Abstract
A novel NIR (near infrared) active photothermal agent, CNTs-PAMAM-Ag2S has been synthesized by covalent grafting of polyamidoamine (PAMAM) to carbon nanotubes (CNTs) and subsequent deposition of Ag2S nanocrystallites. The potential of CNTs-PAMAM-Ag2S as a NIR active photothermal agent was preliminarily accessed by its electronic absorption spectrum measured in UV–vis–NIR region. The CNTs-PAMAM-Ag2S exhibited excellent photothermal effect and photothermal conversion efficiency of 26% under illumination with 980 nm laser, the efficiency was found to be higher than popular gold and copper based photothermal agents. Temperature attained by CNTs-PAMAM-Ag2S during measurement of its photothermal effect was 64.7 °C at 1 g/mL, which far exceeds the temperature tolerance level of cancer cells. So that CNTs-PAMAM-Ag2S could destroy the cancer cells in an effective manner. Furthermore, it was found the linear dependence of photothermal effect of CNTs-PAMAM-Ag2S over its concentration. CNTs-PAMAM-Ag2S possessed excellent stability against photo-bleaching and photo-corrosiveness. In consideration of its outstanding photothermal effect and photothermal conversion efficiency, CNTs-PAMAM-Ag2S could be a promising photothermal agent to employ in future photothermal therapy.
Keywords: Carbon nanotubes, Polyamidoamine, Ag2S, Photothermal effect, Near infrared
1. Introduction
Currently it is highly desired to develop the novel therapeutic strategies with high efficiency, specificity and precision to treat cancer as cancer has become one of the major threats to human. The conventional clinical therapies presently in use to destroy cancer cells such as surgical removal, chemotherapy and radiotherapy persist with series of critical barriers like lower efficacy and higher toxicity [1,2]. On account of sequenced challenges associated with classical therapies use of near infrared (NIR) light-triggered therapy (photothermal therapy, PTT) has gained recent attention owing to its uncompromised benefits viz., non-invasiveness, safety, and efficiency, [3–8]. The PTT consists of conversion of photon energy into thermal energy in presence of a photo-absorbing agent called photothermal agent under exposure to NIR radiations [9–11]. After delivery of photothermal agent into tumor or cancerous cells results in transfer of NIR radiations into thermal energy, elevate local temperature of cancer cell, and ablaze [12, 13]. PTT is a promising therapeutic method to treat cancer owing to its efficiency, accuracy and safety [3–8]. Additional is use of NIR radiations as this light is considered to be safer light source imposing negligible damage to normal tissues, deeper tissue penetration, and higher precise spatial/temporal resolution of body tissue [3,14,15]. Therefore, success of PTT relies exclusively on photothermal agent executed in therapy. To this contest, series of nanomaterials have been designed and their suitability as photothermal transducers in terms of photothermal effect (PTE) under exposure to NIR radiations were reported [3–8,16]. Among reported photothermal agents, carbon nanotubes (CNTs) are especially interesting due to their unique physical and chemical properties, and excellent biocompatibility [17–20]. Due to photothermal efficiency and deep penetration ability through biological tissues, CNTs have been extensively applied in PTT [17–20]. The benefit in use of CNTs is ease of surface functionalization for customized target. While several polymers have been explored to functionalize CNTs, however, we are more inclined to explore the use of polyamidoamine (PAMAM) due to its unique structure and excellent biocompatibility [21,22]. Nevertheless, application of PAMAM in PTT has been least explored, so its further exploration as a phothermal agent is a current critical requirement.
Ag2S is an important class of chalcogenide, which exhibit narrow band gap and strong absorption coefficient [6,23]. Ag2S persist with needful vital properties such as low cytotoxicity and high stability in aqueous solution that prevents the release of siliver ions into biological surroundings [6]. Moreover, Ag2S absorbs strongly to the NIR radiations, high anti-photobleaching, elevated photoconversion efficiency and ideal inertness [5]. However, irregular morphology and nonhomogeneous size distribution of Ag2S has restricted its amazing photo-responsive properties and hampered its wide range of applications in biomedical field, especially as photothermal agent. Therefore acute challenge still persisting with rational design to produce homogeneous Ag2S nanocrystallites having efficient absorption of NIR radiations and their elevated photothermal conversion efficiency. In this consideration, herein we report the modification of Ag2S nanocrystallites for their effective absorption of NIR radiations and impressive conversion to thermal energy by depositing them over nanostructured framework of PAMAM grafted to CNTs. Thus created CNTs-PAMAM-Ag2S demonstrated exceptional PTE and photothermal conversion efficiency by illuminating to 980 nm NIR laser.
2. Experimental section
2. 1. Materials
All reagents were purchased from Sigma-Aldrich and used without further purification unless otherwise noted and the aqueous solutions were prepared using ultrapure water obtained from Milli-Q Plus system (Millipore).
2.2. Preparation of CNTs-PAMAM-Ag2S
Pristine CNTs were modified to carboxylated and acylated form before grafting of PAMAM [24]. Then acylated CNTs were utilized in covalent grafting of fourth generation PAMAM by repeated process of Michael addition using methylmethacrylate to the surface amino groups and amidation of terminal ester groups with ethylenediamine [24]. Followed by Ag2S nanocrystallites were deposited over PAMAM grafted CNTs (CNTs-PAMAM) by dispersing them in 25 mL methanol and addition of silver nitrate (0.01mol L−1). Then 20 mL of Na2S in methanol (0.01 mol L−1) was added and the resulting mixture was stirred under ambient condition for 6 h to yield CNTs-PAMAM-Ag2S.
2.3. Preparation of Ag2S nanocrystallites
The Ag2S nanocrystallites were prepared according to similar procedure mentioned for CNTs-PAMAM-Ag2S without using CNTs-PAMAM.
2.4. Photothermal effect
The photothermal effect (PTE) of the samples was evaluated using 980 nm NIR diode laser system (Armlaser Inc. USA) with an output power of 1 and 2 W/cm2. In each experiment, 1 mL aqueous dispersion of the sample was transferred into 1 × 1 ×4 cm3 quartz cuvette and illuminated with NIR laser. The resulting elevation in temperature mediated by the exposure to NIR radiations was measured using Hanna precision digital thermometer (Model: HI93510) with a thermocouple immersed in aqueous dispersion of sample during experiment.
2.5. Characterization
The XRD patterns were recorded by Scintag X-ray diffractometer, model PAD X, equipped with a Cu Kα photon source (45 kV, 40 mA) and the TEM images were perceived with JEOL 2010 microscope. The UV–vis–NIR absorption spectra of samples were recorded using Jasco V-770 spectrophotometer.
3. Results and discussion
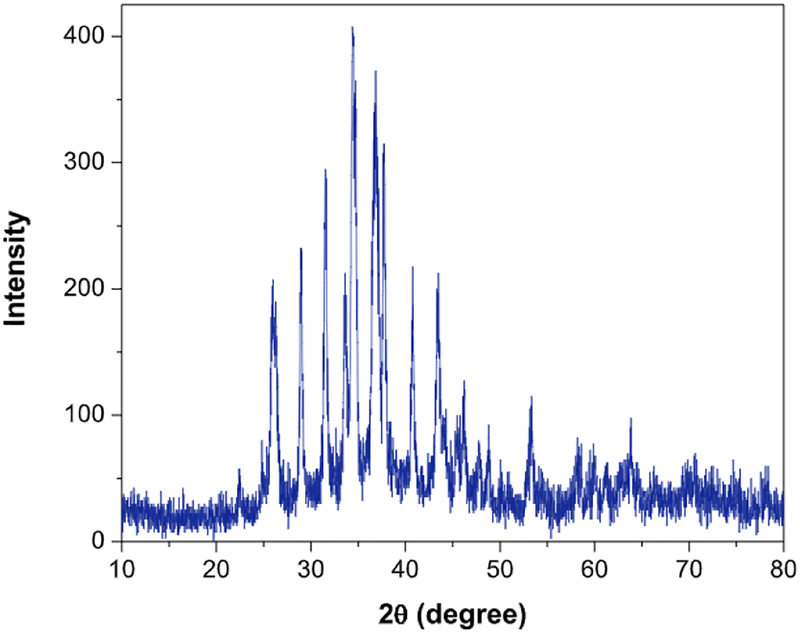
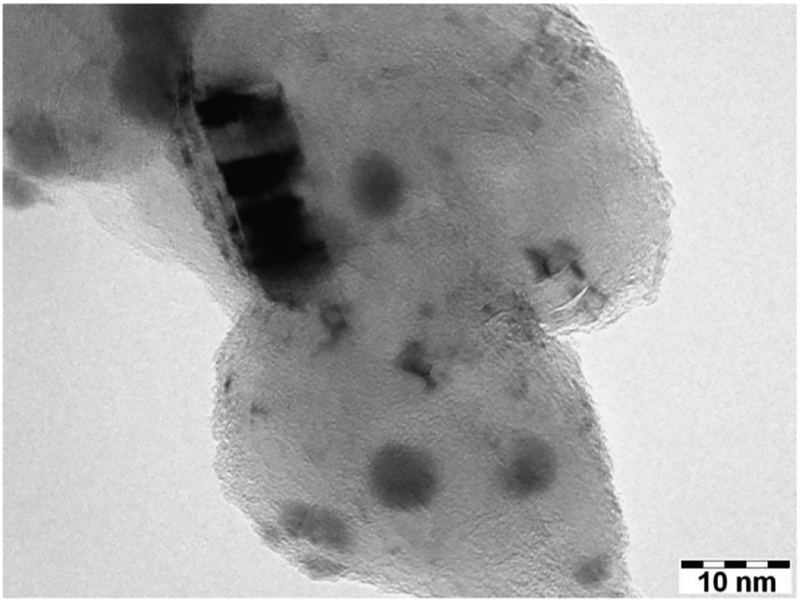
The XRD of CNTs-PAMAM-Ag2S (Fig. 1) demonstrated the peak corresponding to (0 0 2) plane of graphitic shells of CNTs at 26.3°. In addition, it displayed the distinct peaks resolved at 22.4°, 26.0°, 29.0°, 31.5°, 33.6°, 34.4°, 36.8°, 37.8°, 40.7°, 43.5°, 46.2°, 47.7°, 48.8°, 53.3° and 63.8° owing to (−101), (−111), (111), (−112), (120), (−121), (121), (−103), (031), (200), (−123), (−212), (014), (−213)and (−134) planes of acanthite structured Ag2S nanocrystallites, respectively (JCPDS 14–0072). The TEM image of CNTs-PAMAM-Ag2S shown in Fig. 2 exhibit the deposition of Ag2S nanocrystallites and their uniform distribution over the surface of PAMAM grafted to CNTs. The binding of PAMAM to the surface of CNTs is definable in Fig. 2. The strong adherence of Ag2S nanocrystallites over the surface of PAMAM is perceptible in TEM image. The shape of Ag2S nanocrystallites exists in CNTs-PAMAM-Ag2S was found to be spherical and their diameter was around 9 nm.
Fig. 1.
XRD of CNTs-PAMAM-Ag2S.
Fig. 2.
TEM image of CNTs-PAMAM-Ag2S.
The suitability of CNTs-PAMAM-Ag2S as a NIR active photothermal agent was preliminarily accessed by its electronic absorption spectrum measured in UV–vis–NIR region. The spectrum of CNTs (Fig. 3) has exhibited a shoulder band around 231 nm, which is ascribed to C=C bonds of CNTs [25]. After covalent grafting of PAMAM, the characteristic absorption of CNTs has turned to clear band and its position has shifted to 237 nm in CNTs-PAMAM (Fig. 3). The shifting of 6 nm found in the characteristic band of CNTs-PAMAM compared to CNTs is accounted for strong adherence of PAMAM chains to the surface of CNTs. The spectrum of CNTs-PAMAM-Ag2S (Fig. 3) displayed the characteristic band of CNTs at 237 nm, in addition, it demonstrated an absorption tail between 470 and 530 nm corresponding to monoclinic Ag2S nanocrystallites [6,25]. Compared to CNTs-PAMAM, the characteristic band of CNTs has not shifted in CNTs-PAMAM-Ag2S, which reveals that structure of CNTs-PAMAM has not been affected by deposition of Ag2S nanocrystallites. The significant absorption of aqueous dispersion of CNTs-PAMAM-Ag2S in NIR region is a primary assessment to employ it as a NIR active photothermal agent.
Fig. 3.
UV–vis–NIR spectra of CNTs, CNTs-PAMAM and CNTs-PAMAM-Ag2S.
In consideration of its significant absorbance in NIR region, PTE of CNTs-PAMAM-Ag2S was measured with its aqueous dispersion of 1 mg/mL and compared with PTE of water, CNTs and CNTs-PAMAM (Fig. 4). The total rise in temperature (ΔT) of 8.1 °C was found for water under its continuous irradiation for 7 min with 980 nm laser (Table 1). CNTs and CNTs-PAMAM exhibited the elevation in temperature of 30.2 and 36.5 °C, respectively. PTE of CNTs-PAMAM was 6.3 °C higher than the value measured for CNTs, so, absorption rate of CNTs to NIR radiations and their conversion to thermal energy was significantly improved by conjugation with PAMAM in CNTs-PAMAM. PTE of CNTs-PAMAM was further enhanced by anchoring of Ag2S nanocrystallites. After deposition of Ag2S nanocrystallites over surface of CNTs-PAMAM, PTE of CNTs-PAMAM-Ag2S has increased. Nevertheless, PTE measured for Ag2S nanocrystallites was lower than the PTE of CNTs-PAMAM-Ag2S and higher than that of CNTs.
Fig. 4.
Rise in temperature for aqueous dispersions of samples as a function of time of illumination under exposure to 980 nm laser.
Table 1.
Temperature of samples measured during measurement of photothermal effect using 980 nm laser.
| Sample | Power of laser (W/cm2) | Concentration of aqueous dispersion (mg/mL) | Initial temperature before irradiation (°C) | Final temperature after irradiation for 7 min (°C) | ΔT (Tfinal-Tinitial) (°C) |
|---|---|---|---|---|---|
| Water | 2 | – | 20.8 | 28.9 | 8.1 |
| CNTs | 2 | 1.00 | 20.8 | 51.0 | 30.2 |
| CNTs-PAMAM | 2 | 1.00 | 20.7 | 57.2 | 36.5 |
| Ag2S | 2 | 1.00 | 20.4 | 60.9 | 40.5 |
| CNTs-PAMAM-Ag2S | 2 | 1.00 | 20.5 | 64.7 | 44.2 |
| CNTs-PAMAM-Ag2S | 2 | 0.50 | 20.2 | 62.8 | 42.6 |
| CNTs-PAMAM-Ag2S | 2 | 0.25 | 20.7 | 61.0 | 40.3 |
| CNTs-PAMAM-Ag2S | 1 | 1.00 | 20.7 | 62.4 | 41.7 |
Further the ΔT value recorded for CNTs-PAMAM-Ag2S was 44.2 °C, while it was 30.2, 36.5 and 40.5 °C for CNTs, CNTs-PAMAM and Ag2S nanocrystallites, respectively. So that, systematic enhancement in PTE of CNTs was attained by grafting of PAMAM and successive deposition of Ag2S nanocrystallites. However, PTE measured for water was considerably low, therefore contribution of water in PTE accounted for CNTs, CNTs-PAMAM, Ag2S nanocrystallites and CNTs-PAMAM-Ag2S was negligible. Overall a systematic enhancement in the PTE of CNTs was attained by grafting of PAMAM, and consecutive immobilization of Ag2S nanocrystallites. Therefore, synergetic combination of CNTs, PAMAM and Ag2S was significantly enhanced the PTE of CNTs-PAMAM-Ag2S compared to its individual components.
Further to verify the excellence in PTE of CNTs-PAMAM-Ag2S, its photothermal conversion efficiency (η) was calculated using equation (1) [25–30],
| (1) |
where η is photothermal efficiency, h is heat transfer coefficient and S is surface area of the sample container. Tmax is maximum temperature attained by sample and Tsurr is surrounding temperature (Table 2). I is power of laser source (2000 mW) and A980 is absorbance of aqueous dispersion of samples at an excitation wavelength of 980 nm. Qdis is rate of heat dissipated due to absorption of light by solvent and container.
Table 2.
Properties used in calculation of photothermal conversion efficiency.
| Sample | Tmax (°C) | Tsurr (°C) | Tmax − Tsurr (°C) | A980 | η (%) |
|---|---|---|---|---|---|
| CNTs-PAMAM | 62.1 | 20.3 | 41.8 | 1.021 | 23 |
| CNTs-PAMAM-Ag2S | 64.1 | 20.7 | 43.4 | 1.129 | 32 |
To calculate the value of hS, a dimensionless driving force of temperature, θ is introduced and scaled using the maximum system temperature, Tmax and the surrounding temperature, Tsurr
| (2) |
and the sample system time constant, τs was evaluated using the following equation (3)
| (3) |
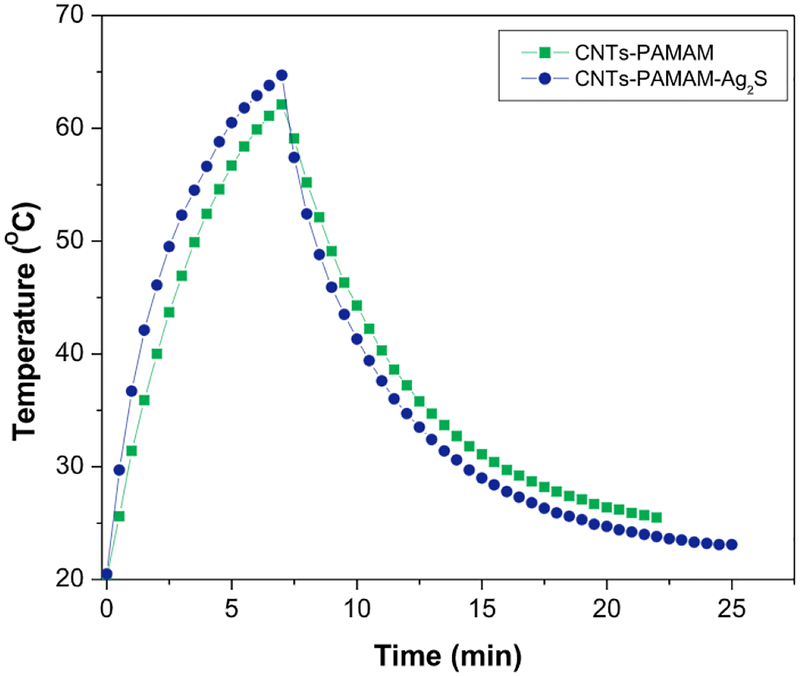
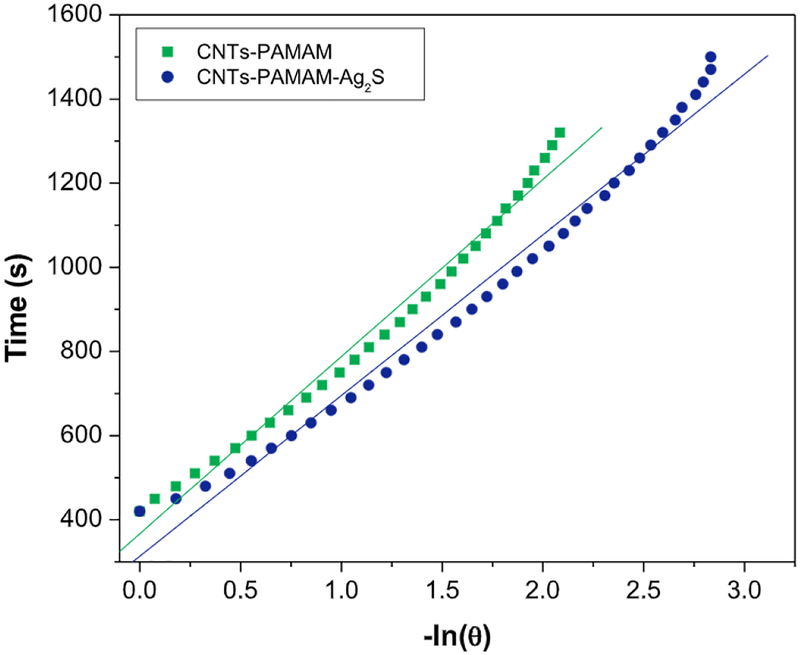
The value of τs was calculated by Figs. 5 and 6, and using its value, the unknown parameter, hS was evaluated with the help of following equation (4).
| (4) |
where mD is mass of DI water (1.01 g) and CD is its heat capacity. The value of Qdis was measured separately using quartz cuvette containing only DI water without any sample and it was found to be 25.9mW.
Fig. 5.
Temperature variation found for aqueous dispersion of CNTs-PAMAM and CNTs-PAMAM-Ag2S under exposure to 980 nm laser followed by its shut off.
Fig. 6.
The plot of time from cooling period versus negative natural logarithm of driving force temperature obtained for CNTs-PAMAM and CNTs-PAMAM-Ag2S using the data shown in Fig. 5.
On basis of above calculation, the value of photothermal conversion efficiency (η) estimated for CNTs-PAMAM-Ag2S was 26%, while it was 23% for CNTs-PAMAM. By anchoring of Ag2S nanocrystallites over CNTs-PAMAM an increment of 3% in the value of η was observed for CNTs-PAMAM-Ag2S. Overall, CNTs-PAMAM-Ag2S exhibited exceptional photothermal conversion efficiency and it is found higher than the value reported for popular Au and Cu based photothermal agents viz., Au nanoshells (25 and 18%) [28,31], Au nanorods (22%) [32], Au nanoparticles (21 and 22%) [17], Cu2-xSe nanoparticles (22%) [33], Cu9S5 nanoparticles (25.7%) [34] and hollow-structured CuS (18%) [7]. Moreover, photothermal conversion efficiency of CNTs-PAMAM-Ag2S was more than Ag2S quantum dots (23.8%) [6].
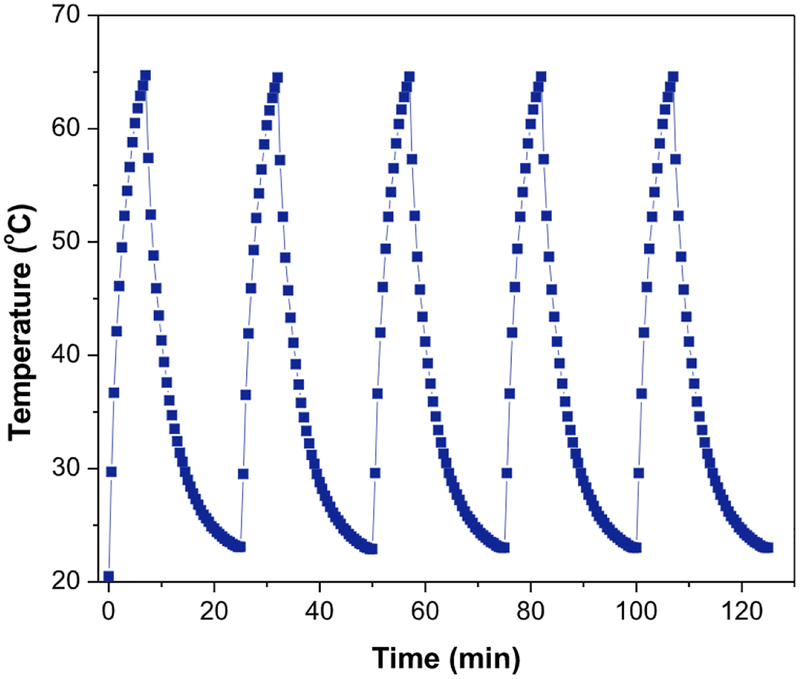
Furthermore stability of CNTs-PAMAM-Ag2S was monitored by measuring its PTE for subsequent five cycles and found that it was constant for all five cycles (Fig. 7). Therefore, CNTs-PAMAM-Ag2S possessed excellent stability against photo-bleaching and photo-corrosiveness. Then to identify the influence of concentration over PTE of CNTs-PAMAM-Ag2S, its PTE was measured in additional two lower concentrations viz., 0.5 and 0.25 mg/mL. It was observed that PTE is linearly dependent on its concentration in such a manner that it improved with increase in concentration (Fig. S1). The ΔT value determined for concentration of 1, 0.5 and 0.25 mg/mL was 44.2, 42.6 and 40.3 °C, respectively.
Fig. 7.
Temerpature variation measured for the aqueous dispersion of CNTs-PAMAM-Ag2S (1mg/mL) for five cycles under irradiation by 980 nm laser.
Generally, the survival temperature level of cancer cells is below 50 °C [34,35]. This temperature was attained by CNTs, CNTs-PAMAM and Ag2S at 6, 4.5 and 3.5 min of irradiation, respectively. However, CNTs-PAMAM-Ag2S conquered the desired temperature of 50 °C within 3 min of exposure to laser. Compared to CNTs, CNTs-PAMAM and Ag2S, CNTs-PAMAM-Ag2S accomplished the needful temperature within a short period of 3 min of irradiation. Nonetheless, rest of the samples were unable to attain the temperature of 50 °C within 3 min of exposure. Therefore, CNTs-PAMAM-Ag2S could produce a strong absorption and heat transfer effect from laser, and this heat could lead to direct destruction of cancer cells. The CNTs-PAMAM-Ag2S can deliver enormous heat within short period to cancer cells and succeed in ablating them in an efficient manner. Overall, CNTs-PAMAM-Ag2S possess strong absorption of 980 nm radiations with large light harvesting ability. In addition, CNTs-PAMAM-Ag2S is feasible at low energy level of laser like 2 W/cm2. Therefore, if CNTs-PAMAM-Ag2S is employed in PTT, it could be viable under low energy of 2 W/cm2 and therapy can complete within short time of about 3 min. So that CNTs-PAMAM-Ag2S persists with strong optical absorption and high efficiency of photothermal conversion, critically needed for success of cancer therapy. In addition, target is also easily perusable using CNTs-PAMAM-Ag2S.
Moreover, to reveal the effect of power of laser source over PTE of CNTs-PAMAM-Ag2S, its PTE was measured under illumination of 980 nm laser having power of 1 W/cm2 and compared it with value obtained with 2 W/cm2 laser (Fig. S2). It was found that PTE of CNTs-PAMAM-Ag2S was reduced by reduction in the power of laser. In particular, ΔT estimated for 1 and 2 W/cm2 laser was 41.7 and 44.2 °C, respectively (Table 1). PTE of CNTs-PAMAM-Ag2S was reduced by 2.5 °C by replacing 2 W/cm2 laser with 1 W/cm2. Hence, PTE of CNTs-PAMAM-Ag2S was found to be dependent on power of laser source and it can be controlled to desired amount by varying the power of laser. In general, due to its significant PTE, CNTs-PAMAM-Ag2S can cause irreversible destruction of cancer cells by denaturation of their protein and damage of cell membrane, led in growth suppression, and ablation of tumors. Hence, CNTs-PAMAM-Ag2S could be a promising photothermal agent to implement in future PTT.
4. Conclusions
In conclusion, an intelligent NIR active photothermal agent, CNTs-PAMAM-Ag2S has been fabricated by immobilization of Ag2S nanocrystallites over the framework of covalently grafted PAMAM to CNTs. The UV–vis–NIR spectrum of CNTs-PAMAM-AgzS revealed its significant absorption in NIR region and it was verified by its excellent PTE and photothermal conversion efficiency under exposure to 980 nm laser. The rational improvement in PTE of CNTs was achieved by grafting of PAMAM and successive anchoring of Ag2S nanocrystallites. The CNTs-PAMAM-Ag2S exhibited higher photothermal conversion efficiency than popularly known gold and copper based photothermal agents. Moreover, PTE of CNTs-PAMAM-Ag2S measured for consecutive five cycles was found to be constant, so it persists with notable antiphoto-bleaching and antiphoto-corrosiveness properties. The linear dependence of PTE over the concentration was observed in CNTs-PAMAM-Ag2S. Overall maximum temperature attained with CNTs-PAMAM-Ag2S by illuminating to 980 nm laser for 7 min was far exceeded the temperature tolerance level of cancer cells. Therefore, on basis its outstanding properties, viz., PTE, photothermal conversion efficiency, antiphoto-bleaching and antiphoto-corrosiveness, CNTs-PAMAM-Ag2S could be a promising candidate to advance the current PTT.
Supplementary Material
Acknowledgments
Authors acknowledge the support from NIH-NIGMS grant 1SC3GM121229-01, the department grant L0002 from Welch Foundation, Texas, United States, and PVAMU faculty research development grant.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.matchemphys.2019.05.040.
References
- [1].Gao F, Sun M, Xu L, Liu L, Kuang H, Xu C, Biocompatible cup-shaped nanocrystal with ultrahigh photothermal efficiency as tumor therapeutic agent, Adv. Funct. Mater 27 (2017) 1700605–1700611. [Google Scholar]
- [2].Ge J, Lan M, Zhou B, Liu W, Guo L, Wang H, Jia Q, Niu G, Huang X, Zhou H, Meng X, Wang P, Lee CS, Zhang W, Han X, A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation, Nat. Commun 5 (2014) 4596–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Song J, Hu H, Jian C, Wu K, Chen X, New generation of gold nanoshell-coated esophageal stent: preparation and biomedical applications, ACS Appl. Mater. Interfaces 8 (2016) 27523–27529. [DOI] [PubMed] [Google Scholar]
- [4].Jiang T, Zhang B, Shen S, Tuo Y, Luo z., Hu Y, Pang z., Jiang X, Tumor microenvironment modulation by cyclopamine improved photothermal therapy of biomimetic gold nanorods for pancreatic ductal adenocarcinomas, ACS Appl. Mater. Interfaces 9 (2017) 31497–31508. [DOI] [PubMed] [Google Scholar]
- [5].Yang T, Tang Y, Liu L, Lv X, Wang Q, Ke H, Deng Y, Yang H, Yang X, Liu G, Zhao Y, Chen H, Size-dependent Ag2S nanodots for second near-infrared fluorescence/photoacoustics imaging and simultaneous photothermal therapy, ACS Nano 11 (2017) 1848–1857. [DOI] [PubMed] [Google Scholar]
- [6].Gao J, Wu C, Deng D, Wu P, Cai C, Direct synthesis of water-soluble aptamer-Ag2S quantum dots at ambient temperature for specific imaging and photothermal therapy of cancer, Adv. Healthc. Mater 5 (2016) 2437–2449. [DOI] [PubMed] [Google Scholar]
- [7].Deng X, Li K, Cai X, Liu B, Wei Y, Deng K, Xie Z, Wu Z, Ma P, Hou Z, Cheng Z, Lin J, A hollow-structured CuS@Cu2S@Au nanohybrid:synergistically enhanced photothermal efficiency and photoswitchable targeting effect for cancer theranostics, Adv. Mater 29 (2017) 1701266–1701275. [DOI] [PubMed] [Google Scholar]
- [8].Huang W, Huang Y, You Y, Nie T, Chen T, High-yield synthesis of multifunctional tellurium nanorods to achieve simultaneous chemo-photothermal combination cancer therapy, Adv. Funct. Mater 27 (2017) 1701388–1701403. [Google Scholar]
- [9].Lv R, Yang P, Hu B, Xu J, Shang W, Tian J, In Situ Growth strategy to integrate up-conversion nanoparticles with ultrasmall CuS for photothermal theranostics, ACS Nano 11 (2017) 1064–1072. [DOI] [PubMed] [Google Scholar]
- [10].Lv R, Yang P, Chen G, Gai S, Xu J, Prasad PN, Dopamine-mediated photothermal theranostics combined with upconversion platform under near infrared light, Sci. Rep 7 (2017) 13562–13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lv R, Yang P, He F, Gai S, Yang G, Lin J, Hollow structured Y2O3:Yb/Er–CuxS nanospheres with controllable size for simultaneous chemo/photothermal therapy and bioimaging, Chem. Mater 27 (2015) 483–496. [Google Scholar]
- [12].Wang Z, Li S, Zhang M, Ma Y, Liu Y, Gao W, Zhang J, Gu Y, Laser-triggered small interfering RNA releasing gold nanoshells against heat shock protein for sensitized photothermal therapy, Adv. Sci 4 (2017) 1600327–1600338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu J, Liu Y, Tang Y, Wang S, Wang C, Li Y, Su X, Tian J, Tian Y, Pan J, Su Y, Zhu H, Teng Z, Lu G, Synergistic Chemo-Photothermal therapy of breast cancer by mesenchymal stem cell-encapsulated Yolk-Shell GNR@HPMO-PTX nanospheres, ACS Appl. Mater. Interfaces 8 (2016) 17927–17935. [DOI] [PubMed] [Google Scholar]
- [14].Liu X, Gao C, Gu J, Jiang Y, Yang X, Li S, Gao W, An T, Duan H, Fu J, Wang Y, Yang X, Hyaluronic acid stabilized iodine-containing nanoparticles with Au nanoshell coating for X-ray CT imaging and photothermal therapy of tumors, ACS Appl. Mater. Interfaces 8 (2016) 27622–27631. [DOI] [PubMed] [Google Scholar]
- [15].Wu C, Li D, Wang L, Guan X, Tian Y, Yang H, Li S, Liu Y, Single wavelength light-mediated, synergistic bimodal cancer photoablation and amplified photothermal performance by graphene/gold nanostar/photosensitizer theranostics, Acta Biomater 53 (2017) 631–642. [DOI] [PubMed] [Google Scholar]
- [16].Tian M, Sun L, Ji S, Zhang L, Liu Z, Construction of a novel fluorinated graphene-based magnetic nanocomposite and its application in cancer photo-chemotherapy, Mater. Lett 196 (2017) 165–167. [Google Scholar]
- [17].Dong X, Sun Z, Wang X, Leng X, An innovative MWCNTs/DOX/TC nanosystem for chemo-photothermal combination therapy of cancer, Nanomed. Nanotechnol 13 (2017) 2271–2280. [DOI] [PubMed] [Google Scholar]
- [18].Kong F, Liu F, Li W, Guo X, Wang Z, Zhang H, Li Q, Luo L, Du Y, Jin Y, You J, Smart carbon nanotubes with laser-controlled behavior in gene delivery and therapy through a non-digestive trafficking pathway, Small 12 (2016) 6753–6766. [DOI] [PubMed] [Google Scholar]
- [19].Zhang M, Wang W, Wu F, Yuan P, Chi C, Zhou N, Magnetic and fluorescent carbon nanotubes for dual modal imaging and photothermal and chemo-therapy of cancer cells in living mice, Carbon 123 (2017) 70–83. [Google Scholar]
- [20].Liang X, Shang W, Chi C, Zeng C, Wang K, Fang C, Chen Q, Liu H, Fan Y, Tian J, Dye-conjugated single-walled carbon nanotubes induce photothermal therapy under the guidance of near-infrared imaging, Cancer Lett 383 (2016) 243–249. [DOI] [PubMed] [Google Scholar]
- [21].Peng Q, He X, Li Y, Wang C, Wang R, Hu P, Yan Y, Sritharan T, Chemically and uniformly grafting carbon nanotubes onto carbon fibers by poly(amidoamine) for enhancing interfacial strength in carbon fiber composites, J. Mater. Chem 22 (2012) 5928–5931. [Google Scholar]
- [22].Bertero A, Boni A, Gemmi M, Gagliardi M, Bifone A, Bardi G, Surface functionalisation regulates polyamidoamine dendrimer toxicity on blood-brain barrier cells and the modulation of key inflammatory receptors on microglia, Nanotoxicology 8 (2014) 158–168. [DOI] [PubMed] [Google Scholar]
- [23].Sheng J, Wang L, Han Y, Chen W, Liu H, Zhang M, Deng L, Liu Y, Dual roles of protein as a template and a sulfur provider: a general approach to metal sulfides for efficient photothermal therapy of cancer, Small 14 (2018) 1702529–1702540. [DOI] [PubMed] [Google Scholar]
- [24].Neelgund GM, Oki A, Photocatalytic activity of CdS and Ag2S quantum dots deposited on poly(amidoamine) functionalized carbon nanotubes, Appl. Catal., B 110 (2011) 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Roper DK, Ahnand W Hoepfner M, Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles, J. Phys. Chem. C 111 (2007) 3636–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wong N, Kam S, O’Connell M, Wisdom JA, Dai H, Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction, Proc. Natl. Acad. Sci. U.S.A 102 (2005) 11600–11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Neelgund GM, Oki A, Influence of carbon nanotubes and graphene nanosheets on photothermal effect of hydroxyapatite, J. Colloid Interface Sci 484 (2016) 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tian Q, Jiang F, Zou R, Liu Q, Chen z., Zhu M, Yang S, Wang J, Wang J, Hu J, Hydrophilic Cu9S5 nanocrystals: a photothermal agent with a 25.7% heat conversion efficiency for PhotothermalAblation of cancer cells in vivo, ACS Nano 5 (2011) 9761–9771. [DOI] [PubMed] [Google Scholar]
- [29].Cui J, Jiang R, Xu S, Hu G, Wang L, Cu7S4 nanosuperlattices with greatly enhanced photothermal efficiency, Small 11 (2015) 4183–4190. [DOI] [PubMed] [Google Scholar]
- [30].Neelgund GM, Oki A, Photothermal effect: an important aspect for the enhancement of photocatalytic activity under illumination by NIR radiation, Mater. Chem. Front 2 (2018) 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang P, Lin J, Li W, Rong P, Wang Z, Wang S, Wang X, Sun X, Aronova M, Niu G, Leapman RD, Nie Z, Chen X, Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy, Angew. Chem. Int. Ed 52 (2013) 13958–13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hessel CM, Pattani VP, Rasch M, Panthani MG, Koo B, Tunnell JW, Korgel BA, Copper selenide nanocrystals for photothermal therapy, Nano Lett 11 (2011) 2560–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pattani VP, Tunnell JW, Nanoparticle-mediated photothermal therapy: a comparative study of heating for different particle types, Lasers Surg. Med 44 (2012) 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ju E, Dong K, Liu z., Pu F, Ren J, Qu X, Tumor microenvironment activated photothermal strategy for precisely controlled ablation of solid tumors upon NIR irradiation, Adv. Funct. Mater 2 (2015) 1574–1580. [Google Scholar]
- [35].Yoo D, Jeong H, Noh SH, Lee JH, Cheon J, Magnetically triggered dual functional nanoparticles for Resistance-Free apoptotic hyperthermia, Angew. Chem. Int. Ed 52 (2013) 13047–13051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.