Abstract
Purpose: To investigate relationships among physical activity, changes in physical function, and health-related quality of life (HRQOL) among patients with pancreatic adenocarcinoma enrolled in a home-based exercise prehabilitation program. Methods: Patients with resectable pancreatic adenocarcinoma receiving preoperative chemotherapy and/or chemoradiation were enrolled on this prospective, single-arm trial and were advised to perform ≥60 minutes each of moderate-intensity aerobic exercise and strengthening exercise weekly. Activity was measured via self-report and accelerometers, including moderate-to-vigorous physical activity (MVPA), light physical activity (LPA), and sedentary activity (SA). Physical function measures at baseline and restaging follow-up included 6-minute walk test (6MWT), 5 times sit-to-stand (5×STS), handgrip strength (HGS), 3-m walk for gait speed (GS), and the PROMIS Physical Function Short Form. HRQOL was measured via the FACT-Hep questionnaire. Results: Fifty participants with mean age 66 years (standard deviation = 8 years) were enrolled. The 6MWT, 5×STS, and GS significantly improved from baseline to restaging follow-up (P=.001, P=.049, and P=.009, respectively). Increases in self-reported aerobic exercise, weekly MVPA, and LPA were associated with improvement in 6MWT (β=.19, P=.048; β=.18, P=.03; and β=.08, P=.03, respectively) and self-reported physical functioning (β=.02, P=.03; β=.03, P=.005; and β=.01, P=.02, respectively). Increased weekly LPA was associated with increased HRQOL (β=.03, P=.02). Increased SA was associated with decreased HRQOL (β=-.02,P=.01). Conclusions: Patients with potentially resectable pancreatic cancer exhibit meaningful improvement in physical function with prehabilitation; physical activity was associated with improved physical function and HRQOL. These data highlight the importance of physical activity during treatment for pancreatic cancer.
Keywords: prehabilitation, pancreatic cancer, preoperative exercise, physical function, outcome measures
Introduction
Pancreatic cancer is a leading cause of cancer-related death.1 Pancreatectomy can improve long-term survival for patients with resectable pancreatic cancer,2 but the complexity of surgery and its sequelae requires robust preoperative health and functional status. Patients with pancreatic cancer are generally older adults in whom frailty and/or sarcopenia are common, and those in whom surgery is anticipated increasingly undergo preoperative chemotherapy and chemoradiation therapy that may further diminish functional status.3-5 It is, therefore, important to develop strategies to optimize physical function and well-being concurrent with preoperative treatment, to improve treatment tolerance and readiness for surgery.
Preoperative exercise, a component of prehabilitation, is an increasingly common strategy to improve the outcomes of cancer treatment. Patients with various cancer diagnoses have demonstrated improvements in fitness, physical functioning, muscle strength, and health-related quality of life (HRQOL) from prehabilitation exercise programs.6,7 Improved fitness measured by cardiopulmonary exercise testing has been demonstrated in prehabilitation for patients undergoing surgery for lung cancer, liver resection for liver metastases, and colorectal cancer resection.8-10 Moreover, improved exercise capacity as measured by improvement in the 6-minute walk test (6MWT) has been demonstrated robustly in multiple cancer populations.11-13 Preservation of and/or improved muscle strength has been documented in patients undergoing surgery for lung, rectal, and prostate cancers.11,14-16 Preoperative exercise interventions have led to improvement in HRQOL among patients with colorectal and liver cancers.9,17 However, few patients cited in these studies received chemotherapy and/or radiation concurrent with prehabilitation.
Patients with pancreatic cancer undergoing preoperative treatment have an average age of 65 years18 and are often frail and/or sarcopenic at the time of diagnosis.19 Frailty, which is a syndrome characterized by weakness, slow gait, weight loss, exhaustion, and low activity, is prevalent among patients undergoing treatment for pancreatic cancer and has been associated with postoperative complications and reduced survival.19,20 Sarcopenia has also been associated with reduced survival, but improved disease-free survival may be observed in patients in whom skeletal muscle is preserved during treatment.5,21 Furthermore, patients receiving preoperative treatment may be at risk for further decline in physical function.22 Poor physical function has been associated with discharge to a rehabilitation, subacute, or long-term acute care facility following surgery.23 Physiologic performance also affects the types of chemotherapy regimens for which patients are eligible. Patients with favorable performance status may receive intensified or combination chemotherapy regimens and thus may have better cancer treatment outcomes.24
The relationships among physical activity and changes in fitness, physical functioning, and HRQOL in this clinical context thus warrant investigation. To date, no studies have examined potential physiologic outcomes related to preoperative exercise among patients with pancreatic cancer, particularly in patients receiving preoperative chemotherapy and/or chemoradiation. The purpose of the current study was to investigate relationships between physical activity and both HRQOL and physical function among patients enrolled in a home-based prehabilitation exercise program while receiving preoperative treatment for pancreatic cancer. We hypothesized that exercise program adherence and physical activity would be positively associated with changes in physical function and HRQOL in these patients.
Methods
Study Setting
This was a single-arm, prospective trial conducted at The University of Texas MD Anderson Cancer Center, a comprehensive cancer center in Houston, TX (ClinicalTrials.gov identifier NCT02295956). The institutional review board approved all study activities (Protocol #2014-0702). Patients presenting with technically resectable pancreatic adenocarcinoma between February 2015 and January 2017 were screened for enrollment, and informed consent was obtained from all participants. Eligibility requirements included intended pancreatectomy for biopsy-positive pancreatic cancer; treatment plan including preoperative chemotherapy and/or chemoradiation followed by rest before final surgical evaluation; English fluency and telephone access; and willingness to engage in follow-up calls every 2 weeks and maintain daily exercise logs. Exclusion criteria included underlying and unstable cardiac or pulmonary disease or symptomatic cardiac disease (New York Heart Association functional class III or IV), acute musculoskeletal injury or fracture that affected exercise ability, intense pain (numeric rating ≥7 out of 10), or other disease that precluded unsupervised exercise.
Following recommendation and approval from medical or surgical oncologists, patients completed the Physical Activity Readiness Questionnaire25 and the Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function 12a Short Form screener question (“Can you walk 25 feet on a level surface, with or without support?”).26 Self-reported loss of balance, chest pain, dizziness, or loss of consciousness during physical activity and inability to walk 25 feet on a level surface were grounds for exclusion. Patients who reported musculoskeletal dysfunction that limited physical activity required clearance from a Physical Medicine and Rehabilitation physician.
Exercise Prehabilitation Program
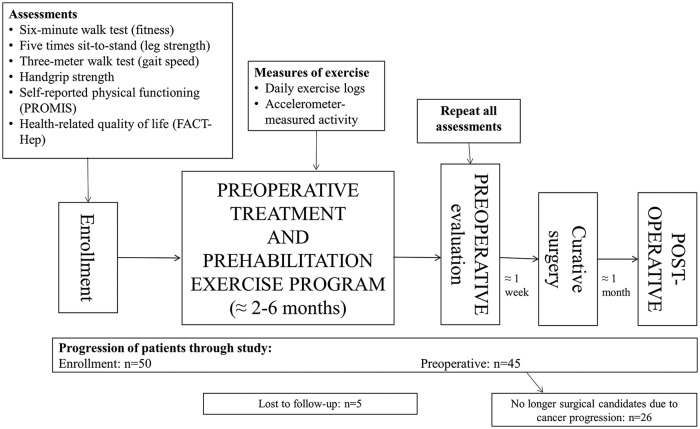
The exercise program has been previously described in detail.27,28 The program was modeled after recommendations for cancer survivors from the American Cancer Society and the American College of Sports Medicine29,30 and modified to accommodate the possible activity limitations of patients undergoing simultaneous chemotherapy and/or chemoradiation. This was a home-based, multimodal exercise program throughout preoperative therapy (chemotherapy and/or chemoradiation and preoperative rest) until preoperative surgical evaluation (Figure 1). Participants were advised to participate in at least 60 minutes per week of preferred, moderate-intensity aerobic exercise (eg, brisk walking, elliptical trainers, or stationary bicycles) and at least 60 minutes per week of full-body strengthening exercises, divided into 2 sessions separated by at least 1 day. Study staff provided in-person demonstrations of the proper form for all strengthening exercises at the time of enrollment. Study staff called participants via phone at least once every 2 weeks to encourage adherence and monitor for adverse events. Participants completed daily exercise logs to record minutes of aerobic and strengthening exercise.
Figure 1.
Study schema and progression of patients. Preoperative treatment included chemotherapy and/or chemoradiation followed by a preoperative rest period. Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System Physical Function 12a Short Form; FACT-Hep, Functional Assessment of Cancer Therapy–Hepatobiliary.
Physical activity was objectively measured using accelerometers (ActiGraph GT3X+; ActiGraph, LLC, Pensacola, FL; 2011) that patients were encouraged to wear for 2 consecutive weeks at the approximate midpoint of each phase of treatment. Calculations of weekly moderate-to-vigorous physical activity (MVPA), light physical activity (LPA), and sedentary activity (SA) were based on the Freedson adult cutpoints as previously described.28,31 At least 10 hours of wear time on at least 7 days during each targeted 2-week period were required to include an accelerometer wear period in analyses. Analyses in the current study included weekly minutes of MVPA, LA, and SA averaged across each patient’s treatment phases. For example, a patient who underwent chemotherapy, chemoradiation therapy, and off-treatment “rest” prior to follow-up restaging would have 3 wear periods, with average activity from those 3 periods included in analyses.
Furthermore, all patients enrolled met with a registered dietitian. The dietitian provided individualized nutrition recommendations including, but not limited to, estimated daily calorie and protein goals and strategies for management of treatment or disease-related side effects. All patients were encouraged to consume a high-protein meal, snack, or supplement drink within an hour of completion of strengthening exercises.
Outcome Measures
Outcome measures were obtained at the time of enrollment (baseline) and at follow-up restaging visits during which final determinations about surgical resection were made. Objective physical function was measured by the 6MWT, 5 times sit-to-stand test (5 × STS), handgrip strength (HGS), and 3-m walk test. The 6MWT, which was conducted per the guidelines of American Thoracic Society,32 is a safe and validated physical measure for submaximal exercise capacity and has been validated in cancer populations, including patients with colorectal and lung cancer.33-36 A change in 6MWT distance of 20 m was considered to be clinically meaningful.33,34,37 Lower limb strength was measured by the 5 × STS, which is a test wherein patients are instructed to rise from sitting in a standard height chair to standing 5 consecutive times with their arms crossed over their chests.38 HGS, measured via handheld dynamometry (Jamar hydraulic hand dynamometer),39 has been used in multiple populations. HGS is a predictor of cancer-related fatigue, postoperative complications, and mortality.40-43 The 3-m walk test was conducted to determine gait speed, which has been shown to be a predictor of treatment complications and survival in cancer populations.44,45 Self-reported functional status was recorded via the PROMIS Physical Function 12a Short Form.26 This is a validated questionnaire that asks 12 questions about basic mobility, ability to exercise, ability to perform housework, and self-care management.
Health-related quality of life was measured using the Functional Assessment of Cancer Therapy–Hepatobiliary (FACT-Hep) questionnaire, which is a validated and reliable tool.46 The FACT-Hep consists of the 27-question FACT-General (FACT-G) subscale and the 18-question hepatobiliary subscale. The FACT-G measures well-being in 4 domains: physical, social/family, emotional, and functional. The hepatobiliary subscale measures the severity of hepatobiliary-specific symptoms and consists of 18 questions designed to evaluate the severity of hepatobiliary cancer-specific symptoms. A high score on the FACT-G subscale indicates high HRQOL, and a high score on the hepatobiliary subscale indicates low disease-related symptoms. The instrument has shown strong validity, consistency, and reliability.46
The determination of frailty was based on Fried’s frailty criteria47 and measured by self-reported weight loss, gait speed (3-m walk test), HGS, self-reported physical activity (recorded via the International Physical Activity Questionnaire), and self-reported fatigue on 2 items from the Center for Epidemiologic Studies Depression Scale. As described in methods previously reported, sarcopenia was determined using sliceOmatic v5.0 software (Tomo Vision, Magog, Canada) to process computed tomography images of the abdomen and pelvis obtained for routine clinical care.5 Sarcopenia was defined as skeletal muscle index ≤38.9 cm2/m2 for women and skeletal muscle index ≤55.4 cm2/m2 for men.48
Analyses
Descriptive statistics were used to summarize patient characteristics and to quantify self-reported exercise, accelerometer physical activity, physical function outcome measures, and HRQOL. Owing to nonnormal distributions, Wilcoxon signed rank tests were used to compare physical function outcome measures and HRQOL between baseline and follow-up restaging, and Mann-Whitney U tests were used to compare weekly volumes of exercise and physical activity and baseline and follow-up values of outcome measures by sarcopenia and frailty status.
Multivariable regression models were used to assess associations of exercise and physical activity with outcome measures. Evaluation of HRQOL outcome measures included the FACT-Hep, FACT-G subscale, and the 4 FACT-G domains (Physical Well-Being, Social Well-Being, Emotional Well-Being, and Functional Well-Being). All multivariable regression models were adjusted for age, sex, exercise program duration, and baseline value of outcome measures on a theoretical basis and on the basis of evidence suggesting that these covariates are associated with differences in exercise or physical activity. All analyses were performed using SPSS Statistics Version 24 (IBM Corp, Armonk, NY; 2016), and P < .05 was considered statistically significant.
Results
Table 1 reports sociodemographic and clinical characteristics of the 50 patients who were enrolled. Concurrent with exercise, 13 patients (26%) received chemotherapy alone, 25 (50%) received chemoradiation alone, and 12 (24%) received chemotherapy followed by chemoradiation. The mean duration of the prehabilitation exercise program was 16 weeks (standard deviation = 9 weeks). Twenty-four patients (48%) underwent surgical resection with curative intent after showing stable or improved disease at preoperative restaging. Forty-two patients self-reported a mean of 126 minutes (standard deviation = 83 minutes) of weekly aerobic exercise and 39 minutes (standard deviation = 33 minutes) of strengthening exercise activity. Mean (standard deviation) weekly accelerometer-measured MVPA, LPA, and SA (measured in 44 patients) were 158.7 (146.7), 923.7 (294.5), and 4462.9 (620.2) minutes, respectively (Supplementary Table 1, available online). There was no significant difference in average weekly exercise or physical activity volume by baseline sarcopenia or frailty status (all P > .05). There was no significant difference in objective or self-reported outcome measures by sarcopenia status, and no significant difference in objective outcome measures by frailty status (all P > .05). Patients who were frail at baseline reported significantly lower physical functioning (PROMIS score) and lower HRQOL (FACT-Hep, FACT-G, and Hepatobiliary symptom subscale scores) than patients who were not frail (all P < .05).
Table 1.
Baseline Characteristics of Participants (N = 50).
| Variable | n (%) |
|---|---|
| Mean ± standard deviation age at enrollment | 66 ± 8 years |
| Female | 24 (48) |
| Mean ± standard deviation BMI at baseline | 27.6 ± 5.3 kg/m2 |
| Normal weight (18.5 ≤ BMI < 25) | 18 (36) |
| Overweight (25 ≤ BMI < 30) | 18 (36) |
| Obese (BMI ≥ 30) | 14 (28) |
| Sarcopenic | 28 (56) |
| Frail | 8 (16) |
| ECOG performance status | |
| 0 | 14 (28) |
| 1 | 32 (64) |
| 2 | 4 (8) |
Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group.
Table 2 shows HRQOL, physical function, and skeletal muscle at baseline and at the preoperative restaging visit. Mean (standard deviation) baseline physical function of the 50 participants was as follows: 6MWT 462.5 (82.7) meters, 5 × STS 11.4 (4.2) seconds, HGS 35.7 (11.8) kg, and 3-meter walk test 1.17 (0.2) meters per second. The 6MWT, 5 × STS, and 3-m walk test values significantly improved from baseline to the preoperative follow-up visit (P = .001, P = .049, P = .009, respectively). The improvement in the 6MWT was clinically meaningful.33,34,37 There were no differences in baseline or follow-up 6MWT, 5 × STS, HGS, or 3-m walk test by sarcopenia or frailty status (all P > .05).
Table 2.
Outcome Measures at Baseline Assessment and at the Preoperative Follow-up Visit (Reported in 45 Patients).
| Mean ± Standard Deviation | |||
|---|---|---|---|
| Variable | Baseline | Follow-up | P a |
| Six-minute walk test, meters | 462.5 ± 82.7 | 488.2 ± 93.1 | .001 |
| Five times sit-to-stand test, seconds | 11.4 ± 4.2 | 10.6 ± 3.6 | .049 |
| Handgrip strength, kilograms | 35.7 ± 11.8 | 35.7 ± 10.5 | .9 |
| Three-meter walk test, meters per second | 1.17 ± 0.2 | 1.22 ± 0.2 | .009 |
| PROMIS Physical Function score | 49.6 ± 5.3 | 46.6 ± 8.0 | .5 |
| FACT-Hep score | 137.9 ± 21.0 | 142.3 ± 21.9 | .09 |
| FACT-G | 84.0 ± 15.0 | 85.5 ± 14.5 | .09 |
| Hepatobiliary symptom subscale | 54.1 ± 9.7 | 56.7 ± 9.1 | .5 |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System Physical Function 12a Short Form; FACT-Hep, Functional Assessment of Cancer Therapy–Hepatobiliary; FACT-G, Functional Assessment of Cancer Therapy–General subscale.
Bold values indicate statistical significance.
Table 3 shows the associations among patient-reported exercise (aerobic and strengthening), and accelerometer-measured MVPA, LPA, and SA time and changes in outcome measures based on multivariable regression models. Self-reported aerobic exercise (β = .19, P = .048), weekly accelerometer-measured MVPA minutes (β = .18, P = .03), and weekly accelerometer-measured LPA minutes (β = .08, P = .03) were positively associated with improvement in the 6MWT during the preoperative period. Self-reported aerobic exercise (β = .02, P = .03), weekly MVPA minutes (β = .03, P = .005), and weekly LPA minutes (β = .01, P = .02) were also positively associated with improvement in perceived physical functioning during the preoperative period. Weekly LPA was positively associated with increased HRQOL as measured by the FACT-Hep (β = .03, P = .02), FACT-G (β = .02, P = .009), the Emotional Well-Being domain (β = .004, P = .04), and the Functional Well-Being domain (β = .009, P = .003) during the preoperative period. Increasing SA was associated with a decrease in HRQOL as measured by the FACT-Hep (β = −.02, P = .01), the FACT-G (β = −.009, P = .04), the hepatobiliary subscale (β = −.007, P = .01), and self-perceived physical function (β = −.007, P = .002). There were no significant associations between patient-reported aerobic or strengthening exercise activity and the FACT-G or any of the 4 domains of FACT-G. However, accelerometer-measured weekly MVPA was positively associated with the Physical Well-Being domain of the FACT-G (β = .01, P = .04). There were no significant associations between any self-reported exercise or accelerometer physical activity variables and changes in the 5 × STS, HGS, or 3-m walk test (all P > .05).
Table 3.
Associations Among Exercise, Physical Activity Variables, and Sedentary Time and Changes in Outcome Measures (in Minutes per Week)a.
| Δ6-Minute Walk Test, m | ΔSelf-Reported Physical Functioning, PROMIS Score | ΔHealth-Related Quality of Life, FACT-Hep Score | ΔHealth-Related Quality of Life, FACT-G Score | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | β | P | β | P | β | P | β | P |
| Patient-reported aerobic exercise | .19 | .048 | .02 | .03 | .004 | .92 | .004 | .9 |
| Patient-reported strengthening exercise | .15 | .57 | .008 | .77 | .09 | .30 | .02 | .8 |
| Accelerometer-measured MVPA | .18 | .03 | .03 | .005 | .03 | .25 | .02 | .2 |
| Accelerometer-measured LPA | .08 | .03 | .01 | .02 | .03 | .02 | .02 | .009 |
| Accelerometer-measured SA | −.04 | .08 | −.007 | .002 | −.02 | .01 | −.009 | .04 |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System Physical Function 12a Short Form; FACT-Hep, Functional Assessment of Cancer Therapy–Hepatobiliary; FACT-G, Functional Assessment of Cancer Therapy–General subscale; MVPA, moderate-to-vigorous physical activity; LPA, light physical activity; SA, sedentary activity.
Multivariable regression models were adjusted for age, sex, exercise program duration, and baseline value of the outcome measure. Bold values indicate statistical significance.
Discussion
Patients with pancreatic cancer who are prescribed chemotherapy and/or radiation treatment in anticipation of surgical resection are often elderly, frail, and sarcopenic, and these preoperative therapies, though important from an oncologic perspective, may further depress functional status. There is a strong need for prehabilitation in this context. In this pragmatic trial of preoperative exercise prescribed concurrent with active cancer therapy, patients with pancreatic cancer had a statistically and clinically significant improvement in their submaximal exercise capacity as measured by the 6MWT, improvement in their leg strength as measured by the 5 × STS test, and improvement in their gait speed as measured by the 3-m walk test. Physical activity was positively associated with improvements in physical function and HRQOL. In contrast, SA was associated with reductions in HRQOL, including both hepatobiliary symptoms and physical well-being. These data demonstrate the potential for exercise prescriptions to preserve or improve physical functioning during preoperative therapy.
In general, prehabilitation is used to optimize preoperative physical function prior to potentially morbid operations. In the current study, physical outcome measures were used to monitor the functional trajectory of patients receiving treatment. The 6MWT is a validated test of submaximal exercise capacity. Preoperative 6MWT distance has been associated with intraoperative and postoperative complications.37,49,50 Improvement in the preoperative 6MWT distance has been associated with decreased postoperative pulmonary complications and reduced length of stay in patients with lung cancer.13 In patients with colorectal cancer, improvement in the 6MWT distance preoperatively also predicts improved functional recovery back to baseline.12 In the current study, patients had a statistically significant and clinically meaningful improvement in their 6MWT distance during the preoperative period. This is quite remarkable as all of these patients received chemotherapy and/or chemoradiation concurrently with their home-based exercise program.
Other metrics further suggest that the physical function of patients improved during treatment. Among these was significant improvement in lower limb strength. The 5 × STS test measures proximal hip and leg strength, which is essential for mobilizing out of bed postoperatively. The 5 × STS results have been shown to be a predictor of falls and a significant predictor of activities of daily living and disability.51 The baseline average gait speed in our study, as measured by the 3-m walk test, was low, but it also improved during the preoperative period. Faster gait speed predicts fewer treatment complications and improved survival in cancer populations.44,45 Improvement in gait speed may mitigate the disability associated with sarcopenia.52 Although HGS did not change during the preoperative period, we note that maintenance of HGS, as an important indicator of functional fitness and ability to perform activities of daily living, may be an important outcome of prehabilitation during preoperative cancer treatments. A recent study examining sarcopenia and HGS during preoperative treatment for esophageal cancer found significant loss of HGS from baseline to follow-up.53
Physical activity was positively associated with improvement in the 6MWT and self-reported physical functioning. However, we did not detect a significant association between physical activity and HGS, 5 × STS, or the 3-m walk test. This may be due to lack of adherence to the strengthening exercise regimen or lack of effective strength training with patients exercising independently and unsupervised at home. Overall, none of the favorable associations we observed linking exercise and physical activity to outcomes in this study involved strengthening exercise. Although home-based strengthening exercise was safe in this study (ie, no adverse events were reported), it may be important to improve strengthening exercise adherence by targeting social support or self-efficacy.54 With a more effective home strength-training regimen, we may be better able to investigate its specific role in improving perioperative outcomes. We observed significantly lower self-reported physical functioning and HRQOL among patients who exhibited frailty at baseline; future interventions may benefit from identifying frail individuals and tailoring exercise regimens and support systems to address specific functional limitations and HRQOL domains.
Finally, it is notable that accelerometer-measured LPA (and not accelerometer-measured MVPA or self-reported exercise adherence) was positively associated with HRQOL, and increased accelerometer-measured SA was associated with reduced HRQOL scores in multiple domains. This suggests that patients undergoing preoperative treatment for pancreatic cancer may benefit from simple recommendations or programming that help them avoid being sedentary. Improving perioperative HRQOL using preoperative exercise has been identified as an intervention target for colorectal cancer survivors,55 and given our findings, the same target should be applied for pancreatic cancer survivors.
The limitations of the current study are primarily associated with its single-arm design and absence of a control group. Furthermore, there was wide variability in treatment courses and durations among patients in this pragmatic study, reflecting the real-life clinical care of patients with resectable pancreatic cancer. However, this variability imposed statistical limitations that future studies should attempt to control with advanced matching techniques and stratification. Fewer than half of patients in this study underwent surgical resection. The postoperative sample size and variability in the period before which participants could comfortably perform postoperative assessments precluded our ability to collect meaningful data describing recovery from surgery. Future prehabilitation trials should aim to collect postoperative assessment data consistently to demonstrate the potential relationship between preoperative exercise and postoperative recovery.
The strengths of our study are also worthy of note. These include its inclusion of both self-reported and objective exercise data to quantify program adherence and general physical activity. Objective monitoring with accelerometers helped corroborate self-reported exercise data, which is widely known to be subject to recall and favorability biases.56,57 We also used validated functional outcome measures to detect changes in physical function. Our study provided important initial evidence of the benefits of exercise while undergoing preoperative treatment for pancreatic cancer. Through a simple, home-based exercise program, we showed that exercise can contribute to important improvements in perioperative function and well-being among patients with pancreatic cancer.
Conclusion
Patients with pancreatic cancer participating in a prehabilitation exercise program improved physical function during preoperative treatment for pancreatic cancer. Increased physical activity was associated with improved HRQOL and functional outcomes. The findings in the current study are widely applicable in the clinical setting. Establishing formal programs to encourage preoperative exercise may improve important perioperative outcomes for these patients. It is important to study this further with randomized, controlled studies to demonstrate the effects of exercise on physical function and cancer outcomes.
Supplemental Material
Supplemental material, Supplementary_Table_1_PancOutcomes for Home-Based Exercise Prehabilitation During Preoperative Treatment for Pancreatic Cancer Is Associated With Improvement in Physical Function and Quality of Life by An Ngo-Huang, Nathan H. Parker, Eduardo Bruera, Rebecca E. Lee, Richard Simpson, Daniel P. O’Connor, Maria Q. B. Petzel, Rhodora C. Fontillas, Keri Schadler, Lianchun Xiao, Xuemei Wang, David Fogelman, Sunil K. Sahai, Jeffrey E. Lee, Karen Basen- Engquist and Matthew H. G. Katz in Integrative Cancer Therapies
Acknowledgments
We would like to acknowledge Scientific Publications in the Research Medical Library at the University of Texas MD Anderson Cancer Center for their review of this manuscript. This study used the Patient-Reported Outcomes, Survey, and Population Research Shared Resource, which is supported by a Cancer Center Support Grant (CA 016672, Principal Investigator: R. DePinho; The University of Texas MD Anderson Cancer Center) from the National Cancer Institute, National Institutes of Health. Patient Reported Outcomes Measurement Information System (PROMIS) was funded with cooperative agreements from the National Institutes of Health Common Fund Initiative (U54AR057951, U01AR052177, U54AR057943, U54AR057926, U01AR057948, U01AR052170, U01AR057954, U01AR052171, U01AR052181, U01AR057956, U01AR052158, U01AR057929, U01AR057936, U01AR052155, U01AR057971, U01AR057940, U01AR057967, and U01AR052186). The contents of this article use data developed under PROMIS. These contents do not necessarily represent an endorsement by the US Federal Government or PROMIS. See www.nihpromis.org for additional information on the PROMIS initiative.
Footnotes
Authors’ Note: The data presented in this article were previously presented as a poster at the American Society for Preventive Oncology Annual Meeting (Parker NH, Ngo-Huang A, Basen-Engquist K, Petzel MQB, Fogelman D, Lee RE, O’Connor DP, Martinez VA, Katz MHG. Physical activity is associated with improved quality of life and functional fitness among patients receiving preoperative therapy for pancreatic cancer. American Society for Preventive Oncology Annual Meeting, New York, NY, April 2018).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Knox Family Foundation; Center for Energy Balance in Cancer Prevention & Survivorship, Duncan Family Institute for Cancer Prevention and Risk Assessment; Texas Chapter of the American College of Sports Medicine; Cancer Prevention & Research Institute of Texas Training Grant/MD Anderson Cancer Prevention Research Training Program (RP170259, Dr Shine Chang, Principal Investigator); the Bettie Willerson Driver Cancer Research Fund, and the National Institutes of Health/National Cancer Institute (Award Number P30CA016672; used the Clinical Trials Support Resource and the Biostatistics Resource Group).
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Trial Registration: This trial was registered at clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/NCT02295956, registration NCT02295956.
ORCID iD: An Ngo-Huang  https://orcid.org/0000-0003-4797-4147
https://orcid.org/0000-0003-4797-4147
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Saad AM, Turk T, Al-Husseini MJ, Abdel-Rahman O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer. 2018;18:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vreeland TJ, Katz MHG. Timing of pancreatic resection and patient outcomes: is there a difference? Surg Clin North Am. 2018;98:57-71. [DOI] [PubMed] [Google Scholar]
- 3. Sahai SK. Perioperative assessment of the cancer patient. Best Pract Res Clin Anaesthesiol. 2013;27:465-480. [DOI] [PubMed] [Google Scholar]
- 4. Cooper AB, Holmes HM, des Bordes JK, et al. Role of neoadjuvant therapy in the multimodality treatment of older patients with pancreatic cancer. J Am Coll Surg. 2014;219:111-120. [DOI] [PubMed] [Google Scholar]
- 5. Cooper AB, Slack R, Fogelman D, et al. Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22:2416-2423. [DOI] [PubMed] [Google Scholar]
- 6. Carli F, Feldman LS. From preoperative risk assessment and prediction to risk attenuation: a case for prehabilitation. Br J Anaesth. 2019;122:11-13. [DOI] [PubMed] [Google Scholar]
- 7. Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92:715-727. [DOI] [PubMed] [Google Scholar]
- 8. Pouwels S, Fiddelaers J, Teijink JA, Woorst JF, Siebenga J, Smeenk FW. Preoperative exercise therapy in lung surgery patients: a systematic review. Respir Med. 2015;109:1495-1504. [DOI] [PubMed] [Google Scholar]
- 9. Dunne DF, Jack S, Jones RP, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg. 2016;103:504-512. [DOI] [PubMed] [Google Scholar]
- 10. West MA, Loughney L, Lythgoe D, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. 2015;114:244-251. [DOI] [PubMed] [Google Scholar]
- 11. García RS, Yáñez-Brage MI, Moolhuyzen EG, Riobo MS, Paz AL, Mate JMB. Preoperative exercise training prevents functional decline after lung resection surgery: a randomized, single-blind controlled trial. Clin Rehabil. 2017;31:1057-1067. [DOI] [PubMed] [Google Scholar]
- 12. Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937-947. [DOI] [PubMed] [Google Scholar]
- 13. Lai Y, Su J, Qiu P, et al. Systematic short-term pulmonary rehabilitation before lung cancer lobectomy: a randomized trial. Interact Cardiovasc Thorac Surg. 2017;25:476-483. [DOI] [PubMed] [Google Scholar]
- 14. Heldens AF, Bongers BC, de Vos-Geelen J, van Meeteren NL, Lenssen AF. Feasibility and preliminary effectiveness of a physical exercise training program during neoadjuvant chemoradiotherapy in individual patients with rectal cancer prior to major elective surgery. Eur J Surg Oncol. 2016;42:1322-1330. [DOI] [PubMed] [Google Scholar]
- 15. Singh F, Newton RU, Baker MK, et al. Feasibility of presurgical exercise in men with prostate cancer undergoing prostatectomy. Integr Cancer Ther. 2017;16:290-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillis C, Fenton TR, Sajobi TT, et al. Trimodal prehabilitation for colorectal surgery attenuates post-surgical losses in lean body mass: a pooled analysis of randomized controlled trials. Clin Nutr. 2019;38:1053-1060. [DOI] [PubMed] [Google Scholar]
- 17. Muller-Nordhorn J, Roll S, Böhmig M, et al. Health-related quality of life in patients with pancreatic cancer. Digestion. 2006;74:118-125. [DOI] [PubMed] [Google Scholar]
- 18. Cooper AB, Parmar AD, Riall TS, et al. Does the use of neoadjuvant therapy for pancreatic adenocarcinoma increase postoperative morbidity and mortality rates? J Gastrointest Surg. 2015;19:80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ngo-Huang A, Holmes HM, des Bordes JKA, et al. Association between frailty syndrome and survival in patients with pancreatic adenocarcinoma. Cancer Med. 2019;8:2867-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Augustin T, Burstein MD, Schneider EB, et al. Frailty predicts risk of life-threatening complications and mortality after pancreatic resections. Surgery. 2016;160:987-996. [DOI] [PubMed] [Google Scholar]
- 21. Choi MH, Yoon SB, Lee K, et al. Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018;9:326-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Romanus D, Kindler HL, Archer L, et al. Does health-related quality of life improve for advanced pancreatic cancer patients who respond to gemcitabine? Analysis of a randomized phase III trial of the cancer and leukemia group B (CALGB 80303). J Pain Symptom Manage. 2012;43:205-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahvi DA, Pak LM, Urman RD, Gold JS, Whang EE. Discharge destination following pancreaticoduodenectomy: a NSQIP analysis of predictive factors and post-discharge outcomes. Am J Surg. 2019;218:342-348. [DOI] [PubMed] [Google Scholar]
- 24. Boeck S, Hinke A, Wilkowski R, Heinemann V. Importance of performance status for treatment outcome in advanced pancreatic cancer. World J Gastroenterol. 2007;13:224-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci. 1992;17:338-345. [PubMed] [Google Scholar]
- 26. Jensen RE, Potosky AL, Reeve BB, et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res. 2015;24:2333-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ngo-Huang A, Parker NH, Wang X, et al. Home-based exercise during preoperative therapy for pancreatic cancer. Langenbecks Arch Surg. 2017;402:1175-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parker NH, Ngo-Huang A, Lee RE, et al. Physical activity and exercise during preoperative pancreatic cancer treatment. Support Care Cancer. 2019;27:2275-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmitz KH, Courneya KS, Matthews C, et al. ; American College of Sports Medicine. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426. [DOI] [PubMed] [Google Scholar]
- 30. American Cancer Society. ACS guidelines on nutrition and physical activity. https://www.cancer.org/healthy/eat-healthy-get-active/acs-guidelines-nutrition-physical-activity-cancer-prevention/guidelines.html. Accessed November 25, 2019. [PubMed]
- 31. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc, accelerometer. Med Sci Sports Exerc. 1998;30:777-781. [DOI] [PubMed] [Google Scholar]
- 32. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-117. [DOI] [PubMed] [Google Scholar]
- 33. Pecorelli N, Fiore JF, Jr, Gillis C, et al. The six-minute walk test as a measure of postoperative recovery after colorectal resection: further examination of its measurement properties. Surg Endosc. 2016;30:2199-2206. [DOI] [PubMed] [Google Scholar]
- 34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. [DOI] [PubMed] [Google Scholar]
- 35. Schmidt K, Vogt L, Thiel C, Jäger E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34:631-636. [DOI] [PubMed] [Google Scholar]
- 36. Granger CL, McDonald CF, Parry SM, Oliveira CC, Denehy L. Functional capacity, physical activity and muscle strength assessment of individuals with non-small cell lung cancer: a systematic review of instruments and their measurement properties. BMC Cancer. 2013;13:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moriello C, Mayo NE, Feldman L, Carli F. Validating the six-minute walk test as a measure of recovery after elective colon resection surgery. Arch Phys Med Rehabil. 2008;89:1083-1089. [DOI] [PubMed] [Google Scholar]
- 38. Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills. 2006;103:215-222. [DOI] [PubMed] [Google Scholar]
- 39. Ordan MA, Mazza C, Barbe C, et al. Feasibility of systematic handgrip strength testing in digestive cancer patients treated with chemotherapy: the FIGHTDIGO study. Cancer. 2018;124:1501-1506. [DOI] [PubMed] [Google Scholar]
- 40. Kilgour RD, Vigano A, Trutschnigg B, et al. Cancer-related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle. 2010;1:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen CH, Ho C, Huang YZ, Hung TT. Hand-grip strength is a simple and effective outcome predictor in esophageal cancer following esophagectomy with reconstruction: a prospective study. J Cardiothorac Surg. 2011;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sato S, Nagai E, Taki Y, et al. Hand grip strength as a predictor of postoperative complications in esophageal cancer patients undergoing esophagectomy. Esophagus. 2018;15:10-18. [DOI] [PubMed] [Google Scholar]
- 43. Kilgour RD, Vigano A, Trutschnigg B, Lucar E, Borod M, Morais JA. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer. 2013;21:3261-3270. [DOI] [PubMed] [Google Scholar]
- 44. Pamoukdjian F, Lévy V, Sebbane G, et al. Slow gait speed is an independent predictor of early death in older cancer outpatients: results from a prospective cohort study. J Nutr Health Aging. 2017;21:202-206. [DOI] [PubMed] [Google Scholar]
- 45. Verweij NM, Schiphorst AH, Pronk A, van den Bos F, Hamaker ME. Physical performance measures for predicting outcome in cancer patients: a systematic review. Acta Oncol. 2016;55:1386-1391. [DOI] [PubMed] [Google Scholar]
- 46. Heffernan N, Cella D, Webster K, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol. 2002;20:2229-2239. [DOI] [PubMed] [Google Scholar]
- 47. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. [DOI] [PubMed] [Google Scholar]
- 48. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997-1006. [DOI] [PubMed] [Google Scholar]
- 49. Hayashi K, Yokoyama Y, Nakajima H, et al. Preoperative 6-minute walk distance accurately predicts postoperative complications after operations for hepato-pancreato-biliary cancer. Surgery. 2017;161:525-532. [DOI] [PubMed] [Google Scholar]
- 50. Keeratichananont W, Thanadetsuntorn C, Keeratichananont S. Value of preoperative 6-minute walk test for predicting postoperative pulmonary complications. Ther Adv Respir Dis. 2016;10:18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang F, Ferrucci L, Culham E, Metter EJ, Guralnik J, Deshpande N. Performance on five times sit-to-stand task as a predictor of subsequent falls and disability in older persons. J Aging Health. 2013;25:478-492. [DOI] [PubMed] [Google Scholar]
- 52. Perez-Sousa MA, Venegas-Sanabria LC, Chavarro-Carvajal DA, et al. Gait speed as a mediator of the effect of sarcopenia on dependency in activities of daily living. J Cachexia Sarcopenia Muscle. 2019;10:1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guinan EM, Doyle SL, Bennett AE, et al. Sarcopenia during neoadjuvant therapy for oesophageal cancer: characterising the impact on muscle strength and physical performance. Support Care Cancer. 2018;26:1569-1576. [DOI] [PubMed] [Google Scholar]
- 54. Parker NH, Lee RE, O’Connor DP, et al. Supports and barriers to home-based physical activity during preoperative treatment of pancreatic cancer: a mixed-methods study [published online October 7, 2019]. J Phys Act Health. doi: 10.1123/jpah.2019-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cheema FN, Abraham NS, Berger DH, Albo D, Taffet GE, Naik AD. Novel approaches to perioperative assessment and intervention may improve long-term outcomes after colorectal cancer resection in older adults. Ann Surg. 2011;253:867-874. [DOI] [PubMed] [Google Scholar]
- 56. Grimm EK, Swartz AM, Hart T, Miller NE, Strath SJ. Comparison of the IPAQ-Short Form and accelerometry predictions of physical activity in older adults. J Aging Phys Act. 2012;20:64-79. [DOI] [PubMed] [Google Scholar]
- 57. Trost SG, O’Neil M. Clinical use of objective measures of physical activity. Br J Sports Med. 2014;48:178-181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table_1_PancOutcomes for Home-Based Exercise Prehabilitation During Preoperative Treatment for Pancreatic Cancer Is Associated With Improvement in Physical Function and Quality of Life by An Ngo-Huang, Nathan H. Parker, Eduardo Bruera, Rebecca E. Lee, Richard Simpson, Daniel P. O’Connor, Maria Q. B. Petzel, Rhodora C. Fontillas, Keri Schadler, Lianchun Xiao, Xuemei Wang, David Fogelman, Sunil K. Sahai, Jeffrey E. Lee, Karen Basen- Engquist and Matthew H. G. Katz in Integrative Cancer Therapies