Abstract
To date, the success of conventional chemotherapy, radiotherapy, and targeted biological therapies in cancer treatment is not satisfactory. The main reasons for such outcomes rely on low target selectivity, primarily in chemo- and radiotherapy, ineffectiveness to metastatic disease, drug resistance, and severe side effects. Although immune checkpoint inhibitors may offer better clinical promise, success is still limited. Since cancer is a complex systemic disease, the need for new therapeutic modalities that can target or block several steps of cancer cell characteristics, modulate or repolarize immune cells, and are less toxic to healthy tissues is essential. Of these promising therapeutic modalities are pleiotropic natural products in which scorpion venom (SV) is an excellent example. SV consists of complex bioactive peptides that are disulfide-rich of different peptides’ length, potent, stable, and exerts various multi-pharmacological actions. SV peptides also contain ion channel inhibitors. These ion channels are dysregulated and overexpressed in cancer cells, and play essential roles in cancer development and invasion, as well as depolarizing immune cells. Furthermore, SV has been found to induce cancer cell apoptosis, and inhibit cancer cells proliferation, invasion, metastasis, and angiogenesis. In the current review, we are presenting data that show the pleiotropic effect of SV against different types of human cancer as well as revealing one potential anticancer agent, Rhopalurus princeps venom. Furthermore, we are addressing what is needed to be done to translate these potential cancer therapeutics to the clinic.
Keywords: angiogenesis, apoptosis, cell arrest, cytotoxicity, immunomodulation, ion channels, metastasis, polarization
Introduction
Cancer is a devastating term that people afraid to hear; besides, it is the second leading cause of death worldwide. The incidence of cancer cases worldwide is on the rise, where 17 million new cases of cancer were diagnosed in 2018, and 9.6 million people died because of cancer in the same year. Furthermore, cancer incidence is projected to increase by 62% in 2040.1 Thus, the future burden will increase yearly as the numbers, and the projections are apparent.
Cancer development is a multistep process and it starts with an initiation step involving DNA mutation. Such mutation could either be inherited or acquired following chronic inflammation, exposure to chemicals or viruses yielding to molecular events that lead to cellular transformation. This transformation gains the abnormal cell(s) specific characteristics. Of these characteristics: 1) evading immunosurveillance and programmed cell death; 2) becoming dysregulated regarding specific genes, proteins and ion channel expression, and go through uncontrolled cell proliferation; 3) stimulating and sustaining new blood vessels, angiogenesis, for their growth; and 4) ability to invade tissues and metastasize to other locations in the body.2–4 In addition, the immune cells that are recruited to tumor site become depolarized and lose their normal function and may become enhancers for tumor growth.3 All in all, these changes yield to the development of a heterogeneous tumor microenvironment.
To date, the success of conventional chemotherapy, radiotherapy, and targeted biological therapies in cancer treatment is not satisfactory. The main reasons for such outcomes rely on low target selectivity, primarily in chemo- and radiotherapy, ineffectiveness to metastatic disease, drug resistance, and severe side effects. In contrast, immune checkpoint inhibitors are now showing better promising results.5 However, success in cancer therapy is still limited.
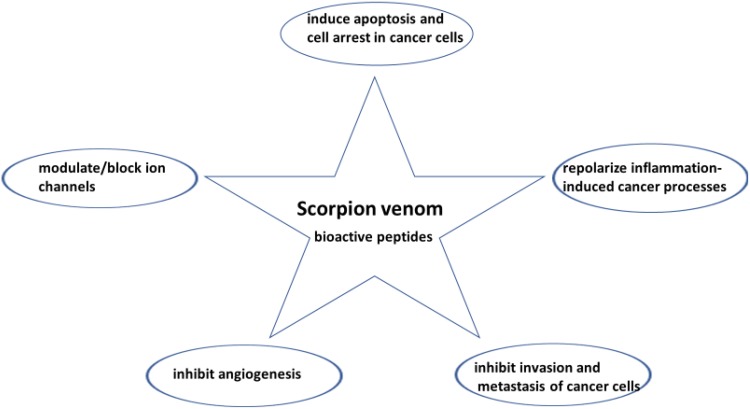
Since cancer is a complex systemic disease, the need for new therapeutic modalities that can target or block several steps of cancer cell characteristics, modulate or repolarize immune cells, and are less toxic to healthy tissues is essential.3,5 Of these promising therapeutic modalities are pleiotropic natural products in which they are multi-targeting and have fewer side effects.6 An excellent example of a pleiotropic natural product is the scorpion venom (SV). SV consists of complex bioactive peptides that are disulfide-rich of different peptides’ length, potent, stable, and exerts various multi-pharmacological actions (Figure 1). Research studies have demonstrated that SV peptides block/modulate ion channels. These ion channels are dysregulated and overexpressed in cancer cells and play essential roles in cancer development and invasion, as well as depolarizing immune cells. Furthermore, SV has pleiotropic anticancer activities such as inducing apoptosis, and inhibiting cancer cells proliferation, invasion, metastasis, along with angiogenesis. Besides, substantial evidence exists that SV is an immunomodulator. In the current review, we are presenting data that show the pleiotropic effect of SV against different types of human cancer as well as revealing one potential anticancer agent, Rhopalurus princeps venom. Furthermore, we are addressing what is needed to be done to translate these potential cancer therapeutics to the clinic.
Figure 1.
The multi-pharmacological actions of scorpion venom bioactive peptides on cancer cells.
Ion Channels and Cancer Cells
It has been proven that tumor cells use ion channels to support their proliferation, adhesion and invasion processes.4 These processes occur by ion channels interplay with changes in cell morphology and volume. In particular, this has been shown to be the mechanism of glioma cell growth and invasion mainly via the electrochemical efflux of Cl− and K+ as KCl and water, which is mediated by an increase in intracellular Ca+2 ions, leading to cell shrinkage that enables the cell to invade the brain tissue.7,8
Furthermore, expression of ion channels has been demonstrated to be altered, dysregulated, or overexpressed in several types of cancers. For instance, chloride channels have been found to be dysregulated in multiple cancer types; either downregulated such as human colorectal cancer9 or altered as in glioma10 to regulate glioblastoma cell invasiveness.11 On the other hand, potassium channels such as voltage-gated potassium channels (Kv) and calcium-activated potassium channels (KCa) and their subtypes have been overexpressed or dysregulated in multiple cancers including glioblastoma,12,13 breast, colon, prostate cancer14 and lymphoma.15 Similarly, voltage-gated sodium channels (VGSCs; Nav1.5, Nav1.6, Nav1.7) have been found to be overexpressed in astrocytoma, breast, colon, cervical, prostate and non-small cell lung cancers.16–20 Lastly, calcium channels as voltage-gated Ca+2 channels or transient receptor potential (TRPC) ion channels are overexpressed in breast, liver, stomach, ovarian and glioma tumors.21,22 Furthermore, when these Ca+2 channels were silenced, tumor proliferation is reduced.22,23
SV is a complex mixture of substances, among which are inorganic salts, free amino acids, heterocyclic components, peptides, and proteins. The most distinctive component of SV is peptides that modify, modulate or block ion channels, specifically modify the gating mechanism of Na+-channels, block K+-channel and Cl− or modulate the function of calcium channel.24 Thus, this opens the implication of using SV peptides in cancer therapy and development research to evaluate their potential in the clinical setting.
Anticancer Effects of SV and Their Peptides
Proliferation Inhibition and Inducing Apoptosis
Several species-specific SV have been tested in preventing or blocking the proliferation of several types of human cancer cell lines (Table 1). This anti-proliferative mechanism of action of SV or their purified peptides can be summarized into inducing apoptosis, cell arrest at Go/G1 phase, and inhibition of DNA synthesis.
Table 1.
In vitro Anticancer Effects of SV or Peptides on Human Cancer Cells
| Species | Venom/Peptide | Action | Target | Ref. |
| Androctonus crassicauda | Whole venom | Inhibits proliferation of human neuroblastoma (SH-SY5Y) and human breast (MCF-7) cancer cell lines | Induces apoptosis through increasing nitric oxide production, caspase-3 activity and depolarizing mitochondrial membrane and arrests S phase | 35 |
| Androctonus crassicauda | Non-disulphide-bridged peptides (NDBP), AcrAP1 and AcrAP2, and their high cationic analogues (HC-AcrAP) (0.001-100 µM) | HC-AcrAP analogues inhibit the proliferation of human lung adenocarcinoma (H460), breast carcinoma (MB435s) breast tumorigenic (MCF-7) and prostate carcinoma (PC-3) cell lines (IC50 2-3.6 µM) | Probably inducing cell lysis | 68 |
| Androctonus bicolor Androctonus crassicauda | Whole venom (500-1000 µg/ml) |
Inhibits proliferation of human breast (MDA-MB-231), and colorectal (HCT-8) cancer cells | Induces apoptosis more than necrotic death, and arrests cells at G0/G1 phase, upregulate p53, downregulate Bcl-xL | 29-31 |
| Androctonus crassicauda, Androctonus bicolor | Whole venom (20-100 µg/ml) |
Inhibits cell motility, and prevents colony mitosis of human breast, (MDA-MB-231), and colorectal (HCT-8 and HCT116) cancer cells | Two mechanisms proposed are i) a decrease in the expression of MMPs, and ii) a reduction in the phosphorylation levels of FAK, which is involved in cell migration and invasion. | 31 |
| Androctonus mauritanicus | Gonearrestide peptide (18 aa, 2192 Da) 2-200 µM |
Inhibits the proliferation of human colon cancer cell line HCT116 in dose-dependent | Arrests cancer cell cycle in G1 phase via modulating cell cycle checkpoint proteins (down-regulate CDK4, and upregulate cyclin D3, p21, p27) | 36 |
| Buthus martensii Karsch | BmK 70-80kDa SVCIII (1-50 µg/ml) |
Inhibits proliferation of human acute monocytic leukemia cell line (THP-1) and the human T lymphoma (Jurkat cell line) and induces cell cycle arrest at G1 phase | Inhibits NF-κB activation through inhibition of IκBα phosphorylation, degradation and p65 nuclear translocation | 44 |
| BmK 50-60 aa 65 kDa (10-200 µg/ml) | Inhibits proliferation of DU145 human prostate cancer cell line | Enhances expression of apoptotic gene, Bax, reduces anti-apoptotic, Bcl-2, expression, and arrests cancer cells at G1/S | 28 | |
| BmK AGAP 71.42KDa with 66 aa (5-60 µM) (IC50 = 40µM for MCF-7 and 50µM for MDA-MB-231 cells) | Inhibits proliferation, stemness, sphere formation, colony formation epithelial-mesenchymal transition, migration and invasion of human breast MCF-7 and MDA-MB-231 cells | Reduces gene and protein expression of Oct4, SOX2, Nanog, N-cadherin, Snail and PTX3, and increased the expression of E-cadherin | 47 | |
| Buthus tumulus | Iberiotoxin (IbTX) (34 aa, 36.07 kDa) | Inhibits growth and proliferation of human cervical cancer (HeLa) and human ovarian cancer (A2780) cell lines | Blocks calcium-activated potassium channels KCNMA1 (KCa1.1, BK) *Found to be expressed in cervical cancer-hormone dependent | 42-43 |
| Centruroides margartatus | Margatoxin (MgTX, 39 aa, 41.92 kDa) | Inhibits proliferation of human lung adenocarcinoma cells and decreases tumor volume in vivo following intra-tumoral injection | Blocks Kv1.3, and increases expression level of p21Waf1/Cip1 and decreases the expression level of Cdk4 and cyclin D3. | 61 |
| Heterometrus bengalensis | Bengalin 72kDa IC50 3.7 and 4.1 µg/ml |
Inhibits proliferation of human leukemic cells (U937) and K562 chronic myelogenous leukemia (K562) and has no effect on normal human lymphocytes | Increases expression of Bax/Bcl-2 ratio, caspase 3 and 9, reduces mitochondrial membrane potential, heat shock proteins 70 and 90 | 32 |
| Leiurus quinquestriatus | Chlorotoxin (ClTx) (36 aa, MW 4.02 KDa) | Inhibits migration and invasion of human glioma (D54-MG and CCF-STTG-1) cells | Binds to matrix metalloproteinase-2 (MMP-2) and modulates the surface expression of an active MMP-2 and increase the uptake of Cl-channel receptors (CIC-3) | 7, 53 |
| Leiurus quinquestriatus | Whole venom (20-100 µg/ml) | Inhibits cell motility, and prevents colony mitosis of human breast, (MDA-MB-231) and colorectal (HCT-8 and HCT116) cancer cells | Two mechanisms proposed are i) a decrease in the expression of MMPs, and ii) a reduction in the phosphorylation levels of FAK, which is involved in cell migration and invasion | 31 |
| Odontobuthus doriae | Whole venom (20-100 µg/ml) | Inhibits proliferation and inhibits DNA synthesis in human breast cancer cell line, MCF-7 | Depolarizes mitochondria, activates Caspase-3, and depletes antioxidant activities | 26 |
| Inhibits proliferation and inhibits DNA synthesis in human neuroblastoma cells, SH-SY5Y | 27 | |||
| Rhopalurus junceus | Whole venom (100-1000 µg/ml) | Inhibits proliferation on a panel of human cancer cell lines (HeLa, SiHa, Hep-2, NCI-H292, A549, MDA-MB-231, MDA-MB-468, HT-29), but has no effect on hematologic malignant cell lines (U937, K562, Raji) or normal cells (MRC-5, MDCK, Vero) | Increased expression of P53, Bax, Caspase 3, 8, & 9 and reduced Bcl-2 in HeLa (apoptosis > necrosis) whereas reduced expression p53, did not affect Bax but reduced Bcl-2 in A549 (necrosis>apoptosis) reflecting concentration used >IC50 or <IC50. | 25 |
| Tityus discrepans | Neopladine peptides 1 (29.918kDa) and 2 (30.388 kDa) | Both peptides induce apoptosis in human breast carcinoma SKBR3 cells | Bind to SKBR3 cell surface and induce FasL and BcL-2 expression | 33 |
| Tityus serrulatus | TsAp-1 and TsAp-2 (17 aa) High Cationic TsAP-1 and TsAP-2 | High cationic analogue of TsAP-1 and 2 induce high anti-proliferative activity against several human cell lines: squamous carcinoma (H157), lung adenocarcinoma (H838), prostate adenocarcinoma (PC-3); breast carcinoma (MCF-7), glioblastoma (U251-MG) | 67 |
Several studies have shown that the whole SV from Androctonus crassicauda, Androctonus bicolor, Leiurus quinquestriatus, Odontobuthus doriae, and Rhopalurus junceus species reduced proliferation of human brain, breast, colorectal, lungs, cervix, and larynx cancer cell lines (Table 1). However, Rhopalurus junceus whole SV was tested against hematopoietic tumor and lymphoma cell lines and showed no activity.25 Regardless of the latter, the reduction of proliferation in the above cancer cell lines was due primarily due to inducing apoptosis specifically by increasing the expression of apoptotic gene bax, and bax/bcl (anti-apoptotic gene) ratio, reducing bcl gene expression, increasing caspase enzymes 3, 8, 9 activities, and inducing Fas-L expression.26–33
Furthermore, SV from either Androctonus bicolor or Androctonus crassicauda has shown to arrest cancer cells in S phase, Go/G1 phase or downregulating cyclin genes expression.29,34,35 Following the improvement of proteomic approaches to study peptides and their anticancer activities, a high throughput platform has been utilized to identify a panel of novel potential anticancer peptides in scorpion venoms and to predict their putative functions and targeted signaling pathways. Li et al36 have demonstrated a panel of potential anticancer peptides from Androctonus mauritanicus and Androctonus australis venoms and from these, Gonearrestide, a peptide from Androctonus mauritanicus, has been found to exhibit the best anti-proliferative activity on human colon cancer cell line HCT116 by modulating cell cycle checkpoint proteins (Table 1). Similarly, in vivo administration of SV from Androctonus amoreuxi reduced Ehrlich ascites carcinoma and solid Ehrlich tumor size, and increased survival of mice-bearing intraperitoneal Ehrlich carcinoma37 as well as topical administration of Leiurus quinquestriatus SV reduced skin carcinogenesis incidence in mice.38
Iberiotoxin (IbTX) is a toxin isolated from Buthus tumulus scorpion and has been found to inhibit calcium-activated potassium channels (KCNMA1). These potassium ion channels were found to be over-expressed in different cancer types such as glioma,39 as well as hormone-influenced like breast, prostate, ovarian and cervix cancer.40–42 It has been shown that iberiotoxin decreases HeLa cell proliferation and human ovarian cancer (A2780) cell lines (Table 1).42,43
Several types of peptides from Buthus martensii Karsch (BmK) have been tested in vitro and in vivo models. In one study, a peptide initially identified as 70–80 kDa fraction, termed by fractionation SVCIII, has shown anti-proliferative effect on human acute monocytic leukemia cell line (THP-1) and the human T lymphoma (Jurkat cell line), but not on normal human peripheral blood lymphocytes, and also induced cell cycle arrest at G1 phase by inhibiting the expression of cyclin D protein.44 The latter was associated with anti–inflammatory activities of the peptide in inhibiting NF-κB activation through inhibition of IκBα phosphorylation, degradation, and p65 nuclear translocation. The SVCIII fraction probably contains the BmK analgesic peptide, BmK AGAP. AGAP, which belongs to a group of long-chain scorpion peptides and has a molecular mass of 7142 Da with 66 amino acid residues, binds to VGSC and has been purified and tested mainly for its pain management.39,45,46 A very recent study by Kampo et al47 demonstrated in detail AGAP mechanism of action against two human breast cancer cell lines (Tables 1 and 2). BmK AGAP at concentrations of 5–60 µM inhibited proliferation, sphere formation, colony formation, stemness, and epithelial-mesenchymal transition (EMT) of human breast MCF-7 and MDA-MB-231 cell lines. These phenomena were related to: a) reduction in expression of specific genes and proteins related to cancer cells proliferation (PTX3), stemness (PTX3, Oct4, SOX2, Nanog), EMT (PTX3, N-cadherin, Snail) and b) increase in the expression of E-cadherin (EMT) as well. Furthermore, one of the VGSC subunits, Nav 1.5, which is overexpressed in breast cancer and associated with tumor progression,48 has been shown to mediate BmK AGAP regulatory action on PTX3 expression/activation and thus reducing the activation of NF-κB and tumor necrosis factor (TNF)-α.47 Following intraperitoneal administration of 0.5 or 1 mg/kg of rBmK AGAP into mice bearing subcutaneous xenograft of a human breast MCF-7 or MDA-MB-231 cell lines, tumor growth (volume and weight) was significantly reduced at both doses in comparison to PBS-treated mice (Table 2). These changes were accompanied with a decrease in PTX3, N-cadherin, Snail-1, Oct4, Sox2, β-catenin, pGSK3-β, Nav 1.5, and p65/NF-κB expression and an increase in E-cadherin and GSK3-β in the excised tumors from mice treated with rBmK AGAP.47 In the same study, the authors proved that PTX3 is highly expressed in breast cancer and its expression is associated with cancer stemness and EMT, and BmK AGAP downregulate PTX3 expression via inhibiting or binding to Nav1.5. This inhibition or binding resulted in a downstream reduction of NF-κB and -Wnt/beta-catenin signaling pathway and inflammatory cytokines production. These results demonstrate the potential of Bmk AGAP in treating inflammation-induced cancers.
Table 2.
In vivo Anticancer Effects of SV or Peptides on Human Cancer Cells
| Species | Venom/Peptide | Action | Target | Ref. |
|---|---|---|---|---|
| Androctonus amoreuxi | Whole venom i.p. 0.22 mg/kg/day for 13 days (20% LD dose) |
Reduced Ehrlich ascites carcinoma and solid Ehrlich tumor size, and increased survival of mice-bearing intraperitoneal tumor | Downregulates expression of Ki-67 and VEGF and increased expression of caspase-3 | 37 |
| Androctonus mauritanicus | Gonearrestide peptide (18 aa, 2192 Da) 50 and 100 µM peritumoral injection for 2 weeks | Reduced tumor growth of human colon cancer cell line HCT116 in dose-dependent | Modulates cell cycle checkpoint proteins (down-regulate CDK4, and upregulate cyclin D3, p21, p27) | 36 |
| Buthus martensii | BmK AGAP 71.42KDa with 66 aa (0.5 and 1 mg/kg) injected i.p. for 20 days at 48 h intervals | Reduced tumor growth of MDA-MB231 cells in mice | Reduces gene and protein expression in ex vivo tumors of Oct4, SOX2, Nanog, N-cadherin, Snail and PTX3, and increased the expression of E-cadherin | 47 |
| Leiurus quinquestriatus | Whole venom 17.5, 35, 52.5 µg topical twice a week for 16 weeks | Decreased skin carcinogenesis incidence in mice | Downregulates expression of Ki-67, NF-kB, Cox-2, Bcl-2, VEGF and proinflammatory cytokines (TNF-α and IL-6) | 38 |
| Leiurus quinquestriatus | TAM-601 (synthetic ClTx) at 10 µg TAM-601 at 2, 10, or 100 mg/kg (i.v.), 3x/week for 2 weeks |
Blocks angiogenesis in chick chorioallantoic membrane growing human tumors Reduces microvessel count in mice using Matrigel plug assay |
Inhibits VEGF, PDF, TNF-α action on vascularization and blocks MMP-2 activity | 54 |
Angiogenesis Inhibition
Angiogenesis, formation of new blood vessels, is a characteristic of solid cancer cells. Cancer cells overexpress growth factors, vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF), that drive the formation of new blood vessels. SV and their peptides have shown to inhibit the expression of VEGF. For instance, intraperitoneal administration of SV from Androctonus amoreuxi has resulted in downregulating the expression of VEGF in the tumor tissue and this was accompanied with a downregulation of the proliferative marker Ki-67 and reduction in the solid tumor size.37 Also, topical administration of Leiurus quinquestriatus SV reduced skin carcinogenesis incidence in mice, which was associated with regulating several protein expressions; one of them was VEGF.
Chlorotoxin (ClTx), which was originally isolated from Leiurus quinquestriatus, is a 36 amino acid in length and has been found to bind glioma cells but not healthy glial brain cells and inhibits Cl− fluxes across glioma membranes.49–51 Similarly, the synthetic peptide of CITx, TM601, was found to be selective to glioma cells but not to non-transformed neurons, astrocytes, or fibroblasts.52 Although TM601 binds to glioma cells, it is non-toxic to cancer cells. However, it has been shown to block angiogenesis in vitro and in vivo.8,53 TM601 treatment of human tumors (SK-Mel melanoma, PC-3 prostate, and U87-MG glioblastoma) grown on the chick chorioallantoic membrane decreased tumor vascularization in chick.54 In addition, TM601 has shown to reduce microvessel count in mice using the Matrigel plug assay. These results were related to inhibition of the VEGF and platelet-derived growth factor.
Invasion and Metastasis Inhibition
SV isolated from either Androctonus crassicauda, Androctonus bicolor, or Leiurus quinquestriatus has been found to interrupt metastasis of breast and colorectal cancer cell lines.31 The authors proposed that SV might decrease the expression of matrix metalloproteinases (MMPs), and/or reduce the phosphorylation levels of FAK, a marker involved in cell migration and invasion.31 In addition, CITx, has been found to inhibit migration and invasion of human glioma (D54-MG and CCF-STTG-1) cells.7,53 Furthermore, glioma cell invasion in the brain was found to require secretion and flux of Cl− ions through their membrane,51 and the activation of MMPs that are highly expressed on glioma and not on normal brain cells.55 Deshane et al53 studied this phenomenon in more details, and their study revealed that ClTx selectively binds to MMP-2, but not to other MMP isoforms, and this binding forms a ClTx-MMP2 complex. This complex reduces the activity of MMP-2, followed by complex internalization inside the glioma cells along with Cl− ion channels, into caveolar rafts, which depletes the available membrane-associated Cl− channels and reduces the expression of MMP-2 on these cells, thereby decreasing invasion process of glioma inside the brain.51 In other in vitro studies, it has been shown that ClTx reduced the migration ability of glioma cells through tight extracellular spaces in the brain tissue by MMP2 inhibition. Blocking MMP2 and reducing Cl− channel expression prevented glioma cells from shrinking (prevented Cl and other ions efflux) and releasing MMP2 to the extracellular matrix.56 Clinically and following local delivery of 131I-TM601 to the resection cavity in patients with recurrent glioblastoma, gamma camera and Single Photon Emission Computed Tomography (SPECT) scans have shown that the peptide is retained at the tumor cavity site for up to 8 days after drug administration.57,58
Like ClTx, BmK peptides have also exhibited an effect on cell migration and metastasis. BmK peptide is a chlorotoxin-like peptide (BmKCTx) and shares amino acid sequence homology of ClTx. BmKCTx, as a Cl−channel blocker, was found to bind to human glioma (SHG SHG-44) cells and inhibit their proliferation at an effective concentration 29 times less than on normal astrocytes,59 and inhibits the invasion and migration of rat glioma (C6) cells by antagonizing MMP −2.59,60 Also as mentioned earlier, the BmK AGAP, which binds to the subunit of VGSC (Nav 1.5), not only inhibited in vitro and in vivo proliferation, stemness, sphere formation, colony formation, epithelial-mesenchymal transition (EMT), but also migration and invasion of human breast MCF-7 and MDA-MB-231 cells.47
SV Peptides, Ion Channels and Immunomodulation
We have presented studies that show the immunomodulatory effect of SV on proinflammatory markers such as NFκB, TNF-α and IL-6.30,38,44,47,54 It has been documented that blocking specific ion channels results in regulating inflammation. In general, charged ions move across hydrophobic membranes via ion channels. However, in immune cells, Ca++, as a divalent cation, has essential second messenger roles in regulating intracellular signaling pathways and lymphocytes activation, whereas monovalent cations (Na+, K+) mainly regulate the negative membrane potential (Vm), which indirectly controls the influx of calcium and immune cell signaling/activation. Therefore, regulating or blocking ion channels would affect signal transduction in immune cells and thus modulate their functions.
VGSCs (mentioned above) are generally responsible for the rising phase of the action potential in most electrically excitable cells and thus crucial in generating impulses and propagation. However, VGSCs have been found to be expressed in non-excitable cells, such as immune cells, and cancer cells.48 VGSCs comprise a multi-gene family of at least nine different functional members (NaV1.1–1.9) coding for the pore-forming α-subunits. There are also four auxiliary β-subunits, of which one or two at a time can associate with an α-subunit and modulate channel expression and activity in the plasma membrane. For instance, BmK AGAP, which binds to Nav 1.5, downregulate PTX3 expression, which resulted in a downstream reduction of NF-κB and Wnt/beta-catenin signaling pathway and inflammatory cytokines production.47 Similarly, BmK fraction of 70–80 KDa was found to inhibit NF-κB activation through inhibition of IκBα phosphorylation, degradation, and p65 nuclear translocation.44 Margatoxin (MgTx) isolated from Centruroides margartatus blocks Kv1.3.61 Kv1.3 has an essential role in Th17 activation, proliferation, and cytokine production, and blocking Kv1.3, attenuates experimental autoimmune encephalomyelitis, autoimmune diabetes and in Kv1.3 -/- mice become resistant to autoimmune diabetes and rheumatoid arthritis.62
Therefore, SV peptides play a significant immunomodulatory action on immune cells and cytokine expression that repolarize inflammation-induce cancer processes. Such immunomodulatory action is an essential pathway to regulate cancer development and growth.
Polarization of SV Venom
In late 2008, Dr. Mikaelian filed a patent on polarizing diluted SV solution that makes the venom more stable, results-consistent, and have more efficacy in regards improving immune response, treating and preventing cancer as well as relieving pain and reducing the adverse events of chemotherapy and radiation.63 The polarization step involves placing the SV solution in an electromagnetic chamber of the MRT-06 polarization machine for 5 hrs, where a monopole field is created by an AC power source. In the patent, the author showed the significance of the polarized Rhopalurus junceus, a species of Buthidae, venom on reducing cancer cell proliferation, tumor size in vivo in a dose-dependent manner, as well as pain.
Rhopalurus princeps (Rp) Venom
Anticancer Effects of Rp Venom
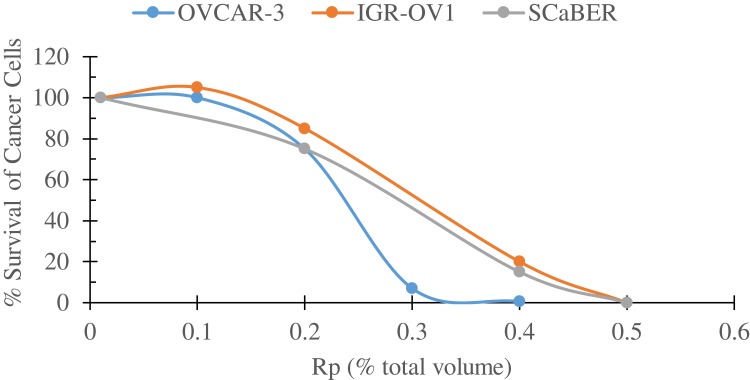
Following the patent, several studies have been performed on a different species of Buthidae family, Rhopalurus princeps (Rp) venom and have been tested in vitro on different human cancer cell lines as well as on normal cells (unpublished data). For instance, Rp venom has been tested on the human prostate cancer cell line, BxPC3, and found that polarized Rp venom significantly inhibited BxPC3 proliferation in a concentration- (≥500 µg/mL) and time-dependent (≥48 hr) manner. In other cell lines, polarized Rp SV has eradicated in vitro human ovarian (OVCAR3 and IGR-OV1) and bladder (SCaBER) cell lines in a concentration-dependent manner (Figure 2). On the other hand, high concentrations of the Rp venom did not exhibit cytotoxicity on dermal fibroblasts or normal ARPE-19 cell line.
Figure 2.
Survival curves of three human cancer cell lines; bladder squamous cell carcinoma cell line (SCaBER, obtained from ATCC), and two ovarian cancer cell lines, OVCAR-3 and IGR-OV1 (obtained from NCI), following a 24 hr incubation with increasing volumes of polarized Rhopalurus princeps venom (Rp). Cell were plated on 24-well plates (5.0 × 104 cells/well) in a volume of 500 ul medium per well and incubated for 24-hr. Then, the cells were treated with the indicated volumes of the polarized Rp for an additional day. Cell viability was determined by the MTT colorimetric assay. These set of experiments were repeated twice.
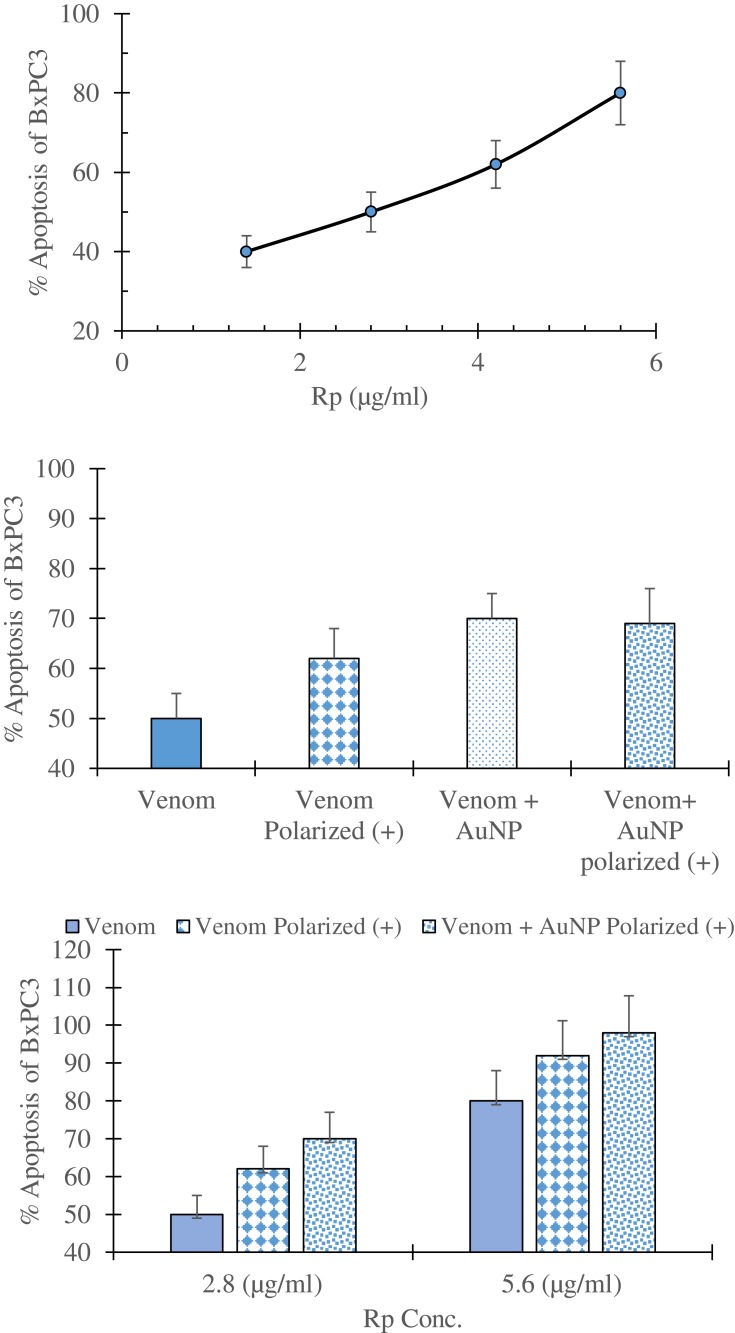
In apoptosis assay, Rp venom induced apoptosis in the BxPC3 cell line at very low concentrations (Figure 3A). Furthermore, the polarization of Rp venom significantly enhanced the incidence of apoptosis in BxPC3 (Figure 3B and C). However, adding AuNP, a delivery agent, to nonpolarized Rp venom increased the apoptotic activity but did not significantly change the percent apoptosis to the polarized venom (Figure 3B and C), indicating that polarization of SV has a similar impact to AuNP on Rp venom-inducing apoptosis. These results suggest that anticancer activity of Rp venom is mainly by inducing apoptosis rather than inhibiting metabolic pathways of cancer cells64 and secondly polarization of Rp venom induces higher apoptotic effects on cancer cells.
Figure 3.
Percent induced apoptosis in human prostate cancer cell line, BxPC3 (obtained from ATCC), following a 24-hr incubation with (A) increasing concentrations of Rhopalurus princeps venom (Rp); (B) with a single concentration (2.8 µg/mL) of Rp venom, polarized Rp, Rp+AuNP, Rp+AuNP polarized; and (C) with two different Rp concentrations that are: Rp venom, polarized Rp or Rp+AuNP polarized. Venom was conjugated with 50-nm AuNP (Cytodiagnostics, Burlington, Ontario, Canada) and then polarized. Apoptosis was measured using the EMD Millipore ApopTag plus peroxide in situ Apoptosis detection kit (Danvers, Mass.). These set of experiments were repeated twice.
Structural Analysis of Rp Venom and in silico Studies
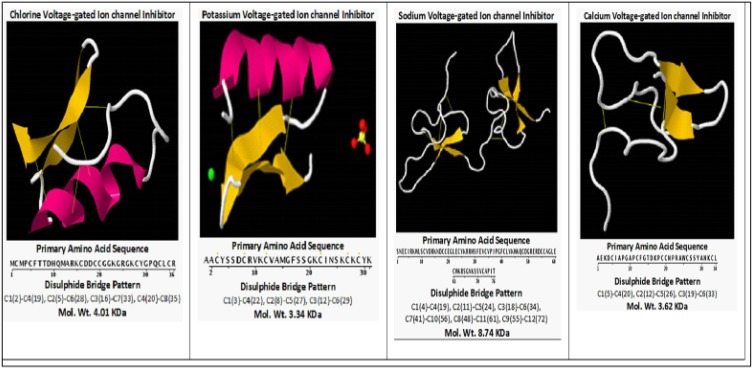
Several studies have been performed to characterize Rp venom. Mass Spectrometry Analysis (MALDI-TOF) revealed 176 molecular mass fragments ranging from ~500–32,000 Da (Table 3). Following BLAST analysis, one of the peptides in Rp venom, termed (Rp-ClTx), resembles in sequence and amino acid length the chlorotoxin peptide from Leiurus quinquestriatus hebraeus – the Death Stalker scorpion (Lqq-CITx) and others (Table 4).65–67 Rp-ClTx shares the highest sequence identity (97%) with Lqq-CITx. The in silico study revealed that Rp-CITX has 4-stranded antiparallel beta-sheets (residues 22–24; 34–36; 59–62; and 71–74) with four disulfide bridges, C1(2)-C4(19), C2(5)-C6(28), C3(16)-C7(33), and C4(20)-C8(35), and are cross-linking the alpha-helix to the beta-sheets.68 The fourth disulfide bridge (C1(2)-C4(19) links the small N-terminal beta-strand to the rest of the molecule (Figure 4).
Table 3.
MALDI-TOF Analysis of Rhopalurus princeps Venom
| Mass Range (m/z) | Number of Fragments | Daltons |
|---|---|---|
| 480–5000 | 30 | 494.5–4247.6 |
| 600–25,000 | 75 | 819.3–17,596.8 |
| 800–60,000 | 71 | 1979.1–31,840.8 |
Table 4.
Sequence Similarities of Rp-ClTx with Other Chlorotoxin-Like Peptides
| Name* | Size | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bs14 | C | G | P | C | F | T | K | D | P | E | T | E | K | K | C | A | E | C | C | G | G | I | G | R | – | – | C | F | G | P | Q | C | L | C | N | R | G | Y | 36 |
| Lqq-CITX** | C | M | P | C | F | T | T | D | H | Q | M | A | R | K | C | D | D | C | C | G | G | K | G | R | G | K | C | Y | G | P | Q | C | L | C | R | 36 | |||
| Rp-CITX** | C | M | P | C | F | T | T | D | H | Q | M | A | R | K | C | D | D | C | C | G | G | L | G | R | G | K | C | Y | G | P | Q | C | L | C | R | 36 | |||
| AmmP2 | C | G | P | C | F | T | T | D | P | Y | T | E | S | K | C | A | T | C | C | G | G | R | G | K | – | – | C | V | G | P | Q | C | L | C | N | R | I | 35 | |
| BmKCL1 | C | G | P | C | F | T | T | D | A | N | M | A | R | K | C | R | E | C | C | G | G | I | G | K | – | – | C | F | G | P | Q | C | L | C | N | R | I | 35 | |
| CITx-b | C | G | F | C | F | T | T | D | H | Q | T | E | Q | K | C | A | E | C | C | G | G | I | G | K | – | – | C | Y | G | P | Q | C | L | C | – | R | G | - | 34 |
| CITx-c | C | G | F | C | F | T | T | D | R | Q | M | E | Q | K | C | A | E | C | C | G | G | I | G | K | – | – | C | Y | G | P | Q | C | L | C | – | R | G | 34 | |
| CITx-d | C | G | P | C | F | T | T | D | H | Q | T | E | Q | K | C | A | E | C | C | G | G | I | G | K | – | – | C | Y | G | P | Q | C | L | C | – | - | - | - | 32 |
| Put-1 | E | K | D | C | I | A | P | G | A | P | C | F | G | T | D | K | P | C | C | N | P | R | A | W | C | S | S | Y | A | N | K | C | L | 34 |
Notes: *The bold C (Cys amino acids) residues are present in 8 locations of all tested chlorotoxin-like peptides, except for Put-1, which form the 4-disulfide bridges. **The highlighted red amino acid sequence shows the similarity of Rp-ClTX to Lqq-ClTX, which accounts for 97%.
Figure 4.
Three dimensional structures of chloride, calcium, potassium and sodium ion channel inhibitors present in Rhopalurus princeps venom (Rp) showing the α-helix, β-sheets and the disulphide bridges in each inhibitor.
Furthermore, other ion channel peptides inhibitors have been found in the Rp venom. The calcium voltage-gated peptide inhibitor consists of 34 amino acids, including six cysteines and three disulfide bonds.69 This inhibitor belongs to the inhibitory cysteine knot structural family that includes a compact disulfide-bonded core from which four loops emerge. The disulfide bonds are as follows: C1(5)-C4(20), C2(12)-C5(26), C3(19)-C6(33). This peptide contains a 2-stranded antiparallel beta-sheets (residues 26–27 and 32–33) as the only secondary structures. Similarly, the potassium channel peptide inhibitor has 31 amino acids in including six cysteines and three disulfide bonds (Figure 4). The structure consists of a double-stranded antiparallel β-sheet (sequences 19–23 and 26–30) and a single helix (sequences 4–15) covering one face of the α-sheet.68 The cysteine side chains connecting the β-sheet and the helix form the core of the molecule. The disulfide bonds are as follows: C1(3)-C4(22), C2(8)-C5(27), C3(12)-C6(29) (Figure 4). Finally, sodium channel peptide inhibitor is also present in Rp venom and consists of 76 amino acids in including twelve cysteines and six disulfide bonds.70 The six disulfide bonds are as follows: C1(4)-C4(19), C2(11)-C5(24), C3(18)-C6(34), C7(41)-C10(56), C8(48)-C11(61), C9(55)-C12(72). This peptide inhibitor contains 4-stranded antiparallel beta-sheets (residues 22–24; 34–36; 59–62; and 71–74) as the only secondary structures.
Concluding Remarks
In the present review, we show the pleiotropic effect of SV and their peptides in a) inhibiting the proliferative characteristics of cancer cells by mainly inducing apoptosis without affecting healthy cells such as lymphocytes proliferative capabilities, b) interrupting the invasion and metastatic capabilities of cancer cells, c) inhibiting angiogenesis and d) modulating the immune cell function by lowering the inflammatory cascade that occurs in some cancer types. Thus, these encouraging results signify the potential of SV peptides in targeting multiple events in cancer cells development and metastasis.
However, it has to be addressed that the concentrations used to show an inhibitory effect for some whole SV were high (IC50 > 500 µg/mL), whereas the IC50 concentrations significantly became low when specific peptides isolated, or recombinant type were used. Interestingly, some recombinant peptides became more anti-proliferatively potent when more cationic amino acids were introduced in the peptide (Table 1).71–73 The latter phenomenon could be initiated by increasing the electrostatic interaction between cationic peptides and the negative membrane charges of tumor cells. The high negative charge of tumor cells is mainly due to the presence of phosphatidylserine, a negatively charged amino acid, on the outer surface of the plasma membrane of tumor cells. Similarly, the polarization of Rp venom showed better anticancer potential in increasing the apoptosis in cancer cells.
A recent review by Marshall & Djamgoz5 pointed out several novel immunotherapy co-targets in pre-clinical development, and one of these targets is ion channels. Since ion channels dysregulation are a factor in cancer development, targeting them would be a unique tool multi-faceted advantages for T cell-based immunotherapy.5 Therefore, we believe that the immunomodulatory effects of SV and their specific peptides would benefit the immune-oncology research programs mainly with the checkpoint inhibitors such as PD-1 and CTLA-4 blockade in reducing tumor microenvironment immunosuppression, immune-checkpoint resistance and enhancing cancer patient response.
Before clinical efficacy is established, non-clinical safety is an evident marker of the success of any pharmaceutical agent in translation medicine. Synthetic ClTx peptide (tozuleristide, or BLZ-100) has shown to be safe and has not exhibit any adverse events in rats and non-human primates following a single intravenous dose of 28 mg/kg and 20 mg/kg, respectively.74 Furthermore, in Phase 1 clinical trial, no adverse event was observed following an intravenous dose of up to 30 mg.75 These targeted and promising results of SV open the venue for full non-clinical safety profiles to be performed before clinical translation into cancer therapy.
Disclosure
AGM is the Chief Executive Officer of Medolife Corporation and owns a patent on SV polarization. ET and XMZ are scientists working at Medolife Corporation. KZM is employed by OncoTherapeutica, Inc. The authors report no other conflicts of interest in this work.
References
- 1.Cancer Research UK. Available from: https://www.cancerresearchuk.org/. Assessed 14 February, 2020.
- 2.Balwit JM, Hwu P, Urba WJ, Marincola FM. The iSBTc/SITC primer on tumor immunology and biological therapy of cancer: a summary of the 2010 program. J Transl Med. 2011;9:18. doi: 10.1186/1479-5876-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alshaker HA, Matalka KZ. IFN-γ, IL-17 and TGF-β involvement in shaping the tumor microenvironment: the significance of modulating such cytokines in treating malignant solid tumors. Cancer Cell Int. 2011;11:33. doi: 10.1186/1475-2867-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litan A, Langhans SA. Cancer as a channelopathy: ion channels and pumps in tumor development and progression. Front Cell Neurosci. 2015;9:86. doi: 10.3389/fncel.2015.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall HT, Djamgoz MBA. Immuno-oncology: emerging targets and combination therapies. Front Oncol. 2018;8:315. doi: 10.3389/fonc.2018.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poornima P, Kumar JD, Zhao Q, Blunder M, Efferth T. Net-work pharmacology of cancer: from understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Pharmacol Res. 2016;111:290–302. doi: 10.1016/j.phrs.2016.06.018 [DOI] [PubMed] [Google Scholar]
- 7.Mcferrin MB, Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006;2(1):39–49. doi: 10.1017/S1740925X06000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sontheimer H. An unexpected role for ion channels in brain tumor metastasis. Exp Biol Med. 2008;233:779–791. doi: 10.3181/0711-MR-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bustin SA, Li SR, Dorudi S. Expression of the Ca2C-activated chloride channel genes CLCA1 and CLCA2 is downregulated in human colorectal cancer. DNA Cell Biol. 2001;20:331–338. doi: 10.1089/10445490152122442 [DOI] [PubMed] [Google Scholar]
- 10.Ullrich N, Sontheimer H. Cell cycle-dependent expression of a glioma-specific chloride current: proposed link to cytoskeletal changes. Am J Physiol. 1997;273:C1290–C1297. doi: 10.1152/ajpcell.1997.273.4.C1290 [DOI] [PubMed] [Google Scholar]
- 11.Turner KL, Sontheimer H. Cl− and K+ channels and their role in primary brain tumour biology. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130095. doi: 10.1098/rstb.2013.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YF, Hu J, Zhang JH, Wang SL, Wu CF. Isolation purification, and N terminal partial sequence of an anti-tumor-analgesic peptide from the venom of the Chinese scorpion Buthus martensii Karsch. Prep Biochem Biotechnol. 2002;32:317–327. doi: 10.1081/PB-120015456 [DOI] [PubMed] [Google Scholar]
- 13.Northcott PA, Dubuc AM, Pfister S, Taylor MD. Molecular subgroups of medulloblastoma. Expert Rev Neurother. 2012;12:871–884. doi: 10.1586/ern.12.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comes N, Bielanska J, Vallejo-Gracia A, et al. The voltage-dependent K.C/channels KV1.3 and KV1.5 in human cancer. Front Physiol. 2013;4:283. doi: 10.3389/fphys.2013.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comes N, Serrano-Albarrás A, Capera J, et al. Involvement of potassium channels in the progression of cancer to a more malignant phenotype. Biochim Biophys Acta. 2015;1848(10 Pt B):2477–2492. doi: 10.1016/j.bbamem.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 16.Chioni AM, Shao D, Grose R, Djamgoz MB. Protein kinase A and regulation of neonatal NaV1.5 expression in human breast cancer cells: activity-dependent positive feedback and cellular migration. Int J Biochem Cell Biol. 2010;42:346–358. doi: 10.1016/j.biocel.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 17.Brisson L, Driffort V, Benoist L, et al. NaV1.5 NaC channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J Cell Sci. 2013;126:4835–4842. doi: 10.1242/jcs.123901 [DOI] [PubMed] [Google Scholar]
- 18.Driffort V, Gillet L, Bon E, et al. Ranolazine inhibits NaV1.5-mediated breast cancer cell invasiveness and lung colonization. Mol Cancer. 2014;13:264. doi: 10.1186/1476-4598-13-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing D, Wang J, Ou S, et al. Expression of neonatal NaV1.5 in human brain astrocytoma and its effect on proliferation, invasion and apoptosis of astrocytoma cells. Oncol Rep. 2014;31:2692–2700. doi: 10.3892/or.2014.3143 [DOI] [PubMed] [Google Scholar]
- 20.Fraser SP, Ozerlat-Gunduz I, Brackenbury WJ, et al. Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130105. doi: 10.1098/rstb.2013.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aydar E, Yeo S, Djamgoz M, Palmer C. Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: a potential target for breast cancer diagnosis and therapy. Cancer Cell Int. 2009;9:23. doi: 10.1186/1475-2867-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding X, He Z, Zhou K, et al. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J Natl Cancer Inst. 2010;102:1052–1068. doi: 10.1093/jnci/djq217 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Wang H, Qian Z, et al. Low voltage- activated T-type Ca2C channel inhibitors as new tools in the treatment of glioblastoma: the role of endostatin. Pflugers Arch. 2014;466:811–818. doi: 10.1007/s00424-013-1427-5 [DOI] [PubMed] [Google Scholar]
- 24.Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, Valdivia HH, Possani LD. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328–342. doi: 10.1016/j.toxicon.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Díaz-García A, Morier-Díaz L, Frión-Herrera Y, et al. In vitro anticancer effect of venom from cuban scorpion Rhopalurus junceus against a panel of human cancer cell lines. J Venom Res. 2013;4:5–12. [PMC free article] [PubMed] [Google Scholar]
- 26.Zargan J, Umar S, Sajad M, et al. Scorpion venom (Odontobuthus doriae) induces apoptosis by depolarization of mitochondria and reduces S-phase population in human breast cancer cells (MCF-7). Toxicol in Vitro. 2011;25(8):1748–1756. doi: 10.1016/j.tiv.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 27.Zargan J, Mir S, Umar S, et al. Scorpion (Odontobuthus doriae) venom induces apoptosis and inhibits DNA synthesis in human neuroblastoma cells. Mol Cell Biochem. 2010;348(1–2):173–181. doi: 10.1007/s11010-010-0652-x [DOI] [PubMed] [Google Scholar]
- 28.Zang YY, Wu LC, Wang ZP, et al. Anti-proliferation effect of polypeptide extracted from scorpion venom on human prostate cancer cells in vitro. J Clin Med Res. 2009;1(1):24–31. doi: 10.4021/jocmr2009.01.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Asmari AK, Riyasdeen A, Abbasmanthiri R, Arshaduddin M, Al-Harthi FA. Scorpion (Androctonus bicolor) venom exhibits cytotoxicity and induces cell cycle arrest and apoptosis in breast and colorectal cancer cell lines. Indian J Pharmacol. 2016;48(5):537–543. doi: 10.4103/0253-7613.190742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Asmari AK, Riyasdeen A, Islam M. Scorpion venom causes upregulation of p53 and downregulation of Bcl-xL and BID protein expression by modulating signaling proteins Erk1/2 and STAT3, and DNA damage in breast and colorectal cancer cell lines. Integr Cancer Ther. 2018;17(2):271–281. doi: 10.1177/1534735417704949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Asmari AK, Islam M, Al-Zahrani AM. In vitro analysis of the anticancer properties of scorpion venom in colorectal and breast cancer cell lines. Oncol Lett. 2016;11(2):1256–1262. doi: 10.3892/ol.2015.4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta SD, Gomes A, Debnath A, Saha A, Gomes A. Apoptosis induction in human leukemic cells by a novel protein Bengalin, isolated from Indian black scorpion venom: through mitochondrial pathway and inhibition of heat shock proteins. Chem Biol Interact. 2010;183:293–303. doi: 10.1016/j.cbi.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 33.D’Suze G, Rosales A, Salazar V, Sevcik C. Apoptogenic peptides from Tityus discrepans scorpion venom acting against the SKBR3 breast cancer cell line. Toxicon. 2010;56(8):1497–1505. doi: 10.1016/j.toxicon.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 34.Al-Asmari AK, Riyasdeen A, Islam M. Scorpion venom causes apoptosis by increasing reactive oxygen species and cell cycle arrest in MDA-MB-231 and HCT-8 cancer cell lines. J Evid Based Integr Med. 2018;23:2156587217751796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zargan J, Sajad M, Umar S, et al. Scorpion (Androctonus crassicauda) venom limits growth of transformed cells (SH-SY5Y and MCF-7) by cytotoxicity and cell cycle arrest. Exp Mol Pathol. 2011;91(1):447–454. doi: 10.1016/j.yexmp.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 36.Li B, Lyu P, Xi X, et al. Triggering of cancer cell cycle arrest by a novel scorpion venom-derived peptide-Gonearrestide. J Cell Mol Med. 2018;22(9):4460–4473. doi: 10.1111/jcmm.2018.22.issue-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salem ML, Shoukry NM, Teleb WK, Abdel-Daim MM, Abdel-Rahman MA. In vitro and in vivo antitumor effects of the Egyptian scorpion Androctonus amoreuxi venom in an Ehrlich ascites tumor model. Springer Plus. 2016;5:570. doi: 10.1186/s40064-016-2269-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Asmari AK, Khan AQ. Investigation of in vivo potential of scorpion venom against skin tumorigenesis in mice via targeting markers associated with cancer development. Drug Des Devel Ther. 2016;10:3387–3397. doi: 10.2147/DDDT.S113171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Chang Y, Reinhart PH, Sontheimer H, Chang Y. Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J Neurosci. 2002;22:1840–1849. doi: 10.1523/JNEUROSCI.22-05-01840.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bloch M, Ousingsawat J, Simon R, et al. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene. 2007;26:2525–2534. doi: 10.1038/sj.onc.1210036 [DOI] [PubMed] [Google Scholar]
- 41.Oeggerli M, Tian Y, Ruiz C, et al. Role of KCNMA1 in breast cancer. PLoS One. 2012;7:e41664. doi: 10.1371/journal.pone.0041664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramírez A, Vera E, Gamboa-Domínguez A, Lambert P, Gariglio P, Camacho J. Calcium-activated potassium channels as potential early markers of human cervical cancer. Oncol Lett. 2018;15(5):7249–7254. doi: 10.3892/ol.2018.8187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han X, Wang F, Yao W, et al. Heat shock proteins and p53 play a critical role in K+ channel-mediated tumor cell proliferation and apoptosis. Apoptosis. 2007;12(10):1837–1846. doi: 10.1007/s10495-007-0101-9 [DOI] [PubMed] [Google Scholar]
- 44.Song X, Zhang G, Sun A, et al. Scorpion venom component III inhibits cell proliferation by modulating NF-kappaB activation in human leukemia cells. Exp Ther Med. 2012;4(1):146–150. doi: 10.3892/etm.2012.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao J, Kang N, Liu Y, Song S, Wu C, Zhang J. Purification and characterization of an analgesic peptide from Buthus martensii Karsch. Biomed Chromatogr. 2007;21:1266–1271. doi: 10.1002/(ISSN)1099-0801 [DOI] [PubMed] [Google Scholar]
- 46.Ma R, Cui Y, Zhou Y, et al. Location of the analgesic domain of scorpion toxin BmK AGAP by mutagenesis of disulfide bridges. Biochem Biophys Res Commun. 2010;394:330–334. doi: 10.1016/j.bbrc.2010.02.179 [DOI] [PubMed] [Google Scholar]
- 47.Kampo S, Ahmmed B, Zhou T, et al. Scorpion venom analgesic peptide, BmK AGAP inhibits stemness, and epithelial-mesenchymal transition by down-regulating PTX3 in breast cancer. Front Oncol. 2019;9:21. doi: 10.3389/fonc.2019.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels. 2012;6:352–361. doi: 10.4161/chan.21910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ullrich N, Sontheimer H. Biophysical and pharmacological characterization of chloride currents in human astrocytoma cells. Am J Physiol. 1996;270:C1511–C1521. doi: 10.1152/ajpcell.1996.270.5.C1511 [DOI] [PubMed] [Google Scholar]
- 50.Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998;58:4871–4879. [PubMed] [Google Scholar]
- 51.Soroceanu L, Manning TJ Jr, Sontheimer H. Modulation of glioma cell migration and invasion using Cl(-) and K(+) ion channel blockers. J Neurosci. 1999;19:5942–5954. doi: 10.1523/JNEUROSCI.19-14-05942.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyons SA, O’Neal J, Sontheimer H. Chlorotoxin, a scorpion derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39:162–173. doi: 10.1002/(ISSN)1098-1136 [DOI] [PubMed] [Google Scholar]
- 53.Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278(6):4135–4144. doi: 10.1074/jbc.M205662200 [DOI] [PubMed] [Google Scholar]
- 54.Jacoby DB, Dyskin E, Yalcin M, et al. Potent pleiotropic anti-angiogenic effects of TM601, a synthetic chlorotoxin peptide. Anticancer Res. 2010;30(1):39–46. [PubMed] [Google Scholar]
- 55.Sawaya RE, Yamamoto M, Gokaslan ZL, et al. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis. 1996;14:35–42. doi: 10.1007/BF00157684 [DOI] [PubMed] [Google Scholar]
- 56.Dardevet L, Rani D, Aziz TA, et al. Chlorotoxin: a helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins (Basel). 2015;7(4):1079–1101. doi: 10.3390/toxins7041079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mamelak AN, Rosenfeld S, Bucholz R, et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J Clin Oncol. 2006;24(22):3644–3650. doi: 10.1200/JCO.2005.05.4569 [DOI] [PubMed] [Google Scholar]
- 58.Wu X S1, Jian XC, Yin B, He ZJ. Development of the research on the application of chlorotoxin in imaging diagnostics and targeted therapies for tumors. Chin J Cancer. 2010;29(6):626–630. doi: 10.5732/cjc.009.10359 [DOI] [PubMed] [Google Scholar]
- 59.Fu YJ, Yin LT, Liang AH, et al. Therapeutic potential of chlorotoxin like neurotoxin from the Chinese scorpion for human gliomas. Neurosci Lett. 2007;412:62–67. doi: 10.1016/j.neulet.2006.10.056 [DOI] [PubMed] [Google Scholar]
- 60.Fu YJ, An N, Chan KG, et al. A model of BmK CT in inhibiting glioma cell migration via matrix metalloproteinase 2 from experimental and molecular dynamics simulation study. Biotechnol Lett. 2011;33:1309–1317. doi: 10.1007/s10529-011-0587-7 [DOI] [PubMed] [Google Scholar]
- 61.Jang SH, Cho ISY, Ryu PD, Lee SY. Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur J Pharmacol. 2011;651:26–32. doi: 10.1016/j.ejphar.2010.10.066 [DOI] [PubMed] [Google Scholar]
- 62.Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annu Rev Immunol. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mikaelian A. Polarized scorpion venom solution and method for making polarized venom solution. US 2009/0123558 A1 and US8097284B2. 2009 14 May and 2012 17 Jan.
- 64.Frankfurt OS, Krishan A. Apoptosis-based drug screening and detection of selective toxicity to cancer cells. Anticancer Drugs. 2003;14(7):555–561. doi: 10.1097/00001813-200308000-00008 [DOI] [PubMed] [Google Scholar]
- 65.DeBin JA, Maggio JE, Strichartz GR. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Physiol. 1993;264:361–369. doi: 10.1152/ajpcell.1993.264.2.C361 [DOI] [PubMed] [Google Scholar]
- 66.Goudet C, Chi CW, Tytgat J. An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon. 2002;40:1239–1258. doi: 10.1016/S0041-0101(02)00142-3 [DOI] [PubMed] [Google Scholar]
- 67.Ali SA, Alam M, Abbasi A, et al. Structure-activity relationship of chlorotoxin-like peptides. Toxins (Basel). 2016;8(2):36. doi: 10.3390/toxins8020036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Possani LD, Merino E, Corona M, Bolivar F, Becerril B. Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie. 2000;82:861–868. doi: 10.1016/S0300-9084(00)01167-6 [DOI] [PubMed] [Google Scholar]
- 69.Valdivia HH, Kirby MS, Lederer WJ, Coronado R. Scorpion toxins targeted against the sarcoplasmic reticulum Ca+2-release channel of skeletal and cardiac muscle. Proc Natl Acad Sci USA. 1992;89:12185–12189. doi: 10.1073/pnas.89.24.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Possani LD, Becerril B, Delepierre M, Tytgat J. Scorpion toxins specific for Na+-channels. Eur J Biochem. 1999;264:287–300. doi: 10.1046/j.1432-1327.1999.00625.x [DOI] [PubMed] [Google Scholar]
- 71.Du Q, Hou X, Ge L, et al. Cationicity-enhanced analogues of the antimicrobial peptides, AcrAP1 and AcrAP2, from the venom of the scorpion, Androctonus crassicauda, display potent growth modulation effects on human cancer cell lines. Int J Biol Sci. 2014;10:1097–1107. doi: 10.7150/ijbs.9859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo X, Ma C, Du Q, et al. Two peptides, TsAP-1 and TsAP-2, from the venom of the Brazilian yellow scorpion, Tityus serrulatus: evaluation of their antimicrobial and anticancer activities. Biochimie. 2013;95(9):1784. doi: 10.1016/j.biochi.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 73.Pedron CN, de Oliveira CS, da Silva AF, et al. The effect of lysine substitutions in the biological activities of the scorpion venom peptide VmCT1. Eur J Pharm Sci. 2019;136:104952. doi: 10.1016/j.ejps.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 74.Parrish-Novak J, Byrnes-Blake K, Lalayeva N, et al. Nonclinical profile of BLZ-100, a tumor-targeting fluorescent imaging agent. Int J Toxicol. 2017;36(2):104–112. doi: 10.1177/1091581817697685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patil CG, Walker DG, Miller DM, et al. Phase 1 safety, pharmacokinetics, and fluorescence imaging study of Tozuleristide (BLZ-100) in adults with newly diagnosed or recurrent gliomas. Neurosurgery. 2019:nyz125. doi: 10.1093/neuros/nyz125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cancer Research UK. Available from: https://www.cancerresearchuk.org/. Assessed 14 February, 2020.