ABSTRACT
Protein-tyrosine kinase 6 (PTK6) sequestrated by its substrate paraspeckle component 1 (PSPC1) in nucleus is a tumor suppressor. PSPC1 functions as a contextual determinant of oncogenic subcellular translocations to synergize PSPC1/β-catenin and PTK6 tumorigenesis. Besides, PSPC1 C-terminal interacting domain (PSPC1-CT131), a dual inhibitor of PSPC1 and PTK6, suppresses cancer progression.
KEYWORDS: Oncogenic subcellular translocation, EMT, CSC, metastasis, HCC
Auto-commentary
Tumor metastasis, the main cause of cancer patient death, is a complicated process involving the intravasation of reprogrammed cancer cells from a primary site into the vasculature or lymphatics followed by entering circulation, extravasation, and expansion of tumor growth at distant organs.1 Emerging evidence suggested that cancer cells with the reprogramming capabilities acquired the malignant features of cancer epithelial-to-mesenchymal transition (EMT) and stemness to sustain the pro-metastatic tumor microenvironment for promoting tumor metastasis led to poor outcomes of cancer patients.2,3 Therefore, targeting the metastasis driver genes could be the “Achilles heel” to abrogate tumor progression and prolong life of cancer patients. We recently reported that paraspeckle component 1 (PSPC1) acts as a master activator of metastatic reprogramming by activating EMT, stemness, and transforming growth factor beta 1 (TGF-β1) pro-metastatic switch.4 High PSPC1 expression is associated with the late stages of cancer patients and poor patient survival including patients with human hepatocellular carcinoma (HCC),4 the most common type of primary liver cancer occurring mainly in developing countries. However, strategies to target PSPC1 and the associated molecular mechanisms to halt tumor metastasis remain unclear. Our recent study provides new evidence that PSPC1 endows the switch of protein-tyrosine kinase 6 (PTK6) and β-catenin oncogenic subcellular translocations in tumor progression. Moreover, C-terminal interacting domain of PSPC1 (PSPC1-CT131), an innovative dual inhibitor of PSPC1 and PTK6 based on the interacting domains, is developed to abrogate the oncogenic translocations of PTK6 and β-catenin and suppress tumor progression in HCC models.5
We identified PTK6 as a PSPC1-interacting protein by proteomic approach in HCC cell lines. PTK6 is a nonreceptor tyrosine kinase activated by phosphorylation and forming a complex with the C-terminal proline-rich domain of PSPC1 in the nucleus. It is known that PTK6 functions as a double‐edged sword in association with its subcellular localizations to determine its oncogenic functions, a tumor suppressor in the nucleus and an oncogene in the cytoplasm.6 Therefore, nuclear PTK6 sequestered by the tyrosine-phosphorylated substrate PSPC1 acts as a tumor suppressor to abrogate the PSPC1 oncogenic effects.
To explore the oncogenic consequences of PTK6 phosphorylation and interaction with PSPC1, we identified two tyrosine residues in the interacting C-terminal domain of PSPC1 and predicted Y523 residue (PSPC1-Y523) is tyrosine-phosphorylated by the PTK6 based on 3D structural docking. Indeed, we observed that expression of phosphorylation-defective mutant PSPC1-Y523F is more oncogenic, in compared to that of expressing PSPC1, to enhance cell migration, invasion, EMT, and stemness in HCC cells and tumor metastasis in orthotopic HCC mouse models. Moreover, expression of PSPC1-Y523F not only fails to pull down PSPC1 but also facilitates subcellular translocation of PTK6 from nucleus to cytoplasm. After validation with immunofluorescent staining of PTK6 subcellular localization, we concluded that expression of PSPC1-Y523F facilitates active PTK6 (p-PTK6) translocation to cytosol and membrane to synergize PSPC1 oncogenic progression including enhancing cell motility, stemness, and metastasis (Figure 1a).
Figure 1.
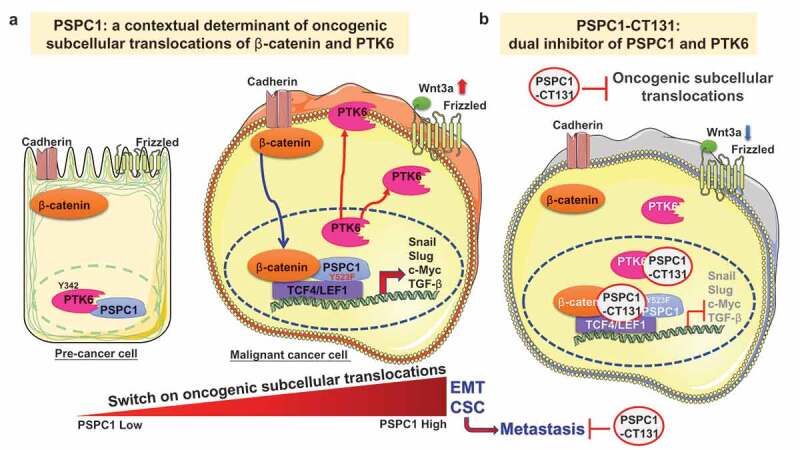
PSPC1 is a contextual determinant of tumor progression. (a) Paraspeckle component 1 (PSPC1) is a contextual determinant of oncogenic subcellular translocations of β-catenin and PTK6 to activate Wnt3a autocrine signaling. PSPC1 upregulation or PSPC1 523 residue mutation from tyrosine to phenylalanine (PSPC1-Y523F) could dictate oncogenic subcellular translocations to synergize PSPC1 interacted with β–catenin and cytoplasmic protein-tyrosine kinase 6 (PTK6) tumorigenic effects to facilitate tumor progression and metastasis. In pre-malignant cancer cells with low PSPC1 expression, PTK6 was sequestered by its tyrosine-phosphorylated substrate PSPC1 in the nucleus to suppress tumorigenic features. However, in advanced cancer cells with high PSPC1 expression, PTK6 could translocate to cytoplasm and cellular membrane as an oncogene and cytoplasmic β–catenin will translocate to the nuclear to preferentially interact with PSPC1 to facilitate synergistic oncogenic effects such as epithelial-to-mesenchymal transition (EMT), Wnt3a autocrine signaling and stemness (CSC, cancer-like stem cells) toward metastasis. (b) PSPC1-CT131 is a dual inhibitor of PSPC1 and PTK6. Treatments of C-terminal interacting domain of PSPC1 (PSPC1-CT131), a dual inhibitor of PSPC1 and PTK6, could target PSPC1 and PTK6 to suppress PTK6 and β-catenin oncogenic subcellular translocations to prolong the survival of mice in an orthotopic hepatocellular carcinoma (HCC) mouse model. Thus, PSPC1-CT131 is an innovative anticancer agent for improving cancer therapy in clinical interventions.
To better understand the molecular mechanisms of synergistic oncogenic effects of PSPC1 and cytoplasmic PTK6, we applied the hepatocyte growth factor (HGF)-induced EMT in Huh7 cells as a model for exploring the PSPC1 and PTK6 oncogenic axis and the downstream Wnt/β-catenin signaling pathway.7 HGF-treated Huh7 cells augment PSPC1 expression to induce EMT, to increase expression of cytoplasmic and membrane PTK6 with reduced PSPC1 and PTK6 nuclear interaction, to stimulate nuclear translocation of β-catenin with enhanced PSPC1 and β-catenin nuclear interaction, and to upregulate Wnt3a autocrine signaling for potentiating PSPC1 and PTK6 synergistic oncogenic progression in HCC cells (Figure 1a). With confirmation of PSPC1/PTK6/β-catenin dynamic interaction in orthotopic HCC mouse model by immunohistochemistry (IHC) staining, we concluded that PSPC1 is the contextual determinant for not only the TGF-β1 pro-metastatic switch but also the oncogenic subcellular translocations of PTK6 and β-catenin to synergize oncogenic tumor progression.5,8
Since the nuclear sequestration of PTK6 with PSPC1-Y523 phosphorylation kept cancer cells in pre-malignant status, we suggest that phosphorylated-Y523 of PSPC1 (p-Y523-PSPC1) could be a biomarker for predicting tumor progression. We generated an antibody specifically against PSPC1-Y523 phosphorylation and demonstrated that nuclear expression of p-PTK6 or reducing expression of p-Y523-PSPC1 is associated with better prognosis of HCC patients in compared to the corresponding controls. Our results indicated that detection of diminishing PSPC1-Y523 phosphorylation could be an early biomarker for the warning of tumor progression in therapeutic interventions of HCC patients.
With PSPC1-CT131 containing proline-rich domain serving as the molecular docking target for PSPC1 and the SH3 domain of PTK6, we provide lines of evidence to demonstrate that PSPC1-CT131 might sequester PSPC1 and PTK6 in nucleus and abrogate the aberrant nucleocytoplasmic shuttling to suppress tumor progression (Figure 1b).5,9 Indeed, PSPC1-CT131 is a dual inhibitor to interact with PSPC1 and p-PTK6 in nucleus to diminish their synergized tumor progression. Transcriptome analysis revealed that PSPC1-CT131 treatment abolished the functional synergism of the oncogenic PSPC1 and PTK6 axes in several gene signatures downstream of PSPC1 and PTK6 pathways.6,7,10 In preclinical studies, treatments with PSPC1-CT131 showed a significant reduction in tumor volume and metastatic tumor nodules with validation of IHC analysis for the PSPC1/PTK6/β-catenin axis. PSPC1-CT131 also prolonged survival of mice compared to the untreated group. Thus, PSPC1-CT131 via targeting PSPC1 and PTK6 to suppress EMT, stemness, TGF-β1, and Wnt/β-catenin autocrine signaling might be an innovative inhibitor for exploring clinical interventions. Taken together, PSPC1 is the contextual determinant of PTK6 and β-catenin reciprocal oncogenic subcellular translocations to augment Wnt3a autocrine signaling. The development and future optimization of PSPC1-CT131 highlight the first-in-class dual inhibitor to target nucleocytoplasmic shuttling by diminishing the synergistic PSPC1/β-catenin and PTK6 oncogenic effects for tumor suppression (Figure 1b).
Funding Statement
Our work was supported by Academia Sinica and Ministry of Science and Technology of Taiwan (MOST 107-0210-01-19-01, 106-0210-01-15-02, 107-2321-B-001-025 and 104-2320-B-001-009-MY3).
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- 1.Lambert AW, Pattabiraman DR, Weinberg RA.. Emerging biological principles of metastasis. Cell. 2017;168:1–3. PMID: 28187288. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuelten CH, Parent CA, Montell DJ. Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat Rev Cancer. 2018;18:296–312. PMID: 29546880. doi: 10.1038/nrc.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–226. PMID: 30594349. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Yeh H-W, Hsu E-C, Lee -S-S, Lang Y-D, Lin Y-C, Chang C-Y, Lee S-Y, Gu D-L, Shih J-H, Ho C-M, et al. PSPC1 mediates TGF-β1 autocrine signalling and Smad2/3 target switching to promote EMT, stemness and metastasis. Nat Cell Biol. 2018;20:479–491. PMID: 29593326. doi: 10.1038/s41556-018-0062-y. [DOI] [PubMed] [Google Scholar]
- 5.Lang Y-D, Chen H-Y, Ho C-M, Shih J-H, Hsu E-C, Shen R, Lee Y-C, Chen J-W, Wu C-Y, Yeh H-W, et al. PSPC1-interchanged interactions with PTK6 and β-catenin synergize oncogenic subcellular translocations and tumor progression. Nat Commun. 2019;10. PMID: 31844057. doi: 10.1038/s41467-019-13665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel RK, Lukong KE. Tracing the footprints of the breast cancer oncogene BRK – past till present. Biochim Biophys Acta Bioenerg. 2015;1856:39–54. PMID: 25999240. doi: 10.1016/j.bbcan.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. PMID: 15542433. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Yeh HW, Lee -S-S, Chang C-Y, Lang Y-D, Jou Y-S. A New Switch for TGFbeta in Cancer. Cancer Res. 2019;79:3797–3805. PMID: 31300476. doi: 10.1158/0008-5472.Can-18-2019. [DOI] [PubMed] [Google Scholar]
- 9.Conforti F, Wang Y, Rodriguez JA, Alberobello AT, Zhang Y-W, Giaccone G. Molecular pathways: anticancer activity by inhibition of nucleocytoplasmic shuttling. Clin Cancer Res. 2015;21(20):4508–4513. PMID: 26324742. doi: 10.1158/1078-0432.Ccr-15-0408. [DOI] [PubMed] [Google Scholar]
- 10.Yeh HW, Jou YS. PSPC1 potentiates TGF-beta-dependent metastatic dissemination. Mol Cell Oncol. 2018;5:e1472058. PMID: 30250921. doi: 10.1080/23723556.2018.1472058. [DOI] [PMC free article] [PubMed] [Google Scholar]


