Abstract
Phenylarsonic feed additives were once widely used in poultry and swine production around the world, which brought significant and unnecessary health risk to consumers due to elevated residues of arsenic species in animal tissues. They also increased the risk to ecosystems via releases of inorganic arsenic through their environmental transformation. Out of concern for the negative impacts on human and ecosystem health, China, one of the world’s largest poultry and swine producing countries, recently banned the use of phenylarsonic feed additives in food animal production. This ban, if fully enforced, will result in reduction of approximately 1160 cancer cases per year from the consumption of chicken meat alone, and avoid an annual economic loss of nearly 0.6 billion CNY according to our risk analysis. Furthermore, the inventory of anthropogenic arsenic emissions in China will be cut by approximately one-third with the phase-out of phenylarsonic feed additives. This ban is also expected to lead to significant reduction in the accumulation of arsenic in the soils of farmlands fertilized by poultry and swine wastes and, consequently, lower the accumulation of arsenic in food crops grown on them, which could have even greater public health benefits. But effective enforcement of the ban is crucial, and it will require detailed supervision of veterinary drug production and distribution, and enhanced surveillance of animal feeds and food products. Furthermore, control of other major anthropogenic sources of arsenic is also necessary to better protect human health and the environment.
Graphical Abstract
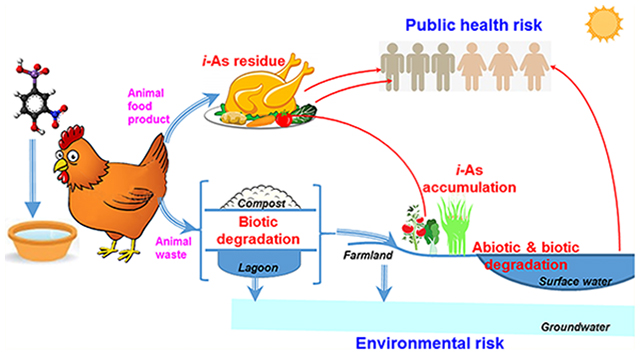
1. INTRODUCTION
Phenylarsonic acid compounds, primarily roxarsone (3-nitro-4-hydroxyphenylarsonic acid, ROX) and p-arsanilic acid (4-aminobenzenearsonic acid, p-ASA), had been widely used in poultry and swine production worldwide as feed additives to treat coccidiosis, promote growth, and improve tissue pigmentation (Table S1). Their disease prevention and growth promotion effects were first discovered in the 1940s and 1950s and were later approved for use in poultry and swine production.1–3 The use of phenylarsonic feed additives became widespread as concentrated animal feeding operations (CAFOs) took over the poultry and swine industry worldwide.
Despite the well-known effects of inorganic arsenic (i-As) on human respiratory, cardiovascular, nervous, and hematopoietic systems from long-term exposure and the epidemiological evidence linking its exposure to human lung and skin cancer, it was not classified as a human carcinogen until 1980.4,5 On the other hand, organic arsenic compounds are much less toxic than i-As, and no epidemiological data are available on their exposure and cancer so far. Like many veterinary drugs, the majority of phenylarsonic feed additives fed to animals are excreted with urine and feces in unchanged forms, but residues would inevitably occur in animal tissues.6 The metabolism of phenylarsonic feed additives in animal bodies has received virtually no attention until the early 2000s.7 As a result, no human health concern was raised for several decades while they were adopted by animal farmers worldwide.
Concerned with the potential transformation to i-As in chickens and the associated public health risk, European Union banned the use of ROX in 1999, although phenylarsonic feed additives had never been approved in Europe.8 Subsequent studies in the U.S. found that chickens fed with ROX had increased levels of i-As in livers and muscles compared to the unexposed controls.7,9,10 With the revelation that ROX was a key source of arsenic contamination of poultry, its sale in the U.S. and Canada was voluntarily suspended in 2011. The U.S. Food and Drug Administration (FDA) subsequently withdrew the approval of ROX, p-ASA, and carbarsone for use on food-producing animals in 2013.11 The only approved phenylarsonic feed additive left, nitarsone, was banned in the U.S. two years later.12 Following the move in the U.S., several countries, including Malaysia, Canada, and Australia, also took actions in banning phenylarsonic feed additives, although their use was still allowed in many countries, including Argentina, Brazil, Chile, Mexico, and Vietnam. By officially banning the use of phenylarsonic feed additives on May 1, 2019, China became the latest country joining the global phase-out efforts.13
2. HUMAN HEALTH RISK OF FOODBORNE ARSENIC RESIDUES BROUGHT BY PHENYLARSONIC FEED ADDITIVES
Once fed to animals, phenylarsonic feed additives move throughout animal bodies via absorption, distribution, metabolism, and excretion (ADME), processes that determine the levels and speciation of their residues in animal tissues. To date, little is known about the metabolism of phenylarsonic feed additives, which may possibly lead to residues of i-As in animal tissues. N-acetyl-4-hydroxy-m-arsanilic acid (N-AHPAA) and 3-amino-4-hydroxyphenylarsonic acid (3-AHPAA) have been detected as in the livers of chickens fed with ROX, suggesting it could be metabolized in animal bodies.14–17 Recently, with the help of an advanced analytical strategy that complemented inductively coupled plasma mass spectroscopy with electrospray ionization mass spectrometry, 3-nitro-4-hydroxyphenylmethylarsonic acid (methyl-ROX), 3-amino-4-hydroxyphenylmethylarsonic acid (methyl-3AHPAA), and N-acetyl-4-hydroxy-phenylmethylarsonic acid (methyl-NAHAA) were identified in the livers of ROX-fed chickens.18,19 The discovery of these methylated metabolites of phenylarsenicals provides new insight on the metabolism of phenylarsonic feed additives in animal bodies, although there are still a number of unidentified arsenic-containing metabolites.19 Despite the detection of i-As as a degradation product of ROX by chicken gut microbiota,20,21 there is an overall lack of understanding on the release of i-As from its metabolic transformation in animal bodies (Figure 1).
Figure 1.
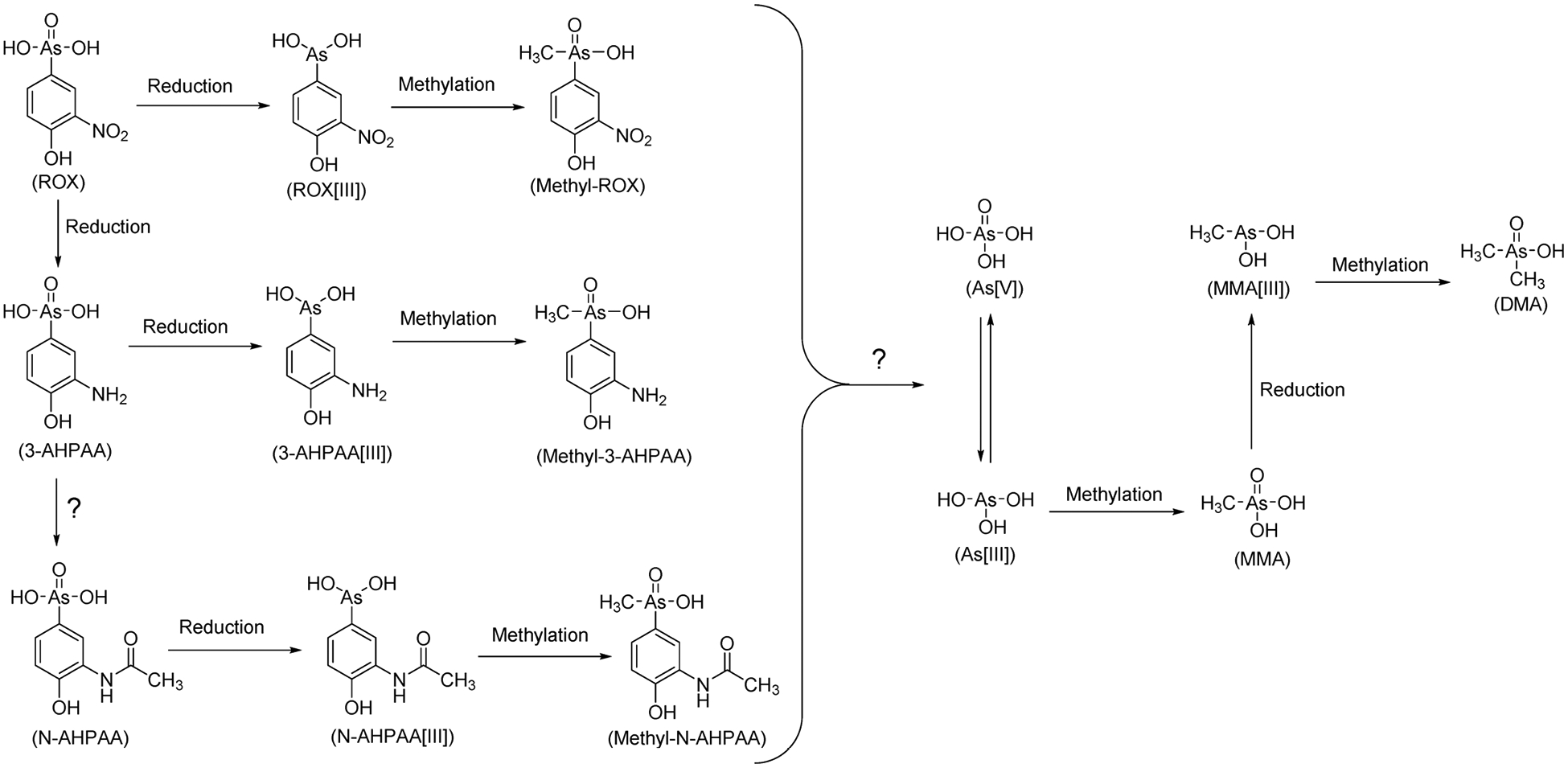
Potential transformation pathways of ROX in animal bodies based on the metabolites detected in the livers of ROX-fed chickens.14–18 Arsenite (As[III]), arsenate (As[V]), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA) are commonly detected as background in the livers of chickens both with and without ROX administration.
While the pharmaceutical activity and toxicity of phenylarsonic feed additives in humans received little attention,22 the negative health consequences of i-As exposure has gradually become well-known. Several studies conducted in the U.S. consistently found that the tissues of chickens fed with phenylarsonic feed additives contained higher levels of i-As (and total arsenic as well) compared to the unexposed controls.7,9,10,23 A 35-day feeding experiment conducted in Canada found that the contents of i-As and several organic arsenic species in the breasts of ROX-fed chickens were significantly higher than those in the control chickens, and the levels of arsenite and ROX were still significantly elevated after a 7-day withdrawal period.17 A survey of the chicken tissues from live poultry markets in Guangdong province of southern China between 2013 and 2014 showed that the geometric mean content of total arsenic in the chicken meats from urban markets was 1.7 times higher than that in the products from rural markets.24,25 This could be attributed to the fact that most chickens sold on the urban markets were raised in CAFOs (and thus received phenylarsonic feed additives), while those on the rural markets were primarily raised in traditional backyard farms.25 A later study also found elevated levels of total arsenic in the chicken meats from markets of 10 provincial capital cities in China, with p-ASA and ROX detected in more than 90% of the samples analyzed for arsenic speciation and i-As occurred at greater levels in the cities that had chicken meats containing higher contents of p-ASA or ROX.26
On the basis of the limited data available, i-As was believed to account for, on the average, 65% of the total arsenic in poultry meats in the evaluation of dietary arsenic intake in the U.S. three decades ago,27 while reassessment of the data a decade later revealed that the average fraction of i-As was actually 41%.28 A market basket study in the U.S. found that the shares of i-As were 24.5 and 12.2% of the total arsenic in the meats of chickens raised with unknown policy on ROX use and without its administration, respectively, while the share was only 9.8% in organic chicken products.10 In contrast, the feeding experiment in Canada showed that i-As accounted for <10% of the total arsenic residue in the meats of chickens treated with ROX, while arsenobetaine (approximately 80%) was the predominant arsenic species due to the fish meal-containing feed.17 ROX, i-As, and unidentified organic arsenic species were detected at comparable levels in the livers of chickens from urban markets of Guangdong, China, resulting in a share of i-As at around 30%.25 The mean shares of arsenite and arsenate in the total arsenic contents of chicken meats from markets of 10 Chinese cities were 11.6 and 23.0%, respectively, which corresponds to a mean share of 34.6% for i-As.26 These results consistently show that the use of phenylarsonic feed additives increased the contents of both i-As and total arsenic in chicken tissues.
Potentially significant public health risk can result from the elevated i-As contents in the tissues of animals fed with phenylarsonic feed additives. Consumption of the meats of chickens and turkeys raised in conventional farms (where the use of phenylarsonic feed additives was not prohibited) increased the incremental lifetime cancer risk (ILCR) of lung and bladder cancer by 37 and 3.1 per million population, respectively, in the U.S.10,23 On the basis of the average content of i-As in the chicken meats from local markets, lifetime consumption would result in 141 and 46 additional cases of lung and bladder cancer per million adults living in the urban and rural areas of Guangdong province in China, respectively.25 Although the chicken consumption rates of urban and rural populations in Guangdong were only 60% and 30% of that of the U.S. population, their ILCR from i-As exposure through chicken consumption was approximately 7.6 and 2.5 times higher, respectively, because of the use of phenylarsonic feed additives in chicken production at the time.24,25 Considerable geographic variation in the cancer risk of i-As exposure from chicken consumption was observed in the 10 provincial capital cities of China: an increase of 117 bladder and lung cancer cases per 1 000 000 adults would occur in Kunming, while only 9 cancer cases (per 1 000 000 adults) would occur in Shanghai.26 Clearly, significant public health risk could arise from the elevated levels of i-As in animal food products because of the widespread use of phenylarsonic feed additives in poultry production.
3. ENVIRONMENTAL RISK OF PHENYLARSONIC FEED ADDITIVES RELEASED WITH ANIMAL WASTES
The phenylarsonic feed additives excreted by animals could enter the environment through multiple pathways during the storage and disposal of animal wastes, e.g., weather-exposed lagoons or windrows and land application of animal wastes. Their environmental transformation could result in the releases of the more toxic and mobile i-As, which elevates the risk to ecosystems.29–34 Rather slow biotransformation of ROX has been observed during anaerobic composting of manure, with the speciation of arsenic shifting from ROX to primarily arsenate in a month.32 Relatively fast transformation of ROX by Clostridium species in chicken litter enrichments under anaerobic conditions was also reported, with 3-AHPAA and i-As being the main degradation products.21 Methanogenic sludge could cause fast transformation of ROX to 3-AHPAA under anaerobic conditions,31 while slow biotransformation of 3-AHPAA and p-ASA also occurred under methanogenic and sulfate-reducing conditions, with only 19–28% of the arsenic from the transformed amino-substituted phenylarsonic acids released as i-As.31 Under reducing conditions, reduction of the nitro group is expected, while cleavage of the C–As bond is unlikely based on the compound’s electronic structure.21 Later studies found that the C–As bond in trivalent ROX, p-ASA, and nitarsone could be cleaved by a bacterial gene, arsI, which is an Fe(II)-dependent extradiol dioxygenase, from the environmental isolate Bacillus sp. MD1.35,36 Exoelectrogenic bacteria could effectively use ROX as an electron acceptor for anaerobic respiration (producing arsenite), with extracellular and intracellular reductions occurring simultaneously.33 The widely occurring facultative anaerobic microbe, Shewanella putrefaciens, was found to be capable of causing cleavage of the C–As bond in trivalent phenylarsonic feed additives under aerobic conditions.37 An aerobic bacterial strain of the genus Enterobacter isolated from an arsenic-contaminated paddy soil also caused relatively fast degradation of ROX, producing N-AHPAA, 3-AHPAA, arsenate, arsenite, and a sulfur-containing arsenic species (AsC9H13N2O6S).38 Overall, literature data indicate that phenylarsonic feed additives could be bio-transformed under anaerobic conditions, and certain microbes in the environment could also cause their degradation with the production of i-As under aerobic conditions, although the mechanism of i-As liberation from the aromatic ring is still not well understood (Figure 2a).
Figure 2.
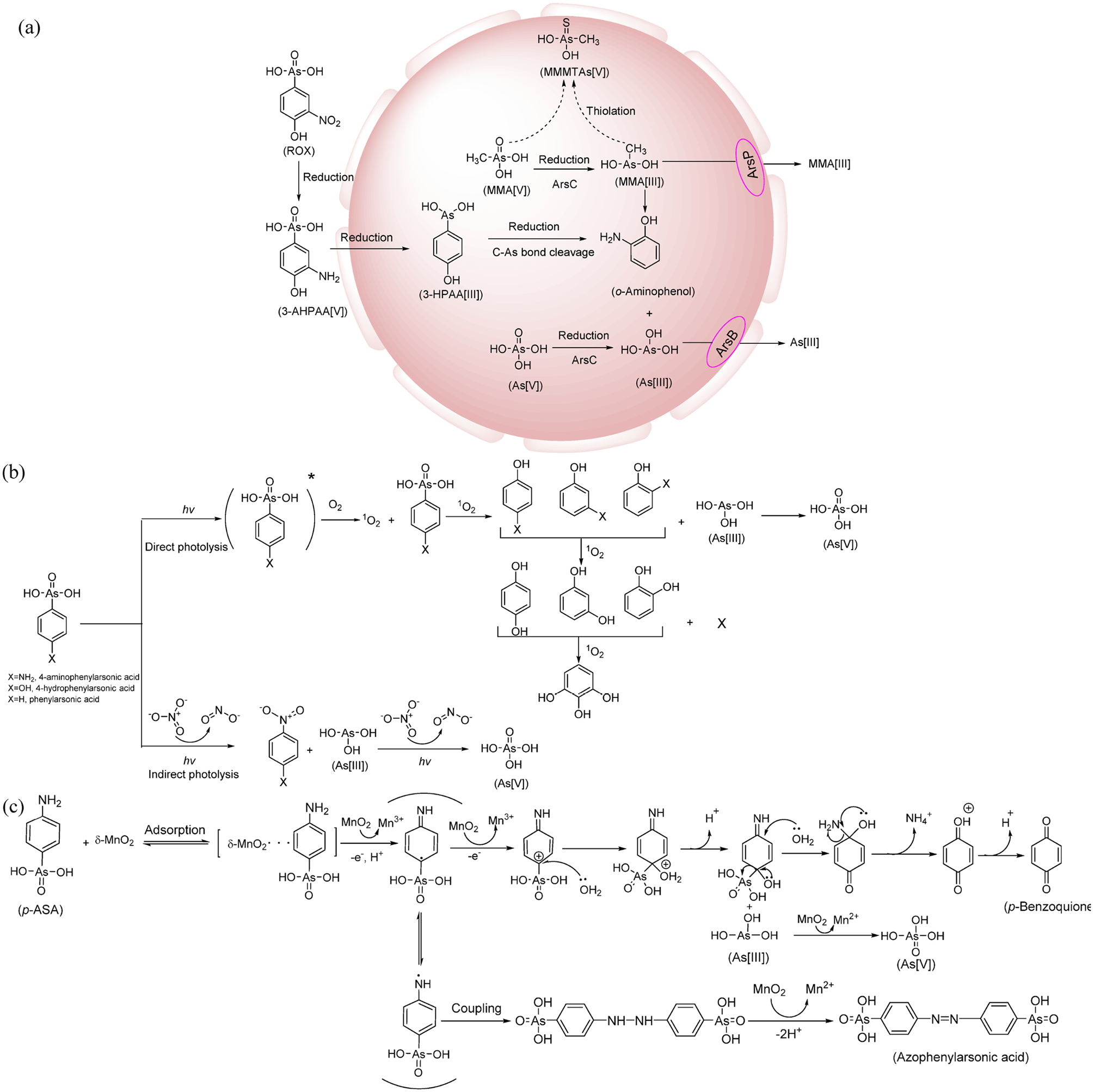
Summary of the major transformation pathways of phenylarsonic feed additives in the environment: (a) biodegradation (where MMMTAs stands for monomethyl monothioarsonic acid),21,31,33,37,38 (b) direct and indirect photodegradation,30,39,40 and (c) abiotic reductive transformation.42–44
Being quite water-soluble, phenylarsonic feed additives could be easily leached out of animal wastes by rainwater and irrigation water and reach surface water bodies. Under UV irradiation, ROX and p-ASA in water degraded quickly and produced i-As, with both direct photolysis and indirect photolysis contributing to the overall degradation.30,39 Under sunlight irradiation, p-ASA also underwent fast photodegradation, releasing i-As.40 Direct photolysis contributed little to its solar photodegradation, while self-sensitized formation of singlet oxygen was primarily responsible for the transformation.40,41 p-ASA could adsorb onto the surface of birnessite, a commonly occurring manganese oxide in surface soils, and then undergo degradation quickly under slightly acidic conditions (pH 4.0–6.2).42 While most arsenic was released as i-As with the cleavage of C–As bond, about a quarter of the arsenic was not mineralized due to the formation of a self-coupling product, azophenylarsonic acid.42 Similarly, manganese–iron binary oxide could also adsorb p-ASA and ROX and mediate their heterogeneous oxidation.43
Unlike biodegradation, abiotic transformation of phenylarsonic feed additives in the environment always leads to partial or complete cleavage of the C–As bond and corresponding release of i-As (Figures 2b and 2c). The half-lives of their photodegradation under natural sunlight are on the order of hours,40,41 and those of the degradation mediated by manganese oxides are on the same order.42,44 In contrast, their biodegradation involving the releases of i-As occurs very slowly under anaerobic conditions,21,31,45 although releases of i-As from ROX biodegradation could be facilitated by a bacteria strain isolated from arsenic-contaminated soils.38 Overall, biodegradation dominates the transformation of phenylarsonic feed additives when the animal wastes undergo stabilization in surface lagoons or compost piles, while the release of i-As from their degradation is likely controlled by abiotic processes once they enter the surface water and surface soils. These findings are consistent with the field observations on the occurrence of p-ASA and i-As in the surface water and surface soils of a swine farming zone in southern China.34,46
4. USE HISTORY OF PHENYLARSONIC FEED ADDITIVES IN CHINA AND THEIR PHASE-OUT
Following the practices in developed countries, the Ministry of Agriculture (MOA) of China approved the use of p-ASA in chicken and swine production in 1993, and ROX three years later (Table S1). Their use in poultry and swine farming has expanded quickly since then, along with the transition from the traditional backyard farms to CAFOs in the country’s poultry and swine production.47 Meanwhile, China’s chemical industry experienced headlong expansion in the early 2000s, which rendered p-ASA and ROX, like many other veterinary drugs, easily available at low costs from quite a few manufacturers. The use of veterinary drugs, including phenylarsonic feed additives, in poultry and swine production generated significant economic paybacks to the manufacturers, feed producers, and animal farmers. On the other hand, their use was largely unchecked because of the overall lack of supervision on the production, distribution, and end use over the past three decades.48,49 Being one of the largest producers of chickens and pigs, together with spotty supervision of veterinary drugs, the volume of phenylarsonic feed additives used in China’s food animal production was probably the highest in the world. For example, it was estimated that a total of 6000 tonnes of arsenic entered soils due to the use of phenylarsonic feed additives in the country’s poultry and swine farming in 2004.50 With less than 1.5% and 0.1% of the domestically produced pork and chicken exported, respectively (Tables S2 and S3), both the human health and environmental impact of phenylarsonic feed additive use is expected to be within the country.
With a better understanding of the public health risk posed by elevated i-As residues in the tissues of animals fed with phenylarsonic feed additives, as well as their impact on the environment once released with the animal wastes over the past two decades, regulatory control on their production and use in China was gradually tightened (Table S4). Maximum residue limits were established for total arsenic in poultry and swine products soon after the approval of p-ASA use in poultry and swine production, while administrative scrutiny requirements were later set for the manufacturers. Local governments even took more aggressive measures: for example, Fujian, a major livestock and poultry production province in China, banned p-ASA and ROX as feed additives for pigs and chickens in 2005 and 2008, respectively.51,52 Phenylarsonic feed additives were included in the program for re-evaluation of approved veterinary drugs in the early 2010s, while a recommendation on banning their use on food-producing animals was made in 2017 based on considerations of the safety of animals, animal food products, environment, and public health.53 In early 2018, the national action plan for phasing out phenylarsonic feed additives was officially announced, outlawing their use in China’s food animal production by May 1, 2019.13
5. PUBLIC HEALTH AND ENVIRONMENTAL BENEFITS OF THE PHENYLARSONIC FEED ADDITIVE BAN IN CHINA
Previous studies have indicated that the residues of i-As present in chicken meats did not pose appreciable non-carcinogenic risk to the consumers.10,17,23–25 As a result, only the carcinogenic risk associated with the exposure to i-As in chicken meats was considered.54 For easy comparison, the risk associated with chicken consumption before and after the discontinuation of phenylarsonic feed additive use in China was estimated for adults only. The ILCR of i-As exposure, which refers to the incremental increase in cancer cases in the exposed population compared to the unexposed one, was calculated by multiplying the lifetime average daily dose (LADD, mg/kg/day) of i-As by its corresponding cancer slope factor (CSF, (mg/kg/day)−1):54
| (1) |
LADD was estimated by multiplying the content of i-As in chicken meat (CiAs, mg/kg) by the daily intake rate (IR, kg/day) for individuals and divided by the body weight (BW, 60 kg):
| (2) |
The mean contents of i-As in the chicken meats on urban and rural markets before the ban were assumed to be 10.2 and 5.4 μg/kg, respectively, based on the results of our previous studies in Guangdong province.24,25 After full implementation of the ban on phenylarsonic feed additives, the mean content of i-As in chicken meats on both urban and rural markets was assumed to be lowered to 0.7 μg/kg, the same value for the chickens raised in CAFOs without using phenylarsonic feed additives in the U.S.10,23 Table S5 summarizes the daily intake rates of chicken meat for urban and rural residents and the respective population sizes for 31 provinces and municipalities in China. For i-As, CSF values of 1.5 and 25.7 (mg/kg/day)−1 were used for estimating the risk of skin cancer and combined lung and bladder cancer, respectively (see more details in Supporting Information). The overall carcinogenic risk of i-As exposure was also estimated by combining the corresponding risk of the three cancer types caused by i-As exposure, that is, bladder, lung, and skin cancers (the risk was calculated for bladder and lung cancer combined), following the approach used in the assessment of the public health risk of arsenic in drinking water.55
Table 1 summarizes the results for risk estimation and cancer cases avoided by the policy of banning phenylarsonic feed additives in different provinces and municipalities. Overall, i-As exposure through chicken consumption would result in approximately 81.9 and 34.8 additional lung and bladder cancer cases, as well as 4.78 and 2.03 additional cases of skin cancer, per 1 000 000 persons for the urban and rural residents, respectively, before phenylarsonic feed additives were banned. With higher arsenic contents in chicken products on the urban markets and greater chicken ingestion rates, the urban populations faced much higher cancer risk compared to the rural populations. For individual provinces and municipalities, the mean ILCR of lung and bladder cancer ranged from 6.15 × 10−6 to 1.92 × 10−4 for the urban populations, and 4.10 × 10−7 to 1.04 × 10−4 for the rural populations. In particular, the urban residents in Hainan, Guangxi, Guangdong, Chongqing, and Shanghai, and the rural residents in Guangdong have mean ILCR (lung and bladder cancer) greater than 10−4, which is considered as serious and deserves significant attention.56 Once the use of phenylarsonic feed additives is fully discontinued, the mean ILCR (lung and bladder cancer) from i-As exposure due to chicken consumption in all provinces and municipalities would drop below 10−4, mostly in the range of 10−6 to 10−5, which is considered as tolerable. Nationwide, phasing out the use of phenylarsonic feed additives would reduce the additional lung and bladder cancer cases per 1 000 000 persons to 5.62 and 4.51 for the urban and rural residents, respectively. Meanwhile, additional skin cancer risk as estimated by cases per 1 000 000 persons would be lowered to 0.33 and 0.26 for the urban and rural populations, respectively. Overall, based on the current population size in China, it is estimated that approximately 81 100 cases of lung, bladder, and skin cancers combined could be avoided due to the elimination of phenylarsonic feed additive use in chicken farming over the next 70 years following the ban (approximately 1160 cancer cases per year).
Table 1.
Estimated ILCR from i-As Exposure through Chicken Meat Consumption before and after the Ban on Phenylarsonic Feed Additives Is Implemented and the Corresponding Reduction in the Number of Cancer Cases Avoided in Different Provinces and Municipalities of China
| combined lung and bladder cancer | skin cancer | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ILCRbeforea (×10−6) | ILCRafterb (×10−6) | cancer cases avoidedc | ILCRbefore (×10−6) | ILCRafter (×10−6) | cancer cases avoided | |||||||||
| province | urban | rural | urban | rural | urban | rural | total | urban | rural | urban | rural | urban | rural | total |
| Beijing | 48.0 | 17.7 | 3.29 | 2.3 | 840 | 45 | 885 | 2.80 | 1.04 | 0.19 | 0.13 | 49 | 3 | 52 |
| Tianjin | 50.0 | 18.1 | 3.43 | 2.34 | 601 | 42 | 643 | 2.92 | 1.05 | 0.20 | 0.14 | 35 | 2 | 38 |
| Hebei | 42.9 | 14.3 | 2.95 | 1.85 | 1654 | 420 | 2074 | 2.51 | 0.83 | 0.17 | 0.11 | 97 | 25 | 121 |
| Shanxi | 24.0 | 7.65 | 1.65 | 0.990 | 475 | 105 | 580 | 1.40 | 0.45 | 0.10 | 0.06 | 28 | 6 | 34 |
| Inner Mongolia | 49.0 | 19.6 | 3.36 | 2.54 | 716 | 164 | 880 | 2.86 | 1.14 | 0.20 | 0.15 | 42 | 10 | 51 |
| Liaoning | 51.2 | 14.9 | 3.51 | 1.93 | 1405 | 184 | 1590 | 2.99 | 0.87 | 0.20 | 0.11 | 82 | 11 | 93 |
| Jilin | 41.9 | 22.2 | 2.88 | 2.87 | 601 | 227 | 828 | 2.45 | 1.29 | 0.17 | 0.17 | 35 | 13 | 48 |
| Heilongjiang | 47.4 | 26.0 | 3.25 | 3.37 | 993 | 348 | 1341 | 2.77 | 1.52 | 0.19 | 0.20 | 58 | 20 | 78 |
| Shanghai | 106 | 59.3 | 7.30 | 7.69 | 2101 | 154 | 2255 | 6.21 | 3.46 | 0.43 | 0.45 | 123 | 9 | 132 |
| Jiangsu | 94.9 | 37.5 | 6.51 | 4.86 | 4880 | 819 | 5699 | 5.54 | 2.19 | 0.38 | 0.28 | 285 | 48 | 333 |
| Zhejiang | 96.2 | 40.1 | 6.60 | 5.20 | 3446 | 632 | 4078 | 5.61 | 2.34 | 0.39 | 0.30 | 201 | 37 | 238 |
| Anhui | 86.0 | 44.7 | 5.90 | 5.79 | 2680 | 1131 | 3812 | 5.02 | 2.61 | 0.34 | 0.34 | 156 | 66 | 222 |
| Fujiand | 6.15 | 7.82 | 6.15 | 7.82 | 0 | 0 | 0 | 0.36 | 0.46 | 0.36 | 0.46 | 0 | 0 | 0 |
| Jiangxi | 85.6 | 29.7 | 5.87 | 3.85 | 2012 | 542 | 2554 | 5.00 | 1.73 | 0.34 | 0.22 | 117 | 32 | 149 |
| Shandong | 54.2 | 22.8 | 3.72 | 2.95 | 3059 | 781 | 3840 | 3.16 | 1.33 | 0.22 | 0.17 | 179 | 46 | 224 |
| Henan | 54.7 | 18.8 | 3.75 | 2.44 | 2442 | 779 | 3221 | 3.19 | 1.10 | 0.22 | 0.14 | 143 | 45 | 188 |
| Hubei | 54.8 | 17.7 | 3.76 | 2.30 | 1786 | 371 | 2156 | 3.20 | 1.03 | 0.22 | 0.13 | 104 | 22 | 126 |
| Hunan | 88.1 | 48.4 | 6.05 | 6.28 | 3074 | 1313 | 4387 | 5.14 | 2.83 | 0.35 | 0.37 | 179 | 77 | 256 |
| Guangdong | 162 | 104.2 | 11.1 | 13.5 | 11741 | 3055 | 14796 | 9.43 | 6.08 | 0.65 | 0.79 | 685 | 178 | 864 |
| Guangxi | 168 | 85.2 | 11.5 | 11.1 | 3764 | 1840 | 5604 | 9.81 | 4.97 | 0.67 | 0.64 | 220 | 107 | 327 |
| Hainan | 192 | 77.6 | 13.2 | 10.1 | 960 | 263 | 1223 | 11.2 | 4.53 | 0.77 | 0.59 | 56 | 15 | 71 |
| Chongqing | 118 | 35.2 | 8.12 | 4.57 | 2172 | 339 | 2511 | 6.91 | 2.06 | 0.47 | 0.27 | 127 | 20 | 147 |
| Sichuan | 100 | 45.0 | 6.86 | 5.83 | 3926 | 1599 | 5525 | 5.83 | 2.62 | 0.40 | 0.34 | 229 | 93 | 322 |
| Guizhou | 62.4 | 15.3 | 4.28 | 1.98 | 958 | 257 | 1214 | 3.64 | 0.89 | 0.25 | 0.12 | 56 | 15 | 71 |
| Yunnan | 68.1 | 31.9 | 4.67 | 4.13 | 1421 | 710 | 2131 | 3.97 | 1.86 | 0.27 | 0.24 | 83 | 41 | 124 |
| Tibet | 42.7 | 0.410 | 2.93 | 0.05 | 41 | 1 | 42 | 2.49 | 0.02 | 0.17 | 0.00 | 2 | 0 | 2 |
| Shaanxi | 31.9 | 7.32 | 2.19 | 0.95 | 646 | 106 | 752 | 1.86 | 0.43 | 0.13 | 0.06 | 38 | 6 | 44 |
| Gansu | 43.5 | 17.1 | 2.99 | 2.21 | 494 | 209 | 703 | 2.54 | 1.00 | 0.17 | 0.13 | 29 | 12 | 41 |
| Qinghai | 35.3 | 12.2 | 2.42 | 1.58 | 104 | 30 | 134 | 2.06 | 0.71 | 0.14 | 0.09 | 6 | 2 | 8 |
| Ningxia | 51.1 | 26.1 | 3.51 | 3.38 | 188 | 65 | 253 | 2.98 | 1.52 | 0.20 | 0.20 | 11 | 4 | 15 |
| Xinjiang | 64.7 | 20.1 | 4.44 | 2.60 | 728 | 216 | 944 | 3.78 | 1.17 | 0.26 | 0.15 | 42 | 13 | 55 |
| total | 79.3 | 33.6 | 5.62 | 4.51 | 59908 | 16747 | 76655 | 4.63 | 1.96 | 0.33 | 0.26 | 3497 | 978 | 4474 |
Estimated ILCR before the ban on phenylarsonic feed additives is implemented.
Estimated ILCR after the ban on phenylarsonic feed additives is implemented.
Expected number of cancer cases avoided over the next 70 years calculated from multiplying the estimated reduction of carcinogenic risk (ILCRbefore – ILCRafter) with the corresponding size of population at risk.
With the use of phenylarsonic feed additives in chicken farming banned in Fujian province back in 2008, the public health risk from i-As exposure through chicken consumption is not expected to change with the implementation of the nationwide ban.
It has been estimated that the 983 678 new cases of lung cancer in 2015 caused economic losses of 489 billion CNY in China (approximately 0.5 million CNY per case), which resulted from hospitalization, short- and long-term disability, and premature death.57 On the basis of the number of new cancer cases (approximately 1160) that will be avoided with the ban on phenylarsonic feed additives in chicken farming, an annual economic savings of nearly 0.6 billion CNY is expected. Also, the reduction in the incidence of cancer and related premature mortality has a broad range of societal beneficial impacts in addition to the cost savings.58 Because phenylarsonic feed additives had played an important role in animal disease treatment and prevention, as well as growth promotion in poultry and swine production, their withdrawal or replacement (by other veterinary drugs) might bring additional costs to the producers. Nonetheless, the monthly wholesale and retail prices of broilers sold in the U.S. between 2007 and 2018 indicate that the complete withdrawal of arsenic-based feed additives did not significantly impact the prices of chicken products on the markets or the production costs (Figure S1a). Although a nationwide ban on phenylarsonic feed additives was only implemented in May 2019, their use on chickens had been banned in Fujian province since 2008.51,52 The farm-gate prices of fast- and medium-growing chickens, which are the major breeds raised in CAFOs, on the markets in Fujian were not significantly different from those of national averages over the past year (Figure S1b). Together, these data consistently indicate that phasing out the use of phenylarsonic feed additives added little cost to the poultry producers but could represent significant public health (and economic) benefits through reducing i-As exposure of the general population. It should be noted that the above analyses were carried out for chickens only due to the availability of data, but a similar finding is expected for pigs.
It was estimated that a total of 5.75 × 104 tonnes of arsenic was emitted into the environment in China in 2010 (1.98 × 104, 3.37 × 104, and 4.0 × 103 tonnes to water, soil, and air, respectively), with 2.93 × 104 tonnes contributed by animal wastes (1.26 × 104 and 7.16 × 103 tonnes from swine and poultry wastes, respectively).59 The use of phenylarsonic feed additives is well documented to result in swine and poultry wastes with elevated levels of arsenic.29,30,32,34 A national survey revealed that 13.7% of commercial animal manure-based compost products had total arsenic contents above the regulatory limit (15 mg/kg) for organic fertilizers in China.60 The arsenic contents of swine and poultry wastes in the provinces with high production volumes (i.e., with many CAFOs) were often an order of magnitude higher than those in the provinces with very low production volumes (e.g., western China, where pigs and chickens are predominantly raised in traditional backyard farms).59 Thus, it is reasonable to speculate that the ban on the use of phenylarsonic feed additives would reduce the arsenic emissions from swine and poultry wastes by roughly 90%. This would translate into reduction of approximately one-third of total arsenic emissions, assuming emissions from all sources had grown at the same rates since 2010. The above preliminary estimation indicates that a significant environmental benefit will accrue by the ban on phenylarsonic feed additives in China.
With the phenylarsonic feed additives administered to the farmed animals almost completely excreted into the urine and feces, their phase-out would lead to immediate reduction in the arsenic contents of animal wastes, and correspondingly, the inputs of arsenic to the farmlands receiving these wastes. Studies have shown that the arsenic contents in rice and vegetables are significantly correlated with its levels in the soils, and the mean transfer factor of arsenic from soil to rice (0.04) is more than 10 times greater than that for wheat or barley.61,62 Table 2 summarizes the accumulation of arsenic in farmland soils and the corresponding increases in the arsenic contents in rice in the absence of the prohibiting policy on phenylarsonic feed additives in China. The annual accumulation rates of arsenic in the agricultural soils receiving manure-based fertilizers range from 0.009 to 0.228 mg/kg in different provinces. The actual accumulation rates of arsenic in farmland soils could well be greater as manure (and its compost) is often spread repeatedly on the farmlands located within 5–15 km radius of CAFOs because of the high transportation cost.47,63 As a result of soil-to-crop transfer, the arsenic contents of rice grown on the farmlands fertilized by animal wastes increase at rates of 0.35–9.15 μg/kg/year. Currently, the rice produced in China has arsenic contents ranging from 0.065 to 0.274 mg/kg, with a mean of 0.114 mg/kg.64 The continued use of phenylarsonic feed additives would cause the arsenic contents in the rice grown on farmlands receiving swine and poultry wastes to double within 30–70 years in all the major rice production provinces of southern China (e.g., Jiangsu, Zhejiang, Anhui, Jiangxi, Hunan, Guangdong, and Sichuan). There is no doubt that banning the use of arsenic-based feed additives would significantly reduce the arsenic intake for the population consuming cereal grains (particularly rice) and vegetables produced in the farmlands fertilized by the animal wastes from swine and poultry CAFOs.
Table 2.
Estimated Increases in the Contents of Arsenic in Farmland Soils Fertilized by Animal Wastes from Swine and Poultry CAFOs in Different Provinces and Municipalities of China and the Corresponding Increases in the Contents of Arsenic in Rice Grown on These Farmlands in the Absence of the Ban on Phenylarsonic Feed Additives (Based on Food Animal Production in 2016 and Literature Results)
| province | pigs farmeda (million) | Chickens farmeda (million) | area of farmlandsa (km2) | As in manure applied to farmlandsb (tonnes) | area of farmlands receiving manurec (km2) | increment in soil As contentd (mg/kg/year) | increment in As content of rice grown the impacted farmlandse (μg/kg/year) |
|---|---|---|---|---|---|---|---|
| Beijing | 2.75 | 38.8 | 1514 | 18.0 | 303 | 0.229 | 9.15 |
| Tianjin | 3.75 | 79.1 | 4792 | 24.9 | 958 | 0.100 | 4.00 |
| Hebei | 34.3 | 607.7 | 87166 | 227 | 17433 | 0.050 | 2.00 |
| Shanxi | 7.49 | 96.4 | 37208 | 48.8 | 7442 | 0.025 | 1.01 |
| Inner Mongolia | 9.09 | 109.5 | 79219 | 59.1 | 15844 | 0.014 | 0.57 |
| Liaoning | 26.1 | 916.2 | 40641 | 180 | 8128 | 0.085 | 3.40 |
| Jilin | 16.2 | 415.6 | 56763 | 109 | 11353 | 0.037 | 1.48 |
| Heilongjiang | 18.4 | 214.5 | 124265 | 120 | 24853 | 0.019 | 0.74 |
| Shanghai | 1.71 | 17.1 | 2947 | 11.1 | 589 | 0.072 | 2.89 |
| Jiangsu | 28.5 | 714.6 | 76769 | 191 | 15354 | 0.048 | 1.92 |
| Zhejiang | 11.7 | 149.4 | 22744 | 76.2 | 4549 | 0.064 | 2.58 |
| Anhui | 28.7 | 781.5 | 88936 | 194 | 17787 | 0.042 | 1.68 |
| Fujian | 17.2 | 570.6 | 23273 | 118 | 4655 | N/Af | N/Af |
| Jiangxi | 31.0 | 506.6 | 55607 | 204 | 11121 | 0.071 | 2.82 |
| Shandong | 46.6 | 1878.3 | 109732 | 325 | 21946 | 0.057 | 2.28 |
| Henan | 60.0 | 934.2 | 144723 | 394 | 28945 | 0.052 | 2.09 |
| Hubei | 42.2 | 522.0 | 78435 | 275 | 15687 | 0.067 | 2.70 |
| Hunan | 59.2 | 426.7 | 87933 | 380 | 17587 | 0.083 | 3.33 |
| Guangdong | 35.3 | 973.9 | 48308 | 239 | 9662 | 0.095 | 3.80 |
| Guangxi | 32.8 | 822.4 | 61453 | 220 | 12291 | 0.069 | 2.76 |
| Hainan | 5.30 | 153.2 | 8233 | 35.9 | 1647 | 0.084 | 3.36 |
| Chongqing | 20.5 | 249.3 | 36007 | 133 | 7201 | 0.071 | 2.85 |
| Sichuan | 69.3 | 677.8 | 97286 | 448 | 19457 | 0.088 | 3.54 |
| Guizhou | 17.6 | 104.0 | 55968 | 113 | 11194 | 0.039 | 1.55 |
| Yunnan | 33.8 | 217.0 | 71645 | 216 | 14329 | 0.058 | 2.32 |
| Tibet | 0.18 | 1.7 | 2579 | 1.18 | 516 | 0.009 | 0.35 |
| Shaanxi | 11.4 | 52.8 | 42769 | 72.9 | 8554 | 0.033 | 1.31 |
| Gansu | 6.70 | 40.2 | 42538 | 42.9 | 8508 | 0.019 | 0.78 |
| Qinghai | 1.38 | 4.6 | 5613 | 8.79 | 1123 | 0.030 | 1.20 |
| Ningxia | 0.96 | 10.9 | 12752 | 6.24 | 2550 | 0.009 | 0.38 |
| Xinjiang | 4.71 | 86.5 | 58675 | 31.1 | 11735 | 0.010 | 0.41 |
Notes:
All data for the year of 2016, which are obtained from the national data released by the National Bureau of Statistics of China (http://data.stats.gov.cn/easyquery.htm?cn=E0103).
The amount of As in manure applied to farmlands is estimated based on following assumptions: (i) each pig emits 0.84 g of As over its lifespan due to the use of phenylarsonic feed additives in farming, while each chicken emits 0.04 g of As over its lifespan due to the use of phenylarsonic feed additives in farming;45,72 and (ii) 75% of the swine manure and 60% of the chicken manure generated are spread on farmlands as organic fertilizers.73
The area of farmlands fertilized by animal manures is estimated as the 20% of total farmland area in each province.74
The increment of soil As content is estimated with the assumption that the As discharged with animal manure accumulates in the surface soil layer of 0–20 cm, and the bulk density of soil is 1300 kg/m3.
The increment of As content in rice grown on the impacted farmlands is estimated using a soil-to-rice transfer factor of 0.04,61 with the assumption that all arsenic species discharged into soils are completely transformed to i-As
N/A: The use of phenylarsonic feed additives in swine and chicken farming was already banned in Fujian province in the 2010s.
A previous study in 2011 estimated the ILCR of i-As from food intake for the adult population in China is 1060 per 1 000 000 persons, with rice, vegetables, and aquatic products being the major contributors.65 Rice is the single largest source of total daily intake of i-As for the populations in both southern China (64.1%) and northern China (41.7%), which is similar to the cases in other Asian countries (Figure S2). In comparison, meat consumption accounted for approximately 4.81 and 3.86% of the total daily intake of i-As for the populations of southern and northern China, respectively.65 Because of the lack of sufficient data, assessment of the overall contribution of the phenylarsonic feed additive phase-out on reduction of the public health risk from decreased i-As intake through food consumption is not possible. Nonetheless, it is expected that the reduction in arsenic contents of cereal grains (particularly rice) and vegetables grown on farmlands fertilized by animal wastes brought by the phase-out could have even greater impact on arsenic exposure of the Chinese population compared to the reduction in the arsenic levels in swine and poultry meats.
6. RECOMMENDATIONS
The nationwide ban on the use of phenylarsonic feed additives in poultry and swine farming is expected to eliminate an important source of arsenic pollution to agricultural soils, and significantly reduce the public health risk of the general population by directly reducing the i-As contents in poultry and swine products, and lowering the arsenic contents in food crops grown on farmlands impacted by animal wastes from poultry and swine CAFOs as well (Figure S3). Nonetheless, substantial human and ecosystem health benefits would materialize only if the use of phenylarsonic feed additives in China’s poultry and swine farming could be fully curtailed by implementation of the ban. Accordingly, the following measures are recommended to facilitate the effective and efficient phase-out of these veterinary drugs and further enhance the environmental and human health benefits.
6.1. Strengthening Supervision on Veterinary Drug Production and Distribution to Eliminate Supply.
Broadly speaking, phenylarsonic feed additives are just a small group of veterinary drugs (primarily antimicrobials and steroids) used on food-producing animals in China.47,48 While veterinary drugs have played important roles in maintaining animal health and reducing the costs of production in CAFOs of various scales, their misuse and overuse often resulted in animal food products bearing drug residues exceeding the tolerable limits and have even caused some food safety incidents between the late 1990s and early 2010s.49 To fight the abuse of veterinary drugs and promote rational use, a total of 227 veterinary drugs have been banned for nontherapeutic use since March 2014.66 However, the results of national surveillance conducted between 2015 and 2017 showed that overall 7.4% of the animal food products inspected had drug residues at unsafe levels or contained residues of drugs prohibited for nontherapeutic use.67 The high occurrence rates of veterinary drug violations clearly indicate the institutional failures of current veterinary drug management.
Institutional reform of the administration of veterinary drugs in China is necessary, while immediate actions should be taken to strengthen the supervision and management of veterinary drug production and distribution. Although eliminating the use of phenylarsonic feed additives in food animal production would contribute significantly to the protection of human and ecosystem health, the experience with management of other veterinary drugs suggests that regulatory ban is probably far from enough to achieve the intended outcome.48,49,67 Production of phenylarsonic feed additives was outlawed on May 1, 2018, while their distribution and use became illegal after May 1, 2019.13 Nonetheless, driven by both profits and demand, some manufacturers and distributors might continue to produce and supply these drugs (illegally). The animal farmers are often not aware of the public health and environmental risk brought by the use of phenylarsonic feed additives. Thus, a crack-down on the illegal production and distribution of veterinary drugs can be much more effective than inspections at individual animal farms, as indicated by the success in combating clenbuterol abuse in China in the early 2010s.68
6.2. Enhancing Surveillance of Animal Feeds and Animal Food Products to Ensure Compliance.
Surveillance and compliance monitoring on animal feeds and animal food products are important in detecting and deterring potential abuse of veterinary drugs by the feed producers and animal farmers. China’s MOA and General Administration of Quality Supervision, Inspection and Quarantine (AQSIQ) conduct national inspections on animal feeds and feed additives twice a year. In general, the overuse and misuse of veterinary drugs have been significantly improved over the past two decades, but some prohibited veterinary drugs were still detected (in 2.2% of the samples), according to the most recent survey results.69 It should be noted that only a total of 620 and 6804 batches of animal feed additives and animal feeds were inspected, respectively, while their total production volumes were 10.9 and 228 million tonnes in 2018. Obviously, the current surveillance program is far from comprehensive and veterinary drug abuse could be easily missed. The same argument also applies to the surveillance program on drug residues in food products of animal origins.67 Thus, the monitoring programs on feed additives and animal feeds, and animal food products should be significantly expanded in terms of the sampling frequency and the number of samples inspected to verify the effectiveness of the ban and safeguard the safety of these products. The increased cost would be well worth the price for improved human health and the environment.
With the use of phenylarsonic feed additives banned, their potential abuse can be easily checked by measuring the total arsenic contents in animal feeds and animal food products. Phenylarsonic feed additives were allowed at rather high levels (p-ASA, 100 mg/kg; ROX, 25 or 50 mg/kg) in swine and chicken feeds, despite the 2.0 mg/kg standard for i-As (Table S4). Thus, speciation analysis, which involves rather complicated extraction and analysis procedures, had to be carried out by compliance monitoring before the ban. A similar problem also existed for the food safety standards with respect to arsenic residues. The Maximum Levels of Contaminants in Foods (GB 2762–2005) stipulated that the i-As contents in livestock and poultry meats should be below 0.05 mg/kg, while the National Food Standards issued in 2013 set an upper limit of 0.5 mg/kg for total arsenic contents in animal food products. These standards caused confusion and were not necessarily consistent (e.g., the share of i-As in the total arsenic residue could be greater than 10% in chicken tissues), although they did seek to accommodate the use of phenylarsonic feed additives in animal agriculture and account for the lower toxicity of phenylarsenicals.
Controversies in food safety standards had also occurred. For example, a maximum residue limit of 0.5 mg/kg for total arsenic in chicken eggs was set in 1999, while a 2001 regulation prohibited the use of phenylarsonic feed additives on egg-producing chickens (Table S4). Thus, a total limit of 0.05 mg/kg (the same as that for i-As) would have been more appropriate for chicken eggs to ensure that phenylarsonic feed additives are not fed to layer chickens. With the use of phenylarsonic feed additives banned in food animal production, the Chinese national standards on arsenic for animal feeds and animal food products should now be revised and set on the basis of total arsenic only, which would greatly simplify and facilitate surveillance and compliance monitoring.
6.3. Reducing Arsenic Pollution to Protect Public Health.
Exposure to arsenic is recognized as a major global public health concern, and the provisional tolerable weekly intake (PTWI) of 15 μg/kg-bw for i-As has been withdrawn since 2011 as there is no safe dose for its exposure.70 In general, consumption of arsenic-contaminated groundwater, which occurs naturally, is the main route of human exposure to i-As. It was estimated that up to 19.6 million people in China, primarily in Xinjiang, Inner Mongolia, Henan, Shandong, and Jiangsu, are at risk from drinking groundwater containing arsenic at unsafe levels (i.e., >10 μg/L).71 Ingestion of food is typically the most important route of arsenic exposure for the general population not exposed to contaminated water. Food crops, which can uptake arsenic from contaminated soils and irrigation water, account for 75.6% and 87.3% of total daily i-As intake of the urban and rural populations in China, respectively.65
The ban on phenylarsonic feed additives would greatly reduce the arsenic contents in poultry and swine products, and in crops grown on farmlands fertilized by animal wastes in the long run (Figure S3). Nonetheless, the historical use of inorganic and organic arsenic pesticides, along with mining and other related industrial activities, have significantly elevated the soil arsenic levels in China. Therefore, it is necessary to step up the control on other major anthropogenic sources of arsenic pollution, such as metal mining, smelting, and metallurgical industries, coal combustion, and industrial use of arsenic, to reduce arsenic pollution and thus i-As exposure of the general population through ingestion of cereal grains and vegetables, and the other routes as well.
Supplementary Material
ACKNOWLEDGMENTS
The constructive comments of the anonymous reviewers on an earlier version of this manuscript are greatly appreciated. This work was supported in parts by the National Natural Science Foundation of China (Grant Nos. 41673089, 41877112, and 41725015), the National Key Research and Development Program of China (2016YFD0800302), the Iowa Environmental Health Sciences Research Center (NIH P30 ES005605), and the 111 Program (B14001).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.9b04296.
Assessment of public health risk from i-As exposure through chicken consumption in China before and after enforcement of the phenylarsonic feed additive ban, impact of phenylarsonic feed additive phase-out on market prices of chickens, summary of the chemical structures of phenylarsonic feed additives, makeup of the chicken and pork markets in China, summary of the major regulatory rules on the production and use of phenylarsonic feed additives, population and per capita daily intake rate of chicken meat for all provinces and municipalities in China, prices of chickens with and without the ban on phenylarsonic feed additive use in poultry production, contribution of various foods to the total daily intake of As, and schematic illustration on the fate of phenylarsonic feed additives used in animal farming (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Bridges JH; Hale F; Kunkel HO; Lyman CM The effects of bacitracin, penicillin and arsanilic acid on growth rate and feed efficiency in swine. J. Anim. Sci 1954, 13, 912–917. [Google Scholar]
- (2).Hanson LE; Carpenter LE; Aunan WJ; Ferrin EF The use of arsanilic acid in the production of market pigs. J. Anim. Sci 1955, 14, 513–524. [Google Scholar]
- (3).Morehouse NF; Mayfield OJ The effect of some aryl arsonic acids on experimental coccidiosis infection in chickens. J. Parasitol 1946, 32, 20–24. [PubMed] [Google Scholar]
- (4).International Agency for Research on Cancer (IARC). Some Metals and Metallic Compounds (Evaluation of Carcinogenic Risk of Chemicals To Man, Vol. 23); IARC: Lyon, France, 1980. [Google Scholar]
- (5).Pershagen G The carcinogenicity of arsenic. Environ. Health Perspect 1981, 40, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Morrison JL Distribution of arsenic from poultry litter in broiler chickens, soil, and crops. J. Agric. Food Chem 1969, 17 (6), 1288–1290. [Google Scholar]
- (7).Lasky T; Sun W; Kadry A; Hoffman M K Mean total arsenic concentrations in chicken 1989–2000 and estimated exposures for consumers of chicken. Environ. Health Perspect 2004, 112 (1), 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).European Commission (EC). Council Directive 1999/29/EC of 22 April 1999 on the Undesirable Substances and Products in Animal Nutrition. Off. J. Eur. Comm 1999, L115/32–L115/46. [Google Scholar]
- (9).Kawalek JC; Carson M; Conklin S; Lancaster V; Howard K; Ward J; Farrell D; Myers M; Swain H; Jeanettes P; Frobish S; Matthews S; McDonald M Final Report on Study 275.30. Provide Data on Various Arsenic Species Present in Broilers Treated with Roxarsone: Comparison with Untreated Birds; U.S. Food and Drug Administration: Laurel, MD, 2011. [Google Scholar]
- (10).Nachman KE; Baron PA; Raber G; Francesconi KA; Navas-Acien A; Love DC Roxarsone, inorganic arsenic, and other arsenic species in chicken: a U.S.-based market basket sample. Environ. Health Perspect 2013, 121 (7), 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).U.S. Food and Drug Administration. FDA’s Response to the Citizen Petition, FDA-2009-p-0594; Food and Drug Administration: Silver Spring, MD, 2013. [Google Scholar]
- (12).U.S. Food and Drug Administration. FDA Announces Pending Withdrawal of Approval of Nitarsone; Food and Drug Administration: Silver Spring, MD, 2015. [Google Scholar]
- (13).Ministry of Agriculture. Ban on the Use of Three Veterinary Drugs, Olaquindox, p-Arsanilic Acid, and Roxarsone, on Food-producing Animals, Announcement No. 2638) Ministry of Agriculture: Beijing, China, 2018. [Google Scholar]
- (14).Conklin SD; Shockey N; Kubachka K; Howard KD; Carson MC Development of an ion chromatography-inductively coupled plasma-mass spectrometry method to determine inorganic arsenic in liver from chickens treated with Roxarsone. J. Agric. Food Chem 2012, 60 (37), 9394–9404. [DOI] [PubMed] [Google Scholar]
- (15).Peng H; Hu B; Liu Q; Yang Z; Lu X; Huang R; Li X; Zuidhof MJ; Le XC Liquid chromatography combined with atomic and molecular mass spectrometry for speciation of arsenic in chicken liver. J. Chromatogr. A 2014, 1370, 40–49. [DOI] [PubMed] [Google Scholar]
- (16).Yang Z; Peng H; Lu X; Liu Q; Huang R; Hu B; Kachanoski G; Zuidhof MJ; Le XC Arsenic metabolites, including N-Acetyl-4-hydroxy-m-arsanilic acid, in chicken litter from a roxarsone-feeding study involving 1600 chickens. Environ. Sci. Technol 2016, 50 (13), 6737–6743. [DOI] [PubMed] [Google Scholar]
- (17).Liu Q; Peng H; Lu X; Zuidhof MJ; Li X-F; Le XC Arsenic species in chicken breast: Temporal variations of metabolites, elimination kinetics, and residual concentrations. Environ. Health Perspect 2016, 124 (8), 1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Peng H; Hu B; Liu Q; Li J; Li XF; Zhang H; Le C Methylated phenylarsenical metabolites discovered in chicken liver. Angew. Chem., Int. Ed 2017, 56 (24), 6773–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Liu Q; Lu X; Peng H; Popowich A; Tao J; Uppal JS; Yan X; Boe D; Le XC Speciation of arsenic - A review of phenylarsenicals and related arsenic metabolites. TrAC, Trends Anal. Chem 2018, 104, 171–182. [Google Scholar]
- (20).Chovanec P; Stolz JF; Basu P A proteome investigation of roxarsone degradation by Alkaliphilus oremlandii strain OhILAs. Metallomics 2010, 2 (2), 133–139. [DOI] [PubMed] [Google Scholar]
- (21).Stolz JF; Perera E; Kilonzo B; Kail B; Crable B; Fisher E; Ranganathan M; Wormer L; Basu P Biotransformation of 3-nitro-4-hydroxybenzene arsonic acid (roxarsone) and release of inorganic arsenic by Clostridium species. Environ. Sci. Technol 2007, 41 (3), 818–823. [DOI] [PubMed] [Google Scholar]
- (22).Basu P; Ghosh RN; Grove LE; Klei L; Barchowsky A Angiogenic potential of 3-nitro-4-hydroxy benzene arsonic acid (roxarsone). Environ. Health Perspect 2008, 116 (4), 520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Nachman KE; Love DC; Baron PA; Nigra AE; Murko M; Raber G; Francesconi KA; Navas-Acien A Nitarsone, inorganic arsenic, and other arsenic species in turkey meat: Exposure and risk assessment based on a 2014 U.S. market basket sample. Environ. Health Perspect 2017, 125 (3), 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Hu Y; Zhang W; Chen G; Cheng H; Tao S Public health risk of trace metals in fresh chicken meat products on the food markets of a major production region in southern China. Environ. Pollut 2018, 234, 667–676. [DOI] [PubMed] [Google Scholar]
- (25).Hu Y; Zhang W; Cheng H; Tao S Public health risk of arsenic species in chicken tissues from live poultry markets of Guangdong province, China. Environ. Sci. Technol 2017, 51 (6), 3508–3517. [DOI] [PubMed] [Google Scholar]
- (26).Zhao D; Wang J; Yin D; Li M; Chen X; Juhasz AL; Luo J; Navas-Acien A; Li H; Ma LQ Arsanilic acid contributes more to total arsenic than roxarsone in chicken meat from Chinese markets. J. Hazard. Mater 2020, 383, 121178. [DOI] [PubMed] [Google Scholar]
- (27).Levine T; Rispin A; Marcus WL; Chen C; Gibb H Special Report on Ingested Inorganic Arsenic: Skin Cancer; Nutritional Essentiality, Report EPA/625/3–87/013; U.S. Environmental Protection Agency: Washington, DC, 1988. [Google Scholar]
- (28).Yost LJ; Schoof RA; Aucoin R Intake of inorganic arsenic in the North American diet. Hum. Ecol Risk Assess. 1998, 4 (1), 137–152. [Google Scholar]
- (29).Arai Y; Lanzirotti A; Sutton S; Davis JA; Sparks DL Arsenic speciation and reactivity in poultry litter. Environ. Sci. Technol 2003, 37 (18), 4083–4090. [DOI] [PubMed] [Google Scholar]
- (30).Bednar AJ; Garbarino JR; Ferrer I; Rutherford DW; Wershaw RL; Ranville JF; Wildeman TR Photodegradation of roxarsone in poultry litter leachates. Sci. Total Environ 2003, 302 (1), 237–245. [DOI] [PubMed] [Google Scholar]
- (31).Cortinas I; Field JA; Kopplin M; Garbarino JR; Gandolfi AJ; Sierra-Alvarez R Anaerobic biotransformation of roxarsone and related N-substituted phenylarsonic acids. Environ. Sci. Technol 2006, 40 (9), 2951–2957. [DOI] [PubMed] [Google Scholar]
- (32).Garbarino JR; Bednar AJ; Rutherford DW; Beyer RS; Wershaw RL Environmental Fate of Roxarsone in Poultry Litter. I. Degradation of Roxarsone during Composting. Environ. Sci. Technol 2003, 37 (8), 1509–1514. [DOI] [PubMed] [Google Scholar]
- (33).Han J; Zhang F; Cheng L; Mu Y; Liu D; Li W; Yu H Rapid release of arsenite from roxarsone bioreduction by exoelectrogenic bacteria. Environ. Sci. Technol. Lett 2017, 4 (8), 350–355. [Google Scholar]
- (34).Liu X; Zhang W; Hu Y; Hu E; Xie X; Wang L; Cheng H Arsenic pollution of agricultural soils by concentrated animal feeding operations (CAFOs). Chemosphere 2015, 119, 273–281. [DOI] [PubMed] [Google Scholar]
- (35).Fisher E; Dawson AM; Polshyna G; Lisak J; Crable B; Perera E; Ranganathan M; Thangavelu M; Basu P; Stolz JF Transformation of inorganic and organic arsenic by Alkaliphilus oremlandii sp. nov. strain OhILAs. Ann. N. Y. Acad. Sci 2008, 1125, 230–241. [DOI] [PubMed] [Google Scholar]
- (36).Pawitwar SS; Nadar VS; Kandegedara A; Stemmler TL; Rosen BP; Yoshinaga M Biochemical characterization of ArsI: A novel C-As lyase for degradation of environmental organoarsenicals. Environ. Sci. Technol 2017, 51 (19), 11115–11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Chen J; Rosen BP Organoarsenical biotransformations by Shewanella putrefaciens. Environ. Sci. Technol 2016, 50 (15), 7956–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Huang K; Peng H; Gao F; Liu Q; Lu X; Shen Q; Le XC; Zhao F-J Biotransformation of arsenic-containing roxarsone by an aerobic soil bacterium Enterobacter sp. CZ-1. Environ. Pollut 2019, 247, 482–487. [DOI] [PubMed] [Google Scholar]
- (39).Zhu X; Wang Y; Liu C; Qin W; Zhou D Kinetics, intermediates and acute toxicity of arsanilic acid photolysis. Chemosphere 2014, 107, 274–281. [DOI] [PubMed] [Google Scholar]
- (40).Xie X; Hu Y; Cheng H Mechanism, kinetics, and pathways of self-sensitized sunlight photodegradation of phenylarsonic compounds. Water Res 2016, 96, 136–147. [DOI] [PubMed] [Google Scholar]
- (41).Xie X; Zhang Z; Hu Y; Cheng H A mechanistic kinetic model for singlet oxygen mediated self-sensitized photo-oxidation of organic pollutants in water. Chem. Eng. J 2018, 334, 1242–1251. [Google Scholar]
- (42).Wang L; Cheng H Birnessite (δ-MnO2) mediated degradation of organoarsenic feed additive p-arsanilic acid. Environ. Sci. Technol 2015, 49 (6), 3473–3481. [DOI] [PubMed] [Google Scholar]
- (43).Joshi TP; Zhang G; Jefferson WA; Perfilev AV; Liu R; Liu H; Qu J Adsorption of aromatic organoarsenic compounds by ferric and manganese binary oxide and description of the associated mechanism. Chem. Eng. J 2017, 309, 577–587. [Google Scholar]
- (44).Joshi TP; Zhang G; Cheng H; Liu R; Liu H; Qu J Transformation of para arsanilic acid by manganese oxide: Adsorption, oxidation, and influencing factors. Water Res 2017, 116, 126–134. [DOI] [PubMed] [Google Scholar]
- (45).Garbarino JR; Bednar AJ; Rutherford DW; Beyer RS; Wershaw RL Environmental fate of roxarsone in poultry litter. I. Degradation of roxarsone during composting. Environ. Sci. Technol 2003, 37 (8), 1509–1514. [DOI] [PubMed] [Google Scholar]
- (46).Liu X; Zhang W; Hu Y; Cheng H Extraction and detection of organoarsenic feed additives and common arsenic species in environmental matrices by HPLC-ICP-MS. Microchem. J 2013, 108, 38–45. [Google Scholar]
- (47).Hu Y; Cheng H; Tao S Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ. Int 2017, 107, 111–130. [DOI] [PubMed] [Google Scholar]
- (48).Hu Y; Cheng H Use of veterinary antimicrobials in China and efforts to improve their rational use. J. Glob. Antimicrob. Resist 2015, 3 (2), 144–146. [DOI] [PubMed] [Google Scholar]
- (49).Hu Y; Cheng H Health risk from veterinary antimicrobial use in China’s food animal production and its reduction. Environ. Pollut 2016, 219, 993–997. [DOI] [PubMed] [Google Scholar]
- (50).Chen F; Long X; Yi J Methods for determination of p-arsanilic acid and roxarsone in animal feeds. Hunan Siliao 2014, 4, 22–25 (in Chinese). [Google Scholar]
- (51).Fujian Provincial Bureau of Quality and Technical Supervision. Safety and Quality Standards for Swine Feeds, Fujian Provincial Standard DB35/562–2005; Fuzhou, China, 2005. [Google Scholar]
- (52).Fujian Provincial Bureau of Quality and Technical Supervision. Safety and Quality Standards for Feeds of Swine, Chicken, and Duck, Fujian Provincial Standard DB35/562–2008; Fuzhou, China, 2008. [Google Scholar]
- (53).Chinese Veterinary Pharmacopoeia Commission. Notification on Recommendation to Ban the use of p-Arsanilic Acid and Two Other Veterinary Drugs as Feed Additives on Food-producing Animals, Document 14, 2017; Beijing, China, 2017. [Google Scholar]
- (54).U.S. Environmental Protection Agency (USEPA). Risk Assessment Guidance for Superfund, Volume 1, Human Health Evaluation Manual (Part B, Development of Risk-Based Preliminary Remediation Goals), Report EPA/540/R–92/003; Washington, DC, 1991. [Google Scholar]
- (55).National Research Council (NRC). Arsenic in Drinking Water; National Academic Press: Washington DC, 1999. [Google Scholar]
- (56).Rosenthal A; Gray GM; Graham JD Legislating acceptable cancer risk from exposure to toxic chemicals. Ecology L. Q 1992, 19, 269–362. [Google Scholar]
- (57).Fan S; Mao Z; Lee AH; Hu T Economic costs of lung cancer in China. Int. J. Oncol. Res 2018, 1, 007. [Google Scholar]
- (58).Abt CC The social costs of cancer. Soc. Indic. Res 1975, 2 (2), 175–190. [Google Scholar]
- (59).Chen Q Emissions Inventory of Arsenic in China. M.S. Thesis, Nanjing University, Nanjing, China, 2013. (in Chinese). [Google Scholar]
- (60).Yang X; Li Q; Tang Z; Zhang W; Yu G; Shen Q; Zhao F-J Heavy metal concentrations and arsenic speciation in animal manure composts in China. Waste Manage. 2017, 64, 333–339. [DOI] [PubMed] [Google Scholar]
- (61).Williams PN; Villada A; Deacon C; Raab A; Figuerola J; Green AJ; Feldmann J; Meharg AA Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol 2007, 41 (19), 6854–6859. [DOI] [PubMed] [Google Scholar]
- (62).Mcbride MB; Shayler HA; Russell-Anelli JM; Spliethoff HM; Marquez-Bravo LG Arsenic and lead uptake by vegetable crops grown on an old orchard site amended with compost. Water, Air, Soil Pollut 2015, 226 (8), 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Food and Agriculture Organization (FAO). Production Systems Management: Livestock–environment Interactions. Available at http://www.fao.org/ag/againfo/resources/documents/brief_notes/BNlivest-enviro10.pdf (last accessed on Oct. 6, 2019). [Google Scholar]
- (64).Liang F; Li Y; Zhang G; Tan M; Lin J; Liu W; Li Y; Lu W Total and speciated arsenic levels in rice from China. Food Addit. Contam., Part A 2010, 27 (6), 810–816. [DOI] [PubMed] [Google Scholar]
- (65).Li G; Sun G-X; Williams PN; Nunes L; Zhu Y-G Inorganic arsenic in Chinese food and its cancer risk. Environ. Int 2011, 37 (7), 1219–1225. [DOI] [PubMed] [Google Scholar]
- (66).Ministry of Agriculture (MOA). Administrative Measures on Veterinary Prescription Drugs and Veterinary Non-prescription Drugs; Beijing, China, 2013. [Google Scholar]
- (67).Hu Y; Cheng H Elevated antimicrobial residues in animal food products call for institutional changes on veterinary drug management and animal food product surveillance in China. Int. J. Antimicrob. Agents 2018, 51 (1), 165–166. [DOI] [PubMed] [Google Scholar]
- (68).Yan H; Xu D; Meng H; Shi L; Li L Food poisoning by clenbuterol in China. Qual Assur. Saf. Crops Foods 2015, 7, 27–35. [Google Scholar]
- (69).Ministry of Agriculture and Rural Affairs (MOA). Public Announcement on the Results of National Animal Feed Quality and Safety Supervision and Sampling Inspections; Beijing, China, 2019. [Google Scholar]
- (70).World Health Organization (WHO); Food and Agriculture Organization of the United Nations; Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Food Additives and Contaminants, 72nd Report of the Joint FAO/WHO Expert Committee on Food Additives (WHO Technical Report Series 959); World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- (71).Rodríguez-Lado L; Sun G; Berg M; Zhang Q; Xue H; Zheng Q; Johnson CA Groundwater arsenic contamination throughout China. Science 2013, 341 (6148), 866–868. [DOI] [PubMed] [Google Scholar]
- (72).Zhang X Progress in the application of organic arsenic feed additives and their toxicity and environmental behavior. Xiandai Xufu Shouyi 2011, 10, 36–38 (in Chinese). [Google Scholar]
- (73).Xuan M; Xu Z; Wu G; OH W; Li J; He W Analysis of utilization of fecal resources in large scale livestock and poultry breeding in China. Noneye Ziyuan Yu Huanjing Xuebao 2018, 35, (2), 126–132 (in Chinese). [Google Scholar]
- (74).Wu S; Liu H; Huang H; Lei Q; Wang H; Zhai L; Liu S; Zhang Y; Hu Y Analysis on the amount and utilization of manure in livestock and poultry breeding in China. Gongcheng Kexue 2018, 20, (5), 103–111 (in Chinese). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


