Abstract
Background
We determined the levels of 11 soluble immune mediators in oral washings of AIDS Clinical Trials Group A5254 participants with varying degrees of plasma viremia and CD4 T-cell counts to characterize the mucosal immune response at different stages of HIV-1 infection.
Methods
A5254 was a multicenter, cross-sectional study in people with HIV (PWH) recruited into 4 strata based on CD4 count and levels of plasma viremia: stratum (St) A: CD4 ≤200 cells/mm3, HIV-1 RNA (viral load [VL]) >1000 cps/mL; St B: CD4 ≤200, VL ≤1000; St C: CD4 >200, VL >1000; St D: CD4 >200, VL ≤1000. Oral/throat washings were obtained from all participants. Soluble markers were tested in oral/throat washings using a multibead fluorescent platform and were compared across strata. Linear regression was used to determine the associations between cytokines and HIV-1 in plasma and oral fluid.
Results
St A participants had higher levels of interleukin (IL)-1β, IL-6, IL-17, tumor necrosis factor alpha (TNFα), and interferon gamma (IFNγ) compared with St B and D (P = .02; P < .0001) but were not different from St C. IL-8, IL-10, and IL-12 were elevated in St A compared with the other 3 strata (P = .046; P < .0001). Linear regression demonstrated that oral HIV-1 levels were associated with IL-1β, IL-6, IL-8, and TNFα production (R > .40; P < .001) when controlling for CD4 count and opportunistic infections.
Conclusions
Our results show that high levels of oral HIV-1, rather than low CD4 counts, were linked to the production of oral immune mediators. Participants with AIDS and uncontrolled viremia demonstrated higher levels of pro- and anti-inflammatory soluble immune mediators compared with participants with lower HIV-1 RNA. The interplay of HIV-1 and these immune mediators could be important in the oral health of PWH.
Keywords: HIV-1, cytokines, humans, immune response, saliva
HIV-1 infection disrupts oral mucosal immunity, but the pathogenesis of this immune dysregulation remains unclear. Progressive HIV-1 infection and subsequent immune deficiency increase susceptibility to a variety of oral opportunistic infections (OIs) including oral candidiasis, hairy leukoplakia, and Kaposi’s sarcoma [1]. In healthy individuals, oral mucosal immunity is maintained by a combination of cellular and molecular components [2]. Epithelial cells maintain the mucosal barrier, express pattern recognition receptors, and secrete cytokines and host defense peptides in response to pathogenic stimuli [2]. A variety of immune cells also aid in the maintenance of mucosal immunity and the oral microbiome [3].
During HIV-1 infection, the saliva is known to harbor viral particles, cytokines, and anti-HIV-1 antibodies [4]. HIV-1 infection is believed to cause significant oral immune dysregulation by altering local cytokine expression, leading to alterations in local innate immunity and, subsequently, a poor immune response to infectious exposures [2]. Multiple variables have been shown to impact the oral production of soluble immune mediators during HIV-1 infection, including ART use, CD4 T-cell count, HIV-1 viral load (VL), smoking status, opportunistic infections, and time from HIV-1 diagnosis [5–11]. However, the relative contribution of these factors to the oral cytokine response remains undefined.
We aimed to formally assess the effects of HIV-1 viremia and CD4 T cells on oral immunity using oral/throat wash samples, plasma HIV-1 VL, CD4 T-cell counts, and demographic data obtained through the AIDS Clinical Trials Group (ACTG) A5254 protocol. This cross-sectional study evaluated whether nonprofessional oral health specialists could accurately diagnose common oral lesions in this population [12]. A follow-up study by Dittmer et al. [13] using this same study population showed increased detection rates of cytomegalovirus (CMV) and herpes simplex–1 virus (HSV-1), but not Epstein-Barr virus (EBV) or human herpesvirus 8 (HHV8 aka Kaposi’s sarcoma–associated herpesvirus) in the oral cavity of those with AIDS and an HIV-1 VL >1000 copies/mL [13]. Only oral CMV shedding was directly associated with plasma HIV-1 VL when controlling for other variables [13].
In the present study, using the previously obtained clinical data and cryopreserved oral/throat wash specimens from ACTG A5254 [12], we determined the association between levels of plasma and oral HIV-1 RNA and CD4 T-cell count on the expression of the oral soluble mediators interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, interferon gamma (IFNγ), and tumor necrosis factor alpha (TNFα). To provide a comprehensive evaluation, we measured both pro- and anti-inflammatory cytokines that are associated with innate immunity and different T-helper cell responses (Th1, Th2, Th17) so we could better characterize the effects of these variables on local mucosal immunity. We also evaluated the impact of oral co-infection with Candida, CMV, EBV, HSV-1, and HHV-8 on soluble mediator levels.
METHODS
Study Design
ACTG A5254 is a multicenter cross-sectional study that enrolled PWH aged 18 years or older with or without prior use of ART across 5 clinical sites in the United States and 1 in Haiti. Participants had plasma HIV-1 RNA VL and CD4 T-cell counts obtained during enrollment. Those receiving ART needed to be on the same regimen for at least 12 weeks before enrollment. Participants not taking ART must have stopped all ART for at least 30 days before study entry. OI prophylaxis was not included in the inclusion criteria, but use of systemic antifungal therapy within 90 days of enrollment was an exclusion criterion. The institutional review board or ethics committee of each participating institution approved the study, and each patient gave written informed consent. Detailed information on study design and population characteristics has been previously published [12].
Participants were divided into 4 strata according to their screening CD4 T-cell count and HIV-1 RNA VL regardless of whether they were on ART (Table 1).
Table 1.
Study Participant Demographics
| N = 288 | A (n = 148) | B (n = 82) | C (n = 29) | D (n = 29) | |
|---|---|---|---|---|---|
| Age, median, y | 44 | 41 | 47 | 38 | 45 |
| Sex, No. (%) | |||||
| Male | 193 (67) | 83 (56) | 70 (85) | 20 (69) | 22 (75) |
| Female | 95 (33) | 65 (44) | 12 (15) | 9 (31) | 7 (25) |
| Race, No. (%) | |||||
| Black, non-Latino | 207 (72) | 132 (89) | 48 (59) | 18 (62) | 12 (42) |
| White, non-Latino | 49 (17) | 7 (5) | 6 (7) | 7 (24) | 5 (17) |
| Latino | 26 (9) | 6 (4) | 26 (32) | 4 (14) | 11 (38) |
| other | 6 (2) | 3 (2) | 2 (2) | 0 (0) | 1 (3) |
| Intravenous drug use, No. (%) | |||||
| Never used | 245 (85) | 133 (90) | 63 (77) | 24 (83) | 23 (79) |
| Current use | 3 (1) | 2 (1) | 1 (1) | 0 (0) | 1 (3) |
| Past use | 40 (14) | 13 (9) | 18 (22) | 5 (17) | 5 (18) |
| Currently on ART, No. (%) | 193 (67) | 77 (52) | 80 (98) | 6 (21) | 29 (100) |
| History of an AIDS-defining illness, No. (%) | 23 (8) | 7 (5) | 10 (12) | 3 (10) | 2 (7) |
Abbreviation: ART, antiretroviral therapy.
Stratum A: CD4 ≤200 cells/mm3, plasma HIV-1 VL >1000 copies/mL
Stratum B: CD4 ≤200 cells/mm3, plasma HIV-1 VL ≤1000 copies/mL
Stratum C: CD4 >200 cells/mm3, plasma HIV-1 VL >1000 copies/mL
Stratum D: CD4 >200 cells/mm3, plasma HIV-1 VL ≤1000 copies/mL
A 1-minute oral/throat wash using 10 mL of sterile saline was collected and frozen in aliquots at –80°C. Oral/throat wash samples were used to measure oral HIV-1 RNA and soluble immune mediators. CD4 counts and levels of HIV-1 RNA in both plasma and oral fluid were measured in Clinical Laboratory Improvement Amendments (CLIA)-certified laboratories at the individual ACTG-participating clinical lab sites in A5254.
Evaluation of Oral Soluble Immune Mediators, Coinfections, and OIs
Cryopreserved oral/throat wash specimens were thawed, and oral cytokine concentrations of IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, IFNγ, and TNFα were analyzed using an electrochemiluminescence platform (Meso Scale Discovery, Rockville, MD, USA). Real-time quantitative polymerase chain reaction was used to detect multiple herpesviruses (CMV, EBV, KSHV, HSV-1), as described previously [13]. Oral examination was also performed for OIs (eg, candidiasis, hairy leukoplakia) by an Oral Health Specialist at each participating ACTG clinical site. In all participants, 2.5 mL of the oral/throat wash specimen was extracted and cultured for the presence of Candida as previously described [12].
Statistical Analysis
Cytokine differences between each stratum were evaluated using the Kruskal-Wallis test with the Dunn’s post-test adjusted for multiple comparisons to accommodate the non-normal distribution of the data. GraphPad Prism statistical analysis software was used to perform these analyses and generate figures. Linear regression analysis was used to model the relationship between cytokine production and oral or plasma HIV VL while controlling for CD4 count, oral candidiasis, and human herpesviruses. The VL and cytokines were log-transformed for this analysis. P values <.05 and R values ≥.40 were considered significant.
RESULTS
Sample Characteristics
Of the 328 participants who were enrolled in A5254, 288 participants with available specimens and oral HIV-1 RNA were included in this analysis (Table 1). The median age was 44 years, with a female:male ratio of ~2:1. The participants predominately identified as black, non-Latino (72%), and reported no history of intravenous drug use (85%). The majority of participants were receiving ART (67%) at study entry, and only 8% reported a history of an AIDS-defining illness. Fewer participants were enrolled in strata C and D (CD4 >200), as the aim of the initial study was to recruit those at higher risk of oral OIs. The overall number of HIV-1-related oral OIs was high among all participants, with rates of oral candidiasis at 47%, hairy leukoplakia at 12%, and Kaposi’s sarcoma at 10%.
Differences in Oral Production of Immune Mediators Among the Strata
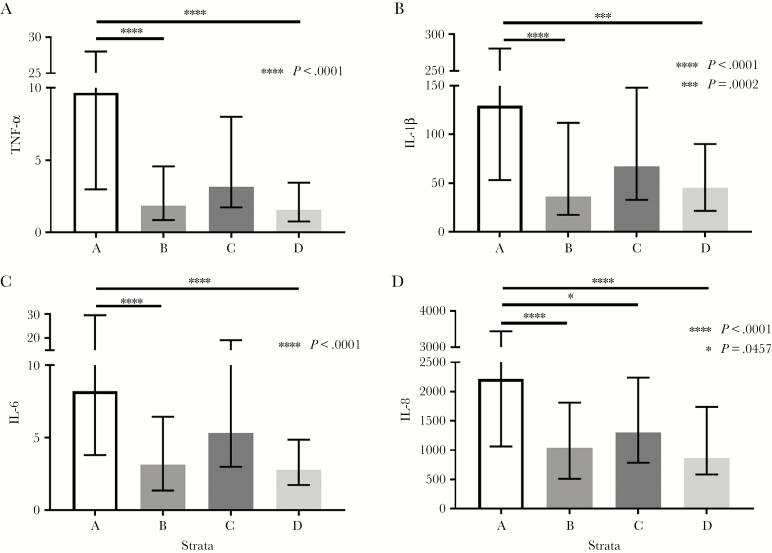
The pro-inflammatory immune mediators TNFα, IL-1β, IL-6, and IL-8 were significantly higher in stratum A compared with stratum B or stratum D (P < .001) (Figure 1A–D). Strata A and B consisted of participants with CD4 ≤200 and were only differentiated by a plasma HIV-1 RNA >1000 copies/mL in stratum A and ≤1000 copies/mL in stratum B. In contrast, stratum D includes only those study participants with CD4 >200 and plasma HIV-1 RNA ≤1000 copies/mL. IL-8 production was also significantly increased in stratum A compared with stratum C (P = .045), which both had viremia of >1000 but were differentiated by a CD4 ≤200 in stratum A and CD4 >200 in stratum C. Despite differences in CD4 counts, no differences were observed between Strata B and D, both of which had viremia <1000 cps/mL.
Figure 1.
Differences in oral production of tumor necrosis factor alpha (TNFα), interleukin (IL)-1β, IL-6, and IL-8 between the strata.
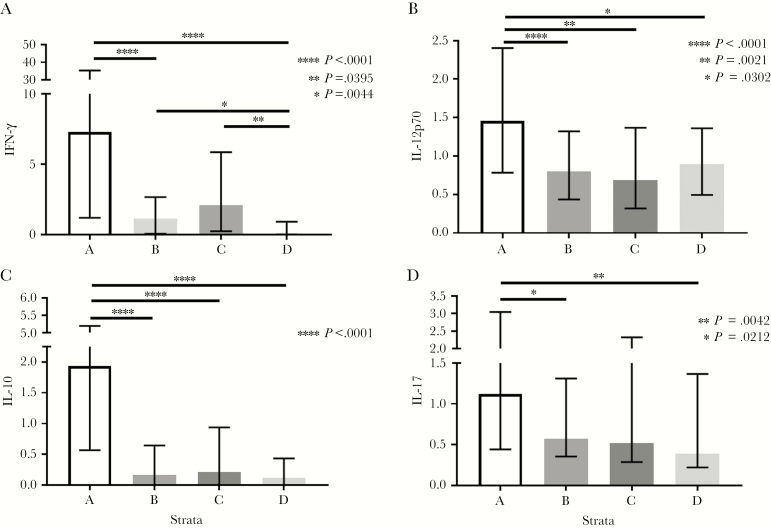
There were increased levels of both IL-12 and IFNγ in stratum A compared with stratum B (both P < .001) or stratum D (P = .03 and P < .001, respectively) (Figure 2A, B). Similarly, IL-12 production was higher in stratum A compared with stratum C (P = .002). IFNγ was the only cytokine with higher levels in stratum B and stratum C compared with stratum D (P = .004 and P = .039, respectively) (Figure 2B). IL-17, an important cytokine in maintaining gut mucosal immunity [14], was also elevated in stratum A compared with stratum B (P = .004) or stratum D (P = .021) (Figure 2C). Meanwhile, levels of IL-10, an anti-inflammatory cytokine, were significantly increased in stratum A when compared with the other 3 strata (P < .001) (Figure 2D). There were no substantial differences noted among the strata in levels of IL-2, IL-4, or IL-13 (data not shown).
Figure 2.
Differences in oral production of interferon gamma, interleukin (IL)-12p70, IL-10, and IL-17 between the strata.
Relationship Between Plasma HIV-1 VL and Oral Immune Mediator Production
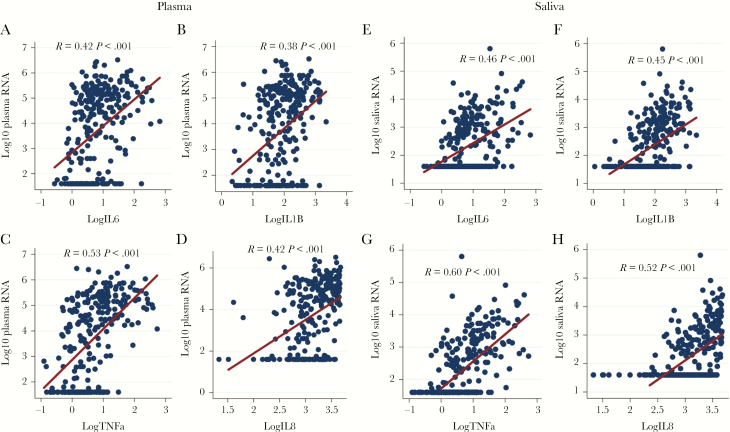
The differences in soluble markers among the strata suggest that HIV-1 viremia influences cytokine dysregulation more than differences in CD4 T-cell counts. We therefore used linear regression modeling to evaluate the relationship between plasma HIV-1 VL and oral immune mediator levels while controlling for CD4 T-cell counts, oral candidiasis, and human herpesvirus shedding. The strongest associations were observed between plasma HIV-1 RNA and TNFα (R = .53), IL-1β (R = .38), IL-6 (R = .42), and IL-8 (R = .42), all with P values <.001 (Figure 3A–D). No significant associations were found between plasma HIV-1 VL and IL-12, IFNγ, IL-17, IL-10, IL-4, IL-13, or IL-2 (data not shown).
Figure 3.
Linear regression modeling demonstrates a positive association between plasma and oral HIV-1 RNA and production of interleukin (IL)-6, IL-1β, tumor necrosis factor alpha (TNFα), and IL-8 when controlling for CD4 count and opportunistic infections.
Relationship Between Oral HIV-1 VL and Oral Cytokine Production
As oral HIV-1 RNA strongly correlated with plasma HIV-1 viremia (R = .76; P < .001), we determined whether levels of HIV-1 in oral fluid are also associated with levels of the immune mediators. The strongest associations were noted between oral HIV-1 VL and the production of TNFα (R = .60), IL-1β (R = .45), IL-6 (R = .46), and IL-8 (R = .52) when controlling for CD4 T-cell count, OIs, and co-infections (all P < .001) (Figure 3E–H). Although associations were similar, the correlations of soluble immune mediator levels with oral HIV-1 RNA were greater than with plasma HIV-1 RNA. No significant associations were identified between oral HIV-1 VL and IL-12, IFNγ, IL-17, IL-10, IL-4, IL-13, or IL-2 (data not shown).
Relationship Between CD4 T-Cell Count, Coinfections and OIs, and Oral Cytokine Production
Additional linear regression analyses were performed to evaluate associations between CD4 T-cell counts and oral cytokine production while controlling for HIV-1 VL, coinfections, and OIs. No associations were identified among these 3 parameters. We also evaluated if OIs (oral candidiasis) and human herpesvirus shedding (CMV, EBV, HHV-8, HSV-1) were significantly impacting production of specific cytokines. Linear regressions were performed comparing each OI or coinfection to the individual cytokines, while controlling for HIV-1 VL and CD4 counts, but no specific associations were found (data not shown). We then performed this same analysis using only strata B and D, as the majority of these participants were on ART (98%–100%) and therefore represented a sample more pertinent to the current HIV treatment era. However, again no OIs or coinfections were found to be significantly associated with any specific cytokine production in this population (data not shown).
DISCUSSION
We provide a comprehensive evaluation of the pathogenic effects of plasma and oral HIV-1 VL on oral mucosal cytokine production at different CD4 T-cell counts. We evaluated the oral mucosal production of 11 different soluble immune mediators as well as oral HIV-1 VL, oral candidiasis, and 4 human herpesviruses (CMV, EBV, HSV-1, HHV-8). In participants with poorly controlled HIV-1 infection (stratum A: CD4 ≤200, HIV-1 VL >1000), there was a significant increase in the oral production of the majority of cytokines tested, including IL-1β, IL-6, IL-8, IL-10, IL-12, IL-17, IFNγ, and TNFα. These cytokines are associated with both pro- and anti-inflammatory immune responses, and many of them correspond to different CD4 T-helper cell responses, including Th1 (IL-12, IFNγ), Th2 (IL-10), and Th17 (IL-17) [2, 15].
This aberrant increase of both pro- and anti-inflammatory cytokines appears counterintuitive but likely reflects the severe degree of mucosal immune dysregulation in this patient population. IL-10, an anti-inflammatory cytokine, serves as a critical regulator of inflammation, and its production is stimulated by many of the same pattern recognition receptors (PRRs) that drive early pro-inflammatory immune responses [16]. This dysregulated oral cytokine production provides a potential mechanistic explanation for the high risk of oral OIs and coinfections in this group.
Differences among the strata suggest that HIV-1 VL is a significant factor in driving this aberrant cytokine production. This was evidenced by the dramatic decrease in cytokine levels from stratum A to stratum B. Participants in these strata all had CD4 counts ≤200; however, those in stratum B were essentially all (ie, 98%) on “effective” ART with controlled plasma viremia <1000 cps/mL. This significant decrease in cytokine production between stratum A and stratum B was seen in all cytokines, which were elevated in stratum A (IL-1β, IL-6, IL-8, IL-10, IL-12, IL-17, IFNγ, TNFα). A similar decrease in production was seen in each of these cytokines between stratum A and stratum D. All participants in stratum D were also well controlled on ART, with plasma viremia <1000 cps/mL and CD4 counts >200.
Our comparisons of oral cytokine levels, CD4 T-cell counts, and both oral and blood HIV-1 VL during “effective” ART are of particular importance. A recent cross-sectional study by Elizondo et al. [10] compared gingival crevicular fluid cytokine production based on duration of ART (non-ART, ART-naïve [ART <1 year], short-term ART [>1 year and <6 years], and long-term ART [>6 years]) and found increased IL-6, IL-7, IL-10, and IL-12 in the gingival crevicular fluid of the non-ART group compared with the others. In contrast to our results, they did not find differences in other pro-inflammatory cytokines (IL-1β, IL-8, IL-17, TNFα, IFNγ) among these groups [10]. They concluded that HIV-1 viremia and ART are important factors in oral cytokine expression.
Lomeli-Martinez et al. [11] showed that participants with lower CD4 counts (CD4 ≤200) not on ART had higher levels of IFNγ, IL-10, IL-6, and IL-4 compared with those with CD4 counts >200 on ART. They also described increased oral production of TNFα, IFNγ, IL-10, and IL-4 in participants with CD4 counts ≤200 compared with those with CD4 >200, even when both groups were on ART. There were differences in HIV-1 plasma VL between these groups, but they did not evaluate for associations between plasma viremia and oral cytokine production. Similar cross-sectional studies by Nittayanta et al. [6, 7] compared production of oral immune mediators among PWH grouped based on duration of ART. In contrast to the prior studies, they showed increased production of IL-8 and human β-defensin in participants on short- and long-term ART compared with no ART; however, they reported no differences in TNFα or IL-6 production [6, 7]. They also found no difference in cytokine production in individuals with plasma HIV-1 VL <50 cps/mL compared with those with HIV-1 VL ≥50 cps/mL.
These previous studies combined with our results highlight the complex involvement of multiple variables in the production of oral immune mediators; however, ART and HIV-1 VL appear to be crucial in this process. Other variables such as Candida and herpesvirus infections have also consistently been shown to affect oral mucosal immunity [3, 8, 11]. Therefore, to more effectively evaluate the association between oral and plasma HIV-1 VL in terms of individual cytokine production, we performed linear regression analysis to control for key variables such as CD4 count, OIs (candidiasis), and herpesvirus infections (CMV, EBV, HSV-1, HHV-8). Linear regression identified TNFα, IL-1β, IL-6, and IL-8 as having the strongest associations with both oral and plasma HIV-1 VL using an R value >.40 as significant. IL-10, IL-12, IL-17, and IFNγ all had much weaker associations with HIV-1 VL, with R values around .10. Spear et al. performed a similar study in 2005 and found no significant associations between saliva HIV-1 RNA and oral cytokine production (IL-1β, IFN-γ, IL-10, IL-6, TNF-α) [9]. However, they did not perform a linear regression analysis and were not able to control for potential confounders. They also had a smaller sample size and detected saliva HIV-1 RNA in only 20 participants, potentially explaining the discrepancy between our results.
Interactions within the cytokine network are complex, but TNFα, IL-1β, IL-6, and IL-8 share common features that could explain their strong association with HIV-1 viremia in our study. They are relatively nonspecific, pro-inflammatory cytokines that are released early in the innate immune response [17]. They are secreted by immune cells, along with epithelial cells, keratinocytes, and fibroblasts [17]. Thus, we hypothesize that a high viral burden in the oral mucosa would persistently stimulate local PRRs on epithelial and immune cells, driving the production of these early pro-inflammatory mediators.
A notable limitation of our study is that 52% of the participants in stratum A were reportedly taking ART but had HIV-1 VLs >1000. Although this is a confounder for our results in stratum A, it is important to note that strata B and D provide a more accurate representation of participants on “effective” ART, with >98% on ART, and control of HIV-1 viremia (<1000 cps/mL). The earlier described studies demonstrate conflicting results on ART use and changes in mucosal cytokine production [6, 7, 10, 11]. Our results describe pertinent differences in oral immune mediators between participants with uncontrolled viremia on or off ART (stratum A) compared with those taking ART with effectively controlled HIV-1 virus replication (strata B and D). Based on these findings and the linear regression results, plasma and oral HIV-1 VL impact production of immune mediators. Future studies should consider not only ART status but control of plasma and oral HIV-1 VL when evaluating oral mucosal immunity.
Additionally, the improvements in mucosal cytokine production between stratum A and stratum B are supported by clinical observations described in the initial paper by Shiboski et al. [12]. The prevalence of oral candidiasis was 71% in stratum A compared with only 22% in stratum B, which was similar to the combined prevalence of oral candidiasis in strata C and D (22%) [12]. Oral candidiasis often resolves rapidly with the initiation of ART; this may be explained by the rapid resolution in aberrant cytokine production demonstrated between stratum A and stratum B. Both CMV and HSV-1 oral shedding were significantly decreased in stratum B compared with stratum A, indicating that improvement in cytokine dysregulation could assist in immune-mediated suppression of herpesvirus reactivations [13].
Other notable limitations of our study include the small sample sizes in strata C and D. The primary objective of the original study was to evaluate the prevalence of oral OIs, and therefore a higher number of individuals with CD4 counts ≤200 were enrolled in strata A and B. Detailed evaluation of periodontal health was not objectively graded in our study, and we were unable to evaluate the effect of periodontal disease on oral cytokine expression. Due to the cross-sectional design, clinical data were available from only 1 visit, and only oral/throat wash samples were collected as part of the research protocol. Additionally, per the original research protocol, a cutoff of plasma HIV-1 VL >1000 cps/mL was chosen to delineate the strata, rather than viral suppression by commercial assays, which could limit the generalizability of the results. Finally, a large number of participants in stratum A were enrolled in Haiti compared with the participants in the other strata, who were largely enrolled from US domestic sites; this represents a potential confounder.
HIV-1 is associated with compromised oral mucosal immunity [1]. Our study demonstrates severe oral immune dysregulation in participants with poorly controlled viremia and low CD4 counts, which likely contributes to the increased prevalence of oral OIs and coinfection with herpesviruses in this population. This oral immune dysfunction is directly associated with the amount of HIV-1 virus present both locally and systemically, although multiple other variables including CD4 count, OIs, and herpesvirus coinfections are also involved. There was a notable improvement in oral cytokine dysregulation with ART use and suppression of HIV-1 viremia, leading to clinical restoration of mucosal immunity. These improvements decreased the risk of oral candidiasis and reduced the shedding of human herpesviruses. However, it is well established that systemic immunity does not return to a baseline level with HIV-1 viral suppression or normalization of the CD4 count [3]. Subtle immune deficits persist in the oral mucosa, as evidenced by higher rates of periodontal disease and HPV oral infections, even in those with controlled HIV-1 infection on ART [18, 19]. Future studies on the mechanisms of chronic inflammation and their impact on the oral microbiome in well-controlled HIV-1 infection are essential.
Acknowledgments
We would like to express our sincere appreciation to the A5254 study participants and to the investigative teams of the ACTG clinical research sites that enrolled participants in protocol A5254.
Sites and contributors to protocol A5254. Jean William Pape, MD, Patrice Sévère, MD, Rode Secours, MD, Daphné Bernard, MD, and Maria Linda Aristhomène, RN—Les Centres Gheskio (Gheskio-INLR) CRS (Site 30022; ACTG CTU Grant U01 AI069421). Caroline Shiboski, DDS, MPH, PhD, Sivappiriyai Veluppillai, DDS, Amanda Hutton Parrott, DPT, NP, and Jay Dwyer, RN—UCSF AIDS CRS (Site 801) (ACTG CTU Grant UM1 AI069496). Judith A. Aberg, MD, Karen Cavanagh, RN, Alexander Ross Kerr, DDS, MSD, Sonal S. Shah, DDS, and Manley Lammarre, RDH—New York University HIV/AIDS CRS (Site 401; ACTG CTU Grant UM1AI069532). Jennifer Webster-Cyriaque, DDS, PhD, Jonathan Oakes, BA, Dirk P. Dittmer, PhD, and Lauren Patton, DDS—Chapel Hill CRS (Site 3201; ACTG CTU Grant UM1 AI069423-08, CTSA Grant 1UL1TR001111, Center for AIDS Research Grant P30 AI50410, RO1 DE018304). Jeffrey Lennox, MD, Dale Maddox, RN, and David A. Reznik, DDDS—The Ponce de Leon Ctr. CRS (Site 5802; Emory University HIV/AIDS CTU Grant 5UO1AI069418, Center for AIDS Research Grant P30 AI050409, Clinical and Translational Science Award Grant UL1 RR025008). Michael Lederman, MD, Jane Baum, RN, Mahmoud Ghannoum, PhD, Nancy Isham, and Richard Jurevic—Case CRS (Site 2501; ACTG CTU Grant AI069501).
Financial support. This study was supported in part by the National Institutes of Health (grant UM1-AI106701), by the National Institutes of Health Cooperative Agreement U01AI068636 from the National Institute of Allergy and Infectious Diseases and the National Institute of Dental and Craniofacial Research, and by internal funding from the Department of Medicine of the University of Pittsburgh School of Medicine.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Challacombe SJ, Naglik JR. The effects of HIV infection on oral mucosal immunity. Adv Dent Res 2006; 19:29–35. [DOI] [PubMed] [Google Scholar]
- 2. Heron SE, Elahi S. HIV infection and compromised mucosal immunity: oral manifestations and systemic inflammation. Front Immunol 2017; 8:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nittayananta W, Tao R, Jiang L, et al. Oral innate immunity in HIV infection in HAART era. J Oral Pathol Med 2016; 45:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campo J, Perea MA, del Romero J, et al. Oral transmission of HIV, reality or fiction? An update. Oral Dis 2006; 12:219–28. [DOI] [PubMed] [Google Scholar]
- 5. Leigh JE, Steele C, Wormley FL Jr, et al. Th1/Th2 cytokine expression in saliva of HIV-positive and HIV-negative individuals: a pilot study in HIV-positive individuals with oropharyngeal candidiasis. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 19:373–80. [DOI] [PubMed] [Google Scholar]
- 6. Nittayananta W, Amornthatree K, Kemapunmanus M, et al. Expression of oral cytokines in HIV-infected subjects with long-term use of antiretroviral therapy. Oral Dis 2014; 20:e57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nittayananta W, Kemapunmanus M, Amornthatree K, et al. Oral human β-defensin 2 in HIV-infected subjects with long-term use of antiretroviral therapy. J Oral Pathol Med 2013; 42:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Black KP, Merrill KW, Jackson S, Katz J. Cytokine profiles in parotid saliva from HIV-1-infected individuals: changes associated with opportunistic infections in the oral cavity. Oral Microbiol Immunol 2000; 15:74–81. [DOI] [PubMed] [Google Scholar]
- 9. Spear GT, Alves ME, Cohen MH, et al. Relationship of HIV RNA and cytokines in saliva from HIV-infected individuals. FEMS Immunol Med Microbiol 2005; 45:129–36. [DOI] [PubMed] [Google Scholar]
- 10. Elizondo JE, Rocha-Pizaña MD, Treviño AC, et al. Potential gingival crevicular fluid and serum biomarkers by stage of HIV infection. Cytokine 2017; 91:96–103. [DOI] [PubMed] [Google Scholar]
- 11. Lomeli-Martinez SM, Valentin-Goméz E, Varela-Hernández JJ, et al. Candida spp. determination and Th1/Th2 mixed cytokine profile in oral samples from HIV+ patients with chronic periodontitis. Front Immunol 2019; 10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiboski CH, Chen H, Secours R, et al. ; Oral HIV/AIDS Research Alliance, Subcommittee of the AIDS Clinical Trial Group High accuracy of common HIV-related oral disease diagnoses by non-oral health specialists in the AIDS clinical trial group. PLoS One 2015; 10:e0131001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dittmer DP, Tamburro K, Chen H, et al. Oral shedding of herpesviruses in HIV-infected patients with varying degrees of immune status. AIDS 2017; 31:2077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pandiyan P, Younes SA, Ribeiro SP, et al. Mucosal regulatory T cells and T helper 17 cells in HIV-associated immune activation. Front Immunol 2016; 7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slavinsky J 3rd, Myers T, Swoboda RK, et al. Th1/Th2 cytokine profiles in saliva of HIV-positive smokers with oropharyngeal candidiasis. Oral Microbiol Immunol 2002; 17:38–43. [DOI] [PubMed] [Google Scholar]
- 16. Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010; 10:170–81. [DOI] [PubMed] [Google Scholar]
- 17. Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol 2016; 138:984–1010. [DOI] [PubMed] [Google Scholar]
- 18. Amornthatree K, Sriplung H, Mitarnun W, Nittayananta W. Effects of long-term use of antiretroviral therapy on the prevalence of oral Epstein-Barr virus. J Oral Pathol Med 2012; 41:249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gillison ML. Oropharyngeal cancer: a potential consequence of concomitant HPV and HIV infection. Curr Opin Oncol 2009; 21:439–44. [DOI] [PubMed] [Google Scholar]