Supplemental Digital Content is available in the text.
Keywords: acute kidney injury, critical illness, fibroblast growth factor 23, intensive care unit, Klotho, major adverse kidney events
Objectives:
Klotho and fibroblast growth factor-23 were recently postulated as candidate biomarkers and/or therapeutic targets in acute kidney injury. We examined whether urine Klotho and serum intact fibroblast growth factor-23 levels were differentially and independently associated with major adverse kidney events in critically ill patients with and without acute kidney injury.
Design:
Single-center, prospective, case-control study.
Setting:
ICU in a tertiary medical center.
Patients:
Fifty-four acute kidney injury patients and 52 controls without acute kidney injury admitted to the ICU.
Interventions:
None.
Measurements and Main Results:
Acute kidney injury was defined by Kidney Disease: Improving Global Outcomes criteria and included only acute kidney injury stage greater than or equal to 2. Controls were matched by age, gender, and baseline estimated glomerular filtration rate. Paired serum and urine samples were obtained 24–48 hours after acute kidney injury diagnosis (cases) or ICU admission (controls). The primary outcome was 90-day major adverse kidney events, which was the composite of all-cause death, dependence on renal replacement therapy, or a 50% or higher decrease in estimated glomerular filtration rate from baseline. Major adverse kidney events, which was the composite of all-cause death, dependence on renal replacement therapy, or a 50% or higher decrease in estimated glomerular filtration rate from baseline. Major adverse kidney events developed in 44 patients (41.5%). Patients in whom major adverse kidney events developed had more comorbidity, higher acuity of illness scores, and more prevalent acute kidney injury. Levels of urine Klotho adjusted by creatinine were lower, and serum intact fibroblast growth factor-23 levels were higher in acute kidney injury patients versus ICU controls. In adjusted models, the highest versus lowest tertile of urine Klotho/creatinine was independently associated with an overall 95% lower risk of major adverse kidney events (81% lower risk in patients with acute kidney injury). The highest versus lowest tertile of serum intact fibroblast growth factor-23 was associated with more than 300% higher risk of major adverse kidney events.
Conclusions:
Urine Klotho/creatinine levels were significantly lower and serum intact fibroblast growth factor-23 levels were significantly higher in critically ill patients with acute kidney injury versus matched controls without acute kidney injury. When measured in the first 48 hours of ICU admission or acute kidney injury diagnosis, urine Klotho/creatinine independently associated with major adverse kidney events, particularly in patients with acute kidney injury. These results show promise for testing these biomarkers—individually or in combination—as part of novel risk prediction models of renal outcomes in the ICU.
Acute kidney injury (AKI) is a detrimental syndrome that occurs in approximately 50% of critically ill patients admitted to ICUs (1, 2). AKI is associated with high morbidity, mortality (3, 4), and risk of chronic kidney disease (CKD) (5, 6) and end-stage renal disease (ESRD) (7). Discovery and validation of AKI biomarkers have mostly focused on early AKI recognition (8, 9), and little is known about their utility to predict AKI recovery or subsequent CKD/ESRD risk post-AKI. Kidney function examination within 90 days postdischarge in survivors of AKI associates with subsequent risk of ESRD and has been proposed as a surrogate endpoint for ESRD after AKI (10).
αKlotho (referred to as “Klotho”) is primarily expressed in the kidney (11) and has emerged as a potential therapeutic agent for AKI in preclinical studies (12). Transmembrane Klotho is a coreceptor for fibroblast growth factor (FGF)-23. The extracellular domain of transmembrane Klotho is cleaved by proteases (13–15) and released into the circulation (soluble Klotho) and can be measured in blood and urine (16, 17). Soluble Klotho is not filtered by the glomerulus, but traffics via transcytosis across the renal tubules, from the basolateral to the luminal side, exerts actions in the urine, and is then excreted in the urine (17). Preclinical data have shown that Klotho protein replacement or transgenic overexpression (18–20) can attenuate AKI, promote recovery, and prevent CKD post-AKI (18, 21).
Elevation of plasma FGF23 levels has been described in AKI (22, 23) and associates with adverse outcomes post-AKI in critically ill and cardiac surgery patients (24–26). Impaired renal clearance or increased production from bone or other organs is potential origins of this elevation (27).
The purpose of the present study was to examine whether urine Klotho and serum intact FGF23 levels are different in critically ill patients with versus without AKI and if these biomarker levels associate with major adverse kidney events (MAKE). To our knowledge, this is the first study in critically ill patients in which concomitant measurements of urine Klotho and serum intact FGF23 levels were done.
METHODS
Study Design and Participants
Single-center, prospective study of 54 AKI patients and 52 matched controls without AKI admitted to the ICU at the University of Texas Southwestern Hospital from January 2015 to January 2016. AKI was defined by Kidney Disease: Improving Global Outcomes (KDIGO) criteria (28). Only patients with AKI stage greater than or equal to 2 were included in the study as cases. Controls were frequency matched by age (10-yr intervals), gender, and two-category baseline estimated glomerular filtration rate (eGFR, calculated using CKD-Epidemiology Collaboration (CKD-EPI) equation, ≥ 90 and 60–89 mL/min/1.73 m2) (29). Baseline serum creatinine (SCr) was defined as the most recent SCr within the 6-month period before ICU admission.
Inclusion criteria included the following: adults greater than or equal to 18 years old, admission to the ICU, and baseline eGFR greater than or equal to 60 mL/min/1.73 m2. Exclusion criteria consisted of prior kidney or any other solid organ transplant, ESRD, evidence of AKI before ICU admission, or the presence of uroepithelial tumors. The protocol was approved by the Institutional Review Board. Informed consent was obtained for all study participants.
Sample Collection
A single-timepoint paired serum and urine samples were obtained 24–48 hours after AKI diagnosis (cases) or ICU admission (controls). Standardized techniques for serum and urine collection, transport, processing, and storage were employed. Biospecimens were centrifuged at 1,000g, 4°C for 10 minutes. Serum and urine supernatant were aliquoted in codified nonsiliconized cryovials and stored at –80°C until biomarker measurements were done by laboratory personnel blinded to the study design and data. Urine samples were not obtained in seven patients due to anuria.
Laboratory Analyses
Urine Klotho was measured by immunoblotting urine with monoclonal anti-Kl1 antibody KM2076 (TransGenic, Kobe, Japan) with calibration against Klotho standards (30). Urine Klotho was normalized to creatinine concentration in the same spot urine sample. The intra-assay coefficient of variation is 8%. Serum intact FGF23 was measured using the human Intact FGF23 Enzyme-Linked Immunosorbent Assay kit (Immutopics-Quidel, San Diego, CA). Urine creatinine was measured by capillary electrophoresis (31). Other reported analytes were measured as part of standard of care.
Clinical Data
Data pertaining to demographics, kidney function, comorbidity, Charlson index, and critical illness parameters were obtained. Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores were calculated integrating data from the first day of ICU admission. For both APACHE II and SOFA, the points related to SCr were subtracted from the total score. Anemia was defined as admission hematocrit less than 39% for men and less than 36% for women.
Study Outcome
The observation period was from study enrollment until death or 90 days for survivors. The primary outcome was 90-day MAKE, which consisted of the composite of all-cause death, renal replacement therapy (RRT) dependence, or the decrease in eGFR of greater than or equal to 50% from baseline (32). Secondary outcomes consisted of mechanical ventilation days and hospital days and were examined only inhospital survivors to offset the competing risk of death.
Statistical Analysis
Categorical data were reported as percentages, and continuous data were reported as means ± sd or median (25th–75th percentile). Comparisons across biomarker tertiles for categorical variables were made using Fisher exact test. For continuous variables, analysis of variance was used for Gaussian and Kruskal-Wallis test for non-Gaussian distributed data. Comparisons between AKI versus no-AKI and MAKE-90 versus no MAKE-90 for categorical variables were made using Fisher exact test. For continuous variables, t test was used for Gaussian and Wilcoxon rank-sum test for non-Gaussian distributed data. Biomarker data were non-Gaussian distributed and were therefore natural log transformed.
Multivariable logistic regression models were constructed for MAKE-90 as the dependent variable and to evaluate the following biomarkers as independent variables: urine Klotho, urine Klotho adjusted by urine creatinine (urine Klotho/creatinine), serum intact FGF23, and serum intact FGF23 divided by urine Klotho adjusted by urine creatinine (FGF23-to-Klotho/creatinine ratio). Model 1 included age, sex, and Charlson index. Model 2 included variables in Model 1 plus nonrenal APACHE II score. Only one of two acuity of illness scores (APACHE II or SOFA) was included due to collinearity between variables. Secondary outcomes (hospital days and mechanical ventilation days) were examined only in those who survived the hospitalization utilizing negative binomial regression adjusted for Model 2. The two-way interaction between AKI status and each study biomarker for its association with MAKE-90 was evaluated and found to be nonsignificant (p > 0.1 for all biomarkers).
Spearman correlation analysis was performed for each study biomarker and preselected biochemical parameters measured less than 48 hours apart from the study biomarkers. Two-sided p values of less than 0.05 indicated statistical significance. SAS 9.4 (SAS Institute, Cary, NC) was used for statistical analyses.
Sensitivity Analyses
We examined an alternative and more sensitive definition for the primary outcome of MAKE-90, which consisted of the composite of all-cause death, RRT dependence, or the decrease in eGFR of greater than or equal to 25% from baseline (rather than 50%) (32).
RESULTS
Clinical Characteristics
A total of 106 patients were included in the study, 54 with AKI stage greater than or equal to 2 and 52 without AKI (controls). When comparing characteristics according to tertiles of urine Klotho/creatinine, we observed that patients in the lowest tertile of urine Klotho/creatinine had more frequently diabetes, anemia, and dipstick proteinuria greater than or equal to 30 mg/dL. Concordantly, patients in the lowest tertile of urine Klotho/creatinine had higher Charlson index scores and higher SOFA and APACHE II scores, along with worse critical illness parameters when compared with their counterparts (Table 1). As depicted in Supplementary Table 1 (Supplemental Digital Content 1, http://links.lww.com/CCX/A52), patients in the highest tertile of FGF23 had more frequently anemia and dipstick proteinuria greater than or equal to 30 mg/dL, higher Charlson scores, and higher APACHE II scores.
TABLE 1.
Characteristics of Patients According to Tertiles of Urine Klotho/Creatinine
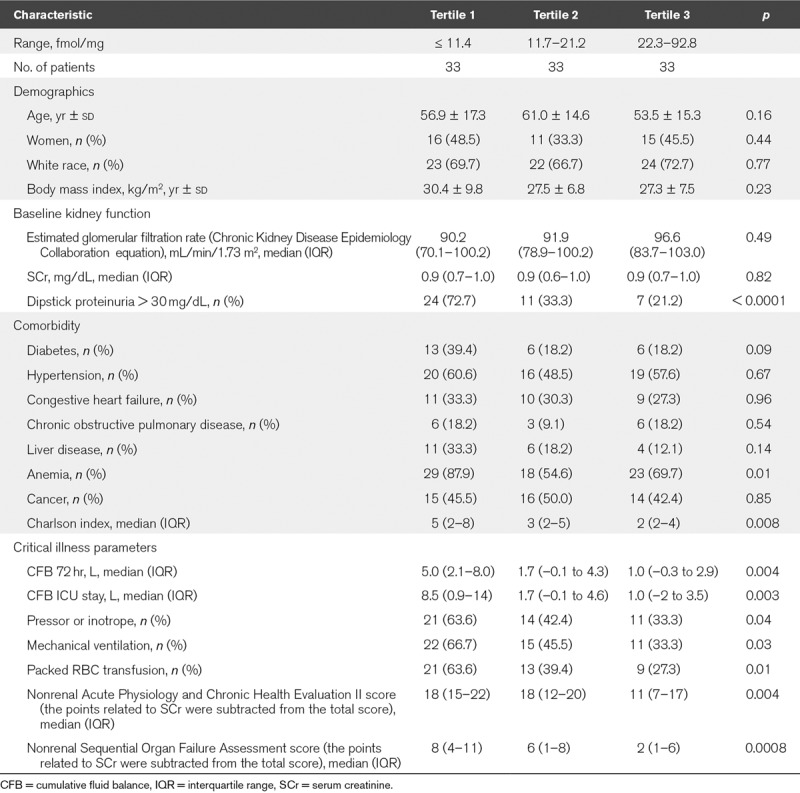
Characteristics according to AKI status are reported in Supplementary Table 2 (Supplemental Digital Content 2, http://links.lww.com/CCX/A53). There were no differences in age, gender, or baseline eGFR as per study design. However, patients with AKI had more frequently anemia, liver disease, and higher Charlson scores. Similarly, patients with AKI had higher SOFA and APACHE II scores along with worse critical illness parameters when compared with those without AKI.
Study Outcomes
A total of 44 patients (41.5%) developed MAKE-90: 33 (75%) died, three (6.8%) became RRT dependent, and eight (18.2%) survived, were RRT independent but had a decrease in eGFR of greater than or equal to 50% from baseline by 90 days postenrollment. Patients in whom MAKE-90 developed had higher Charlson scores and higher critical illness scores (Supplementary Table 3, Supplemental Digital Content 3, http://links.lww.com/CCX/A54). MAKE-90 occurred more frequently in patients who suffered from AKI (70.4% vs 29.6%; p < 0.0001).
Study Biomarkers According to AKI Status
Urine Klotho (pM) and urine Klotho/creatinine (fmol/mg of creatinine) levels were significantly lower in patients with AKI versus without AKI (median [25th–75th percentile], 6.0 [4.9–45.0] vs 23.0 [5.0–67.4]; p < 0.0001; and 9.2 [3.0–33.6] vs 25.0 [6.0–92.8]; p < 0.0001, respectively). Serum intact FGF23 (pg/mL) was significantly higher in patients with AKI versus without AKI (139.4 [10.5–4,844.4] vs 17.4 [4.9–196.0]; p < 0.0001). Similarly, FGF23-to-Klotho/creatinine ratios were significantly higher in patients with AKI versus without AKI (Fig. 1).
Figure 1.
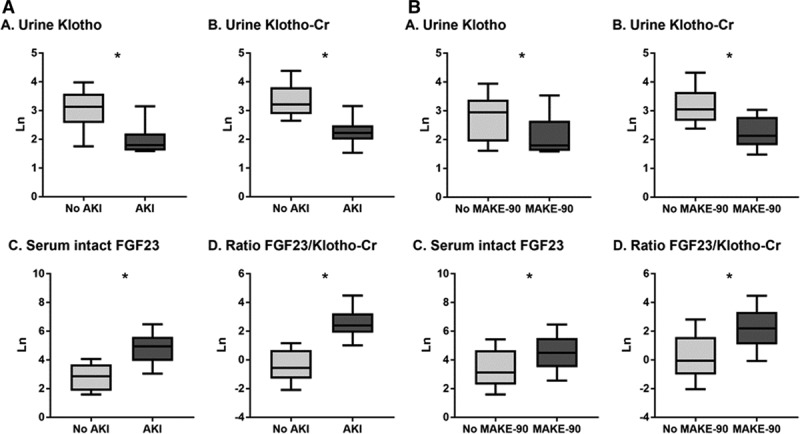
Study biomarkers according to (A) acute kidney injury (AKI) status and (B) major adverse kidney event (MAKE)-90 outcome. All biomarker measurements are natural log-transformed (Ln). Cr = creatinine, FGF = fibroblast growth factor.
Association of Study Biomarkers With MAKE-90
Urine Klotho (pM) and urine Klotho/creatinine (fmol/mg of creatinine) levels were significantly lower in patients who had MAKE-90 versus those who did not (median [25th–75th percentile], 6.0 [4.9–54.6] vs 19.0 [4.9–67.4]; p = 0.0008; and 8.4 [3.0–28.7] vs 21.2 [4.7–92.8]; p < 0.0001, respectively). Serum intact FGF23 (pg/mL) was significantly higher in patients who had versus who did not have MAKE-90 (89.2 [4.9–3,151.0] vs 22.9 [4.9–4,844.4]; p = 0.0004). Similarly, FGF23-to-Klotho/creatinine ratios were significantly higher in patients who had versus who did not have MAKE-90 (Fig. 1).
In adjusted models, each one-fold higher urine Klotho/creatinine levels was associated with an 83% (95% CI, 60–93%) lower risk of developing MAKE-90. In contrast, each one-fold higher serum intact FGF23 levels was associated with a 90% (95% CI, 17–210%) higher risk of developing MAKE-90. Similarly, each one-fold increase in the FGF23-to-Klotho/creatinine ratio was associated with a 172% (95% CI, 49–396%) higher risk of developing MAKE-90 (Table 2). When biomarker levels were stratified in tertiles, the highest tertile of urine Klotho/creatinine (vs the lowest tertile) was independently associated with a 95% (95% CI, 75–99%) lower risk of developing MAKE-90. In contrary, the highest tertile of both serum intact FGF23 and the FGF23-to-Klotho/creatinine ratio (vs the lowest tertile) was associated with higher risk of MAKE-90 (Table 2 and Fig. 2).
TABLE 2.
Association of Biomarkers With Major Adverse Kidney Event-90 Outcome (Death, Renal Replacement Therapy Dependency, 50% Decrease in Estimated Glomerular Filtration Rate)
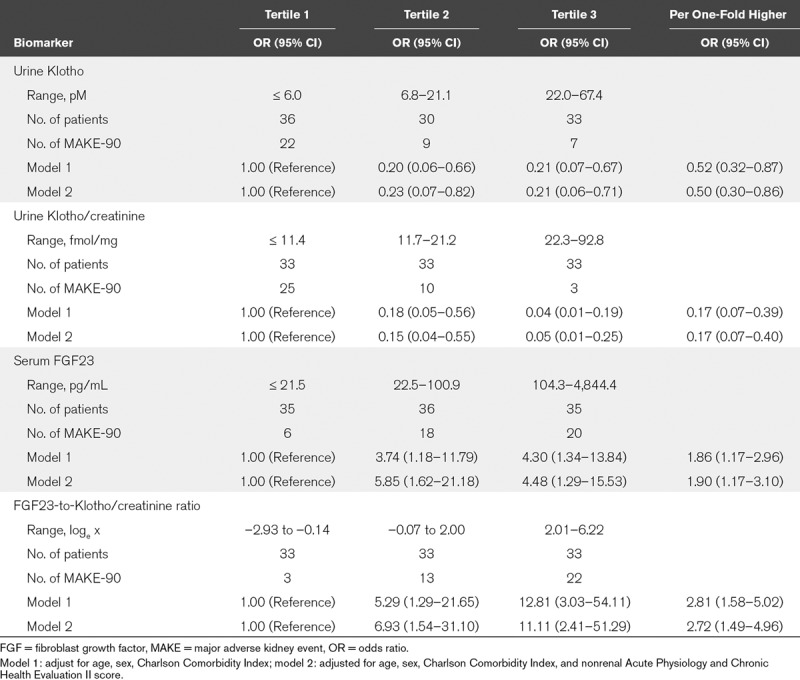
Figure 2.
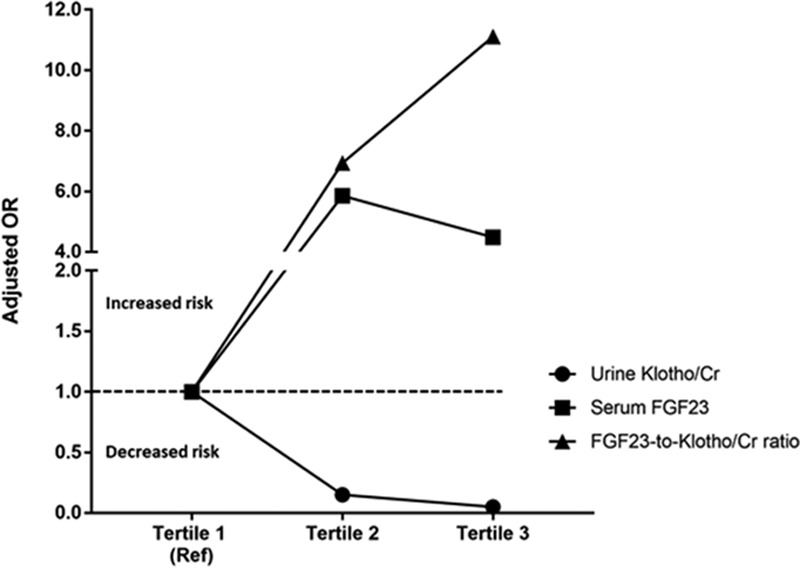
Association of biomarkers with major adverse kidney event-90 outcome (death, renal replacement therapy dependency, 50% decrease in estimated glomerular filtration rate). The adjusted odds ratios for the higher tertiles (tertiles 2 and 3) of each biomarker are represented in reference to the lowest tertile (tertile 1). Cr = creatinine, FGF = fibroblast growth factor, OR = odds ratio.
There was no statistical interaction between the AKI status and the association between the study biomarkers and MAKE-90 (p > 0.1 for each biomarker). Notably, urine Klotho/creatinine was the only biomarker significantly associated with MAKE-90 in patients with AKI (Supplementary Table 4, Supplemental Digital Content 4, http://links.lww.com/CCX/A55). There were 16 patients (25.8%) who had AKI but did not develop MAKE-90. In these patients, urine Klotho/creatinine was significantly higher and serum intact FGF23 lower, when compared with those who developed MAKE-90 (median [25th–75th percentile], 26.3 [12.4–92.8] vs 8.4 [3.0–28.7]; p < 0.0001; and 17.4 [4.9–196.0] vs 89.2 [4.9–3,151.0]; p < 0.0001, respectively).
Association of Study Biomarkers With Secondary Outcomes
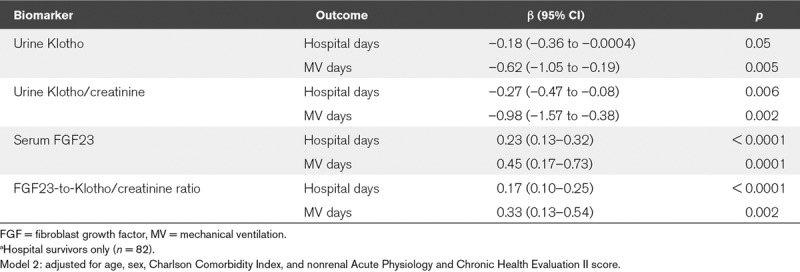
In adjusted models, urine Klotho and urine Klotho/creatinine levels were inversely correlated with total hospital days and mechanical ventilation days in hospital survivors. In contrast, serum intact FGF23 and the FGF23-to-Klotho/creatinine ratio were positively correlated with total hospital days and mechanical ventilation days in hospital survivors (Table 3). The median length of hospital stay was 10.5 days (1–63 d) for the 82 patients who survived the hospitalization.
TABLE 3.
Associations of Biomarkers With Secondary Outcomesa
Correlation Between Study Biomarkers and Biochemical Parameters
Urine Klotho/creatinine levels were positively correlated with serum calcium, serum albumin, and serum bicarbonate levels (r = 0.047–0.184; p < 0.05) and negatively correlated with serum phosphate and serum magnesium. In contrast, serum intact FGF23 levels were positively correlated with serum phosphate levels (r = 0.379; p < 0.001) and negatively correlated with serum albumin, serum bicarbonate, and arterial pH (Supplementary Fig. 1, Supplemental Digital Content 5, http://links.lww.com/CCX/A56).
Sensitivity Analyses
Similar results were obtained when the MAKE-90 definition was modified to the composite of all-cause death, RRT dependence, or the decrease in eGFR of greater than or equal to 25% (rather than 50%) from baseline. In adjusted models, each one-fold higher urine Klotho/creatinine levels was associated with an 83% (95% CI, 60–93%) lower risk of MAKE-90. Each one-fold higher serum intact FGF23 levels was associated with a 110% (95% CI, 26–251%) higher risk of MAKE-90. Similar associations to the primary analysis were observed when biomarker levels were stratified in tertiles (Supplementary Table 5, Supplemental Digital Content 6, http://links.lww.com/CCX/A57).
DISCUSSION
The new finding of our study is that both urine Klotho/creatinine and serum intact FGF23 levels associate with MAKE-90 in critically ill patients admitted to the ICU. Specifically, we found that higher urine Klotho/creatinine levels were associated with a decreased risk of MAKE-90, whereas higher serum intact FGF23 levels were associated with an increased risk of MAKE-90 in this subset of critically ill patients. These findings are timely and relevant because increased circulating FGF23 levels (33–35) and reduced Klotho expression (18, 36–38) have been consistently described in experimental models of AKI. Although higher circulating FGF23 levels have been previously associated with human AKI (22, 23) and increased morbidity and mortality in critically ill patients (25, 26, 39), data related to soluble Klotho levels are scarce (40). Our study builds upon evidence that serum intact FGF23 and urine Klotho/creatinine levels may inform novel risk prediction models focusing on renal outcomes in critically ill patients with and without AKI. In addition, our study underpins the relevance of ongoing experimental work targeting therapies to modify circulating levels or downstream effects of FGF23 and Klotho pathways in AKI and critical illness.
Low renal Klotho messenger RNA (mRNA) and protein levels have been described in experimental AKI (18, 36–38). Interestingly, in murine models of post–ischemia-reperfusion injury (IRI) compounded by high-phosphate diet starting 2 weeks after IRI (CKD was detected functionally and histologically ≈22 wk post-IRI), renal Klotho mRNA and protein expression showed a subacute and progressive decline starting at day 14 post-IRI, particularly in mice exposed to prolonged ischemia (41). In the latter model, administration of recombinant Klotho protein given for 4 consecutive days beginning 24 hours after IRI accelerated kidney recovery, reduced kidney fibrosis, enhanced endogenous renal Klotho mRNA and protein expression, and attenuated the occurrence of CKD (41). Multiple mechanisms of Klotho-related renoprotection have been postulated such as suppression of apoptosis (38, 42) and cell senescence (43, 44), antifibrosis (37, 45–48), and up-regulation of autophagy (41, 49) in renal tubular cells.
Elevated plasma FGF23 levels have been reported in murine models of AKI (33–35, 50). Nonetheless, the mechanisms of FGF23 elevation in AKI are not known. Impaired excretion or catabolism of FGF23 by the kidneys or increased production from bone or other organs could be reasonable explanations for this elevation (33–35, 50–52). Measurement of intact FGF23—the biologically active form—is less represented than FGF23 assays with C-terminal reagents (nondiscriminatory between full length and C-terminal fragments) in human AKI literature. Elevation of FGF23 in AKI can augment myofibroblast activation and promote fibrosis via activation of transforming growth factor-β pathways (53).
Data related to urine Klotho measurements in human AKI are scarce. Hu et al (18) demonstrated lower urine Klotho/creatinine levels in hospitalized AKI patients when compared with healthy volunteers (4.85 ± 1.69 vs 25.38 ± 4.08 fmol/mg of creatinine; p < 0.01). In contrast, Torregrosa et al (54) reported that urine Klotho/creatinine levels were not different in patients with AKI (cardiac surgery or coronary angiography related) versus no AKI. It is important to note that the Klotho assays used were different. Nonetheless, concerns regarding a reliable soluble Klotho assay have precluded large-scale studies (30).
Elevation of plasma FGF23 levels has been described in human AKI (22, 23). In a cohort of 250 adult patients undergoing cardiac surgery, Leaf et al (23) reported that plasma C-terminal FGF23 levels were differentially elevated starting at the end of cardiopulmonary bypass in patients who did versus who did not develop postoperative AKI. In critically ill patients, elevated levels of C-terminal FGF23 have been also reported to be independently associated with incident AKI (26, 39). In a recent post hoc analysis, critically ill patients with the highest versus lowest quartiles of plasma C-terminal and intact FGF23 were found to have higher 60-day mortality (25).
Our study has important strengths that need to be delineated. First, we included patients with well-characterized AKI (KDIGO stage ≥ 2) and controls in the ICU that were matched on clinical parameters that can potentially affect both the independent and dependent variables in the analysis. Second, we used SCr and urine output criteria to define AKI, which is particularly appropriate in the ICU setting. Third, we adjusted our multivariable analyses for appropriate confounders, including objective and comprehensive comorbidity and critical illness scores. Fourth, we measured—for the first time—serum intact FGF23 and urine Klotho/creatinine using paired biospecimens of critically ill patients, which highlights their bidirectional relationship in human AKI and their association with adverse outcomes. Fifth, we measured urine Klotho using a previously reported and validated method that is available and can be replicated (30). Finally, our study tested a relevant 90-day renal outcome which is a recommended endpoint because it relates to the risk of CKD/ESRD post-AKI (32).
Our study also has limitations. First, although our study sample is representative of a heterogeneous ICU population, our study design is also susceptible to selection bias because we included patients with AKI stage greater than or equal to 2 and corresponding matched controls for prespecified clinical characteristics. Further, our sample size is relatively small for the examination of associations of study biomarkers with MAKE-90 according to AKI status. Nonetheless, we did not find a significant interaction between study biomarkers and AKI status for MAKE-90. Second, we did not clinically adjudicate etiology of AKI which can also provide important information for risk stratification of adverse outcome. Third, although we adjusted for confounders by comprehensive comorbidity and critical illness scores, residual confounding by unmeasured covariates may not have been completely eliminated. Finally, our study did not specifically test the utility (performance) of urine Klotho/creatinine or serum intact FGF23 for the prediction of MAKE-90 but identified an important bidirectional relationship between the levels of these biomarkers measured early during the course of ICU admission or AKI diagnosis and subsequent adverse renal outcomes.
CONCLUSIONS
Urine Klotho/creatinine levels were significantly lower and serum intact FGF23 levels were significantly higher in critically ill patients with AKI versus matched controls without AKI. When measured in the first 48 hours of ICU admission or AKI diagnosis, urine Klotho/creatinine independently associated with MAKE, particularly in patients with AKI. These results show promise for testing these biomarkers—individually or in combination—as part of novel risk prediction models of renal outcomes in the ICU. Nonetheless, our results need to be validated in a larger sample of critically ill patients with and without AKI to further underpin the impact of Klotho for diagnostics and therapeutics in AKI and critical illness.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by the University of Texas Southwestern Medical Center O’Brien Kidney Research Core Center (P30 DK079328-06), the National Center for Advancing Translational Sciences (UL1TR001105), and the National Institutes of Health (R01 DK092461-04S1).
The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or the University of Texas Southwestern.
Dr. Neyra was supported by the Ben J. Lipps Research Fellowship Program of American Society of Nephrology Foundation for Kidney Research, the Truelson Fellowship Fund, and the Seldin-Pak Center of Metabolic Research at University of Texas Southwestern Charles and Jane Pak Center of Mineral Metabolism and Clinical Research. He is currently supported by an Early Career Pilot Grant from the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR001998. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Drs. Neyra, Toto, and Moe helped with study concept and design. Drs. Neyra and Mescia helped with patient screening, recruitment, and biospecimen collections. Ms. Pastor helped with sample processing and biomarker measurements. Drs. Neyra, Hu, Toto, and Moe helped with analysis and interpretation of data. Dr. Neyra helped with drafting of the manuscript. Drs. Neyra, Hu, Toto, and Moe helped with critical revision of the manuscript for important intellectual content. Drs. Li, Ortiz-Soriano, and Adams-Huet helped with statistical analysis. Drs. Neyra, Toto, and Moe helped with administrative, technical, and material support. Drs. Neyra and Moe helped with study supervision.
REFERENCES
- 1.Mandelbaum T, Scott DJ, Lee J, et al. Outcome of critically ill patients with acute kidney injury using the acute kidney injury network criteria. Crit Care Med 2011; 39:2659–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neyra JA, Manllo J, Li X, et al. ; AKICI Study Group: Association of de novo dipstick albuminuria with severe acute kidney injury in critically ill septic patients. Nephron Clin Pract 2014; 128:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 2008; 3:844–861 [DOI] [PubMed] [Google Scholar]
- 4.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 2010; 21:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 2011; 79:1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 2012; 81:442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald R, Quinn RR, Adhikari NK, et al. ; University of Toronto Acute Kidney Injury Research Group: Risk of chronic dialysis and death following acute kidney injury. Am J Med 2012; 125:585–593 [DOI] [PubMed] [Google Scholar]
- 8.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013; 17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014; 189:932–939 [DOI] [PubMed] [Google Scholar]
- 10.Grams ME, Sang Y, Coresh J, et al. Candidate surrogate end points for ESRD after AKI. J Am Soc Nephrol 2016; 27:2851–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato Y, Arakawa E, Kinoshita S, et al. Establishment of the anti-klotho monoclonal antibodies and detection of klotho protein in kidneys. Biochem Biophys Res Commun 2000; 267:597–602 [DOI] [PubMed] [Google Scholar]
- 12.Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol 2012; 8:423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CD, Tung TY, Liang J, et al. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry 2014; 53:5579–5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloch L, Sineshchekova O, Reichenbach D, et al. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 2009; 583:3221–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CD, Podvin S, Gillespie E, et al. Insulin stimulates the cleavage and release of the extracellular domain of klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 2007; 104:19796–19801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mencke R, Harms G, Moser J, et al. Human alternative Klotho mRNA is a nonsense-mediated mRNA decay target inefficiently spliced in renal disease. JCI Insight 2017; 2:pii: 94375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MC, Shi M, Zhang J, et al. Renal production, uptake, and handling of circulating αklotho. J Am Soc Nephrol 2016; 27:79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 2010; 78:1240–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiura H, Yoshida T, Mitobe M, et al. Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant 2010; 25:60–68 [DOI] [PubMed] [Google Scholar]
- 20.King GD, Chen C, Huang MM, et al. Identification of novel small molecules that elevate klotho expression. Biochem J 2012; 441:453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi M, Flores B, Gillings N, et al. alphaKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol 2015; 27:2331–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JR, Katz R, Ix JH, et al. Fibroblast growth factor-23 and the long-term risk of hospital-associated AKI among community-dwelling older individuals. Clin J Am Soc Nephrol 2014; 9:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leaf DE, Christov M, Jüppner H, et al. Fibroblast growth factor 23 levels are elevated and associated with severe acute kidney injury and death following cardiac surgery. Kidney Int 2016; 89:939–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leaf DE, Wolf M, Waikar SS, et al. FGF-23 levels in patients with AKI and risk of adverse outcomes. Clin J Am Soc Nephrol 2012; 7:1217–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leaf DE, Siew ED, Eisenga MF, et al. Fibroblast growth factor 23 associates with death in critically ill patients. Clin J Am Soc Nephrol 2018; 13:531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leaf DE, Jacob KA, Srivastava A, et al. Fibroblast growth factor 23 levels associate with AKI and death in critical illness. J Am Soc Nephrol 2017; 28:1877–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neyra JA, Moe OW, Hu MC. Fibroblast growth factor 23 and acute kidney injury. Pediatr Nephrol 2015; 30:1909–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Inter Suppl 2012; 2:1–138 [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker SL, Pastor J, Carranza D, et al. The demonstration of αklotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 2015; 30:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinellu A, Caria MA, Tavera C, et al. Plasma creatinine and creatine quantification by capillary electrophoresis diode array detector. Anal Biochem 2005; 342:186–193 [DOI] [PubMed] [Google Scholar]
- 32.Kellum JA, Zarbock A, Nadim MK. What endpoints should be used for clinical studies in acute kidney injury? Intensive Care Med 2017; 43:901–903 [DOI] [PubMed] [Google Scholar]
- 33.Christov M, Waikar SS, Pereira RC, et al. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int 2013; 84:776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toro L, Barrientos V, León P, et al. Erythropoietin induces bone marrow and plasma fibroblast growth factor 23 during acute kidney injury. Kidney Int 2018; 93:1131–1141 [DOI] [PubMed] [Google Scholar]
- 35.Mace ML, Gravesen E, Nordholm A, et al. Kidney fibroblast growth factor 23 does not contribute to elevation of its circulating levels in uremia. Kidney Int 2017; 92:165–178 [DOI] [PubMed] [Google Scholar]
- 36.Sugiura H, Yoshida T, Tsuchiya K, et al. Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 2005; 20:2636–2645 [DOI] [PubMed] [Google Scholar]
- 37.Sugiura H, Yoshida T, Shiohira S, et al. Reduced klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol 2012; 302:F1252–F1264 [DOI] [PubMed] [Google Scholar]
- 38.Panesso MC, Shi M, Cho HJ, et al. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 2014; 85:855–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rygasiewicz K, Hryszko T, Siemiatkowski A, et al. C-terminal and intact FGF23 in critical illness and their associations with acute kidney injury and in-hospital mortality. Cytokine 2018; 103:15–19 [DOI] [PubMed] [Google Scholar]
- 40.Neyra JA, Hu MC. Potential application of klotho in human chronic kidney disease. Bone 2017; 100:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi M, Flores B, Gillings N, et al. Αklotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol 2016; 27:2331–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 2011; 22:124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem 2008; 389:233–241 [DOI] [PubMed] [Google Scholar]
- 44.Liu F, Wu S, Ren H, et al. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 2011; 13:254–262 [DOI] [PubMed] [Google Scholar]
- 45.Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 2011; 286:8655–8665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, Li Y, Zhou D, et al. Loss of klotho contributes to kidney injury by derepression of wnt/β-catenin signaling. J Am Soc Nephrol 2013; 24:771–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan X, Nie L, He T, et al. Klotho suppresses renal tubulo-interstitial fibrosis by controlling basic fibroblast growth factor-2 signalling. J Pathol 2014; 234:560–572 [DOI] [PubMed] [Google Scholar]
- 48.Huang JS, Chuang CT, Liu MH, et al. Klotho attenuates high glucose-induced fibronectin and cell hypertrophy via the ERK1/2-p38 kinase signaling pathway in renal interstitial fibroblasts. Mol Cell Endocrinol 2014; 390:45–53 [DOI] [PubMed] [Google Scholar]
- 49.Bian A, Neyra JA, Zhan M, et al. Klotho, stem cells, and aging. Clin Interv Aging 2015; 10:1233–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mace ML, Gravesen E, Hofman-Bang J, et al. Key role of the kidney in the regulation of fibroblast growth factor 23. Kidney Int 2015; 88:1304–1313 [DOI] [PubMed] [Google Scholar]
- 51.Hassan A, Durlacher K, Silver J, et al. The fibroblast growth factor receptor mediates the increased FGF23 expression in acute and chronic uremia. Am J Physiol Renal Physiol 2016; 310:F217–F221 [DOI] [PubMed] [Google Scholar]
- 52.Smith ER, Tan SJ, Holt SG, et al. FGF23 is synthesised locally by renal tubules and activates injury-primed fibroblasts. Sci Rep 2017; 7:3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith ER, Holt SG, Hewitson TD. FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-β autoinduction. Int J Biochem Cell Biol 2017; 92:63–78 [DOI] [PubMed] [Google Scholar]
- 54.Torregrosa I, Montoliu C, Urios A, et al. Urinary klotho measured by ELISA as an early biomarker of acute kidney injury in patients after cardiac surgery or coronary angiography. Nefrologia 2015; 35:172–178 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


