Abstract
Background
Different techniques have been described to reduce morbidity during caesarean section. After the baby has been born by caesarean section and the placenta has been extracted, temporary removal of the uterus from the abdominal cavity (exteriorisation of the uterus) to facilitate repair of the uterine incision has been postulated as a valuable technique. This is particularly so when exposure of the incision is difficult and when there are problems with haemostasis. Several clinical trials have been done, with varying results, including substantial reduction in the rate of postoperative infection and morbidity with extra‐abdominal closure of the uterine incision, and less associated peri‐operative haemorrhage. Subsequent studies suggest that the method of placental removal rather than method of closure of the uterine incision influences peri‐operative morbidity.
Objectives
To evaluate the effects of extra‐abdominal repair of the uterine incision compared to intra‐abdominal repair.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (September 2003), the Cochrane Central Register of Controlled Trials (The Cochrane Library, 2003, Issue 3) and PubMed (1966 to 2003). We updated the search of the Cochrane Pregnancy and Childbirth Group's Trials Register on 12 January 2011 and added the results to the awaiting classification section.
Selection criteria
Randomised controlled trials involving a comparison of uterine exteriorisation with intra‐abdominal repair of the uterine incision in women undergoing caesarean section.
Data collection and analysis
Two reviewers independently assessed the trials identified for inclusion. We compared categorical data using relative risks and 95% confidence intervals and continuous data using the weighted mean difference with 95% confidence intervals. We tested for statistical heterogeneity between trials using the I squared test. Where no significant heterogeneity (greater than 50%) existed, we pooled data using a fixed effect model. If significant heterogeneity existed, a random effects model was used.
Main results
Six studies were included, with 1294 women randomised overall, and 1221 women included in the analysis. There were no statistically significant differences between the groups in most of the outcomes identified, except for febrile morbidity and length of hospital stay. With extra‐abdominal closure of the uterine incision, febrile morbidity was lower (relative risk 0.41, 95% confidence interval (CI) 0.17 to 0.97), and the hospital stay was longer (weighted mean difference 0.24 days, 95% CI 0.08 to 0.39).
Authors' conclusions
There is no evidence from this review to make definitive conclusions about which method of uterine closure offers greater advantages, if any. However, these results are based on too few and too small studies to detect differences in rare, but severe, complications.
[Note: The 12 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Plain language summary
Extra‐abdominal versus intra‐abdominal repair of the uterine incision at caesarean section
There is not enough evidence to say if closing the cut in the womb after caesarean section is better done within the abdomen or outside.
In order to perform a caesarean section, the mother's abdomen and then the uterus need to be cut in order for the baby to be born. These cuts then need to be stitched up (sutured). It has been suggested that it might be easier to bring the uterus outside the abdomen in order to suture it and then return it to its place, rather than suturing it in position. The review of six trials found that there was not enough evidence to say if this was better for the mother or not. More research is needed.
Background
Caesarean section is one of the most frequently performed major surgical procedures worldwide. It accounts for between 1% and 70% of deliveries depending on the facility or country assessed. Rates in the United Kingdom for 2000 were reported as 21% in England and 24% in Wales and in Northern Ireland (Thomas 2001). Similar rates have been reported for the United States of America (Curtin 1997) and China (Cai 1998). In Latin America, estimates from a survey of selected hospitals ranged from 1.6% in a Haitian hospital to 40% in Chile, and more than 50% in most private hospitals in Argentina, Brazil, Chile, Colombia, Mexico and Paraguay (Belizan 1999). Rates for West and East African countries ranged from 0.3% in Niger to 10.5% in Kenya (Beukens 2001).
Many variations in the technique of caesarean section have been devised, with the purpose of shortening the operating time, making the operation easier and more efficient, reducing costs, decreasing the risk of adverse effects, and shortening postoperative morbidity and duration of hospital stay. While details of operative technique are not more important than the question of whether or not there is a valid indication for the operation, these proposed variations are also important, and must be evaluated by randomised comparisons.
After the baby has been born by caesarean section and the placenta has been extracted, either spontaneously (placenta separates spontaneously from the wall of the uterus) or by manual removal (separation of the placenta from the uterine wall by hand), temporary removal of the uterus from the abdominal cavity (exteriorisation of the uterus) to facilitate repair of the uterine incision has been postulated as a valuable technique. This is particularly so when exposure of the incision is difficult and there may be complications such as tearing of the uterine angle (rupture of part of the uterine wall) or problems with haemostasis (reducing the flow of blood). Many surgeons believe that it is easier to repair the exteriorised uterus, and thus that bleeding may be reduced with this method (Cosgrove 1958). However, opposition to uterine exteriorisation, particularly with epidural or spinal analgesia, arose from concerns about nausea and vomiting with uterine traction, haemodynamic instability (instability of the blood circulatory system), exposure of the fallopian tubes to unnecessary trauma, potential infection, possible rupture of the utero‐ovarian veins upon replacing the uterus and pulmonary embolism (Carrie 1990; Stock 1985). Antibiotics are frequently prescribed, either pre‐ or postoperatively (peri‐operative antibiotics).
The lack of agreement on the site of uterine repair is reflected in variations in practice. In a pilot study in Hull Maternity Hospital, UK, 46% of uterine incisions were repaired with exteriorisation of the uterus, and 54% intraperitoneally (PS Eccersley, personal communication, cited by Wahab 1999). Although limited work has been done on this subject, there have been a few randomised controlled trials, with varying results. Earlier works showed a substantial reduction in the rate of postoperative infection and morbidity with exteriorisation of the uterus. There was also less associated peri‐operative haemorrhage (bleeding during the surgical period) (Hershey 1978). However, Magann (Magann (M) 1993a; Magann (M) 1993b; Magann (M) 1995) suggested, in a series of studies, that the method of placental removal (i.e. spontaneous versus manual) rather than exteriorisation of the uterus influenced peri‐operative haemorrhage and postoperative infection rates. More recent studies have found that although there were no significant differences in haemodynamic parameters, exteriorisation of the uterus was associated with a smaller reduction in postoperative haematocrit values (Edi‐Osagie 1998; Wahab 1999). These authors feel that exteriorisation of the uterus at caesarean section is a valid option, as demonstrated by clinical and statistical evidence.
This is one of a series of reviews of individual aspects of caesarean section technique. More detailed background and reference to related reviews is given in the review 'Techniques for caesarean section' (Hofmeyr 2008).
Objectives
To evaluate the effects of extra‐abdominal repair of the uterine incision, compared to intra‐abdominal repair.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing uterine exteriorisation with intra‐abdominal repair of the uterine incision at caesarean section.
Types of participants
Women undergoing caesarean section, either elective or emergency.
Types of interventions
For the experimental group, the surgeon will have been requested to exteriorise the uterus following delivery of the baby and placenta. For the control group, the uterus will have been repaired intra‐abdominally. Where other interventions such as spontaneous versus manual removal of placenta, and use of peri‐operative antibiotics versus placebo are randomly allocated, the effects of uterine exteriorisation alone will be assessed, if possible. Studies evaluating a package of interventions from which extra‐abdominal repair cannot be isolated are considered in a separate review ('Techniques for caesarean section' (Hofmeyr 2008)).
Types of outcome measures
Primary outcomes
Blood loss (as gauged by differences in pre‐ and postoperative haemoglobin or haematocrit levels) ‐ this would impact directly on the health and well‐being of the woman. Mild to moderate blood loss would lead to symptoms such as a feeling of tiredness or weakness, palpitations, anxiety and dizziness or black‐outs. More severe blood loss would lead to hypovolemic shock (a state of shock due to lowered blood volume) and possibly even death.
Postoperative sepsis (as defined by trial authors) ‐ infection following the surgical procedure will influence well‐being of the woman, pain following the surgery, healing of the wound and amount of time spent in the hospital. If managed correctly and timeously, postoperative sepsis may resolve without complications.
Secondary outcomes
Duration of operation Intraoperative pain Postoperative pain Analgesia use Nausea or vomiting Operative complications including exposure of the fallopian tubes to unnecessary trauma and possible rupture of the utero‐ovarian veins upon replacing the uterus into the abdominal cavity Blood transfusion Intra‐operative blood loss (estimated or measured) Postoperative haemoglobin level Postoperative anaemia, as defined by trial authors Postoperative pyrexia Postoperative infection requiring additional antibiotic therapy Wound complications (haematoma, infection, breakdown) Deep vein thrombosis and pulmonary embolism Time to mobilisation Time to oral intake Time to return of bowel function Time to breastfeeding initiation Length of postoperative hospital stay Unsuccessful breastfeeding, as defined by trial authors Mother not satisfied ‐ this was analysed as the woman's perception of intra‐ and postoperative discomfort Caregiver not satisfied Cost
Outcomes were included if clinically meaningful; reasonable measures taken to minimise observer bias; missing data insufficient to materially influence conclusions; data available for analysis according to original allocation, irrespective of protocol violations; data available in format suitable for analysis.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (September 2003). We updated this on 12 January 2011 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched the CENTRAL (The Cochrane Library 2003, Issue 3) and PubMed (1966 to 2003) using the search strategy detailed in Appendix 1.
Searching other resources
We searched the reference lists of relevant recent papers by hand.
We did not apply any language restrictions.
Data collection and analysis
We extracted data on trial methodology from published trial reports. We assessed the quality of each study and excluded studies when appropriate before we analysed the results or incorporated them into the meta‐analysis, in order to minimise the chances of selection bias. Assessment of the quality of the studies was based on: allocation concealment (scored as adequate, unclear or inadequate); generation of random allocation sequence (adequate, unclear or inadequate); blinding of participants; blinding of caregivers; blinding of outcome assessment; completeness of data collection, including differential withdrawal of participants or loss to follow up from different groups; analysis of randomised participants in randomised groups (analysis by intention to treat). We contacted authors of published abstracts or unpublished data for further details of the study methodology and results, so that their data could be included where appropriate.
We extracted data onto data forms and checked them for accuracy.
We performed statistical analyses using the Review Manager software (RevMan 2000). We compared categorical data using relative risks and 95% confidence intervals. We compared continuous data using the weighted mean difference with 95% confidence intervals. We tested statistical heterogeneity between trials using the I2 statistic. If there was no significant heterogeneity (greater than 50%), we pooled data using a fixed effect model. If we found significant heterogeneity, we used a random effects model.
Results
Description of studies
Risk of bias in included studies
In all the trials included, the method of randomisation was clearly explained. In five studies, there were double randomisations of the participants included in both arms of the study (Hershey 1978; Magann (M) 1993a; Magann (M) 1993b; Magann (M) 1995; Wahab 1999), with consequent subgrouping of the data. The types of participants, interventions and outcomes were clearly defined, in all the studies. However, allocation concealment in four studies was unclear (Edi‐Osagie 1998; Hershey 1978; Magann (M) 1993a; Wahab 1999).
Two studies stated that analysis was done by intention to treat (Edi‐Osagie 1998; Wahab 1999). Protocol violations occurred in three studies involving 92 women in total (Hershey 1978 ‐ 78 (20%); Magann (M) 1993b ‐ 12 (10%); Wahab 1999 ‐ 2 (0.01%)), of which 73 women were excluded, by the authors, from the analysis. There were no indications of protocol violations in the other studies.
In the three studies reported by Magann et al (Magann (M) 1993a; Magann (M) 1993b; Magann (M) 1995), women were randomised into four groups: uterine exteriorisation with spontaneous placental removal, in situ repair with spontaneous placental removal, uterine exteriorisation with manual placental removal, in situ repair with manual placental removal. Subgroup analysis for manual and spontaneous placental removal were added post‐hoc to the review in order to be able to include these data. Data from these subgroups were identified as (M) and (S). Thus the subgroup with manual removal of the placenta and uterine exteriorisation is compared with the subgroup with manual removal of the placenta and in situ repair, etc. Additionally, as there were two studies done in 1993 by the same author, these have been identified as Magann (M) 1993a and Magann (M) 1993b.
We excluded one trial (Wallace 1984) because it did not meet the inclusion criteria for this review (seeCharacteristics of excluded studies.
(Twelve reports from an updated search in January 2011 have been added to Studies awaiting classification.)
Effects of interventions
There were six studies included in this review. A total of 1294 women were randomised, and 1221 of these results were analysed as 73 women were excluded/disqualified for various reasons (78 women excluded due to protocol violations in total, although in 17 of these women the change in peri‐operative haematocrit was analysed (Group X) Hershey 1978; 12 women were excluded prior to analysis due to infection, Magann (M) 1993b). We used a random effects model for four outcomes with significant heterogeneity between results (drop in haematocrit, drop in haemoglobin, endometritis and duration of hospital stay).
Meta‐analysis of the results showed that febrile morbidity (symptoms due to a temperature of above 37.5 degrees Celsius on at least two consecutive readings, done at least six hours apart) was less common in the uterine exteriorisation group (relative risk 0.41, 95% confidence interval (CI) 0.17 to 0.97) in the one study that reported this outcome (Hershey 1978). This finding was statistically significant. In four studies, the length of hospital stay was only marginally longer with uterine exteriorisation (weighted mean difference 0.24 days, 95% CI 0.08 to 0.39), which may not have much clinical significance. With regard to wound complications, the incidence is much higher in one study (Edi‐Osagie 1998), suggesting that the trialists' definition of wound infection may have been different from other authors.
There were no statistically significant differences between the groups for the other outcomes. For most outcomes, relatively few studies contributed data.
The occurrence of uterine angle tear was documented in only one study, where it occurred in one woman in each group (Edi‐Osagie 1998).
Discussion
So far, few clinical trials have been conducted comparing uterine exteriorisation with intra‐abdominal closure of the uterus. The quality of the trials is not high overall, particularly with regard to the large number of exclusions from the analysis. The existing data do not provide clinicians with adequate answers regarding the benefits or risks of either method. Furthermore, these data have been produced over 20 years with very little concordance among the authors about which method of uterine closure is better. Therefore, it is not possible to make definitive conclusions about which method of uterine closure offers greater advantages, if any. Additionally, in three of the six trials reviewed, women were randomised to method of placental removal (either spontaneous or manual), and these results analysed within the groups, which makes it difficult to ascertain whether either method is superior. Methods concerning closure of the uterine incision need to be considered with regards to the benefits or harm in order to be able to offer the best available surgical care to women undergoing caesarean section.
This review attempted to bridge the gap that existed regarding the quality and quantity of data available on this topic. We noted that, of the six studies reviewed, three had been conducted by the same author (Magann (M) 1993a; Magann (M) 1993b; Magann (M) 1995).
Meta‐analysis of various outcomes shows that, apart from febrile morbidity and length of hospital stay, there were overall no statistically significant differences between the groups, despite conflicting evidence in the various trials. There is therefore no clear evidence in favour of either method.
It must be noted that the clinical trials included in this review are relatively small, and most of the outcomes identified could only be assessed with data from a few studies. There is thus a possibility of type 2 statistical error (failure to identify a true difference).
The possibility of rare complications, such as tearing of the ovarian veins, which are unlikely to be reflected in randomised trials, should be borne in mind when interpreting the trial data. This information can be collected using large retrospective non‐randomised studies, although interpretation of these results may be problematic.
Authors' conclusions
Implications for practice.
There is no good evidence from this review to support one intervention above the other when it comes to considering extra‐abdominal and intra‐abdominal repair of the uterine incision. This may be due to the fact that these results are based on too few and too small studies to detect differences in rare, but serious, complications.
Implications for research.
There is a need for further research in this area, as no large randomised controlled clinical trials have been done to assess the benefits and risks of uterine exteriorisation. All the trials that have been done are relatively small and measure few outcomes. Additionally, all of the trials were conducted in high‐income countries, where there is access to high care facilities and the risks of caesarean section are small. There are no data available for low resource settings, where there may be restricted access and management options, which would have a direct impact on patient care.
[Note: The 12 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 12 January 2011 | Amended | Search updated. Twelve new reports added to Studies awaiting classification. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 3 November 2008 | Amended | Converted to new review format. |
Notes
This review updates the previously published Cochrane review entitled 'Uterine exteriorization versus intraperitoneal repair at caesarean section', which was first published on The Cochrane Library, Issue 1, 1995. A new protocol to update this review was published in Issue 3, 2003, from which this review has been developed.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser. In addition, the authors would like to acknowledge the help and support received from Dr R Kulier ‐ Geneva Foundation for Medical Education and Research and the International Association for Maternal and Neonatal Health (Post‐Graduate Study Grant), Geneva, SWITZERLAND.
Appendices
Appendix 1. Search strategy
Authors searched CENTRAL (The Cochrane Library, 2003, Issue 3) and PubMed (1966 to 2003) using the following strategy:
(exteriorization or exteriorisation or extra‐abdominal or extraabdominal or exp Cesarean Section [methods]) and (uterus or uterine)
Data and analyses
Comparison 1. Uterine exteriorization versus intraperitoneal repair at caesarean section.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Operative blood loss (ml) | 6 | 504 | Mean Difference (IV, Fixed, 95% CI) | 17.11 [‐23.15, 57.37] |
| 1.1 with manual removal of placenta | 3 | 252 | Mean Difference (IV, Fixed, 95% CI) | 26.75 [‐34.54, 88.05] |
| 1.2 with spontaneous separation of placenta | 3 | 252 | Mean Difference (IV, Fixed, 95% CI) | 9.79 [‐43.59, 63.18] |
| 1.3 placental management not stated | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Postoperative drop in haematocrit | 3 | 324 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.48, 0.54] |
| 2.1 with manual removal of placenta | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐3.52, 0.52] |
| 2.2 with spontaneous separation of placenta | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.47, 1.47] |
| 2.3 placental management not stated | 1 | 224 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐0.90, ‐0.70] |
| 3 Postoperative drop in haemoglobin levels (g/dl) | 2 | 482 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.62, 0.65] |
| 4 Febrile morbidity for more than 3 days | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.17, 0.97] |
| 5 Endometritis | 3 | 592 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.64, 2.60] |
| 6 Wound complications (infection, haematoma, breakdown) | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.53, 1.46] |
| 7 Nausea/vomiting (intra‐operative) | 3 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.78, 1.80] |
| 8 Postoperative sepsis | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.19, 4.57] |
| 9 Duration of operation | 9 | 1281 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐2.31, 3.95] |
| 10 Satisfaction with operation | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.82, 1.04] |
| 11 Length of hospital stay (postoperative) | 4 | 766 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.08, 0.39] |
| 12 Pain (intra‐operative) | 2 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.79, 2.27] |
| 13 Failure of procedure | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.16, 1.28] |
| 14 Patients requiring blood transfusion | 2 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.43, 3.19] |
| 15 Deep vein thrombosis | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.12, 68.42] |
1.1. Analysis.
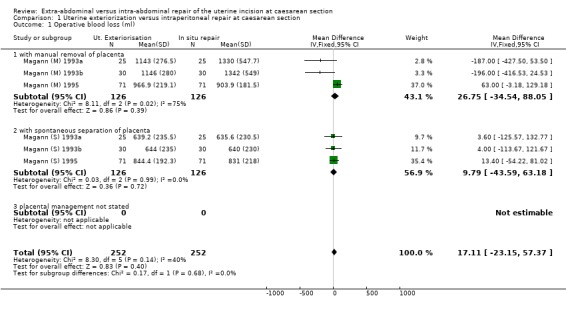
Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 1 Operative blood loss (ml).
1.2. Analysis.
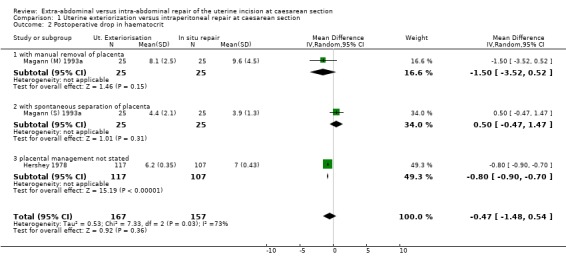
Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 2 Postoperative drop in haematocrit.
1.3. Analysis.
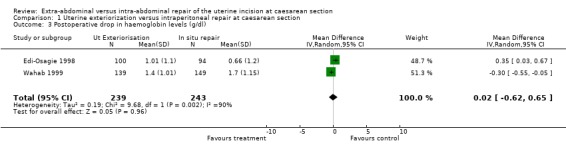
Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 3 Postoperative drop in haemoglobin levels (g/dl).
1.4. Analysis.

Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 4 Febrile morbidity for more than 3 days.
1.5. Analysis.
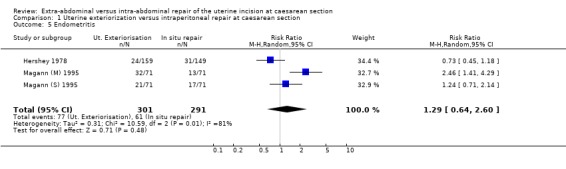
Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 5 Endometritis.
1.6. Analysis.

Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 6 Wound complications (infection, haematoma, breakdown).
1.7. Analysis.
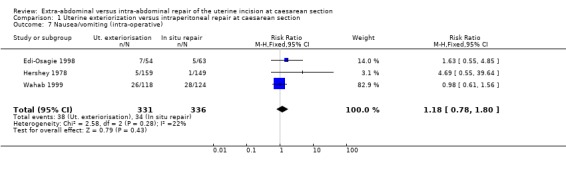
Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 7 Nausea/vomiting (intra‐operative).
1.8. Analysis.
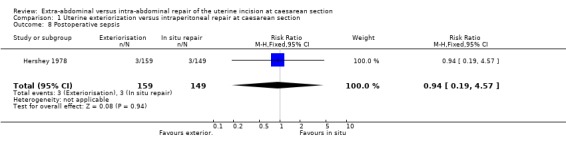
Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 8 Postoperative sepsis.
1.9. Analysis.

Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 9 Duration of operation.
1.10. Analysis.
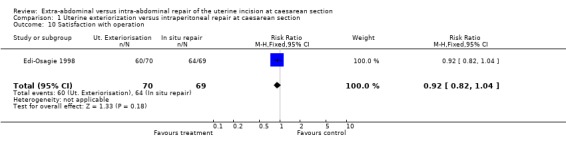
Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 10 Satisfaction with operation.
1.11. Analysis.
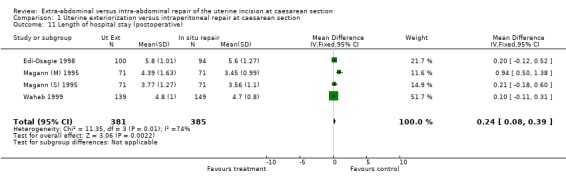
Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 11 Length of hospital stay (postoperative).
1.12. Analysis.

Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 12 Pain (intra‐operative).
1.13. Analysis.

Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 13 Failure of procedure.
1.14. Analysis.
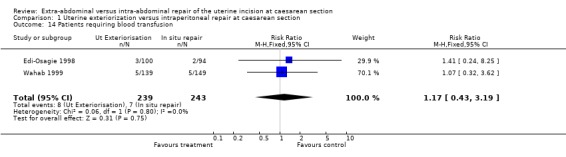
Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 14 Patients requiring blood transfusion.
1.15. Analysis.

Comparison 1 Uterine exteriorization versus intraperitoneal repair at caesarean section, Outcome 15 Deep vein thrombosis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Edi‐Osagie 1998.
| Methods | Randomised controlled trial. Randomisation by a table of random numbers, and allocations kept secure in sealed envelopes. Not stated whether or not these were opaque. Analysis by "intention to treat" principle. | |
| Participants | 194 women included. Exclusion of those with placenta previa, placental abruption, chorioamnionitis. | |
| Interventions | 2 groups ‐ uterine exteriorisation = 100 women. In situ repair = 94 women. All received prophylactic antibiotics. Pain relief by patient controlled analgesia ‐ morphine for the first 24‐36 hours, then rectal diclofenac/codeine‐ paracetamol combination PRN. | |
| Outcomes | 1. Hosp. stay. 2. Febrile morbidity. 3. UTI. 4. + HVS. 5. + wound swab. 6. Haemorrhage/blood transfusion. 7. Deep vein thrombosis. 8. Hematuria. 9. Pain and vomiting ‐ intra/postoperative. 10. Late puerperal pain. 11. Peri‐operative Hb change (Day 1 and Day 3 Hb). 12. Satisfaction with operation. 13. Failure of procedure. 14. Assessment of abdominal scar. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hershey 1978.
| Methods | "all...were randomly assigned". No details of method of randomisation were given. | |
| Participants | 386 consecutive caesarean sections, in a county hospital. 78 (20%) excluded, as they required extensive surgical manipulations. | |
| Interventions | 2 groups ‐ 1. Experimental (159): eventration of uterus following delivery of fetus and placenta. 2. Control (149): repair of the uterus intraperitoneally. Subgroup formed within the 2 groups (1A and 2A), of those women with intact membranes at the time of operation. High morbidity subgroup identified (group Y), which contained patients with > 3 febrile days and / > 6 postoperative days in hospital. | |
| Outcomes | 1. Febrile days (excluding first 24 hours, when a temperature of 100.4 F or greater was recorded). 2. Postoperative infection. 3. Postoperative days in hospital. 4. Drop in haematocrit (patients with third trimester bleeding excluded from analysis). 5. Duration of operation. 6. Additional morbidity/wound infections 6‐8 weeks postoperatively. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Magann (M) 1993a.
| Methods | Randomised prospective study. Four groups of cards prepared from a random number table. | |
| Participants | 100 women having a caesarean section. Women with a bleeding diathesis, abnormal placentation, or prior postpartum haemorrhage were excluded. | |
| Interventions | 4 groups formed: Group 1 ‐ In situ repair, spontaneous placental removal. Group 2 ‐ Exteriorisation of the uterus, spontaneous placental removal. Group 3 ‐ In situ repair, manual placental removal. Group 4 ‐ Exteriorisation of the uterus, manual placental removal. After delivery of the fetus, iv pitocin infused. | |
| Outcomes | 1. Blood loss (measured in suction apparatus, drapes, sponges and pads). 2. Postoperative haematocrit drop (Pre‐ and 48 hr. postoperative levels measured). | |
| Notes | Women recruited to the Magann studies were doubly randomised in trials with factorial design. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Magann (M) 1993b.
| Methods | Random group assignment by card selection, from sealed opaque envelopes. Group appointment from random number table. | |
| Participants | 120 women undergoing caesarean section. Exclusion of women with chorioamnionitis, those who refused, those who received antenatal steroid/insulin therapy. | |
| Interventions | 4 groups formed: Group 1 ‐ In situ repair, spontaneous placental removal. Group 2 ‐ Exteriorisation of the uterus, spontaneous placental removal. Group 3 ‐ In situ repair, manual placental removal. Group 4 ‐ Exteriorisation of the uterus, manual placental removal. No antibiotics received by any group of participants. Pelvis irrigated with normal saline prior to closure of abdominal wound in all cases. | |
| Outcomes | 1. Infectious morbidity (as gauged by: maternal temp > 38 C on 2 occasions, 6 hours apart, excluding the first 24 hours: uterine tenderness: foul smelling lochia: blood and urine cultures. 2. Duration of operation. |
|
| Notes | Women recruited to the Magann studies were doubly randomised in trials with factorial design. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Magann (M) 1995.
| Methods | Computer generated random sequence, sealed in opaque envelopes. | |
| Participants | 284 women undergoing caesarean section. Exclusion of women who refused, chorioamnionitis, history of previous caesarean section without labour. | |
| Interventions | Participants divided into 4 equal groups (71). Group 1 ‐ In situ repair, spontaneous placental removal. Group 2 ‐ Exteriorisation of uterus, spontaneous placental removal. Group 3 ‐ In situ repair, manual placental removal. Group 4 ‐ Exteriorisation of the uterus, manual placental removal. All patients received prophylactic antibiotics. | |
| Outcomes | 1. Operative blood loss (measured in suction apparatus, surgical drapes and sponges). 2. Endometritis (temperature of 38 C on 2 occasions, 6 hours apart, excluding the first 24 hours; uterine tenderness: foul smelling lochia). | |
| Notes | Women recruited to the Magann studies were doubly randomised in trials with factorial design. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Magann (S) 1993a.
| Methods | Randomised prospective study. Four groups of cards prepared from a random number table. | |
| Participants | 100 women having a caesarean section. Women with a bleeding diathesis, abnormal placentation, or prior postpartum haemorrhage were excluded. | |
| Interventions | 4 groups formed: Group 1 ‐ In situ repair, spontaneous placental removal. Group 2 ‐ Exteriorisation of the uterus, spontaneous placental removal. Group 3 ‐ In situ repair, manual placental removal. Group 4 ‐ Exteriorisation of the uterus, manual placental removal. After delivery of the fetus, iv pitocin infused. | |
| Outcomes | 1. Blood loss (measured in suction apparatus, drapes, sponges and pads). 2. Postoperative haematocrit drop (Pre‐ and 48 hr postoperative levels measured). | |
| Notes | Women recruited to the Magann studies were doubly randomised in trials with factorial design. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Magann (S) 1993b.
| Methods | Random group assignment by card selection, from sealed opaque envelopes. Group appointment from random number table. | |
| Participants | 120 women undergoing caesarean section. Exclusion of women with chorioamnionitis, those who refused, those who received antenatal steroid/insulin therapy. | |
| Interventions | 4 groups formed: Group 1 ‐ In situ repair, spontaneous placental removal. Group 2 ‐ Exteriorisation of the uterus, spontaneous placental removal. Group 3 ‐ In situ repair, manual placental removal. Group 4 ‐ Exteriorisation of the uterus, manual placental removal. No antibiotics received by any group of participants. Pelvis irrigated with normal saline prior to closure of abdominal wound in all cases. | |
| Outcomes | 1. Infectious morbidity (as gauged by: maternal temp > 38 C on 2 occasions, 6 hours apart, excluding the first 24 hours: uterine tenderness: foul smelling lochia: blood and urine cultures. 2. Duration of operation. |
|
| Notes | Women recruited to the Magann studies were doubly randomised in trials with factorial design. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Magann (S) 1995.
| Methods | Computer generated random sequence, sealed in opaque envelopes. | |
| Participants | 284 women undergoing caesarean section. Exclusion of women who refused, chorioamnionitis, history of previous caesarean section without labour. | |
| Interventions | Participants divided into 4 equal groups (71). Group 1 ‐ In situ repair, spontaneous placental removal. Group 2 ‐ Exteriorisation of uterus, spontaneous placental removal. Group 3 ‐ In situ repair, manual placental removal. Group 4 ‐ Exteriorisation of the uterus, manual placental removal. All patients received prophylactic antibiotics. | |
| Outcomes | 1. Operative blood loss (measured in suction apparatus, surgical drapes and sponges). 2. Endometritis (temperature of 38 C on 2 occasions, 6 hours apart, excluding the first 24 hours; uterine tenderness: foul smelling lochia). | |
| Notes | Women recruited to the Magann studies were doubly randomised in trials with factorial design. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wahab 1999.
| Methods | Randomised controlled trial. Randomisation by closed, numbered envelope technique, after anaesthetic technique established. Also, independent randomisation for 3 anaesthetic techniques used. Surgeons and anaesthetists blinded. Analysis by "intention to treat" principle. | |
| Participants | 316 women randomised, although only 288 included in analysis. (? 112 in pilot study, included in interim analysis.) Exclusion: Pre‐/postoperative blood specimens not taken, technical problems with anaesthetic, any change in standard operative procedure. |
|
| Interventions | 1. Group 1 (139) Uterine exteriorisation. 2. Group 2 (149) Intra‐abdominal repair of the uterus. |
|
| Outcomes | 1. Peri‐operative drop in Hb. 2. Duration of operation. 3. Duration of hospital stay/maternal morbidity. 4. Patient's perception of discomfort (intra‐operatively) 5. Nausea, vomiting and pain scores. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hosp: hospital hr: hour HVS: high vaginal swab IV: intravenous temp: temperature UTI: urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Wallace 1984 | We felt that the methods of uterine repair in this study compared extra‐peritoneal closure, rather than exteriorisation of the uterus, with intra‐peritoneal closure. |
Contributions of authors
D Jacobs‐Jokhan wrote the protocol and the review. GJ Hofmeyr commented on and revised earlier drafts of the protocol and the review.
Sources of support
Internal sources
University of the Witwatersrand, Johannesburg, South Africa.
External sources
World Health Organization (Long‐term Institutional Development Grant), Switzerland.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Edi‐Osagie 1998 {published data only}
- Edi‐Osagie ECO, Hopkins RE, Ogbo V, Lockhat‐Clegg F, Ayeko M, Akpala WO, et al. Uterine exteriorisation at caesarean section: influence on maternal morbidity. British Journal of Obstetrics and Gynaecology 1998;105:1070‐8. [DOI] [PubMed] [Google Scholar]
Hershey 1978 {published data only}
- Hershey DW, Quilligan EJ. Extraabdominal uterine exteriorization at cesarean section. Obstetrics & Gynecology 1978;52:189‐92. [PubMed] [Google Scholar]
Magann (M) 1993a {published data only}
- Magann EF, Dodson MK, Allbert JR, McCurdy CM, Martin RW, Morrison JC. Blood loss at time of caesarean section by method of placental removal and exteriorisation versus in situ repair of the uterine incision. Surgery, Gynecology and Obstetrics 1993;177:389‐92. [PubMed] [Google Scholar]
Magann (M) 1993b {published data only}
- Magann EF, Dodson MK, Harris RL, Floyd RC, Martin JN, Morrison JC. Does method of placental removal or site of uterine incision repair alter endometritis after cesarean delivery ?. Infectious Diseases in Obstetrics and Gynaecology 1993;1:65‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magann (M) 1995 {published data only}
- Magann EF, Washburne JF, Harris RL, Bass JD, Duff WP, Morrison JC. Infectious morbidity following Cesarean delivery by method of placental removal and site of uterine repair. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 2):301. [PubMed] [Google Scholar]
- Magann EF, Washburne JF, Harris RL, Bass JD, Duff WP, Morrison JC. Infectious morbidity, operative blood loss and length of the operative procedure after cesarean delivery by method of placental removal and site of uterine repair. Journal of the American College of Surgeons 1995;181:517‐20. [PubMed] [Google Scholar]
Magann (S) 1993a {published data only}
- Magann EF, Dodson MK, Allbert JR, McCurdy CM, Martin RW, Morrison JC. Blood loss at time of cesarean section by method of placental removal and exteriorization vs in situ repair of the uterine incision. Surgery, Gynecology and Obstetrics 1993;177:389‐92. [PubMed] [Google Scholar]
Magann (S) 1993b {published data only}
- Magann EF, Dodson MK, Harris RL, Floyd RC, Martin JN, Morrison JC. Does method of placental removal or site of uterine incision repair alter endometritis after cesarean delivery ?. Infectious Diseases in Obstetrics and Gynaecology 1993;1:65‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magann (S) 1995 {published data only}
- Magann EF, Washburne JF, Harris RL, Bass JD, Duff WP, Morrison JC. Infectious morbidity following Cesarean delivery by method of placental removal and site of uterine repair. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 2):301. [PubMed] [Google Scholar]
- Magann EF, Washburne JF, Harris RL, Bass JD, Duff WP, Morrison JC. Infectious morbidity, operative blood loss and length of the operative procedure after cesarean delivery by method of placental removal and site of uterine repair. Journal of the American College of Surgeons 1995;181:517‐20. [PubMed] [Google Scholar]
Wahab 1999 {published data only}
- Wahab MA, Karantzis P, Eccersley PS, Russell IF, Thompson JW, Lindow SW. A randomised, controlled study of uterine exteriorisation and repair at caesarean section. British Journal of Obstetrics and Gynaecology 1999;106:913‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Wallace 1984 {published data only}
- Wallace RI, Eglinton GS, Yonekura ML, Wallace TM. Extraperitoneal cesarean section: a surgical form of infection prophylaxis?. American Journal of Obstetrics and Gynecology 1984;148(2):172‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Coronis 2007 {published data only}
- The CORONIS Trial Collaborative Group. The CORONIS trial. International study of caesarean section surgical techniques: a randomised fractional, factorial trial. BMC Pregnancy and Childbirth 2007;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coutinho 2008 {published data only}
- Coutinho IC, Ramos de Amorim MM, Katz L, Bandeira de Ferraz AA. Uterine exteriorization compared with in situ repair at cesarean delivery: a randomized controlled trial. Obstetrics & Gynecology 2008;111(3):639‐47. [DOI] [PubMed] [Google Scholar]
Ding 1997 {published data only}
- Ding Y, Zhu F, Tao G. Clinical observation of improved passing peritoneum extraperitoneal cesarean sectron. Bulletin of Hunan Medical University 1997;22(5):434‐6. [PubMed] [Google Scholar]
Ezechi 2005 {published data only}
- Ezechi OC, Kalu BK, Njokanma FO, Nwokoro CA, Okeke GC. Uterine incision closure at caesarean section: a randomised comparative study of intraperitoneal closure and closure after temporary exteriorisation. West African Journal of Medicine 2005;24(1):41‐3. [DOI] [PubMed] [Google Scholar]
Lager 2007 {published data only}
- Lager JC, Spielman FJ, Boggess KA, Mayer D, Salo‐Coombs V. Exteriorization of the uterus and intraoperative nausea: a randomized, blinded trial [abstract]. Anesthesiology 2007;106(Suppl 1):15. [Google Scholar]
Magann 1993 {published data only}
- Magann EF, Dodson MK, Harris RL, Floyd RC, Martin JN, Morrison JC. Does method of placental removal or site of uterine incision repair alter endomyometritis after cesarean delivery?. American Journal of Obstetrics and Gynecology 1993;168:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nafisi 2006 {published data only}
- Nafisi S, Mohammadzadeh S. Influence of uterine exteriorization versus in situ repair on post‐cesarean maternal pain [abstract]. International Journal of Obstetric Anesthesia 2006;15 Suppl 1:S15. [DOI] [PubMed] [Google Scholar]
Nafisi 2007 {published data only}
- Nafisi S. Influence of uterine exteriorization versus in situ repair on post‐cesarean maternal pain: a randomized trial. International Journal of Obstetric Anesthesia 2007;16(2):135‐8. [DOI] [PubMed] [Google Scholar]
Orji 2008 {published data only}
- Orji EO, Olaleye AO, Loto OM, Ogunniyi SO. A randomised controlled trial of uterine exteriorisation and non‐exteriorisation at caesarean section. Australian and New Zealand Journal of Obstetrics and Gynaecology 2008;48(6):570‐4. [DOI] [PubMed] [Google Scholar]
Siddiqui 2005 {published data only}
- Siddiqui M, Goldszmidt E, Tharmaratnam U, Kingdom J, Windrim J, Carvalho J. Complications of exteriorized versus in situ uterine repair at cesarean section under spinal anesthesia [abstract]. Anesthesiology 2005;102(Suppl 1):13. [Google Scholar]
Siddiqui 2007 {published data only}
- Siddiqui M, Goldszmidt E, Fallah S, Kingdom J, Windrim R, Carvalho JC. Complications of exteriorized compared with in situ uterine repair at cesarean delivery under spinal anesthesia: a randomized controlled trial. Obstetrics & Gynecology 2007;110(3):570‐5. [DOI] [PubMed] [Google Scholar]
Sood 2003 {published data only}
- Sood AK. Exteriorization of uterus at cesarean section. Journal of Obstetrics and Gynecology of India 2003;53(4):353‐8. [Google Scholar]
Additional references
Belizan 1999
- Belizan J, Althabe F, Barros F, Alexander S. Rates and implication of cesarean sections in Latin America: ecological study. BMJ 1999;319:1397‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beukens 2001
- Beukens P. Over‐medicalisation of maternal care in developing countries. In: Brouwere Van Lerberghe editor(s). Safe motherhood strategies: a review of the evidence. Antwerp: ITG Press, 2001:195‐206. [Google Scholar]
Cai 1998
- Cai WW, Marks JS, Chen CH, Zhuang YX, Morris L, Harris JR. Increased caesarean section rates and emerging patterns of health insurance in Shangai, China. American Journal of Public Health 1998;88:777‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrie 1990
- Carrie LEF. Spinal and epidural blockade for caesarean section. In: Reynolds F editor(s). Epidural and spinal blockade in obstetrics. London: Bailliere Tindall, 1990:139‐50. [Google Scholar]
Cosgrove 1958
- Cosgrove RA. Caesarean section. Clinical Obstetrics and Gynaecology 1958;1:951‐2. [DOI] [PubMed] [Google Scholar]
Curtin 1997
- Curtin SC. Rates of caesarean birth and vaginal birth after previous caesarean section, 1991‐1995. Monthly Vital Statistics Report 1997; Vol. 45, issue 11 Suppl 3:1‐10.
Hofmeyr 2008
- Hofmeyr GJ, Mathai M, Shah AN, Novikova N. Techniques for caesarean section. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004662.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2000 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Stock 1985
- Stock RJ, Skelton H. Fatal pulmonary embolism occurring two hours after exteriorisation of the uterus for repair, following caesarean section. Military Medicine 1985;150:549‐51. [PubMed] [Google Scholar]
Thomas 2001
- Thomas J, Panjothy S. The National Sentinel Caesarean Section Audit Report. London: Royal College of Obstetricians and Gynaecologists, London, 2001. [Google Scholar]
References to other published versions of this review
Wilkinson 1996
- Wilkinson C, Enkin MW. Uterine exteriorization versus intraperitoneal repair at caesarean section. Cochrane Database of Systematic Reviews 1996, Issue 1. [DOI: 10.1002/14651858.CD000085] [DOI] [PubMed] [Google Scholar]


