Abstract
Summary
Background:
Development and validation of a quantitative radiomic risk score (QuRiS) and associated nomogram (QuRNom) for early-stage non-small cell lung cancer (ES-NSCLC) that is prognostic of disease-free survival (DFS) and predictive of the added benefit of adjuvant chemotherapy (ACT) following surgery.
Methods:
QuRiS was developed using radiomic texture features derived from within and outside the primary lung nodule on chest CT scans using a cohort D1 of 329 patients from the Cleveland Clinic. A LASSO-Cox regularization model was used for data dimension reduction, feature selection, and QuRiS construction. QuRiS was independently validated on D2(N=114; University of Pennsylvania) and D3(N=82; TCIA). QuRNom was constructed by integrating QuRiS with T-, N-Descriptors, and LVI. The added benefit of ACT using QuRiS and QuRNom was validated by comparing patients who received ACT against patients who underwent surgery alone in D1-D3. To explore the underlying morphologic basis of the QuRiS, we explored associations with corresponding whole-slide tissue scans (WSIs) and mRNA sequencing data using subsets of D1 and D3.
Findings:
QuRiS consisted three intra- and ten peri-tumoral CT-radiomic features and was found to be significantly associated with DFS (D1: HR=1.60 [1.10–2.20];p<·05; D2:HR=2.70 [1.40–5.10]; p<·01; D3:HR=2.70 [1.20–5.70];p<·01). Patients were partitioned into three risk groups (QH, QI, QL) based off their corresponding QuRiS score. High QuRiS group, QH, patients were observed to have significantly prolonged survival with ACT when compared to surgery alone (D1: HR=0·27[0.07–0.95],p<0.05; D2+D3: HR=0·08[0.01–0.42],p<0.01). For developed QuRNom, the actual efficacy of ACT was predictive of nomogram-estimated survival benefit (D1: HR= D1:0·25 [0·12–0·55], D3: HR=0·13 [0·004–0·99]). QuRiS features were found to be associated with the spatial arrangement of TILs and cancer nuclei on corresponding WSIs (D1: Rho=0·23,p<0·05, N=70). They were also observed to have an association with biological pathways implicated in chemotaxis (D3,p<0·05, N=86) and other immune specific biological pathways.
Interpretation:
QuRiS and QuRNom were validated as being prognostic of DFS and predictive of the added benefit of ACT.
Keywords: Lung Cancer, Adjuvant chemotherapy, Predictive Biomarker, Radiomics, Nomogram, Risk-Stratification, Disease Free Survival, Pathomics, Radiogenomics
Introduction
International Association for the study of Lung Cancer (IASLC)(1) derived pathological staging system is considered the gold standard in treatment planning in non-small cell lung cancers (NSCLC). Stages I, II NSCLC are considered early stage (ES), and surgical resection is considered the standard of care doe these patients(2). In addition, adjuvant cisplatin-based chemotherapy (ACT) is currently recommended for Stage-II patients, while stage-I patients continue to be treated with surgery alone(2–5). These results are based on large prospective clinical trials showing a 5-year overall survival (OS) benefit of 4% for International Adjuvant Lung Cancer Trial (IALT, N=1867)(4) and 15% for JBR.10 (N=482)(5) in the ACT group as compared to surgery alone. The Lung Adjuvant Cisplatin Evaluation (LACE)(6) –a pooled meta-analysis that includes 4584 patients across five clinical trials revealed both a 5-year survival absolute benefit of 5·4% from ACT and the relative benefit with HR (ACT vs no ACT) of 0.89 (95% CI: 0.82– 0.95, p = 0.005). Interestingly, some other clinical trials, including the Big Lung Trial (BLT; N=381)(7) and Adjuvant Lung Cancer Project Italy (ALPI; N=1088)(8) have shown no statistically significant benefit for either 5-year OS or disease-free survival (DFS). This seems to suggest the need for a biomarker that could identify the patients who would benefit from ACT.
More importantly, stratified subgroup analysis of these trials based on the 8th edition TNM stage meanwhile has shown that ACT does not show significant improved OS in stage-IB (T2aN0M0) patients (HR=0·93; 95% CI:0·78–1·10). The CALGB9633 trial (N=344)(9) which included only stage-IB patients showed no improved survival benefit with ACT (HR=0·83(95% CI:0·64–1·08, P=0·12) with a median follow-up time of 74 months. Based on the lack of significant survival benefit demonstrated in Stage-I (and sometimes detrimental survival in Stage-IA: HR>1) with ACT, currently ACT is not the recommended treatment. For stage IB patients, ASCO guidelines do not recommend ACT following surgery in routine practice. However, National Comprehensive Cancer Network (NCCN) guidelines suggest that ACT is an appropriate option when considering high-risk factors like poorly differentiated tumors, vascular invasion, wedge resection, tumor size >4 cm, and pleural vascular invasion.
Even after curative resection, about 30 to 40% of Stage-I patients tend to recur with observation only. This appears to suggest that these are high-risk patients who are at an increased risk of disease recurrence and, therefore, might benefit from ACT. Along with traditionally clinicopathologic factors, single and multi-gene based expression assays have shown prognostic value in ES-NSCLC, but only a select few have been shown to be predictive of identifying which patients might derive added benefit from ACT(10,11).
Recently there has been an increasing interest in the use of computed tomography (CT) based radiomics(12) (computer-extracted quantitative imaging features derived from radiographic images) in lung cancer for disease diagnosis and prognosis(12–16). A few groups have recently shown that radiomics based prognostic biomarkers can predict disease recurrence and survival in the context of NSCLC, but most of them are limited in clinical utility by being “black-box” approaches, lacking an underlying biological rationale, especially deep learning-based models. Although Radiomics can be considered as a back-box approach itself, they offer the opportunity to study the association between specific image features with underlying morphologic and molecular attributes of the disease, thereby providing a stronger correlative link with the underlying tumor biology. In our work, we have tried to explore biological underpinning by exploring the association with genomic and pathomic analysis. More critically; none of these approaches has been evaluated in their ability to predict the added benefit of adjuvant chemotherapy in ES-NSCLC.
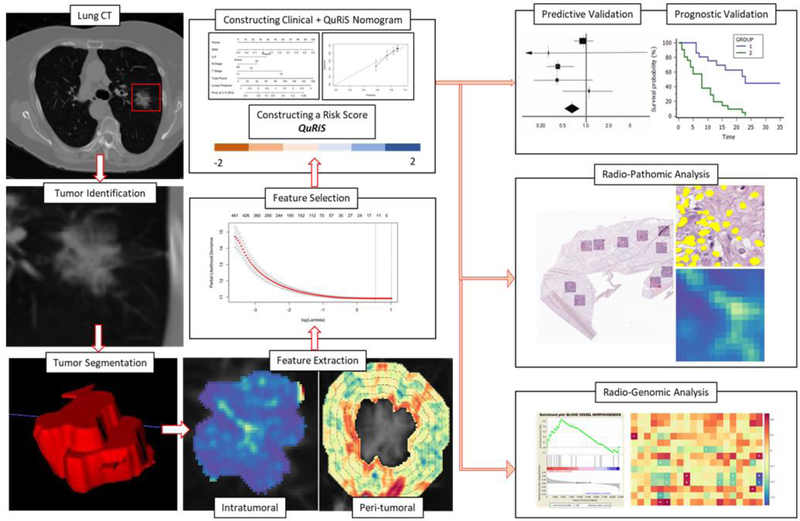
In this study, we have constructed a quantitative Radiomic Risk Score (QuRiS), which employs 13 quantitative texture features from within and outside the primary lung nodule derived from routine CT scans to predict DFS and response to ACT for Stage I, II NSCLC patients. Multiple studies(17,18) have suggested that for clinical decision making, signatures derived from a combination of biomarkers may be more prognostic and predictive compared to considering individual analytical approaches. Consequently, in this work, we constructed a nomogram (QuRNom) integrating QuRiS with T-, N- descriptor, and lymphovascular invasion for DFS estimation. Using a total of 525 ES-NSCLC patients derived from three independent sites, treated either with surgery+ACT or surgery alone, we sought to demonstrate that QuRiS and QuRNom were both a) prognostic of DFS and b) associated with the added benefit of ACT across multiple sites for ES-NSCLC. Additionally, to evaluate the morphological underpinning of the radiomic features, we studied the association between QuRiS features with the spatial architecture and arrangement of cancer nuclei and tumor-infiltrating lymphocytes(TILs) derived from Hematoxylin and Eosin (H&E) tissue images of corresponding surgical specimens. We also investigated the association between QuRiS features and underlying biological pathways in cancer progression by using mRNA sequencing gene-expression data.
PATIENTS AND METHODS
Study design and data sources
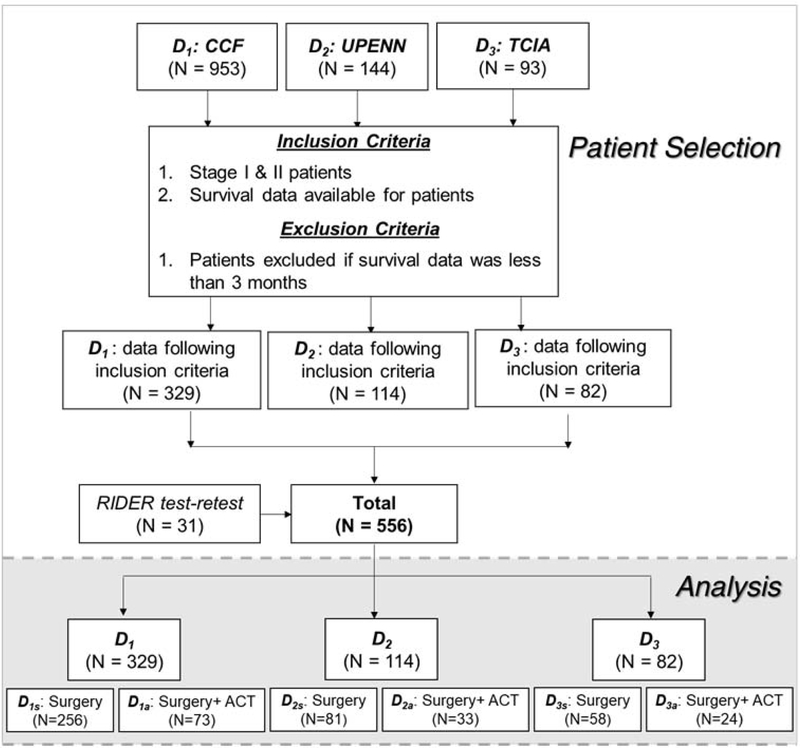
An institutional review board (IRB) approved protocol was used for this retrospective cohort study with an informed consent requirement waived by the IRB. Retrospective chart review of patients admitted in the Cleveland Clinic Foundation (CCF) with NSCLC between 2005–15 yielded 700 patients. All resected stage I and II NSCLC with available diagnostic CT scans were included in the present study after pathological confirmation (CONSORT flow diagram, Fig 1.), rendering 329 ES-NSCLC (D1) patients suitable for the analysis, after applying necessary inclusion and exclusion criteria. Out of these, 73 patients received ACT.
Figure 1:
Data source and CONSORT diagram for patient selection
Further multi-site validation was carried out using two independent cohorts D2 (N=114) obtained from a retrospective chart review of N=128 NSCLC cases continuously admitted at the University of Pennsylvania (UPenn) from 2005–15 applying the necessary inclusion and exclusion criteria. TCIA dataset was used to obtain D3. After applying the essential inclusion and exclusion criteria, including the availability of diagnostic CT scans on the Cancer Imaging Archive (TCIA), which yielded N=82. In D2, 33 out of 114 patients received ACT (D2a), while 81 patients underwent surgery alone (D2s). In D3, 58 patients underwent surgery alone (D3s), while 24 out of 82 patients received ACT (D3a).
The primary endpoint for the study was DFS which was measured from the date of surgery to the time of disease relapse (tumor recurrence within or immediately adjacent to the treated field, mediastinal relapse, distant relapse) or the time of death, whichever was earlier and was censored at the date of last follow-up for patients who were alive and did not relapse(19). For stage I and II cancers, DFS specifically focuses on disease-specific survival since these patients have comparatively longer OS. Therefore, in this study, we specifically focused on the analysis with respect to DFS.
Procedures
Radiomic features were extracted to capture textural heterogeneity from within and outside and the nodules from the chest CT scans for all early-stage lung cancer patients (Supplementary-1 for CT scan parameters). An experienced cardiothoracic radiologist (PR) with 12 years of experience identified the target nodule on CT scans in D1, D2. A second cardio-thoracic radiologist (RG) with 22 years of experience identified the corresponding nodules on the scans in D3. The nodule was further manually segmented across all the sections where it was visible in the axial view using a manual annotation tool in open-source software (3D Slicer, version 4·6: NIH-funded; https://www.slicer.org). These annotations were considered for intranodular and perinodular radiomic analysis.
Following annotations, the peritumoral mask was selected using morphological dilation and erosion operations (supplement-1). A radial distance of 15mm outside the nodule in an annular 3-mm ring shaped fashion was selected for extracting peritumoral features.
Quantitative feature extraction
Radiomic features
Radiomic features from Gabor, Haralick, Laws, Laplace, and Collage feature families were extracted from each annotated region. To ensure the stability and reproducibility of radiomic features, we used the test-retest Reference Image Database to Evaluate Response (RIDER) lung CT dataset(20). Details are described in supplement-1.
Constructing the QuRiS Risk Score
The least absolute shrinkage and selection operator (LASSO) method, which is suitable for high-dimensional data(21), was used to select the most useful predictive features from the discovery cohort D1s (N=256). QuRiS was calculated for each patient via a linear combination of selected features that were weighted by their respective coefficients. The value of the tuning parameter in the LASSO-Cox model (lambda) was averaged out by 10-fold cross-validation to minimize error. (supplement-1)
Statistical Analysis
To show the incremental value of QuRiS to significant clinicopathologic factors for personalizing DFS prediction, a QuRiS based nomogram, QuRNom, was constructed using the discovery cohort(22). The QuRNom comprised the radiomics model as well as significant clinic-pathological risk factors found on multivariate Cox Regression analysis.
For prognostic stratification, QuRiS was divided into two groups using DFS and hazard ratios using the discovery cohort D1 [procedure for selecting the threshold in supplement-1]. The prognostic performance was validated using Kaplan-Meier (KM) survival analysis, log-rank test, hazard ratio, and C-index, along with its confidence interval (CI). Univariate analysis of QuRiS and the clinic-pathologic variables was carried out. Multivariable Cox-regression analysis was performed to determine the relationships between the various covariates and 3-year DFS while controlling for baseline factors. Further subset analysis involved determining survival differences in different stages. To measure nomogram performance, Harrell’s Concordance indices (CIndex) were calculated from the nomogram for QuRiS, clinical factors alone, and QuRiS+clinical factors. The calibration plot for the nomogram was plotted by reviewing the plots of nomogram-predicted survival probabilities with KM estimated probabilities. Bootstraps with 1,000 resamples were used to quantify model overfitting and calculate Kaplan-Meier estimates. A decision curve analysis was performed to assess the clinical usefulness of the QuRiS nomogram by estimating the net benefits at different thresholds.
For predictive validation, QuRis was divided into three different risk groups (QH, QI, QL), the thresholds of which were identified by using D1a and D1s and hazard ratios to predict 3-years’ DFS. Forest plots were constructed to show the HRs comparing DFS between the ACT group and the surgery alone group in all the cohorts amongst the QuRis risk groups. For predictive validation of combined QuRNom, nomogram estimated survival probabilities were used for comparing HRs between ACT and surgery alone groups.
For finding the histomorphometric correlation of QuRiS, features explaining TIL-nuclei interplay were extracted from whole-slide tissue scans (WSIs)(23) as explained in supplement-2. Radiogenomic analysis was performed using mRNA sequencing data obtained from Illumina Genome Analyzer sequencing(24). The detailed procedure is explained in supplement-3. The statistical analysis was performed using R version 3·5·3. The statistical methods are further detailed in the supplementary materials.
RESULTS
D1 (N=330) from CCF was used as the discovery cohort to train QuRiS and to construct QuRNom. For external validation of QuRiS and QuRNom as prognostic as well as predictive biomarkers, D2 and D3 were used. Table-1 describes the individual cohorts along with baseline demographics and treatment-related information.
Table 1:
Dataset Description
| Variables | Sub-variables | CCF (D1) (N%) | UPenn (D2) (N%) | TCGA (D3) (N%) | P- value |
|---|---|---|---|---|---|
| Number of patients | 329 | 114 | 82 | ||
| Treatment | Surgery Alone | 256 (D1s) (77·81%) | 81 (D2s) (71·05%) | 58 (D3s) (70·73%) | p>0·05 |
| ACT | 73 (D1a) (22·18%) | 33 (D2a) (28·94%) | 24 (D2a) (29·26%) | ||
| Age at Dx | <=65 | 86 (26·14%) | 46 (40·35%) | 34 (41·46%) | p>0·05 |
| >65 | 142 (43·16%) | 68 (59·65%) | 48 (58·53%) | ||
| Unknown | 101 (30·69%) | 0 (0%) | 0 (0%) | ||
| Gender | Male | 115 (34·95%) | 38 (33·33%) | 56 (68·29%) | p>0·05 |
| Female | 113 (34·34%) | 76 (66·66%) | 26 (31·70%) | ||
| Unknown | 101 (30·69%) | 0 (0%) | 0 (0%) | ||
| Smoking Status | Current | 85 (25·83%) | 6 (5·26%) | 17 (20·73%) | p>0·05 |
| Previous Use | 209 (63·52%) | 82 (71·93%) | 47 (57·32%) | ||
| Never Used | 32 (9·73%) | 26 (22·80%) | 18 (21·95%) | ||
| Unknown | 3 (0·91%) | 0 (0%) | 0 (0%) | ||
| Stage | Stage 1 | 276 (83·89%) | 89 (78·07%) | 57 (69·51%) | p>0·05 |
| Stage 2 | 53 (16·11%) | 33 (28·94%) | 25 (30·48%) | ||
| LVI | Present | 98 (29·79%) | Unknown | 5 (6·09%) | P>0·05 |
| Absent | 214 (65·04%) | 74 (90·24%) | |||
| Unknown | 17 (5·16%) | 3 (3·65%) | |||
| N-descriptor | 0 | 255 (77·51%) | Unknown | 69 (84·14%) | p>0·05 |
| 1 | 46 (13·98%) | 13 (15·85%) | |||
| Unknown | 28 (8·51%) | 0 (0%) | |||
| T- descriptor | 1 | 158 (48·02%) | Unknown | 38 (46·34%) | p>0·05 |
| 2 | 88 (26·74%) | 31 (37·80%) | |||
| 3 | 8 (2·43%) | 13 (15·85%) | |||
| Unknown | 75 (22·79%) | 0 (0%) | |||
| Margin Status | Negative | 323 (98.17%) | 144 (100%) | Unknown | P<0.05 |
| Positive | 6 (1.82%) | 0 (0.00%) | |||
| Tumor | RUL | 128 (38.90%) | 35 (30.70%) | 26 (31.71%) | P<0.05 |
| Location | RLL | 45 (13.67%) | 22 (19.29%) | 11 (13.41%) | |
| RML | 15 (4.56%) | 4 (3.51%) | 8 (9.75%) | ||
| LUL | 94 (28.57%) | 32 (28.07%) | 23 (28.05%) | ||
| LLL | 47 (14.28%) | 21 (18.42%) | 14 (17.07%) | ||
| Type of | Lobectomy | 64 (19.45%) | 55 (48.24%) | Unknown | P<0.05 |
| surgery | Sublobar | 234 (71.12%) | 49 (42.98%) | ||
| Pneumonectomy | 15 (4.56%) | 1 (0.88%) | |||
| segmentectomy | 16 (4.86%) | 0 (0.00%) | |||
| EGFR | Mutatnt | 25 (7.59%) | 36 (31.58%) | 17 (20.73%) | P<0.05 |
| Mutation | Wildtype | 95 (28.87%) | 78 (68.42%) | 54 (65.85%) | |
| Unknown | 209 (63.52%) | 0 (0.00%) | 11 (13.41%) | ||
| KRAS | Mutatant | 2 (0.61%) | 32 (28.07%) | 17 (20.73%) | P<0.05 |
| Mutation | Wildtype | 3 (0.91%) | 82 (71.93%) | 54 (65.85%) | |
| Unknown | 324 (98.48) | 0 (0.00%) | 11 (13.41%) | ||
| Histology | Adenocarcinoma | 211 (64·13%) | 114 (100%) | 82 (100%) | P>0·05 |
| Squamous cell carcinoma | 93 (28·26%) | 0 (0.00%) | 0 (0%) | ||
| Other | 21 (6·38%) | 0 (0.00%) | 0 (0%) | ||
| Unknown | 4 (1·21%) | 0 (0.00%) | 0 (0%) | ||
| Recurrence | Recurrence | 71 (21·58%) | 25 (21·92%) | 26 (31·70%) | p>0·05 |
| Non-Recurrence | 258 (78·41%) | 89 (78·07%) | 56 (68·29%) |
QuRiS and QuRNom independently predict DFS in training and validation sets
The thirteen most discriminative features were selected to construct the QuRiS using LASSO analysis. QuRiS could predict DFS on D1 (HR = 1·60 [95% CI: 1·10–2·20], N = 329) D2 (HR = 2·70 [95% CI:1·20–5.70], N=114) and D3 (HR = 2·70 [95% CI: 1·40–5.10], N=82). Within the patients who received surgery alone, we noticed two definite groups with high and low risk (Fig. 3b). Whereas, in the ACT group, patients showed no significant difference between high- and low-risk groups, potentially suggesting that this cohort had a subset of patients that received benefit from chemotherapy and had better survival (Fig. 3c).
Figure 3:
Kaplan-Meier survival curves for QuRiS on patients a) for entire cohorts, b) patients who received surgery alone on (c) patients with surgery + ACT for differentiating long-term and short-term DFS. The threshold was developed using training cohort ai) D1 (N=329). bi) Subset analysis on patients who received surgery alone D1s (N=256) and ci) patients who received surgery + ACT D1a (N=73). The similar performance was observed using the same threshold on two independent validation cohorts. Validation cohort-1 using aii) D2 (N=144) bii) D2s (N=81) and cii) D2a (N=33). Validation cohort-2 using aii) D3 (N=82) bii) D3s (N=58) and cii) D3a (N=25). In the surgery alone group, there were two distinctive subsets, one with poor disease-free survival indicating high risk of recurrence. QuRiS was able to define high-risk group from this clinically defined low-risk cohort who received surgery and potentially would benefit from adjuvant-chemotherapy. In the ACT subgroup analysis, we did not see any significant difference between high-risk and low-risk groups, potentially suggesting this group had a subset of patients who received benefit from ACT and had better survival.
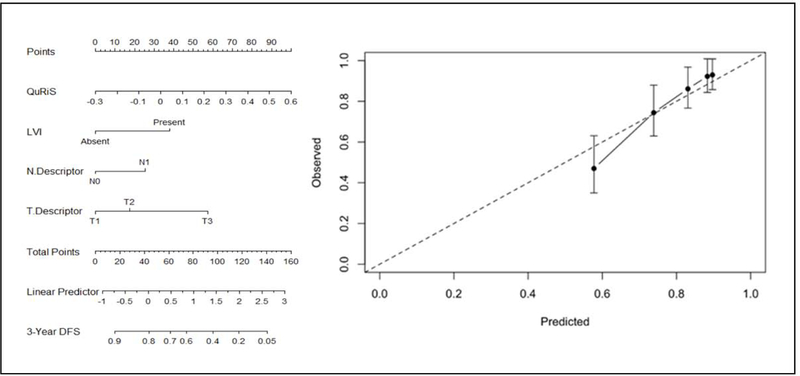
Univariate analysis on the training cohort revealed T-descriptor, N-descriptor, LVI status, pathological stage, and margin status to be the prognostic factors amongst all clinicopathologic factors (supplement-1). We included T-, N- descriptor, and LVI status along with QuRiS to construct QuRNom for predicting 3-year DFS. (Fig. 4). We could not include margin status as another independent parameter in the nomogram since almost all the patients had a negative margin. The stage was mainly a representation of T- and N- descriptors hence excluded from the final model.
Figure 4:
Use of constructed QuRiS and clinical nomogram to estimate DFS for stage I, II lung cancer along with the assessment of the model calibration. a) The nomogram was developed using D1s (N=257) using QuRiS, lymphovascular invasion, N-descriptor and T-descriptor. b) Calibration curve for QuRNom which depicts the calibration of the model in terms of the agreement between the predicted DFS and observed DFS. The y-axis represents the actual DFS and x-axis represents observed DFS. The black solid line represents the performance of the nomogram, of which a closer fit to the diagonal dotted line represents a better prediction.
The calibration curve for QuRNom for predicting 3-year DFS demonstrated agreement between prediction survival and actual survival. The Hosmer-Lemeshow test yielded a non-significant statistic (P >0·05), suggesting that there was no departure from a fit. The C-index for QuRNom was 0·74 (95% CI, 0·72–0·76), which was confirmed via bootstrap validation. QuRNom showed significantly superior performance to that of the 8th edition TNM classification (C-Index-0·65; 95% CI, 0·64–0·66) and a combined clinical-pathologic model (C-Index 0·71; 95% CI, 0·690·72).
QuRiS and QuRNom predicts added benefit of adjuvant chemotherapy in training and validation sets
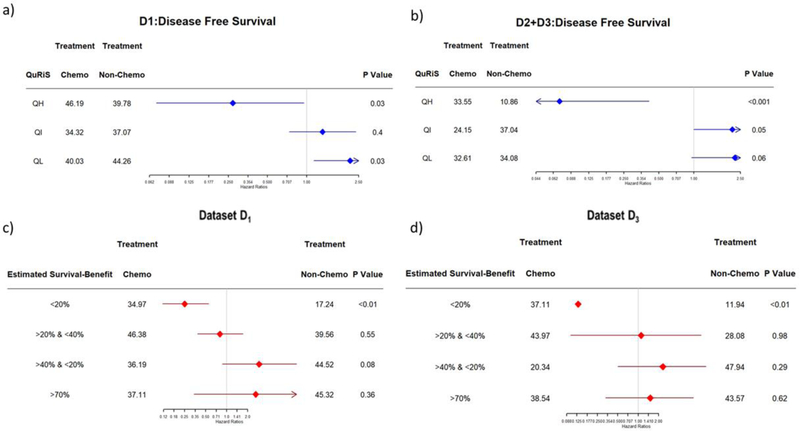
For predictive validation, QuRiS was classified into three risk groups (High (QH), Intermediate (QI), and Low (QL)) based on hazard ratios from the D1 cohort (supplement-1 for threshold selection).
In the QH, patients showed significantly prolonged survival when patients received ACT, whereas there was no added benefit and in fact, even showed negative effects of ACT on the QL group in both training and validation cohorts (Table-2 and Fig. 5(a)). Stratified subgroup analysis within stage 1b showed a similar difference in treatment-related effects between different QuRiS groups [supplement-1].
Table 2:
Treatment interaction with the QuRiS for DFS in patients with stage I and II lung cancer. High QuRiS score was associated with significant benefit from ACT, whereas low QuRiS in fact showed negative effect of ACT in training as well as both validation groups.
| D1 | D2 + D3 | ||||
| QuRiS | HR (Surgery Alone Vs. Adj. Chemo) | P-Value | QuRiS | HR (Surgery Alone Vs. Adj. Chemo) | P-Value |
| QH | 0.27 [0.07–0.95] | 0.03 | QH | 0.07 [0.01–0.41] | <0.001 |
| QI | 1.32 [0.73–2.38] | 0.4 | QI | 2.13 [0.99–4.54] | 0.06 |
| QL | 2.15 [1.13–2.15] | 0.02 | QL | 2.24 [0.95–5.30] | 0.06 |
Figure 5:
Hazard ratio comparing DFS between ACT and surgery alone group based on QuRiS a) on training and b) Validation cohorts. Fig 5c) and d) represents the nomogram estimated survival benefit from ACT. The estimated survival benefit of ACT was calculated as the difference between nomogram estimated 3-year DFS rates between ACT and surgery alone groups. In c) training as well as d) validation sets, the nomogram-estimated survival benefit from ACT was positively associated with the magnitude of improved DFS among patient treated with ACT.
Further, combined QuRNom was used for predicting the added benefit of ACT. The QuRNom estimated survival benefit was used for differentiating patients into different subgroups (Group1: survival benefit <20% (QNH), Group2: survival benefit >20% & <40% (QNIH), Group3: survival benefit >40% & <70% (QNIL), Group4: survival benefit >70% (QNL)). QNH showed an improved median DFS of about 17 months in D1 (HR=0·27, p=0·02) and 27 months in D3 (HR=0·09, p=0·01). Meanwhile, in the two lowest risk groups QNIL and QNL, the ACT cohorts showed poorer survival as compared to surgery alone.
Histological and genomic correlations of the QuRiS score
Histomorphometric analysis revealed QuRiS to have a statistically significant correlation with the clustering patterns of the cancerous cells observed on H&E stained whole-slide scans (D1:N=70, Pearson correlation coefficient (rho) = 0·23; p<0·01). Individual radiomic feature analysis with the SpaTIL feature revealed intratumoral Haralick feature to be associated with various features explaining the TIL-nuclei interplay. (supplement-2)
In the radiogenomic analysis (D3: N=82), QuRiS was observed to be related to biological pathways implicated in angiogenesis, proliferation, cellular differentiation, T-Cell, and Lymphocyte activation as well as chemotaxis. Although these terms had less than 100 overrepresented genes, the fold enrichment change of all these relevant pathways was greater than 2. Specifically, the intratumoral Haralick energy feature was observed to be inversely correlated with macrophage chemotaxis (Supplement-3).
Discussion
Current treatment guidelines don’t recommend the addition of ACT following curative resection in Stage-IA NSCLC, while the potential use of ACT in Stage-IB is fraught with controversy, mainly due to ambiguous results from large-scale prospective clinical trials investigating the added benefit of ACT within patients treated with curative resection. Meanwhile, in Stage-II NSCLC, ACT is currently recommended universally without any possibility of risk stratification. This sets up the need for a predictive biomarker, which could potentially identify patients who are at a higher risk of recurrence, even with clinicopathological defined low stage cancers (Stage-I). These patients would be ideal candidates for ACT following resection. Meanwhile, identifying patients who are at low risk of recurrence, even in-clinic pathologically defined higher stage NSCLC, might save them from the toxic side effects of ACT, primarily since it would provide no additional benefit over resection.
The current space of biomarkers in NSCLC includes largely prognostic signatures that are reliant on clinical factors like ctDNA, tumor macrophages as well as single-, multi-gene expression, and next-generation sequencing. For instance, Kratz et al.(25) developed a 14-gene expression signature using qPCR analysis on DNA of 361 non-squamous NSCLC patients. The assay was independently validated on N=433 patients (5-year OS of 71·4% in low-risk while 49·2% in high-risk;p=0·0003) and N=1006 patients (5-year OS of 74·1% in low-risk, and 44·6% in high-risk) with Stage I-III non-squamous NSCLC. However, for maximum clinical benefit, signatures need to be not only prognostic but predictive of benefit to added ACT to be decision support for patient management. The few existing predictive signatures are based on gene-expression assays with its usual limitations of being expensive, time-consuming and disrupting clinical workflow in needing a tissue to be shipped to a central laboratory. Zhu et al.(10) developed a 15-gene predictive signature using N=133 patients (62 surgery, 71 surgery+ACT) from JBR·10 using tissue microarray samples. The signature developed from patients who received surgery alone when applied to the cohort of patients who received ACT; the high-risk patients showed improved OS (HR=0·33;p<0·001) while the low-risk patients,in fact, showed negative OS trends (HR=3·67;P=0·013).
In this work, we presented the first of its kind CT-radiomics based test (QuRiS), which is also predictive of benefit to ACT, as well as associated with survival in ES-NSCLC. QuRiS comprises 13 radiomic textural features, three features from the intra-nodular region, and fourteen from the peri-nodular region characterizing the tissue microenvironment (TME). The TME is the immediate cellular area outside the tumor and typically includes a plethora of cells, including fibroblasts, dendritic cells, and inflammatory markers. Recent work has suggested that this area embeds information that relates to drug resistance and the effectiveness of chemo- and immunotherapies(26–29). The resection status of the tumors can have a role in the solicitation of the immune response as the tumors need to be completely resected with no remaining microscopic tumor cells for the immune system to work. Previous investigations of radiomic features from the TME have shown their utility in diagnostic and prognostic lung cancer domain. The selected most discriminative features from the TME included Haralick and CoLIAge features from 15mm outside the tumor, capturing textural heterogeneity, which was found to be overexpressed in the poor responders (high-risk patients). This could potentially reflect the increased heterogeneity in rapidly dividing tumors, which were the more aggressive tumor variants with more chaotic and disordered growth.
QuRiS was also developed to predict benefit to ACT in ES-NSCLC patients. The QuRiS high-risk group showed statistically significant DFS benefit in internal as well as external validation sets with an estimated 70% survival benefit with an improved median DFS of about 17 months in D1 (HR=0·27;p=0·02), 24 months in D2 (HR=0·15,p=0·03) and 27 months in D3 (HR=0·09,p=0·01) for patients who received ACT as compared to surgery alone. In the QuRiS low-risk groups, however, all three cohorts showed an increase in HR without approaching statistical significance (>1, see Table 2) for the patients who received ACT. This seems to suggest that at least in the QuRiS low-risk groups, there is no added benefit of instituting potentially toxic ACT.
Looking within the individual stages revealed that QuRiS was prognostic even within stage-1 (Supplement-1). The IALT and JBR·10 trials were one of the firsts large randomized clinical trials to demonstrate that ACT, after complete surgical resection, improved 5-year overall survival compared to observation alone. But even within these trials, there were no statistically significant differences in survival of Stage I patients treated with ACT vs. surgery alone (Stage IA, in fact, showed an HR>1 i.e. a detrimental effect with ACT possibly due to toxicity). The results of our QuRiS based analysis seem to be a possible interpretation of the clinical trial results, as there seem to be two distinct risk groups (as shown by QuRiS) within clinically defined risk groups.
We believe this is one of the first instances of a radiomics based risk model that is both prognostic as well as predictive of ACT benefit in ES-NSCLC patients treated primarily with curative resection. Huang et. Al.(15) published a radiomic signature using a LASSO Cox-regression model constructed using 132 radiomic textures features from the histogram and grey-level co-occurrence matrix families, from within the node from N=282 ES-NSCLC patients. The authors found that incorporating the radiomics signature into a radiomics-based nomogram (radiomics and significant clinicopathologic factors) resulted in better performance(P<·0001) for the estimation of DFS(C-index:0·72;95%CI:0·71,0·73) than with only the clinical-pathologic nomogram(C-index:0·691;95%CI:0·68,0·70). Meanwhile, He et. Al.(30) showed that in N=186 NSCLC patients, a Random Forest-based classifier using intranodular radiomic features could predict patients who died versus those who were alive with an AUC=0·93. The present study differs from these works in i) utilizing perinodular radiomic features up to 15mm outside the tumor in addition to intranodular features; ii) utilizing multiple independent validation sets for testing the developed radiomic signature and iii) demonstrating the ability of the signature and constructed nomogram to predict benefit to ACT as well besides being prognostic.
One of the ways our study also differed from previous related works was the interrogation and subsequent discovery of a biological as well as the underlying genomic rationale behind the QuRiS score and the associated prognostic radiomic features. Using quantitative histomorphometric features related to the spatial interplay and arrangement of cancer nuclei and TIL clusters, we found that QuRiS was correlated with a spatial feature that represented the clustering pattern of cancerous cells, with more chaotic and closer arrangement of clusters in the QuRiS high-risk group, as compared to the relative lack of cancer nuclei clustering in the low-risk group. Further, on gene ontology analysis by using the DEGs among QuRiS risk groups, QuRiS was found to be significantly correlated with biological pathways related particularly to cellular differentiation and angiogenesis, among others. Potential markers of angiogenesis, including microvessel density found on histological analysis, have shown to be prognostic across multiple cancer types. QuRiS association with angiogenesis pathways seems to suggest that the perinodular texture features might also be driven by more disordered and heterogeneous blood vessel formation in high-risk patients, as compared to more well-formed and ordered blood vessel arrangements for patients with good survival. Similarly, the higher intranodular texture feature in high-risk patients is possibly due to the presence of cellular invasion and poor differentiation in the more aggressive tumor variant, regulated by the biological pathway of cellular differentiation.
The study did have its limitations. Firstly, QuRiS was not implicitly tested for pre-analytic sources of variations, including scanner manufactures, reconstruction kernels, slice thicknesses. However, our study, since it involved cohorts from multiple institutions, had multiple scanners, kernels, and slice thicknesses. Secondly, the developed nomogram, QuRNom, showed prognostic and predictive performance but was not explicitly demonstrated to have a causal association. Third, there was no way to control for the time between imaging study and treatment received. While not explicitly evaluated in this study, these temporal could affect QuRiS performance. Fourth, the treatment cohorts (surgery and surgery+ACT) were not homogeneously determined since it was not obtained from a controlled clinical trial setting. In order to ready QuRiS for clinical use, patients would need to be prospectively and randomly assigned to either the ACT or surgery cohorts based on QuRiS scores, followed by survival analysis of these patients.
In summary, we developed a 13-feature based quantitative radiomic risk score (QuRiS), which was prognostic and predictive of benefit to adjuvant chemotherapy following curative resection in ES-NSCLC. Further multi-site validation including retrospective validation of archived samples from completed clinical trials followed by large prospective clinical trial evaluation, could validate QuRiS as a non-invasive biomarker to risk-stratify patients and be a prognostic and predictive companion test for ES-NSCLC, especially with its low-cost footprint that could possibly be non-disruptive of clinical workflow.
Supplementary Material
Figure 2:
Overall workflow and pipeline of the project. The first step involved identifying and annotating the primary nodule on the CT scan. Intratumoral and peritumoral textural features were extracted using MATLAB 2016. For the peritumoral region, features were extracted from 0–15mm region outside the tumor and divided into five 3mm peritumoral rings. Feature statistics including mean, median, standard deviation, skewness, kurtosis and range were calculated for each of the individual annular rings. Top features were selected using LASSO feature selection method and used for constructing QuRiS. QuRNom was constructed using prognostic clinical features and QuRiS. QuRiS and QuRNom were validated for prognostic performance and predicting added benefit of adjuvant-chemotherapy. Associations between QuRiS features and spatial patterns of TILs on whole-slide tissue scans were also evaluated, as were associations with mRNA data and underlying immune specific biological pathways.
Figure 6:

Radiomic-Pathomic-Genomic association. a) CT scan f) corresponding whole-slide tissue scan i) corresponding mRNA sequencing data. b) Intratumoral and c) Peritumoral Radiomic Haralick textural feature for low-risk nodule with g) representation of SpaTIL feature on corresponding whole-slide scan. d) and e) represents intratumoral and peritumoral Haralick feature for high-risk nodule with h) corresponding SpaTIL representation. QuRiS predicted high risk patients had a more chaotic and disturbed microarchitecture observed on CT scans. For these patients, the corresponding whole slide tissue images revealed very dense, tightly bound cancer nuclear clusters. j) represents corresponding gene set enrichment analysis for these patients. On genomic level, QuRiS was observed to have correlation with chemotaxis.
Research in context.
Evidence before this study
We did a comprehensive search on PubMed and Google Scholar for papers published before October 1, 2019, with the terms (“texture analysis” OR “radiomics” OR “computational imaging” OR “CT-radiomics” OR “radiomics nomogram”) AND (“NSCLC” OR “non-small cell lung cancer” OR “resectable NSCLC” “benefit to adjuvant chemotherapy” OR “prognostic” OR “risk score”), with no language restrictions. To the best of our knowledge, there are no published studies so far that have used radiomics to predict benefit to adjuvant chemotherapy in non-small cell lung cancer. A single study looked to develop and validate a nomogram using radiomics to predict the benefit of adjuvant radiotherapy for patients with resected gastric cancer. Studies in NSCLC using radiomics have focused on prognosis as it predicts the risk of recurrence following resection, with 3 studies looking at developing a nomogram. None of the studies have also looked at genomic and histologic correlations of the radiomic feature analysis
Added-value of this study
To the best of our knowledge, our study is the first to develop and validate a radiomics based nomogram to predict added benefit of adjuvant chemotherapy in two independent validation cohorts and also investigates the correlation of the radiomic imaging features with multimodality, multi-scale data including the spatial architecture of immune and cancer cells on tissue pathology as well as biological pathways of cancer progression through mRNA sequencing data.
Implications of all the available evidence
Our study seems to indicate the potential for a non-invasive biomarker for risk assessment and predicting the added benefit of chemotherapy in resectable non-small cell lung cancer. Especially in the clinically defined low-risk group, integrating our model with the previously described low-risk group would benefit in the treatment planning.
Acknowledgments
The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers 1U24CA199374-01, R01CA202752-01A1 R01CA208236-01A1, R01 CA216579-01A1, R01 CA220581-01A1, 1U01 CA239055-01, 1P20 CA233216-01, National Center for Research Resources under award number 1 C06 RR1246301, VA Merit Review Award IBX004121A from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, the DOD Prostate Cancer Idea Development Award (W81XWH-15-1-0558), the DOD Lung Cancer Investigator-Initiated Translational Research Award (W81XWH-18-1-0440), the DOD Peer Reviewed Cancer Research Program (W81XWH-16-1-0329), National Institute of Diabetes and Digestive and Kidney Diseases (1K25 DK115904-01A1), the Ohio Third Frontier Technology Validation Fund the Wallace H. Coulter Foundation Program in the Department of Biomedical Engineering and the Clinical and Translational Science Award Program (CTSA) at Case Western Reserve University, Department of Defense Peer Reviewed Cancer Research Program (PRCRP) Career Development Award, Dana Foundation David Mahoney Neuroimaging Program
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs, the Department of Defense, or the United States Government.
Role of funding source
No funders had access to any data or any role in data collection, analysis, interpretation, or in writing the report. The corresponding author had access to all of the data and the final responsibility to submit for publication.
Conflict of interest
AM reports grants from National Cancer Institute of the National Institutes of Health, grants from National Center for Research Resources, grants from VA Merit Review Award, grants from DOD Lung Cancer Investigator-Initiated Translational Research Award, during the conduct of the study; grants from DOD Prostate Cancer Idea Development Award, grants from DOD Peer Reviewed Cancer Research Program, grants from National Institute of Diabetes and Digestive and Kidney Diseases , grants from the Ohio Third Frontier Technology Validation Fund, grants from the Wallace H. Coulter Foundation Program in the Department of Biomedical Engineering and the Clinical and Translational Science Award Program (CTSA) at Case Western Reserve University, grants from Department of Defense Peer Reviewed Cancer Research Program (PRCRP) Career Development Award, grants from Dana Foundation David Mahoney Neuroimaging Program, outside the submitted work; In addition, Dr. Madabhushi has a patent RECURRENCE PROGNOSIS AND PREDICTION OF ADDED BENEFIT OF ADJUVANT CHEMOTHERAPY IN EARLY STAGE NON-SMALL CELL LUNG CANCER WITH RADIOMIC FEATURES ON BASELINE COMPUTED TOMOGRAPHY (CT) pending.
VV reports grants, personal fees and other from Merck, Bristol-Myers Squibb, Genentech, AstraZeneca, Celgene, Novartis, Amgen, Fulgent Genetics, Reddy Labs, Alkermes, Nektar Therapeutics, Novocure, and Foundation Medicine. , outside the submitted work; In addition, Dr. Velcheti has a patent RECURRENCE PROGNOSIS AND PREDICTION OF ADDED BENEFIT OF ADJUVANT CHEMOTHERAPY IN EARLY STAGE NON-SMALL CELL LUNG CANCER WITH RADIOMIC FEATURES ON BASELINE COMPUTED TOMOGRAPHY (CT) pending.
MDF reports grants from NIH (P50CA174523), outside the submitted work.
KB and PV has a patent RECURRENCE PROGNOSIS AND PREDICTION OF ADDED BENEFIT OF ADJUVANT CHEMOTHERAPY IN EARLY STAGE NON-SMALL CELL LUNG CANCER WITH RADIOMIC FEATURES ON BASELINE COMPUTED TOMOGRAPHY (CT) pending.
Ethics Committee Approval
The study conformed to HIPAA guidelines was approved by the Institutional Review Board (IRB) at University Hospitals Cleveland Medical Center, IRB No 02-13-42C. Informed consent requirement was waived as the study utilized archival tissue.
Acronyms:
- CT
Computed Tomography
- ES
Early-Stage
- NSCLC
Non-Small cell lung cancer
- ACT
Adjuvant-chemotherapy
- IASLC
International Association for the study of Lung Cancer
- IALT
International Adjuvant Lung Cancer Trial
- JBR.10
Phase III randomized trial of adjuvant cisplatin and vinorelbine versus observation in stage IB or II NSCLC
- LACE
Lung Adjuvant Cisplatin Evaluation
- ALPI
Adjuvant Lung Cancer Project Italy
- BLT
Big Lung Trial
- ASCO
American Society of Clinical Oncology
- NCCN
National Comprehensive Cancer Network
- OS
Overall-Survival
- DFS
Disease-Free Survival
- HR
Hazard Ratio
- KM
Kaplan-Meier
- CI
Confidence Interval
- C-index
Concordance Index
- IRB
Institutional Review Board
- CCF
Cleveland Clinic Foundation
- UPenn
University of Pennsylvania
- TCIA
The Cancer Imaging Archive
- RIDER
Reference Image Database to Evaluate Response
- Intratumoral
Inside the annotated tumor region on CT scan
- Peritumoral
Immediate outside tumor region on CT scan
- LASSO
The least absolute shrinkage and selection operator
- QuRiS
Quantitative Radiomic Risk Score
- QH
High-QuRiS group
- QI
Intermediate-QuRiS group
- QL
Low-QuRiS group
- QuRNom
Constructed Clinical + Quantitative Radiomic Risk Score Nomogram
- QNH
High-QuRNom group
- QNIH
Intermediate-High QuRNom group
- QNIL
Intermediate-Low QuRNom group
- QNL
Low-QuRNom group
- H&E tissues
Hematoxylin and Eosin Tissues
- WSIs
Whole-Slide Tissue Scans
- TILs
Tumor Infiltrating Lymphocytes
- SpaTIL
Spatial architecture and arrangement of tumor-infiltrating lymphocytes
- TME
Tissue Microenvironment
- DEG
Differentially Expressing Genes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016. January;11(1):39–51. [DOI] [PubMed] [Google Scholar]
- 2.Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of Non-small Cell Lung Cancer Stage I and Stage II: ACCP Evidence-Based Clinical Practice Guidelines (2nd Edition). Chest. 2007. September 1;132(3, Supplement):234S–242S. [DOI] [PubMed] [Google Scholar]
- 3.Arriagada R, Dunant A, Pignon J-P, Bergman B, Chabowski M, Grunenwald D, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010. January 1;28(1):35–42. [DOI] [PubMed] [Google Scholar]
- 4.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon J-P, Vansteenkiste J, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004. January 22;350(4):351–60. [DOI] [PubMed] [Google Scholar]
- 5.Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005. June 23;352(25):2589–97. [DOI] [PubMed] [Google Scholar]
- 6.Pignon J-P, Tribodet H, Scagliotti GV, Douillard J-Y, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol Off J Am Soc Clin Oncol. 2008. July 20;26(21):3552–9. [DOI] [PubMed] [Google Scholar]
- 7.Waller D, Peake MD, Stephens RJ, Gower NH, Milroy R, Parmar MKB, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004. July 1;26(1):173–82. [DOI] [PubMed] [Google Scholar]
- 8.Scagliotti GV, Fossati R, Torri V, Crinò L, Giaccone G, Silvano G, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst. 2003. October 1;95(19):1453–61. [DOI] [PubMed] [Google Scholar]
- 9.Strauss GM, Herndon JE, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant Paclitaxel Plus Carboplatin Compared With Observation in Stage IB Non–Small-Cell Lung Cancer: CALGB 9633 With the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008. November 1;26(31):5043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu C-Q, Ding K, Strumpf D, Weir BA, Meyerson M, Pennell N, et al. Prognostic and Predictive Gene Signature for Adjuvant Chemotherapy in Resected Non–Small-Cell Lung Cancer. J Clin Oncol. 2010. September 7;28(29):4417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roepman P, Jassem J, Smit EF, Muley T, Niklinski J, van de Velde T, et al. An Immune Response Enriched 72-Gene Prognostic Profile for Early-Stage Non–Small-Cell Lung Cancer. Clin Cancer Res. 2009. January 1;15(1):284–90. [DOI] [PubMed] [Google Scholar]
- 12.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2015. November 18;278(2):563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bera K, Velcheti V, Madabhushi A. Novel Quantitative Imaging for Predicting Response to Therapy: Techniques and Clinical Applications. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Annu Meet. 2018. May 23;(38):1008–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He L, Huang Y, Yan L, Zheng J, Liang C, Liu Z. Radiomics-based predictive risk score: A scoring system for preoperatively predicting risk of lymph node metastasis in patients with resectable non-small cell lung cancer. Chin J Cancer Res. 2019. August 1;31(4):641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Liu Z, He L, Chen X, Pan D, Ma Z, et al. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non—Small Cell Lung Cancer. Radiology. 2016. June 27;281(3):947–57. [DOI] [PubMed] [Google Scholar]
- 16.Hosny A, Parmar C, Coroller TP, Grossmann P, Zeleznik R, Kumar A, et al. Deep learning for lung cancer prognostication: A retrospective multi-cohort radiomics study. PLoS Med [Internet]. 2018. November 30 [cited 2019 Aug 22];15(11). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6269088/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang T, Xu R, Schiller JH, Bonomi P, Johnson DH. Clinical Model to Predict Survival in Chemonaive Patients With Advanced Non–Small-Cell Lung Cancer Treated With Third-Generation Chemotherapy Regimens Based on Eastern Cooperative Oncology Group Data. J Clin Oncol. 2005. January 1;23(1):175–83. [DOI] [PubMed] [Google Scholar]
- 18.Yuan S-Q, Wu W-J, Qiu M-Z, Wang Z-X, Yang L-P, Jin Y, et al. Development and Validation of a Nomogram to Predict the Benefit of Adjuvant Radiotherapy for Patients with Resected Gastric Cancer. J Cancer. 2017. September 29;8(17):3498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauguen A, Pignon J-P, Burdett S, Domerg C, Fisher D, Paulus R, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re-analysis of meta-analyses of individual patients’ data. Lancet Oncol. 2013. June 1;14(7):619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RIDER Collections - The Cancer Imaging Archive (TCIA) Public Access - Cancer Imaging Archive Wiki [Internet]. [cited 2018 Dec 3]. Available from: https://wiki.cancerimagingarchive.net/display/Public/RIDER+Collections
- 21.Friedman J, Hastie T, Tibshirani R, Simon N, Narasimhan B, Qian J. glmnet: Lasso and Elastic-Net Regularized Generalized Linear Models [Internet]. 2018. [cited 2018 May 23]. Available from: https://CRAN.R-project.org/package=glmnet
- 22.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015. April 1;16(4):e173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corredor G, Wang X, Zhou Y, Lu C, Fu P, Syrigos K, et al. Spatial Architecture and Arrangement of Tumor-Infiltrating Lymphocytes for Predicting Likelihood of Recurrence in Early-Stage Non-Small Cell Lung Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2019. March 1;25(5):1526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakr S, Gevaert O, Echegaray S, Ayers K, Zhou M, Shafiq M, et al. Data for NSCLC Radiogenomics Collection [Internet]. The Cancer Imaging Archive; 2017. [cited 2019 Aug 29]. Available from: https://wiki.cancerimagingarchive.net/x/W4G1AQ [Google Scholar]
- 25.Kratz JR, He J, Van Den Eeden SK, Zhu Z-H, Gao W, Pham PT, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet Lond Engl. 2012. March 3;379(9818):823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khorrami M, Prasanna P, Gupta A, Patil P, Velu PD, Thawani R, et al. Changes in CT radiomic features associated with lymphocyte distribution predict overall survival and response to immunotherapy in non-small cell lung cancer. Cancer Immunol Res [Internet]. 2019. January 1 [cited 2019 Nov 18]; Available from: https://cancerimmunolres.aacrjournals.org/content/early/2019/11/12/2326-6066.CIR-190476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braman N, Prasanna P, Whitney J, Singh S, Beig N, Etesami M, et al. Association of Peritumoral Radiomics With Tumor Biology and Pathologic Response to Preoperative Targeted Therapy for HER2 (ERBB2)–Positive Breast Cancer. JAMA Netw Open. 2019. April 5;2(4):e192561–e192561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morimoto J, Nakajima T, Suzuki H, Nagato K, Iwata T, Yoshida S, et al. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2016. July;152(1):64–72.e1. [DOI] [PubMed] [Google Scholar]
- 29.Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg. 2016;23(4):567–72. [DOI] [PubMed] [Google Scholar]
- 30.He B, Zhao W, Pi J-Y, Han D, Jiang Y-M, Zhang Z-G, et al. A biomarker basing on radiomics for the prediction of overall survival in non–small cell lung cancer patients. Respir Res [Internet]. 2018. [cited 2019 Aug 30];19. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6180390/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.