Abstract
Objective:
Quadriceps muscle weakness is common in knee osteoarthritis (OA). While pain, disuse, and atrophy are commonly cited causes for muscle weakness in OA, emerging evidence suggests changes in muscle quality also occur. Alterations in muscle quality are not well understood, but likely include both cellular and morphologic adaptions. The purpose of this study was to conduct the first cellular-level analysis of the vastus lateralis in adults with moderate knee OA.
Methods:
Vastus lateralis biopsies were obtained from 24 subjects with moderate knee OA and 15 healthy controls. Quadriceps strength, muscle fiber cross sectional area (CSA), fiber type distribution, extracellular matrix (ECM) content, satellite cell abundance, and profibrotic gene expression were assessed.
Results:
Relative to controls, quadriceps strength was significantly lower in OA subjects (OA 62.23, 50.67–73.8 Nm vs 91.46, 75.91–107.0 Nm, P=0.003) despite no difference in fiber CSA. OA subjects had significantly fewer Type I fibers (OA 41.51, 35.56–47.47% vs 53.07, 44.86–61.29%, P=0.022) and more hybrid IIa/x fibers (OA 24.61, 20.61–28.61% vs 16.4, 11.60–21.20%, P=0.009). Significantly greater ECM content, lower satellite cell density, and higher profibrotic gene expression was observed with OA, and muscle collagen content was inversely correlated to strength and SC density.
Conclusion:
Lower quadriceps function with moderate OA may not result from fiber size impairments, but is associated with ECM expansion. Impaired satellite cell density, high profibrotic gene expression, and a slow-to-fast fiber type transition may contribute to reduced muscle quality in OA. These findings can help guide therapeutic interventions to enhance muscle function with OA.
Keywords: osteoarthritis, atrophy, fibrosis, quadriceps weakness, knee
Introduction
Knee osteoarthritis (OA) is a leading cause of disability worldwide [1–4]. While the development of knee OA is multifactorial, alterations in loading of the knee are believed to be an important contributor [5, 6]. The quadriceps muscle plays a central role in modulating loads across the knee joint, and weakness contributes to pain and loss of function in people with knee OA [7–9]. The quadriceps muscle can be up to 40% weaker in OA patients compared to healthy individuals [10–12]. Quadriceps weakness is generally attributed to pain and atrophy due to inactivity [12–14]. However, the relationship between pain and atrophy to reduced strength in knee OA is not always strong [13, 14]. For example, even when controlling for pain and activity levels, patients with knee OA have weaker quadriceps than controls [15], and the progression of OA-mediated muscle weakness appears dissociated from muscle mass [16]. Weakness of the quadriceps muscle is observed early in the disease process and may precede disease onset [11, 12]. The early manifestation of strength loss in patients with OA suggests that pain and atrophy from disuse cannot fully explain the reported muscle weakness, and that other cellular factors may play a role.
Muscle fiber type composition (i.e. the distribution of slow-contracting/oxidative muscle fibers versus fast-contracting/glycolytic muscle fibers) is a powerful indicator of global muscle health [17]. In general, the presence of “hybrid” muscle fibers co-expressing Type IIa fast-twitch and Type IIx super-fast twitch (Type IIa/x) myosin heavy chains (MyHC) is indicative of poor muscle health [17, 18], and may contribute to impaired muscle function. Limited work in orthopedic populations, such as those with anterior cruciate ligament (ACL) reconstruction, indicates that knee joint injury results in unfavorable slow-to-fast fiber type transitions in the vastus lateralis that are not reversed after rehabilitation [19]. In people with advanced-stage knee OA, a high prevalence of hybrid fibers has also been reported in the vastus lateralis [20]. The precise mechanisms that mediate fiber type transitioning are still being explored; however, elucidating whether fiber type transitioning is a consequence of early disease pathology, or is secondary to atrophy and/or inactivity is important for understanding the etiology of muscular deficits in people with OA.
Although contractile components of muscle fibers (e.g. MyHCs) are fundamental for determining overall muscle function other components within the muscle also play an important role in force transmission and contractile performance. For instance, the amount of extracellular matrix (ECM), or non-contractile tissue, within skeletal muscle can negatively affect whole muscle strength [21]. Pathological conditions are often characterized by excessive ECM accumulation [22], which results in poor muscle quality. Whether fibrotic deposition contributes to muscle dysfunction in OA is unknown. Specialized cell populations such as muscle stem cells, called satellite cells, are responsible for muscle repair and regeneration [23–26], but also play an important role in regulating the ECM [21, 27]. It is conceivable that alterations to the ECM and/or satellite cells in moderate OA may contribute to muscle strength deficits that occur independent from muscle atrophy.
The purpose of this study was to characterize cellular properties of muscle from individuals presenting with symptomatic moderate radiographic OA. We hypothesized that features other than muscle fiber size, such as increased abundance of IIa/x MyHC hybrid muscle fibers, elevated profibrotic gene expression and ECM content, and/or decreased satellite cell density may contribute to functional deficits in OA. Cellular-level insight can help guide early preventative countermeasures for strength loss with OA, thereby improving muscle performance and quality of life at the onset of disease. Identifying muscular perturbations in early-stage OA may also help develop targeted therapeutics for treating profound muscle weakness observed in late-stage OA [16, 28].
Methods
Study Subjects and Muscle Biopsies
Subjects provided their written informed consent from a protocol approved by the institutional review board at the University of Kentucky and Wake Forest University, in accordance with the Declaration of Helsinki. Subjects with OA for the present study were recruited from those entering the Strength Training for Arthritis Trial (START) at Wake Forest University. Complete details of the study including the inclusion and exclusion criteria have been published [29]. Briefly, OA subjects had to be ambulatory and over 55 years of age with knee pain and mild-moderate radiographic knee OA based on semi-flexed knee radiographs with grade II or grade III OA on the Kellgren Lawrence (KL) scale [30]. Subjects with lateral>medial tibiofemoral OA or patellofemoral OA of grade 3 on a 0–3 scale (assessed using the sunrise view of the patellofemoral joint) were excluded. Special care was taken to ensure that healthy, control subjects were similar to OA subjects in regard to activity, age and BMI; however, no matching was performed. Control subjects underwent the same standing semi-flexed bilateral anteroposterior and patellofemoral x-rays as the subjects with OA and had to have a KL grade of ≤1 in both knees and no evidence of patellofemoral OA. To maintain consistency, the same rheumatologist (RFL) read all x-rays. Activity level in both groups was assessed by the Physical Activity Scale for the Elderly (PASE). Comparable healthy control subjects were from the University of Kentucky Center for Muscle Biology: Normal Tissue Bank. All muscle strength data were processed and analyzed at Wake Forest University, whereas all muscle biopsy samples were processed and analyzed at the University of Kentucky, Center for Muscle Biology.
Vastus lateralis muscle biopsies were obtained via the Bergstrom technique [31] and processed for analyses. For RNA, muscle (~100 mg) was immediately frozen in liquid nitrogen followed by storage at −80°C until RNA extraction was performed. For histology/immunohistochemistry (IHC), muscle samples (~50 mg) were cleaned of fat and connective tissue, then mounted onto a piece of cork with mounting medium (1 part tragacanth (VWR):1.5 part O.C.T (TedPella)). Mounts were snap frozen in liquid nitrogen-cooled isopentane and stored at −80°C until sectioning. Usable muscle mounts for IHC were obtained from 15 control and 20 OA subjects.
Strength Measurements
Maximum unilateral concentric isokinetic quadriceps strength was measured using a HUMAC NORM isokinetic dynamometer (CSMi, Stoughton, MA, USA). Knee extensor strength was tested through a joint arc from 90° to 30° of knee flexion. The average force was calculated between joint angles of 40–80°. Trials were spaced by rest periods of 30–60 seconds. The three maximal reproducible trials were averaged from five trials for each test.
Quantitative real-time rtPCR
Muscle biopsy RNA was extracted by homogenizing frozen samples in QIAzol Lysis Reagent (QIAGEN, Hilden, Germany, 79306) and RNA was precipitated and washed using the RNeasy kit (QIAGEN, 74104). RNA quality and integrity was assessed using the Agilent 2100 Bioanalyser (Agilent Technologies, Santa Clara, CA). High quality RNA was obtained from 15 control subjects and 22 OA subjects. Reverse transcription was performed with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, 170–8890). Quantitative real-time reverse transcription polymerase chain reaction (rtPCR) was performed using SYBR Select Master Mix (Thermo-Fisher, 4472908). Gene expression was normalized to the geometric mean of four housekeeping genes: 18S RNA, β-actin (ACTβ), β−2 microglobulin (β2M), and Phosphoglycerate kinase (PGK1), using the 2ΔΔCt method. Primer pairs are presented in supplemental Table 1.
Histology and Immunohistochemistry (IHC)
Frozen muscle mounts were sectioned at −23 to −25°C. Sections from 15 control and 20 OA subjects were cut at 7μm, mounted onto charged slides and allowed to air-dry at room temperature for at least an hour prior to storing at −20°C. Prior to staining, slides were removed from −20°C and air dried at room temperature for 10–15 minutes. All samples were subject to all IHC analyses. However, IHC staining of certain samples was unsuccessful for some analyses; therefore, sample sizes vary by staining procedure.
Fiber typing and cross sectional area measurements were performed as follows. Briefly, sections were rehydrated with phosphate buffered saline (PBS) followed by overnight incubation with isoform-specific MyHC primary antibodies against: Type I (1:100)(Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA; BA.D5 concentrate), Type IIa (neat)(DSHB, SC.71 supernatant), Type IIx (neat)(DSHB, 6H1 supernatant) and Laminin (1:100)(Sigma Aldrich, St. Louis, MO, USA; L9393). Sections were washed then incubated for 1 hour with fluorophore conjugated secondary antibodies (1:200): goat anti-mouse (GtαMs) IgG2b AlexaFluor647 (A-21242), GtαMs IgG1 AlexaFluor488 (A-21121), GtαMs IgM AlexaFluor555 (A-21426) (Thermo-Fisher, Carlsbad, CA, USA) and GtαRab (rabbit) AMCA (Vector Laboratories, Burlingame, CA, USA; CI-1000). Sections were washed, post-fixed in methanol and cover slipped with Vectashield Mounting Medium (Vector Laboratories, H-1000).
For fiber type-specific satellite cell analysis, sections were fixed in ice cold (−20°C) acetone and washed with PBS. Endogenous peroxidases were blocked with 3% hydrogen peroxide. Sections were then blocked for 1 hour in 2.5% normal horse serum (NHS)(Vector Laboratories, S-2012) followed by overnight incubation with primary antibodies against: Pax7 (1:100)(DSHB, Pax7 concentrate), Type I MyHC (1:75)(DSHB, BA.D5 concentrate) and Laminin (1:100)(Sigma Aldrich, L9393). Sections were washed and incubated with GtαMs IgG1 biotin (1:1000)(Jackson ImmunoResearch Laboratories, West Grove, PA, USA; 115-065-205) for 90 minutes, followed by 4 washes then a 1 hour incubation with: streptavidin horseradish peroxidase (1:500)(Thermo-Fisher, S-911; 2.5 μg/μl stock), GtαRab IgG AlexaFluor488 (1:250)(Thermo-Fisher, A-11034) and GtαMs IgG2b AlexaFluor647 (1:250)(Thermo-Fisher, A-21242). Sections were incubated for 20 minutes with TSA AlexaFluor594 (1:200) in amplification diluent (Thermo-Fisher, T20950), washed and cover slipped with Vectashield with DAPI to visualize nuclei (Vector Laboratories, H-1200).
Wheat Germ Agglutinin (WGA), which binds to glycosaminoglycans, was used to quantify ECM [27, 32] Sections were fixed for 20 min with 4% paraformaldehyde, washed with PBS, and incubated with Texas Red conjugated WGA (1:50 of 1mg/ml) for 2 hours (Thermo-Fisher, W21405). Sections were then washed and cover slipped with Vectashield. Collagen I and III content in the ECM was assessed using Sirius Red staining. Sections were fixed with Bouin’s for 15 min at 56°C. Sections were then washed with distilled water and incubated in 0.1% Sirius red in saturated picric acid for 2 hours (Electron Microscopy Sciences (EMS), Hatfield, PA, USA, 26357–02). Following Sirius red staining, sections were washed in 0.5% acetic acid in distilled water, dehydrated with 95% then 100% ethanol (1–3 dips each) and equilibrated for 10–15 minutes in Xylenes. Slides were mounted with Cytoseal 60 (EMS, 18007).
Image Capture and Quantification
Fluorophores were imaged using an AxioImager M1 (Zeiss; Oberkochen, Germany), and brightfield images were acquired with an Olympus BX61VS (Olympus; Shinjuku, Tokyo, Japan). Muscle cross sectional area was determined by manually outlining muscle fibers on entire cross sections using Zen image analysis software (Zeiss). Fiber type distribution was quantified manually by a trained technician. All event counts were collected in Zen. Threshold analysis of WGA and Sirius Red staining was done according to previously published methods [27]. Briefly, for WGA analysis, regions of interest (ROI) that did not include fibrotic swathing were chosen (at least 3 ROIs/sample), a background correction was applied, and images were converted to grayscale. A threshold of 0–215 was used for each image and the percent of the total ROI area positive within the threshold was measured. For fibrotic swath ranking, images were given a generic label and two blinded, independent researchers were asked to rank biopsies from most swathing (23) to least swathing (1). We then compared swath rankings to the percentage of WGA+ area. For Sirius Red analysis, whole section images were acquired, uploaded into PhotoShop (Adobe, San Jose, CA, USA), and non Red+ pixels were selected then deleted. Images were then opened in Image J and ROIs were chosen. ROIs were converted to grayscale and the Sirius Red+ area was measured by threshold as described above. Satellite cells were identified as Pax7+/DAPI+ and expressed relative to fiber number. To identify satellite cells associated with specific fiber types, satellite cells located under the laminin border of each fiber type (MyHC Type I or II) were counted.
Rigor and Reproducibility
A number of steps were employed to enhance the scientific rigor of the experiments. Rigor between Wake Forest University and the University of Kentucky was maintained through onsite review of muscle strength and standardized biopsy protocols. In addition, the same techniques and equipment were used at both sites. Control subjects were chosen to represent a similar activity level, age range and BMI of OA subjects. Isotype, no primary antibody and negative and positive controls were performed in each experiment to validate results, minimize non-specific background staining and tissue autofluorescence, and assure antibody specificity, sensitivity, and lack of cross-reactivity. Quantification was semi-automated and analyses were performed blinded to study design. Operators were spot checked with characterized tissue samples to minimize inter-analysis and inter-individual variability and assure reproducibility.
Statistical Analysis
The assumption that data were sampled from a Gaussian distribution was tested using D’Agostino-Pearson normality test. For all analyses except PCR, data was found to be normally distributed, warranting the use of a parametric t-test. Since no matching was performed an unpaired t-test was chosen. Additionally, no assumption of equal variances was made and groups had unequal sample sizes, so Welch’s correction was applied. Differences in all muscle characteristics between control and OA groups were analyzed by unpaired t-tests with Welch’s correction. Pearson’s product moment correlations were used to determine relationships of muscle characteristics to muscle strength. Gene expression data (PCR) did not pass the D’Agostino-Pearson normality test, warranting the use of nonparametric statistical test. A Mann-Whitney U test was used to determine differences in relative mRNA expression between control and OA groups and Spearman Rho correlation was used to analyze the relationship of relative mRNA expression to Sirius red staining and knee extensor strength. Statistical analyses were performed using Prism 7 (GraphPad Software Inc, La Jolla CA). All normally distributed data are expressed as mean ± 95% Confidence Interval (CI). Gene expression data is shown as median and interquartile range (IQR). All tests were two-tailed, α < 0.05.
Results
Thirty-nine subjects participated in this study: 24 with OA and 15 healthy controls. Group demographics are presented in Table 1. Similarly, activity level was not statistically different between OA and control groups, determined by PASE (160.4, 123.0–197.8 vs 175.3, 143.0–209.7, P=0.507) [Fig. 1(A)]. However, OA subjects had significantly weaker quadriceps than the control subjects (62.23, 50.67–73.8 Nm vs 91.46, 75.91–107.0 Nm, P=0.003) [Fig. 1(B)]. Despite quadriceps weakness, there was no significant difference in muscle fiber CSA or fiber type-specific CSA [Fig. 1(C) and (D)].
Table I.
Patient Demographics expressed as mean ± standard deviation, and range
| Measure | Healthy Control (CON) | Osteoarthritis (OA) |
|---|---|---|
| Age | 63.7 ± 6.9, 53.9–74.3 years | 60.2 ± 5.5, 52.0–73.0 years |
| BMI | 26.9 ± 2.6, 23.3–34.2 kg/m2 | 28.4 ± 3.9, 20.6–37.5 kg/m2 |
| Sex | F = 10; M = 5 | F =1 0; M = 14 |
Fig. 1.
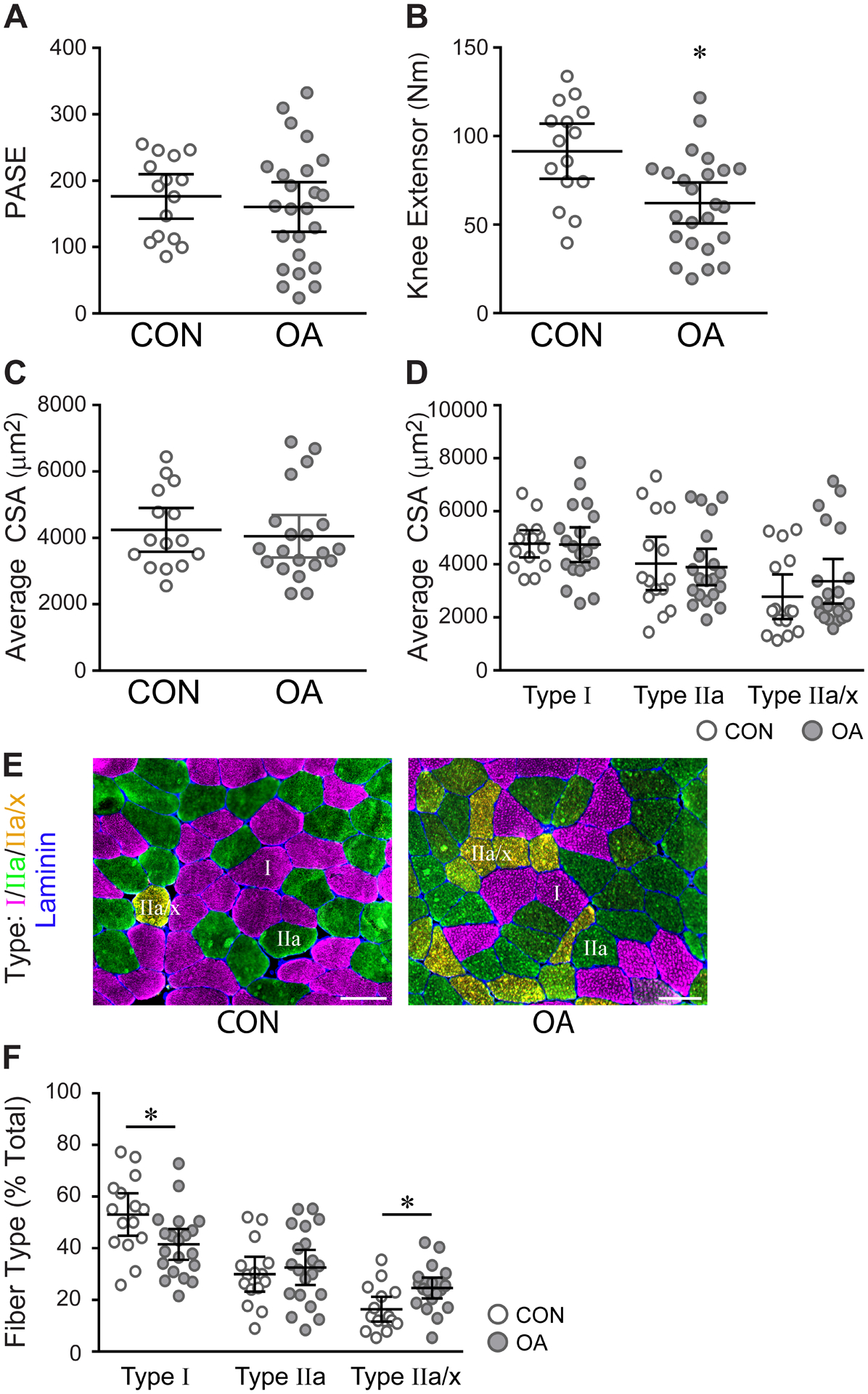
Subjects with mild to moderate radiographic and symptomatic OA have reduced quadriceps strength in the absence of muscle atrophy. A) PASE results showing comparable activity levels between control (CON, n=15) and OA (n=24) subjects. B) Quadriceps strength measured by knee extensor. CON (n=15), OA (n=24); *P=0.0034. C) CSA of all fibers within muscle biopsies. CON (n=15), OA (n=20). D) CSA classified by fiber type. CON (n=15), OA (n=20). E, F) Immunohistochemical analysis of fiber type distribution; MyHC Type I is pink, IIa is green, IIa/x is orange, and Laminin is blue. CON (n=15), OA (n=20); Type I fibers, *P=0.0222, Type IIa/x fibers, *P=0.0091. Representative images were obtained at 20x. Scale bars = 100 μm. All data are expressed as mean ± 95% CI, analyzed by unpaired t test with Welch’s correction.
OA patients had significantly fewer Type I muscle fibers (41.51, 35.56–47.47 % to vs 53.07, 44.86–61.29 %, P=0.02) [Fig. 1(E) and (F)]. Fewer Type I fibers coincided with a greater percentage of hybrid fibers co-expressing IIa and IIx MyHC isoforms (Type IIa/x) (24.61, 20.61–28.61% vs 16.4, 11.6–21.2%, P=0.009) [Fig. 1(E) and (F)]. Fiber CSA positively correlated to quadriceps strength in both OA and control (r=0.51, 0.22 – 0.72, P=0.002) [SFig. 1(A)]. This correlation was significant for both Type I (r=0.34, 0.003 – 0.602, P=0.048) and IIa (r=0.52, 0.23 – 0.73, P=0.001) fibers, but not hybrid IIa/x fibers (r=0.26, −0.08 – 0.55, P=0.129) [Fig. 1(B) and (C)]. The percentage of hybrid IIa/x fibers negatively correlated to strength (r=−0.35, −0.61 – −0.02, P=0.039) [SFig. 1 (D)]. Hybrid Type I/IIa fibers were present in muscles from both OA and control groups, but were rare (<2% of total fibers), and thus excluded from analyses [SFig. 1(E)].
A significantly greater percentage of muscle area positively stained with WGA in OA muscles compared to control (7.33, 5.98–8.67 % of area vs 4.70, 3.74–5.65 % of area, P=0.002) [Fig. 2(A)]. In addition, large swaths of fibrotic (WGA+) tissue were observed in several OA subjects [Fig. 2(B)]. We found that biopsies with the most WGA+ ECM surrounding individual muscle fibers also had the greatest fibrotic swathing (r=0.88, 0.74 – 0.95, P<0.0001) [Fig. 2(C)]. Areas of WGA+ fibrotic swathing also contained collagen, staining positive with Sirius Red [SFig. 2(A)]. Similar to WGA staining, the percentage of Sirius Red+ area surrounding muscle fibers was significantly elevated in OA muscles (4.34, 3.88–4.79 % of area vs 3.31, 2.83–3.79 % of area, P=0.002) [Fig. 3(A) and (B)], and samples with more Sirius Red+ area had higher average swath rankings (r=0.58, 0.22 – 0.81, P=0.004) [SFig. 2(B)]. Subjects with more Sirius Red+ muscle area also had weaker quadriceps (r=−0.55, −0.76 – −0.25, P=0.001) [Fig. 3(C)]. Fibrotic swathing also correlated to strength, where samples with higher average swath ranks had weaker quadriceps (r=−0.40, −0.70 – 0.01, P=0.056) [SFig. 2(C)].
Fig. 2.
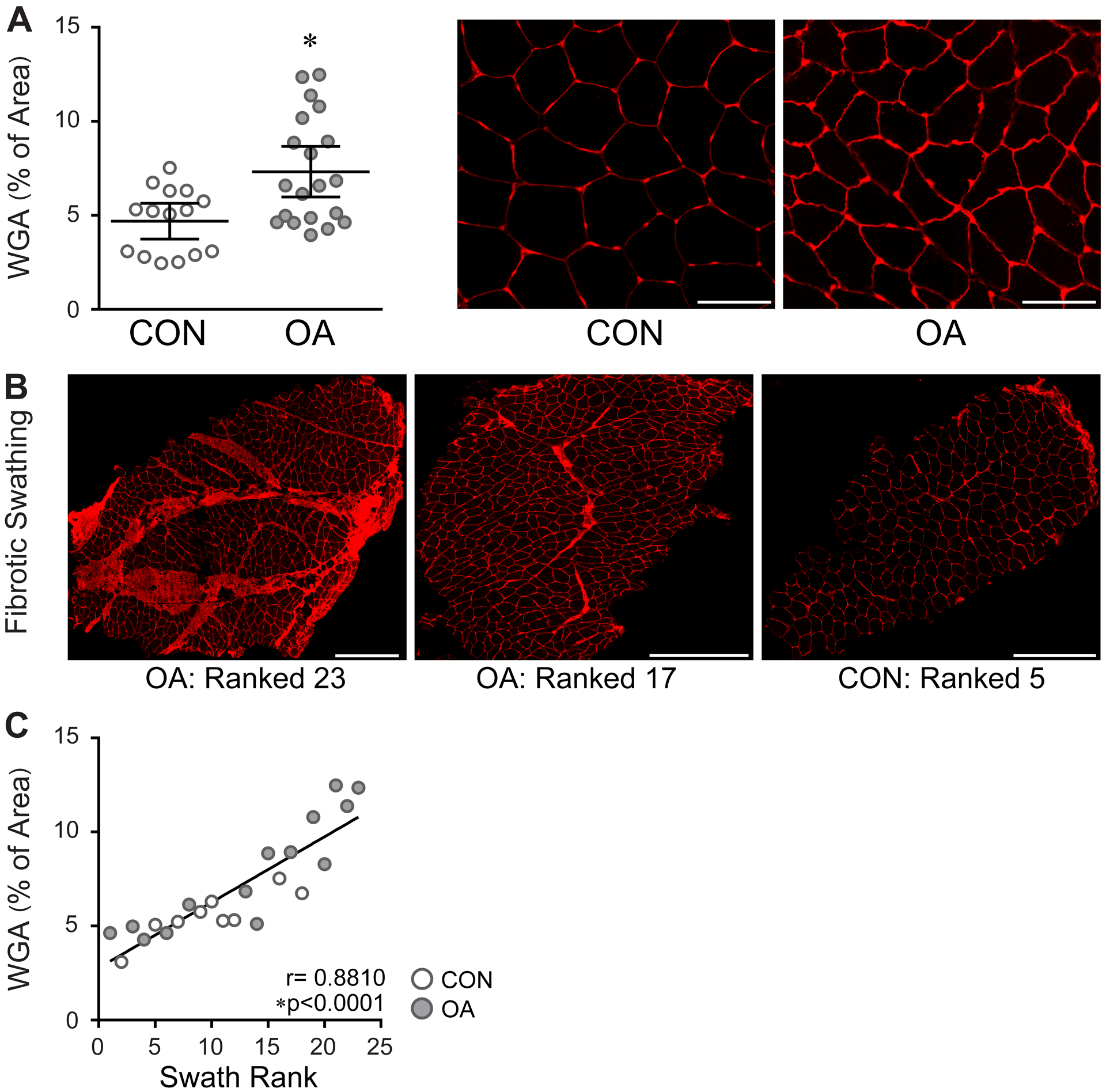
Pathogenic fibrosis in the quadriceps muscle of OA subjects. A) Bar graph (left) and representative images (right) showing increased WGA staining in between muscle fibers in OA. Data are expressed as mean ± 95% CI; control (CON, n=15) and OA (n=20). Data analyzed by unpaired t test with Welch’s correction, *P=0.0020. Images were acquired at 20x. Scale bars = 100 μm. B) Representative images from sections of whole muscle biopsies showing large swaths of fibrotic tissue in OA muscles. Images acquired at 20x. Scale bars = 500 μm. C) Relationship between WGA surrounding muscle fibers and fibrotic Swath rank. Data were analyzed by two-tailed Pearson’s Product moment correlation.
Fig. 3.
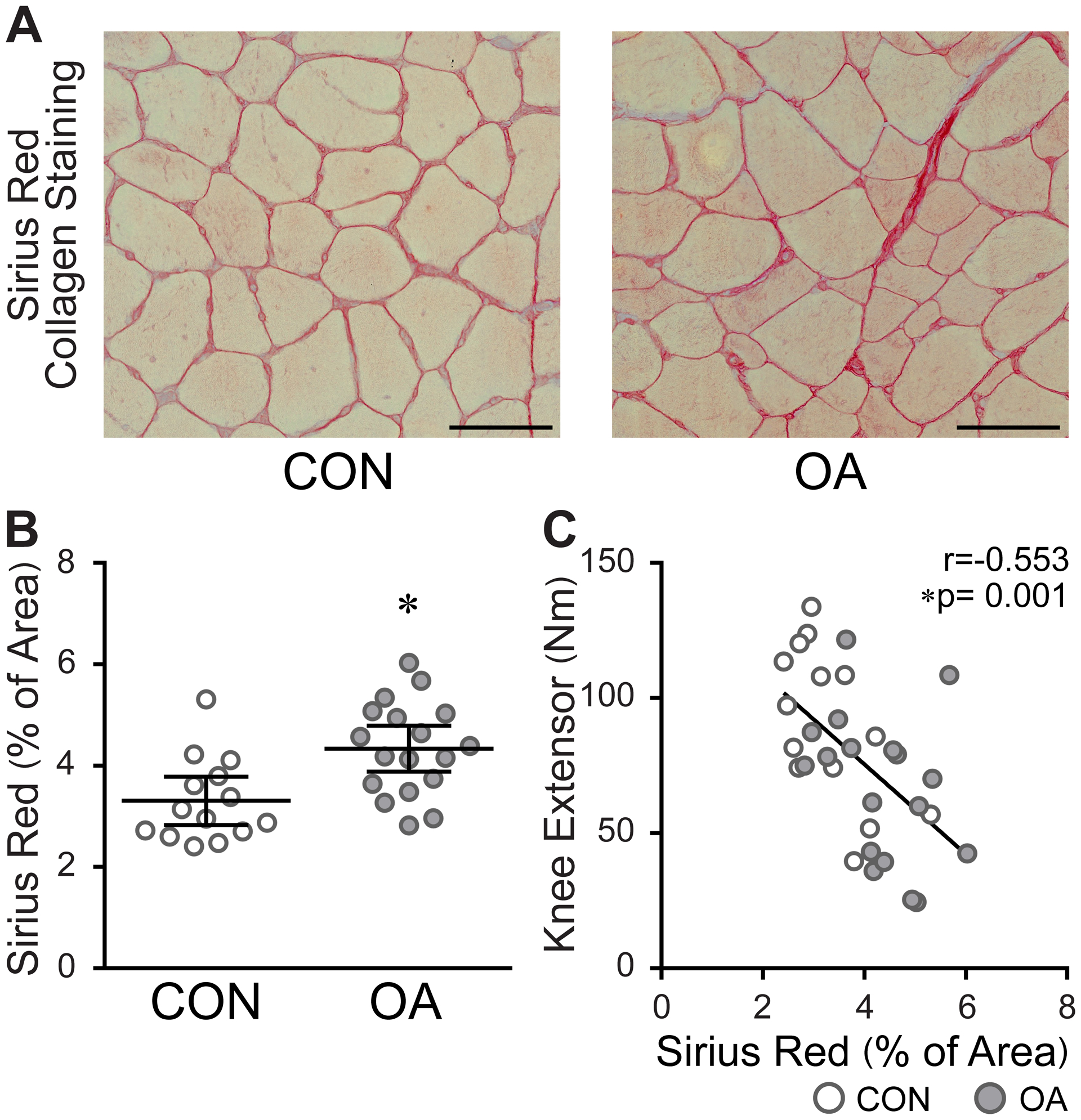
Aberrant collagen deposition in the ECM of OA muscle inversely correlates to strength. A) Representative images of Sirius Red staining for collagen. Images were acquired at 20x. Scale bars = 100 μm. B) Bar graph quantification of Sirius Red staining surrounding muscle fibers. Data are expressed as mean ± 95% CI; control (CON, n=14) and OA (n=18). Data analyzed by unpaired t test with Welch’s correction, *P=0.0023. C) Subjects with greater collagen deposition between muscle fibers have weaker quadriceps. CON (n=14) and OA (n=18). Data analyzed by two-tailed Pearson’s Product moment correlation.
The distributions of gene expression for both Connective Tissue Growth Factor (CTGF) (4.37, 3.36–6.56 vs 1.915, 1.71–3.87, P=0.007) and Transforming Growth Factor beta (TGFβ) (21.21, 10.25–47.91 vs 6.99, 1.93–18.76, P=0.015) differed in the vastus lateralis of OA subjects relative to control subjects [Fig. 4(A)]. The expression levels of both CTGF and TGFβ mRNAs positively correlated to the percentage of Sirius Red+ muscle area (r=0.54, 0.22 – 0.76, P=0.002 and r=0.45, 0.099 – 0.697, P=0.012 respectively) [Fig. 4 (B)]. Subjects with higher levels of CTGF and TGFβ also had weaker quadriceps (r=−0.61, −0.78 – −0.34, P <0.0001 and r=−0.45, −0.683 – 0.142, P=0.005 respectively) [Fig. 4 (C)].
Fig. 4.
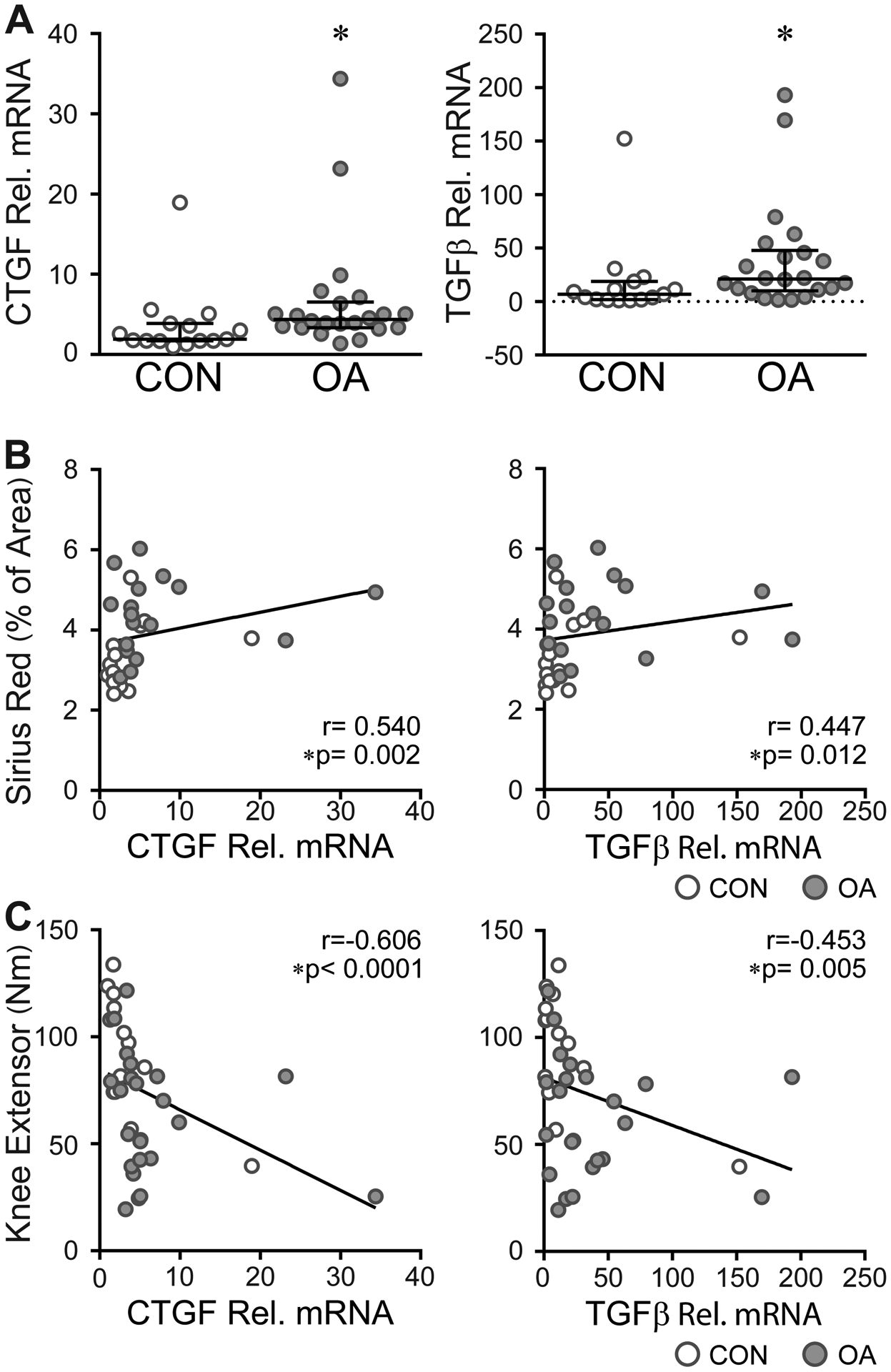
Differences in the expression of profibrotic genes in OA muscle. A) Bar graph showing the relative (rel.) mRNA expression of the profribrotic genes CTGF (left) and TGFβ (right) in control (CON) and OA muscle. Data are expressed as median with IQR; control (CON, n= 15) and OA (n= 22). Data analyzed by Mann-Whitney test, CTGF *P=0.0070, TGFβ *P=0.0152. B) Profibrotic gene expression is positively correlated to collagen deposition. CON (n=14), OA (n=18). C) Subjects with higher profibrotic gene expression have weaker quadriceps muscles. CON (n=15), OA (n=22). B-C) Data analyzed by two-tailed Spearman rho correlation.
Satellite cell density (Pax7+ per 100 fibers) was significantly reduced with OA relative to control (6.87, 5.79–7.95 vs 10.25, 8.44–12.06, P=0.002) [Fig. 5(A) and (B)], primarily due to a reduction in satellite cells associated with Type II muscle fibers in OA patients (5.297, 4.30–6.30 vs 7.13, 5.96–8.29, P=0.017) [Fig. 5 (C)]. There was a trend toward a reduction of satellite cells associated with Type I fibers as well; however, this reduction was not statistically significant (9.20, 7.56–10.84 vs 11.75, 9.38–14.12, P=0.068) [Fig. 5 (C)]. Overall satellite cell density inversely correlated to the percentage of Sirius Red+ muscle area (r=−0.41, −0.67 – −0.06, P=0.025) [Fig. 5 (D)]. This correlation was also significant for Type I (r=−0.41, −0.68 – −0.06, P=0.026) and Type II satellite cells (r=−0.44, −0.70 – −0.09, P=0.016) [Fig. 5 (E)] individually.
Fig. 5.
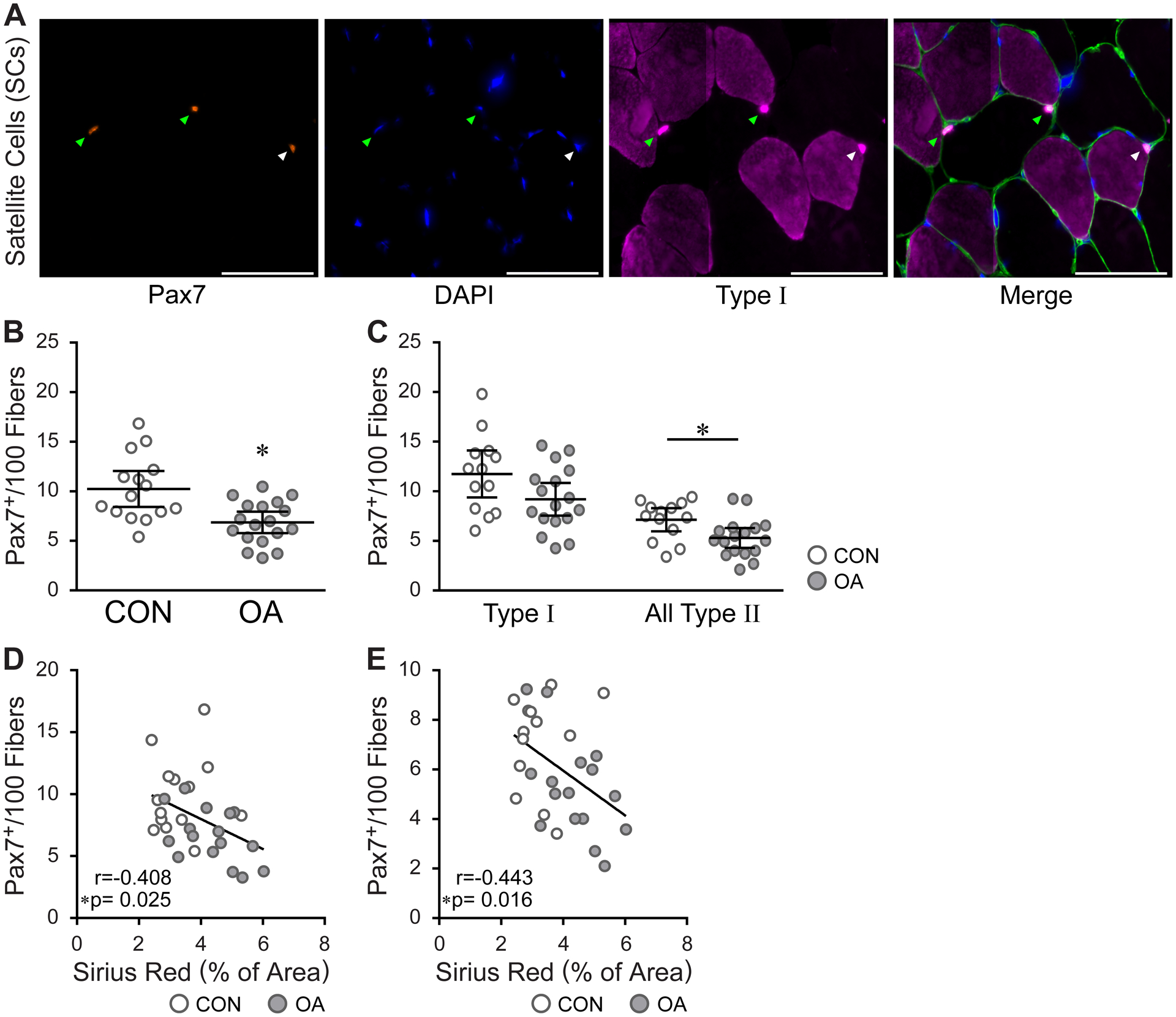
Decreased satellite cell number in the quadriceps of adults with knee OA. A) IHC showing fiber type specific identification of satellite cells; Pax7 (satellite cells, orange), DAPI (nuclei, blue), MyHC Type I (pink) and Laminin (green). Green arrowheads point to SCs (Pax7+) associated with myosin heavy chain MyHC Type II fibers, white arrowheads point to MyHC Type I SCs. Images were acquired at 20x. Scale bars = 100 μm. B) Bar graph quantification showing a reduction in satellite cell abundance in muscles from OA subjects. Control (CON, n=15), OA (n=18); *P=0.0023. C) Decreased abundance of satellite cells associated with Type II fibers in the quadriceps of OA subjects. CON (n=13), OA (n=17); Type I satellite cells, P=0.0683, Type II satellite cells, *P=0.0165. B-C) Data are expressed as mean ± 95% CI and analyzed by unpaired t test with Welch’s correction. D, E) Total satellite cell abundance (D) and Type II associated satellite cell abundance (E) are inversely correlated to ECM collagen content. Data analyzed by two-tailed Pearson’s Product moment correlation. D) CON (n=15), OA (n=18) and (E) CON (n=13), OA (n=17).
Discussion
The purpose of this study was to establish a cellular and molecular profile that provides insight into the pathology of muscle weakness resulting from mild to moderate radiographic and symptomatic knee OA. Muscle fiber CSA was similar in OA subjects compared to activity- and age-matched controls, but quadriceps strength was lower in the OA subjects. OA patients had fewer Type I fibers and more hybrid IIa/x fibers compared to healthy controls, pointing to a pathologically-mediated fiber type transition. OA subjects also presented with significantly more ECM, which may contribute to comparatively lower muscle strength. Fewer satellite cells in OA subjects, concomitant with greater profibrotic gene expression, is consistent with expanded ECM. This study provides the first cellular evidence for reduced whole muscle quality in moderate OA. This information may help guide future therapeutics aimed at reducing OA-mediated muscle weakness and dysfunction.
Only one previous report has assessed fiber type frequency in OA subjects relative to controls [20]. In agreement with our findings, the authors showed significantly more Type IIa/x hybrid fibers in OA. Additionally, we observed fewer Type I fibers in OA muscles relative to controls. Shifts in the frequency of muscle fiber types with OA are not without functional implications, as ~50% of the muscle fibers in a healthy vastus lateralis are Type I. A slow-to-fast fiber type transition could affect the performance of activities that require submaximal efforts [33]. A preponderance of Type IIa/x hybrid fibers is generally associated with disuse-mediated atrophy [34] and/or sedentary behavior [18]. However, since atrophy was not apparent in these OA subjects and activity level was the same relative to controls, these data indicate that the observed fiber type transition has a different etiology. Following extensive rehabilitation and recovery after ACL injury, elevated Type IIa/x fiber percentage persists in the vastus lateralis, suggesting that knee joint alterations may result in persistent and pathological fiber type changes within the muscle [19]. Interestingly, we found that Type IIa/x percentage negatively correlated with quadriceps strength. Type IIa/x expression could point to early denervation events, which may contribute to muscle weakness [28]. Neurogenic muscle atrophy and subsequent fiber type grouping has also been shown in end-stage OA [35]. While more work is necessary to determine the cause of OA-mediated fiber type transitioning, it appears that the OA fiber type profile is symptomatic of intrinsic pathological changes within the muscle, and could contribute to overall muscle dysfunction.
In contrast to previous reports in end-stage knee OA [28, 36], muscle fiber atrophy does not appear characteristic of moderate OA. However, we report for the first time that ECM is significantly expanded in subjects with moderate OA relative to controls, potentially limiting the regenerative capacity of the muscle. In support of this we found a negative association between quadriceps strength and fibrotic collagen deposition. In agreement with these findings, it was recently shown in an animal model that excessive fibrosis can impair whole muscle strength [21]. Furthermore, differences in gene transcription of CTGF and TGFβ in OA patients suggest a signaling pathway potentially activated in OA that may contribute to increased fibrosis. TGFβ is a hormone-like cytokine that is a key regulator of ECM remodeling, and CTGF cooperates with TGFβ to promote collagen production from fibroblasts [37]. Collectively, these data suggest that mild to moderate radiographic OA is characterized by a profibrotic environment. We speculate that the pro-fibrotic milieu in in this stage of OA may contribute to atrophy that manifests in the later stages of OA [28, 36]. Whether physical training is able to reverse or slow the accumulation of collagen in the ECM is not yet known. OA-mediated ECM deposition could potentially influence the results of intervention programs in this population, which emphasizes the need for early treatment of OA.
Satellite cell density was significantly lower in subjects with OA, and overall muscle collagen content was inversely related to satellite cell density. Evidence from animal models indicates a strong reciprocal relationship between satellite cells and ECM components [21, 24, 27, 38], particularly fibroblasts and the collagens they secrete [27, 38]. The current study provides the first human evidence that fewer satellite cells is associated with greater collagen content. In addition to ECM alterations, changes in satellite cell density in OA patients could affect skeletal muscle adaptive potential and limit the effectiveness of strength training interventions. A significant reduction in Type II satellite cell density with OA could also contribute to Type II specific impairments observed in later stages of OA [20, 39]. Recent work has suggested that satellite cell phenotype may dictate the regenerative potential of muscle in patients with OA [40]. Along with decreased satellite cell abundance, alterations in the phenotype of satellite cell populations may be an important contributing factor to muscle alterations in late stage OA [40, 41]. Therapeutic targets that address satellite cell phenotype, abundance and ECM deposition may prove effective at increasing quadriceps strength within this cohort.
This study is not without study design constraints and limitations. As this study is cross sectional nature we are unable to establish a cause and effect relationship between the observed cellular alterations in muscle and subsequent atrophy in later stages of the disease. Further we acknowledge that while the subjects had similar PASE scales it is possible that there could be variation in the amounts and types activities the two groups were selecting. Future work, matching subjects using activity trackers maybe one method of addressing this limitation. Lastly, due the small sample size there were wide CIs for some of the performed analyses.
Our findings emphasize the need for more in depth studies extending beyond muscle atrophy to improve our understanding of alterations within the quadriceps in early OA. Determining cellular changes within muscle that contribute to the weakness associated with OA is fundamental to the development of effective therapeutic interventions that can increase the force generating capacity of the quadriceps and counteract pathology. These interventions may also have an important chondroprotective effect on an already damaged joint [42]. Future studies should consider the effects of the cellular adaptations reported here on whole muscle properties such as endurance and power.
Supplementary Material
Acknowledgements
The authors thank Ryan Williamson, Colleen Benish, and Seth Walsh Blackmore for assistance with WGA staining and muscle CSA analysis. The authors also thank Doug Long for recruitment and testing of subjects at the University of Kentucky Center for Muscle Biology. The authors also thank Jovita Newman, START project manager at Wake Forest University, for facilitating the coordination and processing of data between the two testing sites. Lastly, the authors would like to thank Daniel Beavers from Wake Forest University for the statistical advice and manuscript review.
Role of funding source
This study was supported by National Institutes of Health grants 3R01AR059105-03S1, AR062069, and UL1TR000117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing Interests
The authors have no competing interests.
References
- 1.Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morb Mortal Wkly Rep 2001; 50: 120–125. [PubMed] [Google Scholar]
- 2.Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morb Mortal Wkly Rep 2009; 58: 421–426. [PubMed] [Google Scholar]
- 3.Allen KD, Golightly YM. Epidemiology of osteoarthritis: state of the evidence. Current opinion in rheumatology 2015; 27: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neogi T. The Epidemiology and Impact of Pain in Osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2013; 21: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maly MR. Abnormal and cumulative loading in knee osteoarthritis. Curr Opin Rheumatol 2008; 20: 547–552. [DOI] [PubMed] [Google Scholar]
- 6.Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol 2006; 18: 514–518. [DOI] [PubMed] [Google Scholar]
- 7.Segal NA, Glass NA. Is quadriceps muscle weakness a risk factor for incident or progressive knee osteoarthritis? Phys Sportsmed 2011; 39: 44–50. [DOI] [PubMed] [Google Scholar]
- 8.O’Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis 1998; 57: 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurley MV. Quadriceps weakness in osteoarthritis. Curr Opin Rheumatol 1998; 10: 246–250. [DOI] [PubMed] [Google Scholar]
- 10.Messier SP, Loeser RF, Hoover JL, Semble EL, Wise CM. Osteoarthritis of the knee: effects on gait, strength, and flexibility. Arch Phys Med Rehabil 1992; 73: 29–36. [PubMed] [Google Scholar]
- 11.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, et al. Quadriceps Weakness and Osteoarthritis of the Knee. Annals of Internal Medicine 1997; 127: 97–104. [DOI] [PubMed] [Google Scholar]
- 12.Bennell KL, Hunt MA, Wrigley TV, Lim BW, Hinman RS. Role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am 2008; 34: 731–754. [DOI] [PubMed] [Google Scholar]
- 13.Hassan BS, Doherty SA, Mockett S, Doherty M. Effect of pain reduction on postural sway, proprioception, and quadriceps strength in subjects with knee osteoarthritis. Ann Rheum Dis 2002; 61: 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riddle DL, Stratford PW. Impact of pain reported during isometric quadriceps muscle strength testing in people with knee pain: data from the osteoarthritis initiative [corrected] [published erratum appears in PHYS THER 2011; 11(1):1696]. Physical Therapy 2011; 91: 1478–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conroy MB, Kwoh CK, Krishnan E, Nevitt MC, Boudreau R, Carbone LD, et al. Muscle strength, mass, and quality in older men and women with knee osteoarthritis. Arthritis Care Res (Hoboken) 2012; 64: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segal NA, Findlay C, Wang K, Torner JC, Nevitt MC. The longitudinal relationship between thigh muscle mass and the development of knee osteoarthritis. Osteoarthritis and Cartilage 2012; 20: 1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. International review of cytology 1997; 170: 143–223. [DOI] [PubMed] [Google Scholar]
- 18.Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, et al. Muscle fiber type is associated with obesity and weight loss. American Journal of Physiology-Endocrinology and Metabolism 2002; 282: E1191–E1196. [DOI] [PubMed] [Google Scholar]
- 19.Noehren B, Andersen A, Hardy P, Johnson DL, Ireland ML, Thompson KL, et al. Cellular and Morphological Alterations in the Vastus Lateralis Muscle as the Result of ACL Injury and Reconstruction. J Bone Joint Surg Am 2016; 98: 1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callahan DM, Tourville TW, Miller MS, Hackett SB, Sharma H, Cruickshank NC, et al. Chronic disuse and skeletal muscle structure in older adults: sex-specific differences and relationships to contractile function. Am J Physiol Cell Physiol 2015; 308: C932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, et al. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 2014; 28: 1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serrano AL, Muñoz-Cánoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Experimental cell research 2010; 316: 3050–3058. [DOI] [PubMed] [Google Scholar]
- 23.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 2011; 138: 3647–3656. [DOI] [PubMed] [Google Scholar]
- 24.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 2011; 138: 3625–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepper C, Partridge TA, Fan C-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011; 138: 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 2011; 138: 3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy. Cell Stem Cell 2017; 20: 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callahan DM, Tourville TW, Slauterbeck JR, Ades PA, Stevens-Lapsley J, Beynnon BD, et al. Reduced rate of knee extensor torque development in older adults with knee osteoarthritis is associated with intrinsic muscle contractile deficits. Exp Gerontol 2015; 72: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messier SP, Mihalko SL, Beavers DP, Nicklas BJ, DeVita P, Carr JJ, et al. Strength Training for Arthritis Trial (START): design and rationale. BMC Musculoskelet Disord 2013; 14: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellgren J, Lawrence J. Radiological assessment of osteo-arthrosis. Annals of the rheumatic diseases 1957; 16: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergström J. Muscle electrolytes in man. Scandinavian Journal of Clinical Laboratory Investigation 1962; 68: 7–110. [Google Scholar]
- 32.Emde B, Heinen A, Godecke A, Bottermann K. Wheat germ agglutinin staining as a suitable method for detection and quantification of fibrosis in cardiac tissue after myocardial infarction. Eur J Histochem 2014; 58: 2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zierath JR, Hawley JA. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS biology 2004; 2: e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagley J, Murach K, Trappe S. Microgravity-Induced Fiber Type Shift in Human Skeletal Muscle. Gravitational and Space Biology 2012; 26: 34–40. [Google Scholar]
- 35.Fink B, Egl M, Singer J, Fuerst M, Bubenheim M, Neuen‐Jacob E. Morphologic changes in the vastus medialis muscle in patients with osteoarthritis of the knee. Arthritis & Rheumatism 2007; 56: 3626–3633. [DOI] [PubMed] [Google Scholar]
- 36.Levinger I, Levinger P, Trenerry MK, Feller JA, Bartlett JR, Bergman N, et al. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis & Rheumatism 2011; 63: 1343–1348. [DOI] [PubMed] [Google Scholar]
- 37.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, et al. Role and interaction of connective tissue growth factor with transforming growth factor‐β in persistent fibrosis: a mouse fibrosis model. Journal of cellular physiology 1999; 181: 153–159. [DOI] [PubMed] [Google Scholar]
- 38.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nature communications 2013; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Callahan DM, Miller MS, Sweeny AP, Tourville TW, Slauterbeck JR, Savage PD, et al. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol 2014; 592: 4555–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scimeca M, Bonanno E, Piccirilli E, Baldi J, Mauriello A, Orlandi A, et al. Satellite Cells CD44 Positive Drive Muscle Regeneration in Osteoarthritis Patients. Stem Cells Int 2015; 2015: 469459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scimeca M, Piccirilli E, Mastrangeli F, Rao C, Feola M, Orlandi A, et al. Bone Morphogenetic Proteins and myostatin pathways: key mediator of human sarcopenia. Journal of Translational Medicine 2017; 15: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennell KL, Hunt MA, Wrigley TV, Lim BW, Hinman RS. Muscle and exercise in the prevention and management of knee osteoarthritis: an internal medicine specialist’s guide. The Medical clinics of North America 2009; 93: 161–177, xii. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


