ABSTRACT
Vascularisation is essential for the development of tailored, tissue-engineered organs and tissues due to diffusion limits of nutrients and the lack of the necessary connection to the cardiovascular system. To pre-vascularize, endothelial cells and supporting cells can be embedded in the scaffold to foster an adequate nutrient and oxygen supply after transplantation. This technique is applied for tissue engineering of various tissues, but there have been few studies on the use of different cell types or cells sources. We compare the effect of supporting cells from different sources on vascularisation. Fibrin gels and agarose-collagen hydrogels were used as scaffolds. The supporting cells were primary human dermal fibroblasts (HDFs), human nasal fibroblasts (HNFs), human mesenchymal stem cells from umbilical cord’s Wharton’s jelly (WJ MSCs), adipose-derived MSCs (AD MSCs) and femoral bone marrow-derived MSCs (BM MSCs). The tissue constructs were incubated for 14 days and analyzed by two-photon laser scanning microscopy. Vascularisation was supported by all cell types, forming branched networks of tubular vascular structures in both hydrogels. In general, fibrin gels present a higher angiogenic promoting environment compared to agarose-collagen hydrogels and fibroblasts show a high angiogenic potential in co-culture with endothelial cells. In agarose-collagen hydrogels, vascular structures supported by AD MSCs were comparable to our HDF control in terms of volume, area and length. BM MSCs formed a homogeneous network of smaller structures in both hydrogels. This study provides data toward understanding the pre-vascularisation properties of different supporting cell types and sources for tissue engineering of different organs and tissues.
KEYWORDS: 3D cell culture, co-cultures, hydrogels, human mesenchymal stem cells, vascularisation
Impact statement
Tissue engineered transplants offer a chance to counteract the shortage of donor organs. The supply of these artificially produced tissues with nutrients and oxygen plays a decisive role after transplantation. The introduction of pre-vascularisation into engineered transplants could help to solve this problem. A pre-established network of vascular structures can foster the supply of nutrients and oxygen to the engineered tissue. In this work, the formation of vessels (vascularisation) is induced in vitro by endothelial cells in co-culture with supporting cells such as fibroblasts and mesenchymal stem cells in different scaffolds.
Introduction
Tissue engineering approaches like bioartificial grafts are promising to counteract the shortage of donor organs. However, for larger tissues, the formation of blood vessels is required to ensure a sufficient nutrient and oxygen supply to human tissues and organs. Until now, this poses a major challenge in tissue engineering due to tissue thickness of complex organs and an oxygen diffusion limit of 100–200 μm.1 To overcome this issue, pre-vascularisation of the grafts enables a better integration in vivo.
Angiogenesis is the formation of vascular structures from existing vessels containing differentiated endothelial cells.2 New blood vessels emerge from existing ones in response to hypoxia, which initiates the production of nitric oxide (NO). The NO molecule interacts with a transcription factor to regulate the expression of the pro-angiogenic vascular endothelial growth factor (VEGF), the production of angiopoietins 1 and 2 (Ang-1 and Ang-2) and NO synthase. VEGF increases the permeability of the vessel and simultaneously initiates the degradation of the extracellular matrix (ECM).3 In addition, the reorganization of adhesion molecules such as platelet adhesion molecule-1 (PECAM-1/CD31) and vascular endothelial cadherin (CD144) is necessary to facilitate cell disintegration.1 In response, the tissue becomes more permeable (including the supporting cells known as pericytes), leading to the destabilization of the blood vessels.3 Pericytes are involved in the processes that control endothelial cell proliferation and the synthesis of ECM components.4 The loss of ECM causes the endothelial cells to detach from their integrin-binding sites, which in return leads to endothelial cell migration.1
Fibroblasts are motile cells of mesenchymal origin that play a supportive role in vascularisation, and one of their tasks in vivo is the synthesis of ECM, which comprises matrix proteins, growth factors, biochemical mediators and proteases. In combination with endothelial cells, fibroblasts enhance vascularisation, initiate vasodilatory expansion and improve the mechanical properties of the vasculature.3 The co-culture of human umbilical vein endothelial cells (HUVECs) with fibroblasts revealed that the latter can regulate the migration of endothelial cells. The co-cultures with fibroblasts express higher levels of the angiogenic factors VEGF and TGF-β1 compared to HUVEC mono-cultures.5
The co-culture of HUVECs and HDFs has expediently been used as a control to compare co-cultures with different supporting cells, allowing the investigation of different types of support cells to determine their impact on differentiation.6,7
MSCs are multipotent adult stem cells from connective tissue and can differentiate into adipocytes, myocytes, chondroblasts and osteoblasts.8 The main sources of human MSCs are adipose tissue, bone marrow, cord blood, placental tissue and Wharton’s jelly of the umbilical cord. MSCs play a key role in angiogenesis and wound healing. The isolation of multipotent MSCs is a straightforward procedure, making these cells ideal for therapeutic tissue regeneration. Furthermore, when combining AD MSCs and BM MSCs and endothelial cells vascular-like structures developed in scaffolds made from the polymer poly-N-isopropylacrylamide after 21 days of culture.9,10
In tissue engineering, scaffolds mimic the ECM and support cellular proliferation and differentiation to generate new tissues. Scaffolds can be biodegradable, but they must provide mechanical stability and allow cell adhesion.11 Hydrogels are ideal scaffolds for the engineering of soft tissues due to their mechanical properties and tissue-like characteristics, including their water content, biocompatibility and efficient transport of nutrients and metabolites. Fibrin, collagen and agarose are frequently used in tissue engineering approaches.12,13 Fibrin is a natural polymer composed of the monomeric plasma protein fibrinogen, which is synthesized in the liver and can be isolated autologously from the patient.14 Furthermore, it promotes cell division, facilitates cell attachment and migration along RGD (arginine-glycine-aspartic acid) adhesion sequences and encourages vascularisation.11 Collagen is a water-insoluble and fibrous ECM protein involved in the construction of connective tissues. It is the most abundant protein in mammals and is involved in the differentiation of osteoblasts and fibroblasts.11 Type I collagen is the most common form of collagen and is therefore often used as a scaffold in tissue engineering. Furthermore, collagen is biodegradable, non-cytotoxic, compatible with synthetic polymers and easy to modify.15 Agarose is a natural, biocompatible material that is non-cytotoxic and is compatible with other hydrogels such as collagen.15 By combining the two natural polymers collagen and agarose, collagen’s lack of mechanical resilience can be compensated by the small-pore-size branched network of agarose.16,17 Fibrin and agarose-collagen hydrogels are known to possess pro-angiogenic properties. Their rheological properties have been analyzed. Characterization of mixed blends (0.5% agarose, 0.5% collagen) revealed a significant increase in storage and elastic shear modulus compared to those of agarose and type I collagen separately.6
In this study, we compared the degree of in vitro vascularisation in co-cultures of HUVECs with MSCs from different sources by studying the formation of vascular structures. Fibrin gels and agarose–collagen hydrogels were used as scaffolds, and the co-cultures were incubated for 14 days before analysis by two-photon laser scanning microscopy (TPLSM). The impact of different MSCs and fibroblasts was compared as a step toward understanding the pre-vascularisation properties of different supporting cell types and sources for tissue engineering of different organs and tissues.
Results
Agarose–collagen type l hydrogels
We first investigated the influence of different supporting cell types in co-culture with HUVECs in agarose-collagen hydrogels. The evaluation was carried out by TPLSM, followed by statistical analysis (Figure 1). The co-culture of HUVECs and HDFs is known to promote vascularisation and served as a positive control.6 All co-cultures were established with three different supporting cell donors and each experiment was carried out in triplicate. One representative image per cell type is shown in Figure 1. Branched and elongated structures were observed in all co-cultures (Figure 1(a-e)). However, co-cultures with HDFs (Figure 1(a)) showed particularly extensive and widespread structures, whereas the vessel-like structures formed by HNFs (Figure 1(b)) were less branched and shorter. In co-cultures with WJ MSCs (Figure 1(c)) and AD MSCs (Figure 1(d)) only few vessel-like structures with branches were formed, less than for the fibroblasts. Co-cultures with BM MSCs (Figure 1(e)) formed a comprehensive network of fine structures that evolved homogeneously throughout the hydrogel.
Figure 1.

a-e: TPLSM images of CD31-stained AGR0.5COLL0.5 of HUVECs co-cultured with HDFs (a), HNFs (b), WJ MSCs (c), AD MSCs (d) and BM MSCs (e) after 14 days of cultivation at 37°C and 5% CO2. Scale bar: 50 µm. Statistical evaluation of the four parameters: area of the vascular structures (f), structure volume (g), length of the structures (h) and number of branching points (i) in the structures .The statistical significance is provided with * shows the significance of the individual values with respect to the positive control. ELISA results with eight angiogenesis cytokines: TNF-α, IGF-1, VEGF, IL-6, bFGF, TGF-ß, EGF and Leptin (j). The absorbance was measured at a wavelength of 450 nm.
Statistical analysis of the co-cultures revealed that structures formed by the different donors did not differ significantly (results not shown). The co-culture with HNFs had significant lower values compared to the positive control for the parameters cell surface area and structure length in agarose-collagen hydrogels (Figure 1(f–i)). The co-culture with BM MSCs had significant lower values compared to the positive control regarding cell surface area and structure volume. In contrast, the co-culture of BM MSCs had significant and up to eight times higher values for branching points (Figure 1(i)). There was no significant difference between the different MSC sources in terms of cell structure length. The vascular structures formed by MSCs from bone marrow contained up to eight times more branching points than MSCs from other sources but significantly lower values for the structure volume.
A multiplex ELISA was carried out after culture for 14 days (Figure 1(j)). This assay was used to detect secreted pro-angiogenic factors in the culture medium. The factors TNF-α, IGF-1, VEGF, bFGF and EGF were detected in similar concentrations in all co-cultures. IL-6 was up to two times higher in the MSC samples compared to both fibroblast types. TGF-ß of BM MSC co-cultures was twice as high value compared to all other samples. Leptin level was up to two times higher in the co-cultures with fibroblasts than for MSCs.
Permeability of the formed vessels was proven visually by cross sections of the structures. A representative cross-section of a co-culture with HUVECs and HNFs in agarose-collagen hydrogel is shown in Figure 2 (for all figures see supplementary section). The parameters and the morphological evaluation are additionally supported by the calculated values of the inner diameter of the structures (Figure 3). The mean diameters of the different structures in agarose collagen gels range between 1.27 and 8.48 µm. The co-cultures with BM MSC form the smallest diameters with 1.27 µm and the other co-cultures lie between 7–9 µm.
Figure 2.

Cross sections of a co-culture with HUVECs and HNFs in a CD31-stained AGR0.5COLL0.5 hydrogel. Scale bar: 50 µm.
Figure 3.

Calculated diameter in agarose-collagen hydrogels of HUVECs co-cultured with HDFs, HNFs, WJ MSCs, AD MSCs and BM MSCs after 14 days of cultivation at 37°C and 5% CO2.
Fibrin gels
Above-mentioned experiments were performed under the same conditions with fibrin gels. Highly branched and elongated structures were observed in all co-cultures (Figure 4(a–e)). In the co-cultures with dermal fibroblasts (Figure 4(a)) a wide and long branched network was formed. In contrast, in the co-cultures with HNFs shorter structures were built but with extensive distribution within the hydrogel (Figure 4(b)). WJ MSC (Figure 4(c)) and AD MSC co-cultures (Figure 4(D)) formed a few elongated, vessel-like structures with some branching points. BM MSCs (Figure 4e) produced a comprehensive network of fine structures that evolved homogeneously throughout the hydrogel, similar to the behavior in agarose-collagen gels.
Figure 4.

a-e: TPLSM images of CD31-stained fibrin gels of HUVECs co-cultured with HDFs (a), HNFs (b), WJ MSCs (c), AD MSCs (d) and BM MSCs (e) after 14 days of cultivation at 37°C and 5% CO2. Scale bar: 50 µm. Statistical evaluation of the four parameters: area of the vascular structures (f), structure volume (g), length of the structures (h) and number of branching points (i) in the structures .The statistical significance is provided with * shows the significance of the individual values with respect to the positive control. ELISA results with eight angiogenesis cytokines: TNF-α, IGF-1, VEGF, IL-6, bFGF, TGF-ß, EGF and Leptin (j). The absorbance was measured at a wavelength of 450 nm.
The morphological observations were supported by the quantitative investigation of the four parameters stated above (Figure 4(f–i)). The vessel surface area (Figure 4(f)) was significantly lower for WJ MSC and BM MSC co-cultures compared to the positive control. The structure volume (Figure 4(g)) was significantly higher for the structures formed by HDFs than the other co-cultures. The length of the vascular structures was comparable in dermal and nasal fibroblasts. Structures formed by the BM MSCs behaved similarly in the fibrin and agarose-collagen hydrogels, with a significant difference in the number of branches in the fibrin gel compared to the other cell sources (factor 10 higher). The results of the ELISA assay (Figure 4(j)) showed that TNF-α, IGF-1, VEGF, bFGF, EGF and leptin were detected in similar concentrations in all co-cultures. IL-6 was expressed up to three times as high in WJ MSCs and AD MSCs compared to the other samples. TGF-ß had a double higher value in BM MSCs compared to the others.
An example of a cross-section of a co-culture with HUVECs and HNFs in a CD31-stained fibrin gel culture is shown in Figure 5 (for all figures see supplementary section). The same tendency as in the agarose-collagen hydrogels was observed in the fibrin gels: The mean diameters of the different structures in fibrin gels range between 1.45 and 8.9 µm. The co-cultures with BM MSC form the thinnest diameters with 1.45 µm and the others co-cultures range between 7–9 µm (Figure 6).
Figure 5.

Cross sections of a co-culture with HUVECs and HNFs in a CD31-stained fibrin gel. Scale bar: 50 µm.
Figure 6.
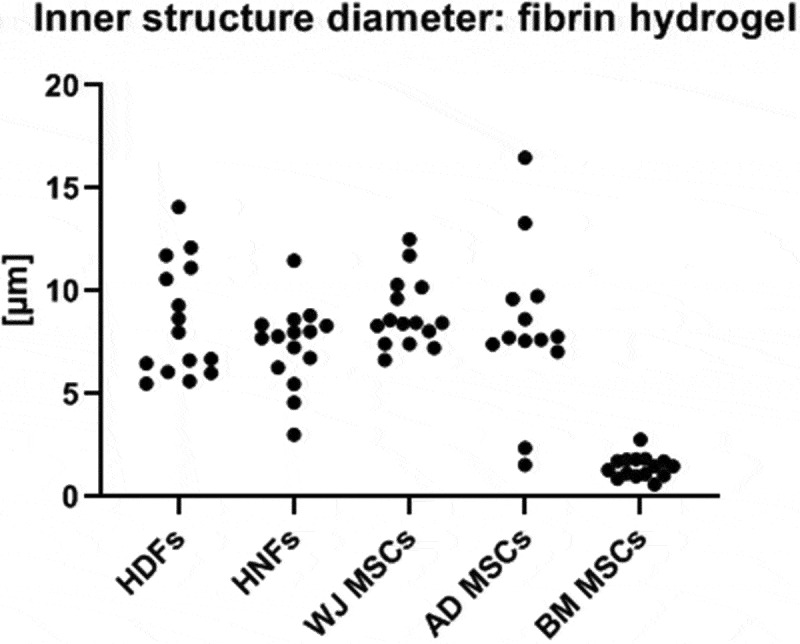
Calculated diameter in fibrin hydrogels of HUVECs co-cultured with HDFs, HNFs, WJ MSCs, AD MSCs and BM MSCs after 14 days of cultivation at 37°C and 5% CO2.
Discussion
In this study, we were able to detect vascular like structures in all tested co-cultures and hydrogels after a culture period of 14 days. By comparing the parameters cell surface area, structure volume, structure length, branching points numbers, the inner diameter of the structures and eight different pro-angiogenic cytokines, the vascularisation potential of the different cell types in both gels was quantitatively determined.
The microstructural arrangement of scaffolds plays a key role in capillary formation by co-cultures. Collagen-based hydrogels have an ordered structure of undulating collagen fibers.18 By strengthening the fibers with agarose, the density of the matrix can be increased, which might inhibit endothelial cell proliferation and migration. Fibrin gels, on the other hand, have a lower mechanical stability, but in turn have a higher gel porosity, which ensures cell maturation and increased nutrient supply to the cells.19 The analysis of porous hydrogels with different chemical properties (such as collagen I, III and IV and fibrin) has shown that the degree of vascularisation is not only dependent on the chemical properties of the materials, but also on the pore size. A positive correlation was observed by Sieminski et al. between implant pore size and vascularisation rate20 and the coarsely porous structure of the fibrin matrix favors migration and proliferation of endothelial cells. In different types of fibrin gels, the vessel length was dependent on the density of the biomaterial, with less dense and therefore more porous materials leading to longer vessel-like structures. 21 Despite their strong angiogenic potential, pure fibrin gels are less suitable as bioprintable scaffolds for fabrication of complex geometries due to their slow polymerization and low mechanical strength.6 However, recent technological advances now allow the bioprinting of fibrinogen by embedding the printed hydrogel within a secondary hydrogel that serves as a temporary and biocompatible support.22 One further disadvantage of fibrin gels is the fast degradation after a few days induced by proteases.11 The degradation process can be slowed by adding the protease inhibitor tranexamic acid, which is non-cytotoxic.23 Our experiments revealed that fibrin gels induce the formation of significantly more structures, but capillary-like structures could also be detected in directly printable agarose-collagen hydrogels. Focussing on future personalized biofabrication, the formation of capillary-like structures within the agarose-collagen scaffolds is a promising progress.6 Roa et al. cultured HUVECs and MSCs in different concentration ratios in a collagen/fibrin gel for 7 days. Vessel-like structures could be detected and their length was measured. However, no MSCs cell source is indicated in this study.24 In another study by Freiman et al., AD MSCs and microvascular endothelial cells, were cultivated in a 3D porous and biodegradable scaffold of PLLA/PLGA for up to 14 days and evaluated according to their vessel length and complexity.25 PLLA/PLGA are synthetic materials compared to the natural (bio)-polymers like agarose, collagen and fibrin. Even though MSCs from different tissues were used to test their potential as vascularisation, the different studies cannot be compared due to use of different scaffolds and culture periods. Thus, our study is a novel insight in this field, with regard to compare natural scaffolds with different cell sources in vitro.
Our results show that not only dermal fibroblasts but also other cell types can function as supporting cells to promote formation of vascular structures in agarose-collagen hydrogels and fibrin gels. We found that the source of the supporting cells influence the degree of vascularisation, confirming the assumption that also nasal fibroblasts exert an angio-inductive influence on endothelial cells.6 Similarly, Lee et al. have co-cultured lung fibroblasts with HUVECs successfully to promote the development of vascular structures for 14 days and to develop a vascular network.26 The observations are consistent with previous studies showing that fibroblasts promote endothelial cell proliferation and migration.3,27 These studies demonstrated that fibroblasts, particularly dermal fibroblasts, can stimulate vascularisation via the secretion of growth factors such as VEGF under suitable growth conditions.
The therapeutic potential of human MSCs (particularly those derived from adipose tissue and bone marrow) is promising in terms of the ability to harvest autologous cell material.28 WJ MSCs are used as an alternative cell source for stem cell therapy, because the isolation of these cells, unlike BM MSCs, is feasible without painful intervention. However, WJ MSCs are rarely available as autologous cell source. We found that MSCs from all sources induced vascularisation in co-culture systems in both agarose-collagen hydrogels and in fibrin gels, though in a lesser extend compared to fibroblasts. MSCs are known to promote wound healing, during which the expression of vascularisation markers such as VEGF and Ang-1 increases.29 The hypoxia-induced emergence of new blood vessels induces the accumulation of MSCs, which is associated with the increased secretion of the pro-angiogenic factors VEGF, bFGF and Ang-1. Noteworthy were the high-level expression of the pro-angiogenic factor TGF-ß of the mesenchymal stem cells demonstrated by ELISA, especially of BM MSCs. The co-cultures with BM MSCs produced by far the highest number of branches in both hydrogels (factor 8–10) and is characterized by a fine, area-cornering network of structures. The vessel sprouting process is facilitate by releasing ECM-bound angiogenic TGF-ß: Due to its angiogenic effects on endothelial cells via the TGF-ß/ALK1 pathway, it induces endothelial cell proliferation and migration, thus promoting the activation state of angiogenesis.30,31 Furthermore VEGF and TGF-β are often co-expressed in tissues in which vascularisation occurs. Briefly: TGF-ß is secreted by supporting cells such as fibroblasts, which in turn leads to the activation of endoglin, an accessory TGF-ß receptor, highly expressed during angiogenesis and essential for the ALK1 signaling pathway, leading to Smad1/5 responses and indirectly inhibits TGF‐β/ALK5 signaling pathway, thereby promoting the activation state of the endothelium.32 In addition, the angiogenic potential of BM MSCs in combination with HUVECs seeded in Matrigel has also been demonstrated in a study by Zhang et al: A tubular cell network was shown after only several hours.33 We found that WJ MSCs are suitable as a source of supporting cells for co-cultures, but that AD MSCs and BM MSCs show a higher, but not significant, potential to promote vascularisation after 14 days, and thus increase the formation of vessel-like structures in both hydrogels. In a study by Batsali et al., vessel-like structures have been produced by the co-culture of WJ MSCs with endothelial cells, and pro-angiogenic factors such as VEGF and Ang-1 were detected in the culture medium.34 McLaughlin et al. have shown that AD MSCs and BM MSCs induced vascular-like structures in poly-N-isopropylacrylamide scaffolds after culture for 21 days.9 Furthermore, AD MSCs in a co-culture with endothelial cells promoted endothelial cell migration and vessel formation via the secretion of the pro-angiogenic factors VEGF and PDGF.35 In general, a higher number of branching points means a broader branched network of capillary-like structures, as shown in the BM MSC co-cultures. Furthermore, the parameters area and volume of the structures correlate with the length, which is calculated from the two values. Thus, an even more precise statement can be made about the degree of vascularisation, e.g. HDFs co-cultures in fibrin gels, which have a higher cell surface and structure volume compared to HNFs, but the total length of the structures in both approaches does not differ significantly. The two co-cultures therefore form a capillary-like network, which exhibits different morphological properties, but without differences between the inner diamonds of the structures.
A limitation of our study is the donor anonymity from whom tissue samples were used. In future studies, cells with gender and age information of the donor available should be used, for example commercially available and genotyped cells from biotechnology companies. Furthermore, it should be considered that the possibility of donor variability for MSCs remains.36
Human capillaries have an average diameter between 5–10 µm depending on the tissue they are located in. In both of our tested hydrogels, mean diameters of the formed structures are in the same range for all supporting cell types despite BM MSCs For these, the diameter was much smaller and the structures have to be considered nonfunctional.37,38 On the other hand, Loughlin et al. were able to show in an in vivo study that BM MSC, after a cultivation period of 21 days on a synthetic scaffold, exhibits the formation of vascular structures and can thus play a supportive role in wound healing and angiogenesis.39
Conclusion
In summary, we found that vascularisation was supported by all tested cell types, and branched networks of tubular vascular structures were formed in both hydrogels. All the tested cell sources are suitable as support cells for the induction of vascularisation in co-cultures with HUVECs. In general, fibroblasts have a high angiogenic potential in co-culture with endothelial cells. In agarose collagen hydrogels, the vascular structures supported by AD-MSCs were comparable to our HDF control in volume, area and length. BM MSCs in both hydrogels formed a homogeneous, area-wide network of smaller structures with up to ten times more branching points than the other cell types. In summary, we conclude that both AD MSCs and BM MSCs are suitable for further pre-vascularisation studies due to their suitability in terms of expression levels of vascularisation-related marker (AD MSCs) and their capacity to form a wide-spread network (BM MSCs).
Further challenges are the connections to a suitable perfusion system in vitro and finally to a suitable system in the host circulatory system during implantation in vivo. Our data provide the first step toward understanding the pre-vascularisation properties of different supporting cell types and sources for further development of biological transplants.
Material and methods
Isolation and culture of primary cells
The primary human cells were isolated as approved by the local ethics committee of the Medical Faculty of RWTH Aachen University (EK 218/14). HUVECs and WJ MSCs were isolated from umbilical cords provided by the Clinic for Gynecology and Obstetrics (RWTH Aachen University Hospital). HDFs and AD MSCs were isolated from skin and underlying fat tissue, respectively, provided by the Clinic for Oral and Maxillofacial Surgery (RWTH Aachen University Hospital). HNFs were isolated from nasal turbinate and provided by the Clinic for Otorhinolaryngology and Plastic Surgery of the Head and Throat (RWTH Aachen University Hospital). BM MSCs were isolated from femoral heads provided by the Orthopedic Clinic (RWTH Aachen University Hospital). The cells were seeded at a density of 5,000 cells/cm2 and cultivated in the appropriate medium. The fibroblasts were cultivated with Dulbecco’s Modified Eagle’s medium (DMEM, Gibco) with 10% fetal calf serum (FCS, Thermo Fisher Scientific) and 1% antibiotic/antimycotic solution (ABM, Gibco). The endothelial cells were cultivated in endothelial cell growth medium (EBM-2 Medium with EGM-2 SingleQuot Kit, Lonza). The identification of HUVECs and fibroblasts was shown in previous studies for the same isolation procedures.7,40 The MSCs were cultivated in Mesenpan medium (PAN-Biotec) with 2% FCS and 1% ABM solution (Table 1). Isolation of MSCs was verified by flow cytometry according to Dominici et al., 2016 with cells in passage. 2–345 As displayed in the supplementary material, MSCs from all tissues were positive for CD90, CD73 and CD105 with ≥ 97.2%. The markers CD45, CD34, CD11b, CD79alpha and HLA-DR surface molecules were negative (≥ 98.7%). These results comply with the minimal criteria for cells to be classified as MSCs according to Domini et al., 201645
Table 1.
Overview about the the different cell types, cell sources, isolation methods, used culture medium, and passages.
| Cell type | Cell source | Isolation method | Culture medium | Passage used | Abbreviation |
|---|---|---|---|---|---|
| Endothelial cells | Umbilical cord vein | 7 | EGM-2 (Lonza) | 5 | HUVECs |
| Fibroblast | Skin tissue | 7 | DMEM (Gibco) | 4 | HDFs |
| Fibroblast | Nasal turbinate tissue | 41 | DMEM (Gibco) | 3 | HNFs |
| Mesenchymal stem cell | Wharton’s jelly, umbilical cord | 42 | Mesenpan (PAN-Biotech) | 2 | WJ MSCs |
| Mesenchymal stem cell | Adipose derived tissue | 43 | Mesenpan (PAN-Biotech) | 2 | AD MSCs |
| Mesenchymal stem cell | Bone marrow, femoral head | 44 | Mesenpan (PAN-Biotech) | 3 | BM MSCs |
Hydrogel molding process
Cells were trypsinized (Trypsin EDTA, Pan Biotech), counted (Neubauer chamber, Blaubrand) and molded in hydrogels (as described below) in 24-well plates (Greiner Bio-One) with a final volume of 350 µL and a cell concentration of 1.5 × 106 or 3.0 x 106/mL hydrogel. The samples were incubated for 14 days at 37°C and 5% CO2 in EGM-2 medium supplemented with 0.16 mg/mL tranexamic acid (Cyclokapron, Pfizer), medium change was performed every second day. Day 14 was chosen based on preliminary results, showing the highest degree in formation of capillary like structures and the largest internal diameter. All experiments were carried out using cells from three different donors and three technical replicates in each hydrogel.
Agarose–type l collagen blend
The final agarose concentration of 5 mg/mL hydrogel blend was achieved by diluting an agarose stock solution (22 mg/mL, low gelling temperature, Sigma-Aldrich) followed by storage at 45°C. The type l collagen solution was prepared from 4 mL collagen stock (9.9 mg/mL, FibriCol, Advanced BioMatrix), 500 µL 10x DMEM (Thermo Fischer Scientific), 500 µL DMEM (Gibco) and 100 µL 1 M NaOH (Sigma-Aldrich). The corresponding cells for the co-cultures were added to the collagen solution. Finally, the agarose solution was added and the hydrogel was molded into a 24-well plate. Polymerization was induced by incubation for 10 min at 4°C.
Fibrin gel
Fibrin hydrogels were molded with a final concentration of 5 mg/mL fibrinogen (VWR), 3 U/mL thrombin (Sigma-Aldrich) and 3.75 mM CaCl2 and the corresponding cell co-culture suspension into the wells. The polymerization was induced by incubating for 30 min at 37°C and 5% CO2.
Immunohistochemistry and quantitative analysis
The samples were stained and analyzed according to established protocols. 6 Briefly, the samples were fixed in ice-cold methanol for 30 min and stained with mouse anti-CD31 (PECAM-1, 1:100; Sigma-Aldrich) and Alexa Fluor® 594 goat anti-mouse IgG (1:400, Acris Antibodies). For quantitative evaluation, the samples were imaged using a two-photon laser scanning microscope (TPLSM) and images processed and evaluated with Imaris 9.0.0 software (Bitplane Inc. South Winsor, USA). First, the threshold value was set. In the next step, the background noise was reduced by selecting a filter set, such that only vascular structures were considered for the quantitative evaluation. The values for surface area and volume of the entire structures was then recorded. The length of the vascular structures was determined by the following assumptions: The individual sections of the structures were approximated as cylindrical objects with diameter d and length L, assuming that d ≪ L. With data on volume V and surface area A of the structures, calculation of the structure length L is possible by transforming both equations:
In addition, the software was used to measure the wall thickness of the structures and the inner lumen diameter of the structures. By measuring the large and small half-axis of the cross-section of the structures and using the diameter formula for ellipsoids, the average diameter was calculated for the cross-sections of the structures.
Statistics
Data were analyzed by an one-way analysis of variance with Turkey`s post hoc tests using SPSS Statistics 24 (IBM). A value of p < .05 was considered as statistically significant.
ELISA
The influence of the different co-cultures on vascularisation was determined by sandwich enzyme-linked immunosorbent assay (ELISA) using a multiplex ELISA kit (Angiogenesis ELISA Strip Profiling Assay, Signosis) to detect various proangiogenic factors such as tumor necrosis factor alpha (TNF-α), insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), interleukin 6 (IL-6), basic fibroblast growth factor (bFGF), transforming growth factor beta (TGF-β), epidermal growth factor (EGF) and leptin. Two experiments were carried out, first with the co-cultures of HDFs and HNFs and secondly with the MSCs. The results were evaluated according to manufacturer’s instructions.
Supplementary Material
Funding Statement
This study was supported by Deutsche Forschungsgemeinschaft (Grant No. JO 764/4-2 and FI 975/23-2).
List of abbreviations
- AD MSCs
Adipose-derived mesenchymal stem cells
- Ang
Angiopoietin
- bFGF
Basic fibroblast growth factor
- BM MSCs
Aemoral bone marrow-derived mesenchymal stem cells
- DMEM
Dulbecco’s Modified Eagle’s medium
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EGM
Endothelial cell growth medium
- FCS
Foetal calf serum
- HDFs
Human dermal fibroblasts
- HNFs
Human nasal fibroblasts
- HREs
Human respiratory epithelial cells
- HUVECs
Human umbilical vein endothelial cells
- IGF-1
Insulin-like growth factor 1
- IL-6
Interleukin 6
- MSCs
Mesenchymal stem cells
- NaOH
Sodium hydroxide
- NO
Nitric oxide
- PECAM-1
Platelet adhesion molecule-1
- RGD
Arginine-glycine-aspartic
- TNF-α
Tumor necrosis factor alpha
- TPLSM
Two-photon laser scanning microscopy
- TGF-β
Transforming growth factor beta
- VEGF
Vascular endothelial growth factor
- WJ MSCs
Mesenchymal stem cells from umbilical cord’s Wharton’s jelly
Acknowledgments
The authors would like to thank the RWTH Aachen University Hospital for providing human tissue for cell isolation: the Department of Gynaecology and Perinatal Medicine (Prof. Dr. Stickeler) for providing umbilical cords, the Department for Otorhinolaryngology and Plastic Surgery of the Head and Throat (Prof. Dr. Westhofen and Dr. Ilgner) for providing nose shell tissue, the Orthopaedic Clinic for providing femoral heads (PD Dr. Rath) and the Clinic for Oral and Maxillofacial Surgery (Prof. Dr. Hölzle and PD Dr. Lethaus) for providing skin and fat tissue for this study. We thank Dr. Richard Twyman for editing this paper, Felicitas Kempf and Bernhard Lüttgenau for the statistical and mathematical assistance. This work was supported by the Core Facility ‘‘Two-Photon Imaging’’ [Interdisciplinary Centre for Clinical Research (IZKF Aachen)] within the Faculty of Medicine at RWTH Aachen University and by the Flow Cytometry Facility, a core facility of the Interdisciplinary Center for Clinical Research (IZKF) Aachen within the Faculty of Medicine at RWTH Aachen University. We thank Marie Hauser (BioTex) for her support with the FACS analysis.
Competing interests
The authors declare that they have no competing financial interests.
Declarations
Ethics approval and consent to participate
The primary human cells were isolated as approved by the local ethics committee of the Medical Faculty of RWTH Aachen University, Germany (EK 218/14) after informed written consent.
References
- 1.Carmeliet P, Jain RK.. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 3.Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell. 2011;22(20):3791–800. doi: 10.1091/mbc.e11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fam NP, Verma S, Kutryk M, Stewart DJ. Clinician guide to angiogenesis. Circulation. 2003;108(21):2613–18. doi: 10.1161/01.CIR.0000102939.04279.75. [DOI] [PubMed] [Google Scholar]
- 5.Cheung JW, Jain D, McCulloch CA, Santerre JP. Pro-angiogenic character of endothelial cells and gingival fibroblasts cocultures in perfused degradable polyurethane scaffolds. Tissue Eng Part A. 2015;21(9–10):1587–99. doi: 10.1089/ten.tea.2014.0548. [DOI] [PubMed] [Google Scholar]
- 6.Kreimendahl F, Kopf M, Thiebes AL, Duarte Campos DF, Blaeser A, Schmitz-Rode T, Apel C, Jockenhoevel S, Fischer H, et al. Three-dimensional printing and angiogenesis: tailored agarose-type I collagen blends comprise three-dimensional printability and angiogenesis potential for tissue-engineered substitutes. Tissue Eng Part C Methods. 2017;23(10):604–15. doi: 10.1089/ten.tec.2017.0234. [DOI] [PubMed] [Google Scholar]
- 7.Helmedag MJ, Weinandy S, Marquardt Y, Baron JM, Pallua N, Suschek CV, Jockenhoevel S, et al. The effects of constant flow bioreactor cultivation and keratinocyte seeding densities on prevascularized organotypic skin grafts based on a fibrin scaffold. Tissue Eng Part A. 2015;21(1–2):343–52. doi: 10.1089/ten.tea.2013.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold F, West DC. Angiogenesis in wound healing. Pharmacol Ther. 1991;52(3):407–22. doi: 10.1016/0163-7258(91)90034-J. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin MM, Marra KG. The use of adipose-derived stem cells as sheets for wound healing. Organogenesis. 2013;9(2):79–81. doi: 10.4161/org.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards SS, Zavala G, Prieto CP, Elliott M, Martinez S, Egana JT, Bono MR, Palma V. Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton’s jelly in dermal regeneration. Angiogenesis. 2014;17(4):851–66. doi: 10.1007/s10456-014-9432-7. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B. 2008;14(2):199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 12.Kopf M, Campos DF, Blaeser A, Sen KS, Fischer H. A tailored three-dimensionally printable agarose-collagen blend allows encapsulation, spreading, and attachment of human umbilical artery smooth muscle cells. Biofabrication. 2016;8(2):025011. doi: 10.1088/1758-5090/8/2/025011. [DOI] [PubMed] [Google Scholar]
- 13.San Martin S, Alaminos M, Zorn TM, Sanchez-Quevedo MC, Garzon I, Rodriguez IA, Campos A. The effects of fibrin and fibrin-agarose on the extracellular matrix profile of bioengineered oral mucosa. J Tissue Eng Regen Med. 2013;7(1):10–19. doi: 10.1002/term.v7.1. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich M, Heselhaus J, Wozniak J, Weinandy S, Mela P, Tschoeke B, Schmitz-Rode T, Jockenhoevel S. Fibrin-based tissue engineering: comparison of different methods of autologous fibrinogen isolation. Tissue Eng Part C Methods. 2013;19(3):216–26. doi: 10.1089/ten.tec.2011.0473. [DOI] [PubMed] [Google Scholar]
- 15.Antoine EE, Vlachos PP, Rylander MN. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng Part B Rev. 2014;20(6):683–96. doi: 10.1089/ten.teb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulrich TA, Jain A, Tanner K, MacKay JL, Kumar S. Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials. 2010;31(7):1875–84. doi: 10.1016/j.biomaterials.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 17.Kreimendahl F, Ossenbrink S, Kopf M, Westhofen M, Schmitz-Rode T, Fischer H, Jockenhoevel S, Thiebes AL. Combination of vascularization and cilia formation for three-dimensional airway tissue engineering. J Biomed Mater Res A. 2019;107(9):2053–62. doi: 10.1002/jbm.a.v107.9. [DOI] [PubMed] [Google Scholar]
- 18.Lake SP, Hald ES, Barocas VH. Collagen-agarose co-gels as a model for collagen-matrix interaction in soft tissues subjected to indentation. J Biomed Mater Res A. 2011;99(4):507–15. doi: 10.1002/jbm.a.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6(30):1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sieminski AL, Gooch KJ. Biomaterial-microvasculature interactions. Biomaterials. 2000;21(22):2232–41. doi: 10.1016/S0142-9612(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 21.Kniazeva E, Putnam AJ. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. Am J Physiol Cell Physiol. 2009;297(1):C179–C87. doi: 10.1152/ajpcell.00018.2009. [DOI] [PubMed] [Google Scholar]
- 22.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1(9):e1500758. doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cholewinski E, Dietrich M, Flanagan TC, Schmitz-Rode T, Jockenhoevel S. Tranexamic acid–an alternative to aprotinin in fibrin-based cardiovascular tissue engineering. Tissue Eng Part A. 2009;15(11):3645–53. doi: 10.1089/ten.tea.2009.0235. [DOI] [PubMed] [Google Scholar]
- 24.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15(2):253–64. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freiman A, Shandalov Y, Rozenfeld D, Shor E, Segal S, Ben-David D, Meretzki S, Egozi D, Levenberg S. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res Ther. 2016;7:5. doi: 10.1186/s13287-015-0251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JW, Choi YJ, Yong WJ, Pati F, Shim JH, Kang KS, Kang I-H, Park J, Cho D-W. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication. 2016;8(1):015007. doi: 10.1088/1758-5090/8/1/015007. [DOI] [PubMed] [Google Scholar]
- 27.Kunz-Schughart LA, Schroeder JA, Wondrak M, van Rey F, Lehle K, Hofstaedter F, Wheatley DN. Potential of fibroblasts to regulate the formation of three-dimensional vessel-like structures from endothelial cells in vitro. Am J Physiol Cell Physiol. 2006;290(5):C1385–C98. doi: 10.1152/ajpcell.00248.2005. [DOI] [PubMed] [Google Scholar]
- 28.King A, Balaji S, Keswani SG, Crombleholme TM. The Role of Stem Cells in Wound Angiogenesis. Adv Wound Care (New Rochelle). 2014;3(10):614–25. doi: 10.1089/wound.2013.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–59. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 30.Clapp C, Thebault S, Jeziorski MC, Martinez De La Escalera G. Peptide hormone regulation of angiogenesis. Physiol Rev. 2009;89(4):1177–215. doi: 10.1152/physrev.00024.2009. [DOI] [PubMed] [Google Scholar]
- 31.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, Dijke PT. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. Embo J. 2004;23(20):4018–28. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775(1):21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, Lee YW, Rui YF, Cheng TY, Jiang XH, Li G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 2013;4(3):70. doi: 10.1186/scrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batsali AK, Kastrinaki MC, Papadaki HA, Pontikoglou C. Mesenchymal stem cells derived from Wharton’s Jelly of the umbilical cord: biological properties and emerging clinical applications. Curr Stem Cell Res Ther. 2013;8(2):144–55. doi: 10.2174/1574888X11308020005. [DOI] [PubMed] [Google Scholar]
- 35.Marfia G, Navone SE, Di Vito C, Ughi N, Tabano S, Miozzo M, Tremolada C, Bolla G, Crotti C, Ingegnoli F, et al. Mesenchymal stem cells: potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis. 2015;11(4):183–206. doi: 10.1080/15476278.2015.1126018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell AL, Lefavor R, Durand N, Glover L, Zubair AC. Modifiers of mesenchymal stem cell quantity and quality. Transfusion. 2018;58(6):1434–40. doi: 10.1111/trf.14597. [DOI] [PubMed] [Google Scholar]
- 37.Moya ML, Hsu YH, Lee AP, Hughes CC, George SC. In vitro perfused human capillary networks. Tissue Eng Part C Methods. 2013;19(9):730–37. doi: 10.1089/ten.tec.2012.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skalak R, Branemark PI. Deformation of red blood cells in capillaries. Science. 1969;164(3880):717–19. doi: 10.1126/science.164.3880.717. [DOI] [PubMed] [Google Scholar]
- 39.O’Loughlin A, Kulkarni M, Creane M, Vaughan EE, Mooney E, Shaw G, Murphy M, Dockery P, Pandit A, O’Brien T, et al. Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer. Diabetes. 2013;62(7):2588–94. doi: 10.2337/db12-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinandy S, Laffar S, Unger RE, Flanagan TC, Loesel R, Kirkpatrick CJ, van Zandvoort M, Hermanns-Sachweh B, Dreier A, Klee D, et al. Biofunctionalized microfiber-assisted formation of intrinsic three-dimensional capillary-like structures. Tissue Eng Part A. 2014;20(13–14):1858–69. doi: 10.1089/ten.tea.2013.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goto Y, Noguchi Y, Nomura A, Sakamoto T, Ishii Y, Bitoh S, Picton C, Fujita Y, Watanabe T, Hasegawa S, et al. In vitro reconstitution of the tracheal epithelium. Am J Respir Cell Mol Biol. 1999;20(2):312–18. doi: 10.1165/ajrcmb.20.2.3062. [DOI] [PubMed] [Google Scholar]
- 42.Schneider RK, Pullen A, Kramann R, Bornemann J, Knuchel R, Neuss S, Perez-Bouza A. Long-term survival and characterisation of human umbilical cord-derived mesenchymal stem cells on dermal equivalents. Differentiation. 2010;79(3):182–93. doi: 10.1016/j.diff.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Cholewa D, Stiehl T, Schellenberg A, Bokermann G, Joussen S, Koch C, Walenda T, Pallua N, Marciniak-Czochra A, Suschek CV, et al. Expansion of adipose mesenchymal stromal cells is affected by human platelet lysate and plating density. Cell Transplant. 2011;20(9):1409–22. doi: 10.3727/096368910X557218. [DOI] [PubMed] [Google Scholar]
- 44.Lauria I, Kramer M, Schroder T, Kant S, Hausmann A, Boke F, Leube R, Telle R, Fischer H. Inkjet printed periodical micropatterns made of inert alumina ceramics induce contact guidance and stimulate osteogenic differentiation of mesenchymal stromal cells. Acta Biomater. 2016;44:85–96. doi: 10.1016/j.actbio.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans RJ, Keating A, Prockop DJ, Horwitz EM, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


