Abstract
Background
Tooth bleaching causes a significant decrease in the bonding strength between the resin and human enamel. Nevertheless, the effects of different antioxidant types on the immediate bonding strength of resin and bleached enamel were significantly different. Therefore, the objective of this study was to compare the effects of 2 antioxidants for enhancing the bond strength of the resin to bleached enamel.
Material/Methods
There were 48 enamel blocks performed from 48 recently extracted maxillary central incisors. There were 8 groups: NC (negative control, no bleached specimens restored without antioxidants); NA (no antioxidant, bleached specimens bonded immediately without any antioxidants); SA30, SA60, and SA120 (bleached specimens accepted the management of 10% sodium ascorbate (SA) for 30 minutes, 60 minutes, and 120 minutes, respectively, before restored); PC30, PC60, and PC120 (bleached specimens received treatment of 5% proanthocyanidins (PC) for 30 minutes, 60 minutes, and 120 minutes, respectively, before restored). We measured the micro-tensile bond strength of specimens and used 2-way ANOVA to analyze the data.
Results
The mean±standard deviation bond strength measured were: NC, 29.99±4.00; NA, 14.90±1.97; SA30, 18.60±2.20; SA60, 22.57±2.71; SA120, 26.15±3.85; PC30, 16.78±2.29; PC60, 19.13±2.24, PC120, 23.90±2.01 MPa. In addition, the fracture types were mainly of an adhesive mode (88.75%), followed by mixed (7.5%), and cohesive (3.75%).
Conclusions
10% sodium ascorbate provided a comparatively more promising improvement for immediate bond strength than 5% proanthocyanidins when the same duration of antioxidant was applied.
MeSH Keywords: Adhesives, Antioxidants, Bleaching Agents, Proanthocyanidins
Background
With the improvement of people’s aesthetic concept, more and more people have received bleaching treatment of discolored teeth [1–3]. However, scholars reported that tooth bleaching could decrease the bond strength of resin to bleached enamel (BS-RBE), primarily when bonding is conducted at once after the tooth bleaching [1–23].
Delaying the bonding time is a common method to improve the BS-RBE, with the waiting period ranging from 1 day to 28 days [9,24–26]. However, sometimes, cases are too urgent to wait for their teeth restoration [23]. In order to prevent the delay of recovery treatment after bleaching, various antioxidant agents, including sodium ascorbate (SA) [16,21,27], proanthocyanidins (PC) [28,29], green tea extract [4,5,7,13,16], pine bark extract [30], aloe extract [5,16], and grape seed extract [16,31], have been attempted, among which, SA and PC have shown promising results [4,5,14,28,29]. Nevertheless, the results of antioxidants with different types and durations are quite different [4,5,8–10,32,33]. Consequently, we conducted the present in vitro study to compare the effects of 10% SA and 5% PC on the immediate BS-RBE.
Material and Methods
The study was approved by the ethics committee of the Stomatological Hospital of Jilin University and signed the informed consent document.
Dental specimen preparation
Forty-eight intact incisors were collected, not including caries, tetracycline, and dental fluorosis (Oral and Maxillofacial Surgery Clinic Office of Stomatological Hospital of Jilin University, China). An enamel slab specimen with 5×5 mm made from maxillary central incisors was extracted within 1 month by using a dental high-speed turbine handpiece at low speed under water irrigation. The surface of all specimens was polished to #1000 by silicon carbide abrasive paper (Hubei poses with Abrasive Belt Group Co., Ltd., China) under water irrigation to obtain a 5 mm2 flat enamel area. Crack-free teeth in crowns were identified under a stereomicroscope (SEM), which were then stored in a 1% chloramine T solution (Tianjin Guangfu Fine Chemical Research Institute, China) at 4°C.
Soft tissue on the surface of specimens was removed by ultrasonic vibration cleaning. Then, 48 samples were averagely and randomly categorized as the following 8 groups. NC (negative control): specimens were bonded without bleaching; NA (no antioxidant): bleached specimens were restored immediately without any antioxidant treatment; SA30, SA60, and SA120: bleached specimens received the management of 10% SA (Beijing Solebo Technology Co., Ltd. China) before restoration for 30 minutes, 60 minutes, and 120 minutes, respectively; PC30, PC60, and PC120: bleached specimens were treated with 5% PC (Shanghai Yuanye biological Co., Ltd. China) before restoration for 30 minutes, 60 minutes and 120 minutes, respectively.
Bleaching procedure
All specimens in the 8 groups were bleached by utilizing 35% hydrogen peroxide (VIVA Technology International Co., Ltd., USA) on the surface of enamel with a 1 to 2 mm thin layer, with the light of oral whitening lamp as close as possible perpendicularly illuminating the surface of the enamel. The bleaching agent was removed after 10 minutes and then reapplied. Specimens were bleached for 3 times for 30 minutes in total, 10 minutes each time. Specimens, after each bleaching, were rinsed for 1 minute, wiped by a cotton ball, and dried by blowing machine (Shanghai Jinghong Experimental Equipment Co., Ltd. China).
Treatment with antioxidants
Bleached specimens assigned to SA30, SA60, and SA120, were exposed to 10% SA gel for 30, 60, and 120 minutes, respectively, while those in PC30, PC60, and PC120 were exposed to 5% PC for 30, 60, and 120 minutes, respectively. After antioxidant treatment, the enamel surface was rinsed using saline irrigation for 30 seconds (Basic Laboratory, School of Stomatology, Jilin University, China).
Restoration with composite resin
Each prepared specimen was etched by 37% phosphoric acid for the 20 seconds, rinsed for 30 seconds, followed by an air dry for 20 seconds. Modified acrylate adhesive (GE LiaHao New Material Co., Ltd., China) was applied over the etched enamel and then spread with a gentle wind. A transparent polypropylene tube of 4 mm x 4 mm in size was placed in the central area of enamel to limit the bonding range, and then light-cured for 20 seconds. The resin matrix was divided into 3 layers for stacking up to the height of the tube orifice, with 40 seconds each time. The bonded specimens were stored for 24 hours in normal saline at room temperature, and then were embedded in transparent epoxy resin (Shenzhen Hengda Technology Co., Ltd., China) to prevent fracture during cutting.
Micro tensile testing
The specimens were cut into 24 small strip sticks with a size of 1×1×8 mm (Figure 1) using a low speed cutting machine (Shenyang Kejing Automation Equipment Co., Ltd., China) underwater washing. Ten sticks were randomly chosen and confirmed to be defect-free and crack-free through SEM for micro-tensile bond strength (MTBS) testing.
Figure 1.
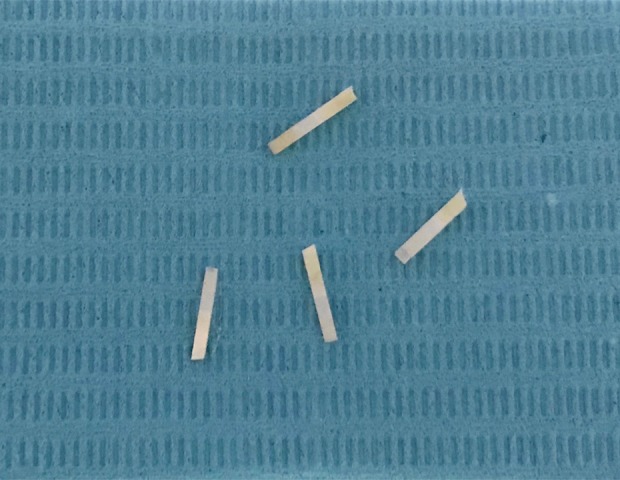
Sticks, performed with the size of 1×1×8 mm, were prepared for micro-tensile bond strength (MTBS) testing.
A universal test machine (Shimadzu Corporation, Japan) (Figure 2) was used to measure MTBS by stretching at a speed of 1 mm/minute, and the SEM was utilized to identify the fracture types. The micrometer (Guilin Measuring Tools and Cutting Tools Factory, China) was used to calculate the actual bond area of the specimens, and the MTBS was conducted according to the formula of MTBS=F/m2.
Figure 2.

Micro-tensile bond strength (MTBS) testing was conducted at 1 mm/minute using a universal testing machine.
The sticks were polished from teeth surface to resin matrix surface with 600, 800, and 1000 mesh silicon carbide water sandpaper, respectively, under irrigation. SEM was used to observe the surfaces of the sticks. The measured surfaces were treated with 37% phosphoric acid etchant for 3 seconds, then rinsed with saline and dried by an air compressor, and then the gradient dehydration of sticks was conducted in 50%, 70%, 90%, and 100% ethanol for 10 minutes, respectively. The sticks were dried in a blast drying chamber at 50°C for 4 hours and then fixed on the loading table. Finally, vacuum gold spraying coating was prepared by ion sputtering, along with the observation of the micro-morphology of bonding interface under scanning electron microscopy.
Statistical analysis
Statistical analysis of the MTBS values of the 8 groups was conducted by SPSS version 24 (IBM Corp., USA) through carrying out a 2-way ANOVA test. Shapiro-Wilk was applied to test the normality of data, and Levene was used to checking the homogeneity of variance. Multiple comparisons of the Tukey test were made at different treatment times of 10% SA solution and 5% PC solution (P<0.05).
Results
Commonly, the fracture types were classified as adhesive fracture, cohesive fracture, and mixed fracture, according to El Zohairy et al. [34]. To be more specific, the adhesive fracture was defined as fracture occurring between enamel and resin, and the cohesive fracture was regarded as the only enamel substrate failure, and the mixed fracture was viewed as the coexistence of enamel substrate and resin materials fractures. In addition, the mean±standard deviation (SD) bond strength measured as follows: NC, 29.99±4.00; NA, 14.90±1.97; SA30, 18.60±2.20; SA60, 22.57±2.71; SA120, 26.15±3.85; PC30, 16.78±2.29; PC60, 19.13±2.24, PC120, 23.90±2.01 MPa (Figure 3).
Figure 3.
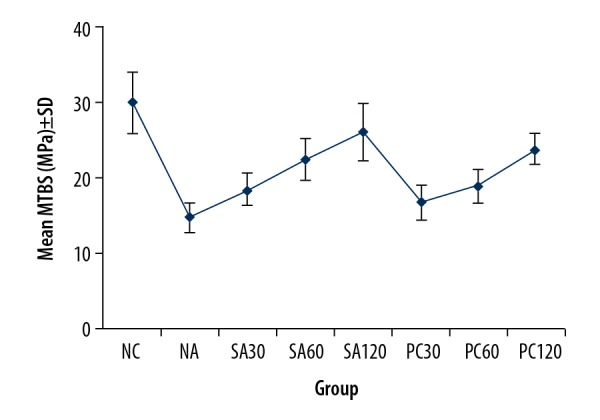
The mean value of micro-tensile bond strength in different groups was measured, with micro-tensile bond strength (MTBS) in figure representing micro-tensile bond strength.
Two-way ANOVA variance analysis demonstrated that the duration, as well as type of antioxidant treatment, exerted significant impacts on the MTBS (P<0.05), without the interaction between the types of antioxidants and the duration of therapy on the MTBS (P>0.05). In other words, a more BS-RBE was observed in 10% SA than 5% PC (P<0.05). Besides, extending the use time of antioxidants can improve the MTBS significantly (P<0.05).
Tukey, multiple comparison test results suggested that the MTBS of specimens after 10% SA treatment for 30, 60, and 120 minutes were of significant difference (P<0.05). Meanwhile, it had no significant difference when treated with 5% PC treatment for 30 and 60 minutes (P>0.05), while it had a substantial difference when treated with 120 minutes (P<0.05).
The fracture types, as listed in Table 1, were also detected. SEM showed that fracture types (Figure 4) mainly showed an adhesive mode (88.75%), followed by mixed (7.5%), and cohesive (3.75%) mode.
Table 1.
Fracture modes found in the experimental groups, N (%).
| Group | Adhesive fractures | Cohesive fractures | Mix fractures | Total number of sticks |
|---|---|---|---|---|
| 1 | 7 (8.75%) | 2 (2.5%) | 1 (1.25%) | 10 |
| 2 | 9 (11.25%) | 0 (0.00%) | 1 (1.25%) | 10 |
| 3 | 9 (11.25%) | 0 (0.00%) | 1 (1.25%) | 10 |
| 4 | 9 (11.25%) | 0 (0.00%) | 1 (1.25%) | 10 |
| 5 | 9 (11.25%) | 0 (0.00%) | 1 (1.25%) | 10 |
| 6 | 9 (11.25%) | 1 (1.25) | 0 (0.00%) | 10 |
| 7 | 10 (12.50%) | 0 (0.00%) | 0 (0.00%) | 10 |
| 8 | 9 (11.25%) | 0 (0.00%) | 1 (1.25%) | 10 |
| Total | 71 (88.75%) | 3 (3.75%) | 6 (7.5%) | 80 |
Figure 4.
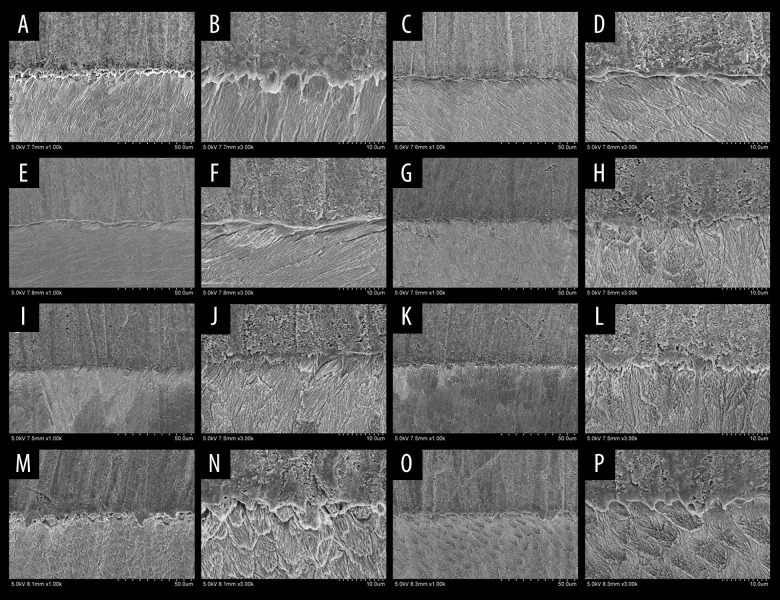
Scanning electron microscopy pictures after bonding tests of all groups. (A, B) The negative control (NC) group showed that the resin-enamel interface was compact, without crack, along with the closely chimeric resin process and enamel. (C, D) The no antioxidant (NA) group demonstrated that the interface between enamel and resin cement was loose and cracked. (E, F) The sodium ascorbate at 30 minutes (SA30) showed that the interface of group 3 was denser than group 2, but there were still cracks. (G, H) The sodium ascorbate at 60 minutes (SA60) group and (I, J) the sodium ascorbate at 120 minutes (SA120) illustrated that the interface between enamel and resin was compact and crack-free, along with chimeric the resin process and enamel. (K, L) The proanthocyanidins at 30 minutes (PC30), and (M, N) the proanthocyanidins at 60 minutes (PC60) showed that the interface between enamel and resin was clear, but the band was loose. There were scattered cracks resin protrusions. (O, P) The proanthocyanidins at 120 minutes (PC120) showed that the interface between enamel and resin cement was not clear, along with the solid bond, without crack. The formation of resin protrusion could be observed.
Discussion
Tooth bleaching is a cosmetic method to change the color of tooth structure through bleaching agent [5,7–9,12,18]. It is advantageous due to its harmlessness to the tooth and surrounding tissues, simple operation, safety, and quick effect. To date, bleaching agents, such as hydrogen peroxide with concentration from 30% to 40% or carbamide peroxide with level from 35% to 37%, have been applied in clinics successfully, getting satisfying aesthetic results [3,4,6,8–10,15,35]. However, most scholars suggested tooth bleaching can decrease the immediate BS-RBE [4–9,15,36]. It is due to bleaching agents damage the surface morphology of enamel. Besides, hydrogen peroxide is decomposed into free radicals and oxygen, which interfere with resin penetration and prevent polymerization of the resin [6,21,37]. Various methods to reverse the decrease of BS-RBE are available, including antioxidant treatment, ethanol pretreatment of enamel surface after bleaching before resin bonding, the utilization of an adhesive containing an organic solvent, and the removal of the enamel surface [14,27,38,39]. However, the removal of surface enamel is disadvantageous in increasing the number of teeth prepared and affecting the pulp status. Ethanol pretreatment of the enamel surface cannot restore the BS-RBE completely, making it still necessary to be combined with postponed rehabilitation. To date, literatures demonstrated that the postponing of 7 to 28 days after enamel bleaching before resin bonding is an effective method [8,9,14,24]. Nevertheless, it increases the number of office visits, thus bringing great inconvenience to patients [23]. In recent years, antioxidant treatment has provided us with promising results to reverse the decline of BS-RBE [3–12,14,19,23,40]. Currently, a variety of antioxidants are used to enhance BS-RBE. However, there is no study comparing the effect of 10% SA and 5% PC on the immediate BS-RBE. Therefore, the purpose of this in vitro comparative study was to estimate the impact exerted by 10% SA and 5% PC on immediate BS-REB.
Bleached group versus unbleached group
In our study, we found that the bleached groups (NA, SA30, SA60, SA120, PC30, PC60, and PC120) have lower MTBS than the unbleached group (NC group). Gonzalez-Lopez S et al. [35] reported that bleaching changed the composition of organic in enamel, and decreased the content of phosphate and carbonate, thus making the enamel surface rough and irregular. Our experimental results are consistent with the conclusions drawn by Gonzalez-Lopez et al. [35]. Besides, scholars also suggested that different concentrations, duration of application, and pH value of bleaching agents may cause different severities of damage to the enamel surface. Wang et al. [20] found that prolonged bleaching time could aggravate the destruction of enamel surface morphology. Besides, several studies have shown that a low pH bleaching agent can worsen enamel demineralization [22]. Therefore, the mild hydrogen peroxide with a concentration of 35% was chosen in our experiment, with a total bleaching time of 30 minutes, to minimize the damage to the enamel surface.
Antioxidant group versus non-antioxidant group
According to the previous studies, the BS-RBE with antioxidants was significantly higher than that without [12–14]. Furthermore, De Carvalho HC et al. [12] found the most useful BS-RBE when the concentration of SA was 10%. However, the BS-RBE decreased with the concentration increasing to 20% and 30%. Meanwhile, PC is an antioxidant with vigorous antioxidant activity as well as a water-soluble natural, free radical scavenger, almost without side effects [28,29,32,33]. It was applied successfully in many dental treatments. Thus, SA with 10% concentration and 5% PC was chosen in this experiment. In our study, the specimens of the NA group which received the resin bond immediately after bleaching, without receiving any antioxidant treatment, demonstrated the lowest MTBS values at the resin-enamel interface. We argue that this result could be attributed to residual oxygen produced by hydrogen peroxide on the superficial enamel layer, which prevents the polymerization of the bonding agents. This view was in line with what is proposed in literatures [9,18,21,31].
10% SA group versus 5% PC group
Both 10% SA and 5% PC treated with bleached enamel for 120 minutes could achieve satisfying composite bond strength with 26.15±3.85 MPa and 23.90±2.01 MPa, respectively, which were higher than clinical restoration (>17–20 MPa) [41]. Besides, it was also found that 10% of SA is superior to 5% PC in reverse bond strength when the same duration of antioxidants treatment was performed. We suggested that it is related to the different molecular weights and the solubility of the 2 antioxidants. The molecular weight of PC is much higher than that of SA, which makes it more difficult for the PC to penetrate enamel than SA, thus achieving weak antioxidants and free radical scavenging effects. Moreover, the characteristics of PC with low solubility and high viscosity may lead to less than 5% of dissolved PC in solution. Ultimately, the adherence of undissolved PC to the surface of enamel inhibits the role played by antioxidants, thus hindering subsequent bonding operations.
Duration of antioxidant application
In the present study, the antioxidants application with 120 minutes has better MTBS values than that with 30 minutes and 60 minutes. Consequently, it is recommended that 10% of SA for 120 minutes may serve as a useful clinical alternative for patients in need of immediate esthetic rehabilitations after bleaching. Based on previous studies, the authors reported that the reparation was performed at once with 10% SA for 1 hour after bleaching, without discolor or shape change after 1 year. However, antioxidants are limited in that they gradually oxidize with time and thus become less reductive [42]. To overcome this shortcoming, 2 groups (SA120 and PC120) in this study were treated with antioxidants for 120 minutes.
Evaluation of materials bond properties
Numerous methods have been used to evaluate the bond properties of materials, including bond strength test, fracture type, SEM to observe the cohesion, micro-leakage test, and analytical chemistry [4,5,9,14,40,43–45].
Bond strength refers to the maximum load per unit area when the bond interface breaks. Each bond strength test method has its characteristics. Usually, the bond strength test, depending on the area of the bond interface, can be classified into 2 categories, namely, macro-bond strength test and micro-bond strength test. The macroscopic bond strength test (bond area >3 mm2) is prone to cohesive failure during force loading, thus affecting the outcomes of the bond strength test. In contrast, the micro-bond strength test (bond area <3 mm2) is not easy to bring about cohesion damage when compared to the macro-test, which can reflect the BS-RBE accurately [43,44]. In this study, because of the small size of the specimens, the micro-bond strength test was selected to avoid excessive cohesive stress affecting the research results.
According to previous studies, the evaluation methods of adhesive capacity include mainly Micro-shear bond strength (MSBS) test and micro-tensile bond strength (MTBS) test [36,43,44,46,47]. MSBS test has the advantages of simple specimen preparation and universal fixture. However, it is not uniform during the process of force loading, and the measured bond strength may be lower than the actual situation [43]. Meanwhile, the MTBS test has the advantages of saving tooth tissue, reducing regional differences, detecting irregular bonding interface, uniform stress distribution, and convenient observation of SEM after fracture [44]. Thus, it can better represent BS-RBE. Although the MTBS test has the shortcomings of difficult specimen making and accidental breakage of specimens, they can be prevented effectively by making specimen after embedding. Therefore, the MTBS test was utilized to access the BS-RBE in the present study.
Typically, the shape of the specimens is classified as funnel, dumbbell, and strip. Among them, funnel-shaped and dumbbell-shaped loading forces concentrate more on the bonding interface than strip-shaped loading forces, but the specimen is easy to fracture in the preparation process. Besides, it is difficult to achieve the same shape of all specimens, and irregular shape specimens are difficult to fix on the material testing machine. These factors increase the difficulty of the experiment. Therefore, a rectangular specimen with a square bonding interface was selected in this experiment. There are 3 types of fracture interface: adhesive fracture, cohesive fracture, and mixed fracture, with the type of fracture interface capable of reflecting the bond strength indirectly. The bond strength at the interface is higher for both cohesive fracture interface and mixed fracture interface. In this experiment, only cohesive fracture and mixed fracture in the unbleached-bonded group account for a high proportion, which is in line with the mechanical test results. Also, to facilitate the analysis of fracture interface types, only the enamel-resin bonding interface was studied in this experiment.
The bonding interface, as well as the morphology, density, and bonding interface of resin protrusions, can directly be observed under scanning electron microscopy [5,6,10,23,48]. In this study, adhesive type fractures accounted for 88.75%, followed by cohesive for 3.75%. Hence, we followed the recommendations proposed by Roulet et al. [49] as possible as we can in this study to prevent variability and standardize procedures.
Although the encouraging results were obtained in this study, it still involves many limitations. During the preparation process of MTBS specimens, it is difficult to control the area of bonding interface at 1 mm2. In fact, the actual area of the specimens in the study was between 1 mm2 and 2.5 mm2, which could meet the requirements of the MTBS test. However, the results may be affected by different areas of the specimens. Besides, no micro-leakage test or aging test was conducted in this study. The micro-leakage analysis also serves as an essential parameter to evaluate the bonding interface tightness, and the aging test can determine the long-term bonding effect of bonding materials. Moreover, it is necessary to further study the combination of antioxidant treatment with other methods, such as treated with alcohol to bleached enamel, to achieve a better bonding effect.
Conclusions
This study had a number of findings: 1) The immediate BS-RBE was decreased significantly by tooth bleaching. 2) Both 10% SA and 5% PC can significantly enhance the immediate BS-RBE. 3) 10% SA provided a comparatively more promising improvement for immediate bond strength than 5% PC when the same duration of antioxidant was applied. 4) The MTBS value increases significantly with the prolonged duration of antioxidant treatment. 5) The bond strength was restored approximately to unbleached enamel when bleached enamel was treated with 10% SA for 120 minutes. 6) As this is an in vitro study, conclusions should be drawn carefully, and further in vivo studies should be conducted to support the clinical application of this method.
Abbreviations
- SA
sodium ascorbate
- PC
proanthocyanidins
- MTBS
micro-tensile bond strength
- MSBS
micro-shear bond strength
- ANOVA
analysis of variance
- SEM
stereomicroscope
- BS-RBE
bond strength of resin to bleached enamel
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Oliveira LSJ, Silva PFD, Figueiredo FED, et al. In vitro evaluation of tooth discoloration induced by regenerative endodontic therapy and the effectiveness of the walking bleach technique. Int J Esthet Dent. 2019;14(3):300–9. [PubMed] [Google Scholar]
- 2.Alqahtani MQ. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent J. 2014;26(2):33–46. doi: 10.1016/j.sdentj.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moosavi H, Maleknejad F, Hoseinipour Z, et al. Antioxidant agents and their effects on shear bond strength of bleached enamel. J Contemp Dent Pract. 2013;14(5):871–75. doi: 10.5005/jp-journals-10024-1418. [DOI] [PubMed] [Google Scholar]
- 4.Rana R, Kaushik M, Sharma R, et al. Comparative evaluation of effects of natural antioxidants on the shear bond strength of composite resin to bleached enamel. Indian J Dent Res. 2019;30(1):112–16. doi: 10.4103/ijdr.IJDR_397_17. [DOI] [PubMed] [Google Scholar]
- 5.Nari-Ratih D, Widyastuti A. Effect of antioxidants on the shear bond strength of composite resin to enamel following extra-coronal bleaching. J Clin Exp Dent. 2019;11(2):e126–32. doi: 10.4317/jced.55359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karadas M, Demirbuga S. Influence of a short-time antioxidant application on the dentin bond strength after intracoronal bleaching. Microsc Res Tech. 2019;82(10):1720–27. doi: 10.1002/jemt.23337. [DOI] [PubMed] [Google Scholar]
- 7.Bansal M, Kaur P, Cyriac AR, et al. Impact of J different antioxidants on the bond strength of resinbased composite on bleached enamel-an in vitro study. Contemp Dent Pract. 2019;20(1):64–70. [PubMed] [Google Scholar]
- 8.Feiz A, Mosleh H, Nazeri R. Evaluating the effect of antioxidant agents on shear bond strength of tooth-colored restorative materials after bleaching: A systematic review. J Mech Behav Biomed Mater. 2017;71:156–64. doi: 10.1016/j.jmbbm.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Turkmen C, Guleryuz N, Atali PY. Effect of sodium ascorbate and delayed treatment on the shear bond strength of composite resin to enamel following bleaching. Niger J Clin Pract. 2016;19(1):91–98. doi: 10.4103/1119-3077.164328. [DOI] [PubMed] [Google Scholar]
- 10.Kilinc HI, Aslan T, Kilic K, et al. Effect of delayed bonding and antioxidant application on the bond strength to enamel after internal bleaching. J Prosthodont. 2016;25(5):386–91. doi: 10.1111/jopr.12303. [DOI] [PubMed] [Google Scholar]
- 11.Kavitha M, Selvaraj S, Khetarpal A, et al. Comparative evaluation of superoxide dismutase, alpha-tocopherol, and 10% sodium ascorbate on reversal of shear bond strength of bleached enamel: An in vitro study. Eur J Dent. 2016;10(1):109–15. doi: 10.4103/1305-7456.175693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Carvalho HC, Guiraldo RD, Poli-Frederico RC, et al. Correlation between antioxidant activity and bonding strength on bleached enamel. Acta Biomater Odontol Scand. 2016;2(1):102–7. doi: 10.1080/23337931.2016.1222283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger SB, Guiraldo RD, Lopes MB, et al. Effects of green tea on the shear bond strength of orthodontic brackets after in-office vital bleaching. Gen Dent. 2016;64(3):72–75. [PubMed] [Google Scholar]
- 14.Whang HJ, Shin DH. Effects of applying antioxidants on bond strength of bleached bovine dentin. Restor Dent Endod. 2015;40(1):37–43. doi: 10.5395/rde.2015.40.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramonian R, Mathai V, Christaine Angelo JB, Ravi J. Effect of three different antioxidants on the shear bond strength of composite resin to bleached enamel: An in vitro study. J Conserv Dent. 2015;18(2):144–48. doi: 10.4103/0972-0707.153076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharafeddin F, Farshad F. The effect of aloe vera, pomegranate peel, grape seed extract, green tea, and sodium ascorbate as antioxidants on the shear bond strength of composite resin to home-bleached enamel. J Dent (Shiraz) 2015;16(4):296–301. [PMC free article] [PubMed] [Google Scholar]
- 17.Suneetha R, Pavithra S, Thomas J, et al. An in vitro comparative study of shear bond strength of composite resin to bleached enamel using synthetic and herbal antioxidants. J Int Oral Health. 2014;6(6):77–81. [PMC free article] [PubMed] [Google Scholar]
- 18.Ozelin AA, Guiraldo RD, Carvalho RV, et al. Effects of green tea application time on bond strength after enamel bleaching. Braz Dent J. 2014;25(5):399–403. doi: 10.1590/0103-6440201300015. [DOI] [PubMed] [Google Scholar]
- 19.Arumugam MT, Nesamani R, Kittappa K, et al. Effect of various antioxidants on the shear bond strength of composite resin to bleached enamel: An in vitro study. J Conserv Dent. 2014;17(1):22–26. doi: 10.4103/0972-0707.124113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Zhu Y, Li J, et al. Efficacy of cold light bleaching using different bleaching times and their effects on human enamel. Dent Mater J. 2013;32(5):761–66. doi: 10.4012/dmj.2013-109. [DOI] [PubMed] [Google Scholar]
- 21.Sharafeddin F, Motamedi M, Modiri S. Effect of immediate application of promaganate peel, grape seed and green tea extracts on composite shear bond strength of in-office bleached enamel. Research Journal of Biological Sciences. 2013;8:83–87. [Google Scholar]
- 22.Sa Y, Sun L, Wang Z, et al. Effects of two in-office bleaching agents with different pH on the structure of human enamel: An in situ and in vitro study. Oper Dent. 2013;38(1):100–10. doi: 10.2341/11-173-L. [DOI] [PubMed] [Google Scholar]
- 23.Miranda TA, Moura SK, Amorim VH, et al. Influence of exposure time to saliva and antioxidant treatment on bond strength to enamel after tooth bleaching: An in situ study. J Appl Oral Sci. 2013;21(6):567–74. doi: 10.1590/1679-775720130035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbosa CM, Sasaki RT, Florio FM, Basting RT. Influence of time on bond strength after bleaching with 35% hydrogen peroxide. J Contemp Dent Pract. 2008;9(2):81–88. [PubMed] [Google Scholar]
- 25.Dishman MV, Covey DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater. 1994;10(1):33–36. doi: 10.1016/0109-5641(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 26.Santana FR, Pereira JC, Pereira CA, et al. Influence of method and period of storage on the microtensile bond strength of indirect composite resin restorations to dentine. Braz Oral Res. 2008;22(4):352–57. doi: 10.1590/s1806-83242008000400012. [DOI] [PubMed] [Google Scholar]
- 27.Vidhya S, Srinivasulu S, Sujatha M, Mahalaxmi S. Effect of grape seed extract on the bond strength of bleached enamel. Oper Dent. 2011;36(4):433–38. doi: 10.2341/10-228-L. [DOI] [PubMed] [Google Scholar]
- 28.Liu R, Fang M, Xiao Y, et al. The effect of transient proanthocyanidins preconditioning on the cross-linking and mechanical properties of demineralized dentin. J Mater Sci Mater Med. 2011;22(11):2403–11. doi: 10.1007/s10856-011-4430-4. [DOI] [PubMed] [Google Scholar]
- 29.Silva AP, Goncalves RS, Borges AF, et al. Effectiveness of plant-derived proanthocyanidins on demineralization on enamel and dentin under artificial cariogenic challenge. J Appl Oral Sci. 2015;23(3):302–9. doi: 10.1590/1678-775720140304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aksakalli S, Ileri Z, Karacam N. Effect of pine bark extract on bond strength of brackets bonded to bleached human tooth enamel. Acta Odontol Scand. 2013;71(6):1555–59. doi: 10.3109/00016357.2013.776108. [DOI] [PubMed] [Google Scholar]
- 31.Abraham S, Ghonmode WN, Saujanya KP, et al. Effect of grape seed extracts on bond strength of bleached enamel using fifth and seventh generation bonding agents. J Int Oral Health. 2013;5(6):101–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Leme-Kraus AA, Aydin B, Vidal CM, et al. Biostability of the proanthocyanidins-dentin complex and adhesion studies. J Dent Res. 2017;96(4):406–12. doi: 10.1177/0022034516680586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fawzy AS, Priyadarshini BM, Selvan ST, et al. Proanthocyanidins-loaded nanoparticles enhance dentin degradation resistance. J Dent Res. 2017;96(7):780–89. doi: 10.1177/0022034517691757. [DOI] [PubMed] [Google Scholar]
- 34.El Zohairy AA, Saber MH, Abdalla AI, Feilzer AJ. Efficacy of microtensile versus microshear bond testing for evaluation of bond strength of dental adhesive systems to enamel. Dent Mater. 2010;26(9):848–54. doi: 10.1016/j.dental.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Lopez S, Torres-Rodriguez C, Bolanos-Carmona V, et al. Effect of 30% hydrogen peroxide on mineral chemical composition and surface morphology of bovine enamel. Odontology. 2016;104(1):44–52. doi: 10.1007/s10266-014-0189-7. [DOI] [PubMed] [Google Scholar]
- 36.Trakiniene G, Petraviciute G, Smailiene D, et al. Impact of fluorosis on the tensile bond strength of metal brackets and the prevalence of enamel microcracks. Sci Rep. 2019;9(1):5957. doi: 10.1038/s41598-019-42325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai SC, Mak YF, Cheung GS, et al. Reversal of compromised bonding to oxidized etched dentin. J Dent Res. 2001;80(10):1919–24. doi: 10.1177/00220345010800101101. [DOI] [PubMed] [Google Scholar]
- 38.Barghi N, Godwin JM. Reducing the adverse effect of bleaching on composite-enamel bond. J Esthet Dent. 1994;6(4):157–61. doi: 10.1111/j.1708-8240.1994.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 39.Freire A, Durski MT, Ingberman M, et al. Assessing the use of 35 percent sodium ascorbate for removal of residual hydrogen peroxide after in-office tooth bleaching. J Am Dent Assoc. 2011;142(7):836–41. doi: 10.14219/jada.archive.2011.0273. [DOI] [PubMed] [Google Scholar]
- 40.Kunt GE, Yilmaz N, Sen S, Dede DO. Effect of antioxidant treatment on the shear bond strength of composite resin to bleached enamel. Acta Odontol Scand. 2011;69(5):287–91. doi: 10.3109/00016357.2011.568958. [DOI] [PubMed] [Google Scholar]
- 41.Calamia JR, Calamia CS. Porcelain laminate veneers: Reasons for 25 years of success. Dent Clin North Am. 2007;51(2):399–417. ix. doi: 10.1016/j.cden.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Muraguchi K, Shigenobu S, Suzuki S, Tanaka T. Improvement of bonding to bleached bovine tooth surfaces by ascorbic acid treatment. Dent Mater J. 2007;26(6):875–81. doi: 10.4012/dmj.26.875. [DOI] [PubMed] [Google Scholar]
- 43.Placido E, Meira JB, Lima RG, et al. Shear versus micro-shear bond strength test: A finite element stress analysis. Dent Mater. 2007;23(9):1086–92. doi: 10.1016/j.dental.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Della Bona A, van Noort R. Shear vs. tensile bond strength of resin composite bonded to ceramic. J Dent Res. 1995;74(9):1591–96. doi: 10.1177/00220345950740091401. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki RT, Florio FM, Basting RT. Effect of 10% sodium ascorbate and 10% alpha-tocopherol in different formulations on the shear bond strength of enamel and dentin submitted to a home-use bleaching treatment. Oper Dent. 2009;34(6):746–52. [PubMed] [Google Scholar]
- 46.Pashley DH, Sano H, Ciucchi B, et al. Adhesion testing of dentin bonding agents: A review. Dent Mater. 1995;11(2):117–25. doi: 10.1016/0109-5641(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 47.Maletin A, Markovic D, Neskovic I, et al. Application of a novel modification of the microbond test for evaluation of adhesive bond strength between fiber posts and dual-cure dental resin cement. Med Sci Monit. 2019;25:3397–405. doi: 10.12659/MSM.914151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda CB, Pagani C, Benetti AR, Matuda Fda S. Evaluation of the bleached human enamel by Scanning Electron Microscopy. J Appl Oral Sci. 2005;13(2):204–11. doi: 10.1590/s1678-77572005000200021. [DOI] [PubMed] [Google Scholar]
- 49.Roulet JF, Van Meerbeek B. Editorial: Statistics: A nuisance, a tool, or a must? J Adhes Dent. 2007;9:287–88. [Google Scholar]