Abstract
While transition of donor programs to national control is increasingly common, there is a lack of evidence about the consequences of transition for private health care providers. In 2015, President’s Emergency Plan for AIDS Relief (PEPFAR) identified 734 facilities in Uganda for transition from PEPFAR support, including 137 private not-for-profits (PNFP) and 140 private for-profits (PFPs). We sought to understand the differential impacts of transition on facilities with differing ownership statuses. We used a survey conducted in mid-2017 among 145 public, 29 PNFP and 32 PFP facilities reporting transition from PEPFAR. The survey collected information on current and prior PEPFAR support, service provision, laboratory services and staff time allocation. We used both bivariate and logistic regression to analyse the association between ownership and survey responses. All analyses adjust for survey design. Public facilities were more likely to report increased disruption of sputum microscopy tests following transition than PFPs [odds ratio (OR) = 5.85, 1.79–19.23, P = 0.005]. Compared with public facilities, PNFPs were more likely to report declining frequency of supervision for human immunodeficiency virus (HIV) since transition (OR = 2.27, 1.136–4.518, P = 0.022). Workers in PFP facilities were more likely to report reduced time spent on HIV care since transition (OR = 6.241, 2.709–14.38, P < 0.001), and PFP facilities were also more likely to discontinue HIV outreach following transition (OR = 3.029, 1.325–6.925; P = 0.011). PNFP facilities’ loss of supervision may require that public sector supervision be extended to them. Reduced HIV clinical care in PFPs, primarily HIV testing and counselling, increases burdens on public facilities. Prior PFP clients who preferred the confidentiality and service of private facilities may opt to forgo HIV testing altogether. Donors and governments should consider the roles and responses of PNFPs and PFPs when transitioning donor-funded health programs.
Keywords: HIV/AIDS, private health providers, President’s Emergency Plan for AIDS Relief, development assistance for health, Uganda, sub-Saharan Africa
Key Messages
As donor funding for human immunodeficiency virus (HIV) declines, private providers of HIV care face an uncertain future.
Between 2014 and 2017, President’s Emergency Plan for AIDS Relief withdrew support to >700 public, private for-profit and private not-for-profit facilities in Uganda.
Compared with transitioned public facilities, not-for-profits lost more support but for-profits disengaged from HIV care to a greater extent.
Donors and governments should consider private providers when transitioning HIV programs to national control.
Introduction
The private sector has played a large and diverse role in the response to human immunodeficiency virus (HIV)/AIDS in Uganda and many other low-and-middle-income countries (LMICs). That role has been shaped, in part, by donor HIV programs, including the President’s Emergency Plan for AIDS Relief (PEPFAR). While the inclusion of private providers in the HIV response has been encouraged by some (Rao et al., 2011), others have warned about the risks of drug resistance from unregulated private providers (Brugha, 2003), or raised questions about the sustainability of provision in private facilities following donor withdrawal (Kakaire et al., 2016; Zakumumpa et al., 2016a). We were unable to identify any prior studies that compared the effects of donor transition on HIV providers of differing ownership status in an LMIC setting.
The private sector in Uganda is large, and accounts for 47% of the health workforce, including 60% of clinical officers and 80% of doctors (O’Hanlon et al., 2017). The boundaries between the public and private sectors are often blurred, as health providers, medical doctors in particular, commonly work in both the private and public sectors (Paina, 2014). The facility-based private sector in Uganda ranges in size from single owner-operated clinics to large networks of hospitals and health centres (HC). We restrict this discussion of HIV service provision by private providers to licensed private not-for-profit (PNFP) and private for-profit (PFP) facilities staffed by trained medical personnel who perform clinical HIV services, as this is the type of provider that PEPFAR engages with. The Ugandan AIDS Commission estimated Uganda’s HIV/AIDS expenditure on private providers (including for-profit and not-for-profit clinics, HCs, pharmacies and hospitals) to be 180 billion Ugandan Shillings (USHs) in 2009/2010 (∼US$95 million using the December 2009 exchange rate of 1890.38 USH per USD), which is significantly greater than HIV/AIDS spending in the public sector (116 billion USHs or US$61 million) (Uganda AIDS Commission, 2012).
Private providers in Uganda are divided between a well-organized PNFP sector and a weakly-regulated PFP sector. The PNFP sector is predominantly faith-based and structured along faith-lines. The Uganda Protestant Medical Bureau (UPMB), Uganda Catholic Medical Bureau (UCMB), Uganda Muslim Medical Bureau (UMMB) and the Orthodox Church of Uganda Medical Bureau (OUMB) are the primary umbrella organizations for PNFP providers. These four bureaus supervised 645 PNFP facilities in 2014 (O’Hanlon et al., 2017). PNFPs are distributed across the country, including remote rural areas (O’Hanlon et al., 2017). PNFPs are integrated into the Government of Uganda’s health planning process and receive some financial support from government through primary health care (PHC) grants (Boulenger and Criel, 2012; Ssennyonjo et al., 2018). While PNFP facilities should receive supportive supervision from district health teams (DHTs) (Ministry of Health, The Republic of Uganda, 2016), the frequency and nature of such support is not well documented. In contrast, the formal relationship between PFPs and government is nearly non-existent (Asiimwe, 2008). Most licensed and clinically staffed PFP facilities are located in urban areas of Uganda, with Kampala having nearly half the national total in 2005 (Mandelli et al., 2005). Many PFPs are directly owned by health professionals, especially medical doctors, the majority of whom also work in the public sector (Mandelli et al., 2005).
Across LMICs, the role of the private sector in provision of health care is highly variable, both by country and by type of service (Kagawa et al., 2012; Olivier et al., 2015; Grépin, 2016). In 2008–10, women in Uganda were more likely to seek care from the private sector (both PFP and PNFP) for family planning and childhood fever/cough or diarrhoea than in any of 12 other LMICs; however, for HIV testing, Ugandan women were more on par with their peers in other LMICs (Johnson and Cheng, 2014). According to Demographic and Health Surveys (DHS) conducted from 2004–08, the proportion of women receiving their most recent HIV test from a private provider (PFP or PNFP) ranged from 10% in Rwanda to 58% in Haiti, with 28.5% of women and 36.4% of men in Uganda being tested by a private facility (Wang et al., 2011). A more recent study using the 2011 Uganda DHS puts the share of women tested in the private sector in Uganda at a more modest 18% (Johnson and Cheng, 2014). According to DHIS2 data, 28% of people in Uganda receiving antiretroviral therapy (ART) in 2015 obtained it from a private provider, almost entirely from PNFPs (O’Hanlon et al., 2017).
There is an income gradient in the use of private health services in Uganda: while only 18% of all women received an HIV test from a private (PNFP or PFP) provider in 2008–10, 31% of urban women and 29% of the richest quintile of women did so (O’Hanlon et al., 2017). As a result, more than half of women receiving a test from a private provider were among the highest wealth quintile in 2008–10 (Johnson and Cheng, 2014). While HIV care is ostensibly free in public facilities in Uganda, PFPs can charge for services, and PNFPs may as well. The relatively affluent demographic using private providers has led some to argue that PNFPs should consider co-payment for ART in the event of funding cutbacks (Kakaire et al., 2016), and a pilot model for a fee-based, after-hours ART clinic in Kampala has been studied (Twimukye et al., 2017).
Donor-supported HIV programs have encouraged the growth of private HIV service provision in many settings, with PEPFAR being particularly eager to do so, with the emphasis between PNFP and PFP sectors varying from country to country (PEPFAR, 2005; Sturchio and Cohen, 2012). For example, out of five countries examined by Sulzbach et al. (2011), PNFP facilities’ share of HIV expenditures increased in four (Kenya, Malawi, Rwanda and Zambia) following the expansion of donor HIV programs between 2002–03 and 2005–06 (Sulzbach et al., 2011). The share of expenditures going to PFPs increased in two countries but declined marginally in three countries (Sulzbach et al., 2011). Coutinho et al. (2012) identified Uganda as a leader in private sector involvement in HIV service delivery and indigenous non-governmental organizations as primary recipients PEPFAR funding in 2007–10 (Coutinho et al., 2012). By 2014, 25% of facilities supported by PEPFAR in Uganda were privately owned, including 481 PNFPs and 160 PFPs.
Having multiple, uncoordinated private providers can fragment the HIV response. As donor funding for HIV declines (Kates et al., 2017), transitions of HIV programs to national control and co-financing of the HIV response are likely to increase. Without incentives and support from donor organizations, the future role of private providers in the HIV response in Uganda and other LMICs is uncertain.
There is limited research on the private sector in HIV transitions. Prior empirical research on transition has tended to focus on macro-level impacts (Patcharanarumol et al., 2013; Bennett et al., 2015; Vogus and Graff 2015; Binagwaho et al., 2016; Burrows et al., 2016) or to lump together private and public providers. A few notable exceptions deal with the transfer of patients from private clinics following donor withdrawal. In South Africa, PEPFAR transition resulted in patients being transferred from a large HIV specialty clinic in a PNFP Hospital to public primary HCs across Durban (Cloete et al., 2014; Katz et al., 2015) or, in one pilot, to private general practitioners (Igumbor et al., 2014). Interruptions of care during private-to-public transfers have been reported in many cases (Cloete et al., 2014; Freeman et al., 2014; Katz et al., 2015), and the transfers appear to have negatively affected satisfaction with care (Katz et al., 2015). However, none of the studies examined impacts on private facilities during transition.
The PEPFAR geographic prioritization policy
The PEPFAR geographic prioritization (GP) in Uganda presents an opportunity to study the differential effects that loss of donor support has on private and public facilities transitioned from PEPFAR. As a part of a strategy to prioritize resources to highest burden regions and populations within countries, the GP process prioritized support to 1384 facilities, maintained support for 419, and identified another 734 facilities for transition (PEPFAR, 2015), including 137 PNFP and 140 PFPs. Under the GP, these facilities were expected to lose site-level support for supervision, training and on-site laboratories, outreach and health worker incentives, but retain above-site support through commodity supply chains and laboratory hubs. It was unclear to what extent private facilities would receive support for HIV services from government to replace lost PEPFAR support or be able to continue to access central commodity supply chains and laboratory hubs. The objective of this article is to understand how the experiences and responses of private facilities (both PNFPs and PFPs) transitioned from PEPFAR differ from those of transitioned public facilities.
Materials and methods
This study is nested within a mixed methods evaluation of the PEPFAR GP in Kenya (Rodríguez et al., 2018) and Uganda (Wilhelm et al., 2018). The parent study has three components: documentation of PEPFAR GP process; quantitative analysis of trends in HIV and maternal, neonatal and child health (MNCH) services, staffing and health systems; and in-depth qualitative research through case studies. Prior findings from the parent study identified impacts of transition on human resources (HIV supervision, training, worker time allocation for HIV and non-HIV care), service delivery (HIV outreach, facility in-charges perceptions of quality of care) and laboratory networks [disruption of viral load (VL) and sputum tuberculosis (TB) testing].
In Uganda, we conducted a cross-sectional facility survey in July and August of 2017, roughly 9 months after the median transition date and 4 months after the official end of the GP process reported by United States Agency for International Development (USAID). For logistical reasons, we limited the sampling area for this survey to 42 districts (out of the 112 districts that existed in 2014): 40 districts in Northern and Eastern Uganda, as well as two urban districts, Kampala and Wakiso, in Central Uganda. Kampala and Wakiso contain more than half of the PFPs transitioning from PEPFAR.
We constructed the sample frame from a list supplied by USAID of PEPFAR-supported facilities. Only facilities supported by USAID-contracted Implementing Partners (IPs) were included in the survey. We also excluded all facilities identified for scale-up (i.e. increased support). From the sample frame, we selected districts using a stratified random sampling design with three strata: (1) 100% selection of all districts containing transitioning HC IVs and/or Hospitals as well as Kampala and Wakiso, (2) random sampling of 11 out of 18 remaining districts that were designated for transition or maintenance and (3) random sampling of 6 out of 14 Scale-Up districts in our sampling region, which also contain some facilities designated for transition on the basis of having ‘low volume’ of HIV services. We sampled all facilities within selected districts that were identified as maintenance (constant support) or transition (withdrawal of support), except for Kampala/Wakiso, where we selected a 40% sample of transition facilities due to the large number of PFP facilities in these districts. This sampling methodology was guided by the goal of the parent study to achieve a 2:1 ratio of transition to maintenance facilities from across Northern, Eastern and Central Uganda. The 2:1 ratio was selected to provide enough power for comparisons between transition facilities (e.g. by ownership) as well as between transition and maintenance facilities.
Using this process, we selected 275 facilities. We assumed a 9% non-response rate to achieve a final sample of ∼250. Two facilities that were selected for longitudinal case studies by the parent study but not randomly selected for the facility survey—one PNFP and one PFP—were purposively added to the survey sample and weighted accordingly. Enumerators were able to complete surveys at 262 facilities. Of the 15 facilities that could not be surveyed, 9 had closed permanently, 2 were closed for construction, 2 facilities were identified as duplicate records, 1 (a PFP facility) refused to participate in the survey and 1 was not accessible due to road conditions.
Of surveyed facilities, 206 reported having been transitioned, 20 reported continuing to receive PEPFAR support and 36 claimed to have had no PEPFAR support within the past 3 years. This was contrary to what was expected, due both to the 36 sites claiming to have no recent PEPFAR support and the larger than expected proportion of sites reporting transition. From follow-up interviews with IPs and USAID, we determined that as many as 60 of the transitioned facilities were experiencing a break in support between IPs lasting for about 12 months. As the objective of this study was to study facilities’ responses to loss of support, we decided to use facility-reported transition status, and included the roughly 60 facilities that were designated for maintenance and may have been in an extended break in funding as transition. In addition, these facilities reported similar processes to those intended for transition. To examine differences between transitioned PFPs, PNFPs and public facilities, we restrict the analysis in this paper to the 206 facilities reporting transition.
In smaller facilities, survey interviews were conducted with facility in-charges or their representatives. In larger facilities, multiple respondents (e.g. facility in-charge, head of the HIV clinic, head of the maternity ward and a financial officer) contributed to different components of the survey. Enumerators asked about conditions before and after the facility’s transition date in the areas of service delivery, laboratory, commodities, human resources and finances. In addition, enumerators sought 1–3 staff that provide HIV care (including potentially the primary respondent) present on the day of the survey to answer a short individual questionnaire on changes in worker time allocation and receipt of incentives (bonuses/salary top-ups, outreach allowances or other support). These individual interviews were conducted in private to improve confidentiality. A total of 429 health worker interviews were collected from transition facilities (304 in public, 71 in PNFPs and 54 in PFPs).
Given the large number of potential differences by ownership type and the increased risk of making type I errors with multiple comparisons, we pre-specified 17 hypotheses (Supplementary Table S1). Rather than using the Bonferroni correction approach (i.e. dividing alpha = 0.05 by the number of hypothesis tests), which is conservative when outcomes are correlated, we use the Benjamini–Hochberg (BH) method (Benjamini and Hochberg, 1995) to control the false detection rate to 5%.
Compared with public facilities, we hypothesized that transitioned PNFPs would be more likely to lose supervision, access to training and access to lab networks. Public facilities are more closely linked to national support structures, including referral labs, training programs and DHTs, whereas PNFPs may have been more reliant on PEPFAR IPs to provide or facilitate access to these resources. However, we expected that PNFPs, which have a strong mandate to provide care to all in need, and greater flexibility than public facilities to manage staff and deploy funding, would continue to offer HIV services at a level comparable to public facilities.
We also expected PFPs to lose more support than transitioned public facilities, even though many PFPs reported never having received supervision. We hypothesized that PFPs are more profit-motivated and have less of a social mission than PNFPs, thus as they lose subsidized inputs for HIV services this may diminish their profits leading them to respond to loss of PEPFAR support by disengaging from HIV services (particularly HIV testing, which accounts for the bulk of their HIV services), following transition.
We aimed to isolate differences in outcomes that are due to ownership by controlling for other factors. Public, PFP and PNFP facilities lost differing amounts of support in transition. We constructed a ‘transition impact index’ that sought to quantify the amount of support lost by an individual facility as a data reduction tool to limit the number of variables required to control for differences in transition strength.
In constructing the index, we used four ordinal variables: (1) the number of types of IP support for HIV reported lost (for training, supervision, outreach, ART, laboratory), (2) the number of HIV services (HIV testing and counselling, PMTCT, ART, outreach) for which the in-charge identified the PEPFAR IP as primary source of support prior to transition but not after transition, (3) the change in frequency of HIV supervision since transition (−1 decreased, 0 same and 1 increased), with facilities reporting no previous support imputed to ‘same’ and (4) The types of non-salary incentives provided by the IP to at least one worker in the facility prior to transition (0 = none provided, 1 = bonuses or outreach allowances provided and 2 = both provided), almost all of which was lost during transition. We attempted to include loss of salaries paid by the IP, both as a proportion of workers and as a binary variable for any salaries lost; however, salaries had a high degree of uniqueness and added little to the index. Principle component analysis with a polychoric correlation matrix resulted in a single factor model that explained 50.7% of variance. We used exploratory factor analysis to determine factor loadings and create an index score for each facility (Supplementary Table S2).
The index can be used to identify transition status with a high degree of accuracy (AUC = 0.981). The diversity of scores for transition facilities is large, suggesting a considerable variation in amounts of support lost (Figure 1). Among transition facilities, the impact index was independently associated with several outcomes, including discontinuation of outreach and workers reporting less time on HIV care. However, the index is only a rough measure of the loss of support during transition. Since the index contains information on supervision frequency, we omit it from the analysis of supervision frequency.
Figure 1.
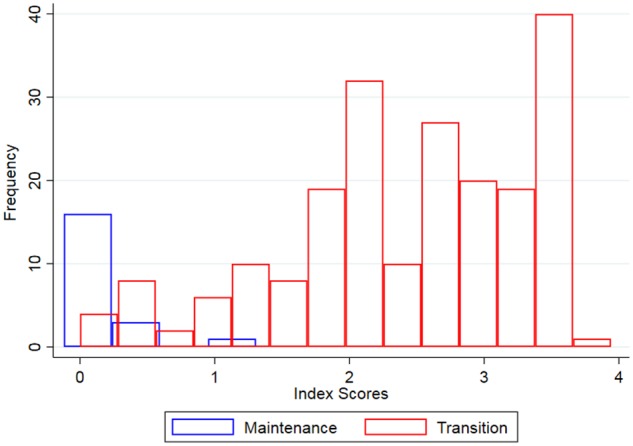
Histogram of transition impact index scores.
In addition to the transition index, we include other covariates, including facility level (health centre—HC II or clinic, HC III, HC IV or hospital), number of HIV workers prior to transition, number of months since transition and an index of transition preparedness. Ten districts were selected by PEPFAR for transition of all facilities during GP, regardless of their volume (‘Central Support Districts’). These districts commonly had low PEPFAR presence and are mostly located in the sparsely populated Karamoja region. New districts, those created within 10 years of the survey, tend to have less capacity than more established districts. We adjust for district status (new vs established) in the analysis.
We created the preparedness index by taking an unweighted average of 14 questions about the facility’s preparedness for transition in domains of communication (to facility, to patients, between facilities), consistency (of HIV and MNCH services, reporting systems and outreach to key populations) before and after transition, and capacity (of facility, management, staff) for transition, each rated on a 5-item Likert scale, excluding don’t know/not applicable. Higher scores indicate a higher level of self-rated preparedness.
We compare responses across ownership categories and perform pairwise weighted chi-square tests of the significance of differences in proportions. We also use logistic regression to compare outcomes across facility ownership types, adjusting for covariates. The two methods are complementary. The unadjusted proportions provide perspective on changes taking place among transition facilities as a whole, while logistic regression better isolates the effect of ownership. In both methods, we accounted for survey design using stratification, clustering, finite population correction and sampling weights. All analyses were performed using Stata 15 (StataCorp, 2017).
Results
Of the 206 facilities reporting transition, the majority were public (N = 145, 61.5%). Private facilities were split among PFPs (N = 32, 23.9%) and PNFPs (N = 29, 14.6%; Table 1). Transitioned public facilities in our survey tended to be higher level (i.e. HC IV/Hospital) than either PNFPs or PFPs. Public facilities were also more likely to be located in districts formed since 2007 and in Central Support Districts. While 81% of public facilities offered ART prior to transition and 65% of PNFPs did so, only 6% of PFPs reported offering ART. HIV supervision was more frequent for PNFPs than for public or PFPs, half of which reported no HIV supervision at all. The mean transition impact index score was highest for public facilities (2.58), followed by PNFPs (2.25) and lowest for PFPs (1.88). Preparedness index scores, with higher scores indicating better preparedness, were significantly higher for PNFPs (3.73) than for public (3.35) or PFP facilities (3.25). PNFPs and Public facilities both have an unweighted median transition date of September 2016, but half of PFPs had transitioned on or before April 2015.
Table 1.
Weighted descriptive statistics of surveyed health facilities
| Public | PNFP | PFP | |
|---|---|---|---|
| N (weighted %) | N (weighted %) | N (weighted %) | |
| Total | 145 | 29 | 32 |
| Facility levela | |||
| HC II or clinic | 22 (19) | 6 (25) | 22 (71) |
| HC III | 104 (69) | 20 (65) | 9 (25) |
| HC IV or hospital | 12 (12) | 1 (10) | 1 (4) |
| District status | |||
| Central Support District | 78 (31) | 7 (15) | 2 (3) |
| New district (since 2007) | 35 (17) | 1 (3) | 0 (0) |
| Services offered (at baseline) | |||
| % | % | % | |
| HTC | 97 | 100 | 98 |
| HIV outreach | 87 | 95 | 72 |
| ART | 81 | 65 | 6 |
| Public | PNFP | PFP | |
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Number of workers providing HIV care (baseline) | 5.61 (5.16–6.06) | 5.64 (4.28–7.01) | 3.94 (3.20–4.68) |
| Transition impact index | 2.58 (2.42–2.74) | 2.25 (2.04–2.46) | 1.88 (1.63–2.12) |
| Transition preparedness index | 3.35 (3.26–3.45) | 3.73 (3.45–4.01) | 3.25 (3.08–3.41) |
| Supervision frequency for HIVa | |||
| >1 time per month | 10 (7) | 3 (12) | 1 (4) |
| Monthly | 45 (30) | 10 (40) | 3 (9) |
| 1–2 times per quarter | 67 (45) | 13 (38) | 11 (32) |
| 1–3 times per year | 2 (1) | 0 (0) | 0 (0) |
| Don’t know | 2 (1) | 0 (0) | 2 (5) |
| Never | 18 (15) | 3 (10) | 15 (51) |
| Transition date distribution (unweighted) | |||
| 10th percentile | May 2015 | March 2015 | February 2014 |
| Median | September 2016 | September 2016 | April 2015 |
| 90th percentile | March 2017 | March 2017 | September 2016 |
Counts of facilities may not sum to the total number of facilities due to missing data.
Table 2 presents the results of bivariate analysis. PNFPs were more likely to report a decline in on-site supervision frequency following transition than public facilities. PFP workers were more likely to report declining time spent on HIV clinical care compared with public. Public facilities were significantly more likely to report disruption of VL testing than PNFPs. Other unadjusted comparisons were not statistically significant.
Table 2.
Bivariate comparison of survey responses across facility types
| Weighted proportion reporting outcome % (95% CI)with weighted chi-square test P-values |
Weighted chi-square test |
|||||
|---|---|---|---|---|---|---|
| Outcomes | PNFP | PNFP vs public | Public | Public vs PFP | PFP | Public vs PNFP vs PFP |
| P-value | P-value | P-value | ||||
| Decline in HIV supervision frequency | 68.5 (53.2–80.6) | 0.040 | 51.8 (46.0–57.5) | 0.159 | 36.4 (19.4–57.7) | 0.038 |
| Discontinued outreach | 46.8 (30.7–63.7) | 0.546 | 52.0 (44.4–59.5) | 0.797 | 54.2 (37.6–70.0) | 0.769 |
| Workers report less time on HIV services | 33.8 (23.1–46.5) | 0.082 | 23.5 (18.6–29.2) | <0.001 | 59.6 (42.7–74.4) | <0.001 |
| Workers report less time on non-HIV services | 13.5 (6.8–25.2) | 0.648 | 11.5 (8.5–15.4) | 0.072 | 20.8 (11.7–34.3) | 0.155 |
| Workers report less time on training | 35.7 (25.3–47.6) | 0.545 | 39.2 (33.5–45.3) | 0.667 | 42.3 (28.7–57.1) | 0.709 |
| IP providing supervision after transition | 3.4 (1.0–11.1) | 0.056 | 10.9 (8.0–14.7) | 0.494 | 14.3 (6.6–28.1) | 0.153 |
| In-charge reports less time on HIV and more time on MNCH | 12.6 (7.3–21.1) | 0.056 | 21.0 (17.8–24.7) | 0.733 | 23.5 (12.0–40.9) | 0.319 |
| Increased disruption of viral load testing | 8.8 (3.6–19.9) | 0.016 | 24.3 (19.3–30.0) | n/a | n/a | n/a |
| Increased disruption of sputum testing | 13.0 (5.4–27.9) | 0.068 | 27.4 (22.6–32.9) | 0.078 | 7.6 (1.4–32.8) | 0.058 |
HIV, human immunodeficiency virus; MNCH, maternal, neonatal and child health; n/a, not applicable; PFP, private for-profit; PNFP, private not-for-profit. Bold values provide direct information about statistical significance.
Table 3 presents the logistic regression results for inputs to health facilities. Relative to equivalent public facilities, PNFPs were significantly more likely to report a decline in the frequency of on-site supervision they receive for HIV [odds ratio (OR) = 2.266, 1.136–4.518, P = 0.022]. Notably, higher-level facilities were also more likely to report declining frequency of supervision.
Table 3.
Changes in inputs to health facilities during transition
| Outcomes | Decline in HIV supervision frequency | IP providing supervision after transition | Increased disruption of sputum testing | Increased disruption of viral load testing |
|---|---|---|---|---|
| OR (95% CI), P-value | OR (95% CI), P-value | OR (95% CI), P-value | OR (95% CI), P-value | |
| Ownership | ||||
| PNFP vs Government | 2.266 * (1.136–4.518), 0.022 | 0.304 (0.070–1.331), 0.109 | 0.242 * (0.065–0.900), 0.035 | 1.038 (0.371–2.902), 0.941 |
| PFP vs Government | 0.706 (0.279–1.790), 0.446 | 1.155 (0.483–2.761), 0.735 | 0.171 ** (0.052–0.558), 0.005 | n/ac |
| Level | ||||
| HC III vs HC II | 1.418 (0.812–2.476), 0.207 | |||
| IV/Hospital vs HC II | 3.329** (1.619–6.845), 0.002 | Omittedb | ||
| IV/Hospital vs HC II/HC III | 1.859 (0.576–5.998), 0.284 | 0.930 (0.107–8.069), 0.945 | ||
| Transition impact index | Excludeda | 0.612 ** (0.428–0.876), 0.009 | 0.506 *** (0.365–0.702), <0.001 | 1.578 * (1.055–2.361), 0.028 |
| Preparedness index | 0.859 (0.574–1.284), 0.441 | 0.514 ** (0.318–0.833), 0.009 | 0.982 (0.710–1.358), 0.907 | 0.578 (0.324–1.030), 0.062 |
| Months since transition | 1.010 (0.984–1.038), 0.433 | 1.000 (0.970–1.030), 0.989 | 1.011 (0.985–1.038), 0.373 | 1.000 (0.948–1.055), 0.998 |
| Number of HIV workers prior to transition | 0.982 (0.908–1.063), 0.645 | 1.103 (0.993–1.225), 0.065 | 0.998 (0.943–1.057), 0.944 | 1.120 ** (1.056–1.187), 0.001 |
| Central Support District | 0.878 (0.606–1.272), 0.473 | 1.128 (0.616–2.066), 0.683 | 0.659 (0.341–1.275), 0.203 | 2.165 * (1.103–4.248), 0.027 |
| New district | 0.807 (0.618–1.053), 0.109 | 1.303 (0.652–2.608), 0.437 | 1.048 (0.438–2.512), 0.911 | 0.608 * (0.412–0.896), 0.014 |
| Constant | 1.619 (0.258–10.16), 0.592 | 1.827 (0.243–13.71), 0.542 | 1.791 (0.114–28.02), 0.911 | 0.241 (0.010–5.633), 0.358 |
| N | 170 | 206 | 160 | 151 |
The transition impact index uses information about supervision frequency, making it tautologically associated with the outcome ‘Change in Frequency of HIV Supervision’. We omitted the index from the analysis.
The level HC IV/Hospital is a perfect predictor of not having IP involvement following transition. Therefore, we removed level from the analysis for this outcome.
Few PFPs in our facility survey sample provided ART services, such as viral load testing.
HIV, human immunodeficiency virus; HC, health centre; n/a, not applicable; PFP, private for-profit; PNFP, private not-for-profit.
P < 0.05
P < 0.01
P < 0.001. Bold values provide direct information about statistical significance.
Contrary to expectations, IPs were not significantly more likely to continue making supervision visits post-transition for either PNFPs or PFPs vs public. PFP and PNFP facilities were less likely than public facilities to report increased disruption of sputum TB testing. However, there were no significant differences in the proportion reporting increased disruption of VL testing among PNFPs and public facilities.
PFPs were significantly more likely than public facilities to discontinue HIV outreach after transition (OR = 3.029, 1.325–6.925, P = 0.011) but PNFPs were not (Table 4). Discontinuation of outreach was also associated with the amount of support lost during transition and length of time since transition. In addition, PFPs workers were more likely than public to report declining time spent on both HIV (OR = 6.241, 2.709–14.38, P < 0.001) and non-HIV (OR = 3.012, 1.061–7.817, P = 0.025) clinical care. PNFPs workers were also more likely to report less time on HIV clinical care (OR = 2.117, 1.054–4.255, P = 0.036). Neither PFP nor PNFP workers were significantly more likely to report reduced time in training since transition compared with public workers.
Table 4.
Health facility responses to transition
| Outcomes | Discontinue outreach | Workers report reduced time on HIV services | Workers report reduced time on non-HIV services | Workers report reduced time on training |
|---|---|---|---|---|
| OR (95% CI), P-value | OR (95% CI), P-value | OR (95% CI), P-value | OR (95% CI), P-value | |
| Ownership | ||||
| PNFP vs Government | 1.087 (0.518–2.281), 0.819 | 2.117 * (1.054–4.255), 0.036 | 1.429 (0.665–3.069), 0.344 | 1.146 (0.524–2.508), 0.721 |
| PFP vs Government | 3.029 * (1.325–6.925), 0.011 | 6.241 *** (2.709–14.38), <0.001 | 3.012 * (1.161–7.817), 0.025 | 2.393 (0.915–6.261), 0.073 |
| Level | ||||
| HC III vs HC II | 1.016 (0.525–1.966), 0.960 | 1.161 (0.578–2.332), 0.663 | 0.569 (0.266–1.216), 0.138 | 1.061 (0.616–1.827), 0.824 |
| IV/Hospital vs HC II | 1.361 (0.568–3.260), 0.473 | 0.917 (0.345–2.438), 0.856 | 1.165 (0.402–3.372), 0.769 | 1.867 (0.938–3.714), 0.073 |
| Transition impact index | 2.480 *** (1.821–3.376), <0.001 | 1.731 ** (1.247–2.404), 0.002 | 0.994 (0.703–1.406), 0.974 | 1.184 (0.924–1.517), 0.172 |
| Preparedness index | 1.285 (0.838–1.970), 0.237 | 0.699 (0.463–1.055), 0.085 | 1.000 (0.665–1.501), 0.998 | 0.598 * (0.386–0.926), 0.023 |
| Time since transition | 1.026 * (1.002–1.050), 0.032 | 1.000 (0.973–1.026), 0.975 | 1.028 (0.988–1.071), 0.166 | 1.029 * (1.004–1.054), 0.023 |
| Number of HIV workers prior to transition | 0.929 (0.850–1.014), 0.095 | 0.976 (0.889–1.072), 0.602 | 1.126 * (1.009–1.258), 0.036 | 0.959 (0.893–1.031), 0.247 |
| Central Support District | 0.820 (0.424–1.585), 0.540 | 0.381 ** (0.204–0.714), 0.004 | 0.859 (0.377–1.955), 0.705 | 0.818 (0.518–1.292), 0.373 |
| New district | 1.108 (0.489–2.512), 0.797 | 1.787 (0.839–3.802), 0.126 | 2.519 (0.921–6.892), 0.070 | 1.384 (0.769–2.489), 0.265 |
| Constant | 0.084 * (0.010–0.681), 0.022 | 0.287 (0.055–1.491), 0.131 | 0.113 * (0.02–0.648), 0.017 | 3.656 (0.529–25.26), 0.179 |
| N | 179 | 427 | 426 | 428 |
P < 0.05
P < 0.01
P < 0.001.
HIV, human immunodeficiency virus; HC, health centre; PFP, private for-profit; PNFP, private not-for-profit. Bold values provide direct information about statistical significance.
Adjusting for multiple comparisons using the BH method with a false positive rate of 5%, only two of the seven statistically significant findings at the 5% level are retained (Supplementary Table S3). The findings determined insignificant by the BH method were ‘Decline in HIV Supervision Frequency’, ‘Workers report less time on HIV’ and ‘Increased disruption of sputum testing’ in PNFPs as well as ‘Discontinue outreach’ and ‘Workers report less time on non-HIV services’ in PFPs.
Discussion
We compared the experiences of PFPs, PNFPs and public facilities transitioned from PEPFAR support and confirm that they had distinct experiences and responses to transition. Controlling for other factors, PNFPs were more likely to lose HIV supervision. Public facilities had more disruption of sputum testing, but not VL testing. PFP workers were more likely to report declining time on HIV clinical care following transition, and PFPs were more likely to report discontinuing HIV outreach. However, only the findings for sputum testing and time spent on HIV were significant after adjustment for multiple testing.
The loss of supervision by PNFPs may have long-term impacts on their ability to provide quality HIV care and keep up with changing treatment protocols. Many PNFPs are located in rural areas with few or no alternative sources of HIV care. If sustaining supervision in PNFPs is considered important to Uganda’s HIV response, DHTs can expand the supervision that they provide to public facilities to PNFPs, as recommended by current guidelines (Ministry of Health, The Republic of Uganda, 2016), so as to compensate for lost visits from PEPFAR IPs. Alternatively, PNFP umbrella organizations (e.g. UPMB) can be supported to provide better supervision to their members. ‘Bridge funding’ by donors may facilitate supervision of private health facilities post-transition.
The unadjusted difference for VL testing may be confounded by the transition impact index or location factors (CSD or new district status), and hence not related to ownership. However, for sputum testing disruption, adjustment increases the difference between PFPs and PNFPs and public, suggesting that public facilities, compared with similar PFPs and PNFPs, were more likely to experience disruption of sputum testing than comparable private facilities. It is possible that private facilities, which retain patient fees, are better able to ensure that samples are transported and results transmitted than public facilities, which do not formally collect patient fees.
Neither PFPs nor PNFPs were more likely to report less time on training compared with public facilities. However, the OR for PFPs was large, though not significant (OR = 2.393, P = 0.073). Since trainings are often infrequent, it is possible that not enough time had elapsed to allow detectable differences in training between ownership types to emerge.
PFPs were more likely to report discontinuing HIV outreach; however, the unadjusted proportion of PFPs discontinuing outreach was not much higher than for public facilities (54.2% vs 52.0%). Adjustment for the transition impact index made the difference: PFPs were more likely to discontinue HIV outreach when compared with public facilities with similar (i.e. low) transition impact index scores.
Taking the discontinuation of outreach together with reduced worker time allocation for HIV clinical care, PFPs were more likely than comparable public facilities to disengage from HIV care. This finding mirrors that of Zakumumpa et al. (2016b), which found that PFPs were more likely to discontinue ART despite having PEPFAR support and access to a high-burden (urban) population. The challenges noted for PFPs providing ART included low retention in care (Kyayise et al., 2008) as well as weak internal service capacity, personnel management and related shortages, and their profit-maximization orientation (Zakumumpa et al., 2016b). While is it not clear whether reduced HIV-related activity in PFPs is due to supply or demand factors, without PEPFAR support, PFPs’ limitations in term of staffing and their profit-maximization goals may make them less able or willing to conduct outreach and provide testing compared with public and PNFP facilities, which have more staff and receive some PHC funding for outreach activities. Qualitative research conducted by the parent study suggests that at least one PFP responded to the loss of PEPFAR support by introducing user fees for HIV testing and ceasing outreach, which reduced demand (H. Zakumumpa, 2019, personal communication, 25 February). PFPs are mostly located in urban areas near to public facilities that have mostly continued to receive PEPFAR support. PFP clients either received testing in nearby public facilities, where services are nominally free, or they opted not to test at all. Shifting the source of care puts more pressure on already crowded public facilities in urban areas. But, it is possible that PFP clients, who value the privacy and quality of service in PFPs (O’Hanlon et al., 2017), may opt to forgo testing rather than test in public facilities, with implications for the HIV epidemic.
There are several limitations to this study. First, the facility survey is not nationally representative and uses both purposive and probabilistic sampling. In particular, the PFP sample comes predominantly from Kampala and Wakiso districts and consists of facilities that were previously supported by just one IP. Thus, trends for PFPs may be due to geographic or IP-specific factors correlated with ownership. Secondly, the sample size of PNFP and PFP facilities was relatively small, limiting the power to detect important differences. Multiple comparisons further limited our ability to measure differences without increasing the risk of type I errors. Using the BH method with a 5% FDR, we had to exclude 5 out of 7 results that were individually significant at the 5% level.
Third, we are uncertain of the transition status of as many as 60 facilities that may be experiencing an extended gap in support between IPs. These facilities report transition and seem to be reacting to it similarly to other transition facilities. However, it is possible that these facilities are more expectant of returning support than other transitioned facilities and, therefore, less likely to respond to transition. The facilities in a gap between IPs are mostly public with a small number of PNFPs, which may bias comparisons with PFPs. This could explain why PFPs discontinued HIV outreach more frequently than comparable public facilities.
Another limitation comes from our survey itself. Our survey did not collect information on supervision frequency if facilities reported that they don’t receive supervision for HIV. Though intended to capture facilities that had never received supervision for HIV, the screener question (‘Do you receive supervision visits for HIV/MNCH services?’) may have been understood as ‘Do you currently receive supervision visits for HIV/MNCH services?’, which would exclude facilities that lost supervision entirely. This might have resulted in an underestimate of the change in supervision frequency, particularly for PFPs; thus any bias introduced would be conservative.
Lastly, the facility survey is subject to recall and response bias. It was not possible to verify all self-reported responses using facility records without placing undue burden on health workers and facilities. It is possible that respondents are more or less willing to disclose changes they have experienced depending on their facility’s ownership. For example, PFPs may be more willing to report discontinuation of HIV outreach whereas public and PNFP facility in-charges may not want to acknowledge no longer being able to provide a service.
In the future, evaluations of HIV transitions should include population-based care-seeking data and measures of the cost of care to assess whether demand or supply-side factors are affecting private participation in HIV services following transition. It is vital to know if former PFP clients are now seeking care at public or PNFP facilities or forgoing care entirely. Clinical outcomes (e.g. ART adherence) could also add to the picture, provided such data can be obtained from private facilities. Qualitative research can also explore the differing motivations and resources that cause private and public facilities to respond to transition differently. As the private sector plays a large role in many countries’ HIV response, research from other settings is needed. Future studies should also include prospective evaluation designs, with coordination between researchers, donors and countries.
Conclusions
Despite the limitations, this study is the first that we can identify to empirically address ownership type as a modifier of transition impacts and responses. Transition seems to affect facilities differently by ownership, and facilities with differing ownership respond to transition in distinct ways. Therefore, facility ownership should be considered by donors, as well as government agencies during planning and implementation of transition policies. In particular, loss of supervision for HIV may have more of an impact on PNFPs than on public facilities. Mechanisms to fill gaps left by donor programs should be considered in transition planning, including encouraging the public sector to extend supervision to PNFPs or establishing private supervision mechanisms. Transition planning should also recognize that PFPs will likely introduce or increase fees for HIV testing following transition and expect a subset of PFP clients to respond by either shifting to other facilities or forgoing care altogether.
Supplementary Material
Acknowledgements
We would like to thank Daniela Rodríguez, Freddie Ssengooba and Moses Mukuru for their input in the survey design. We also appreciate the efforts of survey enumerators who worked long days to collect the data used in this study as well as the facility respondents who took time to participate in the survey. This study was funded by the United States Agency for International Development though a Project SOAR grant.
Conflict of interest statement. None declared.
Ethical approval. The study was approved by the Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health (00007208). Local ethical approval was provided by the Uganda National Council of Science and Technology's Research Ethics Committee (SS 4263).
References
- Asiimwe D. 2008. Identification of Priority Research Questions within the Areas of: Health Financing; Human Resources for Health and the Role of Non-State Sector. Kampala, Uganda: Makerere Institute of Social Research. [Google Scholar]
- Benjamini Y, Hochberg Y.. 1995. Controlling the false discovery rate—a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 57: 289–300. [Google Scholar]
- Bennett S, Singh S, Rodriguez D. et al. 2015. Transitioning a large scale HIV/AIDS prevention program to local stakeholders: findings from the Avahan transition evaluation. PLoS One 10: e0136177.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binagwaho A, Kankindi I, Kayirangwa E. et al. 2016. Transitioning to country ownership of HIV programs in Rwanda. PLoS Medicine 13: e1002075.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenger D, Criel B.. 2012. The difficult relationship between faith-based health care organisations and the public sector in sub-Saharan Africa: the case of contracting experiences in Cameroon, Tanzania, Chad and Uganda. Studies in Health Services Organisation & Policy 29: 1–236. [Google Scholar]
- Brugha R. 2003. Antiretroviral treatment in developing countries: the peril of neglecting private providers. BMJ 326: 1382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows D, Oberth G, Parsons D, Mccallum L.. 2016. Transitions from donor funding to domestic reliance for HIV responses: recommendations for transitioning countries. APMGlobal Health; Aidspan.
- Cloete C, Regan S, Giddy J. et al. 2014. The Linkage outcomes of a large-scale, rapid transfer of HIV-infected patients from hospital-based to community-based clinics in South Africa. Open Forum Infectious Diseases 1: ofu058.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A, Roxo U, Epino H, Muganzi A, Dorward E, Pick B.. 2012. The expanding role of civil society in the global HIV/AIDS response: what has the President's Emergency Program For AIDS Relief's role been? Journal of Acquired Immune Deficiency Syndrome 60 Suppl 3: S152–7. [DOI] [PubMed] [Google Scholar]
- Freeman A, Kiumbu M, Mwamba B. et al. 2014. Patient outcomes in Lubumbashi, Democratic Republic of Congo after a disruption in HIV care due to decreased global fund appropriations. AIDS and Behavior 18: 2135–43. [DOI] [PubMed] [Google Scholar]
- Grépin KA. 2016. Private sector an important but not dominant provider of key health services in low- and middle-income countries. Health Affairs 35: 1214–21. [DOI] [PubMed] [Google Scholar]
- Igumbor J, Pascoe S, Rajap S, Townsend W, Sargent J, Darkoh E.. 2014. A South African public-private partnership HIV treatment model: viability and success factors. PLoS One 9: e110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Cheng X.. 2014. The role of private health providers in HIV testing: analysis of data from 18 countries. International Journal for Equity in Health 13: 36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa RC, Anglemyer A, Montagu D.. 2012. The scale of faith based organization participation in health service delivery in developing countries: systemic review and meta-analysis. PLoS One 7: e48457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakaire T, Schlech W, Coutinho A, Brough R, Parkes-Ratanshi R.. 2016. The future of financing for HIV services in Uganda and the wider sub-Saharan Africa region: should we ask patients to contribute to the cost of their care? BMC Public Health 16: 896.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J, Wexler A, Lief E.. 2017. Financing the response to HIV in low- and middle-income countries in 2016. Kaiser Family Foundation, UNAIDS. https://www.kff.org/global-health-policy/report/financing-the-response-to-hiv-in-low-and-middle-income-countries-international-assistance-from-donor-governments-in-2015/.
- Katz IT, Bogart LM, Cloete C. et al. 2015. Understanding HIV-infected patients' experiences with PEPFAR-associated transitions at a Centre of Excellence in KwaZulu Natal, South Africa: a qualitative study. AIDS Care 27: 1298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyayise A, Kyeyagalire R, Livesley N, Kirunda I, Tumwesigye B.. 2008. Private-for-Profit HIV/AIDS Care in Uganda: An Assessment http://www.urc-chs.com/sites/default/files/UgandaPFPassessmentfulltechnicalreport_USltr.pdf, 10 Accessed February, 2019.
- Mandelli A, Kyomuhangi LB, Scribner S.. 2005. Survey of Private Health Facilities in Uganda. Bethesda, MD: Abt Associates Inc. [Google Scholar]
- Ministry of Health, The Republic of Uganda. 2016. Primary Health Care Grant Guidelines. [ONLINE] https://health.go.ug/sites/default/files/PHC%20GUIDELINES%20%20%20FY2016-17_Final.pdf, accessed 7 June 2019.
- O’Hanlon B, Nakyanzi A, Musembi V. et al. 2017. Exploring partnership opportunities to achieve universal health access: 2016 Uganda private sector assessment in health. https://www.globalfinancingfacility.org/sites/gff_new/files/Uganda-Private-Sector-Assessment-health.pdf.
- Olivier J, Tsimpo C, Gemignani R. et al. 2015. Understanding the roles of faith-based health-care providers in Africa: review of the evidence with a focus on magnitude, reach, cost, and satisfaction. Lancet 386: 1765–75. [DOI] [PubMed] [Google Scholar]
- Paina L. 2014. Dual Practice in Kampala, Uganda: A Mixed Methods Study of Management and Policy. Doctor of Philosophy, Johns Hopkins University.
- Patcharanarumol W, Thammatacharee N, Kittidilokkul S. et al. 2013. Thailand's HIV/AIDS program after weaning-off the global fund's support. BMC Public Health 13: 1008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEPFAR 2005. The President’s Emergency Plan for AIDS Relief: Community and Faith-Based Organisations. Washington, USA: (PEPFAR) The President’s Emergency Plan for AIDS Relief. http://www.pepfar.gov/reports/progress/76864.htm, accessed 10 February 2019.
- PEPFAR 2015. Uganda Country Operational Plan (COP) 2015 strategic direction summary https://www.pepfar.gov/documents/organization/250305.pdf.
- Rao P, Gabre-Kidan T, Mubangizi DB, Sulzbach S.. 2011. Leveraging the private health sector to enhance HIV service delivery in lower-income countries. Journal of Acquired Immune Deficiency Syndrome 57 Suppl 2: S116–9. [DOI] [PubMed] [Google Scholar]
- Rodríguez D, Mackenzie C, Wilhelm J, Qiu M, Mohan D, Bennett S.. 2018. Effects of PEPFAR’s geographic prioritization on HIV and Non-HIV services and health systems in Kenya: a mixed methods evaluation. International AIDS Conference. Amsterdam, Netherlands: International AIDS Society, 25 July 2018.
- Ssennyonjo A, Namakula J, Kasyaba R, Orach S, Bennett S, Ssengooba F.. 2018. Government resource contributions to the private-not-for-profit sector in Uganda: evolution, adaptations and implications for universal health coverage. International Journal for Equity in Health 17: 130.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- Sturchio J, Cohen G.. 2012. How PEPFAR’s public-private partnerships achieved ambitious goals, from improving labs to strengthening supply chains. Health Affairs 31: 1450–8. [DOI] [PubMed] [Google Scholar]
- Sulzbach S, De S, Wang W.. 2011. The private sector role in HIV/AIDS in the context of an expanded global response: expenditure trends in five sub-Saharan African countries. Health Policy and Planning 26 Suppl 1: i72–84. [DOI] [PubMed] [Google Scholar]
- Twimukye A, King R, Schlech W, Zawedde FM, Kakaire T, Parkes-Ratanshi R.. 2017. Exploring attitudes and perceptions of patients and staff towards an after-hours co-pay clinic supplementing free HIV services in Kampala, Uganda. BMC Health Services Research 17: 580.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda AIDS Commission 2012. National AIDS Spending Assessment—Uganda 2008/9–2009/10. Kampala, Uganda: Ugandan AIDS Commission. [Google Scholar]
- Vogus A, Graff K.. 2015. PEPFAR transitions to country ownership: review of past donor transitions and application of lessons learned to the Eastern Caribbean. Global Health: Science and Practice 3: 274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Sulzbach S, De S.. 2011. Utilization of HIV-related services from the private health sector: a multi-country analysis. Social Science & Medicine 72: 216–23. [DOI] [PubMed] [Google Scholar]
- Wilhelm J, Paina L, Qiu M, Mukuru M, Ssengooba F, Bennett S.. 2018. Impact of PEPFAR geographic pivot on HIV & Non-HIV health services in Uganda. International AIDS Conference. Amsterdam, Netherlands: International AIDS Society, 25 July 2018.
- Zakumumpa H, Taiwo MO, Muganzi A, Ssengooba F.. 2016. Human resources for health strategies adopted by providers in resource-limited settings to sustain long-term delivery of ART: a mixed-methods study from Uganda. Human Resources for Health 14: 63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakumumpa H, Bennett S, Ssengooba F.. 2016. Accounting for variations in ART program sustainability outcomes in health facilities in Uganda: a comparative case study analysis. BMC Health Services Research 16: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


