Abstract
Background:
Previous research reports associations between prenatal exposure to phthalates and childhood behavior problems; however, the neural mechanisms that may underlie these associations are relatively unexplored.
Objective:
This study examined microstructural white matter as a possible mediator of the associations between prenatal phthalate exposure and behavior problems in preschool-aged children.
Methods:
Data are from a subsample of a prospective pregnancy cohort, the Alberta Pregnancy Outcomes and Nutrition (APrON) study (n = 76). Mother-child pairs were included if mothers provided a second trimester urine sample, if the child completed a successful magnetic resonance imaging (MRI) scan at age 3-5 years, and if the Child Behavior Checklist was completed within 6 months of the MRI scan. Molar sums of high (HMWP) and low molecular weight phthalates (LMWP) were calculated from levels in urine samples. Associations between prenatal phthalate concentrations, fractional anisotropy (FA) and mean diffusivity (MD) in 10 major white matter tracts, and preschool behavior problems were investigated.
Results:
Maternal prenatal phthalate concentrations were associated with MD of the right inferior fronto-occipital fasciculus (IFO), right pyramidal fibers, left and right uncinate fasciculus (UF), and FA of the left inferior longitudinal fasciculus (ILF). Mediation analyses showed that prenatal exposure to HMWP was indirectly associated with internalizing (path ab = 0.09, CI.95 = 0.02, 0.20) and externalizing problems (path ab = 0.09, CI.95 = 0.01, 0.19) through MD of the right IFO, and to internalizing problems (path ab = 0.11, CI.95 = 0.01, 0.23) through MD of the right pyramidal fibers.
Discussion:
This study provides the first evidence of childhood neural correlates of prenatal phthalate exposure. Results suggest that prenatal phthalate exposure may be related to microstructural white matter in the IFO, pyramidal fibers, UF, and ILF. Further, MD of the right IFO and pyramidal fibers may transmit childhood risk for behavioral problems.
Keywords: phthalates, diffusion tensor imaging, white matter microstructure, preschool children, behavior problems, APrON Study
1. INTRODUCTION
Phthalates are a group of chemicals used to soften and increase the flexibility of plastic products. They are also used as solvents and additives in consumer goods such as personal-hygiene and cosmetic products, textile and building materials, children’s toys, and food packaging (Benjamin et al., 2017). In North America, exposure to phthalates is ubiquitous (Serrano, Braun, Trasande, Dills, & Sathyanarayana, 2014). Phthalates are classified as endocrine-disrupting chemicals (EDCs), as they are capable of modifying the activity of estrogens, androgens, and thyroid hormones (Schug, Blawas, Gray, Heindel, & Lawler, 2015). Epidemiological evidence suggests that phthalates pose a health hazard in regards to reproductive function, respiratory disease, chronic physical health conditions (e.g., type II diabetes, cancer, etc.), and neurodevelopment (Benjamin et al., 2017). Pregnancy may represent a period of particular risk (Lyche et al., 2009), as phthalates cross the placenta, resulting in fetal phthalate levels that are closely related to maternal levels (Latini et al., 2003).
Systematic evidence has suggested that prenatal exposure to phthalates may have consequences for child neurodevelopment, including adverse effects on psychomotor, cognitive, and behavioral outcomes (Ejaredar, Nyanza, Ten Eycke, & Dewey, 2015; Lee, Kim, Lim, Lee, & Hong, 2018; Zhang, Chen, Huang, Wang, & Wu, 2019). Higher phthalates concentrations in maternal urine during pregnancy have been linked to adverse birth outcomes (e.g., lower birth weight) (Wolff et al., 2008) and alterations in infant neurobehavior (e.g., nonoptimal reflexes) (Yolton et al., 2011). In young children, greater prenatal phthalate exposure has been associated with both internalizing (e.g., emotional reactivity, anxious/depressed, withdrawal) and externalizing (e.g., aggression, attention) problems (Engel et al., 2010; Kim et al., 2009; Whyatt et al., 2012). Less is known about potential sex-differences in child behavioral outcomes following prenatal exposure to phthalates, though some evidence suggests that prenatal phthalate concentrations may be associated with different behavior problems in male children (i.e., aggression, attention, emotional reactivity) compared to female children (i.e., anxious/depressed) (Engel et al., 2010; Whyatt et al., 2012). These findings support the contention that exposure to phthalates during the vulnerable period of fetal development may have enduring behavioral consequences; however, the physiological mechanisms through which phthalate exposure during this period transmits risk have not yet been elucidated (Bowman & Choudhury, 2016).
EDCs may increase the risk of adverse neurodevelopmental outcomes by disrupting the activity of gonadal hormones responsible for the differentiation of neurons and sexually-dimorphic brain development (Braun, 2017; Vrijheid, Casas, Gascon, Valvi, & Nieuwenhuijsen, 2016). Prenatal exposure to EDCs, such as phthalates, may also disrupt the organization of neurochemical and neuroendocrine systems during gestation, which in turn could potentially result in alterations in brain structure and behavioral development during childhood (Gore, Krishnan, & Reilly, 2019; Walker & Gore, 2011). Animal research demonstrates that prenatal exposure to EDCs affects neuronal differentiation and migration during the early embryonic stage, as well as the regulation of genes (e.g., helix-loop-helix transcription factors) critical for brain development (Itoh, Yaoi, & Fushiki, 2012). Additional experiments using rodents have shown that intrauterine exposure to EDCs is associated with sexual differentiation of the brain (Kubo et al., 2003), which in turn results in sex differences in emotional and behavioral responses (Gioiosa, Fissore, Ghirardelli, Parmigiani, & Palanza, 2007; Palanza, Gioiosa, vom Saal, & Parmigiani, 2008). A single investigation has examined the effect of early phthalate exposure on neurodevelopmental outcomes, reporting that perinatal phthalate exposure was related to reduced sized and connectivity of the medial prefrontal cortex, as well as cognitive flexibility deficits in both male and female adult rats (Kougias, Sellinger, Willing, & Juraska, 2018). However, to our knowledge, no prior study has investigated the associations between prenatal phthalate exposure, brain structural differences, and behavioral outcomes in a human population.
Diffusion tensor imaging (DTI) is an advanced magnetic resonance imaging (MRI) technique, which allows for the investigation of the neural micro-environment. DTI uses the directional dependence of water movement to provide inferential information about microstructural features (e.g., fiber diameter, fiber density, myelination) of white matter tracts (Mori, 2007). Two widely used DTI parameters assessing white matter microstructure are fractional anisotropy (FA) and mean diffusivity (MD). FA indicates the directionality of diffusivity within a tract (i.e., how ordered it is); whereas, MD represents the mean diffusivity across all directions of movement (Basser & Jones, 2002).
Early childhood is a period of substantial white matter development, as shown by increases in FA and reductions in MD, which likely reflect increases in myelination and axonal packing (Dubois et al. 2006; Qiu et al. 2015; Reynolds et al. 2019a), and this ongoing maturation of white matter microstructure likely plays a key role in behavioral development (Brown & Jernigan, 2012). Previous studies have reported associations between white matter microstructural development and internalizing and externalizing behavior problems assessed by the Child Behavior Checklist (CBCL) (Albaugh et al., 2017; Loe, Lee, & Feldman, 2013). This study hypothesizes that exposure to phthalates during fetal development could alter white matter microstructure and subsequently impact behavioral outcomes during the preschool years. Therefore, the primary aim of the present observational study was to investigate if the associations between prenatal exposure to phthalates and behavior problems in preschool aged children, as measured by the CBCL, were mediated by microstructural white matter. Additionally, EDC exposure may have differential effects on white matter tracts in male and female children, so analyses also examined the impact of child sex on these associations.
2. MATERIALS AND METHODS
2.1. Participants and Procedure
Seventy-six mother-child pairs were included in the present study. They were recruited from an ongoing prospective pregnancy cohort study, the Alberta Pregnancy Outcomes and Nutrition (APrON) Study (Kaplan et al., 2014). Inclusion criteria were as follows: i) a maternal spot urine sample was provided during the second trimester of pregnancy, ii) the preschool child completed a successful magnetic resonance imaging (MRI) scan with usable results, iii) a parent-report measure of child behavior problems was completed within 6 months (mean months = 2.1 ± 1.7) of the MRI scan, and iv) the preschool child was healthy and typically developing (i.e., the child had not been diagnosed with a neurological or neurodevelopment disorder, Full Scale Intelligence Quotient/FSIQ > 80).
At the time of the urine sample collection, mothers completed questionnaires that provided information on sociodemographic variables (i.e., ethnicity, education, marital status, household income) and gestational characteristics (i.e., body weight/height, tobacco use, physical and mental illnesses). Information on birth outcomes (i.e., birth weight, gestational age, sex) were obtained later from medical records. From 2013 to 2017, when children were 3–5 years of age, they participated in an MRI scan and their mothers completed the Child Behavior Checklist (CBCL). The research protocol was approved by the Conjoint Health Research Ethics Board at the University of Calgary. Written, informed consent was provided prior to the collection of urine samples, MRI scanning, neurodevelopmental assessment, and completion of questionnaires. This work was carried out in accordance with the ethical principles for medical research involving human subjects (World Medical Association, 2013).
2.2. Measures
2.2.1. Maternal Urine Sample
Maternal spot urine samples were collected during the second trimester of pregnancy (mean gestational weeks = 17.0 ± 2.1) in a sterile cup. Following collection, samples were immediately aliquoted into 9mL cryovials and stored at −80 °C. Quality control experiments using liquid chromatography grade water as a surrogate for urine (n = 20 control samples) did not find any contamination during collection, storage, and/or analysis.
2.2.2. Quantification of Phthalate Metabolites and Composites
The current study quantified nine monoester phthalate metabolites that have been previously examined in regards to children’s neuropsychological development (Engel et al., 2010, 2009; Kobrosly et al., 2014). We considered four metabolites of di(2-ethyl-hexyl) phthalate (DEHP): mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxy-hexyl) phthalate (MEHHP), mono(2-ethyl-5-oxyohexyl) phthalate (MEOHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP); two metabolites of dibutyl phthalate (DBP): mono-n-butyl phthalate (MBP) and mono-iso-butyl phthalate (MiBP); and three other common metabolites: mono-benzyl phthalate (MBzP), mono-ethyl phthalate (MEP), and mono-methyl phthalate (MMP). Following the recommended practice for computing molar sums, phthalate metabolites were grouped into categories defined by their molecular weight, as metabolites of high (HMWP; > 250 Da) and low molecular weight phthalates (LMWP; < 250 Da) have similar sources of exposure, molecular structure, and biological activity (Engel et al., 2009; Wolff et al., 2008). For example, high molecular weight phthalates (e.g., MEHHP, MECPP, MEOHP, MEHP) are often used in food packaging, vinyl toys, and vinyl building materials; whereas, low molecular weight phthalates (e.g., MBP) are used as solvents in personal-hygiene and cosmetic products, such as nail polish (Committee on the Health Risks of Phthalates Board on Environmental Studies and Toxicology Division on Earth and Life Studies, 2008). Five metabolites were included in the molar sum of HMWP: MEHP, MEHPP, MEOHP, MECPP, and MBzP. Four metabolites were included in the molar sum of LMWP: MBP, MiBP, MEP, and MMP.
The monoester phthalate metabolites were identified using liquid chromatography-tandem mass spectrometry (QTRAP 5500, AB Sciex, Concord, Canada) at the Alberta Centre for Toxicology, University of Calgary. With the QTRAP operating in negative multiple reaction monitoring (MRM) mode, the detection and quantification of metabolites were based on two MRM transitions combined with the retention time. Phthalate metabolites were separated on a 100 x 2.1 mm BetaSil Phenyl Column (Thermo Scientific, Burlington, Canada) using an Agilent 1200 HPLC system (Agilent Technologies, Mississauga, Canada). The injection volume was 10 μL and a constant column temperature of 40 °C was maintained. The limit of detection (LOD) for all metabolites was 0.10 μg/L, and as per common practice, all values below the LOD were assigned the value of the LOD divided by the square root of 2 (Hornung & Reed, 1990). To account for urine dilution, creatinine-adjusted (μmol/g creatinine) concentrations of the HMWP and LMWP were used for analyses.
2.2.3. MRI Acquisition
All neuroimaging was conducted on a General Electric 3T MR750w system equipped with a 32-channel head coil (General Electric, Waukesha, WI), located at the Alberta Children’s Hospital (ACH), Calgary, Canada. During scanning children were awake watching a movie or naturally asleep. For a total acquisition time of 4:03 minutes, whole brain diffusion weighted images were acquired using a single shot spin echo echo-planar imaging sequence with the following parameters: 1.6 x 1.6 x 2.2 mm resolution (resampled on scanner to 0.78 x 0.78 x 2.2mm), TR = 6750 ms; TE = 79 ms, anterior-posterior phase encoding, 30 gradient encoding directions (b=750s/mm2) and five interleaved non-diffusion weighted images (b=0s/mm2).
2.2.4. DTI Data Processing and Semi-automated Tractography
Eighty-four children participated in MRI scanning. Among the initial group of 84, incidental findings (i.e., previously undiagnosed medical conditions that were unintentionally discovered by the MRI scan) were noted for four participants. Incidental findings include potentially symptomatic or treatable abnormalities (e.g., cysts) and individuals that display these abnormalities are commonly removed from MRI research analyses (Morris et al., 2009), so these four participants were not included in the current study analyses (n = 80). In order to perform reliable DTI analyses, diffusion weighted image (DWIs) volumes from MRI must be corrected for artifacts and distortions (Irfanoglu, Sarlls, Nayak, & Pierpaoli, 2019). All DTI data was visually inspected prior to processing for quality control, which was completed by an investigator blinded to family demographics and child behavior scores. Participant data with artifacts and/or motion corruption were removed (n = 4). This resulted in the final sample of participants (n = 76) included in the current study who had an average of 32 (SD = 3.2) total volumes remaining. Following quality checking, data was processed through version 4.8.6 of ExploreDTI (Leemans et al. 2009) for signal drift, Gibbs ringing, subject motion, and eddy current corrections. In-depth summaries of DTI data processing techniques are available elsewhere (i.e., Alexander, Lee, Lazar, & Field, 2007; Leemans et al., 2009; Soares, Marques, Alves, & Sousa, 2013).
Deterministic semi-automated tractography was undertaken to delineate 10 major white matter tracts: genu, body, and splenium of the corpus callosum, pyramidal fibers, inferior fronto-occipital fasciculus (IFO), inferior longitudinal fasciculus (ILF), cingulum, fornix, superior longitudinal fasciculus including arcuate (SLF), and uncinate fasciculus (UF) (Figure 1). The most representative scan (a 3.68 year old female) for use as the target scan in semi-automated tractography was determined using the tract-based spatial statistics (TBSS; tbss_2_reg script, flag -n) non-linear registration step (Smith et al., 2006) in FSL (Andersson, Jenkinson, & Smith, 2007; Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012; Smith et al., 2004; Woolrich et al., 2009). To identify the target scan, all DTI scans collected prior to September 2017 (when semi-automated tractography commenced) with at least 34 high-quality volumes in a large longitudinal dataset of children aged 2 to 8 years (n = 125) were utilized (Reynolds, Grohs, Dewey, & Lebel, 2019a). The most representative scan (target scan) was selected as the scan that required the least warping to best fit the other scans (Andersson et al., 2007; Jenkinson et al., 2012; Smith et al., 2004; Woolrich et al., 2009). Following the identification of the target scan, inclusion and exclusion regions of interest (ROIs) were drawn on the target participant (for the ROI placements used refer to Reynolds et al. 2019b). The ROIs were created and evaluated by two of the authors (MG, JR) using an iterative evaluation and verification procedure previously described (Lebel, Walker, Leemans, Phillips, & Beaulieu, 2008; Reynolds, Grohs, Dewey, & Lebel, 2019b; Wakana, Jiang, Nagae-Poetscher, Van Zijl, & Mori, 2004). The semi-automated tractography method ensures the ROIs are in the same regions for each participant’s native space scan by normalizing each scan to the target, and then using the inverse of these transformations to warp the ROIs to native space. The delineated tracts were then manually inspected, and spurious fibers were removed as needed. Finally, average tract values for fractional anisotropy (FA) and mean diffusivity (MD) (and axial diffusivity (AD) and radial diffusivity (RD)) were extracted using ExploreDTI for all tracts (where applicable, parameters were extracted for left and right hemisphere tracts separately for a total of 16 isolated white matter tracts).
Figure 1. Isolated white matter tracts.
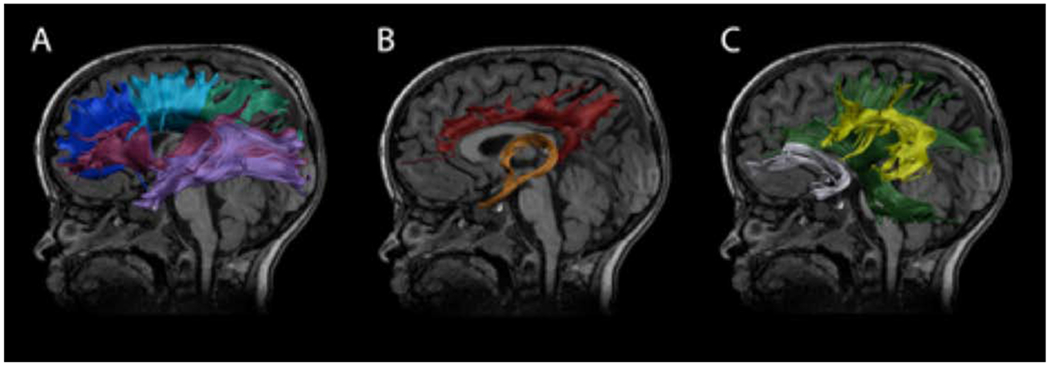
Diffusion parameters were calculated for 10 major white matter tracts: A) dark blue: genu of corpus callosum, light blue: body of corpus callosum, light green: splenium of corpus callosum, magenta: left inferior fronto-occipital fasciculus (IFO), light purple: left inferior longitudinal fasciculus (ILF); B) red: left cingulum bundle, orange: fornix; C) dark green: left pyramidal fibers, yellow: left superior longitudinal fasciculus including the arcuate fasciculus (SFL), grey: left uncincate fasciculus (UF). Tracts are shown on a T1-weighted image from a female 3.68 years of age.
2.2.5. Child Behavior Checklist (CBCL)
Within 6 months of the MRI scan (M = 2.1 months, SD = 1.7 months), mothers completed a checklist of common childhood behavior problems- the Child Behavior Checklist (CBCL; Achenbach and Rescorla 2000). The CBCL asked parents to rate 99 different problems on a three-point rating scale (0 = not at all, 1 = sometimes, 2 = yes). Factor analysis was used to group problems that tended to occur together into seven syndrome scales: emotionally reactive, anxious/depressed, somatic complaints, withdrawn, sleep problems, attention problems, and aggressive behavior. These syndrome scales are scored in terms of two broad groupings of syndromes: internalizing and externalizing problems. The Internalizing Problems scale is comprised of four syndrome scales: emotionally reactive, anxious/depressed, somatic complaints, and withdrawn. The Externalizing Problems scale includes two syndrome scales: attention problems and aggressive behaviors. Previous research has indicated that the CBCL has strong psychometric properties (Achenbach and Rescorla 2000), and that preschool scores on the Internalizing and Externalizing Problems scales are predictive of psychopathology in later childhood and adolescence (Beyer, Postert, Müller, & Furniss, 2012; Mesman & Koot, 2001). In the current study, T scores (i.e., higher T scores indicate more behavior problems) from both the Internalizing and Externalizing Problem scales demonstrated acceptable internal consistency (Cronbach’s α = 0.7).
2.2.6. Covariates
Based on previous examinations of prenatal phthalate exposure and children’s neurobehavioral outcomes (Engel et al. 2010; Kim et al. 2009; Whyatt et al. 2012), potential confounding variables were examined as covariates in the main analyses. Covariates included maternal sociodemographic variables (i.e., ethnicity, education, marital status, household income), gestational characteristics (i.e., body weight/height, tobacco use, physical and mental illnesses), and child characteristics (i.e., birth weight, gestational age at birth, sex, age at assessment).
2.3. Statistical Approach
Skewed phthalate concentration data were log-transformed prior to analyses. There was no missing data and all analyses were performed in version 25.0 of SPSS (IBM Corp., 2017). Initially, exploratory multivariate regression analyses examined the associations between creatinine-adjusted phthalate molar sums, FA and MD from the 16 isolated white matter tracts, and T scores from the CBCL Internalizing and Externalizing Problems scales. Sixty-four regression analyses were conducted to investigate whether maternal HMWP or LMWP concentrations during pregnancy (i.e., the independent variables) were associated with FA or MD of the 16 isolated white matter tracts (i.e., the dependent variables). Following which, 10 regression analyses examined the associations between the white matter tracts found to be significantly associated with prenatal phthalate concentrations (i.e., independent variables) and T scores from the CBCL Internalizing and Externalizing Problem scales (i.e., dependent variables). Based on previous research, these exploratory regression models controlled for child sex and child age at assessment (Chen et al., 2010; Grohs et al., 2019; Reynolds et al., 2019a). As significant sex differences are noted in child white matter microstructure (Reynolds et al. 2019a) and preschool behavior problems (Basten et al., 2016; Chen, 2010), child sex was also examined as a potential modifier of these associations. In line with previous neuroimaging research (Sowell et al., 2008; Zanin et al., 2011), we corrected for multiple comparisons in the regression analyses using the Benjamini-Hochberg procedure to control the false discovery rate (FDR) (Benjamini & Hochberg, 1995; Schwartzman, Dougherty, Lee, Ghahremani, & Taylor, 2009). Given the limited evidence on the effect of prenatal exposure to phthalates on children’s neurobehavioral development and the breadth of data available for the current sample, we adopted an exploratory approach consistent with prior research examining the influence of phthalates on behavior problems in children aged 3 to 5 years (Philippat et al., 2017), and report significant adjusted p-values from 0.05 to 0.1. For all regression models, we report both raw (observed) p-values and q values (adjusted p-values).
Based on the associations from the exploratory regression analyses, the main analyses included mediation models that assessed which independent variables (i.e., prenatal phthalate concentrations, DTI parameters, covariates) significantly predicted the dependent variables (i.e., CBCL T scores) (Field, 2009; MacKinnon, Fairchild, & Fritz, 2007). These models examined the influence of the following covariates: maternal sociodemographic variables (i.e., ethnicity, education, marital status, household income), gestational characteristics (i.e., body weight/height, tobacco use, physical and mental illnesses), and child characteristics (i.e., birth weight, gestational age at birth, sex, age at assessment). Non-significant covariates were dropped from the mediational analyses. Using standardized variables, mediation analyses were conducted using the bootstrapping method (MacKinnon & Fairchild, 2010) in version 3.0 of the PROCESS macro for SPSS (Hayes, 2017). The bootstrapping method is preferable to the casual steps method for testing indirect effects (Baron & Kenny, 1986; Zhao, Lynch, & Chen, 2010), as it does not require a significant main effect from the independent variable to the dependent variable, and is recommended for models with small sample sizes in which there is a distant temporal relationship between the independent variable and dependent variable (Shrout & Bolger, 2002). Given the small sample size (n = 76), and the distant temporal relationship between prenatal exposure to phthalates and behavior outcomes in children at 3-5 years of age, these methods were used to examine the significance of indirect effects in the absence of significant main (i.e., total and direct) effects.
3. RESULTS
3.1. Study Sample Characteristics
At the time of prenatal urine collection, mothers were between the ages of 25-38 years (M = 31.7, SD = 2.7 years), predominantly Caucasian (93.4%), university-educated (76.3%), married (89.5%), and had a median household income of greater than $100,000 CAD. In the present sample, none of the women reported smoking during pregnancy. Some women reported physical health conditions (7.9% reported a thyroid condition, 5.7% reported Celiac disease, and 8.6% reported another physical condition) and/or mental health problems (5.7% reported anxiety and 2.9% reported depression). Children were born between 35-41 weeks of gestation (M = 39.3, SD = 1.3 weeks) and weighed between 2215 and 4300 grams (M = 3337.8, SD = 464.1 grams) (see Table 1 for maternal and child characteristics). Children (51.3% female) were 3-5 years of age at the time of the MRI scan (mean child age = 4.4 ± 0.8 years) and at the time of CBCL completion (mean child age = 4.4 ± 0.7 years).
Table 1.
Maternal and Child Characteristics (n = 76).
| Maternal n (%) | M±SD |
|---|---|
| Ethnicity | |
| Caucasian | 71 (93.4) |
| Chinese | 1 (1.3) |
| Filipino | 2 (2.6) |
| South Asian | 1 (1.3) |
| Other | 1 (1.3) |
| Parity Status | |
| Primiparous | 41 (53.9) |
| Multiparous | 35 (46.1) |
| Education | |
| Less than High School | 1 (1.3) |
| High School Diploma | 3 (3.9) |
| Technical/Trade School | 14 (18.4) |
| University | 36 (47.4) |
| Postgraduate Education | 22 (28.9) |
| Marital Status | |
| Married | 68 (89.5) |
| Common-law | 5 (6.6) |
| Divorced | 1 (1.3) |
| Single | 2 (2.6) |
| Household Income | |
| < $20,000 | 1 (1.3) |
| $20,000 – 39,000 | 3 (3.9) |
| $40,000 – 69,000 | 5 (6.6) |
| $70,000 – 99,000 | 14 (18.4) |
| $100,000 + | 53 (69.7) |
| Child | |
| Sex (% Female) | 51.3 |
| Age at MRI scan (years) | 4.4 ± 0.8 |
| Gestational age at birth (weeks) | 39.3 ± 1.3 |
| Weight at birth (grams) | 3337.8 ± 464.1 |
| WISC- FSIQ a | 109.5 ± 12.1 |
| CBCL Internalizing problems b | 45.9 ± 9.1 |
| CBCL Externalizing problems b | 45.9 ± 9.8 |
Wechsler Preschool and Primary Scale of Intelligence-Fourth Edition:Canadian - Full Scale Intelligence Quotient (WPPSI-IV FSIQ) composite standard score.
Child Behaviour Checklist (CBCL) T Score
3.2. Phthalate Metabolite Concentrations
Median urinary concentrations of phthalate metabolites during pregnancy ranged from 2.64 μg/L (mono-methyl phthalate; MMP) to 68.54 μg/L (mono-ethyl phthalate; MEP) (Table 2). The HMWP (M = 0.30, SD = 0.30 μmol/L) and LMWP (M = 1.57, SD = 3.32 μmol/L) had median molar concentrations of 0.21 μmol/L (0.24 μmol/g creatinine) and 0.56 μmol/L (0.59 μmol/g creatinine), respectively.
Table 2.
Phthalate analyte concentrations in maternal second trimester urine (n = 76).
| Analyte | % > LOD | Concentration Range (μg/L) | 25th Percentile (μg/L) | 50th Percentile (μg/L) | 75th Percentile (μg/L) | Median Molar Concentration (μmol/L) |
|---|---|---|---|---|---|---|
| MEHP a | 98.70 | < LOD - 55.87 | 1.98 | 3.98 | 7.49 | 0.01 |
| MEHHP a | 100 | 0.61 – 93.85 | 5.65 | 13.56 | 19.73 | 0.05 |
| MEOHPa | 100 | 0.60 – 80.90 | 4.70 | 11.64 | 17.28 | 0.04 |
| MECPP a | 100 | 0.87 – 180.99 | 8.72 | 18.62 | 29.97 | 0.06 |
| MBZP a | 100 | 0.31 – 166.58 | 4.12 | 8.94 | 22.70 | 0.03 |
| HMWP b | 98.70 | 0.02 – 1.46 | 0.21 | |||
| HMWP (creatinine-adjusted) c | 98.70 | 0.07 – 1.34 | 0.24 | |||
| MBP a | 100 | 1.18 – 523.62 | 9.09 | 17.94 | 45.06 | 0.08 |
| MIBP a | 98.70 | < LOD - 374.22 | 5.92 | 12.22 | 21.30 | 0.06 |
| MEP a | 100 | 2.80 – 4505.82 | 16.67 | 68.54 | 193.91 | 0.35 |
| MMP a | 98.70 | < LOD - 86.09 | 1.41 | 2.64 | 4.30 | 0.01 |
| LMWP b | 98.70 | 0.02 – 23.79 | 0.56 | |||
| LMWP (creatinine-adjusted) c | 98.70 | 0.14 – 14.32 | 0.59 |
Note: HMWP = high molecular weight phthalates; LMWP = low molecular weight phthalates.
LOD = 0.10 μg/L
μmol/L
umol/g creatinine
3.3. Exploratory Regressions
Sixty-four multivariate regressions examined if maternal HMWP or LMWP concentrations during pregnancy were associated with FA or MD of the 16 isolated white matter tracts (Table 3). Higher maternal HMWP concentrations were associated with greater MD of the right IFO (β = 0.28, p = 0.01), right ILF (β = 0.21, p = 0.05), right pyramidal fibers (β = 0.27, p = 0.01), left UF (β = 0.26, p = 0.02), and right UF (β = 0.33, p = 0.002). However, only the associations between HMWP and MD of the right IFO, right pyramidal fibers, left UF, and right UF were significant at q < 0.10. Maternal HMWP concentrations were not significantly associated with FA of any of the white matter tracts. Higher maternal LMWP concentrations were associated with greater MD of the left IFO (β = 0.24, p = 0.03) and left ILF (β = 0.18, p = 0.05); however, these associations did not survive the correction for multiple comparisons (q > 0.10). Higher maternal LMWP concentrations were associated with reduced FA of the left ILF (β = −0.34, p = 0.001), and this association did survive the correction for multiple comparisons (q < 0.05). Child sex did not significantly modify any of the associations between maternal HMWP or LMWP concentrations and the white matter tract parameters.
Table 3.
Regression coefficients for exploratory analyses examining the associations between maternal prenatal phthalate concentrations and DTI parameters of the 16 isolated white matter tracts.
| HMWP | Raw p-value | q-value | LMWP | Raw p-value | q-value | |
|---|---|---|---|---|---|---|
| Fractional Anisotropy (FA) | ||||||
| Corpus Callosum- Body | −0.01 | 0.90 | 0.96 | −0.07 | 0.54 | 1.23 |
| Corpus Callosum- Genu | −0.10 | 0.38 | 1.00 | −0.02 | 0.86 | 1.06 |
| Corpus Callosum- Splenium | 0.02 | 0.84 | 1.12 | −0.01 | 0.98 | 0.98 |
| Fornix | 0.04 | 0.69 | 1.10 | 0.01 | 0.93 | 0.99 |
| Left Cingulum | 0.02 | 0.88 | 1.08 | −0.08 | 0.48 | 1.53 |
| Right Cingulum | 0.00 | 1.00 | 1.00 | −0.03 | 0.79 | 1.14 |
| Left IFO | 0.10 | 0.30 | 0.97 | −0.04 | 0.71 | 1.27 |
| Right IFO | 0.06 | 0.61 | 1.09 | 0.02 | 0.90 | 1.03 |
| Left ILF | −0.06 | 0.59 | 1.34 | −0.34 | 0.00* | 0.02*** |
| Right ILF | −0.20 | 0.08 | 1.23 | −0.17 | 0.13 | 1.05 |
| Left Pyramidal | 0.04 | 0.72 | 1.04 | −0.04 | 0.69 | 1.39 |
| Right Pyramidal | −0.15 | 0.15 | 1.22 | −0.15 | 0.15 | 0.82 |
| Left SLF | 0.10 | 0.89 | 1.01 | −0.07 | 0.50 | 1.34 |
| Right SLF | 0.13 | 0.21 | 0.85 | 0.13 | 0.21 | 0.85 |
| Left Uncinate | −0.14 | 0.21 | 1.11 | −0.02 | 0.83 | 1.10 |
| Right Uncinate | −0.05 | 0.60 | 1.21 | −0.04 | 0.73 | 1.17 |
| Mean Diffusivity (MD) | ||||||
| Corpus Callosum- Body | 0.08 | 0.45 | 0.89 | 0.20 | 0.06 | 0.34 |
| Corpus Callosum- Genu | 0.11 | 0.35 | 0.81 | 0.08 | 0.51 | 0.63 |
| Corpus Callosum- Splenium | 0.03 | 0.83 | 0.94 | 0.05 | 0.67 | 0.77 |
| Fornix | 0.00 | 1.00 | 1.00 | −0.01 | 0.92 | 0.92 |
| Left Cingulum | 0.02 | 0.87 | 0.93 | 0.19 | 0.08 | 0.30 |
| Right Cingulum | 0.03 | 0.82 | 1.01 | 0.10 | 0.36 | 0.53 |
| Left IFO | 0.16 | 0.14 | 0.38 | 0.24 | 0.03* | 0.43 |
| Right IFO | 0.28 | 0.01* | 0.09** | 0.11 | 0.34 | 0.61 |
| Left ILF | 0.04 | 0.70 | 0.94 | 0.18 | 0.05* | 0.42 |
| Right ILF | 0.21 | 0.05* | 0.16 | 0.12 | 0.28 | 0.55 |
| Left Pyramidal | 0.06 | 0.54 | 0.96 | 0.15 | 0.15 | 0.48 |
| Right Pyramidal | 0.27 | 0.01* | 0.08** | 0.10 | 0.35 | 0.56 |
| Left SLF | 0.07 | 0.65 | 0.95 | 0.14 | 0.18 | 0.49 |
| Right SLF | 0.05 | 0.64 | 1.03 | 0.12 | 0.25 | 0.58 |
| Left Uncinate | 0.26 | 0.02* | 0.10** | 0.09 | 0.43 | 0.57 |
| Right Uncinate | 0.33 | 0.00* | 0.03*** | 0.04 | 0.76 | 0.81 |
Note: FA = fractional anisotropy; MD = mean diffusivity; HMWP = high molecular weight phthalates; LMWP = low molecular weight phthalates; IFO = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; SLF = superior longitudinal fasciculus including the arcuate fasciculus.
raw p-value ≤ 0.05
q ≤ 0.10
q ≤ 0.05
Following which, 10 multivariate regressions were used to examine the associations between the DTI parameters of white matter tracts found to be significantly associated with prenatal phthalate concentrations (i.e., MD of the right IFO, right pyramidal fibers, left UF, and right UF; and FA of the left ILF) and T scores from the CBCL scales (Table 4). Higher MD of the right IFO was associated with more Internalizing Problems (β = 0.31, p = 0.01) and Externalizing Problems (β = 0.32, p = 0.01) on the CBCL. Higher MD of the right pyramidal fibers was also significantly associated with more Internalizing Problems (β = 0.37, p = 0.003) and Externalizing Problems (β = 0.28, p = 0.03) on the CBCL. Finally, higher FA of the left ILF was associated with fewer Internalizing Problems (β = −0.27, p = 0.05) on the CBCL. These associations survived the correction for multiple comparisons (q < 0.10). MD of the left UF and right UF were not associated with Internalizing Problems or Externalizing Problems, nor was FA of the left ILF associated with Externalizing Problems. Child sex did not significantly modify any of the examined associations between the white matter tract parameters and child behavior.
Table 4.
Regression coefficients for exploratory analyses examining the associations between white matter tract DTI parameters and child behavior scale T scores.
| Internalizing Problems | Raw p-value | q-value | Externalizing Problems | Raw p-value | q-value | |
|---|---|---|---|---|---|---|
| Right IFO- MD | 0.31 | 0.01* | 0.02*** | 0.32 | 0.01* | 0.03*** |
| Right Pyramidal- MD | 0.37 | 0.00* | 0.01*** | 0.28 | 0.03* | 0.06** |
| Left Uncinate- MD | 0.14 | 0.27 | 0.34 | −0.02 | 0.84 | 0.84 |
| Right Uncinate- MD | −0.02 | 0.86 | 0.86 | 0.06 | 0.66 | 0.88 |
| Left ILF- FA | −0.27 | 0.05* | 0.08** | −0.18 | 0.20 | 0.20 |
Note: FA = fractional anisotropy; MD = mean diffusivity; IFO = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus.
raw p-value < 0.05
q < 0.10
q < 0.05
3.4. Phthalates and Child Behavior
Four regressions examined if maternal HMWP and LMWP concentrations during pregnancy were associated with T scores from the CBCL scales. Maternal HMWP (β = 0.01, p = 0.90) and LMWP concentrations (β = 0.07, p = 0.37) were unrelated to Internalizing Problems, and maternal HMWP (β = 0.06, p = 0.45) and LMWP concentrations (β = 0.11, p = 0.19) were also unrelated to Externalizing Problems. The inclusion of covariates did not significantly change (i.e., < 10%) the regression coefficients for the analyses examining the associations between maternal prenatal phthalate concentrations and child behavior T scores (Supplemental Material, Table 1). Given that the associations between maternal prenatal phthalate concentrations and child behaviour T scores were not significant (i.e., the main effects), mediation models were used to examine the significance of indirect effects in the absence of significant main effects (Shrout & Bolger, 2002).
3.5. Mediation Models
Based on the significant associations from the exploratory analyses, mediation analyses examined if: i) MD of the right IFO mediated the association between HMWP and preschool Internalizing Problems, ii) MD of the right IFO mediated the association between HMWP and preschool Externalizing Problems, ii) MD of the pyramidal fibers mediated the association between HMWP and preschool Internalizing Problems, iv) MD of the pyramidal fibers mediated the association between HMWP and preschool Externalizing Problems, and v) FA of the left ILF mediated the association between LMWP and preschool Internalizing Problems. The mediation models examined maternal sociodemographic variables, gestational characteristics, and child characteristics as covariates. Only child age emerged as a significant predictor, thus, the other non-significant covariates were not included in the final mediation models. Models controlled for maternal urine sample dilution, by including creatinine-adjusted phthalate concentrations.
The first mediation model examined the association between HMWP and child Internalizing Problems as mediated by MD of the right IFO. There was a non-significant total effect of HMWP on child Internalizing Problems (path c = 0.01, SE = 0.12, t = 0.12, p = 0.90; R2 = 0.01). However, higher prenatal exposure to HMWP was related to greater MD in the right IFO (path a = 0.27, SE = 0.10, t = 2.65, p = 0.01; R2 = 0.18), and greater MD in the right IFO was related to more Internalizing Problems (path b = 0.34, SE = 0.13, t = 2.69, p = 0.01; R2 = 0.10). There was a significant indirect effect; MD of the right IFO emerged as a significant mediator of HMWP on Internalizing Problems (path ab = 0.09, SE = 0.05, CI95 = 0.02, 0.20) (Figure 2). The effect size of this indirect effect (i.e., completely standardized indirect effect) was 0.09 (CI.95 = 0.02, 0.18) (Lachowicz, Preacher, & Kelley, 2018). The direct effect was non-significant (path c’ = −0.08, SE = 0.12, t = −0.67, p = 0.50), indicating indirect-only mediation (Zhao et al., 2010). In this mediation model, child age was a significant predictor of MD of the right IFO (β = −0.30, SE = 0.10, t = −2.86, p = 0.01), but not Internalizing Problems (β = 0.17, SE = 0.12, t = 1.44 p = 0.15).
Figure 2.
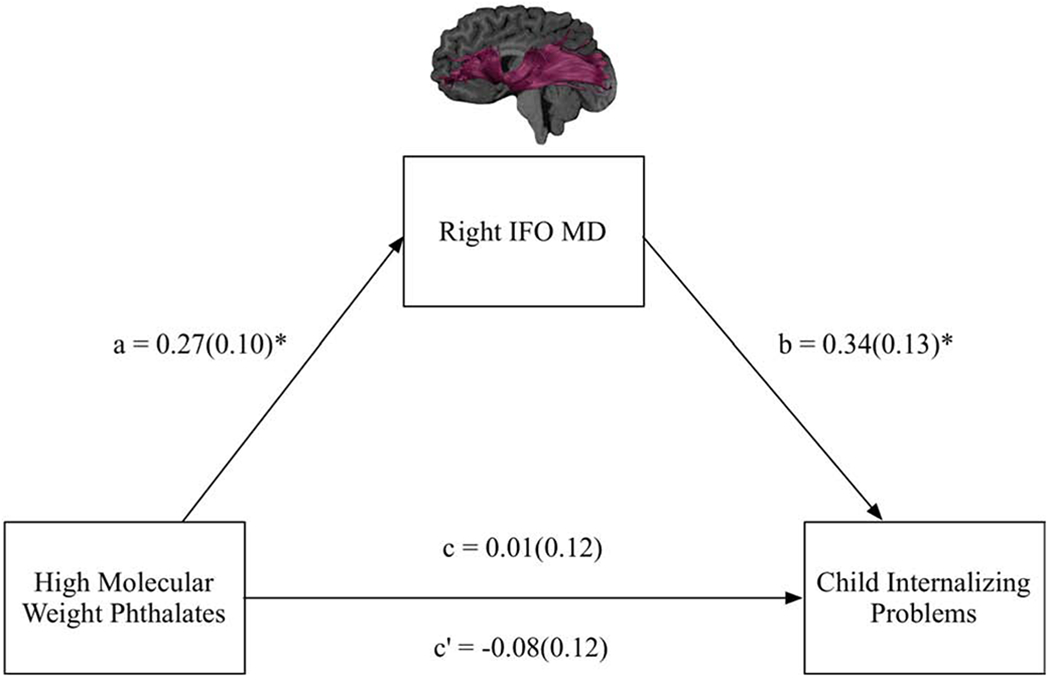
Full model and results for the mediation model in which MD of the right IFO was examined as a mediator of prenatal exposure to high molecular weight phthalates and child internalizing problems. Standardized beta coefficients and standard errors in brackets are reported. Note. a = the effect of the independent variable (high molecular weight phthalates) on the mediating variable (MD of the right IFO); b = the effect of the mediating variable (MD of the right IFO) on the dependent variable (child internalizing problems); c = direct effect; c’ =total effect.
*raw p-values < 0.05.
The second mediation model examined the association between HMWP and child Externalizing Problems as mediated by MD of the right IFO. There was a non-significant total effect of HMWP on child Externalizing Problems (path c = 0.07, SE = 0.12, t = 0.60, p = 0.55; R2 = 0.02). However, higher prenatal exposure to HMWP was related to greater MD in the right IFO (path a = 0.27, SE = 0.10, t = 2.65, p = 0.01; R2 = 0.18), and greater MD in the right IFO was related to more Externalizing Problems (path b = 0.33, SE = 0.13, t = 2.62, p = 0.01; R2 = 0.11). There was a significant indirect effect; MD of the right IFO emerged as a significant mediator of HMWP on Externalizing Problems (path ab = 0.09, SE = 0.05, CI.95 = 0.01, 0.19) (Figure 3). The effect size of this indirect effect was 0.09 (CI.95 = 0.01, 0.18) (Lachowicz et al., 2018). The direct effect was non-significant (path c’ = −0.02, SE = 0.12, t = −0.18, p = 0.86), indicating indirect-only mediation (Zhao et al., 2010). In this mediation model, child age was a significant predictor of MD of the right IFO (β = −0.30, SE = 0.10, t = −2.86, p = 0.01) and Externalizing Problems (β = 0.23, SE = 0.12, t = 1.97, p = 0.05).
Figure 3.
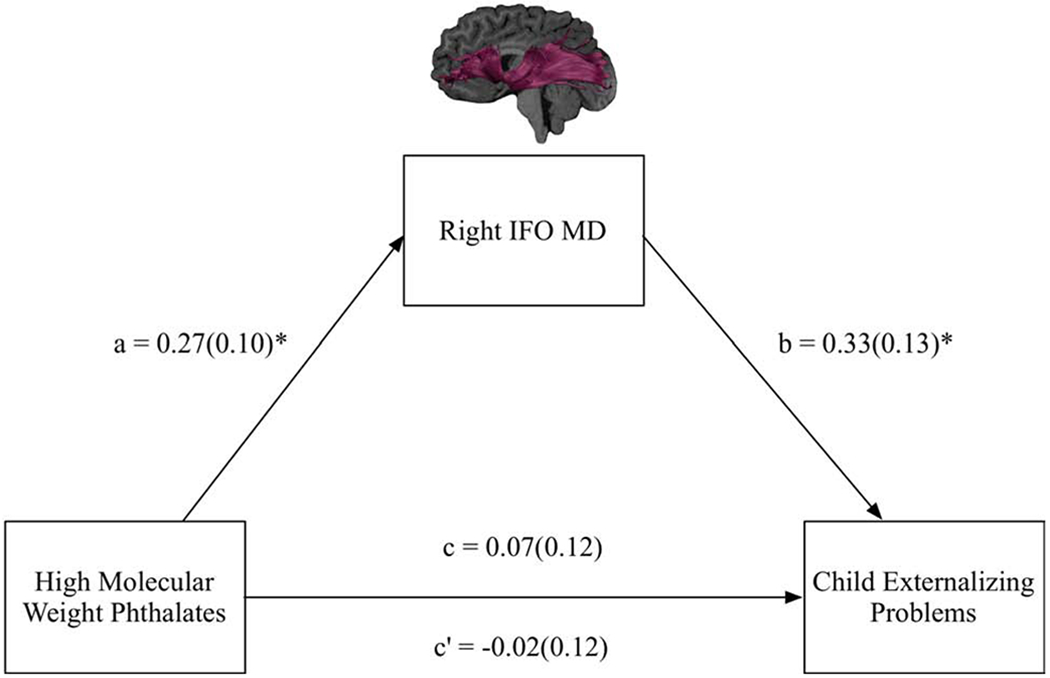
Full model and results for the mediation model in which mean diffusivity (MD) of the right IFO was examined as a mediator of prenatal exposure to high molecular weight phthalates and child externalizing problems. Standardized beta coefficients and standard errors in brackets are reported.
Note. a = the effect of the independent variable (high molecular weight phthalates) on the mediating variable (MD of the right IFO); b = the effect of the mediating variable (MD of the right IFO) on the dependent variable (child externalizing problems); c = direct effect; c’ =total effect.
*raw p-values < 0.05.
The third mediation model examined the association between HMWP and child Internalizing Problems as mediated by MD of the right pyramidal fibers. There was a non-significant total effect of HMWP on child Internalizing Problems (path c = 0.01, SE = 0.12, t = 0.12, p = 0.90; R2 = 0.07). However, higher prenatal exposure to HMWP was related to greater MD in the right pyramidal fibers (path a = 0.26, SE = 0.10, t = 2.60, p = 0.01; R2 = 0.23), and greater MD in the right pyramidal fibers was related to more Internalizing Problems (path b = 0.40, SE = 0.13, t = 3.13, p = 0.003; R2 = 0.12). There was a significant indirect effect; MD of the right pyramidal fibers emerged as a significant mediator of HMWP on Internalizing Problems (path ab = 0.11, SE = 0.06, CI.95 = 0.01, 0.23) (Figure 4). The effect size of this indirect effect was 0.11 (CI.95 = 0.02, 0.22) (Lachowicz et al., 2018). The direct effect was non-significant (path c’ = −0.09, SE = 0.12, t = −0.79, p = 0.43), indicating indirect-only mediation (Zhao et al., 2010). In this mediation model, child age was a significant predictor of MD of the right pyramidal fibers (β = −0.37, SE = 0.10, t = −3.73, p = 0.001), but not Internalizing Problems (β = 0.22, SE = 0.12, t = 1.83, p = 0.07).
Figure 4.
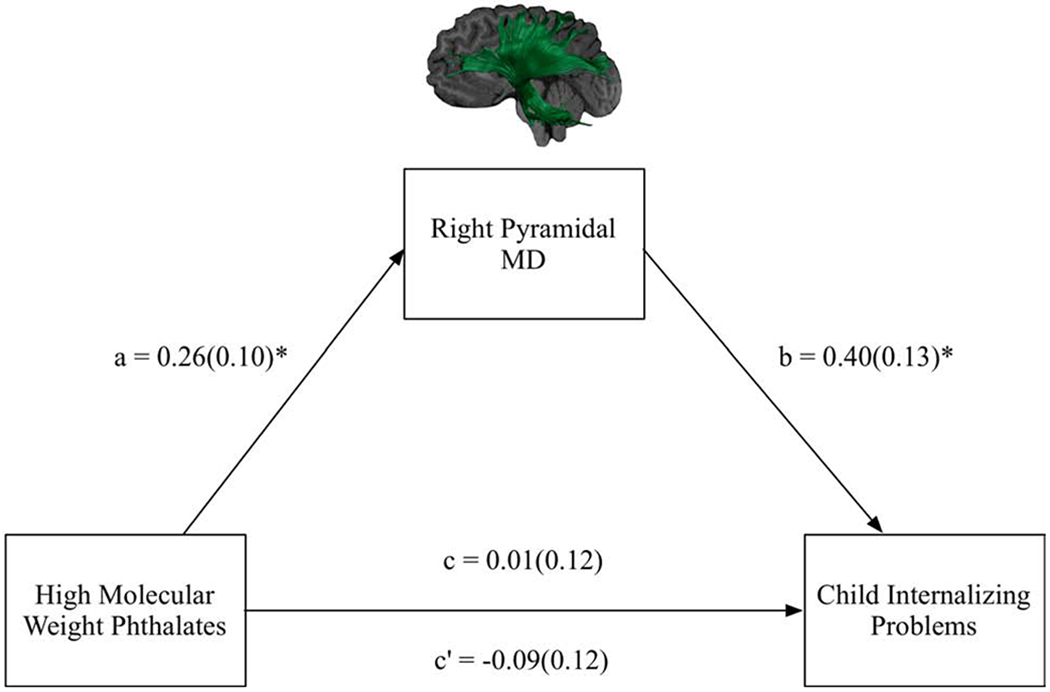
Full model and results for the mediation model in which mean diffusivity (MD) of the right pyramidal fibers was examined as a mediator of prenatal exposure to high molecular weight phthalates and child internalizing problems. Standardized beta coefficients and standard errors in brackets are reported.
Note. a = the effect of the independent variable (high molecular weight phthalates) on the mediating variable (MD of the right pyramidal fibres); b = the effect of the mediating variable (MD of the right pyramidal fibres) on the dependent variable (child internalizing problems); c = direct effect; c’ =total effect.
*raw p-values < 0.05.
The fourth mediation model examined the association between HMWP and child Externalizing Problems as mediated by MD of the right pyramidal fibers. There was a non-significant total effect of HMWP on child Externalizing Problems (path c = 0.07, SE = 0.12, t = 0.60, p = 0.55; R2 = 0.02). Higher prenatal exposure to HMWP was related to greater MD in the right pyramidal fibers (path a = 0.26, SE = 0.10, t = 2.60, p = 0.01; R2 = 0.23), and greater MD in the right pyramidal fibers was related to more Externalizing Problems (path b = 0.28, SE = 0.13, t = 2.14, p = 0.04; R2 = 0.08). However, there was not a significant indirect effect (path ab = 0.07, SE = 0.05, CI.95 = −0.01, 0.20) or direct effect (path c’ = −0.04, SE = 0.12, t = −0.04, p = 0.97), indicating non-mediation (Zhao et al., 2010).
The fifth mediation model examined the association between LMWP and child Internalizing Problems as mediated by FA of the left ILF. There was a non-significant total effect of LMWP on child Internalizing Problems (path c = 0.07, SE = 0.12, t = 0.60, p = 0.55; R2 = 0.03). Higher prenatal exposure to LMWP was related to lower FA in the left ILF (path a = −0.36, SE = 0.09, t = −3.87, p = 0.0002; R2 = 0.37), but lower FA in the left ILF was not significantly related to Internalizing Problems (path b = −0.26, SE = 0.15, t = −1.82, p = 0.07; R2 = 0.04). There was not a significant indirect effect (path ab = 0.09, SE = 0.06, CI.95 = −0.01, 0.21) or direct effect (path c’ = −0.03, SE = 0.13, t = −0.20, p = 0.84), indicating non-mediation (Zhao et al., 2010).
3.6. Post-hoc Analyses
Prenatal exposure to di(2-ethyl-hexyl) phthalate (DEHP), a commonly assessed parent compound, has also been associated with poorer neurodevelopmental outcomes in children (Chen et al., 2019; Huang et al., 2019), and may provide additional relevant information regarding prenatal exposure to phthalates. Four phthalate metabolites were included in the Σ DEHP: MEHP, MEHPP, MEOHP, and MECPP (Messerlian et al., 2016). Thirty-two post-hoc multivariate regression analyses examined if DEHP was associated with FA or MD of the 16 isolated white matter tracts (Supplemental Material, Table 2). Maternal DEHP concentration was significantly associated with MD of the right UF (q ≤ 0.10) and showed associations at levels that approached significance (p ≤ 0.10) with the right IFO and right pyramidal fibers. Given that MD of the right UF was not associated with Internalizing Problems or Externalizing Problems on the CBCL, no further analyses considered DEHP.
Although MD represents the average diffusivity of water across all directions of movement, axial diffusivity (AD; water diffusion along the principal axis of diffusion) and radial diffusivity (RD; the two vectors of diffusion perpendicular to the primary axis of diffusion) may provide additional information about specific processes related to myelination and axonal degeneration (Basser & Jones, 2002; Song et al., 2002). Sixty-four post-hoc multivariate regression analyses were conducted to investigate if HMWP or LMWP were associated with AD or RD of the 16 isolated white matter tracts (Supplemental Material, Table 3). Similar to the findings for HMWP and MD, HMWP was also significantly associated with AD of the right IFO (β = 0.36, q < 0.10) and RD of the right uncinate fasciculus (β = 0.29, q ≤ 0.10). HMWP was also associated with AD and/or RD of other white matter tracts previously associated with MD (i.e., right pyramidal fibers, right ILF, right UF, left UF), but these associations did not survive the correction for multiple comparisons (q > 0.10). Consistent with the findings for LMWP and MD, LMWP was also associated with AD of the left IFO (β = 0.23, p = 0.05) and RD of the left ILF (β = 0.27, p = 0.001), but only the association with RD of the left ILF survived the correction for multiple comparisons (q < 0.10). Six multivariate regressions were conducted to examine associations between these three white matter tracts (i.e., right IFO, right UF, left ILF) and T scores from the CBCL (Supplemental Material, Table 4), but none of these associations were significant (q > 0.10).
4. DISCUSSION
The current study assessed maternal phthalate levels in urine during pregnancy and found similar exposure levels to previously published Canadian biomonitoring data (Health Canada, 2013). Many of the individual phthalate metabolite concentrations in this Canadian sample exhibited lower concentrations than published biomonitoring data from the United States, with the exception of MEHP, MECCP, MiBP, and MMP, which were similar or slightly higher than those reported in the United States (Centers for Disease Control and Prevention, 2009). Although we did not find direct associations between maternal prenatal phthalate concentrations and maternal-reports of preschool behavior problems in our sample of 76 mother-child pairs, our findings revealed that prenatal phthalate concentrations were directly associated with white matter microstructure and indirectly associated with internalizing and externalizing behavior problems. Specifically, our mediation models found evidence for indirect-only mediation (Zhao et al., 2010), which supports the hypothesis that white matter microstructure mediates the association between prenatal phthalate exposure and preschool behavior problems. Although animal studies show that prenatal exposure to EDCs affects brain development (Itoh et al., 2012; Kougias et al., 2018; Masuo & Ishido, 2011), to our knowledge, this is the first study to show that prenatal phthalate exposure is related to brain structure in children.
Our exploratory regression analyses found significant associations between maternal phthalate concentrations during the second trimester of pregnancy and MD of the right pyramidal fibers, MD of the right IFO, MD of the left UF, MD of the right UF, and FA of the left ILF. Furthermore, the subsequent mediation models showed that greater prenatal exposure to high molecular weight phthalates (HMWP) was indirectly associated with more internalizing and externalizing problems in male and female preschool children. However, many of the observed p-values did not survive the correction for multiple comparisons. We can be most confident in the results that withstood correction at FDR of 5% (i.e., the association between HMWP and MD of the right UF and the association between low molecular weight phthalates (LMWP) and FA of the left ILF), while the other results should be interpreted with some caution. Overall, the observed associations between maternal prenatal phthalate concentrations and white matter microstructure, and the subsequent mediation models, suggest that prenatal phthalate exposure impacts the human brain, but will need to be replicated in future studies.
Prior research has used DTI to examine how individual differences in behavior, motor abilities, cognitive functioning, and emotional processes are related to microstructural white matter. Microstructure of the ILF supports perceptual processing and object recognition (Ortibus et al., 2012), and the pyramidal tract is associated with movement coordination and motor function (Lopez, Hemimou, Golse, & Vaivre-Douret, 2018; Ludeman et al., 2008). The IFO and UF are related to speech and language processing (Friederici, 2015), as well as affective functioning. Specifically, the IFO is associated with emotional processing and emotional prosody (Rodrigo et al., 2016; Schmidt et al., 2013), and microstructural alterations (i.e., lower FA, greater MD) in the UF are associated with affective dysregulation (e.g., depressive symptoms) in youth and adults (Vilgis, Vance, Cunnington, & Silk, 2017; Waller, Dotterer, Murray, Maxwell, & Hyde, 2017). However, many major white matter tracts contribute to overlapping functions in pediatric populations, with the cingulum fibers, ILF, SLF, right pyramidal tract, and UF correlating with verbal and performance intelligence quotients (Schmithorst, Wilke, Dardzinski, & Holland, 2005; Tamnes et al., 2010); and the IFO, ILF, and SLF correlating with attention and internalizing problems in children born preterm (Loe, Lee, & Feldman, 2013).
Integrating this neuroimaging research with the current study’s findings could suggest that greater prenatal exposure to HMWP may be associated with microstructural white matter alterations in areas associated with speech and affective functioning (i.e., IFO, UF), as well as tracts associated with motor skills (i.e., pyramidal fibers). Whereas, greater prenatal exposure to LMWP may be associated with microstructural white matter alterations in areas associated with perceptual processing (i.e., ILF). These theoretical associations are consistent with recent evidence reporting that greater prenatal phthalate exposure is associated with motor difficulties (Balalian et al., 2019), cognitive delays (Bornehag et al., 2018; Nakiwala et al., 2018), spatial processing deficits (Braun et al., 2017), and affective problems (Philippat et al., 2017). Thus, the inclusion of neuroimaging data in future studies examining phthalates and other developmental outcomes (e.g., motor, cognitive, affective) could help to undercover potential neurological mechanisms underlying these associations.
DTI parameters, FA and MD, inform both microscopic (i.e., axonal myelination, density, diameter) and macroscopic structural features (i.e., axonal direction, volume of white matter within a voxel) of white matter (Basser & Jones, 2002; Beaulieu, 2002). The finding that MD of two white matter tracts (i.e., right IFO, right pyramidal fibres) mediated the association between prenatal HMWP concentrations and preschool behavior problems, could suggest that prenatal exposure to HMWP may be associated with altered myelination, axon density, and/or fiber coherence in these tracts (Lebel, Treit, & Beaulieu, 2019). Longitudinal research indicates that white matter tracts can be generally categorized by three different patterns of development during early childhood: i) high initial FA and slow development rates in the callosal and pyramidal tracts; ii) low initial FA and rapid development rates in the ILF, IFO, cingulum, and fornix; iii) and low initial FA and slower development rates in the SLF and UF (Reynolds et al. 2019a). Considering the present associations, it is possible that prenatal exposure to HMWP may influence diffusivity and slow development of white matter in the right pyramidal tract, as well as diffusivity and rapid development of the right IFO. Potentially, these alterations could underlie the behavior problems that have been reported in preschool children exposed to higher levels of phthalates prenatally. Our post hoc analyses investigating axial diffusivity (AD) and radial diffusivity (RD) suggest that these measures could provide additional information about white matter differences specific to myelin and axons (Aung, Mar, & Benzinger, 2013; Song et al., 2002) in children exposed prenatally to phthalates. However, as the current study is underpowered to provide more information on these specific processes, future research is needed.
Neural networks of the fetal brain begin to develop early in gestation, progressing towards adult-like organization of white matter tracts during the last trimester of pregnancy (Dubois et al., 2014), with on-going maturation of these fibers continuing throughout childhood, adolescence and into adulthood (Barnea-Goraly et al., 2005; Lebel & Beaulieu, 2011). Although the influence of EDCs on myelination and axonal connections during fetal development and early childhood is not well understood, research suggests that epigenetic changes may mediate the association between early environmental exposures and white matter development (Alavian-Ghavanini & Rüegg, 2018; Dubois et al., 2014). In order to better understand the processes by which prenatal exposure to phthalates could alter brain microstructure, future work that investigates the influence of phthalates on epigenetic signals and neural organization across development is needed.
The current findings have implications for developmental models of psychopathology. Clinical models posit that many behavioral and psychiatric disorders originate during early development and are associated with aberrant white matter microstructure (Fields, 2008; Hüppi, 2008). In line with this developmental hypothesis, the current results could suggest that prenatal phthalate exposure may be associated with alterations in white matter microstructure and that these alterations may underlie childhood internalizing and externalizing problems. Notably, internalizing and externalizing problems during childhood have a high rate of comorbidity (Achenbach, Ivanova, Rescorla, Turner, & Althoff, 2016), but these problems typically have a divergent impact in later life. Internalizing problems are prognostic of later depressive symptomology (Sterba, Prinstein, & Cox, 2007) and externalizing problems incur greater risk for substance use and criminality (Miettunen et al., 2014). Although there are age-specific and sex-specific differences in the prevalence of internalizing and externalizing problems on the CBCL (Mesman, Bongers, & Koot, 2001), child sex did not modify any of our examined associations. Thus, the current results suggest that prenatal exposure to HMWP may affect white matter microstructure, and these alterations may transmit risk for internalizing and externalizing problems, in both male and female preschool children. Given the substantial evidence reporting that perturbations in white matter microstructure can confer risk for psychopathology (Hinton et al., 2019; Lagopoulos et al., 2013; Thomason & Thompson, 2011), additional research is needed to investigate the effects of early environmental exposures on sex-specific trajectories of white matter development and if altered trajectories confer risk for prodromes of mental illness.
4.1. Strengths and Limitations
This study provides a novel examination of a potential neural mediator of the association between prenatal phthalate exposure and child behavior, suggesting that white matter microstructure may serve as a potential mechanism that transmits risk for child internalizing and externalizing problems following greater prenatal phthalate exposure. Another potential strength of this study is that we used molar sums of HMWP and LMWP; molar sums assess exposure to phthalates with similar molecular structures, sources of exposure, and levels of bio-activity (Engel et al., 2010, 2009; Wolff et al., 2008). This allowed us to evaluate whether aggregate prenatal exposures to HMWP and LMWP were associated with white matter microstructure and preschool behavior problems. Post-hoc analyses also examined the associations between DEHP, a parent compound summary measure, which includes some of the metabolites included in HMWP (Spearman’s rho = 0.80), and white matter microstructure; However, no associations between DEHP and any white matter tracts associated with child behavior problems reached significance.
In light of these strengths, there are several limitations that warrant consideration This study specifically hypothesized that molar sums of phthalates based on molecular weight would be associated with child brain microstructure and behavior problems; however, due to its limited sample size and the need to correct for multiple comparisons between correlated variables (i.e., between correlated phthalate exposures), the influence of individual phthalate metabolites on brain structure was not examined. Future studies with larger samples are needed to better delineate the complex relationships between exposure to individual phthalates and biologically-relevant phthalate summary (e.g., molecular weight, parent compound) measures and children’s brain structure. The current study also included a relatively homogeneous and small sample of mother-child pairs of high socioeconomic status (i.e., mothers were predominantly Caucasian, university-educated, married, and had high household incomes), which limits our ability to examine the effect of these potentially important covariates in our analyses, and the generalizability of our findings to other populations. Due to study constraints, phthalate metabolites were assessed via a single spot urine sample collected during the second trimester of pregnancy. Other environmental health studies have analyzed average analyte concentrations from urine samples collected at two or more points (Braun et al., 2011; Harley et al., 2013; Stacy et al., 2017); however, prior work suggests that single spot-sampling demonstrates moderate sensitivity and reflects average exposure (Mahalingaiah et al., 2008; Ye, Wong, Bishop, & Calafat, 2011). Relatedly, we only assessed maternal phthalate concentrations during pregnancy, and do not have any measures of child phthalate exposure during the first years of life, which are an important time for brain development. Furthermore, this study does not lend itself to drawing causal inferences about the relationship between prenatal exposures, neural microstructure, and behavioral outcomes due to the cross-sectional study design. Cross-sectional studies may accurately report associations between DTI parameters and age-specific measures (i.e., child behavior outcomes at specific ages), but they are heavily influenced by inter-subject variability and cannot capture within-subject changes (Lebel et al., 2019). There is pressing need for future longitudinal pediatric studies investigating white matter microstructure and early life exposure to EDCs in order to better understand how early environmental exposures influence neurobehavioral development (Hermoye et al., 2006; Young et al., 2016). Finally, child internalizing and externalizing behaviors were assessed using a parent-report measure. Future research that examines the influence of EDCs on brain structure, which utilizes multi-method assessments of behavioral functioning during childhood may strengthen the current evidence.
4.2. Conclusion
This is the first human study to suggest that prenatal exposure to phthalates is associated with white matter microstructure in children, and further suggests that these differences may underlie behavioral problems in preschool-aged children. Exposure to phthalates is ubiquitous, and therefore pregnant women and their developing fetuses may be constantly exposed. This study provides important new information on the potential effects of prenatal phthalate exposure on children’s brain and behavioral development, effects that could have long-term implications for public health. Future longitudinal research that investigates the associations between pre- and postnatal exposure to phthalates, children’s brain development, and risk for childhood behavior problems and psychopathology in later life are warranted.
Supplementary Material
HIGHLIGHTS.
Prenatal phthalate exposure may impact preschool brain microstructure
Phthalates were related to mean diffusivity of several white matter tracts
Phthalates were related to fractional anisotropy of one white matter tract
High molecular weight phthalates indirectly predicted preschool behavior problems
Acknowledgments
Funding Sources: This cohort was established by an interdisciplinary team grant from Alberta Innovates Health Solutions (formally the Alberta Heritage Foundation for Medical Research). The collection and analysis of data presented in this manuscript were supported by grants from the Canadian Institutes of Health Research (MOP-123535; MOP-136797, IDH-134090), the U.S. National Institutes of Health (Exploration/Development Grant 1R21ES021295-01R21) and the Alberta Children’s Hospital Foundation. Salary support was provided by a Neurodevelopmental Disorders Fellowship from the Alberta Children’s Hospital Foundation (GE). Additional salary support includes a University of Calgary Queen Elizabeth-II Graduate Studentship, Vi Riddell Pediatric Rehabilitation Graduate Studentship, and Alberta Children’s Hospital Research Institute/ Department of Pediatrics Studentship (MNG); a University of Calgary Eyes High Postdoctoral Award, T. Chen Fong Postdoctoral Fellowship in Medical Imaging Science, and Canadian Institutes of Health Research Postdoctoral Fellowship (MFE-164703) (JER); an Alberta Innovates-Health Solutions Scholarship and Faculty of Medicine and Dentistry University of Alberta Medical Science Graduate Program Scholarship (JL); and a Canadian Institutes of Health Research New Investigator Award (CL). The funding sources were not involved in the study design, collection of data, analysis and interpretation of data, writing of the manuscript, and in the decision to submit the article for publication.
We acknowledge the significant contributions of the APrON Study Team whose individual members are: B.J. Kaplan, C.J. Field, R.C. Bell, F.P. Bernier, M. Cantell, L.M. Casey, M. Eliasziw, A. Farmer, L. Gagnon, G.F. Giesbrecht, L. Goonewardene, D. Johnston, L. Kooistra, N. Letourneau, D.P. Manca, L.J. McCargar, M. O’Beirne, V.J. Pop, A.J. Deane, and N. Singhal.
ABBREVIATIONS
- EDC
endocrine disrupting chemicals
- DTI
diffusion tensor imaging
- MRI
magnetic resonance imaging
- FA
fractional anisotropy
- MD
mean diffusivity
- APrON
Alberta Pregnancy Outcomes and Nutrition
- FSIQ
full scale intelligence quotient
- CBCL
Child Behavior Checklist
- DEHP
di(2-ethyl-hexyl) phthalate
- MEHP
mono(2-ethylhexyl) phthalate
- MEHHP
mono(2-ethyl-5-hydroxy-hexyl) phthalate
- MEOHP
mono(2-ethyl-5-oxyohexyl) phthalate
- MECPP
mono(2-ethyl-5-carboxypentyl) phthalate
- DBP
dibutyl phthalate
- MBP
mono-n-butyl phthalate
- MiBP
mono-iso-butyl phthalate
- MBzP
mono-benzyl phthalate
- MEP
mono-ethyl phthalate
- MMP
mono-methyl phthalate
- Σ HMWP
sum of high molecular weight phthalates (MEHP, MEHPP, MEOHP, MECPP, and MBzP)
- Σ LMWP
sum of low molecular weight phthalates (MBP, MiBP, MEP, and MMP)
- LOD
limit of detection
- IFO
inferior fronto-occipital fasciculus
- ILF
inferior longitudinal fasciculus
- SLF
superior longitudinal fasciculus including arcuate
- UF
uncinate fasciculus
- FDR
false discovery rate
- ROI
regions of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: CL’s spouse is an employee of General Electric Healthcare. All other authors declare no actual or potential competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
- AB Sciex. QTRAP® 5500 LC-MS/MS System. Concord, Ontario, Canada: SCIEX. [Google Scholar]
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA preschool forms & profile. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Achenbach Thomas M., Ivanova MY, Rescorla LA, Turner LV, & Althoff RR (2016). Internalizing/externalizing problems: Review and recommendations for clinical and research applications. Journal of the American Academy of Child & Adolescent Psychiatry, 55(8), 647–656. 10.1016/j.jaac.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Alavian-Ghavanini A, & Rüegg J (2018). Understanding epigenetic effects of endocrine disrupting chemicals: From mechanisms to novel test methods. Basic & Clinical Pharmacology & Toxicology, 122(1), 38–45. https://doi.org/10.1111/bcpt.12878 [DOI] [PubMed] [Google Scholar]
- Albaugh MD, Ducharme S, Karama S, Watts R, Lewis JD, Orr C, … Brain Development Cooperative Group. (2017). Anxious/depressed symptoms are related to microstructural maturation of white matter in typically developing youths. Development and Psychopathology, 29(3), 751–758. 10.1017/S0954579416000444 [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, & Field AS (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4(3), 315–329. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2041910/pdf/nihms26625.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, & Smith S (2007). Non-linear registration aka spatial normalisation FMRIB technial report TR07JA2. Retrieved from https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja2/tr07ja2.pdf [Google Scholar]
- Aung WY, Mar S, & Benzinger TL (2013, October). Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging in Medicine, 5(5), 427–440. 10.2217/iim.13.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balalian AA, Whyatt RM, Liu X, Insel BJ, Rauh VA, Herbstman J, & Factor-Litvak P (2019). Prenatal and childhood exposure to phthalates and motor skills at age 11 years. Environmental Research, 171, 416–427. 10.1016/j.envres.2019.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, … Reiss AL (2005). White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex, 15(12), 1848–1854. 10.1093/cercor/bhi062 [DOI] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51(6), 1173–1182. Retrieved from https://journals-scholarsportal-info.libaccess.lib.mcmaster.ca/pdf/00223514/v51i0006/1173_tmvdisrcsasc.xml [DOI] [PubMed] [Google Scholar]
- Basser PJ, & Jones DK (2002). Diffusion-tensor MRI: Theory, experimental design and data analysis - a technical review. NMR in Biomedicine, 15(7–8), 456–467. 10.1002/nbm.783 [DOI] [PubMed] [Google Scholar]
- Basten M, Tiemeier H, Althoff RR, van de Schoot R, Jaddoe VWV, Hofman A, … van der Ende J (2016). The stability of problem behavior across the preschool years: An empirical approach in the general population. Journal of Abnormal Child Psychology, 44(2), 393–404. 10.1007/s10802-015-9993-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine, 15(7–8), 435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, & Faisal PA (2017). Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. Journal of Hazardous Materials, 340, 360–383. 10.1016/jjhazmat.2017.06.036 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x [Google Scholar]
- Beyer T, Postert C, Müller JM, & Furniss T (2012). Prognosis and continuity of child mental health problems from preschool to primary school: Results of a four-year longitudinal study. Child Psychiatry & Human Development, 43(4), 533–543. 10.1007/s10578-012-0282-5 [DOI] [PubMed] [Google Scholar]
- Bornehag C-G, Lindh C, Reichenberg A, Wikström S, Unenge Hallerback M, Evans SF, … Swan SH (2018). Association of prenatal phthalate exposure with language development in early childhood. JAMA Pediatrics, 172(12), 1169 10.1001/jamapediatrics.2018.3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JD, & Choudhury M (2016). Phthalates in neonatal health: friend or foe? Journal of Developmental Origins of Health and Disease, 7(6), 652–664. 10.1017/S2040174416000349 [DOI] [PubMed] [Google Scholar]
- Braun Joe M., Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, & Lanphear BP (2011). Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics, 128(5), 873–882. 10.1542/peds.2011-1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun Joseph M. (2017, March 1). Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nature Reviews Endocrinology, 13(3), 161–173. 10.1038/nrendo.2016.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun Joseph M., Bellinger DC, Hauser R, Wright RO, Chen A, Calafat AM, … Lanphear BP (2017). Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. NeuroToxicology, 58, 75–83. 10.1016/j.neuro.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, & Jernigan TL (2012). Brain development during the preschool years. Neuropsychology Review, 22(4), 313–333. 10.1007/s11065-012-9214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Wang YH, Chen WJ, Hsiung CA, Leon Guo YL, & Julie Wang SL (2019). A benchmark dose study of prenatal exposure to di(2-ethylhexyl) phthalate and behavioral problems in children. International Journal of Hygiene and Environmental Health, 222(6), 971–980. 10.1016/j.ijheh.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Chen JJ (2010). Gender differences in externalising problems among preschool children: implications for early childhood educators. Early Child Development and Care, 180(4), 463–474. 10.1080/03004430802041011 [DOI] [Google Scholar]
- Committee on the Health Risks of Phthalates Board on Environmental Studies and Toxicology Division on Earth and Life Studies. (2008). Phthalates and cumulative risk assessment: The task ahead. Washington, D.C: Retrieved from http://www.nap.edu/catalog/12528.html [Google Scholar]
- Department of Health and Human Services Centres for Disease Control and Prevention. (2009). Fourth national report on human exposure to environmental chemicals. Atlanta, GA: Retrieved from https://www/cdc.gov/exposurereport/pdf/fourthreport.Pdf [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, & Hertz-Pannier L (2014). The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Journal of Neuroscience, 276, 48–71. 10.1016/j.neurosciences.2013.12.044 [DOI] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, & Le Bihan D (2006). Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: A feasibility study using quantitative diffusion tensor imaging and tractography. NeuroImage, 30(4), 1121–1132. 10.1016/j.neuroimage.2005.11.022 [DOI] [PubMed] [Google Scholar]
- Ejaredar M, Nyanza EC, Ten Eycke K, & Dewey D (2015). Phthalate exposure and childrens neurodevelopment: A systematic review. Environmental Research. 142, 51–60. 10.1016/j.envres.2015.06.014 [DOI] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, & Wolff MS (2010). Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environmental Health Perspectives, 118(4), 565–571. 10.1289/ehp.0901470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, & Wolff MS (2009). Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology, 30(4), 522–528. 10.1016/j.neuro.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AP (2009). Discovering statistics using SPSS. SAGE Publications. [Google Scholar]
- Fields RD (2008). White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences, 31(7), 361–370. 10.1016/j.tins.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2015). White-matter pathways for speech and language processing. Handbook of clinical neurology, 129, 177–186. 10.1016/B978-0-444-62630-1.00010-X [DOI] [PubMed] [Google Scholar]
- General Electric. Discovery™ MR750 3.0T. Waukesha, WI: General Electric. [Google Scholar]
- Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, & Palanza P (2007). Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Hormones and Behavior, 52(3), 307–316. 10.1016/j.yhbeh.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Gore AC, Krishnan K, & Reilly MP (2019). Endocrine-disrupting chemicals: Effects on neuroendocrine systems and the neurobiology of social behavior. Hormones and Behavior. 111, 7–22. 10.1016/j.yhbeh.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohs M, Reynolds JE, Liu J, Martin JW, Pollock T, Lebel C, … APrON Study Team. (2019). Prenatal and childhood bisphenol A exposure and brain structure and behaviour of young children. Environmental Health, 18(1), 85 10.1186/s12940-019-0528-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, & Eskenazi B (2013). Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environmental Research, 126, 43–50. 10.1016/j.envres.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach (Second). New York, NY: Guildford Press. [Google Scholar]
- Health Canada. (2013). Second report on human biomonitoring of environmental chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 2 (2009–2011). Ottawa, ON: Retrieved from https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/environmental-contaminants/second-report-human-biomonitoring-environmental-chemicals-canada-health-canada-2013.html [Google Scholar]
- Hermoye L, Saint-Martin C, Cosnard G, Lee S-K, Kim J, Nassogne M-C, … Mori S (2006). Pediatric diffusion tensor imaging: Normal database and observation of the white matter maturation in early childhood. NeuroImage, 29(2), 493–504. 10.1016/j.neuroimage.2005.08.017 [DOI] [PubMed] [Google Scholar]
- Hinton KE, Lahey BB, Villalta-Gil V, Meyer FAC, Burgess LL, Chodes LK, … Zald DH (2019). White matter microstructure correlates of general and specific second-order factors of psychopathology. NeuroImage: Clinical, 22, 101705 10.1016/j.nicl.2019.101705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, & Reed LD (1990). Estimation of average concentration in the presence of mondetectable values. Applied Occupational and Environmental Hygiene, 5(1), 46–51. 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- Huang H-B, Kuo P-H, Su P-H, Sun C-W, Chen WJ, & Wang S-L (2019). Prenatal and childhood exposure to phthalate diesters and neurobehavioral development in a 15-year follow-up birth cohort study. Environmental Research, 172, 569–577. 10.1016/j.envres.2019.02.029 [DOI] [PubMed] [Google Scholar]
- Hüppi PS (2008). Neuroimaging of brain development - discovering the origins of neuropsychiatric disorders? Pediatric Research, 64(4), 325–325. 10.1203/PDR.0b013e31818981ea [DOI] [PubMed] [Google Scholar]
- IBM Corp. (2017). SPSS statistics for Macintosh. Armonk, NY: IBM Corp. [Google Scholar]
- Irfanoglu MO, Sarlls J, Nayak A, & Pierpaoli C (2019). Evaluating corrections for Eddy-currents and other EPI distortions in diffusion MRI: Methodology and a dataset for benchmarking. Magnetic Resonance in Medicine, 81(4), 2774–2787. 10.1002/mrm.27577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Yaoi T, & Fushiki S (2012). Bisphenol A, an endocrine-disrupting chemical, and brain development. Neuropathology, 32(4), 447–457. 10.1111/j.1440-1789.2011.01287.x [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, & Smith SM (2012). FSL. NeuroImage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, Giesbrecht GF, Leung BMY, Field CJ, Dewey D, Bell RC, … APrON Study Team. (2014). The Alberta Pregnancy Outcomes and Nutrition (APrON) cohort study: rationale and methods. Maternal & Child Nutrition, 10(1), 44–60. 10.1111/j.1740-8709.2012.00433.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B-N, Cho S-C, Kim Y, Shin M-S, Yoo H-J, Kim J-W, … Hong Y-C (2009). Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biological Psychiatry, 66(10), 958–963. 10.1016/j.biopsych.2009.07.034 [DOI] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, & Swan SH (2014). Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environmental Health Perspectives, 122(5), 521–528. 10.1289/ehp.1307063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kougias DG, Sellinger EP, Willing J, & Juraska JM (2018). Perinatal exposure to an environmentally relevant mixture of phthalates results in a lower number of neurons and synapses in the medial prefrontal cortex and decreased cognitive flexibility in adult male and female rats. Journal of Neuroscience, 38(31), 6864–6872. 10.1523/JNEUROSCI.0607-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Arai O, Omura M, Watanabe R, Ogata R, & Aou S (2003). Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neuroscience Research, 45(3), 345–356. 10.1016/S0168-0102(02)00251-1 [DOI] [PubMed] [Google Scholar]
- LabX. Agilent 1200 HPLC. Mississauga, Ontario, Canada: Agilent Technologies. [Google Scholar]
- Lachowicz MJ, Preacher KJ, & Kelley K (2018). A novel measure of effect size for mediation analysis. Psychological Methods, 23(2), 244–261. 10.1037/met0000165 [DOI] [PubMed] [Google Scholar]
- Lagopoulos J, Hermens DF, Hatton SN, Battisti RA, Tobias-Webb J, White D, … Hickie IB (2013). Microstructural white matter changes are correlated with the stage of psychiatric illness. Translational Psychiatry, 3(4), e248 10.1038/tp.2013.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, & Mazzeo P (2003). Exposure to di(2-ethylhexyl)phthalate in humans during pregnancy. Neonatology, 83(1), 22–24. 10.1159/000067012 [DOI] [PubMed] [Google Scholar]
- Lebel C, & Beaulieu C (2011). Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience, 31(30), 10937–10947. 10.1523/JNEUROSCI.5302-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Treit S, & Beaulieu C (2019). A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR in Biomedicine, 32(4), e3778 10.1002/nbm.3778 [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, & Beaulieu C (2008). Microstructural maturation of the human brain from childhood to adulthood. NeuroImage, 40(3), 1044–1055. 10.1016/j.neuroimage.2007.12.053 [DOI] [PubMed] [Google Scholar]
- Lee DW, Kim MS, Lim YH, Lee N, & Hong YC (2018). Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: A systematic review and meta-analysis. Environmental Research, 167, 558–566. 10.1016/j.envres.2018.08.023 [DOI] [PubMed] [Google Scholar]
- Leemans A, Jeurissen B, Sijbers J, & Jones DK (2009). ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Retrieved from http://www.exploredti.com
- Loe IM, Lee ES, & Feldman HM (2013). Attention and internalizing behaviors in relation to white matter in children born preterm. Journal of Developmental and Behavioral Pediatrics, 34(3), 156–164. 10.1097/DBP.0b013e3182842122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C, Hemimou C, Golse B, & Vaivre-Douret L (2018). Developmental dysgraphia is often associated with minor neurological dysfunction in children with developmental coordination disorder (DCD). Neurophysiologie Clinique, 48(4), 207–217. 10.1016/j.neucli.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Ludeman NA, Berman JI, Wu YW, Jeremy RJ, Kornak J, Bartha AI, … Glenn OA (2008). Diffusion tensor imaging of the pyramidal tracts in infants with motor dysfunction. Neurology, 71(21), 1676–1682. 10.1212/01.wnl.0000304084.59964.e2 [DOI] [PubMed] [Google Scholar]
- Lyche JL, Gutleb AC, Bergman Å, Eriksen GS, Murk AJ, Ropstad E, … Skaare JU (2009). Reproductive and developmental toxicity of phthalates. Journal of Toxicology and Environmental Health, Part B, 12(4), 225–249. 10.1080/10937400903094091 [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, & Fairchild AJ (2010). A general model for testing mediation and moderation effects. Prevention, 10(2), 87–99. 10.1007/s11121-008-0109-6.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, & Fritz MS (2007). Mediation analysis. Annual Review of Psychology, 58, 593–614. 10.1146/annurev.psych.58.110405.085542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, & Hauser R (2008). Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environmental Health Perspectives, 116(2), 173–178. 10.1289/ehp.10605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuo Y, & Ishido M (2011). Neurotoxicity of endocrine disruptors: Possible involvement in brain development and neurodegeneration. Journal of Toxicology and Environmental Health, Part B, 14(5–7), 346–369. 10.1080/10937404.2011.578557 [DOI] [PubMed] [Google Scholar]
- Mesman J, Bongers IL, & Koot HM (2001). Preschool developmental pathways to preadolescent internalizing and externalizing problems. Journal of Child Psychology and Psychiatry, 42(5), S0021963001007351. 10.1017/S0021963001007351 [DOI] [PubMed] [Google Scholar]
- Mesman J, & Koot HM (2001). Early preschool predictors of preadolescent internalizing and externalizing DSM-IV diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry, 40(9), 1029–1036. 10.1097/00004583-200109000-00011 [DOI] [PubMed] [Google Scholar]
- Messerlian C, Wylie BJ, Mínguez-Alarcón L, Williams PL, Ford JB, Souter IC, … Hauser R (2016). Urinary concentrations of phthalate metabolites and pregnancy loss among women conceiving with medically assisted reproduction. Epidemiology, 27(6), 879–888. 10.1097/EDE.0000000000000525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettunen J, Murray GK, Jones PB, Mäki P, Ebeling H, Taanila A, … Moilanen I (2014). Longitudinal associations between childhood and adulthood externalizing and internalizing psychopathology and adolescent substance use. Psychological Medicine, 44(08), 1727–1738. 10.1017/S0033291713002328 [DOI] [PubMed] [Google Scholar]
- Mori S (Susumu). (2007). Introduction to diffusion tensor imaging. Elsevier Science. 10.1016/B978-0-444-52828-5.X5014-5 [DOI] [Google Scholar]
- Morris Z, Whiteley WN, Longstreth WT, Weber F, Lee YC, Tsushima Y, … Al-Shahi Salman R (2009). Incidental findings on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ (Online), 339(7720), 547–550. 10.1136/bmj.b3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakiwala D, Peyre H, Heude B, Bernard JY, Béranger R, Slama R, … EDEN mother-child study group. (2018). In-utero exposure to phenols and phthalates and the intelligence quotient of boys at 5 years. Environmental Health: A Global Access Science Source, 17(1), 17 10.1186/s12940-018-0359-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortibus E, Verhoeven J, Sunaert S, Caseteels I, De CocK P, & Lagae L (2012). Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: a diffusion tensor imaging study. Developmental Medicine & Child Neurology, 54(1), 38–43. 10.1111/j.1469-8749.2011.04147.x [DOI] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, vom Saal FS, & Parmigiani S (2008). Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environmental Research, 108(2), 150–157. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18949834 [DOI] [PubMed] [Google Scholar]
- Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, … EDEN Mother-Child Study Group. (2017). Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 years. Environmental Health Perspectives, 125(9), 097014 10.1289/EHP1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Mori S, & Miller MI (2015). Diffusion tensor imaging for understanding nrain development in early life. Annual Review of Psychology, 66(1), 853–876. 10.1146/annurev-psych-010814-015340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JE, Grohs MN, Dewey D, & Lebel C (2019a). Global and regional white matter development in early childhood. NeuroImage, 196, 49–58. Retrieved from https://www.sciencedirect.com/science/article/pii/S1053811919302903?via%3Dihub [DOI] [PubMed] [Google Scholar]
- Reynolds J, Grohs M, & Lebel C (2019b). White matter tractography guides. Retrieved from https://figshare.com/articles/White_Matter_Tractography_Guides/7603271/1 [Google Scholar]
- Rodrigo MJ, León I, Góngora D, Hernández-Cabrera JA, Byrne S, & Bobes MA (2016). Inferior fronto-temporo-occipital connectivity: A missing link between maltreated girls and neglectful mothers. Social Cognitive and Affective Neuroscience, 11(10), 1658–1665. 10.1093/scan/nsw080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AT, Hanten G, Li X, Wilde EA, Ibarra AP, Chu ZD, … Levin HS (2013). Emotional prosody and diffusion tensor imaging in children after traumatic brain injury. Brain Injury, 27(13–14), 1528–1535. 10.3109/02699052.2013.828851 [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, & Holland SK (2005). Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Human Brain Mapping, 26(2), 139–147. 10.1002/hbm.20149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Blawas AM, Gray K, Heindel JJ, & Lawler CP (2015). Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology, 156(6), 1941–1951. 10.1210/en.2014-1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman A, Dougherty RF, Lee J, Ghahremani D, & Taylor JE (2009). Empirical null and false discovery rate analysis in neuroimaging. NeuroImage, 44(1), 71–82. 10.1016/j.neuroimage.2008.04.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano SE, Braun J, Trasande L, Dills R, & Sathyanarayana S (2014). Phthalates and diet: A review of the food monitoring and epidemiology data. Environmental Health, 13(1), 43 10.1186/1476-069X-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, & Bolger N (2002). Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods, 7(4), 422–445. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12530702 [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, … Behrens TEJ (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, … Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, & Sousa N (2013). A hitchhiker’s guide to diffusion tensor imaging. Frontiers in Neuroscience, (7 March). 10.3389/fnins.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, & Cross AH (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage, 17(3), 1429–1436. 10.1006/nimg.2002.1267 [DOI] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Horn J. D. Van, Toga AW, … Bookheimer SY (2008). Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. Journal of Neuroscience, 28(6), 1313–1319. 10.1523/JNEUROSCI.5067-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy SL, Papandonatos GD, Calafat AM, Chen A, Yolton K, Lanphear BP, & Braun JM (2017). Early life bisphenol A exposure and neurobehavior at 8 years of age: Identifying windows of heightened vulnerability. Environment International, 107, 258–265. 10.1016/j.envint.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterba SK, Prinstein MJ, & Cox MJ (2007). Trajectories of internalizing problems across childhood: Heterogeneity, external validity, and gender differences. Development and Psychopathology, 19(02), 345–366. 10.1017/S0954579407070174 [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Walhovd KB, Westlye LT, Due-Tønnessen P, & Fjell AM (2010). Intellectual abilities and white matter microstructure in development: A diffusion tensor imaging study. Human Brain Mapping, 31(10), 1609–1625. 10.1002/hbm.20962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermo Scientific™ BetaSil™ Phenyl Column. Burlington, Ontario, Canada: Thermo Fisher Scientific. [Google Scholar]
- Thomason ME, & Thompson PM (2011). Diffusion imaging, white matter, and psychopathology. Annual Review of Clinical Psychology, 7(1), 63–85. 10.1146/annurev-clinpsy-032210-104507 [DOI] [PubMed] [Google Scholar]
- Vilgis V, Vance A, Cunnington R, & Silk TJ (2017). White matter microstructure in boys with persistent depressive disorder. Journal of Affective Disorders, 221, 11–16. 10.1016/j.jad.2017.06.020 [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Casas M, Gascon M, Valvi D, & Nieuwenhuijsen M (2016). Environmental pollutants and child health - a review of recent concerns. International Journal of Hygiene and Environmental Health. 219(4–5), 331–342. 10.1016/j.ijheh.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, Van Zijl PCM, & Mori S (2004). Fiber tract-based atlas of human white matter anatomy. Radiology, 230(1), 77–87. 10.1148/radiol.2301021640 [DOI] [PubMed] [Google Scholar]
- Walker DM, & Gore AC (2011, April). Transgenerational neuroendocrine disruption of reproduction. Nature Reviews Endocrinology. 7(4), 197–207. 10.1038/nrendo.2010.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Dotterer HL, Murray L, Maxwell AM, & Hyde LW (2017). White-matter tract abnormalities and antisocial behavior: A systematic review of diffusion tensor imaging studies across development. NeuroImage: Clinical, 14, 201–215. 10.1016/j.nicl.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, … Factor-Litvak P (2012). Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environmental Health Perspectives, 120(2), 290–295. 10.1289/ehp.1103705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, … Calafat AM (2008). Prenatal phenol and phthalate exposures and birth outcomes. Environmental Health Perspectives, 116(8), 1092–1097. 10.1289/ehp.11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, … Smith SM (2009). Bayesian analysis of neuroimaging data in FSL. NeuroImage, 45(1 Suppl), S173–S186. 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- World Medical Association. (2013) World Medical Association Declaration of Helsinki-ethical principles for medical research involving human subjects. Retrieved from https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [DOI] [PubMed]
- Ye X, Wong L-Y, Bishop AM, & Calafat AM (2011). Variability of urinary concentrations of bisphenol A in pot Sasmples, first morning voids, and 24-hour collections. Environmental Health Perspectives, 119(7), 983–988. 10.1289/ehp.1002701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, & Khoury J (2011). Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicology and Teratology, 33(5), 558–566. 10.1016/j.ntt.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, Morgan BR, Whyte HEA, Lee W, Smith M Lou, Raybaud C, … Taylor MJ (2016). Longitudinal study of white matter development and outcomes in children born very preterm. Cerebral Cortex, 27(8), 4094–4105. 10.1093/cercor/bhw221 [DOI] [PubMed] [Google Scholar]
- Zanin E, Ranjeva J-P, Confort-Gouny S, Guye M, Denis D, Cozzone PJ, & Girard N (2011). White matter maturation of normal human fetal brain. An in vivo diffusion tensor tractography study. Brain and Behavior, 1(2), 95–108. 10.1002/brb3.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chen XZ, Huang X, Wang M, & Wu J (2019). The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts. NeuroToxicology. 73, 199–212. 10.1016/j.neuro.2019.04.007 [DOI] [PubMed] [Google Scholar]
- Zhao X, Lynch JG, & Chen Q (2010). Reconsidering Baron and Kenny: Myths and truths about mediation analysis. Journal of Consumer Research, 37(2), 197–206. 10.1086/651257 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


