Abstract
Purpose: To assess the feasibility, safety, and preliminary effectiveness of a 12-week multimodal Qigong Mind-Body Exercise (QMBE) program for breast cancer survivors with persistent post-surgical pain (PPSP). Methods: This was a single-arm mixed-methods pilot study. Primary outcome measures were feasibility (recruitment, adherence) and safety. Validated self-report questionnaires were used to evaluate a constellation of interdependent symptoms, including pain, fatigue, mood, exercise, interoceptive awareness, and health-related quality of life at baseline and 12 weeks. A subset of the instruments was administered 6 months postintervention. Shoulder range of motion and grip strength were objectively assessed at baseline and 12 weeks. Qualitative interviews were conducted at baseline and 12 weeks. Results: Twenty-one participants were enrolled; 18 and 17 participants, respectively, completed the 12-week and 6-month outcome assessment. No serious adverse events were reported. Statistically significant improvements were observed at 12 weeks in pain severity and interference, fatigue, anxiety, depression, perceived stress, self-esteem, pain catastrophizing, and several subdomains of quality of life, interoceptive awareness, and shoulder range of motion. Changes in pain, fatigue, pain catastrophizing, anxiety, depression, and quality of life were clinically meaningful. Postintervention effects were sustained at 6 months. Conclusions: QMBE is a safe and gentle multimodal intervention that shows promise in conferring a broad range of psychosocial and physical benefits for breast cancer survivors with PPSP. Results support the value of future studies evaluating the impact of QMBE on multiple outcomes relevant to breast cancer survivors with PPSP.
Keywords: Qigong, mind-body exercise, breast cancer, persistent post-surgical pain
Introduction
There are more than 3.5 million breast cancer survivors (BCSs) currently living in the United States, and with improvements in early detection and treatment, this number is growing.1 One common problem in this population is persistent post-surgical pain (PPSP) following lumpectomy or mastectomy. The reported incidence of PPSP ranges from 25% to 60%.1 Pain is typically experienced in the breast area, axilla, arm, and side2,3 and can persist 2 to 3 years following surgery.4 PPSP is increasingly understood as a complex biopsychosocial condition,5 involving not only physiological and musculoskeletal impairments but also elevated levels of psychological distress including anxiety, depression, sleep disturbance, somatization, and catastrophizing.6-9 Given these multiple detrimental impacts, it is not surprising that PPSP has been rated by BCSs as one of the most troubling symptoms.10,11
Common treatment approaches to PPSP include analgesics and physical therapy. However, these approaches are ineffective for many patients12,13 and long-term use of pain medications is often undesirable due to side-effects. Given the complex constellation of symptoms faced by cancer survivors, including those with PPSP, it has been hypothesized that multimodal mind-body therapies that target both physical and psychosocial causes of distress may be more effective than unimodal therapies.14 Qigong mind-body exercise (QMBE) is a traditional Asian therapy that is growing in popularity in the West. QMBE is based on slow intentional movements, coordinated with breathing and a variety of cognitive skills. QMBE aims to strengthen, relax, and integrate the physical body and mind, to improve health and personal development.15 Recent systematic reviews and a meta-analysis concluded that QMBE has the potential to improve fatigue, sleep difficulty, depression, and overall quality of life in cancer patients.14 However, no studies to date have evaluated QMBE for PPSP.
To inform design features of a more definitive future study, this pilot study evaluated the feasibility (recruitment, adherence) and safety of a QMBE program for women with PPSP. We also evaluated responses to a spectrum of physical (pain, shoulder range of motion) and psychosocial (anxiety, depression, pain appraisal, quality of life) outcomes. Qualitative interviews were conducted to explore participants’ perspectives of study feasibility and perceived benefits.
Methods
Study Design
This was a single-arm, pre-post mixed-methods pilot study of a 12-week QMBE intervention for BCS with PPSP. In-person outcome assessments were conducted at baseline and postintervention. A 6-month mail-in questionnaire packet was completed to assess longer term outcomes. The study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board (15-347).
Participants
Women with a history of stage 0 to III breast cancer who had undergone surgical treatment and were experiencing PPSP at least 3 months after completing surgery, chemotherapy, and/or radiation were recruited from breast oncology clinics at the Dana-Farber Cancer Institute in Boston, Massachusetts. Interested patients were screened for eligibility by a research assistant. PPSP was determined by self-report. There was no required pain level for participation. Patients were considered ineligible if they had unstable cardiovascular disease, a psychiatric disorder that would preclude participation, metastatic cancer, a chronic medical condition that might affect upper extremity function (eg, stroke, Parkinson’s disease, multiple sclerosis), planned surgery during the intervention period, were pregnant, currently enrolled in physical therapy, exercised more than 240 minutes per week, or had recently attended regular QMBE, yoga, or Tai Chi classes. Our target sample size was 21. Subjects were recruited between June 2016 and April 2017 and enrolled between April 4 and 18, 2017.
Intervention
The 8 Strands of the Brocades QMBE involves a series of 8 gentle, dynamic upper-body stretching and strengthening movements, integrating multiple potentially therapeutic elements relevant to PPSP, including efficient posture, diaphragmatic breathing, and cognitive skills such as body awareness, focused attention, and imagery (See Supplement 1 for a summary of weekly content, based on protocols used in prior studies16; available online). Participants were asked to attend one 1.25-hour class per week for 12 weeks and to practice at home using an instructional video for 2 to 3 hours per week. Courses were taught by 2 instructors with more than 25 years of experience, including teaching in prior clinical trials. Instructors recorded class attendance and participants recorded their home practice using provided journals.
Outcomes
Feasibility and Safety
To evaluate the feasibility and safety of the QMBE program for this population, we collected data on recruitment and retention rates, class attendance and home practice adherence, and adverse events (AEs). Feasibility was defined a priori as completing recruitment and enrolling our target sample (n = 21) within 12 months, participants attending a minimum of 70% of classes and completing 70% of prescribed home practice, completion of 90% of all outcome assessments, and absence of any serious AEs.
AE data were collected in multiple ways. Weekly class attendance logs included fields for self-report of AEs. Study coordinators inquired about AEs during the regularly scheduled mid-study monitoring call. Participants were instructed to contact the study coordinator if they experienced symptoms of concern. QMBE teachers were instructed to inform the principal investigator of any AEs reported during class.
Clinical Measures
A battery of validated clinical outcomes was selected to span a broad range of symptoms affecting this population (see Table 1 for details). Baseline and 12-week follow-up evaluations were conducted at the Motion Analysis Laboratory at Spaulding Rehabilitation Hospital. A 6-month questionnaire packet including a subset of measures (as noted in Table 1) was sent to participants by mail.
Table 1.
Description of Clinical Self-Reported Outcome Measures.
| Category | Measure | Description |
|---|---|---|
| Pain | Brief Pain Inventory Short Form (BPI)a
• Pain interference • Pain severity |
9-item measure of the severity and impact of pain on daily function1,2 |
| Fatigue | Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F)a | 13-item measure of the intensity of fatigue during the past 7 days3 |
| Physical function | Shoulder ROM (active and passive) • Flexion • Extension • Adduction • Abduction • Internal rotation (0° and 90°) • External rotation (0° and 90° |
Assessed by the study physiatrist (GVD) using standard goniometric techniques |
| Grip strength • Right • Left |
Assessed using a JAMAR Hand Dynamometer. Measurements were recorded to the nearest 0.5 kg and a mean of 3 trials was calculated per hand. | |
| Psychological well-being/mood | Pain Catastrophizing Scale (PCS) • Helplessness • Rumination • Magnification • PCS total score |
13-item measure of catastrophic thinking associated with pain4,5 |
| Rosenberg Self-Esteem Scale (RSE) | 10-item measure of global self-worth; scored on a 4-point scale (1 = strongly agree, 4 = strongly disagree)6 | |
| Hospital Anxiety and Depression Scale (HADS) • Anxiety • Depression |
14-item measure of current feelings of anxiety and depression7 | |
| Perceived Stress Scale (PSS)a | 10-item measure of stress appraisal8,9 | |
| Interoceptive self-awareness | Multidimensional Assessment of Interoceptive Awareness (MAIA) • Noticing • Not distracting • Not worrying • Attention regulation • Emotional awareness • Self-regulation • Body listening • Trusting |
32-item measure of 8 dimensions of mindful body awareness10 |
| Behavior | Godin Leisure-Time Exercise Questionnaire (GLTQ) • Light intensity (min/week) • Moderate intensity (min/week) • Vigorous intensity |
Measure of the frequency of light, moderate, and vigorous intensity leisure-time physical activity11 |
| Self-Efficacy for Exercise scale (SEE) | 13-item measure of the ability to exercise in the face of barriers12 | |
| Quality of life | Functional Assessment of Cancer Therapy Breast Symptom Index (FACT-B+4)a
• Physical well-being • Social well-being • Emotional well-being • Functional well-being • Breast cancer symptoms • FACT-B total score |
42-item measure of physical, emotional, social, and functional well-being, as well as breast cancer specific symptomology13 |
Assessed also at 6 months via mail-in questionnaire packet.
References Cited in Table 1
Mendoza T, Mayne T, Rublee D, Cleeland C. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10:353-361. doi:10.1016/j.ejpain.2005.06.002.
Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309-318.
Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547-561.
Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524-532. doi:10.1037/1040-3590.7.4.524.
Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96:319-324.
Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965.
Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69-77.
Reis RS, Hino AA, Anez CR. Perceived stress scale: reliability and validity study in Brazil. J Health Psychol. 2010;15:107-114. doi:10.1177/1359105309346343.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385-396.
Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS One. 2012;7:e48230. doi:10.1371/journal.pone.0048230.
Godin GS. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Health Fitness J Can. 2011;4:18-22.
Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise scale. Nurs Res. 2000;49:154-159.
Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001;68:273-282.
Qualitative Interviews
At baseline and 12-week follow-up visits, semistructured open-ended interviews were conducted. Interviews lasted approximately 30 minutes each and were conducted after all other data were collected. Questions focused on subjects’ experience with cancer and PPSP, expectations and reasons for joining the study (baseline), and experience with and perceived effectiveness of the intervention (12-week follow-up). Interviews were digitally recorded and transcribed by Scribie Audio/Video Transcription (San Francisco, CA). Transcripts were coded for sought and emergent themes through content analysis.17
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics at baseline. Wilcoxon signed-rank tests were used to assess changes between baseline and 12-week follow-up. Pre-post change score analyses included only subjects who completed outcome measures at both time points. Each difference was calculated by subtracting the estimated score at the earlier time from the later time. Single question missing responses within an assessment were addressed using mean imputation guidelines suggested for the respective questionnaire. To assess the persistence of effects over time, longitudinal linear models were fit with time as the independent predictor for outcomes collected at 6-month follow-up. The assumed covariance structure within patient was variance component. Pairwise differences of each endpoint by time were based on least-squares means. Analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC) and SAS JMP 12 pro (SAS Institute Inc).
Results
Participant Flow
A total of 42 patients were screened for eligibility. Of those, 21 were ineligible (1 did not have breast surgery, 1 metastatic breast cancer, 1 planned surgery during the intervention period, 2 unreachable, 2 no longer interested, 3 did not have PPSP, and 11 not available to attend the scheduled classes) and 21 participants were eligible and enrolled in the study. Informed consent was obtained from each patient at the Dana-Farber Cancer Institute following the Dana Farber/Harvard Cancer Center registration process. Once approval was obtained from the patient’s treating oncologist, each patient was scheduled for a baseline data collection visit at the Spaulding Rehabilitation Hospital Motion Analysis Laboratory within 1 week of enrollment. All 21 participants completed baseline assessments and started the intervention within 3 weeks of baseline. Classes took place from April 26 to July 12, 2017. Two subjects withdrew during the intervention period due to unrelated health issues. One subject did not complete the 12-week follow-up due to scheduling conflicts.
Baseline Characteristics
Participants had a mean age of 54 (SD 10.2) years. Most (81%) were diagnosed with stage I to II breast cancer a mean of 5.1 (SD 2.7) years prior to study enrollment. Six (29%) participants had undergone a mastectomy only; 9 (43%) underwent mastectomy and reconstruction; and 6 (29%) underwent lumpectomy only. The mean number of years since surgery was 4.5 (SD 2.9). Average pain severity at baseline was 3.0 (SD 1.8) on an 11-point scale (see Table 2).
Table 2.
Baseline Characteristics.
| Characteristics | N = 21 | |
|---|---|---|
| Age (years), average ± SD | 54 ± 10.2 | |
| BMI (kg/m2), average ± SD | 31.2 ± 6.5 | |
| Breast cancer stage, n (%) | 0 | 2 (10%) |
| I | 8 (38%) | |
| II | 9 (43%) | |
| III | 2 (10%) | |
| Years since diagnosis, average ± SD | 5.1 ± 2.7 | |
| Years since surgery, average ± SD | 4.5 ± 2.9 | |
| Surgery type, n (%) | Lumpectomy | 6 (29%) |
| Mastectomy | 6 (29%) | |
| Mastectomy with reconstruction | 9 (43%) | |
| BPI: Pain Severity (11-point NRS), average ± SD | 3.0 ± 1.8 | |
| BPI: Pain Interference (11-point NRS), average ± SD | 2.5 ± 2.3 | |
| Past use of physical therapy for PPSP, n (%) | 13 (62%) | |
| Average minutes of exercise per week, average ± SD | Light | 60.2 ± 105.2 |
| Moderate | 40.9 ± 51.2 | |
| Vigorous | 6.4 ± 16.8 | |
| Expectation of pain reduction (11-point NRS), average ± SD | 5.4 ± 2.2 | |
| Race, n (%) | Asian | 1 (5%) |
| Black/African American | 4 (19%) | |
| White | 14 (67%) | |
| More than one race | 1 (4.8%) | |
| Other/unknown | 1 (4.8%) | |
| Education, n (%) | Some college | 2 (10%) |
| College graduate | 6 (29%) | |
| Some postgraduate training | 2 (10%) | |
| Postgraduate degree | 11 (52%) | |
Abbreviations: BMI, body mass index; BPI, Brief Pain Inventory; NRS, Numerical Rating Scale; PPSP, persistent post-surgical pain; SD, standard deviation.
Feasibility and Adherence
Our target sample of 21 was reached within the prespecified 12-month window. Of the 21 enrolled participants, 11 (52%) attended at least 8/12 classes; 14 (67%) attended at least 50% of classes. The mean number of classes attended was 6.52 (54%; SD 3.4; median 8.0, 66.7%). Fourteen participants (67%) successfully completed and returned home practice journals. Of those 14, 5 participants (36%) completed at least 70% of the prescribed home practice. The mean total number of home practice hours completed was 17.0 (SD 7.7; median 15.2), about 70.8% of the prescribed amount. Eighteen participants (86%) successfully completed both the baseline and 12-week outcome assessments. Seventeen participants (81%) returned the 6-month follow-up mail-in questionnaire packet. Sixteen participants (76%) completed both follow-up assessments. At 6-month follow-up, 12 participants (57%) reported that they had continued to practice QMBE an average of 69 minutes per week.
Adverse Events
No serious AEs were reported. The only AE attributable to the intervention, mentioned by 4 study participants, was musculoskeletal discomfort due to overstretching, which was minor, transient, and expected.
Clinical Outcomes
Range of Motion and Grip Strength
There were statistically significant increases in shoulder range of motion postintervention for left shoulder active and passive abduction, adduction, external rotation (0° and 90°), and flexion; right shoulder active abduction, adduction, external rotation (0° and 90°), and flexion; and right shoulder passive abduction, adduction, external rotation (0° only), and flexion (all Ps < .05). No significant differences were observed in grip strength postintervention (see Table 3).
Table 3.
Biopsychosocial Outcomes Postintervention for 18 Subjects Who Completed Baseline and 12-Week Follow-upa.
| Category | Outcome | Baseline (Mean, SD) | 12-Week Follow-up (Mean, SD) | Change (Mean, SD) | P |
|---|---|---|---|---|---|
| Physical | |||||
| Pain | Brief Pain Inventory (BPI) | ||||
| Pain interference | 2.2 (2.0) | 0.7 (1.0) | −1.5 (1.9) | .003 | |
| Pain severity | 2.6 (1.4) | 1.3 (1.3) | −1.3 (1.3) | .0002 | |
| Fatigue | Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) | 32.8 (7.8) | 40.2 (8.8) | 7.4 (7.3) | .001 |
| Physical Function | |||||
| Grip strength | |||||
| Right | 24.8 (5.0) | 25.2 (5.8) | 0.4 (4.1) | .97 | |
| Left | 23.0 (5.7) | 24.4 (5.9) | 1.4 (6.1) | .35 | |
| Shoulder range of motion | |||||
| Left—Active | |||||
| Abduction | 163.7 (12.1) | 175.4 (6.9) | 11.7 (9.5) | <.0001 | |
| Adduction | 35.3 (6.5) | 43.0 (4.0) | 7.7 (8.3) | .003 | |
| Extension | 49.2 (7.8) | 48.2 (2.9) | −1.0 (9.1) | .60 | |
| Flexion | 162.3 (13.6) | 171.4 (8.0) | 9.1 (10.8) | .001 | |
| External rotation 0° | 70.8 (10.7) | 80.6 (8.0) | 9.7 (10.2) | .0005 | |
| External rotation 90° | 79.2 (9.6) | 87.1 (9.2) | 7.8 (9.3) | .003 | |
| Internal rotation | 79.4 (8.6) | 83.6 (5.9) | 4.2 (9.0) | .10 | |
| Left—Passive | |||||
| Abduction | 171.2 (7.8) | 178.6 (2.3) | 7.4 (6.0) | .0002 | |
| Adduction | 39.1 (5.9) | 44.1 (3.0) | 5.1 (7.0) | .010 | |
| Extension | 51.3 (8.4) | 48.2 (2.9) | −3.1 (10.1) | .20 | |
| Flexion | 170.9 (8.0) | 176.1 (4.5) | 5.1 (7.7) | .02 | |
| External rotation 0° | 78.6 (7.4) | 84.7 (6.7) | 6.1 (6.3) | .002 | |
| External rotation 90° | 87.8 (5.7) | 90.6 (6.2) | 2.8 (4.6) | .04 | |
| Internal rotation | 85.7 (8.1) | 87.2 (4.3) | 1.5 (8.7) | .54 | |
| Right—Active | |||||
| Abduction | 166.7 (11.6) | 176.3 (5.3) | 9.6 (9.0) | .0005 | |
| Adduction | 38.2 (5.4) | 43.4 (3.8) | 5.2 (4.2) | .0003 | |
| Extension | 48.3 (8.8) | 49.7 (3.8) | 1.4 (8.5) | .50 | |
| Flexion | 167.1 (11.9) | 173.1 (11.3) | 6.0 (9.3) | .01 | |
| External rotation 0° | 74.3 (11.9) | 82.5 (6.0) | 8.2 (9.5) | .003 | |
| External rotation 90° | 80.7 (8.4) | 88.1 (7.3) | 7.3 (6.9) | .0006 | |
| Internal rotation | 80.6 (11.6) | 81.7 (9.5) | 1.1 (15.3) | .65 | |
| Right—Passive | |||||
| Abduction | 173.5 (7.5) | 178.8 (3.1) | 5.3 (6.6) | .002 | |
| Adduction | 39.9 (4.8) | 44.7 (2.6) | 4.8 (4.3) | .0009 | |
| Extension | 50.7 (8.9) | 49.7 (3.8) | −0.9 (8.8) | .72 | |
| Flexion | 172.1 (7.4) | 177.5 (7.1) | 5.4 (7.6) | .006 | |
| External rotation 0° | 81.1 (8.0) | 86.7 (5.1) | 5.6 (7.0) | .007 | |
| External rotation 90° | 88.3 (5.1) | 90.3 (5.0) | 1.9 (4.9) | .13 | |
| Internal rotation | 84.7 (11.4) | 85.0 (9.7) | 0.3 (16.4) | .86 | |
| Psychosocial | |||||
| Mood | Hospital Anxiety and Depression Scale (HADS) | ||||
| Anxiety | 7.6 (3.8) | 5.8 (2.8) | −1.7 (2.7) | .01 | |
| Depression | 5.3 (3.7) | 3.5 (3.3) | −1.8 (2.9) | .02 | |
| Stress | Perceived Stress Scale (PSS) | 19.0 (7.1) | 14.1 (7.0) | −4.9 (4.4) | .0005 |
| Pain catastrophizing | Pain Catastrophizing Scale (PCS) | ||||
| Helplessness | 5.6 (5.1) | 3.8 (4.5) | −1.8 (3.7) | .05 | |
| Rumination | 5.7 (4.9) | 3.2 (3.1) | −2.5 (3.5) | .01 | |
| Magnification | 3.7 (3.0) | 2.6 (1.9) | −1.1 (2.2) | .05 | |
| PCS Total Score | 14.9 (12.1) | 9.6 (9.0) | −5.3 (7.9) | .01 | |
| Self-esteem | Rosenberg Self-Esteem Scale (RSE) | 21.7 (6.1) | 23.7 (5.5) | 2.1 (3.6) | .04 |
| Exercise self-efficacy | Self-Efficacy for Exercise (SEE) | 50.0 (15.9) | 53.0 (20.9) | 3.0 (20.5) | .71 |
| Exercise amount | Light exercise (min/week) | 70.3 (110.8) | 135.4 (88.8) | 65.2 (125.4) | .006 |
| Moderate exercise (min/week) | 41.1 (50.2) | 71.7 (77.2) | 30.6 (90.5) | .04 | |
| Vigorous exercise (min/week) | 7.9 (18.5) | 21.7 (38.2) | 15.0 (32.7) | .13 | |
| Interoceptive self-awareness | Multidimensional Assessment of Interoceptive Awareness (MAIA) | ||||
| Noticing | 3.2 (1.3) | 3.6 (0.9) | 0.4 (1.0) | .09 | |
| Not distracting | 0.7 (0.7) | 1.1 (1.0) | 0.4 (1.3) | .31 | |
| Not worrying | 1.8 (0.9) | 2.5 (0.8) | 0.7 (1.0) | .007 | |
| Attention regulation | 2.4 (0.7) | 3.3 (0.7) | 0.9 (1.0) | .001 | |
| Emotional awareness | 3.1 (1.1) | 3.9 (0.8) | 0.9 (1.0) | .002 | |
| Self-regulation | 2.2 (1.0) | 3.6 (0.8) | 1.4 (1.1) | <.0001 | |
| Body listening | 1.6 (0.9) | 3.1 (1.1) | 1.4 (1.4) | .0006 | |
| Trusting | 2.1 (1.1) | 3.6 (0.8) | 1.5 (1.2) | <.0001 | |
| Quality of life | |||||
| Functional Assessment of Cancer Therapy Breast Symptom Index (FACT-B+4) | |||||
| Physical well-being | 21.0 (3.9) | 23.4 (3.8) | 2.3 (3.7) | .006 | |
| Social well-being | 22.3 (4.8) | 22.9 (4.7) | 0.6 (3.1) | .42 | |
| Emotional well-being | 16.0 (5.0) | 18.1 (4.1) | 2.1 (3.5) | .03 | |
| Functional well-being | 20.4 (4.6) | 22.3 (4.7) | 1.9 (2.9) | .02 | |
| Breast cancer symptoms | 21.2 (5.4) | 23.4 (4.4) | 2.2 (4.5) | .08 | |
| FACT-B total score | 100.9 (17.8) | 110.1 (18.9) | 9.2 (13.3) | .009 |
Change scores are based on Wilcoxon signed-rank tests. Possible score ranges and interpretation: BPI 0-10, lower scores suggest less pain; FACIT-F 0-52, higher scores reflect better quality of life (QoL); FACT-B+4 total score 0-144, PWB, SWB, FWB 0-28, EWB 0-24, BCS 0-36, higher score suggests better QoL; HADS Anxiety 0-21, depression 0-21, higher scores suggest worse mood; MAIA 0-5 scale, higher scores reflect more interoceptive awareness; PCS total score 0-52, rumination 0-16, magnification 0-12, helplessness 0-24, higher scores suggest higher levels of pain catastrophizing; PSS 0-40, higher scores suggest higher perceived stress; RSE 10-40, higher scores suggest higher self-esteem; SEE 0-90, higher scores suggest higher self-efficacy.
Self-Reported Outcomes
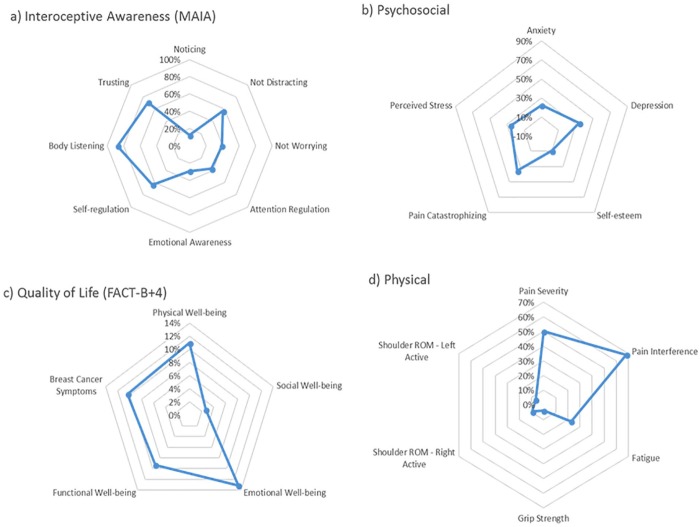
Statistically significant improvements were observed postintervention (at 12-week follow-up) in pain severity (Brief Pain Inventory, P = .0002), pain interference (Brief Pain Inventory, P = .003), fatigue (Functional Assessment of Chronic Illness Therapy–Fatigue, P = .001), anxiety (Hospital Anxiety and Depression Scale, P = .01), depression (Hospital Anxiety and Depression Scale, P = .02), perceived stress (Perceived Stress Scale, P = .0005), self-esteem (Rosenberg Self-Esteem Scale, P = .04), pain catastrophizing (total score and rumination subscale, P = .01), and 6 domains of interoceptive awareness (Multidimensional Assessment of Interoceptive Awareness, P ≤ .01). Participants increased their light exercise by an average of 65.2 minutes and moderate exercise by an average of 30.6 minutes. There were no significant differences observed for exercise self-efficacy. For cancer-related quality of life (Functional Assessment of Cancer Therapy–Breast Cancer [FACT-B+4]), significant improvements were observed at 12 weeks in the physical well-being, emotional well-being, and functional well-being subscales, as well as in the total score (all Ps < .05). Changes in pain, fatigue, pain catastrophizing, anxiety, depression, and quality of life were in range for being clinically meaningful (see Table 3 and Figure 1).
Figure 1.
Representation of magnitudes of changes highlighting broad biopsychosocial impact of Qigong mind-body exercise (QMBE). Values are percent changes from baseline to 12-week follow-up. (a) 8 subscales of the Multidimensional Assessment of Interoceptive Awareness (MAIA). (b) Anxiety and depression: subscales of the Hospital Anxiety and Depression Scale (HADS); perceived stress: Perceived Stress Scale (PSS); pain catastrophizing: Pain Catastrophizing Scale (PCS); self-esteem: Rosenberg Self-Esteem Scale (RSE). (c) 5 subscales of the Functional Assessment of Cancer Therapy–Breast Cancer (FACT-B+4) instrument. (d) Pain severity and pain interference: subscales of the Brief Pain Inventory (BPI); fatigue: Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) instrument; grip strength: average percent change of left and right hands; shoulder range of motion (ROM): average percent change of active abduction, adduction, extension, external rotation at 0° and 90°, flexion, and internal rotation for left and right arms, respectively.
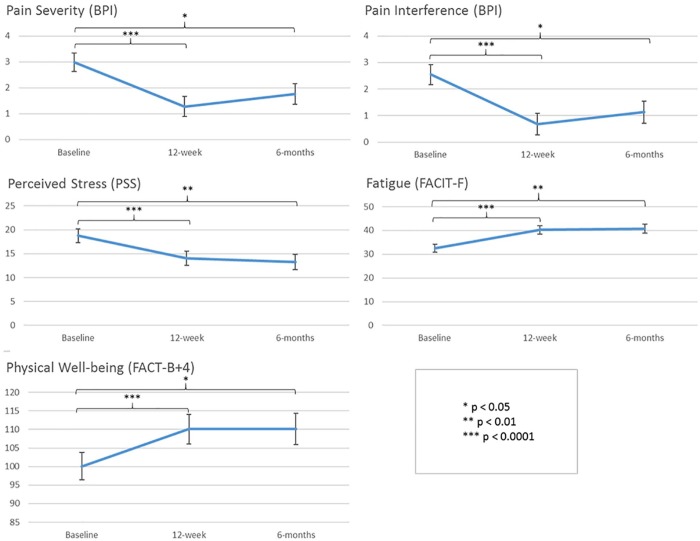
Longitudinal linear models indicated that there were statistically significant improvements in pain severity (P = .03), pain interference (P = .02), perceived stress (P = .006), fatigue (P = .006), and the physical well-being subscale of FACT-B+4 (P = .04) between baseline and 6-months. For the remaining FACT-B+4 subscales, there were no significant differences in scores over time. There were no significant differences between these outcomes at 12 weeks versus 6 months, suggesting that postintervention effects were sustained at 6-month follow-up (see Figure 2).
Figure 2.
Longitudinal linear model results. Least-squares means at baseline, 12-week, and 6-month time points are presented with standard error. P values are from the model t tests. Sample size at baseline = 21, at 12 weeks = 18, and at 6 months = 17. No statistically significant differences were noted between scores at 12-weeks versus 6-month time points in any of the outcomes.
Qualitative Interviews
Qualitative interviews revealed that most participants found participating in the program to be feasible overall. Of the 18 participants who completed the qualitative interviews postintervention (12 weeks), 17 would recommend QMBE to other BCSs and expressed the desire to continue practicing, either in a new class or at home. Barriers that did limit program adherence mentioned by 16 participants included a long or difficult commute, inconvenient timing of the classes, and/or interference of other commitments (eg, work, travel, family obligations). Regarding home practice, the most common barriers to consistent practice included lack of time (n = 6) or difficulty staying focused (n = 6).
Several factors facilitated program adherence. Participants appreciated the adaptability and accessibility of QMBE as an exercise (n = 10), finding that they were able to do the exercises regardless of their level of fitness. The movements could also be done seated, if necessary.
[QMBE] is the most gentle form of exercise that I can think of. It’s easier than walking.
I could see that I actually was doing this, and I could keep up with everybody else.
Participants (n = 12) also noted that QMBE movements could easily be integrated into daily life as they could be done anywhere, anytime, and do not require much physical space. This enabled adherence to the exercises when life became too busy for more formal practice. Most participants (n = 13) reported using the instructional video; providing both a DVD and online link facilitated access while participants were traveling.
[QMBE] was transportable, you could really do it anywhere, it didn’t require any equipment. If you had a free moment, waiting for a train, or a plane, or in a hotel, you could do it on the road.
Practicing in a group was found to be more enjoyable and more motivating than practicing at home for many (n = 13), which facilitated class attendance. Participants described the class atmosphere as welcoming, calming, and non-judgmental (n = 10).
I think the most helpful thing was being in [the classes] with other breast cancer survivors, knowing that we have that connection while we were doing something else.
Participants described a variety of perceived emotional and/or physical benefits from QMBE. Most of the women (n = 16) described feeling more relaxed, calm, and peaceful following practice. Many participants (n = 13) also found that elements of what they learned (eg, deep breathing, stretches) could be used in daily life as a tool for managing stress and coping with worry.
My mind felt freer, clearer. I felt more energized. Just more at peace.
[QMBE] helped me to relax, to calm myself, and to be more aware of my body . . . it’s so relaxing that it actually gave me more emotional stamina and calmness.
Several participants also noticed physical benefits, such as reduction in physical tension, improved strength, flexibility, and/or balance (n = 13). A few (n = 8) noticed a decrease in pain or improved ability to manage pain.
[QMBE] really helped my movement. These are movements that I really wasn’t doing before. So, it increased my mobility.
[QMBE] helped me a lot [with] managing joint pain.
Discussion
The purpose of this pilot study was to evaluate the feasibility, safety, and preliminary effectiveness of a QMBE program for BCS with PPSP. Based on recruitment and adherence rates, as well as participant feedback through qualitative interviews, the intervention was shown to be feasible, well-received, and safe. Preliminary data suggest clinically meaningful improvements in a broad range of both subjectively and objectively assessed outcomes, reflecting the potential of QMBE to have a multifaceted impact in this population, and supporting the value of a more definitive future study.
The intervention was shown to be safe, without any serious AEs reported. Only minor, transient aches and pains were reported by 4 participants, as is expected for any exercise intervention. Recruitment of our target sample was attained within our prespecified 12-month study period, which was our primary measure of feasibility. Twelve weeks was chosen as this is a period of time used in multiple prior Tai Chi and Qigong studies that have reported positive outcomes. It also represents a period of time sufficient to learn and integrate the practices in to a daily practice routine, but not too demanding with regard to commitment to dissuade cancer survivors from enrolling in the study.14 Intervention adherence and completion of outcome assessments fell slightly short of our conservative a priori goals. Our average attendance rate was comparable with that observed in some similar studies of mind-body exercise interventions for BCS, such as yoga18 and Qigong.19 Class attendance and adherence to home practice could be enhanced in a future study by addressing common barriers identified through qualitative interviews, including offering multiple options for class time and/or location. Participants found the exercise to be gentle and accessible. Many of the women incorporated the exercises into their daily lives as a tool for managing stress. Participants also found practicing in a group setting with other BCS to be inspiring and motivating, which facilitated class attendance.
Quantitative measures showed clinically meaningful improvements in a range of biopsychosocial outcomes postintervention in this group of BCS. The 6 most noteworthy responses were pain (interference and severity), fatigue, anxiety, depression, pain catastrophizing, and overall quality of life. Longitudinal linear models revealed that improvements in pain severity, pain interference, perceived stress, fatigue, and the physical well-being subscale of FACT-B+4 were maintained at 6 months, suggesting the effects of the QMBE intervention could persist longer-term. Thus, long-term observation is warranted. Participants’ perceptions of benefits, revealed during qualitative interviews, support that observed quantitative outcome changes were personally meaningful. Several participants described emotional benefits, such as relaxation, stress reduction, and an improved ability to manage emotions, in addition to physical benefits, such as improved mobility, flexibility, and reduced tension and pain, highlighting the biopsychosocial impact of QMBE. QMBE did not eliminate physical symptoms, but provided participants with a tool they could use to manage them, ultimately shifting their relationship with physical discomfort.
Evidence supporting the benefit of mind-body therapies in supportive cancer care is growing14,19-22 and integration of therapies such as meditation and yoga into clinical practice has been recommended for improving anxiety, depression, and quality of life.23 QMBE has generally been less studied than interventions such as meditation and yoga,24 and because most studies have been relatively small, evidence is currently insufficient to draw definitive clinical recommendations.21,23 The existing evidence does suggest a potential benefit for a variety of symptoms faced by BCS. For example, a 12-week randomized controlled trial comparing QMBE with sham Qigong in BCS found a significant reduction in fatigue in the intervention group compared with control.22 Another pilot randomized controlled trial evaluating the effects of an 8-week QMBE intervention compared with gentle exercise or survivorship support for BCS with decreased cognitive function found improvements in both cognitive function and distress in the QMBE group.19 However, the effects of QMBE on chronic pain, particularly PPSP in BCS, are understudied. Evaluating the potential benefit of QMBE for PPSP is in line with the national initiative to identify nonpharmacological treatments for chronic pain.25 Furthermore, the integration of both meditative and stretching components characteristic of QMBE may simultaneously confer both physical and psychological benefits. QMBE is also likely to be gentler and more accessible than many other forms of exercise and offers stress-reduction strategies (eg, breathing techniques and simple stretches) that can be incorporated into daily life.
The results of our study, while preliminary, support the hypothesis that a mind-body exercise intervention, such as QMBE, may be particularly beneficial for BCS experiencing PPSP. Studies have revealed that PPSP is a complex biopsychosocial condition that is associated with psychosocial risk factors2,6,26 and adversely affects emotional well-being and overall quality of life.9,27,28 QMBE may provide benefit by alleviating already existing symptoms of pain and distress associated with PPSP, and also has the potential to prevent the occurrence of PPSP or reduce its duration by impacting psychosocial risk factors such as catastrophizing and anxiety.2,6,29
Our study has several limitations. Interpretation of the clinical effects should be considered preliminary, as this was not a randomized trial and our sample size was small. The generalizability of our study is also limited as the participants were a self-selected group who had an interest in mind-body therapies. Subject selection was not based on pain levels; the participants had relatively low levels of pain at baseline, possibly leading to a floor effect. However, while pain ratings were low, qualitative interviews revealed that even low levels of pain cause significant distress; a more multidimensional approach to measuring the extent of this burden may be more appropriate than a simple pain scale. The pain participants experienced was heterogeneous in terms of type and location. In future studies, PPSP should be more specifically defined and women with clinically significant levels of pain (>3/10)3 should be selected. Several steps could be taken in the future to improve participant adherence to the intervention, such as offering more choices for class time and location.
Future studies might focus on most relevant symptoms or the interdependence of symptoms and the unique role mind-body therapies play in PPSP. Based on our findings, pain, fatigue, anxiety, depression, pain catastrophizing, and overall quality of life are likely to be the most meaningful outcomes. Perceived stress, self-esteem, interoceptive awareness, exercise level, and shoulder range of motion could be included as secondary outcomes. Selecting control groups in a future study will be challenging, given the multimodal nature and broad-spectrum effects of the intervention. One potential approach may be a comparative effectiveness trial comparing QMBE to a standard intervention, such as physical therapy.
Conclusions
Overall, the 12-week QMBE intervention was shown to be feasible and safe for BCS with PPSP. The intervention was well received, with participants describing the classes as enjoyable and noting several perceived benefits following the practice. Improvements in most clinical outcomes were both statistically and clinically significant. QMBE shows promise as an effective intervention for a number of both physical and psychosocial symptoms often faced by this population. Given the biopsychosocial complexity of PPSP, QMBE may be particularly applicable for BCSs facing this syndrome. Adequately powered trials comparing this intervention with standard physical therapy or other exercise modalities are warranted.
Supplemental Material
Supplemental material, QMBE_SUPPLEMENT_1_Qigong_protocol for Qigong Mind-Body Exercise as a Biopsychosocial Therapy for Persistent Post-Surgical Pain in Breast Cancer: A Pilot Study by Kamila Osypiuk, Jennifer Ligibel, Anita Giobbie-Hurder, Gloria Vergara-Diaz, Paolo Bonato, Roxanne Quinn, Winnie Ng and Peter M. Wayne in Integrative Cancer Therapies
Footnotes
Authors’ Note: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Author Peter Wayne is the founder and sole owner of the Tree of Life Tai Chi Center. Peter Wayne’s interests were reviewed and managed by the Brigham and Women’s Hospital and Partner’s HealthCare in accordance with their conflict of interest policies. Remaining authors declare that they have no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Osher Center for Integrative Medicine, the National Center for Complementary and Integrative Health, National Institutes of Health (K24 AT009282), and the Leonard P. Zakim Center for Integrative Therapies and Healthy Living at the Dana-Farber Cancer Institute.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board (Protocol Number 15-347, approval date October 26, 2015).
Informed Consent: Informed consent was obtained from all individual participants included in the study.
ORCID iD: Kamila Osypiuk  https://orcid.org/0000-0002-7500-4562
https://orcid.org/0000-0002-7500-4562
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289. [DOI] [PubMed] [Google Scholar]
- 2. Belfer I, Schreiber KL, Shaffer JR, et al. Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. J Pain. 2013;14:1185-1195. [DOI] [PubMed] [Google Scholar]
- 3. Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer. 2008;99:604-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985-1992. [DOI] [PubMed] [Google Scholar]
- 5. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581-624. [DOI] [PubMed] [Google Scholar]
- 6. Schreiber KL, Martel MO, Shnol H, et al. Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain. 2013;154:660-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bredal IS, Smeby NA, Ottesen S, Warncke T, Schlichting E. Chronic pain in breast cancer survivors: comparison of psychosocial, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852-862. [DOI] [PubMed] [Google Scholar]
- 8. Miaskowski C, Paul SM, Cooper B, et al. Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. Eur J Oncol Nurs. 2014;18:242-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beyaz SG, Ergonenc JS, Ergonenc T, Sonmez OU, Erkorkmaz U, Altintoprak F. Postmastectomy pain: a cross-sectional study of prevalence, pain characteristics, and effects on quality of life. Chin Med J (Engl). 2016;129:66-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lauridsen MC, Overgaard M, Overgaard J, Hessov IB, Cristiansen P. Shoulder disability and late symptoms following surgery for early breast cancer. Acta Oncol. 2008;47:569-575. [DOI] [PubMed] [Google Scholar]
- 11. Smith WC, Bourne D, Squair J, Phillips DO, Chambers WA. A retrospective cohort study of post mastectomy pain syndrome. Pain. 1999;83:91-95. [DOI] [PubMed] [Google Scholar]
- 12. Basen-Engquist K, Hughes D, Perkins H, Shinn E, Taylor CC. Dimensions of physical activity and their relationship to physical and emotional symptoms in breast cancer survivors. J Cancer Surviv. 2008;2:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larsson IM, Ahm Sorensen J, Bille C. The post-mastectomy pain syndrome—a systematic review of the treatment modalities. Breast J. 2017;23:338-343. [DOI] [PubMed] [Google Scholar]
- 14. Wayne PM, Lee MS, Novakowski J, et al. Tai Chi and Qigong for cancer-related symptoms and quality of life: a systematic review and meta-analysis. J Cancer Surviv. 2018;12:256-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein P. Qigong in cancer care: theory, evidence-base, and practice. Medicines (Basel). 2017;4:E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wayne PM, Gagnon MM, Macklin EA, et al. The Mind Body-Wellness in Supportive Housing (Mi-WiSH) study: design and rationale of a cluster randomized controlled trial of Tai Chi in senior housing. Contemp Clin Trials. 2017;60:96-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Creswell JW, Clark VLP. Designing and Conducting Mixed Methods Research. Thousand Oaks, CA: Sage; 2007. [Google Scholar]
- 18. Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25:4387-4395. [DOI] [PubMed] [Google Scholar]
- 19. Myers JS, Mitchell M, Krigel S, et al. Qigong intervention for breast cancer survivors with complaints of decreased cognitive function. Support Care Cancer. 2019;27:1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haller H, Winkler MM, Klose P, Dobos G, Kummel S, Cramer H. Mindfulness-based interventions for women with breast cancer: an updated systematic review and meta-analysis. Acta Oncol. 2017;56:1665-1676. [DOI] [PubMed] [Google Scholar]
- 21. Carlson LE, Zelinski E, Toivonen K, et al. Mind-body therapies in cancer: what is the latest evidence? Curr Oncol Rep. 2017;19:67. [DOI] [PubMed] [Google Scholar]
- 22. Larkey LK, Roe DJ, Weihs KL, et al. Randomized controlled trial of Qigong/Tai Chi Easy on cancer-related fatigue in breast cancer survivors. Ann Behav Med. 2015;49:165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67:194-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stan DL, Collins NM, Olsen MM, Croghan I, Pruthi S. The evolution of mindfulness-based physical interventions in breast cancer survivors. Evid Based Complement Alternat Med. 2012;2012:758641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tait RC, Zoberi K, Ferguson M, et al. Persistent post-mastectomy pain: current status and future directions. J Pain. 2018;19:1367-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaya T, Karatepe AG, Gunaydn R, Yetis H, Uslu A. Disability and health-related quality of life after breast cancer surgery: relation to impairments. South Med J. 2010;103:37-41. [DOI] [PubMed] [Google Scholar]
- 28. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51:75-82. [PubMed] [Google Scholar]
- 29. Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155:232-243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, QMBE_SUPPLEMENT_1_Qigong_protocol for Qigong Mind-Body Exercise as a Biopsychosocial Therapy for Persistent Post-Surgical Pain in Breast Cancer: A Pilot Study by Kamila Osypiuk, Jennifer Ligibel, Anita Giobbie-Hurder, Gloria Vergara-Diaz, Paolo Bonato, Roxanne Quinn, Winnie Ng and Peter M. Wayne in Integrative Cancer Therapies