Abstract
Background and Purpose
Blood pressure (BP) variability may increase the risk of stroke and dementia. It remains inconclusive whether BP variability is associated with cerebral small vessel disease (CSVD), a common and potentially devastating subclinical disease that contributes significantly to both stroke and dementia.
Methods
A systematic review and meta-analysis of prospective cohort studies that examined the association between BP variability and the presence or progression of established markers of CSVD, including white matter hyperintensities, lacunes and microbleeds on magnetic resonance imaging. We searched MEDLINE, EMBASE and Web of Science. Ten studies met the criteria for qualitative synthesis and seven could be included in the meta-analysis. Data were synthetized using random-effect models.
Results
These studies included a total of 2,796 individuals aged 74 (mean) ±4 (SD) years, with a median follow-up of 4.0 years. A one standard-deviation increase in systolic BP variability was associated with increased odds of the presence or progression of white matter hyperintensities (odds ratio [OR]: 1.26; 95% CI: 1.06–1.50). The association of systolic BP variability with the presence of lacunes (OR: 0.93; 95%CI: 0.74–1.16) and the presence of microbleeds (OR: 1.13; 95%CI: 0.89–1.44) were not statistically significant.
Conclusions
A larger BP variability may be associated with a higher risk of having a higher burden of white matter hyperintensities. Targeting large BP variability has the potential to prevent CSVD and thereby reducing the risk of stroke and dementia. The potential issue of reverse causation and the heterogeneity in the assessment of CSVD markers should be better addressed in future studies.
Keywords: blood pressure, blood pressure variability, cerebral small vessel disease, magnetic resonance imaging, systematic review, meta-analysis, cohort studies
Hypertension is an important contributor to cardiovascular disease and mortality globally.1, 2 Increasing evidence also links large BP variability over hours, days, months and even years, to a higher risk of heart disease, stroke and dementia, beyond the effect of BP level per se.3–7 Excessive fluctuation in BP is suggested to be particularly harmful for high flow organs such as the brain.8, 9 This hypothesis concurs with the evidence that the association with BP variability appears stronger for stroke than for heart disease,5 and that BP variability may increase the risk of cognitive decline and dementia.6, 7, 10 It remains largely unknown whether BP variability is associated with subclinical cerebral vascular injuries that may have taken place many years preceding stroke and dementia.
Cerebral small vessel disease (CSVD), in particular, is a group of common and potentially devastating subclinical conditions that contribute significantly to both symptomatic and silent strokes, as well as cognitive impairment and dementia.11, 12 The cumulative damage to small vessels could be crucial in a cascade of events linking BP variability to stroke and dementia. With the development of sensitive non-invasive imaging techniques to detect CSVD in vivo, Identifying the association of BP variability with imaging markers of CSVD, including white matter hyperintensities (WMH), lacunar infarcts and microbleeds, could offer important clinical insights into the early-stage etiology, and thus the prevention of stroke and dementia.13, 14,15 A few prospective cohort studies have examined the association of BP variability with CSVD markers, but these studies are largely limited by small sample sizes and have reported mixed results.10, 16–29 To evaluate the current evidence and its methodological strengths and limitations, we systematically reviewed the literature on the association of BP variability with the presence and progression of CSVD in population-based prospective studies.
Methods
The authors declare that all supporting data are available within the article and the online-only Data Supplement. This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).30
Literature Search and Study Eligibility
We searched Medline (Ovid), Embase and Web of Science up to August 01, 2018, for full-text articles in English, using designed search strategies provided in Supplemental Table I. We included population-based prospective cohort studies in adults (irrespective of hypertension status) that focused on the association of BP variability with the presence or progression of imaging markers of CSVD. Only community-based cohort studies were included. The following studies were excluded: studies among institutionalized individuals and hospital-based patients; studies recruiting patients with pre-existing major neurological diseases at baseline, including dementia, stroke, multiple sclerosis and Parkinson’s disease; studies with median or mean follow-up of less than 12 months. We did not exclude studies conducted in a general population sample that may have a small proportion of participants with major neurological diseases at baseline in order to ensure the representativeness. We performed an additional analysis excluding the two studies with a small proportion of dementia or stroke cases at baseline.
Included studies examined BP variability over time, including hour-to-hour variability derived from ambulatory BP measurement, day-to-day variability usually derived from BP measured at home, and visit-to-visit variability in BP spanning months or years generally measured in cohort settings. These measures were selected because of their highlighted clinical relevance as strong risk indicators for cardiovascular disease and mortality.5 Studies quantified variability in several ways, and if multiple metrics were used to assess BP variability in the same study, metrics were considered according to the following order: coefficient of variation (CV), standard deviation (SD), variation independent of mean, average real variability and residual variability. Coefficient of variation and standard deviation were preferred because these are the most commonly used measures, and because the coefficient of variation assesses the variability after accounting for the average level of BP. We primarily assessed variability in systolic BP because of its stronger association with adverse health outcomes.31 We also reported findings on diastolic BP variability and pulse pressure variability where available.
Studies that examined at least one of the following three established MRI markers of CSVD were included: the burden or volume of WMH, the presence of cerebral microbleeds and lacunes.12, 15, 32 Studies that examined the combined components of any of the above were assessed as a separate outcome.
Data Extraction
A pre-specified data extraction form was used by two independent reviewers (YM/AS) to obtain information on characteristics of the study population (sample size, age, sex, comorbidity at baseline, use of antihypertensive medication, and follow-up duration); exposure assessment (BP measurement, time intervals, calculation methods); outcome assessment (imaging characteristics, definitions of CSVD features); covariate adjustment; and association estimates. Association estimates (hazard ratio, risk ratio and odds ratio for categorical outcomes, and regression coefficient for continuous outcomes) were extracted for each type of exposure measurement and the fully adjusted effect estimates were used for quantitative synthesis, unless there was adjustment for intermediates. The relevant missing information was requested by emailing the corresponding authors. Two independent reviewers (YM/AS) assessed all the titles and abstracts. In case of disagreement, the opinion of a third reviewer (SP) was considered.
Quality Assessment
Risk of bias in study design was assessed by two independent reviewers (YM/AS) using a modified version of the Newcastle Ottawa Scale (NOS), the recommended scale for observational cohort design.33 This modified NOS scale is provided in the Supplement. Specifically, study qualities were assessed from three domains, i.e. selection, comparability, and outcome. A study can be awarded a maximum of one point for each of the three items within the selection and outcome categories, and a maximum of two points for comparability, with a total score of eight points indicating high quality. Any discrepancies were addressed by a joint revaluation of the original article by a third reviewer (SP).
Statistical Analysis
Our primary analysis quantified the association between BP variability and CSVD using random-effect models. Between-study heterogeneity was assessed by I2. Given that several studies reported results on more than one individual markers of CSVD, multi-level linear models were used to allow for the dependence of related outcomes within the same study.34 To allow quantitative synthesis across studies that reported different measures of association, several calculations were performed to obtain the odds ratio of the outcome associated with per standard-deviation increment in BP variability. Specifically, for associations reported by quantiles of BP variability, the estimates were converted to the association with 1-SD increase in BP variability, and it was estimated as 1/2.80 times the log odds ratio for the comparison of the top and bottom quintiles,1/2.54 times the log odds ratio for the comparison of the top and bottom quartiles, or 1/2.18 times for the log odds ratio for the comparison for top and bottom tertiles.35 For studies that assessed the outcome as a continuous variable, which is the case for WMH volume, standardized mean difference and their corresponding SEs were converted to OR per SD increment in BP variability using the approach described by Chinn.36 In studies that provided a continuous volumetric measure for WMH as an outcome, we transformed it to an odds ratio using a standardized formula. 36 This odds ratio is interpreted as the change in the odds of one-SD increase in WMH volume per one-SD increase in BP variability. For one study that reported risk ratio, risk ratio was used as an approximate estimate of risk ratio.26 We also performed the same calculations for diastolic BP variability separately.
We further performed subgroup analyses and meta regression for WMH, the most commonly used marker of CSVD in the included studies. Analyses were stratified by 1) time scales of BP variability measurements (short-term variability over hours or days versus visit-to-visit BP variability), 2) metrics of BP variability (CV, SD and residual variability), 3) length of follow-up (<=5 versus >5 years), 4) risk interval (presence at a single visit and the progression over two scans), 5) score on study quality (NOS scale<=6 versus >6) and quantitative methods (visual rating scale versus volumetric measure). Meta-regression was used to examine the effect of the following factors (defined a priori) on the association between BP variability and CSVD: baseline age, sex, antihypertensive medication and geographical area of the study. Publication bias was intended to be assessed by funnel plots and Egger’s test if more than ten studies were included.
All effect estimates were reported using 95% confidence intervals. All p-values presented are two sided, with a p-value of 0.05 or less considered statistically significant. Statistical analyses were performed using R version 3.4.2 (R Foundation).
Results
Characteristics of the included studies
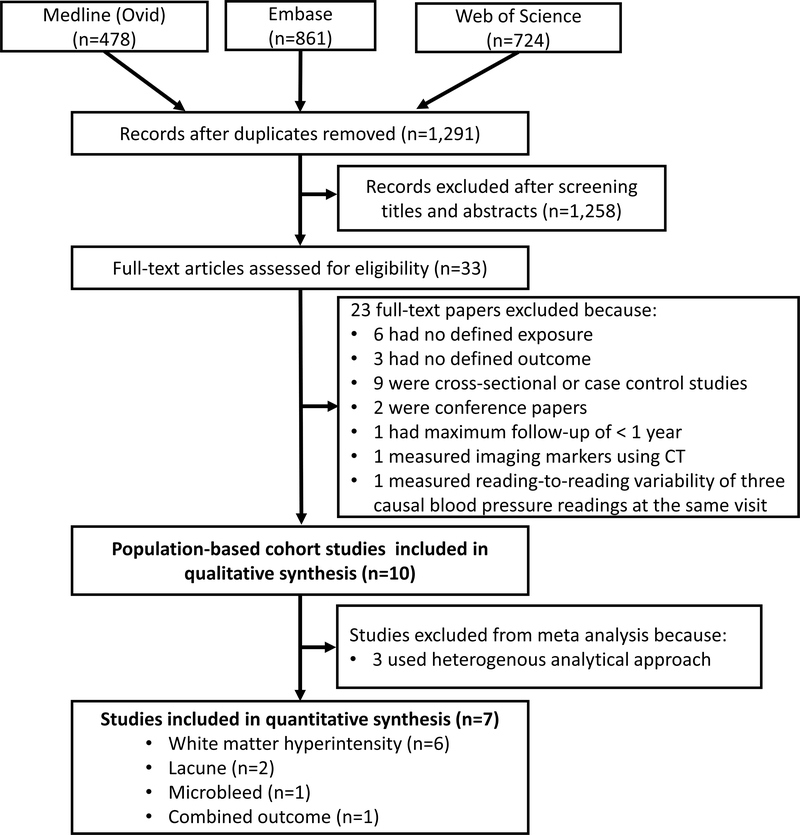
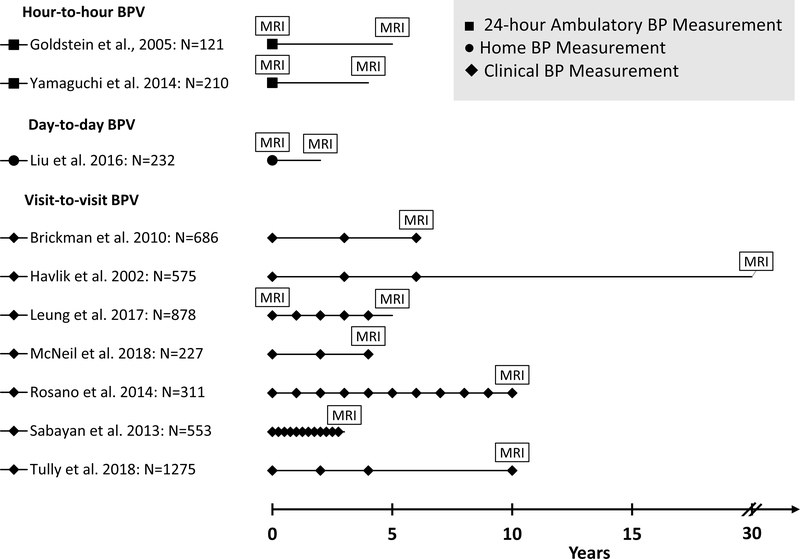
Figure 1 is the study screening and selection flowchart. The search identified 1,291 items, from which 1,258 were excluded after review of the title and abstract. Of the remaining 33 articles that were reviewed in full text, 23 articles were excluded because of the lack of defined exposure or outcome, using cross-sectional or case-control designs (not prospective), conference abstracts, or having less than one year’s follow-up. Ultimately, we included 10 independent population-based prospective cohort studies in our qualitative synthesis. Table 1 summarizes the characteristics of the included studies. Seven studies used similar analytical methods so they could be included in the meta-analysis, reporting on a total of 2,796 participants with a mean age of 74 years. The median follow-up was 4 (range: 2.3–20) years, contributing to 20,546 person-years in total. Figure 2 graphically represents the key design components of these studies, including timing of BP measurements and MRI scanning, and length of follow-up. Seven out of ten studies examined visit-to-visit BP variability and the other three examined day-to-day and hour-to-hour variability. Most studies assessed WMH (n=6) as the indicator for CSVD while studies on lacunes (n=2) and microbleeds (n=1) were limited. Only four studies assessed CSVD both at baseline and subsequent follow-up, and only three examined the progression of CSVD. Overall, the included studies were of reasonable quality; detailed quality assessments are provided in Supplemental Table II.
Figure 1.
PRISMA flow diagram of the study selection process
Table 1.
Study Characteristics
| Study | Location | Age at baseline (years) † | Women (%) | Median follow-up (years) | BP monitor | BP variability‡ | Anti-hypertensive medication (%) | MRI profile | Cerebral small vessel disease | Covariate Adjustments |
|---|---|---|---|---|---|---|---|---|---|---|
| Goldstein et al, 2005 (n=121)16 | USA | 66.2 ± 6.0 (55–79) | 57 | 5 | Ambulatory BP monitor | hour-to-hour (SD) | 0¶ | 1.5T MRI: T2 | WMH: dichotomized by upper 25th percentile of WMH volume (in % of total intracranial volume). | Age |
| Yamaguchi et al, 2014 (n=210)19 | Japan | 70.9 ± 0.9 (70–72) | 55 | 4 | Ambulatory BP monitor | hour-to-hour (SD) | 41** | 0.5T MRI: T1-weighted, T2-weighted, FLAIR | CSVD progression over four years: any WMLs or lacunae on the second MRI that were not visible on the first MRI. WMH was assessed by the Fazekas scale; The presence (yes/no) of lacune was defined as the lesions with a 3- to 15-mm diameter. | Age, sex, BP, hypertension, hyperlipidemia, diabetes, smoking, alcohol drinking |
| Liu et al, 2016 (n=232)20 | China | 84.4 ± 2.5 (≥ 80) | 75 | 2.3 | Home BP monitor | day-to-day (CV) | 22 | 3T MRI: T1-weighted, T2-weighted, FLAIR | Change in WMH volume over 2.3 years (in % of intracranial volume) | Age, sex, BMI, BP, education, smoking, lipid, glucose, alcohol drinking and baseline WMH |
| Brickman et al. 2010 (n=686)28‡ | USA | 80.0 ± 5.5 (≥ 65) | 68 | 6 | Dinamap Pro 100 | visit-to-visit (SD) | 66 | 1.5T MRI: T1-weighted, T2-weighted FLAIR | WMH volume (in % of intracranial volume) | Age, sex, treatment status |
| Havlik et al, 2002 (n=575)24 | USA | 61.6 ± 5.0 (45–64)∥ | 0 | 30 | Standard mercury sphygmomanometer | visit-to-visit (residual variability) | 23 | 1.5T MRI: T1-weighted, T2-weighted | WMH (visual rating scale based on Cardiovascular Health Study criteria) | Age, education, APOE genotype, stroke, and dementia |
| Leung et al. 2017 (n=878)26 | USA | 67.5 ± 4.2 (≥ 65) ∥ | 64 | 6 | Standard mercury sphygmomanometer | visit-to-visit (SD of residuals derived from linear-regression) | 27 | 1.5T MRI: T1-weighted, T2-weighted | New WMH was assessed using visual rating scale according to the Cardiovascular Health Study protocol. The presence (yes/no) of incident lacune was defined as lesions with 3mm or larger in diameter in any direction with specific characteristics. | Age, sex, race, clinic site, smoking, BMI, diabetes, MRI scan interval, antihypertensive medication use |
| McNeil et al. 2018 (n=227)29 | Scotland | 64.5 ± 0.8 (64) | 52 | 4 | Automatic electronic BP monitor | visit-to-visit (CV) | 45 | 1.5T MRI: T2, FLAIR | WMH (Schelten’s scale)37 | Age, sex, systolic and diastolic BP |
| Rosano et al. 2014 (n=311)27§ | USA | 82.9 ± 2.8 (70–79) | 58 | 10 | Standard mercury sphygmomanometer | visit-to-visit (CV) | 62 | 3T MRI: T1-weighted, T2-weighted, FLAIR | WMH volume (in % of intracranial volume) | None |
| Sabayan et al. 2013 (n=553)10 | Ireland, Scotland, Netherlands | 74.9 ± 3.2 (>70) | 44 | 3.2 | Automatic electronic sphygmomanometer | visit-to-visit (SD) | NR | 1.5T MRI: T2-weighted, FLAIR | WMH volume, presence (yes/no) of lacunes, and presence (yes/no) of microbleeds as separate outcomes. | Age, sex, BMI, statin use, smoking, cholesterol, vascular disease, hypertension, diabetes, BP |
| Tully et al. 2018 (n=1,275)22§ | France | 72 (≥65) | 64 | 10 | Automatic electronic sphygmomanometer | visit-to-visit (CV) | 46 | 1.5T MRI: T1-weighted, T2-weighted | WMH volume (normalized by white matter volume) | N/A |
BMI=body mass index, WMH=white matter hyperintensities. MRI= Magnetic resonance imaging.
Mean ± standard deviation (range if available).
SD=standard deviation, CV=coefficient of variation. Residual variability represented the variance of residual from linear regression of SBP on age.
Different analytical approaches.
Estimated age at baseline.
Participants were free from hypertension at baseline.
The proportion of antihypertensive medication use by classes: calcium channel blocker:26.7%; angiotensin receptor blocker:13.3%; angiotensin converting enzyme inhibitor:6.7%; Diuretcs:5.2%; α-Blocker:2.4%; β-Blocker:5.7%; αβ-Blocker:1.9%; Others:3.7%.
NR=not reported.
Figure 2.
Key characteristics of the study design
BP variability and neuroimaging markers of CSVD
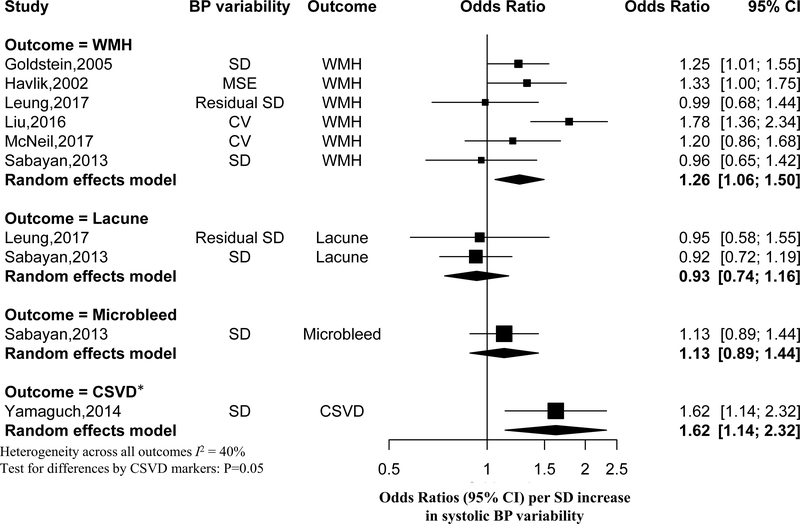
Figure 3 shows the association of systolic BP variability with individual and combined components of CSVD. Overall, the odds ratio of having a higher burden of WMH associated with each one-SD increase in systolic BP variability was 1.26 (95% CI: 1.06–1.50). The odds of having lacunes (OR: 0.93, 95%CI: 0.74–1.16) or having microbleeds (OR: 1.13, 95%CI: 0.89–1.44) did not increase significantly with each one-SD increase in systolic BP variability. The association of systolic BP variability with CSVD appeared to differ by WMH, lacunes and microbleeds (P=0.05). For diastolic BP variability, four out of seven studies reported findings on its association with CSVD markers, and the association was not statistically significant for WMH (OR: 0.96, 95%CI: 0.77–1.21) (Supplemental Figure I). Two studies examined pulse pressure variability but only one study reported detailed results.24, 26 Neither of these two studies identified a statistically significant association of pulse pressure variability with the presence or progression of cerebral small vessel disease.
Figure 3.
The association of systolic BP variability with imaging markers of cerebral small vessel disease
*Defined as new WMH or incident lacunes during the follow-up. SD=standard deviation; MSE=mean squared error (i.e. the variance of residual from linear regression of BP on age); CV=coefficient of variation; WMH=white matter hyperintensities; CSVD=cerebral small vessel disease.
Subgroup analyses and meta-regression
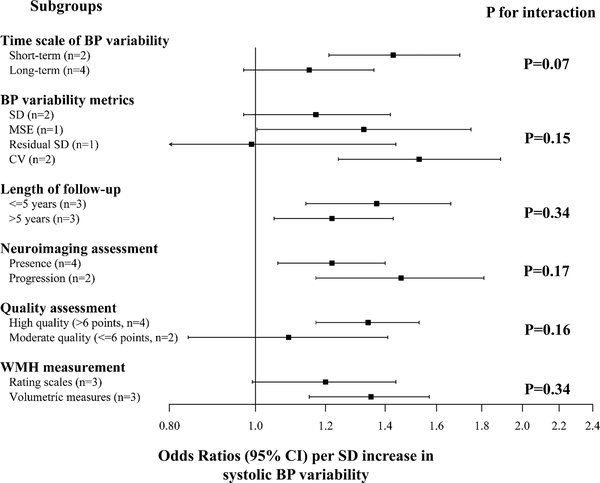
As shown in Figure 4, the subgroup analyses for WMH as the outcome shows that the associations did not differ significantly by time scale of BP variability, metrics of BP variability, length of follow-up, prevalent or incident outcomes. The strength of the association appeared stronger after restricting the analysis to studies with high quality (modified NOS scale >6) (OR:1.34, 95% CI:1.17–1.53) and for WMH assessed by volumetric measures (OR: 1.35, 95%CI: 1.15–1.57). Meta regression did not reveal significant effect modification attributable to age, sex, geographical region (USA, Europe, and Asia) or antihypertensive medication use (All P>0.05). We did not perform a formal detection of publication bias, because such analyses would be statistically underpowered given the small number of studies included (n=7). Our additional analysis excluding the two studies that had a mall proportion of participants with dementia or stroke at baseline showed similar results (Supplemental Figure II). Specifically, the odds ratio of having a higher burden of white matter hyperintensities associated with one-SD increase in systolic BP variability was 1.30 (95%CI: 1.03–1.64).
Figure 4.
The association of systolic BP variability with white matter hyperintensities in subgroup analyses
*SD=standard deviation; MSE=mean squared error (i.e. the variance of residual from linear regression); CV=coefficient of variation.
Discussion
We systematically reviewed the available evidence on the association of BP variability with the presence or progression of neuroimaging markers of CSVD in population-based prospective cohort studies. We found that large systolic BP variability was associated with having a higher burden of neuroimaging markers of CSVD, especially for WMH. The association did not differ significantly by the time scale of BP variability, but appeared to differ by CSVD markers, with limited evidence on lacunes and microbleeds.
Large BP variability is associated with a higher risk of stroke, cognitive impairment and dementia.3–5 The association of larger BP variability with higher burden of WMH was also observed after excluding studies with a small proportion of dementia and stroke cases at baseline. This suggestive relationship may offer an important insight into the early-stage etiology of stroke and dementia, given that these subclinical cerebral vascular injuries may occur many years preceding stroke and dementia. We discuss several possible explanations.12 First, large BP variability could increase pulsation of flow and dampen the smoothing of blood flow as it progresses to small arteries particularly in high-flow organs such as the brain, leading to the damage to brain microvasculature.8 The presence of arterial stiffness could further amplify the harmful effect of wider pressure fluctuations and contribute to WMH.38–40 Second, as suggested by animal studies, large BP variability could inhibit nitric oxide production and impair endothelial function, contributing to “neurovascular unit” injuries, blood-brain-barrier abnormality and thereby small vessel lesions.41–43 This process could be a vicious cycle, as impaired endothelium-dependent synthesis of vasodilating and vasoconstricting substances may affect the maintenance of a stable BP.44, 45 In line with these putative biological pathways, recent trials in patients with lacunar strokes suggest the promising therapeutic effects on small vessel disease progression of oral agents that improve blood-brain barrier integrity, vasodilation, reducing inflammation.46 Further studies that elucidate the potential etiological mechanisms underlying large blood pressure variability may help identify potential therapeutic targets to prevent or slow down CSVD. Alternatively, reverse causation is possible, if subclinical brain changes affect the central autonomic regulation to modulate BP, it could also lead to large BP variability.47
Several methodological issues are prevalent in these previous studies. First, the metrics, numbers and time intervals of BP measurements used to assess BP variability varied largely across studies and relevant data on diastolic BP variability and pulse pressure variability are lacking. Second, the assessment of neuroimaging markers of CSVD is also heterogenous. Most studies assessed WMH while evidence on lacunes and microbleeds is sparse. For the relation between BP variability and WMH, most studies assessed WMH using two approaches, i.e. visual rating scales (such as the Fazekas and Scheltens scales48) and semiautomated volumetric techniques. The association with BP variability appeared stronger for WMH measured by volumetric techniques, which concurs with the suggestive evidence that WMH volume could be more sensitive than rating scales in differentiating clinical symptoms of cognitive decline.49 Last but not least, potential reverse causation remains a major issue. Despite most studies excluded patients with pre-existing major neurological disease at baseline, baseline screening of CSVD was absent in most studies possibly because MRI scans were generally implemented at a later stage of an existing cohort. Such design blurred the temporal order of any observed association because it is possible that pre-existing subclinical vasculature pathology may affect central autonomic regulation, resulting in a large BP variability, and vice versa. The short follow-up particularly in studies on visit-to-visit BP variability further contributes to this issue. Studies that assessed the subsequent progression of CSVD had less potential for reverse causation, but there were few such studies.
Several limitations of our study should be noted. The small number of eligible studies does not allow for the detection of potential effect modifiers or publication bias. Given the nature of summarizing data from different studies, we are limited to the methods used to control confounding in original studies, so we cannot rule out the possibility of residual confounding. However, as noted in Table 1, most studies provide detailed control for confounding, therefore the potential for residual confounding is of no greater magnitude than an epidemiologic study of good quality. The summarized findings are also susceptible to potential reverse causation due to the limitations of included studies. Additionally, the methodological limitations in the included studies may also limit our ability to draw a definite conclusion on the relationship between BP variability and CSVD. Nevertheless, as the first of its kind to systematically review the available literature on BP variability and CSVD, this study offers important insights into the knowledge base and identifies critical knowledge gaps and methodological considerations that may guide future research. Our study assessed evidence originated from population-based cohort studies, which provides some protection against selection bias.
Further studies that bypass the abovementioned major limitations are important to advance the evidence base on BP variability and CSVD. First, it is important to reach a consensus on the assessment of BP variability and to consider the role of antihypertensive treatment in the putative relationships. Studies that elucidate the biological mechanisms underlying BP variability across different time scales are needed to inform its clinical implications. Second, assessing CSVD markers using standardized criteria will improve the comparability of the results across studies. Our understanding on the role of BP variability in small vessel impairment will also be enhanced by future studies that assess the emerging markers of CSVD, such as microbleeds and perivascular space. Finally, given that subclinical brain vascular lesions may be silently present from young adulthood (or earlier),50, 51 a baseline screening of brain lesions is critical to clarify the temporal order of the relationship between BP variability and the development of CSVD.
Summary
The overall evidence suggests that large BP variability is associated with a higher burden of white matter hyperintensities, which may offer important insights into the early-stage etiology of stroke and dementia. Methodological issues such as reverse causation need to be better addressed in future longitudinal studies. If the observed association is causal, the relationship suggests a great potential to prevent cerebral small vessel disease, and thereby stroke and dementia, through maintaining stable blood pressure.
Supplementary Material
Acknowledgement
We thank Dr. Paul Bain at The Francis A. Countway Library of Medicine for his expert advice on the search strategy.
Sources of Funding
This work was partially supported by an unrestricted grant from the Janssen Prevention Center.
Dr Viswanathan is supported by NIH grants R01AG047975, R01AG026484, P50AG005134, K23AG028726.
Footnotes
Disclosures
Dr Viswanathan has been a consultant for Alnylam Pharamceuticals, Genetech Pharmaceuticals, and Roche Pharamaceuticals, but these organizations have no role in this study. Others declare no conflicts of interest.
References
- 1.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. Journal of the American College of Cardiology. 2017:23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. [DOI] [PubMed] [Google Scholar]
- 5.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ. 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oishi E, Ohara T, Sakata S, Fukuhara M, Hata J, Yoshida D, et al. Day-to-day blood pressure variability and risk of dementia in a general japanese elderly population: The hisayama study. Circulation. 2017;136:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alperovitch A, Blachier M, Soumare A, Ritchie K, Dartigues JF, Richard-Harston S, et al. Blood pressure variability and risk of dementia in an elderly cohort, the three-city study. Alzheimers Dement. 2014;10:S330–337. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The age, gene/environment susceptibility--reykjavik study. Brain. 2011;134:3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu TY, Staessen JA, Wei FF, Xu J, Li FH, Fan WX, et al. Blood flow pattern in the middle cerebral artery in relation to indices of arterial stiffness in the systemic circulation. Am J Hypertens. 2012;25:319–324. [DOI] [PubMed] [Google Scholar]
- 10.Sabayan B, Wijsman LW, Foster-Dingley JC, Stott DJ, Ford I, Buckley BM, et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: Prospective cohort study. BMJ. 2013;347:f4600. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantoni L Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 13.Debette S, Schilling S, Duperron M, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: A systematic review and meta-analysis. JAMA neurology. 2018;76:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yilmaz P, Ikram MK, Niessen WJ, Ikram MA, Vernooij MW. Practical small vessel disease score relates to stroke, dementia, and death. Stroke. 2018;49:2857–2865. [DOI] [PubMed] [Google Scholar]
- 15.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and the brain: A 5-year follow-up. Neurology. 2005;64:1846–1852. [DOI] [PubMed] [Google Scholar]
- 17.Filomena J, Riba-Llena I, Vinyoles E, Tovar JL, Mundet X, Castane X, et al. Short-term blood pressure variability relates to the presence of subclinical brain small vessel disease in primary hypertension. Hypertension. 2015;66:634–640; discussion 445. [DOI] [PubMed] [Google Scholar]
- 18.Aribisala BS, Morris Z, Eadie E, Thomas A, Gow A, Valdes Hernandez MC, et al. Blood pressure, internal carotid artery flow parameters, and age-related white matter hyperintensities. Hypertension. 2014;63:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi Y, Wada M, Sato H, Nagasawa H, Koyama S, Takahashi Y, et al. Impact of ambulatory blood pressure variability on cerebral small vessel disease progression and cognitive decline in community-based elderly japanese. Am J Hypertens. 2014;27:1257–1267. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Zhao Y, Zhang H, Chai Q, Cui Y, Diao Y, et al. Excessive variability in systolic blood pressure that is self-measured at home exacerbates the progression of brain white matter lesions and cognitive impairment in the oldest old. Hypertension research : official journal of the Japanese Society of Hypertension. 2016;39:245–253. [DOI] [PubMed] [Google Scholar]
- 21.Gunstad J, Cohen RA, Tate DF, Paul RH, Poppas A, Hoth K, et al. Blood pressure variability and white matter hyperintensities in older adults with cardiovascular disease. Blood pressure. 2005;14:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tully PJ, Debette S, Tzourio C. The association between systolic blood pressure variability with depression, cognitive decline and white matter hyperintensities: The 3c dijon mri study. Psychological medicine. 2017:1–13. [DOI] [PubMed] [Google Scholar]
- 23.Tsukishima E, Saito H, Shido K, Kobashi G, Ying-Yan G, Kishi R, et al. Long-term blood pressure variability and cerebrovascular changes on ct in a community-based elderly population. Journal of epidemiology. 2001;11:190–198. [DOI] [PubMed] [Google Scholar]
- 24.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: The honolulu-asia aging study. Stroke. 2002;33:26–30. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez CE, Pacheco J, Beason-Held LL, Resnick SM. Longitudinal changes in cortical thinning associated with hypertension. J Hypertens. 2015;33:1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung LY, Bartz TM, Rice K, Floyd J, Psaty B, Gutierrez J, et al. Blood pressure and heart rate measures associated with increased risk of covert brain infarction and worsening leukoaraiosis in older adults. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosano C, Abebe KZ, Aizenstein HJ, Boudreau R, Jennings JR, Venkatraman V, et al. Longitudinal systolic blood pressure characteristics and integrity of white matter tracts in a cohort of very old black and white adults. American Journal of Hypertension. 2015;28:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Archives of neurology. 2010;67:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNeil CJ, Myint PK, Sandu AL, Potter JF, Staff R, Whalley LJ, et al. Increased diastolic blood pressure is associated with mri biomarkers of dementia-related brain pathology in normative ageing. Age and ageing. 2018;47:95–100. [DOI] [PubMed] [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. Us population data. Archives of internal medicine. 1993;153:598–615. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, Wardlaw JM. Update on cerebral small vessel disease: A dynamic whole-brain disease. Stroke and Vascular Neurology. 2016;1:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells GA, Shea B, Higgins JP, Sterne J, Tugwell P, Reeves BC. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Research synthesis methods. 2013;4:63–77. [DOI] [PubMed] [Google Scholar]
- 34.Berkey CS, Hoaglin DC, Antczak-Bouckoms A, Mosteller F, Colditz GA. Meta-analysis of multiple outcomes by regression with random effects. Statistics in medicine. 1998;17:2537–2550. [DOI] [PubMed] [Google Scholar]
- 35.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, c-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. Jama. 1998;279:1477–1482. [DOI] [PubMed] [Google Scholar]
- 36.Chinn S A simple method for converting an odds ratio to effect size for use in meta-analysis. Statistics in medicine. 2000;19:3127–3131. [DOI] [PubMed] [Google Scholar]
- 37.Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. Journal of the neurological sciences. 1993;114:7–12. [DOI] [PubMed] [Google Scholar]
- 38.van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: A systematic review and meta-analysis. Neuroscience and biobehavioral reviews. 2015;53:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poels MM, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A, et al. Arterial stiffness and cerebral small vessel disease: The rotterdam scan study. Stroke. 2012;43:2637–2642. [DOI] [PubMed] [Google Scholar]
- 40.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eto M, Toba K, Akishita M, Kozaki K, Watanabe T, Kim S, et al. Reduced endothelial vasomotor function and enhanced neointimal formation after vascular injury in a rat model of blood pressure lability. Hypertension research : official journal of the Japanese Society of Hypertension. 2003;26:991–998. [DOI] [PubMed] [Google Scholar]
- 42.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews. Neuroscience. 2006;7:41–53. [DOI] [PubMed] [Google Scholar]
- 43.Wardlaw JM. Blood-brain barrier and cerebral small vessel disease. Journal of the neurological sciences. 2010;299:66–71. [DOI] [PubMed] [Google Scholar]
- 44.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. [DOI] [PubMed] [Google Scholar]
- 45.Diaz KM, Veerabhadrappa P, Kashem MA, Thakkar SR, Feairheller DL, Sturgeon KM, et al. Visit-to-visit and 24-h blood pressure variability: Association with endothelial and smooth muscle function in african americans. Journal of human hypertension. 2013;27:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blair GW, Appleton JP, Flaherty K, Doubal F, Sprigg N, Dooley R, et al. Tolerability, safety and intermediary pharmacological effects of cilostazol and isosorbide mononitrate, alone and combined, in patients with lacunar ischaemic stroke: The lacunar intervention-1 (laci-1) trial, a randomised clinical trial. EClinicalMedicine. 2019;11:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Convertino VA, Rickards CA, Ryan KL. Autonomic mechanisms associated with heart rate and vasoconstrictor reserves. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2012;22:123–130. [DOI] [PubMed] [Google Scholar]
- 48.Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, et al. A new rating scale for age-related white matter changes applicable to mri and ct. Stroke. 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 49.van Straaten EC, Fazekas F, Rostrup E, Scheltens P, Schmidt R, Pantoni L, et al. Impact of white matter hyperintensities scoring method on correlations with clinical data: The ladis study. Stroke. 2006;37:836–840. [DOI] [PubMed] [Google Scholar]
- 50.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the framingham heart study: A cross-sectional study. Lancet Neurol. 2012;11:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williamson W, Lewandowski AJ, Forkert ND, Griffanti L, Okell TW, Betts J, et al. Association of cardiovascular risk factors with mri indices of cerebrovascular structure and function and white matter hyperintensities in young adults. JAMA. 2018;320:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.