ABSTRACT
In recent years, the demand for functional small-diameter (< 6 mm) artificial vascular grafts has greatly increased due to an increase in the number of patients with vascular heart disease. However, currently, there are no available commercial small-diameter grafts. The objective of this research was to develop a porous silk fibroin (SF)-coated poly(ethylene terephthalate) (PET) graft with a diameter < 6 mm. The graft was compared with a gelatin-coated PET graft because the latter PET graft with a diameter ~ 6 mm was widely used as a commercial vascular graft. Initially, porous SF was prepared using Glyc as the porogen [termed SF(Glyc)] and the PET grafts were prepared through the double-Raschel knitting method. Subsequently, the degradation of the SF coating was monitored using protease XIV in vitro and was compared with that observed in gelatin-coated PET grafts. Finally, these grafts were also implanted into rats for an in vivo comparison. In degradation experiments, after 7 days, the SF was clearly digested by protease XIV, but the gelatin on the graft was still remained at the outer surface. In implantation experiments in rats, the SF(Glyc)-coated PET graft was rapidly degraded in vivo and remodeling to self-tissues was promoted compared with the gelatin-coated PET graft. Thrombus formation and intimal hyperplasia were observed in the gelatin-coated PET graft; however, such side reactions were not observed in the SF(Glyc)-coated PET graft. Thus, the porous SF(Glyc)-coated PET graft with a small diameter < 6 mm may be useful as a commercial vascular graft.
KEYWORDS: Silk fibroin, small-diameter artificial vascular graft, degradation, remodeling, vascular endothelial cell
Introduction
In recent years, the number of patients with cardiovascular diseases has been increasing considerably. Consequently, the need for small-diameter artificial vascular grafts has also increased worldwide.1 The expanded polytetrafluoroethylene or gelatin-coated poly(ethylene terephthalate) (PET) have been used for the commercially available artificial vascular grafts with a diameter ≥ 6 mm, but the limitations of these materials in connection with adverse developments in grafts with diameter < 6 mm have been reported due to thrombus formation and intimal hyperplasia.2,3 These problems are caused by the lack of vascular endothelial cells and mismatch with self-blood vessels.4–6 Thus, in the past decades, studies on the development of small-diameter artificial blood vessels using scaffolding materials that promote remodeling to self-tissues have been conducted.7–9 However, the resulting grafts have not been used yet in the clinical setting.
Silk fibroin (SF) from Bombyx mori has been used in numerous biomedical applications involving drug delivery, tissue engineering, or implantable devices.10 The merits of SF for vascular regeneration (i.e., good cytocompatibility, tailorable biodegradability, suitable mechanical properties and minimal inflammatory reactions) is summarized in an excellent review by Wang et al.11 Our research group has proven, through numerous in vitro and in vivo studies, that SF is suitable for the development of small-diameter artificial vascular grafts.12–25 In addition, this finding has been supported by the work of other research groups highlighting the potential use of SF for the development of such vascular grafts.26,27 We have used SF as both the vascular graft base and the coating materials. Particularly, porous SF prepared using porogen such as poly(ethylene glycol diglycidyl ether) (PGDE) or glycerin (Glyc) has been shown to be useful for coating SF graft bases with small diameter (< 6 mm).19–25 Moreover, Huang et al. reported that good transplantation results have been obtained with a PET graft coated by SF, although porous SF was not investigated as a coating material.28 The PET graft coated with gelatin with a diameter ~ 6 mm has been widely used as commercial artificial vascular graft.1 Therefore, developing a porous SF-coated PET graft with a small diameter and comparing the gelatin-coated and porous SF-coated PET grafts through in vitro and in vivo experiments is of particular interest.
The objective of this research was to develop a porous SF-coated PET graft with a small diameter (< 6 mm). Initially, The porous SF was prepared using Glyc as the porogen [termed SF(Glyc)] and the PET grafts were prepared through the double-Raschel knitting method (a method used for the preparation of the commercial PET graft base). Subsequently, the degradation of the SF coating was monitored using protease XIV in vitro and was compared with that observed in gelatin-coated PET grafts. Finally, these grafts were also implanted into rats for an in vivo comparison.
Results
In vitro degradation of the SF(Glyc) or gelatin coating on the PET grafts
The SEM pictures of the inner and outer surfaces of the PET grafts coated with porous SF(Glyc) or gelatin after degradation with protease XIV are shown in Figure 1.29–32 The pictures of the surfaces were obtained at 0, 3, and 7 days after degradation. The PET graft base without coating yielded an identical picture to that previously reported for the SF graft base without coating.13,15,22,25 At 0 day after degradation (i.e., the stage of no enzyme reaction), the surfaces of the SF(Glyc) and gelatin coatings were different (Figure 1 (A)). Many cracks were observed on the surface of the SF(Glyc)-coated graft, whereas the surface the gelatin-coated graft was smooth. At 3days after degradation, the porous SF(Glyc) coating on the graft was digested using protease XIV and adhered on the surface of the PET fiber as clearly demonstrated in the picture of the expansion (Figure 1 (B)). On the other hand, although the gelatin on the graft was removed from the inner surface of the graft, it remained on the outer surface. At 7days after degradation, a difference was still observed between the surfaces of the porous SF and gelatin coating, although the amounts of the coated materials decreased (Figure 1 (C)). Thus, the degradation behavior using protease XIV was markedly different between SF(Glyc) and gelatin.
Figure 1.
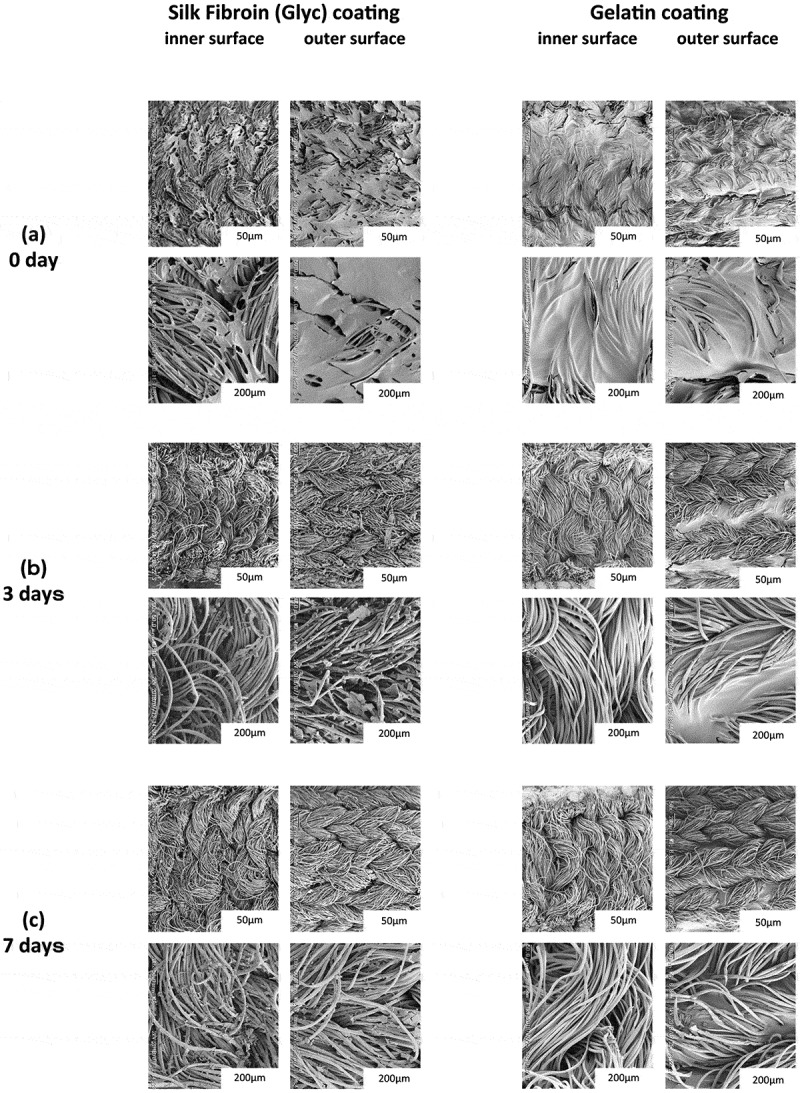
SEM pictures of the morphologies of the inner and outer surfaces of the PET grafts coated with Silk Fibroin (Glyc) or Gelatin at (a) 0 day, (b) 3 days, and (c) 7 days after degradation using protease XIV.
Mechanical properties of 2 types of grafts
The longitude suture retention strength, circumference tensile strength, compressive elastic modulus and water permeability of 2 types of the grafts were measured (Figure 2). There were no significantly differences among these grafts for the longitude suture retention, circumference tensile strength and compressive elastic modulus. The water permeability of SF(Glyc) coated grafts was significantly higher than that of the gelatin coated grafts.
Figure 2.
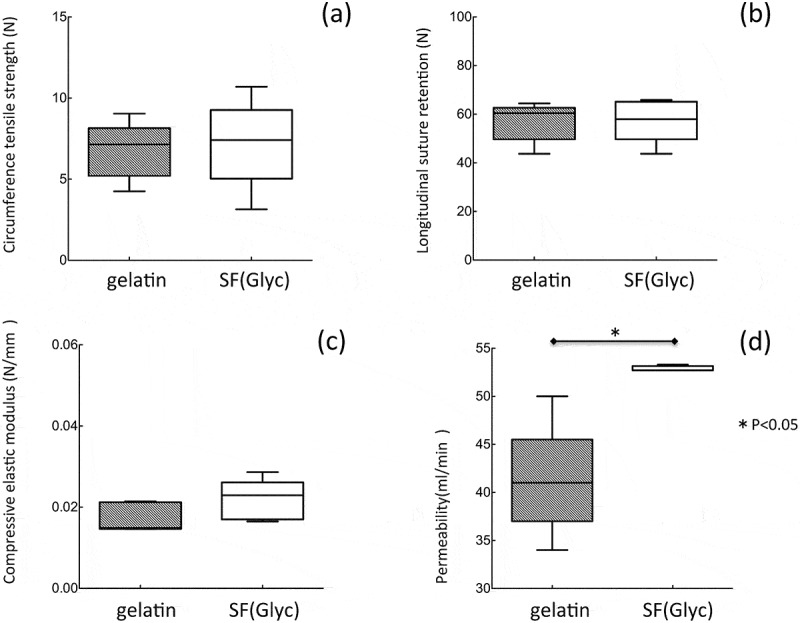
Mechanical properties: (a) circumference tensile strength, (b) longitudinal suture retention, (c) compressive elastic modulus and (d) water permeability of the gelatin- and SF(Glyc)-coated grafts.
Implantation of SF(Glyc)- or gelatin-coated PET grafts into rat
All 24 implantations were successfully performed without complications (e.g., uncontrolled bleeding). After refluxing the blood, bleeding from the gelatin-coated grafts was not observed, with slight leakage of blood from the anastomotic site (Figure 3(A)). On the other hand, mild blood leakage was observed from both the SF(Glyc)-coated graft and the anastomotic site (Figure 3 (B)). The bleeding was stopped by compression with a cotton swab for a few seconds. There was no difference observed among the grafts with respect to the ease of handling and resistance to suture. All rats survived until the scheduled date of removal and were sacrificed at 2 weeks or 3 months after implantation. After 3 months, occlusion was observed in 2 of 6 rats with gelatin-coated grafts. At 2 weeks and 3 months after implantation, all grafts were covered by a thin layer of adipose and surrounding tissue (Figure 4). In addition, peeling of the surrounding tissue from the grafts was easy, with slight bleeding observed from the peeling sites.
Figure 3.
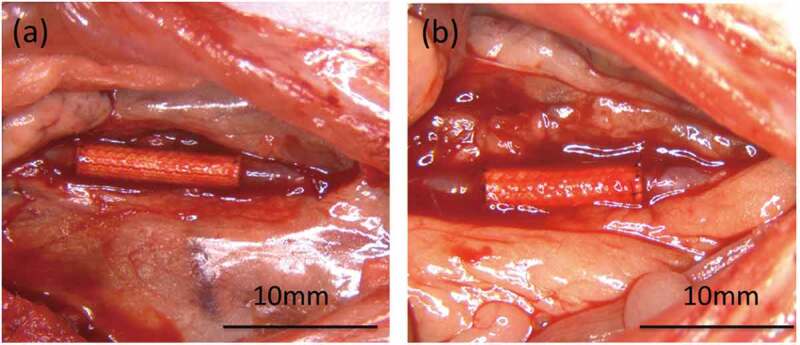
Photograph immediately after releasing the vascular clamp following implantation. (a) The gelatin-coated graft exhibited bleeding primarily from the anastomotic site and (b) the SF(Glyc)-coated graft was exuded from the anastomotic site and the graft. However, hemostasis was achieved in all grafts and there were no deaths occurring due to bleeding.
Figure 4.
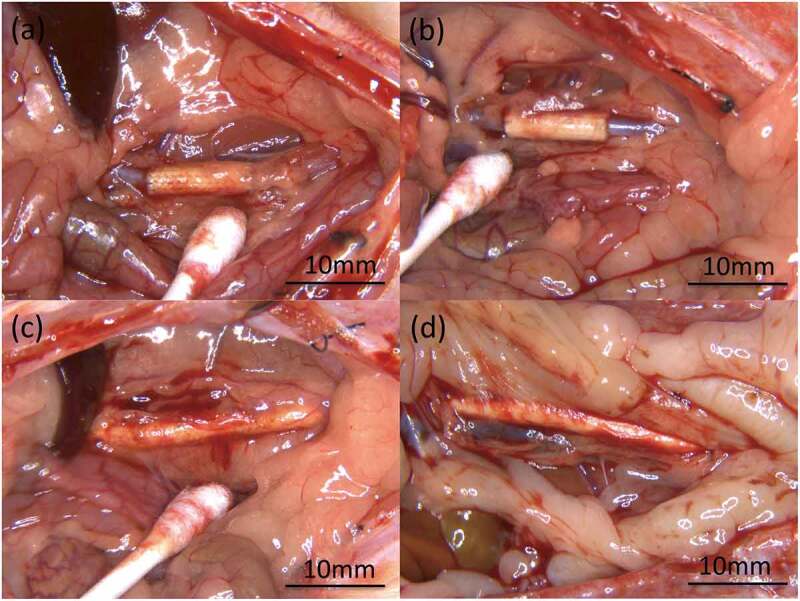
Photographs of microscopic findings during extraction of the grafts: (a) gelatin-coated graft and (b) SF(Glyc)-coated graft at 2 weeks after implantation, (c) gelatin-coated graft and (d) SF(Glyc)-coated graft at 3 months after implantation. Bending, dehiscence, granuloma, hematoma, and formation of aneurysm were not observed. There was no clear difference between the gelatin-coated graft and SF(Glyc)-coated graft in terms of macroscopic findings at the time of excision. However, both grafts at 3 months after implantation exhibited strong adhesion to surrounding tissues compared with that observed at 2 weeks after implantation. Peeling of the surrounding tissue from these grafts was easy and there was no fatal bleeding observed.
Histopathological examination
In the samples obtained at 2 weeks after implantation, HE-stained images showed more inflammatory cells, lymphocytes, macrophages and neutrophils, around and inside the SF(Glyc)-coated grafts compared with the gelatin-coated grafts (Figure 5). In addition, in the SF(Glyc)-coated graft had almost no coating remaining, unlike the gelatin-coated graft which maintained large amounts of coating. The MTC-stained images showed that collagen fibers showed limited infiltration into the gelatin-coated graft and gathered only slightly around the graft (Figure 6), while collagen fibers were gathered around the SF(Glyc)-coated grafts, which were also infiltrated interstices between the PET bases. The α-SMA-stained images showed that smooth muscle cells gathered mainly inside the SF(Glyc)-coated grafts and along the luminal surface of the graft. On the other hand, for the gelatin-coated grafts, the presence of smooth muscle cells could hardly be confirmed inside and outside the graft. The CD31- and EVG-stained images showed that vascular endothelial cells and elastic fibers were not detected in the luminal surfaces of the two types of grafts. The CD68-stained images confirmed a greater presence of macrophages inside the SF(Glyc)-coated graft compared with that observed in the gelatin-coated graft.
Figure 5.
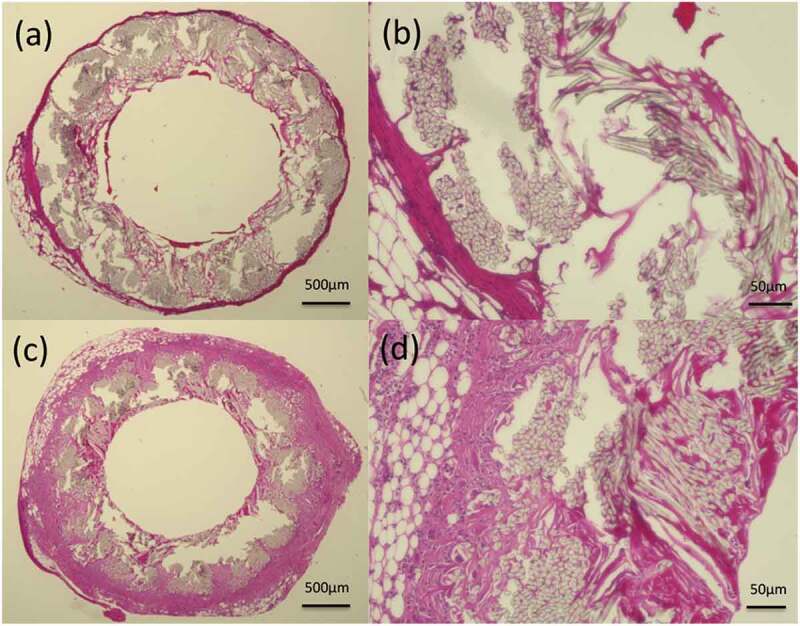
Histological cross-section images of hematoxylin and eosin staining 2 weeks after implantation: (a) gelatin-coated graft and (b) higher magnification of the gelatin-coated graft, (c) SF(Glyc)-coated graft and (d) higher magnification of the SF(Glyc)-coated graft.
Figure 6.
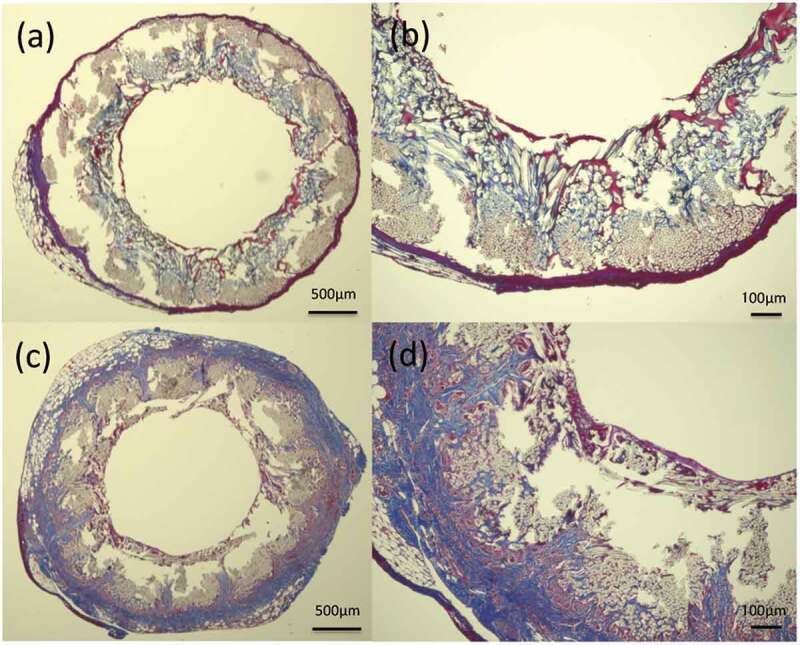
Histological cross-section images of MTC staining 2 weeks after implantation: (a) gelatin-coated graft and (b) higher magnification of the gelatin-coated graft, (c) SF(Glyc)-coated graft and (d) higher magnification of the SF(Glyc)-coated graft. In the gelatin-coated graft, the presence of collagen fibers was hardly confirmed on the outer periphery and inside the graft. In the SF(Glyc)-coated graft, collagen fibers were mainly gathered around the graft. Moreover, it was observed that they penetrated from the outside to the inside of the graft, also entering through the gaps between the polyester fibers.
In the samples obtained at 3 months after implantation, the HE-stained images showed reduction in the presence of inflammatory cells around and inside the two types of grafts. The presence of a thin layer along the luminal surface of the gelatin-coated grafts and a thick layer along the luminal surface of the SF(Glyc)-coated grafts was confirmed (Figure 7). For the gelatin-coated grafts, the amounts of the coating reduced compared with that observed at 2 weeks after implantation, but still slightly remained. In addition, graft occlusion was due to thrombus formation (1 case) and intimal hyperplasia (1 case). The presence of smooth muscle cells and vascular endothelial cells was evaluated in the central part (1 cm) of the graft. Smooth muscle cells mainly gathered along the luminal surface of the two types of grafts (Figure 8). Vascular endothelial cells were not detected in any of the gelatin-coated grafts. However, the presence of these cells was confirmed along the entire area of the central part of the SF(Glyc)-coated grafts.
Figure 7.
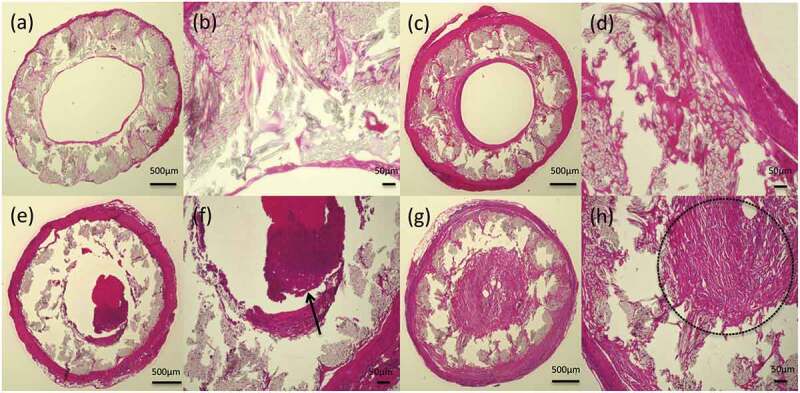
Histological cross-section images of hematoxylin and eosin staining 3 months after implantation: (a) gelatin-coated graft and (b) higher magnification of the gelatin-coated graft, (c) SF(Glyc)-coated graft and (d) higher magnification of the SF(Glyc)-coated graft, (e) gelatin-coated graft with thrombus formed in lumen and (f) thrombus indicated by arrow, (g) gelatin-coated graft with intimal hyperplasia and (h) the part enclosed by the dotted line shows the intimal hyperplasia. A thick-layered structure, consisting of smooth muscle cells and vascular endothelial cells, was found inside the SF(Glyc)-coated graft. Although a thin-layered structure was formed in the gelatin-coated graft, the presence of cells was not observed.
Figure 8.
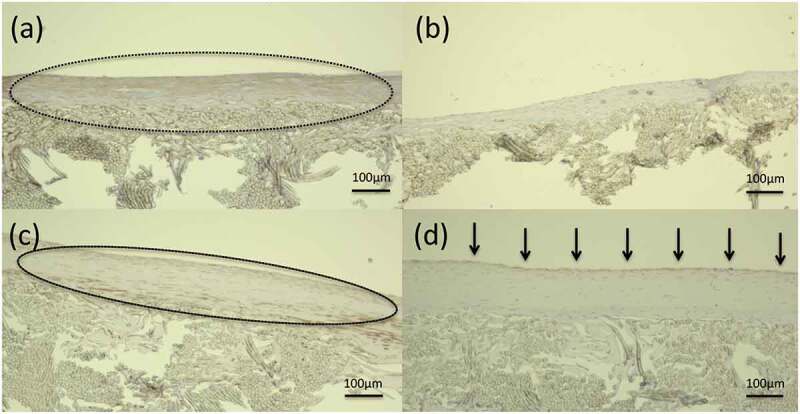
Histological longitudinal images of the central part of the graft at 3 months after implantation: (a) gelatin-coated graft after α-SMA staining and (b) gelatin-coated graft after CD31 staining, (c) SF(Glyc)-coated graft after α-SMA staining, and (d) SF(Glyc)-coated graft after CD31 staining. The part enclosed by the dotted line shows the smooth muscle cells in the intima. Arrows indicate that vascular endothelial cells adhered to the inner surfaces of the grafts. The presence of smooth muscle cells was confirmed in layers inside the central part (1 cm) in both grafts. Vascular endothelial cells were observed continuously without interruption in the central part (1 cm) of the SF(Glyc)-coated graft; however, they were not observed in the gelatin-coated graft.
Image analysis of histopathological examination
Collagen fibers inside the SF(Glyc)- and gelatin-coated grafts were measured and analyzed (Figure 9 (A)). Analysis of tissue infiltration at 3 months after implantation indicated that more collagen fibers infiltrated SF(Glyc)-coated grafts than gelatin-coated grafts. In addition, more tissue infiltration was observed after 3 months of implantation than after 2 weeks of implantation for both types of grafts. The proportion of macrophages in the graft was also measured and analyzed as mentioned earlier in this article (Figure 9(B)). At 2 weeks after implantation, the percentage of macrophages in the SF(Glyc)-coated grafts was higher than that observed in gelatin-coated grafts. For the gelatin-coated grafts, the percentage of macrophages was not different at 2 weeks and 3 months after implantation. However, for the SF(Glyc)-coated grafts, fewer macrophages were observed at 3 months than at 2 weeks after implantation.
Figure 9.
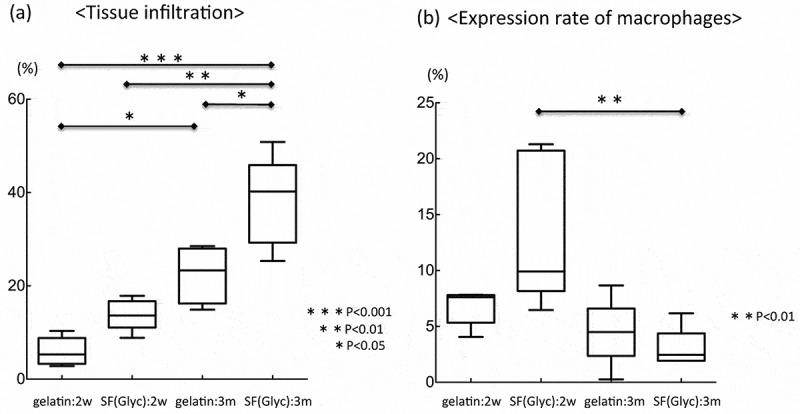
(a) Tissue infiltrations at 2 weeks and 3 months after implantation of the two types of grafts and (b) expression of macrophages at 2 weeks and 3 months after implantation of the two types of grafts. Tissue infiltration at 3 months after implantation was significantly higher in both grafts compared with that observed at 2 weeks after implantation. At 3 months after implantation, tissue infiltration in the SF(Glyc)-coated graft was significantly higher than in the gelatin-coated graft. In the gelatin-coated graft, the expression of macrophages was not significantly different at 2 weeks and 3 months after implantation. However, in the SF(Glyc)-coated graft, the expression of macrophages at 3 months after implantation was significantly lower than that observed at 2 weeks after implantation.
Discussion
SF has proven to be a suitable material for the small-diameter artificial vascular grafts as reported by us and other researchers.11–25 Huang et al. reported that SF-coated PET graft gave excellent results in in vivo experiment.28 Therefore, it seems important to compare the gelatin-coated and SF-coated PET grafts in detail because the gelatin-coated PET graft has been widely used as commercial artificial vescular glaft for further development of silk grafts. In degradation experiments of SF(Glyc)- and gelatin-coated PET grafts with protease XIV in vitro, the aspects of the surfaces are quite different before degradation between two kinds of the grafts. Cracks was occurred on the surfaces of SF(Glyc) coated graft, but smooth surface was observed for gelatin-coated graft. The latter gelatin seems favorable for coating. However, both samples were dried for observation of SEM pictures and the SF(Glyc)-coated PET graft was kept in the hydrated state and therefore such a cracking did not occur till in vivo implantation experiment. After 3 days, the SF was clearly digested by protease XIV. The gelatin on the graft was removed at the inner surface of the graft, but still remained at the outer surface. A similar different picture was obtained after 7 days. Thus it is noted that aspects of the degradation are remarkably different between the SF(Glyc) and gelatin in in vitro experiment using protease XIV.
The quality and amount of these coating materials will affect the strength and handling of the graft.20 Therefore, it is important to carry out physical property tests and investigate before implantation. In the longitude suture retention and circumference tensile tests of this study, 2 types of the grafts had the strength necessary for implantation compared with grafts made previously.13,19 On the other hand, SF(Glyc)-coated grafts was almost the same value as the gelatin-coated grafts in the compressive modulus tests. In the previous study,19 SF coated grafts made with conventional coating methods had more than twice the hardness compared to gelatin-coated grafts. Therefore, the present coating methods has flexibility and it was found that similar flexibility can be imparted to polyester base. In the water permeability test, SF(Glyc)-coated grafts had a higher permeability than gelatin-coated grafts. It is considered that the coating agent was not completely coated on the inner surface of the grafts However, since SF was firmly coated on outer surface, bleeding at the time of implantation could be controlled with mild pressure in this study.
At 2 weeks after implantation, there was no occlusion observed in the gelatin-coated grafts. However, this may be attributed to the length of the graft (1 cm).33 At 3 months after implantation, as reported previously,34–36 occlusion was thought to be due to a deficiency of vascular endothelial cells and turbulence caused by differences in compliance between implanted grafts and native blood vessels. In this study, vascular endothelial cells and smooth muscle cells were confirmed along the entire area of the central part of the SF(Glyc)-coated grafts. These results were superior to those observed with silk-based artificial vascular grafts produced using TG silk.17,21,23 Although materials such as PET do not cause cell adhesion, the SF coating was biodegraded immediately after implantation, resulting in migration of inflammatory cells and promoting remodeling. The gathering of fibroblasts and collagen fibers was confirmed inside and outside the SF(Glyc)-coated grafts at 2 weeks after implantation. Although the presence of vascular endothelial cells was not confirmed in the luminal surface of the graft, a large number of blood vessels along with invasion of collagen fiber were confirmed inside the graft. Moreover, the percentage of macrophages in the SF(Glyc)-coated grafts was higher than that observed in gelatin-coated grafts at 2 weeks after implantation. These results suggested that the SF used as the coating material was biodegraded at an early stage after implantation and the characteristics of SF were fully demonstrated, thereby accelerating the remodeling to self-tissue.
Conclusions
In order to develop a porous SF(Glyc)-coated PET graft with small diameter < 6 mm, several in vitro and in vivo experiments were performed. The implantation experiments in rats showed that the porous SF(Glyc)-coated PET graft was rapidly degraded in vivo and remodeling to self-tissues was promoted, compared with the gelatin-coated PET graft. These results, show that the porous SF(Glyc)-coated PET graft with a small diameter < 6 mm may be useful as a commercial vascular graft.
Materials & methods
Preparation of PET vascular grafts coated with porous SF(Glyc) or gelatin
The tubes of the PET vascular grafts with a 1.5 mm diameter were produced using PET fiber and a computer-controlled double-Raschel knitting machine (Fukui Tateami Kogyo Co., Ltd., Japan).
The preparation of the PET graft coated with SF sponge was as follows. A SF aqueous solution was prepared after removal of silk sericin.12–25 A PTFE rod with a 1.5 mm diameter was inserted into the PET tube with a 1.5 mm diameter. The rod covered with the PET tube was immersed into a pipe filled with a mixed aqueous solution with a 50:50 (w/w %) ratio of SF and Glyc for coating.15,19,20,24,25 The pipe was placed in a desiccator maintained at a reduced pressure of 100 hPa until the absence of air bubbles on the coated surface. Subsequently, the PET graft coated with the mixture of SF and Glyc was frozen at −20°C overnight and then placed in distilled water to remove the Glyc. Subsequently, the PET graft coated with porous SF was placed in a plastic bag with distilled water to avoid the dryness of the sample and then sterilized in an autoclave at 120°C for 20 min. The preparation of the PET graft coated with gelatin was as follows.20,22 An aqueous solution of gelatin was prepared by dissolving MediGelatin (Nippi, Tokyo, Japan) in distilled water to a concentration of 3% (w/v) at 60°C for 4 hr. The gelatin was crosslinked to make it insoluble by immersing the gelatin-coated grafts in 10% glutaraldehyde for 30 min at room temperature, followed by immersion in 70% ethanol for 10 min. This process was repeated three times to remove the glutaraldehyde. The gelatin graft was placed in a plastic bag and sterilized in an autoclave at 120°C for 20 min.
Mechanical properties of PET vascular grafts coated with gelatin and SF
Two types of grafts were observed mechanical properties according to the methods reported previously in detail.13,14,21,22,25 We investigated longitudinal suture retention strength, circumferential tensile strength, and circumferential compressive elastic modulus and water permeability. To measure the longitudinal suture retention strength (N), SF grafts were cut into 20-mm length and suture was passed through 2 mm from the end, pulled by the clamp (3 mm/min) using a 100 N operator cell until the break point, and analyzed using the EZ graph (Shimadzu, Japan). Next, the same EZ graph was used with specific evaluation software to determine circumference tensile strength (N) and compressive elastic moduli (N/mm2). Ring-shaped specimens with an axial length of 10 mm were prepared for each SF graft and the outer diameters were measured. The load cell was 5 N, and the rate of compression was 2 mm/min. The compressive tensile strength was measured when the specimen was compressed using 25% of the value of the inner diameter. The water reservoir was set to apply a hydrostatic pressure to the graft of approximately 120 mmHg. The water permeating through the graft wall was collected and calculated in ml/min.
In vitro degradation of porous SF(Glyc) or gelatin coating on PET grafts
The degradation of the PET vascular grafts coated with SF(Glyc) or gelatin was examined in vitro using the enzymatic digestion of protease XIV prior to the in vivo experiment.29–32 The PET vascular grafts coated with SF(Glyc) or gelatin were incubated in phosphate buffered saline (PBS, DANIBON) solution containing protease XIV (0.1 U/ml: SIGMA) at 37°C. The grafts were removed after 1, 3, 5, and 7 days of incubation and dried. In this experimental condition, the PET graft base was not biodegradable, whereas the SF(Glyc) or gelatin coatings on the graft base were degradable. Because of the limited amounts of the coating materials, it was difficult to monitor changes in these amounts experimentally. Therefore, the morphologies of the inner and outer surfaces of the coated PET grafts were observed using a scanning electron microscope (SEM: VE-7800 Keyence).
Animals
Sprague–Dawley rats (Charles River Laboratories) weighing 200–300 g were used for the in vivo study. All rats were maintained in micro-isolator cages with a 12 h light/dark cycle. All experimental procedures and protocols were approved by the Tokyo University of Agriculture and Technology (TUAT, Approval number: 30–9). The rats were managed and cared for in accordance with the standards established by the TUAT and described in its “Guide for the Care and Use of Laboratory Animals.”
Surgical procedure
Each of the PET grafts coated with SF(Glyc) or gelatin was implanted in the abdominal aorta of 12 rats under a stereoscope (LEICA M60; Leica Microsystems). Half of the rats were sacrificed at 2 weeks after implantation, whereas the remaining half were sacrificed at 3 months after implantation. The dimensions of the PET grafts coated with SF(Glyc) were 1 cm in length and 1.5 mm in inner diameter. The dimensions of the PET grafts coated with gelatin were 3 cm in length and 1.5 mm in inner diameter. For graft implantation, rats were anesthetized using an intraperitoneal injection of pentobarbital (50 mg/kg of body weight). The abdominal aorta was carefully exposed and the aortic branches in this segment were ligated. The aorta was removed and replaced with a PET graft by an end-to-end anastomosis using 9–0 monofilament nylon sutures (BEAR, Japan). The distal and subsequently the proximal vascular clamps were slowly removed, and the flow was restored through the grafts. Graft patency was confirmed visually. No anticoagulant or antiplatelet agents were administered postoperatively. Prior to euthanasia, the rats were perfused with 0.9% saline solution through the left ventricle. The grafts were carefully removed together with the surrounding tissue.
Histopathological examination
The grafts removed at 2 weeks after implantation were cut 4 mm transversely at the center part. The central part of the grafts removed at 3 months after implantation was cut longitudinally (approximately 10 mm) and cut 4 mm transversely toward the cranial and caudal side from the central part. The sutured parts of the remaining native blood vessel and artificial vascular graft were cut transversely (6 mm each). These samples were fixed using ethanol for histological analyzes. Fixed samples were embedded in paraffin and stained with hematoxylin and eosin (HE), Masson’s trichrome (MTC), and Elastica van Gieson (EVG). Sections for immunohistochemical staining were incubated with primary antibodies, including α-smooth muscle actin (α-SMA: clone 1A4; Sigma-Aldrich Inc.), CD68 anti-rat antibody (BIO-RAD Inc.), and CD31 anti-rat antibody (BD biosciences Inc.). These samples were incubated with N-Histofine Simple Stain Rat MAX-PO (Nichirei Biosciences Inc.). Subsequently, color development was performed using the ImmPACT DAB Peroxidase Substrate Kit (Vector laboratories Inc.).
Imaging and statistical analysis
The biological reactions in the stained specimens were visualized using the All-in-One fluorescence microscope (Keyence BZ-9000, Keyence). The area of the PET graft and collagenous fibers inside the graft were also measured, and the percentage of tissue infiltration (area of collagenous fibers inside the graft/area of vascular graft) was calculated. The percentage of tissue infiltration was considered to be the tissue infiltration rate. Moreover, the percentage of macrophages was calculated. Data are presented as mean ± standard error. Comparison of means was performed using one-way analysis of variance, followed by the Bonferroni post-hoc test. Mechanical property data were analyzed using Student’s t-test. Data analysis was performed using the commercial statistics software package GraphPad Prism (Version 5.0a, San Diego, CA, USA). Statistical significance was defined as P < .05.
Acknowledgments
T.A. acknowledges support by a JSPS KAKENHI, Grant-in-Aid for Scientific Research (A), Grant Number JP26248050 and Impulsing Paradigm Change through Disruptive Technologies Program (ImPACT).
References
- 1.Pashneh-Tala S, MacNeil S, Claeyssens F.. The tissue-engineered vascular graft-past, present, and future. Tissue Eng Part B Rev. 2016;22:68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budd JS, Allen KE, Hartley G, Bell PR. The effect of preformed confluent endothelial cell monolayers on the patency and thrombogenicity of small calibre vascular grafts. Eur J Vasc Surg. 1991;5:397–405. [DOI] [PubMed] [Google Scholar]
- 3.Takagi H, Goto SN, Matsui M, Manabe H, Umemoto T. A contemporary meta-analysis of Dacron versus polytetrafluoroethylene grafts for femoropopliteal bypass grafting. J Vasc Surg. 2010;52:232–36. [DOI] [PubMed] [Google Scholar]
- 4.Ballyk PD, Walsh C, Butany J, Ojha M. Compliance mismatch may promote graft-artery intimal hyperplasia by altering suture-line stresses. J Biomech. 1998;31:229–37. [DOI] [PubMed] [Google Scholar]
- 5.Haruguchi H, Teraoka S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review. J Artif Organs. 2003;6:227–35. [DOI] [PubMed] [Google Scholar]
- 6.Kuwabara F, Narita Y, Yamawaki-Ogata A, Satake M, Kaneko H, Oshima H, Usui A, Ueda Y. Long-term results of tissue-engineered small-caliber vascular grafts in a rat carotid arterial replacement model. J Artif Organs. 2012;15:399–405. [DOI] [PubMed] [Google Scholar]
- 7.Pektok E, Nottelet B, Tille JC, Gurny R, Kalangos A, Moeller M, Walpoth BH. Degradation and healing characteristics of small-diameter poly(epsilon-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation. 2008;118:2563–70. [DOI] [PubMed] [Google Scholar]
- 8.Nagaoka Y, Yamada H, Kimura T, Kishida A, Fujisato T, Takakuda K. Reconstruction of small diameter arteries using decellularized vascular scaffolds. J Med Dent Sci. 2014;61:33–40. [PubMed] [Google Scholar]
- 9.Wang W, Hu J, He C, Nie W, Feng W, Qiu K, Zhou X, Gao Y, Wang G. Heparinized PLLA/PLCL nanofibrous scaffold for potential engineering of small-diameter blood vessel: tunable elasticity and anticoagulation property. J Biomed Mater Res A. 2015;103:1784–97. [DOI] [PubMed] [Google Scholar]
- 10.Thurber AE, Omenetto FG, Kaplan DL. In vivo bioresponses to silk proteins. Biomaterials. 2015;71:145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Liu H, Fan Y. Silk fibroin for vascular regeneration. Microsc Res Tech. 2017;80:280–90. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto S, Sumi M, Kajimoto K, Nakazawa Y, Takahashi R, Takabayashi C, Asakura T, Sata M. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J Vasc Surg. 2010;51:155–64. [DOI] [PubMed] [Google Scholar]
- 13.Yagi T, Sato M, Nakazawa Y, Tanaka K, Sata M, Itoh K, Takagi Y, Asakura T. Preparation of double-raschel knitted silk vascular grafts and evaluation of short-term function in a rat abdominal aorta. J Artif Organs. 2011;14:89–99. [DOI] [PubMed] [Google Scholar]
- 14.Nakazawa Y, Sato M, Takahashi R, Aytemiz D, Takabayashi C, Tamura T, Enomoto S, Sata M, Asakura T. Development of small-diameter vascular grafts based on silk fibroin fibers from bombyx mori for vascular regeneration. J Biomater Sci Polym Ed. 2011;22:195–206. [DOI] [PubMed] [Google Scholar]
- 15.Aytemiz D, Sakiyama W, Suzuki Y, Nakaizumi N, Tanaka R, Ogawa Y, Takagi Y, Nakazawa Y, Asakura T. Small-diameter silk vascular grafts (3 mm diameter) with a double-raschel knitted silk tube coated with silk fibroin sponge. Adv Healthc Mater. 2013;2:361–68. [DOI] [PubMed] [Google Scholar]
- 16.Asakura T, Saotome T, Aytemiz D, Shimokawatoko H, Yagi T, Fukayama T, Ozai Y, Tanaka R. Characterization of silk sponge in the wet state using C-13 solid state NMR for development of a porous silk vascular graft with small diameter. RSC Adv. 2014;4:4427–34. [Google Scholar]
- 17.Asakura T, Isozaki M, Saotome T, Tatematsu K-I, Sezutsu H, Kuwabara N, Nakazawa Y. Recombinant silk fibroin incorporated cell-adhesive sequences produced by transgenic silkworm as a possible candidate for use in vascular graft. J Mater Chem B. 2014;2:7375–83. [DOI] [PubMed] [Google Scholar]
- 18.Aytemiz D, Suzuki Y, Shindo T, Saotome T, Tanaka R, Asakura T. In vitro and in vivo evaluation of hemocompatibility of silk fibroin based artificial vascular grafts. Int J Chem. 2014;6:1–14. [Google Scholar]
- 19.Fukayama T, Takagi K, Tanaka R, Hatakeyama Y, Aytemiz D, Suzuki Y, Asakura T. Biological reaction to small-diameter vascular grafts made of silk fibroin implanted in the abdominal aortae of rats. Ann Vasc Surg. 2015;29:341–52. [DOI] [PubMed] [Google Scholar]
- 20.Fukayama T, Ozai Y, Shimokawadoko H, Aytemiz D, Tanaka R, Machida N, Asakura T. Effect of fibroin sponge coating on in vivo performance of knitted silk small diameter vascular grafts. Organogenesis. 2015;11:137–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saotome T, Hayashi H, Tanaka R, Kinugasa A, Uesugi S, Tatematsu K-I, Sezutsu H, Kuwabara N, Asakura T. Introduction of VEGF or RGD sequences improves revascularization properties of Bombyx mori silk fibroin produced by transgenic silkworm. J Mater Chem B. 2015;3:7109–16. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto S, Okamoto H, Haga M, Shigematsu K, Miyata T, Watanabe T, Ogawa Y, Takagi Y, Asakura T. Rapid endothelialization and thin luminal layers in vascular grafts using silk fibroin. J Mater Chem B. 2016;4:938–46. [DOI] [PubMed] [Google Scholar]
- 23.Fukayama T, Ozai Y, Shimokawatoko H, Kimura Y, Aytemiz D, Tanaka R, Machida N, Asakura T. Evaluation of endothelialization in the center part of graft using 3 cm vascular grafts implanted in the abdominal aortae of the rat. J Artif Organs. 2017;20:1–9. [DOI] [PubMed] [Google Scholar]
- 24.Haga M, Yamamoto S, Okamoto H, Hoshina K, Asakura T, Watanabe T. Histological reactions and the In Vivo patency rates of small silk vascular grafts in a canine model. Ann Vasc Dis. 2017;10:132–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka T, Uemura A, Tanaka R, Tasei Y, Asakura T. Comparison of the knitted silk vascular grafts coated with fibroin sponges prepared using glycerin, poly(ethylene glycol diglycidyl ether) and poly(ethylene glycol) as porogens. J Biomater Appl. 2018;32:1239–52. [DOI] [PubMed] [Google Scholar]
- 26.Lovett M, Cannizzaro C, Daheron L, Messmer B, Vunjak-Novakovic G, Kaplan DL. Silk fibroin microtubes for blood vessel engineering. Biomaterials. 2007;28:5271–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Fan H, Wang Y, Toh SL, Goh JC. The interaction between a combined knitted silk scaffold and microporous silk sponge with human mesenchymal stem cells for ligament tissue engineering. Biomaterials. 2008;29:662–74. [DOI] [PubMed] [Google Scholar]
- 28.Huang F, Sun L, Zheng J. In vitro and in vivo characterization of a silk fibroin-coated polyester vascular prosthesis. Artif Organs. 2008;32:932–41. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Ogiso M, Minoura N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials. 2003;24:357–65. [DOI] [PubMed] [Google Scholar]
- 30.Arai T, Freddi G, Innocenti R, Tsukada M. Biodegradation ofBombyx mori silk fibroin fibers and films. J Appl Polym Sci. 2004;91:2383–90. [Google Scholar]
- 31.Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH. In vitro degradation of silk fibroin. Biomaterials. 2005;26:3385–93. [DOI] [PubMed] [Google Scholar]
- 32.Numata K, Cebe P, Kaplan DL. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials. 2010;31:2926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahara A, Somekawa S, Kobayashi N, Hirano Y, Kimura Y, Fujisato T, Yamaoka T. Tissue-engineered acellular small diameter long-bypass grafts with neointima-inducing activity. Biomaterials. 2015;58:54–62. [DOI] [PubMed] [Google Scholar]
- 34.Bos GW, Poot AA, Beugeling T, van Aken WG, Feijen J. Small-diameter vascular graft prostheses: current status. Arch Physiol Biochem. 1998;106:100–15. [DOI] [PubMed] [Google Scholar]
- 35.Ao PY, Hawthorne WJ, Vicaretti M, Fletcher JP. Development of intimal hyperplasia in six different vascular prostheses. Eur J Vasc Endovasc Surg. 2000;20:241–49. [DOI] [PubMed] [Google Scholar]
- 36.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. [DOI] [PubMed] [Google Scholar]


