Abstract
Obstructive sleep apnea (OSA), a chronic sleep disorder characterized by repetitive reduction or cessation of airflow during sleep, is widely prevalent and is associated with adverse neurocognitive sequelae including increased risk of Alzheimer’s disease (AD). In humans, OSA is more common in elderly males. OSA is characterized by sleep fragmentation and chronic intermittent hypoxia (CIH) and recent epidemiological studies point to CIH as the best predictor of neurocognitive sequelae associated with OSA. The sex- and age- specific effects of OSA-associated CIH on specific cell populations such as γ-aminobutyric acid (GABA)-ergic neurons in the hippocampus and the medial prefrontal cortex (mPFC), regions important for cognitive function, remain largely unknown. The present study examined the effect of 35 days of either moderate (10% oxygen) or severe (5% oxygen) CIH on GABAergic neurons in the mPFC and hippocampus of young and aged male and female mice as well as post-accelerated ovarian failure (AOF) female mice. In the mPFC and hippocampus, the number of GABA-labeled neurons increased in aged and young severe CIH males compared to controls but not in young moderate CIH males. This change was not representative of the individual GABAergic cell subpopulations, as the number of parvalbumin-labeled neurons decreased while the number of somatostatin-labeled neurons increased in the hippocampus of severe CIH young males only. In all female groups, the number of GABA-labeled cells was not different between CIH and controls. However, in the mPFC, CIH increased the number of parvalbumin-labeled neurons in young females and the number of somatostatin-labeled cells in AOF females but decreased the number of somatostatin-labeled cells in aged females. In the hippocampus, CIH decreased the number of somatostatin-labeled neurons in young females. CIH decreased the density of vesicular GABA transporter in the mPFC of AOF females only. These findings suggest sex-specific changes in GABAergic neurons in the hippocampus and mPFC with males showing an increase of this cell population as compared to their female counterparts following CIH. Age at exposure and severity of CIH also differentially affect the GABAergic cell population in mice.
Keywords: obstructive sleep apnea, GABA, somatostatin, parvalbumin, vesicular GABA transporter
1. Introduction
Obstructive Sleep Apnea (OSA), a chronic sleep disorder affecting nearly 100 million people worldwide, is characterized by repetitive reduction or cessation of airflow during sleep due to an obstruction in the upper airway (Toth and Bhargava, 2013). The condition can be classified as mild, moderate, or severe depending on the degree of blood oxygen deprivation (Boland et al., 2002). OSA is a risk factor to other serious diseases such as diabetes mellitus, hypertension, cardiac arrhythmias, cognitive impairment, and Alzheimer’s disease (AD) (Dempsey et al., 2010; Emamian et al., 2016; Motamedi et al., 2009; Toth and Bhargava, 2013).
As hypoxia is the main symptom of OSA, induced chronic intermittent hypoxia (CIH) is a common model used to study OSA in animals (Veasey, 2009). The CIH animal model reproduces the frequent hypoxic cycles and oxygen desaturation seen in OSA in humans (Fletcher, 2001). In particular, C57BL/6 mice show spontaneous apnea and irregular breathing in response to acute hypoxia (Toth and Bhargava, 2013). Most CIH protocols expose rodents to hypoxic conditions for 10-40 days (Sforza and Roche, 2016). Previous studies showed that male mice exposed to CIH for 35 days developed hypertension (Coleman et al., 2010; Jackman et al., 2014; Lessard et al., 2010). Also, 28 days of CIH exposure has been shown to induce cognitive dysfunction (Gao et al., 2017) and AD-like abnormally hyperphosphorylated tau (Yagishita et al., 2017) in rodents. Thus, CIH based modeling of OSA in rodents provides an opportunity to study the detrimental consequences of the long-term human condition most directly.
Using proton magnetic resonance spectroscopy (1H MRS), we recently found a decrease in γ-aminobutyric acid (GABA) in the medial prefrontal cortex (mPFC) of OSA patients, substantiating the link between mPFC, GABA concentration and OSA (Pereira et al., 2017). In rodent models, previous research has shown the effect of hypoxia on GABAergic neurons of other brain regions (Anju et al., 2011; Pozdnyakova et al., 2011), establishing that oxygen deficiency impacts the amount of GABA found in the brain. However, the effect of CIH on the GABA neurons of the rodent mPFC is not yet known. The mPFC is a structure crucial to executive function, a critical cognitive function (Vertes, 2004). Two major regions of the mPFC, the prelimbic (PL) and infralimbic (IL) cortices, are both involved in cognition; the PL is associated with behavioral flexibility and the IL is implicated in impulsive behavior as well as habit formation (van Aerde et al., 2008). In addition to mPFC deficits, patients with OSA have cognitive impairment in episodic memory and visuospatial abilities (Kerner and Roose, 2016; Kerner et al., 2017), functions which involve the hippocampus (Burton et al., 2009; Churchwell and Kesner, 2011; Diana et al., 2010; Floresco et al., 1997; Hasselmo and Eichenbaum, 2005; Kennedy and Shapiro, 2004; Kyd and Bilkey, 2003; Preston and Eichenbaum, 2013). Moreover, hypoxia during development can also affect GABAergic neurons in the hippocampus (Wang et al., 2011). Thus, whether CIH affects GABAergic neurons in the hippocampus also is not completely known.
In contrast to their male counterparts, female rodents are less affected by hypoxia (Hinojosa-Laborde and Mifflin, 2005; Sanfilippo-Cohn et al., 2006). Experiments examining CIH exposure for eight weeks showed that male mice but not female mice developed disrupted sleep and wake cycles (Sanfilippo-Cohn et al., 2006). In addition to neurobehavioral differences, oxygen-deprived male rats have shown significant physiological changes such as increase in heart rate, which was not observed in females (Hinojosa-Laborde and Mifflin, 2005). Sex differences with regards the effect of hypoxia on GABAergic neuronal counts in the mPFC or hippocampus has not previously been elucidated.
The present study aims to examine the effects of sex, age, and severity of oxygen deprivation on GABAergic neurons in the mPFC and hippocampus of CIH exposed mice. We examined the counts of GABAergic neurons, GABAergic sub-populations, i.e., parvalbumin (PARV) and somatostatin (SOM) neurons, and processes immunolabeled for vesicular GABA transporter (vGAT) in the brains of young and aged male mice as well as young, aged, and post-accelerated ovarian failure (AOF) female mice following 35 days of either moderate (10%) or severe (5%) CIH. We report the age- and sex-specific effects of moderate and severe CIH on GABAergic neurons and sub-population counts.
2. Materials and Methods
2.1. Animals
All animal procedures were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of Weill Cornell Medicine. Experiments were performed on adult male and female C57BL/6 mice (2 months old at time of arrival; N = 39; Jackson Laboratory, Bar Harbor, ME) and aged adult male and female C57BL/6 mice (18 months old at time of arrival; N=19; National Institute on Aging, Bethesda, Maryland). Four to five mice were housed per cage and mice had access to ad libitum food and water
2.2. AOF induction and Estrous Cycle Determination
To model hormonal levels seen in post-menopausal humans, female mice (50-55 days old; N = 10) were injected with 130 mg/kg 4-vinylcyclohexane diepoxide (VCD) in sesame oil intraperitoneally (i.p.) for 5 sequential days per week for 3 weeks to induce AOF, as described previously (Marques-Lopes et al., 2017; Van Kempen et al., 2014). Control mice were injected with sesame oil alone. AOF mice were randomly assigned to CIH or control groups. Mice were subjected to CIH (see below) 129 days following the initiation of the VCD injection (~6.5 months old), at a post-AOF time point corresponding to ‘post-menopause’ wherein mice have an acyclic, anestrous status, in which ovulation has ceased (Van Kempen et al., 2014). Similar to human post-menopause, this time point is characterized by undetectable levels of estrogen, decreased levels of progesterone as well as elevated serum levels of follicle-stimulating hormone, luteinizing hormone and androstenedione (Lohff et al., 2005; Mayer et al., 2004; Van Kempen et al., 2014; Van Kempen et al., 2011). Vaginal smear cytology (Turner and Bagnara, 1971) and uterine weight (collected at the end of the experiment) were used to assess estrous cycle stage.
2.3. Chronic Intermittent Hypoxia
A CIH system developed by Dr. George J. Delagrammatikas was used to create cyclic hypoxia conditions, as previously described (Coleman et al., 2010; Jackman et al., 2014; Lessard et al., 2010). Briefly, all mice were kept under conditions of 21% oxygen before being separated into CIH and control groups. The gas distribution was controlled by an automated gas delivery system, and oxygen (O2) levels within the cage transitioned from low O2 (10±1%) or (5±1%) following nitrogen (N2) infusion to ambient concentrations (21±1%) following pure O2 infusion. This transition occurred every 90 sec resulting in 20 hypoxic episodes per hour. This rate is comparable to the number of oxygen desaturation events that occur in patients with mild to moderate OSA (Boland et al., 2002). Hypoxia/sham cycling was induced throughout the light (sleep) phase (8:00 am to 4:00 pm; 8 hours). The control cages were infused with air every 90 seconds to maintain oxygen concentrations at 21±1%. During the remaining 16 hours of the day (4:00 pm to 8:00 am), both CIH and sham cages were infused with room air. The protocol was repeated for 35 days during which the mice in each treatment group were housed together (5 per cage) and had free access to food and water. The temperature was kept at 22-24°C. The group housing protocol and procedure were developed to minimize variability due to differences in handling and constraints on mobility (Lee et al., 2009; Liu et al., 2009).
There were six CIH cohorts: 1) young males (~2 months old) with mild (10%) CIH (N = 5) and controls (N = 5), 2) aged males (~18 months old) with 10% CIH (N = 5) and controls (N = 4), 3) young saline-injected females (~6 months old) with 10% CIH (N = 4) and controls (N = 5), 4) AOF females (~6 months old) with 10% CIH (N = 5) and controls (N = 5), 5) aged females (~18 months old) with 10% CIH (N = 5) and controls (N = 5), and 6) young males (~2 months old) with severe (5%) CIH (N = 5) and controls (N = 5).
2.4. Blood Pressure Measurements
Our previous studies (Coleman et al., 2010) in male mice have shown small but significant increases in systolic blood pressure (SBP) following 35 days of 10% CIH. Thus, in the present study, we recorded blood pressure in the female cohorts (described above). For this, we used tail-cuff plethysmography (Model MC4000; Hatteras Instruments, Cary, NC) to measure SBP, as previously described (Coleman et al., 2010). Measurements were taken prior to CIH (baseline) as well as weekly throughout the CIH administration. Measuring SBP through tail-cuff plethysmography has limitations as have been discussed previously (Marques-Lopes et al., 2014). Animals were handled by the same experimenter and at the same time of day throughout the experiment to minimize stress. Mice were euthanized one day after the final SBP measurements were taken (Coleman et al., 2013; Marques-Lopes et al., 2014).
2.5. Antibody characterization
A rat polyclonal antiserum against GABA-glutaraldehyde-hemocyanin conjugates was produced and provided by Dr. Andrew Towle, formerly affiliated with Department of Neurology and Neuroscience at Cornell University Medical College. Preadsorption with GABA-BSA (bovine serum albumin) was used to test the specificity of the antiserum and served to eliminate immunoreactivity. Preadsorption with unconjugated GABA or BSA conjugated to glutamate, ß-alanine, or taurine did not abolish immunoreactivity (Lauder et al., 1986). Immunoreactivity of this antiserum is consistent with the specificity of other GABA-antisera (Lauder et al., 1986). This antiserum has been used previously in light and electron microscope studies (Almey et al., 2016; Bayer and Pickel, 1991; Mazid et al., 2016).
A rabbit polyclonal antibody against rat muscle PARV was provided by Dr. K.G. Baimbridge of the University of British Columbia, Vancouver. Its specificity has been demonstrated through incomplete cross-reactivity with muscle or brain preparations from other species (Mithani et al., 1987). It has been previously used in light and electron microscope studies (Milner and Prince, 1998).
A polyclonal rabbit anti-Somatostatin-14 antibody (Peninsula Laboratories, Catalog. No. T-4103; RRID:AB_518614) was used. It has been previously used in light and electron microscope studies (Peng et al., 2013).
A polyclonal rabbit anti-vGAT (Synaptic Systems, Catalog no. 131 002; RRID:AB_887871) was used (Martens et al., 2008). The manufacturer has demonstrated its specificity using knockout mice (https://www.sysy.com/products/vgat/facts-131002.php). It has previously been utilized in immunohistochemistry and western blot experiments (Bocarsly et al., 2015).
2.6. Tissue preparation
The mice were deeply anesthetized with sodium pentobarbital (150 mg/kg, i.p.) and perfused through the left ventricle of the heart sequentially with ~5 ml heparinized saline followed by 30 ml of 3.75% acrolein and 2% paraformaldehyde in 0.1M phosphate buffer (PB; pH 7.4) (Milner, 2011). The brains were then removed and post-fixed in 2% acrolein and 2% paraformaldehyde in PB for 30 minutes. The brains were cut into 5-mm coronal blocks using a brain mold (Activational Systems Inc., Warren Michigan) and sectioned (40 μm thick) using a VT1000x Vibratome (Leica Microsystems, Buffalo Grove, IL). Sections were stored at −20°C in cyroprotectant solution (30% sucrose, 30% ethylene glycol in PB) until immunocytochemical processing (Milner, 2011).
2.7. Immunocytochemistry
The sections were processed for immunoperoxidase labeling using previously described protocols (Milner, 2011). For this, the sections were washed in PB to remove cryoprotectant. PFC and hippocampal sections from each mouse were marked with punch codes prior to pooling into crucibles. To minimize differences due to immunocytochemical labeling, tissue from animals in the same set were processed together through all procedures (Pierce et al., 1999). However, separate cohorts were run at different times so comparisons were only made between controls and CIH of each group (as described in 2.3).
The sections were incubated in 1% sodium borohydride in PB for 30 minutes to remove unbound aldehydes, and subsequently washed in PB until gaseous bubbles disappeared. Next, the sections were washed in 0.1M Tris-saline (TS; pH 7.6) followed by incubation in 0.5% BSA in TS, to block non-specific binding. The sections were then rinsed in TS and then incubated in the GABA (1: 4000 dilution), PARV (1: 20,000 dilution), SOM (1: 8000 dilution), or vGAT (1: 15000 dilution) antibody in 0.1% BSA in TS for 1 day at room temperature (~23°C), followed by 3–4 days cold (~4°C). The primary antibody diluent for PARV and SOM also included 0.25% Triton-X.
After the allotted 3-4 days, sections were removed from the primary antibody and rinsed with TS. They were then incubated in a biotinylated secondary antibody (GABA: donkey anti-rat IgG (Jackson Immunoresearch Laboratories, Catalog no. 712-065-150, RRID:AB_2340646); SOM and vGAT: goat anti-rabbit IgG (Jackson Immunoresearch Laboratories, Catalog no. 111-065-144, RRID:AB_2337965); PARV: donkey anti-rabbit IgG (Jackson Immunoresearch Laboratories, Catalog no. 711-506-152, RRID:AB_2616595) diluted 1:400 for 30 minutes. The sections were rinsed with TS and then incubated in avidin-biotin complex (ABC) solution (Vector labs, Burlingame, CA) for 30 minutes, followed by TS washes. The ABC reaction product was visualized with 3,3’-diaminobenzidine (DAB) and H2O2 in TS for 6-12 minutes. Following this, sections were washed in TS followed by PB and mounted in 0.05M PB on 1% gelatin coated glass slides. The sections then were dehydrated through a series of alcohols and xylene and cover slipped using DPX mounting media (Sigma-Aldrich, St. Louis, MO).
2.8. Cell counting
An investigator blind to experimental conditions performed cell counts and data analyses. GABA, PARV and SOM cell bodies were quantified in the PL and IL regions of the PFC (between Bregma 1.7 and 1.4) and the dorsal hippocampus (between Bregma −2.1 and −2.5) (Paxinos and Franklin, 2001). Labeled cell bodies had clearly recognizable nuclei. The Paxinos and Franklin Mouse Brain Atlas was used to set landmarks, and cells were counted at 10x from a square of equivalent size (5.8 cm x 3.9 cm) for each PFC region and the dorsal CA1 region of the hippocampus. Cells also were counted in the hilus of the dentate gyrus (DG). For this, labeled cells were counted between the granule cell layers and then the number per unit was determined by dividing by the area of the hilus determined using ImageJ64 (ImageJ, RRID:SCR_003070).
2.9. Densitometry
Densitometry measurements were taken using previously described methods (Pierce et al., 2014). Images from Regions of Interest (ROI) were taken at 4x magnification on a Nikon eclipse 90i light microscope (RRID: SCR_O15572). Densitometry analyses of GABAergic terminal fields using ImageJ64 were performed on the mPFC, the PL and IL of which were roughly divided into four layers (Paxinos and Franklin atlas between Bregma 1.7 and 1.4). Background was subtracted from an area of tissue lacking immunoreactivity.
2.10. Statistics
Data are expressed as mean ± SEM. Cohorts were run individually, so comparisons were made between the CIH and control groups of individual cohorts only. All statistical analyses were conducted on JMP 12 Pro software (JMP, RRID:SCR_014242). Significance was determined through one-way ANOVA or Welch t-test for samples with unequal variances (as determined by Levene’s test).
2.11. Figure preparation
Data were unblinded after the final graphs were generated. Adjustments to brightness, contrast and sharpness on the photographs were made in Adobe Photoshop 9.0 (Adobe Photoshop, RRID:SCR_014199) on an iMac prior to importing into PowerPoint 2011, where final adjustments to brightness, contrast and size were made. Illustrations were created in PowerPoint 2011. Graphs were generated using Prism 7 software (Graphpad Prism, RRID:SCR_002798).
3. Results
3.1. CIH had no influence on blood pressure in females
Unlike males (Coleman et al., 2010), SBP was not significantly different between young females or AOF females following 10% CIH (YF Con: 120 ± 5.0 mmHg; YF 10% CIH: 119 ± 6.8 mmHg; AOF-F Con: 117.9 ± 7.0 mmHg; AOF-F 10% CIH: 117.5 ± 5.8 mmHg). Additionally, there was no difference in SBP between aged females with 10% CIH and controls (AF Con: 107 ± 9.3 mmHg; AF 10% CIH: 107 ± 8.8 mmHg).
3.2. Estrous Cycling and Uterine Weights
Aged females were determined to be in the estrus stage of the estrous cycle, consistent with our previous studies (Marques-Lopes et al., 2017; Marques-Lopes et al., 2014; Van Kempen et al., 2014). Uterine weights were not significantly different between CIH and control females of any cohort, young saline (Con: 0.072 ± 0.019 g; CIH: 0.077 ± 0.011 g), AOF (Con: 0.051 ± 0.007 g; CIH: 0.072 ± 0.007 g), and aged (Con: 0.145 ± 0.026 g; CIH: 0.111 ± 0.008 g).
3.3. Number of GABA neurons increased in mPFC and hippocampus of males only following CIH
GABA-immunoreactive (ir) neurons were found dispersed throughout the mPFC layers, consistent with previous studies in rats (Carr and Sesack, 2000) and gerbils (Brummelte et al., 2007). No difference in the number of GABA-ir neurons in either the PL or IL mPFC was found in moderate CIH (10%)-exposed young males as compared to the control group. However, severe CIH (5%)-exposed young males compared to controls showed an increase (t(9) = 3.24, p = 0.012) in the number of GABA-ir neurons in the IL of the mPFC only (Fig. 1B,E). Additionally, 10% CIH-exposed aged males exhibited a significant increase in the number of GABA-labeled neurons in both the PL and IL regions of the mPFC compared to controls (t(6) = 9.98, p = 0.0105; t(6) = 2.86, p = 0.035 respectively; Fig. 2B,E). No significant difference in the number of GABA-ir neurons in the PL or IL mPFC was observed in any female cohort (young, AOF, or aged) following CIH (Fig. 3B,E).
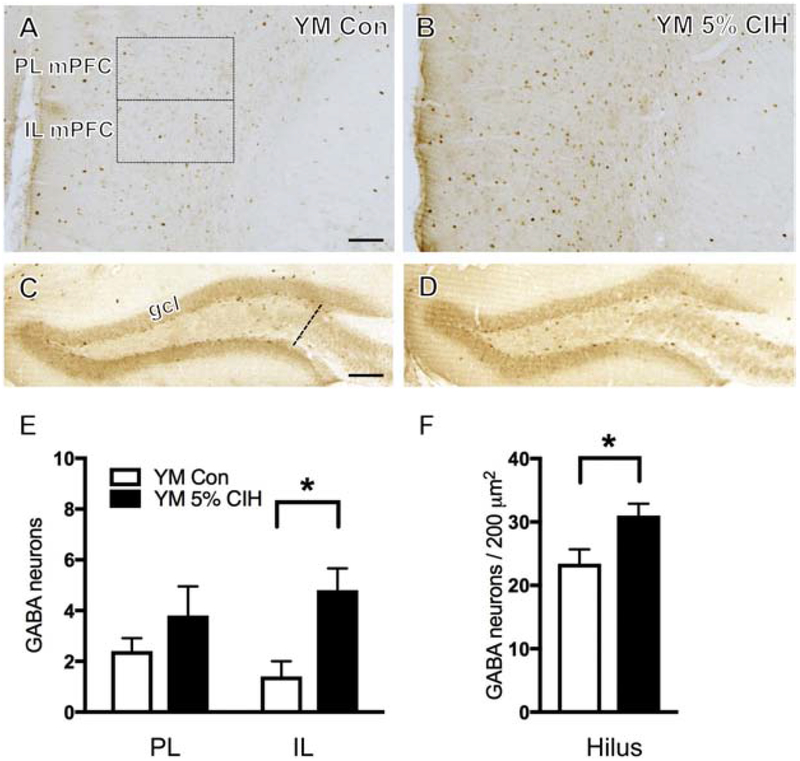
Figure 1. Young male mice show increased number of GABA-labeled neurons in the mPFC and hippocampus following severe CIH.
(A & B) Representative images showing localization of GABA in PL and IL mPFC of a control (A) and a young 5% CIH (B) male mouse. Severe CIH exposure increased the number of GABA-labeled neurons in the IL of the mPFC in young male mice. (C & D) Representative images depicting GABA-labeled neurons in the hilus of the dentate gyrus in a control (C) and a 5% CIH (D) male mouse. Severe CIH increased the number of GABA-labeled neurons in the hilus in young male mice. (E & F) Bar graphs show the relative numbers of GABA-labeled neurons in the PL and IL mPFC (E) and GABA-labeled neurons per area in the hilus (F) in control and young 5% CIH male mice. N = 5 animals per group. Scale bars = 100 μm.
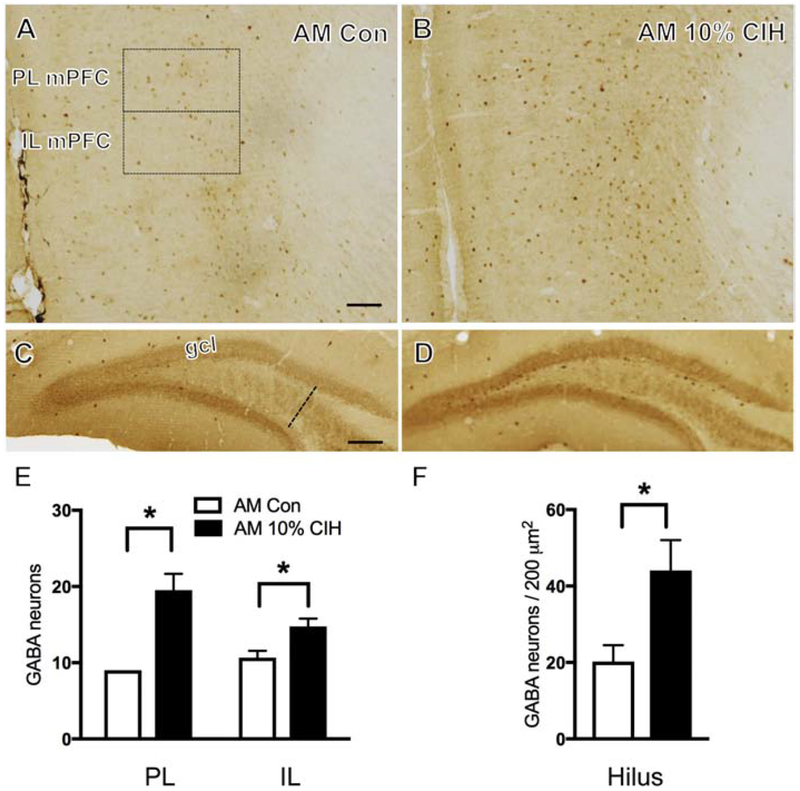
Figure 2. Aged male mice show increased number of GABA-labeled neurons in the mPFC and hippocampus following moderate CIH.
(A & B) Representative photomicrographs illustrating localization of GABA in PL and IL mPFC of a control (A) and an aged 10% CIH (B) male mouse. Moderate CIH exposure resulted in increased number of GABA-labeled neurons in the PL and IL mPFC in aged male mice. (C & D) Representative images showing localization of GABA-labeled neurons in the hilus of the dentate gyrus in control (C) and 10% CIH (D) males. Moderate CIH increased number of GABA-labeled neurons in the hilus in aged male mice. (E & F) Bar graphs show the relative numbers of GABAergic neurons in the PL and IL mPFC (E) and GABAergic neurons per area in the hilus (F) in control and aged 10% CIH male mice. N = 5 animals per group. Scale bars = 100 μm.
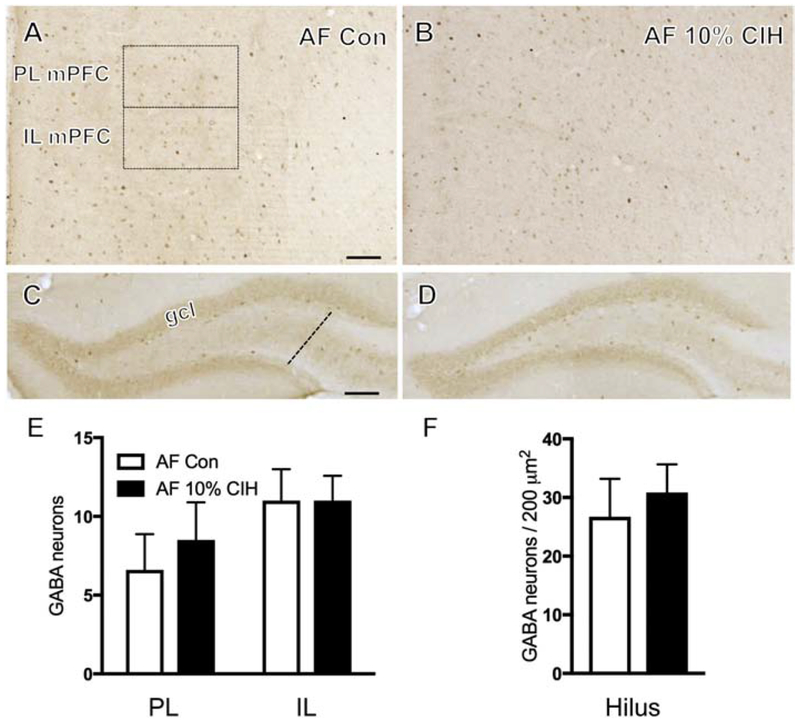
Figure 3. Aged female mice exhibit no change in number of GABA-labeled neurons in the mPFC or hippocampus following moderate CIH.
(A & B) Representative images showing localization of GABA-labeled neurons in PL and IL mPFC of a control (A) and an aged 10%CIH (B) female mouse. Moderate CIH exposure did not affect the number of GABA-labeled neurons in the PL or IL mPFC in aged female mice. (C & D) Representative photomicrographs demonstrating localization of GABA in the hilus of the dentate gyrus in a control (C) and an aged 10% CIH (D) female mouse. Moderate CIH exposure had no effect on the number of GABA-labeled neurons in the hilus in aged female mice. (E & F) Bar graphs show the relative numbers of GABAergic neurons in the PL and IL mPFC (E) and GABAergic neurons per area in the hilus (F) in control and aged 10% CIH female mice. N = 5 animals per group. Scale bars = 100 μm.
Consistent with previous studies in rats (Gamrani et al., 1986), GABA-labeled neurons were scattered throughout strata pyramidale, oriens, and radiatum of the CA1 and CA3 regions of the hippocampus. Additionally, GABA-ir neurons were present in the DG, especially in the sub-granule layer region of the hilus, consistent with previous studies (Gamrani et al., 1986). In the present study, GABA-ir neurons were examined throughout the layers of the CA1 as well as in the hilus layer of the DG. No significant difference in the number of GABA-labeled cells was observed in the CA1 of any cohort, male or female. Findings in the hilus were consistent with those in the mPFC. While no difference was observed in the number of GABA-ir neurons of 10% CIH young male mice, both 5% CIH young and 10% CIH aged mice showed increased GABA (t(8) = 2.48, p = 0.042; t(8) = 2.43, p = 0.046 respectively) in the hilus of the DG compared to control groups (Figs. 1D,F; 2D,F). Following CIH, no significant difference in the number of GABA labeled neurons was found in the hilus of any female cohort (Fig. 3D,F).
3.4. The effect of CIH on the number of PARV neurons varied with sex in mPFC and hippocampus
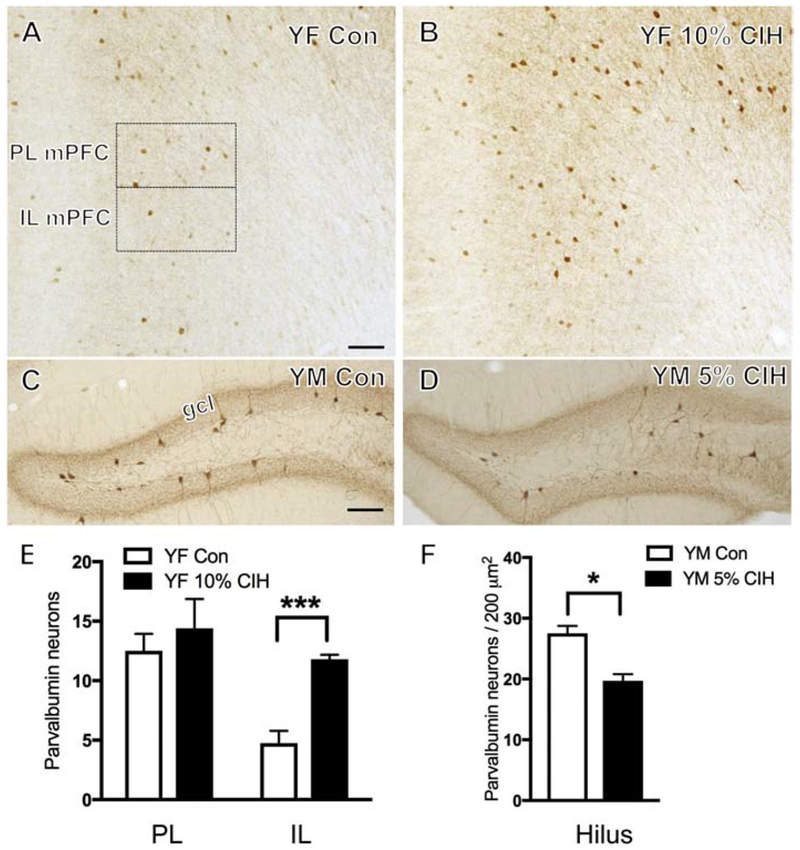
PARV, a calcium binding protein found in a subpopulation of GABAergic neurons (Tanahira et al., 2009), was counted to further examine the effect of CIH on the GABAergic system. PARV cells were sparsely distributed throughout the PL and IL of mPFC, consistent with previous studies in mice (Umeda et al., 2016). No difference in the number of PARV neurons in either the PL or IL of mPFC was found in any of the male cohorts. Additionally, no significant difference was observed in the PL or IL of mPFC of aged or AOF females. However, young 10% CIH females showed a significant increase in the number of PARV neurons compared to controls (t(8) = 7.05, p = 0.0036) in the IL mPFC only (Fig. 4B,E).
Figure 4. Number of PARV-ir neurons increases in young female mPFC and decreases in young male hippocampus following moderate and severe CIH exposure, respectively.
(A & B) Representative images showing localization of PARV-labeled neurons in PL and IL mPFC of a control (A) and a young 10% CIH (B) female mouse. Moderate CIH exposure resulted in increased number of PARV-ir neurons in the IL mPFC in young female mice. (C & D) Representative photomicrographs showing PARV-labeled neurons in the hilus of the dentate gyrus in a control (C) and a young 5% CIH (D) male mouse. Severe CIH exposure decreased the number of PARV-ir neurons in the hilus in young male mice. (E & F) Bar graphs show the relative numbers of PARV-ir neurons in the PL and IL mPFC in control and young 10% CIH females (E) and PARV-ir neurons per area in the hilus in control and young 5% CIH males (F).N = 5 animals per group. Scale bars = 100 μm.
Consistent with previous studies, PARV-ir neurons were most dense near stratum pyramidale of the CA1 and CA3 regions of the hippocampus. Additionally, PARV-labeled neurons were present in the sub-granular DG hilus, in agreement with previous studies in mice (Tanahira et al., 2009) and rats (Milner et al., 2013). No differences in the number of PARV-labeled cells were observed in the CA1 of any cohort. In the hilus, the number of PARV neurons was decreased (t(9) = 4.76, p = 0.001) in 5% CIH males only compared to controls (Fig. 4D,F).
3.5. CIH affected SOM in mPFC of females only
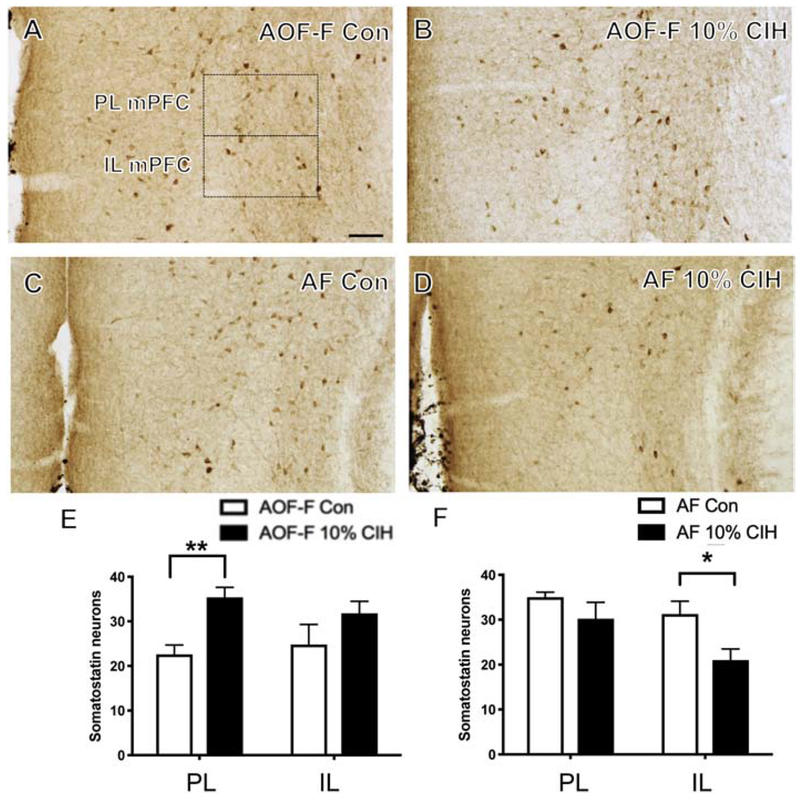
SOM, an inhibitory neuropeptide, is localized to another subset of GABAergic neurons. SOM-containing cells were distributed throughout the PL and IL mPFC, consistent with previous studies in mice (Gaykema et al., 2014). No difference in the number of SOM-labeled neurons was found in the PL or IL of mPFC of any male cohorts. Additionally, no change was observed in the number of SOM-labeled cells in young females. AOF CIH females exhibited more SOM-ir neurons in the PL mPFC (t(9) = 4.15, p = .003) compared to control AOF females (Fig. 5B,E). However, aged CIH females exhibited less SOM-labeled neurons in the IL mPFC (t(8) = 2.57, p = .037) compared to the control aged females (Fig. 5D,F).
Figure 5. Number of SOM-ir neurons increases in AOF females and decreases in aged females in mPFC following CIH exposure.
(A & B) Representative images showing localization of SOM-labeled neurons in the PL and IL mPFC of a control (A) and a 10% CIH (B) AOF female mouse. Moderate CIH increased the number of SOM-ir neurons in the PL mPFC of AOF female mice. (C & D) Representative images showing localization of SOM-labeled neurons in the PL and IL mPFC in a control (C) and an aged 10% CIH (D) female mouse. Moderate CIH decreased the number of SOM-ir neurons in the IL mPFC in aged female mice. (E & F) Bar graphs show the relative numbers of SOM-ir neurons in AOF 10% CIH females (E) and aged 10% CIH females (F) in the PL and IL mPFC. N = 5 animals per group. Scale bar = 100 μm.
3.6. CIH had opposite effects on the number of hippocampal SOM neurons in young females and males
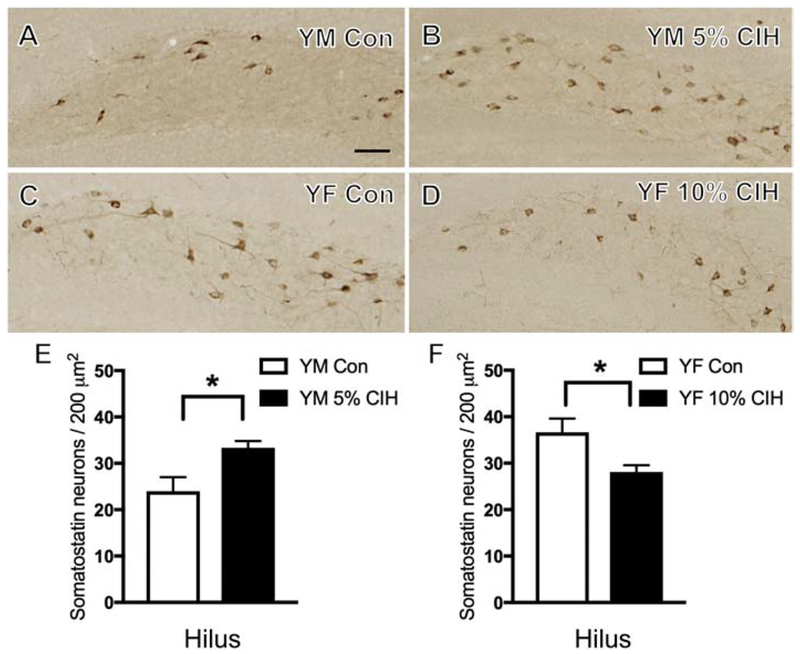
Within the hippocampal regions, SOM labeling was found in scattered interneurons. In CA1, consistent with previous studies in mice (Shimada and Ishikawa, 1989), SOM-ir was mainly restricted to the stratum oriens and the DG hilus. No significant difference in the number of SOM-labeled neurons was found in the CA1 of any cohort, male or female. In the DG hilus, young 5% CIH males compared to controls exhibited increased SOM-containing cells (t(9) = 2.77, p = 0.024; Fig. 6B,E). Young 10% CIH females compared to controls had decreased SOM-labeled neurons (t(8) = 2.78, p = 0.027; Fig. 6D,F). No difference in the number of SOM-containing neurons was observed in the hilus of any other cohort, male or female.
Figure 6. Sex differences in the effect of CIH on the number of hilar SOM-ir neurons in young mice.
(A & B) Representative images showing localization of SOM-labeled neurons in the hilus of the dentate gyrus of a control (A) and a young 5% CIH (B) male mouse. Severe CIH increased the number of SOM-ir neurons in the hilus of young male mice. (C & D) Representative images showing localization of SOM-labeled neurons in the hilus of the dentate gyrus in a control (C) and a young 10% CIH (D) female mouse. Moderate CIH decreased the number of SOM-ir neurons in the hilus in young female mice. (E & F) Bar graphs show the relative numbers of SOM-ir neurons in young 5% CIH males (E) and young 10% CIH females (F) per area in the hilus. N = 5 animals per group. Scale bar = 100 μm.
3.7. CIH altered mPFC v-GAT in AOF females only
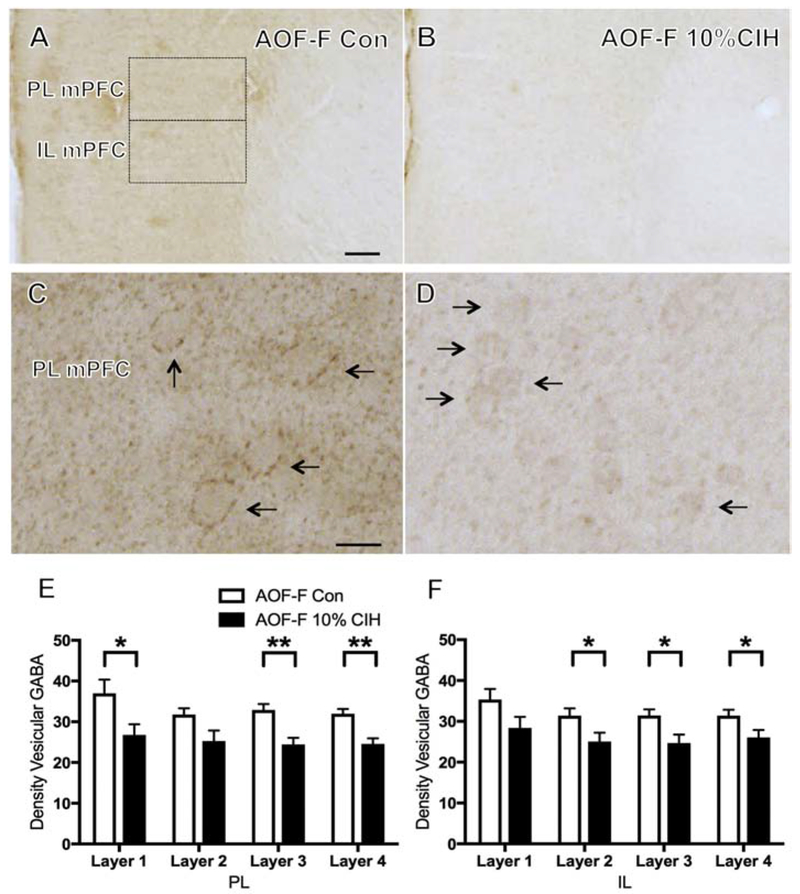
To elucidate the effect of CIH on the GABAergic terminals, the density of v-GAT was assessed in the mPFC and hippocampus. In agreement with previous studies in rats (Henny and Jones, 2008) and rabbits (Chaudhry et al., 1998), v-GAT-labeled processes were heavily stained throughout the PL and IL of mPFC in all cohorts. The mPFC was divided into four layers, as depicted in Figure 6, and each of the four layers of the mPFC was examined independently. No significant difference in the density of v-GAT labeling was observed in any layer of the PL or IL of mPFC of any CIH male cohort. Additionally, no difference in the density of v-GAT labeling in any layer of the PL or IL mPFC was observed in the young or aged females. However, 10% CIH AOF females compared to controls exhibited a decrease in the density of v-GAT immunoreactivity in layers 1, 3, and 4 of the PL mPFC (t(9) = 2.41, p = 0.04; t(9) = 3.87, p = 0.005; t(9) = 4.15, p = 0.003, respectively) as well as a decrease in the density of v-GAT immunoreactivity in layers 2, 3, and 4 of the IL mPFC (t(9) = 2.27, p = 0.053; t(9) = 2.63, p = 0.03; t(9) = 2.26, p = 0.054, respectively; Fig. 7B).
Figure 7. Vesicular GABA transporter decreases in AOF females in mPFC following CIH exposure.
(A & B) Representative images depicting the localization of v-GAT-labeled terminal fields in PL and IL mPFC of an AOF control (A) and an AOF 10% CIH (B) female mouse. Moderate CIH decreased v-GAT-ir density in the PL and IL mPFC in AOF mice. (C & D) Representative images showing higher magnification of v-GAT-ir in the PL of an AOF control (C) and an AOF 10% CIH (D) female mouse. Arrows show examples of vGAT-labeled processes. (E & F) Bar graphs show the relative v-GAT-ir density in the PL (E) and IL (F) of the four layers of mPFC. AOF females with mild CIH show decreased v-GAT-ir density in the PL (significant in layers 1, 3, and 4) and IL (significant in layers 2, 3, and 4) mPFC. N = 5 animals per group. Scale bars = A,B 100 μm; C,D = 20 μm.
Consistent with previous studies, v-GAT-ir was widely distributed in processes in CA1 and CA3 sub-regions of the hippocampus, as well as in the DG in rabbits (Chaudhry et al., 1998) and rats (Sperk et al., 2003). No significant difference in the density of v-GAT labeling was observed in the CA1 or DG hilus in the hippocampus of any cohort, male or female.
4. Discussion
The present study indicates a link between CIH and GABA in the mouse mPFC and hippocampus. Specifically, as age or severity of hypoxia increases, GABA-labeled cells concomitantly increase in males. These findings suggest that when male mice reach a severity of oxygen deprivation or age threshold, they begin to exhibit an increase in the number of GABA-labeled neurons in the mPFC and hippocampus. Female mice do not experience a change in overall number of GABA-labeled neurons, indicating a sex difference that might have implications for clinical OSA. All results observed in the mPFC and hippocampus are summarized in Figure 8.
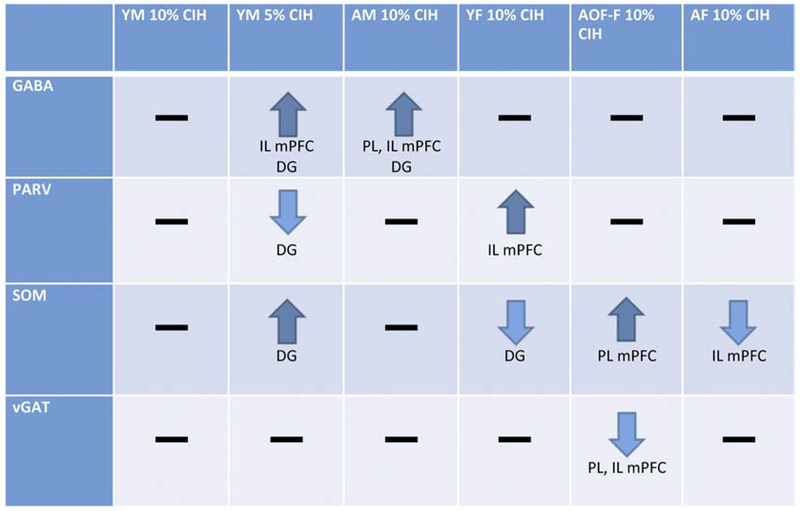
Figure 8. Summary of results:
1) Following moderate CIH, no changes were observed in GABA-labeled neurons in young males. 2) Aged males and severely hypoxic young males showed increased GABA-labeled neurons in the mPFC and hippocampal DG, with deferring effects on GABAergic neuron populations PARV and SOM. 3) Females did not show any changes in GABA-labeled neurons following CIH, but showed differences in GABAergic neuron population SOM following CIH but the direction depended on hormonal state and age.
Previous studies have reported both increase (Madl and Royer, 2000; Pereira et al., 2017; Wood et al., 1968) and decrease (Yuan et al., 2015) in GABAergic neurons after hypoxia. In the context of conflicting evidence from published literature, the present study presents some interesting data showing the age- and sex-specific effects of CIH on GABAergic neurons and sub-populations in the hippocampus and mPFC, two brain regions critically involved in learning and memory. Given the detrimental effects of OSA and CIH on cognition (Yaffe et al., 2011), these findings are of high clinical relevance.
Consistent alterations in GABAergic neuronal counts in mPFC and hippocampus
The observed changes in GABAergic cell counts were found in both the mPFC and hippocampus, brain structures with critical roles in various domains of cognitive functioning (Euston et al., 2012; Preston and Eichenbaum, 2013). Consistent findings in the hippocampus and the mPFC support the largely substantiated idea of a hippocampal-mPFC network and its implications in cognitive processes (Jin and Maren, 2015). A recent study showed that mPFC reduces memory interference by modifying hippocampal encoding, and mPFC inactivation reduced pattern separation in CA1 representations (Guise and Shapiro, 2017). CIH has been widely reported to induce cognitive impairment in rodent models [for review, (Row, 2007)]. Also, in human OSA, oxygen desaturation index (ODI), a measure of CIH, correlates with cognitive decline (Yaffe et al., 2011). Our data of consistent alterations in GABA expression both in the hippocampus and mPFC after CIH exposure hint towards CIH-induced impairments in both these structures and probably their inter-connecting pathways that may contribute to cognitive dysfunction. Previously, CIH was shown to induce neuronal loss in mPFC (Li et al., 2015), and to disrupt adult neurogenesis and synaptic plasticity in the dentate gyrus region of the hippocampus (Khuu et al., 2019). To the best of our knowledge, the present study is the first to show a neuron population-specific (i.e., GABAergic) alteration induced by CIH both in the hippocampus and the mPFC.
Increased GABAergic neurons in both the mPFC and hippocampus of male mice after CIH exposure: compensatory inhibitory neuronal remodeling?
In the present study, interestingly, male mice cohorts exhibited an increase in GABA labeling in both the mPFC and hippocampus. However, they did not display a similar change in v-GAT levels. We therefore speculate that GABA is not being entirely shipped to axon terminals. GABA levels would thus be elevated in the soma, resulting in greater neuronal inhibition. This brings into question the effect of CIH on excitation/inhibition (E/I) balance. Increased GABA cell bodies after CIH exposure, as observed in the present study, could alter the balance between GABA and glutamate, disrupting E/I homeostasis.
Reduction in GABAergic neurons in the hippocampus and the mPFC, contributing to E/I imbalance and network hyperexcitability, has classically been implicated in the pathogenesis of age-related cognitive decline and AD (Mandal et al., 2017; McQuail et al., 2015). Nonetheless, recent mouse model studies suggest compensatory inhibitory neuronal remodeling and increased GABAergic expression in the hippocampus as a consequence of network hyperexcitability in AD and related neurodegenerative and neurocognitive disorders (Palop et al., 2007; Palop and Mucke, 2016). Previously, acute hypoxia was shown to cause hyperexcitability in the hippocampus in mice (Jensen and Wang, 1996; Jensen et al., 1998; Levin and Godukhin, 2005). Also, acute hypoxia (6% O2 for 15 min and 60 min) was reported to increase GABA levels in the brains of mice and rats (Wood et al., 1968). While, to the best of our knowledge, the effect of CIH on mouse hippocampal and mPFC excitability has not been evaluated as yet, our data showing increased hippocampal and mPFC GABA labeling following CIH exposure raises the possibility of compensatory GABAergic inhibitory neuronal remodeling in response to chronic hyperexcitability induced by CIH. As mentioned above, such compensatory remodeling in inhibitory neuronal populations within the hippocampal circuitry following hyperexcitability has been reported in mouse models of AD (Govindpani et al., 2017; Palop et al., 2007), for which OSA is a risk factor (Pan and Kastin, 2014; Polsek et al., 2018).
It is difficult to determine from the present study data whether the increase in the number of GABAergic neurons observed after CIH exposure is due to overproduction of GABAergic neurons, an enhanced cell differentiation process, or an activation of previously undetectable dormant GABAergic neurons. The activity-dependent structural plasticity in inhibitory neurotransmission is accompanied by alterations in GABAergic synapse structure that range from morphological reorganization of postsynaptic density to de novo formation and elimination of inhibitory contacts (Flores and Mendez, 2014; Flores et al., 2015). While we did not study effects of CIH on GABAergic plasticity via electrophysiological recordings or synapse structure, our data regarding increased GABAergic neuron counts suggest altered GABAergic structural plasticity induced by CIH.
Decreased GABA labeling in the mPFC, a structure crucial to executive function, is linked to working memory impairment (Banuelos et al., 2014). The elevated inhibition caused by an increase in mPFC GABA-labeled cell bodies observed after CIH exposure in the present study could be a protective and compensatory mechanism against memory impairment and network hyperexcitability. Nonetheless, the precise causal relationship(s) remain to be evaluated in future studies.
Age and sex-specific differences in GABA-labeled cell bodies following CIH: increased susceptibility of aged males
While young moderate CIH males showed no change in the number of GABA-labeled neurons, young severe CIH males and aged moderate CIH males exhibited an increase in GABA-labeled neurons in the mPFC and DG hilus of the hippocampus. No change in GABA-labeled cells was observed in any female cohort.
Aging is an important factor contributing to the risk of OSA (Edwards et al., 2014). The aging brain is associated with structural, functional and metabolic changes. Age is also linked to the emergence and exacerbation of several neurodegenerative disorders such as mild cognitive impairment (MCI), AD, vascular dementia, and other types of dementia (Savva et al., 2009). At present, the exact causality between these disorders and age-related physiological changes in the brain remains largely unknown; however, a hypoxic environment in brain cells likely plays a role in the development and progression of these disorders (Peers et al., 2007). In fact, a recent study showed higher physiological vulnerability to hypoxic exposure with advancing age in the human brain (Vestergaard et al., 2018). The present study found an age-specific increase in GABA labeling in the hippocampus and mPFC following CIH exposure in mice. These data suggest alterations in E/I homeostasis induced by CIH specifically in the aging brain. Given that these changes were observed in hippocampus and mPFC, brain regions well-known to be involved in cognitive processing, this could provide a possible causal link between OSA and age-related neurocognitive disorders that should be elucidated in future studies.
The sexual dimorphism observed in GABAergic labeling in the brain regions following CIH exposure in the present study is consistent with previous findings, which have reported differential effects of CIH in female mice compared to their male counterparts. Previous studies have addressed sex differences in various effects of hypoxia such as neurobehavioral and physiological changes (Snyder and Cunningham, 2018). Experiments examining neurobehavioral sex differences of hypoxia have shown that male but not female mice have disrupted sleep-wake cycles (Sanfilippo-Cohn et al., 2006). Also, sex-based dimorphism in the effect of CIH on serotoninergic systems has been reported, with increased serotoninergic immunoreactivity in the hypoglossal nucleus and a decreased number of serotoninergic cells in the dorsal raphe nucleus in male but not female mice (Baum et al., 2018). This dimorphism in the neuroplasticity of serotoninergic systems has been suggested to predispose males to a greater alteration of neuronal control of the upper respiratory tract associated with the greater collapsibility of upper airways described in male OSA subjects (Baum et al., 2018). Additionally, another study found increased GABAergic synaptic events in the brain stem lateral paragigantocellular nucleus (involved in REM sleep control) after 4-week CIH exposure both in male and female rats, but to a greater degree in males (Dergacheva, 2015), suggesting a sexual dimorphism in the effect of CIH.
Age-specific divergent effects of CIH on GABA labeling in PL vs IL mPFC
The present study found divergent effects of CIH on GABAergic neurons in PL and IL regions of the mPFC, which substantially differ in their anatomical connections and respective involvement in (1) cognitive activity and (2) visceral/autonomic processes (Vertes, 2004). While moderate CIH did not exert any effect and severe CIH increased the number of GABAergic neurons only in IL in young males, moderate CIH induced a significant increase in the number of GABAergic neurons both in PL and IL mPFC in aged males. This could be explained based on the previous discussion concerning the increased vulnerability of aged brain to hypoxia (Vestergaard et al., 2018). Additionally, given its role in limbic and cognitive activities, selective alteration in GABAergic neuronal population in PL mPFC only in aged mice may provide a shared etiopathological link between CIH and aging and neurocognitive disorders such as MCI and AD.
GABAergic sub-populations PARV and SOM do not present the same increase following CIH
The changes in overall number of GABA-labeled neurons in the mPFC and hippocampus did not reflect changes in GABA subpopulations PARV or SOM. PARV and SOM are estimated to be present in approximately 40% and 24% of GABAergic neurons respectively (Tamamaki et al., 2003). However, these sub-populations are seemingly unaffected by the changes observed in the overall GABAergic system. While it is estimated that there are about 20 different subtypes of GABAergic interneurons, studies have shown that PARV and SOM are present in the most predominant GABAergic interneurons (Gonchar and Burkhalter, 1997). The findings indicate that the changes in overall GABA labeling may be contributable to a different subset of GABA.
CIH results in decreased PARV cell bodies in the hippocampus in males: implications for E/I imbalance
Young severe CIH (5%) males exhibited a decrease in the number of PARV-labeled cells in the hilus of the DG. Previously, non-hypoxic stress was shown to decrease the number of PARV-labeled neurons in the dentate hilus of males by 30% (Czeh et al., 2005; Hu et al., 2010). This decrease was only observed in the severe CIH males, indicating that mild to moderate (10%) CIH is not enough of a stressor to induce this change. As there was an increase in overall GABA labeling in this region, the increased SOM labeling observed in the dentate hilus of this cohort may serve to balance the loss of PARV and increase total GABA.
Previously, CIH associated oxidative stress was shown to reduce PARV neurons in the PFC and lead to neurobehavioral alterations in mice (Yuan et al., 2015). Loss of PARV-immunoreactive interneurons in the brain cortex is an important feature of neuropsychiatric diseases, such as schizophrenia (Lewis et al., 2012). It has been suggested that PARV-mediated inhibition is decreased to compensate for an upstream deficit in pyramidal cell excitation (Lewis et al., 2012). This compensation is thought to rebalance cortical E/I, but at a level insufficient to generate the gamma oscillation power required for high levels of cognitive control (Lewis et al., 2012).
It is well known that during neuronal communication and memory processing, GABAergic activity plays a critical role in neuronal synchronization during theta and gamma activity in certain brain regions (Govindpani et al., 2017; Somogyi and Klausberger, 2005). Interestingly, gamma conductance disruption has also been suggested as a potential mechanism of neuronal network hyperexcitability, contributing to the E/I imbalance in AD (Govindpani et al., 2017; Palop and Mucke, 2010). In fact, it was reported that the hAPP transgenic mice brains exhibited aberrant network hypersynchrony, markedly during periods of reduced gamma oscillations (Verret et al., 2012). It was proposed that this network dysfunction could be caused by deficits in PARV interneurons, as the synaptic firing of these neurons underlies the generation of gamma oscillations (Verret et al., 2012). Indeed, it was found that both hAPP mice, with high Aβ load, and human AD patients expressed decreased Nav1.1 compared with controls (Verret et al., 2012). Nav1.1 is a voltage-gated sodium channel found primarily on PARV interneurons which contributes to the synaptic activity of these cells. It was also reported that restoration of Nav1.1 levels ameliorated memory loss in hAPP mice (Verret et al., 2012). Based on this discussion and our data, we hypothesize that CIH induced PARV interneuron loss could be a major mechanism contributing to OSA and CIH associated cognitive impairment and risk of AD dementia. The precise causal relationship remains to be evaluated in relevant AD mouse models following CIH exposure in future studies.
Estrogen differentially affects PARV and SOM labeling: the estrogen and OSA conundrum
Elevated numbers of PARV-containing neurons were found in the mPFC of young moderate CIH female mice only. As young females exhibit the highest levels of estrogen, this finding supports previous research linking estrogen with elevated calcium binding (Roman-Blas et al., 2009). The observed increase in PARV labeling with no observed change in overall number of GABAergic neurons may indicate that the subset was too small for a change to be observed when labeling the entirety of the GABAergic neuronal population. Alternatively, there may be a decrease in another subset of GABA in this cohort, balancing out the change in PARV. Aged and AOF females did not exhibit a change in PARV labeling. These findings are consistent with a recent study which showed that estrogen protects against CIH-induced cardiorespiratory dysfunction and oxidative stress in the adult brain in rats (Laouafa et al., 2017). It has been suggested that decreased estrogen could be the underlying mechanism linking menopause and occurrence of OSA in women, and hormone replacement therapy could alleviate OSA in postmenopausal women (Keefe et al., 1999). Also, in a study of female rat cardiac myocytes, increased levels of estrogen correlated with an increase in PARV levels, suggesting a link between the two (Wirakiat et al., 2012). The occurrence of OSA is 3-4 times higher in women after versus before menopause (Young et al., 2003), again implicating the role of decreased estrogen in OSA.
Nonetheless, a contradictory relationship between estrogen and OSA has also been suggested (Mirer et al., 2015). An inverse correlation between ovarian hormones and the frequency of OSA has also been reported (Netzer et al., 2003). Additionally, a recent study found that in depressed women in peri- and postmenopause, low estrogen levels correlated with high frequency of OSA (Galvan et al., 2017). While the exact relationship between estrogen and OSA remains to be further evaluated, it seems likely that estrogen could have a protective role against the deleterious effects of OSA and CIH.
Localized in a subset of GABAergic interneurons, SOM plays a role in fine tuning neural activity and in synaptic plasticity and memory formation (Liguz-Lecznar et al., 2016). Hippocampal somatostatin interneurons were reported to control neuronal memory ensembles (Stefanelli et al., 2016). Previously, moderate hypoxia (9% O2 in N2 for two times 8 h) was reported to lead to a slight reduction in SOM expression in the hilus of dorsal and ventral hippocampus (Schwarzer et al., 1996). In the present study, we found increased SOM-labeled cell bodies in the hippocampus (DG) of young severe CIH males that could be a compensatory response to decreased PARV labeling within GABAergic circuitry. While no difference was found in aged females, the young 10% CIH females exhibited a decrease in SOM labeling in the hippocampus. Contrarily, AOF 10% CIH females exhibited an increase in SOM labeling in the PL mPFC compared to controls. Thus, young females with presumably normal estrogen levels exhibited a decrease in SOM labeling in the hippocampus and the AOF females with decreased estrogen showed increased SOM labeling in mPFC. Based on the discussion before regarding the unsolved precise relationship between estrogen and OSA, these findings remain to be explained by future studies.
Decreased v-GAT terminal fields in AOF females exposed to CIH: potential protective effect of estrogen
Analysis of v-GAT, the transporter that gathers GABA from the cell cytoplasm into synaptic vesicles for transport out of the cell (Saito et al., 2010), showed a decrease in mPFC of AOF females only. The decrease in v-GAT terminal fields in the PL and IL mPFC observed in the 10% CIH AOF females was not seen in the other young or aged female cohorts. These data suggest that a lack of estrogen may facilitate a decrease in vesicular GABA following CIH exposure, as the AOF cohort had completely depleted estrogen levels. This substantiates the previously discussed literature that estrogen may have neuroprotective effects against CIH and OSA (Lauoafa et al., 2017; Keefe et al., 1999). A decrease in vesicular GABA at terminal fields coupled with no change in the number of labeled GABAergic soma also indicates that most likely the GABA is produced but not distributed to transporters for secretion into the synapses in the CIH exposed AOF females.
Limitations of the present study: comparison to GABA in human OSA data
The findings of the present study appear to be contradictory to those reported by us in human OSA patients employing proton magnetic resonance spectroscopy (1HMRS) (Pereira et al, 2017). Rather than a decrease in GABA content in mPFC and no change in the hippocampus, as observed in OSA patients (Pereira et al, 2017), this study found an increase in GABAergic neurons both in the mPFC and the hippocampus of male mice following 35-day CIH exposure. The human study (Pereira et al, 2017) reported data from 19 elderly (average age 66.1 yrs, 13 males, 6 females) patients with moderate-to-severe OSA. The human data was from OSA cases that had OSA and thus associated CIH going on for several years. There remains a likely possibility that CIH for such a long time might have induced E/I imbalance and network hyperexcitability with decreased GABA and had overcome the initial compensatory increase in GABA. This initial compensatory increase in GABA both in the hippocampus and mPFC most likely represents the stage captured by the 35-day CIH mouse model. A study in an adult rodent brain model of OSA previously reported a loss of the PARV+ GABAergic interneurons in the mPFC (Yuan et al., 2015), nonetheless other studies found increased GABAergic activity (Madl and Royer, 2000; Wood et al., 1968). These conflicting data in light of the present study raise the possibility that CIH differentially affects GABA in an age- and sex-dependent manner in different brains and different sub-populations of GABAergic neurons. Furthermore, the duration of hypoxic exposure and potential differences between the human and rodent brain response to hypoxia may be other factors that can contribute to the discrepancies. Additionally, the methodological differences between human and mouse studies could have contributed to conflicting data.
While there have been cases of naturally reported OSA in some species, the condition is not frequently reported in non-human animals, and must therefore be induced (Toth and Bhargava, 2013). One major critique is that the CIH induction does not take obesity into account. As OSA is typically characterized by obesity, this may be a key difference between natural and induced CIH. Obesity is believed to contribute to the airway obstruction by narrowing airways through fatty infiltration of the tongue, soft palate, or other surrounding tissue (Motamedi et al., 2009).
Another difference between the CIH model in non-human animals and clinical OSA is the effect on carbon dioxide levels in the blood. Animals exposed to induced CIH are known to develop reduced levels of carbon dioxide in the blood, known as hypocapnia (Dematteis et al., 2009). In contrast, OSA patients develop hypercapnia, with elevated blood carbon dioxide levels (Dematteis et al., 2009; Kanagy, 2009). Different levels of carbon dioxide in the blood may affect other aspects of hypoxia and OSA, or they may be indicative of an underlying distinction between the two conditions.
Clinical relevance of the study
OSA, characterized by sleep fragmentation and CIH, is a common pathological condition with high prevalence worldwide. Gender differences in OSA in humans are well known, with higher occurrence in males (Lin et al., 2008). Also, OSA is found more commonly in elderly males than elderly females (Lim and Pack, 2017; Shochat and Pillar, 2003). While previous preclinical animal model studies of OSA associated CIH have shown sexually dimorphic effects on microglial activation (increased neuroinflammation in males) (Kimyon et al., 2015) and neuroplasticity of the serotonergic system in cardiorespiratory related brain structures (altered in male mice) (Baum et al., 2018), not much is known regarding the sex- and age-specific effects of CIH on GABAergic neuronal circuitry in the hippocampus and mPFC.
OSA is associated with a high risk of adverse neurocognitive and neurovascular sequelae (Culebras and Anwar, 2018; Leng et al., 2017; Polsek et al., 2018). Particularly OSA is associated with an increased risk of cognitive impairment and AD (Blackwell et al., 2015; Leng et al., 2017; Yaffe et al., 2011). Furthermore, CIH may also lead to exacerbation of underlying AD pathology, i.e. Aβ pathology, as OSA patients were found to have higher levels of serum Aβ40 and Aβ42 as compared to controls (Bu et al., 2015). Remarkably, both these levels positively correlated with ODI, a measure of CIH, in OSA patients (Bu et al., 2015).
Both hippocampus and mPFC are known to play essential roles in learning and memory (Jin and Maren, 2015). Also, the role of GABA in memory processes and dysfunctions in neurocognitive disorders have been reviewed before (Ambrad Giovannetti and Fuhrmann, 2019; Lamsa and Lau, 2019). Our findings of sex- and age-specific alterations in GABAergic neurons and specific GABAergic sub-populations in the hippocampus and mPFC regions in mice have wide implications.
Highlights:
Age and hypoxia severity increase neuronal GABA labeling in male mice
Female mice show no changes in GABA labeling following chronic intermittent hypoxia
In young hypoxic mice, DG somatostatin increased in males and decreased in females
Vesicular GABA transporter was affected in accelerated ovarian failure females only
These sex differences might have important implications for clinical OSA patients
Acknowledgments
Support: NIH Grants F32 MH102065 (JDG), DA08259 (TAM, BSM), HL098351 (TAM) & HL096571 (TAM, VMP), DA042943 (VMP), Hope for Depression Research Grant (BSM), NIH grant Paul B. Beeson Emerging Leaders Career Development Award in Aging 1 K76AG054772, 1R01AG064020-01, the BrightFocus Foundation, the DANA Foundation, the Alzheimer’s Drug Discovery Foundation & the Alzheimer’s Association (ACP)
Abbreviations:
- ABC
avidin-biotin complex
- AOF
accelerated ovarian failure
- BSA
bovine serum albumin
- CIH
chronic intermittent hypoxia
- DAB
diaminobenzidine
- DG
dentate gyrus
- E/I
excitation/inhibition
- GABA
gamma aminobutyric acid
- IL
infralimbic mPFC
- ir
immunoreactivity
- MCI
mild cognitive impairment
- mPFC
medial prefrontal cortex
- OSA
obstructive sleep apnea
- ODI
oxygen desaturation index
- PARV
parvalbumin
- PB
phosphate buffer
- PL
prelimbic mPFC
- ROI
region of interest
- SBP
systolic blood pressure
- SOM
somatostatin
- TS
tris-buffered saline
- VCD
4-vinylcyclohexane diepoxide
- v-GAT
vesicular GABA transporter
- 1HMRS
proton magnetic resonance spectroscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interest.
References
- Almey A, Milner TA, Brake WG, 2016. Estrogen receptor alpha and G-protein coupled estrogen receptor 1 are localized to GABAergic neurons in the dorsal striatum. Neuroscience letters 622, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrad Giovannetti E, Fuhrmann M, 2019. Unsupervised excitation: GABAergic dysfunctions in Alzheimer’s disease. Brain research 1707, 216–226. [DOI] [PubMed] [Google Scholar]
- Anju TR, Jayanarayanan S, Paulose CS, 2011. Decreased GABA(B )receptor function in the cerebellum and brain stem of hypoxic neonatal rats: Role of glucose, oxygen and epinephrine resuscitation. Journal of Biomedical Science 18, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, Bizon JL, 2014. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J Neurosci 34, 3457–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum DM, Saussereau M, Jeton F, Planes C, Voituron N, Cardot P, Fiamma MN, Bodineau L, 2018. Effect of gender on chronic intermittent hypoxic Fosb expression in cardiorespiratory-related brain structures in mice. Front Physiol 9, 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer VE, Pickel VM, 1991. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain research 559, 44–55. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Laffan A, Redline S, Ancoli-Israel S, Ensrud KE, Song Y, Stone KL, 2015. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 63, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, McEwen BS, Gould E, 2015. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proceedings of the National Academy of Sciences of the United States of America 112, 15731–15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland LL, Shahar E, Iber C, Knopman DS, Kuo TF, Nieto FJ, 2002. Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: the Sleep Heart Health Study. Journal of sleep research 11, 265–272. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Witte V, Teuchert-Noodt G, 2007. Postnatal development of GABA and calbindin cells and fibers in the prefrontal cortex and basolateral amygdala of gerbils (Meriones unguiculatus). Int J developmental neuroscience 25, 191–200. [DOI] [PubMed] [Google Scholar]
- Bu XL, Liu YH, Wang QH, Jiao SS, Zeng F, Yao XQ, Gao D, Chen JC, Wang YJ, 2015. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Scientific reports 5, 13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton BG, Hok V, Save E, Poucet B, 2009. Lesion of the ventral and intermediate hippocampus abolishes anticipatory activity in the medial prefrontal cortex of the rat. Behavioural brain research 199, 222–234. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J, 1998. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci 18, 9733–9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Kesner RP, 2011. Hippocampal-prefrontal dynamics in spatial working memory: Interactions and independent parallel processing. Behavioural brain research 225, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CG, Wang G, Faraco G, Marques Lopes J, Waters EM, Milner TA, Iadecola C, Pickel VM, 2013. Membrane trafficking of NADPH oxidase p47(phox) in paraventricular hypothalamic neurons parallels local free radical production in angiotensin II slow-pressor hypertension. J Neurosci 33, 4308–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CG, Wang G, Park L, Anrather J, Delagrammatikas GJ, Chan J, Zhou J, Iadecola C, Pickel VM, 2010. Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci 30, 12103–12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culebras A, Anwar S, 2018. Sleep Apnea Is a Risk Factor for Stroke and Vascular Dementia. Current neurology and neuroscience reports 18, 53. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E, 2005. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 30, 67–79. [DOI] [PubMed] [Google Scholar]
- Dematteis M, Godin-Ribuot D, Arnaud C, Ribuot C, Stanke-Labesque F, Pepin JL, Levy P, 2009. Cardiovascular consequences of sleep-disordered breathing: contribution of animal models to understanding the human disease. ILAR journal 50, 262–281. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP, 2010. Pathophysiology of sleep apnea. Physiol Rev 90, 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, 2015. Chronic intermittent hypoxia alters neurotransmission from lateral paragigantocellular nucleus to parasympathetic cardiac neurons in the brain stem. Journal of neurophysiology 113, 380–389. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C, 2010. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. Journal of cognitive neuroscience 22, 1808–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BA, Wellman A, Sands SA, Owens RL, Eckert DJ, White DP, Malhotra A, 2014. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep 37, 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian F, Khazaie H, Tahmasian M, Leschziner GD, Morrell MJ, Hsiung GY, Rosenzweig I, Sepehry AA, 2016. The Association Between Obstructive Sleep Apnea and Alzheimer’s Disease: A Meta-Analysis Perspective. Frontiers in aging neuroscience 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL, 2012. The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EC, 2001. Invited review: Physiological consequences of intermittent hypoxia: systemic blood pressure. Journal of applied physiology (Bethesda, Md. : 1985) 90, 1600–1605. [DOI] [PubMed] [Google Scholar]
- Flores CE, Mendez P, 2014. Shaping inhibition: activity dependent structural plasticity of GABAergic synapses. Front Cell Neurosci 8, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CE, Nikonenko I, Mendez P, Fritschy JM, Tyagarajan SK, Muller D, 2015. Activity-dependent inhibitory synapse remodeling through gephyrin phosphorylation. Proc Natl Acad Sci U S A 112, E65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG, 1997. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci 17, 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan T, Camuso J, Sullivan K, Kim S, White D, Redline S, Joffe H, 2017. Association of estradiol with sleep apnea in depressed perimenopausal and postmenopausal women: a preliminary study. Menopause 24, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamrani H, Onteniente B, Seguela P, Geffard M, Calas A, 1986. Gamma-aminobutyric acid-immunoreactivity in the rat hippocampus. A light and electron microscopic study with anti-GABA antibodies. Brain research 364, 30–38. [DOI] [PubMed] [Google Scholar]
- Gao H, Han Z, Huang S, Bai R, Ge X, Chen F, Lei P, 2017. Intermittent hypoxia caused cognitive dysfunction relate to miRNAs dysregulation in hippocampus. Behav Brain Res 335, 80–87. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Nguyen X-MT, Boehret JM, Lambeth PS, Joy-Gaba J, Warthen DM, Scott MM, 2014. Characterization of excitatory and inhibitory neuron activation in the mouse medial prefrontal cortex following palatable food ingestion and food driven exploratory behavior. Frontiers in Neuroanatomy 8, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A, 1997. Three distinct families of GABAergic neurons in rat visual cortex. Cerebral cortex (New York, N.Y. : 1991) 7, 347–358. [DOI] [PubMed] [Google Scholar]
- Govindpani K, Calvo-Flores Guzman B, Vinnakota C, Waldvogel HJ, Faull RL, Kwakowsky A, 2017. Towards a Better Understanding of GABAergic Remodeling in Alzheimer’s Disease. International journal of molecular sciences 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise KG, Shapiro ML, 2017. Medial Prefrontal Cortex Reduces Memory Interference by Modifying Hippocampal Encoding. Neuron 94, 183–192 e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum HB, 2005. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural networks 18, 1172–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE, 2008. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. European J. Neuroscience 27, 654–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Mifflin SW, 2005. Sex Differences in Blood Pressure Response to Intermittent Hypoxia in Rats. Hypertension 46, 1016–1021. [DOI] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czeh B, Flugge G, Zhang W, 2010. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology 35, 1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman KA, Zhou P, Faraco G, Peixoto PM, Coleman C, Voss HU, Pickel V, Manfredi G, Iadecola C, 2014. Dichotomous effects of chronic intermittent hypoxia on focal cerebral ischemic injury. Stroke 45, 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen FE, Wang C, 1996. Hypoxia-induced hyperexcitability in vivo and in vitro in the immature hippocampus. Epilepsy research 26, 131–140. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Wang C, Stafstrom CE, Liu Z, Geary C, Stevens MC, 1998. Acute and chronic increases in excitability in rat hippocampal slices after perinatal hypoxia In vivo. Journal of neurophysiology 79, 73–81. [DOI] [PubMed] [Google Scholar]
- Jin J, Maren S, 2015. Prefrontal-Hippocampal Interactions in Memory and Emotion. Frontiers in systems neuroscience 9, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagy NL, 2009. Vascular effects of intermittent hypoxia. ILAR journal 50, 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DL, Watson R, Naftolin F, 1999. Hormone replacement therapy may alleviate sleep apnea in menopausal women: a pilot study. Menopause 6, 196–200. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML, 2004. Retrieving Memories via Internal Context Requires the Hippocampus. The Journal of Neuroscience 24, 6979–6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner NA, Roose SP, 2016. Obstructive Sleep Apnea is Linked to Depression and Cognitive Impairment: Evidence and Potential Mechanisms. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 24, 496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner NA, Roose SP, Pelton GH, Ciarleglio A, Scodes J, Lentz C, Sneed JR, Devanand DP, 2017. Association of Obstructive Sleep Apnea with Episodic Memory and Cerebral Microvascular Pathology: A Preliminary Study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 25, 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuu MA, Pagan CM, Nallamothu T, Hevner RF, Hodge RD, Ramirez JM, Garcia AJ 3rd, 2019. Intermittent Hypoxia Disrupts Adult Neurogenesis and Synaptic Plasticity in the Dentate Gyrus. J Neurosci 39, 1320–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimyon R, Smith S, Watters J, 2015. Sex Differences in Microglial Responses to Chronic Intermittent Hypoxia. The FASEB Journal 29. [Google Scholar]
- Kyd RJ, Bilkey DK, 2003. Prefrontal cortex lesions modify the spatial properties of hippocampal place cells. Cerebral cortex (New York, N.Y. : 1991) 13, 444–451. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Lau P, 2019. Long-term plasticity of hippocampal interneurons during in vivo memory processes. Curr Opin Neurobiol 54, 20–27. [DOI] [PubMed] [Google Scholar]
- Laouafa S, Ribon-Demars A, Marcouiller F, Roussel D, Bairam A, Pialoux V, Joseph V, 2017. Estradiol Protects Against Cardiorespiratory Dysfunctions and Oxidative Stress in Intermittent Hypoxia. Sleep 40. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Han VKM, Henderson P, Verdoorn T, Towle AC, 1986. Prenatal ontogeny of the gabaergic system in the rat brain: An immunocytochemical study. Neuroscience 19, 465–493. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Woodske ME, Zou B, O’Donnell CP, 2009. Dynamic arterial blood gas analysis in conscious, unrestrained C57BL/6J mice during exposure to intermittent hypoxia. Journal of applied physiology (Bethesda, Md. : 1985) 107, 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, McEvoy CT, Allen IE, Yaffe K, 2017. Association of Sleep-Disordered Breathing With Cognitive Function and Risk of Cognitive Impairment: A Systematic Review Meta-analysis. JAMA neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard A, Coleman CG, Pickel VM, 2010. Chronic intermittent hypoxia reduces neurokinin-1 (NK(1)) receptor density in small dendrites of non-catecholaminergic neurons in mouse nucleus tractus solitarius. Experimental neurology 223, 634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin S, Godukhin O, 2005. Developmental changes in hyperexcitability of CA1 pyramidal neurons induced by repeated brief episodes of hypoxia in the rat hippocampal slices. Neuroscience letters 377, 20–24. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW, 2012. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LQ, Cao JL, Li L, Han XQ, Wang HY, Liang XM, Wang L, Zhang M, Wang YN, Duan LJ, 2015. [The Effect of Chronic Intermittent Hypoxia on Cognitive Function and Prefrontal Cortex Neurons in Rats]. Sichuan Da Xue Xue Bao Yi Xue Ban 46, 702–705, 725. [PubMed] [Google Scholar]
- Liguz-Lecznar M, Urban-Ciecko J, Kossut M, 2016. Somatostatin and Somatostatin-Containing Neurons in Shaping Neuronal Activity and Plasticity. Front Neural Circuits 10, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DC, Pack AI, 2017. Obstructive Sleep Apnea: Update and Future. Annu Rev Med 68, 99–112. [DOI] [PubMed] [Google Scholar]
- Lin CM, Davidson TM, Ancoli-Israel S, 2008. Gender differences in obstructive sleep apnea and treatment implications. Sleep medicine reviews 12, 481–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ji E-S, Xiang S, Tamisier R, Tong J, Huang J, Weiss JW, 2009. Exposure to cyclic intermittent hypoxia increases expression of functional NMDA receptors in the rat carotid body. Journal of Applied Physiology 106, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohff JC, Christian PJ, Marion SL, Arrandale A, Hoyer PB, 2005. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comparative medicine 55, 523–527. [PubMed] [Google Scholar]
- Madl JE, Royer SM, 2000. Glutamate dependence of GABA levels in neurons of hypoxic and hypoglycemic rat hippocampal slices. Neuroscience 96, 657–664. [DOI] [PubMed] [Google Scholar]
- Mandal PK, Kansara K, Dabas A, 2017. The GABA-Working Memory Relationship in Alzheimer’s Disease. J Alzheimers Dis Rep 1, 43–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Lopes J, Tesfaye E, Israilov S, Van Kempen TA, Wang G, Glass MJ, Pickel VM, Iadecola C, Waters EM, Milner TA, 2017. Redistribution of NMDA Receptors in Estrogen-Receptor-beta-Containing Paraventricular Hypothalamic Neurons following Slow-Pressor Angiotensin II Hypertension in Female Mice with Accelerated Ovarian Failure. Neuroendocrinology 104, 239–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Lopes J, Van Kempen T, Waters EM, Pickel VM, Iadecola C, Milner TA, 2014. Slow-pressor angiotensin II hypertension and concomitant dendritic NMDA receptor trafficking in estrogen receptor beta-containing neurons of the mouse hypothalamic paraventricular nucleus are sex and age dependent. J Comp Neurol 522, 3075–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens H, Weston MC, Boulland J-L, Grønborg M, Grosche J, Kacza J, Hoffmann A, Matteoli M, Takamori S, Harkany T, Chaudhry FA, Rosenmund C, Erck C, Jahn R, Härtig W, 2008. Unique luminal localization of VGAT-C terminus allows for selective labeling of active cortical GABAergic synapses. J Neurosci 28, 13125–13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer LP, Devine PJ, Dyer CA, Hoyer PB, 2004. The follicle-deplete mouse ovary produces androgen. Biology of reproduction 71, 130–138. [DOI] [PubMed] [Google Scholar]
- Mazid S, Hall BS, Odell SC, Stafford K, Dyer AD, Van Kempen TA, Selegean J, McEwen BS, Waters EM, Milner TA, 2016. Sex differences in subcellular distribution of delta opioid receptors in the rat hippocampus in response to acute and chronic stress. Neurobiology of Stress 5, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Frazier CJ, Bizon JL, 2015. Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends in molecular medicine 21, 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Prince SR, 1998. Parvalbumin immunoreactive neurons in the rat septal complex have substantial glial coverage and receive few direct contacts from catecholaminergic terminals. Journal of neuroscience research 52, 723–735. [DOI] [PubMed] [Google Scholar]
- Milner TA, Waters EM, Robinson DC, Pierce JP, 2011. Degenerating processes identified by electron microscopic immunocytochemical methods. Neurodegeneration, Methods and Protocols, 23–59. [DOI] [PubMed] [Google Scholar]
- Mirer AG, Peppard PE, Palta M, Benca RM, Rasmuson A, Young T, 2015. Menopausal hormone therapy and sleep-disordered breathing: evidence for a healthy user bias. Ann Epidemiol 25, 779–784 e771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithani S, Atmadja S, Baimbridge KG, Fibiger HC, 1987. Neuroleptic-induced oral dyskinesias: effects of progabide and lack of correlation with regional changes in glutamic acid decarboxylase and choline acetyltransferase activities. Psychopharmacology 93, 94–100. [DOI] [PubMed] [Google Scholar]
- Motamedi KK, McClary AC, Amedee RG, 2009. Obstructive Sleep Apnea: A Growing Problem. 9, 149–153. [PMC free article] [PubMed] [Google Scholar]
- Netzer NC, Eliasson AH, Strohl KP, 2003. Women with sleep apnea have lower levels of sex hormones. Sleep Breath 7, 25–29. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L, 2007. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L, 2010. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nature neuroscience 13, 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L, 2016. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nature reviews. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, 2014. Can sleep apnea cause Alzheimer’s disease? Neuroscience and biobehavioral reviews 47, 656–669. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, 2001. The Mouse Brain in Stereotaxic Coordinates. Academic Press. [Google Scholar]
- Peers C, Pearson HA, Boyle JP, 2007. Hypoxia and Alzheimer’s disease. Essays Biochem 43,153–164. [DOI] [PubMed] [Google Scholar]
- Peng Z, Zhang N, Wei W, Huang CS, Cetina Y, Otis TS, Houser CR, 2013. A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. The Journal Neurosci 33, 14392–14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Mao X, Jiang CS, Kang G, Milrad S, McEwen BS, Krieger AC, Shungu DC, 2017. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A 114, 10250–10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Kelter DT, McEwen BS, Waters EM, Milner TA, 2014. Hippocampal mossy fiber leu-enkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress. Journal of chemical neuroanatomy 55, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA, 1999. Morphometry of a peptidergic transmitter system: Dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus 9, 255–276. [DOI] [PubMed] [Google Scholar]
- Polsek D, Gildeh N, Cash D, Winsky-Sommerer R, Williams SCR, Turkheimer F, Leschziner GD, Morrell MJ, Rosenzweig I, 2018. Obstructive sleep apnoea and Alzheimer’s disease: In search of shared pathomechanisms. Neuroscience and biobehavioral reviews 86, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdnyakova N, Yatsenko L, Parkhomenko N, Himmelreich N, 2011. Perinatal hypoxia induces a long-lasting increase in unstimulated gaba release in rat brain cortex and hippocampus. The protective effect of pyruvate. Neurochem Int 58, 14–21. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H, 2013. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 23, R764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Blas JA, Castañeda S, Largo R, Herrero-Beaumont G, 2009. Osteoarthritis associated with estrogen deficiency. Arthritis Research & Therapy 11, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row BW, 2007. Intermittent hypoxia and cognitive function: implications from chronic animal models. Advances in experimental medicine and biology 618, 51–67. [DOI] [PubMed] [Google Scholar]
- Saito K, Kakizaki T, Hayashi R, Nishimaru H, Furukawa T, Nakazato Y, Takamori S, Ebihara S, Uematsu M, Mishina M, Miyazaki J, Yokoyama M, Konishi S, Inoue K, Fukuda A, Fukumoto M, Nakamura K, Obata K, Yanagawa Y, 2010. The physiological roles of vesicular GABA transporter during embryonic development: a study using knockout mice. Mol Brain 3, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo-Cohn B, Lai S, Zhan G, Fenik P, Pratico D, Mazza E, Veasey SC, 2006. Sex differences in susceptibility to oxidative injury and sleepiness from intermittent hypoxia. Sleep 29, 152–159. [DOI] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, Medical Research Council Cognitive, F., Ageing S, 2009. Age, neuropathology, and dementia. N Engl J Med 360, 2302–2309. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Sperk G, Rauca C, Pohle W, 1996. Neuropeptide Y and somatostatin immunoreactivity in the rat hippocampus after moderate hypoxia. Naunyn Schmiedebergs Arch Pharmacol 354, 67–71. [DOI] [PubMed] [Google Scholar]
- Sforza E, Roche F, 2016. Chronic intermittent hypoxia and obstructive sleep apnea: an experimental and clinical approach. Hypoxia (Auckland, N.Z.) 4, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada O, Ishikawa H, 1989. Somatostatin-containing neurons in the mouse brain: an immunohistochemical study and comparison with the rat brain. Archives of histology and cytology 52, 201–212. [DOI] [PubMed] [Google Scholar]
- Shochat T, Pillar G, 2003. Sleep apnoea in the older adult : pathophysiology, epidemiology, consequences and management. Drugs Aging 20, 551–560. [DOI] [PubMed] [Google Scholar]
- Snyder B, Cunningham RL, 2018. Sex differences in sleep apnea and comorbid neurodegenerative diseases. Steroids 133, 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T, 2005. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562, 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Heilman J, Furtinger S, Reimer RJ, Edwards RH, Nelson N, 2003. Expression of plasma membrane GABA transporters but not of the vesicular GABA transporter in dentate granule cells after kainic acid seizures. Hippocampus 13, 806–815. [DOI] [PubMed] [Google Scholar]
- Stefanelli T, Bertollini C, Luscher C, Muller D, Mendez P, 2016. Hippocampal Somatostatin Interneurons Control the Size of Neuronal Memory Ensembles. Neuron 89, 1074–1085. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T, 2003. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467, 60–79. [DOI] [PubMed] [Google Scholar]
- Tanahira C, Higo S, Watanabe K, Tomioka R, Ebihara S, Kaneko T, Tamamaki N, 2009. Parvalbumin neurons in the forebrain as revealed by parvalbumin-Cre transgenic mice. Neuroscience research 63, 213–223. [DOI] [PubMed] [Google Scholar]
- Toth LA, Bhargava P, 2013. Animal models of sleep disorders. Comparative medicine 63, 91–104. [PMC free article] [PubMed] [Google Scholar]
- Turner CD, Bagnara JT, 1971. General Endocrinology. W.B. Saunders, Philadelphia. [Google Scholar]
- Umeda K, Iritani S, Fujishiro H, Sekiguchi H, Torii Y, Habuchi C, Kuroda K, Kaibuchi K, Ozaki N, 2016. Immunohistochemical evaluation of the GABAergic neuronal system in the prefrontal cortex of a DISC1 knockout mouse model of schizophrenia. Synapse (New York, N.Y.) 70, 508–518. [DOI] [PubMed] [Google Scholar]
- van Aerde KI, Heistek TS, Mansvelder HD, 2008. Prelimbic and infralimbic prefrontal cortex interact during fast network oscillations. PloS one 3, e2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kempen TA, Gorecka J, Gonzalez AD, Soeda F, Milner TA, Waters EM, 2014. Characterization of Neural Estrogen Signaling and Neurotrophic Changes in the Accelerated Ovarian Failure Mouse Model of Menopause. Endocrinology 155, 3610–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kempen TA, Milner TA, Waters EM, 2011. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain research 1379, 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey S, 2009. Insight from Animal Models into the Cognitive Consequences of Adult Sleep-Disordered Breathing. ILAR journal 50, 307–311. [DOI] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ, 2012. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse (New York, N.Y.) 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Vestergaard MB, Jensen ML, Arngrim N, Lindberg U, Larsson HB, 2018. Higher physiological vulnerability to hypoxic exposure with advancing age in the human brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 271678X18818291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhan L, Zeng W, Li K, Sun W, Xu ZC, Xu E, 2011. Downregulation of hippocampal GABA after hypoxia-induced seizures in neonatal rats. Neurochemical research 36, 2409–2416. [DOI] [PubMed] [Google Scholar]
- Wirakiat W, Udomuksorn W, Vongvatcharanon S, Vongvatcharanon U, 2012. Effects of estrogen via estrogen receptors on parvalbumin levels in cardiac myocytes of ovariectomized rats. Acta histochemica 114, 46–54. [DOI] [PubMed] [Google Scholar]
- Wood JD, Watson WJ, Ducker AJ, 1968. The effect of hypoxia on brain gamma-aminobutyric acid levels. Journal of neurochemistry 15, 603–608. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL, 2011. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. Jama 306, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita S, Suzuki S, Yoshikawa K, Iida K, Hirata A, Suzuki M, Takashima A, Maruyama K, Hirasawa A, Awaji T, 2017. Treatment of intermittent hypoxia increases phosphorylated tau in the hippocampus via biological processes common to aging. Mol Brain 10, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Finn L, Austin D, Peterson A, 2003. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. American journal of respiratory and critical care medicine 167, 1181–1185. [DOI] [PubMed] [Google Scholar]
- Yuan L, Wu J, Liu J, Li G, Liang D, 2015. Intermittent Hypoxia-Induced Parvalbumin-Immunoreactive Interneurons Loss and Neurobehavioral Impairment is Mediated by NADPH-Oxidase-2. Neurochemical research 40, 1232–1242. [DOI] [PubMed] [Google Scholar]