mm Hg [6.7 kPa]), severe hypoxemia (PaO2 ≤45 mm Hg [6.0 kPa] at room air), and a pH range from 7.25 to 7.35. Patients that needed intubation had contraindications for NIV and had a poor short-term prognosis that were not included for analysis. Thirty-nine patients who received NHFT and 43 patients who received NIV, both at least 4 h within the 24 h after admission, were compared. There were no statistical differences in baseline parameters (mean FEV1 47% of pred., mean PaCO2 58 mm Hg [7.7 kPa], mean pH 7.31). No significant difference was observed in the amount of treatment failures, defined as invasive ventilation or switch to other study treatment (28.2% in NHFT vs. 39.5% in NIV group, p = 0.268) or 28-day mortality (15.4% in NHFT vs. 14% in NIV group, p = 0.824). However, NHFT was associated with less airway care interventions, better tolerance, and less nasal facial skin breakdown than NIV. The authors conclude that NHFT is a new potential respiratory support therapy in COPD patients with moderate hypercapnic ARF due to its improved tolerance and comfort in comparison with NIV. However, only a part of these patients were primarily diagnosed with AECOPD (49 of 82 patients), and the patients suffered from severe hypoxemia, which is usually not that severe in sole COPD exacerbations. The additional value of NHFT in hypoxemic patients with ARF is already proven, which is not the case for NIV [41]. Moreover, due to the retrospective nonrandomized design, selection bias was almost surely present.
In the study of Lee et al. [42], the effect of NHFT was prospectively compared to NIV in hospitalized severe AECOPD patients. The included 88 AECOPD patients (mean FEV1 51% of pred.) with moderate hypercapnic ARF (PaCO2 >45 mm Hg [6.0 kPa], 7.25≤ pH <7.35 and PaO2/FiO2 <200 mm Hg [26.7 kPa]) were equally assigned to NHFT and NIV, while the exact grounds and manner of assignment remain unclear. Nevertheless, there were no significant differences in patient characteristics (mean pH 7.32, PaCO2 54.5 mm Hg [7.3 kPa], PaO2/FiO2 134.6 mm Hg [18.0 kPa]) between the groups. Both primary outcomes, namely, intubation rate at day 30 (HFNC 25.0% vs. NIV 27.3%, p = 0.857) and 30-day mortality (HFNC 15.9% vs. NIV 18.2%, p = 0.845) showed no significant differences between groups. Arterial blood gas analysis also showed no significant differences between groups after 6 and 24 h of therapy. Furthermore, underlying etiologies for the AECOPD were very diverse and again hypoxemia was severe. Finally, no data on average therapy settings were provided, making it hard to draw final conclusion about the efficacy of both therapies in this patient group.
Cong et al. [43] performed an RCT to compare the effectiveness of NHFT and NIV in hospitalized AECOPD patients. In total, 168 patients (mean PaO2 of 53.6 mm Hg [7.1 kPa], PaCO2 of 72.5 mm Hg [9.7 kPa], serum pH 7.26 ± 0.09, and FEV1 69% of pred.) were equally randomized to receive either NIV or NHFT. Important exclusion criteria were pneumonia, acute heart failure, and, remarkably, acute respiratory acidosis needing NIV. After treatment with NIV or NHFT, arterial blood gases improved without differences between both treatments. The NHFT group had fewer complications and higher comfort and satisfaction scores than the NIV group. This study implicates that NHFT is as efficient as NIV in improving blood gas parameters in a mixed population of acidotic and nonacidotic AECOPD patients. However, the study lacked clear inclusion criteria regarding blood gas parameters, COPD severity, and the degree of respiratory acidosis, so that is difficult to judge on the population that was included. Furthermore, criteria for termination of the therapy were unclear.
NHFT in Patients Already on NIV
Rittayamai et al. [44] performed a short physiological study to investigate the effects of NHFT at different flow rates in comparison with NIV. Twelve hypercapnic AECOPD patients who were ventilated with NIV for a median of 17 h were included (mean PaCO2 51 mm Hg [6.8 kPa], PaO2 139 mm Hg [18.5 kPa], pH 7.36, and FEV1 of 34% pred.). For the study protocol, the subjects were first ventilated with NIV using their clinical settings for 15 min, after which, NHFT was started at a flow rate of 10 L/min and was subsequently increased to 20, 30, 40, and 50 L/min for 15 min each. The FiO2 during NHFT was titrated to achieve a SpO2 of at least 92% and was kept constant during the entire protocol. The simplified esophageal pressure-time product, measured to quantify inspiratory effort, per minute and respiratory rate were maximally reduced with a NHFT flow rate of 30 L/min; and was, at this flow rate, comparable with NIV. Oxygenation was optimal with very high flow rates of 50 L/min; however, direct comparison with NIV is difficult since the FiO2 was not measured during NIV. No difference in PtCO2 was found during the study protocol. In summary, this short physiological study showed that NHFT with a flow of 30 L/min led to a reduction in inspiratory effort in comparison with lower flow rates, but compared to NIV, NHFT only led to an increase in oxygenation. Limitations of the study included the small physiological and nonrandomized set-up of the study.
NHFT in Patients Who Did Not Tolerate NIV
Bräunlich and Wirtz [45] investigated the effect of NHFT in 38 solely hypercapnic AECOPD patients (mean pCO2 67.6 ± 12.9 mm Hg [9.0 ± 1.7 kPa], pH 7.34 ± 0.04). All included patients did not tolerate NIV and did not fulfil criteria for intubation. Patients were initiated on NHFT with a mean flow rate of approximately 25 L/min. The therapy was terminated when the pH increased to >7.38, the patient did not tolerate the device anymore or had lesser symptoms. The mean treatment time was 195 ± 231 min. With NHFT, pH increased (mean change of 0.052, p = 0.000) and PaCO2 decreased (mean change of −9.1 mm Hg [1.2 kPa], p = 0.001). Analysis in the subset of patients with a baseline pH <7.35 (n = 17) showed an even greater effect in both pH (mean change of 0.082, p = 0.000) and PaCO2 (mean change of −14.2 mm Hg [1.9 kPa], p = 0.002) compared to the patient being more moderately acidotic, which may be explained by the fact that there is more room for improvement in this group. The study shows that NHFT may be an alternative therapy in hypercapnic and moderate-to-nonacidotic AECOPD patients who do not tolerate NIV. However, a major limitation of this study is its retrospective analysis and lack of a control group. Furthermore, it is unusable and probably unnecessary to indicate acute NIV in the nonacidotic AECOPD patients (n = 21), and so comparing NIV with NHFT in this setting is probably not the correct comparison.
In summary, in AECOPD, in comparison to standard oxygen therapy, short-term physiological data suggest that NHFT might be of value as it has an beneficial effect on CO2 removal and might be a better alternative during NIV interruption periods in patients who for example want to eat while still on continuous acute NIV. In patients with acute acidotic hypercapnic respiratory failure who have an indication for acute NIV, there are weak indications that NHFT might be as good to improve gas exchange, prevent intubation and mortality, however, studies have major drawbacks. Table 2 shows an overview of the studies that investigated NHFT in AECOPD patients.
Table 2.
Overview of all studies regarding nasal high-flow therapy in AECOPD patients
| Ref. | Study design | NHFT settings | NHFT use | Population | Patients, n | Main results |
|---|---|---|---|---|---|---|
| NHFT compared to oxygen therapy | ||||||
| [38] | Cross-over RCT: NHFT vs. SNP | Flow 35 L/min, temp 37° C, O2 titrated on baseline SpO2 at study entry (mean FiO2 27±4%) | 30 min | Partly-hypercapnic AECOPD on oxygen suppletion (PtCO2 6.5 kPa, FEV1 27% of pred.) | 24 | Decrease of PtCO2 |
| [39] | Cross-over RCT: NHFT vs. SNP in between NIV periods | Flow 50 L/min, temp 37° C, FiO2 titrated on 90–94% SpO2 (mean FiO2 30%) | 30 min | Non-acidotic AECOPD on NIV for >24 h (PaCO2 7.2 kPa and pH 7.39 during NIV) | 30 | No difference in PaCO2 and pH between NHFT and SNP. Lower inspiratory effort and increased comfort in NHFT group |
| NHFT compared to NIV | ||||||
| [40] | Retrospective observational cohort study: NHFT or NIV | Flow 50 (40–50) L/min, FiO2 30% | ≥4 h in the first 24 h after admission | Hypercapnic, acidotic AECOPD (FEV1 47% of pred., PaCO2 7.7 kPa, mean pH 7.31) | 82 | No difference in treatment failure or 28-day mortality. Less airway care interventions, better tolerance, and less nasal facial skin breakdown |
| [42] | Prospective observational cohort study: NHFT vs. NIV | Flow 35 L/min (up to 45–60 if tolerable), FiO2 titrated on >92% SpO2 | 7.0 (5–10) days | Hypercapnic, acidotic AECOPD (FEV1 51% of pred., pH 7.32, PaCO2 7.3 kPa) | 88 | No difference in intubation rate and mortality after 30 days, and no difference in arterial blood gas levels after 24 h |
| [43] | RCT: NHFT vs. NIV | Flow 30–35 L/min, temp 37° C, FiO2 titrated on SpO2 | 10±5 days | AECOPD (PaO2 7.1 kPa, PaCO2 9.7 kPa, pH 7.26, FEV1 69% of pred.) | 168 | No difference between NIV and NHFT in blood gas analysis. Fewer complications and higher comfort and satisfaction scores |
| NHFT in patients already on NIV | ||||||
| [44] | Prospective physiological study: NHFT (different flow rates) vs. NIV | Flow 10–50 L/min, temp 34–37° C, FiO2 titrated on >92% SpO2 (mean FiO2 35%) | 15 min | Hypercapnic AECOPD stabilised with NIV (PaCO2 6.8 kPa, PaO2 18.5 kPa, pH 7.36 during NIV, FEV1 of 34% pred.) | 12 | No difference in inspiratory effort, respiratory rate and PtCO2 between NIV and NHFT. Improved oxygenation at NHFT with flow rate 50 L/min compared to NIV |
| NHFT if NIV was not tolerated | ||||||
| [45] | Retrospective observational cohort study | Flow 25.8±8.2 L/min, O2 titrated on baseline SpO2 at study entry | 252±251 min | Hypercapnic, partly acidotic AECOPD with NIV failure (pCO2 9.0 kPa, pH 7.34) | 38 | Increase in pH, decrease in PaCO2 |
AECOPD, acute exacerbation of chronic obstructive pulmonary disease; RCT, randomized controlled trial; NHFT, nasal high-flow therapy; SNP, standard nasal prongs; SpO2 oxygen saturation; FiO2, fraction of inspired oxygen; PtCO2, transcutaneous carbon dioxide pressure; FEV1, sforced expiratory volume in 1 s; NIV, noninvasive ventilation; PaCO2, partial arterial carbon dioxide pressure.
Use of NHFT during Exercise Therapy
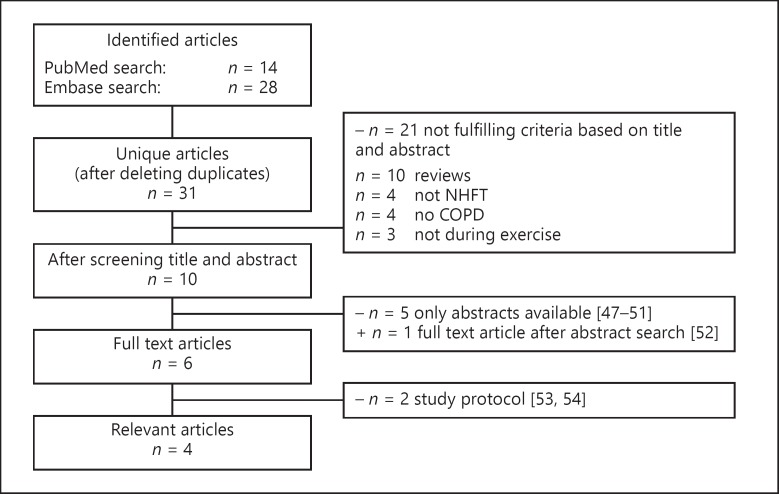
The searches in PubMed and Embase yielded 14 and 28 publications, respectively. After deleting duplicates, 31 unique publications remained. Ten articles were considered relevant for full reading. Of these publications, 5 publications were abstracts and 2 were protocol descriptions. One additional full text article was found after evaluating the abstracts [46, 47, 48, 49, 50, 51, 52, 53]. In total, 4 full text articles were relevant and will be discussed separately (Fig. 3). One study compared NHFT to oxygen therapy, 1 study compared NHFT to standard care (ambient air or oxygen therapy), and the remaining 2 studies compared NHFT to Venturi masks (VM). In all studies, NHFT was performed during exercise training; no studies were found in which NHFT was performed during a pulmonary rehabilitation trajectory.
Fig. 3.
Study selection procedure for exercise therapy studies. COPD, chronic obstructive pulmonary disease; NHFT, nasal high-flow therapy.
NHFT Compared to Oxygen Therapy during a Single Constant Work Rate Test
Chatila et al. [54] studied exercise endurance with HFT compared to conventional low-flow oxygen (LFO) delivery in patients with severe COPD (mean FEV1 23 ± 6% of pred., mean LTOT use of 2.2 ± 1.6 L/min). Patients first performed an unloaded constant work rate test (CWRT) with LFO using a nasal cannula with a mean flow of 3.9 ± 1.8 L/min for a maximum of 12 min, followed by a resting period of 30 min and the same exercise test, only now with HFT at 20 L/min, using a mouthpiece instead of a nasal cannula. Additional oxygen was titrated to deliver an FiO2 that matches the starting FiO2 during LFO, although it remains unclear how this was warranted. Exercise duration increased with the HFT compared to LFO (10.0 ± 2.4 vs. 8.2 ± 4.3 min, p < 0.05), while patients were less dyspneic (p = 0.03) and oxygenation seemed to be better (although measured only in 3 patients). Compared to LFO, respiratory rate, rapid shallow breathing index, and inspiratory time fraction were lower in the high-flow group, while no differences in other respiratory parameters were observed.
NHFT Compared to Standard Care during a Single High-Intensity CWRT
In a recent study, Prieur et al. [55] investigated exercise endurance during a high-intensity (80% of maximal estimated power) CWRT in 19 patients (FEV1 28.7 ± 10.8% of pred., PaO2 69 ± 21 mm Hg [9.2 ± 2.8 kPa], PaO2 44 ± 6 mm Hg [5.9 ± 0.8 kPa]) who were recently discharged (≤7 days) from the hospital after being treated for AECOPD. In a randomized cross-over design, the participants performed 2 high-intensity CWRTs during 2 consecutive days: one with and one without NHFT. Participants on oxygen therapy (n = 9) performed both exercise tests on the same oxygen flow rate, which was titrated based on SpO2 during an exercise test without NHFT performed as a baseline assessment before the other 2 exercise tests. Eventually, no significant difference in endurance time was found (NHF 418.5 s vs. control 485 s, p = 0.119), but heart rate, transcutaneous CO2 pressure, and tissue saturation of the vastus lateralis muscle were all significantly lower at the end of the NHFT exercise test. The authors conclude that NHFT does not improve exercise tolerance in patients recovering from an AECOPD. However, NHFT was perceived as uncomfortable in many patients which may be explained by the fact that the settings were set at the maximal flow rate of 60 L/min with a low temperature and only a 10 min period of familiarization with NHFT was performed. Furthermore, oxygen flow during NHFT in the subgroup of patients on oxygen therapy was the same as during the test without NHFT which may have led to lower FiO2 values during the NHFT test and subsequently a decrease in SpO2, thereby possibly affecting endurance time.
NHFT Compared to Oxygen via VMs during a Single CWRT
Cirio et al. [56] performed a randomized cross-over study to investigate whether NHFT could increase exercise endurance in stable severe COPD patients (FEV1 35 ± 12% of pred., PaO2 73 ± 13 mm Hg [9.7 ± 1.7 kPa], PaCO2 42 ± 5 mm Hg [5.6 ± 0.7 kPa]). Patients performed, in a random order and on 2 separate days, 2 CWRTs at 75% of maximum workload: one with HFNC (HFNC-test) and one with a VM (Control-test). In patients who needed additional oxygen (n = 8), FiO2 was kept constant during both tests (mean FiO2 0.44 ± 0.11). FiO2 values were titrated on maintaining a mean oxygen saturation higher than 88% during an incremental test that was performed 1 day before the 2 constant-load exercise tests. Mean flow during the HFNC test was 58.7 L/min (range 55–60 L/min). It was shown that endurance time was significantly higher during the HFNC-test compared to the Control-test (mean difference of 109 ± 104 s, p < 0.015). Furthermore, at isotime, dyspnea, and leg fatigue scores were significantly reduced during the HFNC-test, while the mean SpO2 significantly increased (p < 0.005), both in the group of patients who used additional oxygen and in the group who did not. This pilot study showed that HFNC can improve exercise endurance in severe COPD patients with exercise limitation, by hypothetically leading to a slower increase in minute ventilation during effort, and thus delayed achievements of the patient's ventilatory reserve capacity. However, minute ventilation was not measured during the study. Furthermore, FiO2 was the same during the start of both tests, but contrary to HFNC during which constant FiO2 values can be delivered, FiO2 during the Control-test with a VM might have led to an unknown drop in FiO2 due to an increase in inspiratory flow above the maximum flow through the VM. This may (partially) explain the difference in oxygenation and dyspnea scores between both tests.
NHFT Compared to Oxygen via VMs during Both an Incremental Test and CWRT
Dell'Era et al. [51] performed a randomized cross-over trial to evaluate the use of NHFT during both an incremental exercise test (IET) and a CWRT. Twenty-eight COPD patients (FEV1 44 ± 19% of pred.) with an indication for oxygen during exercise performed 2 IETs and 2 CWRTs, one with NHFT and one with a VM, with recovery times ranging from 48 to 72 h. NHFT was set to a flow of 50 L/min and both the NHFT and the VM were set to an FiO2 of 40%. Both the maximum speed reached on the IET and the endurance time reached with the CWRT was significantly higher using NHFT than VM (5.9 vs. 5.7 km/h, p = 0.002 and 450 vs. 315 s, p = 0.004 respectively). At isoworkload and isotime during IET and CWRT, respectively, NHFT was associated with less dyspnea and better oxygen saturation. The results of the CWRT are comparable to the study of Cirio et al. [56], but the same limitation also holds: FiO2 values during the tests with a VM could have decreased due to the inspiratory flow of the patient exceeding the flow of the VM.
To summarize, compared to oxygen therapy, there are only studies available assessing the acute effects of NHFT during exercise tests, showing that NHFT may lead to increase in exercise capacity and duration with less dyspnea and better oxygenation, compared to standard oxygen therapy. Table 3 shows an overview of the studies that investigated NHFT during exercise therapy.
Table 3.
Overview of all studies regarding NHFT during exercise therapy
| Ref. | Study design | Type of exercise | NHFT settings | Population | Patients, n | Main results |
|---|---|---|---|---|---|---|
| NHFT compared to oxygen therapy | ||||||
| [54] | Prospective nonrandomized trial: HF vs. low-flow oxygen | CWRT unloaded T | Flow 20 L/min, temp 36° C, FiO2 39±11% | Stable severe COPD (FEV1 23% of pred.) | 10 | Increase in exercise endurance with less dyspnea and better oxygenation during HFT |
| NHFT compared to standard care | ||||||
| [55] | Cross-over RCT: NHFT vs. standard care (ambient air or oxygen therapy) | CWRT at 80% of estimated peak work rate | Flow 60 L/min, temp 31° C, FiO2 0.23±0.03 (n = 9) | Recently discharged with AECOPD (FEV1 29% of pred.) | 19 | No difference in endurance time. Reduced heart rate and nocturnal PtCO2 during NHFT |
| NHFT compared to Venturi mask | ||||||
| [56] | Cross-over RCT: NHFT vs. VM | CWRT at 75% of peak work rate | Flow 58.7 L/min, FiO2 44±11% (n = 8) | Stable severe COPD (FEV1 35% of pred.) with exercise limitation | 12 | Increased endurance time, less dyspnea and leg fatigue, and better oxygenation during NHFT |
| [51] | Cross-over RCT: NHFT vs. VM | IET and CWRT at 90% of maximal speed achieved during the IET | Flow 50 L/min, FiO2 40% | Stable COPD (FEV1 44% of. pred.) | 28 | Increased exercise tolerance during both IET and CWRT with less dyspnea, and better oxygenation during NHFT |
NHFT, nasal high-flow therapy; CWRT, constant work rate test, FiO2, fraction of inspired oxygen; FEV1, forced expiratory volume in 1 s; RCT, randomized controlled trial; AECOPD, acute exacerbation of COPD; PtCO2, transcutaneous carbon dioxide pressure; VM, Venturi mask; IET, incremental exercise test.
Discussion
Long-Term Use of NHFT in Stable COPD Patients
The evidence for long-term use of NHFT in stable COPD patients is limited. Major variation exists in the characteristics of COPD patients included in the trials; NHFT might be beneficial in hypoxemic patients as a replacement of LTOT (1 moderate-quality RCT) but also as an add-on to LTOT in hypercapnic patients (1 moderate-quality cross-over study) or as alternative for NIV in hypercapnic patients (2 cross-over studies with low/moderate quality). Furthermore, the studies differed not only in characteristics of the included patients but also in NHFT settings used, so that we are not sure which settings are optimal. The evidence for clinical use of NHFT in stable COPD is therefore still uncertain, and further studies are needed in well-defined patients groups compared to gold standard treatment control groups. Research in hypoxemic COPD patients should focus on patients who exacerbate frequently to assess the potential clinical impact on admission reduction. For hypercapnic COPD patients, an RCT with adequate selection of patients and adequate treatment settings is needed to show whether NHFT is a (superior) alternative to NIV in reducing hypercapnia and, more importantly, in achieving improvement in patient-related outcomes.
Fortunately, several studies about domiciliary use of NHFT are in progress hopefully providing with answers the coming future. Currently, NHFT is investigated as add-on after their last pulmonary rehabilitation session in order to maintain the benefits of the rehabilitation (ClinicalTrials.gov NCT03882372), as add-on during and after a COPD exacerbation in patients with frequent AECOPDs requiring hospitalisations (ClinicalTrials.gov NCT03564236), and in hypercapnic COPD patients using LTOT (ClinicalTrials.gov NCT03282019).
Use of NHFT during COPD Exacerbations
The evidence for the use of NHFT during COPD exacerbations is very scarce: 2 short physiological randomized cross-over studies with moderate quality compared NHFT to oxygen therapy and 5 (2 observational studies, 1 RCT, 1 physiological study, and 1 retrospective observational study) compared NHFT to NIV. The included patient groups were again very diverse: the underlying etiology of AECOPD differed, severe hypoxemia was a very prevalent finding, and in some studies in which NHFT was compared to NIV, patients included did not seem to qualify for NIV at all [43, 45]. Research in hypercapnic nonacidotic patients on oxygen therapy should focus on the effects of NHFT in relation to patient-related outcomes. In hypercapnic acidotic patients, there is a need for a well-designed RCT with a well-characterized patient group to show whether NHFT is effective or even superior to NIV in stabilizing AECOPD patients. Currently, several studies are ongoing to study the effect of NHFT in acidotic AECOPD patients (ClinicalTrials.gov NCT03014869, NCT03466385, NCT03370666 [37]) and in AECOPD in hypercapnic nonacidotic patients on oxygen therapy (Clinical Trials.gov NCT02439333, NCT03003559).
Use of NHFT during Exercise Therapy
The evidence for the use of NHFT during an exercise training in stable COPD patients is upcoming: 1 prospective nonrandomized trial with low/moderate quality comparing NHFT to oxygen therapy and 2 cross-over RCTs with moderate quality comparing NHFT to VM all showed a beneficial effect of NHFT. Only one cross-over RCT that compared NHFT to standard care (ambient air or oxygen therapy) in patients who were recently discharged with AECOPD showed no difference in exercise endurance. This may be explained by the altered respiratory mechanics in AECOPD patients but could also be explained by differences in the study protocol like the low mean FiO2 during NHFT in comparison with the other studies. Further research should focus on comparing NHFT to VM, LFO, or no respiratory support while making sure that the FiO2 is kept constant between and during different trials. Furthermore, research should focus on whether NHFT has a beneficial effect when added during training as part of a pulmonary rehabilitation program on patient-reported outcomes and eventual unassisted exercise endurance and physical activity. This will provide us with an answer whether assisted exercise training is indeed clinically worthwhile. Multiple studies are planned to investigate the effects of NHFT on exercise endurance, for example, by Vitacca et al. [53] (ClinicalTrials.gov NCT03322787) of which the results will be expected soon.
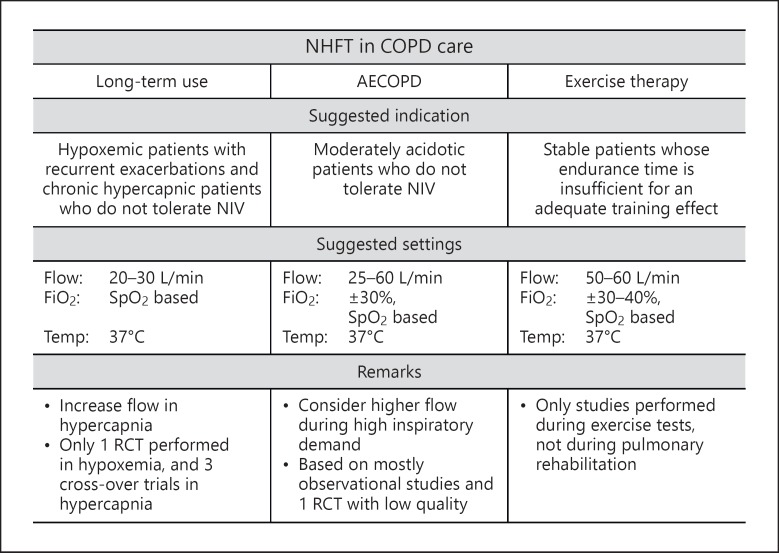
Figure 4 shows an overview of the suggested indications for different aspects of COPD care.
Fig. 4.
Suggested indications of NHFT in COPD care based on available evidence. COPD, chronic obstructive pulmonary disease; AECOPD, acute exacerbations of COPD; NIV, noninvasive ventilation; SpO2, oxygen saturation; RCT, randomized controlled trial.
Conclusion
This review provided an overview of all clinical studies in 3 different aspects of COPD care: long-term use, use during AECOPD treatment, and use during exercise therapy. Studies show promising results, but the evidence for clinical use is still limited. More evidence is therefore needed to incorporate NHFT in standard clinical practice.
Disclosure Statement
Dr. Marieke L. Duiverman reports grants and personal fees from ResMed Ltd., Philips B.V., Vivisol B.V., and grants from Fisher and Paykel Ltd., outside the submitted work.
Author Contributions
M.L.D. designed and conceived the paper, J.E. reviewed, downloaded, and selected the papers for the review, and J.E. and M.L.D. wrote the paper together. Both authors have read and approved the final manuscript.
Appendix 1
Literature Search up to December 9, 2019
1. PUBMED: Long-Term
(“Pulmonary Disease, Chronic Obstructive”[Mesh] OR copd[tiab] OR chronic obstructive lung disease*[tiab] OR chronic obstructive pulmonary disease*[tiab]) AND (high flow[tiab] OR optiflow[tiab]) AND (“Treatment Outcome”[Mesh] OR “Long-term Care”[Mesh] OR long term[tiab] OR domiciliary[tiab] OR stable[tiab] OR outcome*[tiab]).
2. EMBASE: Long-Term
(“chronic obstructive lung disease”/exp OR copd:ab, ti OR “chronic obstructive lung disease*”:ab, ti OR “chronic obstructive pulmonary disease*”:ab, ti) AND (“high flow”:ab, ti OR optiflow:ab, ti) AND (“treatment outcome”/exp OR “long term care”/exp OR “long term”:ab, ti OR domiciliary:ab, ti OR stable:ab, ti OR outcome*:ab, ti).
3. PUBMED: Exacerbation
(“Pulmonary Disease, Chronic Obstructive”[Mesh] OR copd[tiab] OR chronic obstructive lung disease[tiab] OR chronic obstructive pulmonary disease[tiab]) AND (high flow[tiab] OR optiflow[tiab]) AND (“Acute Disease”[Mesh] OR acute[tiab] OR exacerbat*[tiab]).
4. EMBASE: Exacerbation
(“chronic obstructive lung disease”/exp OR copd:ab, ti OR “chronic obstructive lung disease*”:ab, ti OR “chronic obstructive pulmonary disease*”:ab, ti) AND (“high flow”:ab, ti OR optiflow:ab, ti) AND (“acute disease”/exp OR acute:ab, ti OR exacerbat*:ab, ti).
5. PUBMED: Exercise Therapy
(“Pulmonary Disease, Chronic Obstructive”[Mesh] OR copd[tiab] OR chronic obstructive lung disease*[tiab] OR chronic obstructive pulmonary disease*[tiab]) AND (high flow[tiab] OR optiflow[tiab]) AND (“Exercise”[Mesh] OR “Exercise Therapy”[Mesh] OR exercise[tiab] OR rehabilitation[tiab]).
6. EMBASE: Exercise Therapy
(“chronic obstructive lung disease”/exp OR copd:ab, ti OR “chronic obstructive lung disease*”:ab, ti OR “chronic obstructive pulmonary disease*”:ab, ti) AND (“high flow”:ab, ti OR optiflow:ab, ti) AND (“exercise”/exp OR “kinesiotherapy”/exp OR “exercise”:ab, ti OR rehabilitation:ab, ti).
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec;380((9859)):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005 Sep;294((10)):1255–9. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 3.Gauthier A, Bernard S, Bernard E, Simard S, Maltais F, Lacasse Y. Adherence to long-term oxygen therapy in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2019 Jan-Dec;16:1479972318767724. doi: 10.1177/1479972318767724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest. 1999 Sep;116((3)):667–75. doi: 10.1378/chest.116.3.667. [DOI] [PubMed] [Google Scholar]
- 5.Bräunlich J, Köhler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016 May;11:1077–85. doi: 10.2147/COPD.S104616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biselli PJ, Kirkness JP, Grote L, Fricke K, Schwartz AR, Smith P, et al. Nasal high-flow therapy reduces work of breathing compared with oxygen during sleep in COPD and smoking controls: a prospective observational study. J Appl Physiol (1985) 2017 Jan;122((1)):82–8. doi: 10.1152/japplphysiol.00279.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bräunlich J, Seyfarth HJ, Wirtz H. Nasal High-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med. 2015 Sep;10((1)):27. doi: 10.1186/s40248-015-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisani L, Vega ML. Use of nasal high flow in stable COPD: rationale and physiology. COPD. 2017 Jun;14((3)):346–50. doi: 10.1080/15412555.2017.1315715. [DOI] [PubMed] [Google Scholar]
- 9.Bruni A, Garofalo E, Cammarota G, Murabito P, Astuto M, Navalesi P, et al. High Flow Through Nasal Cannula in Stable and Exacerbated Chronic Obstructive Pulmonary Disease Patients. Rev Recent Clin Trials. 2019;14((4)):247–60. doi: 10.2174/1574887114666190710180540. [DOI] [PubMed] [Google Scholar]
- 10.Weinreich U, Storgaard L, Hockey H. Long-term nasal high flow treatment with oxygen in COPD-exacerbations, admissions and mortality. Eur Respir J. 2017;•••:50. [Google Scholar]
- 11.Weinreich U, Storgaard L, Hockey H. Long term high flow heated oxygen treatment in COPD-lung function and physical ability. Eur Respir J. 2017;50:OA4873. [Google Scholar]
- 12.Storgaard L, Weinreich U, Hockey H. Long term high flow humidified oxygen treatment in COPD-effect on blood gases. Eur Respir J. 2017;50 PA3682. [Google Scholar]
- 13.Pisani L, Fasano L, Verhovez A, Comellini V, Nava S. Effects of nasal high flow therapy on PACO2 in COPD patients with stable hypercapnic respiratory failure. A pilot study. Am J Respir Crit Care Med. 2018;197:A5105. [Google Scholar]
- 14.Nilius G, Domanski U, Schroeder M, Franke KJ, Tatkov S. Effects of domiciliary nasal high flow (NHF) in chronic hypercapnic COPD patients on quality of life and gas exchange in a randomized crossover study. Am J Respir Crit Care Med. 2017;195:A5717. [Google Scholar]
- 15.Nagata K, Kikuchi T, Horie T, Shiraki A, Kitajima T, Kadowaki T, et al. Domiciliary high-flow nasal cannula oxygen therapy for stable hypercapnic chronic obstructive pulmonary disease A prospective, multicentre, randomised crossover trial. Eur Respir J. 2017;50:OA4428. doi: 10.1513/AnnalsATS.201706-425OC. [DOI] [PubMed] [Google Scholar]
- 16.Milne R, Hockey H. Long-term humidification therapy improves quality of life and is cost effective for patients with copd or bronchiectasis. Value Health. 2013;16((3)):A236. doi: 10.1016/j.jval.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Meijntz-Valentijn I, Gubbelmans MC, De Vries GJ. Effect of nasal high flow heated oxygen in chronic airway disease on quality of life and hospital admissions. Eur Respir J. 2016;48:PA3744. [Google Scholar]
- 18.Braunlich J, Seyfarth HJ, Hammerschmidt S, Wirtz H. Long term use of nasal high-flow in COPD. Am J Respir Crit Care Med. 2013;187:A3090. [Google Scholar]
- 19.Rea H, McAuley S, Jayaram L, Garrett J, Hockey H, Storey L, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med. 2010 Apr;104((4)):525–33. doi: 10.1016/j.rmed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Storgaard LH, Hockey HU, Laursen BS, Weinreich UM. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis. 2018 Apr;13:1195–205. doi: 10.2147/COPD.S159666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagata K, Kikuchi T, Horie T, Shiraki A, Kitajima T, Kadowaki T, et al. Domiciliary high-flow nasal cannula oxygen therapy for patients with stable hypercapnic chronic obstructive pulmonary disease a multicenter randomized crossover trial. Ann Am Thorac Soc. 2018 Apr;15((4)):432–9. doi: 10.1513/AnnalsATS.201706-425OC. [DOI] [PubMed] [Google Scholar]
- 22.Köhnlein T, Windisch W, Köhler D, Drabik A, Geiseler J, Hartl S, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014 Sep;2((9)):698–705. doi: 10.1016/S2213-2600(14)70153-5. [DOI] [PubMed] [Google Scholar]
- 23.Duiverman ML. Noninvasive ventilation in stable hypercapnic COPD: what is the evidence? ERJ Open Res. 2018 Apr;4((2)):00012–02018. doi: 10.1183/23120541.00012-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bräunlich J, Seyfarth HJ, Wirtz H. Nasal High-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med. 2015 Sep;10((1)):27. doi: 10.1186/s40248-015-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bräunlich J, Dellweg D, Bastian A, Budweiser S, Randerath W, Triché D, et al. Nasal high-flow versus noninvasive ventilation in patients with chronic hypercapnic COPD. Int J Chron Obstruct Pulmon Dis. 2019 Jul;14:1411–21. doi: 10.2147/COPD.S206111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elshof J, Duiverman M. Letter to the editor:“Nasal high-flow versus non-invasive ventilation in patients with chronic hypercapnic COPD”. International Journal of Chronic Obstructive Pulmonary Disease, 2019;14:p. 2117–2118. doi: 10.2147/COPD.S226697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crimi C, et al. High-flow oxygen therapy for treatment of acute exacerbation of copd with bronchiectasis. Eur Respir J. 2017;•••:50. [Google Scholar]
- 28.Do Campo J, Meikle N. Implementation of HFNO and reduction in the number of AECOPD needing NIV in a rural hospital. Intern Med J. 2019;49(S3):10. [Google Scholar]
- 29.Fingleton J, et al. Feasibility of NHF for acute hypercapnic respiratory failure in COPD. Respirology. 2018;23:160. [Google Scholar]
- 30.Hanci P, et al. High flow nasal oxygen therapy in patients with acute exacerbations of COPD. Intensive Care Med Exp. 2018;•••:6. [Google Scholar]
- 31.Lee MK, et al. The efficacy of high-flow nasal cannulae oxygen therapy in severe acute exacerbation of chronic obstructive pulmonary disease: A randomized controlled trial. Eur Respir J. 2016;•••:48. [Google Scholar]
- 32.Patrick H, Pezzella M, Rose D. Taste test: providing patient choice of noninvasive ventilation modalities. Chest. 2013;144((4)):882A. [Google Scholar]
- 33.Rittayamai N, et al. High-flow nasal oxygen cannula in patients with chronic obstructive pulmonary disease requiring ventilator support. Intensive Care Med Exp. 2017;5((2)) [Google Scholar]
- 34.Saeed A, Wagih K, Huusein N. Evaluation of nasal optiflow device in management of COPD patients in acute exacerbations. Eur Respir J. 2015;•••:46. [Google Scholar]
- 35.Song KM, et al. Clinical efficacy of high-flow nasal cannula in patients with acute exacerbation of chronic obstructive pulmonary disease. Respirology. 2017;22:185. [Google Scholar]
- 36.Cortegiani A, Longhini F, Carlucci A, Scala R, Groff P, Bruni A, et al. High-flow nasal therapy versus noninvasive ventilation in COPD patients with mild-to-moderate hypercapnic acute respiratory failure: study protocol for a noninferiority randomized clinical trial. Trials. 2019 Jul;20((1)):450. doi: 10.1186/s13063-019-3514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang S, Zhang G, Liu Z, Yan Q, Meng S, Zhao B, et al. [Effect of high-flow nasal cannula oxygen therapy on diaphragmatic function in patients with acute exacerbation of chronic obstructive pulmonary disease: a prospective randomized controlled trial] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019 May;31((5)):551–5. doi: 10.3760/cma.j.issn.2095-4352.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Pilcher J, Eastlake L, Richards M, Power S, Cripps T, Bibby S, et al. Physiological effects of titrated oxygen via nasal high-flow cannulae in COPD exacerbations: A randomized controlled cross-over trial. Respirology. 2017 Aug;22((6)):1149–55. doi: 10.1111/resp.13050. [DOI] [PubMed] [Google Scholar]
- 39.Longhini F, Pisani L, Lungu R, Comellini V, Bruni A, Garofalo E, et al. High-Flow Oxygen Therapy After Noninvasive Ventilation Interruption in Patients Recovering From Hypercapnic Acute Respiratory Failure: A Physiological Crossover Trial. Crit Care Med. 2019 Jun;47((6)):e506–11. doi: 10.1097/CCM.0000000000003740. [DOI] [PubMed] [Google Scholar]
- 40.Sun J, Li Y, Ling B, Zhu Q, Hu Y, Tan D, et al. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: an observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019 Jun;14:1229–37. doi: 10.2147/COPD.S206567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. FLORALI Study Group. REVA Network High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015 Jun;372((23)):2185–96. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 42.Lee MK, Choi J, Park B, Kim B, Lee SJ, Kim SH, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018 Jun;12((6)):2046–56. doi: 10.1111/crj.12772. [DOI] [PubMed] [Google Scholar]
- 43.Cong L, et al. Original article outcomes of high-flow nasal cannula versunon-invasive positive pressure ventilation for patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Clin Exp Med. 2019;12((8)):10863–7. [Google Scholar]
- 44.Rittayamai N, Phuangchoei P, Tscheikuna J, Praphruetkit N, Brochard L. Effects of high-flow nasal cannula and non-invasive ventilation on inspiratory effort in hypercapnic patients with chronic obstructive pulmonary disease: a preliminary study. Ann Intensive Care. 2019 Oct;9((1)):122. doi: 10.1186/s13613-019-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bräunlich J, Wirtz H. Nasal high-flow in acute hypercapnic exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018 Nov;13:3895–7. doi: 10.2147/COPD.S185001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barneche MF, et al. High flow nasal cannula improves exercise capacity in COPD patients: crossover trial. Eur Respir J. 2018;•••:52. [Google Scholar]
- 47.Hsiao H, et al. Effectiveness of supplemental oxygen during pulmonary rehabilitation. Am J Respir Crit Care Med. 2018;•••:197. [Google Scholar]
- 48.Kelly P, Dikstaal J, Beckert L. Nasal high flow therapy: the effect on exercise performance in patients with COPD. Eur Respir J. 2018;•••:52. [Google Scholar]
- 49.Kelly PT, Beckert LE, Mayo E. Nasal high flow therapy and supplemental oxygen does not augment exercise performance in COPD. Respirology. 2019;24:12. [Google Scholar]
- 50.Rossi V, et al. High flow nasal cannula during walking in severe COPD patients: A randomized controlled trial. Eur Respir J. 2018;•••:52. [Google Scholar]
- 51.Dell'Era S, et al. The High Flow Nasal Cannula Improves the Exercise Capacity in Patients with Chronic Obstructive Pulmonary Disease: Randomized, Crossover Clinical Trial. Rev Am Med Respir. 2019;1:16–26. [Google Scholar]
- 52.Prieur G, Medrinal C, Combret Y, Quesada AR, Prieur F, Quieffin J, et al. Effect of high-flow nasal therapy during acute aerobic exercise in patients with chronic obstructive pulmonary disease after exacerbation: protocol for a randomised, controlled, cross-over trial. BMJ Open Respir Res. 2017 Aug;4((1)):e000191. doi: 10.1136/bmjresp-2017-000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitacca M, Pietta I, Lazzeri M, Paneroni M, Associazione Italiana Riabilitatori Insufficienza Respiratoria (ARIR) and Associazione Italiana Pneumologi Ospedalieri (AIPO) rehabilitation group Effect of high-flow nasal therapy during exercise training in COPD patients with chronic respiratory failure: study protocol for a randomised controlled trial. Trials. 2019 Jun;20((1)):336. doi: 10.1186/s13063-019-3440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chatila W, Nugent T, Vance G, Gaughan J, Criner GJ. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest. 2004 Oct;126((4)):1108–15. doi: 10.1378/chest.126.4.1108. [DOI] [PubMed] [Google Scholar]
- 55.Prieur G, Medrinal C, Combret Y, Dupuis Lozeron E, Bonnevie T, Gravier FE, et al. Nasal high flow does not improve exercise tolerance in COPD patients recovering from acute exacerbation: A randomized crossover study. Respirology. 2019 Nov;24((11)):1088–94. doi: 10.1111/resp.13664. [DOI] [PubMed] [Google Scholar]
- 56.Cirio S, Piran M, Vitacca M, Piaggi G, Ceriana P, Prazzoli M, et al. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respir Med. 2016 Sep;118:128–32. doi: 10.1016/j.rmed.2016.08.004. [DOI] [PubMed] [Google Scholar]