Abstract
Exosomes are a potent means for intercellular communication. However, exosomes have received intensive research focus in immunobiology only relatively recently. Because they transport proteins, lipids and genetic material between cells, they are especially suited to amplify their parental cell's message and overcome the physical constraints of cell-to-cell contact, that is exosome release gives cells the ability to alter distant, non-contiguous cells. As progress is made in this field, it has become increasingly obvious that exosomes are involved in most biological processes. In the immune system, exosomes are fundamental tools used by every immune cell type to fulfil its function and promote inflammation or tolerance. In this review, we first summarize key aspects of immune cell-specific exosomes and their functions. Then, we describe how exosomes have been shown to be indispensable orchestrators of the immune response in two immunological scenarios, namely transplant rejection or tolerance, and tumour evasion or initiation of anti-tumour immune responses.
1 ∣. INTRODUCTION
Long considered cellular debris, exosomes did not gather much consideration for years until relatively recently.1 However, the field of exosome research has rapidly expanded over the last decade, with an exponential increase in the number of publications year after year.2 As our understanding of exosome biology and our tools to study it continue to grow, this trend is projected to persist. Previously ignored, it is now becoming clear that exosomes are key players in most biological processes in multicellular organisms, particularly in immunity. In fact, while exosomes have been the source of many new research questions, they have also provided unequivocal answers to numerous long-standing problems in immunology. There has never been more interest in this area, thus it is increasingly necessary for immunologists to become acquainted with exosome biology.
Exosomes are a type of extracellular vesicle (EV), measuring between 30 to 150 nm in diameter.3 They originate by the inward budding of the endosomal compartment membranes, forming intraluminal bodies, using the machinery of the Endosomal Sorting Complex Required for Transport (ESCRT).4 These intraluminal body-containing endosomes are termed “multi-vesicular bodies” (MVB) and resemble a “nest” of infectious virus particles, except for a lack of uniform shape and size. After fusing with the plasma membrane, the MVB release their content, exosomes, into the extracellular space.5 Exosomes thus require complex cellular machinery for their genesis and secretion. Aside from their size and subcellular origin, exosomes can be identified by expression of defined markers. Of particular interest are the tetraspanins (CD9, CD63 and CD81, among others), which are involved in classic exosome biogenesis, cell membrane adhesion, protein-protein interactions and as molecular carriers.6
The main purpose of the exosome is intercellular communication. Exosomes transport lipids, proteins (both soluble/intraluminal as well as membrane-associated) and genetic material (including messenger RNA, long non-coding RNA [lncRNA], micro RNA [miRNA], etc) between a producing cell and an acquiring cell.7 Although originally thought to carry DNA, recent investigations have shown that DNA is not carried by exosomes, but rather by larger EVs termed “nucleosomes”.7 Because of their size and the fact that exosomes mediate the cell-to-cell spread of macromolecules, their ‘infectious’ nature has given rise to the hypothesis that they share ontogeny with viruses.8-10
This means of intercellular communication is exploited by leucocytes and many cell populations with which they interact to regulate immune responses.11 In this review, we will summarize key effects and mediators of exosome-associated modifications in different immune cell populations, both as exosome producers and as acquirers. Then, we will discuss the central role of exosomes in coordinating the immune response in two scenarios: transplantation and anti-tumour immunity.
2 ∣. T CELL EXOSOMES AND THEIR EFFECTS
T cells release exosomes and other EVs after T cell receptor (TCR) activation, with a variety of effects ranging from immune suppression to activation, depending on the context of exosome release.12 Because exosome release by T cells is TCR-dependent, T cell exosome releases can be antigen-specific, although their effects are not. Some of these EV-mediated effects were first recognized when it was found that stimulated T cells released bioactive Fas ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL) associated with a ‘particulate, ultracentrifugable fraction’. This fraction, composed of EVs, mediated activation-induced cell death.13 Furthermore, FasL and TRAIL were present in defined intracellular compartments, co-localized with exosome-associated proteins (such as CD63) and trafficked to the cell membrane for secretion in vesicles after mitogenic stimulation.14
T cells, especially activated T cells, are also avid exosome acquirers. Here again, the effect of exosome acquisition depends on the exosome source. In rhesus macaques, plasma exosomes can activate resting CD4 T cells, which can result in latent simian immunodeficiency virus (SIV) reactivation in infected quiescent cells.15 Likewise, bystander resting CD8 T cells can be activated by cytotoxic T lymphocyte (CTL)-derived exosomes, thus amplifying the inflammatory response.16
Despite these observations of exosome-mediated T cell activation, the bulk of the literature reports that the predominant result of exosome acquisition by a T cell is suppression. Ultimately, the exosome source and context of exosome production and acquisition will dictate whether the T cell gets activated or suppressed; for example, while mature dendritic cell (DC)-derived exosomes directly activate CD4 T cells and induce a Th1 cytokine expression profile, immature DC-derived exosomes are suppressive and bias them to a Th2 phenotype.17
Exosomes are the latest addition to the arsenal of tools used by regulatory T cells (Tregs) to maintain immune tolerance. Exosomal transfer of miRNAs is central for Treg function. Rab27a- or Rab27b- (genes involved in exosome secretion) or Dicer (miRNA maturation)-deficient Tregs fail to elicit tolerance and to suppress autoimmune Th1 responses.18 Treg-mediated tolerance requires exosomal transfer of the Let-7d miRNA from Tregs to CD4 T cells via exosomes.18 Aside from T cells, Treg exosomes can also target antigen-presenting cells (APCs), skewing them towards a tolerogenic phenotype. Treg exosome-modified DCs alter their cytokine secretion profile and surface expression of immune inhibitory molecules. Here, a key miRNA is 142-3p.19 This is especially interesting, because miR 142-3p transfection has been reported to interfere with antigen presentation by myeloid cells at every step, including decreased phagocytosis, inhibited antigen digestion and epitope processing and increased PD-L1 expression. This ultimately results in failure of APCs to activate T cells.20-22
Treg exosomes also suppress inflammatory responses by carrying immunomodulatory proteins. They express CD73, which catalyses AMP conversion into adenosine, which in turn acts on adenosine receptors on T cells to suppress them.23 Particularly interesting is IL-35, a potent immunosuppressive cytokine composed of the p35 subunit of IL-12 and Epstein-Barr-induced gene 3 protein (Ebi3).24-26 IL-35 is secreted by Tregs27 and is capable of suppressing CD4 and CD8 T cells and inducing T cell exhaustion.28 Furthermore, it expands Treg populations and can convert conventional T cells into induced IL-35 producers, termed iTr35.29 Our laboratory has found that this cytokine is unique in that it requires an exosome to hold its subunits together, in order to be biologically active.30 Additionally, target cells that acquire IL-35 exosomes not only are directly affected by the cytokine (primary suppression), but also can display it on their surface, where it remains active and capable of secondarily suppressing another cell (secondary suppression). Moreover, these non-FoxP3+, “suppressed” T cells may themselves acquire the IL-35 secretor phenotype (termed iTr35 cells).31 Thus, IL-35+ Treg exosomes mediate infectious tolerance, whereby a single tolerance-inducing cell transforms multiple host T effector cells into tolerance-inducing cells, analogous to how a single “passenger” DC in an allograft transforms multiple host DC to surrogate ‘allo’-DCs, inducing myriad effector alloreactive T cells for acute rejection, as will be detailed below.32,33
Interestingly, not only are exosomes a mechanism used by Tregs to maintain tolerance, but also a mechanism used to maintain Treg populations. Thymic EVs induce CD4+CD25+FoxP3+ Tregs, partially via exosomal transport of TGF-β.34 As will be discussed, tumour cells exploit this mechanism as a means to produce immune suppression.35 Mesenchymal stem cell EVs have been reported to expand Treg populations as well.36
3 ∣. B CELLS
B cell EVs were first characterized by Geuze's laboratory in 1996, who discovered they transported major histocompatibility complex (MHC)-II and associated antigens capable of mediating antigen presentation.37 B cells secrete these exosomes upon activation, which can be acquired by CD8α+ DCs, which then prime CTL responses with CD4 T cell and NK cell help.38-40 B cell exosomes can target T cells as well, which might activate them (by delivery of antigens, including allergens)41 or induce their apoptosis (if they are CD5+ killer B cell-derived, FasL+ exosomes).42,43 Thus, as is true for other cell populations, the immunophenotype of the parental B cell dictates the function of the exosome.
On the other hand, specific B regulatory cell (Breg) populations can secrete IL-35,44 which we propose is exosome-associated.30
The B cell exosome field is, as can be seen, largely unexplored, with few articles describing their role in a limited number of immunological contexts. It will surely keep expanding, and efforts are underway to study the tolerogenicity of Breg exosomes in more detail, as new mechanisms of immune suppression are being identified in this novel regulatory population.45,46
4 ∣. DENDRITIC CELL AND MYELOID CELL-DERIVED EXOSOMES
Myeloid APCs, especially DCs, are important exosome producers that heavily influence immune responses, as initially described by Geuze's group.47 DCs engulf antigens, load their peptides into MHC molecules and redirect these peptide-MHC complexes (pMHCs) to the exosome pathway in a LAMP2-dependent fashion.48 DC exosomes are notorious for transporting intact, functional pMHCs, which are acquired by different cell populations, including other APCs. These APCs become ‘cross-dressed’ and now display acquired pMHCs on their surface, and accordingly present them to effectors cells. Although initially it was reported that cross-dressing occurred via cell blebs derived from dying DCs,49 it is now clear that exosomes are the main culprit.50
Because an individual DC can produce myriad exosomes that cross-dress many APCs, antigenic cross-dressing serves the purpose of amplifying immune responses against specific antigens by multiplying the number of cells that display it on their surface. Other components of the exosome cargo, including co-stimulatory or inhibitory molecules and miRNA that alter the immunophenotype of the recipient cell, dictate the context in which the acquired pMHC will be presented and thus skew the resulting antigen-specific immune response to be pro-inflammatory or tolerogenic. Therefore, cross-dressing not only triggers, but also orchestrates the nature of antigen-specific immune responses. For example, in transplantation, cross-dressing by pro-inflammatory donor DC exosomes results in rejection,32,33 but cross-dressing from tolerance-prone grafts (eg non-inherited maternal antigen (NIMA)+ kidney or liver) promotes transplant tolerance 51,52 as will be detailed below.
5 ∣. EXOSOMES IN TOLERANCE AND TRANSPLANT REJECTION
Allorecognition of graft antigens by the recipient immune system was thought to occur via two pathways: direct and indirect. In the direct pathway, specific CD4 and CD8 T cell clones recognize intact, non-self-MHC-I or II molecules expressed on the surface of donor cells. On the other hand, the indirect pathway requires a host APC to engulf and process allogeneic antigens and express them on self-MHC-II molecules for recognition by effector CD4 T cells.53,54 From this, one can deduce that a T cell clone with ‘direct’ specificity will only interact with a donor APC expressing allo-MHC, while an ‘indirect’ T cell will only interact with a host APC expressing alloantigenic peptides on self-MHC.55 Thus, according to this paradigm, each T cell requires a specific APC with no possibility of a single APC priming direct and indirect T cells (for major antigens). This theoretical 4-cell requirement (separate APCs for T helper and T cytotoxic cells) contrasts with the observation that a single host APC can indeed do both functions.
The solution to this paradox came with the realization that there exists a third pathway of allorecognition, that is the semi-direct pathway. First described by Herrera et al,56 the semi-direct pathway consists of transfer of intact allo-MHC from allogeneic cells to host APCs, followed by recognition by direct-like T cells. Thus, host APCs can prime both direct and indirect T cells.
Indeed, without MHC cross-dressing and the semi-direct pathway of allorecognition, priming of direct T cells would require physical contact between host T cells and graft resident APCs. The passenger leucocyte theory stated that this occurs after leucocytes migrate from the graft to the recipient's lymphoid organs, where they engage the recipient's T cells. However, the number of passenger leucocytes found in recipient lymphoid organs after transplantation has been shown to be minimal, yet the number of host APCs cross-dressed by donor exosomes is greater by several orders of magnitude.32 It is now clear that, in vivo, the direct pathway of recognition plays a minor role compared to the semi-direct pathway, which is responsible for initiating allograft-specific immune responses.32,33,57
These studies were performed primarily in skin, heart and pancreatic islet transplant models, that is models where the graft is potently rejected. In this context, donor exosomes participate in priming inflammatory responses towards the graft.29,30 Nevertheless, in other transplant situations, donor exosomes can be tolerogenic. In a mouse liver transplant model, which is tolerated without therapeutic immune suppression, recipient DCs become cross-dressed with donor MHC; however, acquisition of donor exosomes by DCs results in high levels of expression of PD-L1, resulting in allo-MHC presentation in a tolerogenic fashion.58
Another interesting case where exosomes induce tolerance is when the graft alloantigens are NIMAs. This finding ultimately stemmed from Ray Owen's observation in 1954 that Rh-negative daughters of Rh-positive women are less likely to develop Rh antibodies during their pregnancies than Rh-negative daughters of Rh-negative women.59 Thus, pre-and perinatal exposure to antigens present in the mother but absent in the offspring affords tolerance to them throughout life. The underlying mechanisms were unclear until the finding was made that maternal microchimerism—this is, a miniscule population of immune cells transferred from mother to offspring during pregnancy and breastfeeding—is responsible for induction and maintenance of tolerance to NIMAs.60-63 The immunological consequences of this phenomenon translate to better graft outcomes, if the alloantigens are NIMAs.64-68 This begs the question, how is it possible for such an exceedingly small population of maternal microchimeric cells to influence the immune system globally? The answer came with the realization that these cells are potent exosome producers, and thereby cross-dress host DCs, upregulate their endogenous PD-L1 expression (potentially via miRNA transfer) and induce NIMA-specific T cell clonal anergy.51 In conclusion, maternal microchimeric cells educate the offspring's immune system to tolerate NIMAs via exosomes.
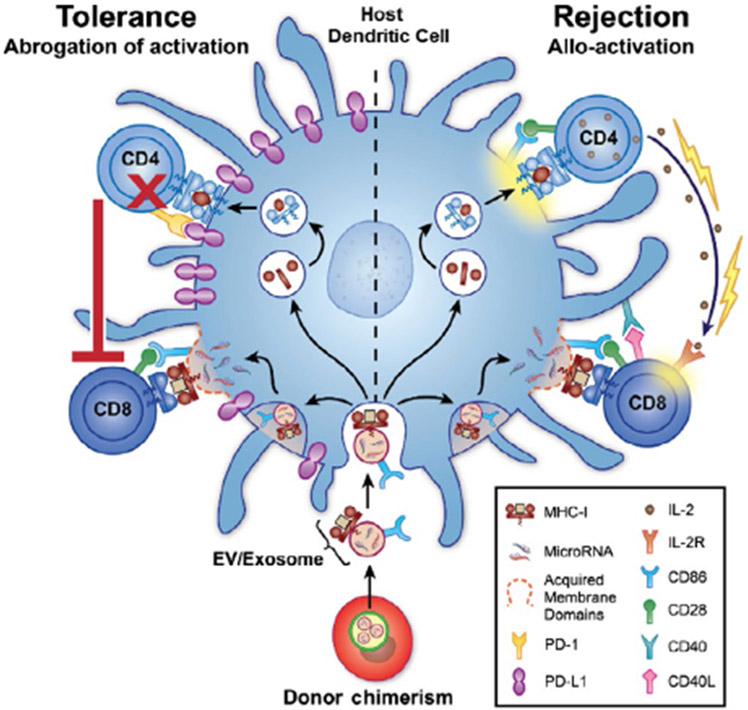
This situation is analogous to that of chimerism-induced allotolerance in transplantation. Chimerism, or the persistence of hematopoietic donor cells in the host, has immunological consequences that include tolerance to donor antigens, another observation that was originally made by Owen in freemartin cows (which share vascular anastomoses during gestation that allow for leucocyte and hematopoietic cell exchange between fraternal twins).63 Starzl's pioneering work described the establishment of donor chimerism from passenger leucocytes after organ transplantation and the beneficial outcomes of this phenomenon.69 Ever since, numerous efforts have been underway to artificially establish donor chimerism in transplant recipients in order to induce organ tolerance without immunosuppressive drugs, including hematopoietic stem cell transplantation concurrently with the solid-organ transplant.70 As with the NIMA paradox, it has been intriguing how donor chimerism could modify allo-specific responses, and again exosomes have been found to be key players (Figure 1). In a rejection situation (Figure 1B), donor passenger DCs release exosomes that cross-dress recipient DCs, resulting in CD8 T cell activation through the semi-direct pathway. At the same time, MHC transported by these exosomes is digested and presented in self-MHC-II to indirect CD4 T cells, which then provide cytokine help to directly primed CD8 T cells. Importantly, these exosomes carry the co-stimulatory molecule CD86, which stimulates T cells primed through both pathways of presentation. On the other hand (Figure 1A), certain exosomes (for example, maternal DC-, or liver transplant-derived) carry tolerogenic miRNAs that upregulate PD-L1 expression. Even though allo-MHC cross-dressing occurs in the presence of exosomal CD86 (which co-localizes with allo-MHC because they are acquired through the same exosome), PD-L1 induced by exosomal nucleic acid, and expressed elsewhere on the host DC surface, produces anergy of indirect pathway CD4 T cells, which then fail to help direct pathway CD8 T cells. This results in abrogation of activation and ultimately tolerance to donor antigens.51
FIGURE 1.
Donor chimerism exosomes cross-dress host dendritic cells and determine allo-activation and rejection (right) or T helper inactivation and tolerance (left) to donor MHC
6 ∣. EXOSOMES IN TUMOUR IMMUNE EVASION
Like many other biological processes, malignant cells hijack exosomes to exploit intercellular communication mechanisms that modify the tumour microenvironment in order to escape the immune system and permit tumour progression. Tumour cells produce vast amounts of exosomes that are internalized by target cells.71,72
Tumour-derived exosomes (TEx) affect the immune response at virtually every step and every cell population, from antigen presentation to effector cell function. Chronic lymphocytic leukaemia TEx can upregulate endogenous PD-L1 expression in monocytes and macrophages by transfer of non-coding Y RNAs,73 while TEx from breast, lung and skin cancer carry functional PD-L1 molecules on their surface.74 TEx can also prevent DC maturation75 and their miRNA cargo can inhibit DC cross-presenting capabilities to tumour-specific CTLs,76 thus inducing APC dysfunction.
TEx inhibit effector T cells at various levels. They carry the ectoenzymes CD39 and CD73 on their surface, which catalyse the stepwise conversion of ATP to adenosine, a potent T cell inhibitor.77,78 Head and neck cancer TEx modify transcription of a wide array of genes in conventional CD4 and CD8 T cells as well as Tregs, including PD1, FOXP3 and CTLA4, among others, which promote T cell dysfunction and exhaustion.79,80 Melanoma TEx also directly induce apoptosis of effector T cells by miRNA-associated downregulation of anti-apoptosis genes81 as well as bioactive FasL delivery.82
Tregs are abundant in the tumour microenvironment and are key contributors to local immunosuppression.83 Tumour cells release exosomes that interact with surface receptors in CD4 T cells to induce and maintain Treg populations in a process that involves exosomic TGF-β.35,84,85 Tumour-associated macrophage exosomes also induce the Treg phenotype, but this is instead due to transfer of miRNAs that target the STAT3 pathway.86
TEx from head and neck cancers induce a CD27−/CD28− suppressive phenotype in CD8 T cells.87 Interestingly, other suppressive CD8 T cell populations are induced by hepatocellular carcinomas and prostate tumours, including exhausted (PD1+/TIM3+) and inhibitory (CTLA4+) cells which contribute to the suppressive tumour microenvironment by exosome/IL-35 production.88,89 Thus, atypical suppressive CD8 T cell populations contribute to tumour immune escape by secretion of immunoregulatory exosomes.
In conclusion, TEx subvert anti-tumour immune responses at the antigen presentation and effector function level, as well as inducing both typical and atypical immune-suppressing cells. Hence, TEx deter effective tumour immunity at every step, making them, and their cell-specific effects, attractive targets for tumour immune therapies.
7 ∣. CONCLUSION
The effects of exosomes in the immune system are pleiotropic. Virtually every immune cell produces and releases exosomes with cargo that is dependent on molecular expression by the parental cell and also subcellular sorting mechanisms that redirect and load specific proteins and genetic material into exosomes for secretion. This greatly amplifies any given cell's influence by permitting it to modify a great number of target cells. Exosomes not only transport molecules, including antigens, but also can fundamentally reprogram the recipient cell, which dictates the context in which the antigen will be encountered by other immune cell populations. Thus, exosomes are particularly suited to skew the direction of immune responses. This has been shown to be especially true in transplantation and tumour immunity.
The field of exosome biology, especially in immunology, is growing at an accelerated pace. There is much enthusiasm to study this vehicle of intercellular communication, and new roles are ascribed to exosomes continuously, including cytokine stabilization and transport. Deeper knowledge of leucocyte exosomes and their effects are sure to translate into therapeutic and diagnostic applications in the future, including tolerance-inducing protocols, vaccine delivery vehicles and liquid biopsies.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rashed M H, Bayraktar E K Helal G, et al. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int J Mol Sci. 2017;18:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Wang Q, Wei X, et al. Global scientific trends on exosome research during 2007–2016: a bibliometric analysis. Oncotarget. 2017;8:48460–48470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meldolesi J Exosomes and ectosomes in intercellular communication. Curr Biol. 2018;28:R435–R444. [DOI] [PubMed] [Google Scholar]
- 4.Juan T, Furthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018;74:66–77. [DOI] [PubMed] [Google Scholar]
- 5.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. [DOI] [PubMed] [Google Scholar]
- 6.Levy S Function of the tetraspanin molecule CD81 in B and T cells. Immunol Res. 2014;58:179–185. [DOI] [PubMed] [Google Scholar]
- 7.Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of Exosome Composition. Cell. 2019; 177(2):428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nolte-‘t Hoen E, Cremer T, Gallo RC, Margolis LB. Extracellular vesicles and viruses: Are they close relatives? Proc Natl Acad Sci. 2016;113:9155–9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen DG, Booth A, Gould SJ, Hildreth J. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem. 2003;278:52347–52354. [DOI] [PubMed] [Google Scholar]
- 10.Izquierdo-Useros N, Puertas MC, Borràs FE, Blanco J, Martinez-Picado J. Exosomes and retroviruses: the chicken or the egg? Cell Microbiol. 2011;13:10–17. [DOI] [PubMed] [Google Scholar]
- 11.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanchard N, Lankar D, Faure F, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. [DOI] [PubMed] [Google Scholar]
- 13.Martínez-Lorenzo MJ, Anel A, Gamen S, et al. Activated human T cells release bioactive fas ligand and APO2 ligand in microvesicles. J Immunol. 1999;163:1274–1281. [PubMed] [Google Scholar]
- 14.Monleón I, Martínez-Lorenzo MJ, Monteagudo L, et al. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167:6736–6744. [DOI] [PubMed] [Google Scholar]
- 15.Hong X, Schouest B, Xu H. Effects of exosome on the activation of CD4+ T cells in rhesus macaques: a potential application for HIV latency reactivation. Sci Rep. 2017;7:15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Jay SM, Wang Y, Wu S-W, Xiao Z. IL-12 stimulates CTLs to secrete exosomes capable of activating bystander CD8+ T cells. Sci Rep. 2017;7:13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tkach M, Kowal J, Zucchetti AE, et al. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017;36:3012–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okoye I, Coomes S, Pelly V, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tung SL, Boardman DA, Sen M, et al. Regulatory T cell-derived extracellular vesicles modify dendritic cell function. Sci Rep. 2018;8:6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naqvi AR, Fordham JB, Ganesh B, Nares S. miR-24, miR-30b and miR-142-3p interfere with antigen processing and presentation by primary macrophages and dendritic cells. Sci Rep. 2016;6:32925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Self-Fordham JB, Naqvi AR, Uttamani JR, Kulkarni V, Nares S. MicroRNA: Dynamic regulators of macrophage polarization and plasticity. Front Immunol. 2017;8:1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naqvi AR, Fordham JB, Nares S. miR-24, miR-30b, and miR-142-3p regulate phagocytosis in myeloid inflammatory cells. J Immunol. 2015;194:1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smyth LA, Ratnasothy K, Tsang JY, et al. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43:2430–2440. [DOI] [PubMed] [Google Scholar]
- 24.Devergne O, Birkenbach M, Kieff E: Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci. 1997;94:12041–12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devergne O, Hummel M, Koeppen H, et al. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niedbala W, Wei X-Q, Cai B, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. [DOI] [PubMed] [Google Scholar]
- 27.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566. [DOI] [PubMed] [Google Scholar]
- 28.Turnis ME, Sawant DV, Szymczak-Workman AL, et al. Interleukin-35 Limits Anti-Tumor Immunity. Immunity. 2016;44:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collison LW, Chaturvedi V, Henderson AL, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan JA, Tomita Y, Jankowska-Gan E et al. Treg-dependent, lymphocyte-derived, IL35-coated extracellular vesicles promote infectious toleran. Cell Rep. 2019. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson BM, Sullivan JA, Burlingham WJ. Interleukin 35: a key mediator of suppression and the propagation of infectious tolerance. Front Immunol. 2013;4:315–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, et al. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol. 2016;1:aaf8759–aaf8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Rojas-Canales DM, Divito SJ, et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest. 2016;126:2805–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G-J, Liu Y, Qin A, et al. Thymus exosomes-like particles induce regulatory T cells. J Immunol. 2008;181:5242–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada J, Onishi H, Suzuki H, et al. Surface-bound TGF-beta1 on effusion-derived exosomes participates in maintenance of number and suppressive function of regulatory T-cells in malignant effusions. Anticancer Res. 2010;30:3747–3757. [PubMed] [Google Scholar]
- 36.Zhang Q, Fu L, Liang Y, et al. Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. J Cell Physiol. 2018;233:6832–6840. [DOI] [PubMed] [Google Scholar]
- 37.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunderson SC, McLellan AD. Role of lymphocyte subsets in the immune response to primary B cell-derived exosomes. J Immunol. 2017;199:2225–2235. [DOI] [PubMed] [Google Scholar]
- 39.Saunderson SC, Schuberth PC, Dunn AC, et al. Induction of exosome release in primary B cells stimulated via CD40 and the IL-4 receptor. J Immunol. 2008;180:8146–8152. [DOI] [PubMed] [Google Scholar]
- 40.Buschow SI, van Balkom B, Aalberts M, Heck A, Wauben M, Stoorvogel W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. 2010;88:851–856. [DOI] [PubMed] [Google Scholar]
- 41.Admyre C, Bohle B, Johansson SM, et al. B cell–derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120:1418–1424. [DOI] [PubMed] [Google Scholar]
- 42.Lundy SK, Klinker MW, Fox DA. Killer B. lymphocytes and their Fas ligand positive exosomes as inducers of immune tolerance. Front Immunol. 2015;6:122 10.3389/fimmu.2015.00122. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klinker MW, Lizzio V, Reed TJ, Fox DA, Lundy SK. Human B cell-derived lymphoblastoid cell lines constitutively produce Fas ligand and secrete MHCII+FasL+ killer exosomes. Front Immunol. 2014;5:144 10.3389/fimmu.2014.00144. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen P, Roch T, Lampropoulou V, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tedder TF. B10 Cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194:1395–1401. [DOI] [PubMed] [Google Scholar]
- 46.Kaku H, Cheng KF, Al-Abed Y, Rothstein TL. A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J Immunol. 2014;193:5904–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denzer K, Kleijmeer MJ, Heijnen HF, et al. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. [DOI] [PubMed] [Google Scholar]
- 48.Leone DA, Peschel A, Brown M, et al. Surface LAMP-2 is an endocytic receptor that diverts antigen internalized by human dendritic cells into highly immunogenic exosomes. J Immunol. 2017;199:531–546. [DOI] [PubMed] [Google Scholar]
- 49.Dolan BP, Gibbs KD Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–6024. [DOI] [PubMed] [Google Scholar]
- 50.Montecalvo A, Shufesky WJ, Beer Stolz D, et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008;180:3081–3090. [DOI] [PubMed] [Google Scholar]
- 51.Bracamonte-Baran W, Florentin J, Zhou Y, et al. Modification of host dendritic cells by microchimerism-derived extracellular vesicles generates split tolerance. Proc Natl Acad Sci U S A. 2017;114:1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ono Y, Perez-Gutierrez A, Nakao T, et al. Graft-infiltrating PD-L1(hi) cross-dressed dendritic cells regulate anti-donor T cell responses in mouse liver transplant tolerance. Hepatology. 2018; 67(4):1499–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hornick P, Lechler R. Direct and indirect pathways of alloantigen recognition: relevance to acute and chronic allograft rejection. Nephrol Dial Transplant. 1997;12:1806–1810. [DOI] [PubMed] [Google Scholar]
- 54.Direct Benichou G. and indirect antigen recognition: the pathways to allograft immune rejection. Front Biosci. 1999;4:D476–D480. [DOI] [PubMed] [Google Scholar]
- 55.Bracamonte-Baran W, Burlingham W. Non-inherited maternal antigens, pregnancy, and allotolerance. Biomed J. 2015;38:39–51. [DOI] [PubMed] [Google Scholar]
- 56.Herrera OB, Golshayan D, Tibbott R, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828–4837. [DOI] [PubMed] [Google Scholar]
- 57.Morelli AE, Bracamonte-Baran W, Burlingham WJ. Donor-derived exosomes: the trick behind the semidirect pathway of allorecognition. Curr Opin Organ Transplant. 2017;22:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ono Y, Perez-Gutierrez A, Nakao T, et al. Graft-infiltrating PD-L1hi cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance. Hepatology. 2018;67:1499–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owen RD, Wood HR, Foord AG, Sturgeon P, Baldwin LG. Evidence for actively acquired tolerance to Rh antigens. Proc Natl Acad Sci USA. 1954;40:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dutta P, Burlingham WJ. Correlation between post transplant maternal microchimerism and tolerance across MHC barriers in mice. Chimerism. 2011;2:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dutta P, Molitor-Dart M, Bobadilla JL, et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood. 2009;114:3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kinder J, Jiang T, Ertelt J, et al. Cross-Generational Reproductive Fitness Enforced by Microchimeric Maternal Cells. Cell. 2015;162:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. [DOI] [PubMed] [Google Scholar]
- 64.Burlingham WJ. Clinical implications of basic science discoveries: microchimerism finds a major role in reproductive success; but does it also contribute to transplant success? Am J Transplant. 2016;16:2795–2799. [DOI] [PubMed] [Google Scholar]
- 65.Burlingham WJ, Grailer AP, Fechner JH Jr, et al. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 1995;59:1147–1155. [PubMed] [Google Scholar]
- 66.Cai J, Lee J, Jankowska-Gan E, et al. Minor H antigen HA-1-specific regulator and effector CD8+ T cells, and HA-1 microchimerism, in allograft tolerance. J Exp Med. 2004;199:1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dutta P, Dart M, Roenneburg DA, Torrealba JR, Burlingham WJ. Pretransplant immune-regulation predicts allograft tolerance. Am J Transplant. 2011;11:1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molitor-Dart ML, Andrassy J, Kwun J, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–6761. [DOI] [PubMed] [Google Scholar]
- 69.Starzl TE, Demetris AJ, Murase N, et al. Chimerism after organ transplantation. Curr Opin Nephrol Hypertens. 1997;6:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strober S Stable mixed chimerism and tolerance to human organ transplants. Chimerism. 2016;6:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Christianson HC, Svensson KJ, van Kuppevelt TH, Li J-P, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci. 2013;110:17380–17385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Messenger SW, Woo SS, Sun Z, et al. A Ca2+-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J Cell Biol. 2018;217:2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haderk F, Schulz R, Iskar M, et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci Immunol. 2017;2(13). eaah5509 10.1126/sciimmunol.aah5509 [DOI] [PubMed] [Google Scholar]
- 74.Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ning Y, Shen K, Wu Q, et al. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol Lett. 2018;199:36–43. [DOI] [PubMed] [Google Scholar]
- 76.Kikete S, Chu X, Wang LI, Bian Y. Endogenous and tumour-derived microRNAs regulate cross-presentation in dendritic cells and consequently cytotoxic T cell function. Cytotechnology. 2016;68:2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–683. [DOI] [PubMed] [Google Scholar]
- 78.Smyth LA, Ratnasothy K, Tsang J, et al. CD73 expression on extracellular vesicles derived from CD4+CD25+Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43:2430–2440. [DOI] [PubMed] [Google Scholar]
- 79.Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016;6:20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye S-B, Li Z-L, Luo D-H, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou JI, Yang YI, Wang WenWen, et al. Melanoma-released exosomes directly activate the mitochondrial apoptotic pathway of CD4+ T cells through their microRNA cargo. Exp Cell Res. 2018;371:364–371. [DOI] [PubMed] [Google Scholar]
- 82.Andreola G, Rivoltini L, Castelli C, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Overacre-Delgoffe AE, Vignali D. Treg Fragility: A Prerequisite for Effective Antitumor Immunity? Cancer Immunol Res. 2018;6:882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-Derived Microvesicles Induce, Expand and Up-Regulate Biological Activities of Human Regulatory T Cells (Treg). PLoS ONE. 2010;5:e11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muller L, Simms P, Hong C-S, et al. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology. 2017;6:e1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou J, Li X, Wu X, et al. Exosomes released from tumor-associated macrophages transfer miRNAs that Induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res. 2018;6:1578–1592. [DOI] [PubMed] [Google Scholar]
- 87.Maybruck BT, Pfannenstiel LW, Diaz-Montero M, Gastman BR. Tumor-derived exosomes induce CD8+ T cell suppressors. J Immunotherapy Cancer. 2017;5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Shen H, He Q, Tian W, Xia A, Lu X-J. Exosomes derived from exhausted CD8+ T cells impaired the anticancer function of normal CD8+ T cells. J Med Genet. 2019;56:29–31. [DOI] [PubMed] [Google Scholar]
- 89.Olson BM, Jankowska-Gan E, Becker JT, et al. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J Immunol. 2012;189:5590–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]