To the Editor—We read with great interest the recent study of an Indonesian cohort who demonstrated persistently negative interferon-gamma release assay (IGRA) despite close exposure to an index tuberculosis (TB) case [1]. The authors termed this phenomenon “early clearance”, but epidemiologically it is the same phenomenon we have characterized in our household contact study in Uganda [2–4], which we have called “resistance to latent M. tuberculosis (Mtb) infection” (resister [RSTR]). Additional studies have been conducted in India [5], and The Gambia [6], with similar study designs. With the increased relevance of this phenotype for TB research [7], it is instructive to examine these studies collectively, to identify commonalities and future directions.
There are 2 important differences between our Uganda study and the Indonesia study. First, the Uganda study had a minimum of 12 months follow-up after diagnosis of the index TB case, whereas the Indonesia study only had 14 weeks (~3 months) follow-up. Although extended observation is logistically challenging and risks loss to follow-up, it is critical for avoiding subject misclassification. In our Uganda study [4], among contacts of active TB patients who were tuberculin skin test (TST)-negative at baseline and converted their TST, we found that >25% of conversion events occurred between 3 and 24 months post enrollment, including 16% between 3 and 6 months. The national TB incidence rate in Indonesia is 395 per 100 000, compared to 253 per 100 000 in Uganda at the national level, although the estimated incidence in our prospective study in Kampala was 740 per 100 000, a higher transmission, urban setting [4]. Because these incidence rates are comparable, there is no compelling reason to expect that later conversion events would be less likely in Indonesia. Shorter follow-up is one possible explanation for the lower total conversion rate in the Indonesia study (26.8%) compared to our Uganda study (55.9%). Moreover, the India study conducted 12 months of follow-up, whereas the Gambia study conducted only 3 months, making additional comparison among cohorts difficult.
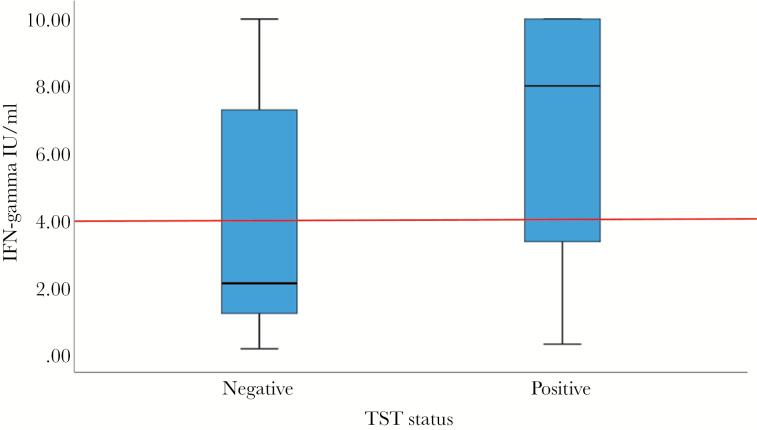
Second, the Indonesia study only utilized IGRA, whereas our study used TST initially [2, 4], and our long-term follow-up study utilized both TST and IGRA [3]. The limitations of both TST and IGRA are well known, including substantial rates of discordance (kappa coefficients of 0.6–0.7 across multiple studies) due to the use of different stimuli and measurement of different immunologic responses. However, our study and others have shown that there is valuable information gained from both assays. A South African cohort study [8] demonstrated that TB incidence was highest in adolescents with concordantly positive TST and Quantiferon (QFT), with lower incidence in adolescents with discordant TST and QFT, and lowest incidence in those with concordantly negative TST and QFT. Data from QFT-positive subjects in our study [3] illustrate variation in the quantitative IGRA values in TST-positive (≥10 mm induration) versus TST-negative individuals (Figure 1). Among QFT-positive individuals, 5% had negative TST. Although the distribution of quantitative QFT values is not significantly different between TST-positive and TST-negative individuals (P = .128), TST-negative individuals have notably lower QFT responses. Using the 4 IU/mL cutoff proposed by others [9], the comparison of QFT-positive versus QFT-negative values by TST status is nearly significant (P = .063). These data, taken together, show that TST adds information to the QFT.
Figure 1.
Quantitative Quantiferon (QFT) values among QFT-positive subjects by tuberculin skin test (TST)-positivity. Boxplots show distributions of QFT-Plus quantitative values in TST-positive (N = 222, TST ≥10-mm induration) and TST-negative (N = 12, TST <10-mm induration) subjects (all human immunodeficiency virus-negative). Comparison of individuals with 0–5 mm versus 5–9.9 mm TST shows similar distributions of interferon (IFN)-gamma values (data not shown). Comparison of quantitative values by Mann-Whitney test, P = .128. Comparison using 4 IU/mL cutoff value as proposed by Andrews et al [9], represented by red line, P = .063.
Finally, it is clear that each group studying this phenotype has developed their own naming convention through their hypothesized perspectives on the meaning of these phenotypes, a point made eloquently by Lalvani and Seshadri [10]. Through our recent study of antibody profiles and T-cell responses to ESAT6/CFP10 [11], we learned that persistently TST/IGRA-negative individuals have Mtb antigen-specific antibody profiles confirming exposure, and their antibody response is different from individuals who are TST/IGRA positive. “Early clearance” implies that these individuals had an Mtb infection, and cleared it, for which there is no direct evidence. A better phenotype for early clearance might be IGRA and/or TST reverters, because sustained QFT converters have been proposed as a model for sustained Mtb infection [9]. On the other hand, our naming convention (“RSTRs”) may be an overstatement. It is possible that these subjects have in fact acquired Mtb infection, but because of their lack of interferon-gamma T-cell responses to Mtb antigens, they are unable to mount the characteristic immune response captured by TST and/or IGRA. Without more data and an assay that directly measures Mtb infection (rather than an immune response to Mtb antigens), the correct nomenclature is difficult to ascertain. In conclusion, further correctly designed studies will be necessary to determine the mechanisms underlying “resistance to classically defined latent Mtb infection.”
Notes
Financial support. Funding for this work was provided by National Institutes of Health Grants R01AI124348 (awarded to W. H. B., C. M. S., and T. R. H.) and UO1-AI-09-001 (awarded to W. H. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Verrall AJ, Alisjahbana B, Apriani L, et al. Early clearance of Mycobacterium tuberculosis: the INFECT case contact cohort study in Indonesia. J Infect Dis 2019. [DOI] [PubMed] [Google Scholar]
- 2. Ma N, Zalwango S, Malone LL, et al. ; Tuberculosis Research Unit (TBRU) Clinical and epidemiological characteristics of individuals resistant to M. tuberculosis infection in a longitudinal TB household contact study in Kampala, Uganda. BMC Infect Dis 2014; 14:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stein CM, Nsereko M, Malone LL, et al. Long-term stability of resistance to latent Mycobacterium tuberculosis infection in highly exposed tuberculosis household contacts in Kampala, Uganda. Clin Infect Dis 2019; 68:1705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stein CM, Zalwango S, Malone LL, et al. Resistance and susceptibility to Mycobacterium tuberculosis infection and disease in tuberculosis households in Kampala, Uganda. Am J Epidemiol 2018; 187:1477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mave V, Chandrasekaran P, Chavan A, et al. ; CTRIUMPH RePORT India Study Team Infection free “resisters” among household contacts of adult pulmonary tuberculosis. PLoS One 2019; 14:e0218034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weiner J, Domaszewska T, Donkor S, Kaufmann SH, Hill PC, Sutherland JS.. Changes in transcript, metabolite and antibody reactivity during the early protective immune response in humans to Mycobacterium tuberculosis infection. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simmons JD, Stein CM, Seshadri C, et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol 2018; 18:575–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahomed H, Hawkridge T, Verver S, et al. The tuberculin skin test versus QuantiFERON TB Gold® in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One 2011; 6:e17984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrews JR, Nemes E, Tameris M, et al. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med 2017; 5:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lalvani A, Seshadri C. Understanding how BCG vaccine protects against Mycobacterium tuberculosis infection: lessons from household contact studies. J Infect Dis 2019. [DOI] [PubMed] [Google Scholar]
- 11. Lu LL, Smith MT, Yu KKQ, et al. IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat Med 2019; 25:977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]