Abstract
PURPOSE
Inflammatory breast cancer (IBC) is an aggressive variant for which axillary lymph node dissection following neoadjuvant chemotherapy (NACT) remains standard of care. But with increasingly effective systemic therapy, it is unclear whether more limited axillary surgery may be appropriate in some IBC patients. We sought to examine whether extent of axillary lymph node (LN) surgery was associated with overall survival (OS) for IBC.
METHODS
Female breast cancer patients with non-metastatic IBC (cT4d) diagnosed 2010–2014 were identified in the National Cancer Data Base. Cox proportional hazards modeling was used to estimate the association between extent of axillary surgery (≤9 vs ≥10 LNs removed) and OS after adjusting for covariates, including post-NACT nodal status (ypN0 vs ypN1–3) and radiotherapy receipt (yes/no).
RESULTS
3,471 patients were included: 597 (17.2%) had cN0 disease, 1,833 (52.8%) had cN1 disease, and 1,041 (30%) had cN2–3 disease. 49.9% of cN0 patients were confirmed to be ypN0 on post-NACT surgical pathology. Being ypN0 (vs ypN1–3) was associated with improved adjusted OS for all patients. Radiotherapy was associated with improved adjusted OS for cN1 and cN2–3 patients but not for cN0 patients. Regardless of ypN status, there was a trend towards improved adjusted OS with having ≥10 (vs ≤9) LNs removed for cN2–3 patients (HR 0.78, 95% CI 0.60–1.01, p=0.06) but not for cN0 patients (p=0.83).
CONCLUSIONS
A majority of IBC patients in our study presented with node-positive disease, and for those presenting with cN2–3 disease, more extensive axillary surgery is potentially associated with improved survival. For cN0 patients, however, more extensive axillary surgery was not associated with a survival benefit, suggesting an opportunity for more personalized care.
Keywords: axillary lymph node dissection, inflammatory breast cancer, neoadjuvant, pathologic complete response, sentinel lymph node biopsy, targeted axillary dissection
Introduction
Inflammatory breast cancer (IBC) is a rare and aggressive clinical variant characterized by rapid-onset, marked skin changes including erythema and peau d’orange, and a worse prognosis relative to other forms of invasive breast carcinoma.1,2 Historically, patients with IBC have been presumed to have nodal involvement at presentation, thus standard of care includes neoadjuvant systemic therapy followed by modified radical mastectomy, i.e., mastectomy plus axillary lymph node dissection (ALND), and chest wall and regional nodal irradiation.3 Although IBC is generally thought to have a poor prognosis, multimodal therapy has led to significant improvements in survival for some IBC patients, specifically those experiencing clinical and radiographic resolution of lymphadenopathy and confirmation of pathologic complete response (pCR).4–7
With increasingly effective systemic therapy – particularly for tumors with HER2 overexpression – and collective efforts to de-escalate potentially morbid locoregional treatments of the axilla, the feasibility and long-term safety of sentinel lymph node biopsy (SLNB) alone in the presence of limited nodal involvement has been proposed for patients undergoing surgery after neoadjuvant chemotherapy (NACT). Several previous studies and trials examining the potential application of post-NACT SLNB in both cN08–10 and cN111–13 patients have excluded patients with IBC, typically because of concerns that dermal lymphatic involvement might preclude successful mapping and/or IBC was simply too high-risk to safely omit ALND. However, not all patients with IBC have clinical evidence of nodal involvement at diagnosis, and it is unclear whether ALND could potentially be avoided in patients who neither present with nor develop nodal metastases.14
Accordingly, we sought to examine whether extent of axillary lymph node (LN) surgery was associated with overall survival in IBC patients, particularly in patients with limited (cN1) or no (cN0) LN disease at presentation and no evidence of nodal disease at surgery after NACT (ypN0).
Methods
Female patients ≥18 years old with non-metastatic IBC (cT4d) diagnosed between 2010 and 2015 who received NACT and underwent breast surgery were identified from the 2004–2016 National Cancer Data Base (NCDB) Participant User File (PUF). 2010 was selected as the beginning of our cohort date to reflect when HER2 coding became standardized in the NCDB, an important consideration given the high rates of breast and nodal pCR achieved among HER2+ patients receiving NACT in combination with anti-HER2 targeted therapy.4,15 Continuation of chemotherapy in the adjuvant setting cannot be accurately discerned in the NCDB, as only the start date of systemic therapy is available; therefore, patients who received both NACT and adjuvant systemic therapy were included in the study but could not be distinguished from those who received all of their chemotherapy in the neoadjuvant setting.
The cohort was divided into three groups based on clinical nodal (cN) status at presentation (cN0, cN1, and cN2–3), which is defined in the NCDB according to imaging studies (excluding lymphoscintigraphy), clinical examination demonstrating characteristics highly suspicious for malignancy, and/or pathologic diagnosis obtained via needle biopsy16. As pathologic and staging information are reported by contributing NCDB sites and not subject to central review, it is not possible to distinguish patients for whom pathological confirmation was performed from those for whom staging was entirely clinical.
Patients with missing stage or survival information or no/unknown number of LNs examined were excluded. Patients with a surgical procedure coded as “none,” “local tumor destruction only,” “not otherwise specified,” or “unknown” were also excluded. As required by the NCDB, patients diagnosed in 2015 were excluded from survival analyses due to insufficient length of follow-up.
With regards to biomarkers, hormone receptor-positive (HR+) was defined as estrogen receptor-positive (ER+) and/or progesterone receptor-positive (PR+) while HR-negative (HR−) was defined as estrogen receptor-negative (ER−) and progesterone receptor-negative (PR−). The cohort was divided into 4 subtypes based on combinations of HR and HER2 status: (1) HR+/HER2−, (2) HR+/HER2+, (3) HR−/HER2+, and (4) HR/HER2− (i.e., triple-negative). Patients were divided into 2 groups based on nodal response to NACT: those with no residual disease in the LNs (ypN0) and those with persistent LN involvement (ypN+, i.e., ypN1 [including ypN1mic], ypN2, and ypN3).
Patient characteristics were summarized with N (%) for categorical variables and median (interquartile range) for continuous variables for all patients. Chi-square or Fisher’s exact tests were used to compare categorical variables and Wilcoxon Rank Sum tests or t-tests were used to compare continuous variables, as appropriate.
Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Kaplan-Meier (KM) curves stratified by cN classification (cN0, cN1, cN2–3) were used to visualize unadjusted OS, and the log-rank test was used to test for a difference between having ≤9 vs ≥10 LNs removed and examined. The NCDB does not capture information on axillary surgery type (i.e., whether SLNB or ALND was the intended operation), thus a threshold of ≥10 LNs was used to represent likely ALND, in keeping with current NCCN guidelines regarding adequacy of ALND and previous publications featuring NCDB data.17,18
Cox proportional hazards regression analyses were used to estimate the association of cN classification at presentation (cN0, cN1, cN2–3), number of LNs examined (≤9 vs ≥10 LNs), and post-NACT nodal response (ypN0 vs ypN+) with OS after adjustment for known covariates including race/ethnicity (non-Hispanic black, non-Hispanic white, non-Hispanic other, and white) and receipt of radiation (yes/no). Interactions were tested between cN stage and number of LNs examined, between cN stage and post-NACT nodal status, between post-NACT nodal status and number of LNs examined, and between post-NACT nodal status and radiation receipt, and these are reported if significant. Additionally, adjusted survival analyses stratified by cN classification (cN0, cN1, cN2–3) were performed, and an interaction was again tested between post-NACT nodal status and number of LNs examined and between post-NACT nodal status and radiation receipt. Finally, we conducted two sensitivity analyses for the Cox survival models: (1) a model in which number of LNs examined was included as a continuous, rather than a binary, variable; and (2) a model limited to cN0 and cN1 patients for which the “LNs removed” variable was divided into 3 levels (1–5, 6–9, ≥10), with the 1–5 LNs level serving as a proxy for SLNB. We limited this latter 3-level sensitivity analysis to cN0–1 patients because we felt it was unlikely that SLNB (or a similarly selective approach to axillary sampling) would be pursued in cN2–3 patients. A robust sandwich covariance estimator was included in all Cox models to account for the correlation of patients treated at the same facility, and hazard ratios are presented. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.5.0. The study was deemed exempt by the Duke University Institutional Review Board due to use of de-identified data.
Results
Patient, disease, and treatment characteristics
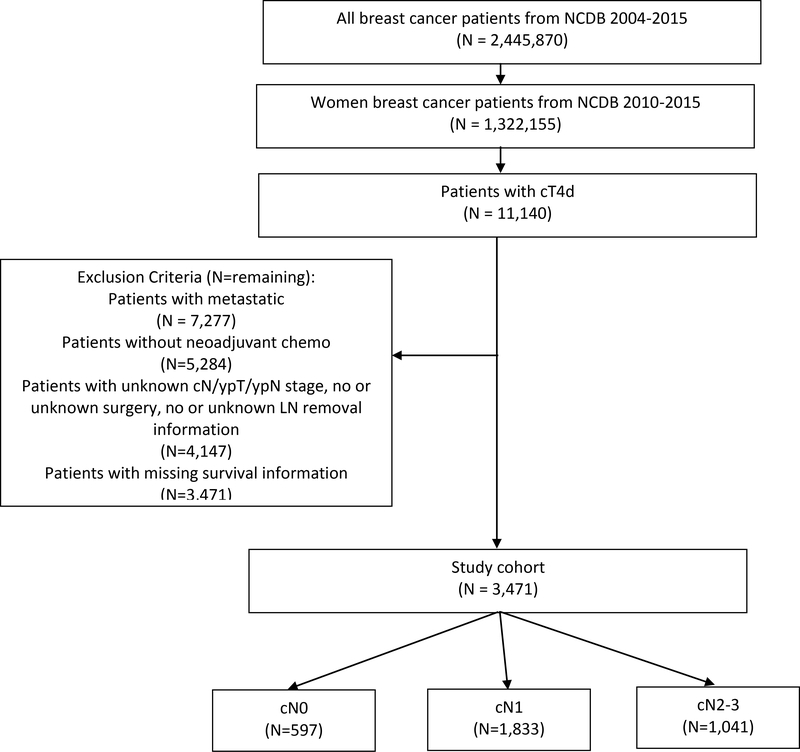
3,471 patients with IBC were included in our analysis (median age 56, Figure 1, Table 1): 597 (17.2%) presented with cN0 disease, 1,833 (52.8%) with cN1 disease, and 1,041 (30%) with cN2–3 disease. Hispanic (35.4%) and non-Hispanic black (36.8%) patients had higher rates of cN2–3 disease than non-Hispanic white patients (27.9%, p<0.001). Notably, 2.2% of patients (n=75) underwent lumpectomy despite mastectomy’s being standard of surgical care for management of the breast in IBC. There was no difference in the rates of cN classification between patients who received lumpectomy vs standard-of-care mastectomy. Likewise, most (84.2%, n=2,921) but not all patients received radiation, with the vast majority of treated patients (n=2,828) receiving it entirely in the adjuvant setting.
Figure 1.
Female Patients with Non-Metastatic Inflammatory Breast Cancer, National Cancer Data Base, 2010–2015
Table 1.
Female Patients with Inflammatory Breast Cancer, National Cancer Data Base, 2010–2015
| All Patients | cN0 | cN1 | cN2–3 | ||
|---|---|---|---|---|---|
| N=3,471 (100%) | N=597 (17.2%) | N=1,833 (52.8%) | N=1,041 (30.0%) | P-Value | |
| Age | |||||
| Median (IQR) | 56 (47 – 64) | 58 (49 – 66) | 55 (47 – 63) | 56 (48 – 64) | <0.001 |
| Race/Ethnicity | |||||
| Hispanic | 246 (7.1%) | 32 (5.4%) | 127 (6.9%) | 87 (8.4%) | <0.001 |
| Non-Hispanic Black | 543 (15.6%) | 80 (13.4%) | 263 (14.3%) | 200 (19.2%) | |
| Non-Hispanic Other | 91 (2.6%) | 18 (3%) | 44 (2.4%) | 29 (2.8%) | |
| Non-Hispanic White | 2463 (71%) | 437 (73.2%) | 1339 (73%) | 687 (66%) | |
| Histology | |||||
| Ductal | 2,301 (66.3%) | 366 (61.3%) | 1,260 (68.7%) | 675 (64.8%) | 0.005 |
| Lobular | 115 (3.3%) | 34 (5.7%) | 50 (2.7%) | 31 (3%) | |
| Mammary | 110 (3.2%) | 23 (3.9%) | 58 (3.2%) | 29 (2.8%) | |
| Metaplastic | 26 (0.7%) | 5 (0.8%) | 11 (0.6%) | 10 (1%) | |
| Missing | 78 (2.2%) | 17 (2.8%) | 40 (2.2%) | 21 (2%) | |
| Other/NOS* | 841 (24.2%) | 152 (25.5%) | 414 (22.6%) | 275 (26.4%) | |
| Grade | |||||
| Unknown | 415 (12%) | 70 (11.7%) | 209 (11.4%) | 136 (13.1%) | <0.001 |
| 1 | 93 (2.7%) | 31 (5.2%) | 36 (2%) | 26 (2.5%) | |
| 2 | 934 (26.9%) | 202 (33.8%) | 518 (28.3%) | 214 (20.6%) | |
| 3 | 2029 (58.5%) | 294 (49.2%) | 1070 (58.4%) | 665 (63.9%) | |
| Receptor Subtype | |||||
| HR+/HER2+ | 586 (16.9%) | 93 (15.6%) | 343 (18.7%) | 150 (14.4%) | 0.005 |
| HR+/HER2− | 1308 (37.7%) | 254 (42.5%) | 665 (36.3%) | 389 (37.4%) | |
| HR−/HER2+ | 598 (17.2%) | 88 (14.7%) | 324 (17.7%) | 186 (17.9%) | |
| TNBC | 892 (25.7%) | 138 (23.1%) | 466 (25.4%) | 288 (27.7%) | |
| Surgery Type | |||||
| Lumpectomy | 75 (2.2%) | 19 (3.2%) | 35 (1.9%) | 21 (2%) | 0.17 |
| Mastectomy | 3396 (97.8%) | 578 (96.8%) | 1798 (98.1%) | 1020 (98%) | |
| LNs Examined | |||||
| Median (IQR) | 11 (7 – 17) | 10 (5 – 15) | 12 (7 – 17) | 12 (7 – 17) | <0.001 |
| Positive LNs | |||||
| Median (IQR) | 4 (2 – 9) | 4 (2 – 8) | 4 (1 – 8) | 6 (2 – 10) | <0.001 |
| Axillary Surgery Extent | |||||
| ≤9 LNs | 1350 (38.9%) | 288 (48.2%) | 674 (36.8%) | 388 (37.3%) | <0.001 |
| ≥10 LNs | 2121 (61.1%) | 309 (51.8%) | 1159 (63.2%) | 653 (62.7%) | |
| Received Endocrine Therapy (HR+ patients only, n=1929 – cN0: 357, cN1: 1020, cN2–3: 552) | |||||
| No | 233 (12.1%) | 47 (13.2%) | 112 (11%) | 74 (13.4%) | 0.22 |
| Yes | 1613 (83.6%) | 288 (80.7%) | 868 (85.1%) | 457 (82.8%) | |
| Received Radiation Therapy | |||||
| No | 543 (15.6%) | 125 (20.9%) | 252 (13.7%) | 166 (15.9%) | <0.001 |
| Yes | 2921 (84.2%) | 471 (78.9%) | 1578 (86.1%) | 872 (83.8%) | |
| Pathological T Classification | |||||
| ypT0 | 693 (20%) | 103 (17.3%) | 384 (20.9%) | 206 (19.8%) | 0.012 |
| ypT1 | 1035 (29.8%) | 184 (30.8%) | 571 (31.2%) | 280 (26.9%) | |
| ypT2 | 461 (13.3%) | 78 (13.1%) | 250 (13.6%) | 133 (12.8%) | |
| ypT3 | 341 (9.8%) | 53 (8.9%) | 182 (9.9%) | 106 (10.2%) | |
| ypT4 | 941 (27.1%) | 179 (30%) | 446 (24.3%) | 316 (30.4%) | |
| Pathological N Classification | |||||
| ypN0 | 1211 (34.9%) | 298 (49.9%) | 630 (34.4%) | 283 (27.2%) | <0.001 |
| ypN1 | 914 (26.3%) | 138 (23.1%) | 573 (31.3%) | 203 (19.5%) | |
| ypN1mic (subset of ypN1) | 145 (4.2%) | 26 (4.4%) | 87 (4.7%) | 32 (3.1%) | |
| ypN2 | 857 (24.7%) | 116 (19.4%) | 407 (22.2%) | 334 (32.1%) | |
| ypN3 | 489 (14.1%) | 45 (7.5%) | 223 (12.2%) | 221 (21.2%) | |
includes 691 patients for whom “inflammatory breast carcinoma” was only histological information provided
Patients who were reported as presenting with cN1 and cN2–3 disease had more extensive axillary surgery than cN0 patients: the median number of LNs examined in clinically node-positive patients was 12, compared to 10 in clinically node-negative patients (p<0.001). In addition, a greater proportion of patients with cN1–3 disease, compared to cN0 patients, had ≥10 LNs removed, which has traditionally been the threshold for adequate axillary clearance (p<0.001).17 15.7% of all patients achieved pCR in both the breast and LNs, with similar rates across cN classifications. 34.9% of all patients had ypN0 status after NACT; notably, half of cN0 patients (49.9%) were found to have no nodal disease at surgery. However, among patients who presented with clinically involved nodes, rates of nodal pCR were lower among both cN2–3 patients (27.2%) and cN1 patients (34.4%, p<0.001).
HR+/HER2− (38.6%) and triple-negative breast cancer (TNBC, 26.4%) made up the largest proportion of IBC subtypes while rates of HR+/HER2+ (17.3%) and HR−/HER2+ (17.7%) subtypes were lower (Supplemental Table 1). There was no significant difference by subtype with regards to number of lymph nodes removed, but both HR+/HER2− and TNBC patients had more positive LNs (median 5 LNs) vs the two HER2+ subtypes (median 3 LNs, p<0.001). Almost ¼ of HR+/HER2+ (22.2%) and approximately 1/3 of HR−/HER2+ patients (33.4%) achieved pCR in the breast and lymph nodes as compared to only 4.5% of HR+/HER2− and 15% of TNBC patients (p<0.001). Notably, a majority of HR−/HER2+ (59%) and almost half of HR+/HER2+ patients (46.8%) were ypN0 on final surgical pathology while only 18.8% of HR+/HER2− and 33.2% of TNBC patients were (p<0.001).
Survival analyses
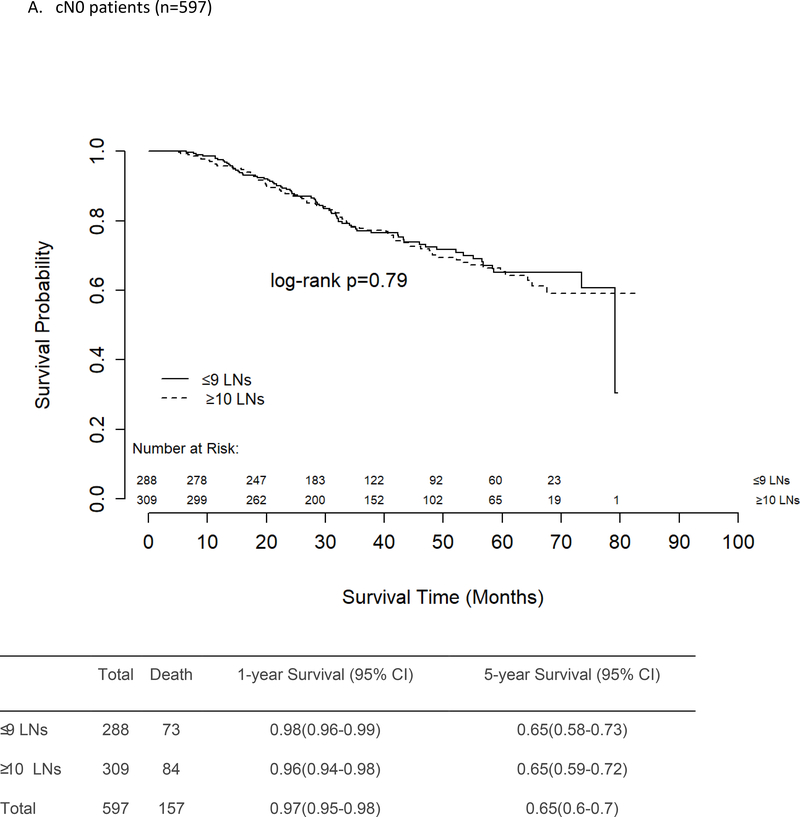
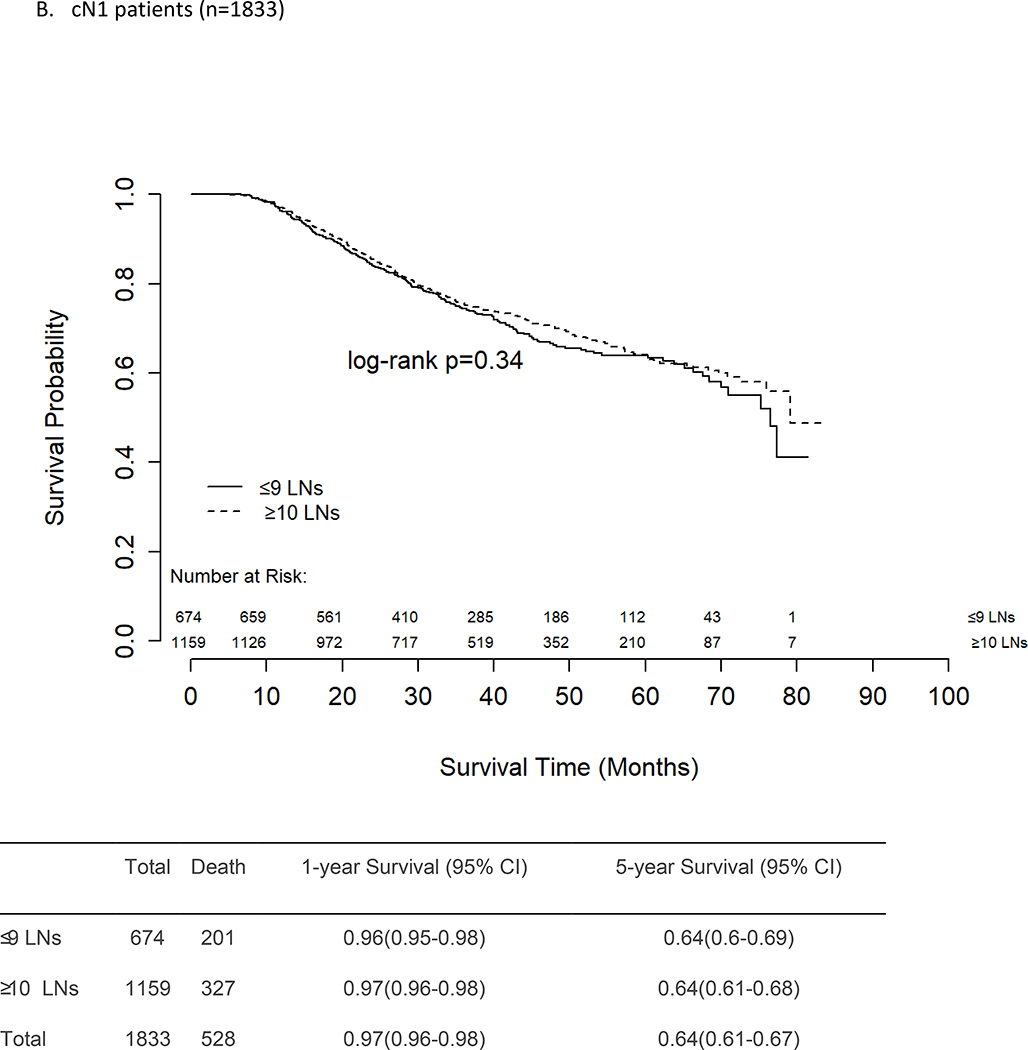
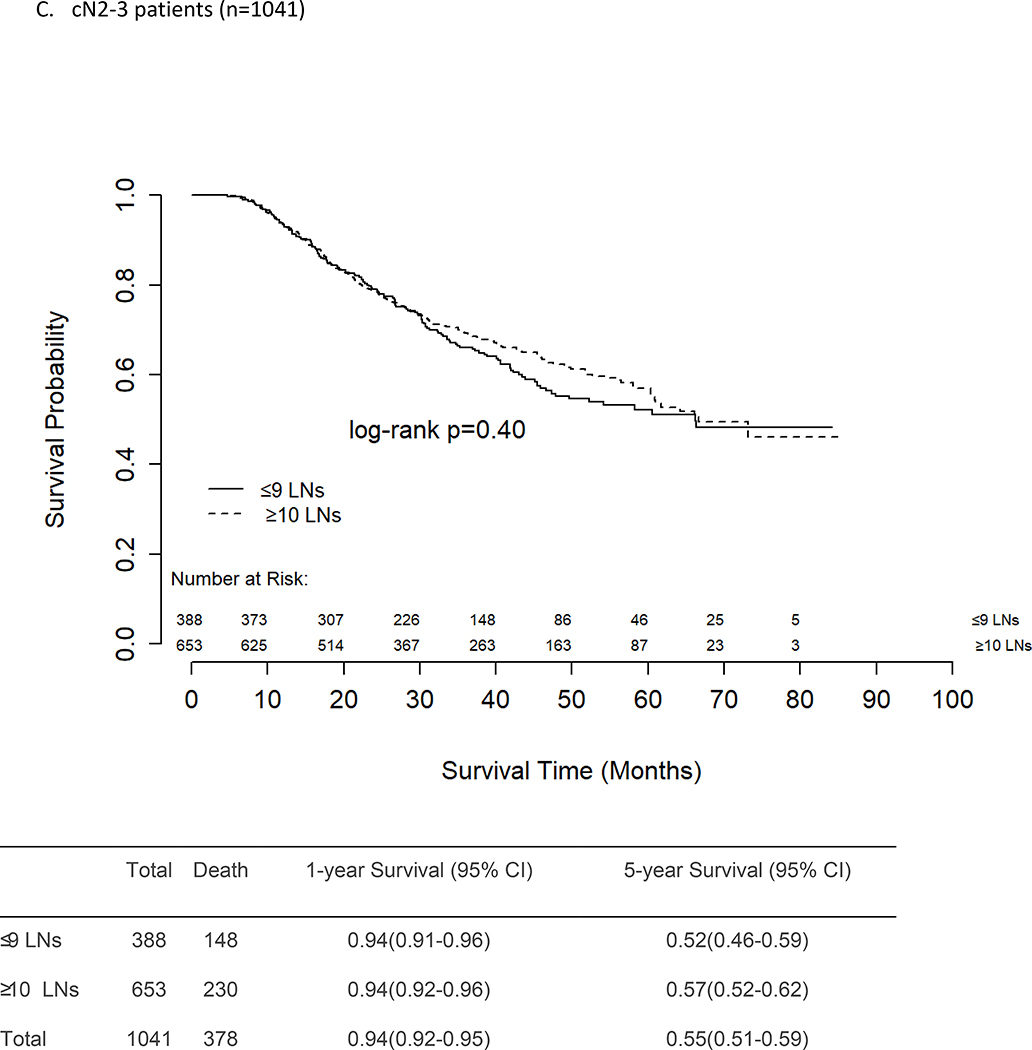
There was no difference in unadjusted OS between patients who had ≤9 vs ≥10 LNs removed and examined, regardless of presenting nodal stage (Figures 2a–c). The 5-year survival rates for cN0 and cN1 patients were similar at 65% and 64%, respectively, for both those who had ≤9 LNs removed and those who had ≥10 LNs removed. For patients with cN2–3 disease, the 5-year OS rates were again similar for those with ≤9 LNs removed (52%) and those with ≥10 LNs removed (57%, p=0.40).
Figure 2.
Unadjusted Overall Survival, Female Patients with Inflammatory Breast Cancer, National Cancer Data Base, 2010–2014
When examining the entire cohort of IBC patients, having ≥10 LNs removed, being ypN0, and receiving radiation were all independently associated with improved survival after adjusting for known covariates (Supplemental Table 2). Older age, higher cN status, black race, and having TNBC were associated with worse survival. There were no significant interactions between cN classification and number of LNs examined, between cN classification and nodal response to NACT, or between nodal response to NACT and radiation receipt. The interaction between response to NACT and number of LNs removed was significant (p=0.03), but we deferred interpretation and extrapolation of this interaction to the analyses stratified by cN status, and none of these interactions, including response to NACT*number of LNs removed, were significant in the stratified analyses.
Among patients who presented with node-negative disease (cN0), ypN0 status was independently associated with improved survival, while older age at diagnosis and TNBC were associated with worse adjusted OS (Table 2). Neither removal of more LNs nor receipt of radiotherapy were independently associated with survival.
Table 2.
Adjusted Overall Survival, Female Patients with Inflammatory Breast Cancer, National Cancer Data Base, 2010–2014a
| cN0 (n=471, events=130) | cN1 (n=1506, events=431) | cN2–3 (n=820, events=295) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-Value | Overall P-Value | HR (95% CI) | P-Value | Overall P-Value | HR (95% CI) | P-Value | Overall P-Value | |
| Age | |||||||||
| Continuous | 1.02 (1.01 – 1.04) | 0.01 | 0.01 | 1.00 (1.00 – 1.01) | 0.27 | 0.27 | 1.00 (0.99 – 1.02) | 0.45 | 0.45 |
| Tumor Response | |||||||||
| ypN+ | -REF- | <0.001 | -REF- | <0.001 | -REF- | <0.001 | |||
| ypN0 | 0.32 (0.21 – 0.49) | <0.001 | - | 0.37 (0.28 – 0.47) | <0.001 | 0.37 (0.25 – 0.54) | <0.001 | ||
| # LNs examined | |||||||||
| ≤9 | -REF- | 0.83 | -REF- | 0.08 | -REF- | 0.06 | |||
| ≥10 | 0.96 (0.68 – 1.37) | 0.83 | 0.83 (0.68 – 1.02) | 0.08 | 0.78 (0.60 – 1.01) | 0.06 | |||
| Race/Ethnicityb | |||||||||
| Non-Hispanic White | - | - | - | -REF- | <0.001 | -REF- | 0.07 | ||
| Hispanic | - | - | - | 0.74 (0.47 – 1.17) | 0.19 | 0.79 (0.48 – 1.3) | 0.35 | ||
| Non-Hispanic Black | - | - | - | 1.51 (1.16 – 1.96) | 0.002 | 1.32 (0.98 – 1.77) | 0.06 | ||
| Non-Hispanic Other | - | - | - | 0.47 (0.17 – 1.34) | 0.16 | 0.53 (0.18 – 1.57) | 0.25 | ||
| Receptor Subtype | |||||||||
| HR+/HER2− | -REF- | 0.001 | -REF- | <0.001 | -REF- | <0.001 | |||
| HR+/HER2+ | 0.84 (0.48 – 1.50) | 0.56 | 0.88 (0.63 – 1.21) | 0.43 | 0.55 (0.35 – 0.86) | 0.01 | |||
| HR−/HER2+ | 1.26 (0.72 – 2.21) | 0.42 | 1.10 (0.78 – 1.55) | 0.59 | 0.76 (0.51 – 1.13) | 0.18 | |||
| TNBC | 2.17 (1.40 – 3.36) | <0.001 | 2.93 (2.32 – 3.70) | <0.001 | 2.60 (1.95 – 3.45) | <0.001 | |||
| Radiation | |||||||||
| No | -REF- | 0.50 | -REF- | <0.001 | -REF- | 0.03 | |||
| Yes | 0.86 (0.55 – 1.34) | 0.50 | 0.49 (0.38 – 0.64) | <0.001 | 0.69 (0.49 – 0.97) | 0.03 | |||
Patients diagnosed in 2015 were excluded from survival analyses due to insufficient follow-up and as recommended by the NCDB.
Race/Ethnicity not included in cN0 model to avoid overfitting given limited number of events. LN, lymph node. NACT, neoadjuvant chemotherapy. ypN+, ypN1–3 (i.e., persistent nodal disease). Interaction for LNs examined*nodal response (ypN0 vs ypN+) was non-significant for all 3 nodal groups: cN0 (p=0.69), cN1 (p=0.07), and cN2–3 (p=0.10). Interaction for Radiation*nodal response (ypN0 vs ypN+) was non-significant for all 3 nodal groups: cN0 (p=0.56), cN1 (p=0.24), and cN2–3 (p=0.88).
There was a trend towards improved survival with ALND in cN1 patients (HR 0.83, 95% CI 0.68–1.02, p=0.08, Table 2); as with cN0 patients, in the analysis with the continuous LN variable, removal of more LNs was not significantly associated with improved survival for cN1 patients (HR 0.98, 95% CI 0.97–1, p=0.46, Supplemental Table 3). But among cN1 and in contrast to cN0 patients, receipt of radiation was associated with improved adjusted survival (HR 0.48, 95% CI 0.37–0.62, p<0.001). This effect was independent of nodal response to NACT, as demonstrated by a non-significant interaction between response to NACT and radiation receipt. Nodal pCR, i.e., ypN0 status in previously node-positive patients, continued to be independently associated with improved survival, while black race and having TNBC were both associated with worse adjusted OS.
Among patients with cN2–3 disease, as with cN1 disease, there was a trend towards improved survival with ALND (HR 0.78, 95% CI 0.60–1.01, p=0.06, Table 2), but when a continuous LN variable was used in the survival model, removal of more LNs was significantly associated with improved survival for cN2–3 patients (HR 0.98, 95% CI 0.96–1, p=0.015), but not for cN1 and cN0 patients (Supplemental Table 3). Both nodal pCR and radiation receipt (HR 0.69, 95% CI 0.49–0.98, p=0.036) were associated with improved survival in cN2–3 patients, as was having HR+/HER2+ disease (vs HR+/HER2−: HR 0.55, 95% CI 0.35–0.86, p=0.009). TNBC was again associated with worse survival (HR 2.67, 95% CI 2.01–3.55, p<0.001).
A sensitivity analysis limited to cN0–1 patients showed no association between survival and extent of axillary surgery (Supplemental Table 4).
Discussion
In our analysis of patients with non-metastatic IBC, most patients presented with LN involvement, but removal of ≥10 LNs (a proxy for ALND) was not associated with improved OS in patients who presented with cN0 disease, despite the observation that 50% of cN0 patients were found to have some nodal involvement on final pathology. Similarly, in cN1 patients, having more LNs removed was not associated with improved survival when LNs were evaluated as a continuous variable, though there was a trend towards significance in the binary LN analysis, independent of treatment response in the nodes. However, for patients presenting with cN2–3 disease, there was a trend towards improved survival associated with receipt of more extensive axillary surgery for both the main model and the sensitivity analysis, and this, too, was true regardless of nodal pCR. Collectively, these findings suggest that while ALND could potentially be omitted for some patients with IBC, it is still warranted for those IBC patients who present with significant LN involvement (i.e., cN2–3), even in the setting of nodal pCR.
As mentioned, there was no significant interaction between post-NACT nodal status and number of LNs removed, demonstrating that the benefit (for cN2–3 patients) or lack of benefit (for cN0 patients) from extensive axillary surgery for survival did not vary between ypN0 and ypN+ patients. While it may seem surprising that patients who presented with a large nodal disease burden would benefit from ALND even if they achieved nodal pCR, this finding has been observed in other studies, and may reflect, in part, differential administration of adjuvant treatment based on response to NACT as determined by more accurate assessment of nodal stage.19 Notably, cN2–3 patients were also the only group of patients for whom having the HR+/HER2+ subtype was associated with improved survival, likely because patients with such extensive nodal disease at presentation have such a poor prognosis that the presence of any favorable prognosticators such as HR+/HER2+ status have a greater impact on survival than they do in less severe disease. Our findings collectively suggest that multimodal therapy addressing both locoregional and systemic disease is especially important in cN2–3 IBC patients, while among cN0 patients, treatment may potentially be de-escalated for some patient groups.
Given our findings that nodal involvement at presentation is common but not universal (8.6% of patients were both cN0 and ypN0), pre-NACT axillary evaluation in those presenting with cN0 disease may potentially allow a few women who never have nodal involvement to avoid ALND. One challenge with axillary staging in IBC is that sentinel lymph node (SLN) mapping may prove challenging or impossible given the dermal lymphatic involvement that is characteristic of the disease. Several of the recent trials and studies that examined the feasibility of SLN mapping after NACT deliberately excluded patients with IBC.8,11,12 Small, retrospective studies that specifically examined SLNB in IBC patients reported successful SLN mapping and detection in 80–85% of patients, and false negative rates of 10–18%, neither of which meet current standards for reliable SLNB performance or accuracy.20,21 A recent single-institution prospective series by DeSnyder et al. found that dual tracer SLN mapping was successful in only 4 of 16 patients (25%), but 3 of those 4 patients had nodal pCR.22 The authors concluded that SLNB was unsuccessful in most IBC patients but that patients with pCR might be able to undergo SLNB if mapping can be successfully achieved; accordingly, they concluded that ALND should remain standard of care for IBC patients.
However, we do not believe that attempts to limit LN removal in node-negative IBC patients should be abandoned simply because conventional methods for identifying SLNs are challenging and often unsuccessful. Rather, we need to be creative in developing new and more effective ways to identify LNs that are representative of the rest of the axilla in IBC patients. Axillary ultrasound is increasingly used for pre-NACT axillary evaluation, but its accuracy is variable. An institutional series by Caretta-Weyer et al. observed a false negative rate (FNR) of 24% in women with cN0 breast cancer who underwent sonographic evaluation, demonstrating that while sonography is a useful adjunct to SLNB, it is not of sufficient accuracy to replace it.23 Another study by Britton et al. examining concordance between sonographically identified and percutaneously biopsied LNs vs surgically excised SLNs in women with clinically negative (cN0) axillae found that 22 of 73 patients with negative percutaneous biopsies had positive SLNs, consistent with a FNR of 30%.24 Furthermore, only 64% of the retrieved SLNs demonstrated evidence of previous percutaneous biopsy. The authors concluded that better methods of both identifying SLNs by imaging and sampling the axilla at the time of surgery are needed.
Targeted axillary dissection (TAD) may be a means through which sonographic identification and percutaneous sampling of abnormal nodes prior to NACT followed by pathologic confirmation of nodal response to NACT at time of surgery can facilitate more accurate axillary staging in IBC patients. Caudle et al. have demonstrated that TAD, which is a combination of excising the previously biopsied LN (localized with a clip at pre-NACT biopsy and a radiographic marker immediately prior to surgery) and any SLNs identified by dual tracer mapping, yields a FNR of approximately 2%.25 In patients with IBC, SLN mapping would remain a challenge, but radiographically identifying, sampling, and clipping a few borderline or abnormal LNs in patients presenting with limited radiographic evidence of LN involvement prior to NACT could allow for these LNs to be targeted for excision at the time of surgery, even if mapping were unsuccessful. Post-NACT, pre-operative localization of these previously sampled nodes could help to ensure adequate excision and reliable axillary sampling after NACT, potentially obviating full ALND and its concomitant morbidity in a subset of IBC patients. Of note, in ACOSOG Z1071, which was designed to examine the feasibility of post-NACT SLNB in the clinically downstaged axilla, the FNR for SLNB was 16.7% (95% CI 2.1% to 48.4%) when the only LN removed was the previously clipped, biopsied node.26 This rate might be expected to improve if the goal of axillary sonographic evaluation and sampling were not simply to identify at least one positive node but rather to identify all potentially abnormal nodes, though we recognize that specificity might also decline with this approach. While the majority (~65%) of IBC patients, as demonstrated in our study, are confirmed to be node-positive at the time of surgery and potentially benefit from axillary clearance, a thoughtful, more targeted approach such as the one described here could potentially allow some IBC patients to avoid the morbidity of ALND without compromising survival. Thus, our study provides support for emerging efforts to improve the feasibility and accuracy of pre- and post-NACT axillary evaluation in patients with IBC.
Limitations
As with all retrospective, observational studies including those using the NCDB, selection bias is a limitation of our study. The NCDB does not report breast cancer-specific survival or locoregional recurrence rates, thus overall survival was the only long-term outcome we were able to examine. Pre- and postoperative histologic and staging information as well as radiation dosage and treatment data are reported by member institutions to the NCDB, but these assessments are not subjected to central review that might otherwise identify and reclassify cases for which these data were incorrect. We recognize that, in general, there may be under-ascertainment of ambulatory services for both chemotherapy and radiotherapy. We included radiation as a binary covariate in our adjusted analyses because the vast majority of IBC radiation recipients will have received post-mastectomy radiation that included nodal irradiation, but we recognize that inaccurate documentation with regards to the inclusion or omission of regional nodal irradiation might have affected the results of our survival analyses. It is important to note that IBC represents a challenging diagnostic entity for which there is no unique histologic criteria common to all IBC diagnoses. Thus, some proportion of patients in this study may have had locally advanced rather than inflammatory breast cancer and may, therefore, have had less aggressive, lower grade tumors with potentially better post-treatment outcomes than true IBC patients. Moreover, not all initial nodal staging was biopsy-proven, thus, our analysis could have included patients with pathologically benign but radiographically or anatomically abnormal lymphadenopathy that were inappropriately considered cN1–3; these patients would be expected to do better than true node-positive patients and could potentially influence survival results. Finally, in using removal of ≥10 LNs to represent ALND and 1–5 LNs to represent SLNB, we are applying thresholds used in previous publications given the coding limitations of the NCDB, but we acknowledge that these cutoffs represent yield rather than surgical intent.18,27 We also recognize that nodal yield is typically lower after NACT and that the 10-LN threshold is based on historical data from patients who underwent upfront surgery rather than NACT.17,19 Thus, we acknowledge the possibility that a higher number of ALNDs (for which ≥10 LNs has been used as a proxy) were performed than is reflected in our data and that <10 LNs were sometimes retrieved even when ALND was intended. Recognizing this limitation, we have also included LN removal as a continuous and 3-level variable (including a proxy for SLNB) in our adjusted Cox survival analyses to help contextualize the trends observed in our models using the binary LN variable.
Conclusions
A majority of patients with IBC present with nodal involvement, and for patients who present with cN2–3 disease, extent of axillary surgery was associated with improved survival in our analysis regardless of nodal status after systemic treatment. However, for patients who presented with cN0 disease, removal of more LNs was not associated with improved survival, suggesting that extensive axillary clearance may potentially be avoided in some IBC patients. Thus, while multimodal therapy has improved survival for all IBC patients and axillary clearance should be performed for IBC patients with residual LN involvement after NACT, locoregional treatment may be of even greater importance in the subset of patients presenting with significant nodal burden. Consideration of ALND omission in cN0, and potentially even cN1 patients who downstage after NACT, will necessitate more reliable techniques for confirming the absence of nodal disease before and after NACT. Accordingly, current methods for evaluating the axilla in IBC patients will need to be improved and refined through prospective investigation.
Supplementary Material
Acknowledgments
Compliance with Ethical Standards
Funding: Dr. Fayanju is currently supported by the National Institutes of Health (NIH) under Award Number 1K08CA241390 (PI: Fayanju) and previously by the National Center for Advancing Translational Sciences (NCATS) of the NIH under Award Number 1KL2TR002554 (PI: Svetkey). Dr. Greenup is supported by the NIH BIRCWH K12HD043446 (PI: Andrews). Dr. Suneja is supported by grants K08CA228631 (PI: Suneja) and P30AI064518 (PI: Weinhold) from the NIH. Dr. Devi is supported by grants from the DoD W81XWH-17–1-0297 (PI: Devi) and the IBC Network Foundation (PI: Devi). This work is also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest:
Dr. Fayanju has no conflicts of interest to disclose.
Mr. Ren has no conflicts of interest to disclose.
Dr. Greenup has no conflicts of interest to disclose.
Dr. Plichta has no conflicts of interest to disclose.
Dr. Rosenberger has no conflicts of interest to disclose.
Dr. Force is a consultant for Genomic Health, Nanostring, and Pfizer.
Dr. Suneja has no conflicts of interest to disclose.
Dr. Devi has no conflicts of interest to disclose.
Dr. King is a consultant for Genomic Health.
Dr. Nakhlis has no conflicts of interest to disclose.
Dr. Hyslop is a consultant for AbbVie.
Dr. Hwang has no conflicts of interest to disclose.
Data availability
The data that support the findings of this study are available from the National Cancer Data Base (NCDB) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the NCDB.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human participants or animals performed by any of the authors as de-identified data were used and the study was granted exempt status by our institutional review board.
References
- 1.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. Journal of the National Cancer Institute. 2005;97(13):966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Uden DJP, van Laarhoven HWM, Westenberg AH, de Wilt JHW, Blanken-Peeters CFJM. Inflammatory breast cancer: An overview. Critical Reviews in Oncology/Hematology. 2015;93(2):116–126. [DOI] [PubMed] [Google Scholar]
- 3.“Inflammatory Breast Cancer.” NCCN Guideline Version 2.2019. https://www.nccn.org/. Accessed 2019 July 01.
- 4.Gianni L, Pienkowski T, Im Y-H, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. The Lancet Oncology. 2016;17(6):791–800. [DOI] [PubMed] [Google Scholar]
- 5.Nahleh ZA, Barlow WE, Hayes DF, et al. SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Research and Treatment. 2016;158(3):485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakhlis F, Regan MM, Warren LE, et al. The Impact of Residual Disease After Preoperative Systemic Therapy on Clinical Outcomes in Patients with Inflammatory Breast Cancer. Annals of surgical oncology. 2017;24(9):2563–2569. [DOI] [PubMed] [Google Scholar]
- 7.Devi GR, Hough H, Barrett N, et al. Perspectives on Inflammatory Breast Cancer (IBC) Research, Clinical Management and Community Engagement from the Duke IBC Consortium. Journal of Cancer. 2019;10(15):3344–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Classe JM, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(5):726–732. [DOI] [PubMed] [Google Scholar]
- 9.Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(12):2694–2702. [DOI] [PubMed] [Google Scholar]
- 10.van der Heiden-van der Loo M, de Munck L, Sonke GS, et al. Population based study on sentinel node biopsy before or after neoadjuvant chemotherapy in clinically node negative breast cancer patients: Identification rate and influence on axillary treatment. European journal of cancer (Oxford, England : 1990). 2015;51(8):915–921. [DOI] [PubMed] [Google Scholar]
- 11.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(3):258–264. [DOI] [PubMed] [Google Scholar]
- 12.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. Jama. 2013;310(14):1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. The Lancet Oncology. 2013;14(7):609–618. [DOI] [PubMed] [Google Scholar]
- 14.Imeokparia FO, Hughes TM, Dossett LA, Jeruss JS, Chang AE, Sabel MS. Axillary Pathologic Complete Response in Inflammatory Breast Cancer Patients: Implications for SLNB? Annals of surgical oncology. 2019;26(10):3374–3379. [DOI] [PubMed] [Google Scholar]
- 15.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. [DOI] [PubMed] [Google Scholar]
- 16.The National Cancer Data Base. The American College of Surgeons. www.ncdb.org.
- 17.“Surgical Axillary Stagng.” NCCN Guideline Version 2.2019. https://www.nccn.org/. Accessed 2019 July 01.
- 18.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(18):2946–2953. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberger LH, Ren Y, Thomas SM, et al. Axillary Lymph Node Dissection in Node-Positive Breast Cancer: Are 10-Nodes Adequate and When is Enough, Enough? Breast Cancer Res Treat.2019. November 18 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hidar S, Bibi M, Gharbi O, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in inflammatory breast cancer. International journal of surgery (London, England). 2009;7(3):272–275. [DOI] [PubMed] [Google Scholar]
- 21.Stearns V, Ewing CA, Slack R, Penannen MF, Hayes DF, Tsangaris TN. Sentinel lymphadenectomy after neoadjuvant chemotherapy for breast cancer may reliably represent the axilla except for inflammatory breast cancer. Annals of surgical oncology. 2002;9(3):235–242. [DOI] [PubMed] [Google Scholar]
- 22.DeSnyder SM, Mittendorf EA, Le-Petross C, et al. Prospective Feasibility Trial of Sentinel Lymph Node Biopsy in the Setting of Inflammatory Breast Cancer. Clinical breast cancer. 2018;18(1):e73–e77. [DOI] [PubMed] [Google Scholar]
- 23.Caretta-Weyer H, Sisney GA, Beckman C, et al. Impact of axillary ultrasound and core needle biopsy on the utility of intraoperative frozen section analysis and treatment decision making in women with invasive breast cancer. The American Journal of Surgery. 2012;204(3):308–314. [DOI] [PubMed] [Google Scholar]
- 24.Britton PD, Provenzano E, Barter S, et al. Ultrasound guided percutaneous axillary lymph node core biopsy: How often is the sentinel lymph node being biopsied? The Breast. 2009;18(1):13–16. [DOI] [PubMed] [Google Scholar]
- 25.Caudle AS, Yang WT, Krishnamurthy S, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(10):1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance). Annals of surgery. 2016;263(4):802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fayanju OM, Ren Y, Thomas SM, et al. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB). Annals of surgery. 2018;268(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.