Abstract
Background
Biochemical tests of placental or feto‐placental function were widely used in the 1960s and 1970s in high‐risk pregnancies to try to predict, and thus try to avoid, adverse fetal outcome.
Objectives
To assess the effects of performing biochemical tests of placental function in high‐risk, low‐risk, or unselected pregnancies.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (10 May 2012).
Selection criteria
Controlled trials (randomized or 'quasi‐randomized') that compare the use of biochemical tests of placental function in pregnancy with non‐use.
Data collection and analysis
Trial quality was assessed and data were extracted by the review author.
Main results
A single eligible trial of poor quality was identified. It involved 622 women with high‐risk pregnancies who had had plasma (o)estriol estimations. Women were allocated to have their (o)estriol results revealed or concealed on the basis of hospital record number (with attendant risk of selection bias). There were no obvious differences in perinatal mortality (relative risk (RR) 0.88, 95% confidence interval (CI) 0.36 to 2.13) or planned delivery (RR 0.97, 95% CI 0.81 to 1.15) between the two groups.
Authors' conclusions
The available trial data do not support the use of (o)estriol estimation in high‐risk pregnancies. The single small trial available does not have the power to exclude a beneficial effect but this is probably of historical interest since biochemical testing has been superseded by biophysical testing in antepartum fetal assessment.
Keywords: Female; Humans; Pregnancy; Biomarkers; Biomarkers/blood; Estriol; Estriol/blood; Fetal Diseases; Fetal Diseases/diagnosis; Placental Function Tests; Placental Function Tests/methods; Pregnancy, High‐Risk; Pregnancy, High‐Risk/blood; Randomized Controlled Trials as Topic
Plain language summary
Biochemical tests of placental function for assessment in pregnancy
Testing women's hormone levels during high‐risk pregnancy has not been shown to benefit women or their babies.
The placenta provides nourishment for the baby in the womb (uterus) during pregnancy. It has been thought that testing women's hormone levels during pregnancy, might show how well the placenta is functioning and whether the baby is growing as would be expected. (Hormones are natural chemicals produced in the body.) The review of one trial (622 women) found some evidence that measuring (o)estriol levels in high‐risk pregnancies did not affect the outcome of the pregnancy.
Background
A wide range of biochemical tests of fetal well‐being were introduced during the 1950s and 1960s, but there was little agreement on their usefulness (Alexander 1989). Greene 1965 listed more than 20 biochemical tests of placental function, but only two found an established role in antepartum assessment: urinary or plasma (o)estriols, and human placental lactogen (Chard 1982). Human placental lactogen is produced by the placenta while oestriol is produced by a biochemical pathway that involves both the placenta and the endocrine system of the fetus. Both hormones tend to demonstrate low (and sometime falling) levels in association with utero‐placental dysfunction manifesting as fetal growth restriction. Although there was little strong evidence to either commend or reject the use of these tests, they fell rapidly out of favour during the 1970s and became superceded by biophysical fetal testing, notably by antepartum cardiotocography and the ultrasound‐based fetal biophysical profile. Ironically, the evidence base for the use of these tests is similarly thin (Alfirevic 2002; Pattison 2002). It was not until 1995 that a systematic review of randomized controlled trials of use of any method of antepartum fetal assessment demonstrated any tangible evidence of benefit ‐ in this case, Doppler assessment of umbilical artery waveforms in high‐risk pregnancies (Alfirevic 1995; Neilson 2002).
It is possible that trials of the effects of using other biochemical tests may take place in the future. Both alpha‐fetoprotein (a fetal product) and human chorionic gonadotropin (a placental hormone) are biochemical tests used to screen for fetal chromosomal disorders in early to mid pregnancy. Both tests have a loose capability of predicting subsequent pregnancy complications including fetal growth restriction and pre‐eclampsia (e.g. Luckas 1998) and could, theoretically, provide the basis for future screening trials.
Objectives
To determine if knowledge of the results of placental or feto‐placental hormone levels are of benefit in improving fetal outcome or obstetric care in high‐risk, low‐risk, or unselected pregnancies.
Methods
Criteria for considering studies for this review
Types of studies
Any randomized or 'quasi‐randomized' controlled trial that assesses the effects of biochemical testing of placental or feto‐placental function in pregnancy and reports clinically meaningful results on an intention to treat basis.
Types of participants
Pregnant women with high‐risk, low‐risk, or unselected pregnancies.
Types of interventions
Biochemical tests that predict adverse pregnancy outcome.
Types of outcome measures
Adverse fetal outcomes, pregnancy complications, obstetric intervention.
Search methods for identification of studies
The Cochrane Pregnancy and Childbirth Group's Trials Register (10 May 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group. Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
No language restrictions were applied.
Data collection and analysis
Reports of identified trials that appeared relevant to the objectives of the review were evaluated for inclusion. Both published and unpublished reports could be included. Attempts would be made to translate identified, non‐English language reports. Primary authors would be contacted for additional details when necessary. Reasons for excluding apparently relevant trials are made explicit.
Included trials were assessed according to the following criteria: (1) adequate concealment of treatment allocation (e.g. sealed, opaque, numbered envelopes); (2) method of allocation to treatment (e.g. by computer randomisation, random number tables); (3) adequate documentation of how exclusions were handled after treatment allocation ‐ to facilitate 'intention to treat' analyses; (4) adequate blinding of outcome assessment, where appropriate; (5) losses to follow up (trials with losses of greater than 25% will be excluded).
Data were entered directly from reports into Review Manager software (RevMan 2000) and statistical analysis performed. For dichotomous data, relative risks (RRs) and 95% confidence intervals (CIs) were calculated. Weighted mean differences (WMDs) and 95% CIs were calculated for continuous data (Clarke 2001).
Heterogeneity between trials is tested using a standard chi squared test. In the presence of significant heterogeneity, a sensitivity analysis is used to explore the influence of high quality trials (fulfilling the criteria above) compared with those of lesser quality.
Results
Description of studies
A single trial of 622 women that met the criteria for this review was found (Duenhoelter 1976) (see table of Characteristics of included studies). Three potentially eligible trials were excluded ‐ seeCharacteristics of excluded studies for details.
Risk of bias in included studies
In the included study participants were allocated into groups by hospital number, with the attendant risk of selection bias.
Effects of interventions
In the Duenhoelter 1976 trial, there were similar rates of perinatal death (relative risk (RR) 0.88, 95% confidence interval (CI) 0.36 to 2.13) and planned delivery (RR 0.97, 95% CI 0.81 to 1.15) in the two groups (oestriol results reported or concealed).
Discussion
Available data from the single, identified trial provide no encouragement for the use of biochemical testing of feto‐placental wellbeing during pregnancy.
Authors' conclusions
Implications for practice.
There is no support from the single available randomized trial for the use of oestriol estimation in high‐risk pregnancies.
Implications for research.
It seems unlikely at the moment that this area will be a major focus for research effort in the future, but innovations in laboratory techniques could change that.
What's new
| Date | Event | Description |
|---|---|---|
| 27 June 2012 | New citation required but conclusions have not changed | Review updated with results of new search. |
| 10 May 2012 | New search has been performed | Search updated. No new trials identified |
History
Protocol first published: Issue 2, 1997 Review first published: Issue 2, 1997
| Date | Event | Description |
|---|---|---|
| 1 October 2009 | New search has been performed | Search updated. One new study identified and excluded (Sharf 1984). |
| 31 October 2008 | Amended | Converted to new review format. |
Acknowledgements
None.
Data and analyses
Comparison 1. Oestriol levels reported versus not reported.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fetal death | 1 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.16, 1.88] |
| 2 Neonatal death | 1 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.39, 6.74] |
| 3 Perinatal death | 1 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.36, 2.13] |
| 4 Induction of labour | 1 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.64, 1.06] |
| 5 Elective caesarean section | 1 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.89, 1.79] |
| 6 Planned delivery | 1 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.15] |
1.1. Analysis.
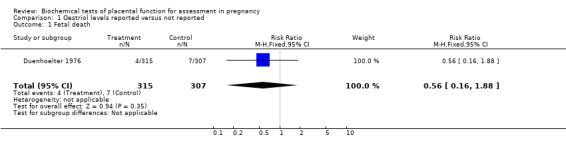
Comparison 1 Oestriol levels reported versus not reported, Outcome 1 Fetal death.
1.2. Analysis.
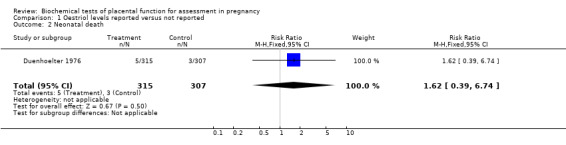
Comparison 1 Oestriol levels reported versus not reported, Outcome 2 Neonatal death.
1.3. Analysis.
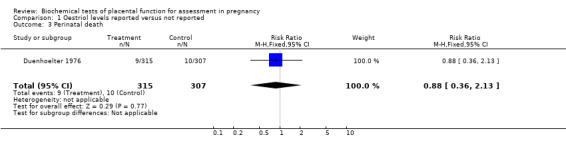
Comparison 1 Oestriol levels reported versus not reported, Outcome 3 Perinatal death.
1.4. Analysis.
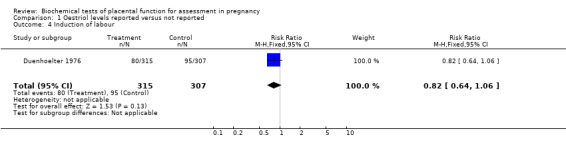
Comparison 1 Oestriol levels reported versus not reported, Outcome 4 Induction of labour.
1.5. Analysis.
Comparison 1 Oestriol levels reported versus not reported, Outcome 5 Elective caesarean section.
1.6. Analysis.
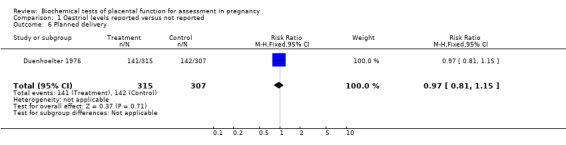
Comparison 1 Oestriol levels reported versus not reported, Outcome 6 Planned delivery.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Duenhoelter 1976.
| Methods | 'Quasi‐randomization' by hospital record number. Group A (reported group), plasma oestrogen levels measured and reported promptly. Group B (concealed group), plasma oestrogen levels measured but values were neither computed nor reported; they were computed and evaluated retrospectively. A total of 4,678 plasma samples were assayed in the 622 women, an average of 7.5 samples per woman. |
|
| Participants | 622 women with high risk pregnancies, including fetal growth restriction, hypertension,
adverse obstetric history. There were 315 in Group A (reported group) and 307 in Group B (concealed group). |
|
| Interventions | Oestriol results revealed or concealed. | |
| Outcomes | Perinatal deaths (stillbirths and neonatal deaths), planned delivery (induction of labour and elective caesarean section). | |
| Notes | The study was conducted at two different sites which dealt with high risk obstetric problems. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Grudzinskas 1990 | Trial abandoned ‐ no data available. |
| Sharf 1984 | 'Patients were divided into two ... groups according to the diagnoses, ages, parity and weeks of gestation' ‐ so unlikely to have been allocated randomly. |
| Spellacy 1975 | Data only available for the 8% of participants who had abnormally low human placental lactogen results. |
Contributions of authors
JP Neilson prepared and maintains the review.
Sources of support
Internal sources
University of Liverpool, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Duenhoelter 1976 {published data only}
- Duenhoelter JH, Whalley PJ, MacDonald PC. An analysis of the utility of plasma immunoreactive estrogen measurements in determining delivery time of gravidas with a fetus considered at high risk. American Journal of Obstetrics and Gynecology 1976;125:889‐98. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Grudzinskas 1990 {unpublished data only}
- Grudzinskas JG. To assess the effects of biochemical placental function testing. Personal Communication 1990.
Sharf 1984 {published data only}
- Sharf M, Eibschitz I, Hakim M, Degani S, Rosner B. Is serum free estriol measurement essential in the management of hypertensive disorders during pregnancy?. European Journal of Obstetrics & Gynecology and Reproductive Biology 1984;17:365‐75. [DOI] [PubMed] [Google Scholar]
Spellacy 1975 {published data only}
- Spellacy WN, Buhi WC, Birk SA. The effectiveness of human placental lactogen measurements as an adjunct in decreasing perinatal deaths. Results of a retrospective and randomized controlled prospective study. American Journal of Obstetrics and Gynecology 1975;121:835‐44. [PubMed] [Google Scholar]
Additional references
Alexander 1989
- Alexander S, Stanwell‐Smith R, Buekens P, Keirse MJNC. Biochemical assessment of fetal well‐being. In: Chalmers I, Enkin MW, Keirse MJNC editor(s). Effective care in pregnancy and childbirth. Vol. 1, Oxford: Oxford University Press, 1989:455‐76. [Google Scholar]
Alfirevic 1995
- Alfirevic Z, Neilson JP. Doppler ultrasonography in high‐risk pregnancies: systematic review with meta‐analysis. American Journal of Obstetrics and Gynecology 1995;172:1379‐87. [DOI] [PubMed] [Google Scholar]
Alfirevic 2002
- Alfirevic Z, Neilson JP. Biophysical profile for fetal assessment in high risk pregnancies. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI] [PubMed] [Google Scholar]
Chard 1982
- Chard T, Klopper A. Placental function tests. Berlin: Springer‐Verlag, 1982. [Google Scholar]
Clarke 2001
- Clarke M, Oxman AD, editors. Cochrane Reviewers’ Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1. Oxford, England: The Cochrane Collaboration, 2000.
Greene 1965
- Greene JW Jr, Duhring JL, Smith K. Placental function test. A review of methods available for assessment of the fetoplacental complex. American Journal of Obstetrics and Gynecology 1965;92:1030‐58. [PubMed] [Google Scholar]
Luckas 1998
- Luckas MJ, Sandland R, Hawe J, Neilson JP, McFadyen IR, Meekins JW. Fetal growth retardation and second trimester maternal serum human chorionic gonadotrophin levels. Placenta 1998;19:143‐7. [DOI] [PubMed] [Google Scholar]
Neilson 2002
- Neilson JP, Alfirevic Z. Doppler ultrasound for fetal assessment in high risk pregnancies. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI] [PubMed] [Google Scholar]
Pattison 2002
- Pattison N, McCowan L. Cardiotocography for antepartum fetal assessment. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI] [PubMed] [Google Scholar]
RevMan 2000 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
References to other published versions of this review
Neilson 1995
- Neilson JP. Hormonal Placental Function Tests [ revised 12 May 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds) Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Neilson 1997
- Neilson JP, Cloherty LJ. Hormonal placental function tests for fetal assessment in high risk pregnancies. Cochrane Database of Systematic Reviews 1997, Issue 2. [DOI] [PubMed] [Google Scholar]


